Abstract
Alkaloids are aromatic hydrocarbons with nitrogen as heteroelements in the ring structure that are responsible for bonding with the metal surface and help to reduce corrosion of metals such as mild steel (MS) in an acidic medium. In this study, the alkaloid of Rhynchostylis retusa (RR) was extracted by solvent extraction method and confirmed by chemical test as well as FTIR spectroscopic test. Extracted alkaloids were tested as green inhibitors for the MS corrosion in a 1.0 M H2SO4 solution. The inhibition efficiency (IE) of alkaloid extracts of RR was studied by the weight loss measurement method and electrochemical polarization method. Results showed that the maximum IE in the gravimetric method was 87.51% in 1000 ppm solution at 6 h immersion time. Open circuit potential (OCP) and potentiodynamic polarization results indicated that the extracted alkaloids acted as a mixed type of inhibitor. IE by polarization method was found to be 93.24% for the sample immersed for 6 h. The temperature effect study reveals that inhibitors can work only below 35 °C. Alkaloids of RR can be successfully extracted and used as corrosion inhibitors for MS in an acidic medium below 35 °C.
1. Introduction
Mild steel (MS) is a versatile ferrous alloy with large applications because of its excellent mechanical properties [1,2,3]. It is used heavily to build a large number of infrastructures, buildings, and industries due to its ease of fabrication and low purchasing cost; however, MS corrosion is a serious matter of concern in acidic environments such as Petrochemical, chemical plants, and gasoline pipelines [4,5]. In addition, processes such as acid pickling, de-scaling, and cleaning of boilers cause extensive corrosion damage to the surface and mechanical properties of MS. Nevertheless, it is a difficult task for industries to protect MS from corrosion, leading to huge economic losses.
To prevent metal loss, various methods such as surface modification, alloy formation, and coatings are adopted [6]; however, such methods are applicable only for those MS which are utilized for infrastructures development, construction of buildings, and industries where it is not in contact with an acid environment. For the protection of MS used in acidic environments, the use of various inhibitors is more often used. Corrosion inhibitors may be either synthetic or natural, which, when added to such an environment, effectively decreases the corrosion rate. Interest in synthetic inhibitors is diminishing due to their toxicity toward human, animal, and aquatic life [7]. On the other hand, natural products as inhibitors are non-toxic, eco-friendly, easily available, and effective. Therefore, natural products are recently studied as corrosion inhibitors [8,9]. Plant extracts contain a large number of phytochemicals that are biodegradable. The inhibitive performances of plant extract are related to their phytochemicals such as alkaloids, tannins, flavonoids, carbohydrates, phenolics, proteins, etc. These metabolites usually bear polar functional groups as well as heteroelements such as Nitrogen, Sulphur, or Oxygen, and triple or double conjugate bonds which act as major adsorption centers [10]. Literatures reveal descriptions of numerous plants exhibiting inhibitive properties for MS in acidic medium such as the crude extracts of Lantana camara [1], Artemisia vulgaris [2], Aloe vera [11], Jasminum nudiflorum [12], Euphorbia royleana [13], Bamboo [14], Ficus hispida [15], Aniba rosaeodora [16], Mansoa alliacea [17], Ginkgo [18], Annona squamosal [19]. The inhibition efficiency of such reported plant extracts is very promising.
Among the heteroelements as corrosion inhibitors, the inhibition efficiency has to be followed the trend O < N < S < P [13]; however, natural products containing Sulphur and phosphorus-containing phytochemicals are hard to find. Therefore, natural product-based research on corrosion inhibitors focuses on nitrogen and oxygen-bearing phytochemicals. Alkaloids contain nitrogen in their structure and oxygen may have a chance to be in the structure of alkaloids in the form of the functional group. Rhynchostylis retusa (RR) has been reported as alkaloids containing plants in their rhizome, leaves, and flowers, Figure 1a,b [20,21]. Rhynchostylis retusa is a monopodial epiphyte, an exotic blooming orchid belonging to the family Orchidaceae that grows in the forest of lower Himalayan ranges. The epiphytic orchid usually grows on trees or shrubs without directly harming the hosts and plays a vital role in ecosystem functioning. It can be found in Nepal and South Asia [22]. In Nepal, commonly it is known as ‘Sunakhari or Sunghava’. It can tolerate the temperature from 3 °C to 31 °C. The flowering time is mainly from May to July. Nevertheless, Rhynchostylis has a very wide host range, Ficus religious is the most common host tree species [22].

Figure 1.
(a) Rhynchostylis retusa in the host tree, (b) Rhynchostylis retusa showing Rhizome, leaf, and flower.
R. retusa is orchid species with beautiful flowers arranged in racemose, an inflorescence consisting of more than 100 pink–spotted white flowers, and has a robust stem of about 25 cm long [23]. It has medicinal properties due to the presence of different phytochemicals constituents such as dibenzyl derivatives, alkaloids, flavonoids, essential oils, etc. The extract of this plant is reported as medicine for the treatment of rheumatic disease, tuberculosis, epilepsy, blood dysentery, menstrual disorders, gout, asthma, skin diseases, external inflammations, and throat inflammation [23].
Therefore, in this work, alkaloid has been extracted from methanol extract of RR and the extracted alkaloid has been characterized and tested as a corrosion inhibitor for MS in 1 M H2SO4 solution using weight loss and electrochemical measurements.
2. Materials and Methods
2.1. MS Sample Preparation
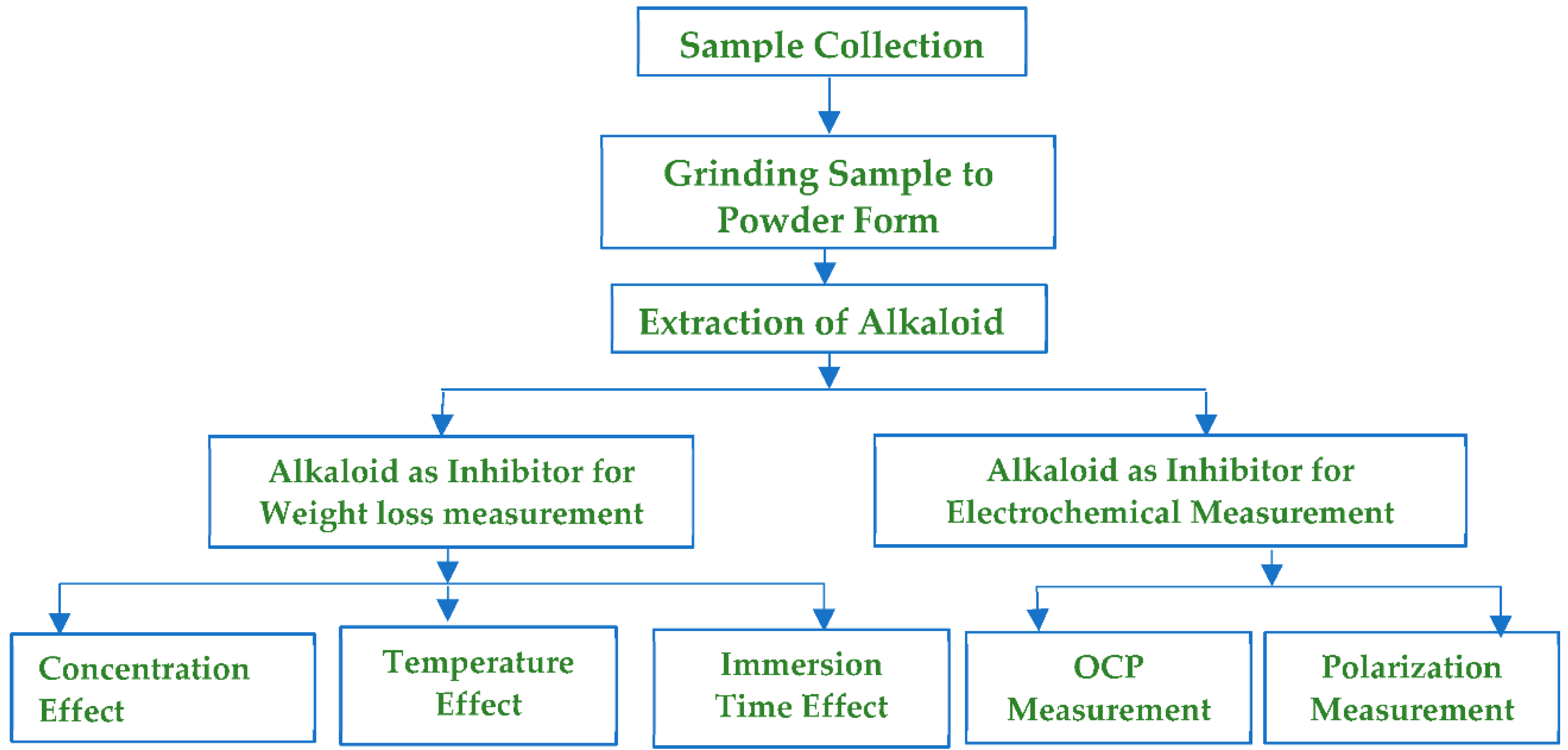
Mild steel sample A516 grade 60 (0.21–0.27% C, 0.13–0.45% Mn, 0.79–1.30% Si, 0.035% P, 0.035% S, and rest of Fe) in the form of a flat sheet was collected from the local market in Kathmandu, Nepal and cut into pieces of dimension (4 × 4 × 0.55) cm3. Before experimenting, each coupon was polished with silicon carbide (SiC) paper of 100–1000 #grits. To ensure the dimension of coupons was measured with a digital Vernier caliper and washed with hexane. Washed coupons were ultrasonicated in ethanol, dried, and placed in a moisture-free desiccator (Experimental schematics are shown in Scheme 1).
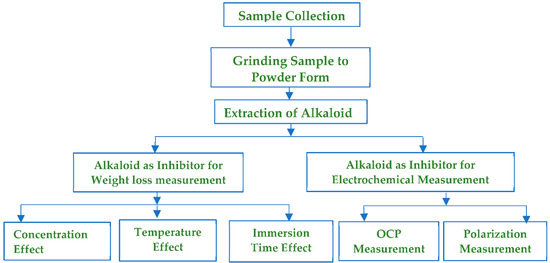
Scheme 1.
Experimental schematics.
