Abstract
Polymer hybrid composites and hybrid polymer composites are distinct but interconnected composite classes, each with unique compositions and design philosophies. The mechanical properties of these composites are vital in advanced materials due to their impacts on performance, durability, and suitability for various applications. The addition of ionic liquids into these composites is a promising innovation in advanced materials. In this short review, various polymer matrices (e.g., thermosets, thermoplastics, and biopolymers), fillers (e.g., inorganic, carbon, organic, and metal), and ionic liquids (e.g., imidazolium- and phosphonium-based) used to fabricate polymer hybrid composites and hybrid polymer composites with added ionic liquids are identified. Furthermore, the addition of ionic liquids into these composites through different methods (e.g., magnetic stirring, mechanical stirring, solid grinding, etc.) is discussed. The influence of ionic liquid addition on the mechanical properties, specifically the tensile properties of these composites, is also shortly reviewed. The changes in the tensile properties, such as the tensile strength, tensile modulus, and elongation at break, of these composites are explained as well. The information presented in this review enhances the understanding of the methods applied to add ionic liquids into polymer hybrid composites and hybrid polymer composites, along with their tensile properties. In short, some ionic liquids have the capacity to enhance the tensile properties of hybrid polymer composites, and several ionic liquids can reduce the tensile properties of polymer hybrid composites.
1. Introduction
Polymer hybrid composites and hybrid polymer composites are two distinct yet interconnected classes of composite materials, each characterized by unique compositions and design philosophies. Polymer hybrid composites predominantly refer to composites in which a polymer matrix is reinforced with various types of fibers or fillers [1]. Here, the focus is on synergistically combining different reinforcing materials within the polymer matrix itself. For instance, a polymer hybrid composite might consist of a polymer matrix reinforced with a combination of a metal–organic framework and graphene oxide [2]. This approach leverages the exceptional mechanical, thermal, and functional properties of the chosen reinforcing materials to enhance the overall composite performance. On the other hand, hybrid polymer composites entail the combination of different polymers within the composite material [3]. This design strategy diverges from the conventional reinforcement-based approach by emphasizing the integration of diverse polymer constituents. For example, a hybrid polymer composite could involve blending two or more polymers, each contributing distinct attributes to the final material [4]. This blending of polymers results in a composite with tailored properties that surpass the individual capabilities of the constituent polymers. Therefore, polymer hybrid composites focus on combining different reinforcing materials within a polymer matrix, while hybrid polymer composites center on blending different polymers with a reinforcing material to achieve materials with combined properties.
The mechanical properties of polymer hybrid composites and hybrid polymer composites hold paramount significance in the field of advanced materials due to their direct influences on material performance, durability, and suitability for specific applications. These properties provide insights into how these composites will behave under various loads and conditions, thereby guiding their optimal utilization. Tensile properties, a pivotal subset of mechanical properties, are particularly crucial in assessing the structural integrity of these composites. The tensile strength, tensile modulus, and elongation at break offer insights into a composite’s ability to withstand pulling forces to determine its strength, stiffness, and ductility [5]. In polymer hybrid composites, where different reinforcing materials synergize within the polymer matrix, understanding the tensile properties illuminates how these materials interact, potentially leading to superior strength-to-weight ratios or enhanced impact resistance [6]. In hybrid polymer composites, where distinct polymers are combined, the tensile properties elucidate the cooperative effects of blended polymers, impacting factors like flexibility, toughness, and overall strength [7]. The accurate measurement of tensile properties guides material design, aiding in the selection of optimal polymer combinations or reinforcement ratios. Ultimately, comprehending the tensile behaviors of both of these composite classes is essential for tailoring materials to diverse applications ranging from aerospace and automotive applications to structural engineering [8].
The addition of ionic liquids into polymer hybrid composites and hybrid polymer composites constitutes a novel and promising avenue in the field of advanced materials. Ionic liquids are unique organic salts that possess intriguing properties and exhibit remarkable thermal stability, electrical conductivity, and tunable properties, which can impart distinct functionalities to these composites [9]. Ionic liquids possess several distinctive characteristics, rendering them encouraging candidates as valuable supplementary substances for polymer composites. A substantial attribute of ionic liquids is their compatibility with a wide range of polymer matrices and reinforcing materials [10]. Moreover, it is possible to tailor the properties of ionic liquids to enhance their efficiency. Nonetheless, the efficiency of ionic liquids can be influenced by various factors, including specific polymer matrices, the types of reinforcing materials, varieties of ionic liquids, and methods of addition. When successfully added, ionic liquids can confer multifaceted benefits. In polymer hybrid composites, they may enhance interfacial adhesion between the matrix and reinforcing materials, thus improving the mechanical properties [11]. In hybrid polymer composites, they can tune the polymer–polymer interactions, resulting in tailored mechanical, electrical, or thermal properties [12]. As research continues to explore the potential of ionic liquids in composite materials, their addition represents an emerging frontier that could revolutionize material design.
At this time, the addition of ionic liquids into polymer hybrid composites and hybrid polymer composites has attracted considerable attention from researchers in the respective fields. However, it is important to note that the addition of ionic liquids into composites is relatively rare and at the forefront of research. Due to their complex nature and potential reactivity with polymers, ionic liquids require meticulous compatibility assessments and tailored processing techniques to ensure their successful integration [13]. The rarity of their addition can be attributed to these challenges and the need for an in-depth understanding of how ionic liquids interact with the polymer matrix and any reinforcing materials [14]. So far, based on the authors’ knowledge, there are only a few short reviews that specifically review the methods and mechanical properties of polymer hybrid composites and hybrid polymer composites with added ionic liquids. Hence, this short review aims to provide an enhanced comprehension of the methods for adding ionic liquids into the composites, with a specific focus on their mechanical properties, particularly their tensile properties. Furthermore, the addition of ionic liquids brings to light fresh perceptions of their potential as supplementary substances for improving the mechanical properties of both polymer hybrid composites and hybrid polymer composites.
2. Polymer Matrices of Polymer Hybrid Composites and Hybrid Polymer Composites with Added Ionic Liquids
The differences between the polymer matrices in polymer hybrid composites and hybrid polymer composites lie in their composition and functional roles. In polymer hybrid composites, the polymer matrix refers to the primary polymeric material into which reinforcing materials, such as fibers or fillers, are incorporated to enhance specific properties. The focus is on the interaction between the primary polymer matrix and the reinforcing materials, and successful implementation requires careful consideration of the processing techniques and additives to ensure uniform dispersion and effective bonding. On the other hand, in hybrid polymer composites, the polymer matrix is itself a blend of different polymers with an incorporated reinforcing material, forming a heterogeneous mixture that combines the unique properties of each polymer component. The challenge here is to achieve a homogeneous blend and compatibility between the chosen polymers to avoid issues like phase separation. Therefore, the key difference lies in whether the matrix is a single polymer with added reinforcements (polymer hybrid composites) or a blend of polymers with added reinforcements (hybrid polymer composites).
