Alkaloid of Rhynchostylis retusa as Green Inhibitor for Mild Steel Corrosion in 1 M H2SO4 Solution
Abstract
:1. Introduction
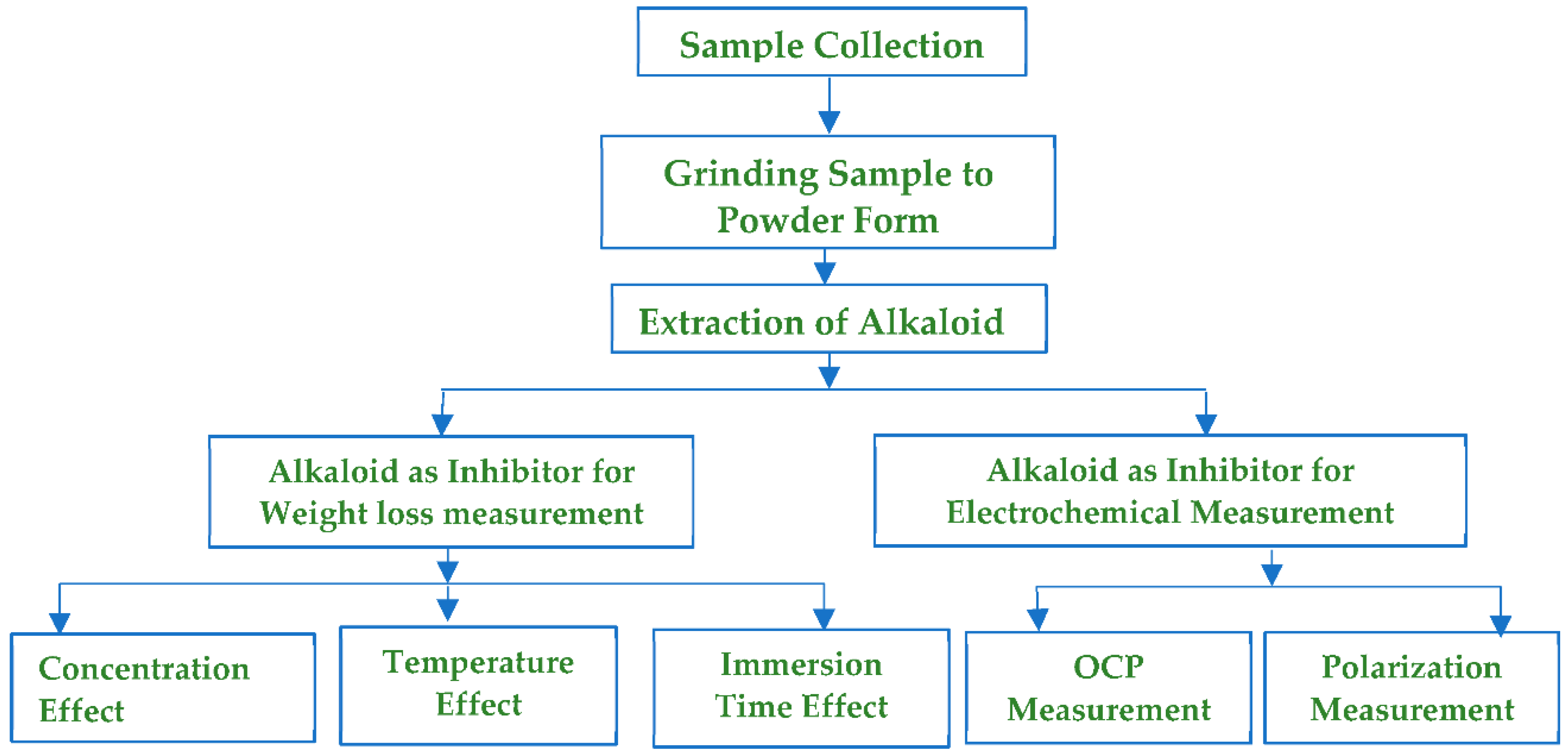
2. Materials and Methods
2.1. MS Sample Preparation
2.2. Preparation of Methanol Extract and Separation of Alkaloids
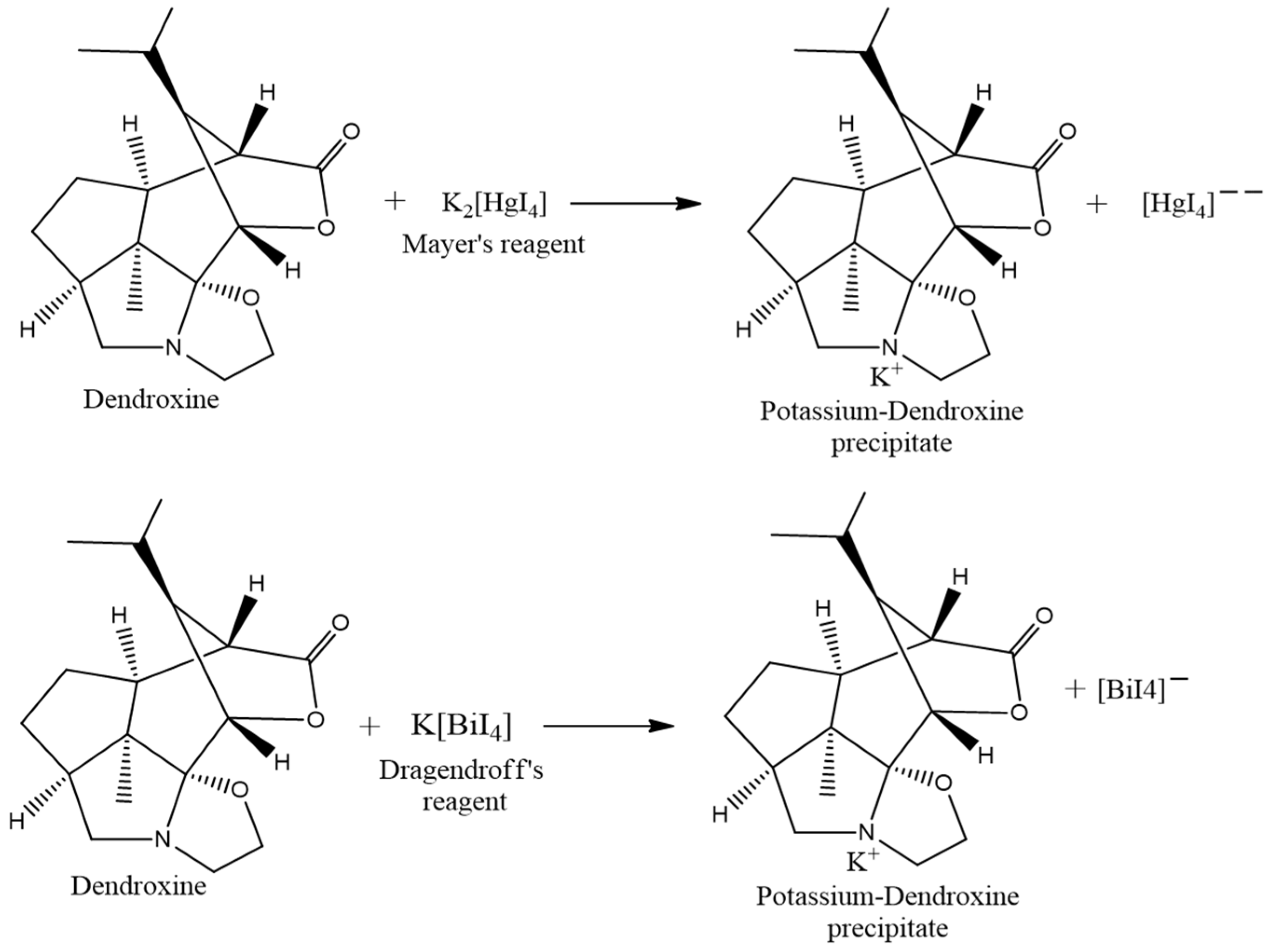
2.3. Test for Alkaloids
2.4. Preparation of Acid and Inhibitor Solution
2.5. Weight Loss Measurement Method
- T = time of immersion in h,
- D = density of mild steel in g/cm3
- Wa = weight loss in the absence of inhibitors
- Wp = weight loss in the presence of inhibitors
2.6. Electrochemical Measurements
2.6.1. Open Circuit Potential
2.6.2. Potentiodynamic Polarization
3. Results
3.1. Test for Alkaloid
3.1.1. Qualitative Chemical Test
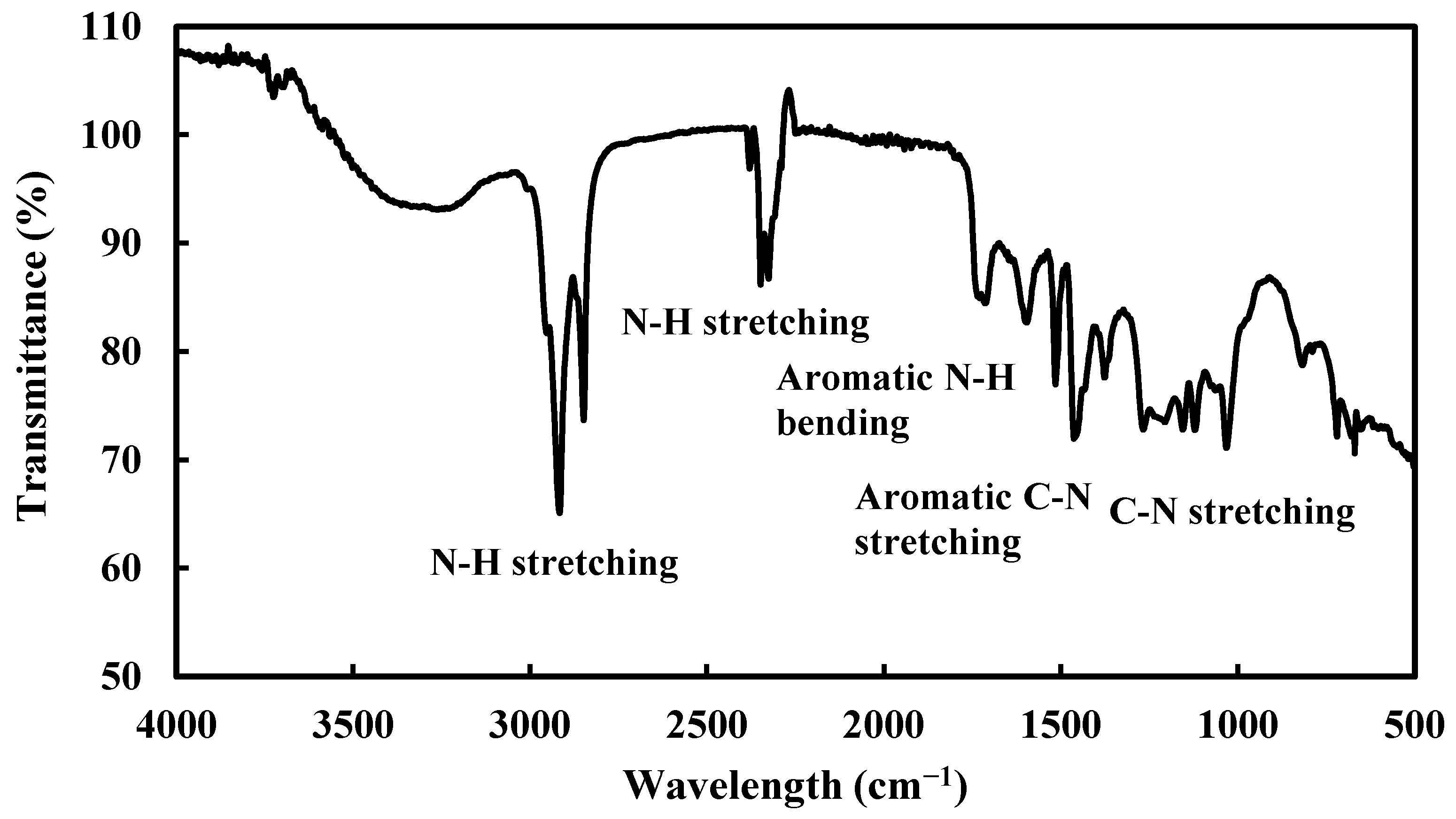
3.1.2. FTIR Spectroscopic Analysis
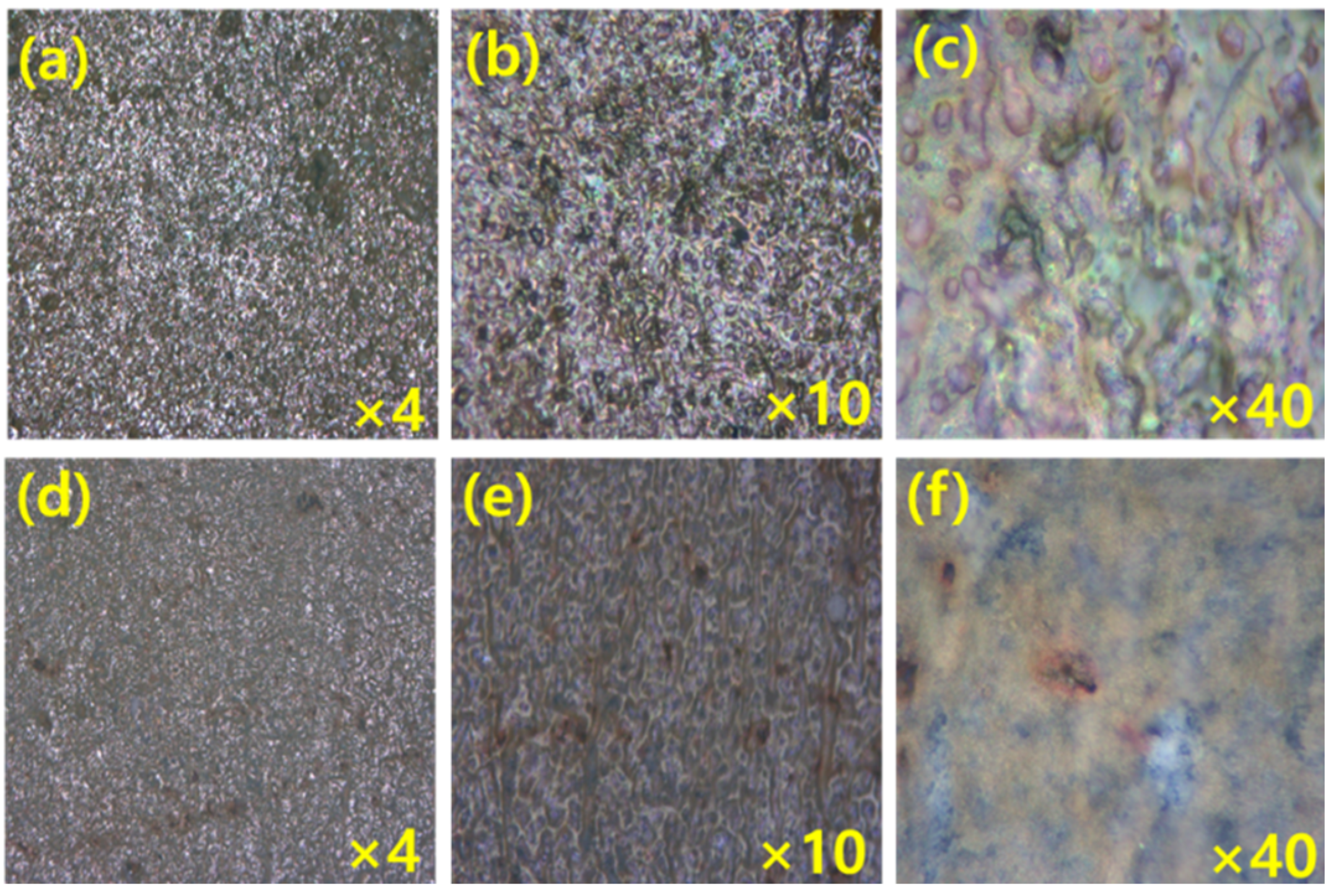
3.2. Effect of Immersion Time
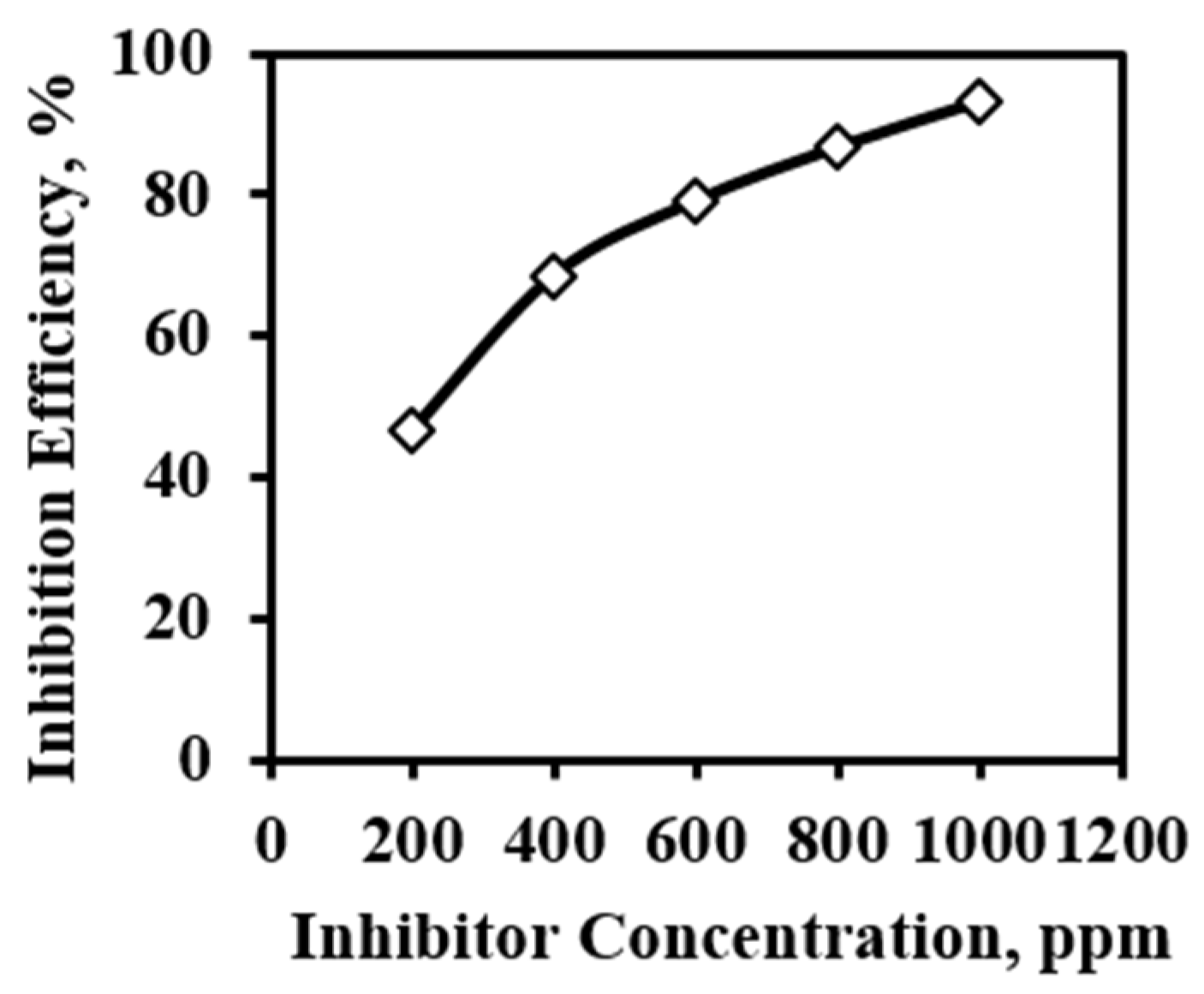
3.3. Effect of Concentration
3.4. Temperature Effect
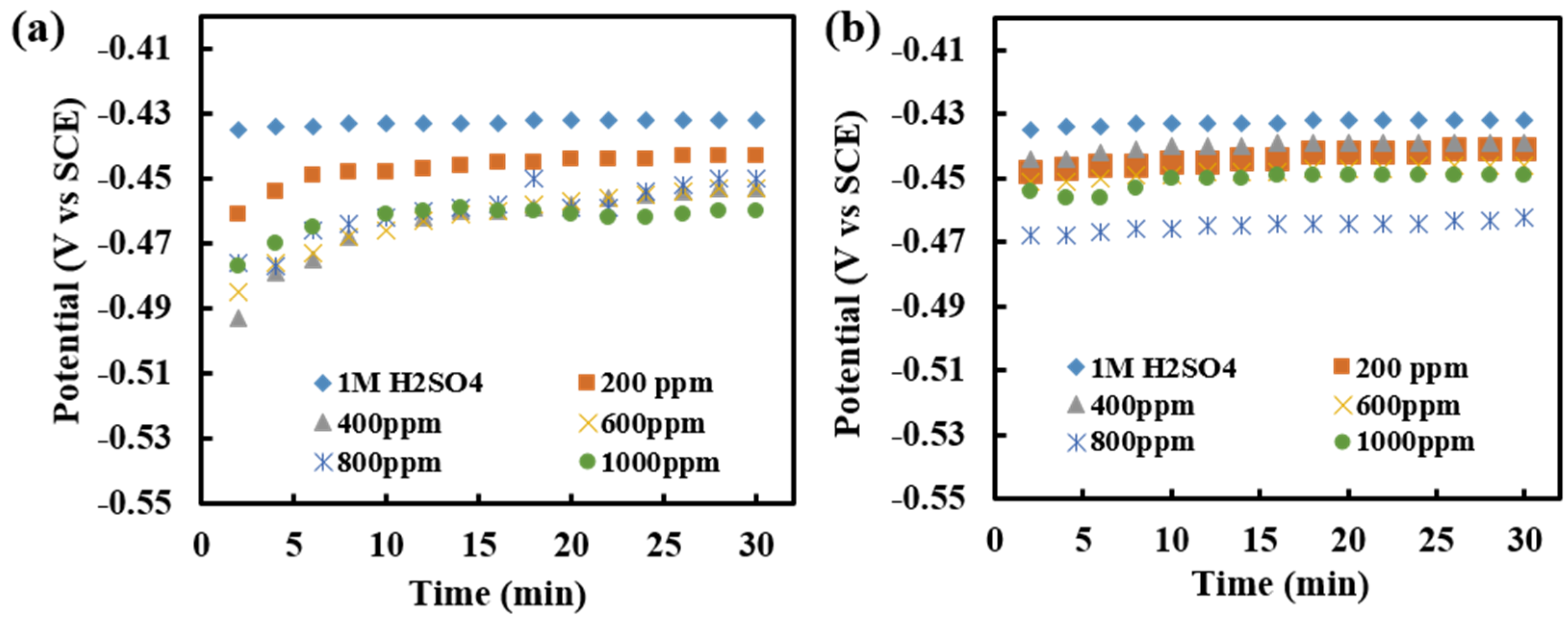
3.5. Open Circuit Potential Measurements
3.6. Polarization Behaviour
