Analysis and Modeling of Sunscreen Ingredients’ Behavior in an Aquatic Environment
Abstract
:1. Introduction
1.1. Sunscreens: Function, Market and Legislation
1.2. Design and Manufacturing of Sunscreen
2. Analysis and Modeling of the Behavior of Sunscreen’s Ingredients in an Aquatic Environment in the Absence of Organisms
2.1. Modeling of the Sunscreen Ingredients’ Behavior in Field Scenarios
2.2. Modeling of the Sunscreen Ingredients’ Behavior in Lab-Scale Studies
2.2.1. Photodegradation of Sunscreen UV Organic Filters under Simulated Natural Conditions
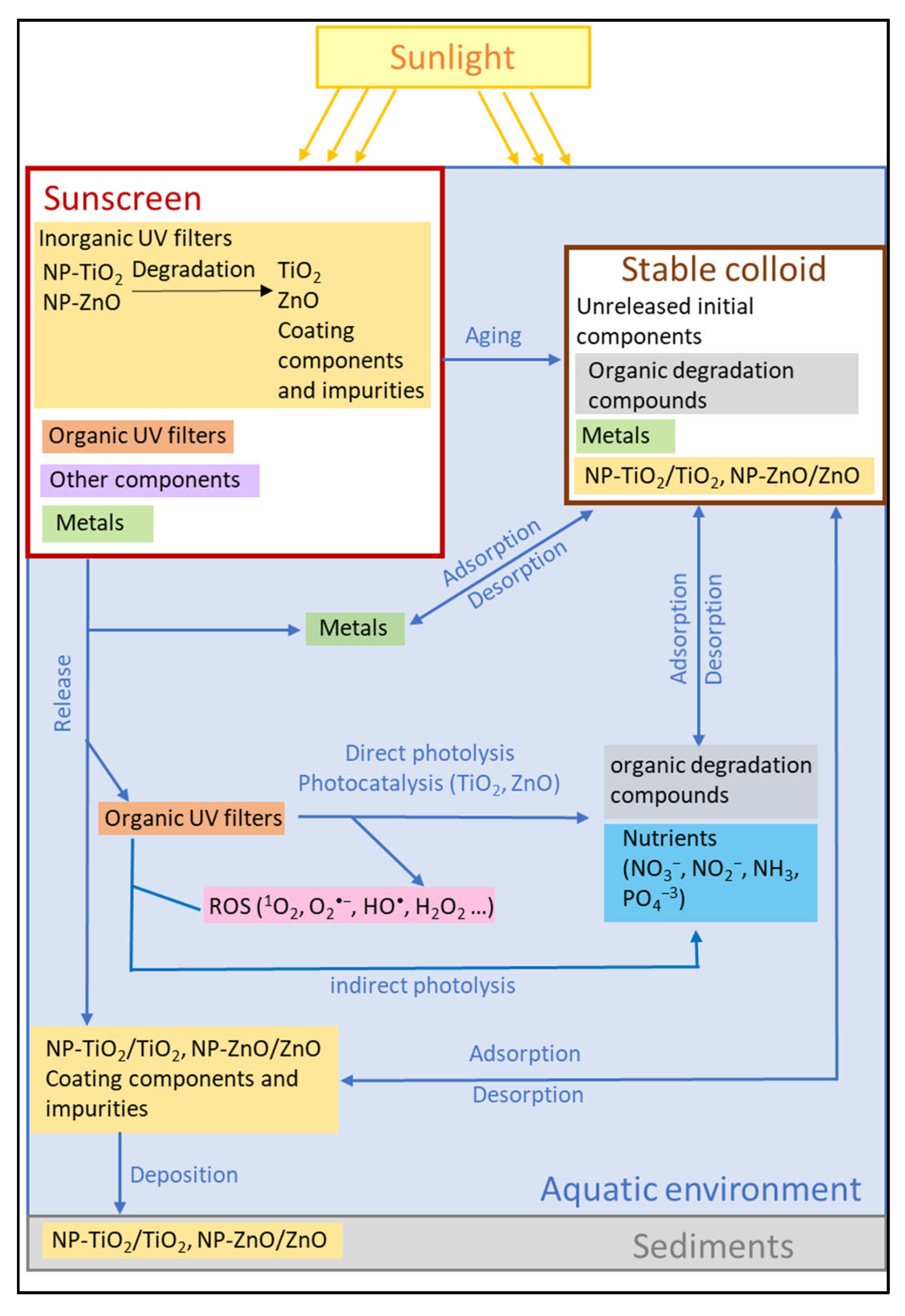
2.2.2. Degradation and Aging of TiO2-NPs Used in Sunscreen
2.2.3. Inorganic Nutrients of Sunscreen
| Model | Modeling Approach and Model Expressions | Chemical | Ref. |
|---|---|---|---|
| Sunscreen ingredients’ modeling in field scenarios | |||
| Substance flow analysis (SFA), material flow analysis (MFA), probabilistic and dynamic probabilistic MFA (ss-DPMFA). Particle-flow analysis (PFA). |
| TiO2-NPs | [19,35,36,37,38,39,40,41,43] |
| Hydrodynamic models (HMs). | Hydrodynamic numerical models describing and estimating the velocity field of three-dimensional currents and turbulent vertical mixing. Combination with water-quality models. | TiO2-NPs, 4-MBC, OC, BP4 | [19,40] |
| Kinetic model of laboratory-scale photodegradation of sunscreen UV organic filters | |||
| BP3, BP4, H-BP, HM-BP, DH-BP, DHM-BP, PABA, OD-PAB, PBSA, 4-MBC | [24,25,32,44,45,47,48,50,52] | |
| Degradation and aging of TiO2-NPs used in sunscreen | |||
| Several kinetic models for NP dissolution coupled with adsorption and aggregation. | Pseudo-first- and second-dissolution kinetic orders. Diffusion or reaction limited agregation models. | TiO2-NPs | [16,20,51,63,67] |
| Inorganic nutrients from sunscreen | |||
| Pseudo-first-order kinetic model.Zero-order kinetics at high nitrogen concentrations. | Organic UV filters with N2 and P compounds. P, SiO2impurities/coatings. | [5,50,68,69,70,71,72] | |
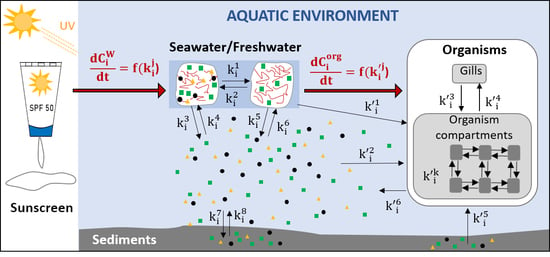
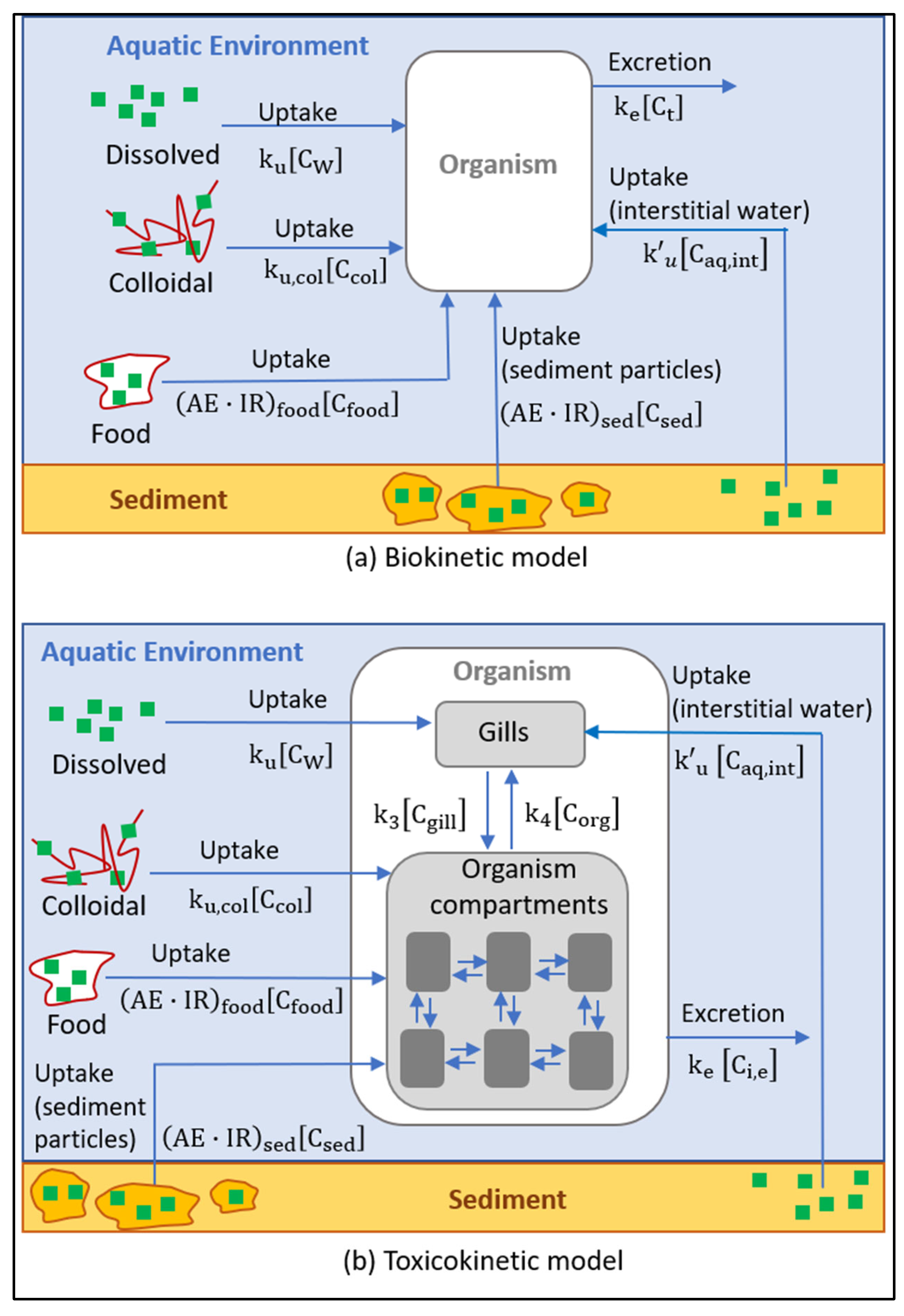
3. Analysis and Modeling of the Behavior of Sunscreen Ingredients in Contact with Aquatic Organisms
Biokinetic and Toxicokinetic Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabanoglu, T. Cosmetics Industry—Statistics & Facts. Available online: https://www.statista.com/topics/3137/cosmetics-industry/#dossierKeyfigures (accessed on 12 January 2022).
