The Impact of Incorporating Grapefruit Seed Skin Particles into 3D-Printed Acrylic Resin on Mechanical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Powder Preparation
2.2. Preparation of the Samples
2.3. Testing Procedures
2.4. Statistical Analysis
3. Results
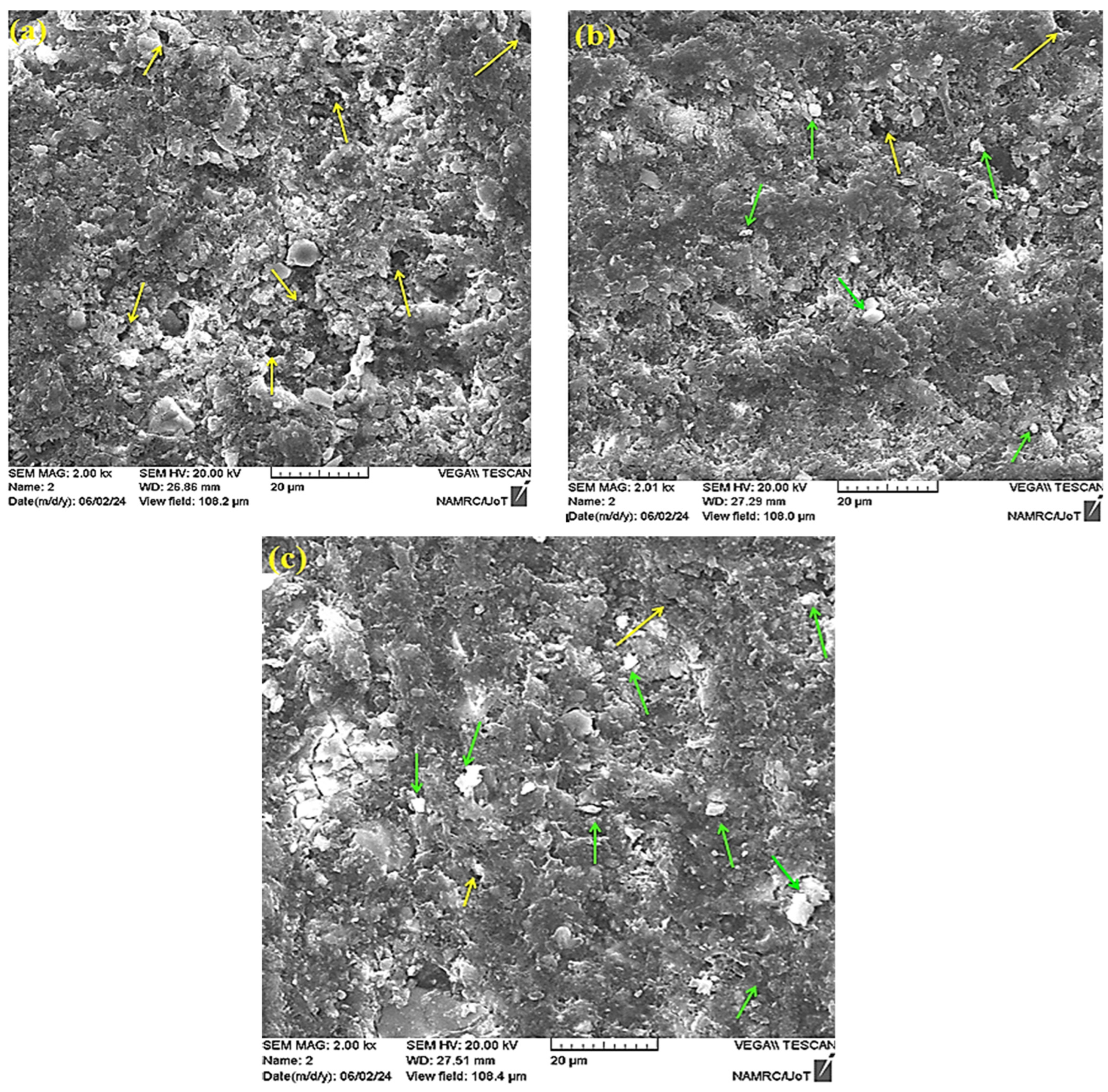
3.1. SEM and Particle Size Analysis
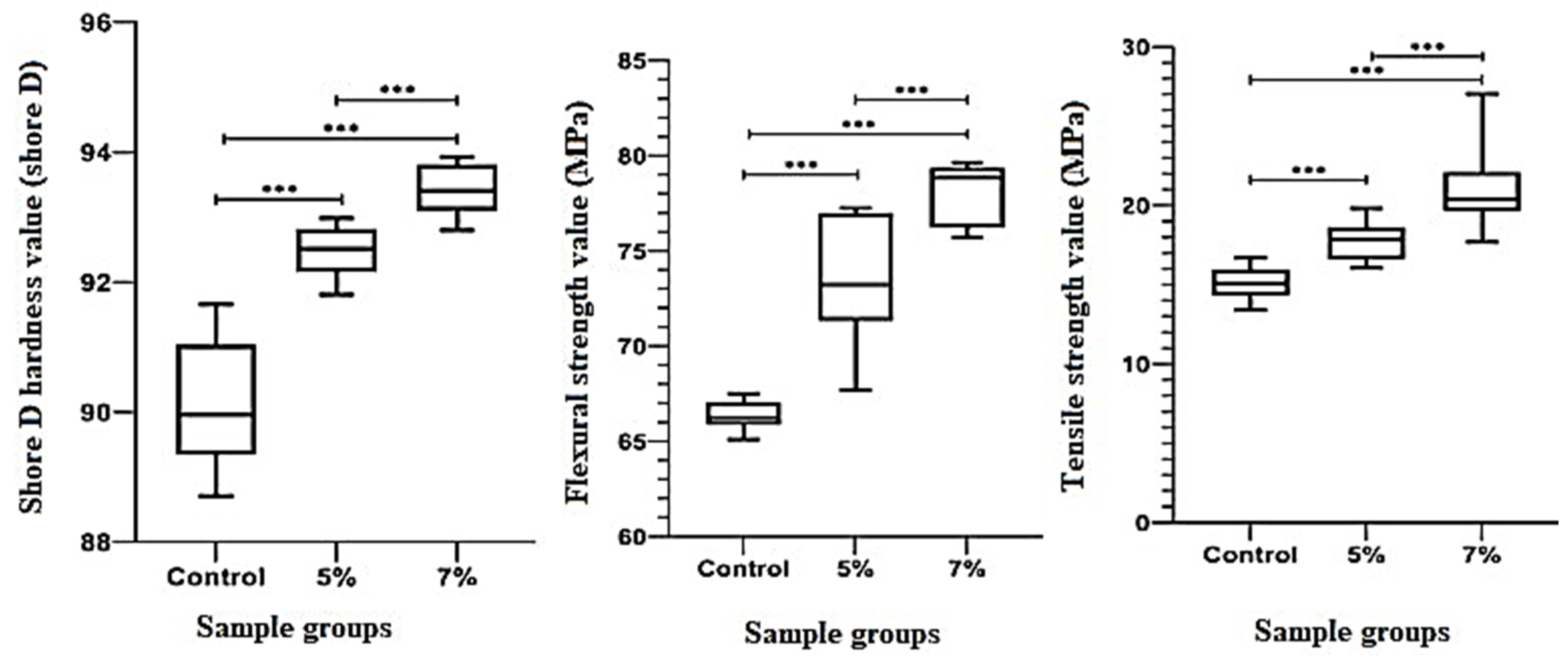
3.2. Hardness, Flexural Strength, and Tensile Strength
3.3. Fracture Modes and Mechanisms
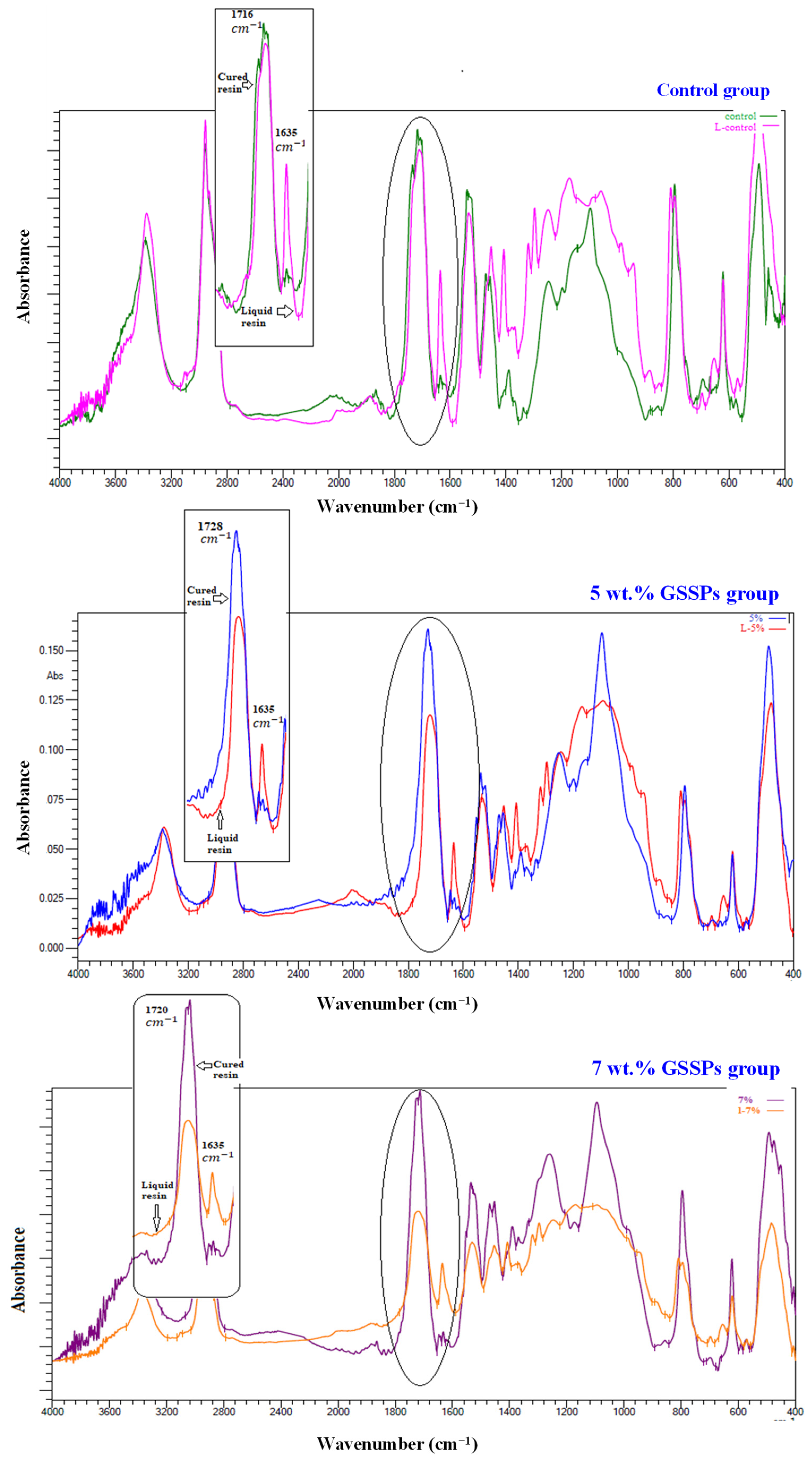
3.4. Fourier Transform Infrared (FTIR) and Degree of Conversion
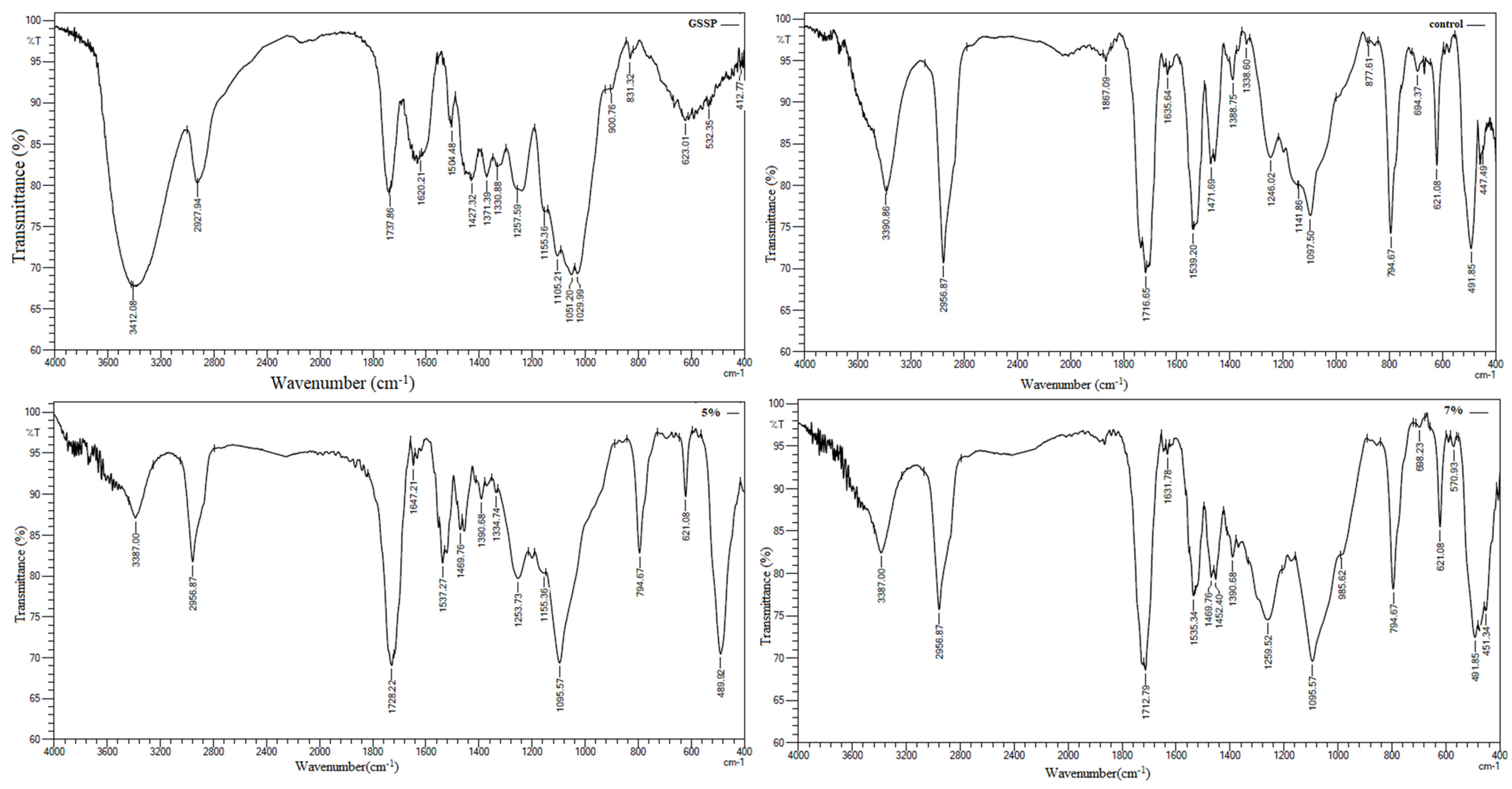
4. Discussion
4.1. 3D Printing
4.2. Mechanical Properties
4.3. Microstructural Characteristics of the Fractured Surface
4.4. Clinical Significance, Limitations, and Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polzer, I.; Schimmel, M.; Müller, F.; Biffar, R. Edentulism as part of the general health problems of elderly adults. Int. Dent. J. 2010, 60, 143–155. [Google Scholar] [PubMed]
- Chhabra, M.; Kumar, M.N.; RaghavendraSwamy, K.N.; Thippeswamy, H.M. Flexural strength and impact strength of heat-cured acrylic and 3D printed denture base resins—A comparative in vitro study. J. Oral Biol. Craniofacial Res. 2022, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent Application No. 638905, 8 August 1984. [Google Scholar]
- Crump, S.S. Apparatus and Method for Creating Three-Dimensional Objects. U.S. Patent Application No. 5,121,3291992, 9 June 1992. [Google Scholar]
- ASTM International. ASTM Committee F42 on Additive Manufacturing Technologies; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Revilla-León, M.; Meyers, M.J.; Zandinejad, A.; Özcan, M. A review on chemical composition, mechanical properties, and manufacturing work flow of additively manufactured