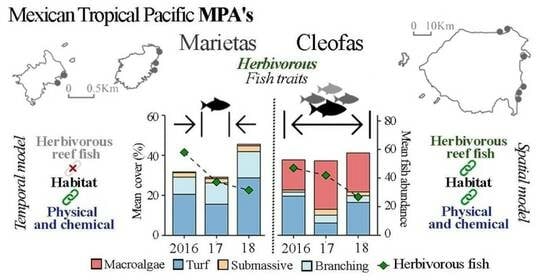
Herbivorous Reef Fish Interaction with the Habitat and Physicochemical Variables in Coral Ecosystems in the Mexican Tropical Pacific
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data
2.2.1. Herbivorous Fish Species and Traits
2.2.2. Habitat Benthic Components
2.2.3. Physicochemical Variables
2.3. Statistical Analyses
2.3.1. Differences between Insular Systems and Temporal Variability of Herbivorous Reef Fish
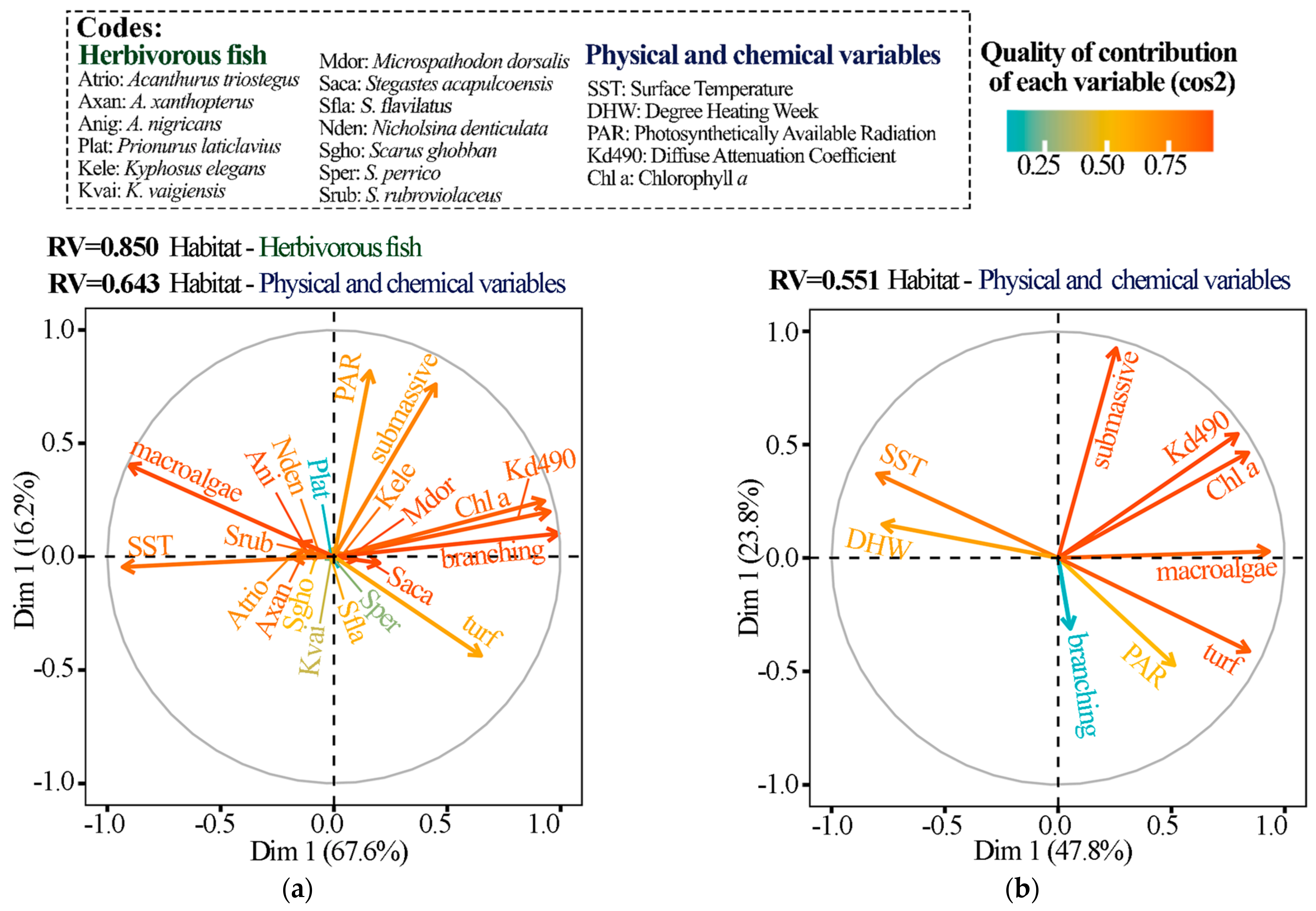
2.3.2. Link between Herbivorous Fish–Habitat–Physicochemical Variables
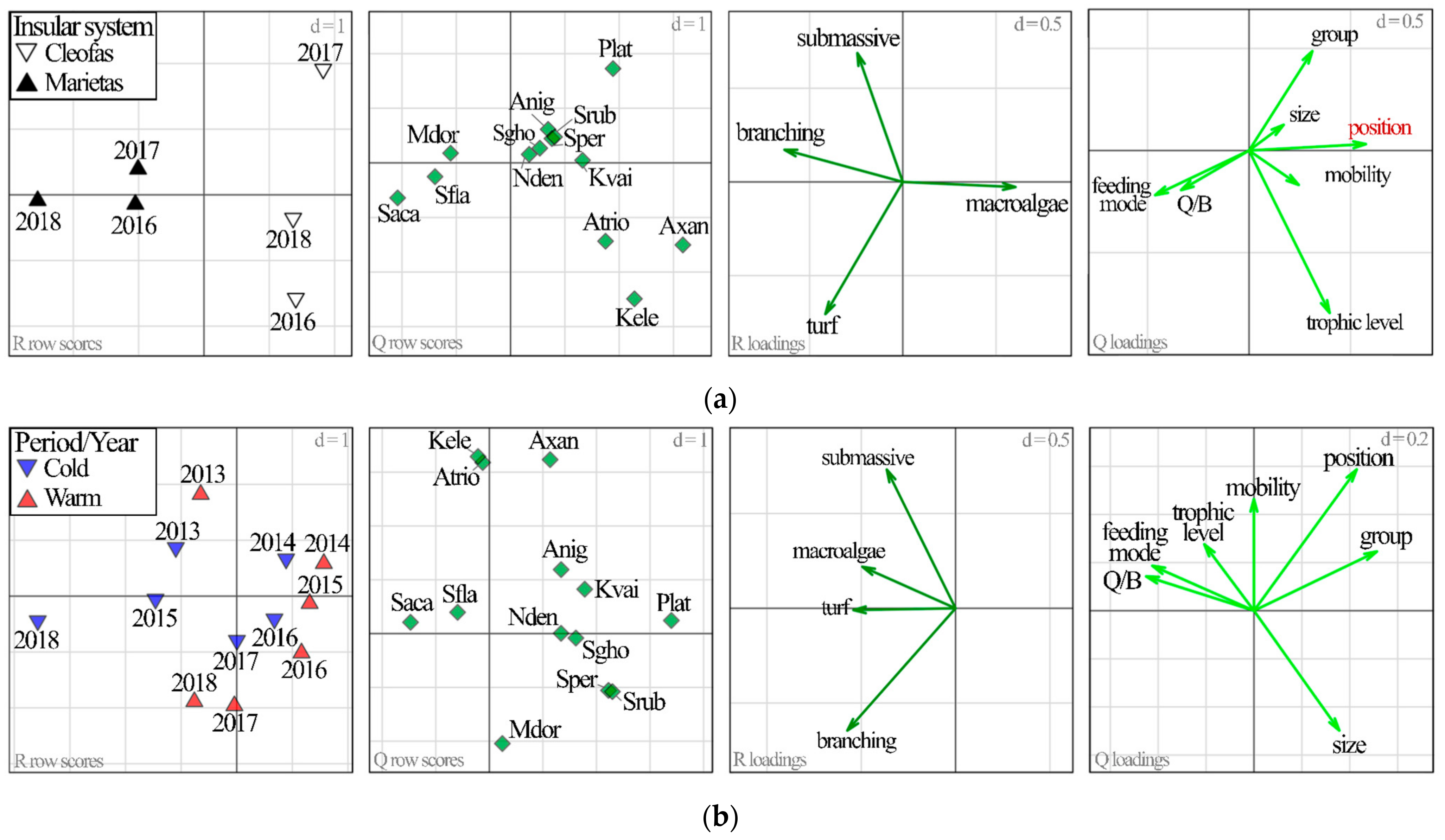
2.3.3. Relationship between Herbivorous Reef Fish Traits and Habitat
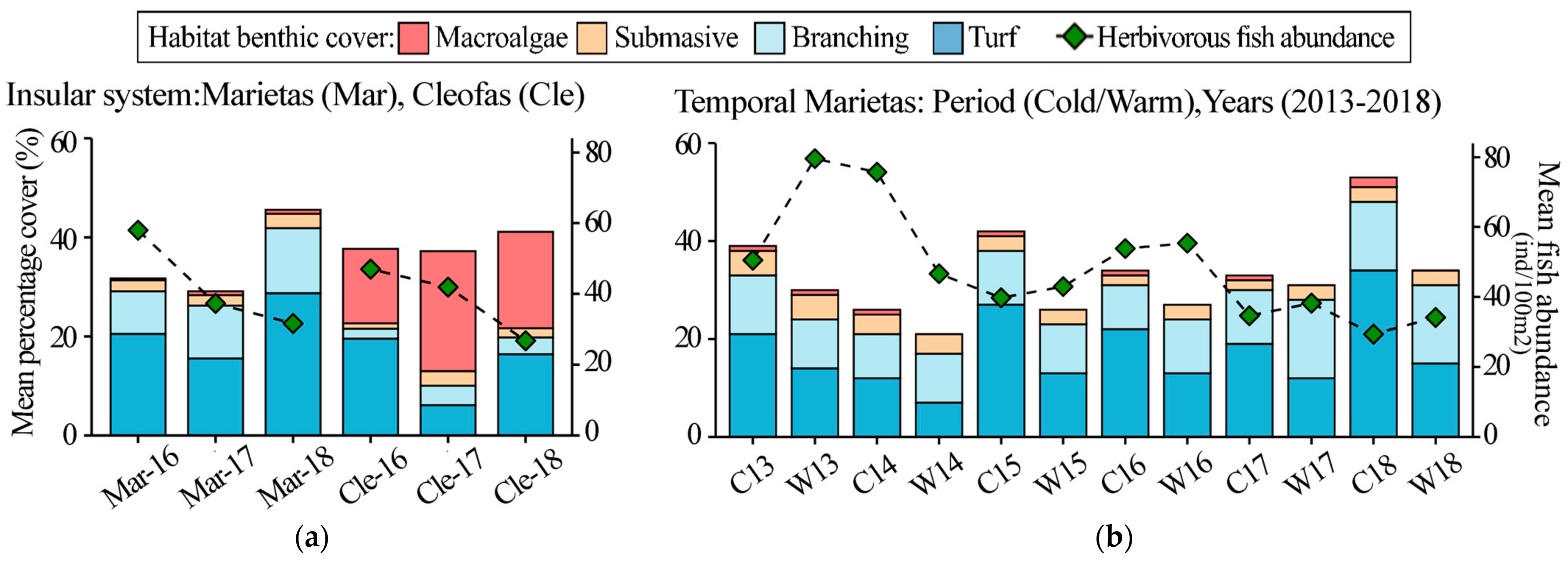
3. Results
3.1. Island Comparison (Marietas vs. Cleofas)
3.2. Temporal Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carpenter, R.C.; Edmunds, P.J. Local and Regional Scale Recovery of Diadema Promotes Recruitment of Scleractinian Corals. Ecol. Lett. 2006, 9, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Mumby, P.J. The Impact of Exploiting Grazers (Scaridae) on the Dynamics of Caribbean Coral Reefs. Ecol. Appl. 2006, 16, 747–769. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, R.; Hoey, A.; Bellwood, D. The Ecosystem Roles of Parrotfishes on Tropical Reefs. Ocean. Mar. Biol. Ann. Rev. 2014, 52, 81–132. [Google Scholar]
- Bonaldo, R.M.; Krajewski, J.P.; Bellwood, D.R. Relative Impact of Parrotfish Grazing Scars on Massive Porites Corals at Lizard Island, Great Barrier Reef. Mar. Ecol. Prog. Ser. 2011, 423, 223–233. [Google Scholar] [CrossRef][Green Version]
- Miller, M.W.; Hay, M.E. Coral-seaweed-grazer-nutrient Interactions on Temperate Reefs. Ecol. Monogr. 1996, 66, 323–344. [Google Scholar] [CrossRef]
