Abstract
Nickel–cobalt alloys were prepared by alloy electrodeposition with a sulfamate bath, and the mechanical properties on the micro-scale were evaluated for the application as micro-components in miniaturized electronic devices. Nickel bromide and a commercially available surface brightener were used as the additives. The cobalt content increased from 21.5 to 60.1 at.% after addition of nickel bromide into the bath, and the grain size refined from 21.1 to 13.2 nm when the surface brightener was used. The mechanical properties on the micro-scale were evaluated by micro-compression test using micro-pillar type specimens fabricated by a focused ion beam system to take the sample size effect into consideration. The yield strength of the nickel–cobalt alloy having an average grain size at 13.9 nm and cobalt content of 66.6 at.% reached 2.37 GPa, revealing influences from the sample size, grain boundary strengthening, and solid solution strengthening effects.
1. Introduction
Nickel–cobalt alloys are important functional materials due to their unique magnetic and mechanical properties, and these characteristics can be well manipulated when electrodeposition is applied [1,2,3,4,5,6,7,8,9]. The nickel–cobalt system has shown advantageous properties compared to other ferrous metals, such as that the magnetic characteristics can be shifted from soft magnetic to permanent magnetic for cobalt rich alloy deposits [10]. Moreover, the electrodeposition process can be integrated with photolithography process for fabrication of components in miniaturized electronic devices, such as microelectromechanical systems (MEMS) devices [11,12,13,14]. For example, nickel–cobalt alloys have been applied as the magnetic component in MEMS actuators [13,15].
In addition to magnetic properties, mechanical property characterization of materials used in the movable component is critical for understanding the reliability and eventually lifetime of the entire device. Mechanical properties of metallic materials are known to have a sample size effect [16,17], where an increase in the strength is observed as the size of the sample used in the mechanical property characterization reaches the micro-scale or smaller. Dimensions of the movable components in miniaturized electronic devices are often on the micro-scale or smaller. Hence, the sample size effect has to be considered in mechanical property characterization.
Mechanical property characterization of electrodeposited materials can be easily conducted by hardness test [18,19]. The hardness is determined from the indentation mark created on the surfaces of the electrodeposited material after applying a constant loading for a length of time. Generally, a large indentation mark is generated on a soft electrodeposited material, and the indentation mark becomes small when the mechanical strength is high. From the size of the indentation mark and the loading force, the hardness can be calculated. The hardness is called micro-hardness [18] or nano-hardness [19] when the indentation mark is on the micrometer or nanometer scale, respectively. In a study on micro-hardness of electrodeposited nickel–cobalt alloys [18], the micro-hardness was reported to be dependent on the current density used in the alloy electrodeposition, with the micro-hardness increasing with increasing current density. However, materials surrounding the indentation mark, especially materials at the bottom of the indentation mark, could also affect generation of the indentation mark [20]. Therefore, it is difficult to include the sample size effect in the clarification of the mechanical properties on the micro-scale by hardness test.
For precise evaluation of the mechanical properties on the micro-scale toward micro-components in miniaturized electronic devices, micro-compression [1,2,21,22,23], micro-tensile [24], and micro-bending [25,26,27] tests have been developed. The micro-mechanical properties of micro-grained nickel [1], nano-grained nickel [2,24,27], nano-grained copper [21], nano-grained gold [25,26], nano-grained gold-copper alloys [22], amorphous nickel-phosphorus alloy [23], and single crystalline nickel–cobalt alloys [28] have been reported in previous studies. On the other hand, there is still very limited reporting on micro-mechanical properties of polycrystalline nickel–cobalt alloys.
Nickel–cobalt alloys applied in MEMS are prepared by alloy electrodeposition, and electrodeposited nickel–cobalt alloys are polycrystals. Mechanical properties of metals are closely related to their average grain size and the composition [18]. The relationship between the average grain size and mechanical strength usually follows the Hall-Petch relationship [29,30], where the strength is inversely proportional to square root of the average grain size. Meanwhile, the strength is dependent on the composition (cobalt content) in a nickel–cobalt alloy since the nickel–cobalt system forms a solid solution that results in a strengthening effect [28]. In addition, the average grain size and composition are easily affected by the electrodeposition conditions, such as the current density, the use of additives (surface brightener), and composition of the bath [5,13,31,32,33]. This implies that the micro-mechanical properties of electrodeposited nickel–cobalt alloys are dependent on the electrodeposition conditions.
In this work, nickel–cobalt alloys were electrodeposited from a sulfamate bath, and the mechanical properties on the micro-scale are evaluated by compression test using micro-pillar type specimens fabricated from the electrodeposited alloys by focused ion beam (FIB). Effects of the electrochemical parameters, such as the current density, on the average grain size and cobalt content are reported. Then, the contributions of the grain boundary strengthening mechanism and solid solution strengthening mechanism on the micro-mechanical properties are studied. The yield strength is determined from the engineering stress-engineering strain curve generated from the micro-compression test, and compared with the micro-hardness determined from micro-Vickers hardness test.
2. Experimental Section
2.1. Electrodeposition of Nickel–Cobalt Alloy
As the substrates, 99.96% copper plates (Kikuya PM Co., Ltd., Tokyo, Japan) with a size of 10 × 10 mm2 were used in the nickel–cobalt alloy electrodeposition. The substrates were pretreated in 0.1 M hydrochloric acid (Kanto Chemical Co., Inc., Tokyo, Japan) for 1 min to remove surface oxides. The anode electrode was a 99.95% platinum plate (Kikuya PM Co., Ltd., Tokyo, Japan). The alloy electrodeposition was performed at 55 °C with a commercially available sulfamate bath (MATEX JAPAN Co., Ltd., Shizuoka, Japan), and the bath was composed of 40 g/L of boronic acid, 240 g/L of nickel sulfamate tetrahydrate and 30 g/L of cobalt sulfamate tetrahydrate. The electrodeposition was conducted by the galvanostatic method with a current density ranging from 5 mA/cm2 to 20 mA/cm2, and constant mixing of the bath was performed by stirring at 200 rpm with a cross-shape magnetic stirrer. Then, 10 g/L of nickel bromide (Sigma-Aldrich Co. LLC, St. Louis, MI, USA) and 0.5 vol.% of a surface brightener (Surf-Bright, NSF-E provided by Nihon Kagaku Sangyo Co., Ltd., Tokyo, Japan) were added to manipulate properties of the electrodeposited alloys.
2.2. Characterization of Nickel–Cobalt Alloys
Crystalline characteristics of the nickel–cobalt alloy were evaluated using an X-ray diffractometer (XRD, Ultima IV, Rigaku Corp., Tokyo, Japan) with a scanning rate of 0.04°/s. The X-rays were generated by a copper target operating at 40 mA and 40 kV. The average grain size was determined by the Scherrer equation. The surface morphology was observed by a scanning electron microscope (SEM, SU4300SE Hitachi Co., Ltd., Tokyo, Japan), and the energy dispersive X-ray spectroscopy system (EDS, EMAX EX-250 Horiba Co., Ltd., Kyoto, Japan) installed in the SEM was used to determine the composition. Micro-hardness of the alloys was appraised by a micro-Vickers hardness testing machine (HMV-G20S, Shimadzu Corp., Kyoto, Japan) at 100 g of loading and a loading time of 15 s.
2.3. Micro-Pillar Fabrication and Micro-Compression Test
Micro-pillar type specimens were fabricated from the nickel–cobalt alloys by a focused ion beam system (FIB, FB2100, Hitachi, Tokyo, Japan) using Ga as the liquid metal ion source. A four-step milling method using different beam currents was applied to produce micro-pillars with desired and precise dimensions [1,2]. The beam currents were 6.64, 1.48 and 0.35 nA for coarse milling and 0.07 nA for fine milling and final polishing. The micro-pillar had a cuboidal shape with dimensions of 20 µm in height and a 10 × 10 µm2 square cross-section. Observations of the micro-pillars were conducted through a scanning ion microscope (SIM) installed in the FIB before and after the micro-compression test.
The micro-compression test was conducted using a test machine especially designed for micro-sized samples. The test machine was equipped with a flat-topped indenter with a diameter of 50 μm on the top surface. The test machine had the load resolution of 10 μN, and the displacement resolution was 5 nm. A constant displacement rate of 0.1 μm/s was used in all micro-compression tests, which gave a constant strain rate at 5 × 10−3 s−1. More details of the test machine are reported in a previous study [34].
