Single Crown Restorations Supported by One-Piece Zirconia Dental Implants: Case Series with a Mean Follow-Up of 58 Months
Abstract
:1. Introduction
2. Materials and Methods
Implant Characteristics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buser, D.; Janner, S.F.; Wittneben, J.G.; Bragger, U.; Ramseier, C.A.; Salvi, G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant. Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef]
- Thoma, D.S.; Ioannidis, A.; Cathomen, E.; Hämmerle, C.H.; Hüsler, J.; Jung, R.E. Discoloration of the peri-implant mucosa caused by zirconia and titanium implants. Int. J. Periodontics Restor. Dent. 2016, 36, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin. Oral Implant. Res. 2008, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, M.; Nishigawa, K.; Miyamoto, Y.; Ohe, G.; Matsuka, Y. Allergic contact dermatitis caused by titanium screws and dental implants. J. Prosthodont. Res. 2016, 60, 213–219. [Google Scholar] [CrossRef]
- Fretwurst, T.; Nelson, K.; Tarnow, D.P.; Wang, H.L.; Giannobile, W.V. Is Metal Particle Release Associated with Peri-implant Bone Destruction? An Emerging Concept. J. Dent. Res. 2018, 97, 259–265. [Google Scholar] [CrossRef]
- Mombelli, A.; Hashim, D.; Cionca, N. What is the impact of titanium particles and biocorrosion on implant survival and complications? A critical review. Clin. Oral Implant. Res. 2018, 29 (Suppl. S18), 37–53. [Google Scholar] [CrossRef]
- Benic, G.I.; Thoma, D.S.; Sanz-Martin, I.; Munoz, F.; Hämmerle, C.H.; Cantalapiedra, A.; Cantalapiedra, A.; Fischer, J.; Jung, R.E. Guided bone regeneration at zirconia and titanium dental implants: A pilot histological investigation. Clin. Oral Implant. Res. 2017, 28, 1592–1599. [Google Scholar] [CrossRef]
- Jung, R.E.; Sailer, I.; Hämmerle, C.H.; Attin, T.; Schmidlin, P. In vitro color changes of soft tissues caused by restorative materials. Int. J. Periodontics Restor. Dent. 2007, 27, 251–257. [Google Scholar]
- Cosgarea, R.; Gasparik, C.; Dudea, D.; Culic, B.; Dannewitz, B.; Sculean, A. Peri-implant soft tissue colour around titanium and zirconia abutments: A prospective randomized controlled clinical study. Clin. Oral Implant. Res. 2015, 26, 537–544. [Google Scholar] [CrossRef]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef]
