Abstract
Iron is a widely used metal due to its low cost and availability, but it is susceptible to corrosion in many circumstances. This corrosion can result in economic and environmental losses, and negatively affect the physical and chemical properties of the metal. This chapter provides a background on iron corrosion in archaeology and introduces various inhibitors used for its protection. It starts with a general overview of corrosion and metallurgy of iron, followed by an in-depth explanation of the mechanisms of iron corrosion in water and air. The chapter concludes with a review of different corrosion inhibitors, focusing on those made from natural plant extracts.
1. Introduction
Corrosion is a major issue in industry, causing the loss of 100 million tons of steel per year, which is nearly 15% of the world’s production. It is a complex phenomenon that depends on several factors such as the environment, composition of the metal, and its properties. Corrosion results in direct costs such as replacement of corroded structures and labor, and indirect costs such as production losses, efficiency loss, product contamination, and more, which add up to billions of dollars in losses annually. Due to the long-term nature of corrosion, it is often underestimated in the design of equipment and structures, leading to further losses [1,2].
Corrosion is an important issue to consider as it can cause significant damage to historical and cultural artifacts. The process of corrosion can cause a loss of important information and details about the artifact, and can also change its appearance. This can make it difficult for archaeologists and historians to understand and preserve the history of a particular site or artifact, making it an important issue to address [3].
Corrosion is a slow process that affects the structural safety of various industries, including transportation, chemical, petrochemical, and construction. The rate of corrosion depends on the environment, the metal’s composition, and its properties. It is often underestimated in design and results in direct costs such as replacement and labor, as well as indirect costs including production loss and efficiency loss. In heritage conservation, corrosion leads to the disappearance of historical artifacts and alters archaeological history [4,5].
2. History of Iron and Metallurgy
Iron is a very abundant metal on the earth’s crust, mostly in the form of oxides. The metallurgy of iron dates back to 1700–1500 BC in regions south of the Caucasus and spread to Europe via Greece and the Danube valley [6]. Iron working was developed in Europe around 800 BC with the Hallstatt civilization and spread to Switzerland and Gaul [7].
The production of iron was traditionally carried out using the direct method or “blast furnace” process, where iron ore was reduced in the presence of wood, coal, and oxygen. This method was widely used until the beginning of the Middle Age and allowed for the production of iron in a single stage using low furnaces. The resulting iron was used to make various tools, weapons, household items, and construction materials [6].
The use of hydraulic power in the 15th century led to the creation of larger blast furnaces and the ability to produce cast iron. Cast iron was used for a variety of purposes, such as chimney caps, cooking pots, cannonballs, and pipes. The process of producing cast iron dates back to 4th century B.C. in China and was first seen in Europe in the late 14th century.
The use of coke in the blast furnace during the 18th century revolutionized iron production by making it more efficient and cost-effective. The decarburization process further purified the iron, allowing for the production of high-quality cast iron products such as domestic objects, steam engine cylinders, and rails.
The indirect process of steelmaking has had a significant impact on the society by leading to increased production capabilities, especially in the fields of artillery and transportation. This has led to a growth in the number of machine tools and the ability to produce parts in larger series.
The use of iron and steel in architecture and engineering became more prominent in the 19th century, leading to the construction of iconic structures such as the Eiffel Tower, Grand Palais, railway stations, and many others. Iron and steel were used in the form of chain-linkages, tie rods, etc., and played an important role in the construction of these buildings, even though their use was often concealed.
Iron and its alloys (such as steel and cast iron) are widely used in various fields, including metal construction, building, public equipment, and transportation. Iron has been an important metal in heritage collections and historical monuments. Many iron-based objects from archaeological excavations have a layer of corrosion products, such as oxides, which can cause an inhomogeneous surface appearance with gray and oxidized areas. These areas are often fragile and susceptible to environmental conditions [4].
To protect iron against degradation, it is important to use methods such as coatings, cathodic protection, and inhibitors. The optimal method of protection depends on the specific conditions and environment in which the iron will be used [8]. The study of corrosion protection and conservation of iron will be discussed in the following sections.
3. Iron Corrosion
Aqueous corrosion and atmospheric corrosion are two types of corrosion that often affect archaeological iron. Aqueous corrosion is caused by the reaction of iron with water and atmospheric corrosion is caused by the reaction of iron with air. These types of corrosion are important to study in the field of archaeology as they often affect the iron artifacts being studied [9].
3.1. Corrosion of Iron in Aqueous Media
3.1.1. Behavior of Iron in Aqueous Medium
The corrosion of iron in an aqueous environment is due to an irreversible oxidation– reduction reaction between the metal and an oxidizing agent contained in the environment.
The oxidation of the metal involves the reduction of the oxidizing agent.
Metal + oxidizing agent → oxidized metal + reduced agent
In wet corrosion, the two main oxidizers are:
- -
- Solvated protons (H+(aq)).
- -
- Dissolved oxygen (O2).
In the case of iron corrosion, for example, if we consider the overall oxidation-reduction Reaction (3), it can be broken down into a partial oxidation reaction or anodic Reaction (1) and a partial reduction reaction or cathodic Reaction (2):
Fe → Fe2+ + 2e−
2H+ + 2e− → H2
Fe + 2H+ → Fe2+ + H2
The anodic and cathodic partial reactions to be considered for iron corrosion in the presence of water are generally:
Anodic partial reaction (oxidation):
Fe → Fe2+ + 2e−
Cathodic partial reactions (reduction):
- -
- In an aerated environment (aerobic):
O2 + 4H+ + 4e− → 2H2O
O2 + 2H2O + 4e− → 4OH−
- -
- In a deaerated (anaerobic) environment:
2H+ + 2e− → H2
2H2O + 2e− → 2OH− + H2
In the case of corrosion in aqueous media, aeration or de-aeration generally corresponds to the presence or absence of dissolved oxygen [10].
3.1.2. Thermodynamic Approach
The Nernst potential, acidity constants, and solubility products are used to predict the thermodynamics of iron corrosion reactions in aqueous media using Pourbaix diagrams [11]. These diagrams show the stability ranges of the chemical species involved, but do not provide information on the reaction kinetics.
Figure 1 gives an example of an equilibrium diagram established by Descostes [12] based on data from Chivot [13,14]. Considering the following species:
Fe, Fe2+, Fe3+, FeOH+, FeOH2+, Fe(OH)2+, HFeO2−, Fe(OH)2, Fe(OH)3−, Fe(OH)3, Fe(OH)4−
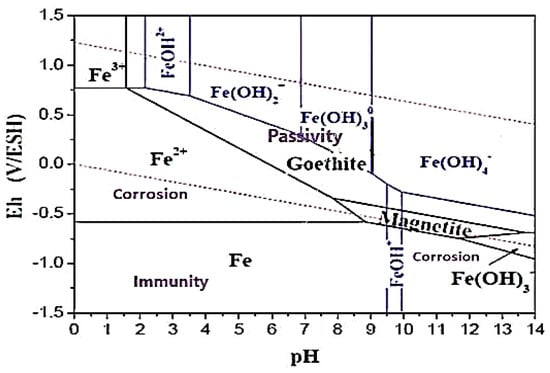
Figure 1.
