Alkaloids of Solanum xanthocarpum Stem as Green Inhibitor for Mild Steel Corrosion in One Molar Sulphuric Acid Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments Used
2.2. Extraction of Alkaloids from S. xanthocarpum Stem
2.3. Chemical Test for Alkaloids
2.4. Preparation of MS Specimen
2.5. Preparation of Inhibitor Solution
2.6. Weight Loss Measurement Method
2.7. Electrochemical Measurement
2.8. Adsorption Isotherm and Kinetic Study
2.9. Surface Morphological Study
3. Results
3.1. Characterization of Alkaloids
3.1.1. Chemical Test of Alkaloids
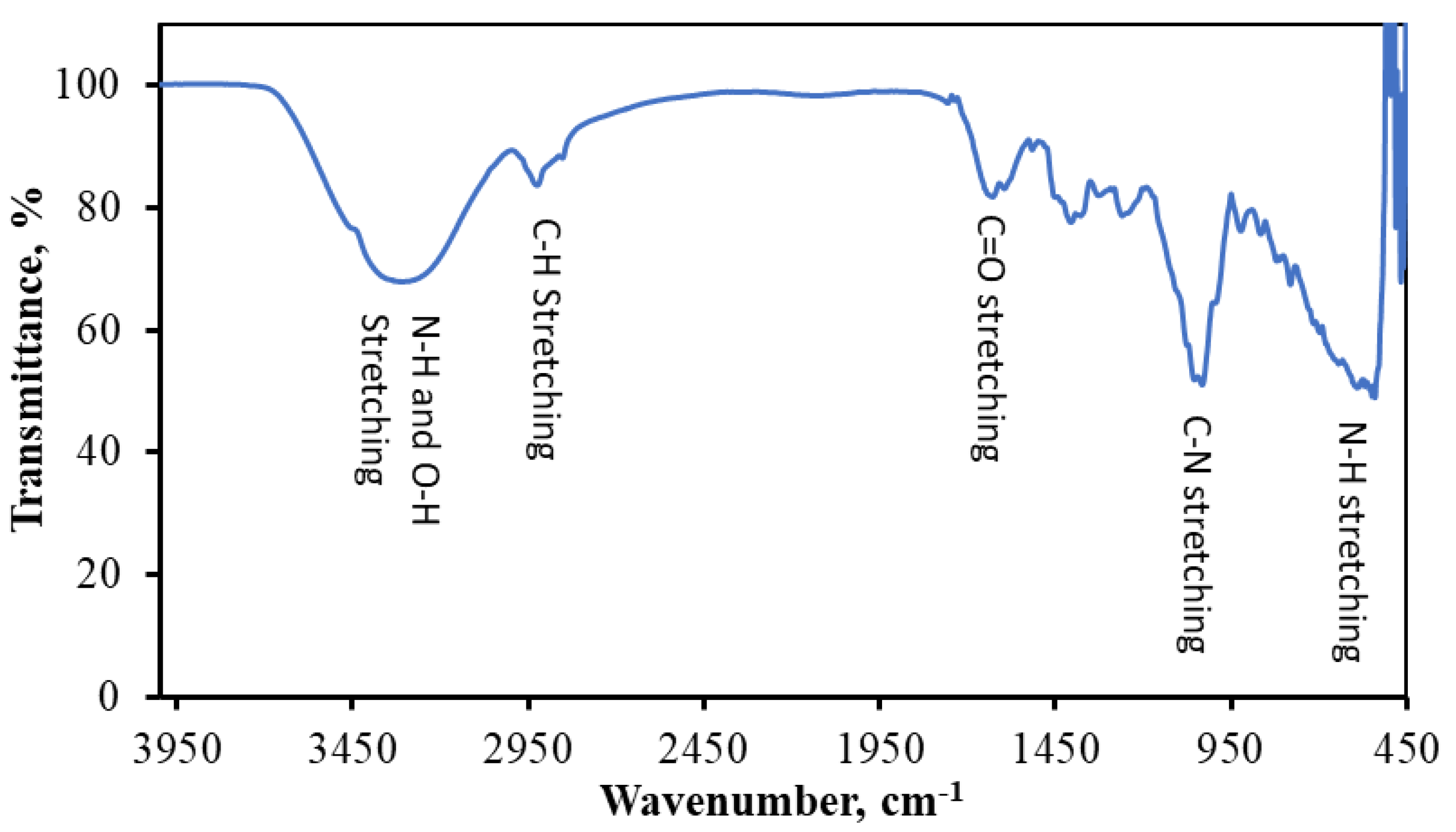
3.1.2. FTIR Spectroscopic Measurement
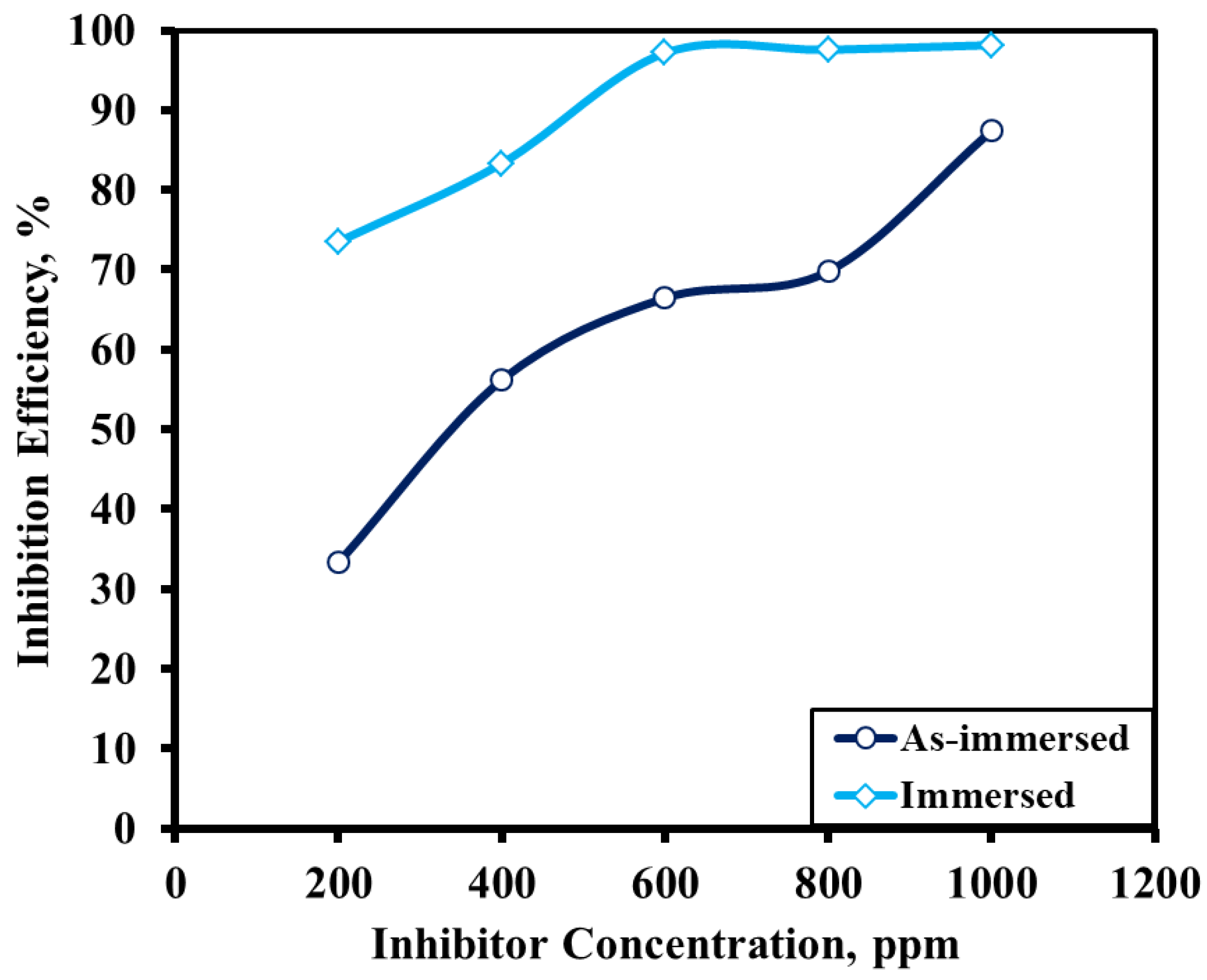
3.2. Findings from Immersion Tests
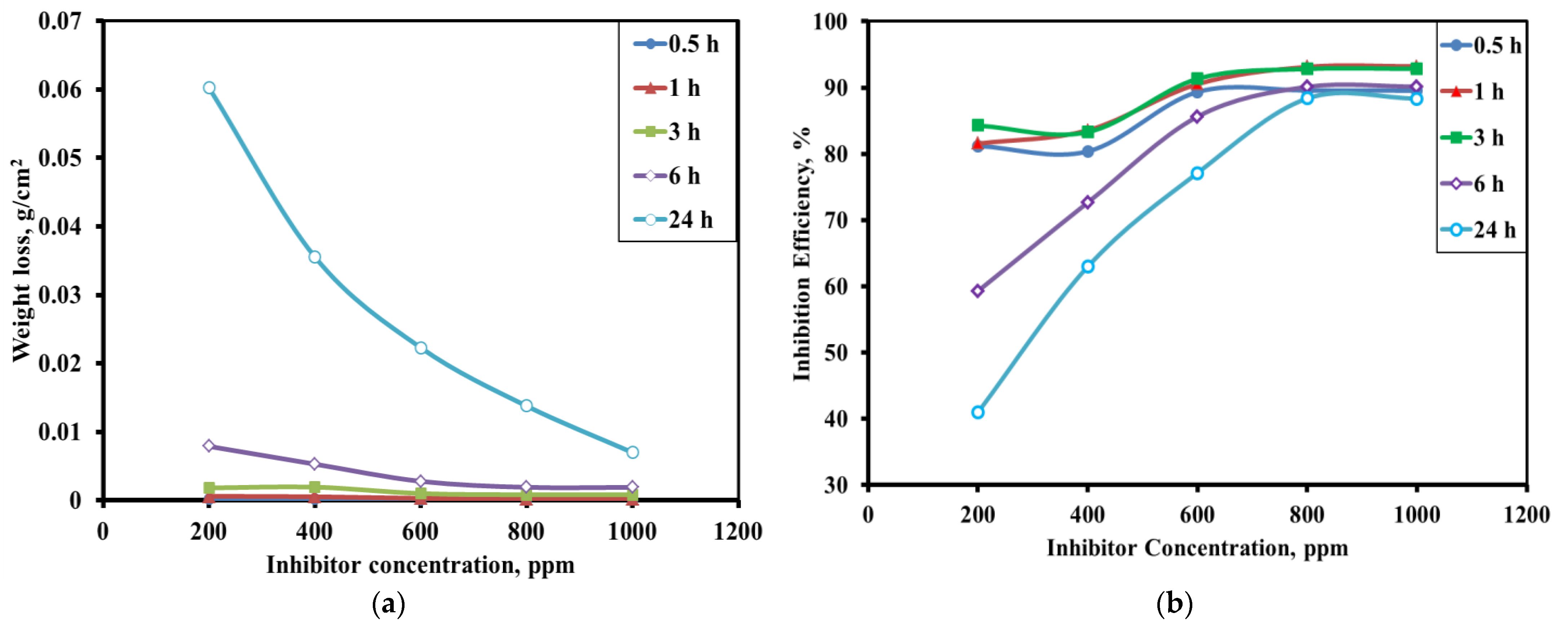
3.2.1. Effect of Alkaloid Concentration
3.2.2. Effect of Immersion Time
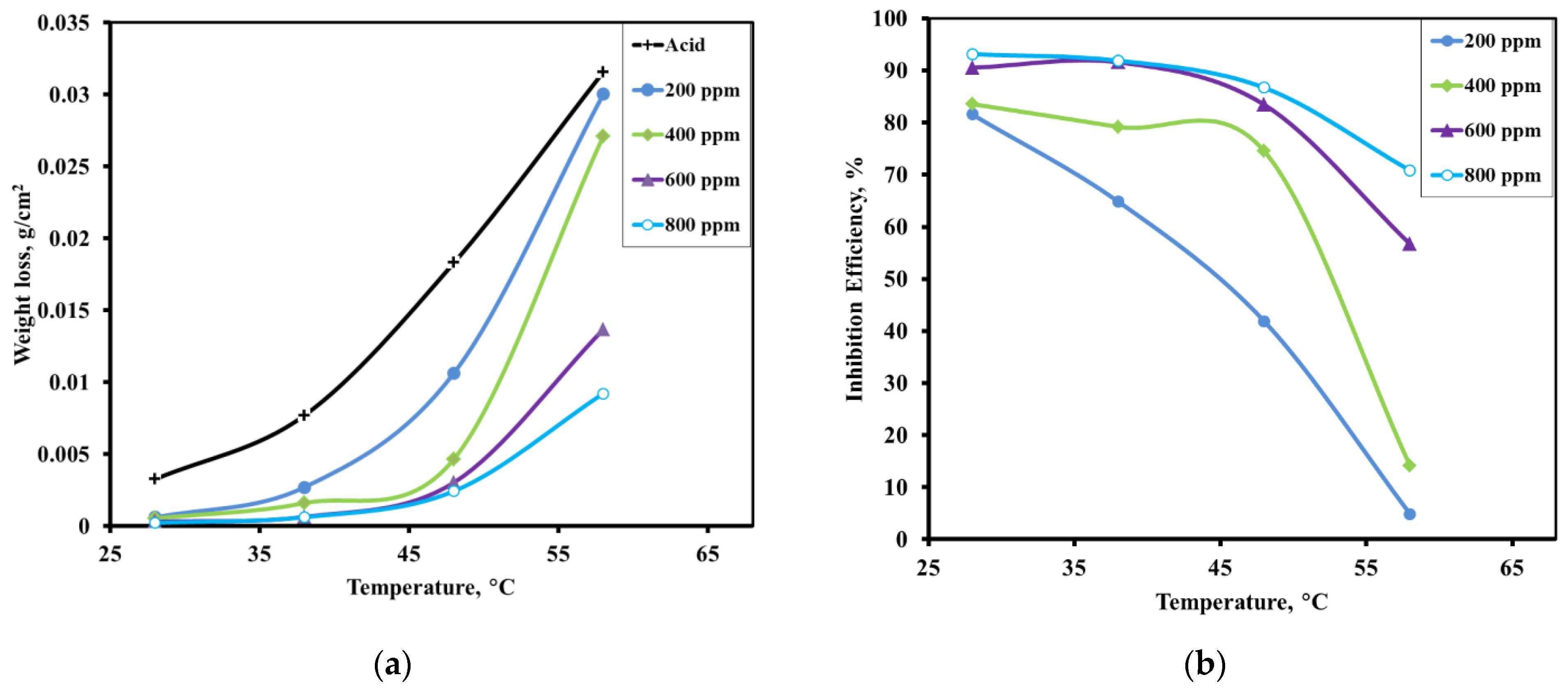
3.2.3. Effect of Temperature
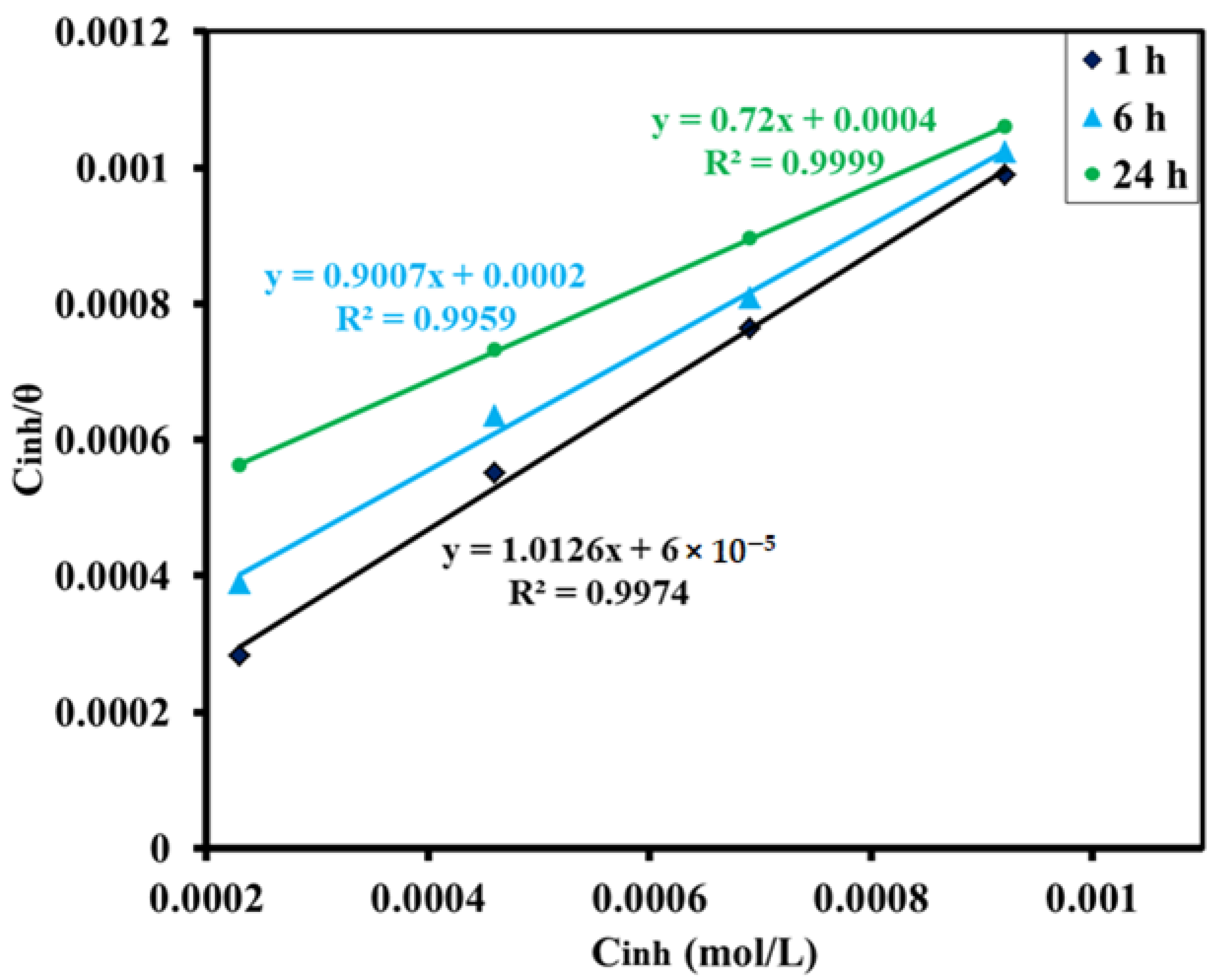
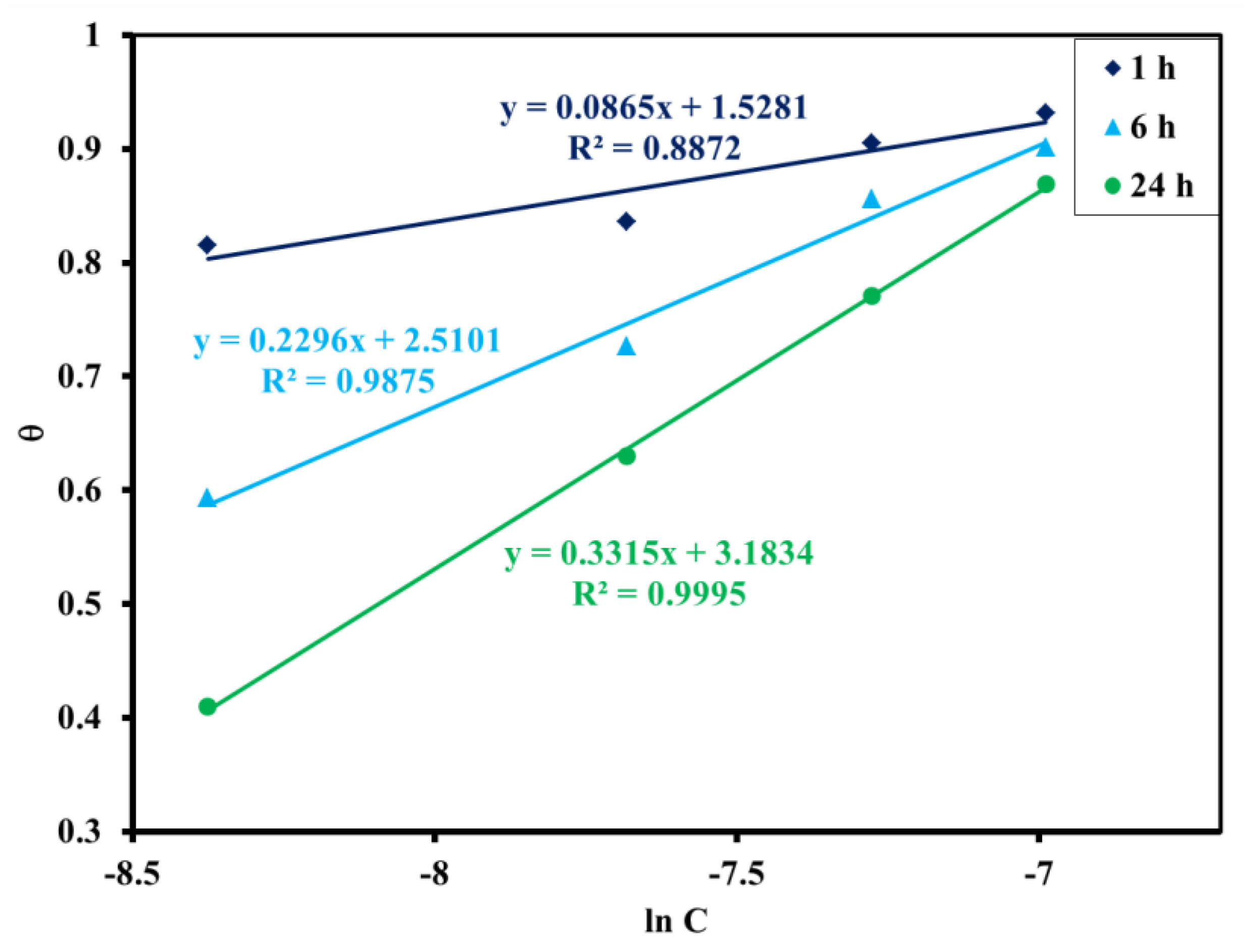
3.2.4. Adsorption Isotherm
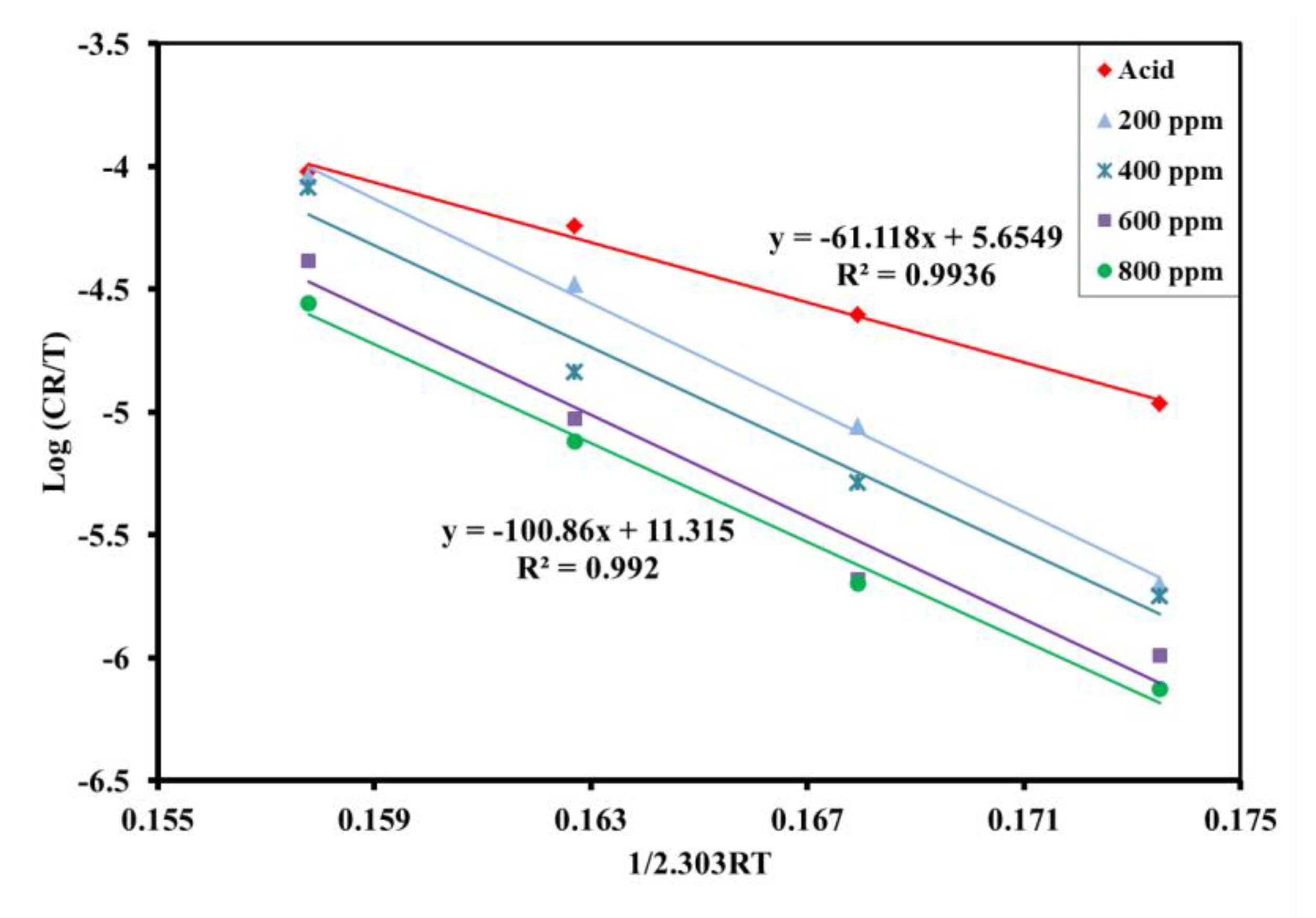
3.2.5. Activation Energy and Corrosion Kinetics
3.2.6. Thermodynamics of Corrosion and Mode of Inhibition
3.3. Electrochemical Measurement
3.3.1. Open-Circuit Potential Curves
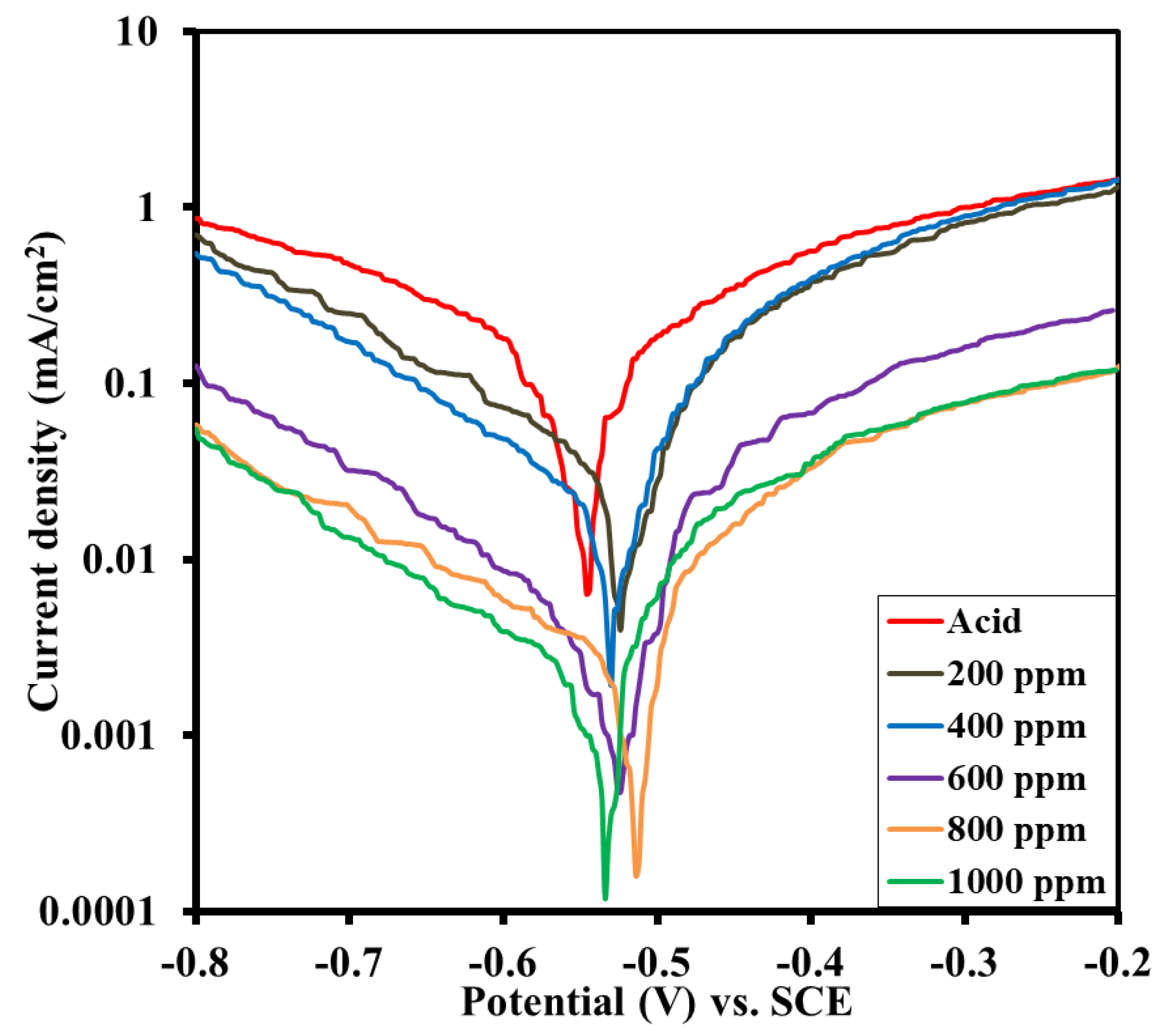
3.3.2. Polarization Curves
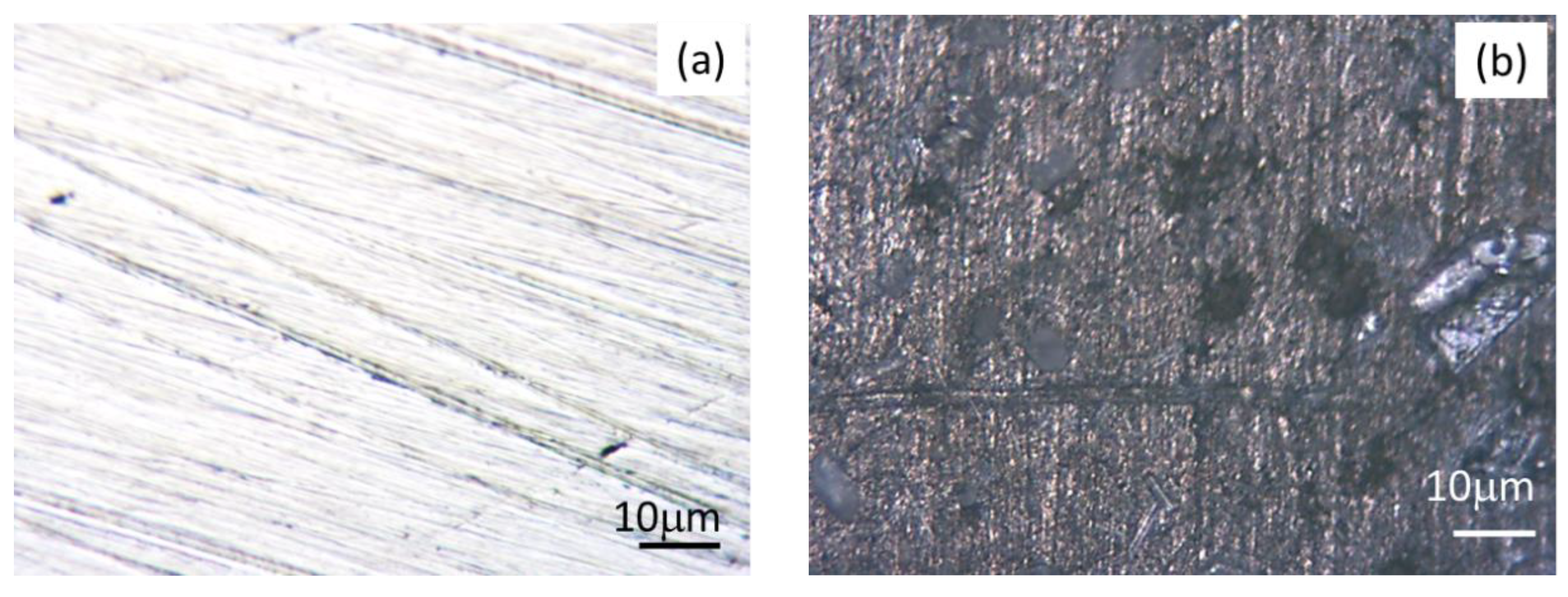
3.4. Surface Morphological Study
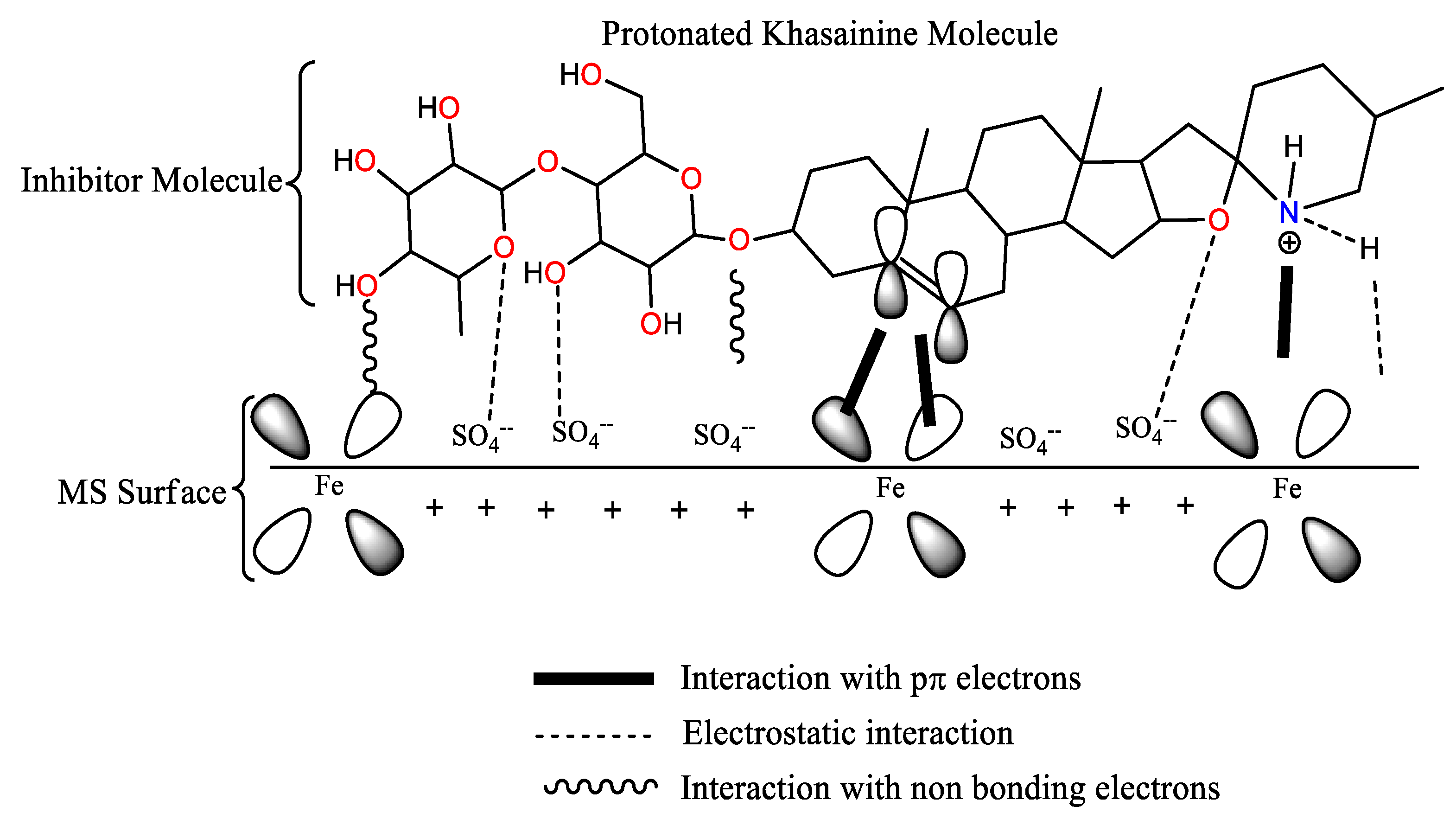
3.5. Mechanism of Corrosion Inhibition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaw, B.; Kelly, R. What Is Corrosion? Electrochem. Soc. Interface 2006, 15, 24. [Google Scholar] [CrossRef]
- Uhlig, H.H.; Revie, R.W. Corrosion and Corrosion Control; John Wiley & Sons: Hoboken, NJ, USA, 1985; ISBN 978-0-471-73279-2. [Google Scholar]
- Bhattarai, J. Frontiers of Corrosion Science; Kshitiz Publisher: Kirtipur, Nepal, 2010; p. 304. [Google Scholar]
- Heusler, K.; Landolt, D.; Trasatti, S. Electrochemical Corrosion Nomenclature (Recommendations 1988). Pure Appl. Chem. 1989, 61, 19–22. [Google Scholar] [CrossRef]
- Krivián, L. Meaning and Measurement of Corrosion Potential. Br. Corros. J. 1991, 26, 191–194. [Google Scholar] [CrossRef]