3.7. Adsorption Isotherm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shrestha, P.R.; Oli, H.B.; Thapa, B.; Choudhary, Y.; Gupta, D.K.; Das, A.K.; Nakarmi, K.B.; Singh, S.; Karki, N.; Yadav, A.P. Bark Extract of Lantana camara in 1M HCl as Green Corrosion Inhibitor for Mild Steel. Eng. J. 2019, 23, 205–211. [Google Scholar] [CrossRef]
- Karki, N.; Choudhary, Y.; Yadav, A.P. Thermodynamic, Adsorption and Corrosion Inhibition Studies of Mild Steel by Artemisia vulgaris Extract from Methanol as Green Corrosion Inhibitor in Acid Medium. J. Nepal Chem. Soc. 2018, 39, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Oli, H.B.; Parajuli, D.L.; Sharma, S.; Chapagain, A.; Yadav, A.P. Adsorption Isotherm and Activation Energy of Inhibition of Alkaloids on Mild Steel Surface in Acidic Medium. Amrit Res. J. 2021, 2, 59–67. [Google Scholar] [CrossRef]
- Obiukwu, O.O.; Opara, I.O.; Oyinna, B.C. Corrosion Inhibition of Stainless Steel Using Plant Extract Vernoniaamygdalina and Azadirachtaindica. Pac. J. Sci. Technol. 2013, 14, 31–35. [Google Scholar]
- Alaneme, K.K.; Olusegun, S.J.; Adelowo, O.T. Corrosion inhibition and adsorption mechanism studies of Hunteria umbellate seed husk extraction mild steel immersed in acidic solutions. Alex. Eng. J. 2016, 55, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Oguzie, E.E.; Li, Y.; Wang, F.H. Effect