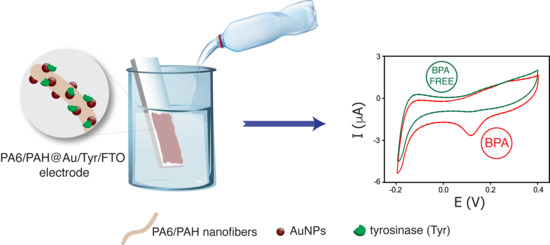
Electrochemical Detection of Bisphenol A by Tyrosinase Immobilized on Electrospun Nanofibers Decorated with Gold Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of the Biosensing Platform
2.3. Characterization and Electrochemical Measurements
3. Results and Discussion
3.1. Choice of Materials for the Biosensing Platform
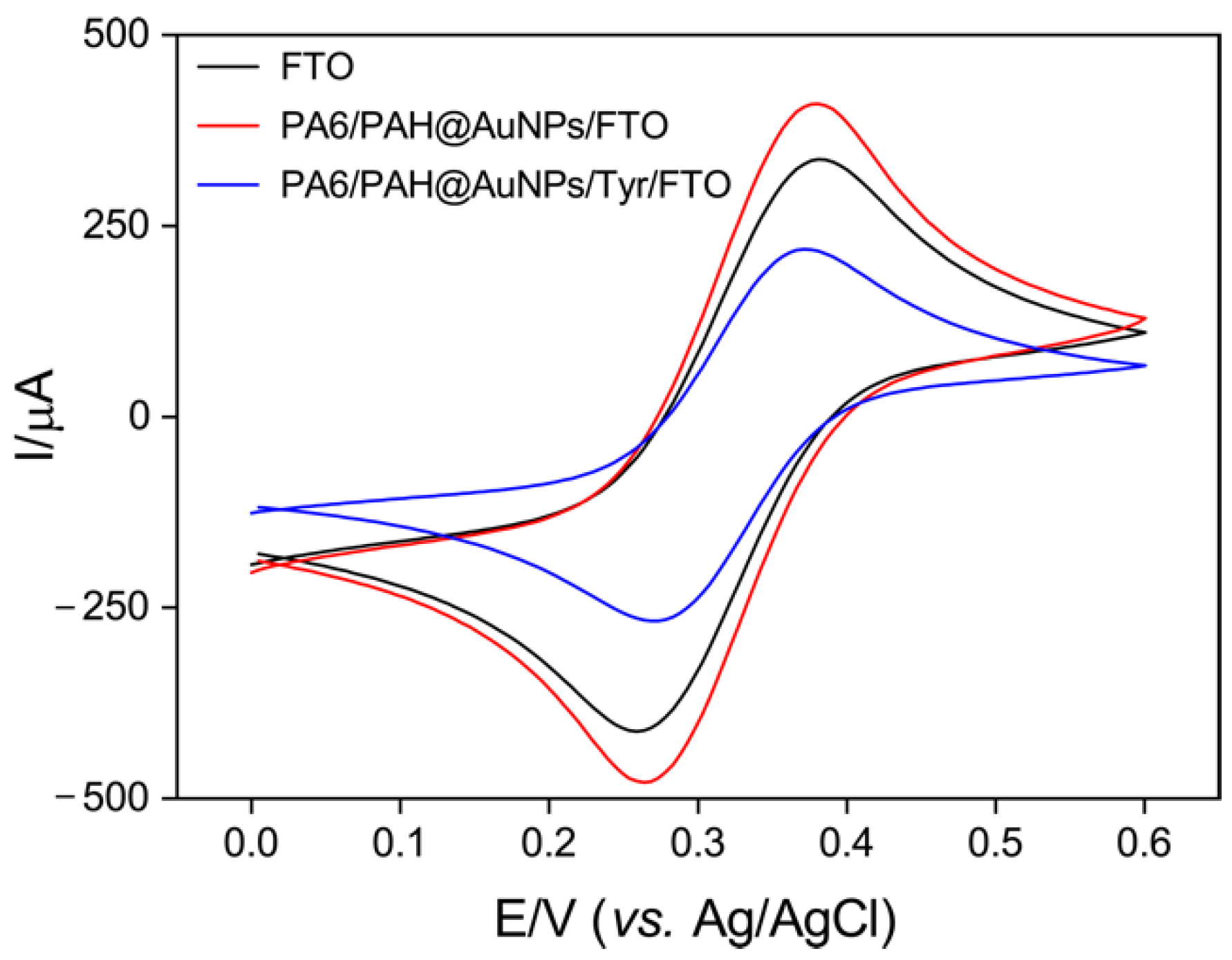
3.2. Characterization of the Biosensing Platform
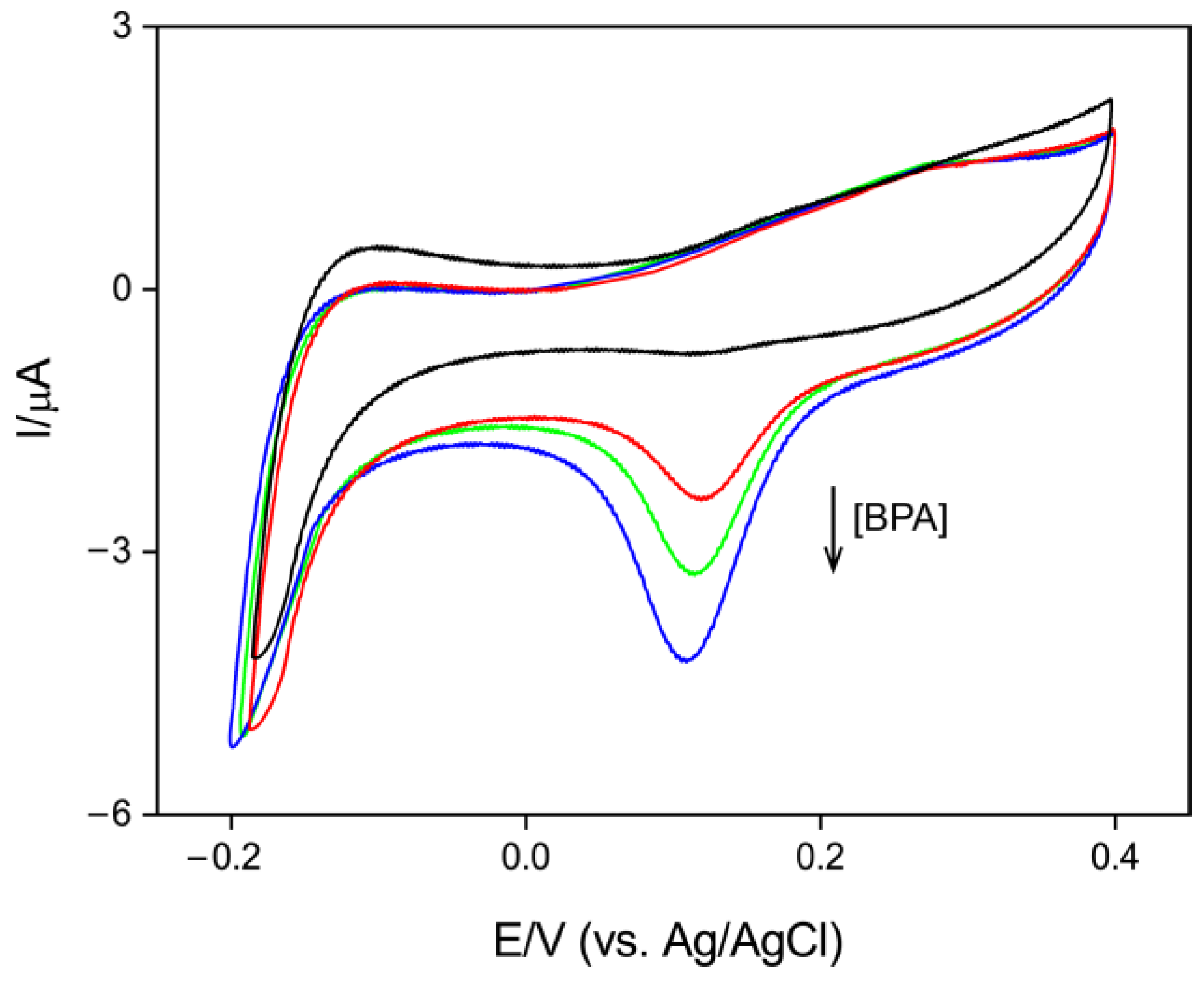
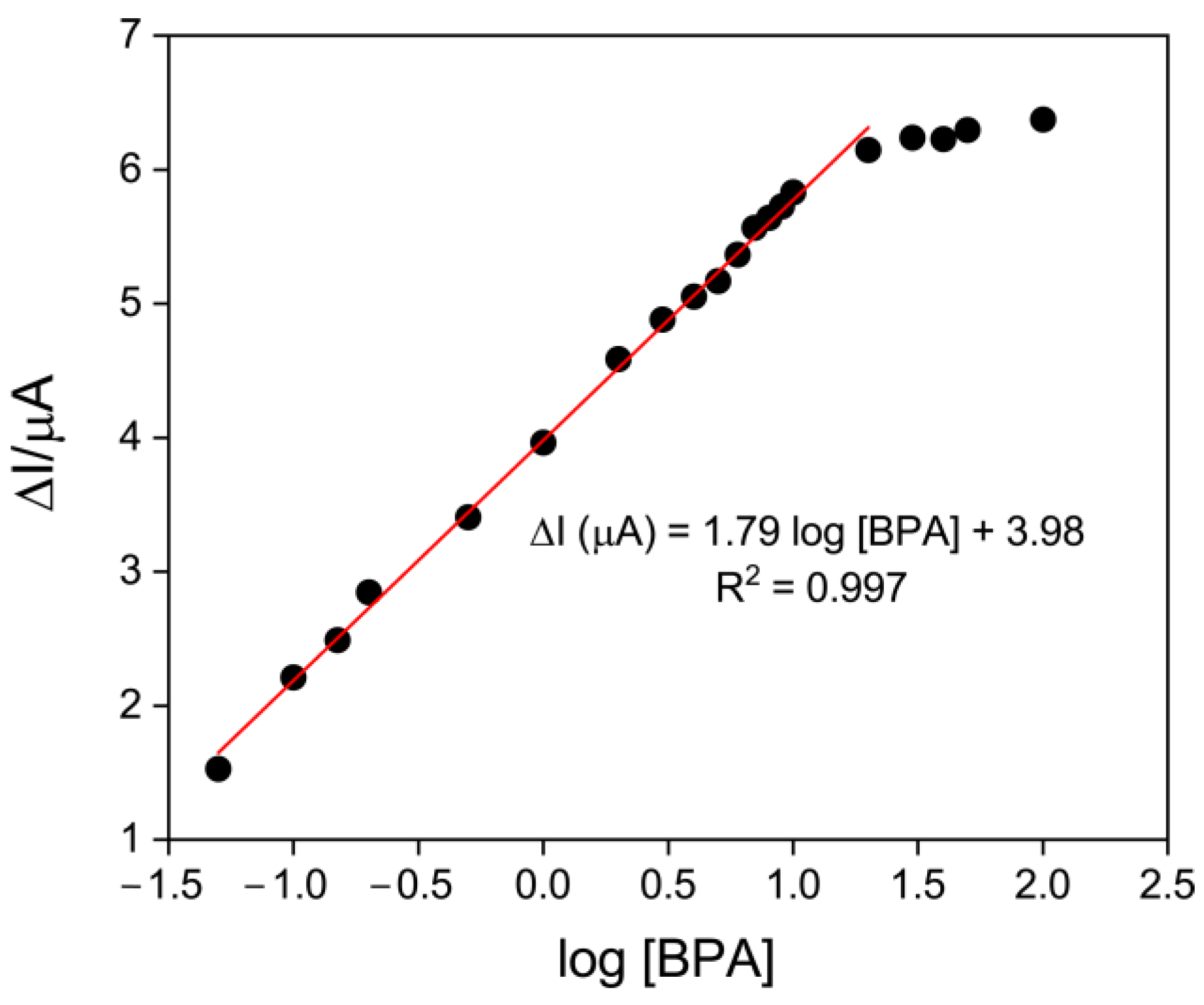
3.3. Electrochemical Detection of BPA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simón-Herrero, C.; Naghdi, M.; Taheran, M.; Kaur Brar, S.; Romero, A.; Valverde, J.L.; Avalos Ramirez, A.; Sánchez-Silva, L. Immobilized laccase on polyimide aerogels for removal of carbamazepine. J. Hazard. Mater. 2019, 376, 83–90. [Google Scholar] [CrossRef]
- Koloti, L.E.; Gule, N.P.; Arotiba, O.A.; Malinga, S.P. Laccase-immobilized dendritic nanofibrous membranes as a novel approach towards the removal of bisphenol A. Environ. Technol. 2018, 39, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Maynard, I.F.N.; Cavalcanti, E.B.; da Silva, L.L.; Martins, E.A.J.; Pires, M.A.F.; de Barros, M.L.; Cardoso, E.; Marques, M.N. Assessing the presence of endocrine disruptors and markers of anthropogenic activity in a water supply system in northeastern Brazil. J. Environ. Sci. Heal. Part A Toxic Hazard. Subst. Environ. Eng. 2019, 54, 891–898. [Google Scholar] [CrossRef]
- Maryšková, M.; Schaabová, M.; Tománková, H.; Novotný, V.; Rysová, M. Wastewater Treatment by Novel Polyamide/Polyethylenimine Nanofibers with Immobilized Laccase. Water 2020, 12, 588. [Google Scholar] [CrossRef]
- Starling, M.C.V.M.; Amorim, C.C.; Leão, M.M.D. Occurrence, control and fate of contaminants of emerging concern in environmental compartments in Brazil. J. Hazard. Mater. 2019, 372, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Alam, A.U.; Howlader, M.M.R. Fabrication of highly sensitive Bisphenol A electrochemical sensor amplified with chemically modified multiwall carbon nanotubes and β-cyclodextrin. Sens. Actuators B Chem. 2020, 320, 128319. [Google Scholar] [CrossRef]