- Revie, R.W. Uhlig’s Corrosion Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 51, ISBN 0-470-08032-9. [Google Scholar]
- Karki, R.; Bajgai, A.K.; Khadka, N.; Thapa, O.; Mukhiya, T.; Oli, H.B.; Bhattarai, D.P. Acacia Catechu Bark Alkaloids as Novel Green Inhibitors for Mild Steel Corrosion in a One Molar Sulphuric Acid Solution. Electrochem 2022, 3, 668–687. [Google Scholar] [CrossRef]
- Oli, H.B.; Thapa Magar, J.; Khadka, N.; Subedee, A.; Bhattarai, D.P.; Pant, B. Coriaria Nepalensis Stem Alkaloid as a Green Inhibitor for Mild Steel Corrosion in 1 M H2SO4 Solution. Electrochem 2022, 3, 713–727. [Google Scholar] [CrossRef]
- Roberge, P.R. Handbook of Corrosion Engineering; McGraw-Hill Education: New York, NY, USA, 2019; ISBN 1-260-11697-2. [Google Scholar]
- Slepski, P.; Gerengi, H.; Jazdzewska, A.; Orlikowski, J.; Darowicki, K. Simultaneous Impedance and Volumetric Studies and Additionally Potentiodynamic Polarization Measurements of Molasses as a Carbon Steel Corrosion Inhibitor in 1M Hydrochloric Acid Solution. Constr. Build. Mater. 2014, 52, 482–487. [Google Scholar] [CrossRef]
- Alkais, A.R.; Edrah, S.M. The Corrosion Inhibition of Mild Steel in Acid Solutions Media by Adsorption of Leaves of Morus nigra L. from Libya. Int. J. Sci. Res. 2016, 5, 730–734. [Google Scholar]
- Parajuli, D.; Sharma, S.; Oli, H.B.; Bohara, D.S.; Bhattarai, D.P.; Tiwari, A.P.; Yadav, A.P. Comparative Study of Corrosion Inhibition Efficacy of Alkaloid Extract of Artemesia Vulgaris and Solanum Tuberosum in Mild Steel Samples in 1 M Sulphuric Acid. Electrochem 2022, 3, 416–433. [Google Scholar] [CrossRef]
- Chapagain, A.; Acharya, D.; Das, A.K.; Chhetri, K.; Oli, H.B.; Yadav, A.P. Alkaloid of Rhynchostylis Retusa as Green Inhibitor for Mild Steel Corrosion in 1 M H2SO4 Solution. Electrochem 2022, 3, 211–224. [Google Scholar] [CrossRef]
- Davis, J.R. Corrosion: Understanding the Basics; ASM International: Metals Park, OH, USA, 2000; ISBN 1-61503-068-9. [Google Scholar]