- Durán, A.; Claro, R. Actividad Alimentaria de Los Peces Herbívoros y Su Impacto En Arrecifes Con Diferente Nivel de Degradación Antrópica. Rev. Biol. Trop. 2009, 57, 687–697. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mora, C.; Aburto-Oropeza, O.; Ayala-Bocos, A.; Ayotte, P.M.; Banks, S.; Bauman, A.G.; Beger, M.; Bessudo, S.; Booth, D.J.; Brokovich, E.; et al. Global Human Footprint on the Linkage between Biodiversity and Ecosystem Functioning in Reef Fishes. PLoS Biol. 2011, 9, e1000606. [Google Scholar] [CrossRef]
- D’Agata, S.; Mouillot, D.; Kulbicki, M.; Andréfouët, S.; Bellwood, D.R.; Cinner, J.E.; Cowman, P.F.; Kronen, M.; Pinca, S.; Vigliola, L. Human-Mediated Loss of Phylogenetic and Functional Diversity in Coral Reef Fishes. Curr. Biol. 2014, 24, 555–560. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Chabanet, P.; Evans, R.D.; Jennings, S.; Letourneur, Y.; Aaron Macneil, M.; Mcclanahan, T.R.; Öhman, M.C.; Polunin, N.V.C.; Wilson, S.K. Extinction Vulnerability of Coral Reef Fishes. Ecol. Lett. 2011, 14, 341–348. [Google Scholar] [CrossRef]
- De Bello, F.; Vandewalle, M.; Reitalu, T.; Lepš, J.; Prentice, H.C.; Lavorel, S.; Sykes, M.T. Evidence for Scale- and Disturbance-Dependent Trait Assembly Patterns in Dry Semi-Natural Grasslands. J. Ecol. 2013, 101, 1237–1244. [Google Scholar] [CrossRef]
- Darling, E.S.; McClanahan, T.R.; Côté, I.M. Life Histories Predict Coral Community Disassembly under Multiple Stressors. Glob. Chang. Biol. 2013, 19, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Norström, A.V.; Nyström, M.; Lokrantz, J.; Folke, C. Alternative States on Coral Reefs: Beyond Coral-Macroalgal Phase Shifts. Mar. Ecol. Prog. Ser. 2009, 376, 293–306. [Google Scholar] [CrossRef]
- Vinebrooke, D.R.; Cottingham, L.K.; Norberg Jon, M.S.; Dodson, I.S.; Maberly, C.S.; Sommer, U. Impacts of Multiple Stressors on Biodiversity and Ecosystem Functioning: The Role of Species Co-tolerance. Oikos 2004, 104, 451–457. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Hoey, A.S.; Wilson, S.K.; Messmer, V.; Graham, N.A.J. Changes in Biodiversity and Functioning of Reef Fish Assemblages Following Coral Bleaching and Coral Loss. Diversity 2011, 3, 424–452. [Google Scholar] [CrossRef]