- Market Report. Global Sunscreen Ingredients Market Report 2021, Featuring Key Players Ashland Inc., BASF, Evonik Industries, l’Oreal SA and Royal DSM. 30 September 2021 08:43 ET | Source: Research and Markets. 2021. Available online: https://www.researchandmarkets.com/categories/personal-care#hmc (accessed on 1 November 2021).
- Tovar-Sánchez, A.; Sánchez-Quiles, D.; Basterretxea, G.; Benedé, J.L.; Chisvert, A.; Salvador, A.; Moreno-Garrido, I.; Blasco, J. Sunscreen products as emerging pollutants to coastal waters. PLoS ONE 2013, 8, e65451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsui, M.M.P.; Leung, H.W.; Wai, T.-C.; Yamashita, N.; Taniyasu, S.; Liu, W.; Lam, P.K.S.; Murphy, M.B. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface waters from different countries. Water Res. 2014, 67, 55–65. [Google Scholar] [CrossRef]
- Rodríguez-Romero, A.; Ruiz-Gutiérrez, G.; Viguri, J.R.; Tovar-Sánchez, A. Sunscreens as a new source of metals and nutrients to coastal waters. Environ. Sci. Technol. 2019, 53, 10177–10187. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Huang, J.; Jiang, X.; Huang, Y.; Zhu, X.; Cai, Z. Environmental fate and toxicity of sunscreen-derived inorganic ultraviolet filters in aquatic environments: A Review. Nanomaterials 2022, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Labille, J.; Catalano, R.; Slomberg, D.; Motellier, S.; Pinsino, A.; Hennebert, P.; Santaella, C.; Bartolomei, V. Assessing sunscreen lifecycle to minimize environmental risk posed by nanoparticulate UV-filters–A Review for safer-by-design products. Front. Environ. Sci. 2020, 8, 1–25. [Google Scholar] [CrossRef]
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Are sunscreens a new environmental risk associated with coastal tourism? Environ. Int. 2015, 83, 158–170. [Google Scholar] [CrossRef] [Green Version]
- FDA. U.S Food & Drug Administration. Protector Solar. 2019. Available online: https://www.fda.gov/drugs/understanding-over-counter-medicines/sunscreen-how-help-protect-your-skin-sun (accessed on 27 February 2021).
- Coronado, M. Ingredient Trends and Innovation in Sun Protection. In-Cosmetic Global. 2017. Available online: https://www.researchgate.net/publication/317545165_Ingredients_Trends_and_Innovation_in_Sun_Protection (accessed on 5 April 2017).
