Infective Endocarditis After Transcatheter Aortic Valve Replacement: A Narrative Review
Abstract
1. Introduction
2. IE in the 21st Century: An Ongoing Evolutionary Challenge
The Clinical Problem: Insight into Epidemiology, Pathophysiology, Clinical Future, and Microbiological Characteristics
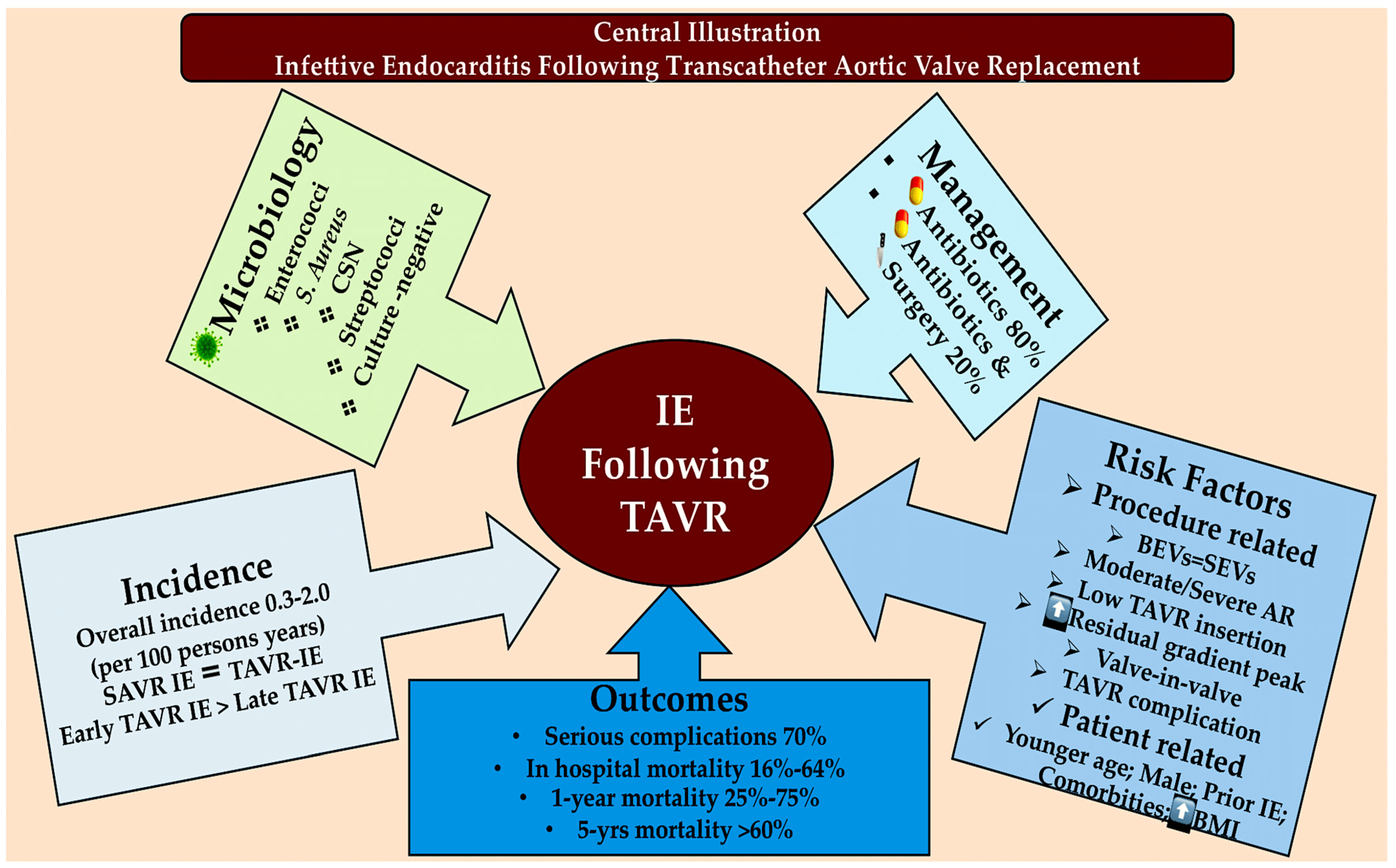
3. New Challenges in the Field of IE: The Management of IE Following TAVR
3.1. Causative Pathogens in the TAVR-IE Trials
3.2. Medical Management
3.3. Surgery on Bioprosthetic Valves for PVE in Patients with TAVR
4. Discussion
4.1. Risk Factors
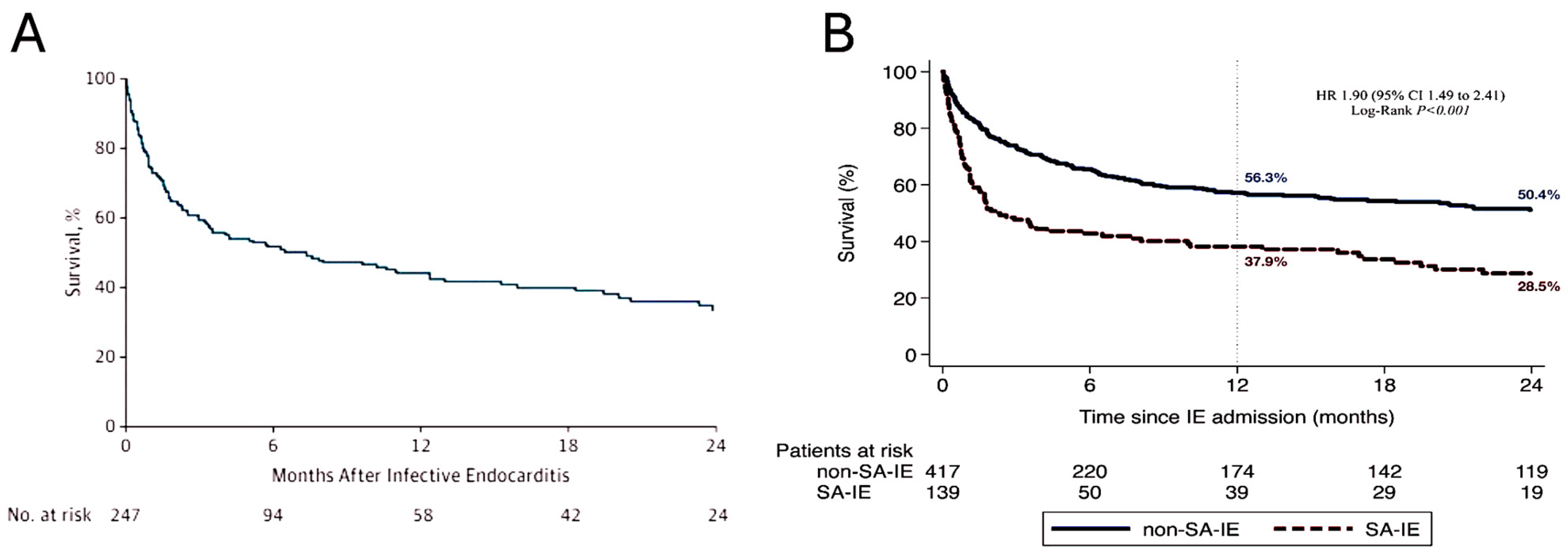
4.2. Outcomes
4.3. Infective Endocarditis Caused by Staphylococcus Aureus After Transcatheter Aortic Valve Replacement: A Nightmare That Will Not End
4.4. Impact of Imaging Techniques
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nappi, F.; Spadaccio, C.; Mihos, C. Infective endocarditis in the 21st century. Ann. Transl. Med. 2020, 8, 1620. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)—Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [PubMed]
- Fowler, V.G.; Durack, D.T.; Selton-Suty, C.; Athan, E.; Bayer, A.S.; Chamis, A.L.; Dahl, A.; Di Bernardo, L.; Durante-Mangoni, E.; Duval, X.; et al. The 2023 Duke-International Society for Cardiovascular Infectious Diseases Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin. Infect. Dis. 2023, 77, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Martuscelli, G.; Bellomo, F.; Avtaar Singh, S.S.; Moon, M.R. Infective Endocarditis in High-Income Countries. Metabolites 2022, 12, 682. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation foraortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef]
- Aung, T.; Poon, K.; Horvath, R.; Coulter, C.; Walters, D.L. A case series of medically managed infective endocarditis after transcatheter aortic valve replacement. Scand. J. Infect. Dis. 2013, 45, 489–493. [Google Scholar] [CrossRef]
- Latib, A.; Naim, C.; De Bonis, M.; Sinning, J.M.; Maisano, F.; Barbanti, M.; Parolari, A.; Lorusso, R.; Testa, L.; Actis Dato, G.M.; et al. TAVR-associated prosthetic valve infective endocarditis: Results of a large, multicenter registry. J. Am. Coll. Cardiol. 2014, 64, 2176–2178. [Google Scholar] [CrossRef] [PubMed]
- Olsen, N.T.; De Backer, O.; Thyregod, H.G.; Vejlstrup, N.; Bundgaard, H.; Søndergaard, L.; Ihlemann, N. Prosthetic valve endocarditis after transcatheter aortic valve implantation. Circ. Cardiovasc. Interv. 2015, 8, e001939. [Google Scholar] [CrossRef] [PubMed]
- Puls, M.; Eiffert, H.; Hünlich, M.; Schöndube, F.; Hasenfuß, G.; Seipelt, R.; Schillinger, W. Prosthetic valve endocarditis after transcatheter aortic valve implantation: The incidence in a single-centre cohort and reflections on clinical, echocardiographic and prognostic features. EuroIntervention 2013, 8, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Amat-Santos, I.J.; Messika-Zeitoun, D.; Eltchaninoff, H.; Kapadia, S.; Lerakis, S.; Cheema, A.N.; Gutiérrez-Ibanes, E.; Munoz-Garcia, A.J.; Pan, M.; Webb, J.G.; et al. Infective endocarditis after transcatheter aortic valve implantation: Results from a large multicenter registry. Circulation 2015, 131, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.B.; Swinski, L.A.; Waternaux, C.M.; Karchmer, A.W.; Buckley, M.J. Risk factors for the development of prosthetic valve endocarditis. Circulation 1985, 72, 31–37. [Google Scholar] [CrossRef]
- Agnihotri, A.K.; McGiffin, D.C.; Galbraith, A.J.; O’Brien, M.F. The prevalence of infective endocarditis after aortic valve replacement. J. Thorac. Cardiovasc. Surg. 1995, 110, 1708–1720. [Google Scholar] [CrossRef]
- Amat-Santos, I.J.; Ribeiro, H.B.; Urena, M.; Allende, R.; Houde, C.; Bédard, E.; Perron, J.; DeLarochellière, R.; Paradis, J.M.; Dumont, E.; et al. Prosthetic valve endocarditis after transcatheter valve replacement: A systematic review. JACC Cardiovasc. Interv. 2015, 8, 334–346. [Google Scholar] [CrossRef]
- Braghieri, L.; Kaur, S.; Black, C.K.; Cremer, P.C.; Unai, S.; Kapadia, S.R.; Mentias, A. Endocarditis after Transcatheter Aortic Valve Replacement. J. Clin. Med. 2023, 12, 7042. [Google Scholar] [CrossRef]
