Abstract
The electrodeposition of Al was investigated in an ionic liquid (IL), with 1-ethyl-3-methylimidazolium tetrachloroaluminate ([EMIm]AlCl4) as the electrolyte with AlCl3 precursor. The [EMIm]AlCl4 electrolyte exhibited a wide and stable electrochemical window from 3.2 to 2.3 V on a glassy carbon electrode when temperature was increased from 30 °C to 110 °C. The addition of AlCl3 into [EMIm]AlCl4 generated significant well-developed nucleation growth loops, and new coupled reduction and oxidation peaks in cyclic voltammograms corresponding to the Al deposition and dissolution, respectively. A calculation model was proposed predicting compositions of anions in AlCl3/[EMIm]AlCl4 system, and [Al2Cl7]− was found to be the active species for Al deposition. In AlCl3/[EMIm]AlCl4 (1:5), the reduction rate constants were 1.18 × 10−5 cm s−1 and 3.37 × 10−4 cm s−1 at 30 °C and 110 °C, respectively. Scanning electron microscope (SEM), energy dispersive spectroscope (EDS), and X-ray diffraction (XRD) microscope results showed that the metallic Al film had been successfully deposited on glassy carbon electrodes through constant-potential cathodic reductions. The [EMIm]AlCl4 was a promising electrolyte directly used for Al deposition.
1. Introduction
Al materials and coatings have been extensively investigated in electronic and automotive industries due to their excellent conductivity, corrosion resistance, and wear tolerance [1,2]. Al can be electrochemically deposited from ionic liquids with an aluminum salt precursor, due to the wide electrochemical window of ionic liquids. The deposits were affected by factors, such as the substrate materials [3], composition of the mixture (AlCl3-to-IL ratio) [4,5,6], operating temperature [7,8,9,10], deposition current density [9,11], substrate pretreatment [11], stirring [7,8,12], and additives [7,13].
The AlCl3-to-IL ratio determines the distribution of various Al anion species and viscosity of the system, which controls reaction kinetics and diffusion process for Al deposition. The AlCl3/[BMIm]Cl mixtures with 2:1 molar ratio resulted in a dull Al deposit whereas the 1.5:1 molar ratio mixture led to a bright Al surface because the AlCl3/[BMIm]Cl at 1.5:1 is more viscous with less aluminum complex ions [4]. Al deposition is the direct result from electrochemical reduction of chloroaluminate complexes [14,15].
In general, the chloroacidity determines reactivity and electrochemistry in the ionic liquid electrolyte. The Al deposition chemistry is complicated because of the chemical nature of different chloroaluminate complexes species including chemical equilibria and interconversion [16,17]. The equilibrium composition was highly dependent on the amount of AlCl3 added to the ionic liquid. The main anions are [AlCl4]−, [Al2Cl7]−, and Cl− in the alkylimidazolium chloride and AlCl3 solutions [18,19,20]. When AlCl3 concentration is high, more complicated anions such as [Al3Cl10]−, [Al4Cl13]−, and [Al2OCl5]− will be formed, which are confirmed by Infrared (IR) spectroscopy and the Nuclear Magnetic Resonance (NMR) spectroscopy [21].
Various ionic liquids have been investigated for Al deposition including 1-methyl-3-ethylimidazolium chloride ([MEIm]Cl) [18,19,20,22,23,24,25], 1-ethyl-3-methylimidazolium chloride ([EMIm]Cl) [3,11,26,27,28,29], 1-butylpyrrolidine [30], 1-butyl-3-methylimidazolium chloride ([BMIm]Cl) [4,7,8,9,31], and 1-(2-methoxyethyl)-3-methylimidazolium chloride ([MoeMIm]Cl) [32]. One class of ionic liquids, imidazolium-based tetrachloroaluminates, has much lower melting points than its chloride counterpart. These ionic liquid systems also received attention in battery applications [33]. Al metal was found as a deposit in an ethylimidazolium-contained system [33,34,35,36,37]. A representative of this class is 1-ethyl-3-methylimidazolium tetrachloroaluminate ([EMIm]AlCl4) with melting point of 9 °C while the melting point for its chloride counterpart [EMIm]Cl is at 77–79 °C. [EMIm]AlCl4 can simplify the preparation process for electrochemical deposition with better control on mixing and heating. Furthermore, it is commercially available on industrial scale and makes Al deposition possible directly at lower temperatures. However, there is only some computational study available for exploring the unique physical properties of the tetrachloroaluminate systems recently [38,39]. However, due to the sensitivity of moisture and oxygen in the experimental performance in ionic liquids, the operations need to be conducted in protective atmosphere such as an inert gas environment. It makes the promotion and scale-up of the process difficult in larger scales [3,30]. The electrochemical characteristics of these systems have not received attention and their potential as a medium for the Al deposition has not been examined.
In this work, the electrochemical window of the [EMIm]AlCl4 will be defined and thermodynamic models for AlCl3/IL systems at 30 °C and 110 °C will be developed. By employing AlCl3/[EMIm]AlCl4 as the electrolyte, electrodepositions will be carried out on glass carbon substrates in the open air, and the products will be characterized for the morphology, composition, and phase information. These results will be of academic and industrial interest in not only the development of metal deposition processes, but also employing the electrochemistry of the [EMIm]AlCl4 as a model system to deepen our understanding of the related process chemistry and electrode reactions.
2. Experimental
2.1. Chemicals and Materials
AlCl3 (99%, Alfa Aesar, Tewksbury, MA, USA) and [EMIm]AlCl4 (C6H11AlCl4N2 ≥ 95%, Sigma Aldrich, St. Louis, MO, USA) were used for Al electrodepositions. [EMIm]AlCl4 is a light-yellow transparent liquid at room temperature. All chemicals were used as received. Glassy carbon disk electrodes were used as working electrode (GC, 1 mm diameter, eDAQ, Colorado Spring, CO, USA). Aluminum wires were used as counterelectrode and reference electrode (1 mm diameter, 99.9995% metal basis, Alfa Aesar). Techne Dri-Block® Digital Block Heater was used for temperature control (±1 °C). A 3 mL glass vial (eDAQ Inc. Colorado Spring, CO, USA) was used for electrodeposition cell. VersaSTAT 4 Potentiostat (Princeton Applied Research, Oak Ridge, TN, USA) with VersaStudio was used for all electrochemical measurements and data acquisition.
2.2. Procedure and Methodology
To determine the stability of [EMIm]AlCl4, cyclic voltammetry (CV) was measured for [EMIm]AlCl4 in the three-electrode electrochemical cell at a series of temperatures including 30 °C, 50 °C, 70 °C, 90 °C, and 110 °C at a scan rate 100 mV s−1.
AlCl3/[EMIm]AlCl4 mixtures were prepared by adding portion of AlCl3 powders into the [EMIm]AlCl4 liquid at the room temperature and heated up to 110 °C for 2 h until homogeneity was reached. As the electrical conductivity for AlCl3/IL (exclusive of Al species) started to decrease when the ratio was higher than 1 [25], a small amount of AlCl3 was added and the final ionic liquid mixture was AlCl3/[EMIm]AlCl4 at a molar ratio of 1:5. In order to determine the Al3+ reduction potential, CVs in AlCl3/[EMIm]AlCl4 (1:5) were measured at various scan rates including 10, 20, 50, 100, and 150 mV s−1 at 30 °C and 110 °C, respectively. The nucleation mechanism was studied by short-time chronoamperometry (CA) measurements. The current-time transients were measured by applying a series of constant cathodic potentials in the kinetic regime. After each current-time transient measurement, a constant potential 1.0 V was applied on the glassy carbon disk working electrode for 5 min to clean the electrode surface and remove the Al layer on the working electrode. All potentials were reported versus the Al reference electrode.
The Al electrodepositions in AlCl3/[EMIm]AlCl4 (1:5) were carried out by long-time CA measurements. The constant potential depositions were controlled a little higher than the Al/Al3+ potential. A polished and clean Al wire working electrode was used for each deposition. The Al deposition samples were washed thoroughly in acetonitrile first and then acetone. The samples were left in air at room temperature for 24 h before any material characterization.