of surface nano crystalization on corrosion and corrosion inhibition of low carbon steel: Synergistic effect of methionine and iodide ion. Electrochim. Acta 2007, 52, 6988–6996. [Google Scholar] [CrossRef]
- Al-Otaibi, M.S.; Al-Mayouf, A.M.; Khan, M.; Mousa, A.A. Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media. Arab. J. Chem. 2014, 7, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Uwah, I.E.; Okafar, P.C.; Ebiekpe, V.E. Inhibitive action of ethanol extracts from Nauclealati folia on the corrosion of mild steel in H2SO4 solutions and their adsorption characteristics. Arab. J. Chem. 2013, 6, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Hussin, M.H.; Kassim, M.J.; Razali, N.N.; Dahon, N.H.; Nasshorudin, D. The effect of Tinosporacrispa extract as a natural mild steel corrosion inhibitor in 1 M HCl solution. Arab. J. Chem. 2016, 9, S616–S624. [Google Scholar] [CrossRef] [Green Version]
- Baran, E.; Cakir, A.; Yaziri, B. Inhibitory effect of Gentianaoliviera extracts on the corrosion of mild steel in 0.5 M HCl: Electrochemical and Phytochemical evaluation. Arab. J. Chem. 2016, 1, 17. [Google Scholar]
- Abiola, O.K.; James, A.O. The effects of Aloe vera extract on corrosion and kinetics of corrosion process of zinc in HCl solution. Corros. Sci. 2010, 52, 661–664. [Google Scholar] [CrossRef]
- Deng, S.; Li, X. Inhibition by Jasminum nudiflorum Lindl. leaves extract of the corrosion of aluminum in HCl solution. Corros. Sci. 2012, 64, 253–262. [Google Scholar] [CrossRef]
- Thapa, B.; Gupta, D.K.; Yadav, A.P. Corrosion Inhibition of Bark Extract of Euphorbia royleana on Mild Steel in 1M HCl. J. Nepal Chem. Soc. 2019, 40, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Deng, S.; Fu, H. Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract. Corros. Sci. 2012, 62, 163–175. [Google Scholar] [CrossRef]
- Muthukrishnan, P.; Prakash, P.; Jeyaprabhu, B.; Shankar, K. Stigmasterol extracted from Ficus hispida leaves as a green inhibitor for the mild steel corrosion in 1M HCl solution. Arab. J. Chem. 2015, 12, 3345–3356. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, M.; Robert, F.; Amusant, N.; Traisnel, M.; Roos, C.; Lebrini, M. Enhanced corrosion resistance of mild steel in 1MHCl solution by alkaloids extract from Anibarosaeodora plant: Electrochemical phytochemical and XPS studies. Electrochim. Acta 2014, 131, 96–105. [Google Scholar] [CrossRef]