- Martínez-Sellés, M.; Bouza, E.; Díez-Villanueva, P.; Valerio, M.; Fariñas, M.C.; Muñoz-García, A.J.; Ruiz-Morales, J.; Gálvez-Acebal, J.; Antorrena, I.; de la Hera Galarza, J.M.; et al. Incidence and clinical impact of infective endocarditis after transcatheter aortic valve implantation. EuroIntervention 2016, 11, 1180–1187. [Google Scholar] [CrossRef]
- Selton-Suty, C.; Célard, M.; Le Moing, V.; Doco-Lecompte, T.; Chirouze, C.; Iung, B.; Strady, C.; Revest, M.; Vandenesch, F.; Bouvet, A.; et al. Preeminence of Staphylococcus aureus in infective endocarditis: A 1-year population-based survey. Clin. Infect. Dis. 2012, 54, 1230–1239. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.; Shalaby, A.; Saba, S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J. Am. Coll. Cardiol. 2006, 48, 590–591. [Google Scholar] [CrossRef] [PubMed]
- Mangner, N.; Woitek, F.; Haussig, S.; Schlotter, F.; Stachel, G.; Höllriegel, R.; Wilde, J.; Lindner, A.; Holzhey, D.; Leontyev, S.; et al. Incidence, predictors, and outcome of patients developing infective endocarditis following transfemoral transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2016, 67, 2907–2908. [Google Scholar] [CrossRef] [PubMed]
- Van Dijck, I.; Budts, W.; Cools, B.; Eyskens, B.; Boshoff, D.E.; Heying, R.; Frerich, S.; Vanagt, W.Y.; Troost, E.; Gewillig, M. Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart 2015, 101, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, J.T.; Van Wijk, W.; Thompson, J.; Vandenbroucke, J.P.; Valkenburg, H.A.; Michel, M.F. Efficacy of antibiotic prophylaxis for pre- vention of native-valve endocarditis. Lancet 1992, 339, 135–139. [Google Scholar] [CrossRef]
- van der Meer, J.M.; Thompson, J.; Valkenburg, H.A.; Michel, M.F. Epidemiology of bacterial endocarditis in the Netherlands. II. antecedent procedures and use of prophylaxis. Arch. Intern. Med. 1992, 152, 1869–1873. [Google Scholar] [CrossRef]
- Strom, B.L.; Abrutyn, E.; Berlin, J.A.; Kinman, J.L.; Feldman, R.S.; Stolley, P.D.; Levison, M.E.; Korzeniowski, O.M.; Kaye, D. Dental and cardiac risk factors for infective endocarditis: A population-based, case-control study. Ann. Intern. Med. 1998, 129, 761–769. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Brennan, M.T.; Sasser, H.C.; Fox, P.C.; Paster, B.J.; Bahrani-Mougeot, F.K. Bacteremia associated with toothbrushing and dental extraction. Circulation 2008, 117, 3118–3125. [Google Scholar] [CrossRef]
- Habib, G.; Badano, L.; Tribouilloy, C.; Prendergast, B.; Vilacosta, I.; Moreillon, P.; de Jesus Antunes, M.; Thilen, U.; Lekakis, J.; Lengyel, M.; et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and by the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur. Heart J. 2009, 30, 2369–2413. [Google Scholar]
- Fagman, E.; Perrotta, S.; Bech-Hanssen, O.; Flinck, A.; Lamm, C.; Olaison, L.; Svensson, G. ECG-gated computed tomography: A new role for patients with suspected aortic prosthetic valve endocarditis. Eur. Radiol. 2012, 22, 2407–2414. [Google Scholar] [CrossRef]
- Saby, L.; Laas, O.; Habib, G.; Cammilleri, S.; Mancini, J.; Tessonnier, L.; Casalta, J.P.; Gouriet, F.; Riberi, A.; Avierinos, J.F.; et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: Increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J. Am. Coll. Cardiol. 2013, 61, 2374–2382. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, M.N.; Roque, A.; Fernández-Hidalgo, N.; Cuéllar-Calabria, H.; Ferreira-González, I.; Gonzàlez-Alujas, M.T.; Oristrell, G.; Gracia-Sánchez, L.; González, J.J.; Rodríguez-Palomares, J.; et al. Improving the diagnosis of infective endocarditis in prosthetic valves and intracardiac devices with 18F-fluorodeoxyglucose positron emission tomography/computed tomography: Initial results at an infective endocarditis referral center. Circulation 2015, 132, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Erba, P.A.; Conti, U.; Lazzeri, E.; Doria, R.; De Tommasi, S.M.; Bandera, F.; Tascini, C.; Menichetti, F.; Dierckx, R.A.; Signore, A.; et al. Added value of 99mTc-HMPAO-labeled leukocyte SPECT/CT in the characterization and management of patients with infectious endocarditis. J. Nucl. Med. 2012, 53, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Rouzet, F.; Chequer, R.; Benali, K.; Lepage, L.; Ghodbane, W.; Duval, X.; Iung, B.; Vahanian, A.; Le Guludec, D.; Hyafil, F. Respective performance of 18F-FDG PET and radiolabeled leukocyte scintigraphy for the diagnosis of prosthetic valve endocarditis. J. Nucl. Med. 2014, 55, 1980–1985. [Google Scholar] [CrossRef]