- Behpour, M.; Ghoreishi, S.; Soltani, N.; Salavati-Niasari, M. The Inhibitive Effect of Some Bis-N, S-Bidentate Schiff Bases on Corrosion Behaviour of 304 Stainless Steel in Hydrochloric Acid Solution. Corros. Sci. 2009, 51, 1073–1082. [Google Scholar] [CrossRef]
- Palou, R.M.; Olivares-Xomelt, O.; Likhanova, N.V. Environmentally Friendly Corrosion Inhibitors. Dev. Corros. Prot. 2014, 19, 431–432. [Google Scholar]
- Karki, N.; Neupane, S.; Gupta, D.K.; Das, A.K.; Singh, S.; Koju, G.M.; Chaudhary, Y.; Yadav, A.P. Berberine Isolated from Mahonia Nepalensis as an Eco-Friendly and Thermally Stable Corrosion Inhibitor for Mild Steel in Acid Medium. Arab. J. Chem. 2021, 14, 103423. [Google Scholar] [CrossRef]
- Shrestha, P.R.; Oli, H.B.; Thapa, B.; Chaudhary, Y.; Gupta, D.K.; Das, A.K.; Nakarmi, K.B.; Singh, S.; Karki, N.; Yadav, A.P. Bark Extract of Lantana Camara in 1M HCl as Green Corrosion Inhibitor for Mild Steel. Eng. J. 2019, 23, 205–211. [Google Scholar] [CrossRef]
- Solomon, M.; Umoren, S.; Udosoro, I.; Udoh, A. Inhibitive and Adsorption Behaviour of Carboxymethyl Cellulose on Mild Steel Corrosion in Sulphuric Acid Solution. Corros. Sci. 2010, 52, 1317–1325. [Google Scholar] [CrossRef]
- Africa, S. Adsorption and Inhibitive Properties of Ethanol Extracts of Musa Sapientum Peels as a Green Corrosion Inhibitor for Mild Steel in H2SO4. Afr. J. Pure Appl. Chem 2008, 2, 046–054. [Google Scholar]
- Thapa, B.; Gupta, D.K.; Yadav, A.P. Corrosion Inhibition of Bark Extract of Euphorbia Royleana on Mild Steel in 1 M HCl. J. Nepal Chem. Soc. 2019, 40, 25–29. [Google Scholar] [CrossRef]
- Karki, N.; Neupane, S.; Chaudhary, Y.; Gupta, D.K.; Yadav, A.P. Berberis Aristata: A Highly Efficient and Thermally Stable Green Corrosion Inhibitor for Mild Steel in Acidic Medium. Anal. Bioanal. Electrochem. 2020, 12, 970–988. [Google Scholar]
- Raja, P.B.; Qureshi, A.K.; Rahim, A.A.; Osman, H.; Awang, K. Neolamarckia Cadamba Alkaloids as Eco-Friendly Corrosion Inhibitors for Mild Steel in 1 M HCl Media. Corros. Sci. 2013, 69, 292–301. [Google Scholar] [CrossRef]
