Computational Mechanics of Polymeric Materials PEEK and PEKK Compared to Ti Implants for Marginal Bone Loss Around Oral Implants
Abstract
1. Introduction
2. Materials and Methods
3. Results
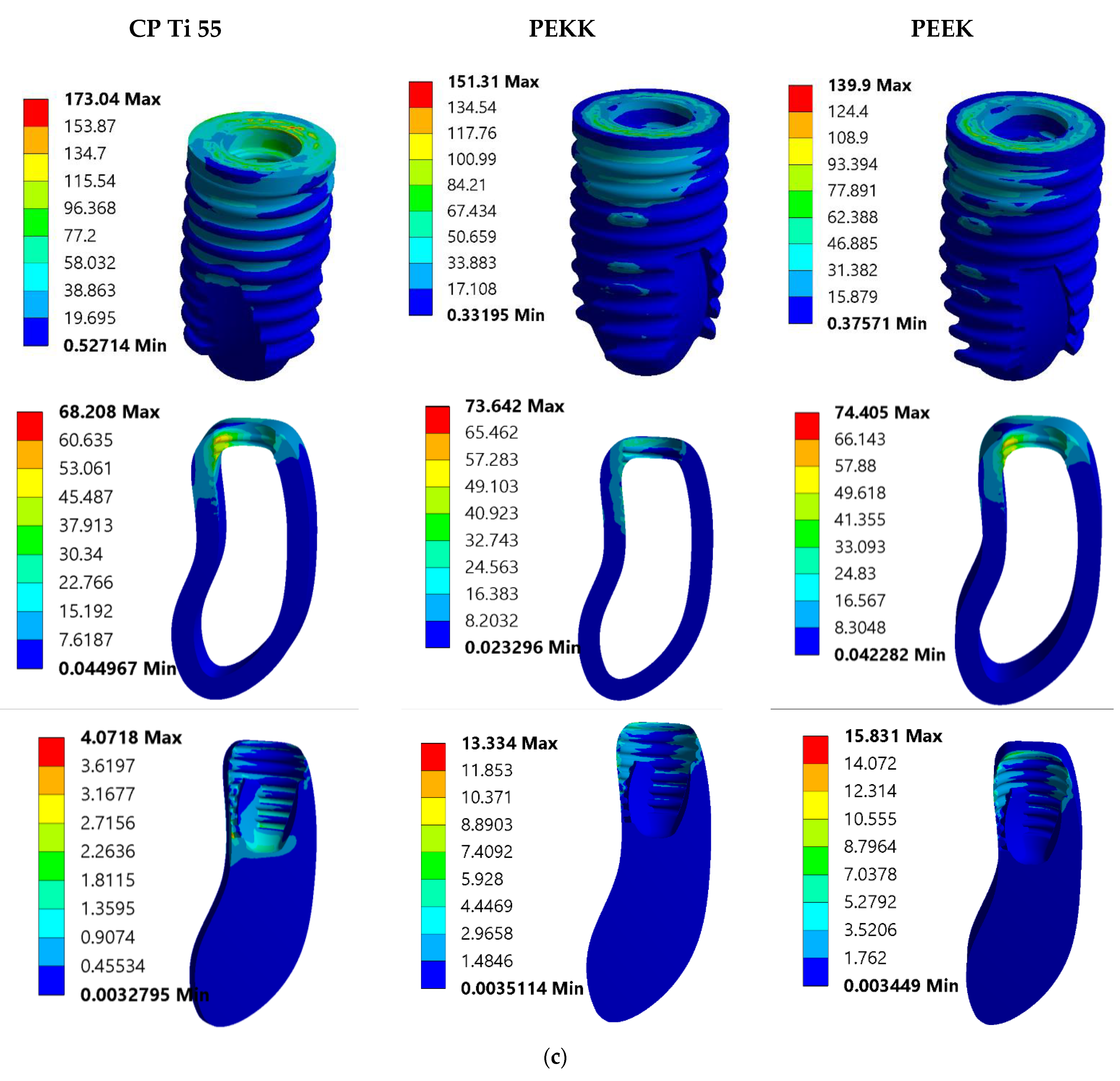
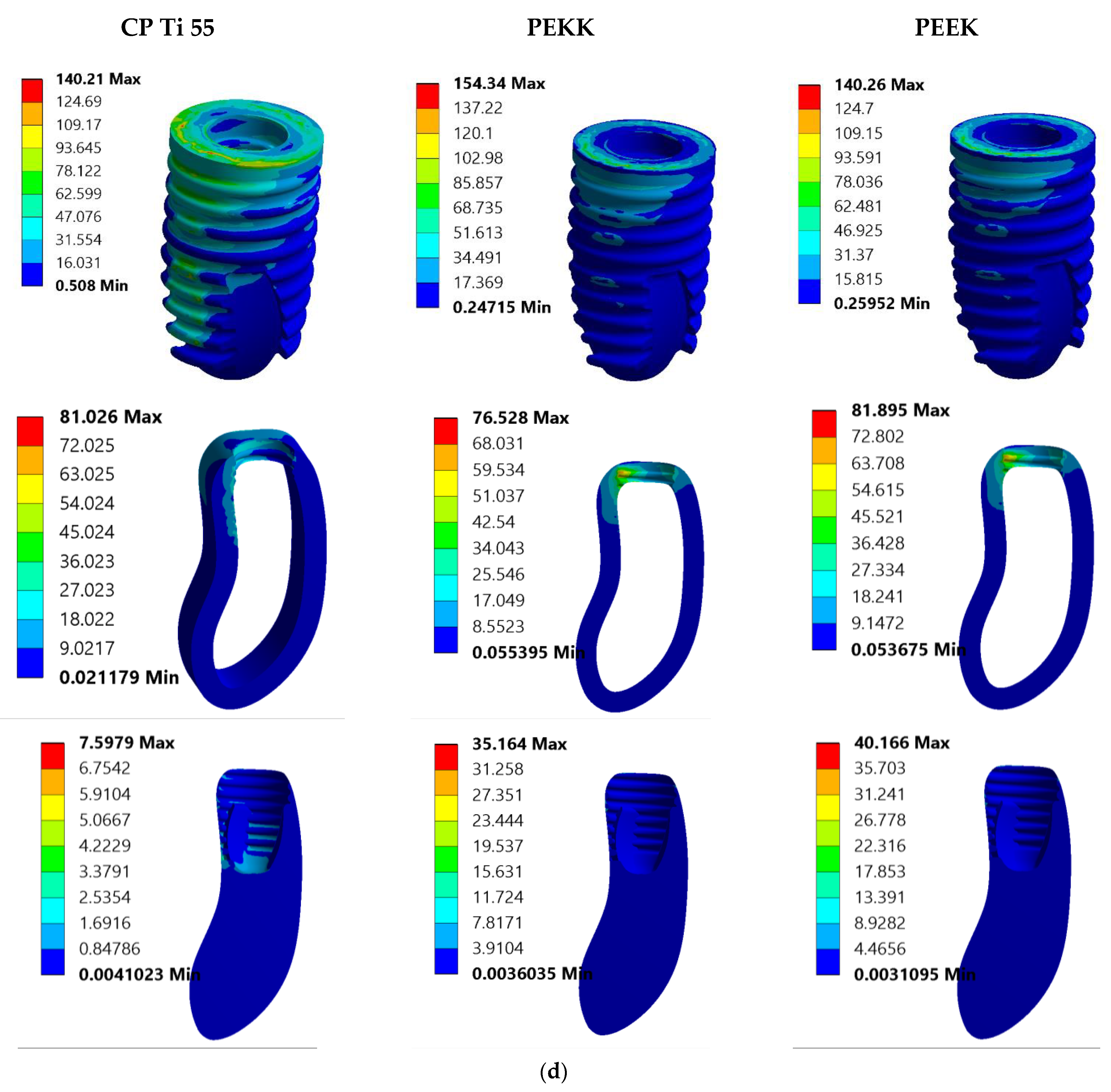
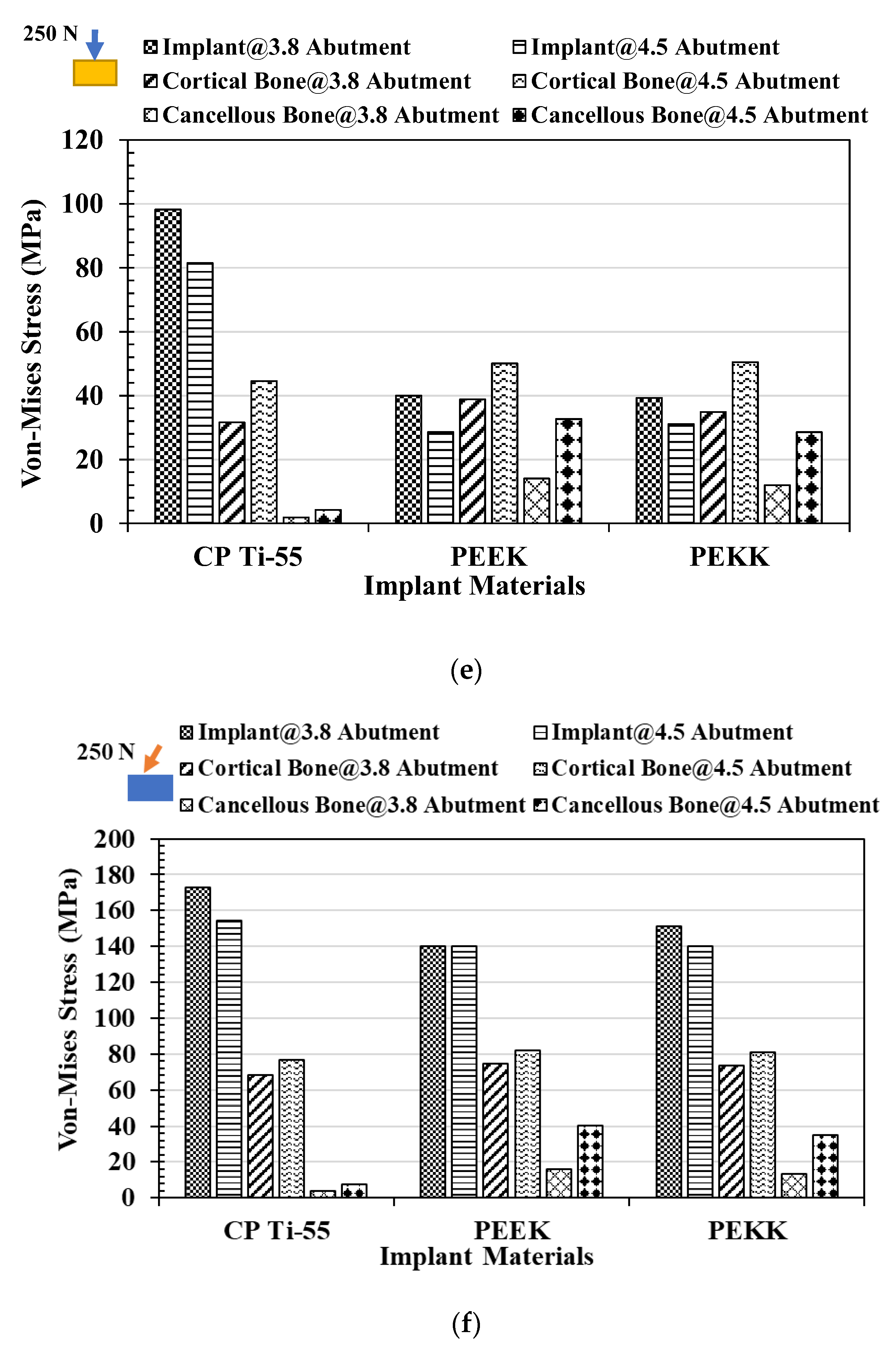
3.1. Von Mises Stress Analysis
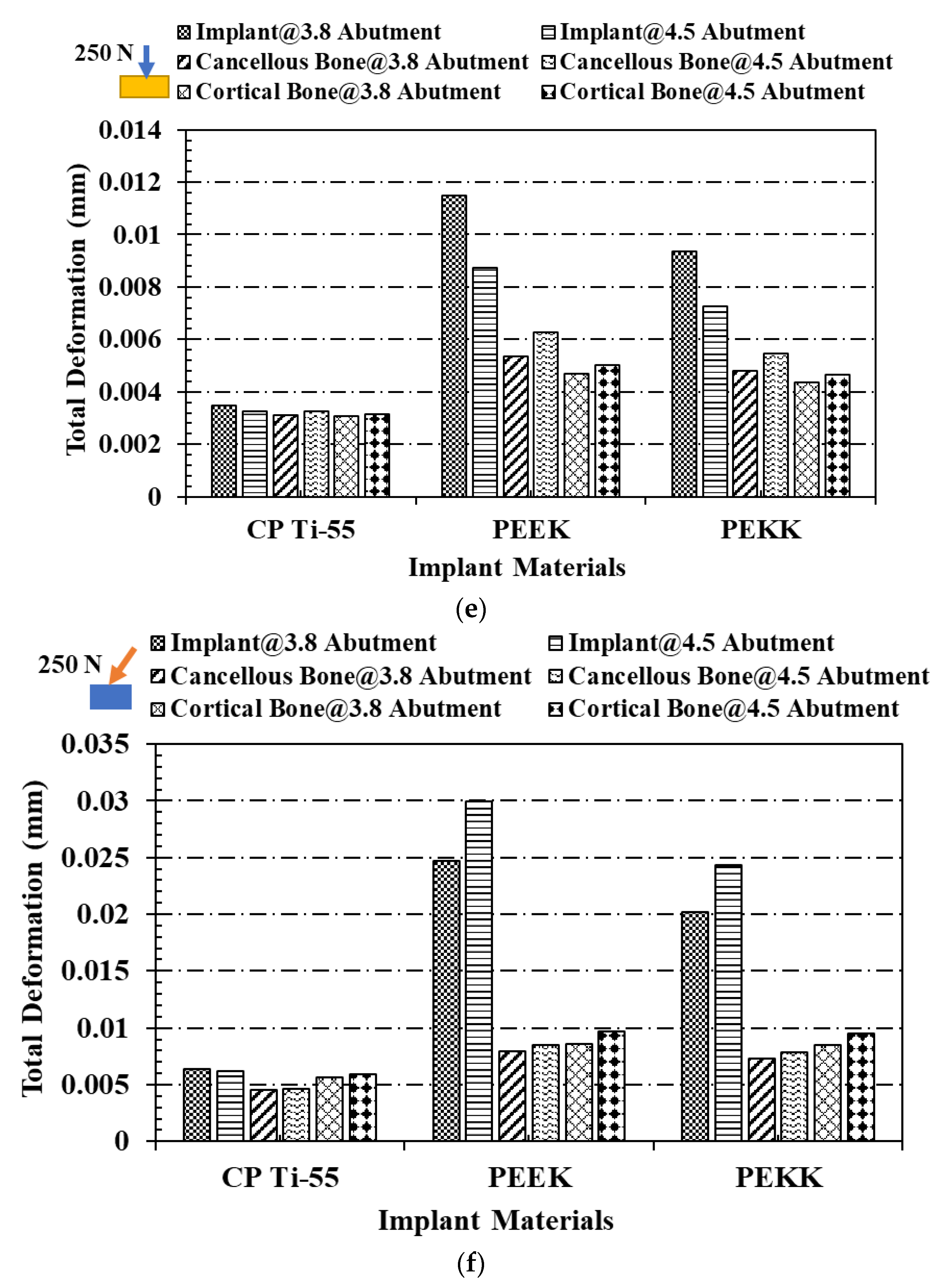
3.2. Total Deformation Analysis
4. Discussion
- The loading criteria in this study were distilled down to a singular force (vertical and oblique individually), and the boundary condition was established as fixed at designated sites.
- Because they were not subject to any kind of flaw population, the virtual materials were thought of as orthotropic and isotropic.
- There was no attempt to mimic sliding contacts, operator mistake, or vertical misalignment of the prosthesis. While the linear contact between screws and polymeric materials used in models may not provide an exact representation of the stress condition under loading, it is standardized and allows for easier comparison.
- Computer-based finite element analysis predicts bone or implant component failure probabilistically. Tissue reaction, blood circulation, growth hormones, microbial colonization, and cleanliness habits are ignored.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paxton, N.C.; Dinoro, J.; Ren, J.; Ross, M.T.; Daley, R.; Zhou, R.; Bazaka, K.; Thompson, R.G.; Yue, Z.; Beirne, S.; et al. Additive manufacturing enables personalised porous high-density polyethylene surgical implant manufacturing with improved tissue and vascular ingrowth. Appl. Mater. Today 2021, 22, 100965. [Google Scholar] [CrossRef]
- Roina, Y.; Auber, F.; Hocquet, D.; Herlem, G. ePTFE-based biomedical devices: An overview of surgical efficiency. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 110, 302–320. [Google Scholar] [CrossRef]
- Kaur, H.; Thakur, A. Applications of poly(methyl methacrylate) polymer in dentistry: A review. Mater. Today Proc. 2022, 50, 1619–1625. [Google Scholar] [CrossRef]
- Etinosa, P.O.; Osuchukwu, O.A.; Anisiji, E.O.; Lawal, M.Y.; Mohammed, S.A.; Ibitoye, O.I.; Oni, P.G.; Aderibigbe, V.D.; Aina, T.; Oyebode, D.; et al. In-depth review of synthesis of hydroxyapatite biomaterials from natural resources and chemical regents for biomedical applications. Arab. J. Chem. 2024, 17, 106010. [Google Scholar] [CrossRef]
- Zol, S.M.; Alauddin, M.S.; Said, Z.; Ghazali, M.I.M.; Hao-Ern, L.; Farid, D.A.M.; Zahari, N.A.H.; Al-Khadim, A.H.A.; Aziz, A.H.A. Description of Poly(aryl-ether-ketone) Materials (PAEKs), Polyetheretherketone (PEEK) and Polyetherketoneketone (PEKK) for Application as a Dental Material: A Materials Science Review. Polymers 2023, 15, 2170. [Google Scholar] [CrossRef]