The Al deposition samples were analyzed using a JEOL JSM-6610LV scanning electron microscope (SEM, Apollo SDD X-Ray spectrometer, 20 kV) for morphology and elemental composition information. The X-ray diffraction (XRD) was performed for the Al deposits from 30° to 90° (2 theta) at 4° min−1 for phase identification (Rigaku SmartLab, Cu Kα radiation, λ = 1.54056 Å, voltage = 40 kV and current = 44 mA).
2.3. Chloroaluminate Complexes Distribution Calculation
Al anions in the AlCl3/[EMIm]AlCl4 solution included [AlCl4]−, [Al2Cl7]−, [Al3Cl10]−, and [Al4Cl13]− [18,19,20]. The distributions of the Al anions for AlCl3/[EMIm]AlCl4 (1:5) at 30 °C and 110 °C were calculated based on using standard entropy and enthalpy (Table 1) [20]. The chloroaluminate species reached equilibrium in the ionic liquid as following reactions [18,19,20]:

Table 1.
Thermodynamic equilibrium parameters for Al2Cl6 equilibria reactions.
The total mole fraction for all species was unity:
where was the modified Temkin ion fraction [40] for the species i, , and was the total molar amount of anions: .
In addition, the charge balance also met the equation:
By assuming a complete dissociation of the IL, it gave .
The materials balance for Al species was
was introduced as the initial molar ratio of AlCl3: .
Combining the mass balance equations and charge balance equation, the following equation was established:
Equations (1)–(8) were solved simultaneously using numerical method within EXCEL for in the range of 0 and 1 at interval 0.0001.
3. Results and Discussion
3.1. Stability Window of [EMIm]AlCl4 at Various Temperatures
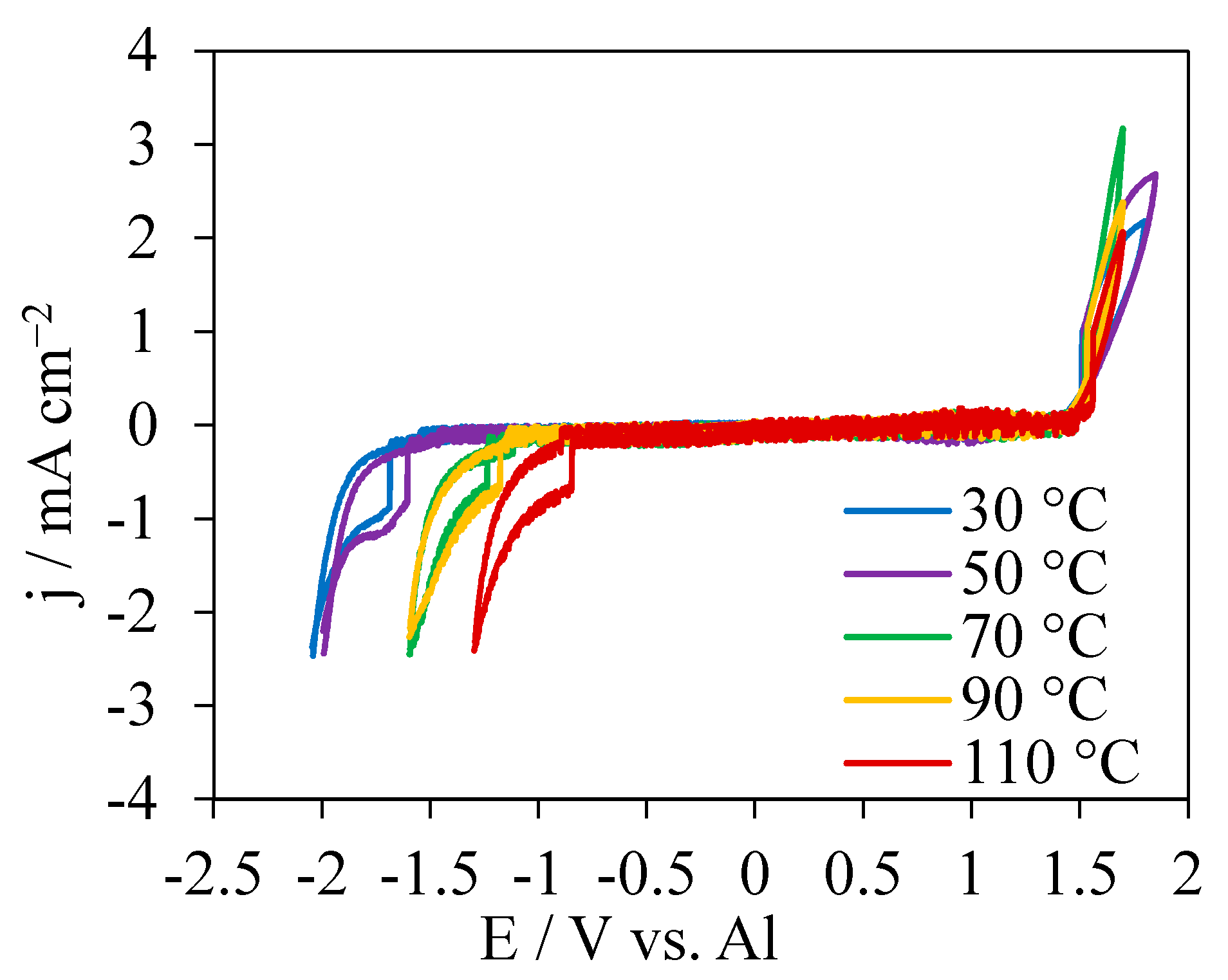
CVs in [EMIm]AlCl4 at different temperatures were measured to determine the stability window (Figure 1). The CVs displayed wide flat zones between the fast-growing oxidation and reduction currents. The onset potentials for oxidation-current growth were very close to 1.5 V which is the oxidation of chloride anions [41,42]. In contrast, the onset potentials for the reduction-current growth were strongly dependent on temperatures, characteristic of a positive shift with increasing temperatures. At the negative potential limits, the cathodic peaks were attributed to the reduction of imidazolium ring [41]. Based on the onset potential differences for the oxidation and reduction current growth, the electrochemical windows of [EMIm]AlCl4 on the GC electrode were determined. The potential windows decreased from 3.2 to 2.3 V as the temperature was increased from 30 °C to 110 °C. These results are consistent with literature data measured under similar conditions [41,43]. No oxidation peak was observed in the electrochemical windows in the CVs indicating that the Al anion [AlCl4]− dissociated from [EMIm]AlCl4 was a very stable Lewis neutral anion and could not lead to Al electrodeposition [44,45,46].
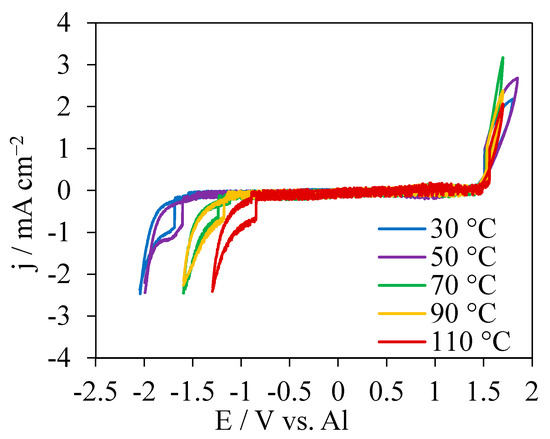
Figure 1.
CVs in [EMIm]AlCl4 at 30 °C, 50 °C, 70 °C, 90 °C and 110 °C at a scan rate 100 mV s−1.
3.2. Cyclic Voltammetry in AlCl3/[EMIm]AlCl4 (1:5)
The advantage of using the [EMIm]AlCl4 is mostly its simple operation. Though [EMIm]AlCl4 can be generated by mixing equimolar [EMIm]Cl and AlCl3, the mixing process requires heating to melt the [EMIm]Cl and continuous stirring to release heat generated by the combination reaction [27]. In contrast, by using [EMIm]AlCl4, it is a liquid at the room temperature and the operation of mixing with AlCl3 does not need extra heating or stirring.
