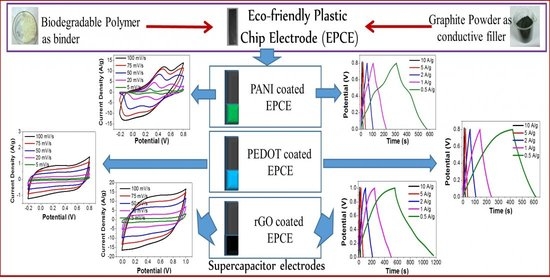
A Biodegradable Polymer-Based Plastic Chip Electrode as a Current Collector in Supercapacitor Application
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
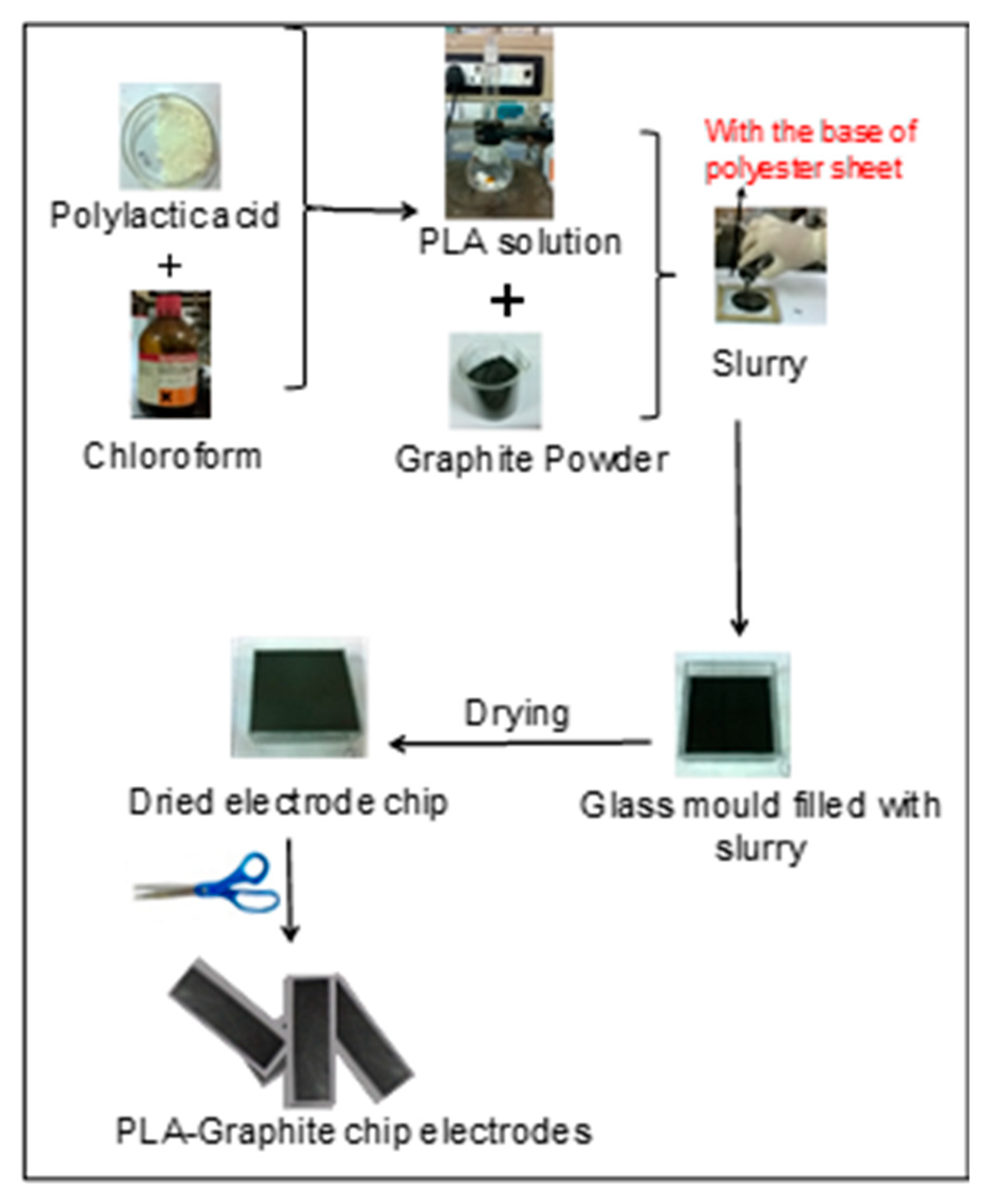
2.2. Preparation of EPCEs of PLA-Graphite (PLAGr)
2.3. Spectroscopic and Morphological Characterizations
2.4. Electrochemical Deposition of PANI on PLA4Gr6 (PLA4Gr6/PANI) Electrodes
2.5. Electrochemical Deposition of PEDOT on PLA4Gr6 (PLA4Gr6/PEDOT) Electrodes
2.6. Electrochemical Characterizations for Supercapacitive Electrodes
2.7. Synthesis of Graphene Oxides (GO), Their Purification and Reduction to rGO (Reduced Graphene Oxide)
3. Results
3.1. Bulk Conductivity of Different Compositions of PLAGr
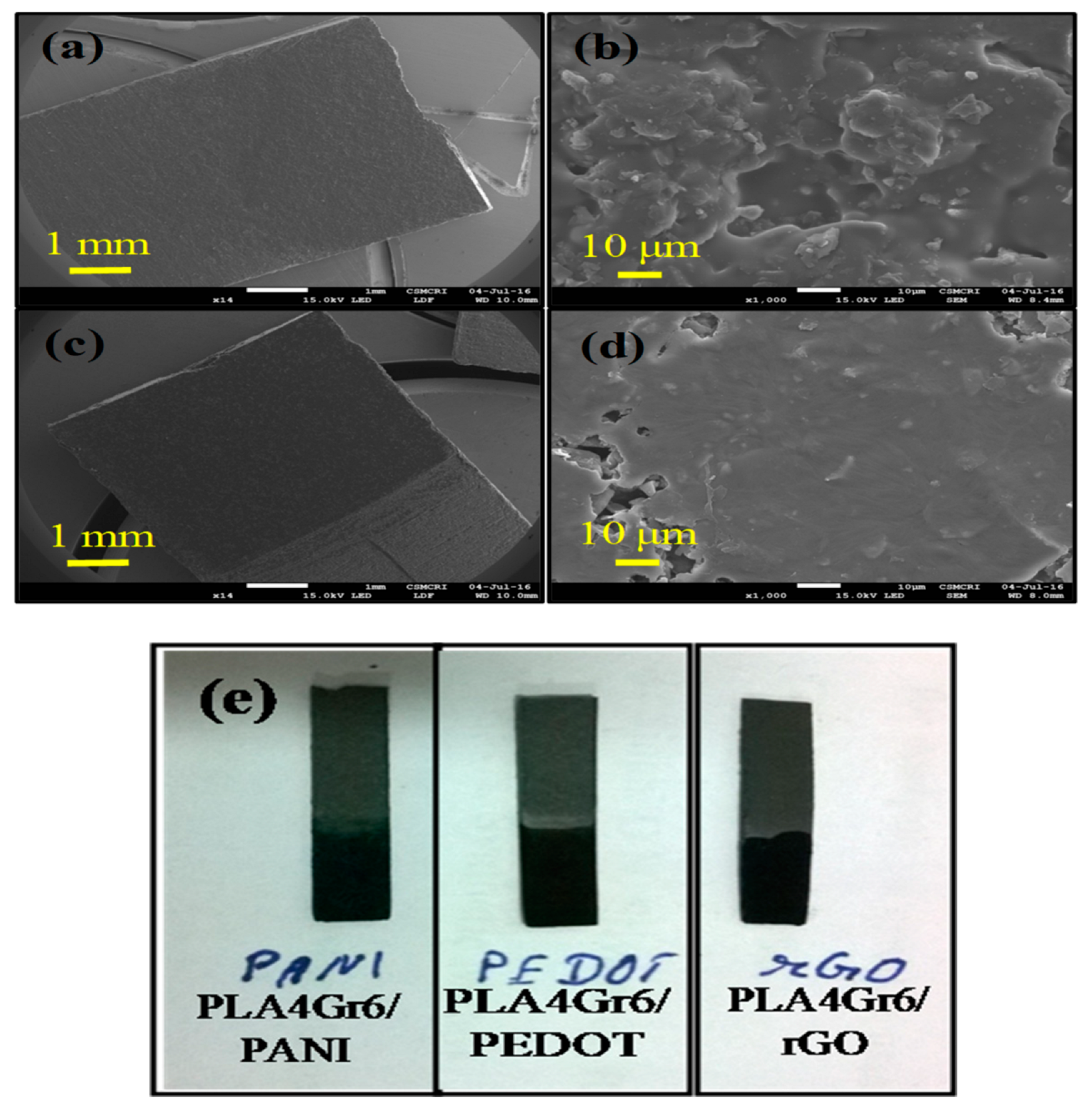
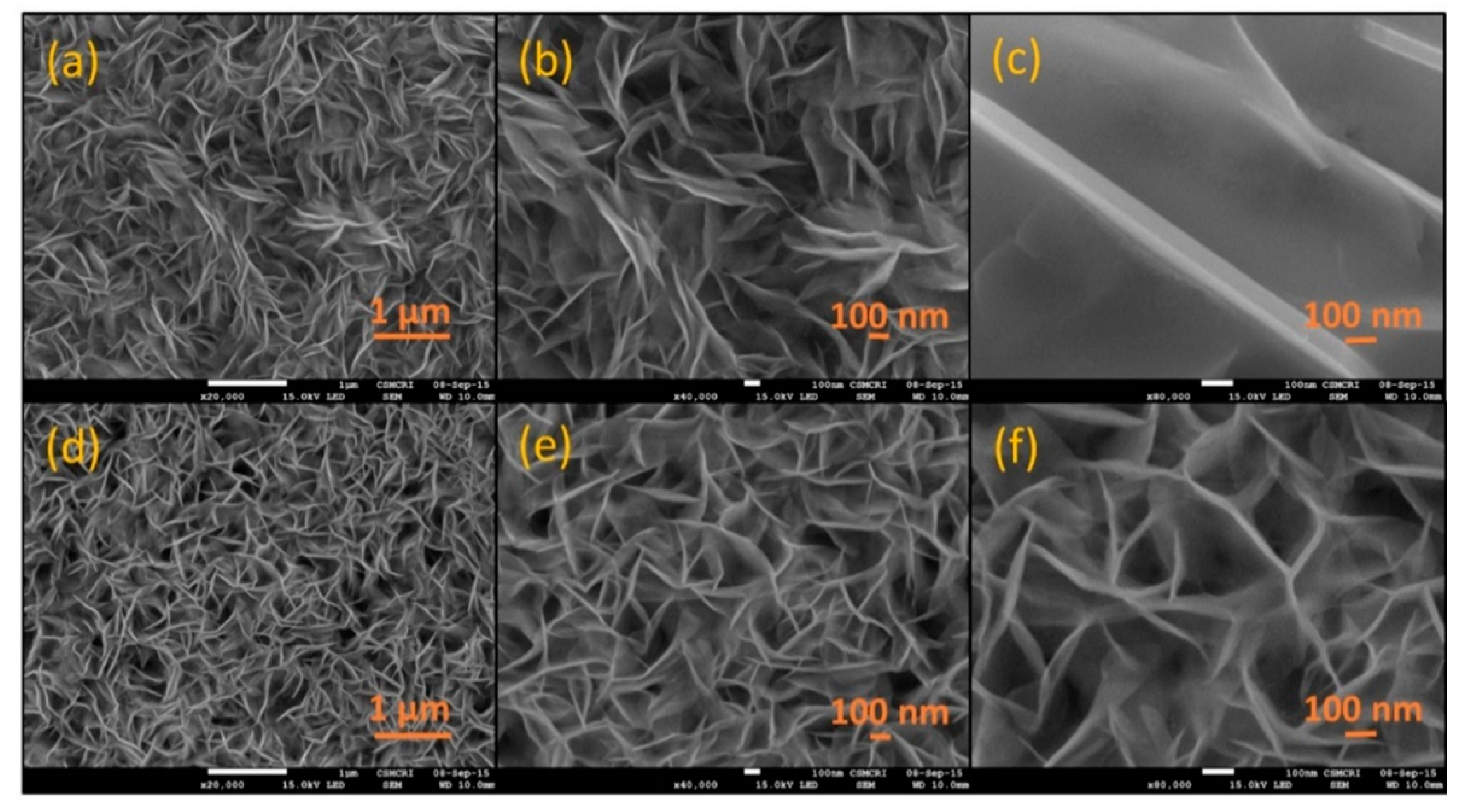
3.2. Scanning Electron Microscopy of PANI and PEDOT Coated Polymer/Graphite Chip Electrodes
3.3. XRD, Raman, SEM, TEM, TGA, FT-IR Characterizations of Prepared GO1 and GO2
3.3.1. XRD of Graphite, GO1, and GO2
3.3.2. Scanning Electron Microscopy
3.3.3. Raman Spectroscopy
3.3.4. Thermogravimetric Analysis
3.3.5. Transmission Electron Microscopy
3.3.6. FT-IR Spectroscopy
3.4. XRD, Raman, TGA, FT-IR and Electrical Conductivity Characterizations of rGO
3.5. Electrochemical Studies of Supercapacitor Electrodes
3.5.1. PANI on Polylactic Acid-Graphite Chip Electrode (PLA4Gr6/PANI)
3.5.2. PEDOT on Polylactic Acid-Graphite Chip Electrodes (PLA4Gr6/PEDOT)
3.5.3. rGO Coated PLA4Gr6 Electrodes (PLA4Gr6/rGO)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vatamanu, J.; Bedrov, D. Capacitive Energy Storage: Current and Future Challenges. J. Phys. Chem. Lett. 2015, 6, 3594–3609. [Google Scholar] [CrossRef] [PubMed]