- Nascimento, C.D.; Pita, M.S.; Fernandes, F.H.N.C.; Pedrazzi, V.; de Albuquerque Junior, R.F.; Ribeiro, R.F. Bacterial adhesion on the titanium and zirconia abutment surfaces. Clin. Oral Implant. Res. 2014, 25, 337–343. [Google Scholar] [CrossRef]
- Roehling, S.; Woelfler, H.; Hicklin, S.; Kniha, H.; Gahlert, M. A retrospective clinical study with regard to survival and success rates of zirconia implants up to and after 7 years of loading. Clin. Implant. Dent. Relat. Res. 2016, 18, 545–558. [Google Scholar] [CrossRef]
- Degidi, M.; Artese, L.; Scarano, A.; Perrotti, V.; Gehrke, P.; Piattelli, A. Inflammatory infiltrate, microvessel density, nitric oxide synthase expression, vascular endothelial growth factor expression, and proliferative activity in peri-implant soft tissues around titanium and zirconium oxide healing caps. J. Periodontol. 2006, 77, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Welander, M.; Abrahamsson, I.; Berglundh, T. The mucosal barrier at implant abutments of different materials. Clin. Oral Implant. Res. 2008, 19, 635–641. [Google Scholar] [CrossRef]
- Kajiwara, N.; Masaki, C.; Mukaibo, T.; Kondo, Y.; Nakamoto, T.; Hosokawa, R. Soft tissue biological response to zirconia and metal implant abutments compared with natural tooth: Microcirculation monitoring as a novel bioindicator. Implant. Dent. 2015, 24, 37–41. [Google Scholar] [CrossRef]
- Liñares, A.; Grize, L.; Muñoz, F.; Pippenger, B.E.; Dard, M.; Domken, O.; Blanco-Carrión, J. Histological assessment of hard and soft tissues surrounding a novel ceramic implant: A pilot study in the minipig. J. Clin. Periodontol. 2016, 43, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Hasim, D.; Cionca, N.; Courvoisier, D.S.; Mombelli, A. A systematic review of the clinical survival of zirconia implants. Clin. Oral Investig. 2016, 20, 1403–1417. [Google Scholar] [CrossRef]
- Vohra, F.; Al-Kheraif, A.A.; Ab Ghani, S.M.; Abu Hassan, M.I.; Alnassar, T.; Javed, F. Crestal bone loss and periimplant inflammatory parameters around zirconia implants: A systematic review. J. Prosthet. Dent. 2015, 114, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Balmer, M.; Payer, M.; Kohal, R.J.; Spies, B.C. EAO Position Paper: Current Level of Evidence Regarding Zirconia Implants in Clinical Trials. Int. J. Prosthodont. 2022, 35, 560–566. [Google Scholar] [CrossRef]
- Gahlert, M.; Röhling, S.; Wieland, M.; Eichhorn, S.; Küchenhoff, H.; Kniha, H. A comparison study of the osseointegration of zirconia and titanium dental implants. A biomechanical evaluation in the maxilla of pigs. Clin. Implant. Dent. Relat. Res. 2010, 12, 297–305. [Google Scholar] [CrossRef]