- Casas-Beltrán, D.A.; Febles-Moreno, K.; Hernandez-Yac, E.; Gallaher, C.M.; Alvarado-Flores, J.; Leal-Bautista, R.M.; Lenczewski, M. Impact of tourist behavior on the discharge of sunscreen contamination in aquatic parks, sinkholes, and beaches of the Mexican caribbean. Appl. Sci. 2021, 11, 6882. [Google Scholar] [CrossRef]
- Watkins, Y.S.D.; Sallach, J.B. Investigating the exposure and impact of chemical UV filters on coral reef ecosystems: Review and research gap prioritization. Integr. Environ. Assess. Manag. 2021, 17, 967–981. [Google Scholar] [CrossRef]
- McCall, M.J.; Gulson, B.; Andrews, D. Consumer use of sunscreens containing nanoparticles. In Nanotechnology Environmental Health and Safety: Risks, Regulation, and Management; Hull, M., Bowman, D., Eds.; Elsevier: Cambridge, MA, USA, 2018; pp. 389–423. [Google Scholar]
- Osmond-McLeod, M.J.; Oytam, Y.; Rowe, A.; Sobhanmanesh, F.; Greenoak, G.; Kirby, J.; McInnes, E.F.; McCall, M.J. Long-term exposure to commercially available sunscreens containing nano-particles of TiO2 and ZnO revealed no biological impact in a hairless mouse model. Part. Fibre Toxicol. 2015, 13, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Calle, I.; Menta, M.; Klein, M.; Séby, F. Screening of TiO2 and Au nanoparticles in cosmetics and determination of elemental impurities by multiple techniques (DLS, SP-ICP-MS, ICP- MS and ICP-OES). Talanta 2017, 171, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Slomberg, D.L.; Catalano, R.; Bartolomei, V.; Labille, J. Release and fate of nanoparticulate TiO2 UV filters from sunscreen: Effects of particle coating and formulation type. Environ. Pollut. 2021, 271, 116263. [Google Scholar] [CrossRef] [PubMed]
- Donia, D.T.; Carbone, M. Fate of the nanoparticles in environmental cycles. Int. J. Environ. Sci. Technol. 2019, 16, 583–600. [Google Scholar] [CrossRef]
- Tsui, M.M.P.; Lam, J.C.W.; Ng, T.Y.; Ang, P.O.; Murphy, M.B.; Lam, P.K.S. Occurrence, distribution, and fate of organic UV filters in coral communities. Environ. Sci. Technol. 2017, 51, 4182–4190. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Bowes, M.J.; Crossley, A.; Jarvie, H.P.; Jurkschat, K.; Jürgens, M.D.; Lawlor, A.J.; Park, B.; Rowland, P.; Spurgeon, D.; et al. An assessment of the fate, behaviour and environmental risk associated with sunscreen TiO2 nanoparticles in UK field scenarios. Sci. Total Environ. 2011, 409, 2503–2510. [Google Scholar] [CrossRef]
- Labille, J.; Feng, J.; Botta, C.; Borschneck, D.; Sammut, M.; Cabie, M.; Auffan, M.; Rose, J.; Bottero, J.Y. Aging of TiO2 nanocomposites used in sunscreen. Dispersion and fate of the degradation products in aqueous environment. Environ. Pollut. 2010, 158, 3482–3489. [Google Scholar] [CrossRef]
- Wang, N.; He, L.; Sun, X.; Li, X.; Li, M. The transformation of Benzophenone-3 in natural waters and AOPs: The roles of reactive oxygen species and potential environmental risks of products. J. Hazard. Mater. 2022, 427, 127941. [Google Scholar] [CrossRef]
- Lead, J.R.; Batley, G.E.; Alvarez, P.J.J.; Croteau, M.-N.; Handy, R.D.; McLaughlin, M.J.; Judy, J.D.; Schirmerh, K. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects—An updated review. Environ. Toxicol. Chem. 2018, 37, 2029–2063. [Google Scholar] [CrossRef] [PubMed]
- Amde, M.; Liu, J.-F.; Tan, Z.-Q.; Bekana, D. Transformation and bioavailability of metal oxide nanoparticles in aquatic and terrestrial environments. A review. Environ. Pollut. 2017, 230, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiao, X.; Zhou, C.; Zhang, Y.; Fu, Z.; Chen, J. Photochemical transformation of sunscreen agent benzophenone-3 and its metabolite in surface freshwater and seawater. Chemosphere 2016, 153, 494–499. [Google Scholar] [CrossRef]
- Vione, D.; Caringella, R.; De Laurentiis, E.; Pazzi, M.; Minero, C. Phototransformation of the sunlight filter benzophenone-3 (2-hydroxy-4-methoxybenzophenone) under conditions relevant to surface waters. Sci. Total Environ. 2013, 463–464, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.C.; Chen, W.Y.; Ju, Y.R.; Tsai, J.W.; Jou, L.J.; Singh, S.; Liao, C.M. Combining bioaccumulation and coping mechanism to enhance long-term site-specific risk assessment for zinc susceptibility of bivalves. Chemosphere 2011, 84, 707–715. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.C.P.; Santos, B.A.M.C.; Castro, H.C.; Rodrigues, C.R. Ethylhexyl methoxycinnamate and butyl methoxydibenzoylmethane: Toxicological effects on marine biota and human concerns. J. Appl. Toxicol. 2022, 42, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.; Torres-Duarte, C.; Cherr, G.; Adams, N. Effects of three zinc-containing sunscreens on development of purple sea urchin (Strongylocentrotus purpuratus) embryos. Aquat. Toxicol. 2020, 218, 105355. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Tsui, M.M.P.; Tan, C.J.; Ma, C.Y.; Yiu, S.K.F.; Wang, L.H.; Chen, T.H.; Fan, T.Y.; Lam, P.K.S.; Murphy, M.B. Toxicological effects of two organic ultraviolet filters and a related commercial sunscreen product in adult corals. Environ. Pollut. 2019, 245, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Naasz, S.; Altenburger, R.; Kühnel, D. Environmental mixtures of nanomaterials and chemicals: The Trojan-horse phenomenon and its relevance for ecotoxicity. Sci. Total Environ. 2018, 635, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Sendra, M.; Sánchez-Quiles, D.; Blasco, J.; Moreno-Garrido, I.; Lubián, L.M.; Pérez-García, S.; Tovar-Sánchez, A. Effects of TiO2 nanoparticles and sunscreens on coastal marine microalgae: Ultraviolet radiation is key variable for toxicity assessment. Environ. Int. 2017, 98, 62–68. [Google Scholar] [CrossRef]
- Kotnik, K.; Kosjek, T.; Žegura, B.; Filipič, M.; Heath, E. Photolytic fate and genotoxicity of benzophenone-derived compounds and their photodegradation mixtures in the aqueous environment. Chemosphere 2016, 147, 114–123. [Google Scholar] [CrossRef]
- Grech, A.; Brochot, C.; Dorne, J.-L.; Quignot, N.; Bois, F.Y.; Beaudouin, R. Toxicokinetic models and related tools in environmental risk assessment of chemicals. Sci. Total Environ. 2017, 578, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Sun, T.; Nowack, B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013, 181, 287–300. [Google Scholar] [CrossRef]
- Gottschalk, F.; Scholz, R.W.; Nowack, B. Probabilistic material flow modeling for assessing the environmental exposure to compounds: Methodology and an application to engineered nano-TiO2 particles. Environ. Modell. Softw. 2009, 25, 320–332. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for different regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.C.; Nowack, B. Exposure modeling of engineered nanoparticles in the environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef] [PubMed]