- Kamal, C.; Sethuraman, M.G. Caulerpin—A Bis-Indole Alkaloid as a Green Inhibitor for the Corrosion of Mild Steel in 1 M HCl Solution from the Marine Alga Caulerpa Racemosa. Ind. Eng. Chem. Res. 2012, 51, 10399–10407. [Google Scholar] [CrossRef]
- Raja, P.B.; Fadaeinasab, M.; Qureshi, A.K.; Rahim, A.A.; Osman, H.; Litaudon, M.; Awang, K. Evaluation of Green Corrosion Inhibition by Alkaloid Extracts of Ochrosia Oppositifolia and Isoreserpiline against Mild Steel in 1 M HCl Medium. Ind. Eng. Chem. Res. 2013, 52, 10582–10593. [Google Scholar] [CrossRef]
- Ikeuba, A.; Okafor, P.; Ekpe, U.; Ebenso, E.E. Alkaloid and Non-Alkaloid Ethanolic Extracts from Seeds of Garcinia Kola as Green Corrosion Inhibitors of Mild Steel in H2SO4 Solution. Int. J. Electrochem. Sci. 2013, 8, 7455–7467. [Google Scholar]
- Ugi, B. Alkaloid and Non Alkaloid Extracts of Solanum Melongena Leaves as Green Corrosion Inhibitors on Carbon Steel in Alkaline Medium. Fountain J. Nat. Appl. Sci. 2014, 3, 1–9. [Google Scholar] [CrossRef]
- Faustin, M.; Maciuk, A.; Salvin, P.; Roos, C.; Lebrini, M. Corrosion Inhibition of C38 Steel by Alkaloids Extract of Geissospermum Laeve in 1 M Hydrochloric Acid: Electrochemical and Phytochemical Studies. Corros. Sci. 2015, 92, 287–300. [Google Scholar] [CrossRef]
- El Hamdani, N.; Fdil, R.; Tourabi, M.; Jama, C.; Bentiss, F. Alkaloids Extract of Retama monosperma (L.) Boiss. Seeds Used as Novel Eco-Friendly Inhibitor for Carbon Steel Corrosion in 1 M HCl Solution: Electrochemical and Surface Studies. Appl. Surf. Sci. 2015, 357, 1294–1305. [Google Scholar] [CrossRef]
- Ngouné, B.; Pengou, M.; Nouteza, A.M.; Nanseu-Njiki, C.P.; Ngameni, E. Performances of Alkaloid Extract from Rauvolfia Macrophylla Stapf toward Corrosion Inhibition of C38 Steel in Acidic Media. ACS Omega 2019, 4, 9081–9091. [Google Scholar] [CrossRef]
- Sadik, K.; Hamdani, N.E.; Hachim, M.; Byadi, S.; Bahadur, I.; Aboulmouhajir, A. Towards a Theoretical Understanding of Alkaloid-Extract Cytisine Derivatives of Retama monosperma (L.) Boiss. Seeds, as Eco-Friendly Inhibitor for Carbon Steel Corrosion in Acidic 1M HCl Solution. J. Theor. Comput. Chem. 2020, 19, 2050013. [Google Scholar] [CrossRef]
