Graphene Quantum Dots-Based Nanocomposites Applied in Electrochemical Sensors: A Recent Survey
Abstract
:1. Introduction
2. Graphene Quantum Dots Synthesis
2.1. Top-Down
2.2. Bottom-Up
2.3. GQDs Doping and Functionalization
3. Strategies for Producing GQDs-Based Nanocomposites
3.1. Electrodeposition
3.2. Electrospinning
3.3. Hydrothermal/Solvothermal Synthesis
3.4. Co-Precipitation Method
3.5. Other Strategies
4. Physicochemical Characterization of GQDs
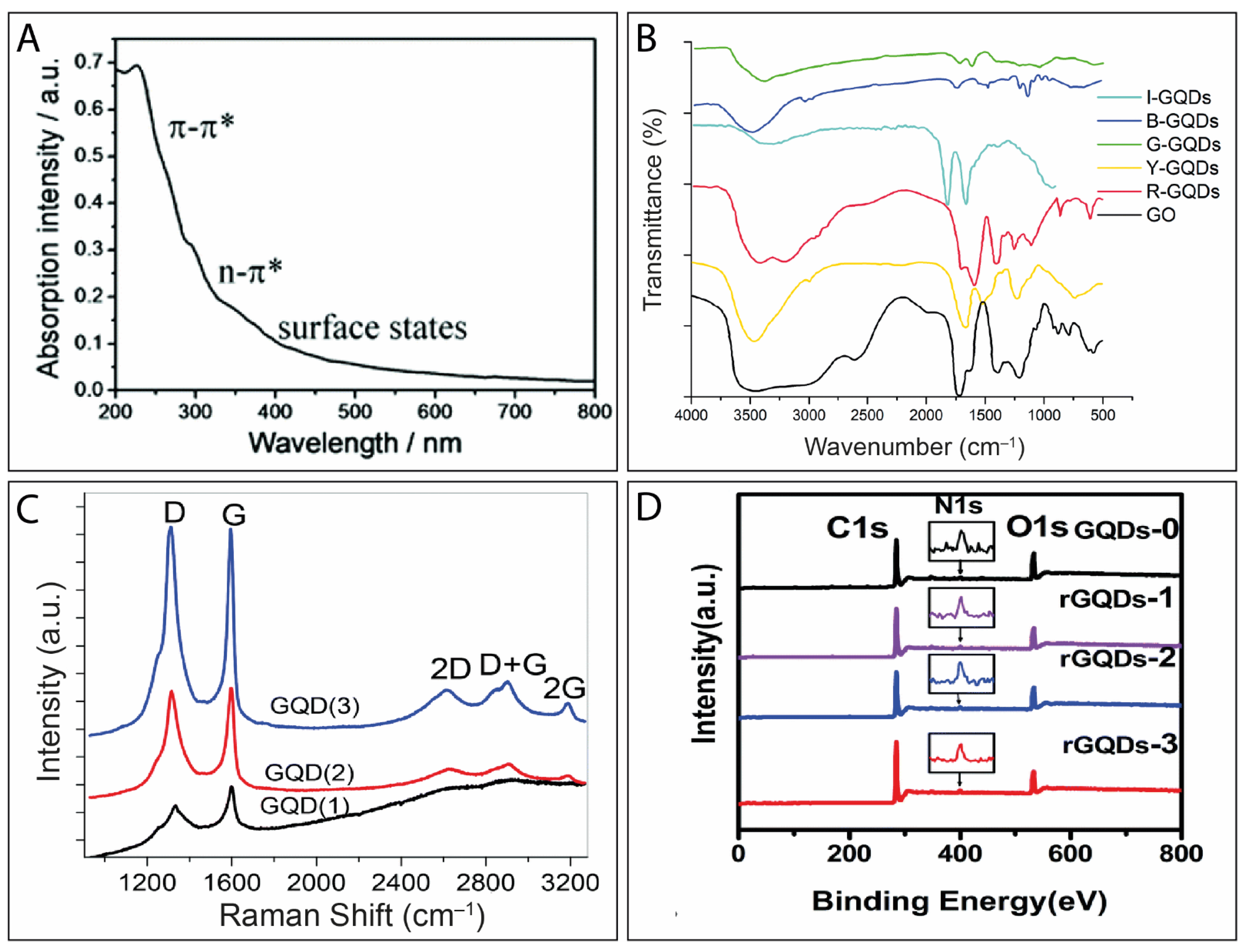

5. Electrochemical Performance of GQDs
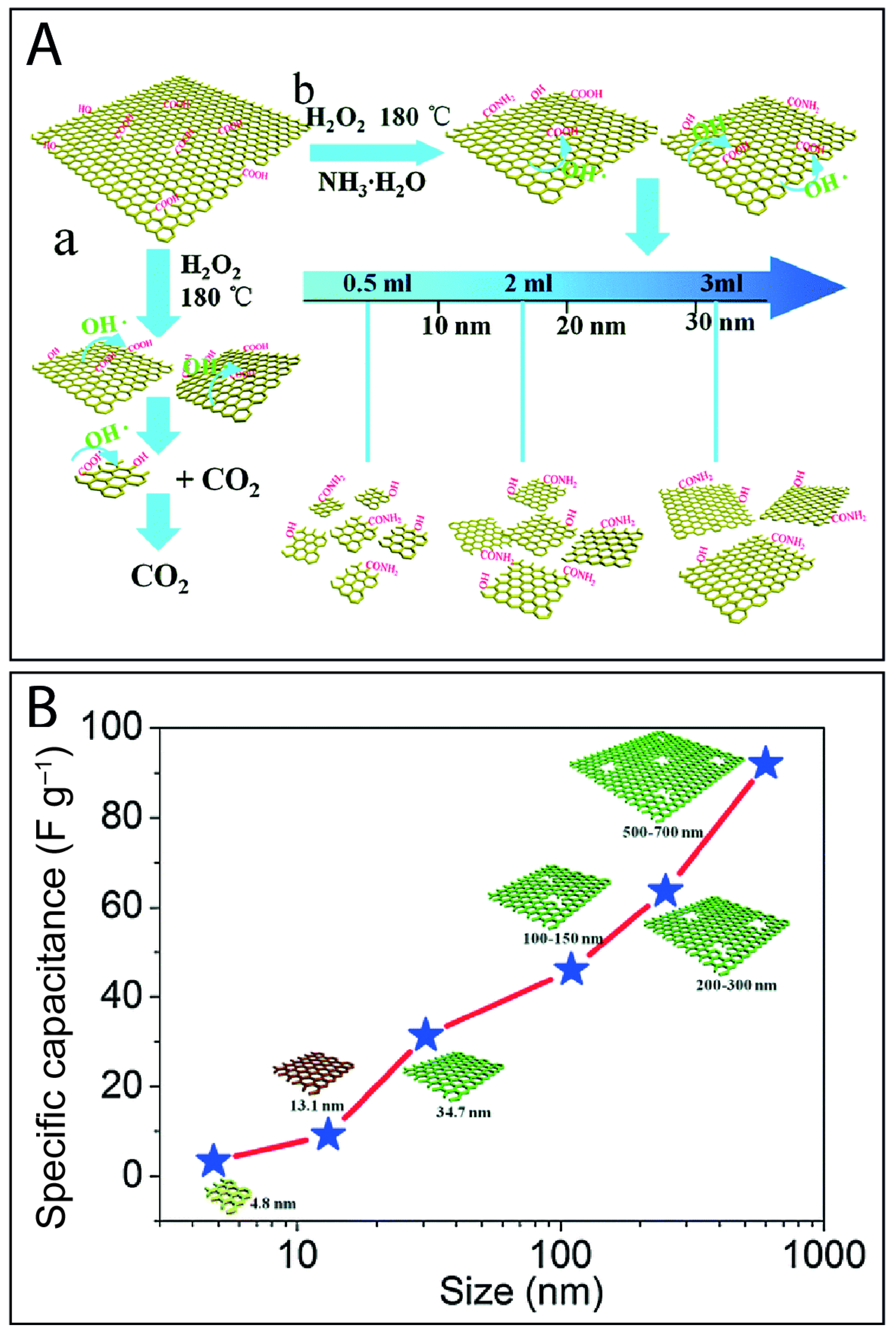
5.1. Effect of the Edges of GQDs on Their Electrochemical Performance
5.2. Effect of the Size of GQDs in Their Electrochemical Performance
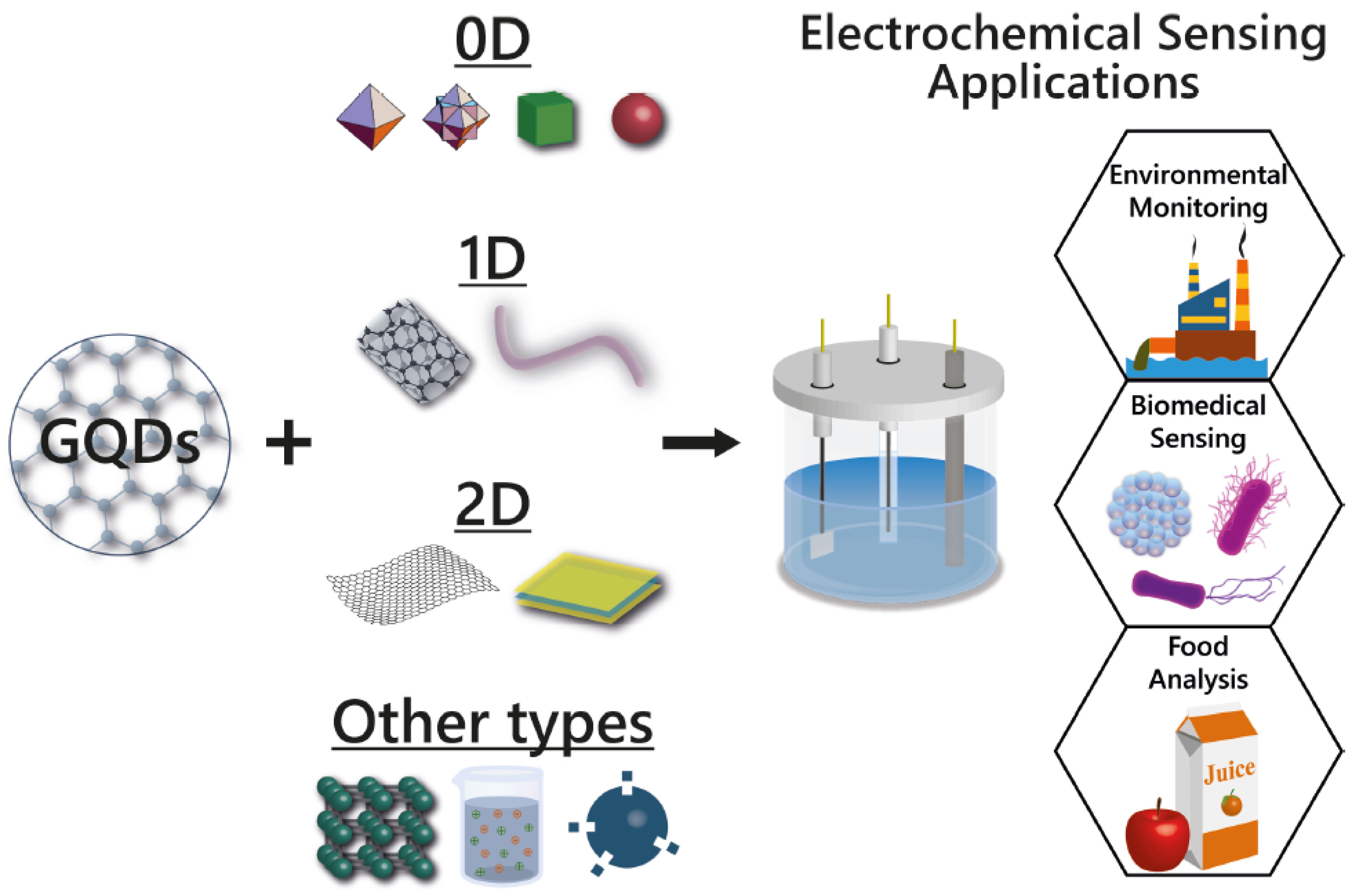
6. GQDs-Based Nanocomposites Applied in Electrochemical Sensors
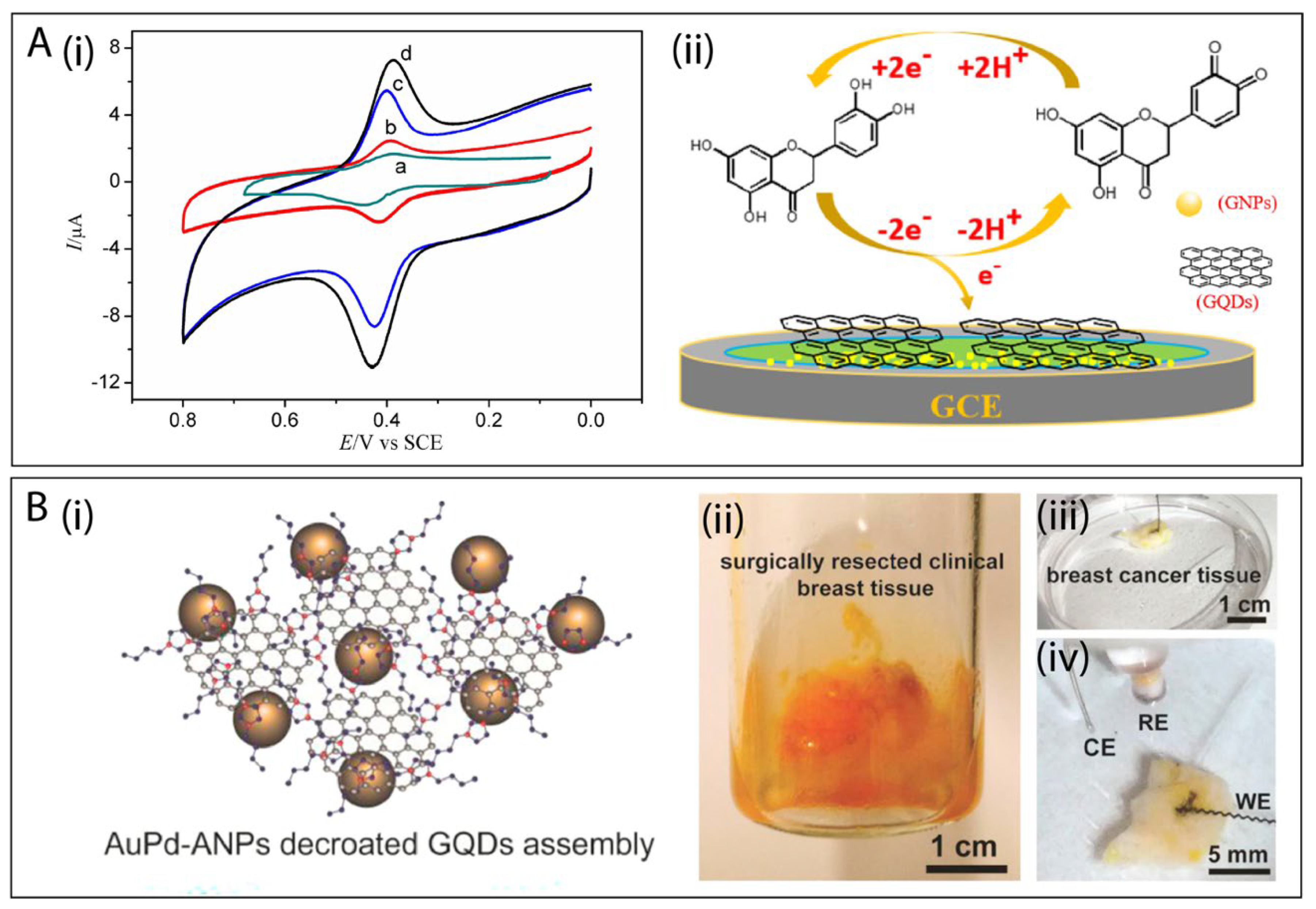
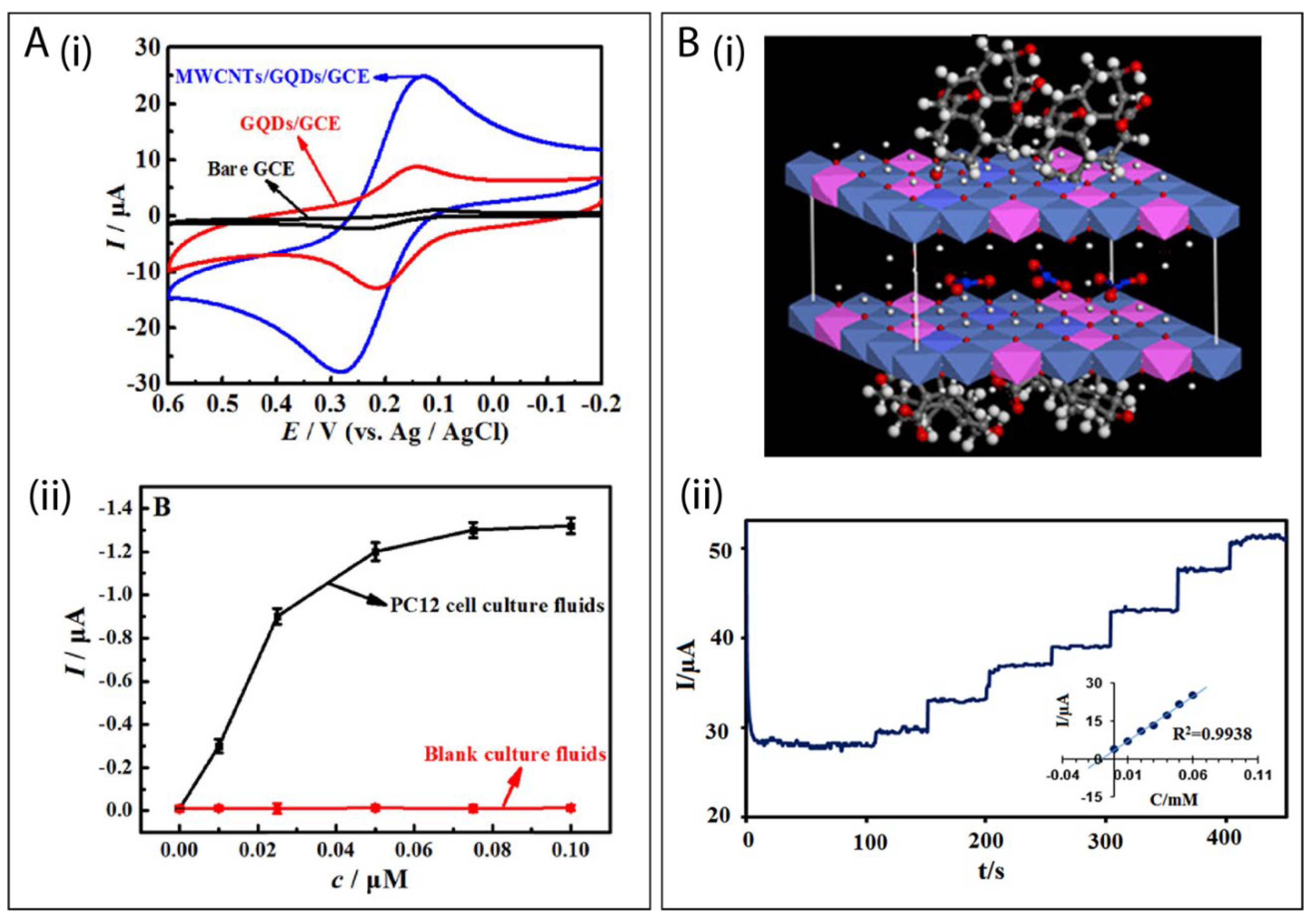
6.1. GQDs Combined with 0D Nanomaterials
6.2. GQDs Combined with 1D Nanomaterials
6.3. GQDs Combined with 2D Nanomaterials
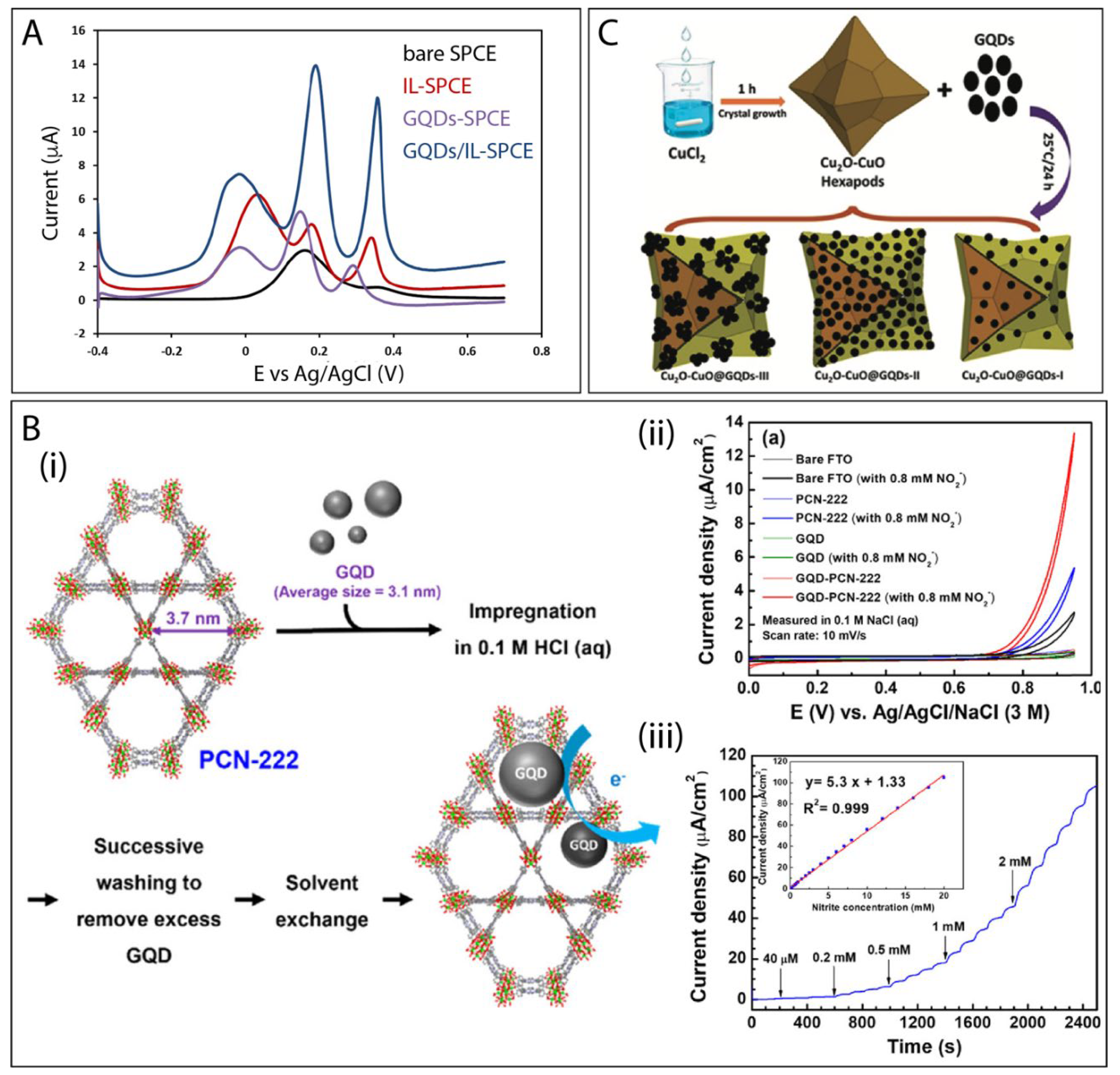
6.4. GQDs Combined with Other Types of Materials
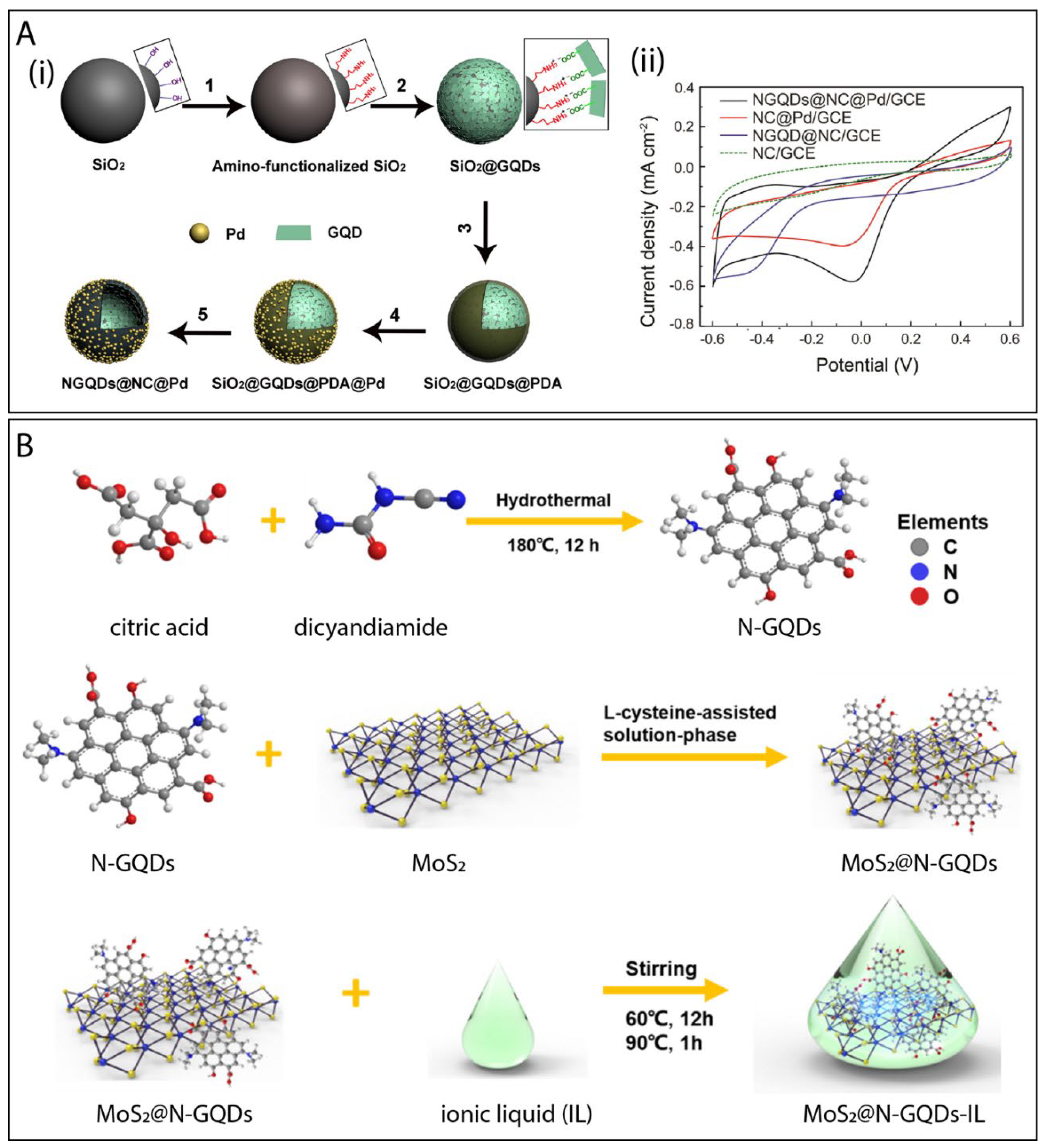
6.5. GQDs Combined with More Than One Type of Material
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Mao, S.; Pu, H.; Chen, J. Graphene oxide and its reduction: Modeling and experimental progress. RSC Adv. 2012, 2, 2643–2662. [Google Scholar] [CrossRef]
- Ponomarenko, L.A.; Schedin, F.; Katsnelson, M.I.; Yang, R.; Hill, E.W.; Novoselov, K.S.; Geim, A.K. Chaotic Dirac Billiard in Graphene Quantum Dots. Science 2008, 320, 356–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facure, M.H.M.; Schneider, R.; Mercante, L.A.; Correa, D.S. A review on graphene quantum dots and their nanocomposites: From laboratory synthesis towards agricultural and environmental applications. Environ. Sci. Nano 2020, 7, 3710–3734. [Google Scholar] [CrossRef]
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent Advances on Graphene Quantum Dots: From Chemistry and Physics to Applications. Adv. Mater. 2019, 31, 1808283. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef]
- Haque, E.; Kim, J.; Malgras, V.; Reddy, K.R.; Ward, A.C.; You, J.; Bando, Y.; Hossain, M.S.A.; Yamauchi, Y. Recent Advances in Graphene Quantum Dots: Synthesis, Properties, and Applications. Small Methods 2018, 2, 1800050. [Google Scholar] [CrossRef]
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Choudhary, R.P.; Shukla, S.; Vaibhav, K.; Pawar, P.B.; Saxena, S. Optical properties of few layered graphene quantum dots. Mater. Res. Express 2015, 2, 95024. [Google Scholar] [CrossRef]
- Jin, S.H.; Kim, D.H.; Jun, G.H.; Hong, S.H.; Jeon, S. Tuning the Photoluminescence of Graphene Quantum Dots through the Charge Transfer Effect of Functional Groups. ACS Nano 2013, 7, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Peng, Z.; Metzger, A.; Lin, J.; Mann, J.A.; Huang, K.; Xiang, C.; Fan, X.; Samuel, E.L.G.; Alemany, L.B.; et al. Bandgap Engineering of Coal-Derived Graphene Quantum Dots. ACS Appl. Mater. Interfaces 2015, 7, 7041–7048. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, H.; Elhaes, H.; Ibrahim, M.A. Tuning electronic properties in graphene quantum dots by chemical functionalization: Density functional theory calculations. Chem. Phys. Lett. 2018, 695, 138–148. [Google Scholar] [CrossRef]
- Lim, C.S.; Hola, K.; Ambrosi, A.; Zboril, R.; Pumera, M. Graphene and carbon quantum dots electrochemistry. Electrochem. Commun. 2015, 52, 75–79. [Google Scholar] [CrossRef]
- Faridbod, F.; Sanati, A.L. Graphene Quantum Dots in Electrochemical Sensors/Biosensors. Curr. Anal. Chem. 2019, 15, 103–123. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon Dots and Graphene Quantum Dots in Electrochemical Biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef] [Green Version]
- Pumera, M.; Ambrosi, A.; Bonanni, A.; Chng, E.L.K.; Poh, H.L. Graphene for electrochemical sensing and biosensing. TrAC Trends Anal. Chem. 2010, 29, 954–965. [Google Scholar] [CrossRef]
- Stetter, J.R.; Penrose, W.R.; Yao, S. Sensors, Chemical Sensors, Electrochemical Sensors, and ECS. J. Electrochem. Soc. 2003, 150, S11. [Google Scholar] [CrossRef]
- Wu, S.; He, Q.; Tan, C.; Wang, Y.; Zhang, H. Graphene-Based Electrochemical Sensors. Small 2013, 9, 1160–1172. [Google Scholar] [CrossRef]
- Asadian, E.; Ghalkhani, M.; Shahrokhian, S. Electrochemical sensing based on carbon nanoparticles: A review. Sens. Actuators B Chem. 2019, 293, 183–209. [Google Scholar] [CrossRef]
- Jadon, N.; Jain, R.; Sharma, S.; Singh, K. Recent trends in electrochemical sensors for multianalyte detection—A review. Talanta 2016, 161, 894–916. [Google Scholar] [CrossRef]
- Benítez-Martínez, S.; Valcárcel, M. Graphene quantum dots in analytical science. Trends Anal. Chem. 2015, 72, 93–113. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots for sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Xie, R.; Wang, Z.; Zhou, W.; Liu, Y.; Fan, L.; Li, Y.; Li, X. Graphene quantum dots as smart probes for biosensing. Anal. Methods 2016, 8, 4001–4006. [Google Scholar] [CrossRef]
- Jie, G.; Zhou, Q.; Jie, G. Graphene quantum dots-based electrochemiluminescence detection of DNA using multiple cycling amplification strategy. Talanta 2019, 194, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Adsetts, J.R.; Nie, Y.; Sun, X.; Ding, Z. Electrochemiluminescence of nitrogen- and sulfur-doped graphene quantum dots. Carbon N. Y. 2018, 129, 45–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Zhou, X.; Wu, X.; Yang, Y.; Wu, H.; Guo, S.; Zhang, J. Graphene quantum dots/gold electrode and its application in living cell H2O2 detection. Nanoscale 2013, 5, 1816–1819. [Google Scholar] [CrossRef]
- Zheng, A.-X.; Cong, Z.-X.; Wang, J.-R.; Li, J.; Yang, H.-H.; Chen, G.-N. Highly-efficient peroxidase-like catalytic activity of graphene dots for biosensing. Biosens. Bioelectron. 2013, 49, 519–524. [Google Scholar] [CrossRef]
- Nirala, N.R.; Khandelwal, G.; Kumar, B.; Vinita; Prakash, R.; Kumar, V. One step electro-oxidative preparation of graphene quantum dots from wood charcoal as a peroxidase mimetic. Talanta 2017, 173, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Nirala, N.R.; Abraham, S.; Kumar, V.; Bansal, A.; Srivastava, A.; Saxena, P.S. Colorimetric detection of cholesterol based on highly efficient peroxidase mimetic activity of graphene quantum dots. Sens. Actuators B Chem. 2015, 218, 42–50. [Google Scholar] [CrossRef]
- Ballesteros, C.A.S.; Mercante, L.; Alvarenga, A.; Moreira Facure, M.H.; Schneider, R.; Correa, D. Recent trends in nanozymes design: From materials and structures to environmental applications. Mater. Chem. Front. 2021. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon Nanozymes: Enzymatic Properties, Catalytic Mechanism, and Applications. Angew. Chem. Int. Ed. 2018, 57, 9224–9237. [Google Scholar] [CrossRef]
- Sun, H.; Ren, J.; Qu, X. Carbon-based Nanozeymes BT—Nanozymology: Connecting Biology and Nanotechnology. In Nanozymology; Yan, X., Ed.; Springer: Singapore, 2020; pp. 171–193. ISBN 978-981-15-1490-6. [Google Scholar]
- Garg, B.; Bisht, T. Carbon Nanodots as Peroxidase Nanozymes for Biosensing. Molecules 2016, 21, 1653. [Google Scholar] [CrossRef]
- Chen, W.; Lv, G.; Hu, W.; Li, D.; Chen, S.; Dai, Z. Synthesis and applications of graphene quantum dots: A review. Nanotechnol. Rev. 2018, 7, 157–185. [Google Scholar] [CrossRef]
