The Influence of Various Superstructure Materials on Stress Distribution for Implant-Supported Prosthesis: Three-Dimensional Finite Element Analysis
Abstract
1. Introduction
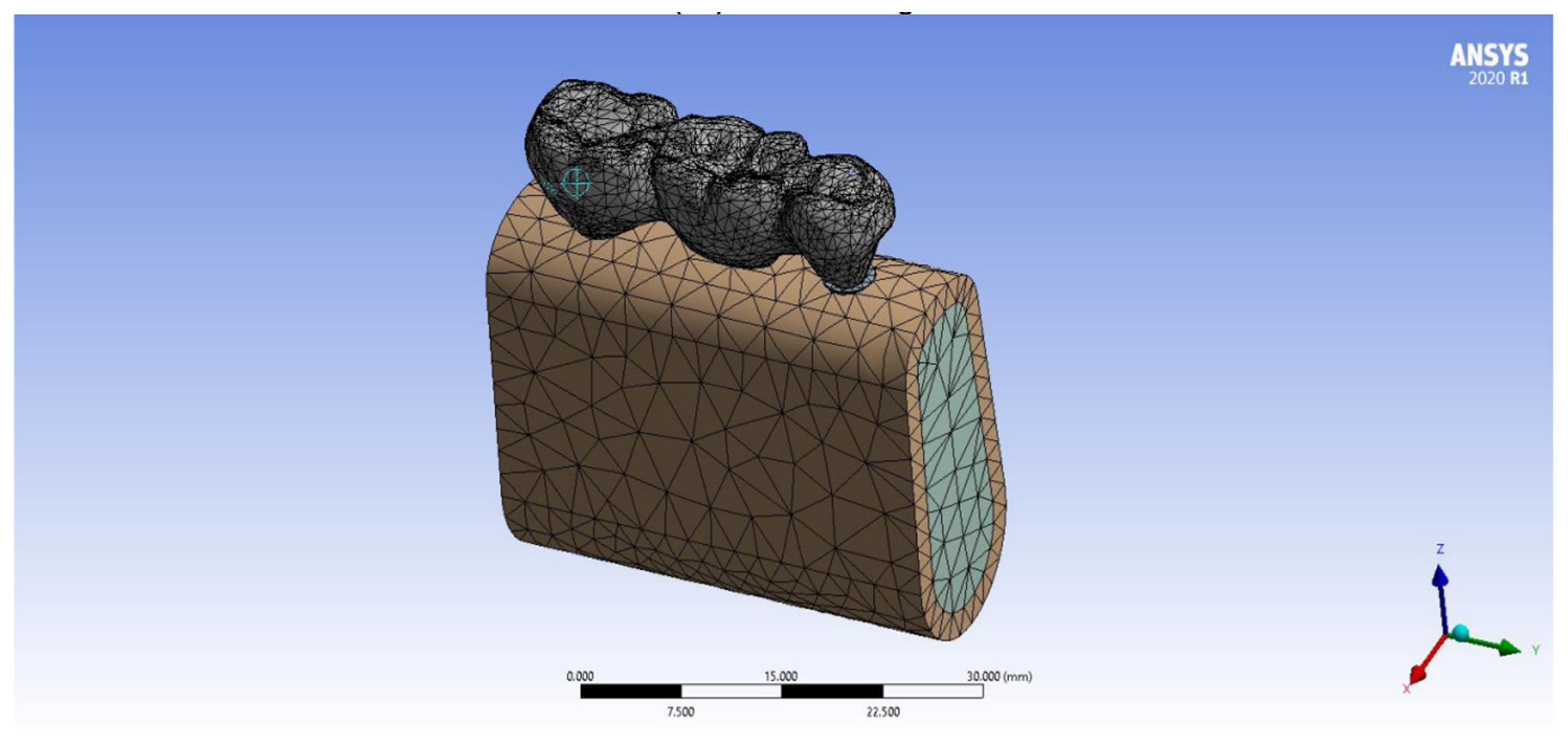
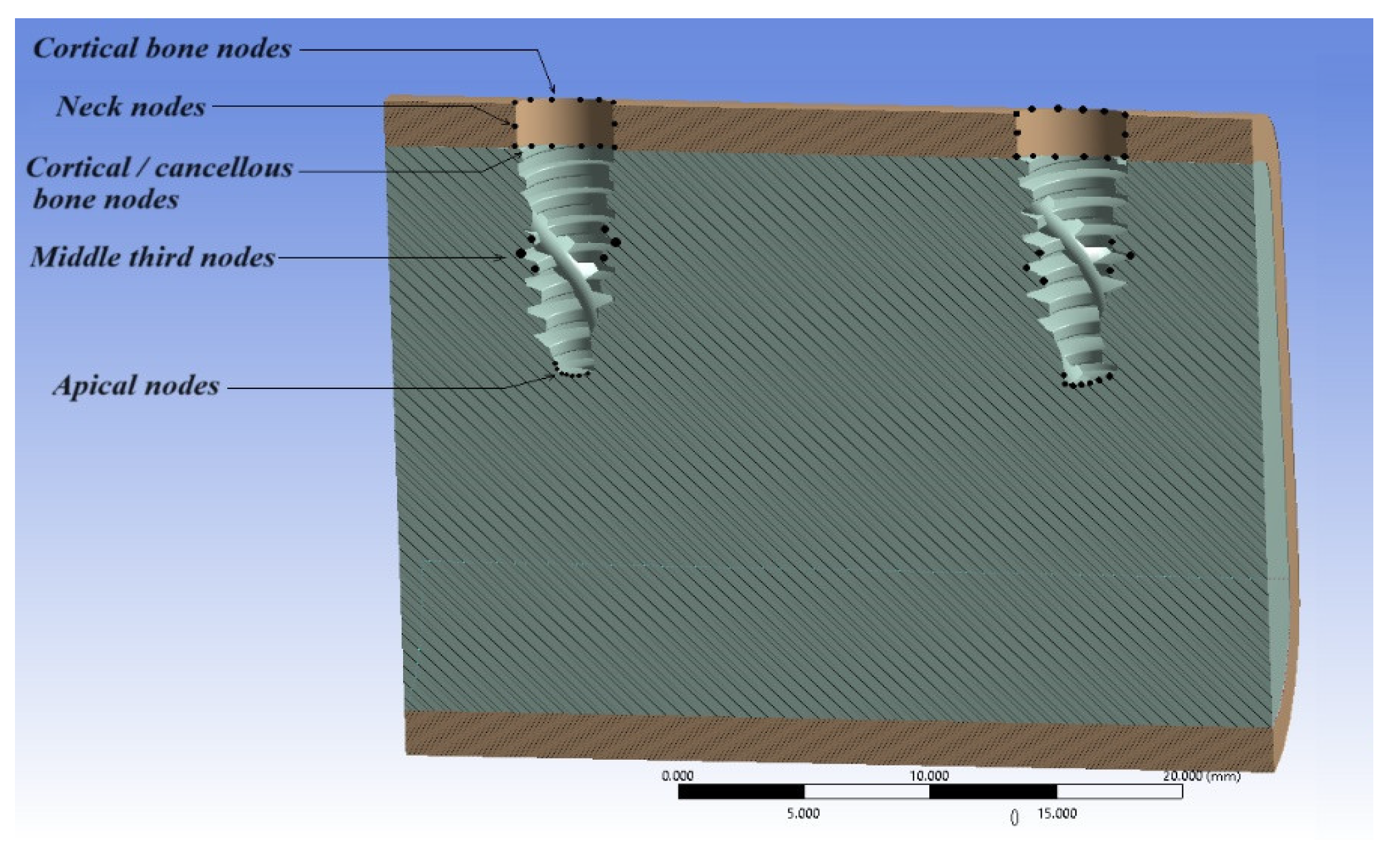
2. Materials and Methods
- Personal computer GPU: NVIDIA GEFORCE RTX 3080 10GB, Ram:64GB, CPU: Core I9 10900K, Power Supply: 850W, Motherboard:Z490 AORUS ELITE AC.
