Effects of Food Concentration and Light Intensity on the Growth of a Model Coral
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Wound-Healing Experiment
2.3. The Culture System
2.4. Light and Feeding Treatments
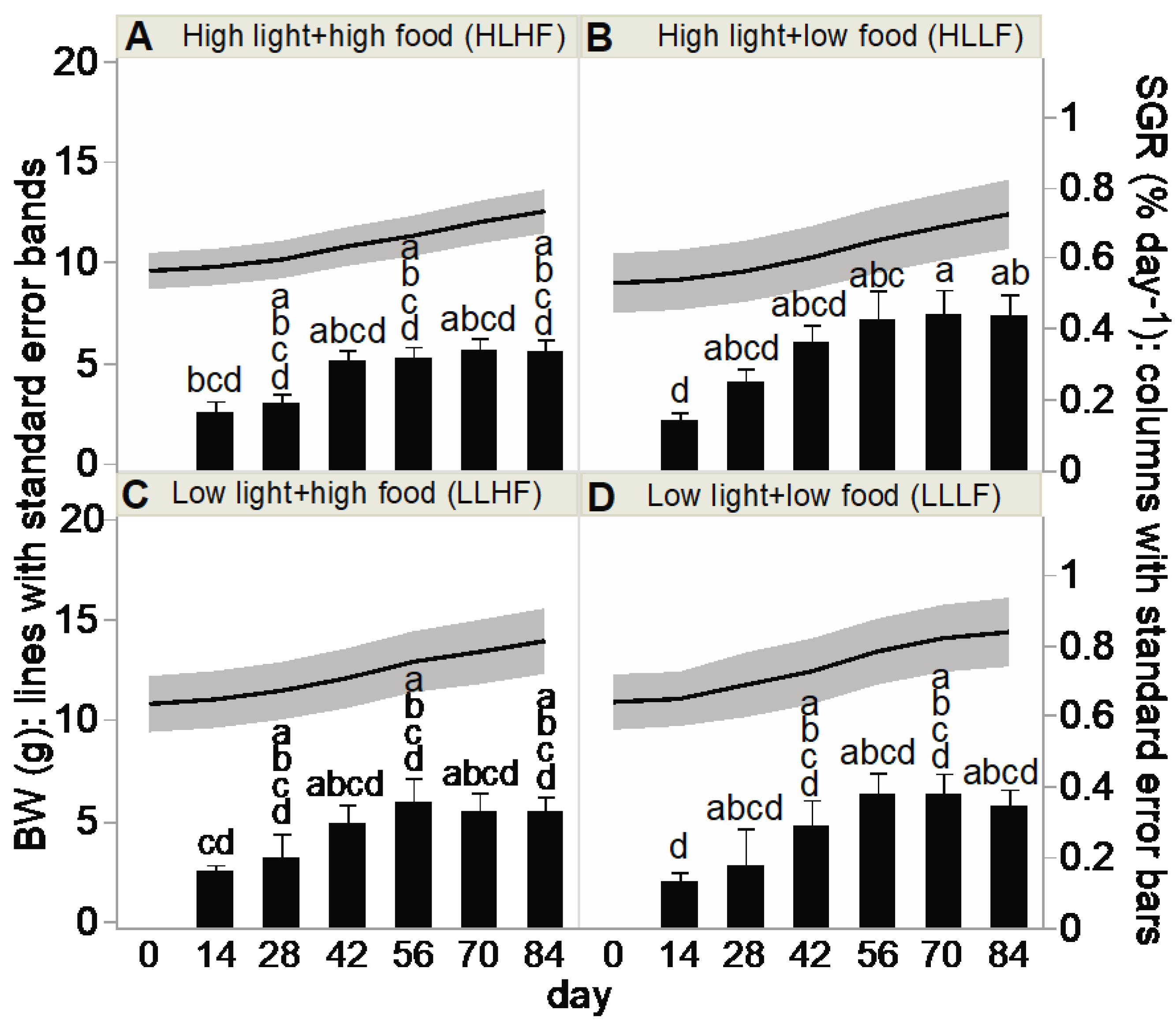
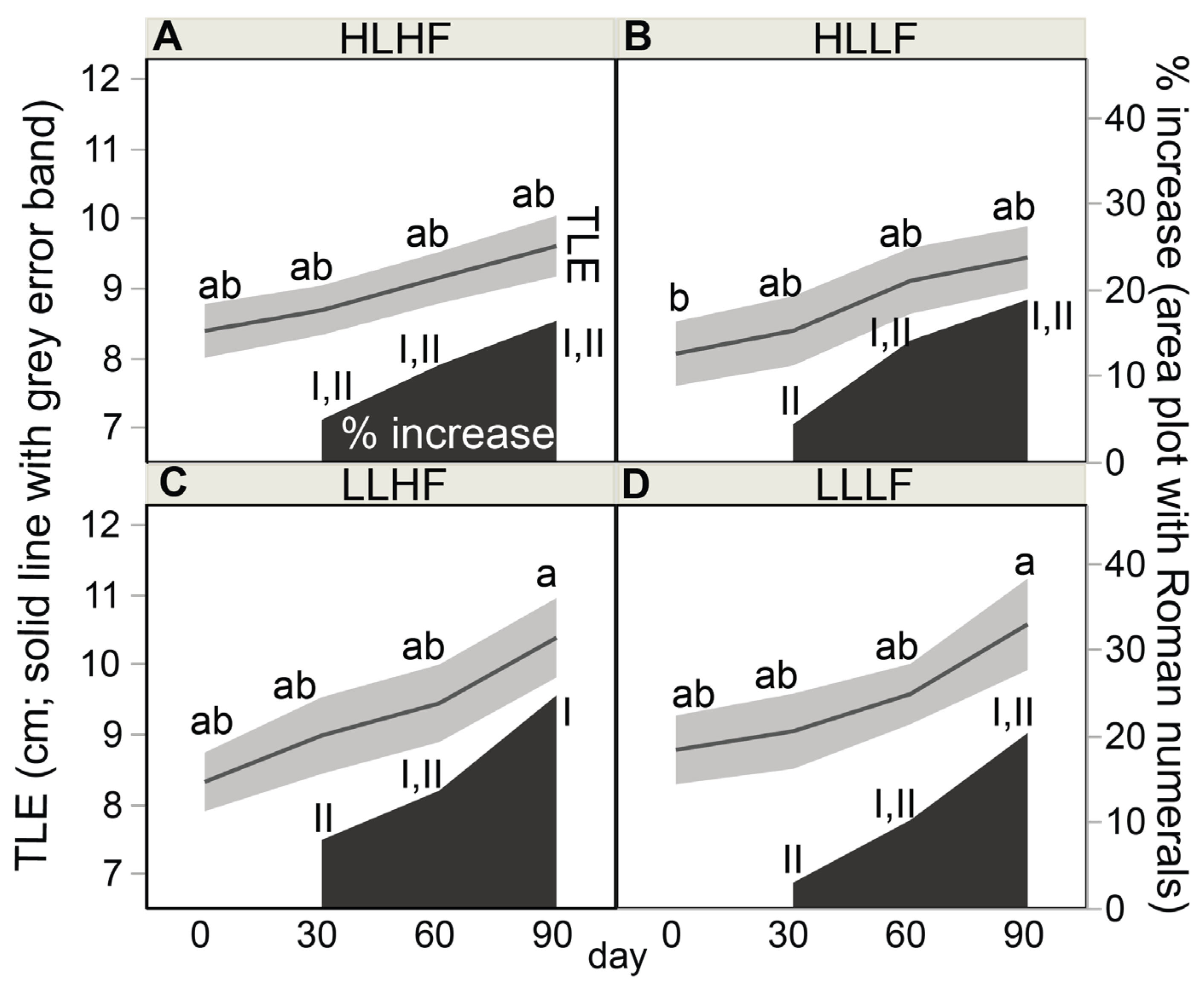
2.5. Buoyant Weight (BW), Specific Growth Rate (SGR), and TLE
2.6. Maximum Quantum Yield of Photosystem II (Fv/Fm)
2.7. Statistical Analyses
3. Results
3.1. Seawater Quality
3.2. Coral Response Variables
4. Discussion
4.1. Coral Growth under Differing Light and Food Regimes
4.2. Optimizing the Growth of P. acuta Ex Situ
4.3. Wound Healing
4.4. RAS-Related Coral Culture Issues and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, A.B.; Tsai, S.; Lin, C. The Coral Hospital. Biopreserv. Biobank. 2019, 17, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, A.B.; Chen, C.S.; Liu, P.J. Decreased green fluorescent protein-like chromoprotein gene expression in specimens of the reef-building coral Pocillopora damicornis undergoing high temperature-induced bleaching. Platax 2014, 11, 1–23. [Google Scholar]
- Mayfield, A.B.; Wang, Y.B.; Chen, C.S.; Chen, S.H.; Lin, C.Y. Compartment-specific transcriptomics in a reef-building coral exposed to elevated temperatures. Mol. Ecol. 2014, 23, 5816–5830. [Google Scholar] [CrossRef] [PubMed]
- Shafir, S.; van Rijn, J.; Rinkevich, B. Coral nubbins as source material for coral biological research: A prospectus. Aquaculture 2006, 259, 444–448. [Google Scholar] [CrossRef]
- Leal, M.C.; Calado, R.; Sheridan, C.; Alimonti, A.; Osinga, R. Coral aquaculture to support drug discovery. Trends Biotechnol. 2013, 31, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Ferrier-Pagès, C.; Petersen, D.; Osinga, R. Coral aquaculture: Applying scientific knowledge to ex situ production. Rev. Aquacul. 2016, 8, 136–153. [Google Scholar] [CrossRef]
- Schubert, P.; Wilke, T. Coral microcosms: Challenges and opportunities for global change biology. In Corals in a Changing World; Chapter 8; IntechOpen: London, UK, 2018. [Google Scholar]
- Huang, Y.L.; Mayfield, A.B.; Fan, T.Y. Effects of feeding on the physiological performance of the stony coral Pocillopora acuta. Sci. Rep. 2020, 10, 19988. [Google Scholar] [CrossRef]