- Kundu, S.; Pillai, V.K. Synthesis and characterization of graphene quantum dots. Phys. Sci. Rev. 2019, 5, 1–35. [Google Scholar] [CrossRef]
- Ozhukil Valappil, M.; Pillai, V.K.; Alwarappan, S. Spotlighting graphene quantum dots and beyond: Synthesis, properties and sensing applications. Appl. Mater. Today 2017, 9, 350–371. [Google Scholar] [CrossRef]
- Bacon, M.; Bradley, S.J.; Nann, T. Graphene quantum dots. Part. Syst. Charact. 2014, 31, 415–428. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Asadi, E.; Fassadi Chimeh, A.; Hosseini, S.; Rahimi, S.; Sarkhosh, B.; Bazli, L.; Bashiri, R.; Vakili Tahmorsati, A.H. A Review of Clinical Applications of Graphene Quantum Dot-based Composites. J. Compos. Compd. 2019, 1, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Jiang, G.; Li, G.; Zhu, J.; Xiao, M.; Zhu, Y.; Gao, R.; Yu, A.; Feng, M.; et al. Graphene Quantum Dots-Based Advanced Electrode Materials: Design, Synthesis and Their Applications in Electrochemical Energy Storage and Electrocatalysis. Adv. Energy Mater. 2020, 10, 2001275. [Google Scholar] [CrossRef]
- Sun, H.; Ji, H.; Ju, E.; Guan, Y.; Ren, J.; Qu, X. Synthesis of Fluorinated and Nonfluorinated Graphene Quantum Dots through a New Top-Down Strategy for Long-Time Cellular Imaging. Chem. Eur. J. 2015, 21, 3791–3797. [Google Scholar] [CrossRef]
- Zhu, X.; Zuo, X.; Hu, R.; Xiao, X.; Liang, Y.; Nan, J. Hydrothermal synthesis of two photoluminescent nitrogen-doped graphene quantum dots emitted green and khaki luminescence. Mater. Chem. Phys. 2014, 147, 963–967. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, S.; Li, C.; Luo, P.; Tao, L.; Wei, Y.; Shi, G. Large scale preparation of graphene quantum dots from graphite with tunable fluorescence properties. Phys. Chem. Chem. Phys. 2013, 15, 9907–9913. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, F.; Wu, C.; Guo, T. Optical properties of fluorescent zigzag graphene quantum dots derived from multi-walled carbon nanotubes. Appl. Phys. Lett. 2014, 104, 63109. [Google Scholar] [CrossRef]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Zhao, C.; Yu, Y.; Yang, J. Graphene quantum dots derived from carbon fibers for oxidation of dopamine. J. Wuhan Univ. Technol. Sci. Ed. 2016, 31, 1294–1297. [Google Scholar] [CrossRef]
- Pan, D.; Guo, L.; Zhang, J.; Xi, C.; Xue, Q.; Huang, H.; Li, J.; Zhang, Z.; Yu, W.; Chen, Z.; et al. Cutting sp 2 clusters in graphene sheets into colloidal graphene quantum dots with strong green fluorescence. J. Mater. Chem. 2012, 22, 3314–3318. [Google Scholar] [CrossRef]
- He, M.; Guo, X.; Huang, J.; Shen, H.; Zeng, Q.; Wang, L. Mass production of tunable multicolor graphene quantum dots from an energy resource of coke by a one-step electrochemical exfoliation. Carbon N. Y. 2018, 140, 508–520. [Google Scholar] [CrossRef]
- Chu, K.; Adsetts, J.R.; He, S.; Zhan, Z.; Yang, L.; Wong, J.M.; Love, D.A.; Ding, Z. Electrogenerated Chemiluminescence and Electroluminescence of N-Doped Graphene Quantum Dots Fabricated from an Electrochemical Exfoliation Process in Nitrogen-Containing Electrolytes. Chem. Eur. J. 2020, 26, 15892–15900. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, G.; Jiang, H.; Chen, L.; Zhang, X. One-step ultrasonic synthesis of graphene quantum dots with high quantum yield and their application in sensing alkaline phosphatase. Chem. Commun. 2015, 51, 948–951. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, Y.; Song, L.; Liu, X.; Hu, Z. Microwave assisted one-pot synthesis of graphene quantum dots as highly sensitive fluorescent probes for detection of iron ions and pH value. Talanta 2016, 150, 54–60. [Google Scholar] [CrossRef]
- Li, L.-L.; Ji, J.; Fei, R.; Wang, C.-Z.; Lu, Q.; Zhang, J.-R.; Jiang, L.-P.; Zhu, J.-J. A Facile Microwave Avenue to Electrochemiluminescent Two-Color Graphene Quantum Dots. Adv. Funct. Mater. 2012, 22, 2971–2979. [Google Scholar] [CrossRef]
- Zhuo, S.; Shao, M.; Lee, S.-T. Upconversion and Downconversion Fluorescent Graphene Quantum Dots: Ultrasonic Preparation and Photocatalysis. ACS Nano 2012, 6, 1059–1064. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Wang, C.; Wu, X.; Yang, Y.; Zheng, B.; Wu, H.; Guo, S.; Zhang, J. Photo-Fenton Reaction of Graphene Oxide: A New Strategy to Prepare Graphene Quantum Dots for DNA Cleavage. ACS Nano 2012, 6, 6592–6599. [Google Scholar] [CrossRef]
- Oh, S.D.; Kim, J.; Lee, D.H.; Kim, J.H.; Jang, C.W.; Kim, S.; Choi, S.-H. Structural and optical characteristics of graphene quantum dots size-controlled and well-aligned on a large scale by polystyrene-nanosphere lithography. J. Phys. D Appl. Phys. 2015, 49, 25308. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.G.; Cole, I.; Li, Q. Structural evolution of graphene quantum dots during thermal decomposition of citric acid and the corresponding photoluminescence. Carbon N. Y. 2015, 82, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, T.; Shokri, M. A new humidity sensor based upon graphene quantum dots prepared via carbonization of citric acid. Sens. Actuators B Chem. 2016, 222, 728–734. [Google Scholar] [CrossRef]
- Wu, X.; Tian, F.; Wang, W.; Chen, J.; Wu, M.; Zhao, J.X. Fabrication of highly fluorescent graphene quantum dots using l-glutamic acid for in vitro/in vivo imaging and sensing. J. Mater. Chem. C 2013, 1, 4676–4684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallaj, T.; Amjadi, M.; Manzoori, J.L.; Shokri, R. Chemiluminescence reaction of glucose-derived graphene quantum dots with hypochlorite, and its application to the determination of free chlorine. Microchim. Acta 2015, 182, 789–796. [Google Scholar] [CrossRef]
- Shehab, M.; Ebrahim, S.; Soliman, M. Graphene quantum dots prepared from glucose as optical sensor for glucose. J. Lumin. 2017, 184, 110–116. [Google Scholar] [CrossRef]
- Zhou, L.; Geng, J.; Liu, B. Graphene Quantum Dots from Polycyclic Aromatic Hydrocarbon for Bioimaging and Sensing of Fe3+ and Hydrogen Peroxide. Part. Syst. Charact. 2013, 30, 1086–1092. [Google Scholar] [CrossRef]
- Chua, C.K.; Sofer, Z.; Šimek, P.; Jankovský, O.; Klímová, K.; Bakardjieva, S.; Hrdličková Kučková, Š.; Pumera, M. Synthesis of Strongly Fluorescent Graphene Quantum Dots by Cage-Opening Buckminsterfullerene. ACS Nano 2015, 9, 2548–2555. [Google Scholar] [CrossRef]
- Kaciulis, S.; Mezzi, A.; Soltani, P.; Pizzoferrato, R.; Ciotta, E.; Prosposito, P. Graphene quantum dots obtained by unfolding fullerene. Thin Solid Films 2019, 673, 19–25. [Google Scholar] [CrossRef]
- Kumar, S.; Aziz, S.K.T.; Girshevitz, O.; Nessim, G.D. One-Step Synthesis of N-Doped Graphene Quantum Dots from Chitosan as a Sole Precursor Using Chemical Vapor Deposition. J. Phys. Chem. C 2018, 122, 2343–2349. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, M.; Lee, X.; Zhang, R.; Wang, K.; Wei, J.; Zhong, M.; Wu, D.; Zhu, H. Direct Synthesis of Graphene Quantum Dots by Chemical Vapor Deposition. Part. Syst. Charact. 2013, 30, 764–769. [Google Scholar] [CrossRef]