current polymers for interim dental restorations. J. Esthet. Restor. Dent. 2019, 31, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Yarborough, A.; Cooper, L.; Duqum, I.; Mendonça, G.; McGraw, K.; Stoner, L. Evidence regarding the treatment of denture stomatitis. J. Prosthodont. 2016, 25, 288–301. [Google Scholar] [CrossRef]
- Nawasrah, A.; AlNimr, A.; Ali, A.A. Antifungal effect of henna against Candida albicans adhered to acrylic resin as a possible method for prevention of denture stomatitis. Int. J. Environ. Res. Public Health 2016, 13, 520. [Google Scholar] [CrossRef]
- Gad, M.; Rahoma, A.; Nawasra, A.; Ammar, M. Influence of henna addition on the flexural strength of acrylic denture base material: An in vitro study. Al-Azhar Dent. J. Girls. 2018, 5, 277–283. [Google Scholar] [CrossRef]
- Ikono, R.; Vibriani, A.; Wibowo, I.; Saputro, K.E.; Muliawan, W.; Bachtiar, B.M.; Mardliyati, E.; Bachtiar, E.W.; Rochman, N.T.; Kagami, H.; et al. Nanochitosan antimicrobial activity against Streptococcus mutans and Candida albicans dual-species biofilms. BMC Res. Notes 2019, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammari, S.S.; Abdul-Ameer, F.M.; Bairam, L.R.; Al-Salihi, Z. The influence of lemongrass essential oil addition into heat cured acrylic resin against Candida albicans adhesion. J. Baghdad Coll. Dent. 2023, 35, 67–75. [Google Scholar] [CrossRef]
- Noori, Z.S.; Al-Khafaji, A.M.; Dabaghi, F. Effect of tea tree oil on candida adherence and surface roughness of heat cure acrylic resin. J. Baghdad Coll. Dent. 2023, 35, 46–54. [Google Scholar] [CrossRef]
- Kang, S.T.; Son, H.K.; Lee, H.J.; Choi, J.S.; Choi, Y.I.; Lee, J.J. Effects of grapefruit seed extract on oxidative stability and quality properties of cured chicken breast. Korean J. Food Sci. Anim. Resour. 2017, 37, 429. [Google Scholar] [CrossRef] [PubMed]
