Doped Polythiophene Chiral Electrodes as Electrochemical Biosensors
Abstract
1. Introduction
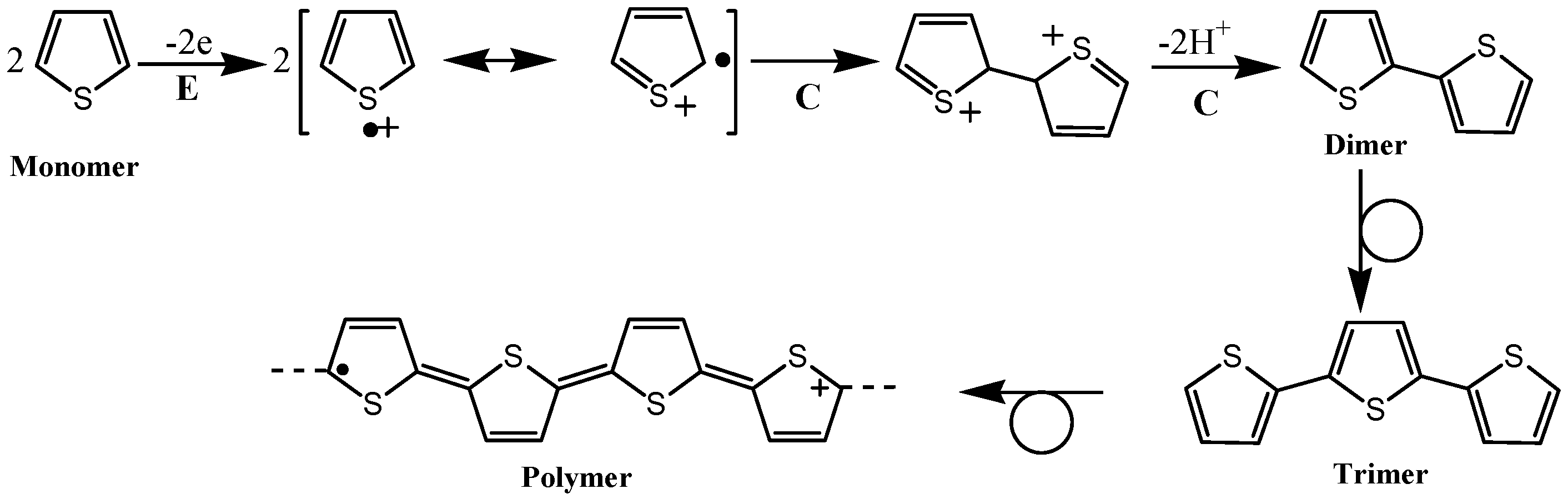
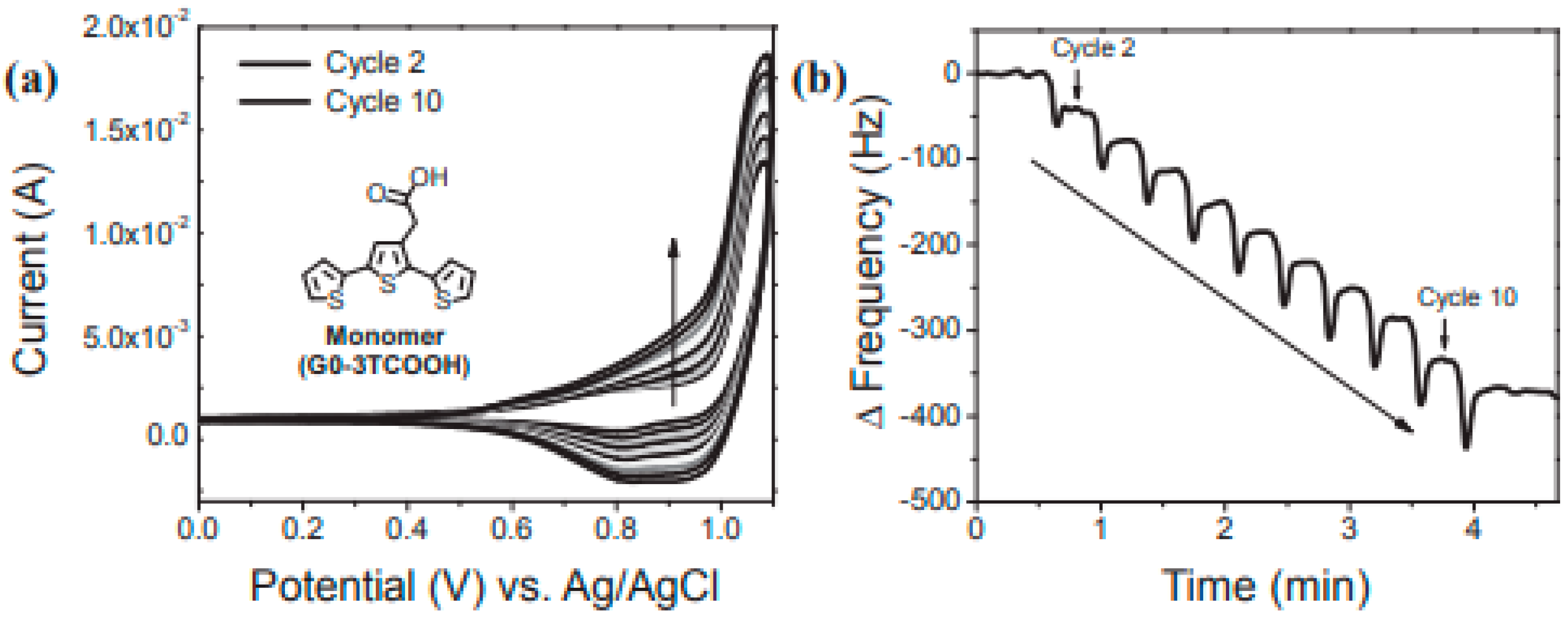
2. Electropolymerization
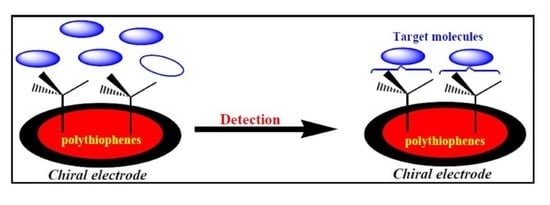
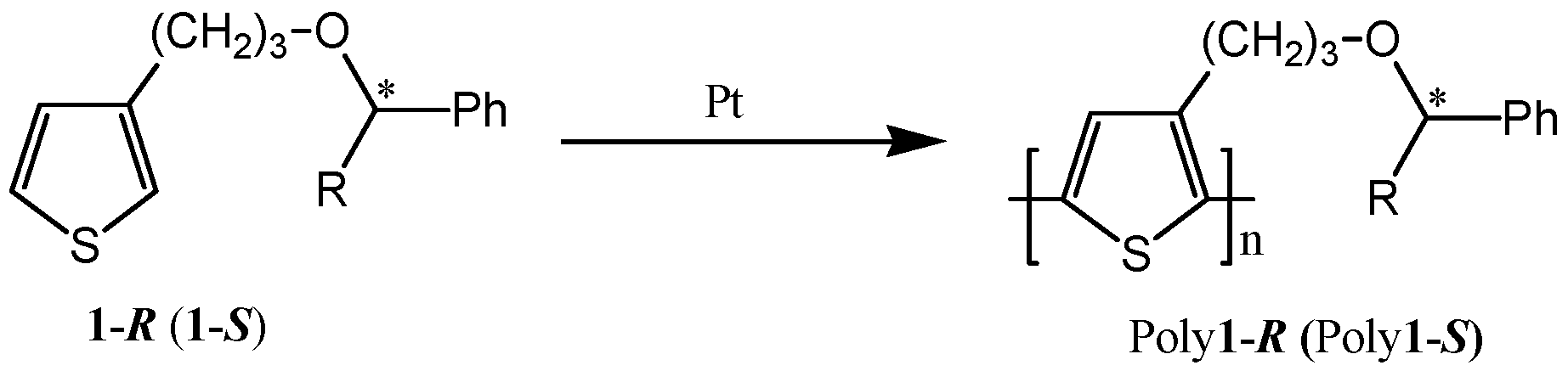
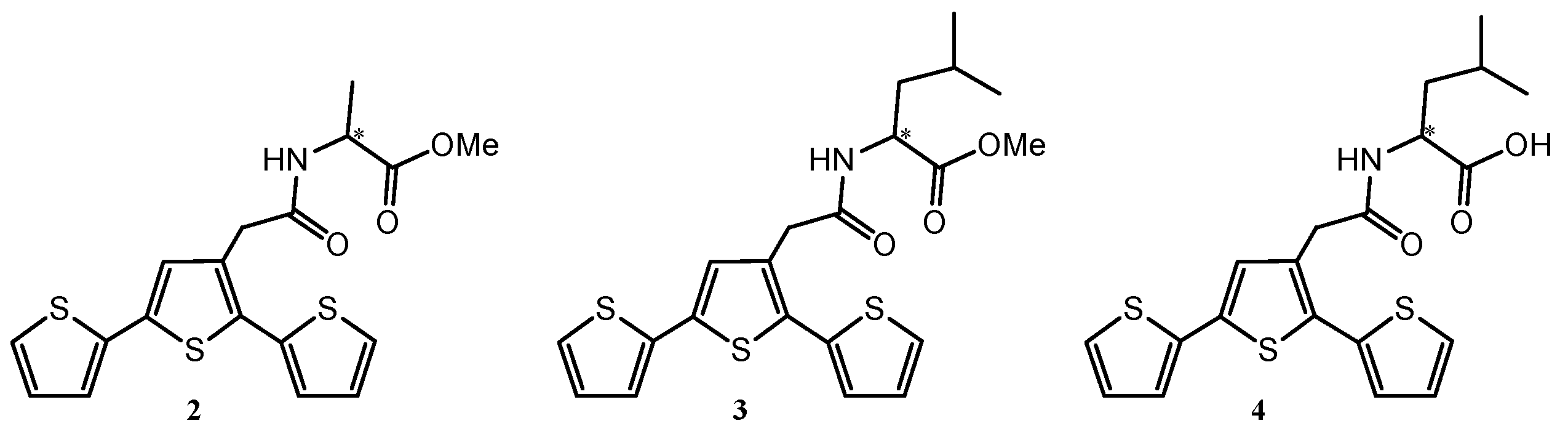
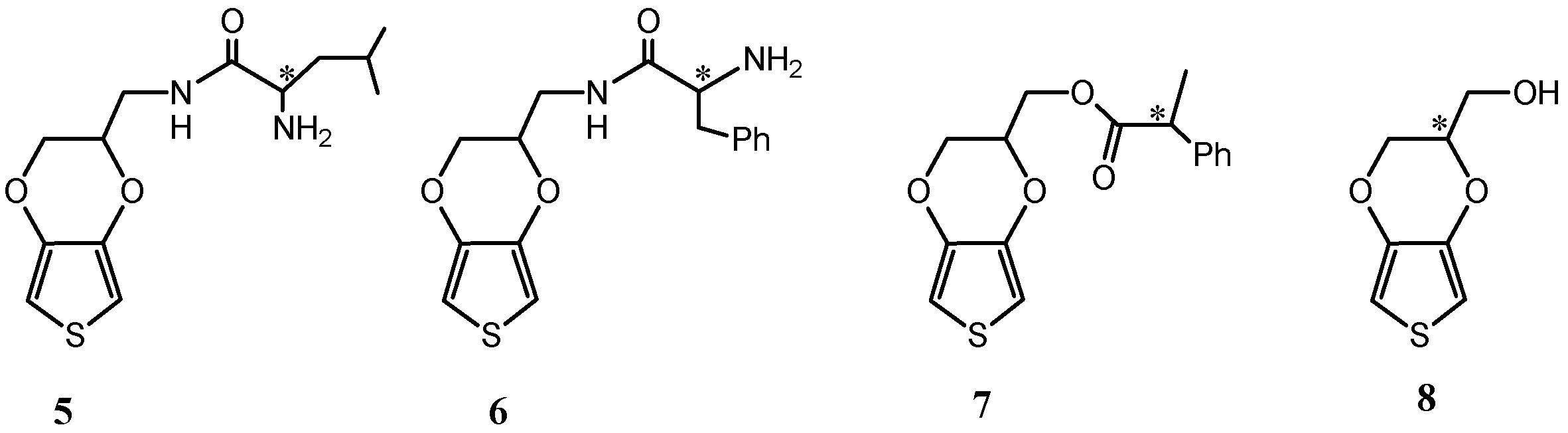
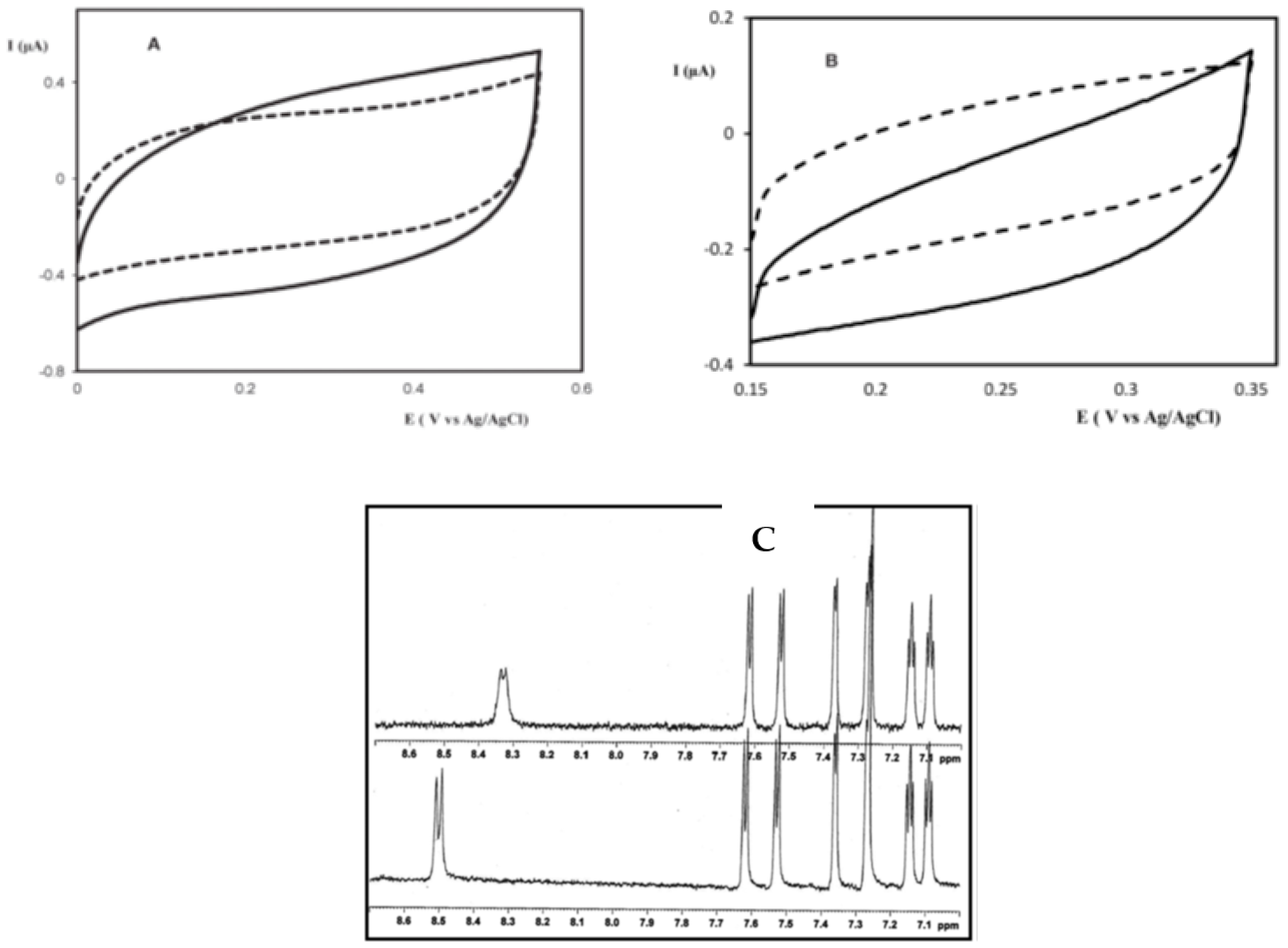
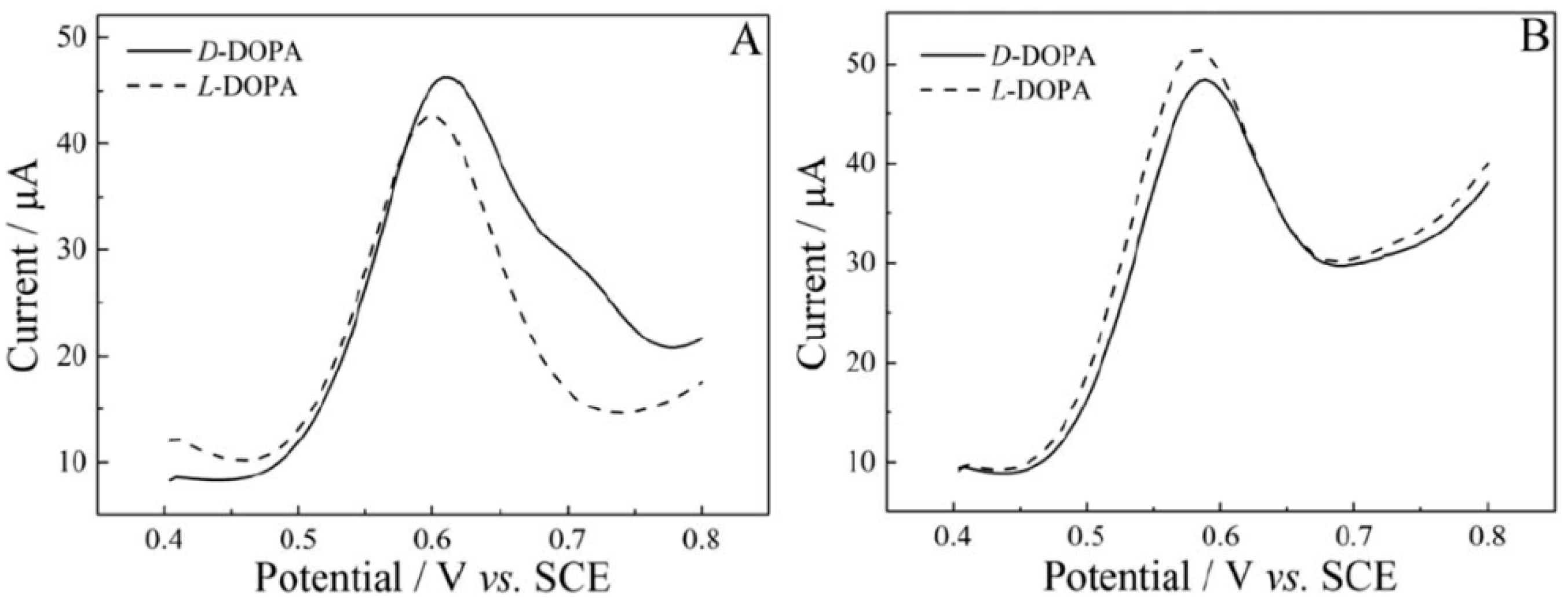
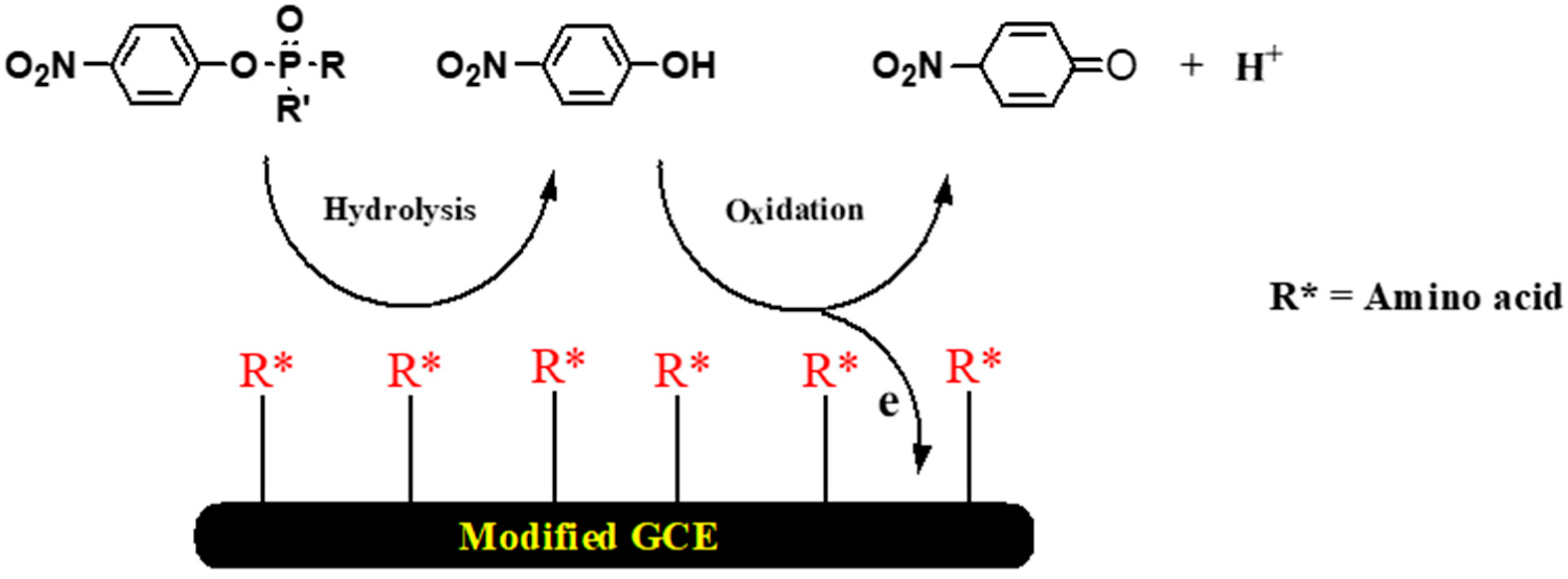
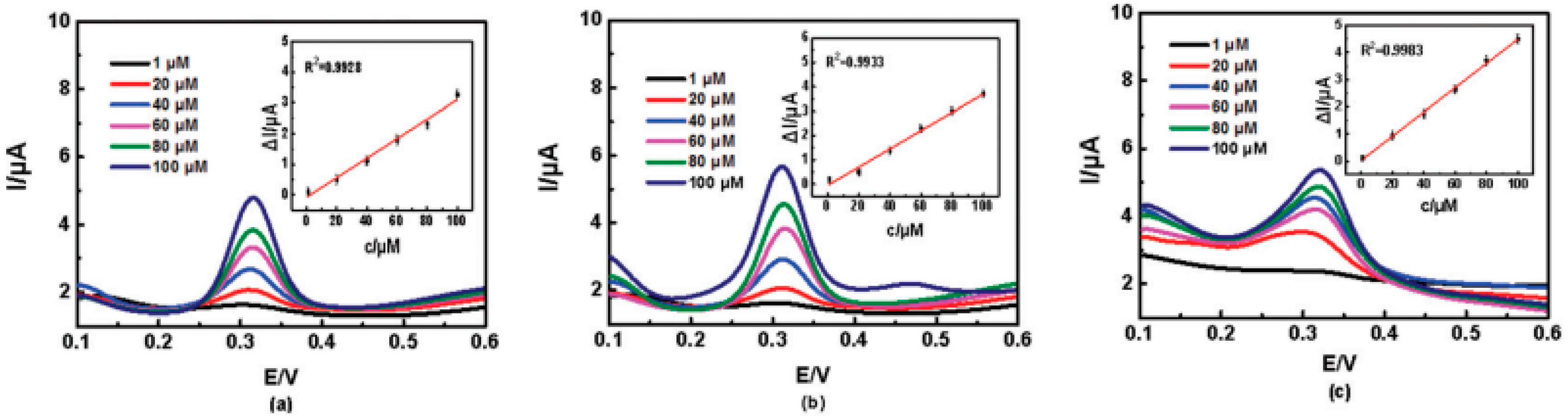
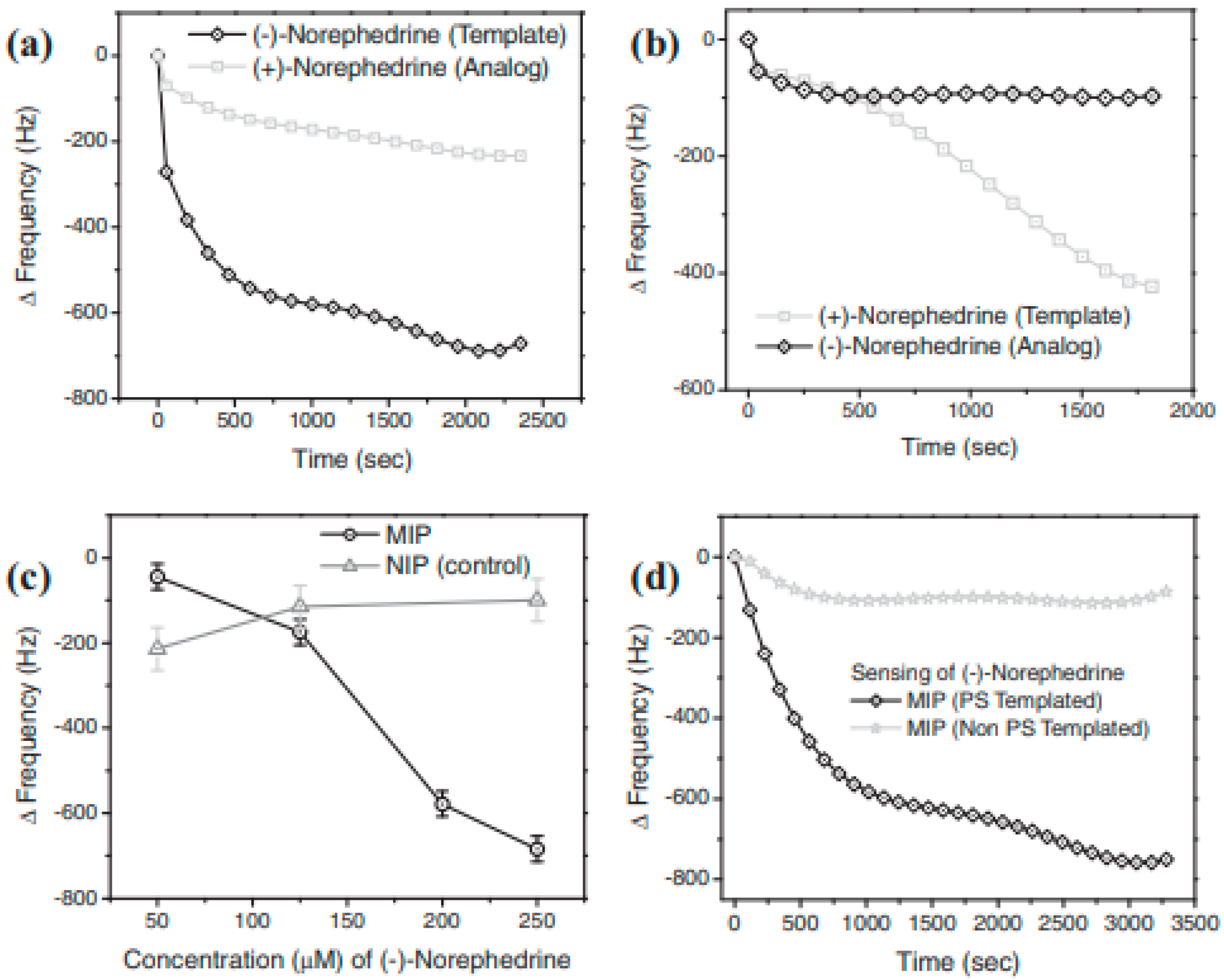
3. Chiral Electrodes Based on Polythiophenes
4. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terán-Alcocer, A.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical sensors based on conducting polymers for the aqueous detection of biologically relevant molecules. Nanomaterials 2021, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Arnaboldi, S.; Grecchi, S.; Magni, M.; Mussini, P. Electroactive chiral oligo- and polymer layers for electrochemical enantiorecognition. Curr. Opin. Electrochem. 2018, 7, 188–199. [Google Scholar] [CrossRef]
- Chahma, M.; Gilroy, J.B.; Hicks, R.G. Linear and branched electroactive polymers based on ethylenedioxythiophene–triarylamine conjugates. J. Mater. Chem. 2007, 17, 4768–4771. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Rincon, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting polymers in the fields of energy, environmental remediation, and chemical–chiral sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar]
- Mandler, D. Chiral self-assembled monolayers in electrochemistry. Curr. Opin. Electrochem. 2018, 7, 42–47. [Google Scholar] [CrossRef]
- Chahma, M.; Lee, J.S.; Kraatz, H.-B. Fc-ssDNA conjugate: Electrochemical properties in a borate buffer and adsorption on gold electrode surfaces. J. Electroanal. Chem. 2004, 567, 283–287. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-assembled monolayers of thiols and dithiols on gold: New challenges for a well-known system. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Combellas, C.; Kanoufi, F.; Pinson, J.; Podvorica, F.I. Sterically hindered diazonium salts for the grafting of a monolayer on metals. J. Am. Chem. Soc. 2008, 130, 8576–8577. [Google Scholar] [CrossRef]
