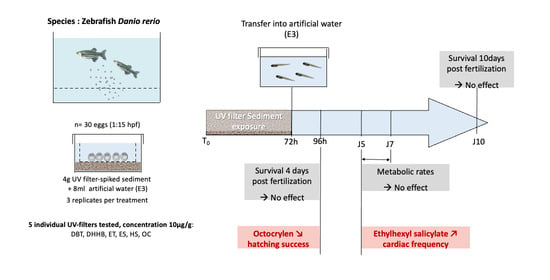
Impact of Egg Exposure to UV Filter-Spiked Sediment on the Survival, Hatching Success, Cardiac Frequency, and Metabolic Scope of Zebrafish Embryos
Abstract
:1. Introduction
| Abbr. | COSING Name | Alternative Names | CAS | Formula | Maximum Concentration in Final Product a | Higher Environmental Concentration Measured in Sediment (in ng·g−1) | References | ||
|---|---|---|---|---|---|---|---|---|---|
| USA | EU | Aus. | |||||||
| DBT | Diethylhexyl butamido triazone | Iscotrizinol Uvasorb HEB | 154702-15-5 | C44H59N7O5 | n.a. | 10% | n.a. | 629 | [10,16] |
| DHHB | Diethylamino hydroxybenzoyl hexyl benzoate | Uvinul A+ | 302776-68-7 | C24H31NO4 | n.a. | 10% | 10% | - | - |
| ET | Ethylhexyl triazone | Uvinul T150 | 88122-99-0 | C48H66N6O6 | n.a. | 5% | n.a. | 2 | [20] |
| ES | 2-Ethylhexyl salicylate | 118-60-5 | C15H22O3 | n.a. | 5% | n.a. | 13.7 | [18,19,20] | |
| HS | Homosalate | Homomenthyl salicylate Sunobel®HMS | 118-56-9 | C16H22O3 | 15% | 10% | 15% | 26 | [14,18,19,20] |
| OC | Octocrylene | 6197-30-4 | C24H27NO2 | 10% | 10% | 10% | 652 | [14,15,16,17,18,19,20] | |
2. Materials and Methods
2.1. Chemicals Used and the Preparation of Spiked Artificial Sediment
2.2. Zebrafish Embryo-Larval Assay
2.3. Survival and Hatching Success
2.4. Cardiac Frequency
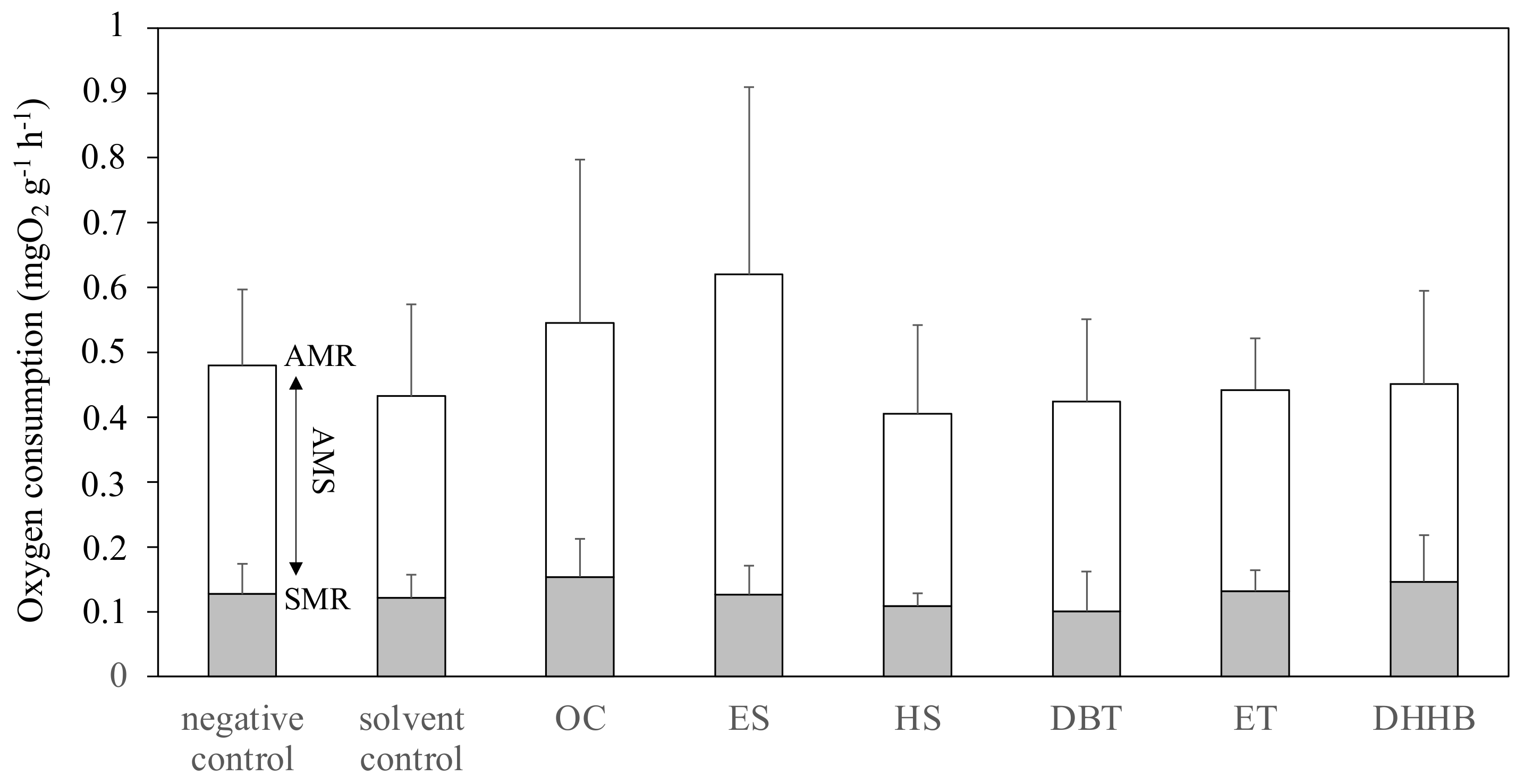
2.5. Static Respirometry
2.6. Statistical Analysis
3. Results and Discussion
3.1. Impact on Survival and Hatching
3.2. Cardiotoxicity
3.3. Aerobic Metabolic Scope
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fent, K.; Zenker, A.; Rapp, M. Widespread occurrence of estrogenic UV-filters in aquatic ecosystems in Switzerland. Environ. Pollut. 2010, 158, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Waldman, R.A.; Grant-Kels, J.M. The role of sunscreen in the prevention of cutaneous melanoma and nonmelanoma skin cancer. J. Am. Acad. Dermatol. 2019, 80, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Giokas, D.L.; Sakkas, V.; Albanis, T.A.; Lampropoulou, D.A. Determination of UV-filter residues in bathing waters by liquid chromatography UV-diode array and gas chromatography–mass spectrometry after micelle mediated extraction-solvent back extraction. J. Chromatogr. A 2005, 1077, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Homem, V.; Alves, A.; Santos, L. A review of organic UV-filters in wastewater treatment plants. Environ. Int. 2016, 86, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.M.; Leung, H.; Kwan, B.K.; Ng, K.-Y.; Yamashita, N.; Taniyasu, S.; Lam, K.S.P.; Murphy, M.B. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in marine sediments in Hong Kong and Japan. J. Hazard. Mater. 2015, 292, 180–187. [Google Scholar] [CrossRef]