- Bahrin, L.G.; Apostu, M.O.; Birsa, L.M.; Stefan, M. The antibacterial properties of sulfur containing flavonoids. Bioorg Med. Chem. Lett. 2014, 24, 2315–2318. [Google Scholar] [CrossRef]
- Bartoloni, J.A.; Murchison, D.F.; Wofford, D.T.; Sarkar, N.K. Degree of conversion in denture base materials for varied polymerization techniques 1. J. Oral Rehabil. 2000, 27, 488–493. [Google Scholar] [CrossRef]
- Farooq Anwar, F.A.; Rehana Naseer, R.N.; Bhanger, M.I.; Samia Ashraf, S.A.; Talpur, F.N.; Aladedunye, F.A. Physico-chemical characteristics of citrus seeds and seed oils from Pakistan. J. Am. Oil Chem. Soc. 2008, 85, 321–330. [Google Scholar] [CrossRef]
- Dunlap, R.A.; Small, D.A.; MacKay, G.R.; O’Brien, J.W.; Dahn, J.R.; Cheng, Z.H. Materials preparation by ball milling. Can. J. Phys. 2000, 78, 211–229. [Google Scholar] [CrossRef]
- Al-Sammraaie, M.F.; Fatalla, A.A.; Atarchi, Z.R. Assessment of the correlation between the tensile and diametrical compression strengths of 3D-printed denture base resin reinforced with ZrO2 nanoparticles. J. Baghdad Coll. Dent. 2024, 36, 44–53. [Google Scholar] [CrossRef]
- Mudhaffer, S.; Althagafi, R.; Haider, J.; Satterthwaite, J.; Silikas, N. Effects of printing orientation and artificial ageing on martens hardness and indentation modulus of 3D printed restorative resin materials. Dent. Mater. 2024, 40, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Fareed Al-Sammraaie, M.; Fatalla, A.A. The effect of ZrO2 NPs addition on denture adaptation and diametral compressive strength of 3D printed denture base resin. Nanomed. Res. J. 2023, 8, 345–355. [Google Scholar]
- Kanmani, P.; Dhivya, E.; Aravind, J.; Kumaresan, K. Extraction and analysis of pectin from citrus peels: Augmenting the yield from Citrus limon using statistical experimental design. Iran. J. Energy Environ. 2014, 5, 303–312. [Google Scholar] [CrossRef]
