Recent Applications of Molecular Structures at Silicon Anode Interfaces
Abstract
:1. Introduction
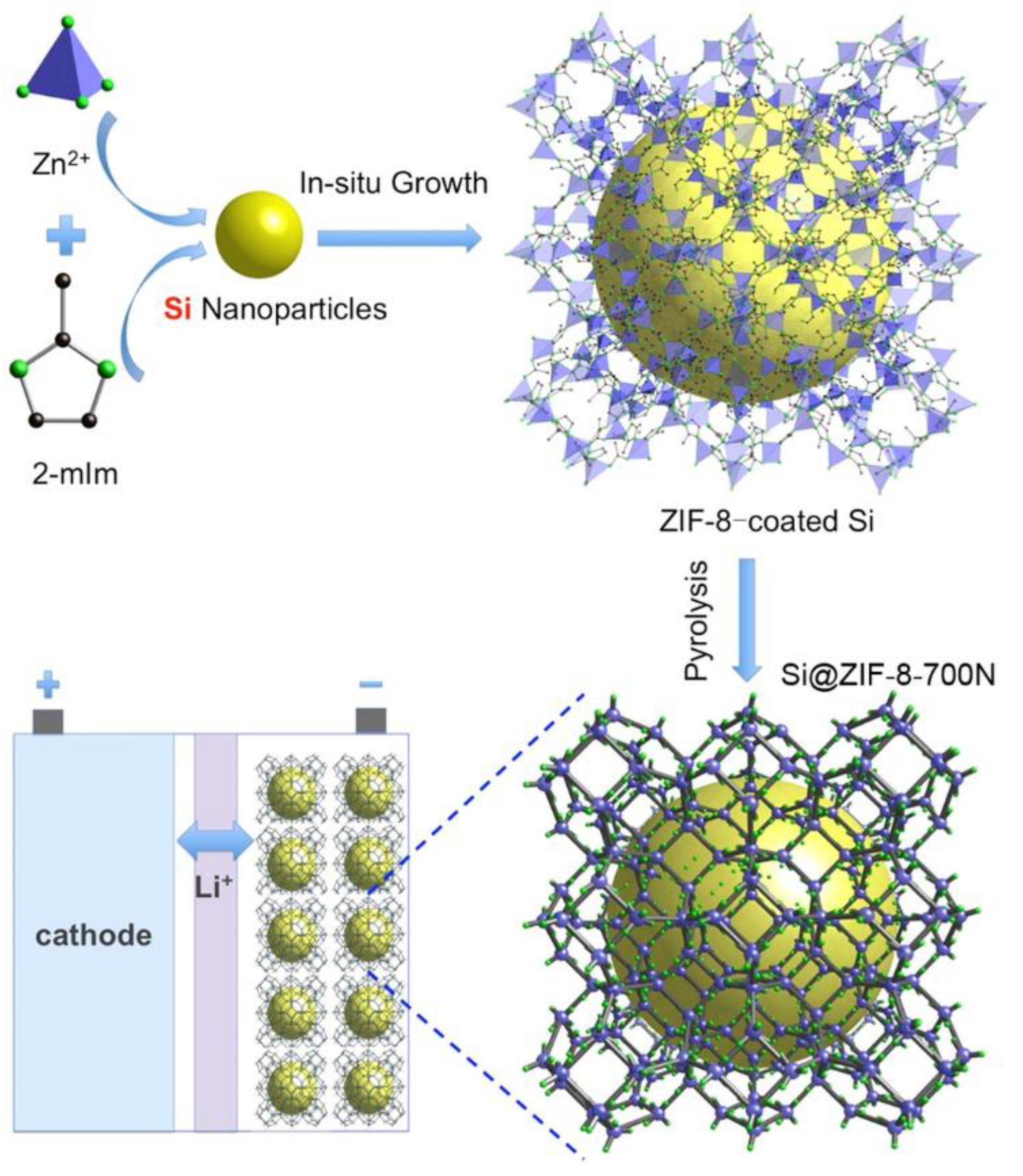
2. Metal–Organic Framework
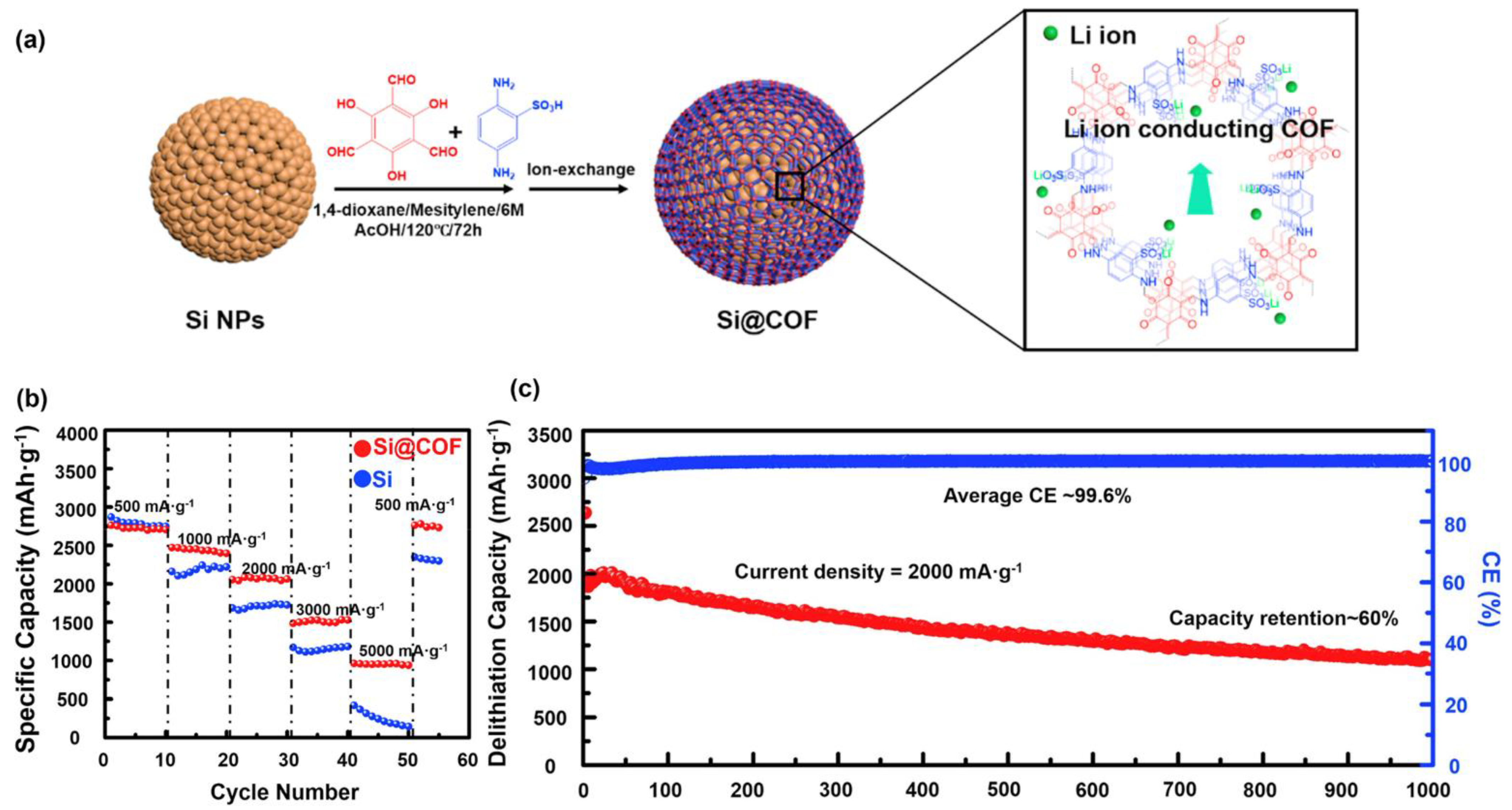
3. Covalent Organic Frameworks
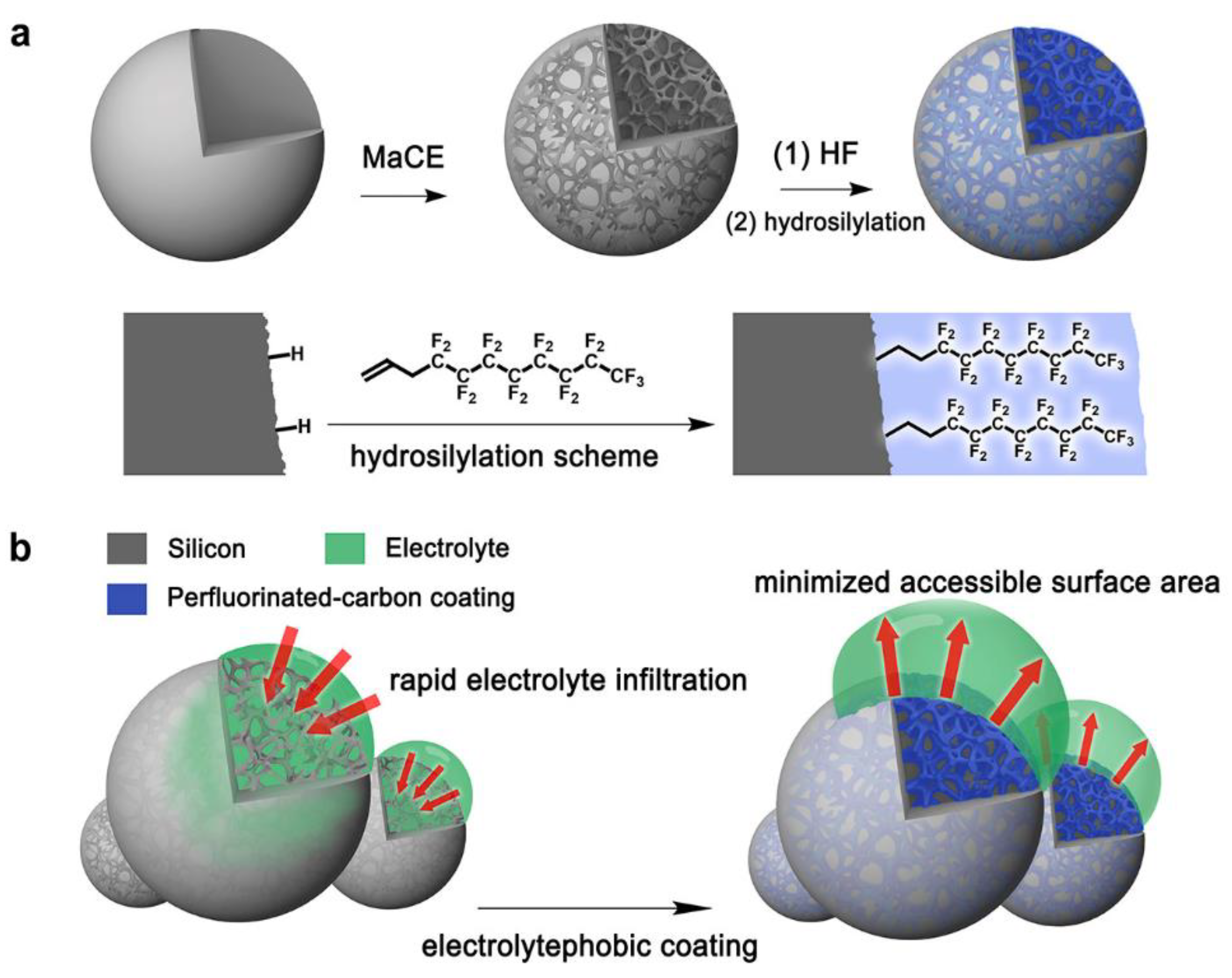
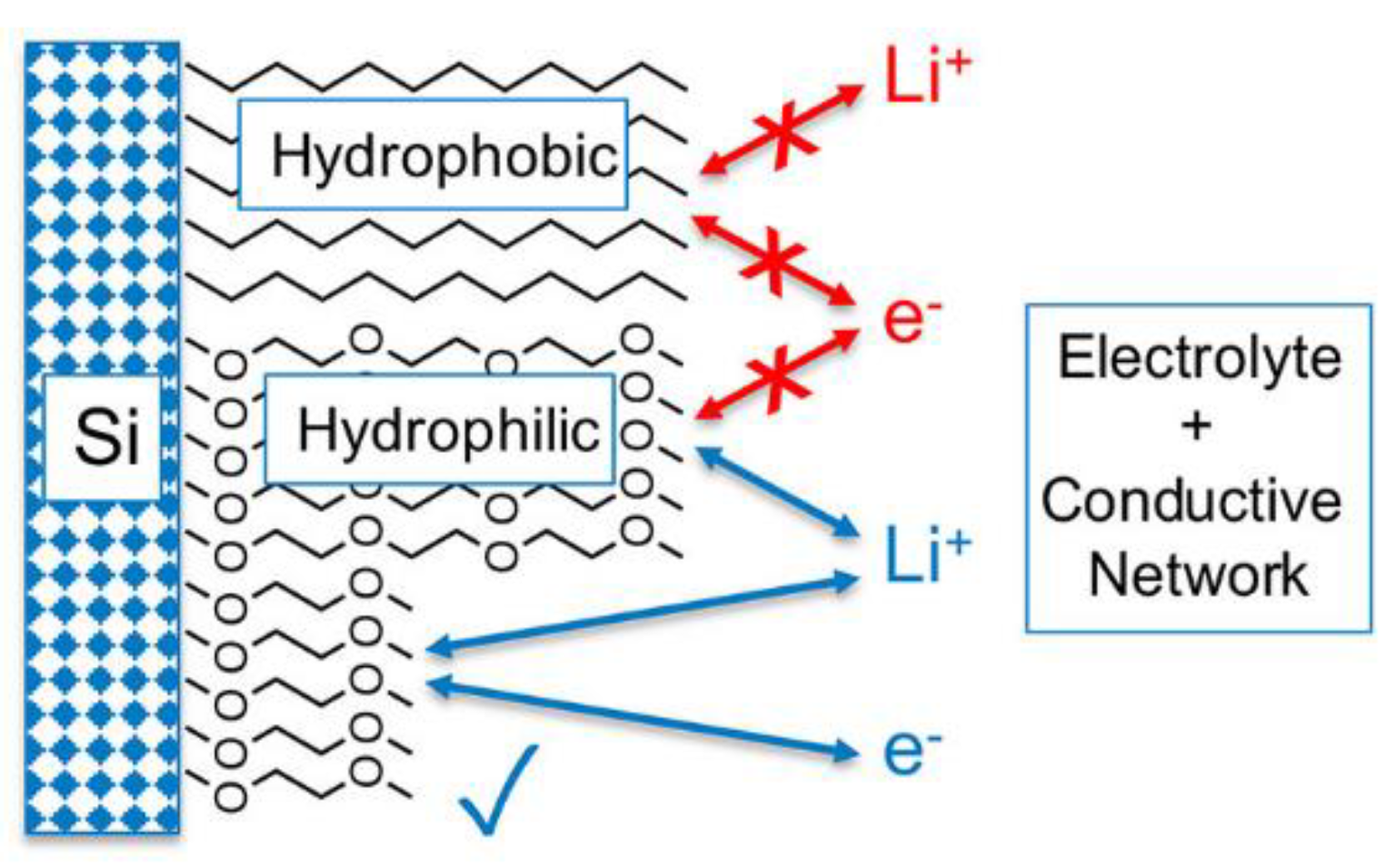
4. Monolayers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McBrayer, J.D.; Rodrigues, M.-T.F.; Schulze, M.C.; Abraham, D.P.; Apblett, C.A.; Bloom, I.; Carroll, G.M.; Colclasure, A.M.; Fang, C.; Harrison, K.L.; et al. Calendar aging of silicon-containing batteries. Nat. Energy 2021, 6, 866–872. [Google Scholar] [CrossRef]
- Yi, R.; Mao, Y.; Shen, Y.; Chen, L. Self-assembled monolayers for batteries. J. Am. Chem. Soc. 2021, 143, 12897–12912. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Lopez, C.V.; Maladeniya, C.P.; Smith, R.C. Lithium-sulfur batteries: Advances and trends. Electrochem 2020, 1, 226–259. [Google Scholar] [CrossRef]
- Liu, Z.; He, X.; Fang, C.; Camacho-Forero, L.E.; Zhao, Y.; Fu, Y.; Feng, J.; Kostecki, R.; Balbuena, P.B.; Zhang, J.; et al. Reversible crosslinked polymer binder for recyclable lithium sulfur batteries with high performance. Adv. Funct. Mater. 2020, 30, 2003605. [Google Scholar] [CrossRef]
- Li, Z.; Fang, C.; Qian, C.; Zhou, S.; Song, X.; Ling, M.; Liang, C.; Liu, G. Polyisoprene captured sulfur nanocomposite materials for high-areal-capacity lithium sulfur battery. ACS Appl. Polym. Mater. 2019, 1, 1965–1970. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, G.; Lau, J.; Liu, G. Recent advances in polysulfide mediation of lithium-sulfur batteries via facile cathode and electrolyte modification. APL Mater. 2019, 7, 080902. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Fang, C.; Zhang, G.; Hubble, D.; Nallapaneni, A.; Zhu, C.; Zhao, Z.; Liu, Z.; Lau, J.; Fu, Y.; et al. A Micelle electrolyte enabled by fluorinated ether additives for polysulfide suppression and Li metal stabilization in Li-S battery. Front. Chem. 2020, 8, 484. [Google Scholar] [CrossRef]
- Li, T.; Bai, X.; Gulzar, U.; Bai, Y.-J.; Capiglia, C.; Deng, W.; Zhou, X.; Liu, Z.; Feng, Z.; Proietti Zaccaria, R. A comprehensive understanding of lithium–sulfur battery technology. Adv. Funct. Mater. 2019, 29, 1901730. [Google Scholar] [CrossRef]
- Phadatare, M.; Patil, R.; Blomquist, N.; Forsberg, S.; Örtegren, J.; Hummelgård, M.; Meshram, J.; Hernández, G.; Brandell, D.; Leifer, K.; et al. Silicon-nanographite aerogel-based anodes for high performance lithium ion batteries. Sci. Rep. 2019, 9, 14621. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Fang, C.; Song, X.; Liu, G. The influence of compact and ordered carbon coating on solid-state behaviors of silicon during electrochemical processes. Carbon Energy 2020, 2, 143–150. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Huang, S.; Wang, S.; Mizota, I.; Liu, X.; Hou, X. Considering critical factors of silicon/graphite anode materials for practical high-energy lithium-ion battery applications. Energy Fuels 2021, 35, 944–964. [Google Scholar] [CrossRef]