- Chang, T.C.; Mayfield, A.B.; Fan, T.Y. Culture systems influence the physiological performance of the soft coral Sarcophyton glaucum. Sci. Rep. 2020, 10, 20200. [Google Scholar] [CrossRef]
- Barton, J.A.; Willis, B.L.; Hutson, K.S. Coral propagation: A review of techniques for ornamental trade and reef restoration. Rev. Aquacul. 2017, 9, 238–256. [Google Scholar] [CrossRef]
- Craggs, J.; Guest, J.; Davis, M.; Simmons, J.; Dashti, E.; Sweet, M.J. Inducing broadcast coral spawning ex situ: Closed system mesocosm design and husbandry protocol. Ecol. Evol. 2017, 7, 11066–11078. [Google Scholar] [CrossRef] [PubMed]
- Osinga, R.; Schutter, M.; Griffioen, B.; Wijffels, R.H.; Verreth, J.A.; Shafir, S.; Henard, S.; Taruffi, M.; Gili, C.; Lavorano, S. The biology and economics of coral growth. Mar. Biotechnol. 2011, 13, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Osinga, R.; Schutter, M.; Wijgerde, T.; Rinkevich, B.; Shafir, S.; Shpigel, M.; Luna, G.M.; Danovaro, R.; Bongiorni, L.; Deutsch, A.; et al. The CORALZOO project: A synopsis of four years of public aquarium science. J. Mar. Biol. Assoc. UK 2012, 92, 753–768. [Google Scholar] [CrossRef]
- Kuanui, P.; Chavanich, S.; Viyakarn, V.; Omori, M.; Fujita, T.; Lin, C. Effect of light intensity on survival and photosynthetic efficiency of cultured corals of different ages. Estuar. Coast. Shelf Sci. 2020, 235, 106515. [Google Scholar] [CrossRef]
- Yuen, Y.S.; Yamazaki, S.S.; Nakamura, T.; Tokuda, G.; Yamasaki, H. Effects of live rock on the reef-building coral Acropora digitifera cultured with high levels of nitrogenous compounds. Aquacul. Eng. 2009, 41, 35–43. [Google Scholar] [CrossRef]
- Bik, H.M.; Alexiev, A.; Aulakh, S.K.; Bharadwaj, L.; Flanagan, J.; Haggerty, J.M.; Hird, S.M.; Jospin, G.; Lang, J.M.; Sauder, L.A.; et al. Microbial community succession and nutrient cycling responses following perturbations of experimental saltwater aquaria. mSphere 2019, 4, e00043-19. [Google Scholar] [CrossRef] [PubMed]
- Ferrier-Pages, C.; Witting, J.; Tambutte, E.; Sebens, K.P. Effect of natural zooplankton feeding on the tissue and skeletal growth of the scleractinian coral Stylophora pistillata. Coral Reefs 2003, 22, 229–240. [Google Scholar] [CrossRef]