- Sinha, A.; Wu, L.; Lu, X.; Chen, J.; Jain, R. Advances in sensing and biosensing of bisphenols: A review. Anal. Chim. Acta 2018, 998, 1–27. [Google Scholar] [CrossRef]
- Kaya, S.I.; Cetinkaya, A.; Bakirhan, N.K.; Ozkan, S.A. Trends in sensitive electrochemical sensors for endocrine disruptive compounds. Trends Environ. Anal. Chem. 2020, 28, e00106. [Google Scholar] [CrossRef]
- Ragavan, K.V.; Rastogi, N.K.; Thakur, M.S. Sensors and biosensors for analysis of bisphenol-A. TrAC Trends Anal. Chem. 2013, 52, 248–260. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Zhang, K.; Van Le, Q.; Jang, H.W.; Kim, S.Y.; Shokouhimehr, M. Recent Advances in Electrochemical Sensors and Biosensors for Detecting Bisphenol A. Sensors 2020, 20, 3364. [Google Scholar] [CrossRef]
- Lu, X.; Sun, J.; Sun, X. Recent advances in biosensors for the detection of estrogens in the environment and food. TrAC Trends Anal. Chem. 2020, 127, 115882. [Google Scholar] [CrossRef]
- Andre, R.S.; Mercante, L.A.; Facure, M.H.M.; Pavinatto, A.; Correa, D.S. Electrospun composite nanofibers as sensors for food analysis. In Electrospun Polymers and Composites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 261–286. ISBN 9780128196113. [Google Scholar]
- Sapountzi, E.; Braiek, M.; Chateaux, J.-F.; Jaffrezic-Renault, N.; Lagarde, F. Recent Advances in Electrospun Nanofiber Interfaces for Biosensing Devices. Sensors 2017, 17, 1887. [Google Scholar] [CrossRef] [PubMed]
- Mercante, L.A.; Scagion, V.P.; Migliorini, F.L.; Mattoso, L.H.C.; Correa, D.S. Electrospinning-based (bio)sensors for food and agricultural applications: A review. TrAC Trends Anal. Chem. 2017, 91, 91–103. [Google Scholar] [CrossRef]
- Li, Y.; Abedalwafa, M.A.; Tang, L.; Li, D.; Wang, L. Electrospun Nanofibers for Sensors. In Electrospinning: Nanofabrication and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 571–601. ISBN 9780323512701. [Google Scholar]