- For modeling, rendering and simulation the implant-supported prosthesis—BLENDER 4.0 program.
- ANSYS finite element program for stress analysis (ANSYS Version 2020 R1).
- For modeling the implant and bone—SolidWorks 2022.
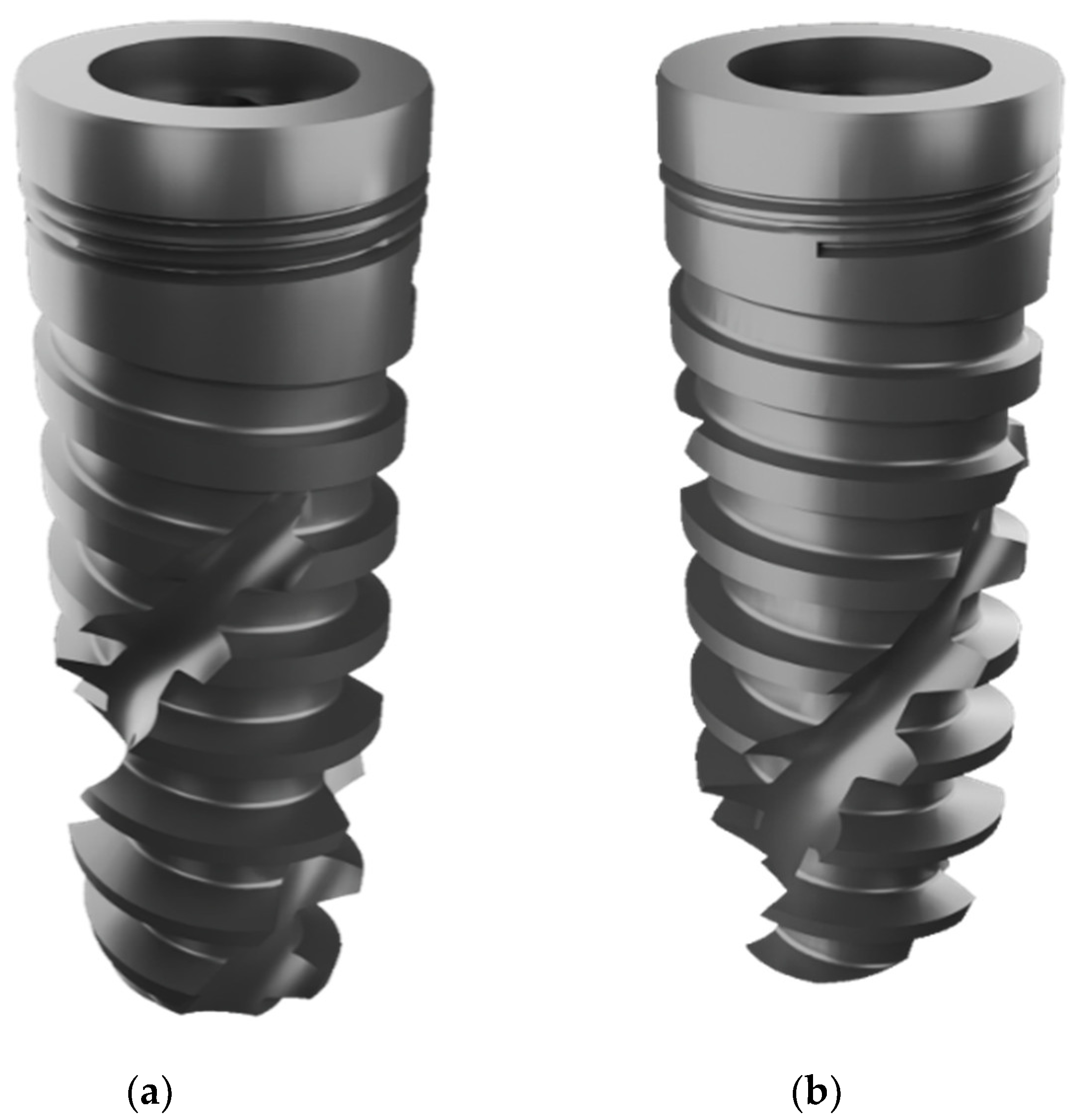
- Screw-shaped dental implant system—The Straumann® BLX Implant, Figure 1.
