Enhancement of the Negative Capacitance Associated with the Dissolution of Silver by Salt Concentrations by Means of Anodic Stripping Voltammetry
Abstract
:1. Introduction
2. Experimental Section
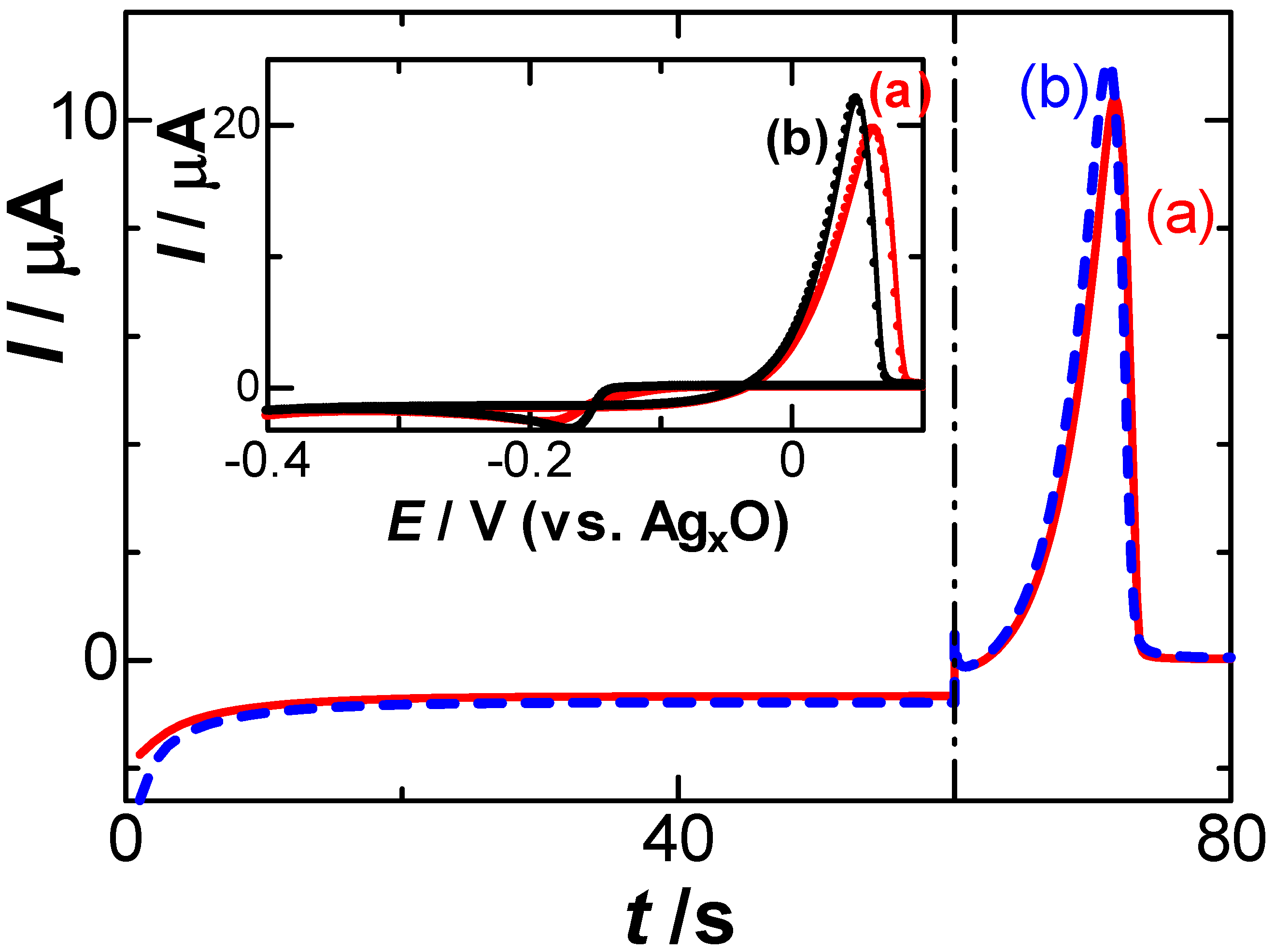
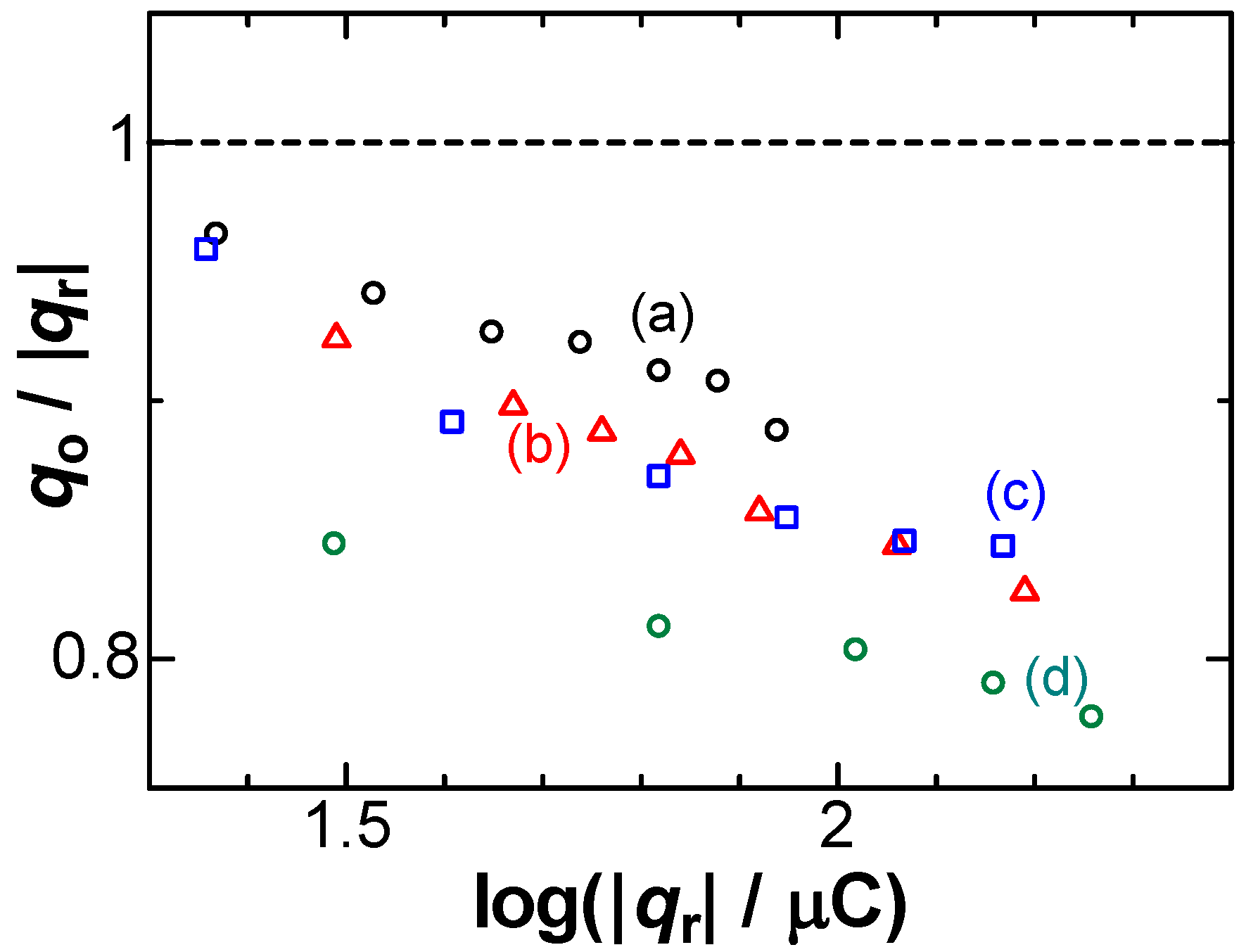
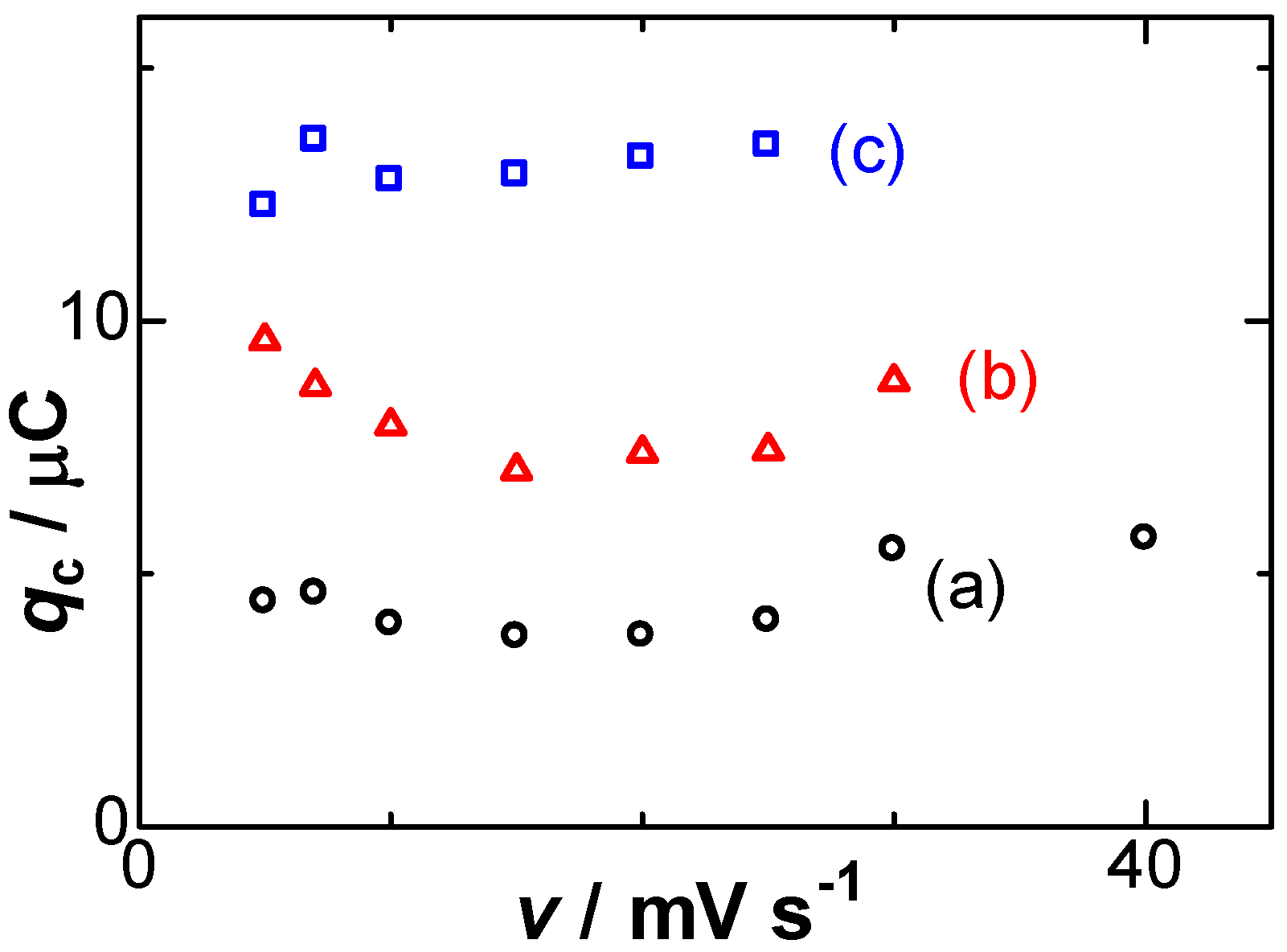
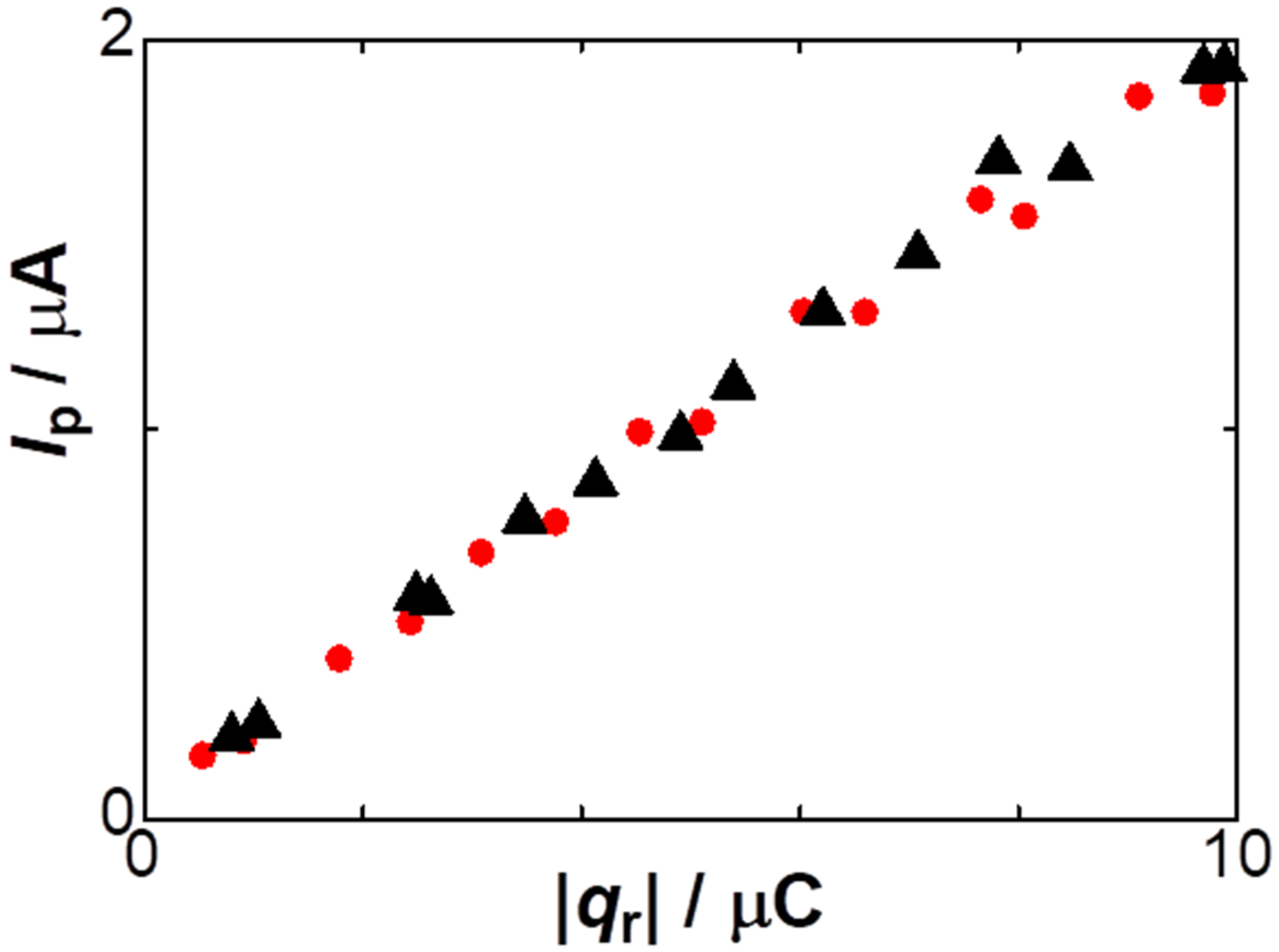
3. Results
4. Theory for Anodic Charge
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Variables
| c- | concentration of NO3−, mol m−3 |
| F | Faraday’s constant, C mol−1 |
| j | total current density, A m−2 |
| jc | capacitive current density, A m−2 |
| jN | current density for Nernstian dispersion, A m−2 |
| qo | charge of dissolved Ag, C |
| qr | deposited charge, C |
| qc | capacitive charge by the dipole, given by ∫(jN2/jc)dt, C |
| R | gas constant, J mol−1 K−1 |
| T | temperature, K |
| t | time, s |
| U | kinetic (motional) energy of a dipole, J |
| utr | transferring velocity of a dipole for random walk, m s−1 |
| v | potential scan rate, V s−1 |
| Γ | surface concentration of Ag+, mol m−2 |
| Γ ο | standard amount at the standard potential, Eo, mol m−2 |
| τ | lifespan, of a dipole before dispersion by diffusion, s |
| τo | lifespan at the standard concentration, co, s |
References
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; John Willey & Sons: New York, NY, USA, 2001; pp. 143–592. [Google Scholar]