- Yang, T.; Zhan, L.; Huang, C.Z. Recent insights into functionalized electrospun nanofibrous films for chemo-/bio-sensors. TrAC Trends Anal. Chem. 2020, 124, 115813. [Google Scholar] [CrossRef]
- Jankowska, K.; Zdarta, J.; Grzywaczyk, A.; Kijeńska-Gawrońska, E.; Biadasz, A.; Jesionowski, T. Electrospun poly(methyl methacrylate)/polyaniline fibres as a support for laccase immobilisation and use in dye decolourisation. Environ. Res. 2020, 184, 109332. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Goodge, K.; Delaney, M.; Struzyk, A.; Tansey, N.; Frey, M. A Comprehensive Review of the Covalent Immobilization of Biomolecules onto Electrospun Nanofibers. Nanomaterials 2020, 10, 2142. [Google Scholar] [CrossRef]
- Fatarella, E.; Spinelli, D.; Ruzzante, M.; Pogni, R. Nylon 6 film and nanofiber carriers: Preparation and laccase immobilization performance. J. Mol. Catal. B Enzym. 2014, 102, 41–47. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Mercante, L.A.; Facure, M.H.M.; Sanfelice, R.C.; Migliorini, F.L.; Mattoso, L.H.C.; Correa, D.S. One-pot preparation of PEDOT:PSS-reduced graphene decorated with Au nanoparticles for enzymatic electrochemical sensing of H2O2. Appl. Surf. Sci. 2017, 407, 162–170. [Google Scholar] [CrossRef]
- Soares, J.C.; Iwaki, L.E.O.; Soares, A.C.; Rodrigues, V.C.; Melendez, M.E.; Fregnani, J.H.T.G.; Reis, R.M.; Carvalho, A.L.; Corrêa, D.S.; Oliveira, O.N. Immunosensor for Pancreatic Cancer Based on Electrospun Nanofibers Coated with Carbon Nanotubes or Gold Nanoparticles. ACS Omega 2017, 2, 6975–6983. [Google Scholar] [CrossRef]
- Migliorini, F.L.; Sanfelice, R.C.; Pavinatto, A.; Steffens, J.; Steffens, C.; Correa, D.S. Voltammetric cadmium(II) sensor based on a fluorine doped tin oxide electrode modified with polyamide 6/chitosan electrospun nanofibers and gold nanoparticles. Microchim. Acta 2017, 184, 1077–1084. [Google Scholar] [CrossRef]
- Mercante, L.A.; Pavinatto, A.; Iwaki, L.E.O.; Scagion, V.P.; Zucolotto, V.; Oliveira, O.N.; Mattoso, L.H.C.; Correa, D.S. Electrospun polyamide 6/poly(allylamine hydrochloride) nanofibers functionalized with carbon nanotubes for electrochemical detection of dopamine. ACS Appl. Mater. Interfaces 2015, 7, 4784–4790. [Google Scholar] [CrossRef] [PubMed]