- Duval, X.; Iung, B.; Klein, I.; Brochet, E.; Thabut, G.; Arnoult, F.; Lepage, L.; Laissy, J.P.; Wolff, M.; Leport, C.; et al. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: A prospective study. Ann. Intern. Med. 2010, 152, 497–504. [Google Scholar] [CrossRef]
- Meshaal, M.S.; Kassem, H.H.; Samir, A.; Zakaria, A.; Baghdady, Y.; Rizk, H.H. Impact of routine cerebral CT angiography on treatment decisions in infective endocarditis. PLoS ONE 2015, 10, e0118616. [Google Scholar] [CrossRef][Green Version]
- Iung, B.; Klein, I.; Mourvillier, B.; Olivot, J.M.; Détaint, D.; Longuet, P.; Ruimy, R.; Fourchy, D.; Laurichesse, J.J.; Laissy, J.P.; et al. Respective effects of early cerebral and abdominal magnetic resonance imaging on clinical decisions in infective endocarditis. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 703–710. [Google Scholar] [CrossRef]
- Barsic, B.; Dickerman, S.; Krajinovic, V.; Pappas, P.; Altclas, J.; Carosi, G.; Casabé, J.H.; Chu, V.H.; Delahaye, F.; Edathodu, J.; et al. Influence of the timing of cardiac surgery on the outcome of patients with infective endocarditis and stroke. Clin. Infect. Dis. 2013, 56, 209–217. [Google Scholar] [CrossRef]
- Hasbun, R.; Vikram, H.R.; Barakat, L.A.; Buenconsejo, J.; Quagliarello, V.J. Complicated left-sided native valve endocarditis in adults: Risk classification for mortality. JAMA 2003, 289, 1933–1940. [Google Scholar] [CrossRef]
- Vilacosta, I.; Graupner, C.; San Román, J.A.; Sarriá, C.; Ronderos, R.; Fernández, C.; Mancini, L.; Sanz, O.; Sanmartín, J.V.; Stoermann, W. Risk of embolization after institution of antibiotic therapy for infective endocarditis. J. Am. Coll. Cardiol. 2002, 39, 1489–1495. [Google Scholar] [CrossRef]
- Thuny, F.; Di Salvo, G.; Belliard, O.; Avierinos, J.F.; Pergola, V.; Rosenberg, V.; Casalta, J.P.; Gouvernet, J.; Derumeaux, G.; Iarussi, D.; et al. Risk of embolism and death in infective endocarditis: Prognostic value of echocardiography: A prospective multicenter study. Circulation 2005, 112, 69–75. [Google Scholar] [CrossRef] [PubMed]
- García-Cabrera, E.; Fernández-Hidalgo, N.; Almirante, B.; Ivanova-Georgieva, R.; Noureddine, M.; Plata, A.; Lomas, J.M.; Gálvez-Acebal, J.; Hidalgo-Tenorio, C.; Ruíz-Morales, J.; et al. Neurological complications of infective endocarditis: Risk factors, outcome, and impact of cardiac surgery: A multicenter observational study. Circulation 2013, 127, 2272–2284. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.; Shalaby, A.; Saba, S. Continued rise in rates of cardiovascular implantable electronic de- vice infections in the United States: Temporal trends and causative insights. Pacing Clin. Electrophysiol. 2010, 33, 414–419. [Google Scholar] [CrossRef]
- Johansen, J.B.; Jørgensen, O.D.; Møller, M.; Arnsbo, P.; Mortensen, P.T.; Nielsen, J.C. Infection after pacemaker implantation: Infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur. Heart J. 2011, 32, 991–998. [Google Scholar] [CrossRef]
- Landolina, M.; Gasparini, M.; Lunati, M.; Iacopino, S.; Boriani, G.; Bonanno, C.; Vado, A.; Proclemer, A.; Capucci, A.; Zucchiatti, C.; et al. Long-term complications related to biventricular defibrillator implantation: Rate of surgical revisions and impact on survival: Insights from the Italian Clinical Service Database. Circulation 2011, 123, 2526–2535. [Google Scholar] [CrossRef]
- Sohail, M.R.; Henrikson, C.A.; Braid-Forbes, M.J.; Forbes, K.F.; Lerner, D.J. Mortality and cost associated with cardio- vascular implantable electronic device infections. Arch. Intern. Med. 2011, 171, 1821–1828. [Google Scholar] [CrossRef]
- Polyzos, K.A.; Konstantelias, A.A.; Falagas, M.E. Risk factors for cardiac implantable electronic device infection: A systematic review and meta-analysis. Europace 2015, 17, 767–777. [Google Scholar] [CrossRef]
- Cahill, T.J.; Prendergast, B.D. Infective endocarditis. Lancet 2016, 387, 882–893. [Google Scholar] [CrossRef]
- Cuervo, G.; Quintana, E.; Regueiro, A.; Perissinotti, A.; Vidal, B.; Miro, J.M.; Baddour, L.M. The Clinical Challenge of Prosthetic Valve Endocarditis: JACC Focus Seminar 3/4. J. Am. Coll. Cardiol. 2024, 83, 1418–1430. [Google Scholar] [CrossRef]