Pourbaix E-pH diagram for iron/water/oxygen systems ([Fe] total = 10−5 mol·L−1, all oxidized phases of iron are considered).
Thus, we can determine:
- -
- A domain of immunity where the metal (Fe) is thermodynamically stable.
- -
- Corrosion areas where the ions resulting from the dissolution of iron either in acidic media (Fe2+ and Fe3+) or in very basic media (HFeO2−) are responsible for the corrosion.
- -
- Areas of passivation (possible) where the formation of a solid compound in a neutral and basic medium (Fe(OH)2 or Fe(OH)3) can be protective (or not).
The stability range of species can vary based on solid phases considered. Goethite has the broadest thermodynamic stability range, but similar diagrams can be created for different compositions of total iron and complexing ions in solution (carbonates, sulfates, etc.,) [15].
3.1.3. Kinetic Approach
The thermodynamic approach can provide information about the likelihood of corrosion reactions, but it does not give information about the speed of the reaction. The speed of the reaction is primarily determined through electrochemical kinetics, which involves measuring corrosion potential, current, polarization resistance, etc. The rate of corrosion can be estimated based on these measurements and is often expressed as mass loss per unit area [10].
The rate of corrosion reaction depends on the rate of charge transfer and the rate of material transport. The slower of the two stages controls the overall rate of the reaction [16].
Accary et al. [17] were the pioneers in conducting studies on the kinetics of corrosion of ferrous alloys. In their 1983 study, they estimated the average rate of corrosion to be between 0.01 and 10 μm/year, based on the measurements of the thickness of corrosion on a collection of 20 ferrous objects. Another study by Soerensen et al. [18], determined the average rate of corrosion by measuring the ratio of the surface area of the corrosion products to that of the metal using X-rays. This study found average corrosion rates ranging from 0.025 to 1.2 μm/year.
Johnson and Francis [19] conducted a study on the global estimate of the corrosion rate of archaeological objects. They found that the corrosion rate can range from a minimum of 0.1 mm per millennium to a maximum of 10 mm per year, depending on the burial conditions. However, the burial conditions are often not well understood, which leads to a wide range of possible corrosion rates [5].
3.1.4. Iron Corrosion Products in Aqueous Media
The layer of corrosion products formed on metal surfaces consists of multiple compounds, the composition of which is dependent on the pH of the environment, the composition of the medium, the concentration and transport conditions of oxygen, and other chemical species present such as chloride, carbonate, sulfate, etc. Factors such as convection and diffusion also play a role in determining the nature of the corrosion products [20].
In the case of corrosion in an aqueous environment with oxygen present, followed by corrosion in a deaerated (anaerobic) environment due to oxygen depletion (for example in storage), we can consider the following hypothetical equilibria between iron and its oxides or hydroxides. Iron/oxide equilibria are stable, while iron/hydroxide equilibria are unstable [10].
Oxidation of iron in the presence of oxygen, aerobic corrosion:
4Fe + 3O2 → 2Fe2O3
4Fe + 6 H2O + 3O2 → 4Fe(OH)3
2Fe + H2O + 3/2O2 → 2FeO(OH)
Reduction of the oxide or hydroxide surface:
4Fe2O3 + Fe → 3Fe3O4
2Fe(OH)3 + 4Fe + 2H2O → 2Fe3O4 + 5H2
After consumption of oxygen, anaerobic corrosion:
3Fe + 4H2O → Fe3O4 + 4H2
Fe + 2H2O → Fe(OH)2 + H2
3Fe(OH)2 → Fe3O4 + 2H2O + H2
Based on these reactions, Misawa et al. and Cornell and Schwertmann [21,22] conducted studies on the formation of compounds resulting from oxidation. These studies established a relatively complex reaction scheme that governs the appearance and evolution of species in aqueous solution, starting from the formation of ferrous ions (Fe2+) (Figure 2):
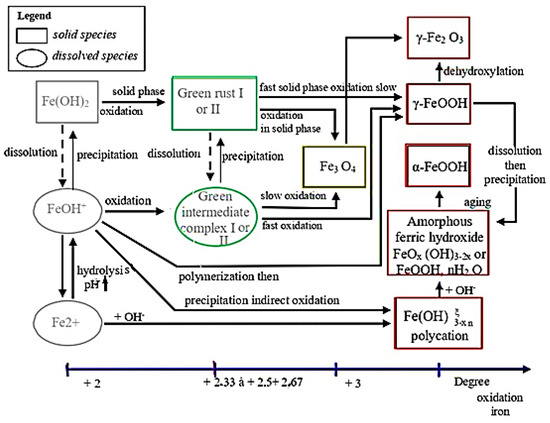
Figure 2.
Reaction scheme governing the appearance and evolution of species in aqueous solution during the iron oxidation process.
For this study, Misawa et al. and Cornell and Schwertmann found that the presence of ferrous ions in solution is dependent on the oxygen level and pH. When the oxygen level is high and the pH is below 6, ferrous ions remain in solution. However, if the pH is above 6, ferrous hydroxide (Fe(OH)2) precipitates and quickly transforms into Fe(OH)3 due to oxidation and hydrolysis.
The oxidation rate of green rusts (Fe(OH)2) determines the formation of lepidocrocite (γ-FeOOH) or magnetite (Fe3O4). Magnetite is a thermodynamically stable phase, but in the presence of iron in an oxidation state between +II and +III, it can evolve into a similar structured phase, maghemite (γ-Fe2O3), at a higher iron oxidation degree.
γ-FeOOH can transform into various forms including γ-Fe2O3, amorphous ferric oxyhydroxide (FeOx(OH)3−2x), or ferrihydrite (FeOOH, n H2O) through different processes such as dehydroxylation or dissolution–precipitation. These phases have poor crystallization and eventually evolve over time to become the more thermodynamically stable phase, goethite α-FeOOH.
In the long term, Fe(OH)3 can transform into goethite α-FeOOH through loss of water. If the oxygen level is low, ferrous ions can react directly with OH anions to form unstable compounds such as Fe(OH)2 or Fe2(OH)3Cl in the presence of chlorine [15].
3.2. Atmospheric Corrosion of Iron
Metals are not only unstable when in contact with or immersed in corrosive solutions, there is another type of corrosion in contact with air, called atmospheric corrosion. This type of corrosion is due to the degradation of metal objects when exposed to air and its elements such as oxygen, humidity, and pollutants. The formation and disappearance of liquid films on metal surfaces contribute to this corrosion. The rate of atmospheric corrosion varies depending on the environment the metal is exposed to, with industrial environments being the most corrosive, followed by urban and rural environments. The difference in the rate of corrosion is due to the presence of pollutants in the atmosphere [10].
Most studies on atmospheric corrosion do not distinguish between outdoor and indoor atmospheric environments. Both environments can cause variations in relative humidity leading to moisture cycling on metal surfaces and result in corrosion. Outdoor atmospheric environments can also result in electrolyte run-off and the presence of a surface water film, while indoor atmospheric corrosion occurs under shelter. Nevertheless, the influence of relative humidity and moisture cycling remains significant in both environments [15].