2.1. Polymer Matrices of Polymer Hybrid Composites with Added Ionic Liquids
Table 1 shows examples of polymer matrices used for the fabrication of polymer hybrid composites with added ionic liquids. It can be seen that there is a predominance of synthetic polymers as preferred polymer matrices over natural polymers. The usage of synthetic polymers is evident through examples like bismaleimide resin [15], epoxy resin [2,8,16,17,18,19], high-density polyethylene [20], polyamide-6 [13], poly(methyl methacrylate) [21], polyurethane [6,9,11,22,23], poly(vinyl alcohol) [24,25], and unsaturated polyester resin [26,27]. These polymers, prized for their versatility and tailored properties, seem to dominate composite fabrication. Figure 1 shows the chemical structures of polyurethane, poly(vinyl alcohol), and unsaturated polyester resin. Comparatively, natural polymers are less frequently represented. The less frequent occurrence of natural polymers in composite fabrication can be attributed to the fact that they sometimes exhibit inherent variability in their properties, which can pose challenges in achieving consistent results during composite manufacturing. As biopolymers, chitosan [1] and natural rubber [28,29] stand as exceptions in this array. Their presence highlights the sustainable trend in material choices, aligned with eco-friendly considerations. This trend toward synthetic polymer matrices can be attributed to their controllable properties, diverse applications, and adaptability to specific needs. However, the dual instance of chitosan and natural rubber underlines the significance of exploring natural alternatives for sustainable composite development. Thus, Table 1 stresses the prevalent preference for synthetic polymers while also hinting at the potential growth of eco-friendly options in polymer hybrid composites with added ionic liquids.

Table 1.
Examples of polymer matrices used for the fabrication of polymer hybrid composites with added ionic liquids.
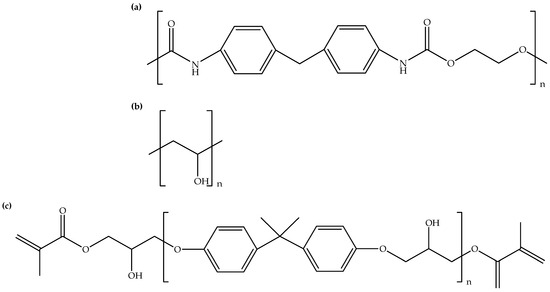
Figure 1.
Chemical structures of (a) PU, (b) PVA, and (c) UPR.
2.2. Polymer Matrices of Hybrid Polymer Composites with Added Ionic Liquids
Table 2 shows examples of polymer matrices used for the fabrication of hybrid polymer composites with added ionic liquids. It can be observed that poly(lactic acid) [12,30,31,32,33] is a more frequently used polymer compared to other synthetic polymers, followed by polypropylene [33,34,35], poly(ε-caprolactone) [31,32], and poly(ethylene-co-vinyl acetate) [3,12]. Figure 2 shows the chemical structures of poly(lactic acid), poly(ε-caprolactone), and poly(ethylene-co-vinyl acetate). The usage of poly(lactic acid) suggests its popularity in sustainable composite fabrication. Its biodegradability and versatility align with the eco-conscious drive-in material selection [36]. Polypropylene, characterized by durability and chemical resistance, is also recurrently used, showcasing its essential role in composite fabrication. Poly(ε-caprolactone) stands out for its biocompatibility and biodegradability, signifying its relevance in specialized applications like biomedical materials. Poly(ethylene-co-vinyl acetate), appreciated for its flexibility and toughness, is applied as a significant contributor to enhance composite properties. Hence, poly(lactic acid) emerges as a notable choice, followed by the consistent presence of polypropylene, poly(ε-caprolactone), and poly(ethylene-co-vinyl acetate). This pattern of usage indicates the preference for certain polymers in specific applications, reflecting a thoughtful selection process based on the desired properties. The diverse range of polymers used in hybrid polymer composites with added ionic liquids features the ongoing innovation in materials science.

Table 2.
Examples of polymer matrices used for the fabrication of hybrid polymer composites with added ionic liquids.
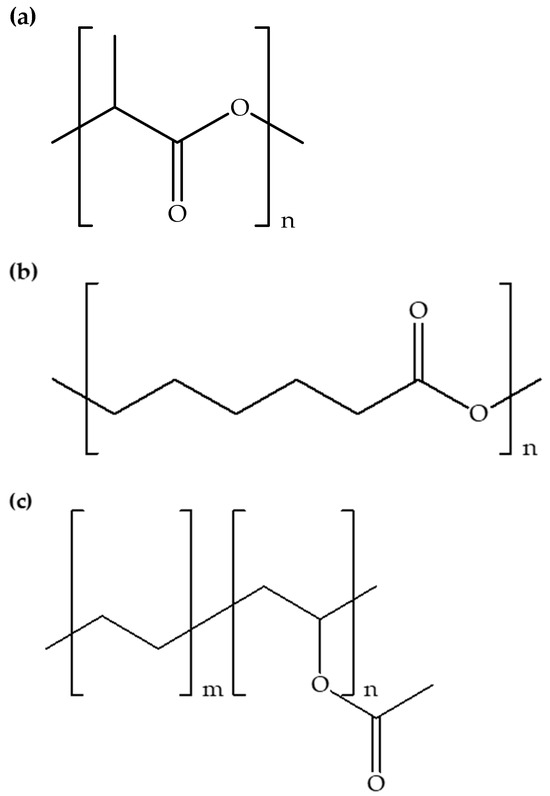
Figure 2.
Chemical structures of (a) PLA, (b) PCL, and (c) EVA.
3. Fillers for Polymer Hybrid Composites and Hybrid Polymer Composites with Added Ionic Liquids
3.1. Fillers for Polymer Hybrid Composites with Added Ionic Liquids
Table 3 shows examples of fillers used for the fabrication of polymer hybrid composites with added ionic liquids. It can be noted that graphene oxide, ammonium polyphosphate, expandable graphite flakes, and multi-walled carbon nanotubes are typically used as fillers for polymer hybrid composites. Graphene oxide is a versatile filler known for its excellent mechanical, thermal, and electrical properties. Its two-dimensional structure and high surface area make it an ideal candidate for reinforcing polymers [1,2,9,17,24,25,27]. Ammonium polyphosphate is a flame-retardant filler used to enhance the fire resistance of polymer composites. It releases non-flammable gases when exposed to high temperatures, thereby reducing the flammability of the composite [9,15,18,26,27]. Expandable graphite flakes are used to improve the thermal and flame-retardant properties of composites. When heated, these flakes expand, creating a barrier that insulates against heat and inhibits flame spread, making them effective flame retardants [9,20,22,27]. Multi-walled carbon nanotubes are known for their exceptional mechanical strength and electrical conductivity. Their high aspect ratios and strong bonding with polymers result in improved structural integrity [8,21,23]. Accordingly, these fillers play crucial roles in tailoring the properties of polymer hybrid composites with added ionic liquids, including mechanical strength, thermal stability, flame resistance, and electrical conductivity, depending on the specific applications and requirements.

Table 3.
Examples of fillers used for the fabrication of polymer hybrid composites with added ionic liquids.