- Sudile, F.; Robert, F.; Roos, C.; Lebrini, M. Corrosion inhibition of Zinc by Mansoaalliacea plant extract in sodium chloride media: Extraction, characterization and electrochemical studies. Electrochim. Acta 2014, 133, 631–638. [Google Scholar] [CrossRef]
- Deng, S.; Li, X. Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros. Sci. 2012, 55, 407–415. [Google Scholar] [CrossRef]
- Lebrini, M.; Robert, F.; Roos, C. Inhibition Effect of Alkaloids Extract from Annonasquamosa Plant on the Corrosion of C38 Steel in Normal Hydrochloric Acid Medium. Int. J. Electrochem. Sci. 2010, 5, 1696–1712. [Google Scholar]
- Bhattacharjee, B.; Islam, S.S. Assessment of Antibacterial and Antifungal Activities of the extracts of Rhynchostylis retusa Blume-A medicinal orchid. World J. Pharm. Pharm. Sci. 2015, 4, 72–87. [Google Scholar]
- Akter, M.; Huda, M.K.; Hoque, M.M. Investigation of secondary metabolites of nine medicinally important orchids of Bangladesh. J. Pharmacogn. Phytochem. 2018, 7, 602–606. [Google Scholar]
- Adhikari, Y.P.; Fischer, A. Distribution of the epiphytic orchid Rhynchostylis retusa under strong human influence in Kathmandu valley, Nepal. Bot. Orient. J. Plant Sci. 2011, 8, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.M.; Bhattacharjee, B. Plant