3. Results and Discussion
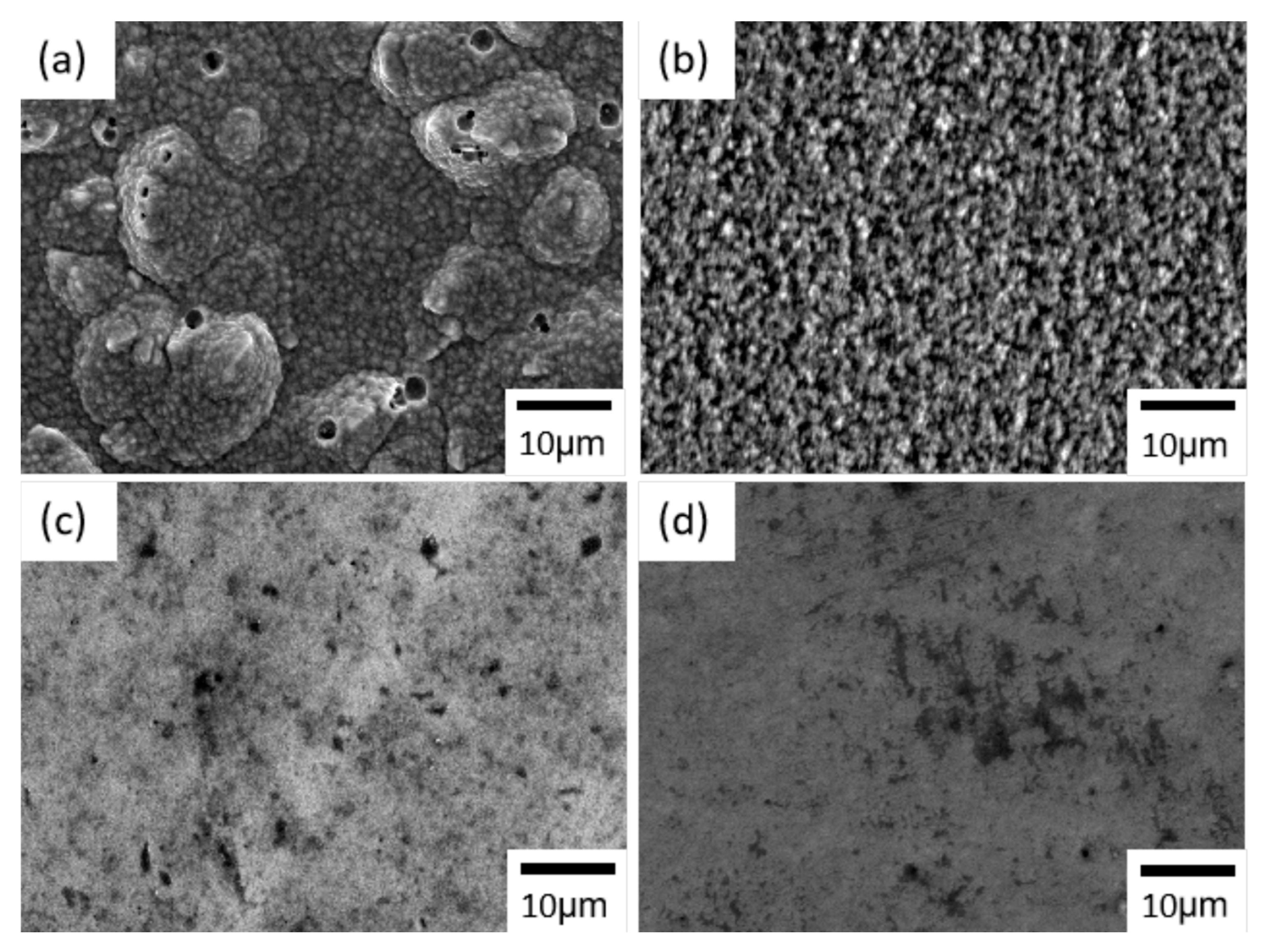
An SEM image of the nickel–cobalt alloy electrodeposited with only the sulfamate bath is shown in Figure 1a. The alloy showed a granular morphology with particle-like structures, and sizes of the particle-like structures were on the order of micrometers. Some circular-shaped defects were also observed. Nickel and cobalt are both non-noble metals, which implies hydrogen evolution would be a side reaction during reduction reactions of nickel and cobalt ions. Hence, the circular-shaped defects were believed to have originated from hydrogen gas bubbles generated and adsorbed on the working electrode.
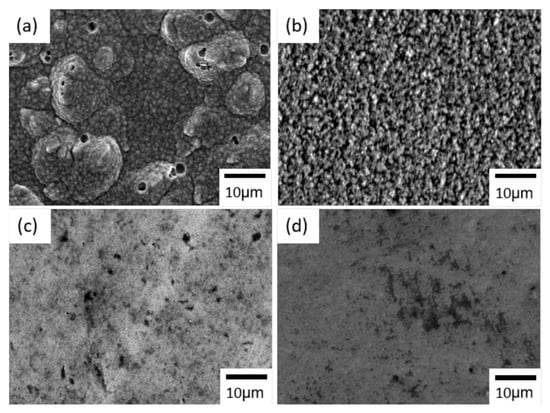
Figure 1.
SEM images of the nickel–cobalt alloys electrodeposited with a current density at 15 mA/cm2 and with the bath containing (a) no additives, (b) nickel bromide, (c) Surf-Bright and (d) both nickel bromide and the Surf-Bright.
After introducing nickel bromide into the sulfamate bath, the alloy surface showed particle-like structures, and sizes of the particle-like structures were also on the micrometer-scale, as shown Figure 1b. The overall condition of the surface became more uniform when compared with the alloy electrodeposited without additives, and no obvious defect was observed. For the nickel–cobalt alloy produced with the bath containing the Surf-Bright, Figure 1c, the surface was also composed of particle-like structures but with Surf-Bright a much smaller size when compared with the alloy electrodeposited from the bath containing nickel bromide. When both nickel bromide and the Surf-Bright were used in the bath, the surface morphology was similar to that of the alloy obtained from the bath containing the Surf-Bright, as shown in Figure 1d.
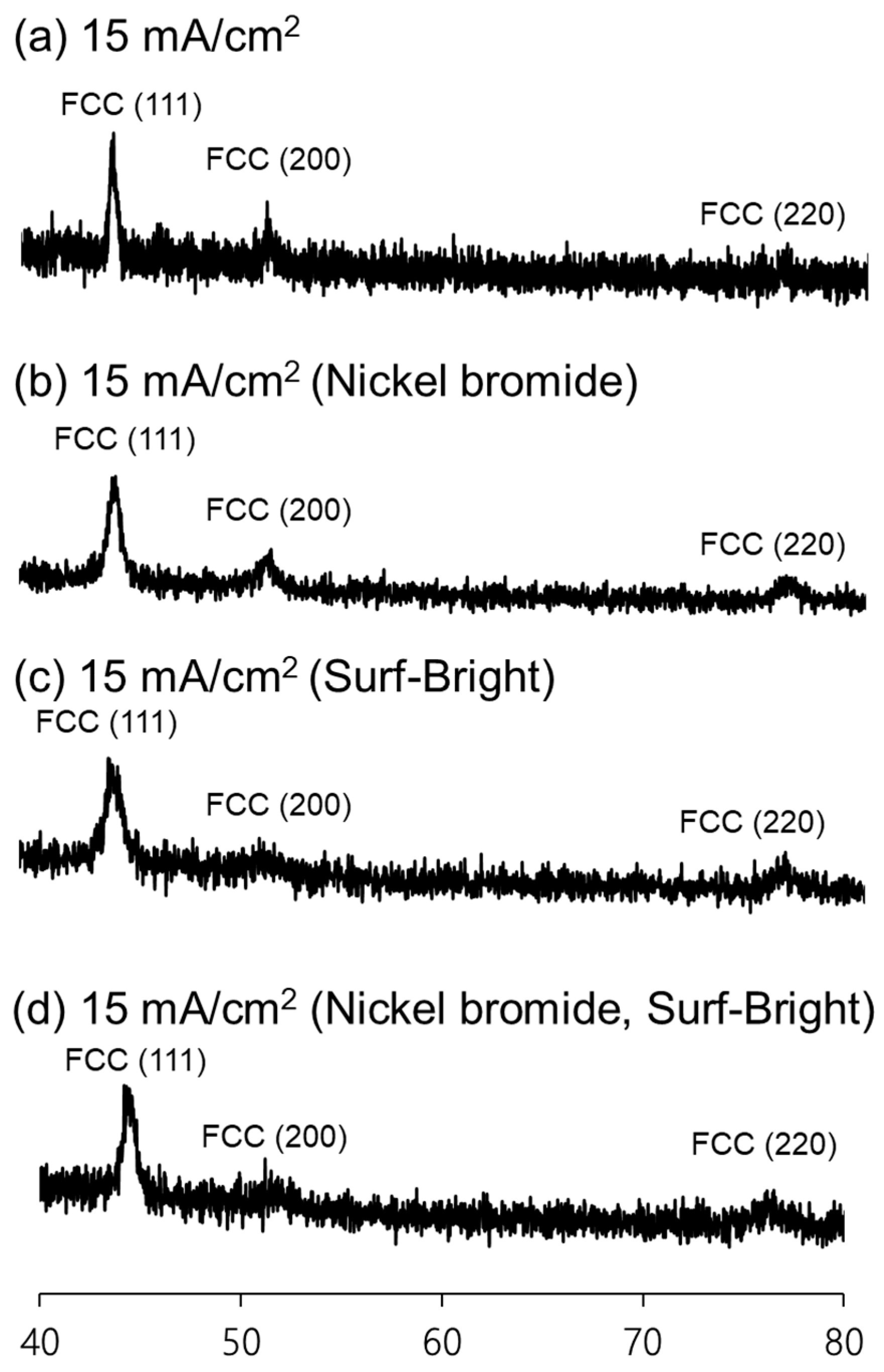
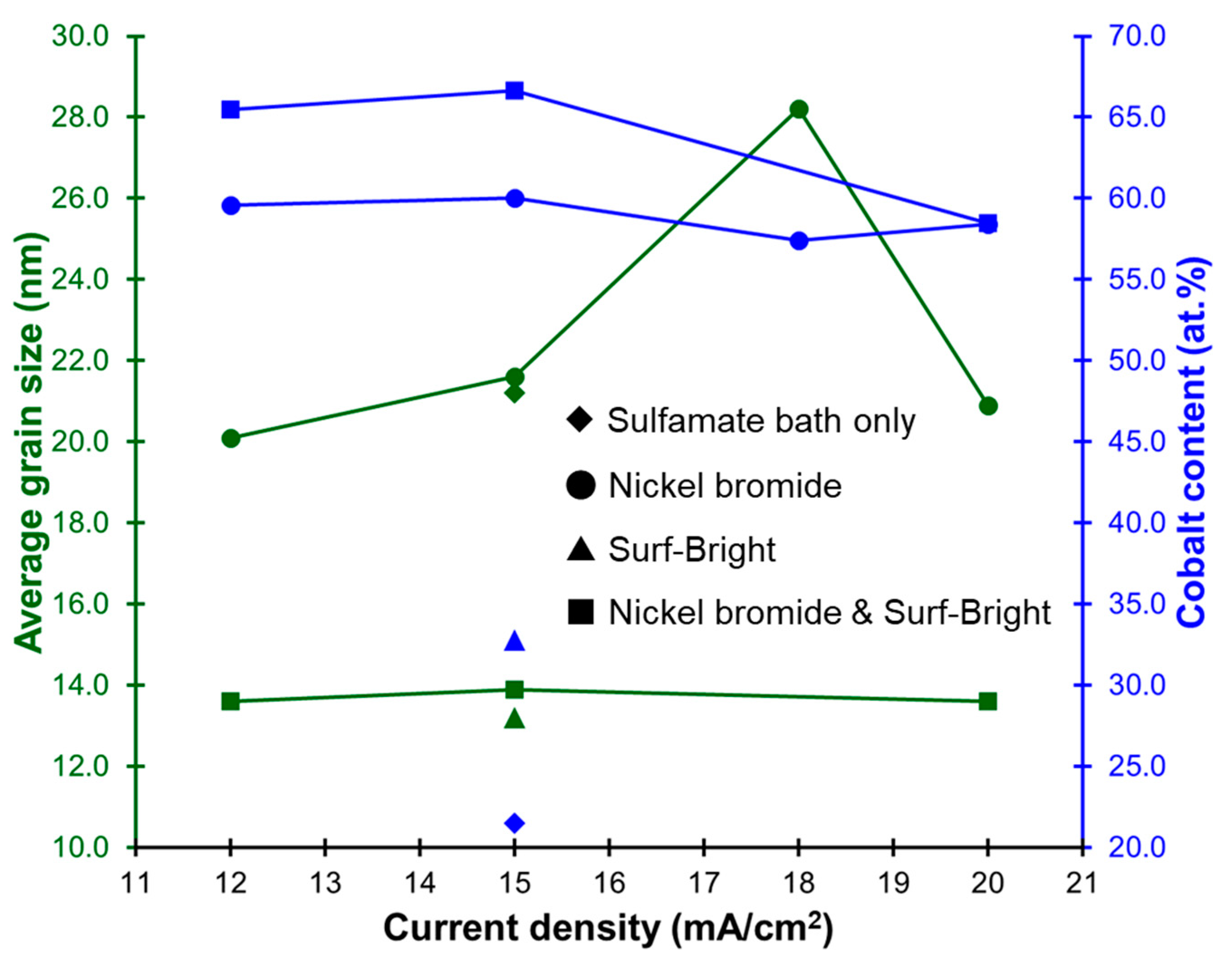
Nickel–cobalt alloys prepared in this study all had XRD peaks corresponding to the (111), (200), and (220) crystal planes in the face-centered cubic (FCC) structure as shown in Figure 2. The (111) XRD peak was the peak with the highest intensity in all nickel–cobalt alloys. The average grain size of the alloys was estimated from the (111) XRD peak using the Scherrer equation. The results are provided in Table 1. The average grain size was 21.1 nm for the alloy electrodeposited at 15 mA/cm2 with the sulfamate bath without additives. After addition of nickel bromide, the average grain size slightly increased to 21.6 nm for the alloy also electrodeposited at 15 mA/cm2. The average grain size obviously decreased to 13.2 nm after addition of the Surf-Bright, and the average grain size slightly increased to 13.9 nm when both nickel bromide and the Surf-Bright were used. The results suggested that nickel bromide caused a very small degree of grain coarsening, and the Surf-Bright was particularly effective in refining the average grain