2.2. Preparation of Methanol Extract and Separation of Alkaloids
All the chemicals used in this experiment were AR grade, collected from a local vendor, and used without purification. Liquor ammonia (25%w/w, sp.gr.0.905), methanol (99.85%, sp.gr.0.8957), tartaric acid (Lobachemie, 99%), and CHCl3 (Fine chemicals, 99.5%, sp.gr. 1.491) were used. For the preparation of methanol extract, Rhizome of Rhynchostylis retusa (RR) was collected from the Godavari, Nepal (27.57′′ N, 85.37′′ E), washed and shade dried. The dried sample was ground into powder. 250 g of powder sample was soaked in hexane for 24 h followed by filtration. The filtrate was discarded. The residue was soaked in methanol for 7 days followed by filtration to obtain extract as filtrate. This filtrate was taken as methanol extract. The pH of a dark brown extract was adjusted to alkaline by using tartaric acid and ammonia solution. Alkaloid was separated using CHCl3 in the separating funnel. The dark green slurry of alkaloid extract was obtained in the organic layer. The organic layer thus obtained was separated, and concentrated using a rotary evaporator under reduced pressure and temperature. The concentrated extract was evaporated up to dry and labeled as alkaloids of RR.
2.3. Test for Alkaloids
Extracted alkaloids were confirmed by qualitative chemical tests and spectroscopic test methods. In the qualitative chemical test, Mayer’s test and Dragendorff’s test methods were applied; for the spectroscopic method, the FTIR method was applied; for the chemical test, reagents of AR grade were applied. FTIR spectra of alkaloids were recorded in this work to find the functional group and N-C bond in the ring structure using an IR prestige 21-FTIR Spectrophotometer, Shimadzu, Japan. FTIR measurement of alkaloids has been carried out for predicting the active sites of molecules that are usually adsorbed on the metal surface.
2.4. Preparation of Acid and Inhibitor Solution
A stock solution of 1 M H2SO4 was prepared by taking 55.6 mL of concentrated H2SO4 (Fisher Scientific, Waltham, MA, USA, 98%, sp. gr. 1.84) in 1000 mL of the volumetric flask containing distilled water and diluted up to mark. This was taken as corrosive media. For the preparation of inhibitor medium, one gram of alkaloid of RR was dissolved in 1 M H2SO4 solution, and it was filtered to remove the un-dissolved extract. The filtrate was taken as 1000 ppm of stock solution. From 1000 ppm stock solution of the inhibitor, solutions of different concentrations were prepared by serial dilutions.
2.5. Weight Loss Measurement Method
The weight of each MS coupon before and after immersion in acid and inhibitor solution was measured using an analytical electronic balance (Ohaus Corporation, Parsippany, NJ, USA, Model: E1RR80). Measurements were carried out for each immersion experiment in inhibitor solution of different concentrations (200, 400, 600, 800, and 1000 ppm) as well as in different intervals of time (0.5, 3, 6, 9, and 24 h). Similarly, the temperature effect was studied by immersing the sample in 600 ppm inhibitor solution at 25, 35, 45, 55, and 65 °C for 6 h.
The corrosion rate (CR) and inhibition efficiency (IE%) were calculated using the formula:
where, K = 87,600, W = weight loss in gram, A = total surface area of coupons in cm2,
- T = time of immersion in h,
- D = density of mild steel in g/cm3
- Wa = weight loss in the absence of inhibitors
- Wp = weight loss in the presence of inhibitors
2.6. Electrochemical Measurements
2.6.1. Open Circuit Potential
The open-circuit potential (OCP) is important to explain the mode of inhibition of alkaloid solution. For a better understanding of the corrosion behavior of MS, the measurement of OCP was conducted at laboratory temperature in both corrosive and inhibitor mediums. The measurement was carried out in the three-electrode system using a Hokuto Denko Potentiostat (HA-151, Hokuto Denko Corporation, Tokyo, Japan). The MS coupon, graphite rod, and saturated calomel electrode (SCE) were used as a working electrode, a counter electrode, and a reference electrode, respectively. The OCP was measured for 30 min as immersed and after 3 h immersion of the MS sample in the inhibitor solution of different concentrations.
2.6.2. Potentiodynamic Polarization
The polarization measurement method was used to evaluate the corrosion current, corrosion potential, and inhibition efficiency. The polarization was carried out in the arrangement as in the OCP measurement process. Before potentiodynamic polarization, a time interval of 30 min was given for each experiment to attain the steady-state OCP; then, the sample was subjected to cathodic and anodic polarization at a scan rate of 1 mV/s after 3 h immersion in acidic inhibitor solutions. The corrosion current densities were obtained by extrapolation of the linear Tafel segment of anodic and cathodic curves.
The corrosion inhibition efficiency and the fraction of surface area coverage (θ) were calculated using the following formulas:
where, and are corrosion current in the absence and presence of inhibitor respectively.
This research work was based on the following experimental schematics.
3. Results
3.1. Test for Alkaloid
3.1.1. Qualitative Chemical Test
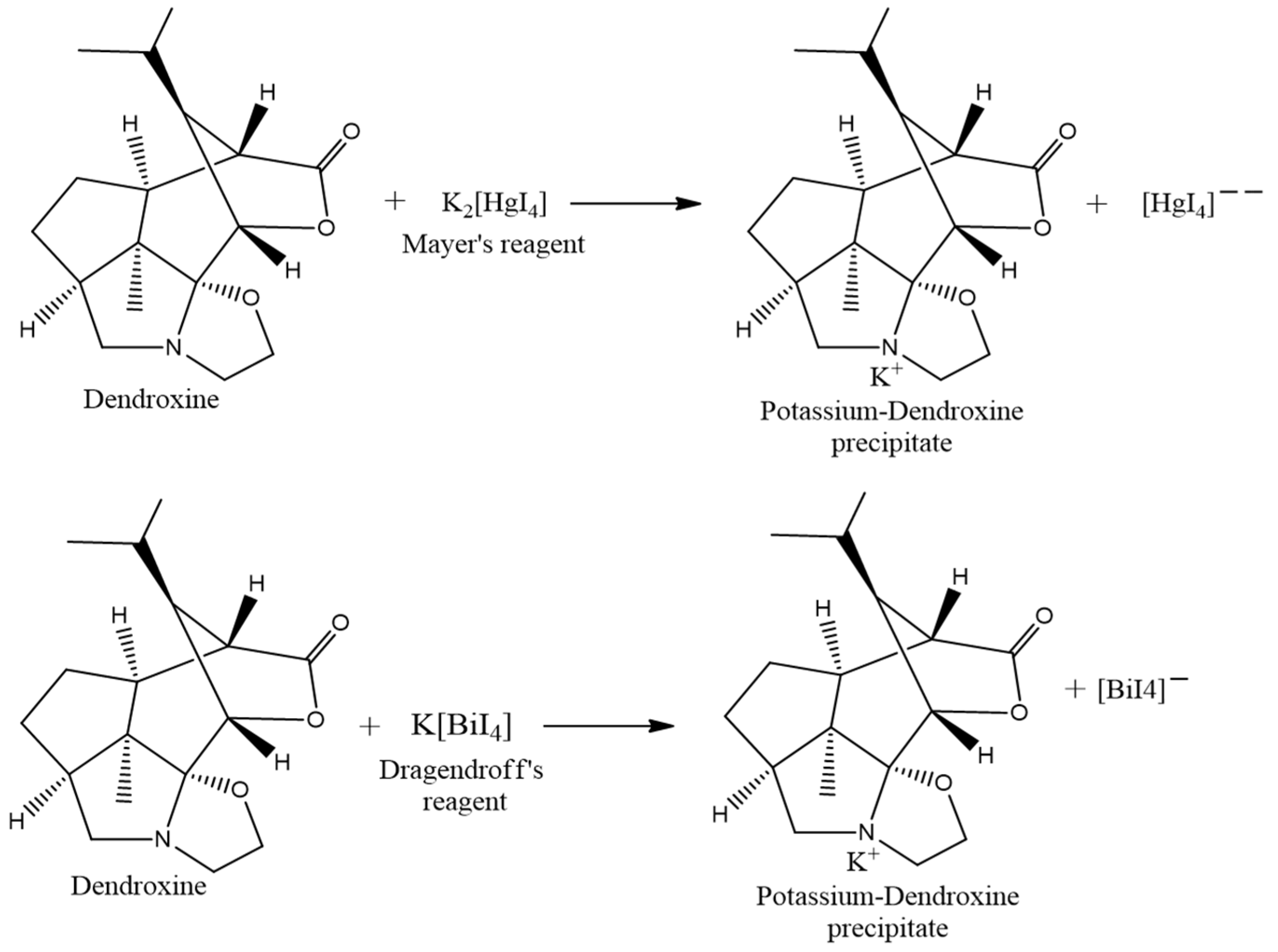
Extracted alkaloids were confirmed by qualitative chemical tests and spectroscopic tests. A qualitative test for confirmation of the presence of alkaloids was performed by Mayer’s and Dragendroff’s test methods. The detailed observations are given in Table 1. The possible chemical reaction with the reagent used is given regarding the dendroxine molecule in Scheme 2 [24].

Table 1.
Phytochemical screening of the extract solution.

Scheme 2.
Possible chemical reactions involved in the chemical test of alkaloids.