- Adenier, A.; Combellas, C.; Kanoufi, F.; Pinson, J.; Podvorica, F.I. Formation of polyphenylene films on metal electrodes by electrochemical reduction of benzenediazonium salts. Chem. Mater. 2006, 18, 2021–2029. [Google Scholar] [CrossRef]
- Assresahegn, B.D.; Brousse, T.; Bélanger, D. Advances on the use of diazonium chemistry for functionalization of materials used in energy storage systems. Carbon 2015, 92, 362–381. [Google Scholar] [CrossRef]
- Lai, J.; Yi, Y.; Zhu, P.; Shen, J.; Wu, K.; Zhang, L.; Liu, J. Polyaniline-based glucose biosensor: A review. J. Electroanal. Chem. 2016, 782, 138–153. [Google Scholar] [CrossRef]
- Thompson, L.A.; Kowalik, J.; Josowicz, M.; Janata, J. Label-free DNA hybridization probe based on a conducting polymer. J. Am. Chem. Soc. 2003, 125, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Lakard, B. Electrochemical biosensors based on conducting polymers: A review. Appl. Sci. 2020, 10, 6614. [Google Scholar] [CrossRef]
- Saheb, A.; Patterson, S.; Josowicz, M. Probing for DNA methylation with a voltammetric DNA detector. Analyst 2014, 139, 786–792. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Magni, M.; Mussini, P.R. Enantioselective selectors for chiral electrochemistry and electroanalysis: Stereogenic elements and enantioselection performance. Curr. Opin. Electrochem. 2018, 8, 60–72. [Google Scholar] [CrossRef]
- Kane-Maguire, L.A.P.; Wallace, G.G. Chiral conducting polymers. Chem. Soc. Rev. 2010, 39, 2545–2576. [Google Scholar] [CrossRef]
- Vacek, J.; Zadny, J.; Storch, J.; Hrbac, J. Chiral electrochemistry: Anodic deposition of enantiopure helical molecules. ChemPlusChem 2020, 85, 1954–1958. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M. Enantioselective electrochemical sensors and biosensors: A mini-review. Electrochem. Commun. 2014, 38, 47–52. [Google Scholar] [CrossRef]
- Lemaire, M.; Delabouglise, D.; Garreau, R.; Guy, A.; Roncali, J. Enantioselective chiral poly(thiophenes). Chem. Commun. 1988, 10, 658–661. [Google Scholar] [CrossRef]
- Mondal, P.C.; Kantor-Uriel, N.; Mathew, S.P.; Tassinari, F.; Fontanesi, C. Chiral conductive polymers as spin filters. Adv. Mater. 2015, 27, 1924–1927. [Google Scholar] [CrossRef]