- Al-Moghrabi, S.; Allemand, D.; Couret, J.M.; Jaubert, J. Fatty acids of the scleractinian coral Galaxea fascicularis: Effect of light and feeding. J. Comp. Physiol. 1995, 165, 183–192. [Google Scholar] [CrossRef]
- Houlbrèque, F.; Tambutté, E.; Ferrier-Pagès, C. Effect of zooplankton availability on the rates of photosynthesis, and tissue and skeletal growth in the scleractinian coral Stylophora pistillata. J. Exp. Mar. Biol. Ecol. 2003, 296, 145–166. [Google Scholar] [CrossRef]
- Houlbrèque, F.; Tambutté, E.; Allemand, D.; Ferrier-Pagès, C. Interactions between zooplankton feeding, photosynthesis and skeletal growth in the scleractinian coral Stylophora pistillata. J. Exp. Biol. 2004, 207, 1461–1469. [Google Scholar] [CrossRef]
- Houlbrèque, F.; Ferrier-Pagès, C. Heterotrophy in tropical scleractinian corals. Biol. Rev. 2009, 84, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Grottoli, A.G.; Rodrigues, L.J.; Palardy, J.E. Heterotrophic plasticity and resilience in bleached corals. Nature 2006, 440, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, T.C. Small Scale Experimental Systems for Coral Research: Considerations, Planning, and Recommendations; NOAA Technical Memorandum NOS NCCOS 165 and CRCP 18; NOAA/National Centers for Coastal Ocean Science: Washington, DC, USA, 2013; 68p.
- Dobson, K.L.; Ferrier-Pagès, C.; Saup, C.M.; Grottoli, A.G. The effects of temperature, light, and feeding on the physiology of Pocillopora damicornis, Stylophora pistillata, and Turbinaria reniformis Corals (sp.). Water 2021, 13, 2048. [Google Scholar] [CrossRef]
- Mayfield, A.B.; Fan, T.Y.; Chen, C.S. Physiological acclimation to elevated temperature in a reef-building coral from an upwelling environment. Coral Reefs 2013, 32, 909–921. [Google Scholar] [CrossRef]
- Schutter, M.; Kranenbarg, S.; Wijffels, R.H.; Verreth, J.; Osinga, R. Modification of light utilization for skeletal growth by water flow in the scleractinian coral Galaxea fascicularis. Mar. Biol. 2011, 158, 769–777. [Google Scholar] [CrossRef]