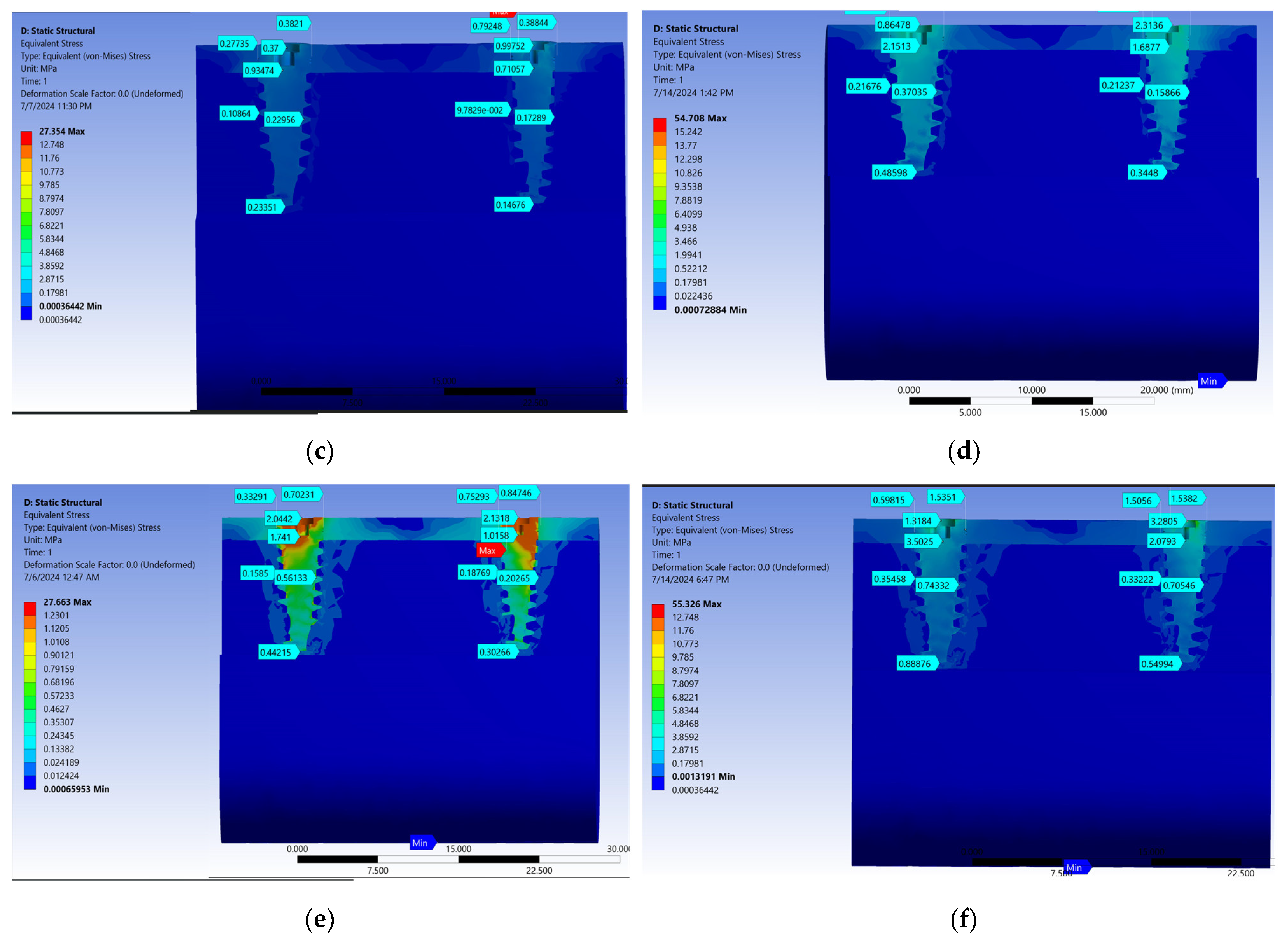
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santiago, J.F.; Verri, F.R.; Almeida, D.A.D.F.; De Souza Batista, V.E.; Lemos, C.A.A.; Pellizzer, E.P. Finite element analysis on influence of implant surface treatments, connection and bone types. Mater. Sci. Eng. C 2016, 63, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Alsmael, M.A.; Al-Khafaji, A.M. Evaluation of High-Performance Polyether Ether Ketone Polymer Treated with Piranha Solution and Epigallocatechin-3-Gallate Coating. BioMed Res. Int. 2024, 2024, 1741539. [Google Scholar] [CrossRef] [PubMed]
- Hamad, T.I.; Fatalla, A.A.; Waheed, A.S.; Azzawi, Z.G.M.; Cao, Y.G.; Song, K. Biomechanical Evaluation of Nano-Zirconia Coatings on Ti-6Al-7Nb Implant Screws in Rabbit Tibias. Curr. Med. Sci. 2018, 38, 530–537. [Google Scholar] [CrossRef]
- KN, C.; Eram, A.; Shetty, N.; Shetty, D.D.; Futane, M.; Keni, L.G. Evaluating Angled Abutments: Three-Dimensional Finite Element Stress Analysis of Anterior Maxillary Implants. Prosthesis 2024, 6, 315–328. [Google Scholar] [CrossRef]
- Muhammed, S.A.; Al-Khafaji, A.M.; Al-Deen, H.H.J.J. The Influence of Strontium Oxide on the Physio-Mechanical Properties of Biomedical-Grade Titanium in Ti-SrO Composites. J. Compos. Sci. 2023, 7, 449. [Google Scholar] [CrossRef]
- Tiwari, R.V.; Shakeel, S.K.; Gulia, S.K.; Biradar, J.M.; Agrawal, P.; Pandey, P.R. Straumann dental implant: A complete review. Eur. J. Mol. Clin. Med. 2022, 9, 579–587. [Google Scholar]
- Fiorillo, L.; Milone, D.; D’Andrea, D.; Santonocito, D.; Risitano, G.; Cervino, G.; Cicciù, M. Finite Element Analysis of Zirconia Dental Implant. Prosthesis 2022, 4, 490–499. [Google Scholar] [CrossRef]
- Zarone, F.; Di Mauro, M.I.; Ausiello, P.; Ruggiero, G.; Sorrentino, R. Current status on lithium disilicate and zirconia: A narrative review. BMC Oral Health 2019, 19, 134. [Google Scholar] [CrossRef]
- Surgery, M.; Ibraheem, N.S.; Al-Adili, S.S. Assessment of dental Assessment of dental implant stability during healing period and determination of the factors that affect implant stability by means of resonance frequency analysis (Clinical study). J. Bagh. Coll. Dent. 2015, 27. Available online: https://jbcd.uobaghdad.edu.iq/index.php/jbcd/article/view/815 (accessed on 31 July 2024).