- Hong, D.G.K.; Oh, J. Recent advances in dental implants. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 33. [Google Scholar] [CrossRef]
- Shemtov-Yona, K.; Rittel, D. An Overview of the Mechanical Integrity of Dental Implants. BioMed Res. Int. 2015, 2015, 547384. [Google Scholar] [CrossRef]
- Ong, G. Periodontal disease and tooth loss. Int. Dent. J. 1998, 48, 233–238. [Google Scholar] [CrossRef]
- Gabiec, K.; Bagińska, J.; Łaguna, W.; Rodakowska, E.; Kamińska, I.; Stachurska, Z.; Dubatówka, M.; Kondraciuk, M.; Kamiński, K.A. Factors Associated with Tooth Loss in General Population of Bialystok, Poland. Int. J. Environ. Res. Public Health 2022, 19, 2369. [Google Scholar] [CrossRef]
- Wiener, R.C.; Shen, C.; Findley, P.A.; Sambamoorthi, U.; Tan, X. The association between diabetes mellitus, sugar-sweetened beverages, and tooth loss in adults. J. Am. Dent. Assoc. 2017, 148, 500–509.e4. [Google Scholar] [CrossRef]
- Kadhim, D.R.; Hamad, T.I.; Fatalla, A.A. Use of Eggshells as Bone Grafts around Commercially Pure Titanium Implant Screws Coated with Nano Calcium Sulfate. Int. J. Biomater. 2022, 2022, 8722283. [Google Scholar] [CrossRef]
- Țap, M.D.; Bîcleşanu, F.C.; Honțaru, O.-S.; Radu, A.-C. Patient Centricity-An Empirical Research on Titanium Dental Implants and Their Adverse Effects on Health Condition. Healthcare 2024, 12, 2207. [Google Scholar] [CrossRef]
- Silva, R.C.S.; Agrelli, A.; Andrade, A.N.; Mendes-Marques, C.L.; Arruda, I.R.S.; Santos, L.R.L.; Vasconcelos, N.F.; Machado, G. Titanium Dental Implants: An Overview of Applied Nanobiotechnology to Improve Biocompatibility and Prevent Infections. Materials 2022, 15, 3150. [Google Scholar] [CrossRef]
- Belkhode, V.; Godbole, S.; Nimonkar, S.; Nimonkar, P.; Pisulkar, S. Comparative evaluation of the efficacy of customized maxillary oral appliance with mandibular advancement appliance as a treatment modality for moderate obstructive sleep apnea patients-protocol for a randomized controlled trial. Trials 2022, 23, 159. [Google Scholar] [CrossRef]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for medical applications. J. Mater. Sci. Mater. Med. 2016, 27, 118. [Google Scholar] [CrossRef]
- AlOtaibi, N.; Naudi, K.; Conway, D.; Ayoub, A. The current state of PEEK implant osseointegration and future perspectives: A systematic review. Eur. Cells Mater. 2020, 40, 1–20. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, J.; Sun, S.; Meng, W.; Zhang, Y.; Wu, J. Current treatment methods to improve the bioactivity and bonding strength of PEEK for dental application: A systematic review. Eur. Polym. J. 2023, 183, 111757. [Google Scholar] [CrossRef]