3.1.2. FTIR Spectroscopic Analysis
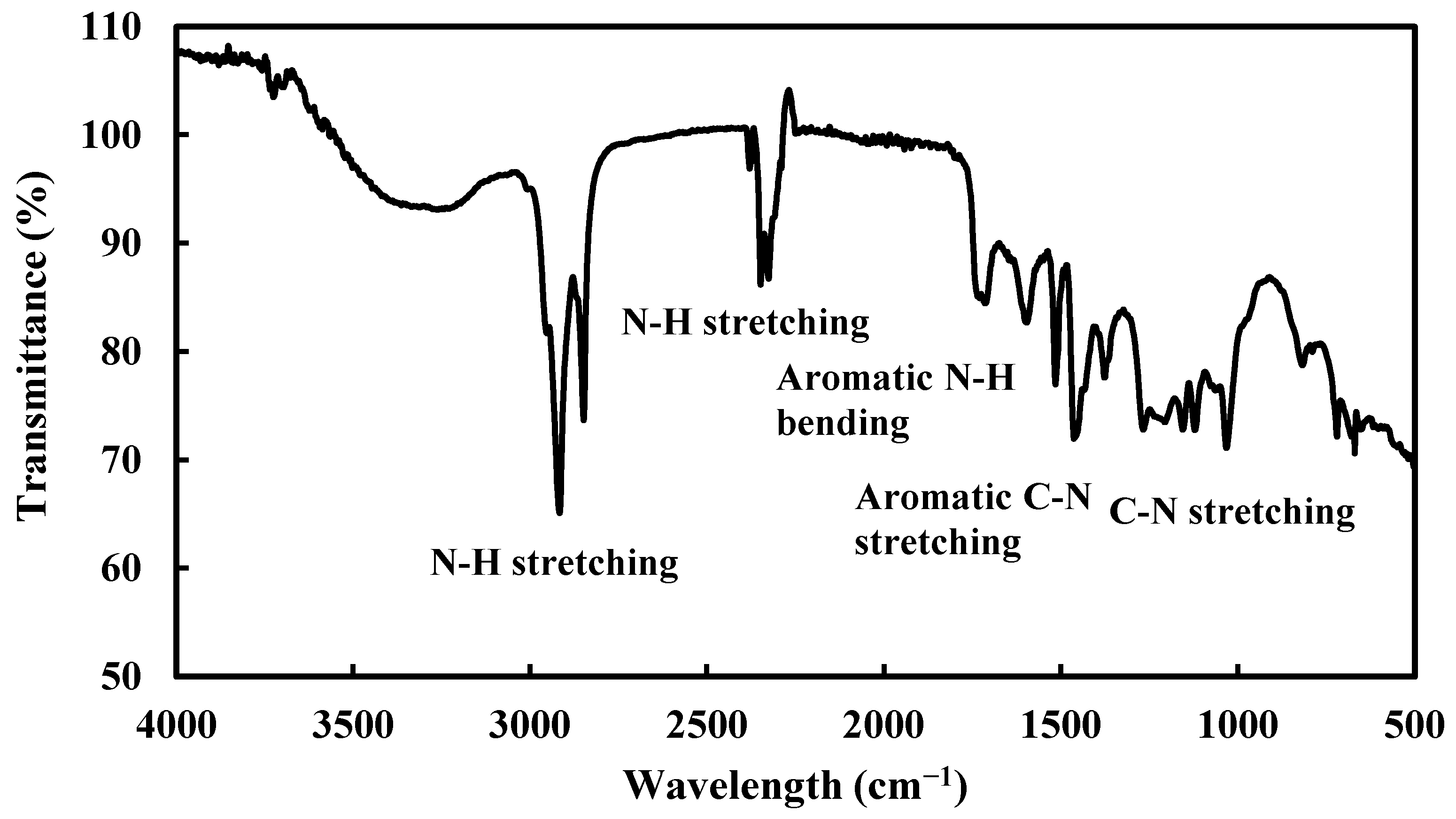
FTIR spectroscopic analysis was carried out to characterize alkaloids, Figure 2. The strong peak at 3000–2700 cm−1 is due to the N-H stretching vibration of primary and secondary ammonium ions, the peak at 2328 cm−1 is secondary amine multiple bonds, and the peak at 1514 cm−1 is due to N-H bending vibration. Peak around 1450 cm−1 is due to aromatic C-N stretching and multiple peaks at 1250–1020 cm−1 are due to C-N stretching of primary, secondary, and tertiary amines [25,26].
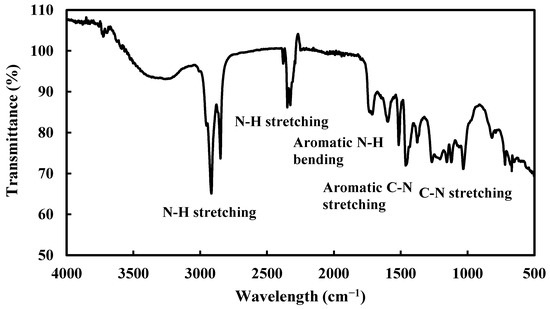
Figure 2.
FTIR spectra of alkaloids extract of RR.
3.2. Effect of Immersion Time
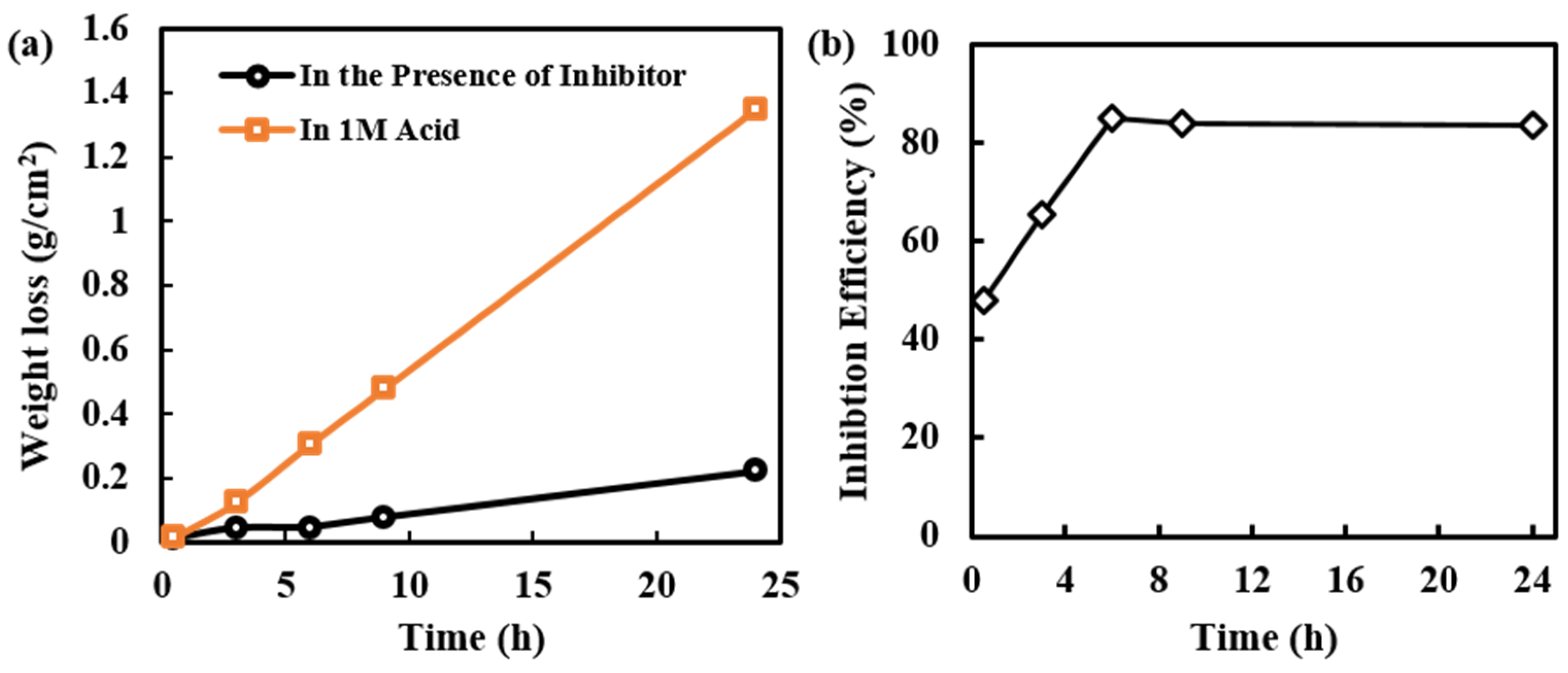
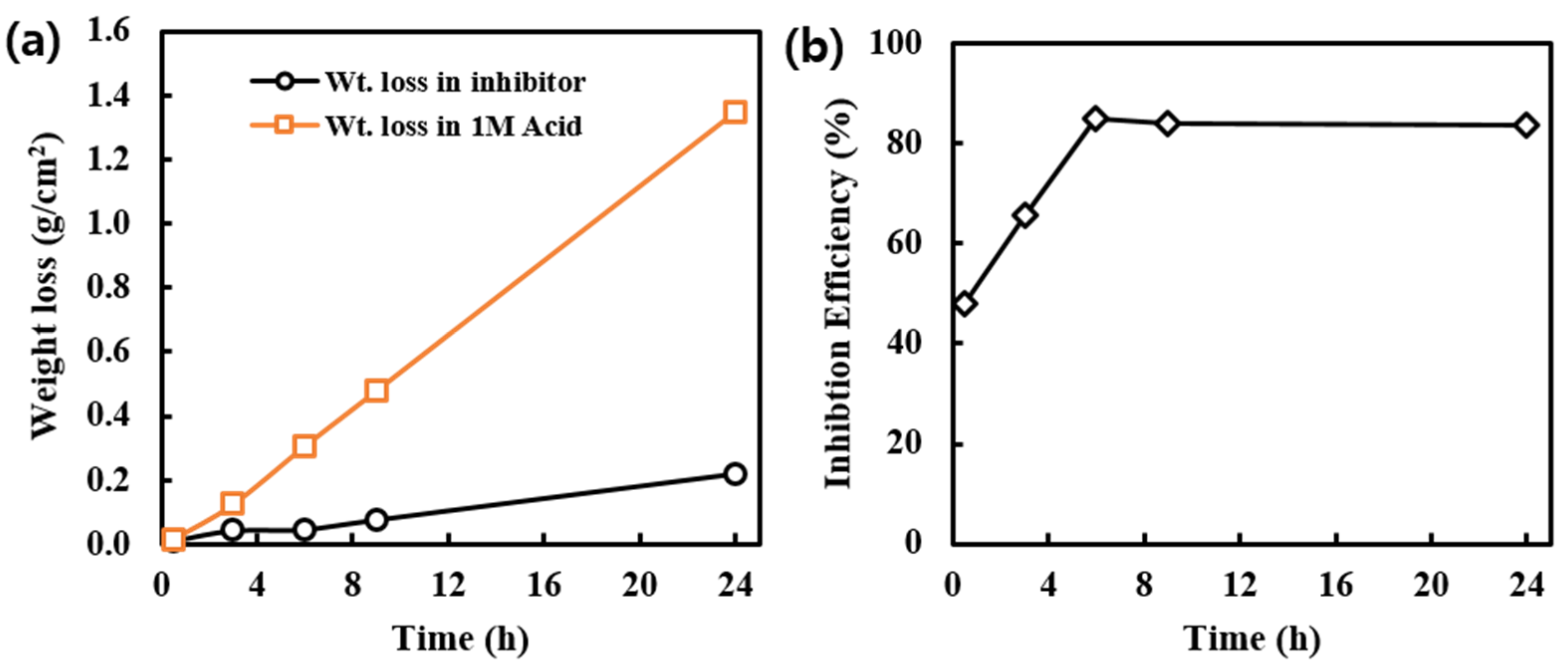
Weight loss of MS and inhibition efficiency of alkaloids extracted from RR at various time intervals are observed and represented in Figure 3a,b, respectively. Initially, the weight loss in acid and the presence of the inhibitor is slightly different; however, later on, the weight loss of MS samples in presence of an inhibitor is significantly decreased. This shows that initially the protective layer of the inhibitor is not completely formed, but later the formation of the protective layer is completed, hence weight loss is minimized. Due to a longer period of exposure to MS in acidic medium desorption can also happen, which results in slight weight loss even in presence of an inhibitor.