- Ercal, P.; Taysi, A.E.; Ayvalioglu, D.C.; Eren, M.M.; Sismanoglu, S. Impact of peri-implant bone resorption, prosthetic materials, and crown to implant ratio on the stress distribution of short implants: A finite element analysis. Med. Biol. Eng. Comput. 2021, 59, 813–824. [Google Scholar] [CrossRef]
- Rudolf, R.; Majerič, P.; Lazić, V.; Raić, K.T. Processing of Cobalt-Chrome Dental Alloys. In Advanced Dental Metallic Materials; Springer: Cham, Switzerland, 2024. [Google Scholar]
- Barua, S.L.; Poduval, T.S.; Rani, S.; Jain, N.; Thakur, S. Stress distribution in bone around an implant-supported three-unit fixed dental prosthesis using two different computer-aided designing/computer-aided milling provisional crown materials: Milled polymethylmethacrylate and milled polyetheretherketone—A finite element analysis. Dent. Res. J. 2023, 20, 33. [Google Scholar]
- Piconi, C.; Sprio, S. Oxide bioceramic composites in orthopedics and dentistry. J. Compos. Sci. 2021, 5, 206. [Google Scholar] [CrossRef]
- Abdelraouf, R.M.; Tsujimoto, A.; Hamdy, T.M.; Alhotan, A.; Jurado, C.A.; Abadir, M.; Habib, N.A. The Effect of Surface Treatments of Presintered Zirconia on Sintered Surfaces. J. Compos. Sci. 2023, 7, 396. [Google Scholar] [CrossRef]
- Magnani, G.; Fabbri, P.; Leoni, E.; Salernitano, E.; Mazzanti, F. New perspectives on zirconia composites as biomaterials. J. Compos. Sci. 2021, 5, 244. [Google Scholar] [CrossRef]
- Vieira, F.R.; Bitencourt, S.B.; Rosa, C.D.D.R.D.; Vieira, A.B.; dos Santos, D.M.; Goiato, M.C. Influence of Different Restoring Materials on Stress Distribution in Prosthesis on Implants: A Review of Finite Element Studies. Eur. J. Dent. 2023, 17, 001–006. [Google Scholar] [CrossRef]
- de Souza Batista, V.E.; Verri, F.R.; de Faria Almeida, D.A.; Santiago Junior, J.F.; Lemos, C.A.A.; Pellizzer, E.P. Evaluation of the effect of an offset implant configuration in the posterior maxilla with external hexagon implant platform: A 3-dimensional finite element analysis. J. Prosthet. Dent. 2017, 118, 363–371. [Google Scholar] [CrossRef]
- Geringer, A.; Diebels, S.; Nothdurft, F.P. Influence of superstructure geometry on the mechanical behavior of zirconia implant abutments: A finite element analysis. Biomed. Tech. 2014, 59, 501–506. [Google Scholar] [CrossRef]
- Ferreira, M.B.; Barão, V.A.; Faverani, L.P.; Hipólito, A.C.; Assunção, W.G. The role of superstructure material on the stress distribution in mandibular full-arch implant-supported fixed dentures. A CT-based 3D-FEA. Mater. Sci. Eng. C 2014, 35, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Bidra, A.; Rungruanganunt, P.; Gauthier, M. Clinical outcomes of full arch fixed implantsupported zirconia prostheses: A systematic review. Eur. J. Oral Implantol. 2017, 10, 35–45. [Google Scholar]
- D’amico, C.; Bocchieri, S.; Sambataro, S.; Surace, G.; Stumpo, C.; Fiorillo, L. Occlusal Load Considerations in Implant-Supported Fixed Restorations. Prosthesis 2020, 2, 252–265. [Google Scholar] [CrossRef]
- Shakir, S.M.; Muhsin, S.A.; Al Marza, R.S. FEM: Mono-implant cement-retained crown with two-different adhesive materials. J. Baghdad Coll. Dent. 2023, 35, 37–48. [Google Scholar] [CrossRef]