- Dhakal, K.; Bohara, D.S.; Bist, B.B.; Oli, H.B.; Bhattarai, D.P.; Singh, S.; Karki, N.; Yadav, A.P. Alkaloids Extract of Alnus Nepalensis Bark as a Green Inhibitor for Mild Steel Corrosion in 1 M H2SO4 Solution. J. Nepal Chem. Soc. 2022, 43, 76–92. [Google Scholar] [CrossRef]
- Singh, A.K. Vegetables. In Wild Relatives of Cultivated Plants in India; Springer: Singapore, 2017; pp. 85–108. [Google Scholar]
- Parmar, S.; Gangwal, A.; Sheth, N. Solanum Xanthocarpum (Yellow Berried Night Shade): A Review. Der. Pharm. Lett. 2010, 2, 373–383. [Google Scholar]
- Kumar, S. Alkaloidal Drugs-A Review. Asian J. Pharm. Sci. Technol. 2014, 4, 107–119. [Google Scholar]
- Karki, N.; Choudhary, Y.; Yadav, A.P. Thermodynamic, Adsorption and Corrosion Inhibition Studies of Mild Steel by Artemisia Vulgaris Extract from Methanol as Green Corrosion Inhibitor in Acid Medium. J. Nepal Chem. Soc. 2018, 39, 76–85. [Google Scholar] [CrossRef]
- Oli, H.B.; Parajuli, D.L.; Sharma, S.; Chapagain, A.; Yadav, A.P. Adsorption Isotherm and Activation Energy of Inhibition of Alkaloids on Mild Steel Surface in Acidic Medium. Amrit Res. J. 2021, 2, 59–67. [Google Scholar] [CrossRef]
- Ijuo, G.; Chahul, H.; Eneji, I. Kinetic and Thermodynamic Studies of Corrosion Inhibition of Mild Steel Using Bridelia Ferruginea Extract in Acidic Environment. J. Adv. Electrochem. 2016, 2, 107–112. [Google Scholar]
- Silverstein, R.M.; Bassler, G.C. Spectrometric Identification of Organic Compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Shukla, S.K.; Ebenso, E.E. Corrosion Inhibition, Adsorption Behavior and Thermodynamic Properties of Streptomycin on Mild Steel in Hydrochloric Acid Medium. Int. J. Electrochem. Sci 2011, 6, 3277–3291. [Google Scholar]
- Andoor, P.A.; Okeoma, K.B.; Mbamara, U.S. Adsorption and Thermodynamic Studies of the Corrosion Inhibition Effect of Rosmarinus officinalis L. Leaves on Aluminium Alloy in 0.25 M HCl and Effect of an External Magnetic Field. Int. J. Phys. Sci. 2021, 16, 79–95. [Google Scholar]