- Molins-Delgado, D.; Díaz-Cruz, M.S.; Barceló, D. Ecological risk assessment associated to the removal of endocrine-disrupting parabens and benzophenone-4 in wastewater treatment. J. Hazard. Mater. 2016, 310, 143–151. [Google Scholar] [CrossRef]
- Montes-Grajales, D.; Fennix-Agudelo, M.A.; Miranda-Castro, W. Occurrence of personal care products as emerging chemicals of concern in water resources: A review. Sci. Total Environ. 2017, 595, 601–614. [Google Scholar] [CrossRef]
- Lozano, C.; Givens, J.; Stien, D.; Matallana-Surget, S.; Lebaron, P. Bioaccumulation and Toxicological Effects of UV-Filters on Marine Species. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Mitchelmore, C.L.; Burns, E.E.; Conway, A.; Heyes, A.; Davies, I.A. A Critical Review of Organic Ultraviolet Filter Exposure, Hazard, and Risk to Corals. Environ. Toxicol. Chem. 2021, 40, 967–988. [Google Scholar] [CrossRef]
- Fagervold, S.K.; Rodrigues, A.S.; Rohée, C.; Roe, R.; Bourrain, M.; Stien, D.; LeBaron, P. Occurrence and environmental distribution of 5 UV filters during the summer season in different water bodies. Water Air Soil Pollut. 2019, 230, 172. [Google Scholar] [CrossRef]
- Rodil, R.; Schrader, S.; Moeder, M. Non-porous membrane-assisted liquid–liquid extraction of UV filter compounds from water samples. J. Chromatogr. A 2009, 1216, 4887–4894. [Google Scholar] [CrossRef]
- Clergeaud, F.; Fagervold, S.K.; Rodrigues, A.M.; Thorel, E.; Stien, D.; Lebaron, P. Transfer of 7 organic UV filters from sediment to the ragworm Hediste diversicolor: Bioaccumulation of benzophenone-3 and further proof of octocrylene metabolism. Pollutants 2022, 2, 23–31. [Google Scholar] [CrossRef]
- Stien, D.; Clergeaud, F.; Rodrigues, A.M.S.; Lebaron, K.; Pillot, R.; Romans, P.; Fagervold, S.; Lebaron, P. Metabolomics reveal that octocrylene accumulates in Pocillopora damicornis tissues as fatty acid conjugates and triggers coral cell mitochondrial dysfunction. Anal. Chem. 2018, 91, 990–995. [Google Scholar] [CrossRef]
- Kameda, Y.; Kimura, K.; Miyazaki, M. Occurrence and profiles of organic sun-blocking agents in surface waters and sediments in Japanese rivers and lakes. Environ. Pollut. 2011, 159, 1570–1576. [Google Scholar] [CrossRef]
- Amine, H.; Gomez, E.; Halwani, J.; Casellas, C.; Fenet, H. UV filters, ethylhexyl methoxycinnamate, octocrylene and ethylhexyl dimethyl PABA from untreated wastewater in sediment from eastern Mediterranean river transition and coastal zones. Mar. Pollut. Bull. 2012, 64, 2435–2442. [Google Scholar] [CrossRef]
- Kaiser, D.; Sieratowicz, A.; Zielke, H.; Oetken, M.; Hollert, H.; Oehlmann, J. Ecotoxicological effect characterisation of widely used organic UV filters. Environ. Pollut. 2012, 163, 84–90. [Google Scholar] [CrossRef]