- Raymundo-Pereira, P.A.; Silva, T.A.; Caetano, F.R.; Ribovski, L.; Zapp, E.; Brondani, D.; Bergamini, M.F.; Marcolino, L.H.; Banks, C.E.; Oliveira, O.N.; et al. Polyphenol oxidase-based electrochemical biosensors: A review. Anal. Chim. Acta 2020, 1139, 198–221. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, Y.; Kim, J.; Yao, L.; Dong, X.; Li, T.; Piao, Y. Multi-layered enzyme coating on highly conductive magnetic biochar nanoparticles for bisphenol A sensing in water. Chem. Eng. J. 2020, 384, 123276. [Google Scholar] [CrossRef]
- Wu, L.; Gao, J.; Lu, X.; Huang, C.; Chen, J. Graphdiyne: A new promising member of 2D all-carbon nanomaterial as robust electrochemical enzyme biosensor platform. Carbon N. Y. 2020, 156, 568–575. [Google Scholar] [CrossRef]
- Wang, H.; Wang, D.; Peng, Z.; Tang, W.; Li, N.; Liu, F. Assembly of DNA-functionalized gold nanoparticles on electrospun nanofibers as a fluorescent sensor for nucleic acids. Chem. Commun. 2013, 49, 5568–5570. [Google Scholar] [CrossRef]
- Pavinatto, A.; Mercante, L.A.; Facure, M.H.M.; Pena, R.B.; Sanfelice, R.C.; Mattoso, L.H.C.; Correa, D.S. Ultrasensitive biosensor based on polyvinylpyrrolidone/chitosan/reduced graphene oxide electrospun nanofibers for 17α–ethinylestradiol electrochemical detection. Appl. Surf. Sci. 2018, 458, 431–437. [Google Scholar] [CrossRef]
- Alkasir, R.S.J.; Ganesana, M.; Won, Y.-H.; Stanciu, L.; Andreescu, S. Enzyme functionalized nanoparticles for electrochemical biosensors: A comparative study with applications for the detection of bisphenol A. Biosens. Bioelectron. 2010, 26, 43–49. [Google Scholar] [CrossRef]
- Oriero, D.A.; Gyan, I.O.; Bolshaw, B.W.; Cheng, I.F.; Aston, D.E. Electrospun biocatalytic hybrid silica-PVA-tyrosinase fiber mats for electrochemical detection of phenols. Microchem. J. 2015, 118, 166–175. [Google Scholar] [CrossRef]