3.2.1. Mechanisms of Atmospheric Corrosion of Iron
When iron is exposed to moist air, it reacts with the oxygen in the air and forms iron oxide (rust) through a process called atmospheric corrosion. This process is an electrochemical reaction, where electrons are transferred between the iron and oxygen atoms in the air. This reaction forms a protective layer of iron oxide on the surface, which helps prevent further corrosion. The accompanying Figure 3 provides a diagram of the atmospheric corrosion process, including the various steps involved in the process and the specific chemical reactions that take place.
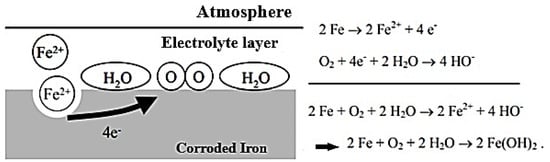
Figure 3.
Diagram of the atmospheric corrosion process at the surface with the reaction balance involved.
The early studies in the 1930s showed that atmospheric corrosion of metals cannot be described by a simple oxidation reaction and that more complex mechanisms are involved. Researchers subsequently highlighted the role of moisture cycling in the corrosion process and demonstrated that the kinetics of electrochemical reactions play a crucial role in atmospheric corrosion [23]. This work expanded the understanding of atmospheric corrosion and showed that it is a multi-faceted process that involves a range of chemical, physical, and electrochemical phenomena. These findings have had a lasting impact on the field of corrosion science and continue to inform current research and understanding of atmospheric corrosion.
Schikorr [24] proposed the “acid regeneration” model, which suggests that iron oxides form in areas of acidic pH and that the presence of sulfur dioxide catalyzes the corrosion reactions through the formation of sulfuric acid, which is regenerated during moisture cycling. This model was later expanded upon by Evans and Taylor [25], who proposed the “electrochemical cycling” model based on electrochemical reactions. This model states that the corrosion process is controlled by a humidification–drying cycle and was experimentally verified by Stratmann et al. This work further advanced our understanding of atmospheric corrosion and showed the important role of electrochemical reactions in the process. The “electrochemical cycling” model is widely accepted today as a key mechanism that governs atmospheric corrosion [26,27].
The humidification–drying cycle in atmospheric corrosion (Figure 4) is divided into three stages, each defined by the evolution of the thickness of the electrolyte film on the surface of the metal object. During the first stage, there is a wetting stage where the metal surface is initially wetted by moisture. The second stage is the wet period, during which the electrolyte film has a significant and constant thickness. The third stage is the drying stage, during which the moisture evaporates and the electrolyte film becomes thinner. These stages play an important role in the electrochemical reactions that take place during atmospheric corrosion, and the progression of the cycles is driven by the relative humidity and temperature conditions in the environment.
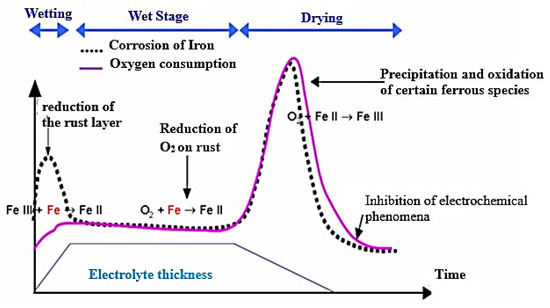
Figure 4.
Stratmann humidification–drying cycle. NB: iron consumption (bold dotted line) oxygen consumption (bold solid line).
Anchor Stage
During the humidification–drying cycle, the formation of an electrolyte film on the metal surface triggers the anodic dissolution of iron, and the electrons produced by the oxidation reaction must be consumed by a reduction reaction. During the wetting phase (Figure 5), a strong dissolution kinetics of iron is observed due to the fact that the rust layer formed is not conductive and therefore oxygen cannot reduce on its surface. This leads to a reduction of the metal surface accessible through a network of tortuous and nanometric pores, which contributes to the overall corrosion process. This highlights the importance of the wetting stage in atmospheric corrosion and the complex interplay between electrochemical reactions, the thickness of the electrolyte film, and the physical and chemical conditions of the environment [3].
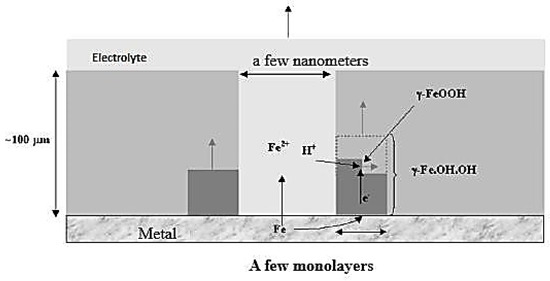
Figure 5.
Representation of the phenomena involved in the wetting phase.
The corrosion product layer itself is responsible for the corrosion, and the anodic dissolution of the metal is balanced by the reduction of a constituent of this layer [28]. Research by Matsushima [15] showed that the reaction of ferric species is dominant in the cathodic reaction on corroded iron in wet corrosion. This finding was later confirmed by Evans and Taylor [25], and Stratmann, [29], further solidifying the importance of the wetting phase in atmospheric corrosion and the role of the corrosion product layer in the electrochemical reactions taking place. This work highlights the complex and multi-faceted nature of atmospheric corrosion and the need for a comprehensive understanding of the various processes involved to effectively prevent and mitigate the effects of corrosion in metal materials.
According to Anthony [30], the phases most likely to reduce during the wetting phase are lepidocrocite, ferrihydrite, and feroxyhyte. However, Monnier stated that lepidocrocite is the most likely phase to reduce and that amorphous phases tend to reduce at lower potentials [31]. As a result, electron consumption during the wetting step may correspond to the reduction of several phases, highlighting the complex nature of the electrochemical reactions taking place during atmospheric corrosion. This work underscores the need for a comprehensive understanding of the various processes involved in atmospheric corrosion, including the role of different phases of corrosion products in the electrochemical reactions and the factors that influence their reduction potential [15].
Wet Stage
In the second stage of the wetting–drying cycle, the electrolyte thickness is assumed to be constant and uniform on the surface of the sample, i.e., on the existing and partially reduced corrosion product layer. Figure 6 shows that the iron and dissolved oxygen consumptions are equal, which indicates that the cathodic reaction is the reduction of dissolved oxygen in the electrolyte and the anodic reaction is the oxidation of the metal substrate. This stage of the humidification–drying cycle highlights the key role that dissolved oxygen plays in atmospheric corrosion, as well as the importance of the corrosion product layer in the overall corrosion process. Understanding the complex interplay between the metal substrate, electrolyte film, and dissolved oxygen is essential to effectively prevent and mitigate the effects of atmospheric corrosion on metal materials [15].
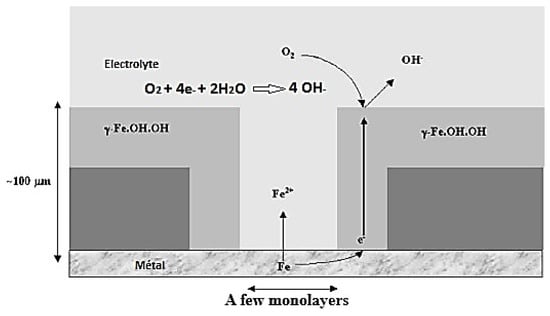
Figure 6.
Representation of the phenomena occurring during the wet phase.