- Dickinson, E.J.F.; Compton, R.G. Influence of the diffuse double layer on steady-state voltammetry. J. Electroanal. Chem. 2011, 661, 198–212. [Google Scholar] [CrossRef]
- Yan, D.; Bazant, M.Z.; Biesheuvel, P.M.; Pugh, M.C.; Dawson, F.P. Theory of linear sweep voltammetry with diffuse charge: Unsupported electrolytes, thin films, and leaky membranes. Phys. Rev. E 2017, 95, 033303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moya, A.A.; Sistat, P. Reaching the limiting current regime by linear sweep voltammetry in ion-exchange membrane systems. J. Membr. Sci. 2018, 555, 134–145. [Google Scholar] [CrossRef]
- Tan, S.-Y.; Perry, D.; Unwin, P.R. Double layer effects in voltammetric measurements with scanning electrochemical microscopy (SECM). J. Electroanal. Chem. 2018, 819, 240–250. [Google Scholar] [CrossRef] [Green Version]
- Newman, J.S. Electrochemical Systems; Prentice-Hall: Upper Saddle River, NJ, USA, 1973; p. 344. [Google Scholar]
- Britz, D. The Laplace Equation and other Steady-State Systems. In Digital Simulation in Electrochemistry; Lecture Notes in Chemistry; Springer: Berlin/Heidelberg, Germany, 1981; Volume 23. [Google Scholar] [CrossRef]
- Guo, Z.; Jiang, X. An Integrated Dual Ultramicroelectrode with Lower Solution Resistance Applied in Ultrafast Cyclic Voltammetry. Anal. Sci. 2005, 21, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Batchelor-McAuley, C.; Ngamchuea, K.; Compton, R.G. Simulated low-support voltammetry: Deviations from Ohm’s Law. J. Electroanal. Chem. 2018, 830–831, 88–94. [Google Scholar] [CrossRef]