- Inroga, F.A.D.; Rocha, M.O.; Lavayen, V.; Arguello, J. Development of a tyrosinase-based biosensor for bisphenol A detection using gold leaf–like microstructures. J. Solid State Electrochem. 2019, 23, 1659–1666. [Google Scholar] [CrossRef]
- Zehani, N.; Fortgang, P.; Saddek Lachgar, M.; Baraket, A.; Arab, M.; Dzyadevych, S.V.; Kherrat, R.; Jaffrezic-Renault, N. Highly sensitive electrochemical biosensor for bisphenol A detection based on a diazonium-functionalized boron-doped diamond electrode modified with a multi-walled carbon nanotube-tyrosinase hybrid film. Biosens. Bioelectron. 2015, 74, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Staszak, M.; Jankowska, K.; Kaźmierczak, K.; Degórska, O.; Nguyen, L.N.; Kijeńska-Gawrońska, E.; Pinelo, M.; Jesionowski, T. The response surface methodology for optimization of tyrosinase immobilization onto electrospun polycaprolactone–chitosan fibers for use in bisphenol A removal. Int. J. Biol. Macromol. 2020, 165, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhou, Y.; Xu, J.; Ai, S.; Cui, L.; Zhu, L. Amperometric biosensor based on tyrosinase immobilized onto multiwalled carbon nanotubes-cobalt phthalocyanine-silk fibroin film and its application to determine bisphenol A. Anal. Chim. Acta 2010, 659, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Furquim, F.C.; Santos, E.N.; Mercante, L.A.; Amaral, M.M.; Pavinatto, A.; Rodrigues, B.V.M. Green and low-cost electrospun membranes from polycaprolactone/graphene oxide for Bisphenol A sensing. Mater. Lett. 2020, 274, 128014. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Lv, S.; Huang, Z.; Cui, L.; Wu, T. An Electrochemical Sensor Based on Nitrogen-doped Carbon Nanofiber for Bisphenol A Determination. Electroanalysis 2016, 28, 439–444. [Google Scholar] [CrossRef]
- Wu, L.; Deng, D.; Jin, J.; Lu, X.; Chen, J. Nanographene-based tyrosinase biosensor for rapid detection of bisphenol A. Biosens. Bioelectron. 2012, 35, 193–199. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Wu, L.; Chen, J. 3D metal-organic framework as highly efficient biosensing platform for ultrasensitive and rapid detection of bisphenol A. Biosens. Bioelectron. 2015, 65, 295–301. [Google Scholar] [CrossRef]