- Gurrappa, I.; Binder, L. Electrodeposition of nanostructured coatings and their characterization—A review. Sci. Technol. Adv. Mater. 2008, 9, 43001. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Karimzadeh, A.; Shadjou, N.; Mokhtarzadeh, A.; Bageri, L.; Sadeghi, S.; Mahboob, S. Graphene quantum dots decorated with magnetic nanoparticles: Synthesis, electrodeposition, characterization and application as an electrochemical sensor towards determination of some amino acids at physiological pH. Mater. Sci. Eng. C 2016, 68, 814–830. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, D.; Scavetta, E.; Gualandi, I. Electrochemical Deposition of Nanomaterials for Electrochemical Sensing. Sensors 2019, 19, 1186. [Google Scholar] [CrossRef] [Green Version]
- Oviedo, O.A.; Negre, C.F.A.; Mariscal, M.M.; Sánchez, C.G.; Leiva, E.P.M. Underpotential deposition on free nanoparticles: Its meaning and measurement. Electrochem. Commun. 2012, 16, 1–5. [Google Scholar] [CrossRef]
- Oviedo, O.A.; Vélez, P.; Macagno, V.A.; Leiva, E.P.M. Underpotential deposition: From planar surfaces to nanoparticles. Surf. Sci. 2015, 631, 23–34. [Google Scholar] [CrossRef]
- Shamsi, A.; Ahour, F.; Sehatnia, B. Nickel oxide nanoparticles decorated graphene quantum dot as an effective electrode modifier for electrocatalytic oxidation and analysis of clozapine. J. Solid State Electrochem. 2018, 22, 2681–2689. [Google Scholar] [CrossRef]
- Xu, Q.; Yuan, H.; Dong, X.; Zhang, Y.; Asif, M.; Dong, Z.; He, W.; Ren, J.; Sun, Y.; Xiao, F. Dual nanoenzyme modified microelectrode based on carbon fiber coated with AuPd alloy nanoparticles decorated graphene quantum dots assembly for electrochemical detection in clinic cancer samples. Biosens. Bioelectron. 2018, 107, 153–162. [Google Scholar] [CrossRef]
- Li, J.; Qu, J.; Yang, R.; Qu, L.; de B. Harrington, P. A Sensitive and Selective Electrochemical Sensor Based on Graphene Quantum Dot/Gold Nanoparticle Nanocomposite Modified Electrode for the Determination of Quercetin in Biological Samples. Electroanalysis 2016, 28, 1322–1330. [Google Scholar] [CrossRef]
- Andre, R.S.; Mercante, L.A.; Facure, M.H.M.; Pavinatto, A.; Correa, D.S. 8—Electrospun composite nanofibers as sensors for food analysis. Electrospun Polym. Compos. 2021, 261–286. [Google Scholar] [CrossRef]
- Correa, D.S.; Mercante, L.A.; Schneider, R.; Facure, M.H.M.; Locilento, D.A. Composite Nanofibers for Removing Water Pollutants: Fabrication Techniques BT—Handbook of Ecomaterials. In Handbook of Ecomaterial; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–29. ISBN 978-3-319-68255-6. [Google Scholar]
- Mercante, L.A.; Scagion, V.P.; Migliorini, F.L.; Mattoso, L.H.C.C.; Correa, D.S. Electrospinning-based (bio)sensors for food and agricultural applications: A review. Trends Anal. Chem. 2017, 91, 91–103. [Google Scholar] [CrossRef]
- Correa, D.S.; Pavinatto, A.; Mercante, L.A.; Mattoso, L.H.C.; Oliveira, J.E.; Riul, A. Chemical sensors based on hybrid nanomaterials for food analysis. Nanobiosensors 2017, 205–244. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Li, C.-W.; Nguyen, N.C.; Nguyen, H.T. A comprehensive review: Electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Adv. 2016, 6, 85495–85514. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, Z.; Sheng, J.; Al-Deyab, S.S.; Yu, J.; Ding, B. Superamphiphobic nanofibrous membranes for effective filtration of fine particles. J. Colloid Interface Sci. 2014, 428, 41–48. [Google Scholar] [CrossRef]
- Wang, G.; Shi, G.; Chen, X.; Yao, R.; Chen, F. A glassy carbon electrode modified with grapheme quantum dots and silver nanoparticles for simultaneous determination of guanine and adenine. Microchim. Acta 2015, 182, 315–322. [Google Scholar] [CrossRef]
- Mahmoudi-Moghaddam, H.; Tajik, S.; Beitollahi, H. A new electrochemical DNA biosensor based on modified carbon paste electrode using graphene quantum dots and ionic liquid for determination of topotecan. Microchem. J. 2019, 150, 104085. [Google Scholar] [CrossRef]
- Talari, F.F.; Bozorg, A.; Faridbod, F.; Vossoughi, M. A novel sensitive aptamer-based nanosensor using rGQDs and MWCNTs for rapid detection of diazinon pesticide. J. Environ. Chem. Eng. 2021, 9, 104878. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, X.; Ji, Y.; Ouyang, Z.; Wen, X.; Li, J.; Su, Z.; Wei, G. Electrospinning graphene quantum dots into a nanofibrous membrane for dual-purpose fluorescent and electrochemical biosensors. J. Mater. Chem. B 2015, 3, 2487–2496. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Yan, T.; Jiang, Z.; Wu, W.; Fang, T. A review: Conventional and supercritical hydro/solvothermal synthesis of ultrafine particles as cathode in lithium battery. Ceram. Int. 2018, 44, 4521–4537. [Google Scholar] [CrossRef]
- Feng, S.-H.; Li, G.-H. Hydrothermal and Solvothermal Syntheses. In Modern Inorganic Synthetic Chemistry, 2nd ed.; Xu, R., Xu, Y.B.T., Eds.; Elsevier: Amsterdam, The Netherland, 2017; Chapter 4; pp. 73–104. ISBN 978-0-444-63591-4. [Google Scholar]
- Tetsuka, H.; Asahi, R.; Nagoya, A.; Okamoto, K.; Tajima, I.; Ohta, R.; Okamoto, A. Optically tunable amino-functionalized graphene quantum dots. Adv. Mater. 2012, 24, 5333–5338. [Google Scholar] [CrossRef]
- Emran, M.Y.; Shenashen, M.A.; Abdelwahab, A.A.; Abdelmottaleb, M.; El-Safty, S.A. Facile synthesis of microporous sulfur-doped carbon spheres as electrodes for ultrasensitive detection of ascorbic acid in food and pharmaceutical products. New J. Chem. 2018, 42, 5037–5044. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Zong, J.; Zhang, J.; Li, C. One-pot hydrothermal synthesis of graphene quantum dots surface-passivated by polyethylene glycol and their photoelectric conversion under near-infrared light. New J. Chem. 2012, 36, 97–101. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Zhao, Y.; Zhou, Y.; Li, C. CdS nanoparticle-functionalized natural cotton cellulose electrospun nanofibers for visible light photocatalysis. Mater. Lett. 2015, 138, 89–91. [Google Scholar] [CrossRef]
- Zhang, T.; Gu, Y.; Li, C.; Yan, X.; Lu, N.; Liu, H.; Zhang, Z.; Zhang, H. Fabrication of Novel Electrochemical Biosensor Based on Graphene Nanohybrid to Detect H2O2 Released from Living Cells with Ultrahigh Performance. ACS Appl. Mater. Interfaces 2017, 9, 37991–37999. [Google Scholar] [CrossRef] [PubMed]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. In Micro and Nano Technologies; Mohan Bhagyaraj, S., Oluwafemi, O.S., Kalarikkal, N., Thomas, S.B.T., Eds.; Woodhead Publishing: Cambridge, UK, 2018; Chapter 5; pp. 121–139. ISBN 978-0-08-101975-7. [Google Scholar]
- Babooram, K. 28—Novel solution routes to ferroelectrics and relaxors. In Handbook of Advanced Dielectric, Piezoelectric and Ferroelectric Materials; Woodhead Publishing Series in Electronic and Optical Materials; Ye, Z.-G., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 852–883. ISBN 978-1-84569-186-8. [Google Scholar]
- Wu, X.; Zhang, Y.; Han, T.; Wu, H.; Guo, S.; Zhang, J. Composite of graphene quantum dots and Fe3O4 nanoparticles: Peroxidase activity and application in phenolic compound removal. RSC Adv. 2014, 4, 3299–3305. [Google Scholar] [CrossRef]
- Ramachandran, S.; Sathishkumar, M.; Kothurkar, N.K.; Senthilkumar, R. Synthesis and characterization of graphene quantum dots/cobalt ferrite nanocomposite. IOP Conf. Ser. Mater. Sci. Eng. 2018, 310, 12139. [Google Scholar] [CrossRef]
- Raeyani, D.; Shojaei, S.; Ahmadi-Kandjani, S. Optical graphene quantum dots gas sensors: Experimental study. Mater. Res. Express 2020, 7, 15608. [Google Scholar] [CrossRef]
- Arunragsa, S.; Seekaew, Y.; Pon-On, W.; Wongchoosuk, C. Hydroxyl edge-functionalized graphene quantum dots for gas-sensing applications. Diam. Relat. Mater. 2020, 105, 107790. [Google Scholar] [CrossRef]