- Kirti; Nandha, N.; Singh, P.S.; Srivastava, D.N. Improved OER Performance on the Carbon Composite Electrode through Tailored Wettability. ACS Appl. Energy Mater. 2021, 4, 9618–9626. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, J.; Zhang, Z.; Chen, X.; Guan, G.; Qiu, L.; Zhang, Y.; Peng, H. Recent Advancement of Nanostructured Carbon for Energy Applications. Chem. Rev. 2015, 115, 5159–5223. [Google Scholar] [CrossRef] [PubMed]
- Shown, I.; Ganguly, A.; Chen, L.-C.; Chen, K.H. Conducting polymer-based flexible supercapacitor. Energy Sci. Eng. 2015, 3, 2–26. [Google Scholar] [CrossRef]
- Ahamed, S.T.; Kulsi, C.; Kirti; Banerjee, D.; Srivastava, D.N.; Mondal, A. Synthesis of multifunctional CdSe and Pd quantum dot decorated CdSe thin films for photocatalytic, electrocatalytic and thermoelectric applications. Surf. Interfaces 2021, 25, 101149. [Google Scholar] [CrossRef]
- Xie, Q.; Bao, R.; Zheng, A.; Zhang, Y.; Wu, S.; Xie, C.; Zhao, P. Sustainable low-cost green electrodes with high volumetric capacitance for aqueous symmetric supercapacitors with high energy density. ACS Sustain. Chem. Eng. 2016, 4, 1422–1430. [Google Scholar] [CrossRef]
- Ghosh, S.; Maiyalagan, T.; Basu, R.N. Nanostructured conducting polymers for energy applications: Towards a sustainable platform. Nanoscale 2016, 8, 6921–6947. [Google Scholar] [CrossRef]
- Holze, R.; Wu, Y. Intrinsically conducting polymers in electrochemical energy technology: Trends and progress. Electrochim. Acta 2014, 122, 93–107. [Google Scholar] [CrossRef]
- Polisetti, V.; Ray, P. Nanoparticles modified Polyacrylonitrile/Polyacrylonitrile–Polyvinylidenefluoride blends as substrate of high flux anti-fouling nanofiltration membranes. J. Appl. Polym. Sci. 2021, 138, 50228. [Google Scholar] [CrossRef]