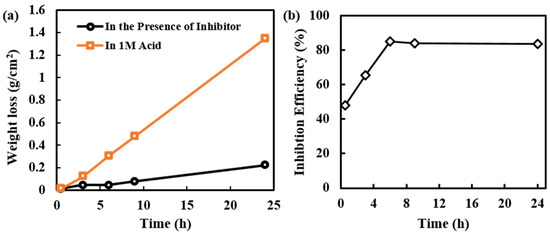
Figure 3.
(a) Variation of weight loss of MS samples in acid and in the presence of inhibitor solution at different intervals of time, and (b) Inhibition efficiency of alkaloid solution at different intervals of time.
The 1000 ppm inhibitor solution was used in this experiment. This concentration seems sufficient for the formation of a protective layer on the MS surface. Initially, there is very small weight loss; however, at a longer period of immersion time, the weight loss has increased. This may be due to the presence of defects on the inhibitor layer on the MS surface. This type of defect may arise due to orientation, size, and interaction among the inhibitor molecules. Even increasing in weight loss, the inhibition efficiency is constant; this is because the exposure of MS surface to the aggressive environment in the presence of inhibitor solution is only from the point of the defect however in absence of inhibitor all the surface area is available for the aggressive environment, and this is much more pronounced than in the presence of an inhibitor.
3.3. Effect of Concentration
Immersion tests have been carried out in 200, 400, 600, 800, and 1000 ppm alkaloid solutions for 6 h to study the effect of inhibitor concentration. The observation showed that at low inhibitor concentration, high weight loss and low efficiency indicate a high corrosion rate. Here, the rate of corrosion decreases by increasing the alkaloid concentration. The maximum efficiency observed is 87.51% in 1000 ppm solution as in Figure 4a.
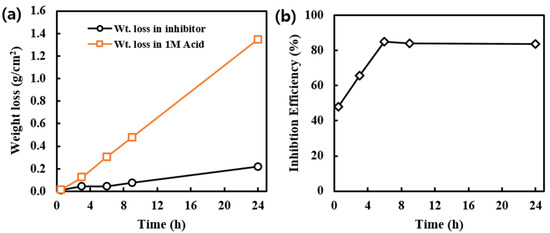
Figure 4.
(a) Variation of weight loss of MS samples in acid and inhibitor solution at fixed intervals of time and (b) Inhibition efficiency of alkaloid solution at different intervals of time.
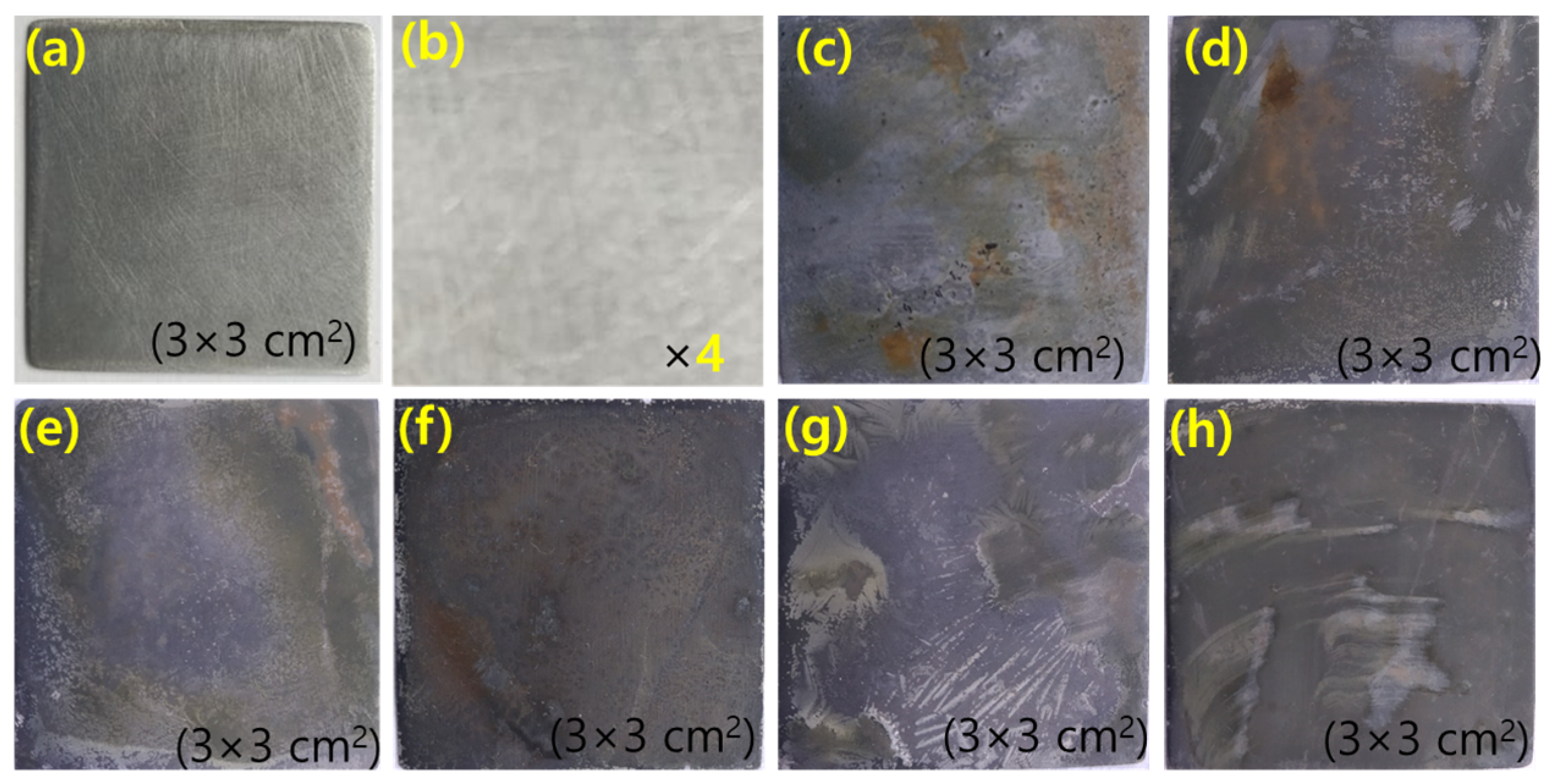
The surface morphology of the MS sample was studied by taking the optical image and microscopic image with a high-performance RXLr-4 microscope. Digital images of polished MS dipped in 1 M H2SO4 solution and the presence of a different concentration of inhibitor recorded are shown in Figure 5. The surface of the MS dipped in 1 M acid is very rough due to the formation of corrosion products but in the presence of an inhibitor, the surface of MS is being coated with inhibitor molecules. The green color of inhibitor molecules on the MS surface indicates the complete adsorption of inhibitor molecules. Due to the adsorption phenomenon, the surface of MS that dipped in the presence of inhibitor solution is smooth.
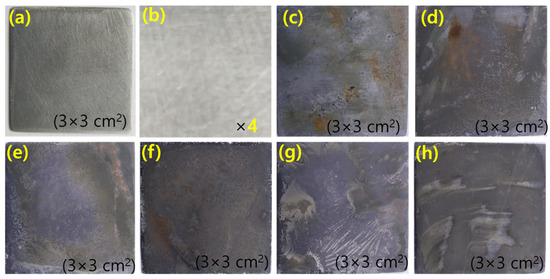
Figure 5.
The digital images of MS surface (a,b) polished MS at different magnification, (c) after dipping in 1 M H2SO4 for 1 h, (d–h) after dipping in 200, 400, 600, 800, 1000 ppm green inhibitor respectively for 1 h at 25 °C.
