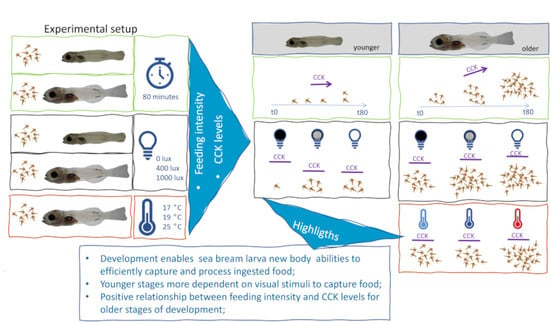
Understanding Fish Larvae’s Feeding Biology to Improve Aquaculture Feeding Protocols
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin and Husbandry Conditions of Fish Larvae
2.2. Experimental Conditions
2.2.1. Trial 1—Feeding Ability over Time
2.2.2. Trial 2—Light Intensity Assay
2.2.3. Trial 3—Temperature
2.3. Sampling
2.4. Analytical
2.4.1. Dry Weight and Total Length
2.4.2. Gut Content
2.4.3. CCK Quantification
2.4.4. Digestive Enzymes
2.5. Data Analysis
3. Results
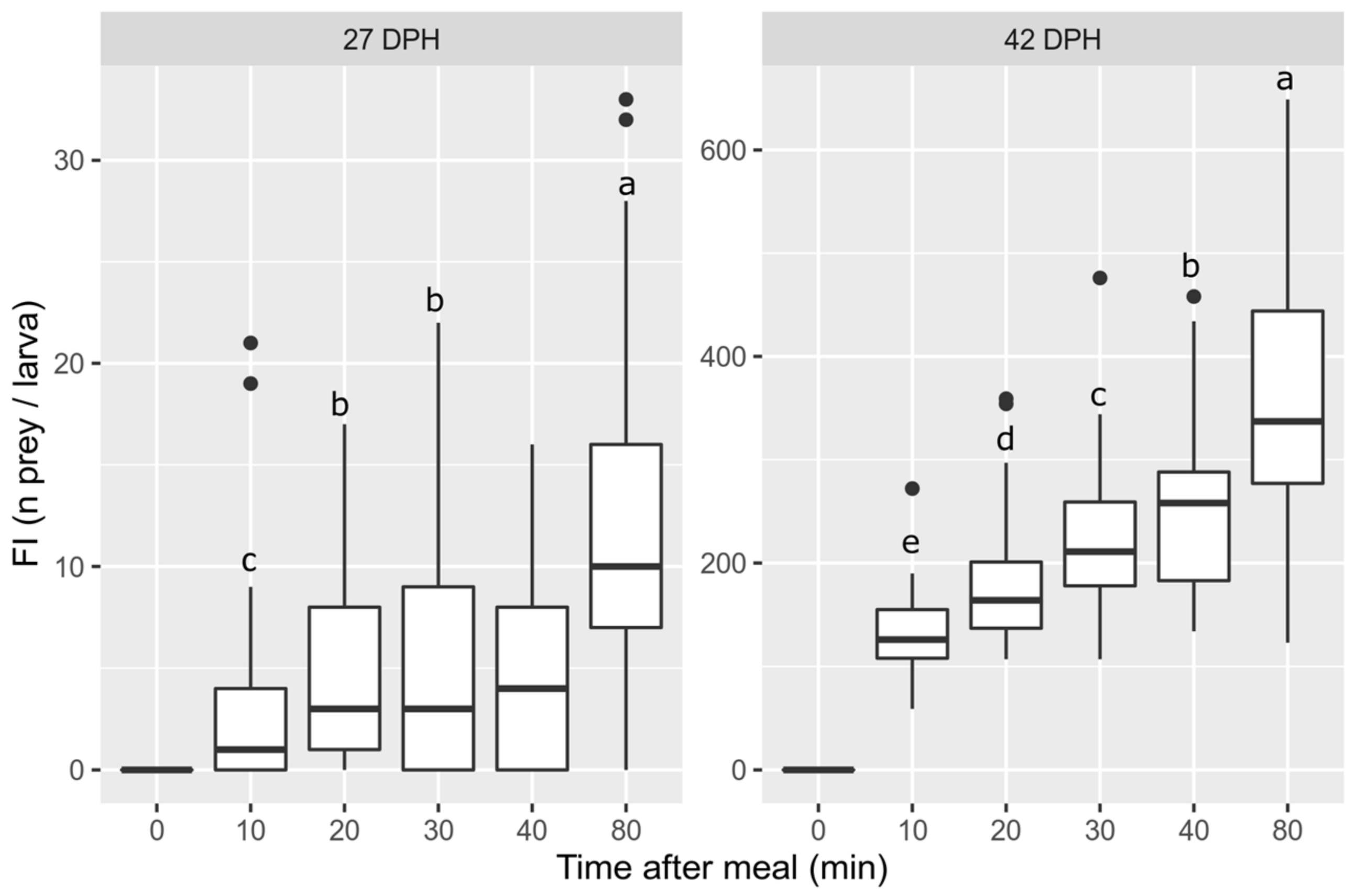
3.1. Feeding Ability over Time
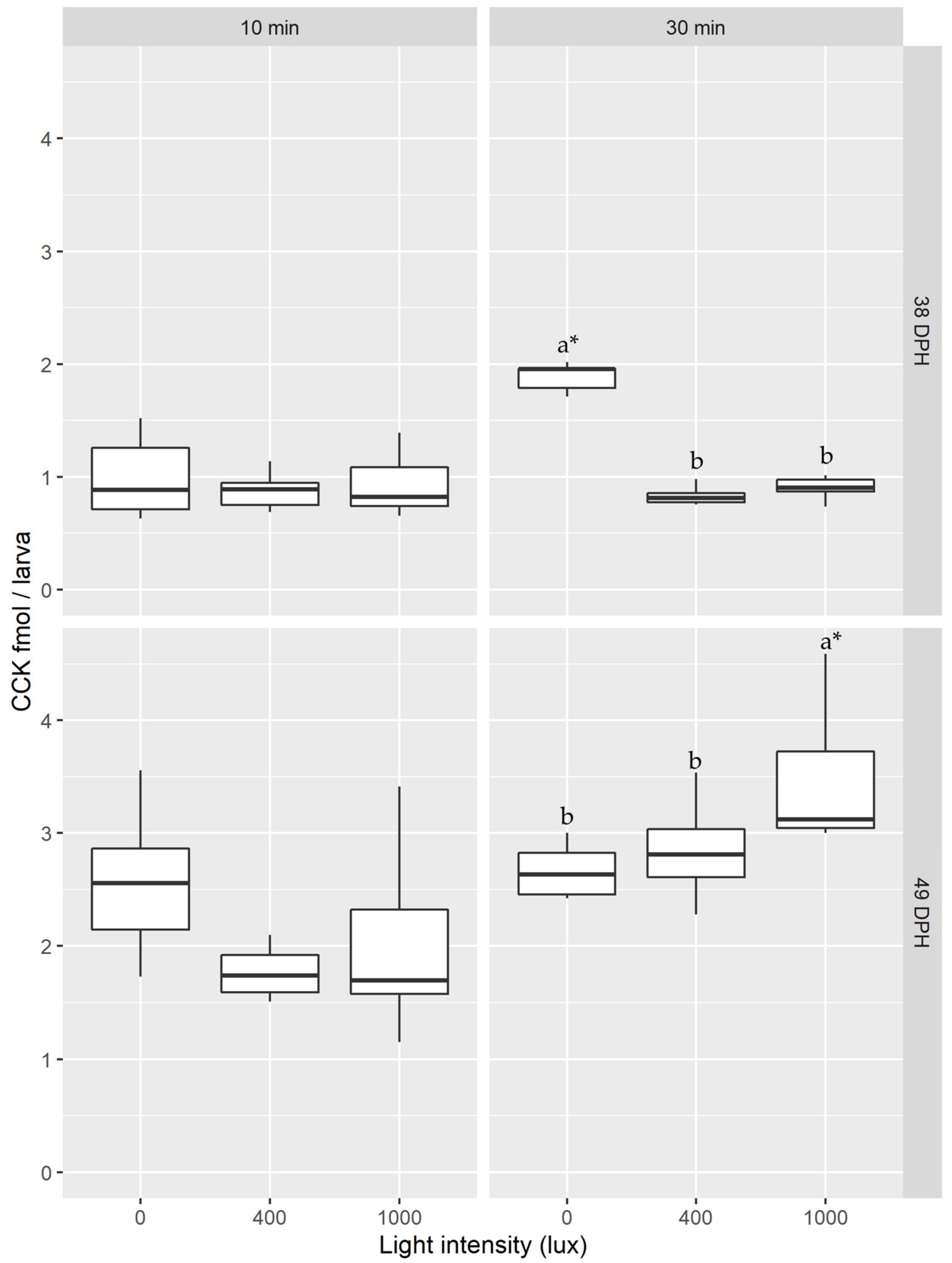
3.2. Light Intensity
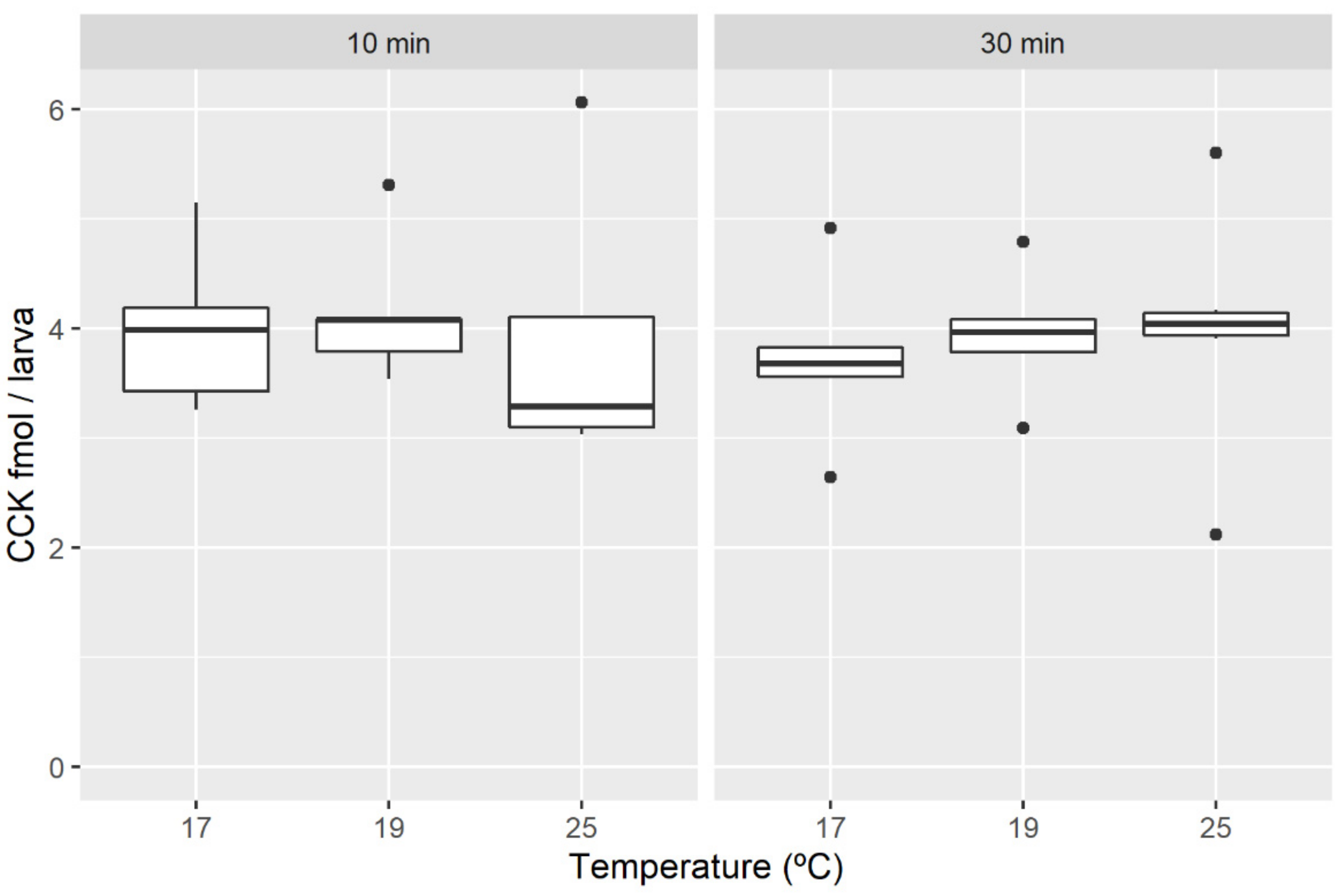
3.3. Temperature
4. Discussion
4.1. Feeding Ability
4.2. Light Intensity
4.3. Temperature
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Houde, E.D.; Schekter, R.C. Feeding by marine fish larvae: Developmental and functional responses. J. Appl. Phycol. 1980, 5, 315–334. [Google Scholar] [CrossRef]
- Pedersen, B.H. The cost of growth in young fish larvae, a review of new hypotheses. Aquaculture 1997, 155, 259–269. [Google Scholar] [CrossRef]
- Power, D.; Einarsdóttir, I.E.; Pittman, K.; Sweeney, G.E.; Hildahl, J.; Campinho, M.A.; Silva, N.; Sæle, Ø.; Galay-Burgos, M.; Smáradóttir, H.; et al. The Molecular and Endocrine Basis of Flatfish Metamorphosis. Rev. Fish. Sci. 2008, 16, 95–111. [Google Scholar] [CrossRef]
- Fuiman, L.A.; Cowan, J.H. Behavior and recruitment success in fish larvae: Repeatability and covariation of survival skills. Ecology 2003, 84, 53–67. [Google Scholar] [CrossRef]
- Rønnestad, I.; Yúfera, M.; Ueberschär, B.; Ribeiro, L.; Saele, Ø.; Boglione, C. Feeding behaviour and digestive physiology in larval fish: Current knowledge, and gaps and bottlenecks in research. Rev. Aquac. 2013, 5, S59–S98. [Google Scholar] [CrossRef]
- Nelson, L.E.; Sheridan, M.A. Gastroenteropancreatic hormones and metabolism in fish. Gen. Comp. Endocrinol. 2006, 148, 116–124. [Google Scholar] [CrossRef]
- Volkoff, H.; Canosa, L.F.; Unniappan, S.; Cerda-Reverter, J.M.; Bernier, N.; Kelly, S.; Peter, R. Neuropeptides and the control of food intake in fish. Gen. Comp. Endocrinol. 2005, 142, 3–19. [Google Scholar] [CrossRef]
- Tillner, R.; Rønnestad, I.; Dhert, P.; Ueberschär, B. The regulatory loop between gut cholecystokinin and tryptic enzyme activitcxuy in sea bass (Dicentrarchus labrax) larvae is influenced by different feeding regimes and trigger substances. Aquaculture 2014, 420, 139–146. [Google Scholar] [CrossRef]
