Ferrocene-Based Porous Organic Polymer (FPOP): Synthesis, Characterization and an Electrochemical Study
Abstract
1. Introduction
2. Results and Discussion
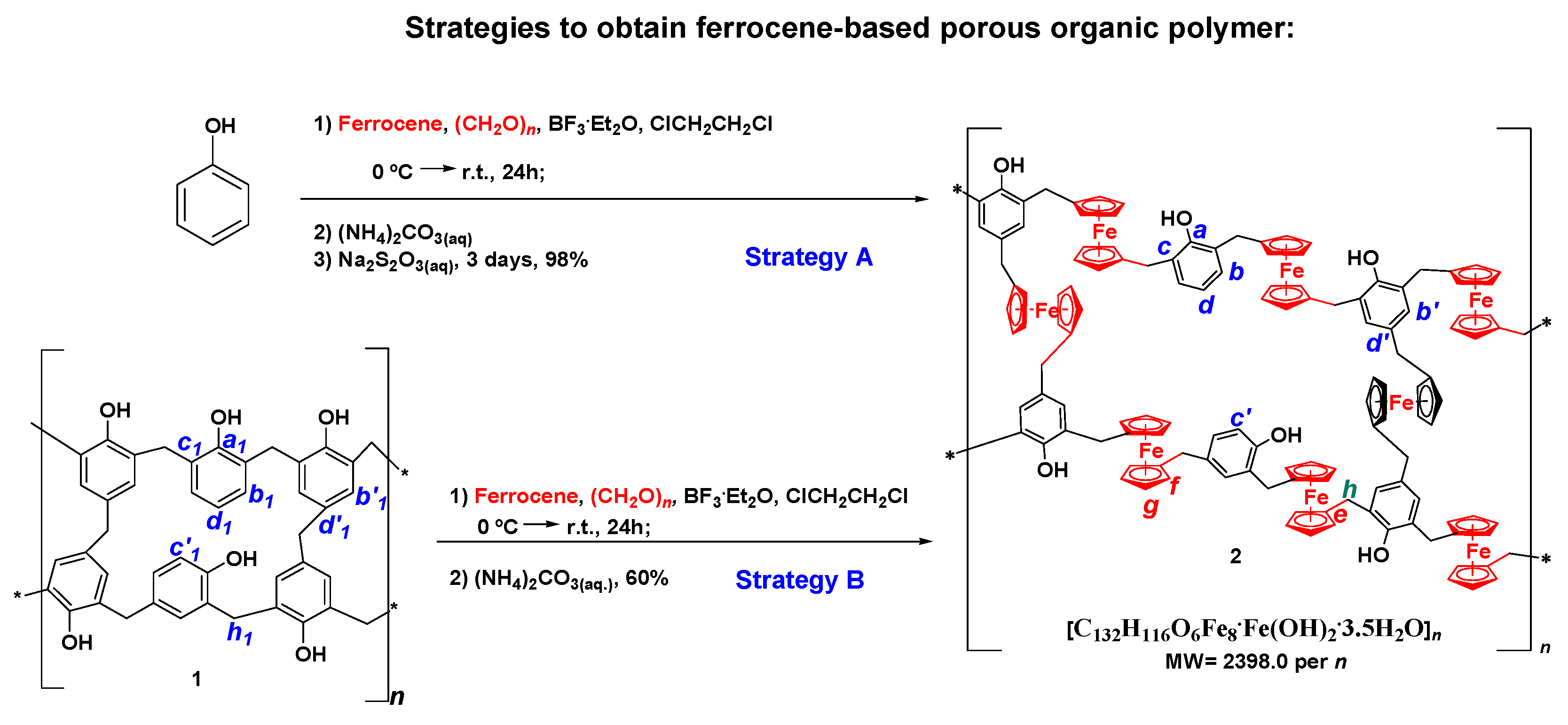
2.1. Synthesis and Characterization of FPOP
- Strategy A consisted of the bottom-up method, using phenol, formaldehyde and ferrocene to obtain the desired FPOP;
- Strategy B was based on a post-synthetic modification approach, where ferrocene was incorporated in the already-formed Bakelite (previously prepared by an acid catalyzed reaction from phenol and formaldehyde).