- Gunderson, L.H. Ecological Resilience—In Theory and Application. Annu. Rev. Ecol. Syst. 2000, 31, 425–439. [Google Scholar] [CrossRef]
- Nyström, M.; Folke, C.; Moberg, F. Coral Reef Disturbance and Resilience in a Human-Dominated Environment. Trends Ecol. Evol. 2000, 15, 413–417. [Google Scholar] [CrossRef]
- Hughes, T.P.; Barnes, M.L.; Bellwood, D.R.; Cinner, J.E.; Cumming, G.S.; Jackson, J.B.C.; Kleypas, J.; Van De Leemput, I.A.; Lough, J.M.; Morrison, T.H.; et al. Coral Reefs in the Anthropocene. Nature 2017, 546, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Arias-González, J.E.; Fung, T.; Seymour, R.M.; Garza-Pérez, J.R.; Acosta-González, G.; Bozec, Y.M.; Johnson, C.R. A Coral-Algal Phase Shift in Mesoamerica Not Driven by Changes in Herbivorous Fish Abundance. PLoS ONE 2017, 12, e0174855. [Google Scholar] [CrossRef]
- Hoey, A.S.; Pratchett, M.S.; Cvitanovic, C. High Macroalgal Cover and Low Coral Recruitment Undermines the Potential Resilience of the World’s Southernmost Coral Reef Assemblages. PLoS ONE 2011, 6, e25824. [Google Scholar] [CrossRef]
- Ferreira, C.E.L.; Floeter, S.R.; Gasparini, J.L.; Ferreira, B.P.; Joyeux, J.C. Trophic Structure Patterns of Brazilian Reef Fishes: A Latitudinal Comparison. J. Biogeo. 2004, 31, 1093–1106. [Google Scholar] [CrossRef]
- Gardner, T.A.; Côté, I.M.; Gill, J.A.; Grant, A.; Watkinson, A.R. Long-Term Region-Wide Declines in Caribbean Corals. Science (1979) 2003, 301, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, J.M.; Connolly, S.R.; Marshall, D.J.; Cohen, A.L. Projecting Coral Reef Futures under Global Warming and Ocean Acidification. Science 2011, 333, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Pratchett, M.S.; Hoey, A.S.; Wilson, S.K. Reef Degradation and the Loss of Critical Ecosystem Goods and Services Provided by Coral Reef Fishes. Curr. Opin. Environ. Sustain. 2014, 7, 37–43. [Google Scholar] [CrossRef]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J. Climate Change, Human Impacts, and the Resilience of Coral Reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef]