- Barron, M.G.; Stehly, G.R.; Hayton, W.L. Pharmacokinetic modeling in aquatic animals l. Models and concepts. Aquat. Toxicol. 1990, 18, 61–86. [Google Scholar] [CrossRef]
- Zheng, Y.; Nowack, B. Size-specific, dynamic, probabilistic material flow analysis of titanium dioxide releases into the environment. Environ. Sci. Technol. 2021, 55, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Lindo-Atichati, D.; Montero, P.; Rodil, R.; Quintana, J.B.; Miró, M. Modeling dispersal of UV filters in estuaries. Environ. Sci. Technol. 2019, 53, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, R.; Molander, S.; Sandén, B.A. Particle flow analysis: Exploring potential use phase emissions of titanium dioxide nanoparticles from sunscreen, paint, and cement. J. Ind. Ecol. 2012, 16, 343–351. [Google Scholar] [CrossRef]
- Musee, N. Simulated environmental risk estimation of engineered nanomaterials: A case of cosmetics in Johannesburg city. Hum. Exp. Toxicol. 2010, 30, 1181–1195. [Google Scholar] [CrossRef]
- Gottschalk, F.; Ort, C.; Scholz, R.W.; Nowack, B. Engineered nanomaterials in rivers—Exposure scenarios for Switzerland at high spatial and temporal resolution. Environ. Pollut. 2011, 159, 3439–3445. [Google Scholar] [CrossRef] [PubMed]
- De Laurentiis, E.; Minella, M.; Sarakha, M.; Marrese, A.; Minero, C.; Mailhot, G.; Brigante, M.; Vione, D. Photochemical processes involving the UV absorber benzophenone-4 (2-hydroxy-4-methoxybenzophenone-5-sulphonic acid) in aqueous solution: Reaction pathways and implications for surface waters. Water Res. 2013, 47, 5943–5953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Ji, Y.; Zeng, C.; Zhang, Y.; Wang, Z.; Yang, X. Aquatic photodegradation of sunscreen agent p-aminobenzoic acid in the presence of dissolved organic matter. Water Res. 2013, 47, 153–162. [Google Scholar] [CrossRef]
- Thio, B.J.R.; Zhou, D.X.; Keller, A.A. Influence of natural organic matter on the aggregation and deposition of titanium dioxide nanoparticles. J. Hazard. Mater. 2011, 189, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Meng, C.; Zeng, C.; Ji, Y.; Yang, X.; Gao, S. The effect of nitrate, bicarbonate and natural organic matter on the degradation of sunscreen agent p-aminobenzoic acid by simulated solar irradiation. Sci. Total Environ. 2011, 409, 5376–5381. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, V.A.; Giokas, D.L.; Lambropoulou, D.A.; Albanis, T.A. Aqueous photolysis of the sunscreen agent octyl-dimethyl-p-aminobenzoic acid: Formation of disinfection byproducts in chlorinated swimming pool water. J. Chromatogr. A. 2003, 1016, 211–222. [Google Scholar] [CrossRef]
- Celeiro, M.; Vignola Hackbarth, F.; Selene, M.A.; de Souza, G.U.; Llompart, M.; Vilar, V.J.P. Assessment of advanced oxidation processes for the degradation of three UV filters from swimming pool water. J. Photochem. Photobiol. A Chem. 2018, 351, 95–107. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, L.; Zhang, Y.; Ferronato, C.; Brigante, M.; Mailhot, G.; Yang, X.; Chovelon, J.M. Photochemical degradation of sunscreen agent 2-phenylbenzimidazole-5-sulfonic acid in different water matrices. Water Res. 2013, 47, 5865–5875. [Google Scholar] [CrossRef] [PubMed]
- Botta, C.; Labille, J.; Auffan, M.; Borschneck, D.; Miche, H.; Cabié, M.; Masion, A.; Rose, J.; Bottero, J.Y. TiO2-based nanoparticles released in water from commercialized sunscreens in a life-cycle perspective: Structures and quantities. Environ. Pollut. 2011, 159, 1543–1550. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, L.; Ferronato, C.; Salvador, A.; Yang, X.; Chovelon, J.M. Degradation of sunscreen agent 2-phenylbenzimidazole-5-sulfonic acid by TiO2 photocatalysis: Kinetics, photoproducts and comparison to structurally related compounds. Appl. Catal. B Environ. 2013, 140–141, 457–467. [Google Scholar] [CrossRef]
- Lu, G.Y.; Ke, C.H.; Zhu, A.; Wang, W.X. Oyster-based national mapping of trace metals pollution in the Chinese coastal waters. Environ. Pollut. 2017, 224, 658–669. [Google Scholar] [CrossRef]
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Sunscreens as a source of hydrogen peroxide production in coastal waters. Environ. Sci. Technol. 2014, 48, 9037–9042. [Google Scholar] [CrossRef] [Green Version]
- Yin, K.; Li, T.; Zhang, T.; Zhang, Y.; Yang, C.; Luo, S. Degradation of organic filter 2-Phenylbenzidazole-5-Sulfonic acid by light-driven free chlorine process: Reactive species and mechanisms. Chem. Eng. J. 2022, 430, 132684. [Google Scholar] [CrossRef]
- Wang, Z.; Khan, M.A.; Al-Othman, A.A.S.; Abdullah, Z.; Sillanpää, M. Pilot-scale study on photodegradation of benzophenone-3 and benzophenone-8 ultraviolet filters enriched synthetic effluent. J. Water Process. Eng. 2021, 44, 102327. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, G.; Zoh, K.D. Benzophenone-3 degradation via UV/H2O2 and UV/persulfate reactions. J. Hazard. Mater. 2021, 403, 123591. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.-Y.; Wang, H.; Yu, K.-P.; Lee, J.; Lin, K.-Y.A. Degradation of sunscreen agent 2-phenylbenzimidazole-5-sulfonic acid using monopersulfate activated by MOF-derived cobalt sulfide nanoplates. J. Water Process. Eng. 2021, 44, 102282. [Google Scholar] [CrossRef]
- Moradi, N.; Amin, M.M.; Fatehizadeh, A.; Ghasemi, Z. Degradation of UV-filter Benzophenon-3 in aqueous solution using TiO2 coated on quartz tubes. J. Environ. Health Sci. Eng. 2018, 16, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Dong, W.; Wang, X.; Bi, W.; Zhai, P.; Li, H.; Nie, M. Degradation of sunscreen agent p-aminobenzoic acid using a combination system of UV irradiation, persulphate and iron(II). Environ. Sci. Pollut. Res. 2016, 23, 4561–4568. [Google Scholar] [CrossRef]
- Abdelraheem, W.H.M.; He, X.; Duan, X.; Dionysiou, D.D. Degradation and mineralization of organic UV absorber compound 2-phenylbenzimidazole-5-sulfonic acid (PBSA) using UV-254nm/H2O2. J. Hazard. Mater. 2015, 282, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Yuan, H.; Zhai, P.; Xue, Y.; Li, H.; Dong, W.; Mailhot, G. Investigation on the degradation of benzophenone-3 by UV/H2O2 in aqueous solution. Chem. Eng. J. 2015, 277, 97–103. [Google Scholar] [CrossRef]