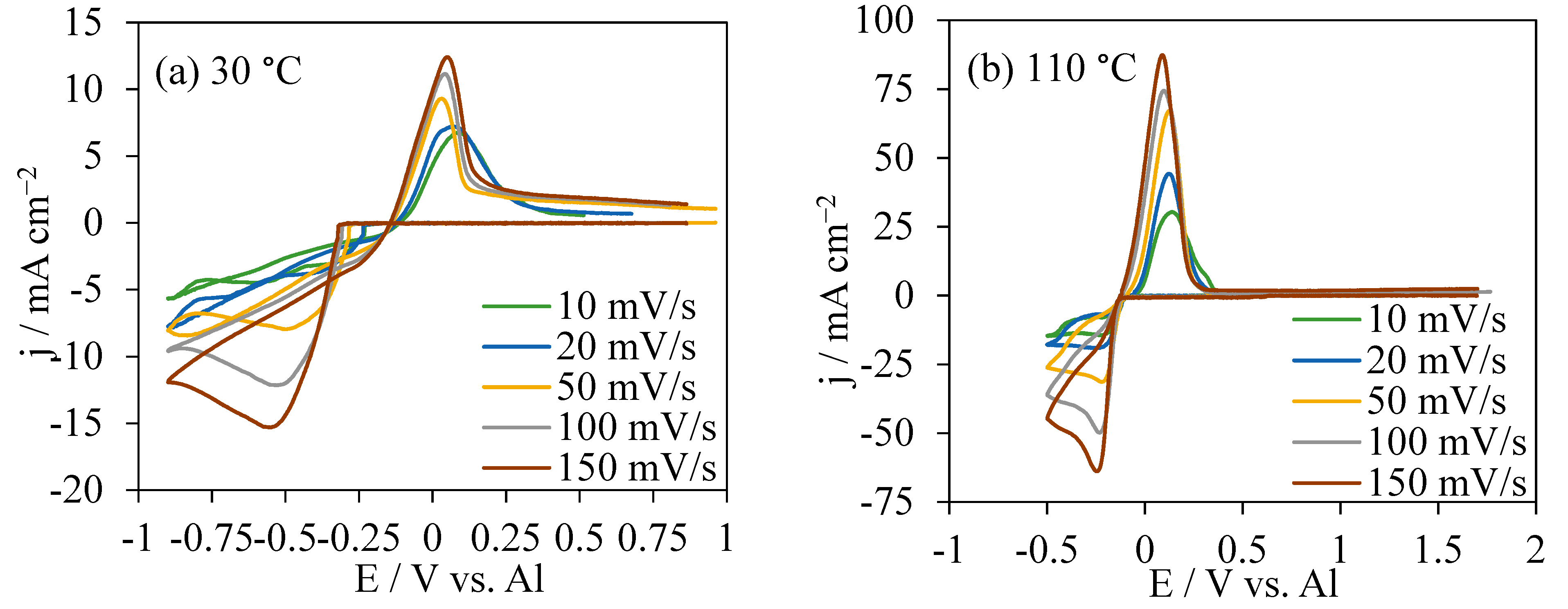
The CVs in AlCl3/[EMIm]AlCl4 (1:5) at 30 °C and 100 °C were shown in Figure 2a,b, respectively. At 30 °C, the onset potentials for Al deposition became higher from −0.25 V to −0.3 V as the scan rates increased from 10 mV s−1 to 150 mV s−1. At 110 °C, the onset potentials for Al deposition at different scan rates were concentrated at approximately −0.14 V, much lower than those in CVs at 30 °C. The potential range for CVs at 30 °C was from OCP to −0.9 V, and the current intensity at 150 mV s−1 was responded at 12.5 to −15.2 mA cm−2, the highest current range. For CVs at the same scan rate 150 mV s−1 at 110 °C, the current response was much higher in a range of −63.8 to 87.2 mA cm−2. At both temperatures, the re-oxidation charges were smaller than the reduction charges. At 30 °C, the Al dissolution was around 26.5% to 36.4% of the charge used in the reduction reaction, compared with the proportion of 61.8% to 79.2% in CVs at 110 °C.
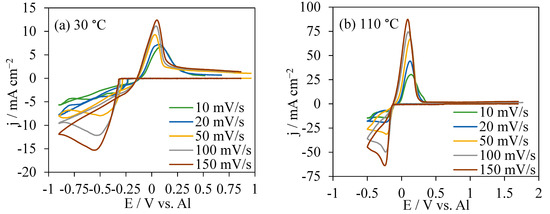
Figure 2.
CVs at various scan rates in AlCl3/[EMIm]AlCl4 (1:5) at 30 °C (a) and 110 °C (b).
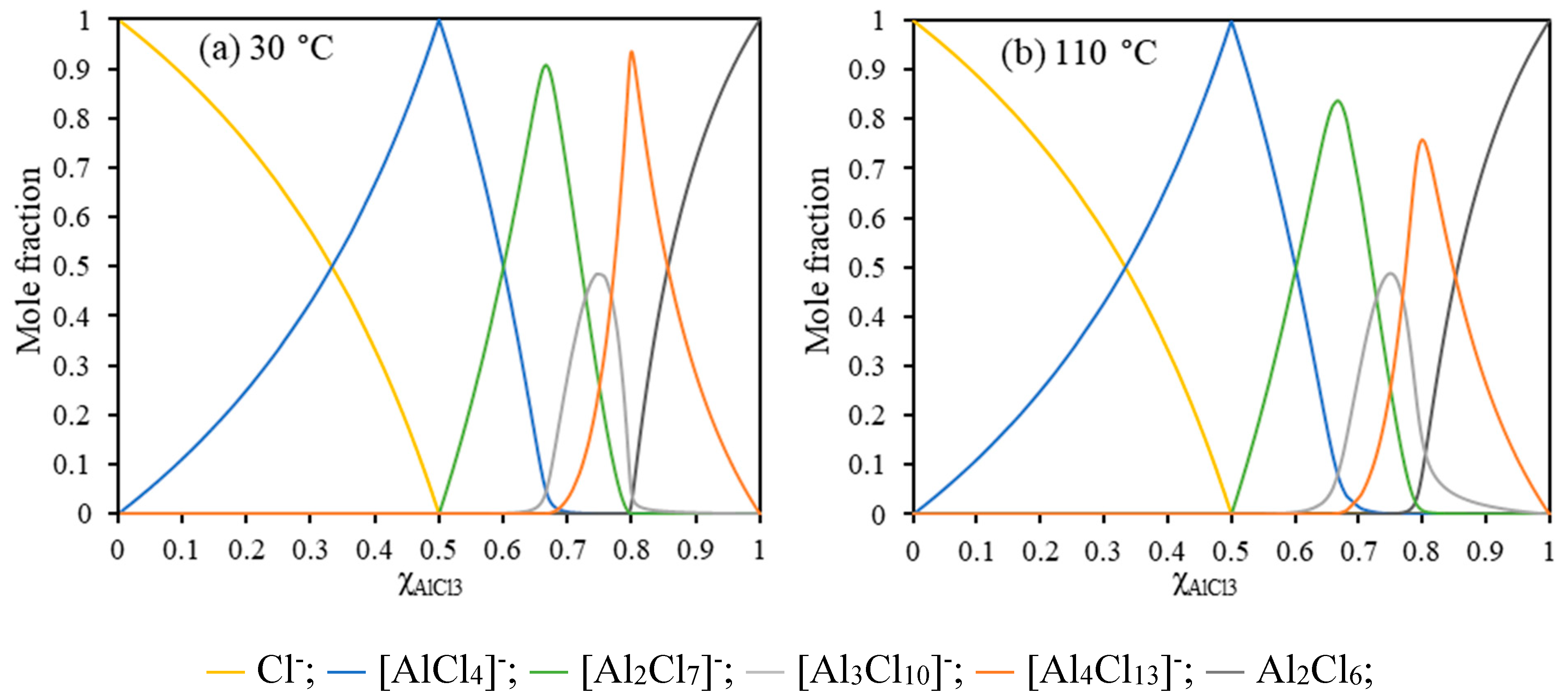
For AlCl3/[EMIm]AlCl4 (1:5, = 0.55), the system is composed of similar aluminum ions at 30 °C and 110 °C with 80.0 mol% [AlCl4]− and 20.0 mol% [Al2Cl7]− at 30 °C while 80.2 mol% [AlCl4]− and 19.8 mol% [Al2Cl7]− at 110 °C (Figure 3). In the system, [AlCl4]−, as the major component, could not be reduced to Al. Moreover, the minor component [Al2Cl7]− was the active species for Al deposition [47,48,49,50]. During the electrolysis, [Al2Cl7]− was reduced to aluminum metal and the generated [AlCl4]− migrating to the anode (Equation (9)) [51,52].