- Salmón, M.; Bidan, G. Chiral polypyrroles from optically active pyrrole monomers. J. Electrochem. Soc. 1985, 132, 1897–1899. [Google Scholar] [CrossRef]
- Ramos, J.C.; Dias, J.M.M.; Souto-Maior, R.M.; Ribeiro, A.S.; Tonholo, J.; Barbier, V.; Penelle, J.; Navarro, M. Synthesis and characterization of poly[(R)-(−) and (S)-(+)-3-(1′-pyrrolyl)propyl-N-(3″,5″-dinitrobenzoyl)-α-phenylglycinate]s as chiral oligomers of pyrrole. Synth. Met. 2010, 160, 1920–1924. [Google Scholar] [CrossRef]
- Takano, N.; Seki, C. Preparation of chiral polypyrrole film-coated electrode incorporating palladium metal and asymmetric hydrogenation of α-keto esters. Electrochemistry 2006, 74, 596–598. [Google Scholar] [CrossRef][Green Version]
- Deore, B.; Yakabe, H.; Shiigi, H.; Nagaok, T. Enantioselective uptake of amino acids using an electromodulated column packed with carbon fibres modified with overoxidised polypyrrole. Analyst 2002, 127, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Syritski, V.; Reut, J.; Menaker, A.; Gyurcsányi, R.E.; Öpik, A. Electrosynthesized molecularly imprinted polypyrrole films for enantioselective recognition of l-aspartic acid. Electrochim. Acta 2008, 53, 2729–2736. [Google Scholar] [CrossRef]
- Yang, J.; Yu, Y.; Wu, D.; Tao, Y.; Deng, L.; Kong, Y. Coinduction of a chiral microenvironment in polypyrrole by overoxidation and camphorsulfonic acid for electrochemical chirality sensing. Anal. Chem. 2018, 90, 9551–9558. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, J.; Zhang, J.; Wu, D.; Tao, Y.; Kong, Y. Single-template molecularly imprinted chiral sensor for simultaneous recognition of alanine and tyrosine enantiomers. Anal. Chem. 2019, 91, 12546–12552. [Google Scholar] [CrossRef] [PubMed]
- Basozabal, I.; Gómez-Caballero, A.; Unceta, N.; Goicolea, M.A.; Barrio, R.J. Voltammetric sensors with chiral recognition capability: The use of a chiral inducing agent in polyaniline electrochemical synthesis for the specific recognition of the enantiomers of the pesticide dinoseb. Electrochim. Acta 2011, 58, 729–735. [Google Scholar] [CrossRef]