- Auffan, M.; Pedeutour, M.; Rose, J.; Masion, A.; Ziarelli, F.; Borschneck, D.; Chaneac, C.; Botta, C.; Chaurand, P.; Labille, J.; et al. Structural degradation at the surface of a TiO2-based nanomaterial used in cosmetics. Environ. Sci. Technol. 2010, 44, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Zachariadis, G.A.; Sahanidou, E. Multi-element method for determination of trace elements in sunscreens by ICP-AES. J. Pharm. Biomed. Anal. 2009, 50, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Zmozinski, A.V.; Pretto, T.; Borges, A.R.; Duarte, Á.T.; Vale, M.G.R. Determination of Pb and Cr in sunscreen samples by high-resolution continuum source graphite furnace atomic absorption spectrometry and direct analysis. Microchem. J. 2016, 128, 89–94. [Google Scholar] [CrossRef]
- Brun, N.R.; Lenz, M.; Wehrli, B.; Fent, K. Comparative effects of zinc oxide nanoparticles and dissolved zinc on zebrafish embryos and eleuthero-embryos: Importance of zinc ions. Sci. Total Environ. 2014, 476–477, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhang, W.; Gao, H.; Li, Y.; Tong, X.; Li, K.; Zhu, X.; Wang, Y.; Chen, Y. Behavior and potential impacts of metal-based engineered nanoparticles in aquatic environments. Nanomaterials 2017, 7, 21. [Google Scholar] [CrossRef]
- Liu, X.; Chen, G.; Erwin, J.G.; Adam, N.K.; Su, C. Release of phosphorous impurity from TiO2 anatase and rutile nanoparticles in aquatic environments and its implications. Water Res. 2013, 47, 6149–6156. [Google Scholar] [CrossRef] [PubMed]
- Low, G.K.C.; McEvoy, S.R.; Matthews, R.W. Formation of nitrate and ammonium ions in titanium dioxide mediated photocatalytic degradation of organic compounds containing nitrogen atoms. Environ. Sci. Technol. 1991, 25, 460–467. [Google Scholar] [CrossRef]
- Jing, J.; Liu, M.; Colvin, V.L.; Li, W.; Yu, W.W. Photocatalytic degradation of nitrogen-containing organic compounds over TiO2. J. Mol. Catal. A Chem. 2011, 351, 17–28. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, S.; Luo, L.; Han, W.; Zhang, J.; Yang, K.; Christie, P. Dissolution and microstructural transformation of ZnO nanoparticles under the influence of phosphate. Environ. Sci. Technol. 2012, 46, 7215–7221. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, G.; Erwin, J.G.; Su, C. Silicon impurity release and surface transformation of TiO2 anatase and rutile nanoparticles in water environments. Environ. Pollut. 2014, 184, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Casado-Martínez, M.C.; Blasco, J.; DelValls, T.A.; González-Castromil, M.A.; Riba, I. Interlaboratory assessment of marine bioassays to evaluate the environmental quality of coastal sediments in Spain. V. Whole sediment toxicity test using juveniles of the bivalve Ruditapes philippinarum. Cienc. Mar. 2006, 32, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Zhang, J.; Fu, J.; Shi, J.; Jiang, G. Biomonitoring: An appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal. Chim. Acta 2008, 606, 135–150. [Google Scholar] [CrossRef]
- Rodríguez-Romero, A.; Jiménez-Tenorio, N.; Basallote, M.D.; de Orte, M.R.; Blasco, J.; Riba, I. Predicting the impacts of CO2 leakage from subseabed storage: Effects of metal accumulation and toxicity on the model benthic organism Ruditapes philippinarum. Environ. Sci. Technol. 2014, 48, 12292–12301. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.F.; Ale, A.; Andrade, V.; Bacchetta, C.; Rossi, A.; Cazenave, J. Metallic, metal oxide, and metalloid nanoparticles toxic effects on freshwater microcrustaceans: An update and basis for the use of new test species. Water Environ. Res. 2021, 93, 2505–2526. [Google Scholar] [CrossRef]
- Wong, S.W.Y.; Zhou, G.J.; Leung, P.T.Y.; Han, J.; Lee, J.S.; Kwok, K.W.H.; Leung, K.M.Y. Sunscreens containing zinc oxide nanoparticles can trigger oxidative stress and toxicity to the marine copepod Tigriopus japonicus. Mar. Pollut. Bull. 2020, 154, 111078. [Google Scholar] [CrossRef]
- Vidal-Liñán, L.; Villaverde-de-Sáa, E.; Rodil, R.; Quintana, J.B.; Beiras, R. Bioaccumulation of UV filters in Mytilus galloprovincialis mussel. Chemosphere 2018, 190, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, C.; Damiani, E.; Marcellini, F.; Falugi, C.; Tiano, L.; Brugè, F.; Danovaro, R. Sunscreen products impair the early developmental stages of the sea urchin Paracentrotus lividus. Sci. Rep. 2017, 7, 7815. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.J.; Bennett, S.; Keller, A.A.; Pease, S.; Lenihan, H.S. TiO2 nanoparticles are phototoxic to marine phytoplankton. PLoS ONE 2012, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moeller, M.; Pawlowski, S.; Petersen-Thiery, M.; Miller, I.B.; Nietzer, S.; Heisel-Sure, Y.; Kellermann, M.Y.; Schupp, P.J. Challenges in current coral reef protection—Possible impacts of UV filters used in sunscreens, a critical review. Front. Mar. Sci. 2021, 8, 665548. [Google Scholar] [CrossRef]
- Benedé, J.L.; Chisvert, A.; Salvador, A.; Sánchez-Quiles, D.; Tovar-Sánchez, A. Determination of UV filters in both soluble and particulate fractions of seawaters by dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry. Anal. Chim. Acta 2014, 812, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Badia-Fabregat, M.; Olivares, A.; Piña, B.; Blánquez, P.; Vicent, T.; Caminal, G.; Díaz-Cruz, M.S.; Barceló, D. Evaluation of fungal- and photo-degradation as potential treatments for the removal of sunscreens BP3 and BP1. Sci. Total Environ. 2012, 427–428, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Law, J.C.F.; Lam, T.K.; Leung, K.S.Y. Risks of organic UV filters: A review of environmental and human health concern studies. Sci. Total Environ. 2021, 755, 142486. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol. Photoimmunol. Photomed. 2019, 35, 442–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health. Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, C.V.M.; Rodríguez-Romero, A.; Fernández, M.; Sparaventi, E.; Medina, M.M.; Tovar-Sánchez, A. Repellency and mortality effects of sunscreens on the shrimp Palaemon varians: Toxicity dependent on exposure method. Chemosphere 2020, 257, 127190. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Bachelot, M.; Boillot, C.; Munaron, D.; Chiron, S.; Casellas, C.; Fenet, H. Bioconcentration of two pharmaceuticals (benzodiazepines) and two personal care products (UV filters) in marine mussels (Mytilus galloprovincialis) under controlled laboratory conditions. Environ. Sci. Pollut. Res. 2012, 19, 2561–2569. [Google Scholar] [CrossRef]