- Fang, C.; Xiao, H.; Zheng, T.; Bai, H.; Liu, G. Organic solvent free process to fabricate high performance silicon/graphite composite anode. J. Compos. Sci. 2021, 5, 188. [Google Scholar] [CrossRef]
- Chae, S.; Choi, S.-H.; Kim, N.; Sung, J.; Cho, J. Integration of graphite and silicon anodes for the commercialization of high-energy lithium-ion batteries. Angew. Chem. Int. Ed. 2020, 59, 110–135. [Google Scholar] [CrossRef] [Green Version]
- Zamfir, M.R.; Nguyen, H.T.; Moyen, E.; Lee, Y.H.; Pribat, D. Silicon nanowires for Li-based battery anodes: A review. J. Mater. Chem. A 2013, 1, 9566–9586. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.-M.; Zhang, Y.-C.; Song, Y.; Wang, G.-K.; Liu, Y.-X.; Wu, Z.-G.; Zhong, B.-H.; Zhong, Y.-J.; Guo, X.-D. A review of rational design and investigation of binders applied in silicon-based anodes for lithium-ion batteries. J. Power Sources 2021, 485, 229331. [Google Scholar] [CrossRef]
- Yuda, A.P.; Koraag, P.Y.E.; Iskandar, F.; Wasisto, H.S.; Sumboja, A. Advances of the top-down synthesis approach for high-performance silicon anodes in Li-ion batteries. J. Mater. Chem. A 2021, 9, 18906–18926. [Google Scholar] [CrossRef]
- Fang, C.; Tran, T.-N.; Zhao, Y.; Liu, G. Electrolyte decomposition and solid electrolyte interphase revealed by mass spectrometry. Electrochim. Acta 2021, 399, 139362. [Google Scholar] [CrossRef]
- Jia, H.; Li, X.; Song, J.; Zhang, X.; Luo, L.; He, Y.; Li, B.; Cai, Y.; Hu, S.; Xiao, X.; et al. Hierarchical porous silicon structures with extraordinary mechanical strength as high-performance lithium-ion battery anodes. Nat. Commun. 2020, 11, 1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Franco Gonzalez, A.; Yang, N.-H.; Liu, R.-S. Silicon anode design for lithium-ion batteries: Progress and perspectives. J. Phys. Chem. C 2017, 121, 27775–27787. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, N.; Yang, D. Designing superior solid electrolyte interfaces on silicon anodes for high-performance lithium-ion batteries. Nanoscale 2019, 11, 19086–19104. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, E.J.; Frisco, S.; Pekarek, R.T.; Stetson, C.; Huey, Z.; Harvey, S.; Li, X.; Key, B.; Fang, C.; Liu, G.; et al. Examining CO2 as an additive for solid electrolyte interphase formation on silicon anodes. J. Electrochem. Soc. 2021, 168, 030534. [Google Scholar] [CrossRef]