- (a)
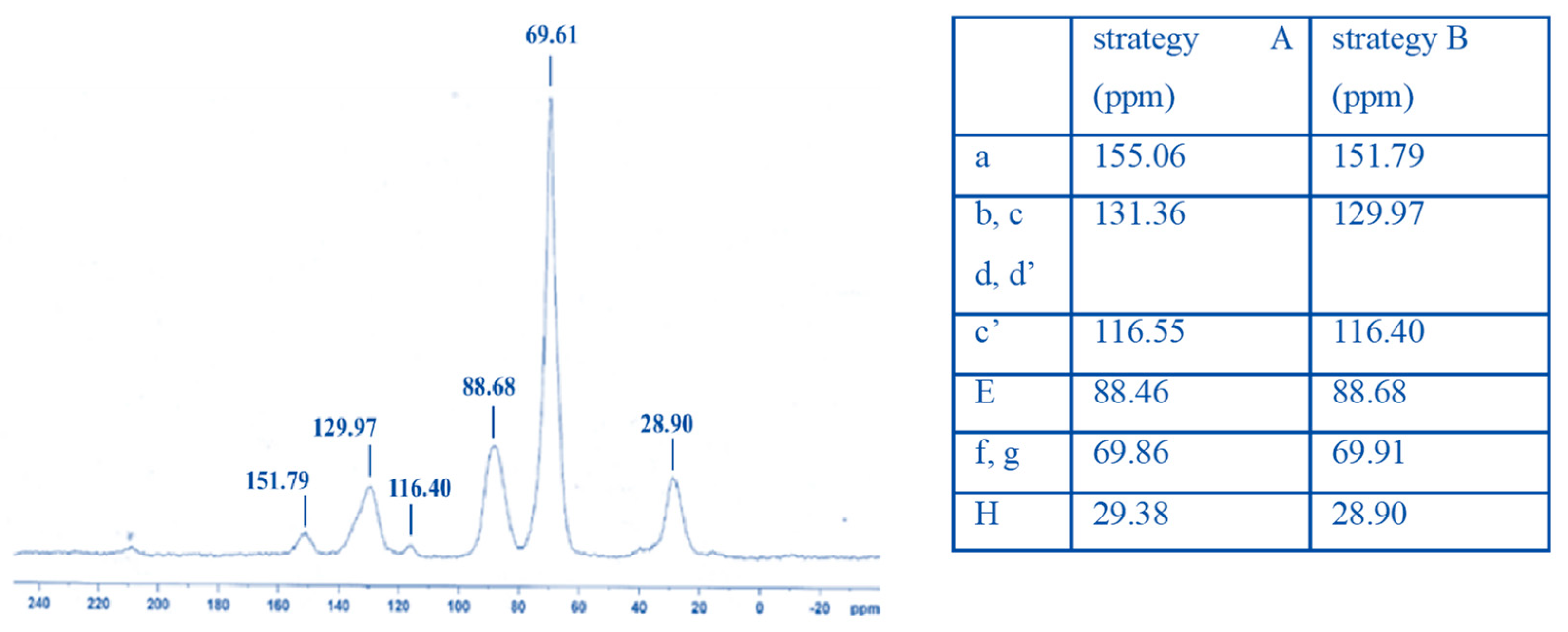
- Characterization by 13C CP-MAS NMR
- (b)
- Characterization by FTIR
- (c)
- Characterization by PXRD
- (d)
- Characterization by BET
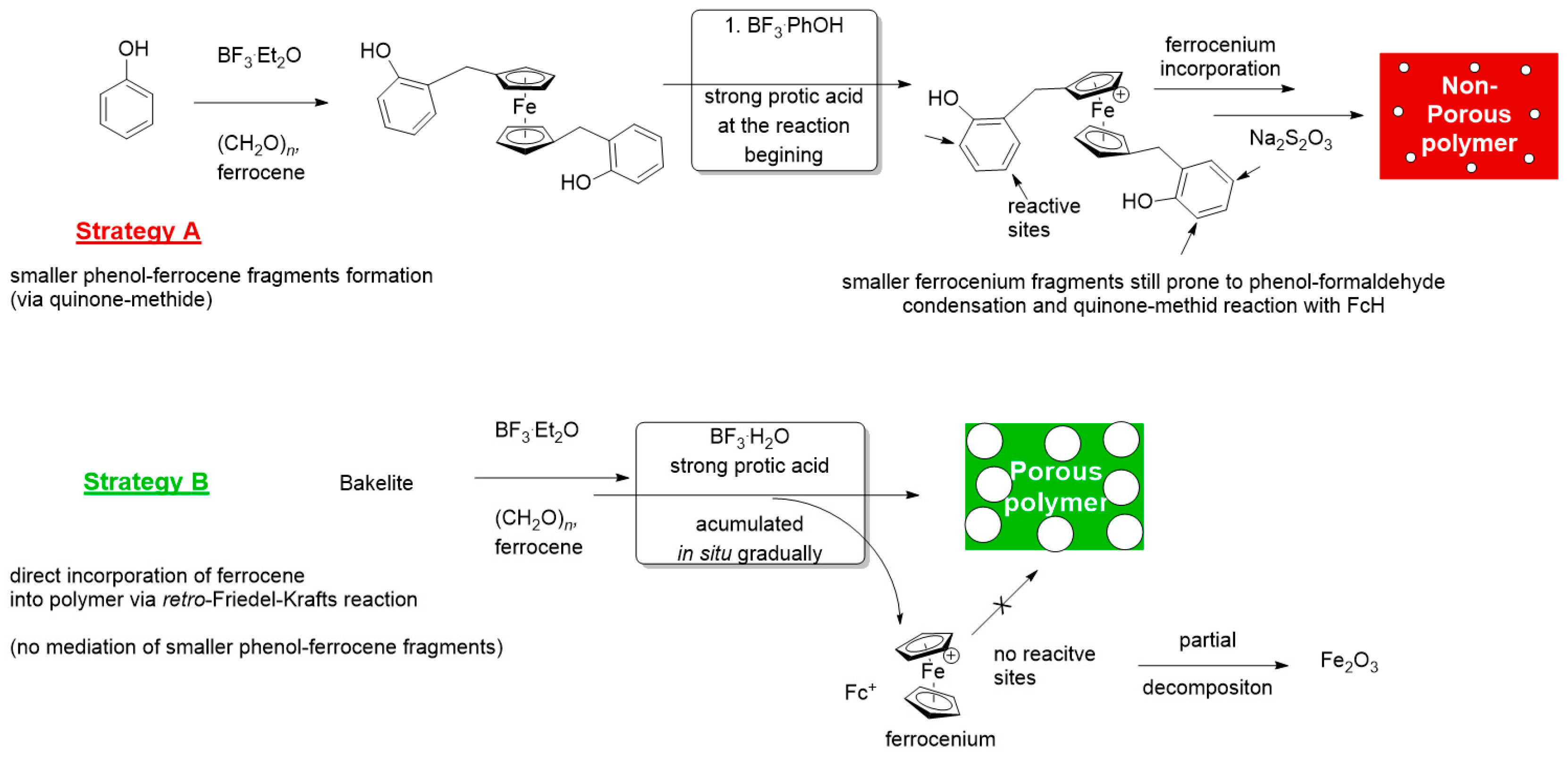
2.2. Differences in Porosity and Proposed Mechanism
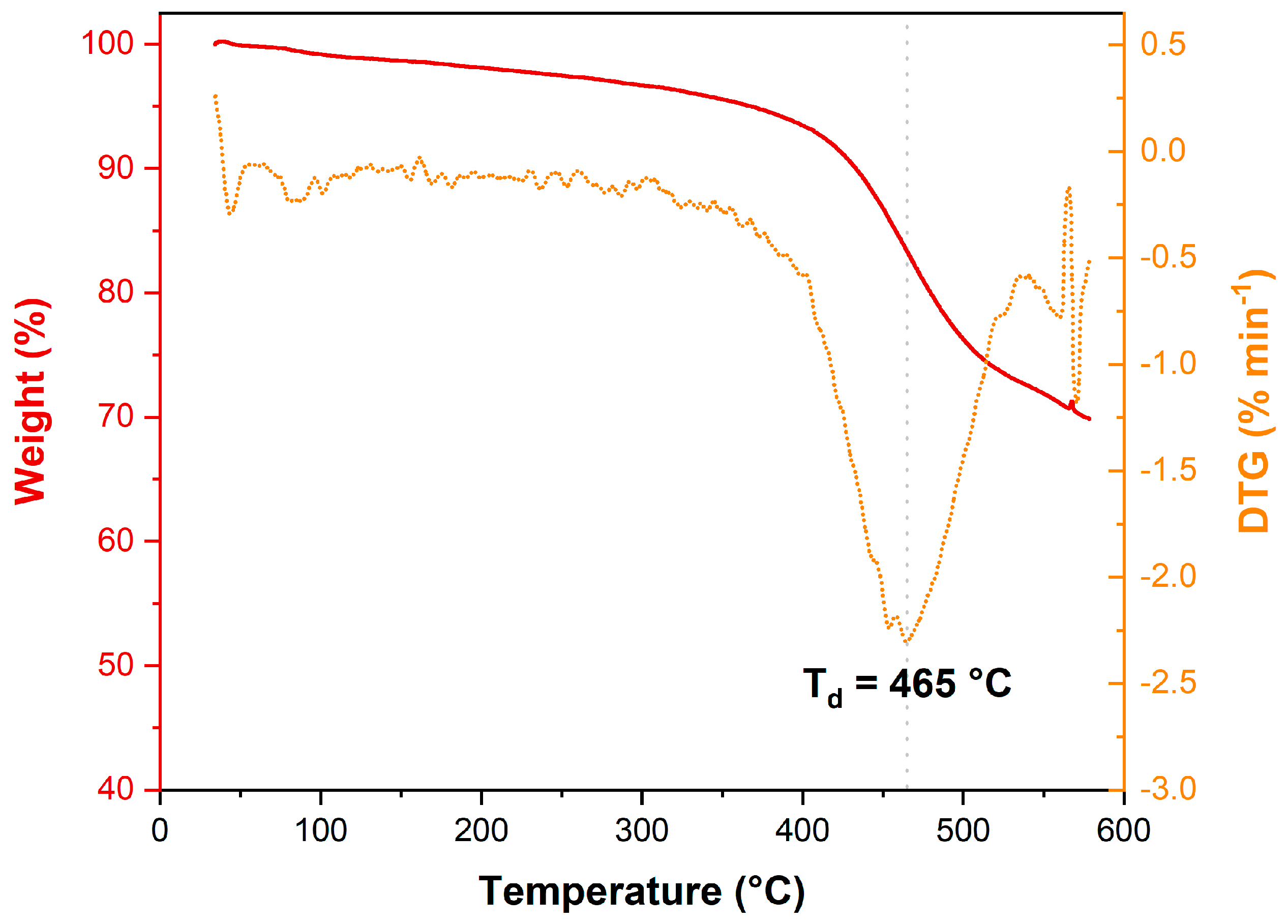
2.3. Calorimetric Analyses
2.4. Electrochemical Studies