size. Surface brighteners are usually suppressors that lead to refinement of the average grain size. Therefore, the grain refinement effect observed after addition of the Surf-Bright was expected. Regarding effects of the current density on the average grain size, no obvious trend was observed in the alloys electrodeposited with the sulfamate bath containing nickel bromide and the bath containing both nickel bromide and the Surf-Bright when the current density changed from 12 to 20 mA/cm2, as shown in Figure 3.
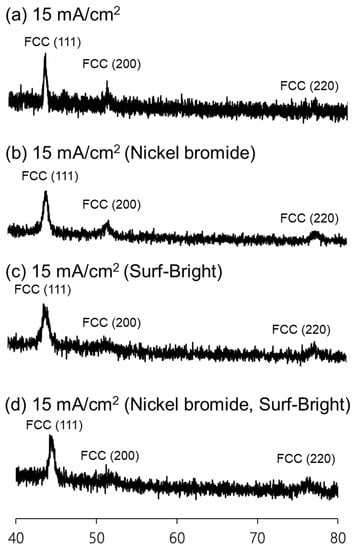
Figure 2.
XRD patterns of the nickel–cobalt alloys electrodeposited with a current density at 15 mA/cm2 and the bath containing (a) no additives, (b) nickel bromide, (c) the Surf-Bright and (d) both nickel bromide and the Surf-Bright.

Table 1.
Effects of the current density on the average grain size, cobalt content, and micro-hardness.
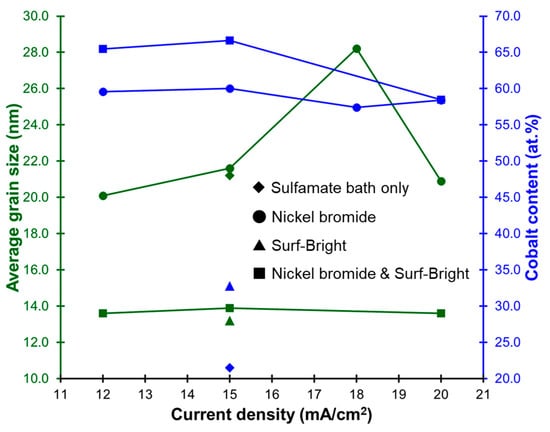
Figure 3.
Relationships between the current density, average grain size and cobalt content.
Effects of the additives on composition of the nickel–cobalt alloys are summarized in Table 1. The alloy electrodeposited with the sulfamate bath at a current density of 15 mA/cm2 contained 21.5 at.% of cobalt. The cobalt content increased to 60.1 and 32.8 at.% after addition of nickel bromide and the Surf-Bright, respectively. The cobalt content further increased to 66.6 at.% when nickel bromide and the Surf-Bright were both used. Bromide ions are expected to act like a weak suppressor in electrodeposition of nickel and cobalt that leads to an increase in the overpotential of reduction reactions, and favor reduction of the less noble constituent cobalt [18]. The atomic ratio of cobalt ions in the sulfamate bath was 10.5 at.%. The cobalt contents in all of the alloys were much higher than 10.5 at.%. A similar trend has been reported [33], where the cobalt content ranged from 23.9 at.% to 45.3 at.% when using a bath containing 20 at.% of cobalt. Again, no obvious current density dependency on the composition was observed as revealed in Figure 3.
Micro-Vickers hardness tests were conducted to evaluate the micro-hardness (Hv) as comparisons with the mechanical properties obtained from the micro-compression test. Values of micro-hardness of the alloys are provided in Table 1. There was no clear relationship between the current density and the micro-hardness in the alloys electrodeposited with the sulfamate bath containing nickel bromide and the bath containing both nickel bromide and the Surf-Bright as the current density varied between 12 and 20 mA/cm2. The mechanical property is expected to be dependent on the average grain size (grain boundary strengthening) and the composition (solid solution strengthening). Since there was no clear current density dependency on either the average grain size or the composition, as shown in Figure 3, this result was expected.