- Ahmed, S.A.S.; Eldosoky, M.A.A.; El-Wakad, M.T. Effect of Stiffness of Single Implant Supported Crowns on the Resultant Stresses: A Finite Element Analysis. Egypt. J. Hosp. Med. 2016, 63, 172–184. [Google Scholar] [CrossRef]
- Gökçimen, G.; Durkan, R.; Deste Gökay, G.; Oyar, P. The effect of different abutment and restorative crown materials on stress distribution in single-unit implant-supported restorations: A 3D finite element stress analysis. J. Prosthodont. 2023, 33, 497–505. [Google Scholar] [CrossRef]
- Al Jabbari, Y.S. Physico-mechanical properties and prosthodontic applications of Co-Cr dental alloys: A review of the literature. J. Adv. Prosthodont. 2014, 6, 138–145. [Google Scholar] [CrossRef]
- Edelhoff, D.; Güth, J.F.; Erdelt, K.; Brix, O.; Liebermann, A. Clinical performance of occlusal onlays made of lithium disilicate ceramic in patients with severe tooth wear up to 11 years. Dent. Mater. 2019, 35, 1319–1330. [Google Scholar] [CrossRef]
- Furtado de Mendonca, A.; Shahmoradi, M.; de Gouvêa, C.V.D.; De Souza, G.M.; Ellakwa, A. Microstructural and Mechanical Characterization of CAD/CAM Materials for Monolithic Dental Restorations. J. Prosthodont. 2018, 28, E587–E594. [Google Scholar] [CrossRef]
- Forster, A.; Ungvári, K.; Györgyey, Á.; Kukovecz, Á.; Turzó, K.; Nagy, K. Human epithelial tissue culture study on restorative materials. J. Dent. 2014, 42, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Ariaans, K.; Heussen, N.; Schiffer, H.; Wienert, A.; Plümäkers, B.; Rink, L.; Wolfart, S. Use of molecular indicators of inflammation to assess the biocompatibility of all-ceramic restorations. J. Clin. Periodontol. 2016, 43, 173–179. [Google Scholar] [CrossRef]
- Baldissara, P.; Llukacej, A.; Ciocca, L.; Valandro, F.L.; Scotti, R. Translucency of zirconia copings made with different CAD/CAM systems. J. Prosthet. Dent. 2010, 104, 6–12. [Google Scholar] [CrossRef]
- Chen, Y.W.; Moussi, J.; Drury, J.L.; Wataha, J.C. Zirconia in biomedical applications. Expert Rev. Med. Devices 2016, 13, 945–963. [Google Scholar] [CrossRef]
- Bacchi, A.; Boccardi, S.; Alessandretti, R.; Pereira, G.K.R. Substrate masking ability of bilayer and monolithic ceramics used for complete crowns and the effect of association with an opaque resin-based luting agent. J. Prosthodont. Res. 2019, 63, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, B.S.; Kim, H.; Cho, S.Y. Occlusal stress distribution and remaining crack propagation of a cracked tooth treated with different materials and designs: 3D finite element analysis. Dent. Mater. 2021, 37, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Sevimay, M.; Usumez, A.; Eskitascioglu, G. The influence of various occlusal materials on stresses transferred to implant-supported prostheses and supporting bone: A three-dimensional finite-element study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 73B, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, D.; Nicita, F.; Risitano, G. A Parametric Study on a Dental Implant Geometry Influence on Bone Remodelling through a Numerical Algorithm. Prosthesis 2021, 3, 157–172. [Google Scholar] [CrossRef]
- Boukhlif, A.; Merdji, A.; Roy, S.; Alkhaldi, H.; Abu-Alshaikh, I.; Della, N.; Cristache, C.M.; Hillstrom, R. Effect of supporting implants inclination on stability of fixed partial denture: A finite element study. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2020, 234, 1162–1171. [Google Scholar] [CrossRef]