- Fang, C.; Lau, J.; Hubble, D.; Khomein, P.; Dailing, E.A.; Liu, Y.; Liu, G. Large-molecule decomposition products of electrolytes and additives revealed by on-electrode chromatography and MALDI. Joule 2021, 5, 415–428. [Google Scholar] [CrossRef]
- Shen, T.; Yao, Z.; Xia, X.; Wang, X.; Gu, C.; Tu, J. Rationally designed silicon nanostructures as anode material for lithium-ion batteries. Adv. Eng. Mater. 2018, 20, 1700591. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Lee, J.H.; Nam, D.H.; Cho, M.; Kim, J.; Chanthad, C.; Lee, Y. Epoxidized natural rubber/chitosan network binder for silicon anode in lithium-ion battery. ACS Appl. Mater. Interfaces 2018, 10, 16449–16457. [Google Scholar] [CrossRef]
- Liu, Z.; Fang, C.; He, X.; Zhao, Y.; Xu, H.; Lei, J.; Liu, G. In situ-formed novel elastic network binder for a silicon anode in lithium-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 46518–46525. [Google Scholar] [CrossRef]
- Jiang, C.; Xiang, L.; Miao, S.; Shi, L.; Xie, D.; Yan, J.; Zheng, Z.; Zhang, X.; Tang, Y. Flexible interface design for stress regulation of a silicon anode toward highly stable dual-ion batteries. Adv. Mater. 2020, 32, 1908470. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Han, J.; Lu, Z.; Wei, D.; Kashani, H.; Watanabe, K.; Chen, M. Ultrastable silicon anode by three-dimensional nanoarchitecture design. ACS Nano 2020, 14, 4374–4382. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Liu, T.; Gao, X.; Li, S.; Ling, M.; Liang, C.; Zheng, J.; Lin, Z. Silicon anode with high initial coulombic efficiency by modulated trifunctional binder for high-areal-capacity lithium-ion batteries. Adv. Energy Mater. 2020, 10, 1903110. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.; Dou, Y.; Cheng, N.; Cui, D.; Du, Y.; Liu, P.; Al-Mamun, M.; Zhang, S.; Zhao, H. A Yolk–shell structured silicon anode with superior conductivity and high tap density for full lithium-ion batteries. Angew. Chem. Int. Ed. 2019, 58, 8824–8828. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Kim, H.; Myung, S.-T.; Sun, Y.-K. Diverting exploration of silicon anode into practical way: A review focused on silicon-graphite composite for lithium ion batteries. Energy Storage Mater. 2021, 35, 550–576. [Google Scholar] [CrossRef]
- Zhou, S.; Fang, C.; Song, X.; Liu, G. Highly ordered carbon coating prepared with polyvinylidene chloride precursor for high-performance silicon anodes in lithium-ion batteries. Batter. Supercaps 2021, 4, 240–247. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef] [Green Version]