regeneration through somatic embryogenesis from leaf and root explants of Rhynchostylis retusa (L.) Blume. Appl. Biol. Res. 2017, 17, 158–165. [Google Scholar] [CrossRef]
- Li, D.; Zhang, P.; Guo, X.; Zhao, X.; Xu, Y. The inhibition of mild steel corrosion in 0.5 M H2SO4 solution by radish leaf extract. R. Soc. Chem. 2019, 9, 40997–41009. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, R.M.; Webster, F.X. Spectrometric Identification of Organic Compounds, 6th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2006; pp. 99–106. [Google Scholar]
- Chhetri, K.; Tiwari, A.P.; Dahal, B.; Ojha, G.P.; Mukhiya, T.; Lee, M.; Kim, T.; Chae, S.H.; Muthurasu, A.; Kim, H.Y. A ZIF-8-derived nanoporous carbon nanocomposite wrapped with Co3O4-polyaniline as an efficient electrode material for an asymmetric supercapacitor. J. Electroanal. Chem. 2020, 856, 113670. [Google Scholar] [CrossRef]
- Lai, C.; Xie, B.; Zou, L.; Zheng, X.; Ma, X.; Zhu, S. Adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by S-allyl-O, O′-dialkyldithiophosphates. Results Phys. 2017, 7, 3434–3443. [Google Scholar] [CrossRef]