- Jassim, R.K. Finite Element Stress Analysis and Radiographic Follow-up of Mandibular Implant Retained—Overdenture [Original]; University of Baghdad: Baghdad, Iraq, 2006. [Google Scholar]
- Jassim, R.K.; Ibrahim, I.K. Finite Element Stress Analysis Study for Stresses around Mandibular Implant Retained Overdenture MIR-OD. J. Baghdad Coll. Dent. 2014, 26, 30–36. [Google Scholar] [CrossRef]
- Phulari, R.G.S. Textbook of Dental Anatomy, Physiology and Occlusion; JP Medical Ltd.: London, UK, 2013. [Google Scholar]
- Guedes, C.A.S.; Matos, R.A.; da Gama Ramos, G.; de Souza Júnior, A.R. Finite element analysis of the stress generated by the type of restorative material in the implant crown system. Braz. J. Health Rev. 2024, 7, 324–347. [Google Scholar] [CrossRef]
- Kilic, E.; Doganay, O. Evaluation of stress in tilted implant concept with variable diameters in the atrophic mandible: Three-dimensional finite element analysis. J. Oral Implant. 2020, 46, 19–26. [Google Scholar] [CrossRef]
- Al-naqshabandi, F.I.; Selivany, B.J.; Al-zahawi, A.R. Biomechanical Behavior of Lithium-Disilicate-Modified Endocrown Restorations: A Three-Dimensional Finite Element Analysis. Ceramics 2023, 6, 2162–2177. [Google Scholar] [CrossRef]
- Faisal, E.; Hamza, T.; Mokhtar, A. Finite Element Analysis of Monolithic PEEK and Zirconia Fixed Dental Prosthesis (In Vitro Study). J. Fundam. Clin. Res. 2023, 3, 1–15. [Google Scholar] [CrossRef]
- Sannino, G.; Pozzi, A.; Schiavetti, R.; Barlattani, A. Stress distribution on a three-unit implant-supported zirconia framework. A 3d finite element analysis and fatigue test. Oral Implantol. 2012, 5, 11. [Google Scholar]
- Byun, S.-H.; Seo, J.-H.; Cho, R.-Y.; Yi, S.-M.; Kim, L.-K.; Han, H.-S.; On, S.-W.; Kim, W.-H.; An, H.-W.; Yang, B.-E. Finite Element Analysis of a New Non-Engaging Abutment System for Three-Unit Implant-Supported Fixed Dental Prostheses. Bioengineering 2022, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- Al-Judy, H.J. Selection of Mandibular Distal Extension Fixed Implant Supported Prosthesis Design Using 3-D Finite Element [Original]; University of Baghdad: Baghdad, Iraq, 2005. [Google Scholar]
- Fatalla, A.A.; Song, K.; Du, T.; Cao, Y. A Three-Dimensional Finite Element Analysis for Overdenture Attachments Supported by Teeth and/or Mini Dental Implants. J. Prosthodont. 2012, 21, 604–613. [Google Scholar] [CrossRef]
- Jain, H.; Kalra, T.; Kumar, M.; Bansal, A.; Jain, D. Three-Dimensional Finite Element Analysis to Evaluate Stress Distribution in Tooth and Implant-Supported Fixed Partial Denture–An In Vitro Study. Dent. J. Adv. Stud. 2020, 8, 084–091. [Google Scholar] [CrossRef]
- Assunção, W.G.; Gomes, É.A.; Barão, V.A.R.; Delben, J.A.; Tabata, L.F.; De Sousa, E.A.C. Effect of superstructure materials and misfit on stress distribution in a single implant-supported prosthesis: A finite element analysis. J. Craniofacial Surg. 2010, 21, 689–695. [Google Scholar] [CrossRef]
- Haroun, F.; Ozan, O. Evaluation of stresses on implant, bone, and restorative materials caused by different opposing arch materials in hybrid prosthetic restorations using the all-on-4 technique. Materials 2021, 14, 4308. [Google Scholar] [CrossRef]