- Plass-Johnson, J.G.; Ferse, S.C.A.; Jompa, J.; Wild, C.; Teichberg, M. Fish Herbivory as Key Ecological Function in a Heavily Degraded Coral Reef System. Limnol. Oceanogr. 2015, 60, 1382–1391. [Google Scholar] [CrossRef]
- Adam, T.C.; Schmitt, R.J.; Holbrook, S.J.; Brooks, A.J.; Edmunds, P.J.; Carpenter, R.C.; Bernardi, G. Herbivory, Connectivity, and Ecosystem Resilience: Response of a Coral Reef to a Large-Scale Perturbation. PLoS ONE 2011, 6, e23717. [Google Scholar] [CrossRef] [PubMed]
- Puk, L.D.; Ferse, S.C.A.; Wild, C. Patterns and Trends in Coral Reef Macroalgae Browsing: A Review of Browsing Herbivorous Fishes of the Indo-Pacific. Rev. Fish Biol. Fish 2016, 26, 53–70. [Google Scholar] [CrossRef]
- Dubuc, A.; Quimbayo, J.P.; Alvarado, J.J.; Araya-Arce, T.; Arriaga, A.; Ayala-Bocos, A.; Julio Casas-Maldonado, J.; Chasqui, L.; Cortés, J.; Cupul-Magaña, A.; et al. Patterns of Reef Fish Taxonomic and Functional Diversity in the Eastern Tropical Pacific. Ecography 2023, 2023, e06536. [Google Scholar] [CrossRef]
- Cowman, P.F.; Parravicini, V.; Kulbicki, M.; Floeter, S.R. The Biogeography of Tropical Reef Fishes: Endemism and Provinciality through Time. Biol. Rev. 2017, 92, 2112–2130. [Google Scholar] [CrossRef]
- Kessler, W.S. The Circulation of the Eastern Tropical Pacific: A Review. Prog. Oceanogr. 2006, 69, 181–217. [Google Scholar] [CrossRef]
- Reyes-Bonilla, H. Coral Reefs of the Pacific Coast of México. In Latin American Coral Reefs; Elsevier: Amsterdam, The Netherlands, 2003; pp. 331–349. [Google Scholar]
- Cisneros-Montemayor, A.M.; Cisneros-Mata, M.A.; Harper, S.; Pauly, D. Extent and Implications of IUU Catch in Mexico’s Marine Fisheries. Mar. Policy 2013, 39, 283–288. [Google Scholar] [CrossRef]
- CONANP. Programa de Conservación y Manejo Parque Nacional Islas Marietas; Mexico, SEMARNAT: México City, México, 2007; pp. 1–155.
- CONANP. Programa de Conservación y Manejo Reserva de la Biósfera Isalas Marias; Mexico, SEMARNAT: México City, México, 2007; pp. 1–116.