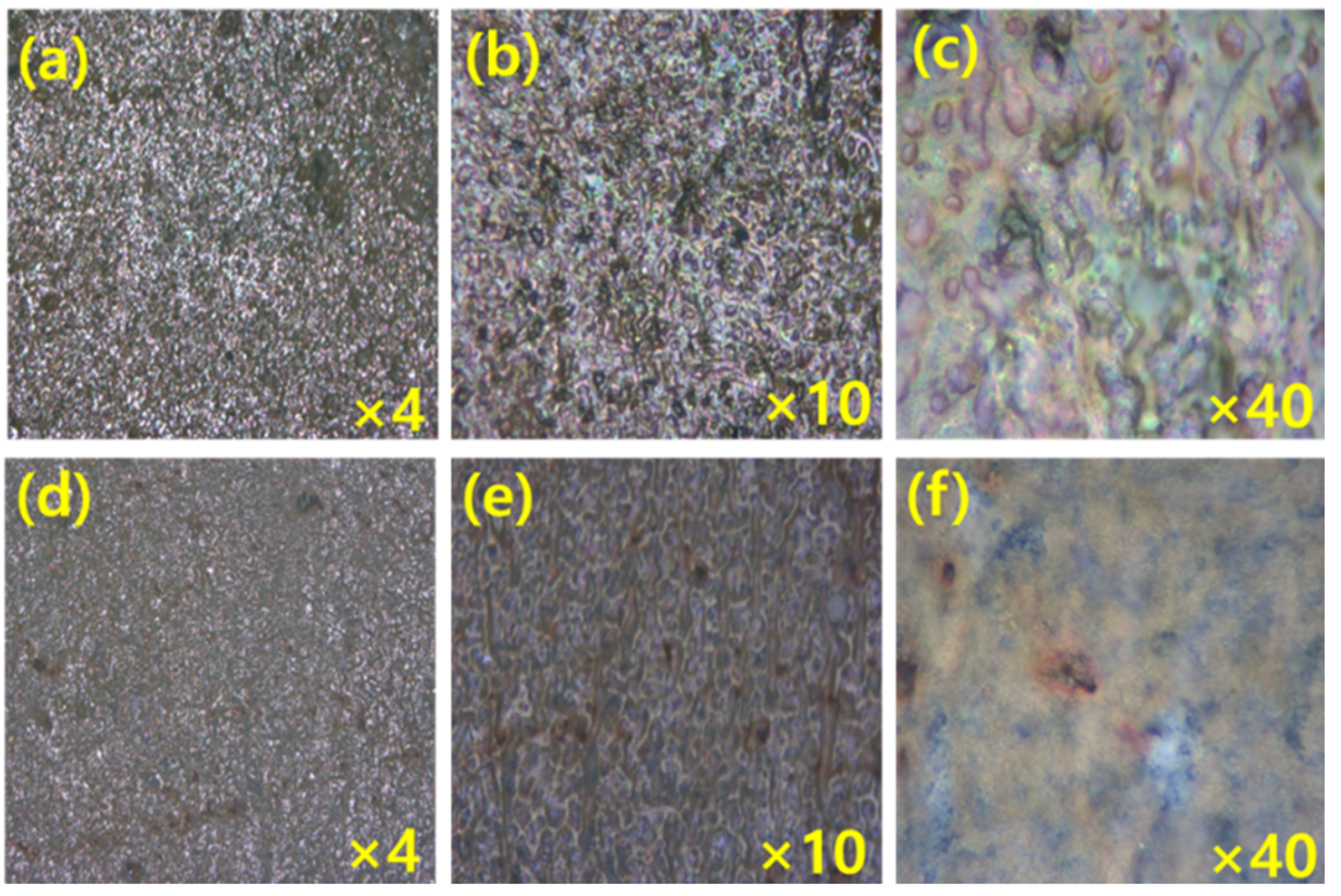
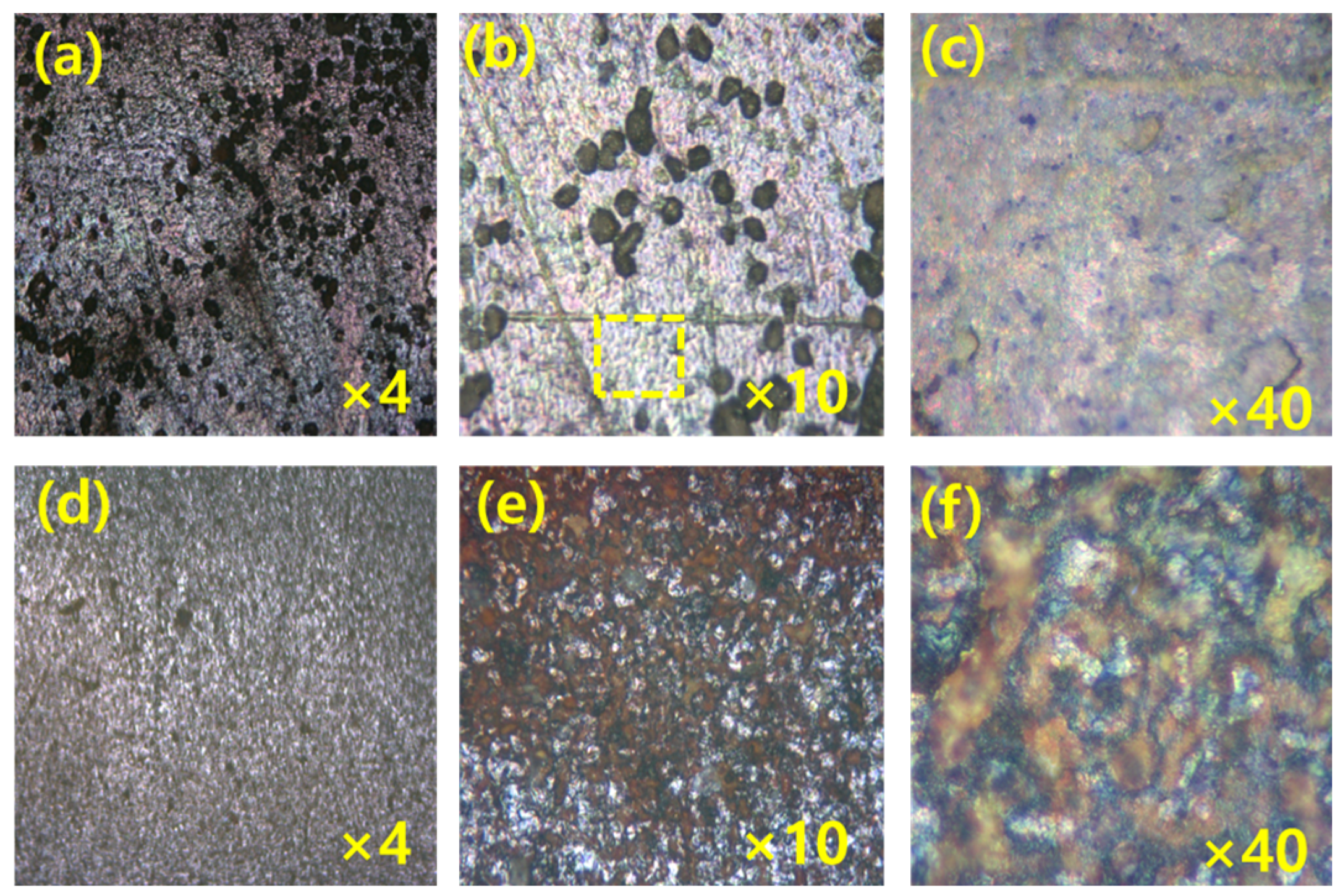
An optical microscopic study was carried out in the presence of acid and green inhibitor solution layer on the MS. The optical images of MS surfaces that are dipped in acid and the presence of green inhibitors in different magnifications are in Figure 6 and Figure 7. The surface of MS dipped in the acid solution only at 0.5 h and 3 h immersion study reveals that there are large pits/grooves on the surface due to dissolution of MS surface by acid molecules. The formation of pits/grooves on the surface is higher in the 3 h immersion sample than in the 1 h immersed sample; however, inhibitor molecules are visible on the MS surfaces which are immersed in the presence of inhibitor. The surface of MS is very smooth for 1 h immersed sample than that of 3 h immersed sample; however, in both cases, inhibitors molecules are adsorbed on the surface, thus reducing the corrosion.

Figure 6.
The MS surface images (a–c) after 1 h dipping, and (d–f) after 3 h dipping in 1 M H2SO4 at different magnifications.
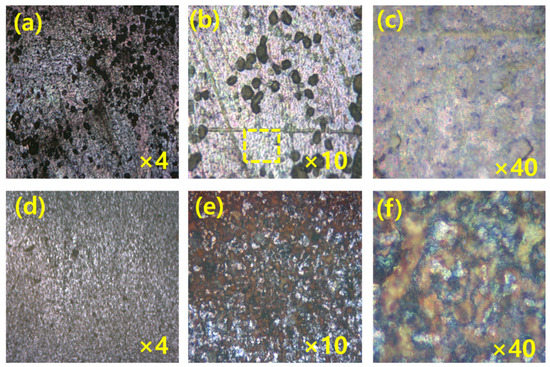
Figure 7.
The MS surface images (a–c) after 1 h dipping, and (d–f) after 3 h dipping at 25 °C in the solution of 1000 ppm green inhibitor at different magnifications.
3.4. Temperature Effect
The inhibition efficiency of the alkaloid inhibitor greatly depends on temperature. This effect was studied by taking reference to 600 ppm inhibitor solution at different temperatures. It is found that inhibition efficiency is almost constant at 25 °C and 35 °C but with the rise in temperature above it, the efficiency decreases, it may be due to desorption of inhibitor molecule from the metal surface, Figure 4b. This gives the key idea that there is physical adsorption between the inhibitor molecule and the MS surface.
3.5. Open Circuit Potential Measurements
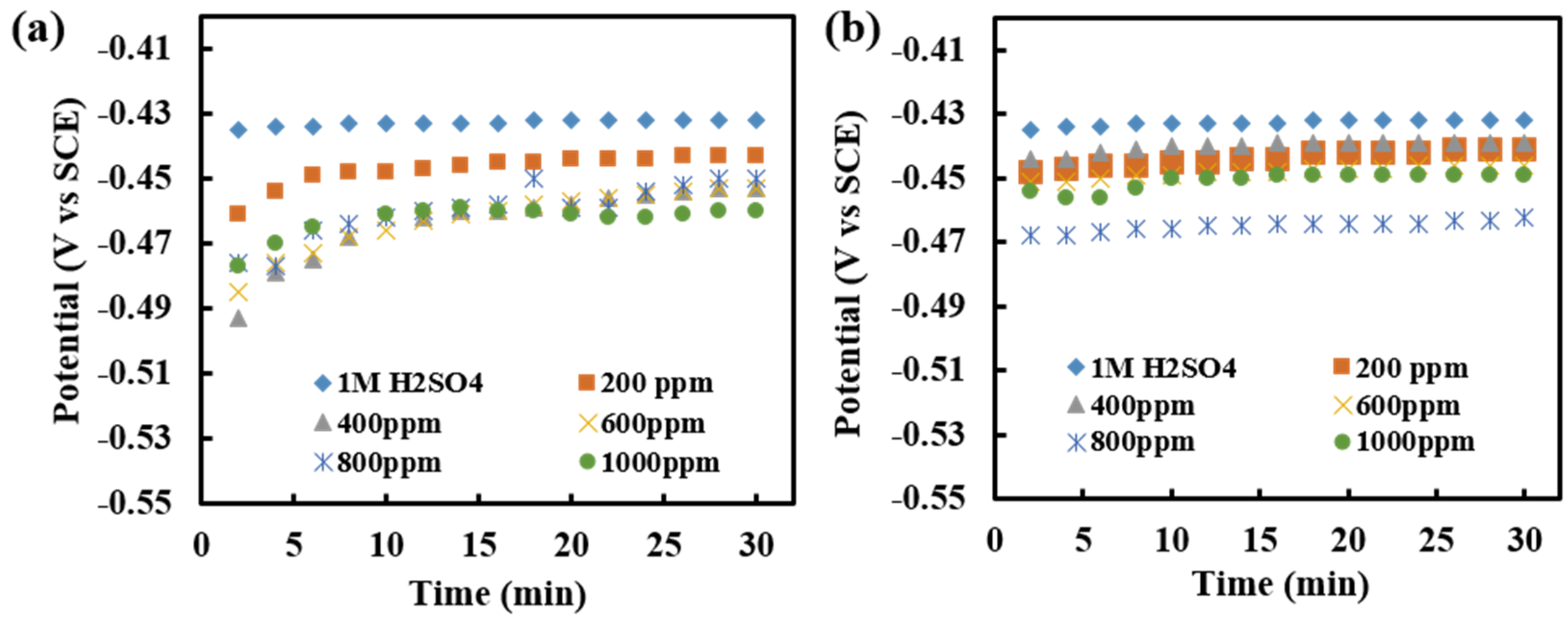
OCP measurement of the MS in 1 M H2SO4 was studied by monitoring changes in OCP with time up to 30 min at laboratory temperature. The change in OCP of MS in acid and at various concentrations of inhibitor solutions was recorded and analyzed. Measurement was carried out as immersed and after 3 h of immersion of MS in inhibitor solution as in Figure 8a,b, respectively.