- Bankoğlu Güngör, M.; Yılmaz, H. Evaluation of stress distributions occurring on zirconia and titanium implant-supported prostheses: A three-dimensional finite element analysis. J. Prosthet. Dent. 2016, 116, 346–355. [Google Scholar] [CrossRef]
- Dhanasekaran, T.; Andonissamy, L.; Abdullah, F.; Paramasivam, Y.S. Effect of different occlusal materials on peri-implant stress distribution with different osseointegration condition: A finite element analysis. J. Indian Prosthodont. Soc. 2024, 24, 292–299. [Google Scholar]
- Makhija, S.K.; Lawson, N.C.; Gilbert, G.H.; Litaker, M.S.; McClelland, J.A.; Louis, D.R.; Gordan, V.V.; Pihlstrom, D.J.; Meyerowitz, C.; Mungia, R.; et al. Dentist material selection for single-unit crowns: Findings from the National Dental Practice-Based Research Network. J. Dent. 2016, 55, 40–47. [Google Scholar] [CrossRef]




| Area | Dimensions, mm | ||
|---|---|---|---|
| Second Premolar | First Molar | Second Molar | |
| Buccolingual diameter of crown | 8 | 10.5 | 10 |
| Buccolingual diameter of crown at cervix | 7 | 9 | 9 |
| Mesiodistal diameter of crown | 7 | 11 | 10.5 |
| Mesiodistal diameter of crown at cervix | 5 | 9 | 8 |
| Cervico-occlusal length of crown | 8 | 7.5 | 7 |
| Material | Young’s Modulus, GPa | Poisson’s Ratio |
|---|---|---|
| Cortical bone [40] | 13.7 | 0.30 |
| Cancellous bone [40] | 1.37 | 0.30 |
| Ti-Zr implant (Roxolid) [40,41] | 100 | 0.30 |
| Lithium disilicate [42] | 95 | 0.30 |
| Cobalt chromium alloy [40] | 218 | 0.30 |
| Zirconia [43] | 200 | 0.31 |
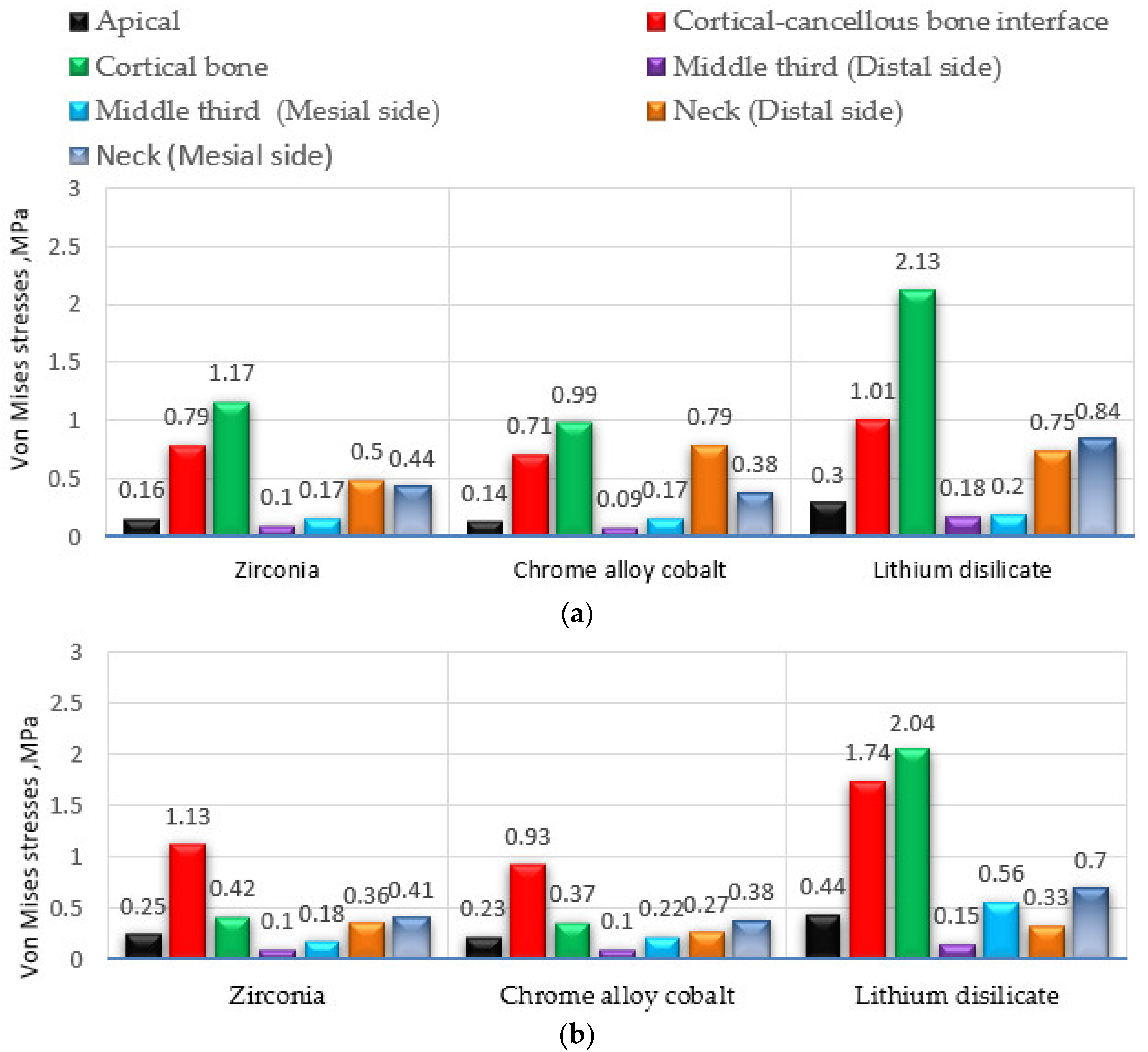
| Material | Max. Equivalent von Mises Stress, MPa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Second Implant-Supported Premolar | Second Implant-Supported Molar | |||||||||||||
| Apical | Cortical-Cancellous Bone Interface | Cortical Bone | Middle Third (Thread for Implant) (Distal Side) | Middle Third (Thread for Implant) (Mesial Side) | Neck (Distal Side) | Neck (Mesial Side) | Apical | Cortical-Cancellous Bone Interface | Cortical Bone | Middle Third (Thread for Implant) (Distal Side) | Middle Third (Thread for Implant) (Mesial Side) | Neck (Distal Side) | Neck (Mesial Side) | |
| Zirconia | 0.16 | 0.79 | 1.17 | 0.10 | 0.17 | 0.50 | 0.44 | 0.25 | 1.13 | 0.42 | 0.10 | 0.18 | 0.36 | 0.41 |
| Chrome alloy cobalt | 0.14 | 0.71 | 0.99 | 0.09 | 0.17 | 0.79 | 0.38 | 0.23 | 0.93 | 0.37 | 0.10 | 0.22 | 0.27 | 0.38 |
| Lithium disilicate | 0.30 | 1.01 | 2.13 | 0.18 | 0.20 | 0.75 | 0.84 | 0.44 | 1.74 | 2.04 | 0.15 | 0.56 | 0.33 | 0.70 |
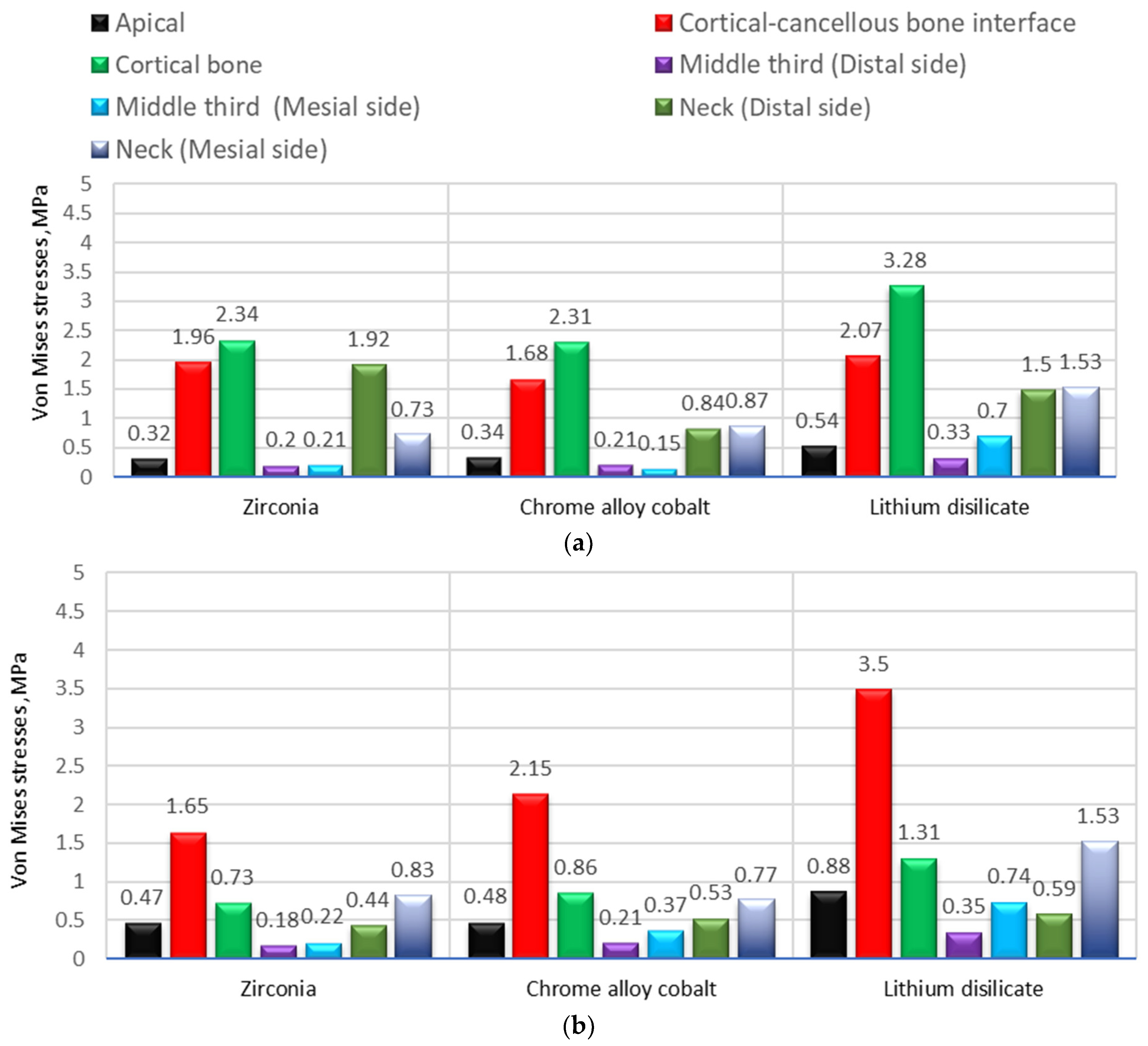
| Material | Max. Equivalent von Mises Stress, MPa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Second Implant-Supported Premolar | Second Implant-Supported Molar | |||||||||||||
| Apical | Cortical-Cancellous Bone Interface | Cortical Bone | Middle Third (Thread for Implant) (Distal Side) | Middle Third (Thread for Implant) (Mesial Side) | Neck (Distal Side) | Neck (Mesial Side) | Apical | Cortical-Cancellous Bone Interface | Cortical Bone | Middle Third (Thread for Implant) (Distal Side) | Middle Third (Thread for Implant) (Mesial Side) | Neck (Distal Side) | Neck (Mesial Side | |
| Zirconia | 0.32 | 1.96 | 2.34 | 0.20 | 0.21 | 1.92 | 0.73 | 0.47 | 1.65 | 0.73 | 0.18 | 0.22 | 0.44 | 0.83 |