- Barneche, D.R.; Floeter, S.R.; Ceccarelli, D.M.; Frensel, D.M.B.; Dinslaken, D.F.; Mário, H.F.S.; Ferreira, C.E.L. Feeding Macroecology of Territorial Damselfishes (Perciformes: Pomacentridae). Mar. Biol. 2009, 156, 289–299. [Google Scholar] [CrossRef]
- McManus, J.W.; Menez, L.A.B.; Kesner-Reyes, K.N.; Vergara, S.G.; Ablan, M.C. Coral Reef Fishing and Coral-Algal Phase Shifts: Implications for Global Reef Status. ICES J. Mar. Sci. 2000, 57, 572–578. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Irz, P.; Lanoiselée, C.; Mouillot, D.; Argillier, C. Evidence That Niche Specialization Explains Species-Energy Relationships in Lake Fish Communities. J. Anim. Ecol. 2008, 77, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Villéger, S.; Miranda, J.R.; Hernández, D.F.; Mouillot, D. Contrasting Changes in Taxonomic vs. Functional Diversity of Tropical Fish Communities after Habitat Degradation. Ecol. Appl. 2010, 20, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Mouillot, D.; Graham, N.A.J.; Villéger, S.; Mason, N.W.H.; Bellwood, D.R. A Functional Approach Reveals Community Responses to Disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Mouillot, D.; Villéger, S.; Scherer-Lorenzen, M.; Mason, N.W.H. Functional Structure of Biological Communities Predicts Ecosystem Multifunctionality. PLoS ONE 2011, 6, e17476. [Google Scholar] [CrossRef]
- Naeem, S.; Duffy, J.E.; Zavaleta, E. The Functions of Biological Diversity in an Age of Extinction. Science 2012, 336, 1401–1406. [Google Scholar] [CrossRef]
- Mouillot, D.; Villéger, S.; Parravicini, V.; Kulbicki, M.; Arias-González, J.E.; Bender, M.; Chabanet, P.; Floeter, S.R.; Friedlander, A.; Vigliola, L.; et al. Functional Over-Redundancy and High Functional Vulnerability in Global Fish Faunas on Tropical Reefs. Proc. Natl. Acad. Sci. USA 2014, 111, 13757–13762. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Schwilk, D.W.; Ackerly, D.D. A trait-based test for habitat filtering: Convex hull volume. Ecology 2006, 87, 1465–1471. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New Multidimensional Functional Diversity Indices for a Multifaceted Framework in Functional Ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Dolédec, S.; Chessel, D.; Ter Braak, C.J.F.; Champely, S. Matching Species Traits to Environmental Variables: A New Three-Table Ordination Method. Environ. Ecol. Stat. 1996, 3, 143–166. [Google Scholar] [CrossRef]
- Dray, S.; Legendre, P. Testing the Species Traits–Environment Relationships: The Fourth-corner Problem Revisited. Ecology 2008, 89, 3400–3412. [Google Scholar] [CrossRef] [PubMed]
- Dray, S.; Choler, P.; Dolédec, S.; Peres-Neto, P.R.; Thuiller, W.; Pavoine, S.; ter Braak, C.J.F. Combining the Fourth-corner and the RLQ Methods for Assessing Trait Responses to Environmental Variation. Ecology 2014, 95, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Beauchard, O.; Veríssimo, H.; Queirós, A.M.; Herman, P.M.J. The Use of Multiple Biological Traits in Marine Community Ecology and Its Potential in Ecological Indicator Development. Ecol. Indic. 2017, 76, 81–96. [Google Scholar] [CrossRef]
- Lavín, M.F.; Fiedler, P.C.; Amador, J.A.; Ballance, L.T.; Färber-Lorda, J.; Mestas-Nuñez, A.M. A Review of Eastern Tropical Pacific Oceanography: Summary. Prog. Oceanogr. 2006, 69, 391–398. [Google Scholar] [CrossRef]
- Wang, C.; Fiedler, P.C. ENSO Variability and the Eastern Tropical Pacific: A Review. Prog. Oceanogr. 2006, 69, 239–266. [Google Scholar] [CrossRef]
- Carballo, J.L.; Bautista, E.; Nava, H.; Cruz-Barraza, J.A.; Chávez, J.A. Boring Sponges, An Increasing Threat for Coral Reefs Affected by Bleaching Events. Ecol. Evol. 2013, 3, 872–886. [Google Scholar] [CrossRef]
- Cruz-García, R.; Rodríguez-Troncoso, A.P.; Rodríguez-Zaragoza, F.A.; Mayfield, A.; Cupul-Magaña, A.L. Ephemeral Effects of El Niño-Southern Oscillation Events on an Eastern Tropical Pacific Coral Community. Mar. Freshw. Res. 2020, 71, 1259–1268. [Google Scholar] [CrossRef]
- Sotelo-Casas, R.C.; Cupul-Magaña, A.L.; Rodríguez-Zaragoza, F.A.; Solís-Marín, F.A.; Rodríguez-Troncoso, A.P. Structural and Environmental Effects on an Assemblage of Echinoderms Associated with a Coral Community. Mar. Biodivers. 2018, 48, 1401–1411. [Google Scholar] [CrossRef]
- Calderon-Aguilera, L.E.; Reyes-Bonilla, H.; Olán-González, M.; Castañeda-Rivero, F.R.; Perusquía-Ardón, J.C. Estimated Flows and Biomass in a No-Take Coral Reef from the Eastern Tropical Pacific through Network Analysis. Ecol. Indic. 2021, 123, 107359. [Google Scholar] [CrossRef]
- McClure, E.C.; Richardson, L.E.; Graba-Landry, A.; Loffler, Z.; Russ, G.R.; Hoey, A.S. Cross-Shelf Differences in the Response of Herbivorous Fish Assemblages to Severe Environmental Disturbances. Diversity 2019, 11, 23. [Google Scholar] [CrossRef]
- Pombo-Ayora, L.; Coker, D.J.; Carvalho, S.; Short, G.; Berumen, M.L. Morphological and Ecological Trait Diversity Reveal Sensitivity of Herbivorous Fish Assemblages to Coral Reef Benthic Conditions. Mar. Environ. Res. 2020, 162, 105102. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D. A Simple Method for Estimating the Food Consumption of Fish Populations from Growth Data and Food Conversion Experiments. Fish. Bull. 1986, 84, 827–840. [Google Scholar]
- Anderson, M.; Gorley, R.; Clarke, K.P. For PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Escofier, B.; Pages, J. Multiple Factor Analysis (AFMULT Package). Comput. Stat. Data Anal. 1994, 18, 121–140. [Google Scholar] [CrossRef]
- Gaertner, J.C.; Mazouni, N.; Sabatier, R.; Millet, B. Spatial Structure and Habitat Associations of Demersal Assemblages in the Gulf of Lions: A Multicompartmental Approach. Mar. Biol. 1999, 135, 199–208. [Google Scholar] [CrossRef]
- Bernier, N.; Gillet, F. Structural Relationships among Vegetation, Soil Fauna and Humus Form in a Subalpine Forest Ecosystem: A Hierarchical Multiple Factor Analysis (HMFA). Pedobiologia 2012, 55, 321–334. [Google Scholar] [CrossRef]
- Robert, P.; Escoufier, Y. A Unifying Tool for Linear Multivariate Statistical Methods: The RV-coefficient. J. R. Stat. Soc. Ser. C Appl. Stat. 1976, 25, 257–265. [Google Scholar] [CrossRef]
- Josse, J.; Pagès, J.; Husson, F. Testing the Significance of the RV Coefficient. Comput. Stat. Data Anal. 2008, 53, 82–91. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.-B. The Ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Hill, M.O.; Smith, A.J.E. Principal Component Analysis of Taxonomic Data with Multi-State Discrete Characters. Taxon 1976, 25, 249–255. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. 2020. Available online: https://www.R-project.org (accessed on 10 March 2020).