- Veerbeek, J.; Huskens, J. Applications of monolayer-functionalized h-terminated silicon surfaces: A review. Small Methods 2017, 1, 1700072. [Google Scholar] [CrossRef] [Green Version]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.-C.; Jiang, H.-L. Metal–organic framework-based hierarchically porous materials: Synthesis and applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef]
- Zhong, M.; Kong, L.; Li, N.; Liu, Y.-Y.; Zhu, J.; Bu, X.-H. Synthesis of MOF-derived nanostructures and their applications as anodes in lithium and sodium ion batteries. Coord. Chem. Rev. 2019, 388, 172–201. [Google Scholar] [CrossRef]
- Xie, L.S.; Skorupskii, G.; Dincă, M. Electrically conductive metal–organic frameworks. Chem. Rev. 2020, 120, 8536–8580. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to metal–organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Cohen, S.M. Postsynthetic methods for the functionalization of metal–organic frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, H.; Yue, L.; Liang, J.; Li, T.; Liu, Q.; Luo, Y.; Kong, X.; Lu, S.; Shi, X.; et al. Recent advances in lithium-based batteries using metal organic frameworks as electrode materials. Electrochem. Commun. 2021, 122, 106881. [Google Scholar] [CrossRef]
- Han, Y.; Qi, P.; Zhou, J.; Feng, X.; Li, S.; Fu, X.; Zhao, J.; Yu, D.; Wang, B. Metal–Organic Frameworks (MOFs) as sandwich coating cushion for silicon anode in lithium ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 26608–26613. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.; Bok, T.; Kim, C.; Na, Y.; Park, S.; Kim, K.S. Mesoporous silicon hollow nanocubes derived from metal–organic framework template for advanced lithium-ion battery anode. ACS Nano 2017, 11, 4808–4815. [Google Scholar] [CrossRef]
- Malik, R.; Loveridge, M.J.; Williams, L.J.; Huang, Q.; West, G.; Shearing, P.R.; Bhagat, R.; Walton, R.I. Porous metal–organic frameworks for enhanced performance silicon anodes in lithium-ion batteries. Chem. Mater. 2019, 31, 4156–4165. [Google Scholar] [CrossRef]
- Nazir, A.; Le, H.T.T.; Min, C.-W.; Kasbe, A.; Kim, J.; Jin, C.-S.; Park, C.-J. Coupling of a conductive Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2 metal–organic framework with silicon nanoparticles for use in high-capacity lithium-ion batteries. Nanoscale 2020, 12, 1629–1642. [Google Scholar] [CrossRef]
- Nazir, A.; Le, H.T.T.; Kasbe, A.; Park, C.-J. Si nanoparticles confined within a conductive 2D porous Cu-based metal–organic framework (Cu3(HITP)2) as potential anodes for high-capacity Li-ion batteries. Chem. Eng. J. 2021, 405, 126963. [Google Scholar] [CrossRef]