- Yong, Q.; Liang, C. Synthesis of an aqueous self-matting acrylic resin with low gloss and high transparency via controlling surface morphology. Polymers 2019, 11, 322. [Google Scholar] [CrossRef]
- Chua, S.C.; Chong, F.K.; Ul Mustafa, M.R.; Mohamed Kutty, S.R.; Sujarwo, W.; Abdul Malek, M.; Show, P.L.; Ho, Y.C. Microwave radiation-induced grafting of 2-methacryloyloxyethyl trimethyl ammonium chloride onto lentil extract (LE-g-DMC) as an emerging high-performance plant-based grafted coagulant. Sci. Rep. 2020, 10, 3959. [Google Scholar] [CrossRef]
- Pandian, S.R.K.; Deepak, V.; Kalishwaralal, K.; Gurunathan, S. Biologically synthesized fluorescent CdS NPs encapsulated by PHB. Enzyme Microb. Technol. 2011, 48, 319–325. [Google Scholar] [CrossRef]
- Sitheeque, M.A.M.; Panagoda, G.J.; Yau, J.; Amarakoon, A.M.T.; Udagama, U.R.N.; Samaranayake, L.P. Antifungal activity of black tea polyphenols (catechins and theaflavins) against Candida species. Chemotherapy 2009, 55, 189–196. [Google Scholar] [CrossRef]
- Gad, M.M.; Al-Harbi, F.A.; Akhtar, S.; Fouda, S.M. 3D-printable denture base resin containing SiO2 nanoparticles: An in vitro analysis of mechanical and surface properties. J. Prosthodont. 2022, 31, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, A.; Jatala, U.W.; Fareed, M.A.; Yazdanie, N. Measurement of residual monomer in autopolymerized acrylic resins by high pressure liquid chromatography. Biomedica 2017, 33, 211. [Google Scholar]