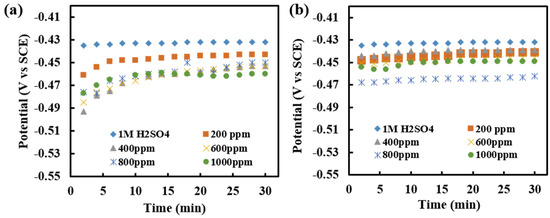
Figure 8.
(a) Recorded OCP with a time of immersion of MS in different concentrations of inhibitor of 1 M H2SO4 in as-immersed condition, and (b) Recorded OCP with the time of immersion of MS in different concentrations of inhibitor of 1 M H2SO4, when measured after 3 h of immersion.
3.6. Polarization Behaviour
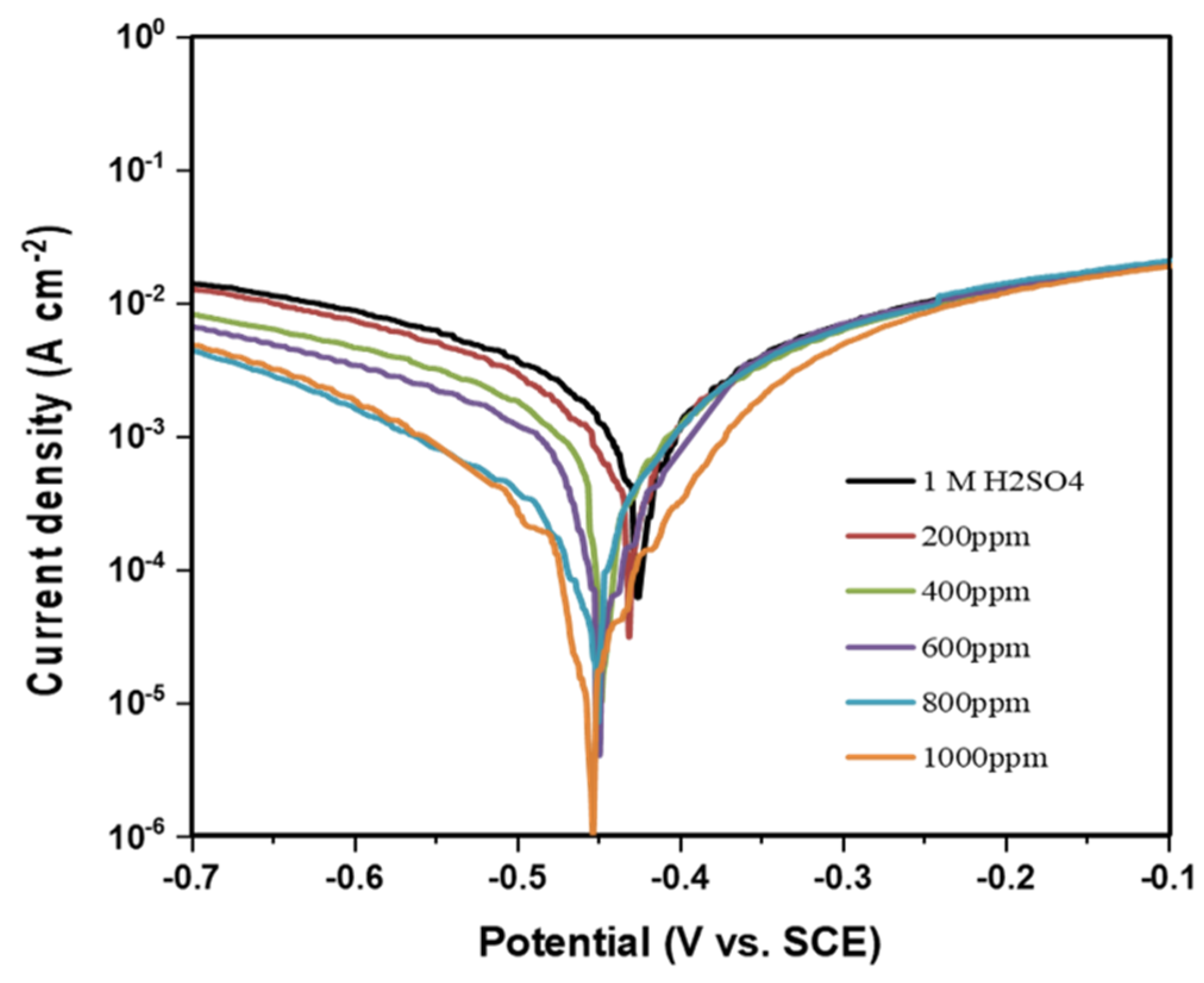
Potentiodynamic polarization measurement of MS sample was measured in three-electrode systems. It is observed that the corrosion current density decreased with the increase in the concentration of inhibitor solution, Figure 9. This may be due to the complete formation of inhibitor film on the MS surface as a result of increasing inhibitor concentration which results in a decrease in corrosion rate. It was further revealed that the efficiency was increased when the sample was immersed for 3 h than as immersed one. This is because it takes time to form an inhibition layer of inhibitor on the MS surface. After the formation of the layer, it prevents corrosion, decreases the current density, and hence increases inhibition efficiency.

Figure 9.
Potentiodynamic polarization curves for MS in 1 M H2SO4 containing different concentrations of inhibitor, when polarization is done immediately after 3 h of immersion.
The polarization curve also indicates that there is a decrease in current density with the addition of inhibitors. In the presence of an inhibitor, the MS surface is covered with inhibitor molecules so that the active site of MS is small. The reaction in this small area produces a small current density. This also indicates that the inhibitor used in this experiment is performing good inhibition. The inhibition efficiency, corrosion potential, fractional surface coverage, and corrosion current densities of the alkaloid are also tabulated in Table 2. The fraction of surface coverage was calculated using Equation (4). The current density in acid only solution is 340 μA/cm2 and the corrosion potential is 0.429 V. With the addition of different concentrations of inhibitor while polarization, the decrease in current density is achieved. The minimum current density is at 1000 ppm inhibitor solution and is equal to 23 μA/cm2 with a corrosion potential of 0.454 V. This decrease in current density is due to the interaction of inhibitor molecule on the MS surface to form a protective barrier and reducing active sites.

Table 2.
Table showing the anodic slope, cathodic slope, and inhibition efficiency for 3 h immersed sample.
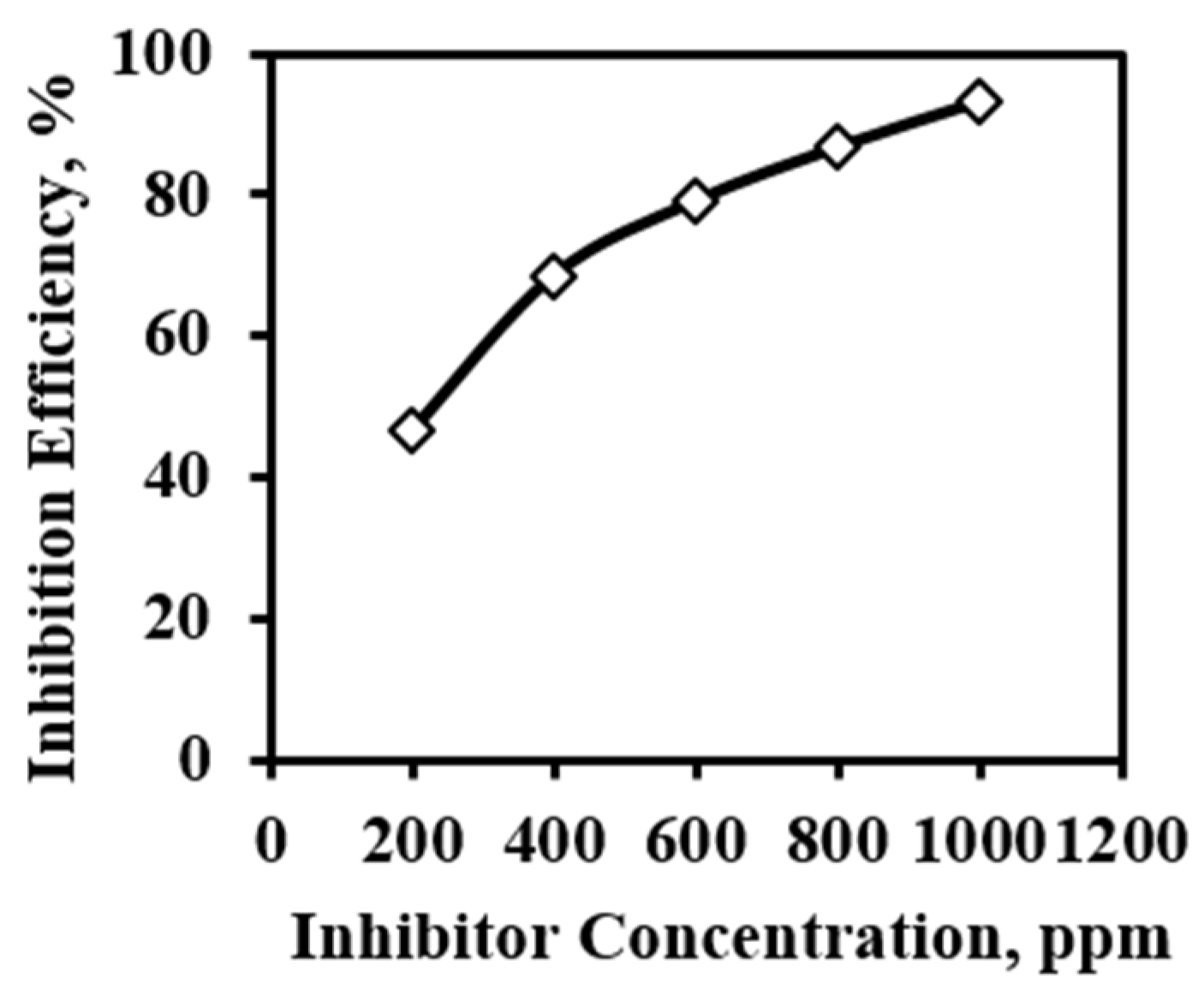
The inhibition effect of used alkaloids is enhanced with increasing concentration. For 3 h immersed sample and all the concentrations of inhibitor, polarization was carried out. The inhibition efficiency was calculated from the polarization method. The inhibition efficiency of the 200 ppm inhibitor is very small whereas the inhibition by 400 ppm solution is in the acceptable range. From this drastic change in the inhibition efficiency, it can be generalized that more than 400 ppm inhibitor concentration may be the effective concentration. To attain steady-state equilibrium between adsorbed and free inhibitor molecules, the inhibitor concentration plays an important role. After 400 ppm, the inhibition gradually increases up to 1000 ppm concentration and inhibition reaches 93.24%. This is because, the higher the concentration of the inhibitor, the inhibitor molecule can have sufficient concentration to get adsorbed and attain an equilibrium state [26]. This adsorbed layer protects the MS from an aggressive environment and reduces the corrosion rate. The detail efficiency variation is shown in Figure 10.