- Yu, Y.; Yue, C.; Lin, X.; Sun, S.; Gu, J.; He, X.; Zhang, C.; Lin, W.; Lin, D.; Liao, X.; et al. ZIF-8 Cooperating in TiN/Ti/Si Nanorods as Efficient Anodes in Micro-Lithium-Ion-Batteries. ACS Appl. Mater. Interfaces 2016, 8, 3992–3999. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yue, C.; Han, Y.; Zhang, C.; Zheng, M.; Xu, B.; Lin, S.; Li, J.; Kang, J. Si nanorod arrays modified with metal–organic segments as anodes in lithium ion batteries. RSC Adv. 2017, 7, 53680–53685. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Zeng, M.; Wu, X.; Zhang, Y.; Wen, J.; Li, J. Three-dimensional cage-like Si@ZIF-67 core-shell composites for high-performance lithium storage. Appl. Surf. Sci. 2020, 510, 145477. [Google Scholar] [CrossRef]
- Gao, R.; Tang, J.; Yu, X.; Tang, S.; Ozawa, K.; Sasaki, T.; Qin, L.-C. In situ synthesis of MOF-derived carbon shells for silicon anode with improved lithium-ion storage. Nano Energy 2020, 70, 104444. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Yu, J.; Yuan, M.; Tan, Q.; Zhong, Z.; Su, F. High-performance Si-Containing anode materials in lithium-ion batteries: A superstructure of Si@Co–NC composite works effectively. Green Energy Environ. 2020. [Google Scholar] [CrossRef]
- Jin, D.; Yang, X.; Ou, Y.; Rao, M.; Zhong, Y.; Zhou, G.; Ye, D.; Qiu, Y.; Wu, Y.; Li, W. Thermal pyrolysis of Si@ZIF-67 into Si@N-doped CNTs towards highly stable lithium storage. Sci. Bull. 2020, 65, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Liu, J.; Jia, D.; Huang, Y.; Luo, J.; Mamat, X.; Yu, Y.; Dong, Y.; Hu, G. Multi-core yolk-shell like mesoporous double carbon-coated silicon nanoparticles as anode materials for lithium-ion batteries. Energy Storage Mater. 2019, 18, 165–173. [Google Scholar] [CrossRef]
- Qiao, Y.; Hu, Y.; Liu, W.; Zhang, H.; Shang, H.; Qu, M.; Peng, G.; Xie, Z. Synergistic carbon coating of MOF-derived porous carbon and CNTs on silicon for high performance lithium-ion batteries. J. Electroanal. Chem. 2021, 888, 115014. [Google Scholar] [CrossRef]
- Qiao, Y.-J.; Zhang, H.; Hu, Y.-X.; Li, W.-P.; Liu, W.-J.; Shang, H.-M.; Qu, M.-Z.; Peng, G.-C.; Xie, Z.-W. A chain-like compound of Si@CNT nanostructures and MOF-derived porous carbon as an anode for Li-ion batteries. Int. J. Miner. Metall. Mater. 2021, 28, 1611–1620. [Google Scholar] [CrossRef]
- Han, Y.; Qi, P.; Feng, X.; Li, S.; Fu, X.; Li, H.; Chen, Y.; Zhou, J.; Li, X.; Wang, B. In situ growth of MOFs on the surface of Si nanoparticles for highly efficient lithium storage: Si@MOF nanocomposites as anode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 2178–2182. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zuo, L.; Chen, S.; Wu, J.; Hou, H.; Wang, L. Porous Nano-Si/carbon derived from zeolitic imidazolate frameworks@Nano-Si as anode materials for lithium-ion batteries. Electrochim. Acta 2015, 173, 588–594. [Google Scholar] [CrossRef]