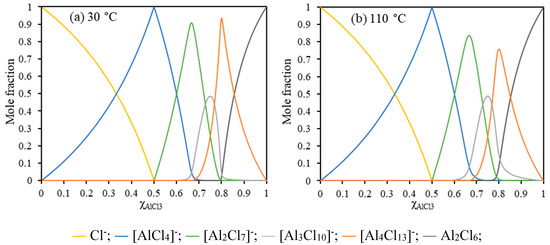
Figure 3.
Calculated Al anion concentrations as a function of at 30 °C (a) and 110 °C (b).
The CVs in AlCl3/[EMIm]AlCl4 (1:5) (Figure 2) at both temperatures exhibited the cathodic and anodic peaks potentials were more separated at higher scan rates. The separations of the Al reduction and re-oxidation peaks were more than 330 mV, which was larger than the critical peak splitting of 282 mV (, n is the number of electrons involved in the charge-transfer step, n = 0.75) [53,54], suggesting an irreversible system.
For an irreversible electrode reaction, the rate constant (k0) for the Al deposition was estimated from the shift of the cathodic peak potential (Epc) with the scan rates in CVs, based on Equation (10) [55]:
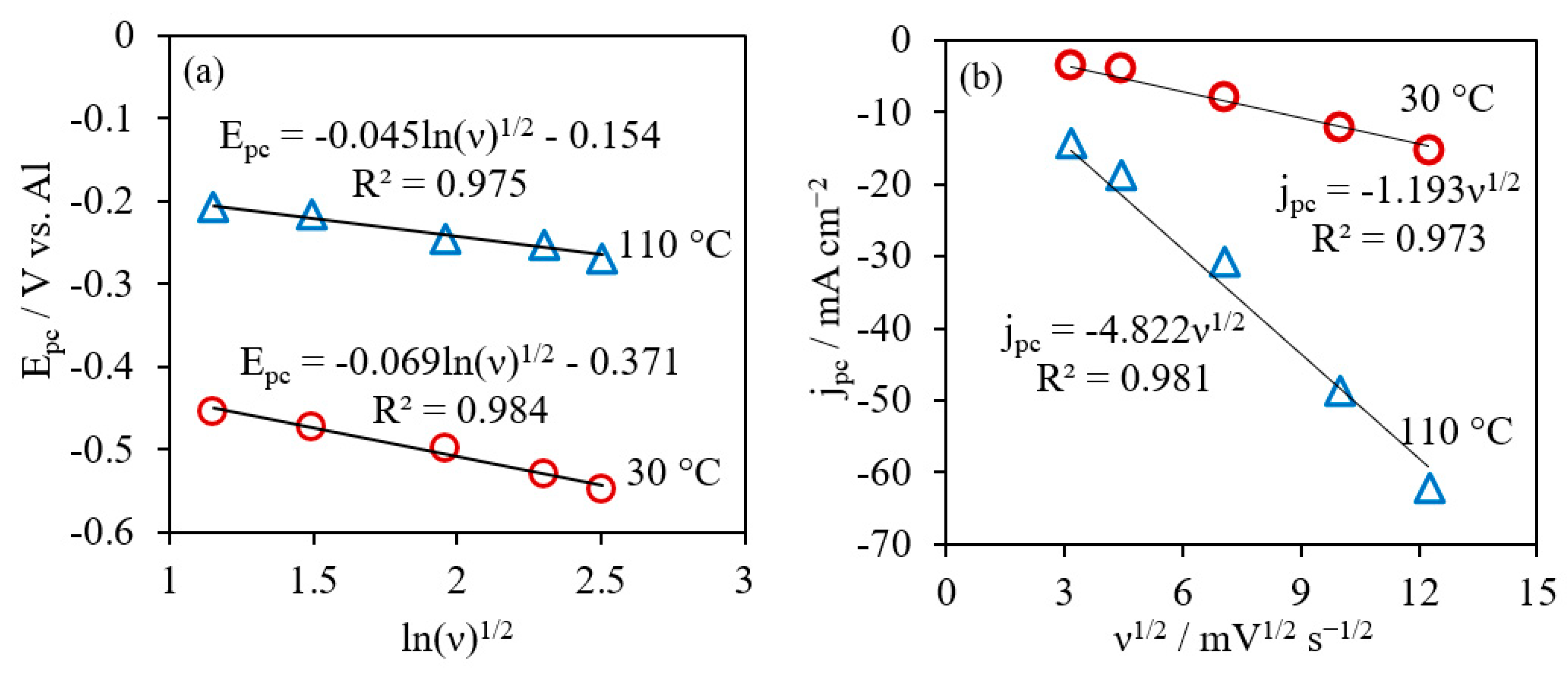
where E0 is the standard potential (E0 = 0 for the current system), F is the Faraday’s constant 96,485 C mol−1, R is the gas constant 8.314 J mol−1 K−1, D is the diffusion coefficient of active species [Al2Cl7]−, and α is the transfer coefficient. Figure 4a showed linear relations of Epc and lnv1/2. The values of could be derived from the slopes of lines at different temperatures, which were 0.38 and 0.74 for reactions at 30 °C and 110 °C, respectively. The diffusion coefficients were calculated from the Randles–Sevcik equation, based on the relationship between the cathodic peak current densities (jpc) correlating with scan rate (v) for an irreversible system in Equation (11) [56]:
where C is the concentration of active species [Al2Cl7]− and n is the number of electrons involved in the charge-transfer step, n = 0.75. The cathodic peak current densities and the square root of scan rate showed good linearity at 30 °C and 110 °C (Figure 4b), indicating that the cathodic reaction was diffusion-controlled. The values of D were estimated to be 9.71 × 10−8 cm2 s−1 at 30 °C and 1.04 × 10−6 cm2 s−1 at 110 °C. In the literature, Lai et al. reported the at 0.44 and the diffusion coefficient of [Al2Cl7]− was about 6.2 × 10−8 cm2 s−1 at 40 °C in AlCl3/[EMIm]Cl with 0.47 mol L−1 of [Al2Cl7]− [50]. Based on the intercept in Figure 4a, the values of the cathodic reduction rate constant were determined as 1.18 × 10−5 cm s−1 and 3.37 × 10−4 cm s−1 at 30 °C and 110 °C, respectively.
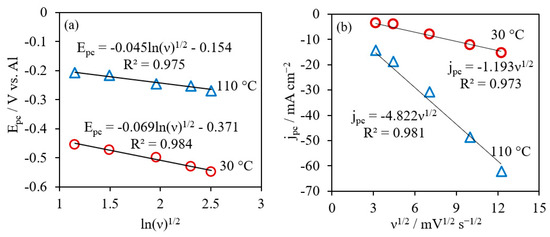
Figure 4.
Plots of cathodic peak potential vs. (a) and plots of cathodic peak current jpc as a function of ν1/2 (b) for AlCl3/[EMIm]AlCl4 (1:5) at 30 °C and 110 °C.