- He, S.; Shang, X.; Lu, W.; Tian, Y.; Xu, Z.; Zhang, W. Electrochemical enantioselective sensor for effective recognition of tryptophan isomers based on chiral polyaniline twisted nanoribbon. Anal. Chim. Acta 2021, 1147, 155–164. [Google Scholar] [CrossRef]
- Feng, Z.; Li, M.; Yan, Y.; Jihai, T.; Xiao, L.; Wei, Q. Several novel and effective methods for chiral polyaniline to recognize the configuration of alanine. Tetrahedron Asymmetry 2012, 23, 411–414. [Google Scholar] [CrossRef]
- Tourillon, G.; Garnier, F. Stability of conducting polythiophene and derivatives. J. Electrochem. Soc. 1983, 130, 2042–2044. [Google Scholar] [CrossRef]
- Joergensen, M.; Norrman, K.; Gevorgyan, S.A.; Tromholt, T.; Andreasen, B.; Krebs, F.C. Stability of Polymer Solar Cells. Adv. Mater. 2012, 24, 580–612. [Google Scholar] [CrossRef] [PubMed]
- So, R.C.; Carreon-Asok, A.C. Molecular design, synthetic strategies, and applications of cationic polythiophenes. Chem. Rev. 2019, 119, 11442–11509. [Google Scholar] [CrossRef]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From fundamental perspectives to applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Yamamoto, T.; Koizumi, T.A. Synthesis of π-conjugated polymers bearing electronic and optical functionalities by organometallic polycondensations and their chemical properties. Polymer 2007, 48, 5449–5472. [Google Scholar] [CrossRef]
- Nilsson, K.P.R.; Johan, D.; Olsson, J.D.; Konradsson, P.; Inganas, O. Enantiomeric substituents determine the chirality of luminescent conjugated polythiophenes. Macromolecules 2004, 37, 6316–6321. [Google Scholar] [CrossRef]
- Chahma, M.; Myles, D.J.T.; Hicks, R.G. Synthesis and characterization of a conducting organomain group polymer, poly[bis((3,4-ethylenedioxy)-2-thienyl) sulfide]: A heteroaromatic relative of poly(p-phenylene sulfide). Macromolecules 2004, 37, 2010–2012. [Google Scholar] [CrossRef]
- Roncali, J. Conjugated poly (thiophenes): Synthesis, functionalization, and applications. Chem. Rev. 1992, 92, 711–738. [Google Scholar] [CrossRef]
- Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3, 4-ethylenedioxythiophene) and its derivatives: Past, present, and future. Adv. Mater. 2000, 12, 481–534. [Google Scholar] [CrossRef]
- Genies, E.M.; Bidan, G.; Diaz, A.F. Spectroelectrochemical study of polypyrrole films. J. Electroanal. Chem. 1983, 149, 101–113. [Google Scholar] [CrossRef]