- Lu, X.; Wang, X.; Wu, L.; Wu, L.; Fu, L.; Gao, Y.; Chen, J. Response Characteristics of Bisphenols on a Metal-Organic Framework-Based Tyrosinase Nanosensor. ACS Appl. Mater. Interfaces 2016, 8, 16533–16539. [Google Scholar] [CrossRef]
- Singh, N.; Ali, M.A.; Suresh, K.; Agrawal, V.V.; Rai, P.; Sharma, A.; Malhotra, B.D.; John, R. In-situ electrosynthesized nanostructured Mn3O4-polyaniline nanofibers- biointerface for endocrine disrupting chemical detection. Sens. Actuators B Chem. 2016, 236, 781–793. [Google Scholar] [CrossRef]
- Mayedwa, N.; Ajayi, R.F.; Mongwaketsi, N.; Matinise, N.; Mulaudzi-Masuku, T.; Hendricks, K.; Maaza, M. Development of a Novel Tyrosinase Amperometric Biosensor Based on Tin Nanoparticles for the Detection of Bisphenol A (4.4-Isopropylidenediphenol) in Water. J. Phys. Conf. Ser. 2019, 1310, 012005. [Google Scholar] [CrossRef]
- Wu, L.; Lu, X.; Niu, K.; Chen, J. Tyrosinase nanocapsule based nano-biosensor for ultrasensitive and rapid detection of bisphenol A with excellent stability in different application scenarios. Biosens. Bioelectron. 2020, 165, 112407. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yao, L.; He, L.; Liu, N.; Piao, Y. Electrochemical Enzyme Biosensor Bearing Biochar Nanoparticle as Signal Enhancer for Bisphenol A Detection in Water. Sensors 2019, 19, 1619. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Park, G.W.; Yoo, Y.J.; Lee, J.S. A perspective on the biotechnological applications of the versatile tyrosinase. Bioresour. Technol. 2019, 289, 121730. [Google Scholar] [CrossRef] [PubMed]

| Electrode | Nanomaterial Type | Linear Range (µM) | LOD (µM) | Reference |
|---|---|---|---|---|
| Tyr-NGP-Chi/GCE | Carbon-based | 0.1–2 | 0.033 | [38] |
| Tyr-GDY-Chi/GCE | Carbon-based | 0.1–3.5 | 0.024 | [27] |
| Tyr/Mag-BCNPs-COOH/MGCE | Nanocomposite | 0.01–1.01 | 0.003 | [26] |
| Tyr-SF-MWNTs-CoPc/GCE | Nanocomposite | 0.05–3 | 0.03 | [35] |
| CuMOF-Tyr-Chi/GCE | Nanocomposite | 0.05–3 | 0.013 | [39,40] |
| Tyr@PANI-Mn3O4/ITO | Nanocomposite | 0.004–0.8 | 0.004 | [41] |