- Ambrosioni, J.; Hernandez-Meneses, M.; Téllez, A.; Pericàs, J.; Falces, C.; Tolosana, J.M.; Vidal, B.; Almela, M.; Quintana, E.; Llopis, J.; et al. The changing epidemiology of infective endocarditis in the twenty-first century. Curr. Infect. Dis. Rep. 2017, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Martínez, A.; Domínguez, F.; Muñoz, P.; Marín, M.; Pedraz, Á.; Fariñas, M.C.; Tascón, V.; de Alarcón, A.; Rodríguez-García, R.; Miró, J.M.; et al. Clinical presentation, microbiology, and prognostic factors of prosthetic valve endocarditis. Lessons learned from a large prospective registry. PLoS ONE 2023, 18, e0290998. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, A.; Linke, A.; Latib, A.; Ihlemann, N.; Urena, M.; Walther, T.; Husser, O.; Herrmann, H.C.; Nombela-Franco, L.; Cheema, A.N.; et al. Association between transcatheter aortic valve replacement and subsequent infective endocarditis and in-hospital death. JAMA 2016, 316, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Del Val, D.; Abdel-Wahab, M.; Mangner, N.; Durand, E.; Ihlemann, N.; Urena, M.; Pellegrini, C.; Giannini, F.; Gasior, T.; Wojakowski, W.; et al. Infective Endocarditis Caused by Staphylococcus aureus After Transcatheter Aortic Valve Replacement. Can. J. Cardiol. 2022, 38, 102–112. [Google Scholar] [CrossRef]
- Del Val, D.; Panagides, V.; Mestres, C.A.; Miró, J.M.; Rodés-Cabau, J. Infective endocarditis after transcatheter aortic valve replacement: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2023, 81, 394–412. [Google Scholar] [CrossRef]
- Hidalgo-Tenorio, C.; Sadyrbaeva-Dolgova, S.; Enríquez-Gómez, A.; Muñoz, P.; Plata-Ciezar, A.; Miró, J.M.; Alarcón, A.; Martínez-Marcos, F.J.; Loeches, B.; Escrihuela-Vidal, F.; et al. EN-DALBACEN 2.0 Cohort: Real-life study of dalbavancin as sequential/consolidation therapy in patients with infective endocarditis due to Gram-positive cocci. Int. J. Antimicrob. Agents 2023, 62, 106918. [Google Scholar] [CrossRef]
- Siciliano, R.F.; Randi, B.A.; Gualandro, D.M.; Sampaio, R.O.; Bittencourt, M.S.; da Silva Pelaes, C.E.; Mansur, A.J.; Pomerantzeff, P.M.A.; Tarasoutchi, F.; Strabelli, T.M.V. Early-onset prosthetic valve endocarditis definition revisited: Prospective study and literature review. Int. J. Infect. Dis. 2018, 67, 3–6. [Google Scholar] [CrossRef]
- Van Valen, R.; De Lind Van Wijngaarden, R.A.F.; Verkaik, N.J.; Mokhles, M.M.; Bogers, A.J.J.C. Prosthetic valve endocarditis due to Propionibacterium acnes. Interact. Cardiovasc. Thorac. Surg. 2016, 23, 150–155. [Google Scholar] [CrossRef]
- Panagides, V.; Abdel-Wahab, M.; Mangner, N.; Durand, E.; Ihlemann, N.; Urena, M.; Pellegrini, C.; Giannini, F.; Scislo, P.; Huczek, Z.; et al. Very early infective endocarditis after transcatheter aortic valve replacement. Clin. Res. Cardiol. 2022, 111, 1087–1097. [Google Scholar] [CrossRef]
- Widmer, D.; Widmer, A.F.; Jeger, R.; Dangel, M.; Stortecky, S.; Frei, R.; Conen, A. Prevalence of enterococcal groin colonization in patients undergoing cardiac interventions: Challenging antimicrobial prophylaxis with cephalosporins in patients undergoing transcatheter aortic valve replacement. J. Hosp. Infect. 2022, 129, 198–202. [Google Scholar] [CrossRef]
- Nappi, F. Current Knowledge of Enterococcal Endocarditis: A Disease Lurking in Plain Sight of Health Providers. Pathogens 2024, 13, 235. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Avtaar Singh, S.S.; Jitendra, V.; Fiore, A. Bridging Molecular and Clinical Sciences to Achieve the Best Treatment of Enterococcus faecalis Endocarditis. Microorganisms 2023, 11, 2604. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F. Native Infective Endocarditis: A State-of-the-Art-Review. Microorganisms 2024, 12, 1481. [Google Scholar] [CrossRef] [PubMed]
- Novick, R.P. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 2003, 48, 1429–1449. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.W. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 2000, 13, 470–511. [Google Scholar] [CrossRef]
- Pillar, C.M.; Gilmore, M.S. Enterococcal virulence—Pathogenicity island of E. faecalis. Front. Biosci. 2004, 9, 2335–2346. [Google Scholar] [CrossRef]
- Gilmore, M.S. The Enterococci: Pathogenesis, Molecular Biology, and Antibiotic Resistance; ASM Press: Washington, DC, USA, 2020; 439p. [Google Scholar]
- Sillanpaa, J.; Xu, Y.; Nallapareddy, S.R.; Murray, B.E.; Hook, M. A family of putative MSCRAMMs from Enterococcus faecalis. Microbiology 2004, 150, 2069–2078. [Google Scholar] [CrossRef]