- Andoor, P.A.; Okeoma, K.B.; Mbamara, U.S. Adsorption and thermodynamic studies of the corrosion inhibition effect of Rosmarinus officinalis L. leaves on aluminum alloy in 0.25 M HCl and effect of an external magnetic field. Int. J. Phys. Sci. 2021, 16, 79–95. [Google Scholar]
- Ituen, E.; Akaranta, O.; James, A. Evaluation of performance of corrosion inhibitors using adsorption isotherm models: An overview. Chem. Sci. Int. J. 2017, 18, 1–34. [Google Scholar] [CrossRef]
- Chhetri, K.; Yadav, A.P. Electrochemical Behavior of Polyaniline (PANI) Doped with Different Nitrate Salts. J. Univ. Grants Comm. 2018, 7, 14–24. [Google Scholar]
- Karki, N.; Neupane, S.; Chaudhary, Y.; Gupta, D.K.; Yadav, A.P. Equisetum hyemale: A new candidate for green corrosion inhibitor family. Int. J. Corros. Scale Inhib. 2021, 10, 206–227. [Google Scholar]






| S.N. | Experiment | Observation | Inference |
|---|---|---|---|
| 1. | Mayer’s Test | The appearance of an orange precipitate. | Alkaloid Presence |
| 2. | Dragendroff’s Test | The appearance of orange-red color. | Alkaloid Presence |
| Medium | Ecorr (V) | Icorr (μA/cm2) | Surface Coverage (θ) | Inhibition Efficiency (%) |
|---|---|---|---|---|
| Acid | 0.429 | 340 | - | - |
| 200 ppm | 0.432 | 182 | 0.4647 | 46.47 |
| 400 ppm | 0.449 | 107 | 0.6853 | 68.53 |