- Fang, J.; Xu, M.J.; Wang, D.J.; Wen, B.; Han, J.Y. Modeling the transport of TiO2 nanoparticle aggregates in saturated and unsaturated granular media: Effects of ionic strength and pH. Water Res. 2013, 47, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Ji, Z. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Science Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Crittenden, J. Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles. Water Res. 2009, 43, 4249–4257. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, L.L.R.; Harvey, K.E.; Ahmed, A.; Harvey, S.C. UV-filter pollution: Current concerns and future prospects. Environ. Monit. Assess. 2021, 193, 840–856. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, A.L.; Blackburn, R.S.; Santillan, C.; Truong, L.; Tanguay, R.L.; Hutchison, J.E. Zinc oxide-induced changes to sunscreen ingredient efficacy and toxicity under UV irradiation. Photochem. Photobiol. Sci. 2021, 20, 1273–1285. [Google Scholar] [CrossRef]
- Vieira Sanches, M.; Oliva, M.; De Marchi, L.; Cuccaro, A.; Puppi, D.; Chiellini, F.; Freitas, R.; Pretti, C. Ecotoxicological screening of UV-filters using a battery of marine bioassays. Environ. Pollut. 2021, 290, 118011. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.; Rogers, N.J.; Apte, S.; Batley, G.; Gadd, G.; Casey, P. Comparative toxicity of tanoparticulate ZnO, Bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef]
- Fan, W.; Liu, L.; Peng, R.; Wang, W.X. High bioconcentration of titanium dioxide nanoparticles in Daphnia magna determined by kinetic approach. Sci. Total Environ. 2016, 569–570, 1224–1231. [Google Scholar] [CrossRef]
- Beiras, R. Bioaccumulation. Marine Pollution Sources, Fate and Effects of Pollutants in Coastal Ecosystems; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 11; pp. 187–204. [Google Scholar] [CrossRef]
- Schäfer, S.; Buchmeier, G.; Claus, E.; Duester, L.; Heininger, P.; Körner, A.; Mayer, P.; Paschke, A.; Rauert, C.; Reifferscheid, G.; et al. Bioaccumulation in aquatic systems: Methodological approaches, monitoring and assessment. Environ. Sci. Eur. 2015, 27, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Tan, Q.G. Applications of dynamic models in predicting the bioaccumulation, transport and toxicity of trace metals in aquatic organisms. Environ. Pollut. 2019, 252, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Curis, E.; Nicolis, I.; Bensaci, J.; Deschamps, P.; Bénazeth, S. Mathematical modeling in metal metabolism: Overview and perspectives. Biochimie 2009, 91, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Landrum, P.F.; Lee II, H.; Lydy, M.J. Toxicokinetics in aquatic systems: Model comparisons and use in hazard assessment. Environ. Toxicol. Chem. 1992, 11, 1709–1725. [Google Scholar] [CrossRef]
- Gestin, O.; Lacoue-Labarthe, T.; Coquery, M.; Delorme, N.; Garnero, L.; Dherret, L.; Ciccia, T.; Geffard, O.; Lopes, C. One and multi-compartments toxico-kinetic modeling to understand metals’ organotropism and fate in Gammarus fossarum. Environ. Int. 2021, 156, 106625. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Feng, J.; Zhu, L. Toxicokinetic-toxicodynamic modeling of cadmium and lead toxicity to larvae and adult zebrafish. Environ. Pollut. 2019, 251, 221–229. [Google Scholar] [CrossRef]
- Chen, W.Y. Toxicokinetic modeling challenges for aquatic nanotoxicology. Front. Mar. Sci. 2016, 2, 114. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Marín, P.; Aierbe, E.; Lorenzo, J.I.; Mubiana, V.K.; Beiras, R.; Blust, R. Dynamic modeling of copper bioaccumulation by Mytilus edulis in the presence of humic acid aggregates. Aquat. Toxicol. 2016, 178, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Zhu, A.; Fang, H.; Dong, Y.; Wang, W.X. Establishing baseline trace metals in marine bivalves in China and worldwide: Meta-analysis and modeling approach. Sci. Total Environ. 2019, 669, 746–753. [Google Scholar] [CrossRef]
- Rainbow, P.S.; Phillips, D.J.H. Cosmopolitan biomonitors of trace metals. Mar. Pollut. Bull. 1993, 26, 593–601. [Google Scholar] [CrossRef]
- Chen, W.Y.; Liao, C.M. Interpreting copper bioaccumulation dynamics in tilapia using systems-level explorations of pulsed acute/chronic exposures. Ecotoxicology 2014, 23, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wang, W.X. Multiple trace element accumulation in the mussel Septifer virgatus: Counteracting effects of salinity on uptake and elimination. Environ. Pollut. 2018, 242, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Kalman, J.; Smith, B.D.; Bury, N.R.; Rainbow, P.S. Biodynamic modelling of the bioaccumulation of trace metals (Ag, As and Zn) by an in faunal estuarine invertebrate, the clam Scrobicularia plana. Aquat. Toxicol. 2014, 154, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Bourgeault, A.; Gourlay-Francéa, C.; Priadic, C.; Ayraultc, S.; Tusseau-Vuillemin, M.H. Bioavailability of particulate metal to zebra mussels: Biodynamic modelling shows that assimilation efficiencies are site-specific. Environ. Pollut. 2011, 159, 3381–3389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, K.; Wang, W.X. Allometry of cadmium and zinc concentrations and bioaccumulation in the scallop Chlamys nobilis. Mar. Ecol. Prog. Ser. 2008, 365, 115–126. [Google Scholar] [CrossRef]
- Wang, W.X.; Fisher, N.S.; Luoma, S.N. Kinetic determinations of trace element bioaccumulation in the mussel Mytilus edulis. Mar. Ecol. Prog. Ser. 1996, 140, 91–113. [Google Scholar] [CrossRef]
- O’Mara, K.; Adams, M.; Burford, M.A.; Fry, B.; Cresswell, T. Uptake and accumulation of cadmium, manganese and zinc by fisheries species: Trophic differences in sensitivity to environmental metal accumulation. Sci. Total Environ. 2019, 690, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Birch, G.F.; Cresswell, T.; Johansen, M.P.; Adams, M.S.; Simpson, S.L. Dietary ingestion of fine sediments and microalgae represent thedominant route of exposure and metal accumulation for Sydney rockoyster (Saccostrea glomerata): A biokinetic model for zinc. Aquat. Toxicol. 2015, 167, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Redeker, E.S.; Bervoets, L.; Blu, R. Dynamic Model for the Accumulation of Cadmium and Zinc from Water and Sediment by the Aquatic Oligochaete, Tubifex tubifex. Environ. Sci. Technol. 2004, 38, 6193–6200. [Google Scholar] [CrossRef]
- Haynes, V.N.; Ward, J.E.; Russell, B.J.; Agrios, A.G. Photocatalytic effects of titanium dioxide nanoparticles on aquatic organisms—Current knowledge and suggestions for future research. Aquat Toxicol. 2017, 185, 138–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
 Field studies;
Field studies;  laboratory studies.
laboratory studies.