- Li, Y.; Zhao, P.; Liang, Q.; Hou, B. Berberine as a Natural Source Inhibitor for Mild Steel in 1 M H2SO4. Appl. Surf. Sci. 2005, 252, 1245–1253. [Google Scholar] [CrossRef]
- Erami, R.S.; Amirnasr, M.; Meghdadi, S.; Talebian, M.; Farrokhpour, H.; Raeissi, K. Carboxamide Derivatives as New Corrosion Inhibitors for Mild Steel Protection in Hydrochloric Acid Solution. Corros. Sci. 2019, 151, 190–197. [Google Scholar] [CrossRef]
- Yaro, A.S.; Khadom, A.A.; Wael, R.K. Apricot Juice as Green Corrosion Inhibitor of Mild Steel in Phosphoric Acid. Alex. Eng. J. 2013, 52, 129–135. [Google Scholar] [CrossRef]
- Bhat, J.I.; Alva, V.D. Meclizine Hydrochloride as a Potential Non-Toxic Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Medium. Arch. Appl. Sci. Res 2011, 3, 343–356. [Google Scholar]
- Qiang, Y.; Zhang, S.; Tan, B.; Chen, S. Evaluation of Ginkgo Leaf Extract as an Eco-Friendly Corrosion Inhibitor of X70 Steel in HCl Solution. Corros. Sci. 2018, 133, 6–16. [Google Scholar] [CrossRef]
- Bhattarai, J. Passivation Behavior of Steel Rod and Wires of Nepal in Acidic and Alkaline Solutions. Nepal J. Sci. Technol. 2008, 9, 157–162. [Google Scholar] [CrossRef][Green Version]
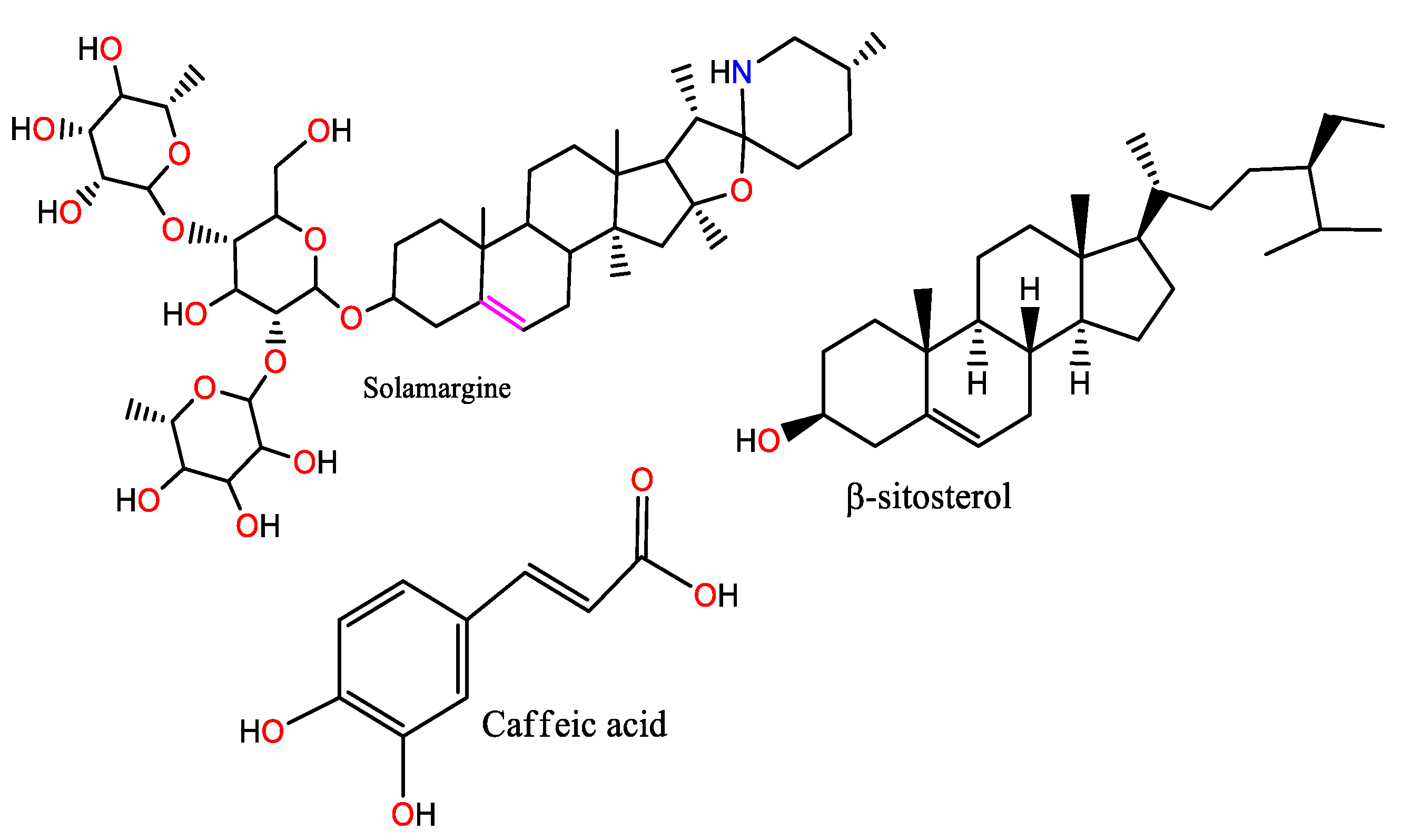
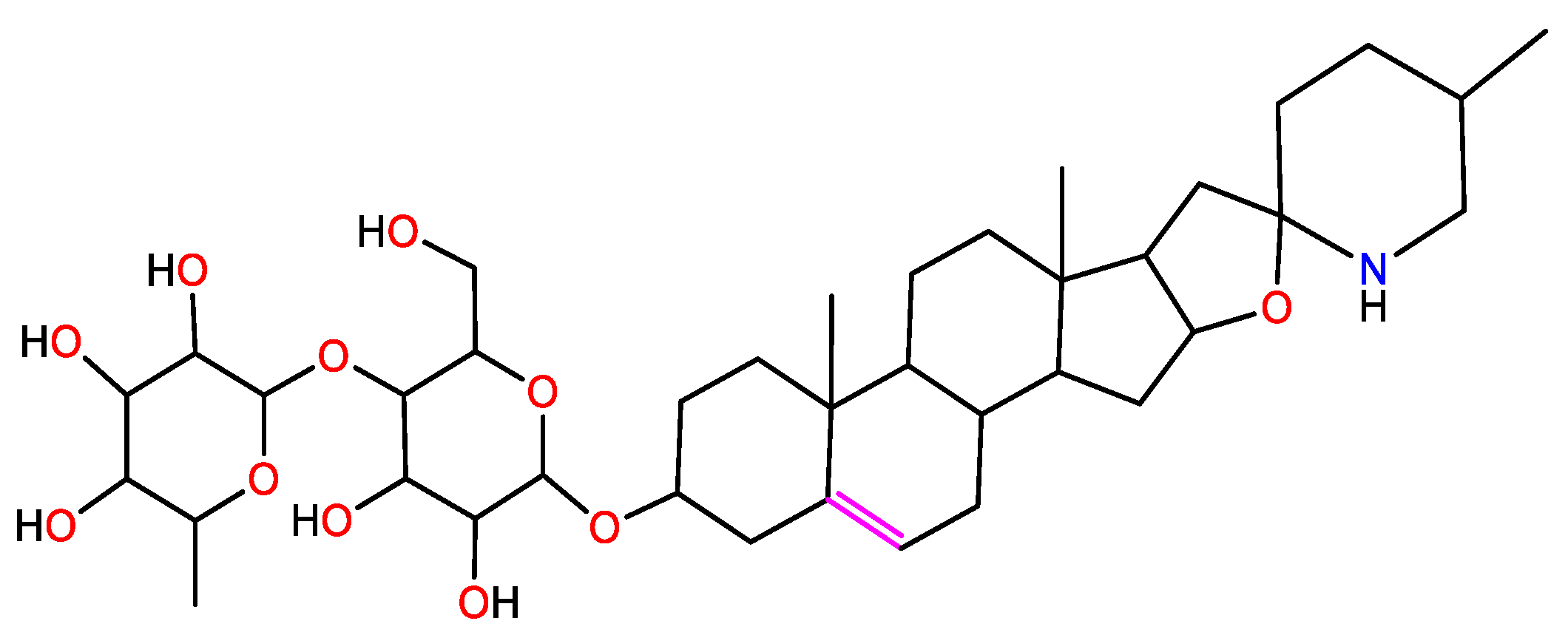

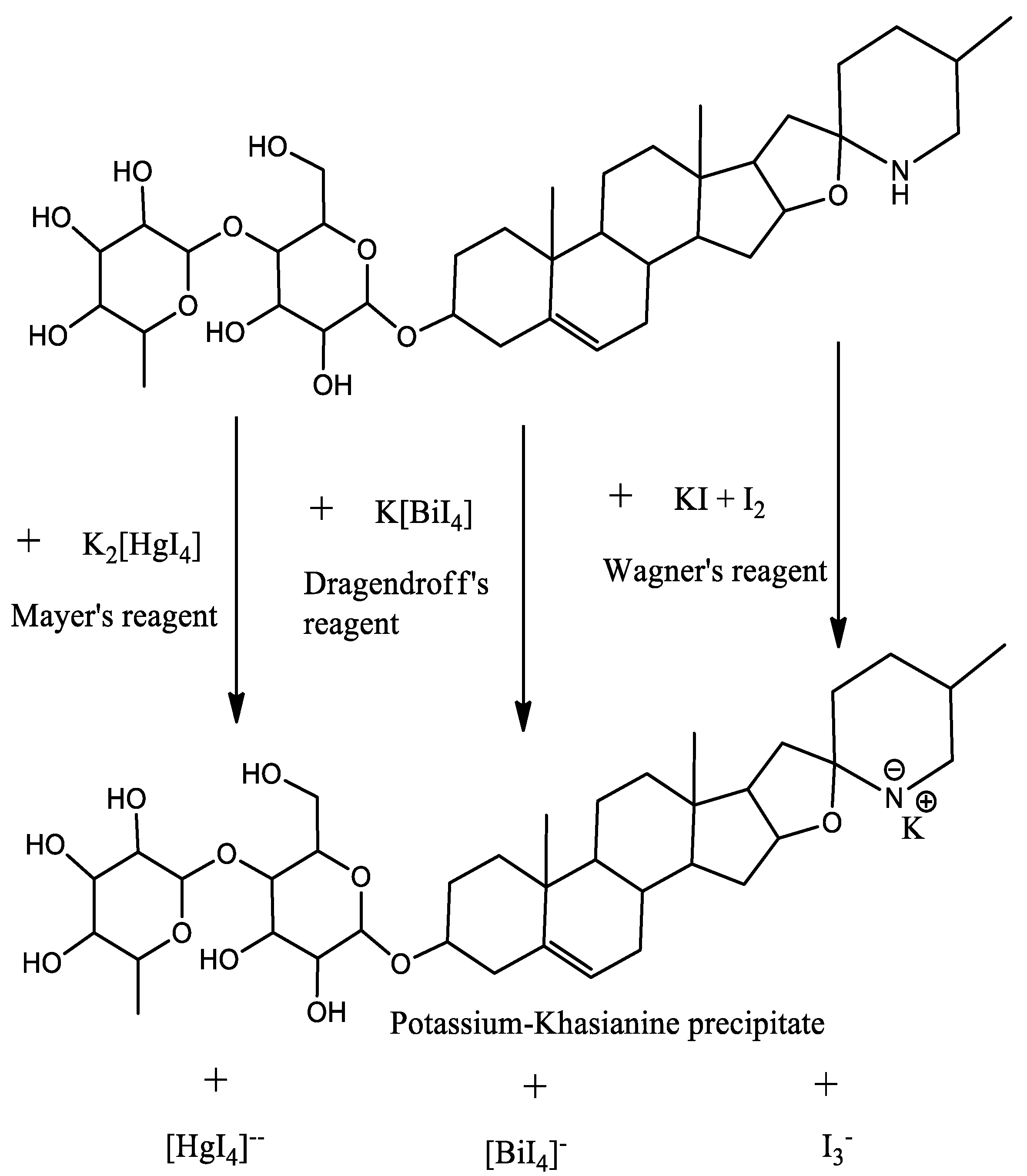






| S.N. | Plant Name | Plant Parts | Medium | Method | Efficiency, % | Reference |
|---|---|---|---|---|---|---|
| 1. | Caulerpa racemosa | Alga alkaloids | 1 M HCl | Weight loss Polarization EIS | 83 80 85 | [24] |
| 2. | Ochrosia oppositifolia | Bark and leaves alkaloids | 1 M HCl | Polarization EIS | 94 89 | [25] |
| 3. | Neolamarckia cadamba | Bark and leaves alkaloids | 1 M HCl | Polarization EIS | 89 83 | [23] |
| 4. | Garcinia kola | Seed alkaloids | 5 M H2SO4 | Hydrogen evolution Polarization | 99.4 98 | [26] |
| 5. | Solanum melongena | Leaves alkaloids | Alkaline medium Sodium trioxocarbonate | Weight loss | 81.1 | [27] |
| 6. | Geissospermum laeve | Whole plant | 1 M HCl | Polarization | 92 | [28] |
| 7. | Retama monosperma | Seed alkaloids | 1 M HCl | EIS | 94.4 | [29] |