- Depprich, R.; Zipprich, H.; Ommerborn, M.; Naujoks, C.; Wiesmann, H.-P.; Kiattavorncharoen, S.; Lauer, H.-C.; Meyer, U.; Kübler, N.R.; Handschel, J. Osseointegration of zirconia implants compared with titanium: An in vivo study. Head Face Med. 2008, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Di Carlo, F.; Quaranta, M.; Piatelli, A. Bone response to zirconia ceramic implants: An experimental study in rabbits. J. Oral Implantol. 2003, 29, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.P.; Weng, D.; Kramer, S.; Biesterfeld, S.; Jahn-Eimermacher, A.; Wagner, W. Osseointegration of one-piece zirconia implants compared with a titanium implant of identical design: A histomorphometric study in the dog. Clin. Oral Implant. Res. 2010, 21, 350–356. [Google Scholar] [CrossRef]
- Mihatovic, I.; Goluvobic, V.; Becker, J.; Schwarz, F. Bone tissue response to experimentalzirconia implants. Clin. Oral Investig. 2017, 21, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Sanon, C.; Chevalier, J.; Douillard, T.; Cattani-Lorente, M.; Scherrer, S.S.; Gremillard, L. A new testing protocol for zirconia dental implants. Dent. Mater. 2015, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Monzavi, M.; Zhang, F.; Meille, S.; Douillard, T.; Adrien, J.; Noumbissi, S.; Nowzari, H.; Chevalier, J. Influence of artificial aging on mechanical properties of commercially and non-commercially available zirconia dental implants. J. Mech. Behav. Biomed. Mater. 2020, 101, 103423. [Google Scholar] [CrossRef]
- Balmer, M.; Spies, B.C.; Vach, K.; Ralf-Joachim, K.; Hämmerle, C.H.F.; Jung, R.E. Three-year analysis of zirconia implants used for single-tooth replacement and three unit fixed dental prostheses: A prospective multicenter study. Clin. Oral Implant. Res. 2018, 29, 290–299. [Google Scholar] [CrossRef]
- Oliva, J.; Oliva, X. 15-Year Post-Market Clinical Follow-up Study of 1828 Ceramic (Zirconia) Implants in Humans. Int. J. Oral Maxillofac. Implant. 2023, 38, 357–366. [Google Scholar] [CrossRef]
- Kiechle, S.; Liebermann, A.; Mast, G.; Heitzer, M.; Möhlhenrich, S.C.; Hölzle, F.; Kniha, H.; Kniha, K. Evaluation of one-piece zirconia dental implants: An 8-year follow-up study. Clin. Oral Investig. 2023, 27, 3415–3421. [Google Scholar] [CrossRef]