- Zambonino-Infante, J.L.; Gisbert, E.; Sarasquete, C.; Navarro, I.; Gutierrez, J.; Cahu, C.L. Ontogeny and physiology of the digestive system of marine fish larvae. In Feeding and Digestive Functions of Fishes; Cyrino, J.E.P., Bureau, D.P., Kapoor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2008; pp. 281–348. [Google Scholar]
- Jönsson, E.; Forsman, A.; Einarsdottir, I.E.; Egnér, B.; Ruohonen, K.; Björnsson, B.T. Circulating levels of cholecystokinin and gastrin-releasing peptide in rainbow trout fed different diets. Gen. Comp. Endocrinol. 2006, 148, 187–194. [Google Scholar] [CrossRef]
- Conceição, L.E.C.; Morais, S.; Dinis, M.T.; Ronnestad, I. Tracer Studies in Fish Larvae. In Feeding and Digestive Functions in Fishes; Cyrino, J.E.P., Bureau, D.P., Kapoor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2008; pp. 349–392. [Google Scholar] [CrossRef]
- Hoskins, L.J.; Volkoff, H. The Comparative Endocrinology of Feeding in Fish: Insights and Challenges. Gen. Comp. Endocrinol. 2012, 176, 327–335. [Google Scholar] [CrossRef]
- Blaxter, J.H.S. Pattern and Variety in Development. In The Physiology of Developing Fish; Hoar, W.S., Randall, B.J., Eds.; Academic Press: San Diego, CA, USA, 1988; Volume XI-part, pp. 1–58. [Google Scholar]
- Moretti, A.; Pedini Fernandez-Criado, M.; Cittolin, G.; Guidastri, R. Manual on Hatchery Production of Seabass and Gilthead Seabream; FAO: Rome, Italy, 1999; Volume 1. [Google Scholar]
- Loew, E.R.; Wahl, C.M. Photoreception. In Fish larval Physiolgy; Finn, R.N., Kapoor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2008; pp. 395–424. [Google Scholar]
- Boeuf, G.; Le Bail, P.Y. Does Light Have an Influence on Fish Growth? Aquaculture 1999, 177, 129–152. [Google Scholar] [CrossRef]
- Salas-Berrios, F.; Valdés-Aguilera, J.; Landaeta, M.F.; Bustos, C.A.; Pérez-Vargas, A.; Balbontín, F. Feeding Habits and Diet Overlap of Marine Fish Larvae from the Peri-Antarctic Magellan Region. Polar Biol. 2013, 36, 1401–1414. [Google Scholar] [CrossRef]
- Vallés, R.; Estévez, A. Light Conditions for Larval Rearing of Meagre (Argyrosomus regius). Aquaculture 2013, 376–379, 15–19. [Google Scholar] [CrossRef]
- Blaxter, J.H.S. Development: Eggs and Larvae. In Fish Physiology; Hoar, W.S., Randall, D.J., Eds.; Academic Press: New York, NY, USA, 1969; Volume III, pp. 177–252. [Google Scholar]
- Blaxter, J.H.S. The Effect of Temperature on Larval Fishes. Neth. J. Zool. 1992, 42, 336–357. [Google Scholar] [CrossRef]