- Kessler, A.; Hickel, R.; Reymus, M. 3D printing in dentistry-state of the art. Oper. Dent. 2020, 45, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Alshamrani, A.A.; Raju, R.; Ellakwa, A. Effect of printing layer thickness and postprinting conditions on the flexural strength and hardness of a 3D-printed resin. Biomed. Res. Int. 2022, 2022, 8353137. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Rawls, H.R.; Esquivel-Upshaw, J.F. Phillips’ Science of Dental Materials E-Book: Phillips’ Science of Dental Materials E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Gondim, B.L.C.; Castellano, L.R.C.; de Castro, R.D.; Machado, G.; Carlo, H.L.; Valença, A.M.G.; de Carvalho, F.G. Effect of chitosan nanoparticles on the inhibition of Candida spp. biofilm on denture base surface. Arch. Oral Biol. 2018, 94, 99–107. [Google Scholar] [CrossRef]
- Chander, N.G.; Venkatraman, J. Mechanical properties and surface roughness of chitosan reinforced heat polymerized denture base resin. J. Prosthodont. Res. 2022, 66, 101–108. [Google Scholar] [CrossRef]
- Alifui-Segbaya, F.; Bowman, J.; White, A.R.; George, R.; Fidan, I. Characterization of the double bond conversion of acrylic resins for 3D printing of dental prostheses. Compend. Contin. Educ. Dent. 2019, 40, 7–11. [Google Scholar]
- Nawasrah, A.; Gad, M.; El Zayat, M. Effect of henna addition on the surface roughness and hardness of polymethylmethacrylate denture base material: An in-vitro study. J. Contemp. Dent. Pr. 2018, 19, 732–738. [Google Scholar] [CrossRef]
- Oleiwi, J.; Hamad, Q.A.; Kadhim, N.N. Study compression, hardness and density properties of PMMA reinforced by natural powder used in denture base applications. Eng. Technol. J. 2019, 37, 522–527. [Google Scholar] [CrossRef]
- AlFuraiji, N.H.; Altaie, S.F.; Qasim, S.S. Evaluating the influence of Ti6Al4V alloy particles on mechanical properties of heat-cured PMMA. J. Baghdad Coll. Dent. 2024, 36, 44–53. [Google Scholar] [CrossRef]