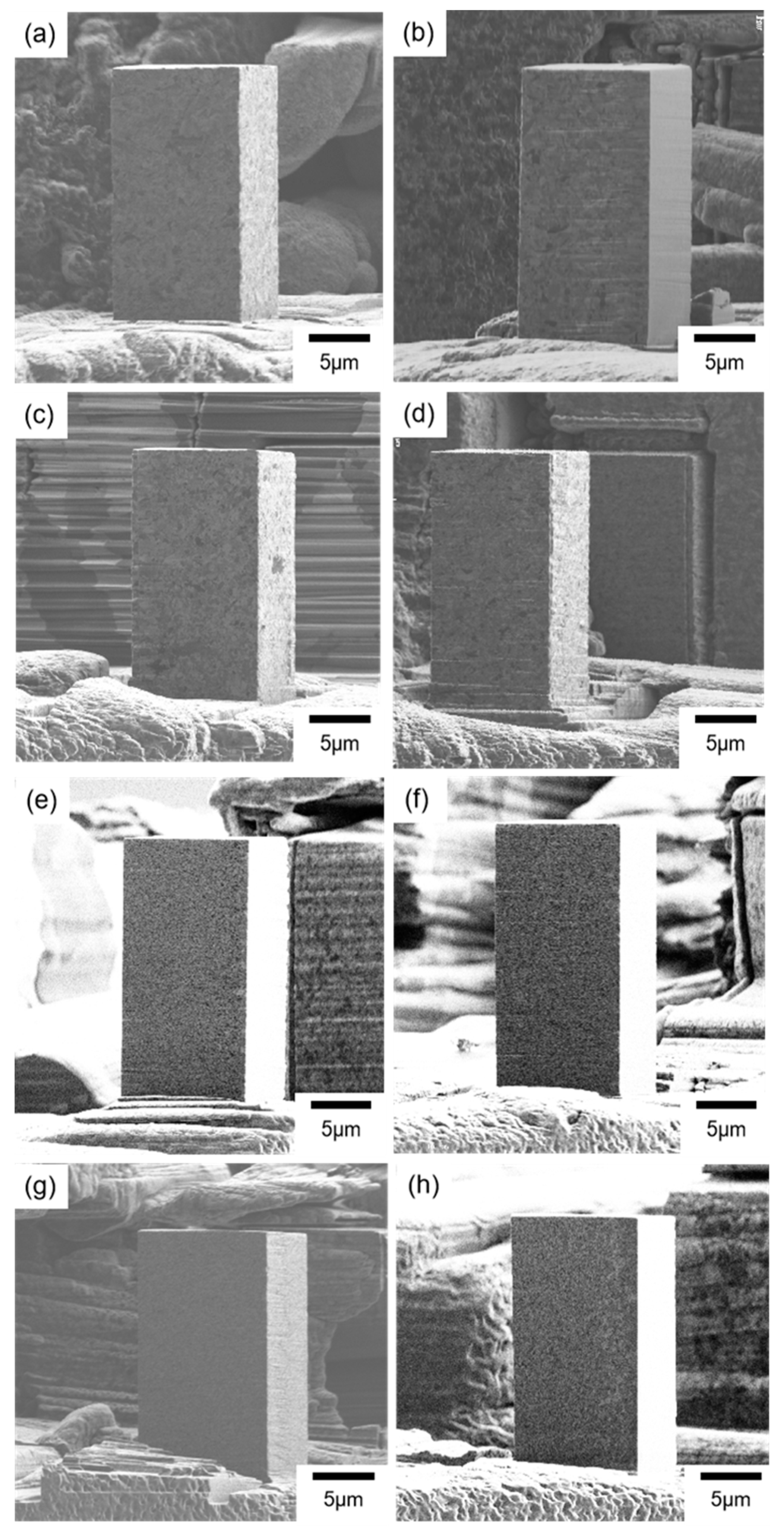
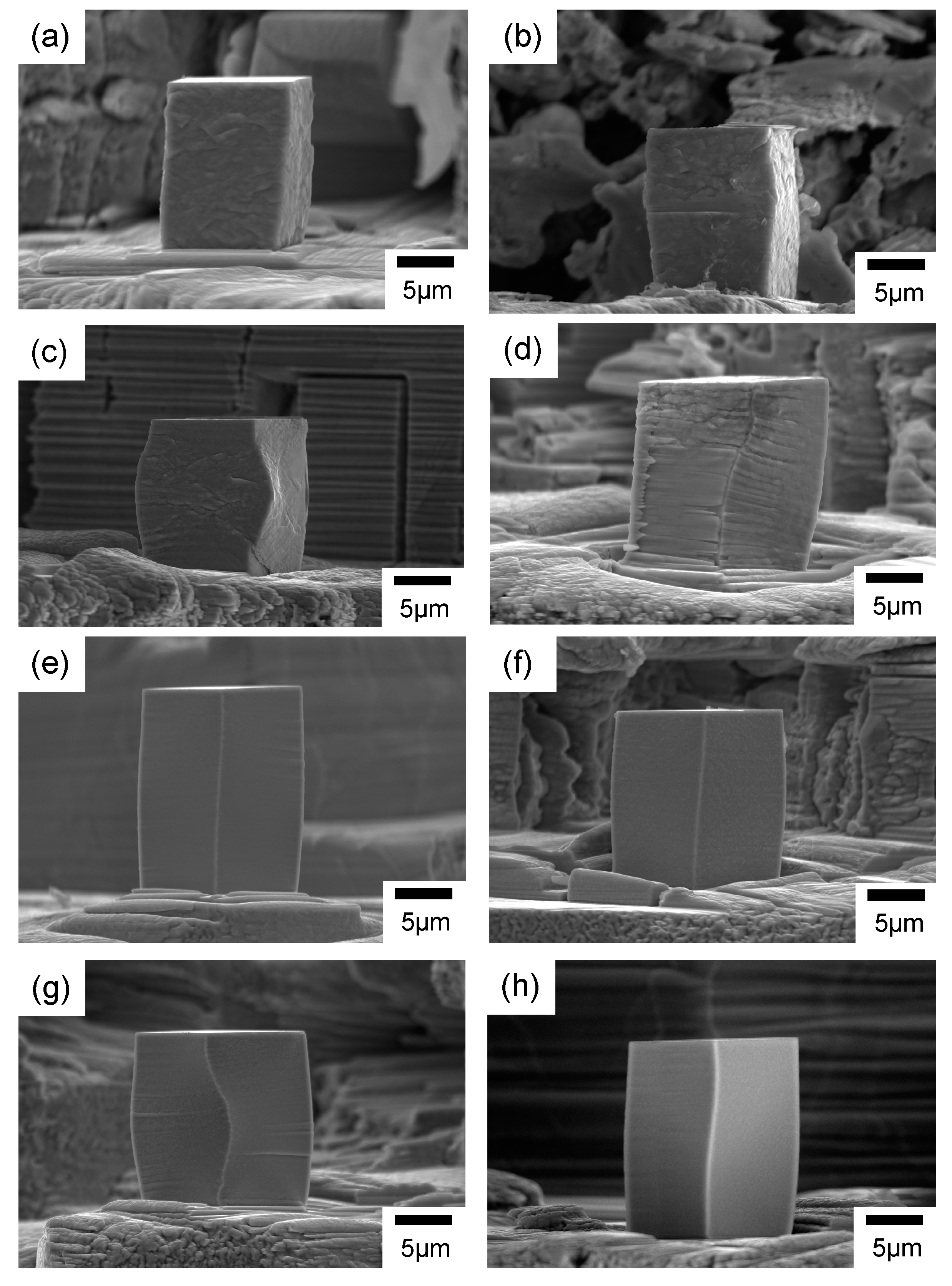
SIM images of micro-pillars fabricated from the alloys are shown in Figure 4. The micro-pillars were all precisely fabricated with a square cross-section, and the aspect ratio of height to length of one side was two. Boundaries of the crystal structure in metallic materials could be observed from the SIM images. On the surfaces of the micro-pillars prepared in this study, camouflage patterns were observed, and these were suggested to be the texture boundaries since the sizes of the patterns were all much larger than the average grain size values estimated from the Scherrer equation. For instance, the average grain size of the alloy electrodeposited at 15 mA/cm2 in the sulfamate bath containing the nickel bromide was 21.6 nm, and the average size of the camouflage patterns was in the hundreds of nanometers level.
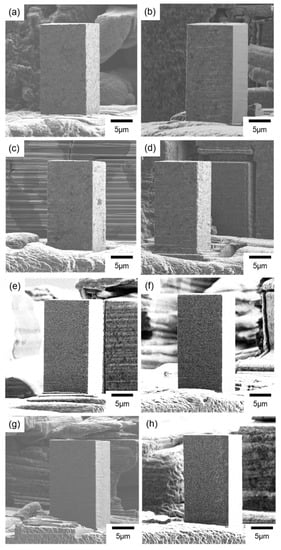
Figure 4.