- Paulsen, I.T.; Banerjei, L.; Myers, G.S.; Nelson, K.E.; Seshadri, R.; Read, T.D.; Fouts, D.E.; Eisen, J.A.; Gill, S.R.; Heidelberg, J.F.; et al. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 2003, 299, 2071–2074. [Google Scholar] [CrossRef]
- Chirouze, C.; Alla, F.; Fowler, V.G.; Sexton, D.J.; Corey, G.R.; Chu, V.H.; Wang, A.; Erpelding, M.L.; Durante-Mangoni, E.; Fernández-Hidalgo, N.; et al. Impact of early valve surgery on outcome of Staphylococcus aureus prosthetic valve infective endocarditis: Analysis in the international collaboration of endocarditis-prospective cohort study. Clin. Infect. Dis. 2015, 60, 741–749. [Google Scholar] [CrossRef]
- Lalani, T.; Chu, V.H.; Park, L.P.; Cecchi, E.; Corey, G.R.; Durante-Mangoni, E.; Fowler, V.G., Jr.; Gordon, D.; Grossi, P.; Hannan, M.; et al. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern. Med. 2013, 173, 1495–1504. [Google Scholar] [CrossRef]
- Pericà, S.J.M.; Llopis, J.; González-Ramallo, V.; Goenaga, M.Á.; Muñoz, P.; García-Leoni, M.E.; Fariñas, M.C.; Pajarón, M.; Ambrosioni, J.; Luque, R.; et al. Outpatient parenteral antibiotic treatment for infective endocarditis: A prospective cohort study from the GAMES cohort. Clin. Infect. Dis. 2019, 69, 1690–1700. [Google Scholar] [CrossRef] [PubMed]
- Iversen, K.; Ihlemann, N.; Gill, S.U.; Madsen, T.; Elming, H.; Jensen, K.T.; Bruun, N.E.; Høfsten, D.E.; Fursted, K.; Christensen, J.J.; et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N. Engl. J. Med. 2019, 380, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, G.B.; Hussain, S.T. Current AATS guidelines on surgical treatment of infective endocarditis. Ann. Cardiothorac. Surg. 2019, 8, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Mangner, N.; del Val, D.; Abdel-Wahab, M.; Crusius, L.; Durand, E.; Ihlemann, N.; Urena, M.; Pellegrini, C.; Giannini, F.; Gasior, T.; et al. Surgical treatment of patients with infective endocarditis after transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 2022, 79, 772–785. [Google Scholar] [CrossRef]
- Santos-Martínez, S.; Alkhodair, A.; Nombela-Franco, L.; Saia, F.; Muñoz-García, A.J.; Gutiérrez, E.; Regueiro, A.; Jimenez-Diaz, V.A.; Rivero, F.; Romaguera, R.; et al. Transcatheter aortic valve replacement for residual lesion of the aortic valve following “healed” infective endocarditis. J. Am. Coll. Cardiol. Interv. 2020, 13, 1983–1996. [Google Scholar] [CrossRef]
- Khan, M.H.; Biondi, N.L.; Zulfiqar, S.; Arif, I.; Das, M.; Budhiraja, M.; Aronow, W.S.; Sukhija, R.; Vimal, M. Challenging case of transcatheter mitral valve-in-valve-in-valve replacement. Am. J. Case Rep. 2023, 24, e938415. [Google Scholar] [CrossRef]
- Brankovic, M.; Hashemi, A.; Ansari, J.; Sharma, A. Transcatheter aortic valve replacement for aortic valve infective endocarditis: A systematic review and call for action. Cardiol. Ther. 2023, 12, 297–306. [Google Scholar] [CrossRef]
- O’Connor, S.A.; Morice, M.C.; Gilard, M.; Leon, M.B.; Webb, J.G.; Dvir, D.; Rodés-Cabau, J.; Tamburino, C.; Capodanno, D.; D’Ascenzo, F.; et al. Revisiting sex equality with transcatheter aortic valve replacement outcomes: A collaborative, patient-level meta-analysis of 11 310 patients. J. Am. Coll. Cardiol. 2015, 66, 221–228. [Google Scholar] [CrossRef]
- Movahed, M.R.; Hashemzadeh, M.; Jamal, M.M. Increased prevalence of infectious endocarditis in patients with type II diabetes mellitus. J. Diabetes Complicat. 2007, 21, 403–406. [Google Scholar] [CrossRef]
- Jerez-Valero, M.; Urena, M.; Webb, J.G.; Tamburino, C.; Munoz-Garcia, A.J.; Cheema, A.; Dager, A.E.; Serra, V.; Amat-Santos, I.J.; Barbanti, M.; et al. Clinical impact of aortic regurgitation after transcatheter aortic valve replacement: Insights into the degree and acuteness of presentation. JACC Cardiovasc. Interv. 2014, 7, 1022–1032. [Google Scholar] [CrossRef]
- Rodbard, S. Blood velocity and endocarditis. Circulation 1963, 27, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Bettmann, M.A.; Bolger, A.F.; Epstein, A.E.; Ferrieri, P.; Gerber, M.A.; Gewitz, M.H.; Jacobs, A.K.; Levison, M.E.; Newburger, J.W.; et al. Nonvalvular cardiovascular device-related infections. Circulation 2003, 108, 2015–2031. [Google Scholar] [CrossRef] [PubMed]