- Hamad, Q.A. Fabrication and Characterization of Denture Base Material by Hybrid Composites from Self Cured PMMA Resin. Ph.D. Thesis, University of Technology, Materials Engineering Department, Hong Kong, China, 2015. [Google Scholar]
- Prpić, V.; Schauperl, Z.; Ćatić, A.; Dulčić, N.; Čimić, S. Comparison of mechanical properties of 3D-printed, CAD/CAM, and conventional denture base materials. J. Prosthodont. 2020, 29, 524–528. [Google Scholar] [CrossRef]
- Tian, K.V.; Nagy, P.M.; Chass, G.A.; Fejerdy, P.; Nicholson, J.W.; Csizmadia, I.G.; Dobó-Nagy, C. Qualitative assessment of microstructure and Hertzian indentation failure in biocompatible glass ionomer cements. J. Mater. Sci. Mater. Med. 2012, 23, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Altarazi, A.; Haider, J.; Alhotan, A.; Silikas, N.; Devlin, H. 3D printed denture base material: The effect of incorporating TiO2 nanoparticles and artificial ageing on the physical and mechanical properties. Dent. Mater. 2023, 39, 1122–1136. [Google Scholar] [CrossRef] [PubMed]
- Lourinho, C.; Salgado, H.; Correia, A.; Fonseca, P. Mechanical properties of polymethyl methacrylate as denture base material: Heat-polymerized vs. 3D-printed—Systematic review and meta-analysis of in vitro studies. Biomedicines 2022, 10, 2565. [Google Scholar] [CrossRef] [PubMed]
- Al-Dwairi, Z.N.; Al Haj Ebrahim, A.A.; Baba, N.Z. A comparison of the surface and mechanical properties of 3D printable denture-base resin material and conventional polymethylmethacrylate (PMMA). J. Prosthodont. 2023, 32, 40–48. [Google Scholar] [CrossRef]
- Diaz-Arnold, A.M.; Vargas, M.A.; Shaull, K.L.; Laffoon, J.E.; Qian, F. Flexural and fatigue strengths of denture base resin. J. Prosthet. Dent. 2008, 100, 47–51. [Google Scholar] [CrossRef]