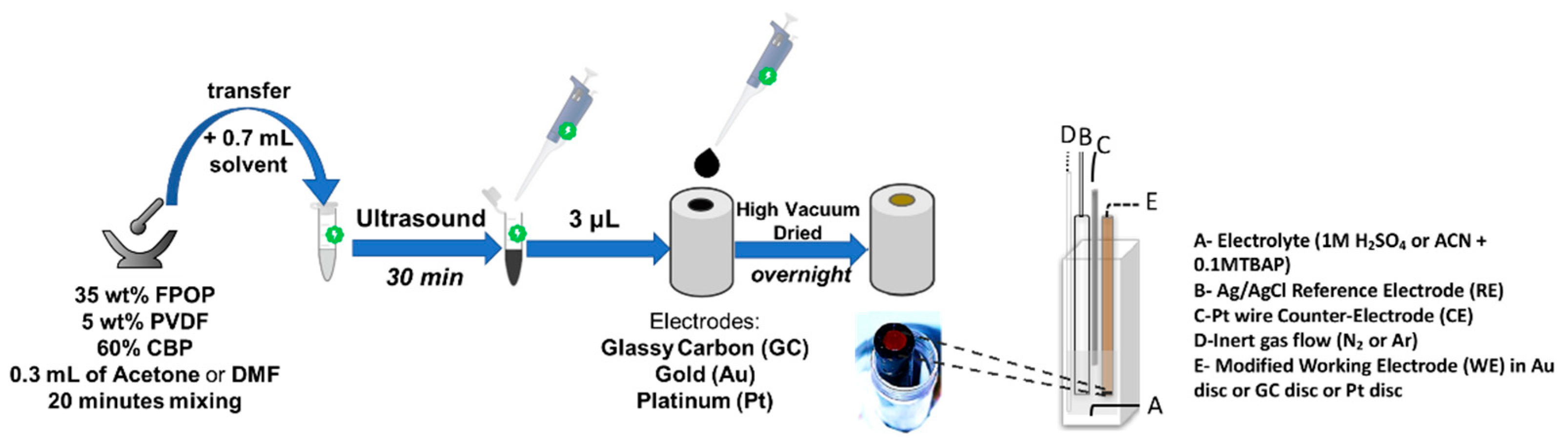
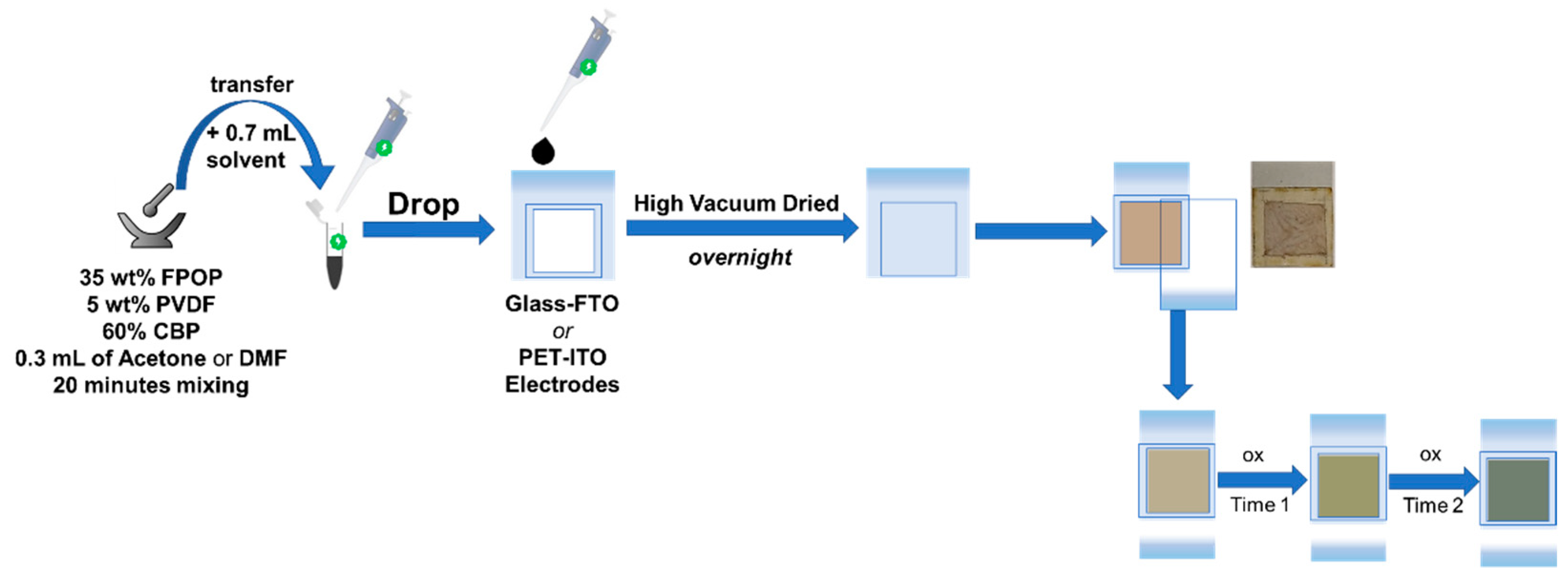
2.4.1. Materials and Methods
- (a)
- Preparation of the electrolyte for ECD (deep eutectic solvent, DES)
- (b)
- Cyclic Voltammetry
- The compound was directly tested in ACN + 0.1 M of TBAP, as it is the conventional electrolyte, allowing a direct comparison with the redox probes which are used for the study of electrochromic organic material.
- The prepared FPOP, deposited on the electrodes, showed the same behavior as ferrocene. Thus, ferrocene was the electrochromic probe model and it was studied under similar conditions.
- Concerning the electrochromic device (ECD), the deep eutectic solvent LiOTf:4EG was selected as the electrolyte for the two-electrode configuration devices.