- Jung, H.-D.; Jang, T.-S.; Lee, J.E.; Park, S.J.; Son, Y.; Park, S.-H. Enhanced bioactivity of titanium-coated polyetheretherketone implants created by a high-temperature 3D printing process. Biofabrication 2019, 11, 045014. [Google Scholar] [CrossRef]
- de Araújo Nobre, M.; Moura Guedes, C.; Almeida, R.; Silva, A. Poly-ether-ether-ketone and implant dentistry: The future of mimicking natural dentition is now! Polym. Int. 2021, 70, 999–1001. [Google Scholar] [CrossRef]
- Johansson, P.; Jimbo, R.; Kozai, Y.; Sakurai, T.; Kjellin, P.; Currie, F.; Wennerberg, A. Nanosized Hydroxyapatite Coating on PEEK Implants Enhances Early Bone Formation: A Histological and Three-Dimensional Investigation in Rabbit Bone. Materials 2015, 8, 3815–3830. [Google Scholar] [CrossRef]
- Li, L.; Zhou, C.; Wei, J.; Ma, J. Quantitative analysis of nFA/PEEK implant interfaces in Beagle dogs. Shanghai Kou Qiang Yi Xue 2014, 23, 166–171. [Google Scholar] [PubMed]
- Belkhode, V.M.; Nimonkar, S.V.; Godbole, S.R.; Nimonkar, P.; Sathe, S.; Borle, A. Evaluation of the effect of different surface treatments on the bond strength of non-precious alloy—Ceramic interface: An SEM study. J. Dent. Res. Dent. Clin. Dent. Prospect. 2019, 13, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Meningaud, J.P.; Spahn, F.; Donsimoni, J.M. After titanium, peek? Rev. De Stomatol. Et De Chir. Maxillo Faciale 2012, 113, 407–410. [Google Scholar] [CrossRef]
- Hoskins, T.J.; Dearn, K.D.; Kukureka, S.N. Mechanical performance of PEEK produced by additive manufacturing. Polym. Test. 2018, 70, 511–519. [Google Scholar] [CrossRef]
- Kewekordes, T.; Wille, S.; Kern, M. Wear of polyetherketoneketones—Influence of titanium dioxide content and antagonistic material. Dent. Mater. 2018, 34, 560–567. [Google Scholar] [CrossRef]
- Polymer Science: A Comprehensive Reference | ScienceDirect. Available online: https://www.sciencedirect.com/referencework/9780080878621/polymer-science-a-comprehensive-reference (accessed on 10 December 2024).
- Alsadon, O.; Wood, D.; Patrick, D.; Pollington, S. Fatigue behavior and damage modes of high performance poly-ether-ketone-ketone PEKK bilayered crowns. J. Mech. Behav. Biomed. Mater. 2020, 110, 103957. [Google Scholar] [CrossRef]
- Elkabbany, A.; Kern, M.; Elkhadem, A.H.; Wille, S.; Amer, A.A.; Chaar, M.S. Retention of metallic and non-metallic double-crown-retained mandibular overdentures on implants: An in-vitro study. J. Prosthodont. Res. 2020, 64, 384–390. [Google Scholar] [CrossRef]