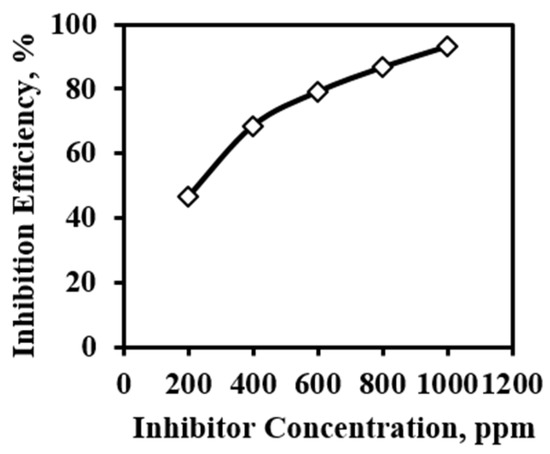
Figure 10.
Inhibition efficiency of inhibitor obtained from the polarization of 3 h immersed MS sample in 1 M H2SO4 in presence of inhibitor.
3.7. Adsorption Isotherm
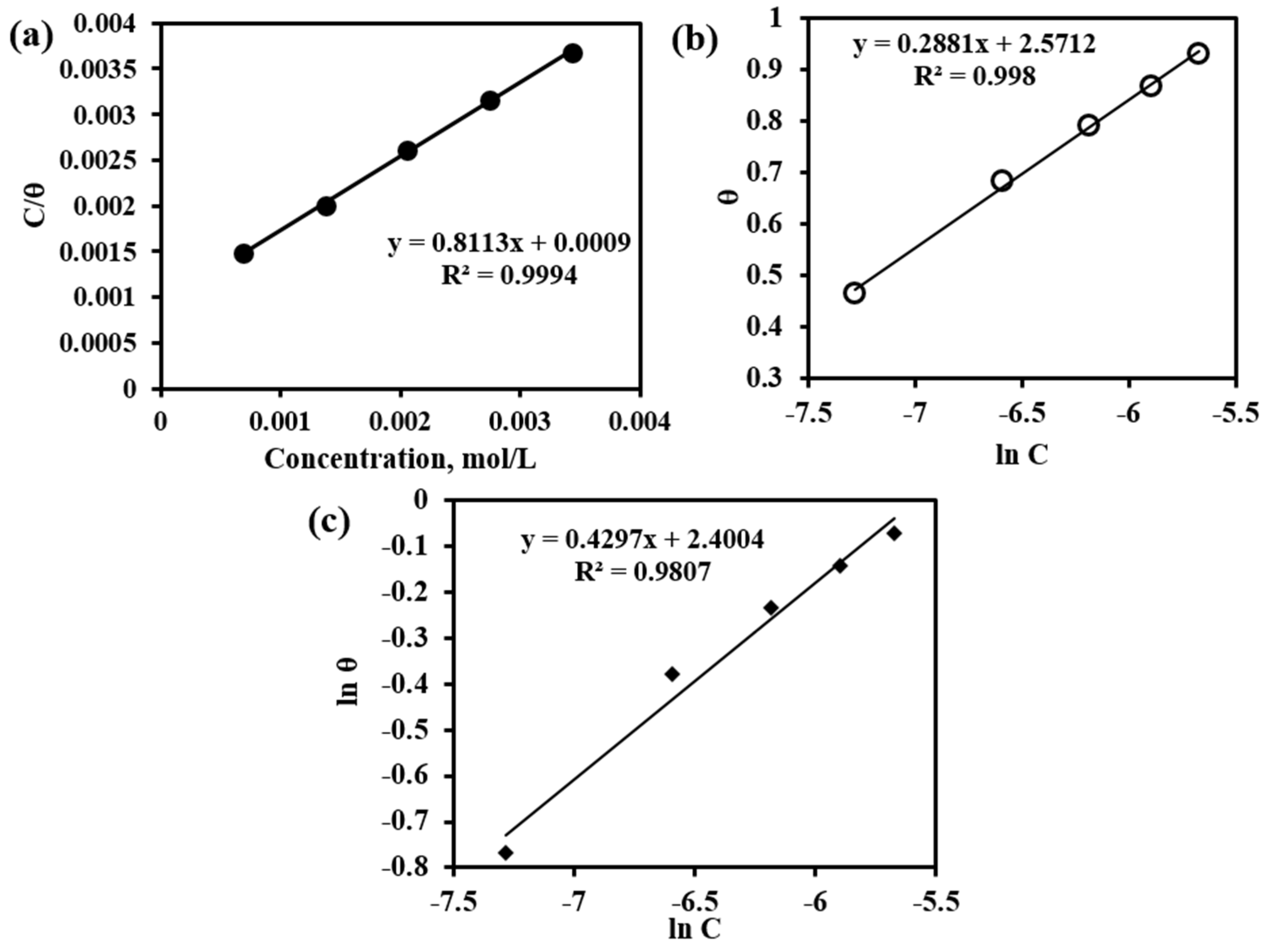
Based on the value of surface coverage and the inhibitor concentration used, various adsorption isotherms have been tested. For reference, the molecular mass of the dendroxine molecule has been used; however, this only is not responsible for the inhibition. Langmuir isotherm has been tested by plotting C/θ versus C (mol/L) from Equation (5) and shown in Figure 11a. The correlation coefficient (R2) value is equal to 0.9994, indicating that the adsorption of inhibitor on the MS surface strongly obeys Langmuir adsorption isotherm and a monolayer of inhibitor formed on the MS surface before multilayer formation. From the value of intercept, the adsorption constant has been calculated and is used for the calculation of the free energy of the adsorption. The free energy of the adsorption has been found at 27.33 kJ/mol. This value is higher than the value of physical adsorption (<20 kJ/mol) and lower than chemical adsorption (>40 kJ/mol) indicating physical dominated chemical adsorption of inhibitor on MS surface [27,28]. This is also in accordance with OCP measurement indicating the mixed type inhibition by inhibitor.
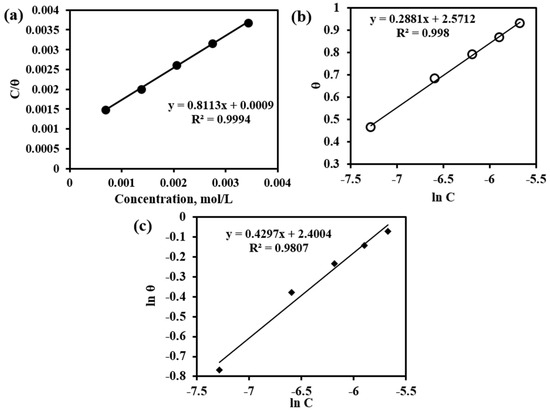
Figure 11.
(a) Langmuir Adsorption Isotherm, (b) Temkin Adsorption Isotherm, and (c) Freundlich Adsorption Isotherm.
A plot of θ versus ln C from Equation (6) gives a straight line with a slope of 0.2881 and an R2 value of 0.998 as in Figure 11b. The value of interaction parameter ‘a’ has been calculated from the intercept and found equal to negative 1.73 indicating the strong interaction between the adsorbed inhibitor molecules. The value of K has been calculated and found to be negative 2.09 indicating the inhibitor molecules are strongly adsorbed on the MS surface [28,29,30].
Freundlich adsorption isotherm has been tested by plotting ln θ versus ln C from Equation (7) as in Figure 11c. A straight line with R2 value of 0.9807 and a slope (1/n) value equal to 0.4297 which is in the range 0 < 1/n < 1 indicates the adsorption process is easy and spontaneous [28,29].
4. Discussion
Recorded data and calculated results of the weight loss measurement method indicate that initially inhibition efficiency (IE) is 48.07% which gradually increases up to 84.91%(at 6 h) and remains almost constant, Figure 3a. The formation of an inhibition layer on the MS surface may take a few minutes. At that duration, the mild steel sample was completely exposed to an acidic environment which leads the corrosion of that surface. The formation of a complete protective layer on the MS surface increases the inhibition efficiency. The efficiency of the inhibitor is not 100% which may be due to the dissolution of the protective layer or may be due to the presence of a vacant site between the large inhibitor molecules.
The effect of concentrations of inhibitor study reveals that, on increasing inhibitor concentration, weight loss gradually decreases and efficiency increases. This shows that IE is high as the concentration is increased. This change may be due to the increase in the covered fraction of the MS surface by the adsorbed molecules. Even if the experiment is performed at optimum inhibitor concentration, the efficiency of the inhibitor may not be 100% due to the desorption process. The temperature may be the next important factor for lowering efficiency. Effect of temperature for inhibition mechanism study reveals that at a higher temperature, the alkaloid molecules in the solution may lose their properties resulting in a decrease in inhibition efficiency. This implies that the adsorption of inhibitors on MS surface is physical adsorption. This claim has been confirmed by free energy calculation [31].
The OCP measurement in the presence of an inhibitor is shifted to a slightly negative direction (<50 mV) as compared to that of the acid solution, Figure 8a,b. This shows that alkaloid extract of RR acts as a mixed type of corrosion inhibitor. The shifting of OCP implies the formation of the protective film of inhibitor on the MS surface. There is a slight increase in potential in the middle, mainly in the 3 h immersed sample, which may be due to the destruction of the film formed at the metal surface due to corrosive attack. After a certain time, the value is constant indicating the reoccurrence of film at the metal surface.