In this stage of the wetting–drying cycle (Figure 6), the oxygen reduction reaction is believed to take place largely on the outer surface of the corrosion product layer, which consists of a conductive reduced phase produced during the wetting step and in contact with the iron meta [31]. This conductive layer allows the electrons produced by the oxidation reaction to be consumed by the reduction reaction, leading to a stable and uniform corrosion rate. On the other hand, the anodic reaction would take place at the bottom of the pores where the metal and the electrolyte are in direct contact, which leads to a decoupling of the anodic and cathodic zones. This separation of the anodic and cathodic zones creates conditions for the formation of local cells, leading to a non-uniform corrosion rate and a more complex overall corrosion process.
Drying Stage
The drying stage of the electrolyte process has two parts: first, as the thickness decreases, reaction speed increases due to improved oxygen diffusion and iron consumption. At a critical electrolyte thickness, there is a sudden drop in iron oxidation and oxygen reduction currents because of water film dislocation [15,25,32]. In the second part of the drying stage, an increase in dissolved species leads to precipitation and surface coverage, resulting in a weak oxygen reduction current and anodic control of the system [28].
During the drying stage (Figure 7), the high concentration of oxygen in the residual electrolyte film causes polarization of the corrosion layer at high potentials, leading to re-oxidation of iron species and the formation of lepidocrocite and goethite [16].
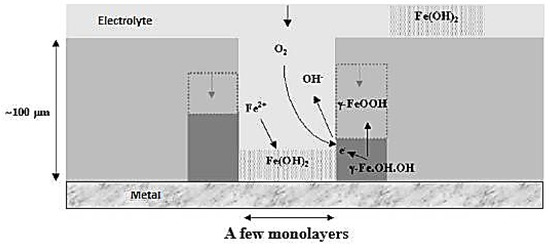
Figure 7.
Representation of the phenomena involved in the drying process.
3.2.2. Products of Atmospheric Corrosion of Iron
Atmospheric corrosion refers to the corrosion of materials caused by the exposure to the atmosphere and its environmental conditions, such as humidity, temperature, presence of pollutants, and pH. The corrosion rate is influenced by the alternation of wet and dry periods, leading to the formation and disappearance of an electrolyte film on the surface. The corrosive power of the atmosphere can be determined by these factors, and the end result is usually a mixture of iron oxides and oxyhydroxides.
Magnetite and maghemite are the two most commonly encountered iron oxides that result from atmospheric corrosion. The corrosion products may also include polymorphic oxyhydroxides of iron, such as goethite (α-FeOOH), lepidocrocite (γ-FeOOH), feroxyhyte (δ-FeOOH), and sometimes akageneite (β-FeOOH) which contains chlorine. In addition, the corrosion layer may also contain ferrihydrite, which is a poorly crystallized and hydrated compound [3]. Table 1 displays the iron oxides and oxyhydroxides present in rust layers that are formed due to atmospheric corrosion.

Table 1.
Iron oxides and oxyhydroxides encountered in rust layers resulting from atmospheric corrosion [15,33,34].
In general, the composition of the rust layer is primarily influenced by atmospheric conditions and the composition of the metal [35]. The rust layer may be relatively homogeneous in some cases [36], but in many cases, it is composed of two parts: an adhesive “sub-layer” containing magnetite and various amorphous oxyhydroxides such as goethite, akageneite, lepidocrocite, and feroxyhyte (δ-FeOOH), and an outer, more porous and crystallized layer containing mainly goethite and lepidocrocite oxyhydroxides [37,38].
Iron (II) and (III) hydroxides can be identified using X-ray diffraction and are unstable in aerated media, undergoing transformation into a green rust-like intermediate before the formation of lepidocrocite or magnetite, which are considered thermodynamically stable phases [39,40,41]. Under atmospheric conditions, the rust layer can reach significant thicknesses (a few millimeters) over a very long period of time (more than 50 years) [16].
The work of J. Monnier [15] has confirmed that lepidocrocite is rarely connected to the metal and that highly reactive phases, such as feroxyhyte and ferrihydrite, are abundant in the corrosion product layers of objects that have undergone long-term corrosion. The reduction and re-oxidation of these phases can result in a mixture of maghemite and magnetite or lead to the reprecipitation of reducible phases such as lepidocrocite, ferrihydrite, and/or feroxyhyte.
4. Protection of Iron by Corrosion Inhibitors
The high cost of annual material degradation has prompted the use of protection methods to save materials and energy, and to meet new requirements, such as the use of non-toxic products.
The protection of materials from corrosive environments can be achieved through various actions at the metallurgical, structural, electrochemical, and environmental levels. Three degrees of action are identified [42]:
- Actions on the material, such as modifying its composition or microstructure or isolating it from its environment through a metallic or organic coating or anodization.
- Actions on the environment, such as incorporating corrosion inhibitors or avoiding moisture accumulation in the structure.
- Actions on the electrochemical corrosion process, such as cathodic protection.
The protection of metal heritage requires consideration of both preservation of surface information and protection against corrosion. Organic coatings, such as varnishes and waxes, can provide protection. Anodic, cathodic, and galvanic protection, as well as the use of inhibitors, are common and effective methods of protection.
4.1. Background
The Romans were aware of corrosion and its effects on metal objects. Pliny the Elder, a Roman naturalist and historian, mentioned in the first century A.D. the use of oil or bitumen for protecting bronze and pitch, gypsum or ceruse for iron from corrosion.
This shows that even in ancient times, people were aware of the need for protection against corrosion and sought methods to preserve their metal objects.
The study of corrosion has a long history, dating back to the 17th century. However, it was not until the 19th century that the means of protecting against corrosion were studied scientifically. The number of references dealing with corrosion inhibitors increased rapidly after 1945, with numerous articles written on the subject in various fields such as aviation, oil refining, and diesel engines. In recent years, there has been a significant increase in works on corrosion inhibition, reflecting technological advancements in the field [43].
4.2. Definition
A corrosion inhibitor is a substance added to a corrosion system to slow down the corrosion rate of a metal without significantly altering the concentration of corrosive agents in the environment. The definition used by the National Association of Corrosion Engineers (NACE) states that an inhibitor is a substance that retards corrosion in low concentration. The international standard ISO 8044 defines an inhibitor as a chemical substance added to the corrosion system in a chosen concentration to decrease the corrosion rate of the metal. The properties of an effective inhibitor include lowering the corrosion rate, stability in the presence of other constituents, stability at temperatures of use, effectiveness at low concentrations, compatibility with non-toxicity standards, and cost-effectiveness [44,45].
4.3. Classification
Inhibitors can be classified based on different criteria such as mechanism of inhibition, application, or chemical nature. This classification helps in better understanding the working of inhibitors and selecting the right inhibitor for a particular corrosion problem. The different classifications of inhibitors provide a comprehensive understanding of the different types of inhibitors and their uses in various corrosion scenarios [46].
The classification of inhibitors (Figure 8) based on their field of application is a useful way to differentiate between inhibitors used in different environments. For example, inhibitors used in acidic media are mainly used to prevent electrochemical attack during pickling processes and in drilling fluids in the oil industry. Inhibitors for neutral media are mainly used to protect cooling circuits. In organic media, a large number of inhibitors are used in engine lubricants and gasoline to protect against corrosion caused by the presence of water and ionic species. This classification helps in selecting the appropriate inhibitor for a specific corrosion problem and ensures maximum protection against corrosion [47].