| 600 ppm | 0.452 | 71 | 0.7912 | 79.12 |
| 800 ppm | 0.451 | 45 | 0.8677 | 86.77 |
| 1000 ppm | 0.454 | 23 | 0.9324 | 93.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapagain, A.; Acharya, D.; Das, A.K.; Chhetri, K.; Oli, H.B.; Yadav, A.P. Alkaloid of Rhynchostylis retusa as Green Inhibitor for Mild Steel Corrosion in 1 M H2SO4 Solution. Electrochem 2022, 3, 211-224. https://doi.org/10.3390/electrochem3020013
Chapagain A, Acharya D, Das AK, Chhetri K, Oli HB, Yadav AP. Alkaloid of Rhynchostylis retusa as Green Inhibitor for Mild Steel Corrosion in 1 M H2SO4 Solution. Electrochem. 2022; 3(2):211-224. https://doi.org/10.3390/electrochem3020013
Chicago/Turabian StyleChapagain, Amrita, Debendra Acharya, Anju Kumari Das, Kisan Chhetri, Hari Bhakta Oli, and Amar Prasad Yadav. 2022. "Alkaloid of Rhynchostylis retusa as Green Inhibitor for Mild Steel Corrosion in 1 M H2SO4 Solution" Electrochem 3, no. 2: 211-224. https://doi.org/10.3390/electrochem3020013
APA StyleChapagain, A., Acharya, D., Das, A. K., Chhetri, K., Oli, H. B., & Yadav, A. P. (2022). Alkaloid of Rhynchostylis retusa as Green Inhibitor for Mild Steel Corrosion in 1 M H2SO4 Solution. Electrochem, 3(2), 211-224. https://doi.org/10.3390/electrochem3020013