- Combi, T.; Miserocchi, S.; Langone, L.; Guerra, R. Polychlorinated biphenyls (PCBs) in sediments from the western Adriatic Sea: Sources, historical trends and inventories. Sci. Total Environ. 2016, 562, 580–587. [Google Scholar] [CrossRef]
- Pintado-Herrera, M.G.; Wang, C.; Lu, J.; Chang, Y.-P.; Chen, W.; Li, X.; Lara-Martin, P.A. Distribution, mass inventories, and ecological risk assessment of legacy and emerging contaminants in sediments from the Pearl River Estuary in China. J. Hazard. Mater. 2017, 323, 128–138. [Google Scholar] [CrossRef]
- Apel, C.; Joerss, H.; Ebinghaus, R. Environmental occurrence and hazard of organic UV stabilizers and UV filters in the sediment of European North and Baltic Seas. Chemosphere 2018, 212, 254–261. [Google Scholar] [CrossRef]
- Sánchez-Brunete, C.; Miguel, E.; Albero, B.; Tadeo, J.L. Analysis of salicylate and benzophenone-type UV filters in soils and sediments by simultaneous extraction cleanup and gas chromatography–mass spectrometry. J. Chromatogr. A 2011, 1218, 4291–4298. [Google Scholar] [CrossRef]
- Thorel, E.; Clergeaud, F.; Jaugeon, L.; Rodrigues, A.M.S.; Lucas, J.; Stien, D.; Lebaron, P. Effect of 10 UV Filters on the Brine Shrimp Artemia salina and the Marine Microalga Tetraselmis sp. Toxics 2020, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Stien, D.; Suzuki, M.; Rodrigues, A.M.S.; Yvin, M.; Clergeaud, F.; Thorel, E.; LeBaron, P. A unique approach to monitor stress in coral exposed to emerging pollutants. Sci. Rep. 2020, 10, 9601. [Google Scholar] [CrossRef] [PubMed]
- Langford, K.H.; Reid, M.J.; Fjeld, E.; Øxnevad, S.; Thomas, K.V. Environmental occurrence and risk of organic UV filters and stabilizers in multiple matrices in Norway. Environ. Int. 2015, 80, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. An overview of UV-absorbing compounds (organic UV filters) in aquatic biota. Anal. Bioanal. Chem. 2012, 404, 2597–2610. [Google Scholar] [CrossRef] [PubMed]
- Molins-Delgado, D.; Muñoz, R.; Nogueira, S.; Alonso, M.B.; Torres, J.P.; Malm, O.; Ziolli, R.L.; Hauser-Davis, R.; Eljarrat, E.; Barceló, D.; et al. Occurrence of organic UV filters and metabolites in lebranche mullet (Mugil liza) from Brazil. Sci. Total Environ. 2018, 618, 451–459. [Google Scholar] [CrossRef]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Lammer, E.; Carr, G.; Wendler, K.; Rawlings, J.; Belanger, S.; Braunbeck, T. Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 196–209. [Google Scholar] [CrossRef]
- Barrionuevo, W.R.; Burggren, W.W. O2 consumption and heart rate in developing zebrafish (Danio rerio): Influence of temperature and ambient O2. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1999, 276, R505–R513. [Google Scholar] [CrossRef] [Green Version]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OCDE: Paris, France, 2013. [Google Scholar] [CrossRef]