- Jugović, B.; Gvozdenović, M.; Stevanović, J.; Trišović, T.; Grgur, B. Characterization of electrochemically synthesized PANI on graphite electrode for potential use in electrochemical power sources. Mater. Chem. Phys. 2009, 114, 939–942. [Google Scholar] [CrossRef] [Green Version]
- Çağlar, M.; Arslan, A.; Kılıç, R.; Hür, E. Electrochemically synthesized Sn2+ doped poly (3-methylthiophene) and poly (3,4-ethylenedioxythiophene) for supercapacitors. Synth. Met. 2015, 206, 8–14. [Google Scholar] [CrossRef]
- Sowmya; Sudhakar, Y.; Selvakumar, M. Supercapacitor studies of electrochemically synthesized multi-layered polyaniline on stainless steel substrate. Ionics 2016, 22, 1729–1739. [Google Scholar] [CrossRef]
- Patra, S.; Barai, K.; Munichandraiah, N. Scanning electron microscopy studies of PEDOT prepared by various electrochemical routes. Synth. Met. 2008, 158, 430–435. [Google Scholar] [CrossRef]
- Mrada, M.; Dhouibia, L. Corrosion prevention of mild steel in a simulated concrete medium by the electro-application of a polyaniline coating. J. Tunis. Chem. Soc. 2017, 19, 359–367. [Google Scholar]
- Rahman, M.; Sarmah, T.; Dihingia, P.; Verma, R.; Sharma, S.; Kirti; Srivastava, D.N.; Pandey, L.M.; Kakati, M. Bulk synthesis of tungsten-oxide nanomaterials by a novel, plasma chemical reactor configuration, studies on their performance for waste-water treatment and hydrogen evolution reactions. Chem. Eng. J. 2022, 428, 131111. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Q.; Wang, X.; Xiang, Q.; Liang, B.; Chen, D.; Shen, G. Flexible asymmetric supercapacitors based upon Co9S8 nanorod//Co3O4@RuO2 nanosheet arrays on carbon cloth. ACS Nano 2013, 7, 5453–5462. [Google Scholar] [CrossRef]