- Amatore, C.; Savéant, J.-M. ECE and disproportionation: Part, V. Stationary state general solution application to linear sweep voltammetry. J. Electroanal. Chem. 1977, 85, 27–46. [Google Scholar]
- Andrieux, C.P.; Audebert, P.; Hapiot, P.; Savéant, J.-M. Identification of the first steps of the electrochemical polymerization of pyrroles by means of fast potential step techniques. J. Phys. Chem. 1991, 95, 10158–10164. [Google Scholar] [CrossRef]
- McTiernan, C.D.; Chahma, M. Synthesis and characterization of alanine functionalized oligo/polythiophenes. New J. Chem. 2010, 34, 1417–1423. [Google Scholar] [CrossRef]
- McTiernan, C.D.; Omri, K.; Chahma, M. Chiral conducting surfaces via electrochemical oxidation of l-leucine-oligothiophenes. J. Org. Chem. 2010, 75, 6096–6103. [Google Scholar] [CrossRef] [PubMed]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods. Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Chahma, M.; McTiernan, C.D.; Abbas, S.A. Characterization of phenomena occurring at the interface of chiral conducting surfaces. New J. Chem. 2014, 38, 3379–3385. [Google Scholar] [CrossRef]
- Hu, D.; Lu, B.; Duan, X.; Xu, J.; Zhang, L.; Zhang, K.; Zhang, S.; Zhen, S. Synthesis of novel chiral l-leucine grafted PEDOT derivatives with excellent electrochromic performances. RSC Adv. 2014, 4, 35597–35608. [Google Scholar] [CrossRef]
- Grenier, R.G.; George, S.J.; Joncheray, T.J.; Meijer, E.W.; Reynolds, J.R. Chiral ethylhexyl substituents for optically active aggregates of π-conjugated polymers. J. Am. Chem. Soc. 2007, 129, 10694–10699. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Lu, B.; Zhang, K.; Sun, X.; Xu, J.; Duan, X.; Dong, L.; Sun, H.; Zhu, X.; Zhen, S. Synthesis of novel chiral l-phenylalanine grafted PEDOT derivatives with electrochemical chiral sensor for 3,4-dihydroxyphenylalanine discrimination. Int. J. Electrochem. Sci. 2015, 10, 3065–3081. [Google Scholar]