- Garrido, S.; Cristóvão, A.; Caldeira, C.; Ben-Hamadou, R.; Baylina, N.; Batista, H.; Saiz, E.; Peck, M.A.; Ré, P.; Santos, A.M.P. Effect of Temperature on the Growth, Survival, Development and Foraging Behaviour of Sardina Pilchardus Larvae. Mar. Ecol. Prog. Ser. 2016, 559, 131–145. [Google Scholar] [CrossRef]
- Moyano, M.; Illing, B.; Peschutter, P.; Huebert, K.B.; Peck, M.A. Thermal Impacts on the Growth, Development and Ontogeny of Critical Speed in Atlantic Herring Larvae. Comp. Biochem. Physiol. A Mol. Integr. 2016, 197, 23–34. [Google Scholar] [CrossRef]
- Dockray, G.J. Gastro-Intestinal Hormones II: Gastrin, Cholecystokinin and Secretin. Hormones in Blood; Academic Press: London, UK, 1979; pp. 189–219. [Google Scholar]
- Walsh, J.H. Gastrointestinal Hormones. In Physiology of Gastrointestinal Tract; Johnson, L.R., Ed.; Raven Press: New York, NY, USA, 1994; pp. 1–67. [Google Scholar]
- Liddle, R.A. Cholecystokinin Cells. Annu. Rev. Physiol. 1997, 59, 221–242. [Google Scholar] [CrossRef]
- Canosa, L.F.; Peter, R.E. Effects of Cholecystokinin and Bombesin on the Expression of Preprosomatostatin-Encoding Genes in Goldfish Forebrain. Regul. Pept. 2004, 121, 99–105. [Google Scholar] [CrossRef]
- Ronnestad, I.; Rojas-Garcia, C.R.; Skadal, J. Retrograde Peristalsis: A Possible Mechanism for Filling the Pyloric Caeca? J. Fish Biol. 2000, 56, 216–218. [Google Scholar] [CrossRef]
- Rønnestad, I.; Gomes, A.S.; Murashita, K.; Angotzi, R.; Jönsson, E.; Volkoff, H. Appetite-Controlling Endocrine Systems in Teleosts. Front. Endocrinol. 2017, 8, 73. [Google Scholar] [CrossRef]
- Kamisaka, Y.; Fujii, Y.; Yamamoto, S.; Kurokawa, T.; Rønnestad, I.; Totland, G.K.; Tagawa, M.; Tanaka, M. Distribution of Cholecystokinin-Immunoreactive Cells in the Digestive Tract of the Larval Teleost, Ayu, Plecoglossus Altivelis. Gen. Comp. Endocrinol. 2003, 134, 116–121. [Google Scholar] [CrossRef]
- Rønnestad, I.; Kamisaka, Y.; Conceição, L.E.C.; Morais, S.; Tonheim, S.K. Digestive Physiology of Marine Fish Larvae: Hormonal Control and Processing Capacity for Proteins, Peptides and Amino Acids. Aquaculture 2007, 268, 82–97. [Google Scholar] [CrossRef]
- Kamisaka, Y.; Drivenes, O.; Kurokawa, T.; Tagawa, M.; Ronnestad, I.; Tanaka, M.; Helvik, J.V. Cholecystokinin MRNA in Atlantic Herring, Clupea Harengus—Molecular Cloning, Characterization and Distribution in the Digestive Tract during the Early Life Stages. Peptides 2005, 26, 385–393. [Google Scholar] [CrossRef]