| 8. | Fauvolfia macrophylla | Bark | 0.5 M H2SO4 1 M HCl | EIS Polarization | 93, 97 92, 93 | [30] |
| 9. | Retama monosperma | Seeds alkaloids | 1 M HCl | Electrochemical | 94.42 | [31] |
| 10. | Rhynchostylus retusa | Rhizome alkaloids | 1 M H2SO4 | Weight loss Polarization | 87.51 93.24 | [13] |
| 11. | Artemisia vulragis | Stem alkaloids | 1 M H2SO4 | Weight loss Polarization | 92.58 88.06 | [12] |
| 12. | Solanum tubersum | Stem alkaloids | 1 M H2SO4 | Weight loss Polarization | 90.79 83.22 | [12] |
| 13. | Alnus nepalensis | Bark alkaloids | 1 M H2SO4 | Weight loss Polarization | 71.94 78.48 | [32] |
| 14. | Acacia catechu | Bark Alkaloids | 1 M H2SO4 | Weight loss Polarization | 93.96 98.54 | [7] |
| 15. | Coriaria nepalensis | Stem Alkaloids | 1 M H2SO4 | Weight loss Polarization | 96.41 97.03 | [8] |
| S.N. | Experiment | Observation | Result | Inference |
|---|---|---|---|---|
| 1. | Mayer’s Test | Orange precipitate | Alkaloids presence | Positive |
| 2. | Dragendroff ’s Test | Orange-red precipitate | Alkaloids presence | Positive |
| 3. | Wagner’s Test | Reddish brown precipitate | Alkaloids presence | Positive |
| Isotherm | Graph | Immersion Time (h) | Slope | Intercept | R2 | Kads | −ΔG (kJ/mol) |
|---|---|---|---|---|---|---|---|
| Langmuir | C/θ vs. C | 1 | 1.0126 | 0.00006 | 0.9974 | 16,666 | 34.37 |
| 6 | 0.9007 | 0.0002 | 0.9959 | 5000 | 31.36 | ||
| 24 | 0.72 | 0.0004 | 0.9999 | 2500 | 29.63 | ||
| Freundlich | ln θ vs. ln C | 1 | 0.0995 | 0.616 | 0.8919 | ||
| 6 | 0.3126 | 2.0945 | 0.9909 | ||||
| 24 | 0.5477 | 3.7131 | 0.9934 | ||||
| Temkin | θ vs. ln C | 1 | 0.0865 | 1.5281 | 0.8872 | ||
| 6 | 0.2296 | 2.5101 | 0.9875 | ||||
| 24 | 0.3315 | 3.1834 | 0.9995 |
| Inhibitor Solution (ppm) | Log (A) | Ea (kJ mol−1) | ΔH° (kJ mol−1) | Ea-ΔH° | ΔS° (J mol−1 K−1) |
|---|---|---|---|---|---|
| 0 | 8.5884 | 63.74 | 61.12 | 2.62 | −89.304 |
| 200 | 15.671 | 108.72 | 106.1 | 2.62 | 46.298 |
| 400 | 15.044 | 105.96 | 103.34 | 2.62 | 34.312 |
| 600 | 14.861 | 106.55 | 103.92 | 2.63 | 30.808 |
| 800 | 14.248 | 103.48 | 100.86 | 2.62 | 19.071 |