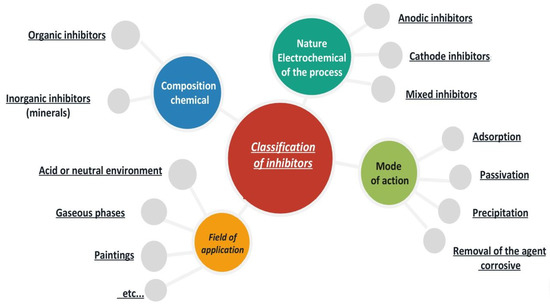
Figure 8.
Classification of corrosion inhibitors.
Inhibitors for paints and gas phases are used to temporarily protect various packaged objects from corrosion during transport and storage. Electrochemically, inhibitors are classified as anodic, cathodic, or mixed inhibitors, depending on their effect on the rate of oxidation and reduction reactions. Anodic inhibitors slow down the oxidation of the metal, cathodic inhibitors slow down the reduction of the oxidant, while mixed inhibitors affect both anodic and cathodic domains. The electrochemical effect of inhibitors on the surface can be explained by various physico-chemical mechanisms. In some cases, the inhibitor forms a physical barrier between the metal and the corrosive medium, as in the case of thick coatings such as waxes and varnishes. In other cases, the inhibitor operates through a pH or redox buffer effect, which can passivate the metal and reduce the corrosion rate. The mode of action of an inhibitor can also involve the formation of surface films due to the precipitation of inorganic salts or poorly soluble organic complexes. These films reduce the accessibility of oxygen to the metal surface and partially block the anodic dissolution [48].
The technique of removing the corrosive agent from the medium is only applicable in closed systems, such as in the closed hot water circuits of thermal power stations. Additionally, it is important to note that many inhibitors act through multiple mechanisms simultaneously, which can increase their effectiveness in preventing corrosion [49].
4.4. Inhibitors Specific to Ferrous Metals in an Acid Medium
Different corrosion inhibitors can effectively protect ferrous metals from corrosion in acidic environments, either individually or in combination. The selection of the appropriate inhibitor depends on various factors such as the type of metal, environment, and specific conditions [50].
4.4.1. Synthetic Inhibitors
Organic inhibitors contain elements such as nitrogen, sulfur, and oxygen that can exchange electrons with the metal and protect it from corrosion. These inhibitors work through a chemical process called spontaneous adsorption and provide good results in inhibiting steel corrosion in acidic medium [51]. Organic inhibitors have several advantages over inorganic inhibitors, as they provide uniform passivation on the metal surface for maximum protection, while inorganic inhibitors form brittle and porous films that can lead to localized corrosion [52]. There is a large body of literature available discussing the use of organic compounds as corrosion inhibitors for ferrous metals in acidic media, as listed in Table 2.

Table 2.
Examples of inhibitors used for the protection of iron and its alloys in acidic media.
The addition of organic compounds to the acid solution generally reduces its aggressiveness, however, these compounds are toxic and harmful to the environment, leading to the need for alternative, eco-friendly, and efficient inhibitors. One such alternative is the use of natural substances, including vegetable oils [58].
4.4.2. Corrosion Inhibitors Based on Natural Substances
The use of natural substances such as vegetable oils is attractive due to their low cost and abundant availability as environmentally friendly and biodegradable compounds. The use of plant extracts as corrosion inhibitors dates back to 1930, with the use of Chelidonium majus and other plants in a pickling bath of H2SO4. The first patent on corrosion inhibition was granted to Baldwin, who used molasses and vegetable oils for pickling steel sheets in acidic media [59].
Currently, many research groups around the world are exploring the use of plant products as corrosion inhibitors for metals and alloys in various corrosive environments [60]. There is an increasing number of publications addressing this topic, as shown in Table 3, which details some of the main green inhibitors for the corrosion of ferrous metals in acidic medium.

Table 3.
Literature review on the use of natural substances as corrosion inhibitors.
5. Conclusions
Corrosion inhibitors are a cost-effective and practical solution for protecting metals and alloys against corrosion. The unique aspect of this method is that the anti-corrosion treatment is applied to the corrosive environment instead of directly to the metal, making it a convenient and inexpensive method in comparison to other corrosion control methods.
There are different types of inhibitors that work based on different mechanisms, leading to varying levels of effectiveness in different environments. Among the inhibitors, plant-based inhibitors have proven to be particularly effective and have gained widespread popularity, especially due to their low toxicity and eco-friendly nature. However, there are still some challenges and problems associated with the use of these inhibitors. Here are some perspectives on the problems of green corrosion inhibitors and future research directions in the field of iron protection [46,47,48,49,50,51]:
- Inhibitor effectiveness: the effectiveness of green corrosion inhibitors can be influenced by several factors, such as the type of metal, the corrosive environment, and the inhibitor concentration. Therefore, further research is needed to optimize the performance of these inhibitors under various conditions.
- Compatibility with other materials: green corrosion inhibitors may not be compatible with other materials used in the metal protection process, such as coatings or paints. Therefore, research efforts should focus on developing inhibitors that are compatible with other materials used in metal protection.
- Environmental impact: although green corrosion inhibitors are considered to be environmentally friendly, their impact on the environment should be carefully evaluated. For example, some natural inhibitors may cause eutrophication in water bodies or have other unintended consequences. Therefore, further research is needed to ensure that these inhibitors do not harm the environment.
- Cost: the cost of green corrosion inhibitors can be high, particularly for large-scale applications. Therefore, research efforts should focus on developing cost-effective inhibitors that can provide effective protection at a lower cost.
Overall, green corrosion inhibitors offer a promising solution for metal protection with reduced environmental impact. However, there are still some challenges that need to be addressed. Future research efforts should focus on developing eco-friendly and cost-effective inhibitors, optimizing their performance under various conditions, exploring new approaches to corrosion protection, and developing smart corrosion inhibitors that can respond to changes in the environment and adjust their effectiveness accordingly.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Popov, B.N. Corrosion Engineering: Principles and Solved Problems, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 2–4. ISBN 978-0-444-62722-3. [Google Scholar]
- ISO 8044:2015; Corrosion of Metals and Alloys—Main Terms and Definitions. International Organization for Standardization: Geneva, Switzerland, 2015.