- Schutter, M.; Crocker, J.; Paijmans, A.; Janse, M.; Osinga, R.; Verreth, A.J.; Wijffels, R.H. The effect of different flow regimes on the growth and metabolic rates of the scleractinian coral Galaxea fascicularis. Coral Reefs 2010, 29, 737–748. [Google Scholar] [CrossRef]
- Schmidt-Roach, S.; Miller, K.J.; Lundgren, P.; Andreakis, N. With eyes wide open: A revision of species within and closely related to the Pocillopora damicornis species complex (Scleractinia; Pocilloporidae) using morphology and genetics. Zool. J. Linnean Soc. 2014, 170, 1–33. [Google Scholar] [CrossRef]
- Tagliafico, A.; Rangel, S.; Kelaher, B.; Christidis, L. Optimizing heterotrophic feeding rates of three commercially important scleractinian corals. Aquaculture 2018, 483, 96–101. [Google Scholar] [CrossRef]
- Ben-Haim, Y.; Zicherman-Keren, M.; Rosenberg, E. Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio Coralliilyticus. Appl. Environ. Microbiol. 2003, 69, 4236–4242. [Google Scholar] [CrossRef]
- Rodríguez-Villalobos, J.C.; Work, T.M.; Calderon-Aguilera, L.E. Wound repair in Pocillopora. J. Invertebr. Pathol. 2016, 139, 1–5. [Google Scholar] [CrossRef]
- Henry, L.-A.; Hart, M. Regeneration from injury and resource allocation in sponges and corals—A review. Int. Rev. Hydrobiol. 2005, 90, 125–158. [Google Scholar] [CrossRef]
- Meesters, E.H.; Wesseling, I.; Bak, R.P.M. Coral colony tissue damage in six species of reef-building corals: Partial mortality in relation with depth and surface area. J. Sea Res. 1997, 37, 131–144. [Google Scholar] [CrossRef]
- Rinkevich, B.; Loya, Y. Reproduction in regenerating colonies of the coral Stylophora pistillata. Environ. Qual. Ecosyst. Stab. 1989, 4, 259–265. [Google Scholar]
- Fong, P.; Lirman, D. Hurricanes cause population expansion of the branching coral Acropora palmata (Scleractinia): Wound healing and growth patterns of asexual recruits. Mar. Ecol. 1995, 16, 317–335. [Google Scholar] [CrossRef]
- Edmunds, P.J.; Yarid, A. The effects of ocean acidification on wound repair in the coral Porites spp. J. Exp. Mar. Biol. Ecol. 2017, 486, 98–104. [Google Scholar] [CrossRef]