- Nyholm, L.; Nyström, G.; Mihranyan, A.; Strømme, M. Toward Flexible Polymer and Paper-Based Energy Storage Devices. Adv. Mater. 2011, 23, 3751–3769. [Google Scholar] [CrossRef]
- Kühnel, R.-S.; Reiter, J.; Jeong, S.; Passerini, S.; Balducci, A. Anodic stability of aluminum current collectors in an ionic liquid based on the (fluorosulfonyl)(trifluoromethanesulfonyl)imide anion and its implication on high voltage supercapacitors. Electrochem. Commun. 2014, 38, 117–119. [Google Scholar] [CrossRef]
- Duffy, N.W.; Baldsing, W.; Pandolfo, A.G. The nickel–carbon asymmetric supercapacitor—Performance, energy density and electrode mass ratios. Electrochim. Acta 2008, 54, 535–539. [Google Scholar] [CrossRef]
- Wu, X.-L.; Wen, T.; Guo, H.-L.; Yang, S.; Wang, X.; Xu, A.-W. Biomass-Derived Sponge-like Carbonaceous Hydrogels and Aerogels for Supercapacitors. ACS Nano 2013, 7, 3589–3597. [Google Scholar] [CrossRef]
- Yu, J.; Wu, J.; Wang, H.; Zhou, A.; Huang, C.; Bai, H.; Li, L. Metallic fabrics as the current collector for high-performance graphene-based flexible solid-state supercapacitor. ACS Appl. Mater. Interfaces 2016, 8, 4724–4729. [Google Scholar] [CrossRef] [PubMed]
- Polisetti, V.; Ray, P. Nano SiO2 and TiO2 embedded polyacrylonitrile/polyvinylidene fluoride ultrafiltration membranes: Improvement in flux and antifouling properties. J. Appl. Polym. Sci. 2021, 138, 49606. [Google Scholar] [CrossRef]