- Dwivedi, D.; Mata, J.P. Archaeometallurgical investigation of ancient artefacts’ degradation phenomenon. NPJ Mater. Degrad. 2019, 3, 35. [Google Scholar] [CrossRef]
- Hollner, S. Development of New Protective Treatments Based on Carboxylic Acid for the Conservation of Iron Objects of Cultural Heritage. Ph.D. Thesis, Université Henri-Poincaré Nancy I, Nancy, France, 2009. [Google Scholar]
- Hammouch, H. Development of a New Protective Treatment Based on Opuntia Ficus Indica for the Conservation of Archaeological Objects of Bronze and Iron. Ph.D. Thesis, Faculty of Science, Ibn Tofail-Kenitra University, Kenitra, Morocco, 2013. [Google Scholar]
- Tylecote, R.F. A History of Metallurgy, 2nd ed.; The Institute of Materials: London, UK, 1992; ISBN 0-9011462-88-8. [Google Scholar]
- France-Lanord, A. History of Iron: An Illustrated Guide to the Iron Museum; Centre de Recherches de L’histoire de la Sidérurgie: Nancy, France, 1977. [Google Scholar]
- Kordas, G. Corrosion Barrier Coatings: Progress and Perspectives of the Chemical Route. Corros. Mater. Degrad. 2022, 3, 376–413. [Google Scholar] [CrossRef]
- Selwyn, L. Overview of archaeological iron: The corrosion problem, key factors affecting treatment, and gaps in current knowledge. In Proceedings of the Metal 2004 National Museum of Australia Canberra ACT, Canberra, Australia, 4–8 October 2004. [Google Scholar]
- David, D. Matériaux: Analogues Archéologiques et Corrosion, Collection Science et Techniques; BIO Intelligence Service: Paris, France, 2002; p. 75. [Google Scholar]
- Pourbaix, M. Atlas of Electrochemical Equilibria; Gauthier—Villars et Cie Editeurs: Paris, France, 1963. [Google Scholar]
- Descostes, M. Evaluation of an Oxidizing Perturbation in Clay Media: Mechanism of Pyrite Oxidation. Ph.D. Thesis, University of Paris VII, Paris, France, 2001. [Google Scholar]
- Chivot, J. Sélection de données thermodynamiques concernant le système Fe-H2O. CEA/FAR SCECF, Technical Report SCECF n°481. 1998. Available online: www.andra.fr/sites/default/files/2017-12/236_0.pdf (accessed on 13 December 2022).
- Chivot, J. Les Diagrammes E-pH Révisés du Système Fer-H2O en Fonction de la Température; ANDRA: Paris, France, 1999. [Google Scholar]
- Monnier, J. Atmospheric Corrosion of Historical Ferrous Alloys under Shelter. Characterization of the System, Mechanisms and Contribution to Modelling. Ph.D. Thesis, Institut de Chimie et des Matériaux, University of Paris-Est, Champs-sur-Marne, France, 2008. [Google Scholar]
- Faiz, H. Etude du Mécanisme de Corrosion Atmosphérique à Long Terme des Aciers: Nouvelles Stratégies de Protection des Aciers du Patrimoine Culturel. Ph.D. Thesis, Faculté des Sciences et Technologies, Université Lorraine, Nancy, France, 2012. [Google Scholar]
- Accary, A.; Haijtink, B. La Paléo-Métallurgie-Outil de Prévision. In Journées de Paléométallurgie; Université de Technologie de Compiègne: Compiègne, France, 1983. [Google Scholar]
- Soerensen, B.; Gregory, D. In Situ Preservation of Artifacts in Nydam Mose, pg. 94 in Metal 98. In Proceedings of the International Conference on Metals Conservation, Draguignan-Figanieres, France, 27–29 May 1998. [Google Scholar]
- Johnson, A.B., Jr.; Francis, B. Durability of Metals from Archaeological Objects, Metal Meteorites and Native Metals; U.S. Department of Energy: Washington, DC, USA, 1980; p. 106.
- Azoulay, I. Long-Term Corrosion of Steels: Physicochemical Properties of Ferrous Hydroxysalts. Ph.D. Thesis, University of La Rochelle, La Rochelle, France, 2013. [Google Scholar]
- Misawa, T.; Hashimoto, K.; Shimodaira, S. The mechanism of formation of iron oxide and oxyhydroxides in aqueous solutions at room temperature. Corros. Sci. 1974, 14, 131–149. [Google Scholar] [CrossRef]
- Cornell, R.; Schwertmann, U. Iron Oxides in the Laboratory; Wiley-VCH: Weinheim, Germany, 2000; 137p. [Google Scholar]
- Vernon, W.H.J.; Hutton, R.S.; Patterson, W.S.; Evans, U.R.; Lee, A.R.; Martin, A.R.; Hudson, J.C.; Coste, J.H. A laboratory study of the atmospheric corrosion of metals. Discussion. Trans. Faraday Soc. 1931, 27, 582–594. [Google Scholar] [CrossRef]
- Schikorr, G. Über den Mechanismus des atmosphärischen Rostens des Eisens. Werkst. Und Korros. 1963, 14, 69–80. [Google Scholar] [CrossRef]
- Evans, U.; Taylor, C. Mechanism of atmospheric rusting. Corros. Sci. 1972, 12, 227–246. [Google Scholar] [CrossRef]
- Stratmann, M.; Bohnenkamp, K.; Engell, H. An electrochemical study of phasetransitions in rust layers. Corros. Sci. 1983, 23, 969–985. [Google Scholar] [CrossRef]
- Stratmann, M. The atmospheric corrosion of iron and steel. Metall. Odlew. 1990, 16, 46–52. [Google Scholar]
- Hoerlé, S.; Mazaudier, F.; Dillmann, P.; Santarini, G. Advances in understanding atmospheric corrosion of iron. II. Mechanistic modelling of wet–dry cycles. Corros. Sci. 2004, 46, 1431–1465. [Google Scholar] [CrossRef]
- Stratmann, M.; Streckel, H. On the atmospheric corrosion of metals which are covered with thin electrolyte layers—I, Verification of the experimental. Corros. Sci. 1990, 30, 681–696. [Google Scholar] [CrossRef]
- Antony, H. Etude Electrochimique des Composés du fer-apport à la Compréhension des Processus Environnementaux. Ph.D. Thesis, Université d’Evry, Evry, France, 2005; 213p. [Google Scholar]
- Dünnwald, J.; Otto, A. An investigation of phase transitions in rust layers using raman spectroscopy. Corros. Sci. 1989, 29, 1167–1176. [Google Scholar] [CrossRef]
- Yamashita, M.; Nagano, H.; Oriani, R. Dependence of corrosion potential and corrosion rate of a low-alloy steel upon depth of aqueous solution. Corros. Sci. 1998, 40, 1447–1453. [Google Scholar] [CrossRef]
- Cornell, R.; Schwertmann, U. The Iron Oxides—Structure, Properties, Occurrences and Uses, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003; 664p. [Google Scholar]
- Reguer, S. Phases Chlorées sur les Objets Archéologiques Ferreux Corrodés Dans les Sols: Caractérisations et Mécanismes de Formation. Ph.D. Thesis, Université Paris Sud-Paris XI, Bures-sur-Yvette, France, 2005. [Google Scholar]