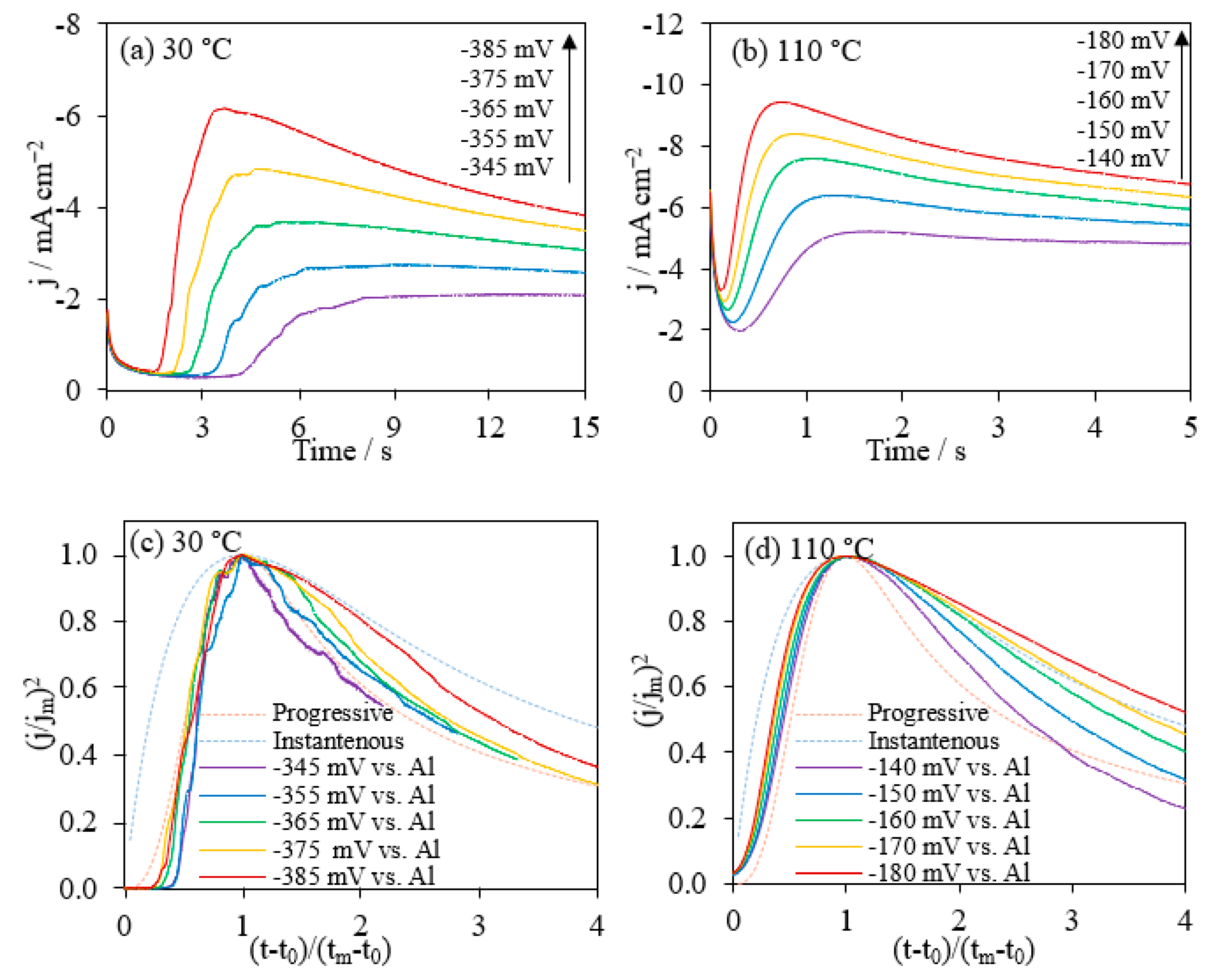
The Al deposits and the nucleation mechanisms can be achieved from the chronoamperometry studies. Al deposition potentials at both 30 °C and 110 °C were chosen as the values achieved at 200 mV s−1 in CVs. In this way, it guaranteed the constant potentials in CA measurements were higher than the Al deposition potentials. The current-time profiles showed an initial slight decrease in current density followed by a sharp increase and gradual decrease to a plateau (Figure 5a,b). The initial decrease was due to the charging of the double layer and a decayed current during the nucleation process [50,57,58]. The current increase was the result of independent nucleus size growing and consequently the increase of total electroactive Al surface area. The following current decrease was because of the overlap of the diffusion zone leading to the formation of one diffusion layer [50]. As the applied potential being more significant, the current density peak became sharper with higher intensity and reached a higher plateau.
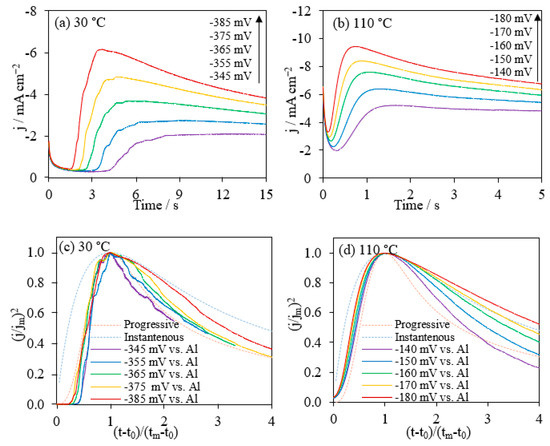
Figure 5.
Current-time transients from potential step experiments on glassy carbon electrodes for AlCl3/[EMIm]AlCl4 (1:5) at 30 °C (a) and 110 °C (b). The comparison of the dimensionless experimental current-time transients (j/jm)2 vs. (t − t0)/(tm − t0) plots (c,d), which derived from subfigures (a,b), respectively, comparing with the theoretical models for the diffusion-controlled three-dimensional nucleation.
Initial stages of metal deposition were usually associated with a three-dimensional (3D) nucleation. For the diffusion controlled 3D nucleation, the instantaneous and progressive nucleation mechanisms were normally expressed in Equations (12) and (13), respectively [59].
where j is the current density at time t, t0 is the induction time, and tm is the time at the maximum current density jm. Figure 5c,d gives a graphic analysis of the Al nucleation mechanism with the data extracted from Figure 5a,b for the Al deposition onto the GC electrode at 30 °C and 110 °C, respectively. The theoretical model curves were generated from Equations (12) and (13). At 30 °C, the nucleation process correlated with the progressive nucleation mechanism in the initial stage. After reaching the maximum current, the deposition was close to the progressive nucleation curve, but the process may be affected by other factors. At 110 °C, the phenomenon was more complicated. It followed the progressive nucleation mechanism for a short time, but the data lines fell on progressive and instantaneous nucleation mechanisms, and in the region in between. This nucleation kinetic was different from literature results for the Al deposition from AlCl3/[EMIm]Cl which exhibited a better fit with the 3D instantaneous nucleation [3,9]. It is possibly the results of electrode surface changes during the depositions. Initially, the electrode surface was flat and small. Next, the surface gradually grew to a hemispheric shape with a large surface area. The changes happened in such a short time that the mass transfer and nucleation kinetics were complicated and could not be simply explained with only one mechanism. In addition, the existence of side reactions during the nucleation, such as the reduction of moisture and oxygen dissolved in the ionic liquid mixture, made the electroplating processes more complicated [60]. Al-contained species would precipitate due to the hydrogen extraction in water electrolysis and oxidized by oxygen generated. Charges were consumed not only by the Al deposition from [Al4Cl7]−, but also by the side reaction discussed above. Therefore, the process resulted in a lower current efficiency and aluminum extraction ratio.