- Webb, K.A.; Khan, I.A.; Nunez, B.S.; Rønnestad, I.; Holt, G.J. Cholecystokinin: Molecular Cloning and Immunohistochemical Localization in the Gastrointestinal Tract of Larval Red Drum, Sciaenops ocellatus (L.). Gen. Comp. Endocrinol. 2010, 166, 152–159. [Google Scholar] [CrossRef]
- Rojas-García, C.R.; Rønnestad, I.; Ueberschär, B. Combined Sensitive Analytical Methods for Cholecystokinin Levels and Tryptic Activity in Individual Fish Larvae. J. Exp. Mar. Biol. Ecol. 2001, 265, 101–115. [Google Scholar] [CrossRef]
- Rojas-García, C.R.; Ronnestad, I. Cholecystokinin and Tryptic Activity in the Gut and Body of Developing Atlantic Halibut Larvae: Evidence for Participation in the Regulation of Protein Digestion. J. Fish Biol. 2002, 61, 973–986. [Google Scholar] [CrossRef]
- Rojas-García, C.R.; Morais, S.; Rønnestad, I. Cholecystokinin (CCK) in Atlantic Herring (Clupea harengus L.)—Ontogeny and Effects of Feeding and Diurnal Rhythms. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 455–460. [Google Scholar] [CrossRef]
- Koven, W.; Rojas-Garcia, C.R.; Finn, R.N.; Tandler, A.; Rønnestad, I. Stimulatory Effect of Ingested Protein and/or Free Amino Acids on the Secretion of the Gastro-Endocrine Hormone Cholecystokinin and on Tryptic Activity, in Early-Feeding Herring Larvae, Clupea Harengus. Mar. Biol. 2002, 140, 1241–1247. [Google Scholar]
- Cahu, C.L.; Ronnestad, I.; Grangier, V.; Zambonino-Infante, J.L. Expression and Activities of Pancreatic Enzymes in Developing Sea Bass Larvae (Dicentrarchus labrax) in Relation to Intact and Hydrolysed Dietary Protein: Involvement of Cholecystokinin. Aquaculture 2004, 238, 295–308. [Google Scholar] [CrossRef]
- Navarro-Guillén, C.; Rønnestad, I.; Jordal, A.E.O.; Moyano, F.J.; Yúfera, M. Involvement of Cholecystokinin (CCK) in the Daily Pattern of Gastrointestinal Regulation of Senegalese Sole (Solea senegalensis) Larvae Reared under Different Feeding Regimes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 203, 126–132. [Google Scholar] [CrossRef]
- Hanel, R.; Tsigenopoulos, C.S. Phylogeny, Evolution and Taxonomy of Sparids with Some Notes on Their Ecology and Biology. In Sparidae, Biology and Aquaculture of Gilthead Sea Bream and other Species; Pavlidis, M.A., Mylonas, C.C., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 51–73. [Google Scholar]
- Basurco, B.; Lovatelli, A.; García, B. Current Status of Sparidae Aquaculture. In Sparidae, Biology and Aquaculture of Gilthead Sea Bream and other Species; Pavlidis, M.A., Constantinos, C., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 1–50. [Google Scholar]