- Nasrazadani, S. The application of infrared spectroscopy to a study of phosphoric and tannic acid interactions with magnetite, goethite and lepidocrocite. Corros. Sci. 1997, 39, 1845–1859. [Google Scholar] [CrossRef]
- Dillmann, P.; Mazaudier, F.; Hoerle, S. Advances in understanding atmospheric corrosion of iron. I. Rust characterisation of ancient ferrous artefacts exposed to indoor atmospheric corrosion. Corros. Sci. 2003, 46, 1401–1429. [Google Scholar] [CrossRef]
- Landolt, D. Treatise on Materials, 12 Corrosion and Chemistry of Metal Surfaces, Polytechnic and University Presses Romandes, 3rd ed.; Collection: Treatise on Materials; EPFL Press: Lausanne, Switzerland, 1993. [Google Scholar]
- Chan-Rosado, G.; Pech-Canul, M. Influence of native oxide film age on the passivation of carbon steel in neutral aqueous solutions with a dicarboxylic acid. Corros. Sci. 2019, 153, 19–31. [Google Scholar] [CrossRef]
- Sumoondur, A.; Shaw, S.; Ahmed, I.; Benning, L.G. Green rust as a precursor for magnetite: An in situ synchrotron based study. Miner. Mag. 2008, 72, 201–204. [Google Scholar] [CrossRef]
- Yamashita, M.; Konishi, H.; Kozakura, T.; Mizuki, J.; Uchida, H. In situ observation of initial rust for-mation process on carbon steel under Na2SO4 and NaCl solution films with wet/dry cycles using synchrotron radiation X-rays. Corros. Sci. 2005, 47, 2492–2498. [Google Scholar] [CrossRef]
- Lair, V.; Antony, H.; Legrand, L.; Chausse, A. Electrochemical reduction of ferric corrosion products and evaluation of galvanic coupling with iron. Corros. Sci. 2006, 48, 2050–2063. [Google Scholar] [CrossRef]
- Aliofkhazraei, M. Chapter 12. Corrosion Resistance Through the Application of Anti- Corrosion Coatings. In Developments in Corrosion Protection; BoD–Books on Demand: Norderstedt, Germany, 2014. [Google Scholar] [CrossRef]
- Serghini Idrissi, M. Study of the Electrochemical Behavior of C38 Steel and UR45N Stainless Steel in Different Media. Ph.D. Thesis, Faculty of Science, Mohammed V University, Rabat, Morocco, 10 December 2016. [Google Scholar]
- Bai, Q.; Bai, Y. Corrosion Prevention and Advanced CP Design. In Subsea Pipeline Design, Analysis, and Installation; Gulf Professional Publishing: Houston, TX, USA, 2014; pp. 451–464. [Google Scholar] [CrossRef]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green Corrosion Inhibitors from Natural Sources and Biomass Wastes. Molecules 2018, 24, 48. [Google Scholar] [CrossRef]
- Zaki, A. Corrosion Control by Inhibition. In Principles of Corrosion Engineering and Corrosion Control; Elsevier: Amsterdam, The Netherlands, 2006; pp. 352–381. [Google Scholar] [CrossRef]
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef]
- Taghavikish, M.; Dutta, N.K.; Choudhury, N.R. Emerging Corrosion Inhibitors for Interfacial Coating. Coatings 2017, 7, 217. [Google Scholar] [CrossRef]
- Askari, M.; Aliofkhazraei, M.; Jafari, R.; Hamghalam, P.; Hajizadeh, A. Downhole corrosion inhibitors for oil and gas production—A review. Appl. Surf. Sci. Adv. 2021, 6, 100128. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M. Corrosion inhibitors for ferrous and non-ferrous metals and alloys in ionic sodium chloride solutions: A review. J. Mol. Liq. 2017, 248, 927–942. [Google Scholar] [CrossRef]
- Chen, L.; Lu, D.; Zhang, Y. Organic Compounds as Corrosion Inhibitors for Carbon Steel in HCl Solution: A Comprehensive Review. Materials 2022, 15, 2023. [Google Scholar] [CrossRef] [PubMed]
- Saji, V.S. A Review on Recent Patents in Corrosion Inhibitors. Recent Patents Corros. Sci. 2010, 2, 6–12. [Google Scholar] [CrossRef]
- Fekry, A.; Ameer, M. Corrosion inhibition of mild steel in acidic media using newly synthesized heterocyclic organic molecules. Int. J. Hydrogen Energy 2010, 35, 7641–7651. [Google Scholar] [CrossRef]
- ESutter, M.; Ammeloot, F.; Pouet, M.J.; Fiaud, C.; Couffignal, R. Heterocyclic compounds used as corrosion inhibitors: Correlation between 13C and 1H NMR spectroscopy and inhibition efficiency. Corros. Sci. 1999, 41, 105–115. [Google Scholar] [CrossRef]
- Abiola, O.; Oforka, N. Adsorption of (4-amino-2-methyl-5-pyrimidinyl methylthio) acetic acid on mild steel from hydrochloric acid solution (HCl)—Part 1. Mater. Chem. Phys. 2004, 83, 315–322. [Google Scholar] [CrossRef]
- Abiola, O.K. Adsorption of 3-(4-amino-2-methyl-5-pyrimidyl methyl)-4-methyl thiazolium chloride on mild steel. Corros. Sci. 2006, 48, 3078–3090. [Google Scholar] [CrossRef]
- Goyal, M.; Kumar, S.; Bahadur, I.; Verma, C.; Ebenso, E.E. Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review. J. Mol. Liq. 2018, 256, 565–573. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Li, Y.; Balcells, M.; Garayoa, R.C.; Fabiano-Tixier, A.-S.; Chemat, F. Vegetable Oils as Alternative Solvents for Green Oleo-Extraction, Purification and Formulation of Food and Natural Products. Molecules 2017, 22, 1474. [Google Scholar] [CrossRef]
- Zaferani, S.H.; Sharifi, M.; Zaarei, D.; Shishesaz, M.R. Application of eco-friendly products as corrosion inhibitors for metals in acid pickling processes—A review. J. Environ. Chem. Eng. 2013, 1, 652–657. [Google Scholar] [CrossRef]
- Zakeri, A.; Bahmani, E.; Aghdam, A.S.R. Plant extracts as sustainable and green corrosion inhibitors for protection of ferrous metals in corrosive media: A mini review. Corros. Commun. 2022, 5, 25–38. [Google Scholar] [CrossRef]
- AOstovari, A.; Hoseinieh, M.; Peikari, M.; Shadizadeh, S.; Hashemi, S. Corrosion inhibition of mild steel in 1M HCl solution by henna extract: A comparative study of the inhibition by henna and its constituents (Lawsone, Gallic acid, α-d-Glucose and Tannic acid). Corros. Sci. 2009, 51, 1935–1949. [Google Scholar] [CrossRef]
- Orubite, K.; Oforka, N. Inhibition of the corrosion of mild steel in hydrochloric acid solutions by the extracts of leaves of Nypa fruticans Wurmb. Mater. Lett. 2004, 58, 1768–1772. [Google Scholar] [CrossRef]
- Chauhan, L.; Gunasekaran, G. Corrosion inhibition of mild steel by plant extract in dilute HCl medium. Corros. Sci. 2007, 49, 1143–1161. [Google Scholar] [CrossRef]
- El-Etre, A. Khillah extract as inhibitor for acid corrosion of SX 316 steel. Appl. Surf. Sci. 2005, 252, 8521–8525. [Google Scholar] [CrossRef]