2.4.2. Electrochemical Studies of Three-Electrode Configuration
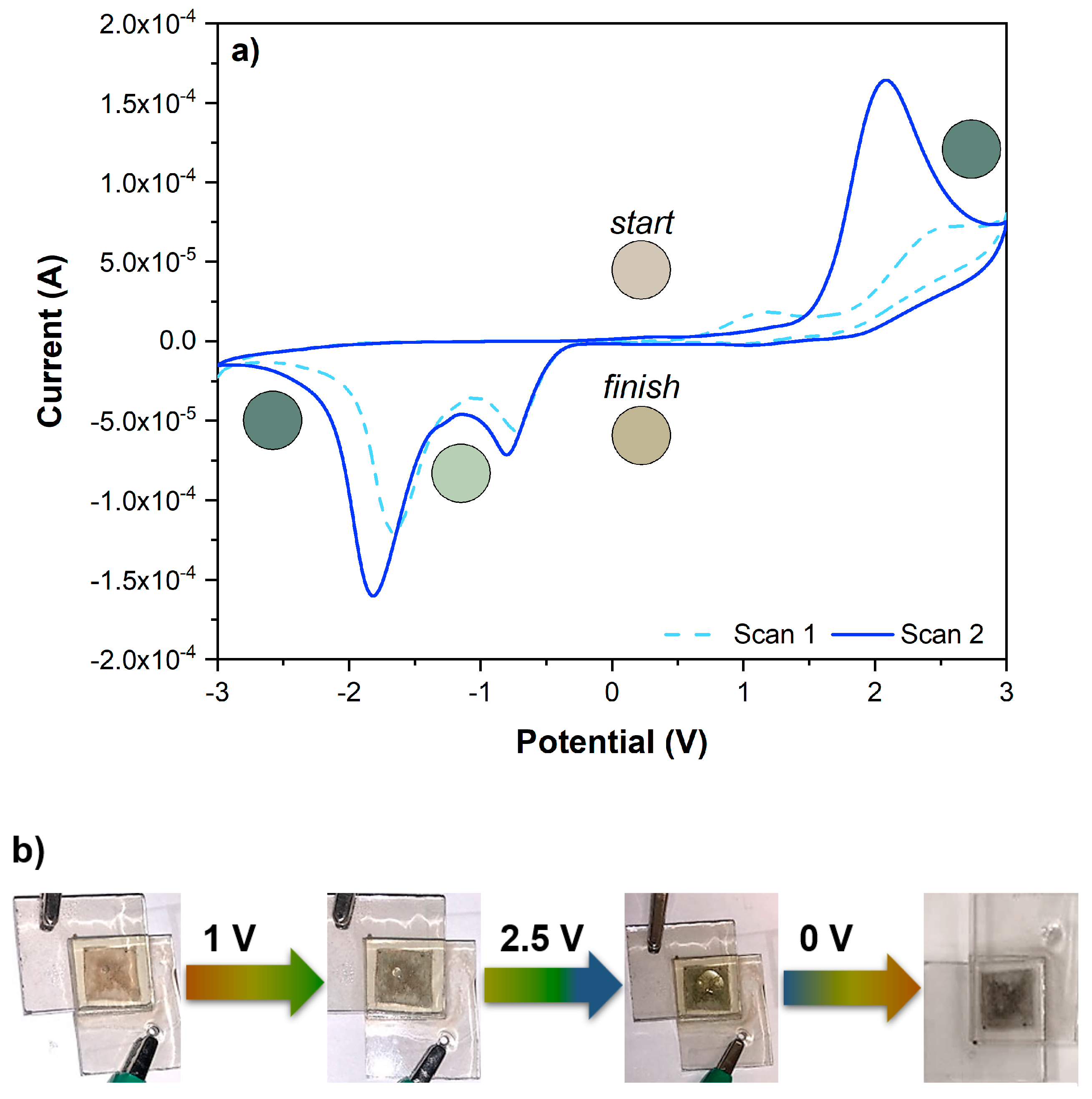
2.4.3. Electrochemical Studies of Two-Electrode Configuration
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hesse, W. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Rhodes, C.J. Plastic Pollution and Potential Solutions. Sci. Prog. 2018, 101, 207–260. [Google Scholar] [CrossRef]
- Farzana, R.; Rajarao, R.; Sahajwalla, V. Reaction Mechanism of Ferrosilicon Synthesis Using Waste Plastic as a Reductant. ISIJ Int. 2017, 57, 1780–1787. [Google Scholar] [CrossRef]
- Farzana, R.; Sahajwalla, V. Novel Recycling to Transform Automotive Waste Glass and Plastics into SiC-Bearing Resource by Silica Reduction. J. Sustain. Metall. 2015, 1, 65–74. [Google Scholar] [CrossRef]
- Farzana, R.; Rajarao, R.; Sahajwalla, V. Transforming Waste Plastic into Reductants for Synthesis of Ferrosilicon Alloy. Ind. Eng. Chem. Res. 2014, 53, 19870–19877. [Google Scholar] [CrossRef]
- Maji, S.; Urakawa, O.; Inoue, T. The Structure and Viscoelasticity of Novolac Resins. Polym. J. 2014, 46, 584–591. [Google Scholar] [CrossRef]
- Pethrick, R.A.; Thomson, B. 13C Nuclear Magnetic Resonance Studies of Phenol-Formaldehyde Resins and Related Model Compounds 2—Analysis of Sequence Structure in Resins. Br. Polym. J. 1986, 18, 380–386. [Google Scholar] [CrossRef]
- Astruc, D. Why Is Ferrocene so Exceptional? Eur. J. Inorg. Chem. 2017, 2017, 6–29. [Google Scholar] [CrossRef]
- Enjamuri, N.; Sarkar, S.; Reddy, B.M.; Mondal, J. Design and Catalytic Application of Functional Porous Organic Polymers: Opportunities and Challenges. Chem. Rec. 2019, 19, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Pietschnig, R. Polymers with Pendant Ferrocenes. Chem. Soc. Rev. 2016, 45, 5216–5231. [Google Scholar] [CrossRef]
- Mattoussi, M.; Matoussi, F.; Raouafi, N. Non-Enzymatic Amperometric Sensor for Hydrogen Peroxide Detection Based on a Ferrocene-Containing Cross-Linked Redox-Active Polymer. Sens. Actuators B Chem. 2018, 274, 412–418. [Google Scholar] [CrossRef]
- Ghimire, G.; Coceancigh, H.; Yi, Y.; Ito, T. Electrochemical Characterization and Catalytic Application of Gold-Supported Ferrocene-Containing Diblock Copolymer Thin Films in Ethanol Solution. ACS Appl. Mater. Interfaces 2017, 9, 2906–2913. [Google Scholar] [CrossRef]
- Gu, H.; Mu, S.; Qiu, G.; Liu, X.; Zhang, L.; Yuan, Y.; Astruc, D. Redox-Stimuli-Responsive Drug Delivery Systems with Supramolecular Ferrocenyl-Containing Polymers for Controlled Release. Coord. Chem. Rev. 2018, 364, 51–85. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Yun, J.; Kong, J. Novel Ferrocene-Containing Organosilicon Polymers and Uniform Microspheres Prepared by Free Radical Copolymerization: Precursors for Magnetic Si-C-Fe-(O) Nanomaterials. Mater. Des. 2018, 144, 86–97. [Google Scholar] [CrossRef]