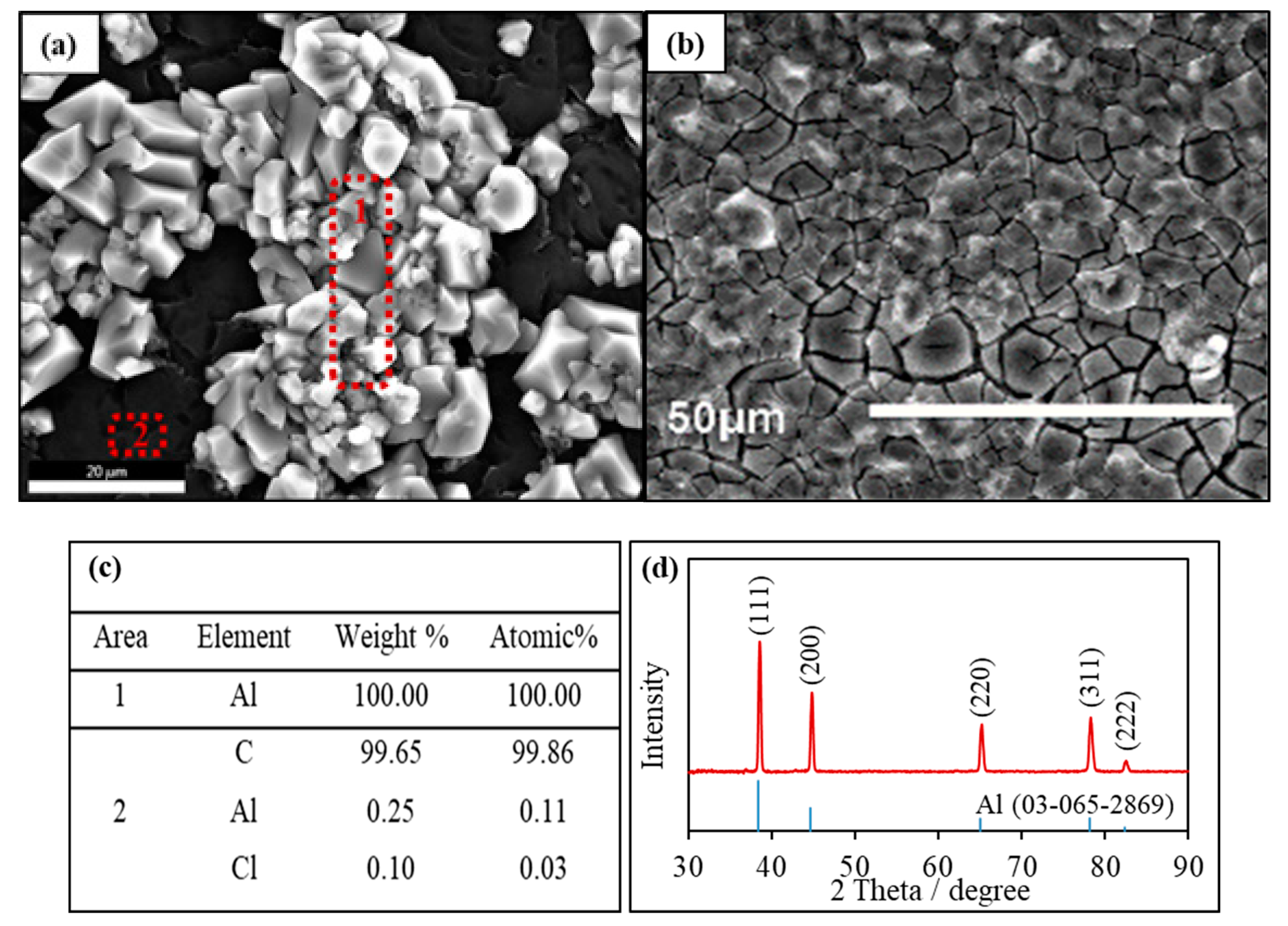
Al electrodepositions were studied at 110 °C on the glassy carbon substrates. Constant potentials were applied at the Al deposition potential defined with CV measurements. Figure 6a,b shows the SEM images for two Al deposits under constant-potential polarization after the charge reached 2.9 C cm−2 and 14.5 C cm−2, respectively. The deposit with less charges exhibited both bright and black regions. Further growth of the deposit layer with more charges lead to the complete coating of the substrate, accompanied by the formation of minor cracks. The elemental analysis (Figure 6c) of the bright zone and the dark zone in Figure 6a confirmed that the polyhedral particles were 100% Al, and the black zone corresponded to the GC substrate. The XRD pattern of the Al layer deposited in Figure 6b was shown in Figure 6d. The peaks were sharp and matched well with the standard Al (JCPDS 03-065-2869), indicative of a well-crystallized face-centered-cubic (fcc) structure based on the patterns in [111], [200], [220], [311] and [222]. The particle size for Al particles is estimated based on the Scherrer equation: . is the shape factor at 0.9; is the X-ray wavelength at 1.5406 ; β is the line broadening at half the maximum intensity in radians, 0.008378; and θ is the Bragg angle. The size of Al particles was calculated at 8.77 nm. Therefore, the result strongly supported the deposition of nano-size metallic Al during the cathode polarization.
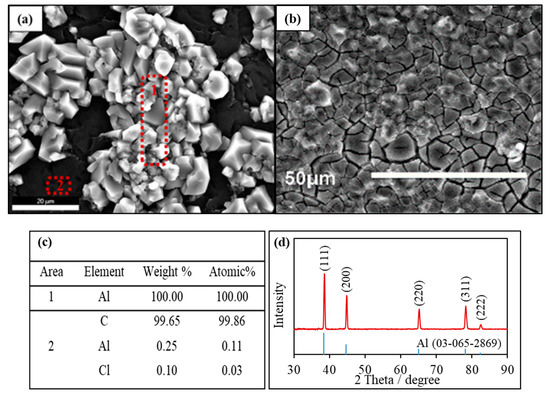
Figure 6.
(a,b) SEM images of Al deposits 110 °C on the glassy carbon electrodes in the AlCl3/[EMIm]AlCl4 ionic liquids with charges of 2.9 and 14.5 C cm−2, respectively; (c) EDS analysis of the Al deposit in panel (a); and (d) XRD pattern of an Al deposit in panel (b).
4. Conclusions
In this work, we explored the potential of the [EMIm]AlCl4 as the ionic liquid electrolyte and AlCl3 as the precursor for the electrodeposition of Al. Because of its wide electrochemical window and low melting point, the [EMIm]AlCl4 is a prospective ionic liquid for the electrodeposition of Al. Thermodynamic models were established to show the composition of Al anions in AlCl3/IL mixtures at 30 °C and 110 °C. It was demonstrated that nano-sized Al was successfully deposited on glassy carbon after AlCl3 was added to the tetrachloroaluminate. The results from this work prove that the AlCl3/[EMIm]AlCl4 mixture is a promising electrodeposition both in developing the process chemistry and improving the control of Al coating.
Author Contributions
Conceptualization, J.J.; methodology, M.S., J.J. and H.Z.; software, M.S.; validation, J.J. and H.Z.; formal analysis, M.S.; resources, J.J.; data curation, M.S.; writing—original draft preparation, M.S. and J.J.; writing—review and editing, H.Z.; project administration, J.J.; funding acquisition, J.J. All authors have read and agreed to the published version of the manuscript.
Funding
The work is supported through the INL Laboratory Directed Research & Development (LDRD) Program under DOE Idaho Operations Office Contract DE-AC07-05ID14517. Publication of this article was funded by the University of Idaho—Open Access Publishing Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data is available for report.