3.2. Fillers for Hybrid Polymer Composites with Added Ionic Liquids
Table 4 shows examples of fillers used for the fabrication of hybrid polymer composites with added ionic liquids. It can be perceived that among the fillers listed, MWCNTs hold significant importance due to their exceptional properties and multifaceted contributions to composite materials. MWCNTs are cylindrical carbon structures with multiple concentric layers [39]. They possess remarkable mechanical strength, thermal conductivity, and electrical properties, making them sought-after reinforcements for polymers [33]. By incorporating MWCNTs into hybrid polymer composites with added ionic liquids, researchers aim to enhance several key characteristics simultaneously. In terms of mechanical performance, MWCNTs act as effective reinforcements, fortifying the composite’s strength and stiffness [12]. Their superior mechanical properties stem from their high aspect ratio and strong interatomic bonds, enabling them to distribute external forces more efficiently throughout the composite structure. Moreover, MWCNTs offer improved electrical conductivity to the composites. Their inherent ability to facilitate electron transport makes them valuable in applications requiring conductivity, such as flexible electronics or electromagnetic shielding [38]. Furthermore, these nanotubes contribute to the enhancement of thermal conductivity [34]. Their efficient heat transfer capabilities help to dissipate heat, which is particularly beneficial in applications requiring effective thermal management. The compatibility of MWCNTs with ionic liquids and polymers is crucial, ensuring proper dispersion and interaction within the composite matrix [31].

Table 4.
Examples of fillers used for the fabrication of hybrid polymer composites with added ionic liquids.
4. Addition of Various Ionic Liquids into Polymer Hybrid Composites and Hybrid Polymer Composites
4.1. Addition of Various Ionic Liquids into Polymer Hybrid Composites
Table 5 shows examples of ionic liquids added into polymer hybrid composites. It can be seen that only two types of ionic liquids, namely imidazolium- and phosphonium-based ionic liquids, have been added to polymer hybrid composites. Imidazolium-based ionic liquids offer versatility, high ionic conductivity, and good solubility, making them adaptable for various applications, though concerns about toxicity and biodegradability persist. On the other hand, phosphonium-based ionic liquids excel in thermal stability, exhibit lower toxicity, and possess a wide liquid temperature range, but they may suffer from higher viscosity and limited ionic conductivity. Additionally, imidazolium-based ionic liquids are typically added more frequently into polymer hybrid composites than phosphonium-based ones. Imidazolium-based ionic liquids may be more cost-effective or accessible for certain scientific studies or industrial applications, further promoting their frequent use. Imidazolium-based ionic liquids have also been extensively studied and characterized over the years [40]. Researchers are often more familiar with their properties, making them readily available for use in experiments and applications. When these specific properties are needed, researchers are more likely to choose imidazolium-based ionic liquids over phosphonium-based ones. In addition, 1-butyl-3-methylimidazolium hexafluorophosphate has been used in multiple studies [9,15,17,18,19,26,27,29], indicating its prevalence in research related to polymer hybrid composites. This ionic liquid likely contributes specific attributes such as hydrophobicity or thermal stability, suggesting its versatility and unique roles in enhancing polymer hybrid composite properties. On the other hand, 1-allyl-3-methylimidazolium chloride [24,25,28,29] and 1-ethyl-3-methylimidazolium tetrafluoroborate [6,16] have appeared in separate studies, indicating their potential for tailoring the performance of polymer hybrid composites. Figure 3 shows the chemical structures of BmimPF6, AmimCl, and EmimBF4.

Table 5.
Examples of ionic liquids added into polymer hybrid composites.
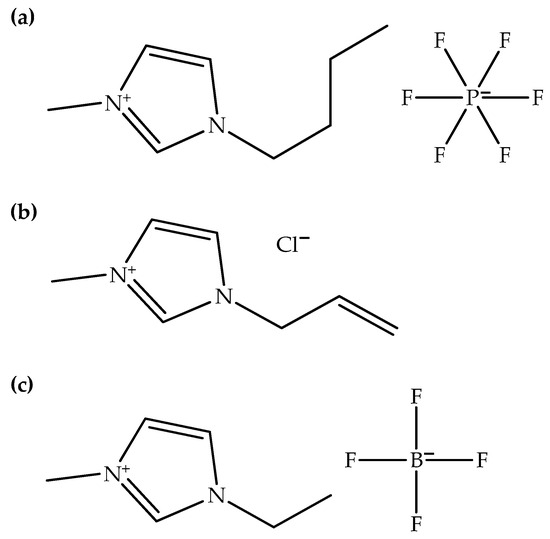
Figure 3.
Chemical structures of (a) BmimPF6, (b) AmimCl, and (c) EmimBF4.
4.2. Addition of Various Ionic Liquids into Hybrid Polymer Composites
Table 6 shows examples of ionic liquids added into hybrid polymer composites. The presence of two distinct types of ionic liquids can be observed, specifically imidazolium- and phosphonium-based ionic liquids, which were likewise added into hybrid polymer composites. Again, imidazolium-based ionic liquids tend to be added with greater frequency compared to their phosphonium-based counterparts. As mentioned earlier, imidazolium-based ionic liquids are preferred in scientific studies or industrial applications due to their cost-effectiveness, accessibility, extensive characterization, and familiarity with their properties. Furthermore, hybrid polymer composites have seen the addition of only a few phosphonium-based ionic liquids. Nevertheless, trihexyl(tetradecyl)phosphonium bistriflimide [33,34,35,37] appears to be a popular choice for addition to hybrid polymer composites, followed by trihexyl(tetradecyl)phosphonium bis(2,4,4-trimethylpentyl)phosphinate [34,35] and 1-vinyl-3-ethylimidazolium bromide [32,38]. Figure 4 shows the chemical structures of TTPTFSI, TTPTMP, and VeimBr. The interest in these ionic liquids likely arises from the specific properties that make them compatible with hybrid polymer composites. This suggests that imidazolium- and phosphonium-based ionic liquids exhibit compatibility with a wide range of polymer types. This can be attributed to the favorable interactions between the ionic liquids and the polymer matrices. Additionally, these ionic liquids can serve as compatibilizers, enhancing the compatibility of immiscible polymer blends [35]. Current research trends in the field of ionic liquids and polymer composites are focused on tailoring ionic liquids for specific polymer systems, involving the design of custom chemical structures and functionalization to enhance compatibility [12,31]. The application of ionic liquids to polymer composites and their role as alternative solvents for polymer processing is expanding [36], contributing to sustainable material development.

Table 6.
Examples of ionic liquids added into hybrid polymer composites.
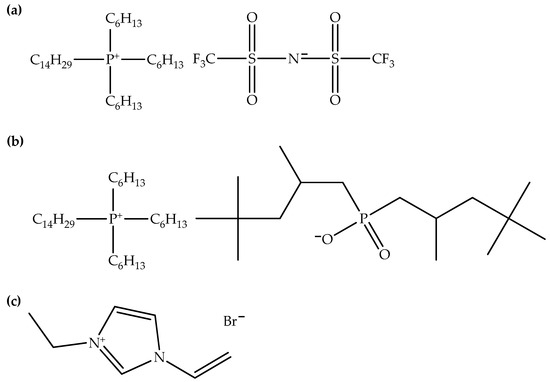
Figure 4.