- Bouyanzer, A.; Hammouti, B.; Majidi, L. Pennyroyal oil from Mentha pulegium as corrosion inhibitor for steel in 1M HCl. Mater. Lett. 2006, 60, 2840–2843. [Google Scholar] [CrossRef]
- Rahim, A.A.; Rocca, E.; Steinmetz, J.; Kassim, M.J.; Adnan, R.; Ibrahim, M.S. Mangrove tannins and their flavanoid monomers as alternative steel corrosion inhibitors in acidic medium. Corros. Sci. 2007, 49, 402–417. [Google Scholar] [CrossRef]
- Amin, M.A.; Abd El-Rehim, S.S.; El-Sherbini, E.E.F.; Bayoumi, R.S. The inhibition of low carbon steel corrosion in hydrochloric acid solutions by succinic acid: Part I. Weight loss, polarization, EIS, PZC, EDX and SEM studies. Electrochim. Acta 2007, 52, 3588–3600. [Google Scholar] [CrossRef]
- El-Etre, A. Inhibition of acid corrosion of carbon steel using aqueous extract of olive leaves. J. Colloid Interface Sci. 2007, 314, 578–583. [Google Scholar] [CrossRef]
- Oguzie, E.E. Evaluation of the inhibitive effect of some plant extracts on the acid corrosion of mild steel. Corros. Sci. 2008, 50, 2993–2998. [Google Scholar] [CrossRef]
- Satapathy, A.; Gunasekaran, G.; Sahoo, S.; Amit, K.; Rodrigues, P. Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros. Sci. 2009, 51, 2848–2856. [Google Scholar] [CrossRef]
- Okafor, P.C.; Ikpi, M.E.; Uwah, I.E.; Ebenso, E.E.; Ekpe, U.J. Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corros. Sci. 2008, 50, 2310–2317. [Google Scholar] [CrossRef]
- AAbdel-Gaber, A.; Abd-El-Nabey, B.; Saadawy, M. The role of acid anion on the inhibition of the acidic corrosion of steel by lupine extract. Corros. Sci. 2009, 51, 1038–1042. [Google Scholar] [CrossRef]
- Da Rocha, J.C.; Gomes, J.A.D.C.P.; D’Elia, E. Corrosion inhibition of carbon steel in hydrochloric acid solution by fruit peel aqueous extracts. Corros. Sci. 2010, 52, 2341–2348. [Google Scholar] [CrossRef]
- Elkhotfi, Y.; Forsal, I.; Rakib, E.M.; Mernari, B. The Inhibition Action of Essential Oil of J. Juniperus Phoenicea on the Corrosion of Mild Steel in Acidic Media. Port. Electrochim. Acta 2018, 36, 77–87. [Google Scholar] [CrossRef]
- Kalaiselvi, P.; Chellammal, S.; Palanichamy, S.; Subramanian, G. Artemisia pallens as corrosion inhibitor for mild steel in HCl medium. Mater. Chem. Phys. 2010, 120, 643–648. [Google Scholar] [CrossRef]
- Abdallah, M. Guar Gum as Corrosion Inhibitor for Carbon Steel in Sulfuric Acid Solutions. Port. Electrochim. Acta 2004, 22, 161–175. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.M.; Abd-El-Nabey, B.A.; Sidahmed, I.M.; El-Zayady, A.M.; Saadawy, M. Inhibitive action of some plant extracts on the corrosion of steel in acidic media. Corros. Sci. 2006, 48, 2765–2779. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, P.; Liang, Q.; Hou, B. Berberine as a natural source inhibitor for mild steel in 1M H2SO4. Appl. Surf. Sci. 2005, 252, 1245–1253. [Google Scholar] [CrossRef]
- Noor, E.A. Temperature effects on the corrosion inhibition of mild steel in acidic solutions by aqueous extract of fenugreek leaves. Int. J. Electrochem. Sci. 2007, 2, 996–1017. [Google Scholar]
- Raja, P.B.; Sethuraman, M. Inhibitive effect of black pepper extract on the sulphuric acid corrosion of mild steel. Mater. Lett. 2008, 62, 2977–2979. [Google Scholar] [CrossRef]
- Ebenso, E.; Oguzie, E. Corrosion inhibition of mild steel in acidic media by some organic dyes. Mater. Lett. 2005, 59, 2163–2165. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Alemu, H.; Umoren, S.A.; Obot, I.B. Inhibition of mild steel corrosion in sulphuric acid using alizarin yellow GG dye and synergistic iodide additive. Int. J. Electrochem. Sci. 2008, 3, 1325–1339. [Google Scholar]
- de Souza, F.; Spinelli, A. Caffeic acid as a green corrosion inhibitor for mild steel. Corros. Sci. 2009, 51, 642–649. [Google Scholar] [CrossRef]
- Shivakumar, M.; Dharmaprakash, M.S.; Manjappa, S.; Nagashree, K.L. Corrosion Inhibition Performance of Lignin Extracted from Black Liquor on Mild Steel in 0.5 M H2SO4 Acidic Media. Port. Electrochim. Acta 2017, 35, 351–359. [Google Scholar] [CrossRef]
- Abbout, S.; Zouarhi, M.; Chebabe, D.; Damej, M.; Berisha, A.; Hajjaji, N. Galactomannan as a new bio-sourced corrosion inhibitor for iron in acidic media. Heliyon 2020, 6, e03574. [Google Scholar] [CrossRef]
- Hammouch, H.; Dermaj, A.; Chebabe, D.; Decaro, P.; Hajjaji, N.; Bettach, N.; Takenouti, H.; Srhiri, A. Opuntia Ficus Indica seed oil: Characterization and application in corrosion inhibition of carbon steel in acid medium. Anal. Bioanal. Electrochem. 2013, 5, 236. [Google Scholar]
- Chellouli, M.; Chebabe, D.; Dermaj, A.; Erramli, H.; Bettach, N.; Hajjaji, N.; Casaletto, M.; Cirrincione, C.; Privitera, A.; Srhiri, A. Corrosion inhibition of iron in acidic solution by a green formulation derived from Nigella sativa L. Electrochim. Acta 2016, 204, 50–59. [Google Scholar] [CrossRef]
- Zouarhi, M.; Chellouli, M.; Abbout, S.; Hammouch, H.; Dermaj, A.; Hassane, S.O.S.; Decaro, P.; Bettach, N.; Hajjaji, N.; Srhiri, A. Inhibiting Effect of a Green Corrosion Inhibitor Containing Jatropha Curcas Seeds Oil for Iron in an Acidic Medium. Port. Electrochim. Acta 2018, 36, 179–195. [Google Scholar] [CrossRef]
- Abbout, S.; Chellouli, M.; Zouarhi, M.; Benzidia, B.; Hammouch, H.; Chebabe, D.; Dermaj, A.; Erramli, H.; Bettach, N.; Hajjaji, N. New formulation based on Ceratonia Siliqua L seed oil, as a green corrosion inhibitor of iron in acidic medium. Anal. Bioanal. Electrochem. 2018, 10, 789–804. [Google Scholar]
- Zouarhi, M.; Abbout, S.; Benzidia, B.; Chellouli, M.; Hammouch, H.; Erramli, H.; Hassane, S.O.S.; Bettach, N.; Hajjaji, N. Evaluation of a New Formulation Derived from Aleurites moluccana Seeds Oil as a Green Corrosion Inhibitor for Iron in Acidic Medium. Anal. Bioanal. Electrochem. 2019, 11, 1651–1668. [Google Scholar]
- Rehioui, M.; Abbout, S.; Benzidia, B.; Hammouch, H.; Erramli, H.; Daoud, N.A.; Badrane, N.; Hajjaji, N. Corrosion inhibiting effect of a green formulation based on Opuntia Dillenii seed oil for iron in acid rain solution. Heliyon 2021, 7, e06674. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).