Acknowledgments
This work is supported through the INL Laboratory Directed Research & Development (LDRD) Program under DOE Idaho Operations Office Contract DE-AC07-05ID14517. The authors thank Jatuporn Burns at the Center for Advanced Energy Studies for performing SEM/EDS and XRD measurements, and assisting data analysis. Publication of this article was funded by the University of Idaho—Open Access Publishing Fund.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, Y.; VanderNoot, T.J. Electrodeposition of Aluminium from Nonaqueous Organic Electrolytic Systems and Room Temperature Molten Salts. Electrochim. Acta 1997, 42, 3–13. [Google Scholar] [CrossRef]
- Tsuda, T.; Stafford, G.R.; Hussey, C.L. Review—Electrochemical Surface Finishing and Energy Storage Technology with Room-Temperature Haloaluminate Ionic Liquids and Mixtures. J. Electrochem. Soc. 2017, 164, H5007–H5017. [Google Scholar] [CrossRef]
- Jiang, T.; Chollier Brym, M.J.; Dubé, G.; Lasia, A.; Brisard, G.M. Electrodeposition of Aluminium from Ionic Liquids: Part I—Electrodeposition and Surface Morphology of Aluminium from Aluminium Chloride (AlCl3)–1-Ethyl-3-Methylimidazolium Chloride ([EMIm]Cl) Ionic Liquids. Surf. Coat. Technol. 2006, 201, 1–9. [Google Scholar] [CrossRef]
- Abbott, A.P.; Qiu, F.; Abood, H.M.A.; Ali, M.R.; Ryder, K.S. Double Layer, Diluent and Anode Effects upon the Electrodeposition of Aluminium from Chloroaluminate Based Ionic Liquids. Phys. Chem. Chem. Phys. 2010, 12, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Endres, F.; Bukowski, M.; Hempelmann, R.; Natter, H. Electrodeposition of Nanocrystalline Metals and Alloys from Ionic Liquids. Angew. Chem. Int. Ed. 2003, 42, 3428–3430. [Google Scholar] [CrossRef]
- Hurley, F.H.; WIer, T.P. The Electrodeposition of Aluminum from Nonaqueous Solutions at Room Temperature. J. Electrochem. Soc. 1951, 98, 207–212. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Zhang, S.; Lu, X. Effect of Nicotinamide on Electrodeposition of Al from Aluminium Chloride (AlCl3)-1-Butyl-3-Methylimidazolium Chloride ([Bmim]Cl) Ionic Liquids. J. Solid State Electrochem. 2014, 18, 257–267. [Google Scholar] [CrossRef]
- Berretti, E.; Giaccherini, A.; Martinuzzi, S.M.; Innocenti, M.; Schubert, T.J.S.; Stiemke, F.M.; Caporali, S. Aluminium Electrodeposition from Ionic Liquid: Effect of Deposition Temperature and Sonication. Materials 2016, 9, 719. [Google Scholar] [CrossRef]
- Tu, X.; Zhang, J.; Zhang, M.; Cai, Y.; Lang, H.; Tian, G.; Wang, Y. Electrodeposition of Aluminium Foils on Carbon Electrodes in Low Temperature Ionic Liquid. RSC Adv. 2017, 7, 14790–14796. [Google Scholar] [CrossRef]
- Stafford, G.R.; Tsuda, T.; Hussey, C.L. The Structure of Electrodeposited Aluminum Alloys from Chloroaluminate Ionic Liquids: Let’s Not Ignore the Temperature. ECS Meet. Abstr. 2014, MA2014-02, 1484. [Google Scholar] [CrossRef]
- Tang, J.; Azumi, K. Improvement of Al Coating Adhesive Strength on the AZ91D Magnesium Alloy Electrodeposited from Ionic Liquid. Surf. Coat. Technol. 2012, 208, 1–6. [Google Scholar] [CrossRef]
- Schaltin, S.; Ganapathi, M.; Binnemans, K.; Fransaer, J. Modeling of Aluminium Deposition from Chloroaluminate Ionic Liquids. J. Electrochem. Soc. 2011, 158, D634–D639. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Q.; Chen, B.; Lu, X.; Zhang, S. Electrodeposition of Bright Al Coatings from 1-Butyl-3-Methylimidazolium Chloroaluminate Ionic Liquids with Specific Additives. J. Electrochem. Soc. 2015, 162, D320–D324. [Google Scholar] [CrossRef]
- Abdul-Sada, A.K.; Greenway, A.M.; Seddon, K.R.; Welton, T. A Fast Atom Bombardment Mass Spectrometric Study of Room-Temperature 1-Ethyl-3-Methylimidazolium Chloroaluminate(III) Ionic Liquids. Evidence for the Existence of the Decachlorotrialuminate(III) Anion. Org. Mass Spectrom. 1993, 28, 759–765. [Google Scholar] [CrossRef]
- Franzen, G.; Gilbert, B.P.; Pelzer, G.; DePauw, E. The Anionic Structure of Room-Temperature Organic Chloroaluminate Melts from Secondary Ion Mass Spectrometry. Org. Mass Spectrom. 1986, 21, 443–444. [Google Scholar] [CrossRef]
- Brown, L.C.; Hogg, J.M.; Swadźba-Kwaśny, M. Lewis Acidic Ionic Liquids. In Ionic Liquids II; Kirchner, B., Perlt, E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 185–224. ISBN 978-3-319-89794-3. [Google Scholar]
- Al Farisi, S.M.; Hertel, S.; Wiemer, M.; Otto, T. Aluminum Patterned Electroplating from AlCl3–[EMIm]Cl Ionic Liquid towards Microsystems Application. Micromachines 2018, 9, 589. [Google Scholar] [CrossRef]
- Fannin, A.A.; King, L.A.; Levisky, J.A.; Wilkes, J.S. Properties of 1,3-Dialkylimidazolium Chloride-Aluminum Chloride Ionic Liquids. 1. Ion Interactions by Nuclear Magnetic Resonance Spectroscopy. J. Phys. Chem. 1984, 88, 2609–2614. [Google Scholar] [CrossRef]
- Dymek, C.J.; Hussey, C.L.; Wilkes, J.S.; Øye, H.A. Thermodynamics of 1-Methyl-3-Ethylimidazolium Chloride—Aluminum Chloride Mixtures. ECS Proc. Vol. 1987, 7, 93–104. [Google Scholar] [CrossRef]
- Øye, H.A.; Jagtoyen, M.; Oksefjell, T.; Wilkes, J.S. Vapour Pressure and Thermodynamics of the System 1-Methyl-3-Ethyl-Imidazolium Chloride—Aluminium Chloride. Mater. Sci. Forum 1991, 73–75, 183–190. [Google Scholar] [CrossRef]
- Zhang, M.; Kamavarum, V.; Reddy, R.G. New Electrolytes for Aluminum Production: Ionic Liquids. J. Miner. Met. Mater. Soc. 2003, 55, 54–57. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Levisky, J.A.; Wilson, R.A.; Hussey, C.L. Dialkylimidazolium Chloroaluminate Melts: A New Class of Room-Temperature Ionic Liquids for Electrochemistry, Spectroscopy and Synthesis. Inorg. Chem. 1982, 21, 1263–1264. [Google Scholar] [CrossRef]
- Hussey, C.L.; Scheffler, T.B.; Wilkes, J.S.; Fannin, A.A. Chloroaluminate Equilibria in the Aluminum Chloride-1-Methyl-3-ethylimidazolium Chloride Ionic Liquid. J. Electrochem. Soc. 1986, 133, 1389–1391. [Google Scholar] [CrossRef]
- Karpinski, Z.J.; Osteryoung, R.A. Determination of Equilibrium Constants for the Tetrachloroaluminate Ion Dissociation in Ambient-Temperature Ionic Liquids. Inorg. Chem. 1984, 23, 1491–1494. [Google Scholar] [CrossRef]
- Fannin, A.A.; Floreani, D.A.; King, L.A.; Landers, J.S.; Piersma, B.J.; Stech, D.J.; Vaughn, R.L.; Wilkes, J.S.; Williams, J.L. Properties of 1,3-Dialkylimidazolium Chloride-Aluminum Chloride Ionic Liquids. 2. Phase Transitions, Densities, Electrical Conductivities, and Viscosities. J. Phys. Chem. 1984, 88, 2614–2621. [Google Scholar] [CrossRef]
- Bakkar, A.; Neubert, V. A New Method for Practical Electrodeposition of Aluminium from Ionic Liquids. Electrochem. Commun. 2015, 51, 113–116. [Google Scholar] [CrossRef]
- Pradhan, D.; Reddy, R.G. Mechanistic Study of Al Electrodeposition from EMIC–AlCl3and BMIC–AlCl3Electrolytes at Low Temperature. Mater. Chem. Phys. 2014, 143, 564–569. [Google Scholar] [CrossRef]
- Hou, Y.; Li, R.; Liang, J. Simultaneous Electropolishing and Electrodeposition of Aluminum in Ionic Liquid under Ambient Conditions. Appl. Surf. Sci. 2018, 434, 918–921. [Google Scholar] [CrossRef]