Chemical structures of (a) TTPTFSI, (b) TTPTMP, and (c) VeimBr.
5. Methods for Adding Ionic Liquids into Polymer Hybrid Composites and Hybrid Polymer Composites
5.1. Methods for Adding Ionic Liquids into Polymer Hybrid Composites
Table 7 shows the methods of adding ionic liquids and the operating temperatures, processing durations, and mixing methods for polymer hybrid composites. The addition of ionic liquids into polymer hybrid composites is a pivotal step in optimizing their properties, with careful consideration given to the compatibility between the ionic liquid and the composite constituents. Traditionally, ionic liquids are added into the composite matrix through processes like stirring, milling, and grinding, where the fillers are intimately mixed with the ionic liquid prior to their addition into the polymer matrix. This sequential method is favored for its effectiveness in ensuring uniform ionic liquid dispersion. However, advanced methods such as ultrasonication and sonication have gained prominence as well, involving the sonication of fillers within a solution containing the ionic liquid [29]. While most of these methods can be executed at room temperature, certain exceptions, such as magnetic stirring [13,26] and sonication [8], may necessitate different temperature conditions. Time considerations are paramount in these processes, with mechanical stirring and solid grinding often completed rapidly, in contrast to ball milling, magnetic stirring, sonication, and ultrasonication, which require extended durations for thorough mixing and dispersion. Table 7 also indicates the mixing methods that are commonly employed in the fabrication of polymer hybrid composites. Mechanical and magnetic stirring are prevalent methods for thermoset hybrid composites [15,18], while melt blending and melt extrusion are generally applied in fabricating thermoplastic hybrid composites [13,20]. In natural polymer hybrid composites, there are two distinctive fabrication methods: thermomechanical kneading [1] and two-roll milling [28].

Table 7.
Methods of adding ionic liquids and the operating temperatures, processing durations, and mixing methods for polymer hybrid composites.
5.2. Methods for Adding Ionic Liquids into Hybrid Polymer Composites
Table 8 shows the methods of adding ionic liquids and the operating temperatures, processing durations, and mixing methods for hybrid polymer composites. The solid grinding method emerges as a prevalent choice for adding ionic liquids into hybrid polymer composites. This method entails the dry grinding of fillers, especially MWCNTs, with an ionic liquid, resulting in the formation of a black paste. Subsequently, this paste is blended with polymer matrices [34]. Furthermore, it is worth noting that mechanical and magnetic stirring methods can also be employed for this particular purpose [3,31]. Another notable method is the direct mixing method, which simplifies the addition of ionic liquids into the composites. This method involves the simultaneous mixing of fillers and ionic liquid directly with the polymer matrices during composite preparation, streamlining the process and enhancing efficiency [30]. In terms of temperature considerations, the majority of methods for adding ionic liquids are carried out at room temperature. However, exceptions exist, particularly for the magnetic stirring method, which requires an elevated temperature [31]. Regarding the processing times, solid grinding typically proceeds rapidly [4,12], whereas mechanical stirring, magnetic stirring, and sonication demand more extended durations to ensure thorough mixing and homogeneity with the fillers [7,31,32]. Table 8 also exhibits the mixing methods utilized to fabricate hybrid polymer composites. Mechanical stirring predominates in the fabrication of hybrid thermoset composites, while melt extrusion and melt blending are commonly applied in the fabrication of hybrid thermoplastic composites. Additionally, the two-roll milling method is frequently employed for hybrid elastomer composites [38].

Table 8.
Methods of adding ionic liquids, operating temperatures, processing durations, and mixing methods for hybrid polymer composites.
6. Mechanical Properties of Polymer Hybrid Composites and Hybrid Polymer Composites with Added Ionic Liquids
6.1. Mechanical Properties of Polymer Hybrid Composites with Added Ionic Liquids
The significance of mechanical properties in polymer hybrid composites and hybrid polymer composites becomes apparent when developing advanced materials. These properties play central roles in determining the overall performance, durability, and suitability of these materials for specific applications. They serve as valuable indicators of how these composites will respond to various loads and conditions, thereby assisting in their optimal utilization. Among these mechanical attributes, tensile properties occupy a pivotal position in evaluating the structural integrity of these composites. The tensile strength, tensile modulus, and elongation at break collectively offer valuable insights into a composite’s capacity to withstand pulling forces to measure its levels of strength and stiffness as well as its degree of ductility.
Table 9 shows the tensile properties of polymer hybrid composites with added ionic liquids. Chen et al. fabricated Chito/rGO/SPT composites with added EmimOAc using the thermomechanical kneading method [1]. The tensile strength of the Chito/rGO/SPT-EmimOAc (20 wt.%) composite was 26.40 MPa, which is substantially lower than that of the Chito/rGO/SPT composite (60.70 MPa). Moreover, the Young’s modulus of the Chito/rGO/SPT-EmimOAc composite was 893 MPa, while that of the Chito/rGO/SPT composite was 1635 MPa. However, the elongation at break of the Chito/rGO/SPT-EmimOAc composite was 78%, which is significantly higher compared to that of the Chito/rGO/SPT composite (19%). The results displayed that the addition of EmimOAc reduced the mechanical strength and stiffness of the Chito/rGO/SPT composite. Nevertheless, this reduction in mechanical properties was accompanied by a substantial improvement in the composite’s ductility, which indicated a higher level of chitosan plasticization facilitated by EmimOAc in the presence of rGO and SPT. The results can help in the design of biopolymer hybrid composites with customized mechanical properties for biomedical, biotechnology, and other high-value applications.

Table 9.
Tensile properties of polymer hybrid composites with added ionic liquids.
Wan et al. fabricated EP/MPP/GO composites with added BmimPF6 using the magnetic stirring method [17]. The tensile strength of the EP/MPP/GO-BmimPF6 (0.1 wt.%) composite was 50.64 MPa, which is moderately higher than that of the EP/MPP/GO composite (45.64 MPa). However, the tensile modulus of the EP/MPP/GO-BmimPF6 composite was 1085 MPa, whereas that of the EP/MPP/GO composite was 1339 MPa. The results showed that the addition of BmimPF6 improved the composite’s resistance to stretching or deformation. This improvement can be attributed to the reinforcing effect of GO-BmimPF6 on the composite’s structural integrity. Nonetheless, this improvement was convoyed by a decrease in stiffness, which might affect the ductility of the composite.
Li et al. fabricated EP/Mo-MOF/GO composites with added BmimBF4 using the magnetic stirring method [2]. The tensile strength of the EP/Mo-MOF/GO-BmimBF4 (3.0 wt.%) composite was 64.31 MPa, which is significantly higher than that of the EP/Mo-MOF/GO composite (51.23 MPa). Moreover, the elongation at break of the EP/Mo-MOF/GO-BmimBF4 composite was 14%, which is marginally higher compared to that of the EP/Mo-MOF/GO composite (11%). The results exhibited that the addition of BmimBF4 contributed positively to enhancing the mechanical properties of the composite. Furthermore, the presence of BmimBF4 resulted in an improved dispersion of the Mo-MOF/GO within the composite and lessened the distinct interface, and Mo-MOF/GO became completely incorporated into the EP matrix. The results also indicated that excellent mechanical properties were obtained, which can broaden the thermoset hybrid composites’ scenario application.