- Henley, I.E.; Fisher, A.C. Computational Electrochemistry: The Simulation of Voltammetry in Microchannels with Low Conductivity Solutions. J. Phys. Chem. B 2003, 107, 6579–6585. [Google Scholar] [CrossRef]
- Atkins, P.; Paula, J.D. Atkin’s Physical Chemistry, 8th ed.; W. H. Freeman and Company: New York, NY, USA, 2006; p. 762. [Google Scholar]
- Ciszkowska, M.; Osteryoung, J.G. Voltammetry of Metals at Mercury Film Microelectrodes in the Absence and the Presence of Varying Concentrations of Supporting Electrolyte. Anal. Chem. 1995, 67, 1125–1131. [Google Scholar] [CrossRef]
- Bond, A.M.; Lay, P.A. Cyclic voltammetry at microelectrodes in the absence of added electrolyte using a platinum quasi-reference electrode. J. Electroanal. Chem. 1986, 199, 285–295. [Google Scholar] [CrossRef]
- Cassidy, J.; Khoo, S.B.; Pons, S.; Fleischmann, M. Electrochemistry at very high potentials: The use of ultramicroelectrodes in the anodic oxidation of short-chain alkanes. J. Phys. Chem. 1985, 89, 3933–3935. [Google Scholar] [CrossRef]
- Daniele, S.; Bragato, C.; Baldo, M.A. Microelectrode voltammetry for the simultaneous reduction of labile complexes of two divalent cations in aqueous solutions with and without supporting electrolyte. J. Electroanal. Chem. 1998, 456, 105–112. [Google Scholar] [CrossRef]