- Athappan, G.; Patvardhan, E.; Tuzcu, E.M.; Svensson, L.G.; Lemos, P.A.; Fraccaro, C.; Tarantini, G.; Sinning, J.M.; Nickenig, G.; Capodanno, D.; et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: Meta-analysis and systematic review of literature. J. Am. Coll. Cardiol. 2013, 61, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Athan, E.; Pappas, P.A.; Fowler, V.G., Jr.; Olaison, L.; Paré, C.; Almirante, B.; Muñoz, P.; Rizzi, M.; Naber, C.; et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007, 297, 1354–1361. [Google Scholar] [CrossRef]
- Scialla, S.; Martuscelli, G.; Nappi, F.; Singh, S.S.A.; Iervolino, A.; Larobina, D.; Ambrosio, L.; Raucci, M.G. Trends in Managing Cardiac and Orthopaedic Device-Associated Infections by Using Therapeutic Biomaterials. Polymers 2021, 13, 1556. [Google Scholar] [CrossRef]
- Nappi, F.; Iervolino, A.; Singh, S.S.A. The New Challenge for Heart Endocarditis: From Conventional Prosthesis to New Devices and Platforms for the Treatment of Structural Heart Disease. BioMed Res. Int. 2021, 2021, 7302165. [Google Scholar] [CrossRef]
- Leone, S.; Noviello, S.; Esposito, S. Combination antibiotic therapy for the treatment of infective endocarditis due to enterococci. Infection 2016, 44, 273–281. [Google Scholar] [CrossRef]
- Rieg, S.; von Cube, M.; Kaasch, A.J.; Bonaventura, B.; Bothe, W.; Wolkewitz, M.; Peyerl-Hoffmann, G.; Deppe, A.C.; Wahlers, T.; Beyersdorf, F.; et al. Investigating the Impact of Early Valve Surgery on Survival in Staphylococcus aureus Infective Endocarditis Using a Marginal Structural Model Approach: Results of a Large, Prospectively Evaluated Cohort. Clin. Infect. Dis. 2019, 69, 487–494. [Google Scholar] [CrossRef]
- Akowuah, E.F.; Davies, W.; Oliver, S.; Stephens, J.; Riaz, I.; Zadik, P.; Cooper, G. Prosthetic valve endocarditis: Early and late outcome following medical or surgical treatment. Heart 2003, 89, 269–272. [Google Scholar] [CrossRef]
- Reardon, M.J.; Adams, D.H.; Kleiman, N.S.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Lee, J.S.; Hermiller, J.B., Jr.; Chetcuti, S.; et al. 2-Year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2015, 66, 113–121. [Google Scholar] [CrossRef]
- Kodali, S.K.; Williams, M.R.; Smith, C.R.; Svensson, L.G.; Webb, J.G.; Makkar, R.R.; Fontana, G.P.; Dewey, T.M.; Thourani, V.H.; Pichard, A.D.; et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N. Engl. J. Med. 2012, 366, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-H.; Kim, Y.-J.; Kim, S.-H.; Sun, B.J.; Kim, D.H.; Yun, S.C.; Song, J.M.; Choo, S.J.; Chung, C.H.; Song, J.K.; et al. Early surgery versus conventional treatment for infective endocarditis. N. Engl. J. Med. 2012, 366, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Butt, J.H.; Ihlemann, N.; de Backer, O.; Søndergaard, L.; Havers-Borgersen, E.; Gislason, G.H.; Torp-Pedersen, C.; Køber, L.; Fosbøl, E.L. Long-term risk of infective endocarditis after transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2019, 73, 1646–1655. [Google Scholar] [CrossRef]
- Alexis, S.L.; Malik, A.H.; George, I.; Hahn, R.T.; Khalique, O.K.; Seetharam, K.; Bhatt, D.L.; Tang, G.H.L. Infective endocarditis after surgical and transcatheter aortic valve replacement: A state of the art review. J. Am. Heart Assoc. 2020, 9, e017347. [Google Scholar] [CrossRef]
- Lanz, J.; Reardon, M.J.; Pilgrim, T.; Stortecky, S.; Deeb, G.M.; Chetcuti, S.; Yakubov, S.J.; Gleason, T.G.; Huang, J.; Windecker, S. Incidence and outcomes of infective endocarditis after transcatheter or surgical aortic valve replacement. J. Am. Heart Assoc. 2021, 10, e020368. [Google Scholar] [CrossRef]
- Habib, G.; Thuny, F.; Avierinos, J.-F. Prosthetic valve endocarditis: Current approach and therapeutic options. Prog. Cardiovasc. Dis. 2008, 50, 274–281. [Google Scholar] [CrossRef]
- Harding, D.; Cahill, T.J.; Redwood, S.R.; Prendergast, B.D. Infective endocarditis complicating transcatheter aortic valve implantation. Heart 2020, 106, 493–498. [Google Scholar] [CrossRef]
- Mentias, A.; Girotra, S.; Desai, M.Y.; Horwitz, P.A.; Rossen, J.D.; Saad, M.; Panaich, S.; Kapadia, S.; Sarrazin, M.V. Incidence, predictors, and outcomes of endocarditis after transcatheter aortic valve replacement in the United States. JACC Cardiovasc. Interv. 2020, 13, 1973–1982. [Google Scholar] [CrossRef]