- Yúfera, M.; Pascual, E.; Polo, A.; Sarasquete, M.C. Effect of Starvation on the Feeding Ability of Gilthead Seabream (Sparus aurata L.) Larvae at First Feeding. J. Exp. Mar. Biol. Ecol. 1993, 169, 259–272. [Google Scholar] [CrossRef]
- Parra, G.; Yúfera, M. Feeding, Physiology and Growth Responses in First-Feeding Gilthead Seabream (Sparus aurata L.) Larvae in Relation to Prey Density. J. Exp. Mar. Biol. Ecol. 2000, 243, 1–15. [Google Scholar] [CrossRef]
- Yúfera, M.; Fernández-Diaz, C.; Pascual, E. Feeding Rates of Gilthead Seabream (Sparus aurata), Larvae on Microcapsules. Aquaculture 1995, 134, 257–268. [Google Scholar] [CrossRef]
- Moyano, F.J.; Díaz, M.; Alarcón, F.J.; Sarasquete, C. Characterization of Digestive Enzyme Activity during Larval Development of Gilthead Seabream (Sparus aurata). Fish Physiol. Biochem. 1996, 15, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Sarasquete, M.C.; Polo, A.; Yufera, M. Histology and Histochemistry of the Development of the Digestive System of Larval Gilthead Seabream, Sparus aurata L. Aquaculture 1995, 130, 79–92. [Google Scholar] [CrossRef]
- Parra, G.; Yúfera, M. Tolerance Response to Ammonia and Nitrite Exposure in Larvae of Two Marine Fish Species (Gilthead Seabream Sparus aurata L. and Senegal Sole Solea Senegalensis Kaup). Aquac. Res. 1999, 30, 857–863. [Google Scholar] [CrossRef]
- Rocha, R.J.; Ribeiro, L.; Costa, R.; Dinis, M.T. Does the Presence of Microalgae Influence Fish Larvae Prey Capture? Aquac. Res. 2008, 39, 362–369. [Google Scholar] [CrossRef]
- Yúfera, M.; Fernández-Díaz, C.; Vidaurreta, A.; Cara, J.B.; Moyano, F.J.; Yufera, M.; Fernandez-Diaz, C.; Vidaurreta, A.; Cara, J.B.; Moyano, F.J. Gastrointestinal PH and Development of the Acid Digestion in Larvae and Early Juveniles of Sparus aurata (Pisces: Teleostei). Mar. Biol. 2004, 144, 863–869. [Google Scholar] [CrossRef]
- Sassa, C.; Kawaguchi, K. Larval Feeding Habits of Diaphus Garmani and Myctophum Asperum (Pisces: Myctophidae) in the Transition Region of the Western North Pacific. Mar. Ecol. Prog. Ser. 2004, 278, 279–290. [Google Scholar] [CrossRef][Green Version]
- Yin, M.C.; Blaxter, J.H.S. Feeding Ability and Survival during Starvation of Marine Fish Larvae Reared in the Laboratory. J. Exp. Mar. Biol. Ecol. 1987, 105, 73–83. [Google Scholar] [CrossRef]
- Ribeiro, L.; Zambonino-Infante, J.L.; Cahu, C.; Dinis, M.T. Development of Digestive Enzymes in Larvae of Solea Senegalensis, Kaup 1858. Aquaculture 1999, 179, 465–473. [Google Scholar] [CrossRef]