- Burmester, E.M.; Breef-Pilz, A.; Lawrence, N.F.; Kaufman, L.; Finnerty, J.R.; Rotjan, R.D. The impact of autotrophic versus heterotrophic nutritional pathways on colony health and wound recovery in corals. Ecol. Evol. 2018, 8, 10805–10816. [Google Scholar] [CrossRef] [PubMed]
- Burmester, E.M.; Finnerty, J.R.; Kaufman, L.; Rotjan, R.D. Temperature and symbiosis affect lesion recovery in experimentally wounded, facultatively symbiotic temperate corals. Mar. Ecol. Prog. Ser. 2017, 570, 87–99. [Google Scholar] [CrossRef]
- Tagliafico, A.; Rudd, D.; Rangel, M.S.; Kelaher, B.P.; Christidis, L.; Cowden, K.; Scheffers, S.R.; Benkendorff, K. Lipid-enriched diets reduce the impacts of thermal stress in corals. Mar. Ecol. Prog. Ser. 2017, 573, 129–141. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Maragos, J.E.; Franzisket, L. Coral growth: Buoyant weight technique. In Coral Reefs: Research Methods. Monographso on Oceanographic Methodology; Stoddart, D.R., Johannes, R.E., Eds.; UNESCO: Paris, France, 1978; pp. 529–542. [Google Scholar]
- Kikuzawa, Y.P.; Toh, T.C.; Ng, C.S.L.; Sam, S.Q.; Taira, D.; Afiq-Rosli, L.; Chou, L.M. Quantifying growth in maricultured corals using photogrammetry. Aquac. Res. 2018, 49, 2249–2255. [Google Scholar] [CrossRef]
- Siebeck, U.; Marshall, N.; Klüter, A.; Hoegh-Guldberg, O. Monitoring coral bleaching using a colour reference card. Coral Reefs 2006, 25, 453–460. [Google Scholar] [CrossRef]
- Conlan, J.A.; Bay, L.K.; Severati, A.; Humphrey, C.; Francis, D.S. Comparing the capacity of five different dietary treatments to optimize growth and nutritional composition in two scleractinian corals. PLoS ONE 2018, 13, e0207956. [Google Scholar] [CrossRef] [PubMed]
- Cunning, R.; Gillette, P.; Capo, T.; Galvez, K.; Baker, A.C. Growth tradeoffs associated with thermotolerant symbionts in the coral Pocillopora damicornis are lost in warmer oceans. Coral Reefs 2015, 34, 155–160. [Google Scholar] [CrossRef]
- Rodrigues, L.J.; Grottoli, A.G. Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnol Oceanogr. 2007, 52, 1874–1882. [Google Scholar] [CrossRef]
- Schutter, M.; van Velthoven, B.; Janse, M.; Osinga, R.; Janssen, M.; Wijffels, R.; Verreth, J. The effect of irradiance on long-term skeletal growth and net photosynthesis in Galaxea fascicularis under four light conditions. J. Exp. Mar. Biol. Ecol. 2008, 67, 75–80. [Google Scholar] [CrossRef]