Yasin et al. fabricated NR/CS/CNC composites with added AmimCl using the two-roll milling method [28]. The tensile strength of the NR/CS/CNC-AmimCl (2.0 phr) composite was 4.65 MPa, which is moderately higher than that of the NR/CS/CNC composite (4.10 MPa). However, the elongation at break of the NR/CS/CNC-AmimCl composite was 205%, which is modestly lower compared to that of the NR/CS/CNC composite (220%). The results demonstrated that the addition of AmimCl had a discernible effect on the strength, which could be due to the improved NR-CS/CNC interfacial interaction. On the other hand, a modest reduction in the elongation of the composite suggested a trade-off between strength and ductility. The results provide a perspective for manufacturing high-performance rubber hybrid composites using novel fillers and an ionic liquid.
Wang et al. fabricated NR/CSDPF composites with added AmimCl using the two-roll milling method [29]. The tensile strength of the NR/CSDPF-AmimCl (0.8 phr) composite was 29.60 MPa, which is moderately higher than that of the NR/CSDPF composite (24.70 MPa). However, the tensile modulus of the NR/CSDPF-AmimCl composite was 10 MPa, while that of the NR/CSDPF composite was 12 MPa. Moreover, the elongation at break of the NR/CSDPF-AmimCl composite was 660%, which is significantly higher compared to that of the NR/CSDPF composite (517%). The results indicated that the addition of AmimCl led to interactions with CSPDF and NR (Figure 5), which enhanced CSPDF dispersion within the NR matrix and facilitated effective stress dissipation. Additionally, during tensile fatigue, the hydrogen bonding between AmimCl and CSDPF was subject to disruption and reformation as the NR chains slid along the CSDPF filler.
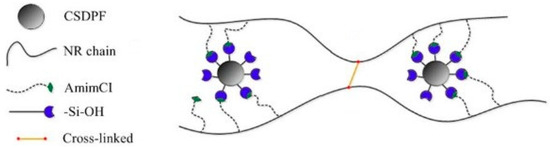
Figure 5.
Schematic of the interactions of AmimCl ionic liquid with CSPDF filler and NR matrix. Adapted with permission from Ref. [29]. 2014, John Wiley & Sons, Inc. (Hoboken, NJ, USA).
Zhang et al. fabricated PA6/MPP/MMT composites with added TDPBr using the melt extrusion method [13]. The tensile strength of the PA6/MPP/MMT-TDPBr (5.0 phr) composite was 50.00 MPa, which is marginally lower than that of the PA6/MPP/MMT composite (53.00 MPa). However, the tensile modulus of the PA6/MPP/MMT-TDPBr composite was 2116 MPa, whereas that of the PA6/MPP/MMT composite was 2077 MPa. Moreover, the strain at break of the PA6/MPP/MMT-TDPBr composite was 2%, which is slightly lower compared to that of the PA6/MPP/MMT composite (3%). The results revealed that the addition of TDPBr modified MMT, which increased the interaction between the MMT-TDPBr and PA6 matrix and consequently improved the dispersion of MMT-TDPBr particles in the matrix. Additionally, the larger increase in the modulus was due to the coordinative effect of MPP between the MMT-TDPBr and PA6 matrix.
Sa et al. fabricated PMMA/rGO/MWCNTs composites with added BmimTFSI using the magnetic stirring method [21]. The tensile strength of the PMMA/rGO/MWCNTs-BmimTFSI (0.5 wt.%) composite was 46.52 MPa, which is significantly higher than that of the PMMA/rGO composite (21.45 MPa). Moreover, the Young’s modulus of the PMMA/rGO/MWCNTs-BmimTFSI composite was 2250 MPa, while that of the PMMA/rGO composite was 1560 MPa. The results exposed that the addition of BmimTFSI had a remarkable impact on the mechanical properties of the composite, which made it considerably stronger, stiffer, and more structurally stable. This can be attributed to the robust cation-π or π–π interactions that occurred between MWCNTs-BmimTFSI and the PMMA matrix.
Sahu et al. fabricated PVA/AgNPs/GO composites with added AmimCl using the magnetic stirring method [25]. The tensile strength of the PVA/AgNPs/GO-AmimCl composite was 34.49 MPa, which is moderately higher than that of the PVA/AgNPs/GO composite (32.36 MPa). However, the elongation at break of the PVA/AgNPs/GO-AmimCl composite was 133%, which is modestly lower compared to that of the PVA/AgNPs/GO composite (149%). The results illustrated that the addition of AmimCl served as a connector between GO and PVA, which lessened the available space and thereby limited the mobility of the PVA chains and facilitated the stress transfer. The interaction between GO-AmimCl and PVA constrained the slipping motion, which reduced the ductility. The results show that the creation of polymer hybrid composites with tailored mechanical properties can be facilitated, which may have potential for biomedical applications.
6.2. Mechanical Properties of Hybrid Polymer Composites with Added Ionic Liquids
Table 10 shows the tensile properties of hybrid polymer composites with added ionic liquids. Gouvêa and Andrade fabricated PHBV/EVA/rGO-ZnO composites with added BmimPF6 using the melt extrusion method [3]. The tensile strength of the PHBV/EVA/rGO-ZnO-BmimPF6 (7.0 wt.%) composite was 4.5 MPa, which is significantly lower than that of the PHBV/EVA/rGO-ZnO composite (7.6 MPa). Moreover, the Young’s modulus of the PHBV/EVA/rGO-ZnO-BmimPF6 composite was 348 MPa, while that of the PHBV/EVA/rGO-ZnO composite was 401 MPa. The results exhibited that the addition of BmimPF6 had a weakening effect on the mechanical properties of the composite. This is due to the higher mobility of PHBV chains, which improved elongation. Therefore, the presence of BmimPF6 resulted in a compromise between the strength and stiffness of the composite. The results also showed that the reduced mechanical properties of the hybrid polymer composites can be widely used in packaging applications.

Table 10.
Tensile properties of hybrid polymer composites with added ionic liquids.
Wang et al. fabricated PLA/EMA-GMA/MWCNTs composites with added CmmimBF4 using the melt blending method [30]. The tensile strength of the PLA/EMA-GMA/MWCNTs-CmmimBF4 (2.0 wt.%) composite was 29.0 MPa, which is similar to that of the PLA/EMA-GMA/MWCNTs composite (29.0 MPa). Moreover, the elongation at break of the PLA/EMA-GMA/MWCNTs-CmmimBF4 composite was 138%, which is significantly higher compared to that of the PLA/EMA-GMA/MWCNTs composite (25%). The results indicated that the addition of CmmimBF4 played a crucial role in enhancing the ability of the composite to deform and stretch, which resulted in increased composite ductility. Furthermore, the even dispersion of MWCNTs mitigated the buildup of stress when the composite underwent tensile testing. Hybrid polymer composites with high flexibility and great reliability can be potentially applied in power electronics, power conditioning, and pulsed power applications.