- Yang, B.; Chen, J.; Cui, L.; Liu, W. Enhanced photocurrent of a ZnO nanorod array sensitized with graphene quantum dots. RSC Adv. 2015, 5, 59204–59207. [Google Scholar] [CrossRef]
- Kepić, D.P.; Marković, Z.M.; Jovanović, S.P.; Peruško, D.B.; Budimir, M.D.; Holclajtner-Antunović, I.D.; Pavlović, V.B.; Todorović Marković, B.M. Preparation of PEDOT:PSS thin films doped with graphene and graphene quantum dots. Synth. Met. 2014, 198, 150–154. [Google Scholar] [CrossRef]
- Zeng, Z.; Xiao, F.-X.; Gui, X.; Wang, R.; Liu, B.; Yang Tan, T.T. Layer-by-layer assembly of nitrogen-doped graphene quantum dots monolayer decorated one-dimensional semiconductor nanoarchitectures for solar-driven water splitting. J. Mater. Chem. A 2016, 4, 16383–16393. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Mokhtari, F.; Shadjou, N.; Eftekhari, A.; Mokhtarzadeh, A.; Jouyban-Gharamaleki, V.; Mahboob, S. Poly arginine-graphene quantum dots as a biocompatible and non-toxic nanocomposite: Layer-by-layer electrochemical preparation, characterization and non-invasive malondialdehyde sensory application in exhaled breath condensate. Mater. Sci. Eng. C 2017, 75, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Xiao, F.-X.; Phan, H.; Chen, S.; Yu, Z.; Wang, R.; Nguyen, T.-Q.; Yang Tan, T.T. Unraveling the cooperative synergy of zero-dimensional graphene quantum dots and metal nanocrystals enabled by layer-by-layer assembly. J. Mater. Chem. A 2018, 6, 1700–1713. [Google Scholar] [CrossRef]
- Xu, S.; Li, F.; Su, B.; Hu, M.Z.; Gao, X.; Gao, C. Novel graphene quantum dots (GQDs)-incorporated thin film composite (TFC) membranes for forward osmosis (FO) desalination. Desalination 2019, 451, 219–230. [Google Scholar] [CrossRef]
- Rahimi, K.; Yazdani, A.; Ahmadirad, M. Graphene quantum dots enhance UV photoresponsivity and surface-related sensing speed of zinc oxide nanorod thin films. Mater. Des. 2018, 140, 222–230. [Google Scholar] [CrossRef]
- Rojas-Andrade, M.D.; Nguyen, T.A.; Mistler, W.P.; Armas, J.; Lu, J.E.; Roseman, G.; Hollingsworth, W.R.; Nichols, F.; Millhauser, G.L.; Ayzner, A.; et al. Antimicrobial activity of graphene oxide quantum dots: Impacts of chemical reduction. Nanoscale Adv. 2020, 2, 1074–1083. [Google Scholar] [CrossRef]
- Udayabhaskar, R.; Mangalaraja, R.V.; Pandiyarajan, T.; Karthikeyan, B.; Mansilla, H.D.; Contreras, D. Spectroscopic investigation on graphene-copper nanocomposites with strong UV emission and high catalytic activity. Carbon N. Y. 2017, 124, 256–262. [Google Scholar] [CrossRef]
- Kadian, S.; Manik, G.; Kalkal, A.; Singh, M.; Chauhan, R.P. Effect of sulfur doping on fluorescence and quantum yield of graphene quantum dots: An experimental and theoretical investigation. Nanotechnology 2019, 30, 435704. [Google Scholar] [CrossRef]
- Zhu, S.; Shao, J.; Song, Y.; Zhao, X.; Du, J.; Wang, L.; Wang, H.; Zhang, K.; Zhang, J.; Yang, B. Investigating the surface state of graphene quantum dots. Nanoscale 2015, 7, 7927–7933. [Google Scholar] [CrossRef] [PubMed]
- Tabish, T.A.; Lin, L.; Ali, M.; Jabeen, F.; Ali, M.; Iqbal, R.; Horsell, D.W.; Winyard, P.G.; Zhang, S. Investigating the bioavailability of graphene quantum dots in lung tissues via Fourier transform infrared spectroscopy. Interface Focus 2018, 8, 20170054. [Google Scholar] [CrossRef]
- Chhabra, V.A.; Kaur, R.; Kumar, N.; Deep, A.; Rajesh, C.; Kim, K.-H. Synthesis and spectroscopic studies of functionalized graphene quantum dots with diverse fluorescence characteristics. RSC Adv. 2018, 8, 11446–11454. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wang, P.; Wang, F.; Fang, Y. Investigation of the microstructures of graphene quantum dots (GQDs) by surface-enhanced raman spectroscopy. Nanomaterials 2018, 8, 864. [Google Scholar] [CrossRef] [Green Version]
- Thang, P.N.; Hung, L.X.; Thuan, D.N.; Yen, N.H.; Hien, N.T.T.; Hanh, V.T.H.; Khang, N.C.; Laverdant, J.; Nga, P.T. Temperature-dependent Raman investigation and photoluminescence of graphene quantum dots with and without nitrogen-doping. J. Mater. Sci. 2021, 56, 4979–4990. [Google Scholar] [CrossRef]
- Dervishi, E.; Ji, Z.; Htoon, H.; Sykora, M.; Doorn, S.K. Raman spectroscopy of bottom-up synthesized graphene quantum dots: Size and structure dependence. Nanoscale 2019, 11, 16571–16581. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, J.; Liu, X.; Li, B.; Wang, X.; Tang, S.; Meng, Q.; Li, Y.; Shi, C.; Hu, R.; et al. Graphene quantum dots with controllable surface oxidation, tunable fluorescence and up-conversion emission. RSC Adv. 2012, 2, 2717–2720. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, W.; Wang, L.; Zhang, J.Z.; Li, Y.; Liu, X.; Li, Y. Optimizing oxygen functional groups in graphene quantum dots for improved antioxidant mechanism. Phys. Chem. Chem. Phys. 2019, 21, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, J.; Tang, S.; Qiao, C.; Wang, L.; Wang, H.; Liu, X.; Li, B.; Li, Y.; Yu, W.; et al. Surface chemistry routes to modulate the photoluminescence of graphene quantum dots: From fluorescence mechanism to up-conversion bioimaging applications. Adv. Funct. Mater. 2012, 22, 4732–4740. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S.; Luk, C.M.; Zeng, S.; Hao, J.; et al. Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano 2012, 6, 5102–5110. [Google Scholar] [CrossRef]
- Dejpasand, M.T.; Saievar-Iranizad, E.; Bayat, A.; Montaghemi, A.; Ardekani, S.R. Tuning HOMO and LUMO of three region (UV, Vis and IR) photoluminescent nitrogen doped graphene quantum dots for photodegradation of methylene blue. Mater. Res. Bull. 2020, 128, 110886. [Google Scholar] [CrossRef]
- Pierrat, P.; Gaumet, J.-J. Graphene quantum dots: Emerging organic materials with remarkable and tunable luminescence features. Tetrahedron Lett. 2020, 61, 152554. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Xie, W.; Wang, Y.; Jia, X.; Nawaz, A.; Song, M.; Gong, J.R. Preparation of blue- and green-emissive nitrogen-doped graphene quantum dots from graphite and their application in bioimaging. Mater. Sci. Eng. C 2021, 119, 111642. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zuo, Y.; Shi, Y.; Han, T.; Lanza, M. Transmission Electron Microscopy-Based Statistical Analysis of Commercially Available Graphene Oxide Quantum Dots. Cryst. Res. Technol. 2020, 55, 1900231. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Q.; Chen, D.; Liu, Z.; Zheng, X.; Xu, A.; Yang, S.; Ding, G. Facile and Highly Effective Synthesis of Controllable Lattice Sulfur-Doped Graphene Quantum Dots via Hydrothermal Treatment of Durian. ACS Appl. Mater. Interfaces 2018, 10, 5750–5759. [Google Scholar] [CrossRef]
- Liu, F.; Jang, M.-H.; Ha, H.D.; Kim, J.-H.; Cho, Y.-H.; Seo, T.S. Facile Synthetic Method for Pristine Graphene Quantum Dots and Graphene Oxide Quantum Dots: Origin of Blue and Green Luminescence. Adv. Mater. 2013, 25, 3657–3662. [Google Scholar] [CrossRef]
- Ritter, K.A.; Lyding, J.W. The influence of edge structure on the electronic properties of graphene quantum dots and nanoribbons. Nat. Mater. 2009, 8, 235–242. [Google Scholar] [CrossRef]