- Bakkar, A.; Neubert, V. Electrodeposition and Corrosion Characterisation of Micro- and Nano-Crystalline Aluminium from AlCl3/1-Ethyl-3-Methylimidazolium Chloride Ionic Liquid. Electrochim. Acta 2013, 103, 211–218. [Google Scholar] [CrossRef]
- Pulletikurthi, G.; Bödecker, B.; Borodin, A.; Weidenfeller, B.; Endres, F. Electrodeposition of Al from a 1-Butylpyrrolidine-AlCl3Ionic Liquid. Prog. Nat. Sci. Mater. Int. 2015, 25, 603–611. [Google Scholar] [CrossRef]
- Nara, S.J.; Harjani, J.R.; Salunkhe, M.M. Friedel—Crafts Sulfonylation in 1-Butyl-3-Methylimidazolium Chloroaluminate Ionic Liquids. J. Org. Chem. 2001, 66, 8616–8620. [Google Scholar] [CrossRef]
- Zein El Abedin, S.; Giridhar, P.; Schwab, P.; Endres, F. Electrodeposition of Nanocrystalline Aluminium from a Chloroaluminate Ionic Liquid. Electrochem. Commun. 2010, 12, 1084–1086. [Google Scholar] [CrossRef]
- Elia, G.A.; Kravchyk, K.V.; Kovalenko, M.V.; Chacón, J.; Holland, A.; Wills, R.G.A. An Overview and Prospective on Al and Al-Ion Battery Technologies. J. Power Sources 2021, 481, 228870. [Google Scholar] [CrossRef]
- Elia, G.A.; Greco, G.; Kamm, P.H.; García-Moreno, F.; Raoux, S.; Hahn, R. Simultaneous X-Ray Diffraction and Tomography Operando Investigation of Aluminum/Graphite Batteries. Adv. Funct. Mater. 2020, 30, 2003913. [Google Scholar] [CrossRef]
- Zhao, Q.; Zachman, M.J.; Al Sadat, W.I.; Zheng, J.; Kourkoutis, L.F.; Archer, L. Solid Electrolyte Interphases for High-Energy Aqueous Aluminum Electrochemical Cells. Sci. Adv. 2018, 4, eaau8131. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, J.; Zhang, X.; Chen, J.; Wang, Z.; Yang, T.; Liu, Z.; Liang, Y.; Wang, B.; Liu, S.; et al. A High-Energy Aqueous Aluminum-Manganese Battery. Adv. Funct. Mater. 2019, 29, 1905228. [Google Scholar] [CrossRef]
- Wu, C.; Gu, S.; Zhang, Q.; Bai, Y.; Li, M.; Yuan, Y.; Wang, H.; Liu, X.; Yuan, Y.; Zhu, N.; et al. Electrochemically Activated Spinel Manganese Oxide for Rechargeable Aqueous Aluminum Battery. Nat. Commun. 2019, 10, 73. [Google Scholar] [CrossRef]
- de Andrade, J.; Böes, E.S.; Stassen, H. A Force Field for Liquid State Simulations on Room Temperature Molten Salts: 1-Ethyl-3-Methylimidazolium Tetrachloroaluminate. J. Phys. Chem. B 2002, 106, 3546–3548. [Google Scholar] [CrossRef]
- de Andrade, J.; Böes, E.S.; Stassen, H. Alkyl Chain Size Effects on Liquid Phase Properties of 1-Alkyl-3-Methylimidazolium Tetrachloroaluminate Ionic Liquids—A Microscopic Point of View from Computational Chemistry. J. Phys. Chem. B 2009, 113, 7541–7547. [Google Scholar] [CrossRef]
- Øye, H.A.; Rytter, E.; Klæboe, P.; Cyvin, S.J. Raman Spectra of KCl-AlCl3Melts and Normal Coordinate Analysis of Al2Cl7-. Acta Chem. Scand. 1971, 25, 559–576. [Google Scholar] [CrossRef]
- Lockett, V.; Sedev, R.; Ralston, J.; Horne, M.; Rodopoulos, T. Differential Capacitance of the Electrical Double Layer in Imidazolium-Based Ionic Liquids: Influence of Potential, Cation Size, and Temperature. J. Phys. Chem. C 2008, 112, 7486–7495. [Google Scholar] [CrossRef]
- Vaughan, J.; Dreisinger, D. Potentiodynamic Polarization of Platinum and Aluminum in AlCl3-[P14,6,6,6]Cl Melts. ECS Trans. 2009, 16, 397–409. [Google Scholar] [CrossRef]
- Suarez, P.A.Z.; Selbach, V.M.; Dullius, J.E.L.; Einloft, S.; Piatnicki, C.M.S.; Azambuja, D.S.; de Souza, R.F.; Dupont, J. Enlarged Electrochemical Window in Dialkyl-Imidazolium Cation Based Room-Temperature Air and Water-Stable Molten Salts. Electrochim. Acta 1997, 42, 2533–2535. [Google Scholar] [CrossRef]
- Gordon, C.M.; Muldoon, M.J.; Wagner, M.; Hilgers, C.; Davis, J.H., Jr.; Wasserscheid, P. Synthesis and Purification. In Ionic Liquids in Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 7–55. ISBN 978-3-527-62119-4. [Google Scholar]
- Nelson, W.M. Green Solvents for Chemistry: Perspectives and Practice; Oxford University Press: Oxford, UK, 2003; ISBN 0-19-803576-4. [Google Scholar]
- Koronaios, P.; King, D.; Osteryoung, R.A. Acidity of Neutral Buffered 1-Ethyl-3-Methylimidazolium Chloride−AlCl3Ambient-Temperature Molten Salts. Inorg. Chem. 1998, 37, 2028–2032. [Google Scholar] [CrossRef]
- Dymek, C.J.; Williams, J.L.; Groeger, D.J.; Auborn, J.J. An Aluminum Acid-Base Concentration Cell Using Room Temperature Chloroaluminate Ionic Liquids. J. Electrochem. Soc. 1984, 131, 2887–2892. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Kar, M.; Pringle, J.M. Synthesis of Ionic Liquids. In Fundamentals of Ionic Liquids; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 81–102. ISBN 978-3-527-34003-3. [Google Scholar]
- Ismail, A.S. Nano-Sized Aluminum Coatings from Aryl-Substituted Imidazolium Cation Based Ionic Liquid. Egypt. J. Pet. 2016, 25, 525–530. [Google Scholar] [CrossRef]
- Lai, P.K.; Skyllas-Kazacos, M. Electrodeposition of Aluminium in Aluminium Chloride/1-Methyl-3-Ethylimidazolium Chloride. J. Electroanal. Chem. Interfacial Electrochem. 1988, 248, 431–440. [Google Scholar] [CrossRef]
- Zhu, G.; Angell, M.; Pan, C.-J.; Lin, M.-C.; Chen, H.; Huang, C.-J.; Lin, J.; Achazi, A.J.; Kaghazchi, P.; Hwang, B.-J.; et al. Rechargeable Aluminum Batteries: Effects of Cations in Ionic Liquid Electrolytes. RSC Adv. 2019, 9, 11322–11330. [Google Scholar] [CrossRef]
- Lin, M.-C.; Gong, M.; Lu, B.; Wu, Y.; Wang, D.-Y.; Guan, M.; Angell, M.; Chen, C.; Yang, J.; Hwang, B.-J.; et al. An Ultrafast Rechargeable Aluminium-Ion Battery. Nature 2015, 520, 324–328. [Google Scholar] [CrossRef]
- Nicholson, R.S. Theory and Application of Cyclic Voltammetry for Measurement of Electrode Reaction Kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Paul, H.J.; Leddy, J. Direct Determination of the Transfer Coefficient from Cyclic Voltammetry: Isopoints as Diagnostics. Anal. Chem. 1995, 67, 1661–1668. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry, 3rd ed.; Wiley: New York, NY, USA, 2006; ISBN 978-0-471-67879-3. [Google Scholar]
- Brett, C.M.A.; Brett, A.M.O. Electrochemistry Principles, Methods, and Applications; Oxford University Press: Oxford, UK, 1993; ISBN 978-0-19-855388-5. [Google Scholar]
- Marsden, K.C.; Pesic, B. Evaluation of the Electrochemical Behavior of CeCl3 in Molten LiCl-KCl Eutectic Utilizing Metallic Ce as an Anode. J. Electrochem. Soc. 2011, 158, F111. [Google Scholar] [CrossRef]
- Serrano, K.; Taxil, P. Electrochemical Nucleation of Uranium in Molten Chlorides. J. Appl. Electrochem. 1999, 29, 505–510. [Google Scholar] [CrossRef]
- Branco, P.D.; Mostany, J.; Borrás, C.; Scharifker, B.R. The Current Transient for Nucleation and Diffusion-Controlled Growth of Spherical Caps. J. Solid State Electrochem. 2009, 13, 565–571. [Google Scholar] [CrossRef]
- Rodríguez-Clemente, E.; Manh, T.L.; Guinto-Pano, C.E.; Romero-Romo, M.; Mejía-Caballero, I.; Morales-Gil, P.; Palacios-González, E.; Ramírez-Silva, M.T.; Palomar-Pardavé, M. Aluminum Electrochemical Nucleation and Growth onto a Glassy Carbon Electrode from a Deep Eutectic Solvent. J. Electrochem. Soc. 2019, 166, D3035–D3041. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).