- Schwitalla, A.D.; Spintig, T.; Kallage, I.; Müller, W.D. Flexural behavior of PEEK materials for dental application. Dent. Mater. 2015, 31, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, A.; Guess, T.M.; Halloran, J.; Tadepalli, S.C.; Morrison, T.M. Considerations for reporting finite element analysis studies in biomechanics. J. Biomech. 2012, 45, 625–633. [Google Scholar] [CrossRef]
- Pankaj, P. Patient-specific modelling of bone and bone-implant systems: The challenges. Int. J. Numer. Methods Biomed. Eng. 2013, 29, 233–249. [Google Scholar] [CrossRef]
- Cowin, S.C. Bone Mechanics Handbook; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Taylor, M.; Prendergast, P.J. Four decades of finite element analysis of orthopaedic devices: Where are we now and what are the opportunities? J. Biomech. 2015, 48, 767–778. [Google Scholar] [CrossRef]
- Afazal, M.; Gupta, S.; Tevatia, A.; Afreen, S.; Chanda, A. Computational Investigation of Dental Implant Restoration Using Platform-Switched and -Matched Configurations. Computation 2023, 11, 79. [Google Scholar] [CrossRef]
- Afazal, M.; Afreen, S.; Chanda, A. Computational modelling and analysis of hard tissue behavior around 0.5 mm and 0.85 mm platform switched abutment using 3D finite element analysis. Forces Mech. 2023, 13, 100243. [Google Scholar] [CrossRef]
- Gačnik, F.; Ren, Z.; Hren, N.I. Modified bone density-dependent orthotropic material model of human mandibular bone. Med Eng. Phys. 2014, 36, 1684–1692. [Google Scholar] [CrossRef]
- Dhatrak, P.; Girme, V.; Shirsat, U.; Sumanth, S.; Deshmukh, V. Significance of Orthotropic Material Models to Predict Stress Around Bone-Implant Interface Using Numerical Simulation. BioNanoScience 2019, 9, 652–659. [Google Scholar] [CrossRef]
- Falcinelli, C.; Valente, F.; Vasta, M.; Traini, T. Finite element analysis in implant dentistry: State of the art and future directions. Dent. Mater. 2023, 39, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.T.; Zanatta, L.C.S.; de Souza Rendohl, E.; Gehrke, S.A. Comparative analysis of stress distribution in one-piece and two-piece implants with narrow and extra-narrow diameters: A finite element study. PLoS ONE 2021, 16, e0245800. [Google Scholar] [CrossRef]
- Xu, X.; He, L.; Zhu, B.; Li, J.; Li, J. Advances in polymeric materials for dental applications. Polym. Chem. 2017, 8, 807–823. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric materials and films in dentistry: An overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Mechanical Performances of PEKK Thermoplastic Composites Linked to Their Processing Parameters. Available online: https://www.researchgate.net/publication/327952758_Mechanical_performances_of_PEKK_thermoplastic_composites_linked_to_their_processing_parameters (accessed on 12 June 2025).