- Galván-Villa, C.M.; Arreola-Robles, J.L.; Ríos-Jara, E.; Rodríguez-Zaragoza, F.A. Ensamblajes de Peces Arrecifales y Su Relación Con El Hábitat Bentónico de La Isla Isabel, Nayarit, México. Rev. Biol. Mar. Oceanogr. 2010, 45, 311–324. [Google Scholar] [CrossRef]
- García-Hernández, V.C.; Reyes-Bonilla, H.; Balart, E.F.; Ríos-Jara, E.; Lluch-Cota, S.E.; Serviere-Zaragoza, E. Comparación de La Diversidad Ecológica y Composición de Especies de Ensambles de Macroalgas, Macroinvertebrados Bentónicos y Peces En Dos Arrecifes Rocosos Tropicales. Rev. Biol. Mar. Oceanogr. 2014, 49, 477–491. [Google Scholar] [CrossRef]
- Ramírez-Ortiz, G.; Calderon-Aguilera, L.E.; Reyes-Bonilla, H.; Ayala-Bocos, A.; Hernández, L.; Fernández Rivera-Melo, F.; López-Pérez, A.; Dominici-Arosamena, A. Functional Diversity of Fish and Invertebrates in Coral and Rocky Reefs of the Eastern Tropical Pacific. Mar. Ecol. 2017, 38, e12447. [Google Scholar] [CrossRef]
- Pérez de-Silva, C.V.; Cupul-Magaña, A.L.; Rodríguez-Troncoso, A.P.; Rodríguez-Zaragoza, F.A. Reef Fish Assemblage in Two Insular Zones within the Mexican Central Pacific. Oceans 2022, 3, 204–217. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hoey, A.S.; Choat, J.H. Limited Functional Redundancy in High Diversity Systems: Resilience and Ecosystem Function on Coral Reefs. Ecol. Lett. 2003, 6, 281–285. [Google Scholar] [CrossRef]
- Hoey, A.S.; Bellwood, D.R. Suppression of Herbivory by Macroalgal Density: A Critical Feedback on Coral Reefs? Ecol. Lett. 2011, 14, 267–273. [Google Scholar] [CrossRef]
- Munsterman, K.S.; Allgeier, J.E.; Peters, J.R.; Burkepile, D.E. A View From Both Ends: Shifts in Herbivore Assemblages Impact Top-Down and Bottom-Up Processes on Coral Reefs Herbivore Identity and Size Influence Top-down and Bottom-up Processes. Ecosystems 2021, 24, 1702–1715. [Google Scholar] [CrossRef]
- Muir, P.R.; Wallace, C.C.; Done, T.; Aguirre, J.D. Limited Scope for Latitudinal Extension of Reef Corals. Science (1979) 2015, 348, 1135–1138. [Google Scholar] [CrossRef]
- Ceccarelli, D.M.; Evans, R.D.; Logan, M.; Mantel, P.; Puotinen, M.; Petus, C.; Russ, G.R.; Williamson, D.H. Long-Term Dynamics and Drivers of Coral and Macroalgal Cover on Inshore Reefs of the Great Barrier Reef Marine Park. Ecol. Appl. 2020, 30, e01635. [Google Scholar] [CrossRef]
- Ceccarelli, D.M.; Jones, G.P.; McCook, L.J. Interactions between Herbivorous Fish Guilds and Their Influence on Algal Succession on a Coastal Coral Reef. J. Exp. Mar. Biol. Ecol. 2011, 399, 60–67. [Google Scholar] [CrossRef]
- Ceccarelli, D.M. Modification of Benthic Communities by Territorial Damselfish: A Multi-Species Comparison. Coral Reefs 2007, 26, 853–866. [Google Scholar] [CrossRef]
- Ceccarelli, D.M.; Jones, G.P.; McCook, L.J. Territorial Damselfishes as Determinants of the Structure of Benthic Communities on Coral Reefs. Oceanogr. Mar. Biol. Annu. Rev. 2001, 39, 355–389. [Google Scholar]
- Kuempel, C.D.; Altieri, A.H. The Emergent Role of Small-Bodied Herbivores in Pre-Empting Phase Shifts on Degraded Coral Reefs. Sci. Rep. 2017, 7, 39670. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.E.L.; Gonçalves, J.E.A.; Coutinho, R.; Peret, A.C. Herbivory by the Dusky Damselfish Stegastes Fuscus (Cuvier, 1830) in a Tropical Rocky Shore: Effects on the Benthic Community. J. Exp. Mar. Biol. Ecol. 1998, 229, 241–264. [Google Scholar] [CrossRef]
- Benkwitt, C.E. Predator Effects on Reef Fish Settlement Depend on Predator Origin and Recruit Density. Ecology 2017, 98, 896–902. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodríguez-Troncoso, A.P.; Carpizo-Ituarte, E.; Cupul-Magaña, A.L. Physiological Response to High Temperature in the Tropical Eastern Pacific Coral Pocillopora Verrucosa. Mar. Ecol. 2016, 37, 1168–1175. [Google Scholar] [CrossRef]
- Santiago-Valentín, J.D.; Rodríguez-Troncoso, A.P.; Bautista-Guerrero, E.; López-Pérez, A.; Cupul-Magaña, A.L. Successful Sexual Reproduction of the Scleractinian Coral Porites Panamensis: Evidence of Planktonic Larvae and Recruitment. Invertebr. Biol. 2019, 138, 29–39. [Google Scholar] [CrossRef]
- Santiago-Valentín, J.D.; Rodríguez-Troncoso, A.P.; Bautista-Guerrero, E.; López-Pérez, A.; Cupul-Magaña, A.L. Settlement Ecology of Scleractinian Corals of the Northeastern Tropical Pacific. Coral Reefs 2020, 39, 133–146. [Google Scholar] [CrossRef]
- Irving, A.D.; Witman, J.D. Positive Effects of Damselfish Override Negative Effects of Urchins to Prevent an Algal Habitat Switch. J. Ecol. 2009, 97, 337–347. [Google Scholar] [CrossRef]