- Bramanti, L.; Iannelli, M.; Fan, T.Y.; Edmunds, P.J. Using demographic models to project the effects of climate change on scleractinian corals: Pocillopora damicornis as a case study. Coral Reefs 2015, 34, 505–515. [Google Scholar] [CrossRef]
- Anderson, K.D.; Cantin, N.E.; Heron, S.F.; Pisapia, C.; Pratchett, M.S. Variation in growth rates of branching corals along Australia’s Great Barrier Reef. Sci Rep. 2017, 7, 2920. [Google Scholar] [CrossRef]
- Tortolero-Langarica, J.A.; Rodríguez-Troncoso, A.P.; Cupul-Magaña, A.L.; Carricart-Ganivet, J.P. Calcification and growth rate recovery of the reef-building Pocillopora species in the northeast tropical Pacific following an ENSO disturbance. PeerJ 2017, 5, e3191. [Google Scholar] [CrossRef]
- Richmond, R.H. Energetic relationships and biogeographical differences among fecundity, growth and reproduction in the reef coral Pocillopora damicornis. Bull. Mar. Sci. 1987, 41, 594–604. [Google Scholar]
- Gantt, S.E.; Keister, E.F.; Manfroy, A.A.; Merck, D.E.; Fitt, W.K.; Muller, E.M.; Kemp, D.W. Wild and nursery-raised corals: Comparative physiology of two framework coral species. Coral Reefs 2023, 42, 299–310. [Google Scholar] [CrossRef]
- Mayfield, A.B.; Fan, T.Y.; Chen, C.S. The physiological impact of ex situ transplantation on the Taiwanese reef-building coral Seriatopora hystrix. J. Mar Biol. 2013, 569369. [Google Scholar] [CrossRef]
- Counsell, C.W.W.; Johnston, E.C.; Sale, T.L. Colony size and depth affect wound repair in a branching coral. Mar. Biol. 2019, 166, 148. [Google Scholar] [CrossRef]
- Edmunds, P.J.; Burgess, S.C. Size-dependent physiological responses of the branching coral Pocillopora verrucosa to elevated temperature and pCO2. J. Exp. Biol. 2016, 219, 3896–3906. [Google Scholar] [PubMed]
- Kramarsky-Winter, E.; Loya, Y. Tissue regeneration in the coral Fungia granulosa: The effect of extrinsic and intrinsic factors. Mar. Biol. 2000, 137, 867–873. [Google Scholar] [CrossRef]