- Tseng, H.C.; Grendell, J.H.; Rothman, S.S. Food, Duodenal Extracts, and Enzyme Secretion by the Pancreas. Am. J. Physiol. 1982, 243, G304–G312. [Google Scholar] [CrossRef] [PubMed]
- Métais, P.; Bieth, J. Détermination de l’a-Amylase Par Une Microtechnique. Ann. Biol. Clin. 1968, 26, 133–142. [Google Scholar]
- Bessey, O.A.; Lowry, O.H.; Brock, M.J. A Method for the Rapid Determination of Alkaline Phosphatase with Five Cubic Millimeters of Serum. J Biol. Chem. 1946, 164, 321–329. [Google Scholar] [CrossRef]
- Maroux, S.; Louvard, D.; Baratti, J. The Aminopeptidase from Hog-Intestinal Brush Border. Biochmica. Biophys. Acta 1973, 321, 282–295. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry, 3rd ed.; W.H. Freeman and Company: New York, NY, USA, 1995. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Pittman, K.; Yúfera, M.; Pavlidis, M.; Geffen, A.J.A.J.; Koven, W.; Ribeiro, L.; Zambonino-Infante, J.L.J.L.; Tandler, A. Fantastically Plastic: Fish Larvae Equipped for a New World. Rev. Aquac. 2013, 5, S224–S267. [Google Scholar] [CrossRef]
- Herbing, I.H. Development of Feeding Structures in Larval Fish with Different Life Histories: Winter Flounder and Atlantic Cod. J. Fish Biol. 2001, 59, 767–782. [Google Scholar] [CrossRef]
- Hunter, J.R. Swimming and Feeding Behaviour of Larval Anchovy Engraulis Mordax. Fish. Bull. 1972, 70, 821–838. [Google Scholar]
- Von Herbing, I.H.; Gallager, S.M. Foraging Behavior in Early Atlantic Cod Larvae (Gadus morhua) Feeding on a Protozoan (Balanion sp.) and a Copepod Nauplius (Psedodiaptomus sp.). Mar. Biol. 2000, 136, 591–602. [Google Scholar] [CrossRef]
- Evans, B.I.; Browman, H.I. Variation in the Development of the Fish Retina. Am. Fish. Soc. Symp. 2004, 40, 145–166. [Google Scholar]
- Brett, J.R.; Groves, T.D.D. Physiological Energetics. In Fish Physiology; Hoar, W.S., Randall, D.J., Brett, J.R., Eds.; Academic Press: London, UK, 1979; Volume VIII, pp. 279–352. [Google Scholar]
- Parra, G.; Yúfera, M. Comparative Energetics during Early Development of Two Marine Fish Species, Solea Senegalensis (Kaup) and Sparus aurata (L.). J. Exp. Biol. 2001, 204, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Castanho, S.; Califano, G.; Soares, F.; Costa, R.; Mata, L.; Pousão-Ferreira, P.; Ribeiro, L. The Effect of Live Feeds Bathed with the Red Seaweed Asparagopsis Armata on the Survival, Growth and Physiology Status of Sparus aurata Larvae. Fish Physiol. Biochem. 2017, 300, 371–1054. [Google Scholar] [CrossRef]