Lopes Pereira et al. fabricated PLA/EVA/MWCNTs composites with added MimbSO3H·Cl using the melt blending method [12]. The tensile strength of the PLA/EVA/MWCNTs-MimbSO3H·Cl (2.5 phr) composite was 24.0 MPa, which is modestly higher than that of the PLA/EVA/MWCNTs composite (21.0 MPa). Moreover, the tensile modulus of the PLA/EVA/MWCNTs-MimbSO3H·Cl composite was 660 MPa, whereas that of the PLA/EVA/MWCNTs composite was 650 MPa. Additionally, the elongation at break of the PLA/EVA/MWCNTs-MimbSO3H·Cl composite was 8%, which is moderately higher compared to that of the PLA/EVA/MWCNTs composite (6%). The results showed that the addition of MimbSO3H·Cl had a discernible impact on the mechanical properties of the composite. This suggests that MimbSO3H·Cl might have contributed to a slightly stiffer composite and enhanced ductility. This change can likely be attributed to improved interfacial adhesion, which is facilitated by the compatibilization process. The results suggested that hybrid polymer composites are a promising option for developing semi-biodegradable conducting materials, particularly for antistatic packaging and diverse applications in the electroelectronic industry.
Huang et al. fabricated PLA/PCL/PU/MWCNTs composites with added BmimmpdBr using the melt blending method [31]. The tensile strength of the PLA/PCL/PU/MWCNTs-BmimmpdBr (0.8 wt.%) composite was 36.1 MPa, which is moderately higher than that of the PLA/PCL/MWCNTs composite (32.3 MPa). Moreover, the elongation at break of the PLA/PCL/PU/MWCNTs-BmimmpdBr composite was 308%, which is significantly higher compared to the PLA/PCL/MWCNTs composite (242%). The results displayed that the addition of BmimmpdBr improved the interfacial adhesion between the immiscible PLA and PCL phases, which aided in stress transfer and increased stress dissipation during the tensile testing. Additionally, the enhanced ductility was due to the compatibilizing effect of BmimmpdBr-functionalized MWCNTs.
Yousfi et al. fabricated PP/PA6/n-Talc composites with added TTPTMP using the melt extrusion method [35]. The tensile strength of the PP/PA6/n-Talc-TTPTMP (2.0 wt.%) composite was 38.5 MPa, which is significantly higher than that of the PP/PA6 blend (28.8 MPa). Moreover, the Young’s modulus of the PP/PA6/n-Talc-TTPTMP composite was 2060 MPa, while that of the PP/PA6 blend was 1880 MPa. Furthermore, the elongation at break of the PP/PA6/n-Talc-TTPTMP composite was 18%, which is slightly higher compared to that of the PP/PA6 blend (17%). The results demonstrated that the addition of TTPTMP led to a substantial boost in stiffness without compromising the ductility of the composite. Additionally, it is believed that the improved mechanical properties primarily stemmed from the strong interactions and excellent dispersion, as well as the exfoliation of n-Talc layers within the minor PA6 phase.
Liu et al. fabricated SR/POE/MWCNTs composites with added VeimBr using the two-roll milling method [38]. The tensile strength of the SR/POE/MWCNTs-VeimBr (7.0 wt.%) composite was 6.4 MPa, which is substantially higher than that of the SR/POE blend (4.5 MPa). However, the elongation at break of the SR/POE/MWCNTs-VeimBr composite was 183%, which is significantly lower compared to that of the SR/POE blend (370%). The results illustrated that the addition of VeimBr participated in the cross-linking of SR, which enhanced the strength of the composite. Nevertheless, while the reinforcement provided by MWCNTs-VeimBr improved the composite’s strength, it concurrently led to a notable reduction in the ductility of the composite. The results offer a clever and efficient approach for fabricating promising hybrid polymer composites suitable for microwave absorption applications.
Zhao et al. fabricated Stch/PBS/MgCl2 composites with added BmimCl using the melt blending method [7]. The tensile strength of the Stch/PBS/MgCl2-BmimCl (8.8 wt.%) composite was 15.8 MPa, which is significantly higher than that of the Stch/PBS/BmimCl blend (10.8 MPa). Furthermore, the Young’s modulus of the Stch/PBS/MgCl2-BmimCl composite was 1183 MPa, while that of the Stch/PBS/BmimCl blend was 687 MPa. Moreover, the elongation at break of the Stch/PBS/MgCl2-BmimCl composite was 20%, which is moderately higher compared to that of the Stch/PBS/BmimCl blend (14%). The results revealed that the addition of BmimCl, particularly in the presence of MgCl2, resulted in substantial improvements in strength and ductility. This is because chloride anions, which are abundant in electrons, can form robust interactions with hydrogen atoms within the Stch/PBS blend (Figure 6), which bolstered the compatibility between the Stch and PBS phases.
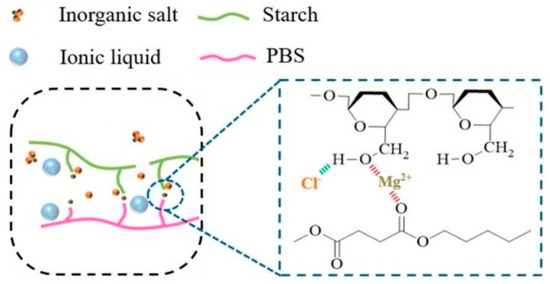
Figure 6.
Schematic of the interactions of BmimCl ionic liquid with MgCl2 salt and Stch/PBS blend [7].
7. Conclusions
In this review, the examples of polymer matrices and fillers of polymer hybrid composites and hybrid polymer composites, examples of ionic liquids added into the polymer composites, and methods for adding ionic liquids are shortly depicted. The mechanical properties, specifically tensile properties such as the tensile strength, tensile modulus, and elongation at break, of polymer hybrid composites and hybrid polymer composites with added ionic liquids are also included in this short review. The ionic liquids that are typically added to polymer composites are imidazolium- and phosphonium-based ionic liquids. Ionic liquids are commonly added to polymer hybrid composites through magnetic and mechanical stirring methods, where fillers are stirred with the ionic liquid before being added into the polymer matrix. In contrast, ionic liquids are generally added to hybrid polymer composites via the solid grinding method, where fillers are dry-ground with the ionic liquid before they are added into the polymer matrices. In the majority of research, the addition of ionic liquids into polymer hybrid composites has led to enhanced tensile strength and elongation at break. These enhancements are attributed to the ability of ionic liquids to interact with fillers, thereby improving the interactions between the filler and polymer matrix and subsequently increasing the strength and ductility of the composites. On the contrary, the addition of ionic liquids into hybrid polymer composites has enhanced the tensile strength, tensile modulus, and elongation at break. This is due to ionic liquids improving the compatibility between the immiscible polymer phases and increasing the interfacial adhesion of the composite constituents, which successively enhances the strength, stiffness, and ductility of the composites. Moreover, the addition of ionic liquids into both types of composites resulted in the improved dispersion of fillers within the polymer matrices, consequently facilitating stress transfer and increasing stress dissipation. Future research in the field should delve into the long-term durability and stability of these composites, assessing their resistance to environmental factors and exploring the potential for imparting multifunctional properties. Additionally, scalability and life cycle assessments are crucial for understanding the feasibility and sustainability of large-scale fabrication. Moreover, applying computational modeling represents a promising avenue for furthering the understanding of polymer hybrid composites and hybrid polymer composites with added ionic liquids.