- Traylor-Knowles, N. Distinctive wound-healing characteristics in the corals Pocillopora damicornis and Acropora hyacinthus found in two different temperature regimes. Mar Biol. 2016, 163, 231. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Zhang, F.; Sun, Y.-F.; Yu, X.-L.; Huang, H. Effects of nitrate enrichment on respiration, photosynthesis, and fatty acid composition of reef coral Pocillopora damicornis larvae. Front. Mar. Sci. 2020, 7, 531. [Google Scholar] [CrossRef]
- Lam, K.W.; McRae, C.J.; Zhang, X.C.; Ye, Y.T.; Qiu, Y.T.; Jiang, M.Q.; Cheng, T.H.; Chen, G.K.; Fan, T.Y. Consistent monthly reproduction and completion of a brooding coral life cycle through ex situ culture. Diversity 2023, 15, 218. [Google Scholar] [CrossRef]
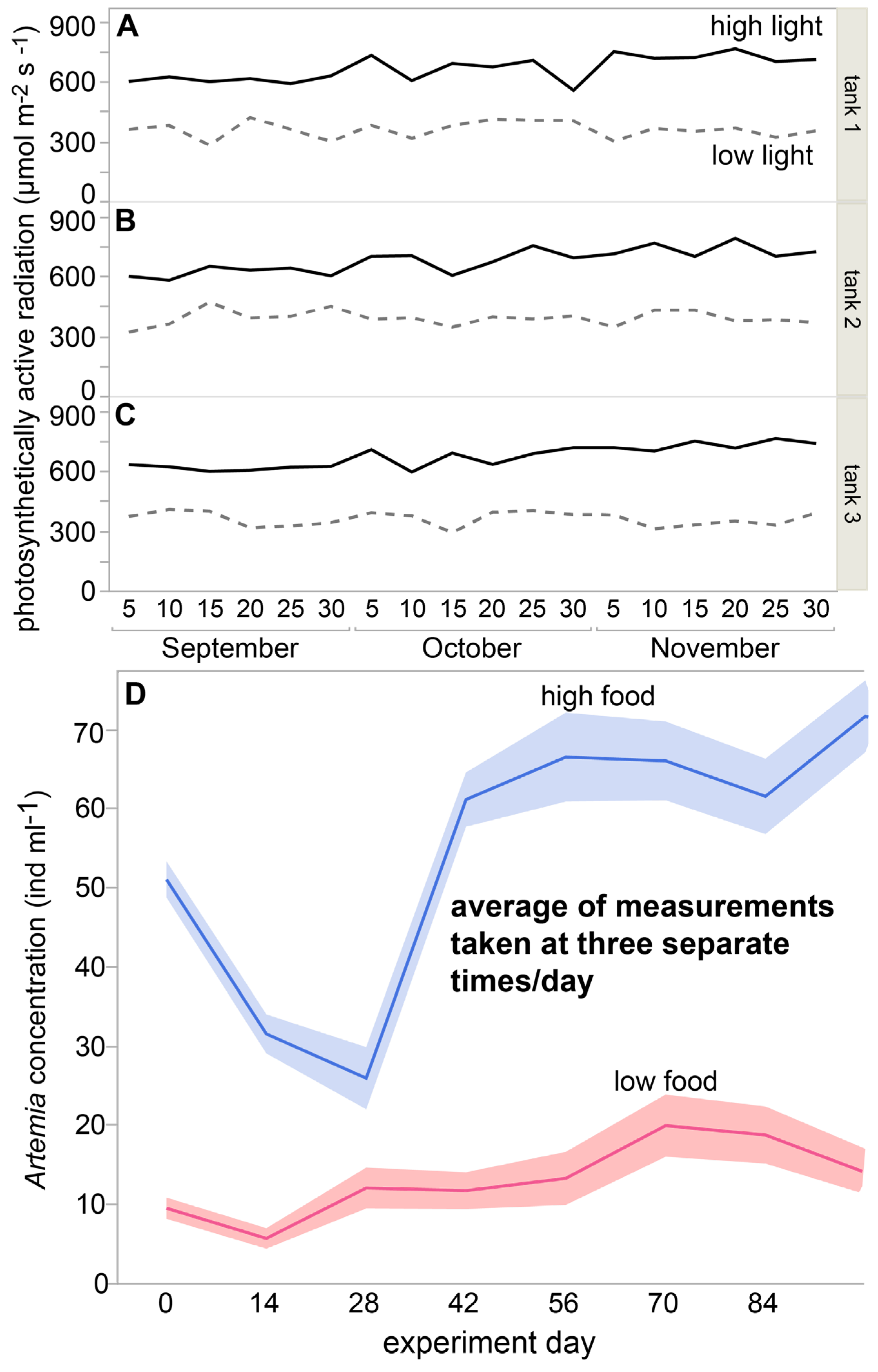
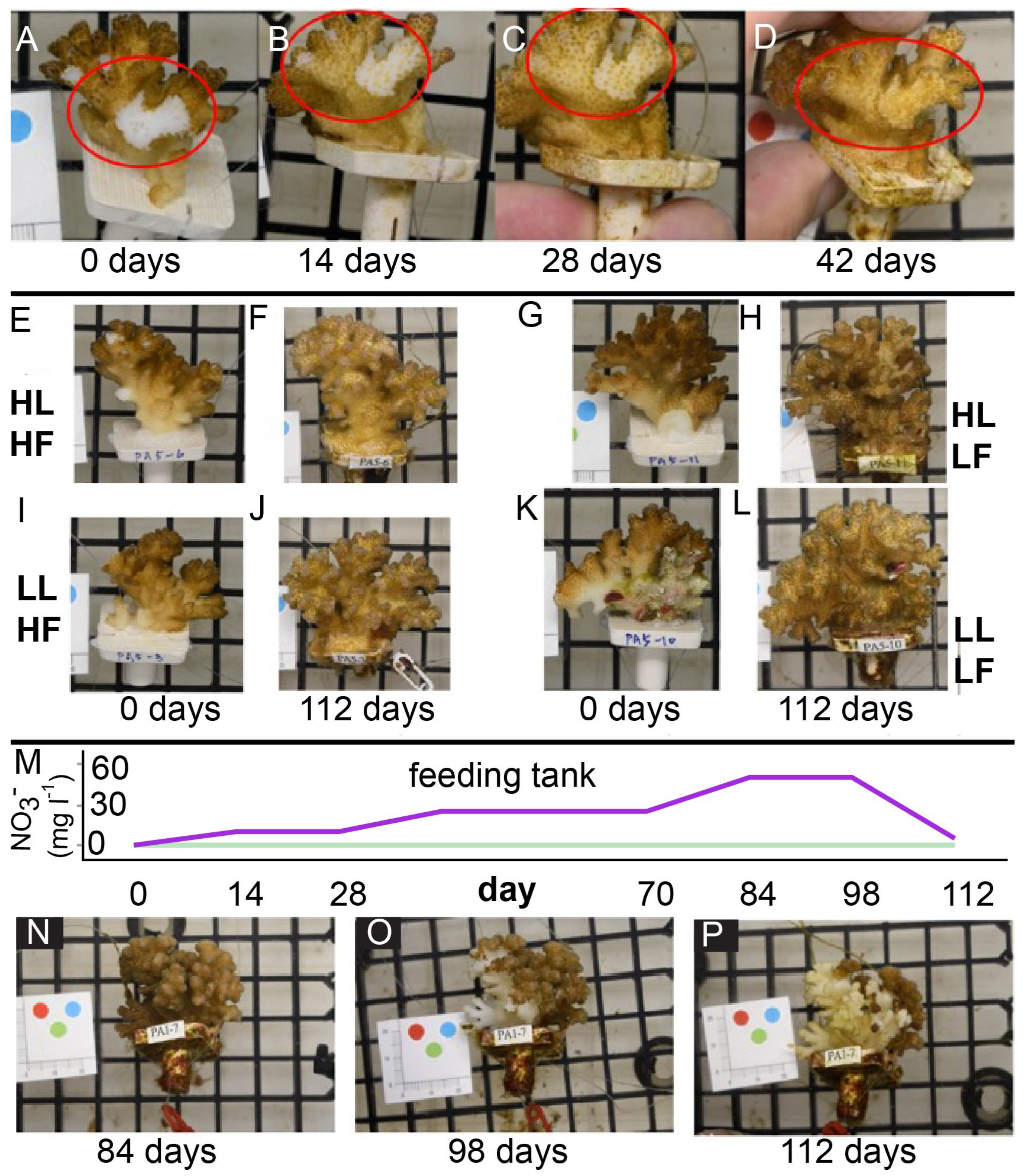
| Parameter | HL | LL | HF | LF | Temp. | Salinity | Flow | NO3− | pH | kH | Ca2+ | Mg2+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tank | μmol m−2 s−1 | Artemia mL−1 | °C | unitless | cm s−1 | mg L−1 | unitless | mg L−1 | ||||
| 1 | 665 | 358 | 0 b | 0 b | 28.3 | 35.0 | 5.4 | 0 b | 8.21 | 6.46 | 477 a | 1351 a |
| 2 | 679 | 390 | 0 b | 0 b | 28.7 | 34.9 | 6.0 | 0 b | 8.20 | 6.56 | 474 a | 1258 ab |
| 3 | 673 | 360 | 0 b | 0 b | 28.7 | 34.8 | 5.1 | 0 b | 8.23 | 6.87 | 438 b | 1204 b |
| Feeding | 0 | 0 | 78 a | 33 a | 28.1 | 35.0 | 2.0 * | 22.2 a | 8.18 | 6.37 | 415 b | 1184 b |
| Source of Variation | df | F | p | Post-Hoc Comparisons |
|---|---|---|---|---|
| Buoyant weight a (BW; g)—first 84 days | ||||
| light | 1 | 1.31 | 0.26 | |
| food | 1 | 0.08 | 0.77 | |
| light × food | 1 | 0.44 | 0.51 | |
| day | 8 | 129.74 | <0.01 | later > earlier |
| light × day | 8 | 0.63 | 0.70 | |
| food × day | 8 | 1.25 | 0.29 | |
| light × food × day | 8 | 0.32 | 0.94 | |
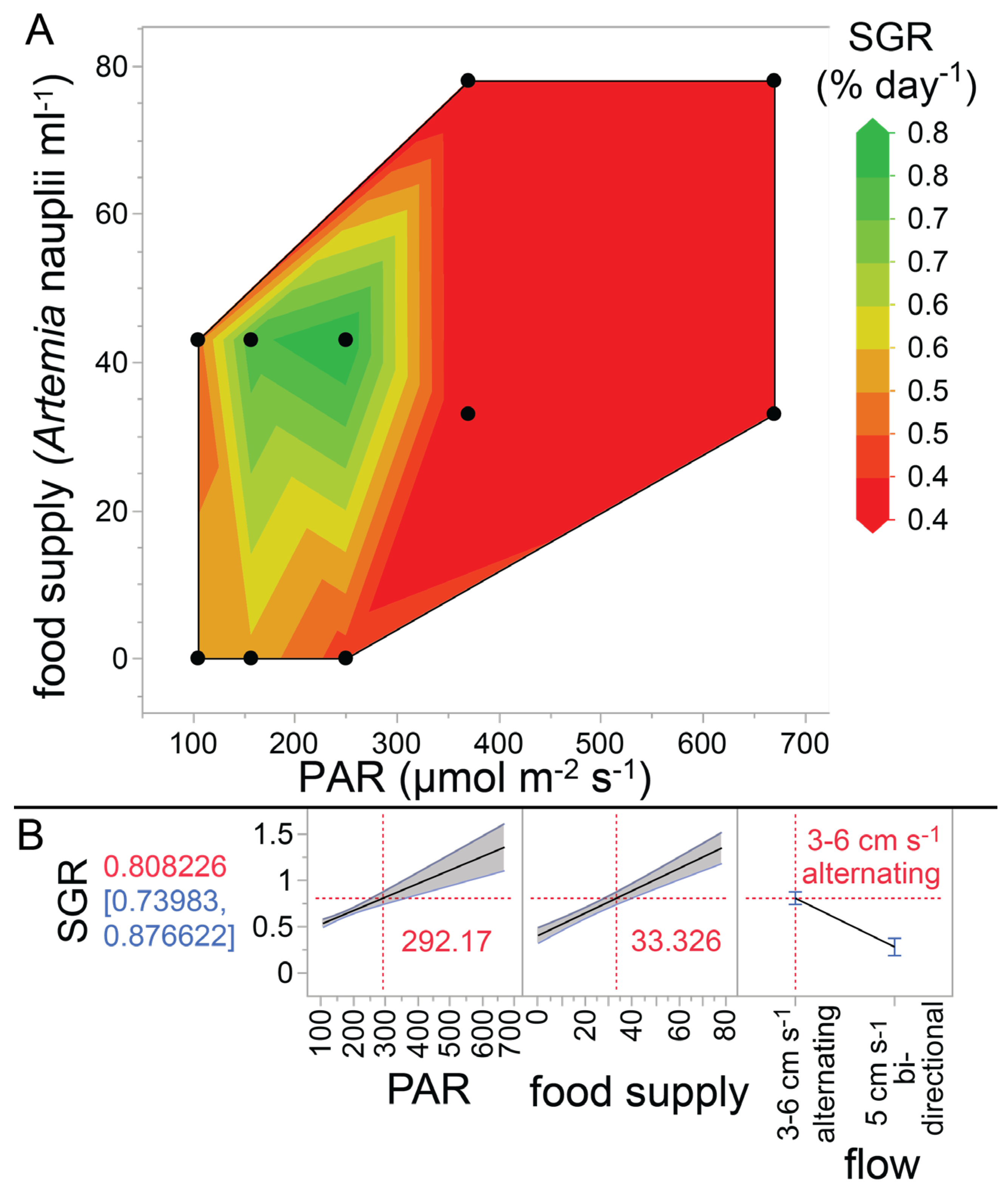
| SGR a (% day−1)—day 84 only | ||||
| light | 1 | 3.04 | 0.09 | |
| food | 1 | 1.70 | 0.20 | |
| light × food | 1 | 1.14 | 0.30 | |
| SGR a —days 14–84 (RM ANOVA) a | ||||
| light | 1 | 1.87 | 0.18 | |
| food | 1 | 0.11 | 0.74 | |
| light × food | 1 | 1.24 | 0.27 | |
| day | 5 | 73.86 | <0.01 | later > earlier |
| light × day | 8 | 1.71 | 0.15 | |
| food × day | 8 | 2.04 | 0.09 | |
| light × food × day | 8 | 0.55 | 0.74 | |
| Total linear extension (TLE; cm)—all times | ||||