| Chrome alloy cobalt | 0.34 | 1.68 | 2.31 | 0.21 | 0.15 | 0.84 | 0.87 | 0.48 | 2.15 | 0.86 | 0.21 | 0.37 | 0.53 | 0.77 |
| Lithium disilicate | 0.54 | 2.07 | 3.28 | 0.33 | 0.70 | 1.50 | 1.53 | 0.88 | 3.50 | 1.31 | 0.35 | 0.74 | 0.59 | 1.53 |
| Fixed Implant Prosthesis under 100 N | Nodes | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | ||
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| Second implant-supported premolar | Zirconia | 7 | 1.5829 | 0.92141 | 0.34826 | 0.7307 | 2.4350 |
| Cobalt chromium alloy | 7 | 1.4914 | 0.83665 | 0.31622 | 0.7177 | 2.2652 | |
| Lithium dislicate | 7 | 2.3886 | 1.40046 | 0.52932 | 1.0934 | 3.6838 | |
| Second implant-supported molar | Zirconia | 7 | 1.8886 | 1.44050 | 0.54446 | 0.5563 | 3.2208 |
| Cobalt chromium alloy | 7 | 1.9014 | 1.39244 | 0.52629 | 0.6136 | 3.1892 | |
| Lithium dislicate | 7 | 2.4086 | 1.70436 | 0.64419 | 0.8323 | 3.9848 | |
| Fixed Implant Prosthesis under 100 N | Levene Statistic | df1 | df2 | Sig. | |
|---|---|---|---|---|---|
| Second implant-supported premolar | Based on Mean | 1.282 | 2 | 18 | 0.302 |
| Second implant-supported molar | Based on Mean | 0.125 | 2 | 18 | 0.883 |
| Fixed Implant Prosthesis under 200 N | Nodes | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | ||
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| Second implant-supported premolar | Zirconia | 7 | 3.2071 | 1.89507 | 0.71627 | 1.4545 | 4.9598 |
| Cobalt chromium alloy | 7 | 3.2057 | 1.97693 | 0.74721 | 1.3774 | 5.0341 | |
| Lithium dislicate | 7 | 4.7771 | 2.85435 | 1.07884 | 2.1373 | 7.4170 | |
| Second implant-supported molar | Zirconia | 7 | 3.5771 | 2.97831 | 1.12570 | 0.8227 | 6.3316 |
| Cobalt chromium alloy | 7 | 3.5214 | 1.92237 | 0.72659 | 1.7435 | 5.2993 | |
| Lithium dislicate | 7 | 5.0629 | 3.36040 | 1.27011 | 1.9550 | 8.1707 | |
| Fixed Implant Prosthesis under 200 N | Levene Statistic | df1 | df2 | Sig. | |
|---|---|---|---|---|---|
| Second implant-supported premolar | Based on Mean | 0.846 | 2 | 18 | 0.446 |
| Second implant-supported molar | Based on Mean | 1.024 | 2 | 18 | 0.379 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jameel, R.M.; Al-Khafaji, A.M. The Influence of Various Superstructure Materials on Stress Distribution for Implant-Supported Prosthesis: Three-Dimensional Finite Element Analysis. Prosthesis 2024, 6, 1133-1148. https://doi.org/10.3390/prosthesis6050082
Jameel RM, Al-Khafaji AM. The Influence of Various Superstructure Materials on Stress Distribution for Implant-Supported Prosthesis: Three-Dimensional Finite Element Analysis. Prosthesis. 2024; 6(5):1133-1148. https://doi.org/10.3390/prosthesis6050082
Chicago/Turabian StyleJameel, Rawan Mufeed, and Aseel Mohammed Al-Khafaji. 2024. "The Influence of Various Superstructure Materials on Stress Distribution for Implant-Supported Prosthesis: Three-Dimensional Finite Element Analysis" Prosthesis 6, no. 5: 1133-1148. https://doi.org/10.3390/prosthesis6050082
APA StyleJameel, R. M., & Al-Khafaji, A. M. (2024). The Influence of Various Superstructure Materials on Stress Distribution for Implant-Supported Prosthesis: Three-Dimensional Finite Element Analysis. Prosthesis, 6(5), 1133-1148. https://doi.org/10.3390/prosthesis6050082