- Rüttiger, C.; Hübner, H.; Schöttner, S.; Winter, T.; Cherkashinin, G.; Kuttich, B.; Stühn, B.; Gallei, M. Metallopolymer-Based Block Copolymers for the Preparation of Porous and Redox-Responsive Materials. ACS Appl. Mater. Interfaces 2018, 10, 4018–4030. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Hupp, J.T.; Nguyen, S.T. Porous Organic Polymers in Catalysis: Opportunities and Challenges. ACS Catalysis 2011, 7, 819–835. [Google Scholar] [CrossRef]
- Zhang, T.; Xing, G.; Chen, W.; Chen, L. Porous organic polymers: A promising platform for efficient photocatalysis. Mater. Chem. Front. 2019, 4, 332–353. [Google Scholar] [CrossRef]
- Zhang, Y.; Riduan, S.N. Functional Porous Organic Polymers for Heterogeneous Catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Kundu, S.K.; Bhaumik, A. A Facile Approach for the Synthesis of Hydroxyl-Rich Microporous Organic Networks for Efficient CO2 Capture and H2 Storage. Chem. Commun. 2017, 53, 2752–2755. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.; Bhanja, P.; Bhaumik, A. A Triazine-Based Covalent Organic Polymer for Efficient CO2 Adsorption. Chem. Commun. 2015, 51, 10050–10053. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Y.-W. Creation and Bioapplications of Porous Organic Polymer Materials. J. Mater. Chem. B 2017, 5, 9278–9290. [Google Scholar] [CrossRef]
- Rai, V.; Singh, R.S.; Blackwood, D.J.; Zhili, D. A Review on Recent Advances in Electrochromic Devices: A Material Approach. Adv. Eng. Mater. 2020, 22, 2000082. [Google Scholar] [CrossRef]
- Jarosz, T.; Gebka, K.; Stolarczyk, A.; Domagala, W. Transparent to Black Electrochromism—The “Holy Grail” of Organic Optoelectronics. Polymers 2019, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Shchegolkov, A.V.; Jang, S.-H.; Shchegolkov, A.V.; Rodionov, Y.V.; Sukhova, A.O.; Lipkin, M.S. A Brief Overview of Electrochromic Materials and Related Devices: A Nanostructured Materials Perspective. Nanomaterials 2021, 11, 2376. [Google Scholar] [CrossRef]
- Yu, F.; Liu, W.; Ke, S.-W.; Kurmoo, M.; Zuo, J.-L.; Zhang, Q. Electrochromic two-dimensional covalent organic framework with a reversible dark-to-transparent switch. Nat. Commun. 2020, 11, 5534. [Google Scholar] [CrossRef] [PubMed]
- Cruz, H.; Jordão, N.; Branco, L.C. Deep Eutectic Solvents (DES) as low-cost and electrolytes for electrochromic devices. Green Chem. 2017, 19, 1653–1658. [Google Scholar] [CrossRef]
- Cruz, H.; Jordão, N.; Amorim, P.; Dionísio, M.; Branco, L.C. Deep Eutectic Solvents as suitable electrolytes for Electrochromic Devices. ACS Sustain. Chem. Eng. 2018, 6, 2240–2249. [Google Scholar] [CrossRef]
- Ahn, S.; Song, Y.-S.; Yoo, B.R.; Jung, I.N. Lewis Acid-Catalyzed Friedel−Crafts Alkylation of Ferrocene with Allylchlorosilanes. Organometallics 2000, 19, 2777–2780. [Google Scholar] [CrossRef]
- Suzaki, Y.; Yoshigoe, Y.; Osakada, K. Cyclic and linear poly(ferrocenylene alkylene)s synthesized from addition-condensation polymerization of ferrocene with aldehydes. J. Polym. Sci. Part A: Polym. Chem. 2013, 51, 3627–3635. [Google Scholar] [CrossRef]