- Burkepile, D.E.; Hay, M.E. Herbivore Species Richness and Feeding Complementarity Affect Community Structure and Function on a Coral Reef. Proc. Natl. Acad. Sci. USA 2008, 105, 16201–16206. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Wilson, S.K.; Jennings, S.; Polunin, N.V.C.; Robinson, J.A.N.; Bijoux, J.P.; Daw, T.M. Lag Effects in the Impacts of Mass Coral Bleaching on Coral Reef Fish, Fisheries, and Ecosystems. Conserv. Biol. 2007, 21, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Mumby, P.J.; Hastings, A.; Edwards, H.J. Thresholds and the Resilience of Caribbean Coral Reefs. Nature 2007, 450, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Gouezo, M.; Fabricius, K.; Harrison, P.; Golbuu, Y.; Doropoulos, C. Optimizing Coral Reef Recovery with Context-Specific Management Actions at Prioritized Reefs. J. Environ. Manag. 2021, 295, 113209. [Google Scholar] [CrossRef] [PubMed]
- Boyer, K.E.; Fong, P.; Armitage, A.R.; Cohen, R.A. Elevated Nutrient Content of Tropical Macroalgae Increases Rates of Herbivory in Coral, Seagrass, and Mangrove Habitats. Coral Reefs 2004, 23, 530–538. [Google Scholar] [CrossRef]
- Fox, R.J.; Bellwood, D.R. Quantifying Herbivory across a Coral Reef Depth Gradient. Mar. Ecol. Prog. Ser. 2007, 339, 49–59. [Google Scholar] [CrossRef]
- Garcia, J.; Saragon, G.; Tessier, A.; Lenfant, P. Herbivorous Reef Fish Movement Ability Estimation in Marine Protected Areas of Martinique. Proc. Gulf Caribb. Fish. Inst. 2011, 63, 254–259. [Google Scholar]
- Eurich, J.G.; McCormick, M.I.; Jones, G.P. Habitat Selection and Aggression as Determinants of Fine-Scale Partitioning of Coral Reef Zones in a Guild of Territorial Damselfishes. Mar. Ecol. Prog. Ser. 2018, 587, 201–215. [Google Scholar] [CrossRef]
- Cheal, A.J.; MacNeil, M.A.; Cripps, E.; Emslie, M.J.; Jonker, M.; Schaffelke, B.; Sweatman, H. Coral-Macroalgal Phase Shifts or Reef Resilience: Links with Diversity and Functional Roles of Herbivorous Fishes on the Great Barrier Reef. Coral Reefs 2010, 29, 1005–1015. [Google Scholar] [CrossRef]
- Bosch, N.E.; McLean, M.; Zarco-Perello, S.; Bennett, S.; Stuart-Smith, R.D.; Vergés, A.; Pessarrodona, A.; Tuya, F.; Langlois, T.; Spencer, C.; et al. Persistent Thermally Driven Shift in the Functional Trait Structure of Herbivorous Fishes: Evidence of Top-down Control on the Rebound Potential of Temperate Seaweed Forests? Glob. Chang. Biol. 2022, 28, 2296–2311. [Google Scholar] [CrossRef]
- Schiettekatte, N.; Brandl, S.J.; Casey, J.M.; Graham, N.A.J.; Barneche, D.R.; Burkepile, D.E.; Allgeier, J.E.; Arias-Gonzaléz, J.E.; Edgar, G.J.; Ferreira, C.E.L. Biological Trade-Offs Underpin Coral Reef Ecosystem Functioning. Nat. Ecol. Evol. 2022, 6, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Streit, R.P.; Bellwood, D.R. To Harness Traits for Ecology, Let’s Abandon ‘Functionality’. Trends Ecol. Evol. 2023, 38, 402–411. [Google Scholar] [CrossRef]
- Welsh, J.Q.; Bellwood, D.R. Herbivorous Fishes, Ecosystem Function and Mobile Links on Coral Reefs. Coral Reefs 2014, 33, 303–311. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Maina, J.M.; Graham, N.A.J.; Jones, K.R. Modeling Reef Fish Biomass, Recovery Potential, and Management Priorities in the Western Indian Ocean. PLoS ONE 2016, 11, e0154585. [Google Scholar]
- McClanahan, T.R.; Graham, N.A.J.; MacNeil, M.A.; Muthiga, N.A.; Cinner, J.E.; Bruggemann, J.H.; Wilson, S.K. Critical Thresholds and Tangible Targets for Ecosystem-Based Management of Coral Reef Fisheries. Proc. Natl. Acad. Sci. USA 2011, 108, 17230–17233. [Google Scholar] [CrossRef] [PubMed]
- Graham, N.A.J.; Nash, K.L. The Importance of Structural Complexity in Coral Reef Ecosystems. Coral Reefs 2013, 32, 315–326. [Google Scholar] [CrossRef]
- Mumby, P.J.; Wolff, N.H.; Bozec, Y.M.; Chollett, I.; Halloran, P. Operationalizing the Resilience of Coral Reefs in an Era of Climate Change. Conserv. Lett. 2014, 7, 176–187. [Google Scholar] [CrossRef]
- Brown, B.E. Coral bleaching: Causes and consequences. Coral Reef 1997, 16, 129–138. [Google Scholar] [CrossRef]
- Victor, B.C.; Wellington, G.M.; Robertson, D.R.; Ruttenberg, B.I. The Effect of the El Niño–Southern Oscillation Event on the Distribution of Reef-Associated Labrid Fishes in the Eastern Pacific Ocean. Bull. Mar. Sci. 2001, 69, 279–288. [Google Scholar]
- Otero, E.; Carbery, K.K. Chlorophyll a and Turbidity Patterns over Coral Reefs Systems of La Parguera Natural Reserve, Puerto Rico. Rev. Biol. Trop. 2005, 53, 25–32. [Google Scholar]
- Day, P.B.; Stuart-Smith, R.D.; Edgar, G.J.; Bates, A.E. Species’ Thermal Ranges Predict Changes in Reef Fish Community Structure during 8 Years of Extreme Temperature Variation. Divers. Distrib. 2018, 24, 1036–1046. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. Fish Base. World Wide Web Electronic Publication. 2019. Available online: https://www.fishbase.se/ (accessed on 1 June 2019).