- del Val, D.; Abdel-Wahab, M.; Linke, A.; Durand, E.; Ihlemann, N.; Urena, M.; Pellegrini, C.; Giannini, F.; Landt, M.; Auffret, V.; et al. Temporal trends, characteristics, and outcomes of infective endocarditis after transcatheter aortic valve replacement. Clin. Infect. Dis. 2021, 73, e3750–e3758. [Google Scholar] [CrossRef]
- Mousa Basha, M.; Al-Kassou, B.; Gestrich, C.; Weber, M.; Beiert, T.; Bakhtiary, F.; Nickenig, G.; Zimmer, S.; Shamekhi, J. Microbial growth on temporary pacemaker leads post-TAVR: Pathogen spectrum and clinical implications. Clin. Res. Cardiol. 2024. ahead-of-print. [Google Scholar] [CrossRef]
- Henrikson, C.A.; Sohail, M.R.; Acosta, H.; Johnson, E.E.; Rosenthal, L.; Pachulski, R.; Dan, D.; Paladino, W.; Khairallah, F.S.; Gleed, K.; et al. Antibacterial Envelope Is Associated with Low Infection Rates After Implantable Cardioverter-Defibrillator and Cardiac Resynchronization Therapy Device Replacement: Results of the Citadel and Centurion Studies. JACC Clin. Electrophysiol. 2017, 3, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Korantzopoulos, P.; Sideris, S.; Dilaveris, P.; Gatzoulis, K.; Goudevenos, J.A. Infection control in implantation of cardiac implantable electronic devices: Current evidence, controversial points, and unresolved issues. Europace 2016, 18, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Fernández Guerrero, M.L.; González López, J.J.; Goyenechea, A.; Fraile, J.; de Górgolas, M. Endocarditis caused by Staphylococcus aureus: A reappraisal of the epidemiologic, clinical, and pathologic manifestations with analysis of factors determining outcome. Medicine 2009, 88, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Nadji, G.; Remadi, J.P.; Coviaux, F.; Mirode, A.A.; Brahim, A.; Enriquez-Sarano, M.; Tribouilloy, C. Comparison of clinical and morphological characteristics of Staphylococcus aureus endocarditis with endocarditis caused by other pathogens. Heart 2005, 91, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Tornos, P.; Iung, B.; Permanyer-Miralda, G.; Baron, G.; Delahaye, F.; Gohlke-Bärwolf, C.; Butchart, E.G.; Ravaud, P.; Vahanian, A. Infective endocarditis in Europe: Lessons from the Euro Heart Survey. Heart 2005, 91, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, T.; Park, L.; Tribouilloy, C.; Cortes, C.; Casillo, R.; Chu, V.; Delahaye, F.; Durante-Mangoni, E.; Edathodu, J.; Falces, C.; et al. Association between valvular surgery and mortality among patients with infective endocarditis complicated by heart failure. JAMA 2011, 306, 2239–2247. [Google Scholar] [CrossRef]
- Mangner, N.; Leontyev, S.; Woitek, F.J.; Kiefer, P.; Haussig, S.; Binner, C.; Mende, M.; Schlotter, F.; Stachel, G.; Höllriegel, R.; et al. Cardiac surgery compared with antibiotics only in patients developing infective endocarditis after transcatheter aortic valve replacement. J. Am. Heart Assoc. 2018, 7, e010027. [Google Scholar] [CrossRef]
- Horgan, S.J.; Mediratta, A.; Gillam, L.D. Cardiovascular imaging in infective endocarditis. Circ. Cardiovasc. Imaging 2020, 13, 8956. [Google Scholar] [CrossRef]
- Dilsizian, V.; Budde, R.P.J.; Chen, W.; Mankad, S.V.; Lindner, J.R.; Nieman, K. Best practices for imaging cardiac device–related infections and endocarditis: A JACC: Cardiovascular Imaging expert panel statement. J. Am. Coll. Cardiol. Imaging 2022, 15, 891–911. [Google Scholar] [CrossRef]
- Lo Presti, S.; Elajami, T.K.; Zmaili, M.; Reyaldeen, R.; Xu, B. Multimodality imaging in the diagnosis and management of prosthetic valve endocarditis: A contemporary narrative review. World J. Cardiol. 2021, 13, 254–270. [Google Scholar] [CrossRef]
- Montané, B.; Chahine, J.; Fiore, A.; Alzubi, J.; Alnajjar, H.; Mutti, J.; Grimm, R.A.; Griffin, B.P.; Xu, B. Diagnostic performance of contemporary transesophageal echocardiography with modern imaging for infective endocarditis. Cardiovasc. Diagn. Ther. 2023, 13, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.; Pizzi, M.N.; Fernández-Hidalgo, N.; Permanyer, E.; Cuellar-Calabria, H.; Romero-Farina, G.; Ríos, R.; Almirante, B.; Castell-Conesa, J.; Escobar, M.; et al. Morpho-metabolic post-surgical patterns of non-infected prosthetic heart valves by [18vF] FDG PET/CTA: “normality” is a possible diagnosis. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Granados, U.; Fuster, D.; Pericas, J.M.; Llopis, J.L.; Ninot, S.; Quintana, E.; Almela, M.; Paré, C.; Tolosana, J.M.; Falces, C.; et al. Diagnostic accuracy of 18F-FDG PET/CT in infective endocarditis and implantable cardiac electronic device infection: A cross-sectional study. J. Nucl. Med. 2016, 57, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Wahadat, A.R.; Tanis, W.; Swart, L.E.; Scholtens, A.; Krestin, G.P.; van Mieghem, N.M.; Schurink, C.A.; van der Spoel, T.I.; van den Brink, F.S.; Vossenberg, T.; et al. Added value of 18F-FDG-PET/CT and cardiac CTA in suspected transcatheter aortic valve endocarditis. J. Nucl. Cardiol. 2021, 28, 2072–2082. [Google Scholar] [CrossRef]