 Field studies;
Field studies;  laboratory studies.
laboratory studies.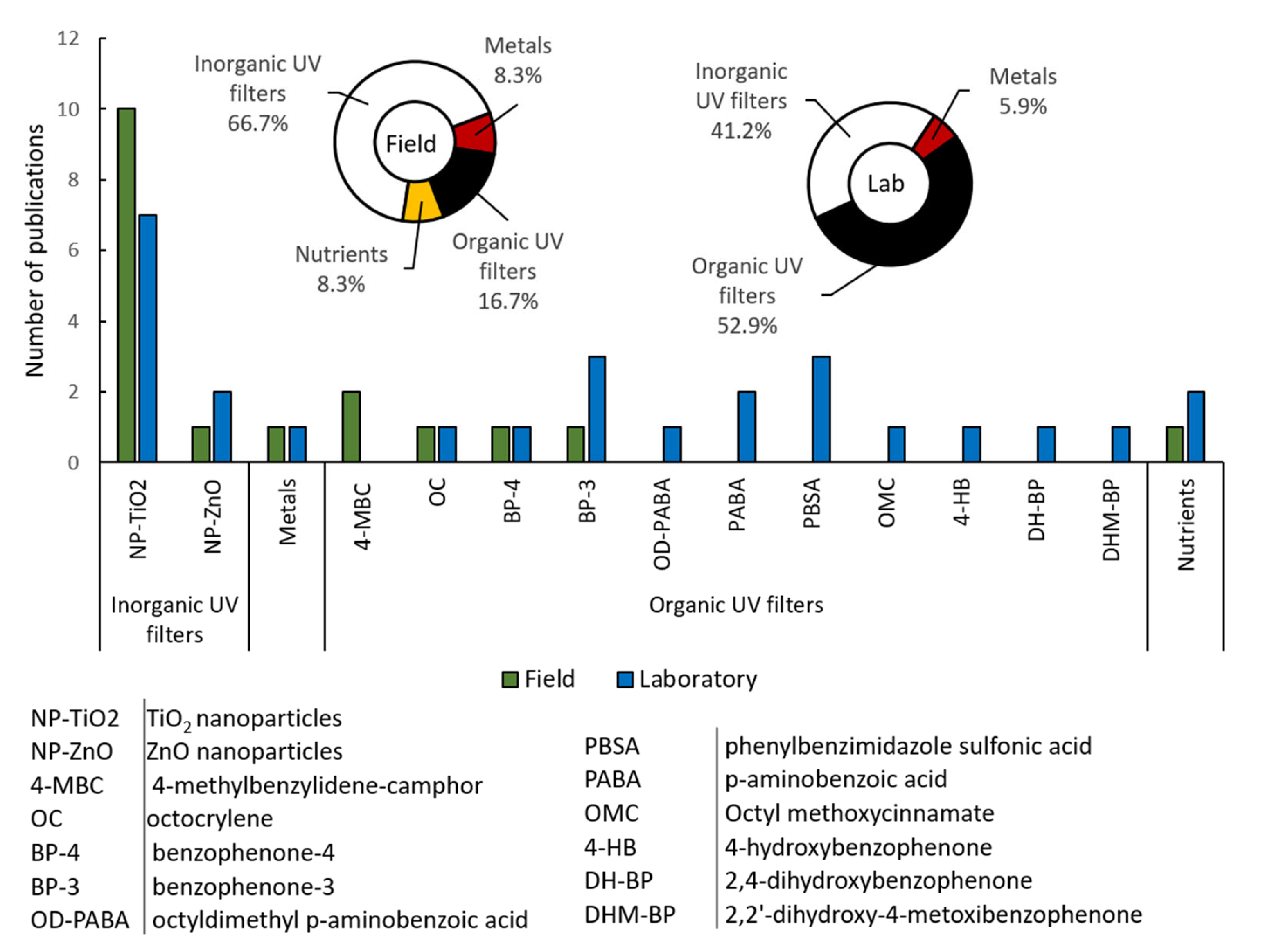
 Field studies;
Field studies;  laboratory studies.
laboratory studies.
 Field studies;
Field studies;  laboratory studies.
laboratory studies.