- Levy-Marchal, C.; Czernichow, P. Cyclosporin A in insulin-dependent diabetes mellitus of recent onset: A pilot study in children. Horm. Res. 1988, 29, 177–184. [Google Scholar] [CrossRef]
- PEEK vs PEKK: Which High Performance Material Should You Choose?—3Dnatives. Available online: https://www.3dnatives.com/en/peek-vs-pekk-240620214/ (accessed on 12 June 2025).
- McMullan, R.; Golbang, A.; Salma-Ancane, K.; Ward, J.; Rodzen, K.; Boyd, A.R. Review of 3D Printing of Polyaryletherketone/Apatite Composites for Lattice Structures for Orthopedic Implants. Appl. Sci. 2025, 15, 1804. [Google Scholar] [CrossRef]
- Alqurashi, H.; Khurshid, Z.; Syed, A.U.Y.; Habib, S.R.; Rokaya, D.; Zafar, M.S. Polyetherketoneketone (PEKK): An emerging biomaterial for oral implants and dental prostheses. J. Adv. Res. 2021, 28, 87–95. [Google Scholar] [CrossRef]
- Buck, E.; Li, H.; Cerruti, M. Surface Modification Strategies to Improve the Osseointegration of Poly(etheretherketone) and Its Composites. Macromol. Biosci. 2020, 20, 1900271. [Google Scholar] [CrossRef] [PubMed]
- Knop, L.; Gandini, L.G.; Shintcovsk, R.L.; Gandini, M.R.E.A.S. Scientific use of the finite element method in Orthodontics. Dent. Press J. Orthod. 2015, 20, 119–125. [Google Scholar] [CrossRef]
- Matsuoka, T.; Nakano, T.; Yamaguchi, S.; Ono, S.; Watanabe, S.; Sato, T.; Yatani, H. Effects of Implant–Abutment Connection Type and Inter-Implant Distance on Inter-Implant Bone Stress and Microgap: Three-Dimensional Finite Element Analysis. Materials 2021, 14, 2421. [Google Scholar] [CrossRef]
- Reddy, M.; Sundram, R.; Eid Abdemagyd, H. Application of finite element model in implant dentistry: A systematic review. J. Pharm. Bioallied Sci. 2019, 11, S85–S91. [Google Scholar] [CrossRef] [PubMed]
- Romanyk, D.L.; Vafaeian, B.; Addison, O.; Adeeb, S. The use of finite element analysis in dentistry and orthodontics: Critical points for model development and interpreting results. Semin. Orthod. 2020, 26, 162–173. [Google Scholar] [CrossRef]
- Menacho-Mendoza, E.; Cedamanos-Cuenca, R.; Díaz-Suyo, A. Stress analysis and factor of safety in three dental implant systems by finite element analysis. Saudi Dent. J. 2022, 34, 579–584. [Google Scholar] [CrossRef]