SIM images of the micro-pillars fabricated from the nickel–cobalt alloys electrodeposited with the bath containing (a–d) nickel bromide, (e) the Surf-Bright, or (f–h) both nickel bromide and the Surf-Bright, and at a current density of (a,f) 12 mA/cm2, (b,e,g) 15 mA/cm2, (c) 18 mA/cm2 and (d,h) 20 mA/cm2.
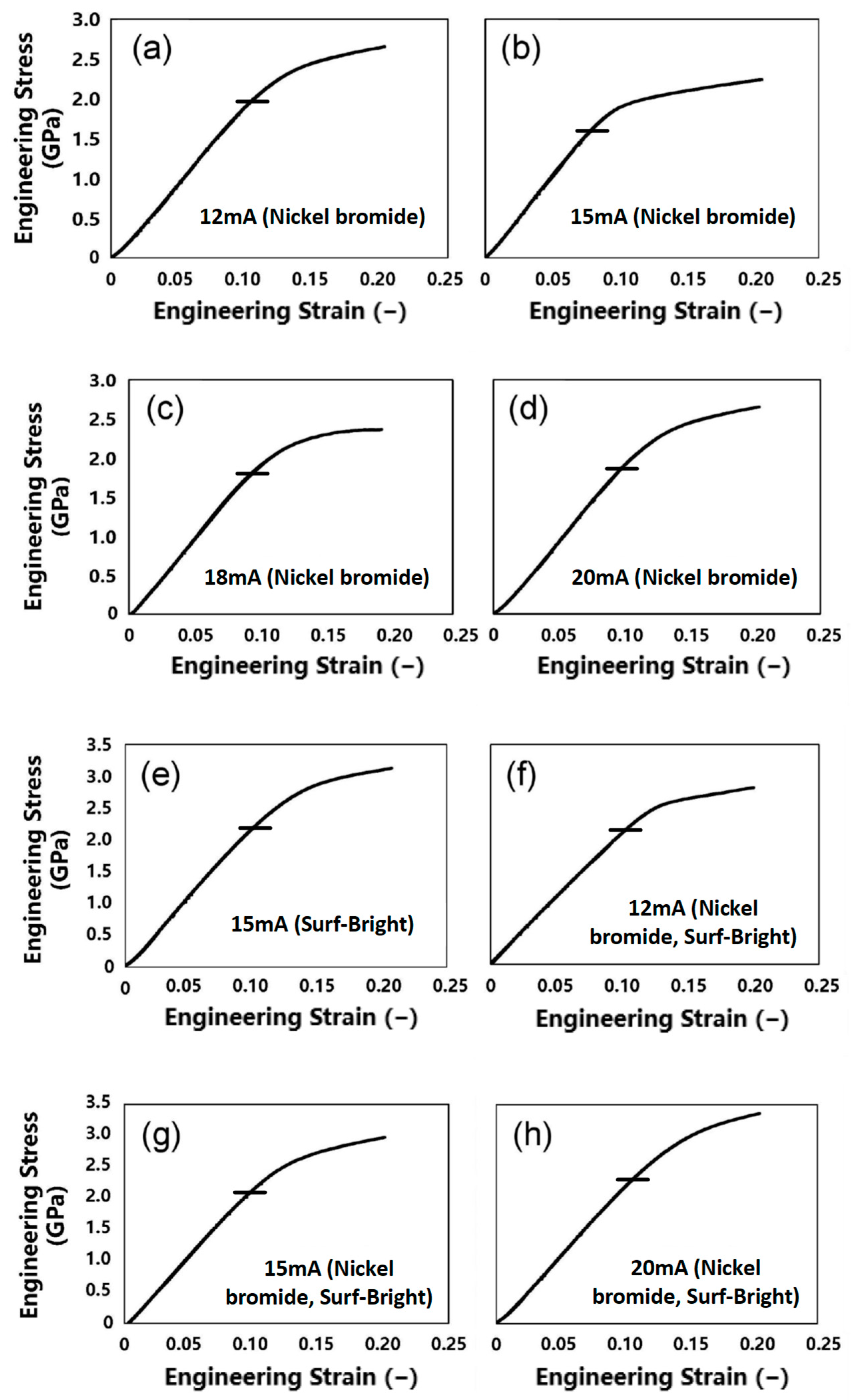
Images of the micro-pillars after the micro-compression test are shown in Figure 5. All micro-pillars showed swelling at some parts of the cross-section after the compression, which is a typical deformation behavior of nanocrystalline metallic materials [1,22]. Engineering stress–engineering strain (SS) curves generated from compression tests of the micro-pillars are shown in Figure 6. The yield point was not clear in all SS curves. Therefore, the yield strength (σy) was determined from the 0.2% offset line of the elastic deformation region [22,35]. A summary of the yield strengths is listed in Table 2. The yield strengths obtained in this study were all higher than bulk electrodeposited nickel–cobalt alloys reported by Li et al. (1200 MPa) [36], and 2~3 folds of the values reported for nickel–ferrum alloys (700 MPa) [37].
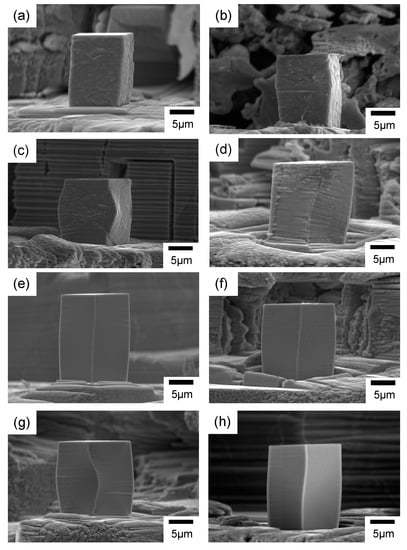
Figure 5.
SEM image of the deformed micro-pillars fabricated from the nickel–cobalt alloys electrodeposited with the bath containing (a–d) nickel bromide, (e) the Surf-Bright, or (f–h) both nickel bromide and the Surf-Bright, and at a current density of (a,f) 12 mA/cm2, (b,e,g) 15 mA/cm2, (c) 18 mA/cm2 and (d,h) 20 mA/cm2.
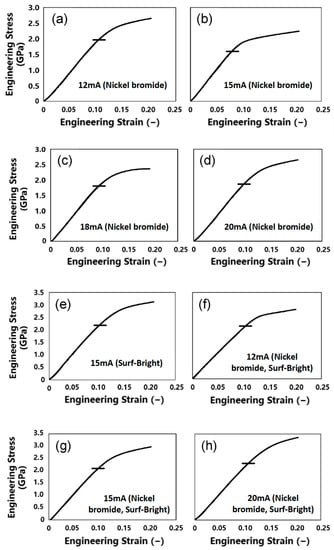
Figure 6.
Engineering stress–strain curves generated from compression of the micro-pillars fabricated from the nickel–cobalt alloys electrodeposited with the bath containing (a–d) nickel bromide, (e) the Surf-Bright, or (f–h) both nickel bromide and the Surf-Bright, and at a current density of (a,f) 12 mA/cm2, (b,e,g) 15 mA/cm2, (c) 18 mA/cm2 and (d,h) 20 mA/cm2.

Table 2.
Summary of the yield strength and Tabor factor.