- Zhang, C.; Zhai, T.; Turng, L.S.; Dan, Y. Morphological, mechanical, and crystallization behavior of polylactide/polycaprolactone blends compatibilized by L-lactide/caprolactone copolymer. Ind. Eng. Chem. Res. 2015, 54, 9505–9511. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Jafarzadeh, S.; Khodaei, D.; Cacciotti, I.; Zargar, M.; Razavi, S.H. Tuning the Physicochemical, Structural, and Antimicrobial Attributes of Whey-Based Poly (L-Lactic Acid)(PLLA) Films by Chitosan Nanoparticles. Front. Nutr. 2022, 9, 880520. [Google Scholar] [CrossRef]
- Perween, M.; Parmar, D.B.; Bhadu, G.R.; Srivastava, D.N. Polymer–graphite composite: A versatile use and throw plastic chip electrode. Analyst 2014, 139, 5919–5926. [Google Scholar] [CrossRef]
- Camalet, J.-L.; Lacroix, J.-C.; Nguyen, T.; Aeiyach, S.; Pham, M.; Petitjean, J.; Lacaze, P.-C. Aniline electropolymerization on platinum and mild steel from neutral aqueous media. J. Electroanal. Chem. 2000, 485, 13–20. [Google Scholar] [CrossRef]
- Kazum, O.; Kannan, M.B. Optimising parameters for galvanostatic polyaniline coating on nanostructured bainitic steel. Surf. Eng. 2016, 32, 607–614. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Shishov, M.; Moshnikov, V.A.; Sapurina, I.Y. Self-organization of polyaniline during oxidative polymerization: Formation of granular structure. Chem. Pap. 2013, 67, 909–918. [Google Scholar] [CrossRef]
- Polisetti, V.; Naidu, S.; Kansara, A.M.; Ray, P.; Singh, P.S. An instant oil separation by octadecyl-polysiloxane-reticulated recyclable superhydrophobic polyester fabric. Environ. Technol. Innov. 2021, 21, 101322. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Shi, M.; Lu, X.; Qin, C.; Li, C.; Ye, M. Fast and Facile Preparation of Graphene Oxide and Reduced Graphene Oxide Nanoplatelets. Chem. Mater. 2009, 21, 3514–3520. [Google Scholar] [CrossRef]