- Belding, S.R.; Limon-Petersen, J.G.; Dickinson, E.J.; Compton, R.G. Cyclic voltammetry in the absence of excess supporting electrolyte offers extra kinetic and mechanistic insights: Comproportionation of anthraquinone and the anthraquinone dianion in acetonitrile. Angew. Chem. 2010, 49, 9242–9245. [Google Scholar] [CrossRef]
- Zhang, C.; Aoki, K.J.; Chen, J.; Nishiumi, T. Blocking of two-electron reduction of non-charged species in the absence of supporting electrolyte at nanoelectrodes. J. Electroanal. Chem. 2013, 708, 101–107. [Google Scholar] [CrossRef]
- Ciszkowska, M.; Stojek, Z. Voltammetry in solutions of low ionic strength. Electrochemical and analytical aspects. J. Electroanal. Chem. 1999, 466, 129–143. [Google Scholar] [CrossRef]
- Aoki, K.J.; Chen, J. Insight of electrolyte-free voltammetry at microelectrodes. Opin. Electrochem. 2018, 10, 67–71. [Google Scholar] [CrossRef]
- Frumkin, A.N. Wasserstoffüberspannung und Struktur der Doppelschicht. Z. Phys. Chem. 1933, 164A, 121–133. [Google Scholar] [CrossRef]
- Van Soestbergen, M. Frumkin-Butler-Volmer Theory and Mass Transfer in Electrochemical Cells. Russ. J. Electrochem. 2012, 48, 570–579. [Google Scholar] [CrossRef] [Green Version]
- White, R.E.; Bockris, J.O.; Conway, B.E.; Yeager, E. Comprehensive Treatise of Electrochemistry; Plenum Press: New York, NY, USA, 1984; Volume 8, pp. 277–282. [Google Scholar]
- Hou, Y.; Aoki, K.J.; Chen, J.; Nishiumi, T. Invariance of double layer capacitance to polarized potential in halide solutions. Univ. J. Chem. 2013, 1, 162–169. [Google Scholar] [CrossRef]