- Wei, Q.; Chen, Y.-M.; Hong, X.-J.; Song, C.-L.; Yang, Y.; Si, L.-P.; Zhang, M.; Cai, Y.-P. Saclike-silicon nanoparticles anchored in ZIF-8 derived spongy matrix as high-performance anode for lithium-ion batteries. J. Colloid Interface Sci. 2020, 565, 315–325. [Google Scholar] [CrossRef]
- Wei, Q.; Chen, Y.-M.; Hong, X.-J.; Song, C.-L.; Yang, Y.; Si, L.-P.; Zhang, M.; Cai, Y.-P. Novel bread-like nitrogen-doped carbon anchored nano-silicon as high-stable anode for lithium-ion batteries. Appl. Surf. Sci. 2020, 511, 145609. [Google Scholar] [CrossRef]
- Majeed, M.K.; Ma, G.; Cao, Y.; Mao, H.; Ma, X.; Ma, W. Metal–organic frameworks-derived mesoporous Si/SiOx@NC nanospheres as a long-lifespan anode material for lithium-ion batteries. Chem. Eur. J. 2019, 25, 11991–11997. [Google Scholar] [CrossRef]
- Zhang, K.; Mao, H.; Gu, X.; Song, C.; Yang, J.; Qian, Y. ZIF-derived cobalt-containing N-doped carbon-coated SiOx nanoparticles for superior lithium storage. ACS Appl. Mater. Interfaces 2020, 12, 7206–7211. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Han, X.; Su, X.; Pang, B.; Luo, Y.; Hu, F.; Zhou, M.; Tao, K.; Xia, Y. Metal-organic frameworks derived porous carbon coated SiO composite as superior anode material for lithium ion batteries. J. Alloys Compd. 2018, 765, 512–519. [Google Scholar] [CrossRef]
- Wang, K.; Pei, S.; He, Z.; Huang, L.-A.; Zhu, S.; Guo, J.; Shao, H.; Wang, J. Synthesis of a novel porous silicon microsphere@carbon core-shell composite via in situ MOF coating for lithium ion battery anodes. Chem. Eng. J. 2019, 356, 272–281. [Google Scholar] [CrossRef]
- Wu, F.; Wang, H.; Shi, J.; Yan, Z.; Song, S.; Peng, B.; Zhang, X.; Xiang, Y. Surface modification of silicon nanoparticles by an “Ink” layer for advanced lithium ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 19639–19648. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, C.; He, J.; Yang, S.; Liu, Z.; Wang, Q. Fe2O3/C-modified Si nanoparticles as anode material for high-performance lithium-ion batteries. J. Alloys Compd. 2019, 795, 284–290. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, X.; Wei, C.; Liang, G.; Huang, Y.; Li, R.; He, Q. A novel Si/Ag@PM@MIL-100 porous double-shell anode materials prepared by in-situ growth with MOF coatings. J. Mater. Sci. Mater. Electron. 2020, 31, 1524–1534. [Google Scholar] [CrossRef]
- Huang, N.; Wang, P.; Jiang, D. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 16068. [Google Scholar] [CrossRef]
- Chen, D.; Huang, S.; Zhong, L.; Wang, S.; Xiao, M.; Han, D.; Meng, Y. In situ preparation of thin and rigid COF film on Li anode as artificial solid electrolyte interphase layer resisting Li dendrite puncture. Adv. Funct. Mater. 2020, 30, 1907717. [Google Scholar] [CrossRef]