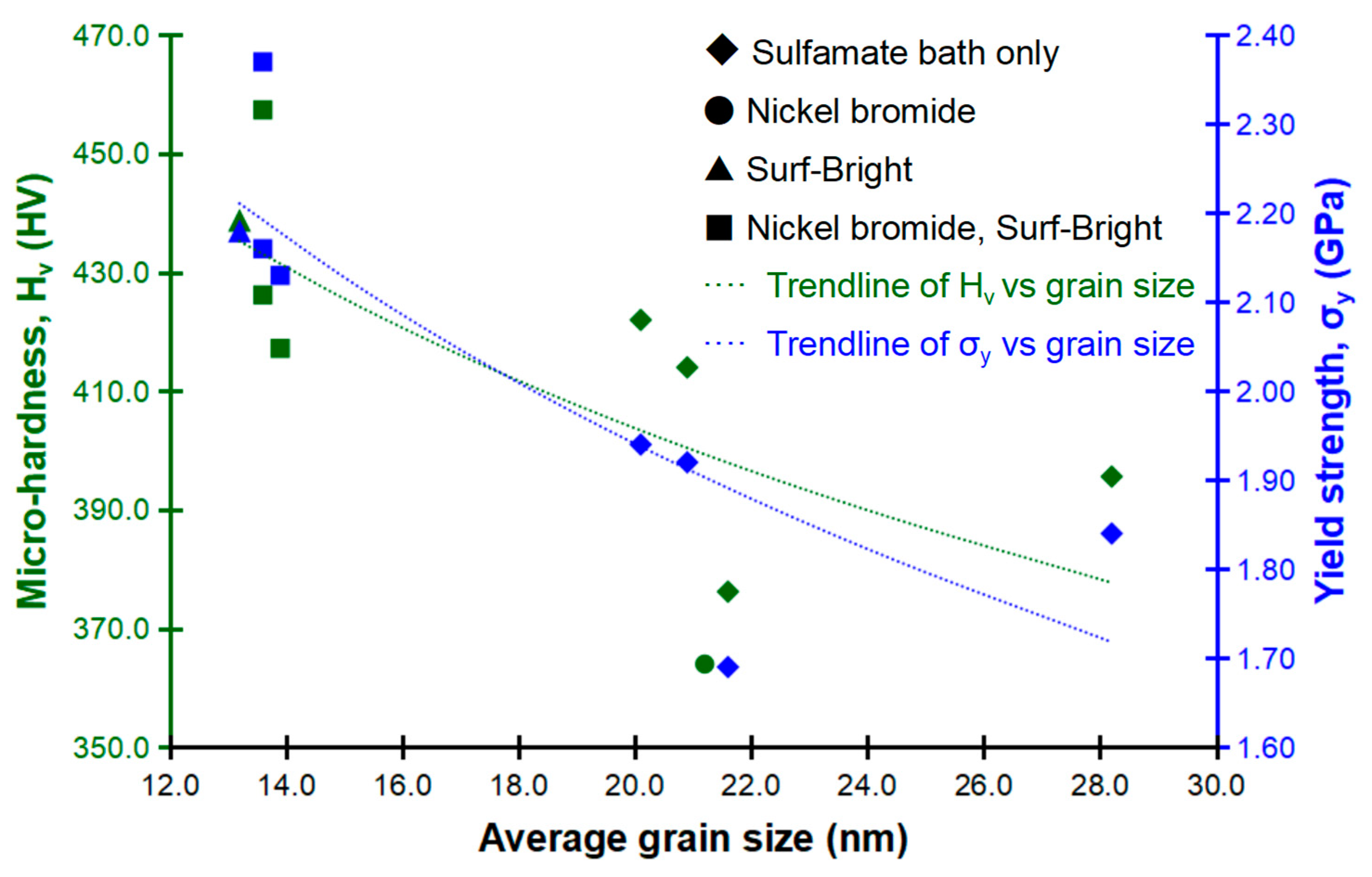
Relationships between the average grain size, micro-hardness and yield strength are shown in Figure 7. Generally, for both of the micro-hardness and yield strength, strengthening was observed following a refinement of the average grain size as illustrated by the dashed trendlines. This trend followed the Hall-Petch relationship well [29,30], and confirmed that the mechanical strength was mostly dependent on the grain boundary strengthening mechanism. Alloys with smaller grain size have fewer dislocations pile up and less stress at grain boundaries, which contributes to a higher mechanical strength. The highest yield strength at 2.37 GPa was obtained from the micro-pillar having the average grain size at 13.6 nm and 58.48 at.% of cobalt, and the highest micro-hardness at 457.4 HV was also obtained from the alloy with the same average grain size and composition. The alloy having the finest average grain size at 13.2 nm had a micro-hardness of 438.8 HV and a yield strength of 2.18 GPa, with both values being the second highest among the alloys/micro-pillars evaluated in this study. This result indicated that the composition, via the solid solution strengthening mechanism, also had a significant contribution to the overall strength since the cobalt content in the alloy with the average grain size at 13.2 nm (32.78 at.%) was much lower than that of the alloy made of 13.6 nm grains (58.48 at.%).
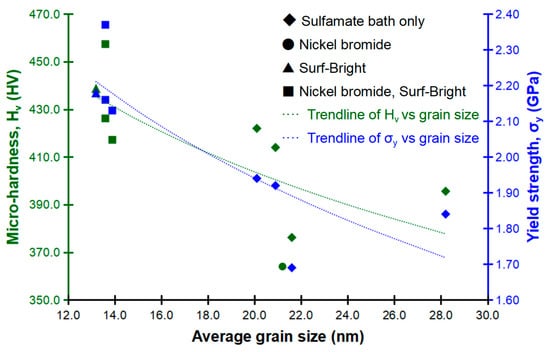
Figure 7.
Relationships between the average grain size, micro-hardness and yield strength.
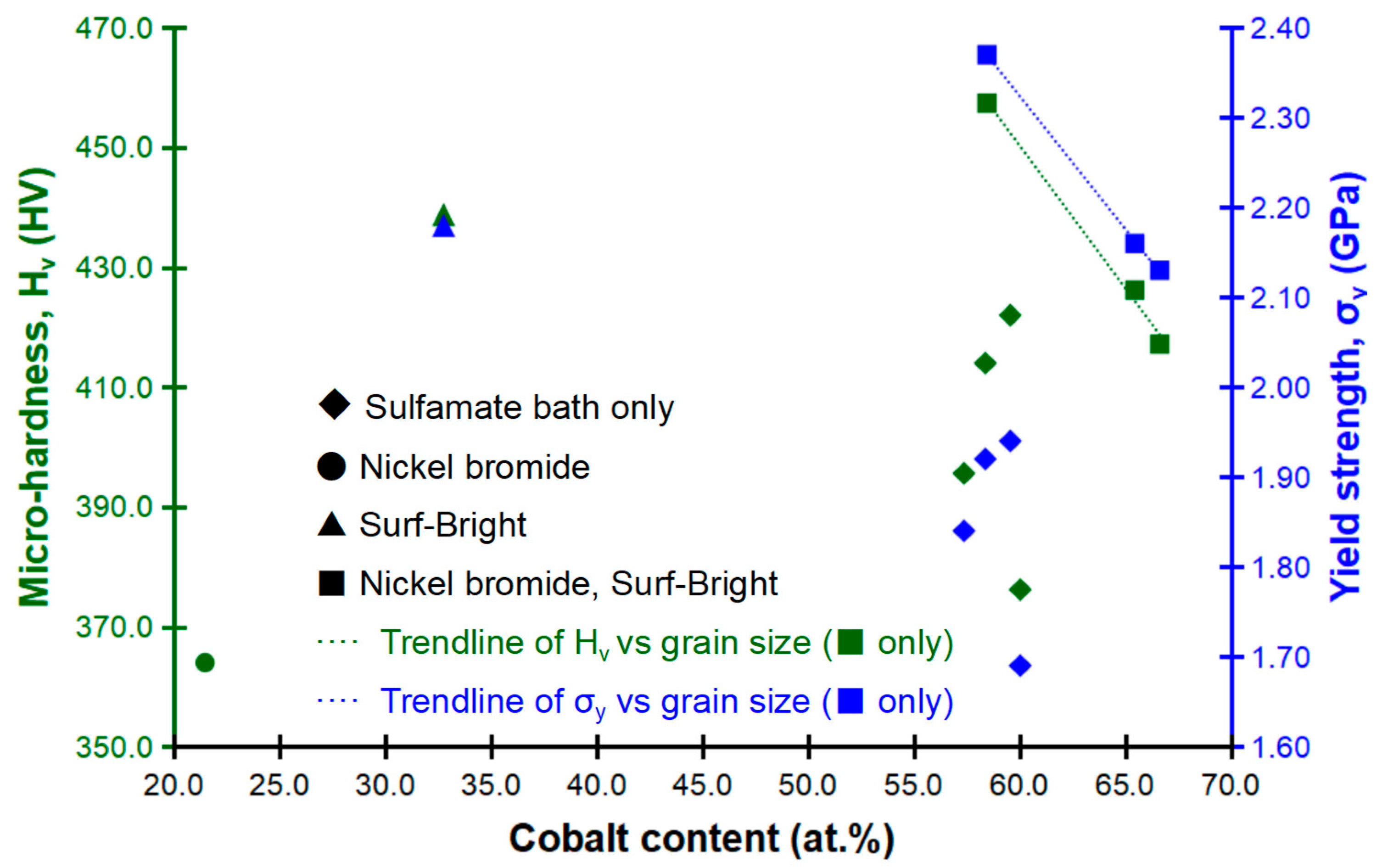
Relationships between the composition, micro-hardness and yield strength are shown in Figure 8. The composition dependency of the mechanical strength was not that obvious. A weak relationship between the composition and the strength could be concluded since a local maximum in the strength was obtained as the composition approached roughly 58 at.% of cobalt. For the three alloys electrodeposited with the two additives (nickel bromide and the Surf-Bright, ■), the average grain size merely changed between 13.6 and 13.9 nm and the cobalt content varied between 58.48 to 66.64 at.% when the current density changed from 12 to 20 mA/cm2. In these three alloys, a decrease in both of the micro-hardness and yield strength was observed following an increase in the cobalt content as indicated by the dashed trendlines in Figure 8.
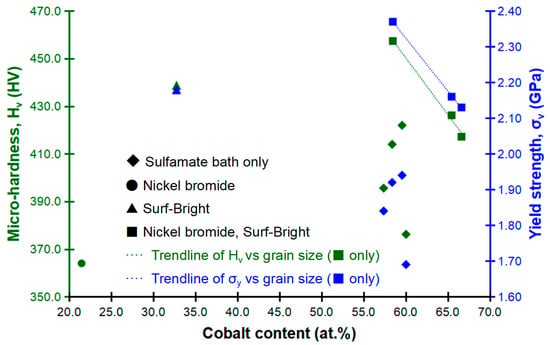
Figure 8.