- Lu, H.F.; Zhang, M.M.; Wu, D.; Huang, J.L.; Zhu, L.L.; Wang, C.M.; Zhang, Q.L. Colorimetric and fluorescent dual-mode sensing of alkaline phosphatase activity in L-02 cells and its application in living cell imaging based on in-situ growth of silver nanoparticles on graphene quantum dots. Sens. Actuators B Chem. 2018, 258, 461–469. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.; Shin, Y.; Yoon, Y.; Kim, D.; Lee, H. Highly transparent and flexible supercapacitors using graphene-graphene quantum dots chelate. Nano Energy 2016, 26, 746–754. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, J.; Gao, K.; Chen, N.; Sun, X.; Ti, D.; Bai, C.; Cui, R.; Qu, L. Graphene quantum dots for energy storage and conversion: From fabrication to applications. Mater. Chem. Front. 2020, 4, 421–436. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, J.; Shen, W.; Zhong, M.; Guo, S. Graphene Quantum Dots Band Structure Tuned by Size for Efficient Organic Solar Cells. Phys. Status Solidi 2019, 216, 1900657. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Chang, K.; Peeters, F.M. Tuning of energy levels and optical properties of graphene quantum dots. Phys. Rev. B Condens. Matter Mater. Phys. 2008, 77, 235411. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Ruffieux, P.; Jaafar, R.; Bieri, M.; Braun, T.; Blankenburg, S.; Muoth, M.; Seitsonen, A.P.; Saleh, M.; Feng, X.; et al. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 2010, 466, 470–473. [Google Scholar] [CrossRef]
- Ruffieux, P.; Wang, S.; Yang, B.; Sanchez-Sanchez, C.; Liu, J.; Dienel, T.; Talirz, L.; Shinde, P.; Pignedoli, C.A.; Passerone, D.; et al. On-surface synthesis of graphene nanoribbons with zigzag edge topology. Nature 2016, 531, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Tamura, N.; Tomai, T.; Oka, N.; Honma, I. Capacity improvement of the carbon-based electrochemical capacitor by zigzag-edge introduced graphene. Appl. Surf. Sci. 2018, 428, 986–989. [Google Scholar] [CrossRef]
- Basak, T.; Basak, T. Theoretical investigation of electronic and optical properties of nitrogen doped triangular shaped graphene quantum dots. J. Phys. Condens. Matter 2020, 32. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Dervishi, E.; Doorn, S.K.; Sykora, M. Size-Dependent Electronic Properties of Uniform Ensembles of Strongly Confined Graphene Quantum Dots. J. Phys. Chem. Lett. 2019, 10, 953–959. [Google Scholar] [CrossRef]
- Ramana, L.N.; Dinh, L.N.M.; Agarwal, V. Influence of surface charge of graphene quantum dots on their uptake and clearance in melanoma cells. Nanoscale Adv. 2021. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Yang, Z.; Weber, J.K.; Liu, X.; Lu, S.; Meng, X.; Xu, J.Y. Particle Size-Dependent Antibacterial Activity and Murine Cell Cytotoxicity Induced by Graphene Oxide Nanomaterials. J. Nanomater. 2016, 2016, 6709764. [Google Scholar] [CrossRef] [Green Version]
- Deng, D.; Yu, L.; Pan, X.; Wang, S.; Chen, X.; Hu, P.; Sun, L.; Bao, X. Size effect of graphene on electrocatalytic activation of oxygen. Chem. Commun. 2011, 47, 10016. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Lang, J.; Yan, X. Insight into the formation mechanism of graphene quantum dots and the size effect on their electrochemical behaviors. Phys. Chem. Chem. Phys. 2015, 17, 14028–14035. [Google Scholar] [CrossRef]
- Ma, Z.; Sa, R.; Li, Q.; Wu, K. Interfacial electronic structure and charge transfer of hybrid graphene quantum dot and graphitic carbon nitride nanocomposites: Insights into high efficiency for photocatalytic solar water splitting. Phys. Chem. Chem. Phys. 2015, 18, 1050–1058. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L.; Ding, Z.; Hu, J.; Liu, J.; Liu, Y. Edge-functionalized graphene quantum dots as a thickness-insensitive cathode interlayer for polymer solar cells. Nano Res. 2018, 11, 4293–4301. [Google Scholar] [CrossRef]
- Sheely, A.; Gifford, B.; Tretiak, S.; Bishop, A. Tunable Optical Features of Graphene Quantum Dots from Edge Functionalization. J. Phys. Chem. C 2021, 125, 9244–9252. [Google Scholar] [CrossRef]
- Sun, J.; Yang, S.; Wang, Z.; Shen, H.; Xu, T.; Sun, L.; Li, H.; Chen, W.; Jiang, X.; Ding, G.; et al. Ultra-High Quantum Yield of Graphene Quantum Dots: Aromatic-Nitrogen Doping and Photoluminescence Mechanism. Part. Syst. Charact. 2015, 32, 434–440. [Google Scholar] [CrossRef]
- Sk, M.A.; Ananthanarayanan, A.; Huang, L.; Lim, K.H.; Chen, P. Revealing the tunable photoluminescence properties of graphene quantum dots. J. Mater. Chem. C 2014, 2, 6954–6960. [Google Scholar] [CrossRef]
- Kim, J.; Suh, J.S. Size-Controllable and Low-Cost Fabrication of Graphene Quantum Dots Using Thermal Plasma Jet. ACS Nano 2014, 8, 4190–4196. [Google Scholar] [CrossRef] [PubMed]
- Bressi, V.; Ferlazzo, A.; Iannazzo, D.; Espro, C. Graphene quantum dots by eco-friendly green synthesis for electrochemical sensing: Recent advances and future perspectives. Nanomaterials 2021, 11, 1120. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, A.; Hasanzadeh, M.; Shadjou, N.; Guardia, M. de la Electrochemical biosensing using N-GQDs: Recent advances in analytical approach. TrAC Trends Anal. Chem. 2018, 105, 484–491. [Google Scholar] [CrossRef]
- Tufa, L.T.; Oh, S.; Tran, V.T.; Kim, J.; Jeong, K.J.; Park, T.J.; Kim, H.J.; Lee, J. Electrochemical immunosensor using nanotriplex of graphene quantum dots, Fe3O4 and Ag nanoparticles for tuberculosis. Electrochim. Acta 2018, 290, 369–377. [Google Scholar] [CrossRef]
- Roushani, M.; Jalilian, Z.; Nezhadali, A. Screen printed carbon electrode sensor with thiol graphene quantum dots and gold nanoparticles for voltammetric determination of solatol. Heliyon 2019, 5, e01984. [Google Scholar] [CrossRef] [Green Version]
- Fajardo, A.; Tapia, D.; Pizarro, J.; Segura, R.; Jara, P. Determination of norepinephrine using a glassy carbon electrode modified with graphene quantum dots and gold nanoparticles by square wave stripping voltammetry. J. Appl. Electrochem. 2019, 49, 423–432. [Google Scholar] [CrossRef]
- Arumugasamy, S.K.; Govindaraju, S.; Yun, K. Electrochemical sensor for detecting dopamine using graphene quantum dots incorporated with multiwall carbon nanotubes. Appl. Surf. Sci. 2020, 508, 145294. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Tong, L.; Tong, Q.X. Graphene Quantum Dots/Multiwalled Carbon Nanotubes Composite-Based Electrochemical Sensor for Detecting Dopamine Release from Living Cells. ACS Sustain. Chem. Eng. 2020, 8, 1644–1650. [Google Scholar] [CrossRef]
- Samuei, S.; Fakkar, J.; Rezvani, Z.; Shomali, A.; Habibi, B. Synthesis and characterization of graphene quantum dots/CoNiAl-layered double-hydroxide nanocomposite: Application as a glucose sensor. Anal. Biochem. 2017, 521, 31–39. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chiang, W.H.; Kurniawan, D.; Yeh, P.C.; Otake, K.I.; Kung, C.W. Impregnation of Graphene Quantum Dots into a Metal-Organic Framework to Render Increased Electrical Conductivity and Activity for Electrochemical Sensing. ACS Appl. Mater. Interfaces 2019, 11, 35319–35326. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Rezayi, M.; Beheshti, H.R.; Boroushaki, M.T. Ultra-sensitive molecularly imprinted electrochemical sensor for patulin detection based on a novel assembling strategy using Au@Cu-MOF/N-GQDs. Sens. Actuators B Chem. 2020, 318, 128219. [Google Scholar] [CrossRef]