- Ai, Q.; Fang, Q.; Liang, J.; Xu, X.; Zhai, T.; Gao, G.; Guo, H.; Han, G.; Ci, L.; Lou, J. Lithium-conducting covalent-organic-frameworks as artificial solid-electrolyte-interphase on silicon anode for high performance lithium ion batteries. Nano Energy 2020, 72, 104657. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, J.; Xu, Z.; Wang, J.; Nuli, Y.; Zhuang, X.; Feng, X. Silicon anodes protected by a nitrogen-doped porous carbon shell for high-performance lithium-ion batteries. Nanoscale 2017, 9, 8871–8878. [Google Scholar] [CrossRef]
- Xue, Y.; Li, X.; Li, H.; Zhang, W. Quantifying thiol–gold interactions towards the efficient strength control. Nat. Commun. 2014, 5, 4348. [Google Scholar] [CrossRef] [Green Version]
- Velpula, G.; Takeda, T.; Adisoejoso, J.; Inukai, K.; Tahara, K.; Mali, K.S.; Tobe, Y.; De Feyter, S. On the formation of concentric 2D multicomponent assemblies at the solution–solid interface. Chem. Commun. 2017, 53, 1108–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Fang, C.; Shelp, R.A.; Zimmt, M.B. Tracking invisible transformations of physisorbed monolayers: LDI-TOF and MALDI-TOF mass spectrometry as complements to STM imaging. Langmuir 2017, 33, 459–467. [Google Scholar] [CrossRef]
- Fang, C.; Zhu, H.; Chen, O.; Zimmt, M.B. Reactive two-component monolayers template bottom-up assembly of nanoparticle arrays on HOPG. Chem. Commun. 2018, 54, 8056–8059. [Google Scholar] [CrossRef] [Green Version]
- Colangelo, E.; Comenge, J.; Paramelle, D.; Volk, M.; Chen, Q.; Lévy, R. Characterizing self-assembled monolayers on gold nanoparticles. Bioconjugate Chem. 2017, 28, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Eves, B.J.; Lopinski, G.P. Formation of organic monolayers on silicon via gas-phase photochemical reactions. Langmuir 2006, 22, 3180–3185. [Google Scholar] [CrossRef]
- Ashuri, M.; He, Q.; Shaw, L.L. Silicon as a potential anode material for Li-ion batteries: Where size, geometry and structure matter. Nanoscale 2016, 8, 74–103. [Google Scholar] [CrossRef]
- Harpak, N.; Davidi, G.; Patolsky, F. Breathing parylene-based nanothin artificial SEI for highly-stable long life three-dimensional silicon lithium-ion batteries. Chem. Eng. J. 2022, 429, 132077. [Google Scholar] [CrossRef]
- Voicu, R.; Boukherroub, R.; Bartzoka, V.; Ward, T.; Wojtyk, J.T.C.; Wayner, D.D.M. Formation, characterization, and chemistry of undecanoic acid-terminated silicon surfaces: Patterning and immobilization of DNA. Langmuir 2004, 20, 11713–11720. [Google Scholar] [CrossRef] [PubMed]