- Reinecke, M.; Muller, C.; Segner, H. An Immunohistochemical Analysis of the Ontogeny, Distribution and Coexistence of 12 Regulatory Peptides and Serotonin in Endocrine Cells and Nerve Fibers of the Digestive Tract of the Turbot, Scophthalmus maximus (Teleostei). Anat. Embryol. 1997, 195, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Kamisaka, Y.; Totland, G.K.; Tagawa, M.; Kurokawa, T.; Suzuki, T.; Tanaka, M.; Ronnestad, I. Ontogeny of Cholecystokinin-Immunoreactive Cells in the Digestive Tract of Atlantic Halibut, Hippoglossus Huppoglossus, Larvae. Gen. Comp. Endocrinol. 2001, 123, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Vollset, K.W.; Folkvord, A.; Browman, H.I. Foraging Behaviour of Larval Cod (Gadus morhua) at Low Light Intensities. Mar. Biol. 2011, 158, 1125–1133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ellertsen, B.; Solemdal, P.; Stromme, T.; Tilseth, S.; Westgard, T.; Moksness, E.; Oiestad, V. Some Biological Aspect of Cod Larvae (Gadus morhua L.). Fisk. Dir. Skr. Ser. Hav. Unders. 1980, 17, 29–47. [Google Scholar]
- Blaxter, J.H.S. Development of Sense Organs and Behaviour in Teleost Larvae with Special Reference to Feeding and Predation Avoidancele. Transations Am. Fish. Soc. 1986, 115, 98–114. [Google Scholar]
- Pavón-Muñoz, T.; Bejarano-Escobar, R.; Blasco, M.; Martín-Partido, G.; Francisco-Morcillo, J. Retinal Development in the Gilthead Seabream Sparus aurata. J. Fish Biol. 2016, 88, 492–507. [Google Scholar] [CrossRef]
- Valen, R.; Eilertsen, M.; Edvardsen, R.B.; Furmanek, T.; Rønnestad, I.; van der Meeren, T.; Karlsen, Ø.; Nilsen, T.O.; Helvik, J.V. The Two-Step Development of a Duplex Retina Involves Distinct Events of Cone and Rod Neurogenesis and Differentiation. Dev. Biol. 2016, 416, 389–401. [Google Scholar] [CrossRef]
- Helvik, J.V.; Drivenes, Ø.; Næss, T.H.; Fjose, A.; Seo, H.-C. Molecular Cloning and Characterization of FIve Opsin Genes from the Marine FLatfish Atlantic Halibut (Hippoglossus hippoglossus). Vis. Neurosci. 2001, 18, 767–780. [Google Scholar] [CrossRef]
- Power, M.L.; Schulkin, J. Anticipatory Physiological Regulation in Feeding Biology. Handb. Behav. Food Nutr. 2011, 50, 829–844. [Google Scholar]
- Einarsson, S.; Davies, P.S.; Talbot, C. Effect of Exogenous Cholecystokinin on the Discharge of the Gallbladder and the Secretion of Trypsin and Chymotrypsin from the Pancreas of the Atlantic Salmon, Salmo salar L. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1997, 117, 63–67. [Google Scholar] [CrossRef]
- Wyatt, T. Some Effects of Food Density on the Growth and Behaviour of Plaice Larvae. Mar. Biol. 1972, 14, 210–216. [Google Scholar] [CrossRef]