- Bali Prasad, B.; Kumar, A.; Singh, R. Synthesis of novel monomeric graphene quantum dots and corresponding nanocomposite with molecularly imprinted polymer for electrochemical detection of an anticancerous ifosfamide drug. Biosens. Bioelectron. 2017, 94, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; El-Wekil, M.M.; Mahnashi, M.H.; Ali, M.F.B.; Alkahtani, S.A. Modification of N,S co-doped graphene quantum dots with p-aminothiophenol-functionalized gold nanoparticles for molecular imprint-based voltammetric determination of the antiviral drug sofosbuvir. Microchim. Acta 2019, 186, 617. [Google Scholar] [CrossRef]
- Sanati, A.L.; Faridbod, F.; Ganjali, M.R. Synergic effect of graphene quantum dots and room temperature ionic liquid for the fabrication of highly sensitive voltammetric sensor for levodopa determination in the presence of serotonin. J. Mol. Liq. 2017, 241, 316–320. [Google Scholar] [CrossRef]
- Kunpatee, K.; Traipop, S.; Chailapakul, O.; Chuanuwatanakul, S. Simultaneous determination of ascorbic acid, dopamine, and uric acid using graphene quantum dots/ionic liquid modified screen-printed carbon electrode. Sens. Actuators B Chem. 2020, 314, 128059. [Google Scholar] [CrossRef]
- Tang, J.; Huang, R.; Zheng, S.; Jiang, S.; Yu, H.; Li, Z.; Wang, J. A sensitive and selective electrochemical sensor based on graphene quantum dots/gold nanoparticles nanocomposite modified electrode for the determination of luteolin in peanut hulls. Microchem. J. 2019, 145, 899–907. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, P.; Wang, C.; Yang, P.; Xie, Y.; Fei, J. Ultra-sensitive amperometric determination of quercetin by using a glassy carbon electrode modified with a nanocomposite prepared from aminated graphene quantum dots, thiolated β-cyclodextrin and gold nanoparticles. Microchim. Acta 2020, 187. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, A.; Ahour, F. Electrochemical Sensing of Thioridazine in Human Serum Samples Using Modified Glassy Carbon Electrode. Adv. J. Chem. A 2020, 4, 22–31. [Google Scholar]
- Mansuriya, B.D.; Altintas, Z. Enzyme-free electrochemical nano-immunosensor based on graphene quantum dots and gold nanoparticles for cardiac biomarker determination. Nanomaterials 2021, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, Y.; Xie, M.; Yang, Q.; Liu, T. The electrochemical behaviors and kinetics of AuNPs/N, S-GQDs composite electrode: A novel label-free amplified BPA aptasensor with extreme sensitivity and selectivity. J. Mol. Liq. 2020, 320, 114384. [Google Scholar] [CrossRef]
- Ahmadi, N.; Bagherzadeh, M.; Nemati, A. Comparison between electrochemical and photoelectrochemical detection of dopamine based on titania-ceria-graphene quantum dots nanocomposite. Biosens. Bioelectron. 2020, 151, 111977. [Google Scholar] [CrossRef]
- Trinadh, T.; Khuntia, H.; Anusha, T.; Bhavani, K.S.; Kumar, J.V.S.; Brahman, P.K. Synthesis and characterization of nanocomposite material based on graphene quantum dots and lanthanum doped zirconia nanoparticles: An electrochemical sensing application towards flutamide in urine samples. Diam. Relat. Mater. 2020, 110, 108143. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Pandey, M.K.; Rajesh; Sumana, G. Electrochemical Aflatoxin B1 immunosensor based on the use of graphene quantum dots and gold nanoparticles. Microchim. Acta 2019, 186, 592. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.; Wang, K.; Mao, H.; You, T. Quantitative detection of nitrite with N-doped graphene quantum dots decorated N-doped carbon nanofibers composite-based electrochemical sensor. Sen. Actuators B Chem. 2017, 252, 17–23. [Google Scholar] [CrossRef]
- Wu, M.; Zhu, J.; Ren, Y.; Yang, N.; Hong, Y.; Wang, W.; Huang, W.; Si, W.; Dong, X. NH2-GQDs-Doped Nickel-Cobalt Oxide Deposited on Carbon Cloth for Nonenzymatic Detection of Glucose. Adv. Mater. Interfaces 2020, 7, 2–7. [Google Scholar] [CrossRef]
- Mishra, P.; Bhat, B.R. A study on the electro-reductive cycle of amino-functionalized graphene quantum dots immobilized on graphene oxide for amperometric determination of oxalic acid. Microchim. Acta 2019, 186, 646. [Google Scholar] [CrossRef]
- Tan, F.; Cong, L.; Li, X.; Zhao, Q.; Zhao, H.; Quan, X.; Chen, J. An electrochemical sensor based on molecularly imprinted polypyrrole/graphene quantum dots composite for detection of bisphenol A in water samples. Sens. Actuators B Chem. 2016, 233, 599–606. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Doong, R.A. Functionalized N-doped graphene quantum dots for electrochemical determination of cholesterol through host-guest inclusion. Microchim. Acta 2018, 185, 562. [Google Scholar] [CrossRef]
- Arvand, M.; Hemmati, S. Analytical methodology for the electro-catalytic determination of estradiol and progesterone based on graphene quantum dots and poly(sulfosalicylic acid) co-modified electrode. Talanta 2017, 174, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Arvand, M.; Hemmati, S. Magnetic nanoparticles embedded with graphene quantum dots and multiwalled carbon nanotubes as a sensing platform for electrochemical detection of progesterone. Sens. Actuators B Chem. 2017, 238, 346–356. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, X.; Geng, L.; Wang, Y. Label-free electrochemical aptasensor for sensitive detection of malachite green based on au nanoparticle/graphene quantum dots/tungsten disulfide nanocomposites. Nanomaterials 2019, 9, 229. [Google Scholar] [CrossRef] [Green Version]
- Rao, H.; Zhao, X.; Liu, X.; Zhong, J.; Zhang, Z.; Zou, P.; Jiang, Y.; Wang, X.; Wang, Y. A novel molecularly imprinted electrochemical sensor based on graphene quantum dots coated on hollow nickel nanospheres with high sensitivity and selectivity for the rapid determination of bisphenol S. Biosens. Bioelectron. 2018, 100, 341–347. [Google Scholar] [CrossRef]
- Mirzaie, A.; Hasanzadeh, M.; Jouyban, A. Cross-linked chitosan/thiolated graphene quantum dots as a biocompatible polysaccharide towards aptamer immobilization. Int. J. Biol. Macromol. 2019, 123, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.M.; Wong, A.; Prado, T.M.; Fava, E.L.; Fatibello-Filho, O.; Sotomayor, M.D.P.T.; Moraes, F.C. Voltammetric determination of ethinylestradiol using screen-printed electrode modified with functionalized graphene, graphene quantum dots and magnetic nanoparticles coated with molecularly imprinted polymers. Talanta 2021, 224, 121804. [Google Scholar] [CrossRef] [PubMed]
- Axin Liang, A.; Huipeng Hou, B.; Shanshan Tang, C.; Liquan Sun, D.; Aiqin Luo, E. An advanced molecularly imprinted electrochemical sensor for the highly sensitive and selective detection and determination of Human IgG. Bioelectrochemistry 2021, 137, 107671. [Google Scholar] [CrossRef]
- Ghiasi, T.; Ahmadi, S.; Ahmadi, E.; Bavil Olyai, M.R.T.; Khodadadi, Z. Novel electrochemical sensor based on modified glassy carbon electrode with graphene quantum dots, chitosan and nickel molybdate nanocomposites for diazinon and optimal design by the Taguchi method. Microchem. J. 2021, 160, 105628. [Google Scholar] [CrossRef]
- Anusha, T.; Bhavani, K.S.; Shanmukha Kumar, J.V.; Brahman, P.K. Synthesis and characterization of novel lanthanum nanoparticles-graphene quantum dots coupled with zeolitic imidazolate framework and its electrochemical sensing application towards vitamin D3 deficiency. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 611, 125854. [Google Scholar] [CrossRef]