- Robertson, D.R.; Allen, G.R. Shorefishes of the Tropical Eastern Pacific: Online Information System, República de Panamá. 2015. Available online: https://biogeodb.stri.si.edu/sftep/ (accessed on 1 June 2019).
- DiSalvo, L.H.; Randall, J.E.; Cea, A. Stomach Contents and Feeding Observations of Some Easter Island Fishes. Atoll Res. Bull. 2007, 548, 1–22. [Google Scholar] [CrossRef]
- Richardson, L.E.; Graham, N.A.J.; Pratchett, M.S.; Hoey, A.S. Structural Complexity Mediates Functional Structure of Reef Fish Assemblages among Coral Habitats. Environ. Biol. Fishes 2017, 100, 193–207. [Google Scholar] [CrossRef]


| PERMANOVA Overall Test | CV (%) | PERMDISP Test | ||||||
|---|---|---|---|---|---|---|---|---|
| Source | Pseudo-F | pPerm | F | pPerm | ||||
| Insular systems - df | ||||||||
| Island (Is) - 1 | 100.880 | <0.001 | 52.601 | 24.789 | <0.001 | |||
| Residual - 178 | 47.399 | |||||||
| Temporal differences - df | ||||||||
| Year (Yr) - 5 | 2.459 | 0.009 | 2.264 | 0.500 | 0.819 | |||
| Period (Pr) - 1 | 8.020 | 0.001 | 3.632 | 1.030 | 0.329 | |||
| Yr × Pr - 5 | 1.316 | 0.228 | 0.981 | 0.643 | 0.877 | |||
| Residual – 348 | 93.124 | |||||||
| Pairwise among years (pPerm) | ||||||||
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |||
| Pairwise among years (t) | 2013 | 0.114 | 0.220 | 0.344 | 0.008 | 0.007 | ||
| 2014 | 1.44 | 0.167 | 0.466 | 0.019 | 0.029 | |||
| 2015 | 1.24 | 1.35 | 0.460 | 0.115 | 0.241 | |||
| 2016 | 1.04 | 0.90 | 0.90 | 0.063 | 0.099 | |||
| 2017 | 2.17 | 1.99 | 1.51 | 1.70 | 0.130 | |||
| 2018 | 2.32 | 1.94 | 1.22 | 1.54 | 1.49 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-de-Anda, D.; Cupul-Magaña, A.L.; Aguilar-Betancourt, C.M.; González-Sansón, G.; Rodríguez-Zaragoza, F.A.; Rodríguez-Troncoso, A.P. Herbivorous Reef Fish Interaction with the Habitat and Physicochemical Variables in Coral Ecosystems in the Mexican Tropical Pacific. Oceans 2024, 5, 21-37. https://doi.org/10.3390/oceans5010002
Morales-de-Anda D, Cupul-Magaña AL, Aguilar-Betancourt CM, González-Sansón G, Rodríguez-Zaragoza FA, Rodríguez-Troncoso AP. Herbivorous Reef Fish Interaction with the Habitat and Physicochemical Variables in Coral Ecosystems in the Mexican Tropical Pacific. Oceans. 2024; 5(1):21-37. https://doi.org/10.3390/oceans5010002
Chicago/Turabian StyleMorales-de-Anda, Diana, Amílcar Leví Cupul-Magaña, Consuelo María Aguilar-Betancourt, Gaspar González-Sansón, Fabián Alejandro Rodríguez-Zaragoza, and Alma Paola Rodríguez-Troncoso. 2024. "Herbivorous Reef Fish Interaction with the Habitat and Physicochemical Variables in Coral Ecosystems in the Mexican Tropical Pacific" Oceans 5, no. 1: 21-37. https://doi.org/10.3390/oceans5010002
APA StyleMorales-de-Anda, D., Cupul-Magaña, A. L., Aguilar-Betancourt, C. M., González-Sansón, G., Rodríguez-Zaragoza, F. A., & Rodríguez-Troncoso, A. P. (2024). Herbivorous Reef Fish Interaction with the Habitat and Physicochemical Variables in Coral Ecosystems in the Mexican Tropical Pacific. Oceans, 5(1), 21-37. https://doi.org/10.3390/oceans5010002