| Tyr-NiNPs/SPCE | Metallic Nanoparticle | 0.9–48 | 0.007 | [30] |
| Tyr/SnNP/GCE | Metallic Nanoparticle | 0.01–0.1 | 0.002 | [42] |
| Nafion/Tyr/Au/SPCE | Metallic Nanoparticle | 0.5–50 | 0.077 | [32] |
| nTyr-Chi/GCE | Nanocapsule | 0.05–2 | 0.012 | [43] |
| BCNPs/Tyr/Nafion/GCE | Polymeric Nanoparticle | 0.02–10 | 0.003 | [44] |
| PA6/PAH@AuNPs/Tyr/FTO | Electrospun Nanofiber | 0.05–20 | 0.011 | This work |
| Compound | Concentration (µM) | % of Signal |
|---|---|---|
| BPA | 5 | 100 |
| phenol | 20 | 20.5 |
| 4-nitrophenol | 20 | 7.6 |
| 4-methoxyphenol | 20 | 4.7 |
| 4-aminophenol | 20 | 1.5 |
| ascorbic acid, uric acid, urea, glucose, KCl, NaCl | 500 | - |
| Real Sample | Recovery (%) | RSD (%) |
|---|---|---|
| Tap Water | 95 | 1.3 |
| Bottled Water | 105 | 2.7 |
| River Water | 92 | 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercante, L.A.; Iwaki, L.E.O.; Scagion, V.P.; Oliveira, O.N., Jr.; Mattoso, L.H.C.; Correa, D.S. Electrochemical Detection of Bisphenol A by Tyrosinase Immobilized on Electrospun Nanofibers Decorated with Gold Nanoparticles. Electrochem 2021, 2, 41-49. https://doi.org/10.3390/electrochem2010004
Mercante LA, Iwaki LEO, Scagion VP, Oliveira ON Jr., Mattoso LHC, Correa DS. Electrochemical Detection of Bisphenol A by Tyrosinase Immobilized on Electrospun Nanofibers Decorated with Gold Nanoparticles. Electrochem. 2021; 2(1):41-49. https://doi.org/10.3390/electrochem2010004
Chicago/Turabian StyleMercante, Luiza A., Leonardo E. O. Iwaki, Vanessa P. Scagion, Osvaldo N. Oliveira, Jr., Luiz H. C. Mattoso, and Daniel S. Correa. 2021. "Electrochemical Detection of Bisphenol A by Tyrosinase Immobilized on Electrospun Nanofibers Decorated with Gold Nanoparticles" Electrochem 2, no. 1: 41-49. https://doi.org/10.3390/electrochem2010004
APA StyleMercante, L. A., Iwaki, L. E. O., Scagion, V. P., Oliveira, O. N., Jr., Mattoso, L. H. C., & Correa, D. S. (2021). Electrochemical Detection of Bisphenol A by Tyrosinase Immobilized on Electrospun Nanofibers Decorated with Gold Nanoparticles. Electrochem, 2(1), 41-49. https://doi.org/10.3390/electrochem2010004