- Yoneda, N.; Aomura, K.; Ohtsuka, H. Studies on BF3-Complex Catalysts in the Alkylation Reaction of Benzene Homologues. Bull. Japan Pet. Inst. 1967, 9, 26–31. [Google Scholar] [CrossRef][Green Version]
- Thakur, R.; Barman, S.; Gupta, R.K. Synthesis of cumene by transalkylation over modified beta zeolite: A kinetic study. Braz. J. Chem. Eng. 2016, 33, 957–967. [Google Scholar] [CrossRef]
- Poljanšek, I.; Krajnc, M. Characterization of Phenol-Formaldehyde Prepolymer Resins by In Line FT-IR Spectroscopy. Acta Chim. Slov. 2005, 52, 238–244. [Google Scholar]
- Ma, C.-C.M.; Wu, H.-D.; Su, Y.-F.; Lee, M.-S.; Wu, Y.-D. Pultruded Fiber Reinforced Novolac Type Phenolic Composite—Processability, Mechanical Properties and Flame Resistance. Compos. Part A Appl. Sci. Manuf. 1997, 28, 895–900. [Google Scholar] [CrossRef]
- Huang, J.; Xu, M.; Ge, Q.; Lin, M.; Lin, Q.; Chen, Y.; Chu, J.; Dai, L.; Zou, Y. Controlled Synthesis of High-Ortho-Substitution Phenol-Formaldehyde Resins. J. Appl. Polym. Sci. 2005, 97, 652–658. [Google Scholar] [CrossRef]
- Křístková, M.; Filip, P.; Weiss, Z.; Peter, R. Influence of Metals on the Phenol–Formaldehyde Resin Degradation in Friction Composites. Polym. Degrad. Stab. 2004, 84, 49–60. [Google Scholar] [CrossRef]
- Vahur, S.; Teearu, A.; Peets, P.; Joosu, L.; Leito, I. ATR-FT-IR Spectral Collection of Conservation Materials in the Extended Region of 4000-80 Cm−1. Anal. Bioanal. Chem. 2016, 408, 3373–3379. [Google Scholar] [CrossRef]
- bt Ruslin, F.; Yamin, B.M. Oxidation of Ferrocene by Thiocyanic Acid in the Presence of Ammonium Oxalate; AIP Publishing LLC: Melville, NY, USA, 2014. [Google Scholar] [CrossRef]
- Sengupta, A.K.; Nandi, A.K. Complex Carbonates of Iron(III). Z. Anorg. Allg. Chem. 1974, 403, 327–336. [Google Scholar] [CrossRef]
- Barreto, W.J.; Barreto, S.R.G.; Moreira, I.; Kawano, Y. Iron Oxide and Pyrocatechol: A Spectroscopy Study of the Reaction Products. Quim. Nova 2006, 29, 1255–1258. [Google Scholar] [CrossRef][Green Version]
- Devaman, R.H.P.; Alagar, M. Synthesis and Characterisation of Iron II Hydroxide Nano Particles. Elixir Nanotechnol. 2013, 61, 16845–16848. [Google Scholar]
- Olvera Venegas, P.N.; Hernández Cruz, L.E.; Lapidus, G.T. Dissolution of Iron from Oxides in a Solution of Citric Acid and Sodium Thiosulfate. Adv. Mater. Res. 2014, 976, 114–118. [Google Scholar] [CrossRef]
- Freitas, S.K.S.; Oliveira, F.L.; Dos Santos, T.C.; Hisse, D.; Merlini, C.; Machado, C.; Esteves, P.M. A Carbocationic Triarylmethane-based Porous Covalent Organic Network. Chem. Eur. J. 2020, 27, 2342–2347. [Google Scholar] [CrossRef]
- Freitas, S.K.S.; Oliveira, F.L.; Merlini, C.; Justo, E.P.; Gioda, A.; Esteves, P.M. Dye-based Covalent Organic Networks (CONs). J. Phys. Mater. 2020, 3, 025011. [Google Scholar]
- Amamoto, Y.; Kikuchi, M.; Masunaga, H.; Sasaki, S.; Otsuka, H.; Takahara, A. Reorganizable Chemical Polymer Gels Based on Dynamic Covalent Exchange and Controlled Monomer Insertion. Macromolecules 2009, 42, 8733–8738. [Google Scholar] [CrossRef]