- Jeong, H.-K.; Lee, Y.P.; Lahaye, R.J.W.E.; Park, M.-H.; An, K.H.; Kim, I.J.; Yang, C.-W.; Park, C.Y.; Ruoff, A.R.S.; Lee, Y.H. Evidence of Graphitic AB Stacking Order of Graphite Oxides. J. Am. Chem. Soc. 2008, 130, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.; Upadhyay, J.; Polychronopoulou, K.; Umer, R.; Das, R. Reduced Graphene Oxide: Effect of Reduction on Electrical Conductivity. J. Compos. Sci. 2018, 2, 25. [Google Scholar] [CrossRef] [Green Version]
- Prakash, R. Electrochemistry of polyaniline: Study of the pH effect and electrochromism. J. Appl. Polym. Sci. 2002, 83, 378–385. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, M.; Fan, L.Z.; Li, P.; Qu, X. Porous polyaniline exhibits highly enhanced electrochemical capacitance performance. Electrochim. Acta 2010, 55, 5819–5822. [Google Scholar] [CrossRef]
- Prasad, K.R.; Munichandraiah, N. Fabrication and evaluation of 450 F electrochemical redox supercapacitors using inexpensive and high-performance, polyaniline coated, stainless-steel electrodes. J. Power Sources 2002, 112, 443–451. [Google Scholar] [CrossRef]
- Gawli, Y.; Banerjee, A.; Dhakras, D.; Deo, M.; Bulani, D.; Wadgaonkar, P.; Shelke, M.; Ogale, S. 3D Polyaniline Architecture by Concurrent Inorganic and Organic Acid Doping for Superior and Robust High Rate Supercapacitor Performance. Sci. Rep. 2016, 6, 21002. [Google Scholar] [CrossRef] [Green Version]
- Bhat, D.K.; Kumar, M.S. N and p doped poly(3,4-ethylenedioxythiophene) electrode materials for symmetric redox supercapacitors. J. Mater. Sci. 2007, 42, 8158–8162. [Google Scholar] [CrossRef]
- Conway, B.E.; Supercapacitors, I.E. Scientific Fundamentals and Technological Applications; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; p. 528. [Google Scholar]