- Böcking, T.; Kilian, K.A.; Gaus, K.; Gooding, J.J. Single-step DNA immobilization on antifouling self-assembled monolayers covalently bound to silicon (111). Langmuir 2006, 22, 3494–3496. [Google Scholar] [CrossRef]
- Min, J.-H.; Bae, Y.-S.; Kim, J.-Y.; Kim, S.S.; Song, S.-W. Self-organized Artificial SEI for Improving the Cycling Ability of Silicon-based Battery Anode Materials. Bull. Korean Chem. Soc. 2013, 34, 1296–1299. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Yang, Z.; Liu, Y.; Johnson, N.; Bloom, I.; Zhang, L.; Zhang, Z. Engineering the Si anode interface via particle surface modification: Embedded organic carbonates lead to enhanced performance. ACS Appl. Energy Mater. 2021, 4, 8193–8200. [Google Scholar] [CrossRef]
- Qian, C.; Zhao, J.; Sun, Y.; Lee, H.R.; Luo, L.; Makaremi, M.; Mukherjee, S.; Wang, J.; Zu, C.; Xia, M.; et al. Electrolyte-phobic surface for the next-generation nanostructured battery electrodes. Nano Lett. 2020, 20, 7455–7462. [Google Scholar] [CrossRef]
- Schulze, M.C.; Carroll, G.M.; Martin, T.R.; Sanchez-Rivera, K.; Urias, F.; Neale, N.R. Hydrophobic versus hydrophilic interfacial coatings on silicon nanoparticles teach us how to design the solid electrolyte interphase in silicon-based Li-Ion battery anodes. ACS Appl. Energy Mater. 2021, 4, 1628–1636. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, B.; Sahore, R.; Zhang, L.; Liu, H.; Zhang, L.; Lu, W.; Zhao, B.; Zhang, Z. Surface-functionalized silicon nanoparticles as anode material for Lithium-Ion battery. ACS Appl. Mater. Interfaces 2018, 10, 44924–44931. [Google Scholar] [CrossRef]
- Kang, S.; Yang, K.; White, S.R.; Sottos, N.R. Silicon composite electrodes with dynamic ionic bonding. Adv. Energy Mater. 2017, 7, 1700045. [Google Scholar] [CrossRef]
- Tan, T.; Lee, P.-K.; Zettsu, N.; Teshima, K.; Yu, D.Y.W. Highly stable lithium-ion battery anode with polyimide coating anchored onto micron-size silicon monoxide via self-assembled monolayer. J. Power Sources 2020, 453, 227874. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, C.; Liu, G. Recent Applications of Molecular Structures at Silicon Anode Interfaces. Electrochem 2021, 2, 664-676. https://doi.org/10.3390/electrochem2040041
Fang C, Liu G. Recent Applications of Molecular Structures at Silicon Anode Interfaces. Electrochem. 2021; 2(4):664-676. https://doi.org/10.3390/electrochem2040041
Chicago/Turabian StyleFang, Chen, and Gao Liu. 2021. "Recent Applications of Molecular Structures at Silicon Anode Interfaces" Electrochem 2, no. 4: 664-676. https://doi.org/10.3390/electrochem2040041
APA StyleFang, C., & Liu, G. (2021). Recent Applications of Molecular Structures at Silicon Anode Interfaces. Electrochem, 2(4), 664-676. https://doi.org/10.3390/electrochem2040041