- Weinstein, A.M.; Klawitter, J.J.; Anand, S.C.; Schuessler, R. Stress Analysis of Porous Rooted Dental Implants. J. Dent. Res. 1976, 55, 772–777. [Google Scholar] [CrossRef]
- Winkelhoff, A.; Cune, M. Zirconia Dental Implants: A Clinical, Radiographic, and Microbiologic Evaluation up to 3 Years. Int. J. Oral Maxillofac. Implant. 2014, 29, 914–920. [Google Scholar] [CrossRef]
- Gowda, E.M.; Iyer, S.R.; Verma, K.; Murali Mohan, S. Evaluation of PEEK composite dental implants: A comparison of two different loading protocols. J. Dent. Res. Rep. 2018, 1. [Google Scholar] [CrossRef]
- Çehreli, M.C.; Akça, K.; İplikçioğlu, H. Force transmission of one- And two-piece morse-taper oral implants: A nonlinear finite element analysis. Clin. Oral Implant. Res. 2004, 15, 481–489. [Google Scholar] [CrossRef]
- Misch, C.E.; Qu, Z.; Bidez, M.W. Mechanical properties of trabecular bone in the human mandible: Implications for dental implant treatment planning and surgical placement. J. Oral Maxillofac. Surg. 1999, 57, 700–706. [Google Scholar] [CrossRef]
- Schwitalla, A.D.; Abou-Emara, M.; Spintig, T.; Lackmann, J.; Müller, W. Finite element analysis of the biomechanical effects of PEEK dental implants on the peri-implant bone. J. Biomech. 2015, 48, 1–7. [Google Scholar] [CrossRef]
- Sarot, J.R.; Contar, C.M.M.; Da Cruz, A.C.C.; De Souza Magini, R. Evaluation of the stress distribution in CFR-PEEK dental implants by the three-dimensional finite element method. J. Mater. Sci. Mater. Med. 2010, 21, 2079–2085. [Google Scholar] [CrossRef]
- Harinee, A.; Rajesh, C.; Anilkumar, S.; Indu, R.; Raghavan, S.M. Comparison of Mechanical Properties of the Implant Materials- Titanium, Zirconium and Peek Using Three Dimensional Finite Element Analysis. Int. J. Adv. Res. 2023, 11, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.D.; Rust-Dawicki, A.M. Preliminary evaluation of titanium-coated PEEK dental implants. J. Oral Implantol. 1995, 21, 176–181. [Google Scholar] [PubMed]