- Hong, J.I.; Yeo, I.H.; Paik, W.K. Conducting polymer with metal oxide for electrochemical capacitor: Poly (3,4-ethylenedioxythiophene) RuOx electrode. J. Electrochem. Soc. 2001, 148, A156–A163. [Google Scholar] [CrossRef]
- Ferraris, J.; Eissa, M.; Brotherston, I.; Loveday, D.; Moxey, A. Preparation and electrochemical evaluation of poly (3-phenylthiophene) derivatives: Potential materials for electrochemical capacitors. J. Electroanal. Chem. 1998, 459, 57–69. [Google Scholar] [CrossRef]
- Liu, K.; Hu, Z.; Xue, R.; Zhang, J.; Zhu, J. Electropolymerization of high stable poly(3,4-ethylenedioxythiophene) in ionic liquids and its potential applications in electrochemical capacitor. J. Power Sources 2008, 179, 858–862. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, G.; Yan, K.; Yan, J.; Wang, J. Binder-free porous PEDOT electrodes for flexible supercapacitors. J. Appl. Polym. Sci. 2015, 132, 1–9. [Google Scholar] [CrossRef]
- Tong, L.; Skorenko, K.H.; Faucett, A.C.; Boyer, S.M.; Liu, J.; Mativetsky, J.M.; Bernier, W.E.; Jones, W.E., Jr. Vapor-phase polymerization of poly (3,4-ethylenedioxythiophene) (PEDOT) on commercial carbon coated aluminum foil as enhanced electrodes for supercapacitors. J. Power Sources 2015, 297, 195–201. [Google Scholar] [CrossRef]
- Rath, T.; Kundu, P.P. Reduced graphene oxide paper based nanocomposite materials for flexible supercapacitors. RSC Adv. 2015, 5, 26666–26674. [Google Scholar] [CrossRef]
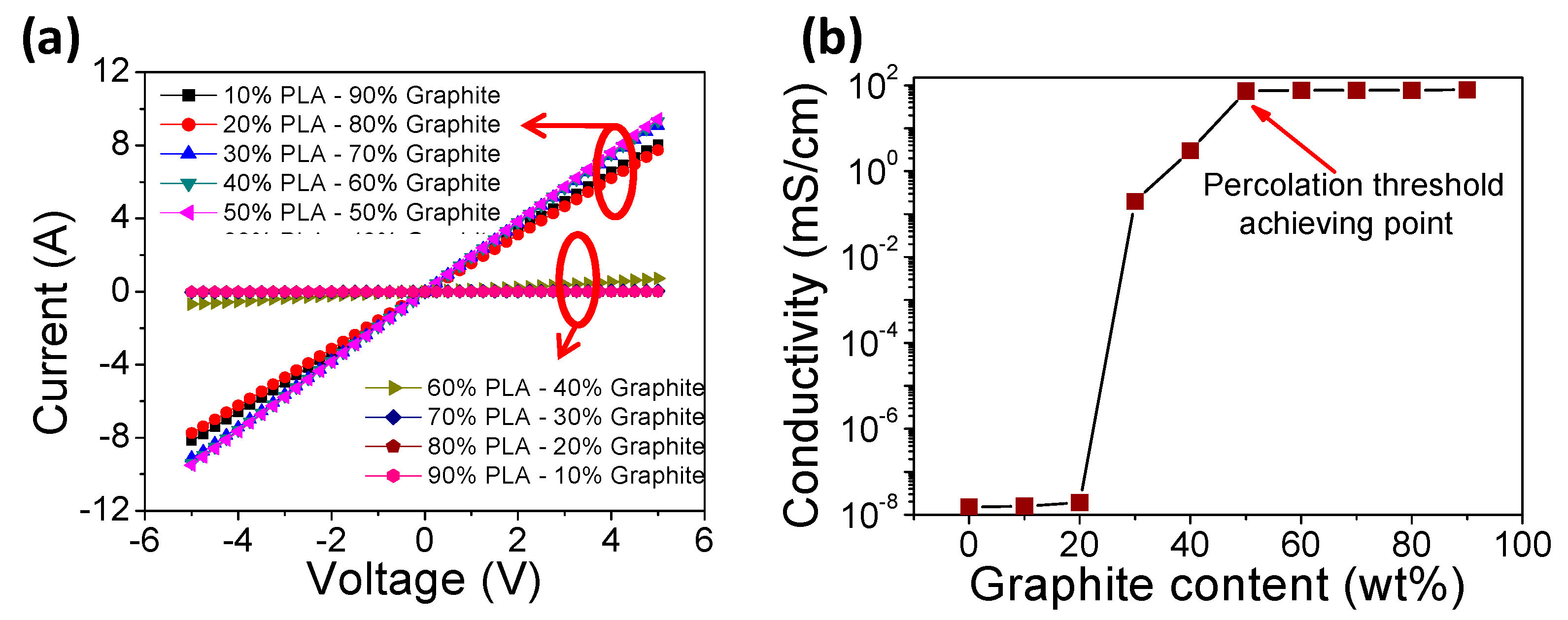




| Sr. No. | Sample Code | Composition of PLAGr Chip (w/w%) | Bulk Conductivity (mS/cm) |
|---|---|---|---|
| 1. | PLA1Gr9 | PLA (10%):Graphite (90%) | 79.0 ± 2 |
| 2. | PLA2Gr8 | PLA (20%):Graphite (80%) | 77.0 ± 1 |