- Spitsbergen, J.M.; Kent, M.L. The State of the Art of the Zebrafish Model for Toxicology and Toxicologic Pathology Research: Advantages and Current Limitations. Toxicol. Pathol. 2003, 31, 62–87. [Google Scholar] [CrossRef]
- Yazar, S.; Gökçek, Y. Assessment of in vitro genotoxicity effect of homosalate in cosmetics. Marmara Pharm. J. 2018, 22, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Coronado, M.; De Haro, H.; Deng, X.; Rempel, M.A.; Lavado, R.; Schlenk, D. Estrogenic activity and reproductive effects of the UV-filter oxybenzone (2-hydroxy-4-methoxyphenyl-methanone) in fish. Aquat. Toxicol. 2008, 90, 182–187. [Google Scholar] [CrossRef]
- Lucas, J.; Logeux, V.; Rodrigues, A.M.S.; Stien, D.; Lebaron, P. Exposure to four chemical UV filters through contaminated sediment: Impact on survival, hatching success, cardiac frequency, and aerobic metabolic scope in embryo-larval stage of zebrafish. Environ. Sci. Pollut. Res. 2021, 28, 29412–29420. [Google Scholar] [CrossRef]
- Le Bihanic, F.; Morin, B.; Cousin, X.; Le Menach, K.; Budzinski, H.; Cachot, J. Developmental toxicity of PAH mixtures in fish early life stages. Part I: Adverse effects in rainbow trout. Environ. Sci. Pollut. Res. 2014, 21, 13720–13731. [Google Scholar] [CrossRef] [Green Version]
- Le Bihanic, F.; Perrichon, P.; Landi, L.; Clérandeau, C.; Le Menach, K.; Budzinski, H.; Cousin, X.; Cachot, J. Development of a reference artificial sediment for chemical testing adapted to the MELA sediment contact assay. Environ. Sci. Pollut. Res. 2014, 21, 13689–13702. [Google Scholar] [CrossRef]
- Lawrence, C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture 2007, 269, 1–20. [Google Scholar] [CrossRef]
- Steffensen, J.F.; Bushnell, P.G.; Schurmann, H. Oxygen consumption in four species of teleosts from Greenland: No evidence of metabolic cold adaptation. Polar Biol. 1994, 14, 49–54. [Google Scholar] [CrossRef]
- Lucas, J.; Schouman, A.; Lyphout, L.; Cousin, X.; Lefrancois, C. Allometric relationship between body mass and aerobic metabolism in zebrafish Danio rerio. J. Fish Biol. 2014, 84, 1171–1178. [Google Scholar] [CrossRef]
- Burggren, W.W. Developing animals flout prominent assumptions of ecological physiology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 141, 430–439. [Google Scholar] [CrossRef]
- Lin, C.; Hui, M.N.; Cheng, S.H. Toxicity and cardiac effects of carbaryl in early developing zebrafish (Danio rerio) embryos. Toxicol. Appl. Pharmacol. 2007, 222, 159–168. [Google Scholar] [CrossRef]
- Lucas, J.; Perrichon, P.; Nouhaud, M.; Audras, A.; Leguen, I.; Lefrançois, C. Aerobic metabolism and cardiac activity in the descendants of zebrafish exposed to pyrolytic polycyclic aromatic hydrocarbons. Environ. Sci. Pollut. Res. 2014, 21, 13888–13897. [Google Scholar] [CrossRef] [Green Version]