- Hou, Y.; Aoki, K.J.; Chen, J.; Nishiumi, T. Solvent Variables Controlling Electric Double Layer Capacitance at Metal|Solution Interface. J. Phys. Chem. C 2014, 118, 10153–10158. [Google Scholar] [CrossRef]
- Zhao, X.; Aoki, K.J.; Chen, J.; Nishiumi, T. Examination of the Gouy–Chapman theory for double layer capacitance in deionized latex suspensions. RSC Adv. 2014, 4, 63171–63181. [Google Scholar] [CrossRef]
- Aoki, K.J. Frequency-dependence of electric double layer capacitance without Faradaic reactions. J. Electroanal. Chem. Spec. Issue Aoki 2016, 779, 117–125. [Google Scholar] [CrossRef]
- Aoki, K.J.; Zhang, C.; Chen, J.; Nishiumi, T. Heterogeneous reaction rate constants by steady-state microelectrode techniques and fast scan voltammetry. J. Electroanal. Chem. 2013, 706, 40–47. [Google Scholar] [CrossRef]
- Dickinson, E.J.F.; Limon-Petersen, J.G.; Rees, N.V.; Compton, R.G. How much supporting electrolyte is required to make a cyclic voltammetry experiment quantitatively “diffusional”? A theoretical and experimental investigation. J. Phys. Chem. C 2009, 113, 11157–11171. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Batchelor-McAuley, C.; Compton, R.G. Anodic stripping voltammetry of silver in the absence of electrolytes: Theory and experiment. J. Electroanal. Chem. 2018, 830–831, 122–130. [Google Scholar] [CrossRef]