Relationships between the composition, micro-hardness and yield strength.
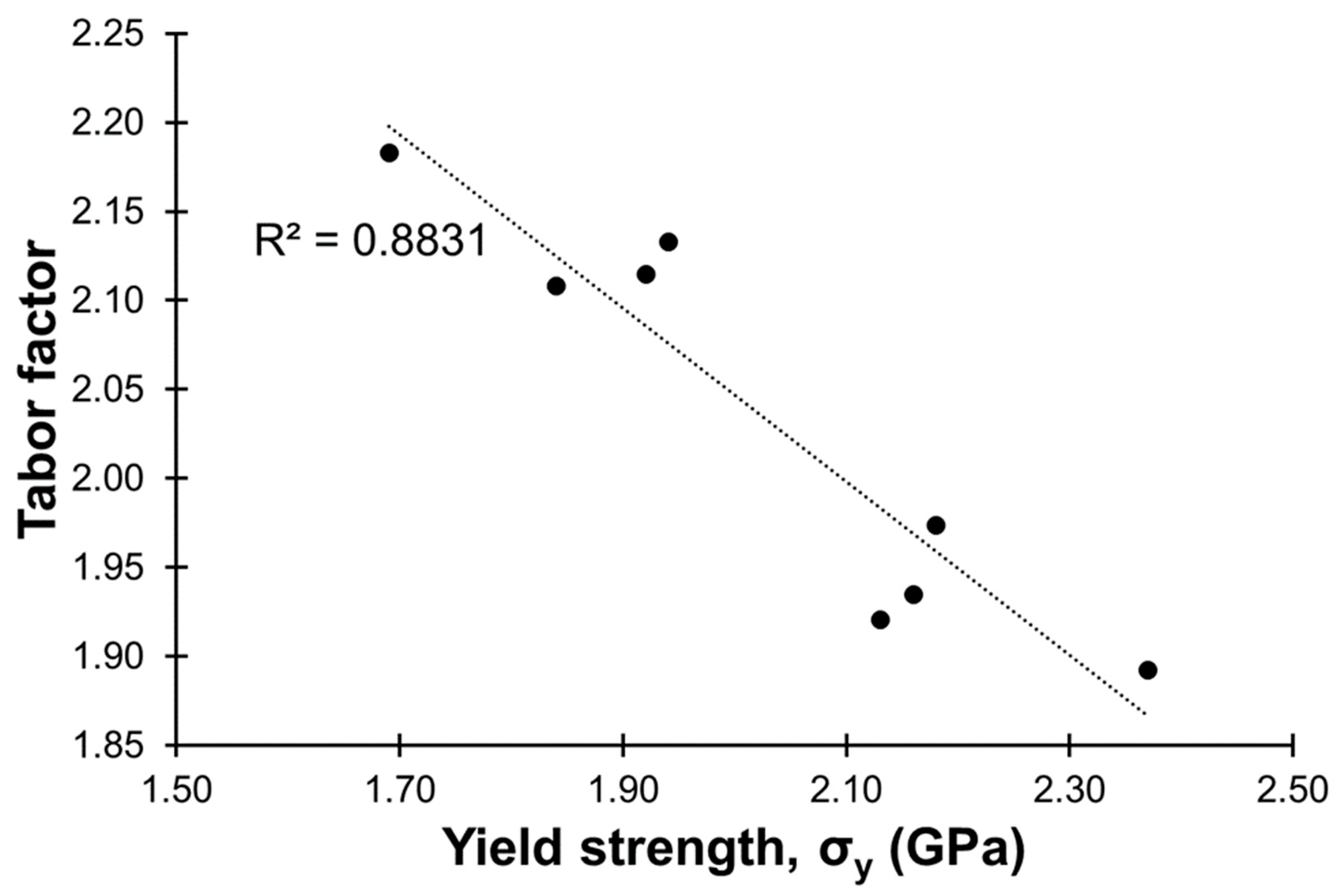
Information on the Tabor factor [38], which is the ratio of the micro-hardness in the unit of MPa by the yield strength also in the unit of MPa (Hv in MPa/σy in MPa), is provided in Table 2. The Tabor factor has been reported to be between 3 and 4 in literature [38]. In this study, the Tabor factors were between ca. 1.9 and 2.2. Figure 9 shows a plotting of the Tabor factor against the yield strength, and a linear relationship (R2 value = 0.88) between the two could be observed. Generally, the Tabor factor became smaller along with an increase in the yield strength.
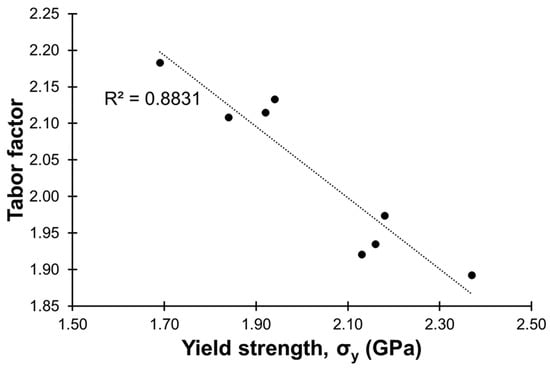
Figure 9.
Relationship between the yield strength and the Tabor factor.
4. Conclusions
Micro-mechanical properties of electrodeposited nickel–cobalt alloys were reported in this study to provide design information of micro-components in MEMS. The nickel–cobalt alloys were prepared by alloy electrodeposition with a sulfamate bath. Refinement in the average grain size was observed after introduction of a Surf-Bright into the bath, and the cobalt content largely increased after addition of nickel bromide. There was no clear relationship between the current density and the average grain size and composition in the alloys. Micro-compression test was conducted using micro-pillar type specimens to investigate the mechanical strength on the micro-scale. The yield strength was found to be highly dependent on the average grain size based on the grain boundary strengthening mechanism. The cobalt content also showed some influences on the yield strength indicating a contribution from the solid solution strengthening mechanism. The highest yield strength at 2.37 GPa was obtained from a micro-pillar fabricated from an alloy electrodeposited with the sulfamate bath containing nickel bromide and the Surf-Bright, with an average grain size of 13.6 nm and composed of 58.48 at.% of cobalt. Micro-mechanical properties of electrodeposited nickel–cobalt alloys reported in this study could make significant contributions to the design of movable micro-components in miniaturized electronic devices.