- Lucas, J.; Lefrancois, C.; Gesset, C.; Budzinski, H.; Labadie, P.; Baudrimont, M.; Coynel, A.; Le Menach, K.; Pardon, P.; Peluhet, L.; et al. Health status of juveniles of European sturgeon intended for reintroduction in Gironde-Garonne-Dordogne catchments. Ecotoxicol. Environ. Saf. 2021, 225, 112720. [Google Scholar] [CrossRef]
- Mccormick, M.I.; Nechaev, I.V. Influence of cortisol on developmental rhythms during embryogenesis in a tropical damselfish. J. Exp. Zoöl. 2002, 293, 456–466. [Google Scholar] [CrossRef]
- Nesan, D.; Vijayan, M.M. Embryo exposure to elevated cortisol level leads to cardiac performance dysfunction in zebrafish. Mol. Cell. Endocrinol. 2012, 363, 85–91. [Google Scholar] [CrossRef]
- Liu, J.; Ye, J.; Chen, Y.; Li, C.; Ou, H. UV-driven hydroxyl radical oxidation of tris(2-chloroethyl) phosphate: Intermediate products and residual toxicity. Chemosphere 2018, 190, 225–233. [Google Scholar] [CrossRef]

| Survival Rate (%) | Hatching Success (%) | |||
|---|---|---|---|---|
| Treatment | Number of Replicates | 96 hpf | 10 dpf | 96 hpf |
| Negative control | 4 | 86.7 ± 6.7 | 85.6 ± 5.1 | 95.75 ± 5.28 |
| Solvent control | 4 | 84.2 ± 7.4 | 84.2 ± 7.4 | 92.5 ± 11.2 |
| OC | 3 | 73.3 ± 20.0 | 72.2 ± 18.4 | 67.1 ± 23.5 * |
| ES | 4 | 78.3 ± 3.3 | 75.8 ± 3.2 | 93.7 ± 8.0 |
| HS | 6 | 78.8 ± 15.6 | 77.8 ± 15.0 | 94.0 ± 4.1 |
| DBT | 3 | 76.7 ± 13.3 | 76.7 ± 15.3 | 80.0 ± 8.7 |
| ET | 3 | 86.7 ± 5.8 | 82.2 ± 3.6 | 96.3 ± 3.6 |
| DHHB | 3 | 77.8 ± 18.9 | 76.7 ± 18.6 | 96.6 ± 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucas, J.; Logeux, V.; Rodrigues, A.M.S.; Stien, D.; Lebaron, P. Impact of Egg Exposure to UV Filter-Spiked Sediment on the Survival, Hatching Success, Cardiac Frequency, and Metabolic Scope of Zebrafish Embryos. Oceans 2022, 3, 84-93. https://doi.org/10.3390/oceans3010008
Lucas J, Logeux V, Rodrigues AMS, Stien D, Lebaron P. Impact of Egg Exposure to UV Filter-Spiked Sediment on the Survival, Hatching Success, Cardiac Frequency, and Metabolic Scope of Zebrafish Embryos. Oceans. 2022; 3(1):84-93. https://doi.org/10.3390/oceans3010008
Chicago/Turabian StyleLucas, Julie, Valentin Logeux, Alice M. S. Rodrigues, Didier Stien, and Philippe Lebaron. 2022. "Impact of Egg Exposure to UV Filter-Spiked Sediment on the Survival, Hatching Success, Cardiac Frequency, and Metabolic Scope of Zebrafish Embryos" Oceans 3, no. 1: 84-93. https://doi.org/10.3390/oceans3010008
APA StyleLucas, J., Logeux, V., Rodrigues, A. M. S., Stien, D., & Lebaron, P. (2022). Impact of Egg Exposure to UV Filter-Spiked Sediment on the Survival, Hatching Success, Cardiac Frequency, and Metabolic Scope of Zebrafish Embryos. Oceans, 3(1), 84-93. https://doi.org/10.3390/oceans3010008