| light | 1 | 2.21 | 0.14 | |
| food | 1 | 0.02 | 0.89 | |
| light × food | 1 | 0.46 | 0.50 | |
| day | 5 | 75.79 | <0.01 | later > earlier |
| light × day | 8 | 1.82 | 0.14 | |
| food × day | 8 | 0.35 | 0.84 | |
| light × food × day | 8 | 0.55 | 0.70 | |
| TLE—first 60 days | ||||
| light | 1 | 0.90 | 0.35 | |
| food | 1 | 0.04 | 0.85 | |
| light × food | 1 | 0.35 | 0.56 | |
| day | 5 | 35.33 | <0.01 | later > earlier |
| light × day | 8 | 0.32 | 0.73 | |
| food × day | 8 | 0.62 | 0.54 | |
| light × food × day | 8 | 0.84 | 0.44 | |
| TLE—% increase over 90 days | ||||
| light | 1 | 0.43 | 0.53 | |
| food | 1 | 0.03 | 0.88 | |
| light × food | 1 | 0.66 | 0.44 | |
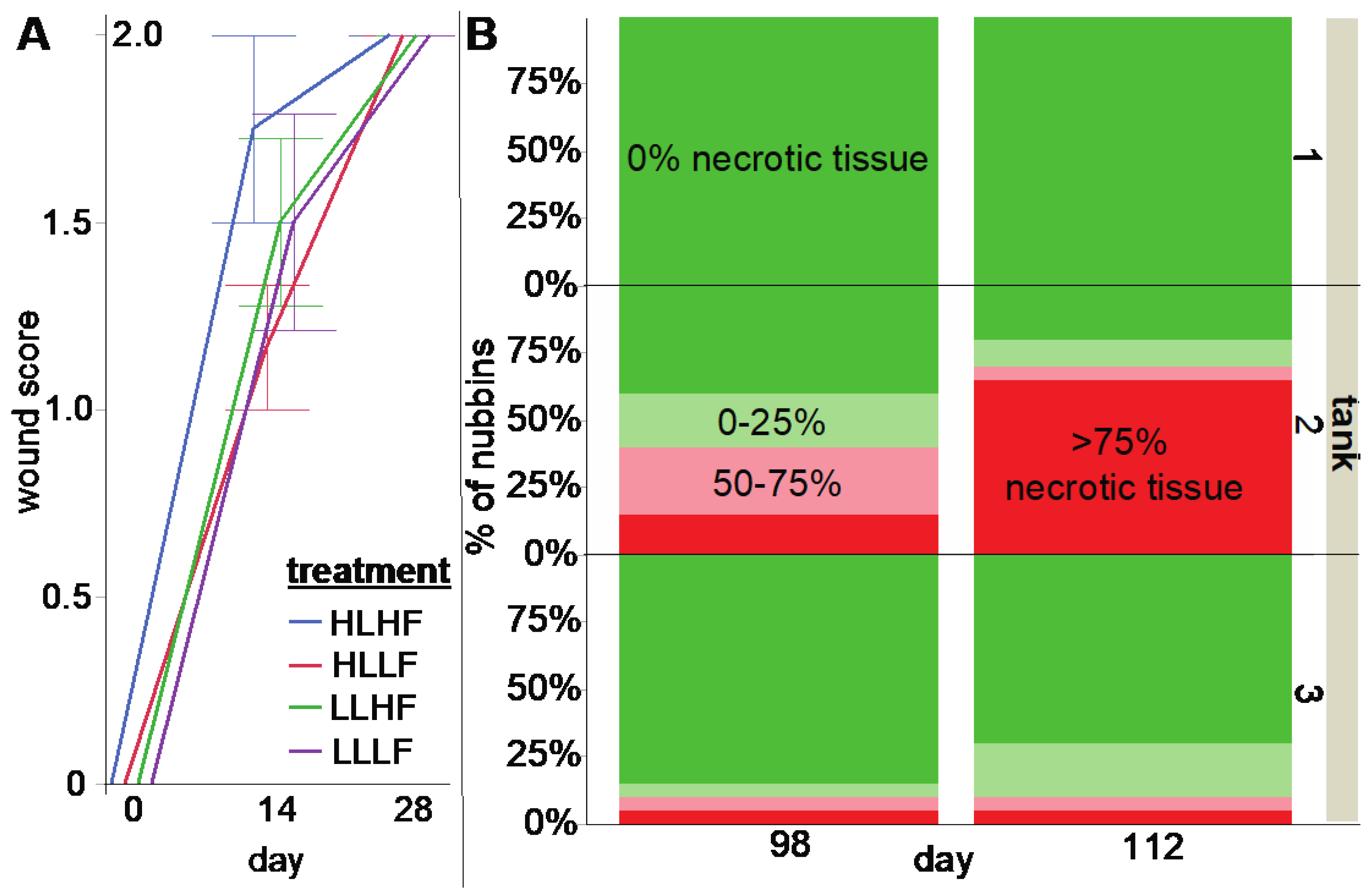
| Tissue sloughing (% area with necrotic tissue) | ||||
| light | 1 | 0.04 | 0.85 | |
| food | 1 | 0.04 | 0.85 | |
| light × food | 1 | 0.13 | 0.73 | |
| day | 5 | 7.91 | <0.01 | earlier > later |
| light × day | 8 | 0.73 | 0.39 | |
| food × day | 8 | 1.21 | 0.27 | |
| light × food × day | 8 | 0.13 | 0.71 | |
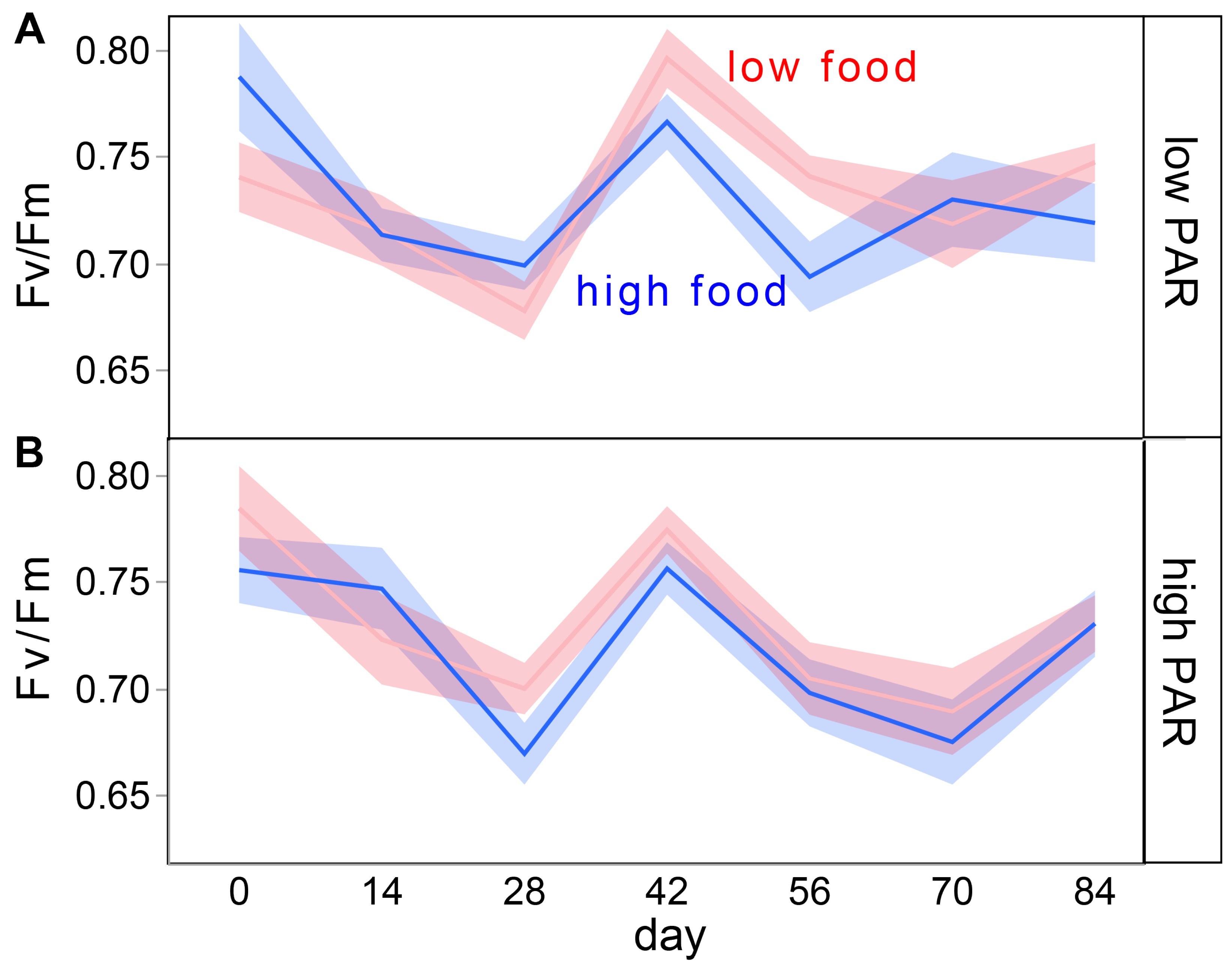
| Fv/Fm a | ||||
| light | 1 | 1.15 | 0.29 | |
| food | 1 | 1.29 | 0.26 | |
| light × food | 1 | 0.36 | 0.55 | |
| day | 8 | 17.93 | <0.01 | day-28 > day-42 |
| light × day | 8 | 1.12 | 0.35 | |
| food × day | 8 | 0.77 | 0.63 | |
| light × food × day | 8 | 1.53 | 0.15 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, T.-Y.; Huang, Y.-L.; Mayfield, A. Effects of Food Concentration and Light Intensity on the Growth of a Model Coral. Oceans 2024, 5, 150-165. https://doi.org/10.3390/oceans5020009
Fan T-Y, Huang Y-L, Mayfield A. Effects of Food Concentration and Light Intensity on the Growth of a Model Coral. Oceans. 2024; 5(2):150-165. https://doi.org/10.3390/oceans5020009
Chicago/Turabian StyleFan, Tung-Yung, Yan-Leng Huang, and Anderson Mayfield. 2024. "Effects of Food Concentration and Light Intensity on the Growth of a Model Coral" Oceans 5, no. 2: 150-165. https://doi.org/10.3390/oceans5020009
APA StyleFan, T.-Y., Huang, Y.-L., & Mayfield, A. (2024). Effects of Food Concentration and Light Intensity on the Growth of a Model Coral. Oceans, 5(2), 150-165. https://doi.org/10.3390/oceans5020009