- Dong, L.; Lu, B.; Duan, X.; Xu, J.; Hu, D.; Zhang, K.; Zhu, X.; Sun, H.; Ming, S.; Wang, Z.; et al. Novel chiral PEDOTs for selective recognition of 3, 4-dihydroxyphenylalanine enantiomers: Synthesis and characterization. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2238–2251. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, Y.; Duan, X.; Zhu, X.; Su, H.; Xu, J. Chiral PEDOT-based enantioselective electrode modification material for chiral electrochemical sensing: Mechanism and model of chiral recognition. Anal. Chem. 2017, 89, 9695–9702. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Benincori, T.; Cirilli, R.; Grecchi, S.; Santagostini, L.; Sannicolò, F.; Mussini, P.R. “Inherently chiral” thiophene-based electrodes at work: A screening of enantioselection ability toward a series of pharmaceutically relevant phenolic or catecholic amino acids, amino esters, and amine. Anal. Bioanal. Chem. 2016, 408, 7243–7254. [Google Scholar] [CrossRef] [PubMed]
- Arnaboldi, S.; Benincori, T.; Cirilli, R.; Kutner, W.; Magni, M.; Mussini, P.R.; Noworyta, K.; Sannicolò, F. Inherently chiral electrodes: The tool for chiral voltammetry. Chem. Sci. 2015, 6, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Wattanakit, C.; Côme, Y.; Lapeyre, V.; Bopp, P.A.; Heim, M.; Yadnum, S.; Nokbin, S.; Warakulwit, C.; Limtrakul, J.; Kuhn, A. Enantioselective recognition at mesoporous chiral metal surfaces. Nat. Commun. 2014, 5, 3325. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Liu, Z.; Sun, L.; Fang, G.; Liu, J.; Wang, S. Electrochemical detection of organophosphorus pesticides based on amino acids-conjugated P3TAA-modified electrodes. Analyst 2020, 145, 8068–8076. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-L.; Gao, Y.; Han, X.X.; Zhao, B. Detection of pesticide residues in food using surface-enhanced Raman spectroscopy: A review. J. Agric. Food Chem. 2017, 65, 6719–6726. [Google Scholar] [CrossRef] [PubMed]
- Pernites, R.B.; Venkata, S.K.; Tiu, B.D.B.; Yago, A.C.C.; Advincula, R.C. Nanostructured, molecularly imprinted, and template-patterned polythiophenes for chiral sensing and differentiation. Small 2012, 8, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Yang, H.; Ding, Y.; Li, L.; Ma, G. A three-dimensional conductive molecularly imprinted electrochemical sensor based on MOF derived porous carbon/carbon nanotubes composites and prussian blue nanocubes mediated amplification for chiral analysis of cysteine enantiomers. Electrochim. Acta 2019, 302, 137–144. [Google Scholar] [CrossRef]