Author Contributions
Conceptualization, A.A.S.; data curation, S.N.A.M.J.; formal analysis, S.N.A.M.J.; funding acquisition, A.A.S. and K.A.; investigation, A.A.S.; methodology, S.N.A.M.J.; project administration, A.A.S.; resources, M.Z.M.Y.; supervision, M.Z.M.Y.; validation, M.Z.M.Y.; writing—original draft preparation, A.A.S.; writing—review and editing, S.N.A.M.J. and K.A. All authors have read and agreed to the published version of the manuscript.
Funding
This short review was funded by the Universiti Putra Malaysia under the Grant Putra IPM Scheme (project number: GP-IPM/2021/9697900).
Data Availability Statement
Not applicable.
Acknowledgments
We would like to express our sincere gratitude to the editors and reviewers for their invaluable contributions and insightful comments. Their expertise and dedication have greatly enhanced the quality and clarity of this short review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Ionic Liquid-Plasticised Composites of Chitosan and Hybrid 1D and 2D Nanofillers. Funct. Compos. Mater. 2021, 2, 14. [Google Scholar] [CrossRef]
- Li, J.; Wu, W.; Duan, R.; Bi, X.; Meng, W.; Xu, J.; Qu, H. Boron-Containing Ionic Liquid Functionalized Mo-MOF/Graphene Oxide Hybrid for Improving Fire Safety and Maintaining Mechanical Properties for Epoxy Resin. Appl. Surf. Sci. 2023, 611, 155736. [Google Scholar] [CrossRef]
- Gouvêa, R.F.; Andrade, C.T. Testing the Effect of Imidazolium Ionic Liquid and Citrate Derivative on the Properties of Graphene-Based PHBV/EVA Immiscible Blend. Polym. Test. 2020, 89, 106615. [Google Scholar] [CrossRef]
- Calheiros Souto, L.F.; Henriques, R.R.; Soares, B.G. Influence of Acidic and Alkaline Environmental Anticorrosive Performance of Epoxy Coatings Based on Polyaniline/Carbon Nanotube Hybrids Modified with Ionic Liquid. Prog. Org. Coat. 2022, 173, 107206. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Abdan, K.; Md. Jamil, S.N.A. Polybutylene Succinate (PBS)/Natural Fiber Green Composites: Melt Blending Processes and Tensile Properties. Phys. Sci. Rev. 2022, 7, 1–13. [Google Scholar] [CrossRef]
- Strąkowska, A.; Członka, S.; Konca, P.; Strzelec, K. New Flame Retardant Systems Based on Expanded Graphite for Rigid Polyurethane Foams. Appl. Sci. 2020, 10, 5817. [Google Scholar] [CrossRef]
- Zhao, Z.; Lei, B.; Du, W.; Zhang, X. The Effects of Inorganic Salts with Different Anions on the Structure and Properties of Starch/Poly (Butylene Succinate) Blends Plasticized with Ionic Liquid. Polymers 2019, 11, 2004. [Google Scholar] [CrossRef]
- Ghafoor, B.; Farooq, U.; Schrekker, H.S.; Amico, S.C. Thermomechanical Properties of Imidazolium Ionic Liquid-Modified Mwcnt/Carbon Fiber/Epoxy Hybrid Composite Laminates. J. Compos. Mater. 2023, 57, 2219–2230. [Google Scholar] [CrossRef]
- Gao, M.; Wang, T.; Chen, X.; Zhang, X.; Yi, D.; Qian, L.; You, R. Preparation of Ionic Liquid Multifunctional Graphene Oxide and Its Effect on Decrease Fire Hazards of Flexible Polyurethane Foam. J. Therm. Anal. Calorim. 2022, 147, 7289–7297. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Abdan, K.; Md. Jamil, S.N.A. Utilization of Ionic Liquids as Compatibilizing Agents for Polymer Blends–Preparations and Properties. Polym. Technol. Mater. 2023, 62, 1008–1018. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Strzelec, K.; Kairytė, A.; Kremensas, A. Melamine, Silica, and Ionic Liquid as a Novel Flame Retardant for Rigid Polyurethane Foams with Enhanced Flame Retardancy and Mechanical Properties. Polym. Test. 2020, 87, 106511. [Google Scholar] [CrossRef]
- Lopes Pereira, E.C.; da Silva, M.E.C.F.; Pontes, K.; Soares, B.G. Influence of Protonic Ionic Liquid on the Dispersion of Carbon Nanotube in PLA/EVA Blends and Blend Compatibilization. Front. Mater. 2019, 6, 234. [Google Scholar] [CrossRef]
- Zhang, R.-C.; Hong, S.M.; Koo, C.M. Flame Retardancy and Mechanical Properties of Polyamide 6 with Melamine Polyphosphate and Ionic Liquid Surfactant-Treated Montmorillonite. J. Appl. Polym. Sci. 2014, 131, 40648. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Jamil, S.N.A.M.; Abdan, K. The Influence of Ionic Liquid Pretreatment on the Physicomechanical Properties of Polymer Biocomposites: A Mini-Review. e-Polymers 2022, 22, 809–820. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, X.; Shi, H.; Hao, J.; Qu, H.; Wang, J. Graphene Nanoplatelets Hybrid Flame Retardant Containing Ionic Liquid and Ammonium Polyphosphate for Modified Bismaleimide Resin: Excellent Flame Retardancy, Thermal Stability, Water Resistance and Unique Dielectric Properties. Materials 2021, 14, 6406. [Google Scholar] [CrossRef] [PubMed]
- Ghamsari, A.K.; Wicker, S.; Woldesenbet, E. Bucky Syntactic Foam; Multi-Functional Composite Utilizing Carbon Nanotubes-Ionic Liquid Hybrid. Compos. Part B Eng. 2014, 67, 1–8. [Google Scholar] [CrossRef]
- Wan, M.; Shen, J.; Sun, C.; Gao, M.; Yue, L.; Wang, Y. Ionic Liquid Modified Graphene Oxide for Enhanced Flame Retardancy and Mechanical Properties of Epoxy Resin. J. Appl. Polym. Sci. 2021, 138, 50757. [Google Scholar] [CrossRef]
- Zhu, T.; Zhou, X.; Guo, G.; Chai, Z.; Gao, M. Synergistic Flame Retardant Effect between Ionic Liquid Functionalized Imogolite Nanotubes and Ammonium Polyphosphate in Epoxy Resin. Polymers 2023, 15, 1455. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, W.; Zhang, M.; Cui, Y.; Zhang, K.; Qu, H. TGA-FTIR and Char Residue Analysis: Studying the Synergistic Flame Retardancy Mechanism of Ionic Liquid-Modified Graphene and Hexaphenoxy Cyclotriphosphazene. J. Therm. Anal. Calorim. 2022, 147, 13253–13259. [Google Scholar] [CrossRef]