| Inhibitor Solution (ppm) | OCP (V) | Icorr (mA/cm2) | Anodic Slope (mA/cm2/V) | Cathodic Slope (mA/cm2/V) | Efficiency (%) |
|---|---|---|---|---|---|
| 0 | −0.552 | 0.0477 | 12.83 | −5.46 | |
| 200 | −0.521 | 0.0318 | 10.87 | −5.19 | 33.33 |
| 400 | −0.518 | 0.0209 | 21.15 | −5.7 | 56.18 |
| 600 | −0.521 | 0.016 | 13.78 | −6.52 | 66.46 |
| 800 | −0.526 | 0.0144 | 17.06 | −7.3 | 69.81 |
| 1000 | −0.532 | 0.006 | 15.01 | −4.15 | 87.42 |
| Inhibitor Solution (ppm) | OCP (V) | Icorr (mA/cm2) | Anodic Slope (mA/cm2/V) | Cathodic Slope (mA/cm2/V) | Efficiency (%) |
|---|---|---|---|---|---|
| 0 | −0.545 | 0.102 | 15.42 | −4.29 | |
| 200 | −0.52 | 0.027 | 10.65 | −5.6 | 73.53 |
| 400 | −0.529 | 0.017 | 14.19 | −5.9 | 83.33 |
| 600 | −0.526 | 0.0029 | 25.26 | −6.32 | 97.16 |
| 800 | −0.52 | 0.0025 | 18.5 | −4.9 | 97.55 |
| 1000 | −0.537 | 0.0019 | 12.5 | −4.8 | 98.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, O.; Thapa Magar, J.; Oli, H.B.; Rajaure, A.; Nepali, D.; Bhattarai, D.P.; Mukhiya, T. Alkaloids of Solanum xanthocarpum Stem as Green Inhibitor for Mild Steel Corrosion in One Molar Sulphuric Acid Solution. Electrochem 2022, 3, 820-842. https://doi.org/10.3390/electrochem3040054
Thapa O, Thapa Magar J, Oli HB, Rajaure A, Nepali D, Bhattarai DP, Mukhiya T. Alkaloids of Solanum xanthocarpum Stem as Green Inhibitor for Mild Steel Corrosion in One Molar Sulphuric Acid Solution. Electrochem. 2022; 3(4):820-842. https://doi.org/10.3390/electrochem3040054
Chicago/Turabian StyleThapa, Onisha, Jamuna Thapa Magar, Hari Bhakta Oli, Anil Rajaure, Durga Nepali, Deval Prasad Bhattarai, and Tanka Mukhiya. 2022. "Alkaloids of Solanum xanthocarpum Stem as Green Inhibitor for Mild Steel Corrosion in One Molar Sulphuric Acid Solution" Electrochem 3, no. 4: 820-842. https://doi.org/10.3390/electrochem3040054
APA StyleThapa, O., Thapa Magar, J., Oli, H. B., Rajaure, A., Nepali, D., Bhattarai, D. P., & Mukhiya, T. (2022). Alkaloids of Solanum xanthocarpum Stem as Green Inhibitor for Mild Steel Corrosion in One Molar Sulphuric Acid Solution. Electrochem, 3(4), 820-842. https://doi.org/10.3390/electrochem3040054