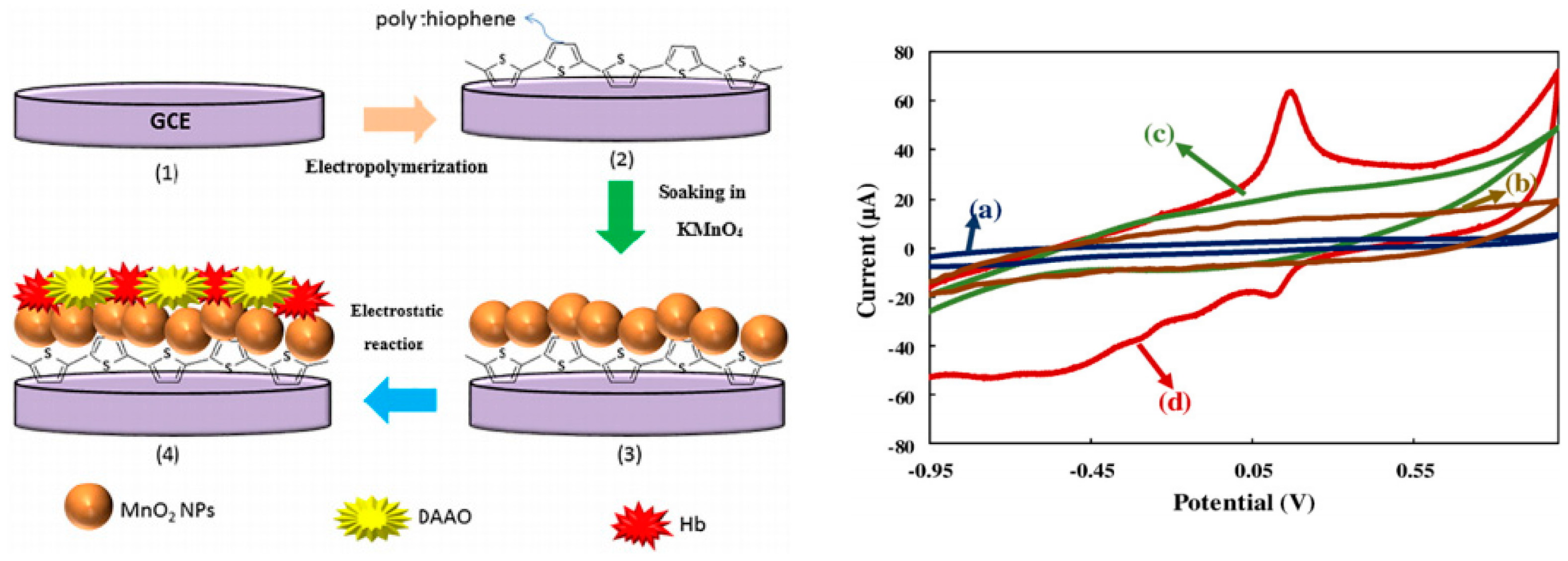
- Shoja, Y.; Rafati, A.A.; Ghodsi, J. Polythiophene supported MnO2 nanoparticles as nano-stabilizer for simultaneously electrostatically immobilization of d-amino acid oxidase and hemoglobin as efficient bio-nanocomposite in fabrication of dopamine bi-enzyme biosensor. Mater. Sci. Eng. C 2017, 76, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Duay, J.; Lee, S.B. Redox exchange induced MnO2 nanoparticle enrichment in poly(3,4-ethylenedioxythiophene) nanowires for electrochemical energy storage. ACS Nano 2010, 4, 4299–4307. [Google Scholar] [CrossRef]
- Li, Q.; Xia, Y.; Wan, X.; Yang, S.; Cai, Z.; Ye, Y.; Li, G. Morphology-dependent MnO2/nitrogen-doped graphene nanocomposites for simultaneous detection of trace dopamine and uric acid. Mater. Sci. Eng. C 2020, 109, 110615. [Google Scholar] [CrossRef] [PubMed]
- Borazjani, M.; Mehdinia, A.; Jabbari, A. An enantioselective electrochemical sensor for simultaneous determination of mandelic acid enantiomers using dexamethasone-based chiral nanocomposite coupled with chemometrics method. J. Electroanal. Chem. 2017, 805, 83–90. [Google Scholar] [CrossRef]
- Niu, X.; Yang, X.; Mo, Z.; Pan, Z.; Liu, Z.; Shuai, C.; Gao, Q.; Liu, N.; Guo, R. Electrochemical chiral recognition at molecular imprinting Au surfaces. J. Electrochem. Soc. 2019, 166, B1126–B1130. [Google Scholar] [CrossRef]
- Ning, G.; Wang, H.; Fu, M.; Liu, J.; Sun, Y.; Lu, H.; Fan, X.; Zhang, Y.; Wang, H. Dual signals electrochemical biosensor for point-of-care testing of amino acids enantiomers. Electroanalysis 2021. [Google Scholar] [CrossRef]
- Zeng, X.; Li, N.; Wang, J. Electrochemical synthesis of (poly) dimethoxyaniline on glassy carbon electrodes and their applications in the detection of l and d-glutamic acids. J. Electrochem. Soc. 2019, 166, B3066–B3071. [Google Scholar] [CrossRef]
- Pandey, I.; Kant, R. Electrochemical impedance based chiral analysis of anti-ascorbutic drug: L-Ascorbic acid and d-ascorbic acid using C-dots decorated conductive polymer nano-composite electrode. Biosens. Bioelectron. 2016, 77, 715–724. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Peng, C.; Zhang, Z.; Chen, J.; Zhou, X.; Jiang, H. Enantiorecognition of tyrosine based on a novel magnetic electrochemical chiral sensor. Electrochim. Acta 2017, 241, 386–394. [Google Scholar] [CrossRef]