- Koch, F.P.; Weng, D.; Krämer, S.; Biesterfeld, S.; Jahn-Eimermacher, A.; Wagner, W. Osseointegration of one-Piece zirconia implants compared with a titanium implant of identical design: A histomorphometric study in the dog. Clin. Oral Implant. Res. 2010, 21, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Majhi, R.; Patro, T.K.; Dhal, A.; Kumar, S.; Guha, P.; Goswami, L.; Goswami, C.; Majhi, R.K.; Garhnayak, L. Evaluation of Osteogenic Potential of a Polysaccharide-Based Hydrogel Coating on Titanium. Cureus 2024, 16, e57785. [Google Scholar] [CrossRef]
- Negi, S. Surfactants as antimicrobial nanocoatings for medical devices and implants. In Next-Generation Antimicrobial Nanocoatings for Medical Devices and Implants; Woodhead Publishing: Cambridge, UK, 2024; pp. 181–204. [Google Scholar] [CrossRef]
- Nedela, O.; Slepicka, P.; Švorcík, V. Surface modification of polymer substrates for biomedical applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- El-Anwar, M.I.; El-Zawahry, M.M.; Ibraheem, E.M.; Nassani, M.Z.; ElGabry, H. New dental implant selection criterion based on implant design. Eur. J. Dent. 2017, 11, 186–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hussein, M.O.; Alruthea, M.S. Evaluation of Bone–Implant Interface Stress and Strain Using Heterogeneous Mandibular Bone Properties Based on Different Empirical Correlations. Eur. J. Dent. 2021, 15, 454–462. [Google Scholar] [CrossRef] [PubMed]



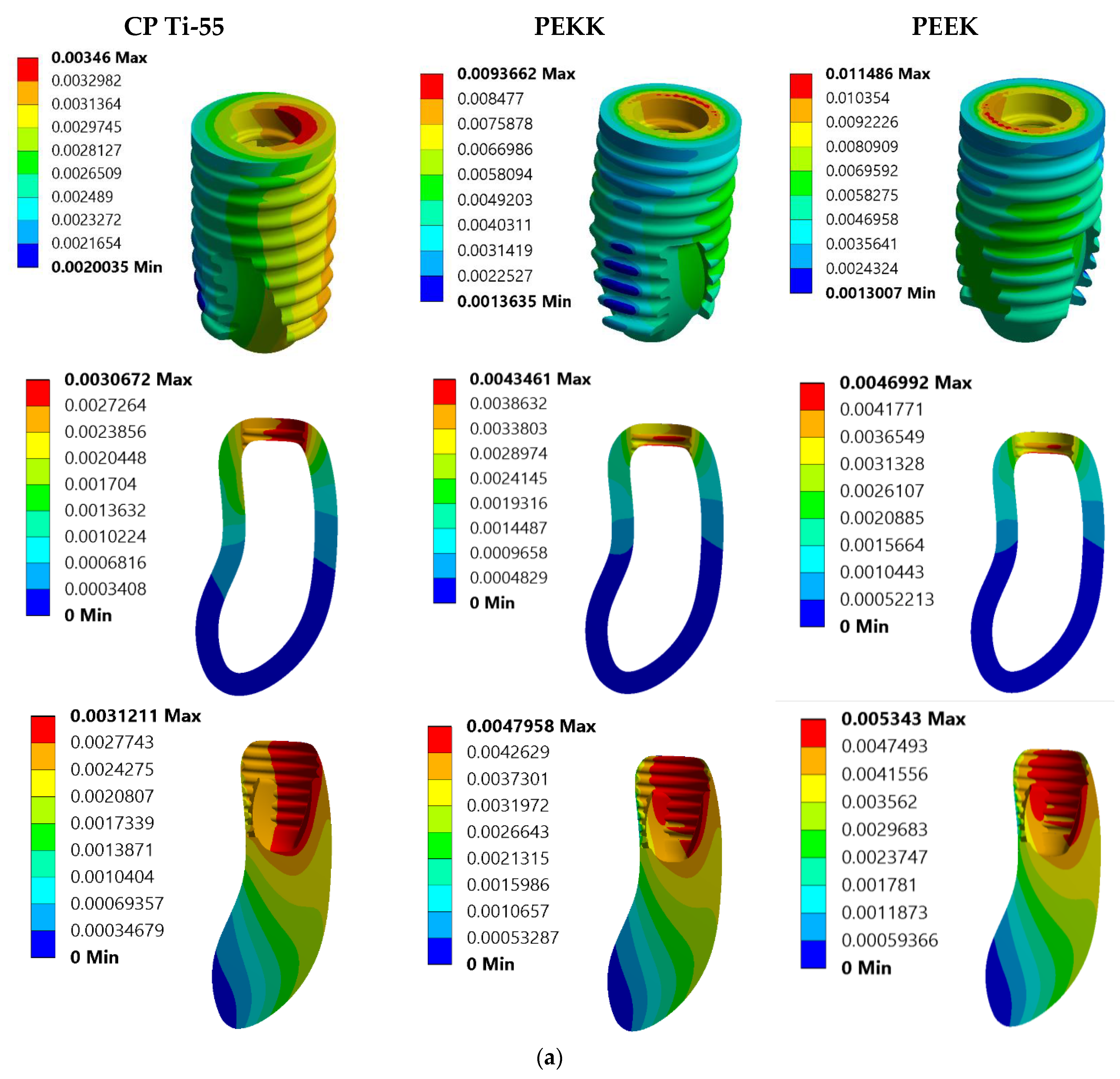
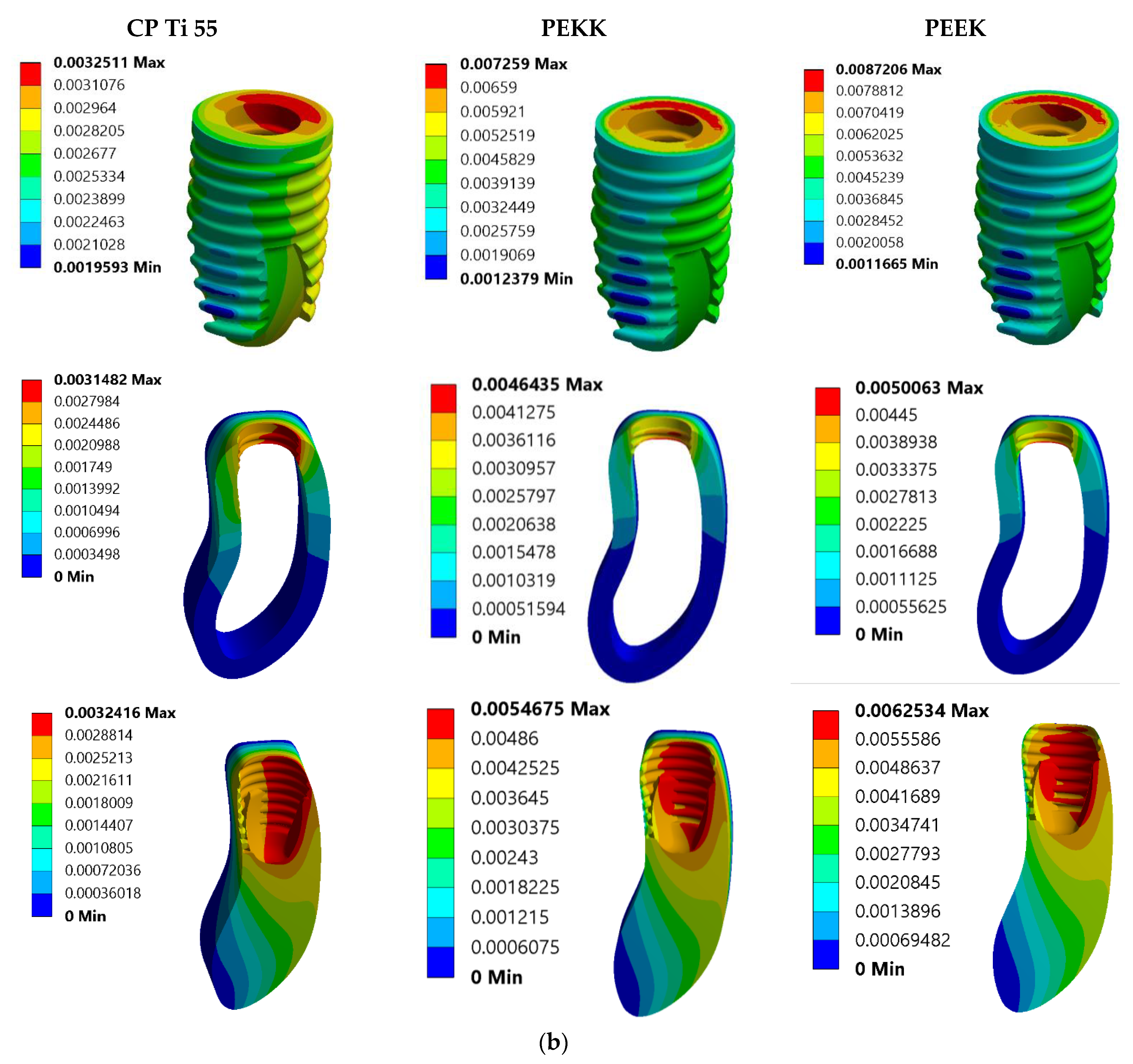
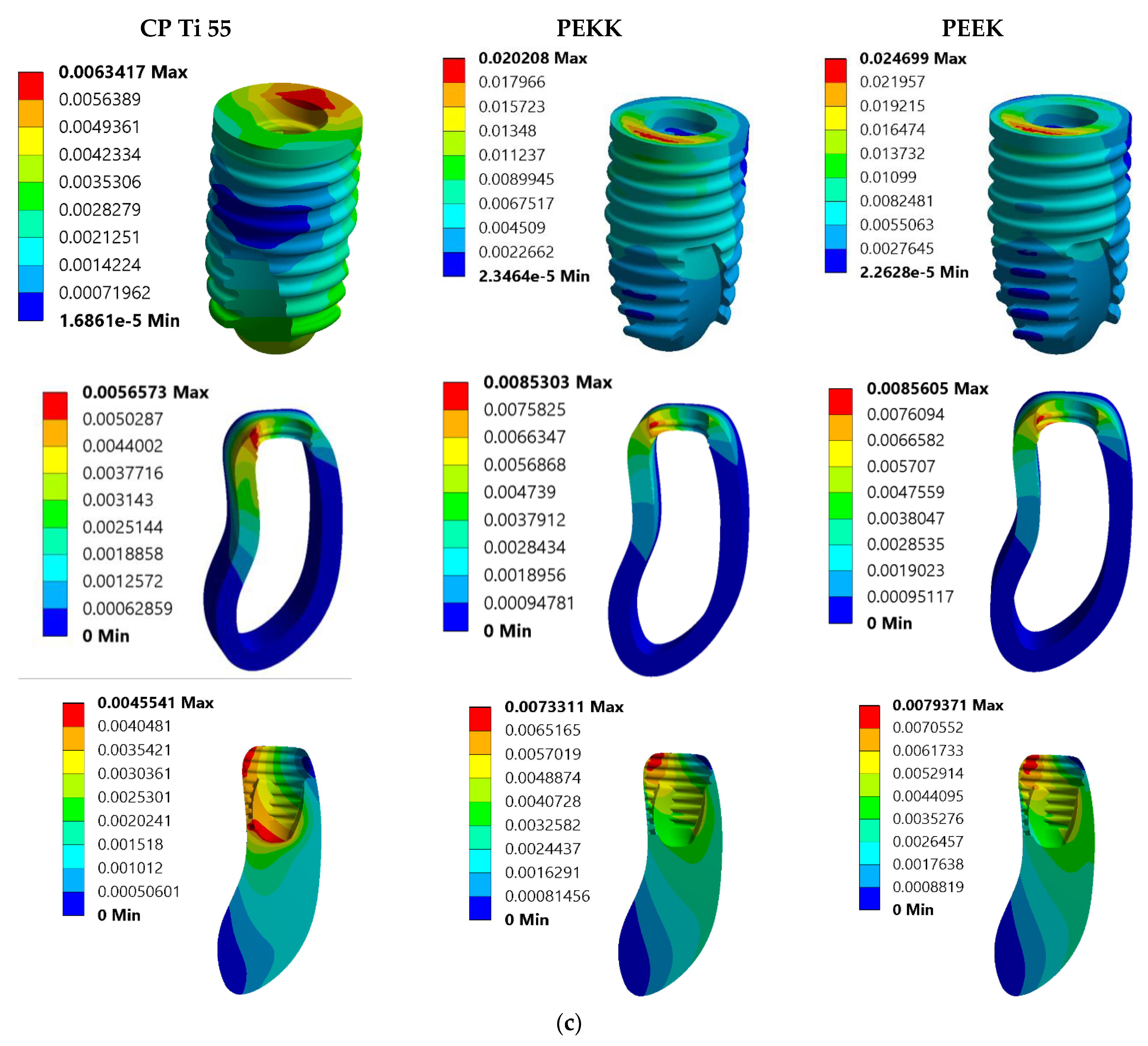
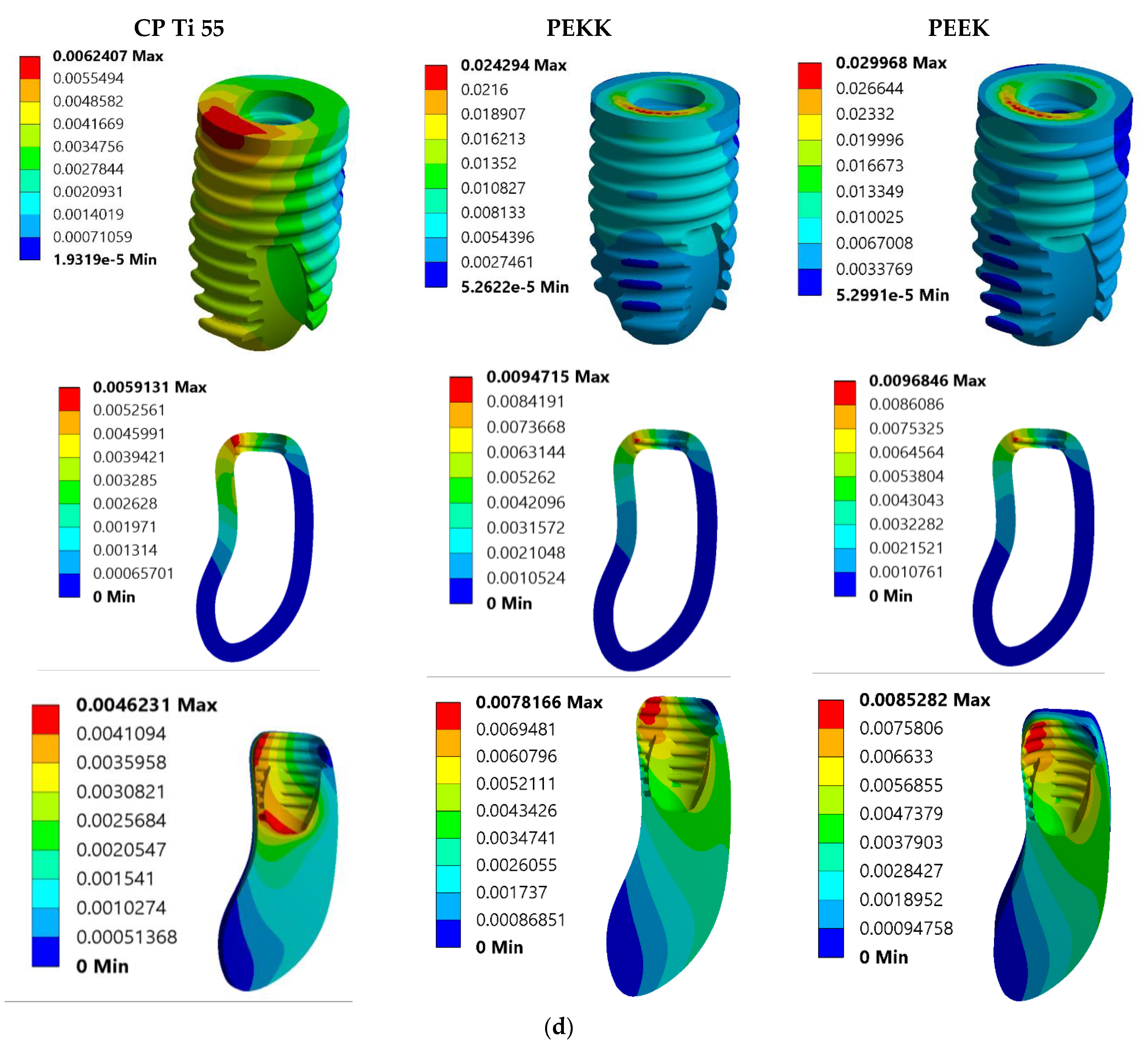
| Model No. | Model Set | Load Condition |
|---|---|---|
| 1. | CP Ti-55, implant with 3.8 mm abutment | 250 N Vertical |
| 2. | CP Ti-55, implant with 3.8 mm abutment | 250 N Oblique |
| 3. | CP Ti-55, implant with 4.5 mm abutment | 250 N Vertical |
| 4. | CP Ti-55, implant with 4.5 mm abutment | 250 N Oblique |
| 5. | PEEK implant with 3.8 mm abutment | 250 N Vertical |
| 6. | PEEK implant with 3.8 mm abutment | 250 N Oblique |
| 7. | PEEK implant with 4.5 mm abutment | 250 N Vertical |
| 8. | PEEK implant with 4.5 mm abutment | 250 N Oblique |