- Polo, A.; Yúfera, M.; Pascual, E. Effects of Temperature on Egg and Larval Development of Sparus aurata L. Aquaculture 1991, 92, 367–375. [Google Scholar] [CrossRef]
- Rombough, P.J. The Effects of Temperature on Embryonic and Larval Development. In Global Warming; Wood, C.M., McDonald, D.G., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 177–224. [Google Scholar] [CrossRef]
- Jordaan, A.; Kling, L.J. Determining the Optimal Temperature Range for Atlantic Cod (Gadus morhua) during Early Life. In Proceedings of the 26th Annual Larval Fish Conference, Bergen, Norway, 22–26 July 2002. [Google Scholar]
- Hofer, R. The Adaptation of Digestive Enzymes to Temperature, Season and Diet in Roach, Rutilus Rutilus and Rudd Scardinius Erythrophthalmus. Proteases. J. Fish Biol. 1979, 15, 373–379. [Google Scholar] [CrossRef]
- Ribeiro, L.; Zambonino-Infante, J.L.; Cahu, C.; Dinis, M.T. Digestive Enzymes Profile of Solea Senegalensis Post Larvae Fed Artemia and a Compound Diet. Fish. Physiol. Biochem. 2002, 27. [Google Scholar] [CrossRef]





| Spawn | Trial | DPH | DW (mg) | TL (mm) | SL (mm) |
|---|---|---|---|---|---|
| A | Feeding over time | 27 | 0.47 ± 0.22 | 8.02 ± 1.18 | 7.1 ± 0.81 |
| A | Light intensity | 38 | 1.56 ± 0.58 | 10.96 ± 1.05 | 9.21 ± 0.82 |
| A | Temperature | 41 | 5.37 ± 1.34 | 13.20 ± 1.39 | 11.06 ± 1.12 |
| B | Feeding over time | 42 | 3.05 ± 1.45 | 12.08 ± 1.08 | 10.02 ± 0.92 |
| B | Light intensity | 49 | 4.41 ± 1.63 | 13.22 ± 0.99 | 10.99 ± 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, L.; Hubert, F.N.; Rodrigues, V.; Rojas-Garcia, C.; Dinis, M.T. Understanding Fish Larvae’s Feeding Biology to Improve Aquaculture Feeding Protocols. Oceans 2022, 3, 94-113. https://doi.org/10.3390/oceans3010009
Ribeiro L, Hubert FN, Rodrigues V, Rojas-Garcia C, Dinis MT. Understanding Fish Larvae’s Feeding Biology to Improve Aquaculture Feeding Protocols. Oceans. 2022; 3(1):94-113. https://doi.org/10.3390/oceans3010009
Chicago/Turabian StyleRibeiro, Laura, François Noel Hubert, Vera Rodrigues, Carlos Rojas-Garcia, and Maria Teresa Dinis. 2022. "Understanding Fish Larvae’s Feeding Biology to Improve Aquaculture Feeding Protocols" Oceans 3, no. 1: 94-113. https://doi.org/10.3390/oceans3010009
APA StyleRibeiro, L., Hubert, F. N., Rodrigues, V., Rojas-Garcia, C., & Dinis, M. T. (2022). Understanding Fish Larvae’s Feeding Biology to Improve Aquaculture Feeding Protocols. Oceans, 3(1), 94-113. https://doi.org/10.3390/oceans3010009