- Cionca, N.; Müller, N.; Mombelli, A. Two-piece zirconia implants supporting all-ceramic crowns: A prospective clinical study. Clin. Oral Implant. Res. 2015, 26, 413–418. [Google Scholar] [CrossRef]
- Borgonovo, A.E.; Censi, R.; Vavassori, V.; Arnaboldi, O.; Maiorana, C.; Re, D. Zirconia implants in esthetic areas: 4-year follow-up evaluation study. Int. J. Dent. 2015, 2015, 415029. [Google Scholar] [CrossRef]
- Afrashtehfar, K.I.; Del Fabbro, M. Clinical performance of zirconia implants: A meta-review. J. Prosthet. Dent. 2020, 123, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Roehling, S.; Schelegel, K.; Woelfler, H.; Gahlert, M. Zirconia compared to titanium dental implants in preclinical studies-a systematic review and meta-analysis. Clin. Oral Implant. Res. 2019, 30, 365–395. [Google Scholar] [CrossRef] [PubMed]
- Andreiotelli, M.; Wenz, H.J.; Kohal, R.J. Are ceramic implants a viable alternative to titanium implants? A systematic literature review. Clin. Oral Implant. Res. 2009, 20 (Suppl. S4), 32–47. [Google Scholar] [CrossRef]
- Kohal, R.J.; Knauf, M.; Larsson, B.; Sahlin, H.; Butz, F. One-piece zirconia oral implants: One-year results from a prospective cohort study. 1. Single tooth replacement. J. Clin. Periodontol. 2012, 39, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Elnayef, B.; Lazaro, A.; Suarez-Lopez Del Amo, F.; Galindo-Moreno, P.; Wang, H.L.; Gargallo-Albiol, J.; Hernandez-Alfaro, F. Zirconia implants as an alternative to titanium: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2017, 32, e125–e134. [Google Scholar] [CrossRef]
- Brüll, F.; van Winkelhoff, J.V.; Cune, M.S. Zirconia dental implants: A clinical, radiographic, and microbiologic evaluation up to 3 years. Int. Oral Maxillofac. Implant. 2014, 29, 914–920. [Google Scholar]
- Osman, R.B.; Morgaine, K.C.; Duncan, W.; Swain, M.V.; Ma, S. Patients’ perspectives on zirconia and titanium implants with a novel distribution supporting maxillary and mandibular overdentures: A qualitative study. Clin. Oral Implant. Res. 2014, 25, 587–597. [Google Scholar] [CrossRef]
- Pieralli, S.; Kohal, R.J.; Jung, R.E.; Vach, K.; Spies, B. Clinical outcomes of zirconia dental implants; a systematic review. J. Dent. Dent. 2017, 9, 38–46. [Google Scholar] [CrossRef]
- Cacaci, C.; Cantner, F.; Mücke, T.; Randelzhofer, P.; Hajto, J.; Beuer, F. Clinical performance of screw-retained and cemented implant-supported zirconia single crowns: 36-month results. Clin. Oral Investig. 2017, 21, 1953–1959. [Google Scholar] [CrossRef]