| 9. | PEKK implant with 3.8 mm abutment | 250 N Vertical |
| 10. | PEKK implant with 3.8 mm abutment | 250 N Oblique |
| 11. | PEKK implant with 4.5 mm abutment | 250 N Vertical |
| 12. | PEKK implant with 4.5 mm abutment | 250 N Oblique |
| Materials | Components | Density (Kg/m3) | Elasticity (MPa) | Rigidity (MPa) | Poisson’s Ratio |
|---|---|---|---|---|---|
| CP Ti-55 | Implant | 4500 | 110,000 | - | 0.37 |
| PEEK | Implant | 1.3 | 3700 | - | 0.4 |
| PEKK | Implant | 1.3 | 5100 | - | 0.4 |
| Ti6AL4V | Abutment | 4428.5 | 105,000 | - | 0.32 |
| Screw | |||||
| Central Bone | Cancellous | E1 = 210 | G12 = 68 | P12 = 0.06 | |
| E2 = 1148 | G13 = 68 | P21 = 0.11 | |||
| E3 = 1148 | G23 = 434 | P13 = 0.06 | |||
| - | - | P31 = 0.09 | |||
| - | - | P23 = 0.32 | |||
| - | - | P32 = 0.33 | |||
| Surrounding Bone | Cortical | E1 = 12,700 | G12 = 5000 | P12 = 0.18 | |
| E2 = 17,900 | G13 = 5500 | P21 = 0.35 | |||
| E3 = 22,800 | G23 = 7400 | P13 = 0.3 | |||
| P31 = 0.5 | |||||
| P23 = 0.28 | |||||
| P32 = 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afazal, M.; Afreen, S.; Anand, V.; Chanda, A. Computational Mechanics of Polymeric Materials PEEK and PEKK Compared to Ti Implants for Marginal Bone Loss Around Oral Implants. Prosthesis 2025, 7, 93. https://doi.org/10.3390/prosthesis7040093
Afazal M, Afreen S, Anand V, Chanda A. Computational Mechanics of Polymeric Materials PEEK and PEKK Compared to Ti Implants for Marginal Bone Loss Around Oral Implants. Prosthesis. 2025; 7(4):93. https://doi.org/10.3390/prosthesis7040093
Chicago/Turabian StyleAfazal, Mohammad, Saba Afreen, Vaibhav Anand, and Arnab Chanda. 2025. "Computational Mechanics of Polymeric Materials PEEK and PEKK Compared to Ti Implants for Marginal Bone Loss Around Oral Implants" Prosthesis 7, no. 4: 93. https://doi.org/10.3390/prosthesis7040093
APA StyleAfazal, M., Afreen, S., Anand, V., & Chanda, A. (2025). Computational Mechanics of Polymeric Materials PEEK and PEKK Compared to Ti Implants for Marginal Bone Loss Around Oral Implants. Prosthesis, 7(4), 93. https://doi.org/10.3390/prosthesis7040093