- Wang, L.; Tricard, S.; Yue, P.; Zhao, J.; Fang, J.; Shen, W. Polypyrrole and graphene quantum dots @ Prussian Blue hybrid film on graphite felt electrodes: Application for amperometric determination of l-cysteine. Biosens. Bioelectron. 2016, 77, 1112–1118. [Google Scholar] [CrossRef]
- Zhao, P.; Ni, M.; Chen, C.; Zhou, Z.; Li, X.; Li, C.; Xie, Y.; Fei, J. Stimuli-enabled switch-like paracetamol electrochemical sensor based on thermosensitive polymer and MWCNTs-GQDs composite nanomaterial. Nanoscale 2019, 11, 7394–7403. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Zhang, L.; Li, J.; Su, Z.; Wei, G. Sequence-Designed Peptide Nanofibers Bridged Conjugation of Graphene Quantum Dots with Graphene Oxide for High Performance Electrochemical Hydrogen Peroxide Biosensor. Adv. Mater. Interfaces 2017, 4, 1600895. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; Takemura, K.; Li, T.C.; Suzuki, T.; Park, E.Y. Electrical pulse-induced electrochemical biosensor for hepatitis E virus detection. Nat. Commun. 2019, 10, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Hu, T.; Liang, R.; Wei, M. Application of Zero-Dimensional Nanomaterials in Biosensing. Front. Chem. 2020, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, R.; Li, Z. Nanohybrid of Co3O4 and histidine-functionalized graphene quantum dots for electrochemical detection of hydroquinone. Electrochim. Acta 2017, 255, 323–334. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Es’haghi, Z. Electrochemical biosensing platform based on molecularly imprinted polymer reinforced by ZnO–graphene capped quantum dots for 6-mercaptopurine detection. Electrochim. Acta 2018, 283, 1170–1177. [Google Scholar] [CrossRef]
- Tran, H.V.; Le, T.A.; Giang, B.L.; Piro, B.; Tran, L.D. Silver nanoparticles on graphene quantum dots as nanozyme for efficient H2O2 reduction in a glucose biosensor. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Ashrafi, H.; Hassanpour, S.; Saadati, A.; Hasanzadeh, M.; Ansarin, K.; Ozkan, S.A.; Shadjou, N.; Jouyban, A. Sensitive detection and determination of benzodiazepines using silver nanoparticles-N-GQDs ink modified electrode: A new platform for modern pharmaceutical analysis. Microchem. J. 2019, 145, 1050–1057. [Google Scholar] [CrossRef]
- Yao, J.; Liu, C.; Liu, L.; Chen, M.; Yang, M. An Electrochemical Sensor for Sensitive Determination of L-cysteine and Its Electrochemical Kinetics on AgNPs/GQDs/GCE Composite Modified Electrode. J. Electrochem. Soc. 2018, 165, B551–B558. [Google Scholar] [CrossRef]
- Shahdost-fard, F.; Roushani, M. Designing an ultra-sensitive aptasensor based on an AgNPs/thiol-GQD nanocomposite for TNT detection at femtomolar levels using the electrochemical oxidation of Rutin as a redox probe. Biosens. Bioelectron. 2017, 87, 724–731. [Google Scholar] [CrossRef]
- Ting, S.L.; Ee, S.J.; Ananthanarayanan, A.; Leong, K.C.; Chen, P. Graphene quantum dots functionalized gold nanoparticles for sensitive electrochemical detection of heavy metal ions. Electrochim. Acta 2015, 172, 7–11. [Google Scholar] [CrossRef]
- Shadjou, N.; Saduooghi, E.; Hasanzadeh, M. Highly sensitive quantification of hydrogen-transmitting coenzyme in physiological pH using silver nanoparticles dispersed on nitrogen doped graphene quantum dots. Microchem. J. 2019, 144, 383–390. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Goncalves, A.; Pereira, S.; Branquinho, R.; Barquinha, P.; Fortunato, E.; Martins, R. Metal oxide nanostructures for sensor applications. Semicond. Sci. Technol. 2019, 34, 043001. [Google Scholar] [CrossRef] [Green Version]
- George, J.M.; Antony, A.; Mathew, B. Metal oxide nanoparticles in electrochemical sensing and biosensing: A review. Microchim. Acta 2018, 185, 358. [Google Scholar] [CrossRef] [PubMed]
- Noy, A.; Artyukhin, A.B.; Misra, N. Bionanoelectronics with 1D materials. Mater. Today 2009, 12, 22–31. [Google Scholar] [CrossRef]
- Nikolaou, P.; Valenti, G.; Paolucci, F. Nano-structured materials for the electrochemiluminescence signal enhancement. Electrochim. Acta 2021, 388, 138586. [Google Scholar] [CrossRef]
- Tian, C.; Wang, L.; Luan, F.; Zhuang, X. An electrochemiluminescence sensor for the detection of prostate protein antigen based on the graphene quantum dots infilled TiO2 nanotube arrays. Talanta 2019, 191, 103–108. [Google Scholar] [CrossRef]
- Su, S.; Chen, S.; Fan, C. Recent advances in two-dimensional nanomaterials-based electrochemical sensors for environmental analysis. Green Energy Environ. 2018, 3, 97–106. [Google Scholar] [CrossRef]
- Su, S.; Chao, J.; Pan, D.; Wang, L.; Fan, C. Electrochemical Sensors Using Two-Dimensional Layered Nanomaterials. Electroanalysis 2015, 27, 1062–1072. [Google Scholar] [CrossRef]
- Ping, J.; Fan, Z.; Sindoro, M.; Ying, Y.; Zhang, H. Recent Advances in Sensing Applications of Two-Dimensional Transition Metal Dichalcogenide Nanosheets and Their Composites. Adv. Funct. Mater. 2017, 27, 1605817. [Google Scholar] [CrossRef]
- Yu, C.; Yang, J.; Zhao, C.; Fan, X.; Wang, G.; Qiu, J. Nanohybrids from NiCoAl-LDH coupled with carbon for pseudocapacitors: Understanding the role of nano-structured carbon. Nanoscale 2014, 6, 3097–3104. [Google Scholar] [CrossRef]
- Sanati, S.; Abazari, R.; Morsali, A. Enhanced electrochemical oxygen and hydrogen evolution reactions using an NU-1000@NiMn-LDHS composite electrode in alkaline electrolyte. Chem. Commun. 2020, 56, 6652–6655. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Jiang, D.; Liu, Q.; Zhu, G.; Mao, H.; Wang, K. Fabrication of graphene oxide decorated with nitrogen-doped graphene quantum dots and its enhanced electrochemiluminescence for ultrasensitive detection of pentachlorophenol. Analyst 2015, 140, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, G.; Asif, M.; Aziz, A.; Dao, A.Q.; Zhang, T.; Iftikhar, T.; Wang, Q.; Liu, H. Facet-energy inspired metal oxide extended hexapods decorated with graphene quantum dots: Sensitive detection of bisphenol A in live cells. Nanoscale 2020, 12, 9014–9023. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.V.; Nigam, A.K.; Batra, A.; Boopathi, M.; Singh, B.; Vijayaraghavan, R. Applications of Ionic Liquids in Electrochemical Sensors and Biosensors. Int. J. Electrochem. 2012, 2012, 165683. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Ivaska, A. Applications of ionic liquids in electrochemical sensors. Anal. Chim. Acta 2008, 607, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Abo-Hamad, A.; Alsaadi, M.A.; Hayyan, M.; Juneidi, I.; Hashim, M.A. Ionic Liquid-Carbon Nanomaterial Hybrids for Electrochemical Sensor Applications: A Review. Electrochim. Acta 2016, 193, 321–343. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, J.; Li, S.; Guo, M.; Fan, Z. An electrochemical sensor for the detection of: P -nitrophenol based on a cyclodextrin-decorated gold nanoparticle-mesoporous carbon hybrid. Analyst 2019, 144, 4400–4406. [Google Scholar] [CrossRef]
- Wei, M.; Tian, D.; Liu, S.; Zheng, X.; Duan, S.; Zhou, C. β-Cyclodextrin functionalized graphene material: A novel electrochemical sensor for simultaneous determination of 2-chlorophenol and 3-chlorophenol. Sens. Actuators B Chem. 2014, 195, 452–458. [Google Scholar] [CrossRef]
- Zhu, G.; Yi, Y.; Chen, J. Recent advances for cyclodextrin-based materials in electrochemical sensing. TrAC Trends Anal. Chem. 2016, 80, 232–241. [Google Scholar] [CrossRef]