| UV Filter | IUPAC Chemical Name (Formula) | Chemical Structure | Regulation(*) (**) | Properties |
|---|---|---|---|---|
| Benzophenone -3 Oxybenzone (BP3, BZ-3) | 2-Hydroxy-4-methoxybenzophenone (C14H12O3) |  | Regulated by maximum contents in the US, Canada, the EU, ASEAN, MERCOSUR, China, India, Japan, Korea, Australia, NZ, and South Africa. Banned in Palau, Thailand marine natural parks, Aruba, Bonaire, the US Virgin Islands, Key West (Florida, US), and Hawaii (US). | CAS number: 131-57-7; molecular weight (MW): 228.24 Density: 1.201 g/cm3 Solubility in water: <0.1 g/100 mL at 20 °C pKa = 7.56; log Kow = 3.52 |
| Benzophenone-4; Sulisobenzone (BP4) | 5-Benzoyl-4-hydroxy-2-methoxybenzene-1-sulfonic acid (C14H12O6S) | 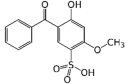 | Regulated by maximum contents in the US, Canada, the EU, ASEAN, MERCOSUR, China, India, Japan, Korea, Australia, NZ, and South Africa. | CAS number: 4065-45-6; MW: 308.31 Solubility in water: 1 g per 4 mL pKa1 = −0.70 (sulfonic acid); pKa2 = 7.56 (hydroxyl); log Kow = 0.37 |
| Octinoxate; Uvinul MC80; Octyl methoxycinnamate (OMC) | (RS)-2-Ethylhexyl (2E)-3-(4-methoxyphenyl) prop-2-enoate (C18H26O3) |  | Regulated by maximum contents in the US, Canada, the EU, ASEAN, MERCOSUR, China, India, Japan, Korea, Australia, NZ, and South Africa. Banned in Palau, Thailand marine natural parks, US Virgin Islands, Key West (Florida), and Hawaii (US). | CAS number: 5466-77-3; MW: 290.40 Density: 1.010 g/cm3 Insoluble in water log Kow = 5.80 |
| Octocrylene; Uvinul N-539 (OC) | 2-Ethylhexyl 2-cyano-3,3-diphenylprop-2-enoate (C24H27NO2) |  | Regulated by maximum contents in the US, Canada, the EU, ASEAN, MERCOSUR, China, India, Japan, Korea, and South Africa. Banned in Palau and US Virgin Islands. | CAS number: 6197-30-4; MW: 361.48 Density: 1.055 g/cm3 Insoluble in water log Kow = 7.35 |
| Enzacamene; 4-Methylbenzylidene camphor (4-MBC) | (3E)-1,7,7-Trimethyl-3-[(4-methylphenyl) methylene]-2-norbornanone (C18H22O) | 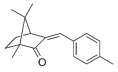 | Regulated by maximum contents in Canada, the EU, ASEAN, MERCOSUR, China, India, Japan, Korea, Australia, NZ, and South Africa. Banned in Palau and Thailand marine natural parks. | CAS number: 36861-47-9; MW: 254.37 Density: 1.064 g/cm3 Insoluble in water log Kow =5.47 |
| Ácido p-aminobenzoico (PABA) | Ácido 4-aminobenzoico (C7H7NO2) |  | Regulated by maximum contents in the US, Canada, the EU, ASEAN, MERCOSUR, China, India, Japan, Korea, Australia, NZ, and South Africa. | CAS number: 150-13-0; MW: 137.14 Density: 1.374 g/cm3 Solubility in water: 1 g/170 mL (25 °C) pKa1 = 2.38; pKa2 = 4.85; log Kow = 0.83 |
| Padimate O; Escalol 507; octyldimethyl PABA (OD-PABA) | 2-ethylhexyl 4-(dimethylamino) benzoate (C17H27NO2) | 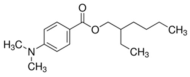 | Permitted UV filters and regulated by maximum contents in the US, Canada, the EU, ASEAN, MERCOSUR, China, India, Japan, Korea, Australia, NZ, and South Africa. | CAS number: 21245-02-3; MW: 277.40 Density: 0.990 g/cm3 Solubility in water: 0.54 mg/L pKa = 2.9; log Kow = 5.77 |
| Ensulizole; phenyl benzimidazole sulfonic acid (PBSA) | 2-Phenyl-3H-benzimidazole-5-sulfonic acid (C13H10N2O3S) | 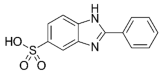 | Regulated by maximum contents in the US, Canada, the EU, ASEAN, MERCOSUR, China, India, Japan, Korea, Australia, NZ, and South Africa. | CAS Number: 27503-81-7 Molecular M.: 274.29 Soluble in water |
| 2,4-Dihydroxy Benzophenone; Benzophenone-1 (DH-BP) | (2,4-dihydroxyphenyl)phenyl-methanone (C13H10O3) | 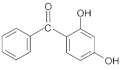 | Regulated by maximum contents in Japan and South Africa. | CAS number: 131-56-6; MW: 214.22 Density: 1.302 g/cm3 Insoluble in water; log Kow = 3.17 |
| Dioxybenzone; Benzophenone-8 (DHM-BP) | 2,2′-Dihydroxy-4-methoxybenzophenone (C14H12O4) | 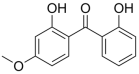 | Regulated by maximum contents in the US, Canada, ASEAN, MERCOSUR, Korea, Australia, NZ, and South Africa. | CAS number: 131-53-3; MW: 244.24 Density: 1.38 g/cm3 Insoluble in water; pKa = 7.11; log Kow = 4.31 |
| TiO2 nanoparticle | Titanium dioxide (TiO2) | 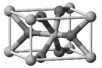 | Regulated by maximum contents in the US, Canada, the EU, ASEAN, MERCOSUR, China, Japan, Korea, Australia, and NZ. | CAS number: 13463-67-7 XRD: rutile, anatase Al(OH)3 or SiO2 coating; TiO2 %: 79–89 |
| ZnO nanoparticle | Zinc oxide (ZnO) | 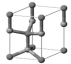 | Regulated by maximum contents in the US, Canada, ASEAN, MERCOSUR, China, Japan, Korea, and NZ. | CAS number: 1314-13-2; XRD: wurtzite SiO2-coated or coated with a silicone derivative. ZnO %: 79.1–81.5 |
| UV Filters/Metals | Water Type | Field/Laboratory | Reference | |
|---|---|---|---|---|
| Mussel | 4-MBC, BP3, BP4, OC, OD-PABA | Seawater | Laboratory | [78] |
| Fish | Cu | Fresh water | Laboratory | [108] |
| Mussel | EHMC | Seawater | Laboratory | [88] |
| Clams/mussels | Zn | Seawater | Field | [26] |
| Oysters/mussels/clams | Ag, Cd, Cr, Cu, Ni, Pb, Ti, Zn | Seawater | Field | [106] |
| Mussel | As, Cd, Cr, Cu, Ni, Se, Pb, Zn | Seawater | Laboratory | [109] |
| Clams | Ag, As, Zn | Seawater | Laboratory | [110] |
| Mussel | Cd, Cr, Cu, Ni, Zn | Fresh water | Field | [111] |
| Scallop | Cd, Zn | Seawater | Laboratory | [112] |
| Mussel | Ag, Am, Cd, Co, Se, Zn | Seawater | Field | [113] |
| Clams/prawns/whiting | Cd, Mn, Zn | Seawater | Laboratory | [114] |
| Oyster | Zn | Seawater | Laboratory | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Gutiérrez, G.; Rodríguez-Romero, A.; Tovar-Sánchez, A.; Viguri Fuente, J.R. Analysis and Modeling of Sunscreen Ingredients’ Behavior in an Aquatic Environment. Oceans 2022, 3, 340-363. https://doi.org/10.3390/oceans3030024
Ruiz-Gutiérrez G, Rodríguez-Romero A, Tovar-Sánchez A, Viguri Fuente JR. Analysis and Modeling of Sunscreen Ingredients’ Behavior in an Aquatic Environment. Oceans. 2022; 3(3):340-363. https://doi.org/10.3390/oceans3030024
Chicago/Turabian StyleRuiz-Gutiérrez, Gema, Araceli Rodríguez-Romero, Antonio Tovar-Sánchez, and Javier R. Viguri Fuente. 2022. "Analysis and Modeling of Sunscreen Ingredients’ Behavior in an Aquatic Environment" Oceans 3, no. 3: 340-363. https://doi.org/10.3390/oceans3030024
APA StyleRuiz-Gutiérrez, G., Rodríguez-Romero, A., Tovar-Sánchez, A., & Viguri Fuente, J. R. (2022). Analysis and Modeling of Sunscreen Ingredients’ Behavior in an Aquatic Environment. Oceans, 3(3), 340-363. https://doi.org/10.3390/oceans3030024