The significant decrease in corrosion current with the addition of an inhibitor reveals that alkaloid acts as a good inhibitor. The polarization curve shows that only the cathodic curve is affected by the alkaloid extract of RR. Alkaloid acts as mixed type inhibitor as corrosion potential shift is less than 85 mV. Calculation shows that inhibition efficiency increases with an increase in the concentration of alkaloid in acid and it is up to 93.24% at 1000 ppm concentration in 3 h immersed sample.
The inhibition efficiency of the inhibitor calculated by polarization measurement is higher in comparison to the weight loss method. Lower the inhibition efficiency in weight loss measurement may be due to handling and systemic errors. The polarization measurement is almost error-free. Hence, the inhibition efficiency calculated by polarization measurement is more reliable.
5. Conclusions
Alkaloids have been extracted successfully from RR by the solvent extraction process. Qualitative chemical test (Dragendorff’s method) supports, and FTIR analysis shows the presence of N-H, O-H, N-O, -C=C- groups in the structure of alkaloid. It has been found that alkaloid inhibition increases with an increase in its concentration. Inhibitor protects mild steel from an aggressive environment by the formation of a protective layer on the surface through the adsorption process. Adsorption of the alkaloid (inhibitor) on the metal surface depends on the contact time, concentration and temperature. Due to this fact, inhibition efficiency is greatly influenced by temperature, concentration, and contact time. OCP measurement reveals that alkaloids extracted from RR inhibit by controlling both anodic and cathodic reactions, i.e., act as a mixed type of inhibitor. The inhibition efficiency of inhibitor by weight loss method is found to be 87.51% for 1000 ppm concentration. Similarly, potentiodynamic polarization shows an inhibition efficiency of 93.24% of the immersed sample for 1000 ppm inhibitor concentration. The alkaloids perform well up to 35 °C.
Author Contributions
Conceptualization, methodology, validation, formal analysis, resources, data curation, writing—review, and editing by A.P.Y., H.B.O. and K.C. The investigation, visualization, and original draft preparation by A.C. and D.A. Formal analysis, and data curation by A.K.D. Supervision, project administration, and funding acquisition by A.P.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University Grants Commission, Sanothimi, Nepal, UGC Masters Research Support 2075/76.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors are thankful to Amrit Campus and the Central Department of Chemistry, Tribhuvan University, Kathmandu, Nepal for laboratory facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shrestha, P.R.; Oli, H.B.; Thapa, B.; Choudhary, Y.; Gupta, D.K.; Das, A.K.; Nakarmi, K.B.; Singh, S.; Karki, N.; Yadav, A.P. Bark Extract of Lantana camara in 1M HCl as Green Corrosion Inhibitor for Mild Steel. Eng. J. 2019, 23, 205–211. [Google Scholar] [CrossRef]
- Karki, N.; Choudhary, Y.; Yadav, A.P. Thermodynamic, Adsorption and Corrosion Inhibition Studies of Mild Steel by Artemisia vulgaris Extract from Methanol as Green Corrosion Inhibitor in Acid Medium. J. Nepal Chem. Soc. 2018, 39, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Oli, H.B.; Parajuli, D.L.; Sharma, S.; Chapagain, A.; Yadav, A.P. Adsorption Isotherm and Activation Energy of Inhibition of Alkaloids on Mild Steel Surface in Acidic Medium. Amrit Res. J. 2021, 2, 59–67. [Google Scholar] [CrossRef]
- Obiukwu, O.O.; Opara, I.O.; Oyinna, B.C. Corrosion Inhibition of Stainless Steel Using Plant Extract Vernoniaamygdalina and Azadirachtaindica. Pac. J. Sci. Technol. 2013, 14, 31–35. [Google Scholar]
- Alaneme, K.K.; Olusegun, S.J.; Adelowo, O.T. Corrosion inhibition and adsorption mechanism studies of Hunteria umbellate seed husk extraction mild steel immersed in acidic solutions. Alex. Eng. J. 2016, 55, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Oguzie, E.E.; Li, Y.; Wang, F.H. Effect of surface nano crystalization on corrosion and corrosion inhibition of low carbon steel: Synergistic effect of methionine and iodide ion. Electrochim. Acta 2007, 52, 6988–6996. [Google Scholar] [CrossRef]
- Al-Otaibi, M.S.; Al-Mayouf, A.M.; Khan, M.; Mousa, A.A. Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media. Arab. J. Chem. 2014, 7, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Uwah, I.E.; Okafar, P.C.; Ebiekpe, V.E. Inhibitive action of ethanol extracts from Nauclealati folia on the corrosion of mild steel in H2SO4 solutions and their adsorption characteristics. Arab. J. Chem. 2013, 6, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Hussin, M.H.; Kassim, M.J.; Razali, N.N.; Dahon, N.H.; Nasshorudin, D. The effect of Tinosporacrispa extract as a natural mild steel corrosion inhibitor in 1 M HCl solution. Arab. J. Chem. 2016, 9, S616–S624. [Google Scholar] [CrossRef] [Green Version]
- Baran, E.; Cakir, A.; Yaziri, B. Inhibitory effect of Gentianaoliviera extracts on the corrosion of mild steel in 0.5 M HCl: Electrochemical and Phytochemical evaluation. Arab. J. Chem. 2016, 1, 17. [Google Scholar]
- Abiola, O.K.; James, A.O. The effects of Aloe vera extract on corrosion and kinetics of corrosion process of zinc in HCl solution. Corros. Sci. 2010, 52, 661–664. [Google Scholar] [CrossRef]
- Deng, S.; Li, X. Inhibition by Jasminum nudiflorum Lindl. leaves extract of the corrosion of aluminum in HCl solution. Corros. Sci. 2012, 64, 253–262. [Google Scholar] [CrossRef]
- Thapa, B.; Gupta, D.K.; Yadav, A.P. Corrosion Inhibition of Bark Extract of Euphorbia royleana on Mild Steel in 1M HCl. J. Nepal Chem. Soc. 2019, 40, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Deng, S.; Fu, H. Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract. Corros. Sci. 2012, 62, 163–175. [Google Scholar] [CrossRef]
- Muthukrishnan, P.; Prakash, P.; Jeyaprabhu, B.; Shankar, K. Stigmasterol extracted from Ficus hispida leaves as a green inhibitor for the mild steel corrosion in 1M HCl solution. Arab. J. Chem. 2015, 12, 3345–3356. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, M.; Robert, F.; Amusant, N.; Traisnel, M.; Roos, C.; Lebrini, M. Enhanced corrosion resistance of mild steel in 1MHCl solution by alkaloids extract from Anibarosaeodora plant: Electrochemical phytochemical and XPS studies. Electrochim. Acta 2014, 131, 96–105. [Google Scholar] [CrossRef]
- Sudile, F.; Robert, F.; Roos, C.; Lebrini, M. Corrosion inhibition of Zinc by Mansoaalliacea plant extract in sodium chloride media: Extraction, characterization and electrochemical studies. Electrochim. Acta 2014, 133, 631–638. [Google Scholar] [CrossRef]
- Deng, S.; Li, X. Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros. Sci. 2012, 55, 407–415. [Google Scholar] [CrossRef]
- Lebrini, M.; Robert, F.; Roos, C. Inhibition Effect of Alkaloids Extract from Annonasquamosa Plant on the Corrosion of C38 Steel in Normal Hydrochloric Acid Medium. Int. J. Electrochem. Sci. 2010, 5, 1696–1712. [Google Scholar]
- Bhattacharjee, B.; Islam, S.S. Assessment of Antibacterial and Antifungal Activities of the extracts of Rhynchostylis retusa Blume-A medicinal orchid. World J. Pharm. Pharm. Sci. 2015, 4, 72–87. [Google Scholar]
- Akter, M.; Huda, M.K.; Hoque, M.M. Investigation of secondary metabolites of nine medicinally important orchids of Bangladesh. J. Pharmacogn. Phytochem. 2018, 7, 602–606. [Google Scholar]
- Adhikari, Y.P.; Fischer, A. Distribution of the epiphytic orchid Rhynchostylis retusa under strong human influence in Kathmandu valley, Nepal. Bot. Orient. J. Plant Sci. 2011, 8, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.M.; Bhattacharjee, B. Plant regeneration through somatic embryogenesis from leaf and root explants of Rhynchostylis retusa (L.) Blume. Appl. Biol. Res. 2017, 17, 158–165. [Google Scholar] [CrossRef]
- Li, D.; Zhang, P.; Guo, X.; Zhao, X.; Xu, Y. The inhibition of mild steel corrosion in 0.5 M H2SO4 solution by radish leaf extract. R. Soc. Chem. 2019, 9, 40997–41009. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, R.M.; Webster, F.X. Spectrometric Identification of Organic Compounds, 6th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2006; pp. 99–106. [Google Scholar]
- Chhetri, K.; Tiwari, A.P.; Dahal, B.; Ojha, G.P.; Mukhiya, T.; Lee, M.; Kim, T.; Chae, S.H.; Muthurasu, A.; Kim, H.Y. A ZIF-8-derived nanoporous carbon nanocomposite wrapped with Co3O4-polyaniline as an efficient electrode material for an asymmetric supercapacitor. J. Electroanal. Chem. 2020, 856, 113670. [Google Scholar] [CrossRef]
- Lai, C.; Xie, B.; Zou, L.; Zheng, X.; Ma, X.; Zhu, S. Adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by S-allyl-O, O′-dialkyldithiophosphates. Results Phys. 2017, 7, 3434–3443. [Google Scholar] [CrossRef]
- Andoor, P.A.; Okeoma, K.B.; Mbamara, U.S. Adsorption and thermodynamic studies of the corrosion inhibition effect of Rosmarinus officinalis L. leaves on aluminum alloy in 0.25 M HCl and effect of an external magnetic field. Int. J. Phys. Sci. 2021, 16, 79–95. [Google Scholar]
- Ituen, E.; Akaranta, O.; James, A. Evaluation of performance of corrosion inhibitors using adsorption isotherm models: An overview. Chem. Sci. Int. J. 2017, 18, 1–34. [Google Scholar] [CrossRef]
- Chhetri, K.; Yadav, A.P. Electrochemical Behavior of Polyaniline (PANI) Doped with Different Nitrate Salts. J. Univ. Grants Comm. 2018, 7, 14–24. [Google Scholar]
- Karki, N.; Neupane, S.; Chaudhary, Y.; Gupta, D.K.; Yadav, A.P. Equisetum hyemale: A new candidate for green corrosion inhibitor family. Int. J. Corros. Scale Inhib. 2021, 10, 206–227. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).