- González-Pelayo, S.; López, E.; Borge, J.; de-los-Santos-Álvarez, N.; López, L.A. Ferrocene-Decorated Phenol Derivatives by Trapping ortho-Quinone Methide Intermediates with Ferrocene. Eur. J. Org. Chem. 2018, 2018, 2858–2862. [Google Scholar] [CrossRef]
- Cruz, H.; Gallardo, I.; Guirado, G. Understanding specific effects on the standard potential shifts of electrogenerated species in 1-butyl-3-methylimidazolium ionic liquids. Electrochim. Acta 2008, 53, 5968–5976. [Google Scholar] [CrossRef]
- Deblase, C.R.; Silberstein, K.E.; Truong, T.T.; Abruña, H.D.; Dichtel, W.R. Β-Ketoenamine-Linked Covalent Organic Frameworks Capable of Pseudocapacitive Energy Storage. J. Am. Chem. Soc. 2013, 135, 16821–16824. [Google Scholar] [CrossRef] [PubMed]
- Scholz, F. Electroanalytical Methods: Guide to Experiments and Applications; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Khan, F.S.T.; Waldbusser, A.L.; Carrasco, M.C.; Pourhadi, H.; Hematian, S. Synthetic, spectroscopic, structural, and electrochemical investigations of ferricenium derivatives with weakly coordinating anions: Ion pairing, substituent, and solvent effects. Dalton Trans. 2021, 50, 7433–7455. [Google Scholar] [CrossRef]
- Wu, M.-S.; Tzu-Ling, L.; Wang, Y.-Y.; Wan, C.-C. Assessment of the wettability of porous electrodes for lithium-ion batteries. J. Appl. Electrochem. 2004, 34, 797–805. [Google Scholar] [CrossRef]


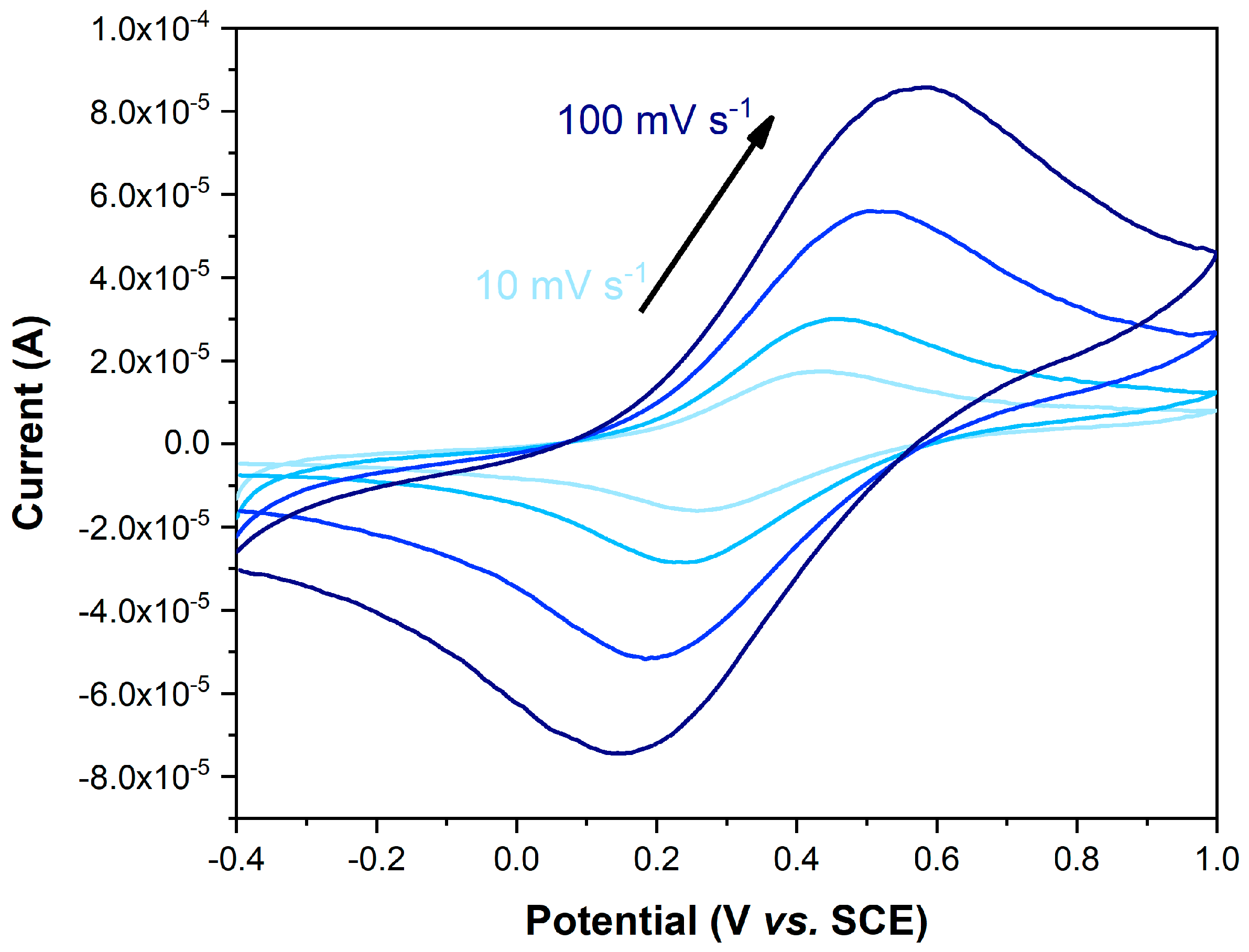
| Entry | Electrolyte | Material | E0 (mV) (a) | Epa (mV) (b) | ΔE (mV) (c) | WE |
|---|---|---|---|---|---|---|
| 1 | ACN + TBAP | Fc | 390 | 430 | 80 | GC |
| 2 | ACN + TBAP | FPOP | 353 | 395 | 85 | GCE/CBP/FPOP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovski, Ž.; Moreira, M.P.; Santos, A.F.M.; Freitas, S.K.S.; Jordão, N.; Maia, R.A.; Nunes, A.V.M.; Branco, L.C.; Cruz, H.; Esteves, P.M. Ferrocene-Based Porous Organic Polymer (FPOP): Synthesis, Characterization and an Electrochemical Study. Electrochem 2022, 3, 184-197. https://doi.org/10.3390/electrochem3010011
Petrovski Ž, Moreira MP, Santos AFM, Freitas SKS, Jordão N, Maia RA, Nunes AVM, Branco LC, Cruz H, Esteves PM. Ferrocene-Based Porous Organic Polymer (FPOP): Synthesis, Characterization and an Electrochemical Study. Electrochem. 2022; 3(1):184-197. https://doi.org/10.3390/electrochem3010011
Chicago/Turabian StylePetrovski, Željko, Mateus P. Moreira, Andreia F. M. Santos, Sunny K. S. Freitas, Noémi Jordão, Renata A. Maia, Ana V. M. Nunes, Luis C. Branco, Hugo Cruz, and Pierre M. Esteves. 2022. "Ferrocene-Based Porous Organic Polymer (FPOP): Synthesis, Characterization and an Electrochemical Study" Electrochem 3, no. 1: 184-197. https://doi.org/10.3390/electrochem3010011
APA StylePetrovski, Ž., Moreira, M. P., Santos, A. F. M., Freitas, S. K. S., Jordão, N., Maia, R. A., Nunes, A. V. M., Branco, L. C., Cruz, H., & Esteves, P. M. (2022). Ferrocene-Based Porous Organic Polymer (FPOP): Synthesis, Characterization and an Electrochemical Study. Electrochem, 3(1), 184-197. https://doi.org/10.3390/electrochem3010011