- Régis, C.; Thy, M.; Mahida, B.; Deconinck, L.; Tubiana, S.; Iung, B.; Duval, X.; Rouzet, F. Absence of infective endocarditis relapse when end-oftreatment fluorodeoxyglucose positron emission tomography/computed tomography is negative. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1480–1488. [Google Scholar] [CrossRef]
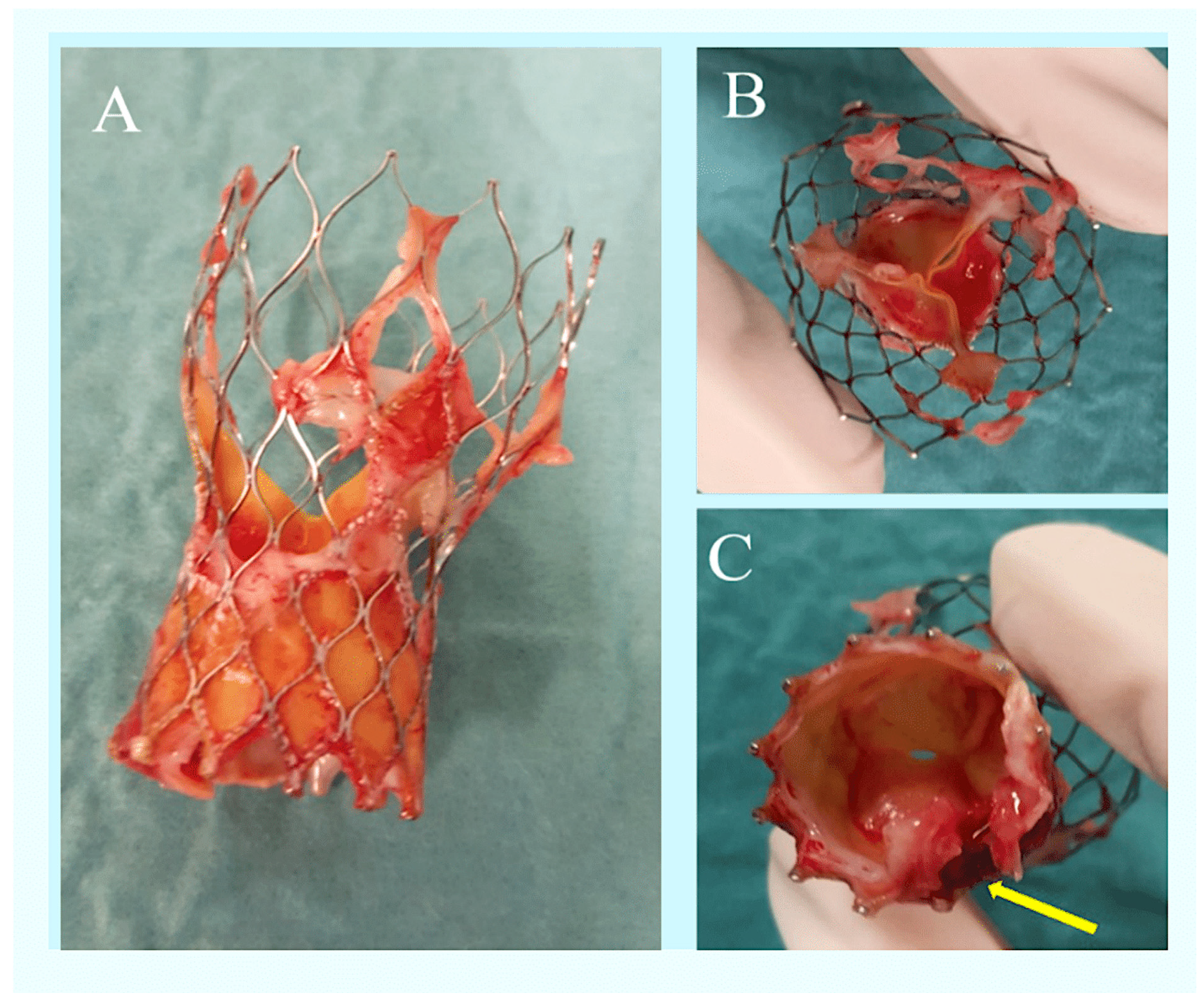

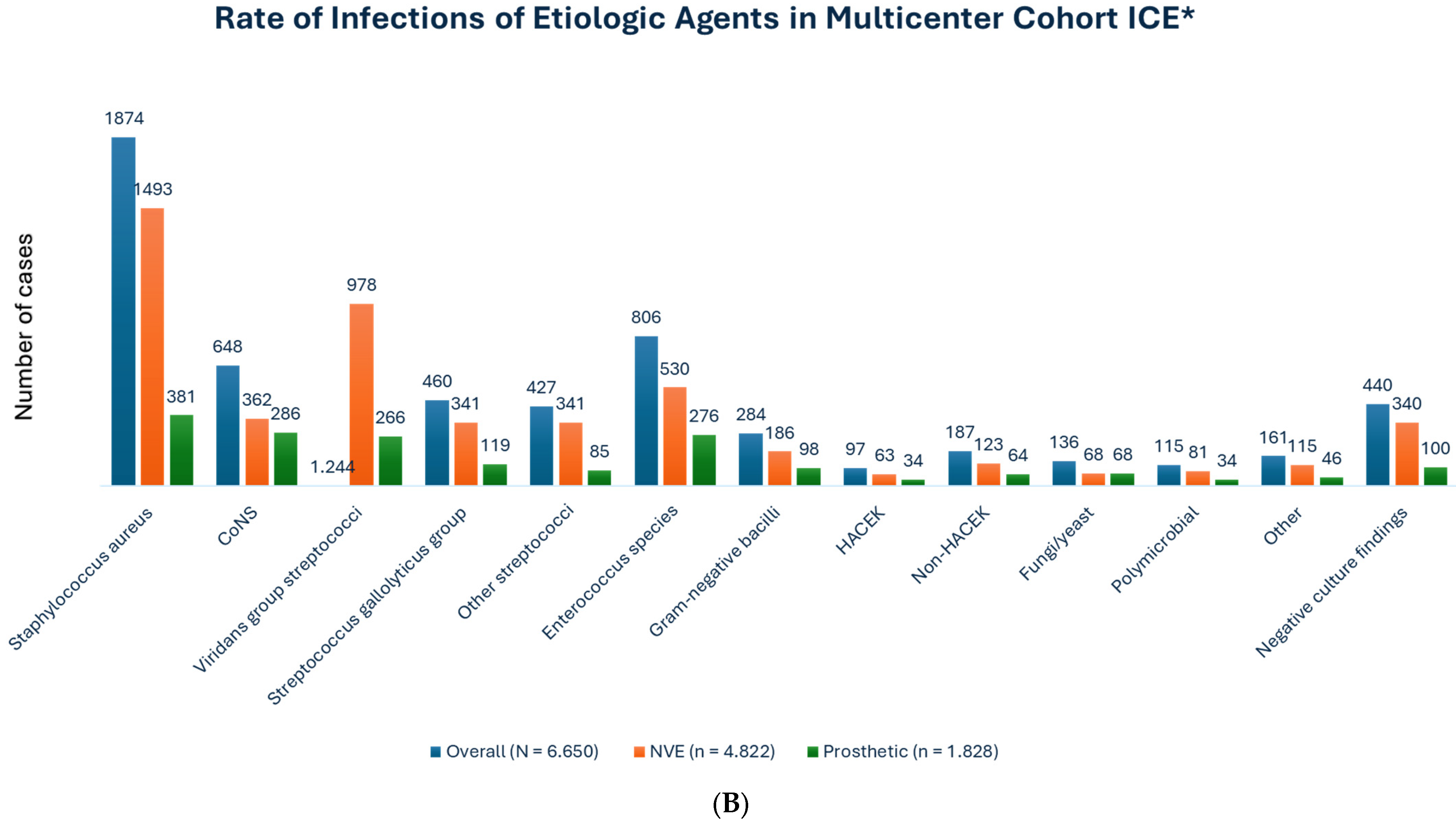
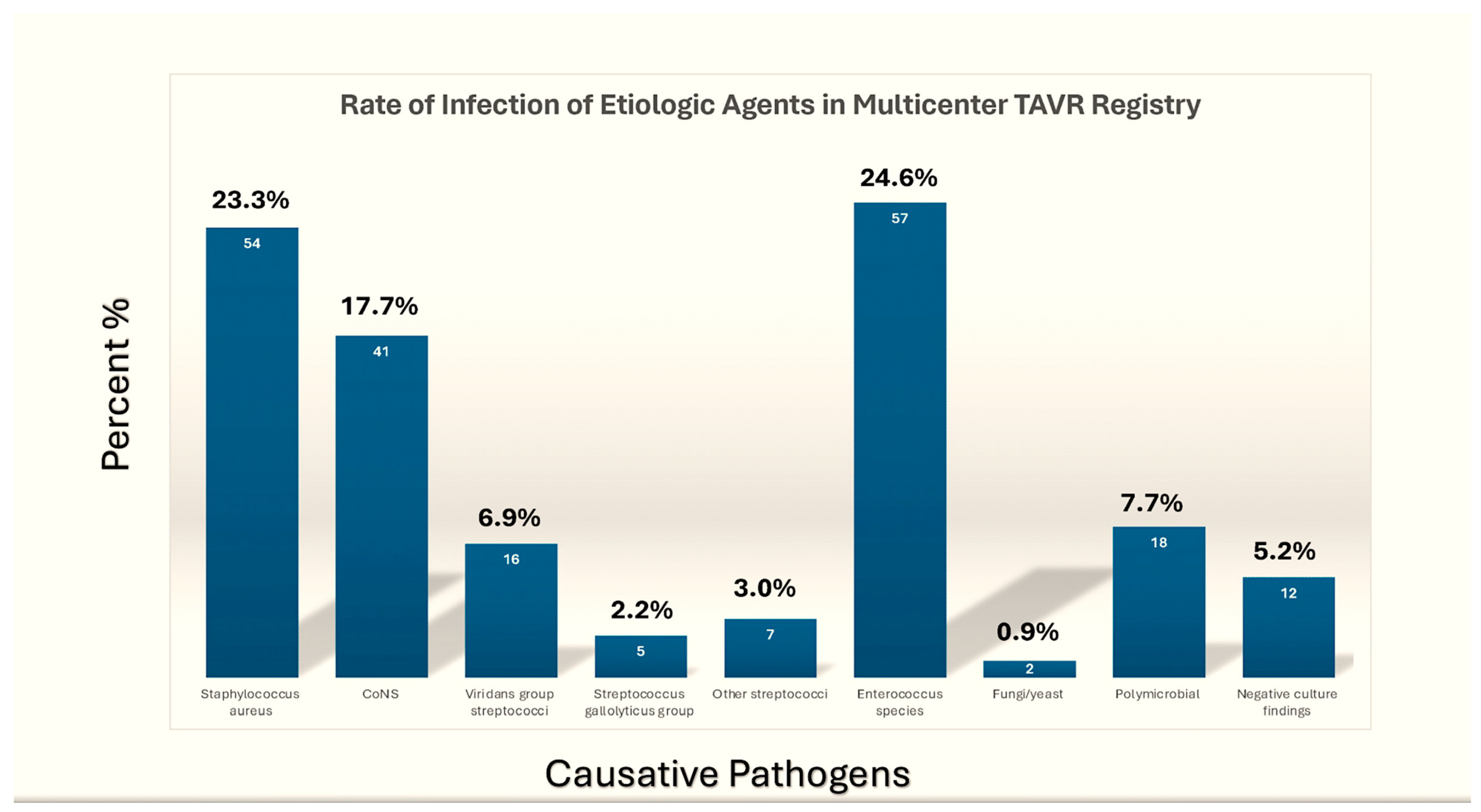
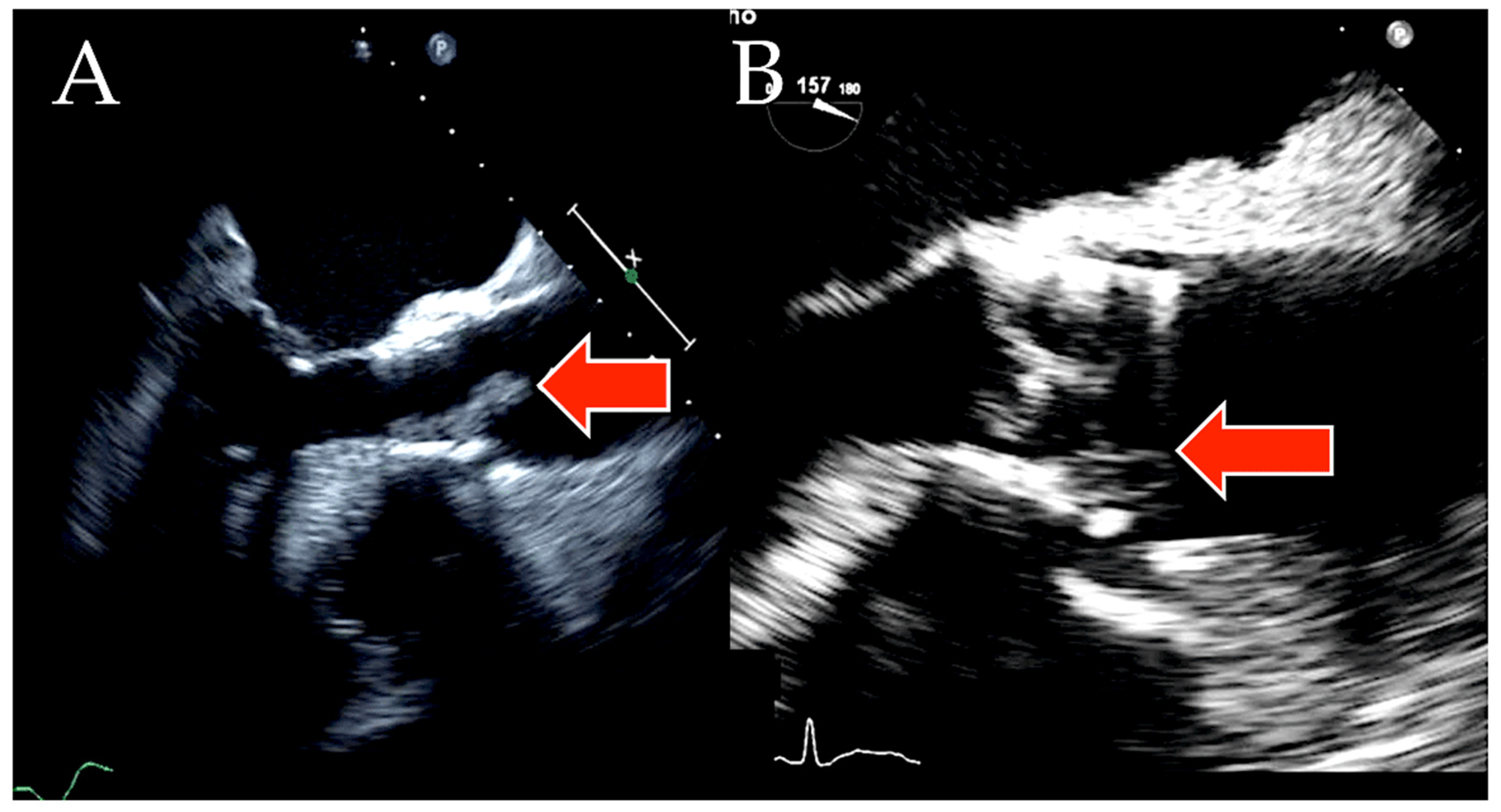
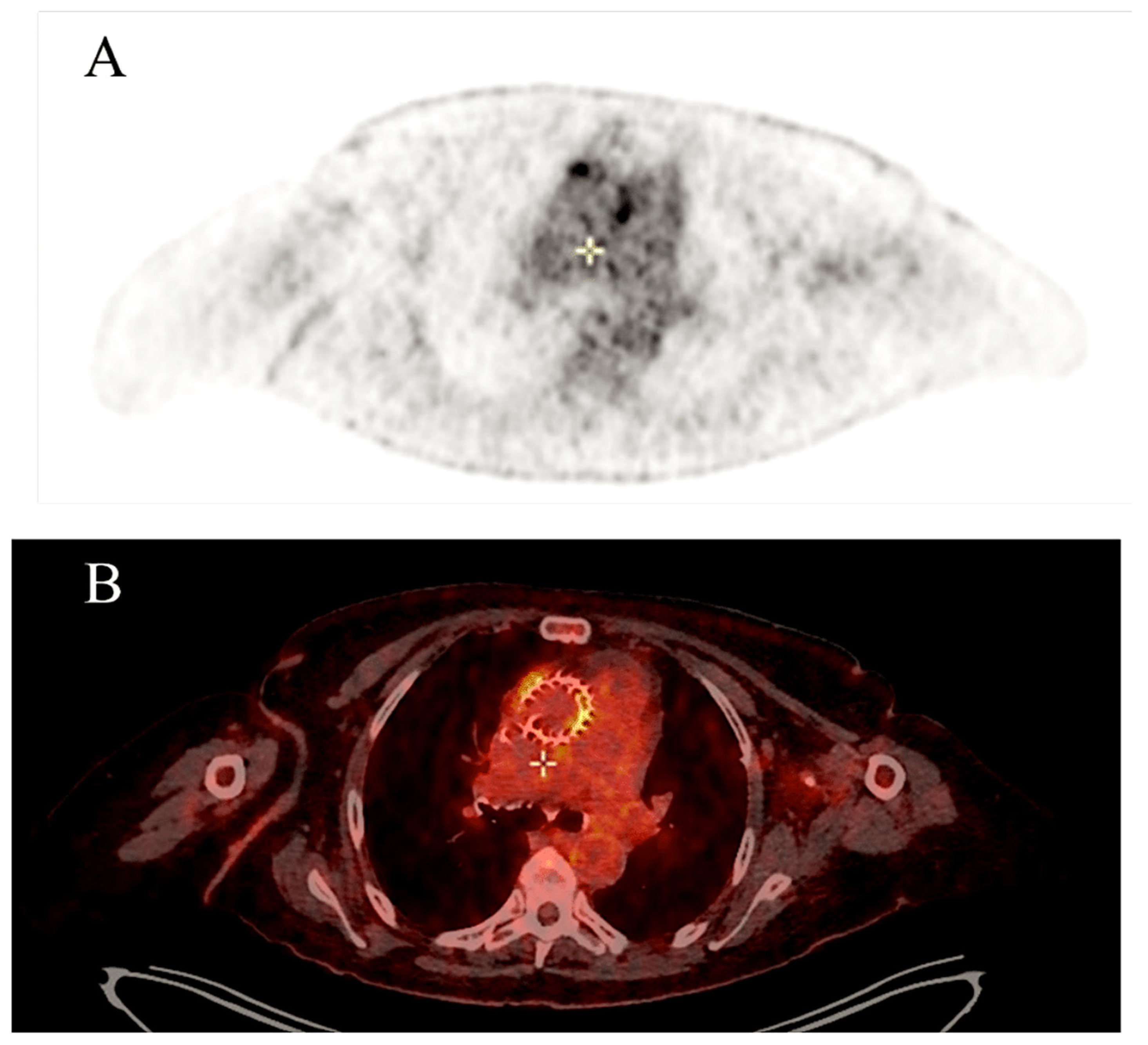
| First Author, Year (Ref. ϕ) | No. of TAVR-IE Patients | Microbiology | 1-Yr Incidence of TAVR-IE | In-Hospital Mortality | 1-Yr Mortality |
|---|---|---|---|---|---|
| PARTNER B, 2010 Leon et al. NEJM [8] | 2 (cohort of 179) | Not indicated | 1.12%γ | Not indicated | 100% |
| PARTNER A, 2011 Smith et al. NEJM [9] | 3 (cohort of 344) | Not indicated | 0.87%γ | Not indicated | 33% |
| Aung et al., 2013 SJID [10] | 4 (cohort of 132) | Enterococci (75%), oral streptococci (25%) | 3.0% | 0% | 0% |
| Latib et al., 2014 JACC [11] | 29 (cohort of 2572) | Enterococci (21%), CoNS (17%), S. aureus (14%), oral streptococci (3.4%) | 0.89%γ | 45% | Not indicated |
| Olsen et al., 2015 CCI 2015 [12] | 18 (cohort of 509) | Enterococci (33%), S. aureus (17%), oral streptococci (17%), CoNS (11%) | 3.1% | 11% | Not indicated |
| Puls et al., 2013 EuroIntervention [13] | 5 (cohort of 180) | Enterococcus (40%), oral streptococci (20%) S. aureus (20%) E. coli (20%) | 2.78% | 40% | 40% |
| Mangner et al., 2016 JACC [23] | 55 (cohort of 1820) | S. aureus (38%), enterococci (31%), CoNS (9.1%), oral streptococci (3.6%) | 2.25%γ | 64% | 75% |
| Amat-Santos et al., 2015 Circulation [14] | 53 (cohort of 7944) | CoNS (24%), Staphylococcus aureus (21%), enterococci (21%), oral streptococci (5.7%) | 0.5% | 47% | 66% |
| Raguiero et al., 2016 JAMA [53] | 250 (cohort of 20,006) | Enterococcus (25%), S. aureus (24%), CoNS (17%) | 1.1% per person/year | 36% | 66.7% (2-yr mortality) |
| del Val et al., 2022 CJC [54] | 604 (cohort of 40,345) | Non-S. aureus (432) S. aureus (141) | Non-S. aureus 6.3 months vs. S. aureus 4.7 months | S. aureus group (47.8% vs. 26.9%) | S. aureus group (71.5% vs. 49.6%) |
| Recommendation | Indications | Applications |
|---|---|---|
| A rapid transfer to OPAT is to be initiated at the 10-day mark following admission. | This indication covers all cases caused by viridans or bovis (gallolyticus) group streptococci or Enterococcus faecalis, provided that the patient is not undergoing cardiac surgery. | The blood cultures taken at 72 h yielded negative results. There were no severe clinical complications, no anticoagulation issues, and a TEE ruled out severe aortic regurgitation and prosthetic dysfunction. |
| The transfer to OPAT is postponed for a minimum of three weeks after admission/surgery. | This indication applies to all cardiac surgery cases that do not fall into any of the following two categories:
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, F. Infective Endocarditis After Transcatheter Aortic Valve Replacement: A Narrative Review. Prosthesis 2024, 6, 1529-1552. https://doi.org/10.3390/prosthesis6060110
Nappi F. Infective Endocarditis After Transcatheter Aortic Valve Replacement: A Narrative Review. Prosthesis. 2024; 6(6):1529-1552. https://doi.org/10.3390/prosthesis6060110
Chicago/Turabian StyleNappi, Francesco. 2024. "Infective Endocarditis After Transcatheter Aortic Valve Replacement: A Narrative Review" Prosthesis 6, no. 6: 1529-1552. https://doi.org/10.3390/prosthesis6060110
APA StyleNappi, F. (2024). Infective Endocarditis After Transcatheter Aortic Valve Replacement: A Narrative Review. Prosthesis, 6(6), 1529-1552. https://doi.org/10.3390/prosthesis6060110