| 3. | PLA3Gr7 | PLA (30%):Graphite (70%) | 76.5 ± 0.5 |
| 4. | PLA4Gr6 | PLA (40%):Graphite (60%) | 76.4 ± 1 |
| 5. | PLA5Gr5 | PLA (50%):Graphite (50%) | 74.0 ± 0.5 |
| 6. | PLA6Gr4 | PLA (60%):Graphite (40%) | 3.0 ± 1 |
| 7. | PLA7Gr3 | PLA (70%):Graphite (30%) | 0.2 ± 0.5 |
| 8. | PLA8Gr2 | PLA (80%):Graphite (20%) | 1.9 × 10−8 ± 0.2 |
| 9. | PLA9Gr1 | PLA (90%):Graphite (10%) | 1.6 × 10−8 ± 0.1 |
| Sr. No. | Frequency (Hz) | Phase Angle (Deg.) | ||
|---|---|---|---|---|
| PLA4Gr6/ PANI | PLA4Gr6/ PEDOT | PLA4Gr6/ rGO | ||
| 1 | 0.01 | 71.30 | - | - |
| 2 | 0.13 | 36.08 | 75.64 | 44.83 |
| 3 | 1.02 | 10.15 | 23.80 | 06.68 |
| 4 | 13.39 | 01.64 | 03.64 | 01.84 |
| 5 | 104.78 | 00.40 | 00.17 | 00.71 |
| 6 | 1371.01 | 00.07 | 00.09 | 00.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirti; Gupta, R.; Srivastava, D.N. A Biodegradable Polymer-Based Plastic Chip Electrode as a Current Collector in Supercapacitor Application. Electrochem 2022, 3, 379-396. https://doi.org/10.3390/electrochem3030026
Kirti, Gupta R, Srivastava DN. A Biodegradable Polymer-Based Plastic Chip Electrode as a Current Collector in Supercapacitor Application. Electrochem. 2022; 3(3):379-396. https://doi.org/10.3390/electrochem3030026
Chicago/Turabian StyleKirti, Rajeev Gupta, and Divesh N. Srivastava. 2022. "A Biodegradable Polymer-Based Plastic Chip Electrode as a Current Collector in Supercapacitor Application" Electrochem 3, no. 3: 379-396. https://doi.org/10.3390/electrochem3030026
APA StyleKirti, Gupta, R., & Srivastava, D. N. (2022). A Biodegradable Polymer-Based Plastic Chip Electrode as a Current Collector in Supercapacitor Application. Electrochem, 3(3), 379-396. https://doi.org/10.3390/electrochem3030026