Author Contributions
Conceptualization, Y.J., T.-F.M.C.; Methodology, Y.J.; Validation, C.-Y.C., X.L., D.Y.; Formal analysis, Y.J.; Investigation, Y.J., T.-F.M.C.; Resources, T.-F.M.C.; Data curation, Y.J.; Writing—original draft preparation, Y.J.; Writing—review and editing, C.-Y.C., T.-F.M.C.; Visualization, Y.J.; Supervision, T.-F.M.C., M.S.; Project administration, T.-F.M.C., M.S.; Funding acquisition, M.M., O.K., R.M., T.-F.M.C., M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JST, CREST Grant Number JPMJCR21C5, Japan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study is based on results obtained from a project commissioned by the New Energy and Industrial Technology Development Organization (NEDO), the Cooperative Research Project of Research Center for Biomedical Engineering.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Yamamoto, T.; Igawa, K.; Tang, H.C.; Chen, C.Y.; Chang, T.F.M.; Nagoshi, T.; Kudo, O.; Maeda, R.; Sone, M. Effects of current density on mechanical properties of electroplated nickel with high speed sulfamate bath. Microelectron. Eng. 2019, 213, 18–23. [Google Scholar] [CrossRef]
- Nagoshi, T.; Chang, T.F.M.; Tatsuo, S.; Sone, M. Mechanical properties of nickel fabricated by electroplating with supercritical CO2 emulsion evaluated by micro-compression test using non-tapered micro-sized pillar. Microelectron. Eng. 2013, 110, 270–273. [Google Scholar] [CrossRef]
- Luo, X.; Chen, C.Y.; Chang, T.F.M.; Hosoda, H.; Sone, M. Crystal Growth of Cobalt Film Fabricated by Electrodeposition with Dense Carbon Dioxide. J. Electrochem. Soc. 2015, 162, D423–D426. [Google Scholar] [CrossRef]
- Homma, T.; Kita, Y.; Osaka, T. Electrochemical Studies on the Deposition Process of Electroless CoNiP Films with Graded Magnetic Properties. J. Electrochem. Soc. 2000, 147, 4138–4141. [Google Scholar] [CrossRef]
- Bai, A.; Hu, C.C. Composition controlling of Co-Ni and Fe-Co alloys using pulse-reverse electroplating through means of experimental strategies. Electrochim. Acta 2005, 50, 1335–1345. [Google Scholar] [CrossRef]
- Luo, J.K.; Pritschow, M.; Flewitt, A.J.; Spearing, S.M.; Fleck, N.A.; Milne, W.I. Effects of Process Conditions on Properties of Electroplated Ni Thin Films for Microsystem Applications. J. Electrochem. Soc. 2006, 153, D155–D161. [Google Scholar] [CrossRef]
- Arai, S.; Saito, T.; Endo, M. Low-Internal-Stress Nickel Multiwalled Carbon Nanotube Composite Electrodeposited from a Sulfamate Bath. J. Electrochem. Soc. 2007, 154, D530–D533. [Google Scholar] [CrossRef]
- Arai, S.; Miyagawa, K. Fabrication of Co-W Alloy/Multiwalled Carbon Nanotube Composite Films by Electrodeposition for Improved Frictional Properties. ECS J. Solid State Sci. Technol. 2013, 2, M39–M43. [Google Scholar] [CrossRef]
- Huang, N.; Kirk, D.W.; Thorpe, S.J.; Liang, C.; Xu, L.; Zhang, S.; Li, W.; Sun, M. Effect of Temperature on Characteristic of Carbon Nanotube Nano-Composite Electrode Supercapacitor. ECS Trans. 2014, 61, 9–13. [Google Scholar] [CrossRef]
- Andricacos, P.C. Electrochemically Deposited Thin Films II; Paunovic, M., Ed.; The Electrochemical Society Proceedings Series; The Electrochemical Society: Pennington, NJ, USA, 1995. [Google Scholar]
- Horiuchi, T.; Furuuchi, Y.; Nakamura, R.; Hirota, K. Micro-gear fabrication using optical projection lithography on copper-clad plastic substrates and electroplating of nickel. Microelectron. Eng. 2006, 83, 1316–1320. [Google Scholar] [CrossRef]
- Li, M.; Luo, W.; Chen, Y.; Zheng, Y.; Cheng, X. Fabrication and nanoindentation characterization of nickel micro-pillar mold for nanoimprint lithography. Microelectron. Eng. 2021, 250, 111636. [Google Scholar] [CrossRef]
- Duch, M.; Esteve, J.; Gómez, E.; Pérez-Castillejos, R.; Vallés, E. Electrodeposited Co-Ni alloys for MEMS. J. Micromech. Microeng. 2002, 12, 400–405. [Google Scholar] [CrossRef]
- Lochel, B.; Maciossek, A. Electrodeposited Magnetic Alloys for Surface Micromachining. J. Electrochem. Soc. 1996, 143, 3343–3348. [Google Scholar] [CrossRef]
- Lee, S.A.; Lee, H.; Pinney, J.R.; Khialeeva, E.; Bergsneider, M.; Judy, J.W. Development of Microfabricated Magnetic Actuators for Removing Cellular Occlusion. J. Micromech. Microeng. 2011, 21, 054006. [Google Scholar] [CrossRef] [PubMed]
- Uchic, M.D.; Dimiduk, D.M.; Florando, J.N.; Nix, W.D. Sample Dimensions Influence Strength and Crystal Plasticity. Science 2004, 305, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.R.; Oliver, W.C.; Nix, W.D. Size dependence of mechanical properties of gold at the micron scale in the absence of strain gradients. Acta Mater. 2005, 53, 1821–1830. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, C.Y.; Chang, T.F.M.; Luo, X.; Yamane, D.; Sone, M. Electrodeposition of Ni-Co Alloys and Their Mechanical Properties by Micro-Vickers Hardness Test. Electrochem 2021, 2, 1–9. [Google Scholar] [CrossRef]
- Valova, E.; Armyanov, S.; Franquet, A.; Petrov, K.; Kovacheva, D.; Dille, J.; Delplancke, J.-L.; Hubin, A.; Steenhaut, O.; Vereecken, J. Comparison of the Structure and Chemical Composition of Crystalline and Amorphous Electroless Ni-W-P Coatings. J. Electrochem. Soc. 2004, 151, C385–C391. [Google Scholar] [CrossRef]
- Manika, I.; Maniks, J. Effect of substrate hardness and film structure on indentation depth criteria for film hardness testing. J. Phys. D Appl. Phys. 2008, 41, 074010. [Google Scholar] [CrossRef]
- Mutoh, M.; Nagoshi, T.; Chang, T.F.M.; Sato, T.; Sone, M. Micro-compression test using non-tapered micro-pillar of electrodeposited Cu. Microelectron. Eng. 2013, 111, 118–121. [Google Scholar] [CrossRef]
- Tang, H.; Chang, T.F.M.; Chai, Y.W.; Chen, C.Y.; Nagoshi, T.; Yamane, D.; Ito, H.; Machida, K.; Masu, K.; Sone, M. Au-Cu Alloys Prepared by Pulse Electrodeposition toward Applications as Movable Micro-Components in Electronic Devices. J. Electrochem. Soc. 2018, 165, D58–D63. [Google Scholar] [CrossRef]
- Hotta, T.; Chang, T.F.M.; Chen, C.Y.; Sawae, H.; Imada, Y.; Mizoguchi, M.; Kudo, O.; Maeda, R.; Sone, M. The Electrochemical Society, Micro-Compression Characterization and Thermal Stability of Electrolessly Plated Nickel Phosphorus Alloy. ECS J. Solid State Sci. Technol. 2021, 10, 035007. [Google Scholar] [CrossRef]
- Kihara, Y.; Nagoshi, T.; Chang, T.F.M.; Hosoda, H.; Sato, T.; Sone, M. Tensile behavior of micro-sized specimen fabricated from nanocrystalline nickel film. Microelectron. Eng. 2015, 141, 17–20. [Google Scholar] [CrossRef]
- Asano, K.; Tang, H.; Chen, C.Y.; Nagoshi, T.; Chang, T.F.M.; Yamane, D.; Konishi, T.; Machida, K.; Masu, K.; Sone, M. Microelectronic Engineering Promoted bending strength in micro-cantilevers composed of nanograined gold toward MEMS applications. Microelectron. Eng. 2018, 196, 20–24. [Google Scholar] [CrossRef]
- Suzuki, K.; Chang, T.F.M.; Hashigata, K.; Asano, K.; Chen, C.Y.; Nagoshi, T.; Yamane, D.; Ito, H.; Machida, K.; Masu, K.; et al. Sample geometry effect on mechanical property of gold micro-cantilevers by micro-bending test. MRS Comm. 2020, 10, 434–438. [Google Scholar] [CrossRef]
- Imamura, H.; Nagoshi, T.; Yoshida, A.; Chang, T.F.M.; Onaka, S.; Sone, M. Evaluation of anisotropic structure in electrodeposited Ni film using micro-sized cantilever. Microelectron. Eng. 2012, 100, 25–27. [Google Scholar] [CrossRef]
- Xiao, Y.; Gan, B.; Sologubenko, A.S.; Spolenak, R.; Wheeler, J.M. Size- and strain rate-dependence of nickel and Ni-Co micropillars with varying stacking fault energy. Mater. Sci. Eng. A 2021, 800, 140266. [Google Scholar] [CrossRef]
- Hall, E.O. The Deformation and Ageing of Mild Steel: III Discussion of Results. Proc. Phys. Soc. Lond. Sect. B 1951, 64, 747. [Google Scholar] [CrossRef]
- Petch, N.J. The orientation relationships between cementite and α-iron. Acta. Cryst. 1953, 174, 25. [Google Scholar] [CrossRef]
- Tanaka, K.; Sakakibara, M.; Kimachi, H. Grain-size effect on fatigue properties of nanocrystalline nickel thin films made by electrodeposition. Procedia Eng. 2011, 10, 542–547. [Google Scholar] [CrossRef][Green Version]
- Goldbach, S.; de Kermadec, R.; Lapicque, F. Electrodeposition of Ni-Co alloys from sulfamate baths. J. Appl. Electrochem. 2000, 30, 277–284. [Google Scholar] [CrossRef]
- Bai, A.; Hu, C.C. Effects of electroplating variables on the composition and morphology of nickel-cobalt deposits plated through means of cyclic voltammetry. Electrochim. Acta 2002, 47, 3447–3456. [Google Scholar] [CrossRef]
- Takashima, K.; Higo, Y.; Sugiura, S.; Shimojo, M. Fatigue crack growth behavior of micro-sized specimens prepared from an electroless plated Ni-P amorphous alloy thin film. Mater. Trans. 2001, 42, 68–73. [Google Scholar] [CrossRef]
- Hosford, W.F. Mechanical Behavior of Materials, 2nd ed.; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar]
- Zhong, Z.; Gu, Y.; Yuan, Y. Microstructural stability and mechanical properties of a newly developed Ni-Fe-base superalloy. Mater. Sci. Eng. A. 2015, 622, 101–107. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Huang, W.; Tian, H. Effects of peak current density on the mechanical properties of nanocrystalline Ni-Co alloys produced by pulse electrodeposition. Appl. Surf. Sci. 2008, 254, 6865–6869. [Google Scholar] [CrossRef]
- Tabor, D. The hardness of solids. Rev. Phys. Tech. 1970, 1, 145. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).