- Li, X.; Liang, D.; Li, K.; Ma, X.; Cui, J.; Hu, Z. Synergistic Effect of a Hypophosphorous Acid-Based Ionic Liquid and Expandable Graphite on the Flame-Retardant Properties of Wood–Plastic Composites. J. Therm. Anal. Calorim. 2021, 145, 2343–2352. [Google Scholar] [CrossRef]
- Sa, K.; Mahakul, P.C.; Nanda, K.K.; Mahanandia, P. Effect of Ionic Liquid Functionalized Carbon Nanotubes on Mechanical, Thermal and Electrical Properties of Carbon Nanotubes-Reduced Graphene Oxide/PMMA Nanocomposites. Chem. Phys. Lett. 2018, 706, 76–81. [Google Scholar] [CrossRef]
- Gebrekrstos Weldemhret, T.; Lee, D.-W.; Tae Park, Y.; Il Song, J. Ionic Liquid-Catalyzed Synthesis of Carbon/Polyurethane Triboelectric Nanocomposites with Excellent Flame Retardancy and Oil Leak Detection. Chem. Eng. J. 2022, 450, 137982. [Google Scholar] [CrossRef]
- Vargas, P.C.; Merlini, C.; Livi, S.; de Nardi Martins, J.; Soares, B.G.; Barra, G.M.O. The Influence of Carbon Nanotubes and Exfoliated Graphite Nanoplatelets Modified by Phosphonium-based Ionic Liquids on Polyurethane Composites. J. Appl. Polym. Sci. 2023, 140, e54289. [Google Scholar] [CrossRef]
- Sahu, G.; Das, M.; Yadav, M.; Sahoo, B.P.; Tripathy, J. Dielectric Relaxation Behavior of Silver Nanoparticles and Graphene Oxide Embedded Poly(Vinyl Alcohol) Nanocomposite Film: An Effect of Ionic Liquid and Temperature. Polymers 2020, 12, 374. [Google Scholar] [CrossRef] [PubMed]
- Sahu, G.; Das, M.; Sethy, C.; Wazalwar, R.; Kundu, C.N.; Raichur, A.M.; Tripathy, J. Ionic Liquid-Assisted Fabrication of Poly(Vinyl Alcohol)/Nanosilver/Graphene Oxide Composites and Their Cytotoxicity/Antimicrobial Activity. Mater. Chem. Phys. 2021, 266, 124524. [Google Scholar] [CrossRef]
- Zhu, T.; Guo, G.; Li, W.; Gao, M. Synergistic Flame Retardant Effect between Ionic Liquid-Functionalized Imogolite Nanotubes and Ammonium Polyphosphate in Unsaturated Polyester Resin. ACS Omega 2022, 7, 47601–47609. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Guo, G.; Chai, Z.; Yi, D.; Qian, L. The Flame Retardancy of Ionic Liquid Functionalized Graphene Oxide in Unsaturated Polyester Resins. Fire Mater. 2022, 46, 743–752. [Google Scholar] [CrossRef]
- Yasin, S.; Hussain, M.; Zheng, Q.; Song, Y. Influence of Ionic Liquid on Rheological Behaviors of Candle Soot and Cellulose Nanocrystal Filled Natural Rubber Nanocomposites. Compos. Commun. 2022, 33, 101214. [Google Scholar] [CrossRef]
- Wang, J.; Jia, H.; Ding, L.; Xiong, X.; Gong, X. The Mechanism of Carbon-Silica Dual Phase Filler Modified by Ionic Liquid and Its Reinforcing on Natural Rubber. Polym. Compos. 2015, 36, 1721–1730. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, Y.; Hu, X.; Wang, F.; Chen, J.; Xu, P.; Ding, Y. Improved Mechanical and Dielectric Properties of PLA/EMA-GMA Nanocomposites Based on Ionic Liquids and MWCNTs. Compos. Sci. Technol. 2020, 200, 108347. [Google Scholar] [CrossRef]
- Huang, B.; Wang, Z.; Tu, J.; Liu, C.; Xu, P.; Ding, Y. Interfacial Distribution and Compatibilization of Imidazolium Functionalized CNTs in Poly(Lactic Acid)/Polycaprolactone Composites with Excellent EMI Shielding and Mechanical Properties. Int. J. Biol. Macromol. 2023, 227, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, P.; Ruan, G.; Xu, P.; Ding, Y. Synergistic Effect of P[MPEGMA-IL] Modified Graphene on Morphology and Dielectric Properties of PLA/PCL Blends. ES Mater. Manuf. 2020, 11, 20–29. [Google Scholar] [CrossRef]
- Soares, B.G.; Cordeiro, E.; Maia, J.; Pereira, E.C.L.; Silva, A.A. The Effect of the Noncovalent Functionalization of CNT by Ionic Liquid on Electrical Conductivity and Electromagnetic Interference Shielding Effectiveness of Semi-biodegradable Polypropylene/Poly(Lactic Acid) Composites. Polym. Compos. 2020, 41, 82–93. [Google Scholar] [CrossRef]
- Lopes Pereira, E.C.; Soares, B.G.; Silva, A.A.; Farias da Silva, J.M.; Barra, G.M.O.; Livi, S. Conductive Heterogeneous Blend Composites of PP/PA12 Filled with Ionic Liquids Treated-CNT. Polym. Test. 2019, 74, 187–195. [Google Scholar] [CrossRef]
- Yousfi, M.; Livi, S.; Dumas, A.; Crépin-Leblond, J.; Greenhill-Hooper, M.; Duchet-Rumeau, J. Ionic Compatibilization of Polypropylene/Polyamide 6 Blends Using an Ionic Liquids/Nanotalc Filler Combination: Morphology, Thermal and Mechanical Properties. RSC Adv. 2015, 5, 46197–46205. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Abdan, K.; Md. Jamil, S.N.A. Preparations and Properties of Ionic Liquid-Assisted Electrospun Biodegradable Polymer Fibers. Polymers 2022, 14, 2308. [Google Scholar] [CrossRef] [PubMed]
- Soares da Silva, J.P.; Soares, B.G.; Silva, A.A.; Livi, S. Double Percolation of Melt-Mixed PS/PBAT Blends Loaded With Carbon Nanotube: Effect of Molding Temperature and the Non-Covalent Functionalization of the Filler by Ionic Liquid. Front. Mater. 2019, 6, 191. [Google Scholar] [CrossRef]
- Liu, C.; Wang, P.; Hu, J.; Cheng, S.; Pan, S.; Xu, P.; Ding, Y. Effect of Phase Morphology on Electromagnetic Interference Shielding Performance of Silicone Rubber/POE Blends Containing ILs Modified MWCNTs. Synth. Met. 2019, 256, 116140. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Nurul, S.; Jamil, A.; Abdan, K. A Brief Review on the Influence of Ionic Liquids on the Mechanical, Thermal, and Chemical Properties of Biodegradable Polymer Composites. Polymers 2021, 13, 2597. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Yusoff, M.Z.M.; Abdan, K.; Jamil, S.N.A.M. Flammability Properties of Polymers and Polymer Composites Combined with Ionic Liquids. e-Polymers 2023, 23, 20230060. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).