- Zou, J.; Yu, J.G. Chiral recognition of tyrosine enantiomers on a novel bis-aminosaccharides composite modified glassy carbon electrode. Anal. Chim. Acta 2019, 1088, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Zhou, Y.; Zhang, G.; Zhang, Y.; Zhang, C.; Hong, S.; Yang, Y.; Dong, C.; Shuang, S. A highly efficient chiral sensing platform for tryptophan isomers based on a coordination self-assembly. Talanta 2019, 195, 306–312. [Google Scholar] [CrossRef] [PubMed]



| Electrode Construction | Detection | LOD | Ref |
|---|---|---|---|
| Leu/PTh/Pt | LeuOMe/AlaOMe | 1 mM | [47] |
| PTh/GCE | 1,4-Dihydroxyphenylalanine | 0.50 mM | [51] |
| Norephedrine/PTh/QCM | Norephedrine | 0.25 mM | [58] |
| His-Ser-Glu/PTh/GCE | Organophosphorus | 0.50 µM | [56] |
| DAAO-Hb/MnO2 NPs/PTh/GCE | Dopamine | 40 nM | [60] |
| DAAO-Hb/MnO2 NPs/GCE | Dopamine | 0.039 µM | [62] |
| PPy-DEX/GR/GCE | Mandelic acid | 0.25 mM | [63] |
| Trp/PPy/Au | l-Tryptophan, d-Tryptophan | 0.012 μM, 0.009 μM | [64] |
| L/D-CNT/PPy/Pt | Amino acids (Tryptophan) | 0.107 nM | [65] |
| PANi/GCE | l-Glutamic acid | 0.011 mM | [66] |
| PANI-FSA/PGE | l-Ascorbic acid | 7.3–4.5 10−4 nM | [67] |
| L-Cys-Au/Fe3O4-NP | l-and d-Tyrosine | 0.021–0.084 μM | [68] |
| GCE | l-Tyrosine, d-Tyrosine | 0.65, 0.86 mM | [69] |
| Cu-β-CD/PLA/MWCN/GCE. | Tryptophan | 3.3 10−7 M | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chahma, M. Doped Polythiophene Chiral Electrodes as Electrochemical Biosensors. Electrochem 2021, 2, 677-688. https://doi.org/10.3390/electrochem2040042
Chahma M. Doped Polythiophene Chiral Electrodes as Electrochemical Biosensors. Electrochem. 2021; 2(4):677-688. https://doi.org/10.3390/electrochem2040042
Chicago/Turabian StyleChahma, M’hamed. 2021. "Doped Polythiophene Chiral Electrodes as Electrochemical Biosensors" Electrochem 2, no. 4: 677-688. https://doi.org/10.3390/electrochem2040042
APA StyleChahma, M. (2021). Doped Polythiophene Chiral Electrodes as Electrochemical Biosensors. Electrochem, 2(4), 677-688. https://doi.org/10.3390/electrochem2040042