- Ihab, N.S.; Hassanen, K.A.; Ali, N.A. Assessment of zirconium oxide nano-fillers incorporation and silanation on impact, tensile strength and color alteration of heat polymerized acrylic resin. J. Bagh Coll. Dent. 2012, 24, 36–42. [Google Scholar]
- Fatalla, A.A. The effect of polyester micro-particles powder addition on some mechanical properties of light cured denture base material. J. Int. Dent. Med. Res. 2018, 11, 495–502. [Google Scholar]
- Song, R.; Zhong, Z.; Lin, L. Evaluation of chitosan quaternary ammonium salt-modified resin denture base material. Int. J. Biol. Macromol. 2016, 85, 102–110. [Google Scholar] [CrossRef] [PubMed]




| Groups | 3D-Printed Liquid Resin (g) | Grapefruit Seed Skin Particles (g) |
|---|---|---|
| 0.0 wt.% (Control) | 100.0 | 0.0 |
| 5.0 wt.% | 95.0 | 5.0 |
| 7.0 wt.% | 93.0 | 7.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulaiman, M.M.; Fatalla, A.A.; Haider, J. The Impact of Incorporating Grapefruit Seed Skin Particles into 3D-Printed Acrylic Resin on Mechanical Properties. Prosthesis 2024, 6, 1420-1436. https://doi.org/10.3390/prosthesis6060103
Sulaiman MM, Fatalla AA, Haider J. The Impact of Incorporating Grapefruit Seed Skin Particles into 3D-Printed Acrylic Resin on Mechanical Properties. Prosthesis. 2024; 6(6):1420-1436. https://doi.org/10.3390/prosthesis6060103
Chicago/Turabian StyleSulaiman, Mira Mohideen, Abdalbseet Ahmad Fatalla, and Julfikar Haider. 2024. "The Impact of Incorporating Grapefruit Seed Skin Particles into 3D-Printed Acrylic Resin on Mechanical Properties" Prosthesis 6, no. 6: 1420-1436. https://doi.org/10.3390/prosthesis6060103
APA StyleSulaiman, M. M., Fatalla, A. A., & Haider, J. (2024). The Impact of Incorporating Grapefruit Seed Skin Particles into 3D-Printed Acrylic Resin on Mechanical Properties. Prosthesis, 6(6), 1420-1436. https://doi.org/10.3390/prosthesis6060103