- Aoki, K.J.; Chen, J.; Zeng, X.; Wang, Z. Decrease in double layer capacitance by Faradaic current. RSC Adv. 2017, 7, 22501–22509. [Google Scholar] [CrossRef] [Green Version]
- Aoki, K.J.; Chen, J.; Tang, P. Capacitive currents flowing in the direction opposite to redox currents. J. Phys. Chem. C 2018, 122, 16727–16732. [Google Scholar] [CrossRef]
- Aoki, K.J.; Chen, J.; Liu, Y.; Jia, B. Peak potential shift for fast cyclic voltammograms owing to capacitance of redox reactions. J. Electroanal. Chem. 2020, 856, 113609. [Google Scholar] [CrossRef]
- Aoki, K.J.; Taniguchi, S.; Chen, J. Participation in negative capacitance of diffusion-controlled voltammograms of hemin. Omega ACS 2020, 45, 29447–29452. [Google Scholar] [CrossRef]
- Liu, Y.; Aoki, K.J.; Chen, J. A loss of charge at reduction of hydrogen ion by fast scan voltammetry. J. Electrochem. Soc. 2022, 169, 036510. [Google Scholar] [CrossRef]
- Aoki, K.J.; Chen, J.; Wang, R. Stripped Charge of Ag less than Deposited one Owing to Negative Capacitance Caused by Redox Reactions. Electroanalysis 2019, 31, 1–9. [Google Scholar] [CrossRef]
- Tang, P.; Aoki, K.J.; Chen, J. Reduction Charge Smaller than the Deposited One in Cathodic Stripping Voltammograms of AgCl. Am. J. Anal. Chem. 2019, 10, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Aoki, K.; Li, C.; Nishiumi, T.; Chen, J. Self-dispersion of mercury metal into aqueous solutions. J. Electroanal. Chem. 2012, 682, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Castellani, M.E.; Anstoter, C.S.; Verlet, J.R.R. On the stability of a dipole-bound state in the presence of a molecule. Phys. Chem. Chem. Phys. 2019, 21, 24286–24290. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. Thermodynamics of Solvation of Ions. Part 5.—Gibbs Free Energy of Hydration at 298.15 K. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Aoki, K.J.; Chen, J. Enhancement of the Negative Capacitance Associated with the Dissolution of Silver by Salt Concentrations by Means of Anodic Stripping Voltammetry. Electrochem 2022, 3, 397-406. https://doi.org/10.3390/electrochem3030027
Wang R, Aoki KJ, Chen J. Enhancement of the Negative Capacitance Associated with the Dissolution of Silver by Salt Concentrations by Means of Anodic Stripping Voltammetry. Electrochem. 2022; 3(3):397-406. https://doi.org/10.3390/electrochem3030027
Chicago/Turabian StyleWang, Ru, Koichi Jeremiah Aoki, and Jingyuan Chen. 2022. "Enhancement of the Negative Capacitance Associated with the Dissolution of Silver by Salt Concentrations by Means of Anodic Stripping Voltammetry" Electrochem 3, no. 3: 397-406. https://doi.org/10.3390/electrochem3030027
APA StyleWang, R., Aoki, K. J., & Chen, J. (2022). Enhancement of the Negative Capacitance Associated with the Dissolution of Silver by Salt Concentrations by Means of Anodic Stripping Voltammetry. Electrochem, 3(3), 397-406. https://doi.org/10.3390/electrochem3030027