- Dong, S.; Bi, Q.; Qiao, C.; Sun, Y.; Zhang, X.; Lu, X.; Zhao, L. Electrochemical sensor for discrimination tyrosine enantiomers using graphene quantum dots and β-cyclodextrins composites. Talanta 2017, 173, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Bi, Q.; Qiao, J.; Shao, S.; Lu, X. Simultaneous determination of three nitroaniline isomers by β-cyclodextrins (β-CDs) and graphene quantum dots (GQDs) composite modified glassy carbon electrodes. Int. J. Electrochem. Sci. 2020, 15, 8552–8562. [Google Scholar] [CrossRef]
- Shadjou, R.; Hasanzadeh, M.; Heidar-poor, M.; Shadjou, N. Electrochemical monitoring of aflatoxin M1 in milk samples using silver nanoparticles dispersed on α-cyclodextrin-GQDs nanocomposite. J. Mol. Recognit. 2018, 31, e2699. [Google Scholar] [CrossRef] [PubMed]
- Younis, S.A.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Rare earth metal–organic frameworks (RE-MOFs): Synthesis, properties, and biomedical applications. Coord. Chem. Rev. 2021, 429, 213620. [Google Scholar] [CrossRef]
- Zamora-Gálvez, A.; Ait-Lahcen, A.; Mercante, L.A.; Morales-Narváez, E.; Amine, A.; Merkoçi, A. Molecularly Imprinted Polymer-Decorated Magnetite Nanoparticles for Selective Sulfonamide Detection. Anal. Chem. 2016, 88, 3578–3584. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, X.; Song, F.; Wang, C.; Chu, F.; Wu, S. A sensing approach for dopamine determination by boronic acid-functionalized molecularly imprinted graphene quantum dots composite. Appl. Surf. Sci. 2017, 423, 810–816. [Google Scholar] [CrossRef]
- Xi, J.; Xie, C.; Zhang, Y.; Wang, L.; Xiao, J.; Duan, X.; Ren, J.; Xiao, F.; Wang, S. Pd Nanoparticles Decorated N-Doped Graphene Quantum Dots@N-Doped Carbon Hollow Nanospheres with High Electrochemical Sensing Performance in Cancer Detection. ACS Appl. Mater. Interfaces 2016, 8, 22563–22573. [Google Scholar] [CrossRef]

| Electrode Material | GQD Preparation Method | Target Analyte | Electrochemical Technique | Linear Range | LOD | Ref. |
|---|---|---|---|---|---|---|
| Combination with 0D nanomaterials | ||||||
| GQDs/GNPs | pyrolysis of CA | luteolin | DPV | 0.01–10 μM | 1.0 nM | [164] |
| NH2-GQDs/Au-β-CD | commercial source | quercetin | DPV | 1–210 nM | 285 pM | [165] |
| NiO/GQD | - | thioridazine | DPV | 2–200 nM | 0.05 nM | [166] |
| anti-cTnI/AuNPs@GQDs | - | cardiac troponin I | SWV | 5–50 pg mL−1 | 0.5 pg mL−1 | [167] |
| AuNPs/N,S-GQDs/aptamer | pyrolysis of CA | bisphenol A | DPV | 0.1–10 μM | 0.03 μM | [168] |
| TC-GQD | hydrothermal method from expresso coffee wastes | dopamine | DPV | 0.3–750 μM | 22 nM | [169] |
| GQDs@La3+@ZrO2 | pyrolysis of CA | flutamide | SWV | 0.00175–15.75 μM | 0.82 nM | [170] |
| AgNPs/GQDs | ultrasonic method from carbon fibers | guanine and adenine | DPV | guanine: 0.015–430 μM adenine: 0.015–390 μM | 10 nM 12 nM | [86] |
| GQD/AuNP | ultrasonic method from carbon black | quercetin | DPV | 0.01–6.0 μM | 2 nM | [79] |
| anti-AFB1/GQDs-AuNPs | pyrolysis of CA | aflatoxin B1 | CV | 0.1–30 ng mL−1 | 0.008 ng mL−1 | [171] |
| Combination with 1D nanomaterials | ||||||
| GQDs-MWCNTs | hydrothermal method from GO | dopamine | DPV | 0.005–100 μM | 0.87 nM | [156] |
| GQDs@MWCNTs | hydrothermal method from glucose | dopamine | DPV | 0.25–250 μM | 95 nM | [155] |
| PVA/GQD/GOx NFs | hydrothermal method from GO | glucose | amperometry | 0.25–24 mM | 10 μM | [89] |
| NGQDs@NCNFs | pyrolysis of CA | nitrite | DPV | 5–300 μM and 400–3000 μM | 3 μM | [172] |
| NH2-GQDs/NiCo2O4 | hydrothermal method from pyrene | glucose | amperometry | 1–159 μM and 159–949 μM | 0.27 μM | [173] |
| Combination with 2D nanomaterials | ||||||
| GQDs/CoNiAl-LDH | pyrolysis of CA | glucose | amperometry | 0.01–14.0 mM | 6 μM | [157] |
| NS-GQD/G | hydrothermal method from rGO | H2O2 | amperometry | 0.4 μM–33 mM | 26 nM | [96] |
| NH2-GQD-GO | hydrothermal method from GO | oxalic acid | amperometry | 0.5–2.0 mM and 2.0–55 mM | 50 μM | [174] |
| Combination with other types of materials | ||||||
| ds-DNA-IL/GQDs | pyrolysis of CA | topotecan | DPV | 0.35–100 μM | 0.1 μM | [87] |
| RTIL-GQDs | pyrolysis of CA | levodopa | SWV | 0.05–250 μM | 10 nM | [162] |
| GQDs/IL | pyrolysis of CA | ascorbic acid (AA), dopamine (DA) and uric acid (UA) | DPV | AA: 25–400 μM DA: 0.2–10 μM UA: 0.5–20 μM | AA: 6.64 μM DA: 0.06 μM UA: 0.03 μM | [163] |
| MIPPy/GQDs | hydrothermal method from GO | bisphenol A | DPV | 0.1–50 µM | 0.04 µM | [175] |
| m-GQDs-MIP | hydrothermal method from GO | ifosfamide | DPASV | 0.25–121.35 ng mL−1 | 0.08 ng mL−1 | [160] |
| GQD-PCN-222 | electrochemical method | nitrite | amperometry | 40–18.000 μM | 6.4 μM | [158] |
| β-CD@N-GQD | hydrothermal method from CA | cholesterol | DPV | 0.5–100 μM | 80 nM | [176] |
| Combination with more than one type of materials | ||||||
| GQDs-PSSA/GO | pyrolysis of CA | estradiol (E2) and progesterone (P4) | DPV | E2: 0.001–6.0 μM P4: 0.001–6.0 μM | 0.23 nM 0.31 nM | [177] |
| Fe3O4@GQD/f–MWCNTs | pyrolysis of CA | progesterone | DPV | 0.01–0.5 and 0.5–3.0 μM | 2.18 nM | [178] |
| AuNPs/GQDs-WS2 | commercial source | malachite green | DPV | 0.01–10 µM | 3.38 nM | [179] |
| hNiNS/GQDs/MIPs | hydrothermal method from rGO | bisphenol S | DPV | 0.1–50 μM | 0.03 μM | [180] |
| Au NSs/GQDs-CS/cysteamine | pyrolysis of CA | ractopamine | DPV | 0.0044 fM–19.55 μM | 0.0044 fM | [181] |
| MIP-AuNPs/N,S@GQDs | pyrolysis of glucose | sofosbuvir | DPV | 1–400 nM | 0.36 nM | [161] |
| (mag@MIP)-GQDs-FG-NF | pyrolysis of CA | ethinylestradiol | SWV | 10 nM–2.5 μM | 2.6 nM | [182] |
| MoS2@N-GQDs-IL MIP | pyrolysis of CA | IgG | DPV | 0.1–50 ng mL−1 | 0.02 ng mL−1 | [183] |
| NMO/GQDs/CS | pyrolysis of CA | diazinon | DPV | 0.1–330 µM | 27 nM | [184] |
| LaNPs-GQDs@ZIF-8 | pyrolysis of CA | vitamin D3 | SWV | 0.00625 μM–1.25 μM | 6.1 nM | [185] |
| PPy/GQDs@PB | pyrolysis of CA | L-cysteine | amperometry | 0.2–50 μM and 50–1000 μM | 0.15 μM | [186] |
| MIP/Au@Cu-MOF/N-GQDs | pyrolysis of CA | patulin | DPV | 0.001–70.0 ng mL−1 | 0.0007 ng mL−1 | [159] |
| PS-PNIPAm-PS/MWCNTs-GQDs | comercial source | paracetamol | DPV | 0.1–7.0 μM and 7.0–103 μM | 66 nM | [187] |
| GQD-PNF-GO | electrolysis of graphite rod | H2O2 | amperometry | 0.01–7.2 mM | 0.055 μM | [188] |
| N,S-GQDs@AuNP-PAni | hydrothermal method with thiourea to citric acid | hepatitis E virus | impedance | 102–107 RNA copies mL−1 | 96.7 RNA copies mL−1 | [189] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facure, M.H.M.; Schneider, R.; Lima, J.B.S.; Mercante, L.A.; Correa, D.S. Graphene Quantum Dots-Based Nanocomposites Applied in Electrochemical Sensors: A Recent Survey. Electrochem 2021, 2, 490-519. https://doi.org/10.3390/electrochem2030032
Facure MHM, Schneider R, Lima JBS, Mercante LA, Correa DS. Graphene Quantum Dots-Based Nanocomposites Applied in Electrochemical Sensors: A Recent Survey. Electrochem. 2021; 2(3):490-519. https://doi.org/10.3390/electrochem2030032
Chicago/Turabian StyleFacure, Murilo H. M., Rodrigo Schneider, Jessica B. S. Lima, Luiza A. Mercante, and Daniel S. Correa. 2021. "Graphene Quantum Dots-Based Nanocomposites Applied in Electrochemical Sensors: A Recent Survey" Electrochem 2, no. 3: 490-519. https://doi.org/10.3390/electrochem2030032
APA StyleFacure, M. H. M., Schneider, R., Lima, J. B. S., Mercante, L. A., & Correa, D. S. (2021). Graphene Quantum Dots-Based Nanocomposites Applied in Electrochemical Sensors: A Recent Survey. Electrochem, 2(3), 490-519. https://doi.org/10.3390/electrochem2030032