- Borgonovo, A.E.; Fabbri, A.; Vavassori, V.; Censi, R.; Maiorana, C. Multiple teeth replacement with endosseous one-piece yttrium-stabilized zirconia dental implants. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e981–e987. [Google Scholar] [CrossRef] [PubMed]
- Vilor-Fernández, V.; García-de-la-Fuente, A.M.; Marichalar-Mendia, X.; Estefanía-Fresco, R.; Aguirre-Zorzano, L.A. Single tooth restoration in the maxillary esthetic zone using a one-piece ceramic implant with 1 year of follow-up: Case series. Int. J. Implant. Dent. 2021, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Glauser, R.; Sailer, I.; Wohlwend, A.; Studer, S.; Schibli, M.; Scharer, P. Experimental zirconia abutments for implant-supported single-tooth restorations in esthetically demanding regions: 4-year results of a prospective clinical study. Int. J. Prosthodont. 2004, 17, 285–290. [Google Scholar] [PubMed]
- Rimondini, L.; Cerroni, L.; Carrassi, A.; Torricelli, P. Bacterial colonization of zirconia ceramic surfaces: An in vitro and in vivo study. Int. J. Oral Maxillofac. Implant. 2002, 17, 793–798. [Google Scholar]
- Rodriguez, A.E.; Monzavi, M.; Yokoyama, C.L.; Nowzari, H. Zirconia dental implants: A clinical and radiographic evaluation. J. Esthet. Restor. Dent. 2018, 30, 538–544. [Google Scholar] [CrossRef]
- Pera, F.; Pesce, P.; Menini, M.; Fanelli, F.; Kim, B.C.; Zhurakivska, K.; Mayer, Y.; Isola, G.; Cianciotta, G.; Crupi, A.; et al. Immediate loading full-arch rehabilitation using transmucosal tissue-level implants with different variables associated: A one-year observational study. Minerva Dent. Oral Sci. 2023. ahead of print. [Google Scholar] [CrossRef]
- Carossa, M.; Alovisi, M.; Crupi, A.; Ambrogio, G.; Pera, F. Full-Arch Rehabilitation Using Trans-Mucosal Tissue-Level Implants with and without Implant-Abutment Units: A Case Report. Dent. J. 2022, 10, 116. [Google Scholar] [CrossRef]
- Fernandes, P.R.E.; Otero, A.I.P.; Fernandes, J.C.H.; Nassani, L.M.; Castilho, R.M.; de Oliveira Fernandes, G.V. Clinical Performance Comparing Titanium and Titanium-Zirconium or Zirconia Dental Implants: A Systematic Review of Randomized Controlled Trials. Dent. J. 2022, 10, 83. [Google Scholar] [CrossRef]
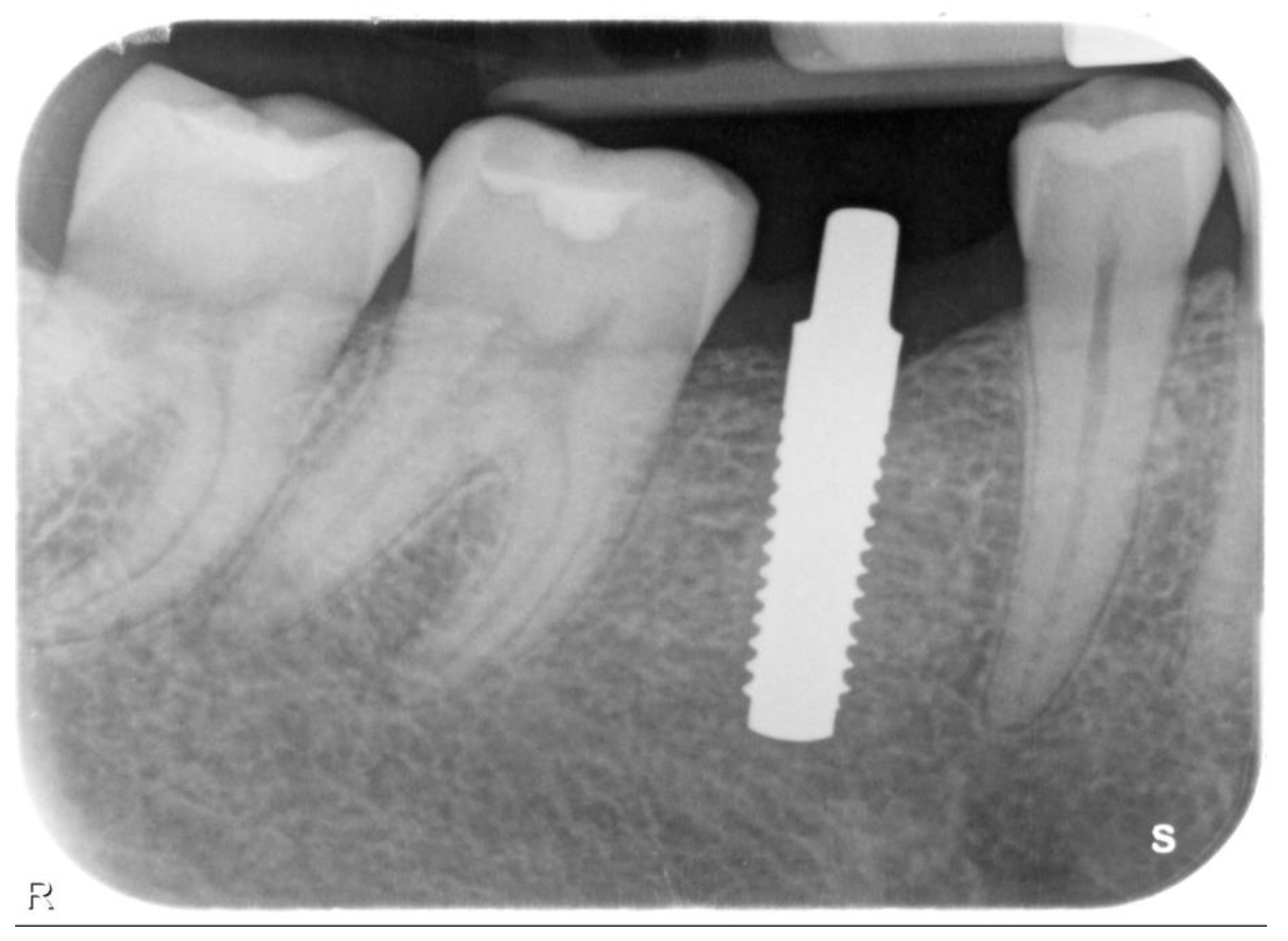
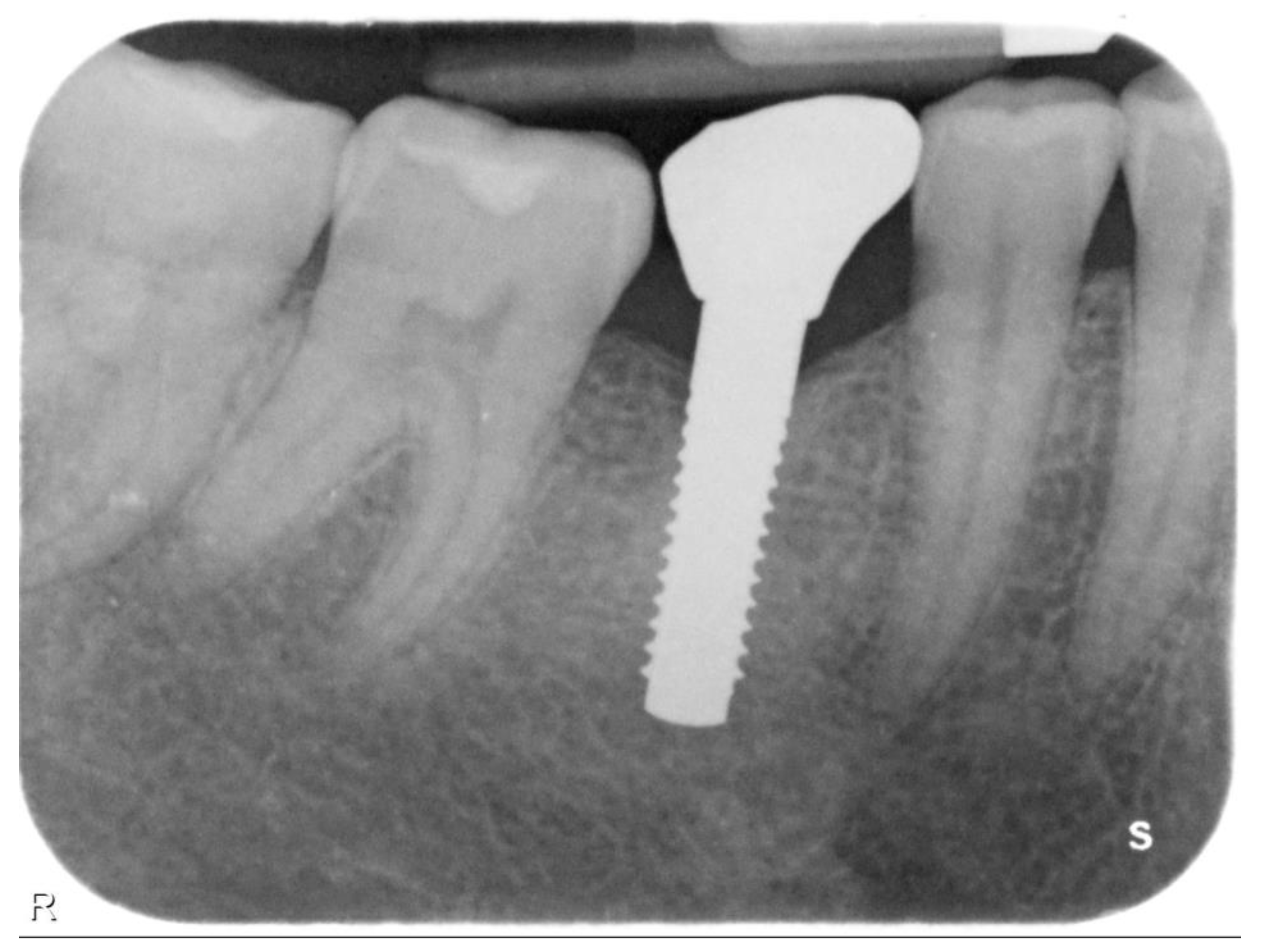
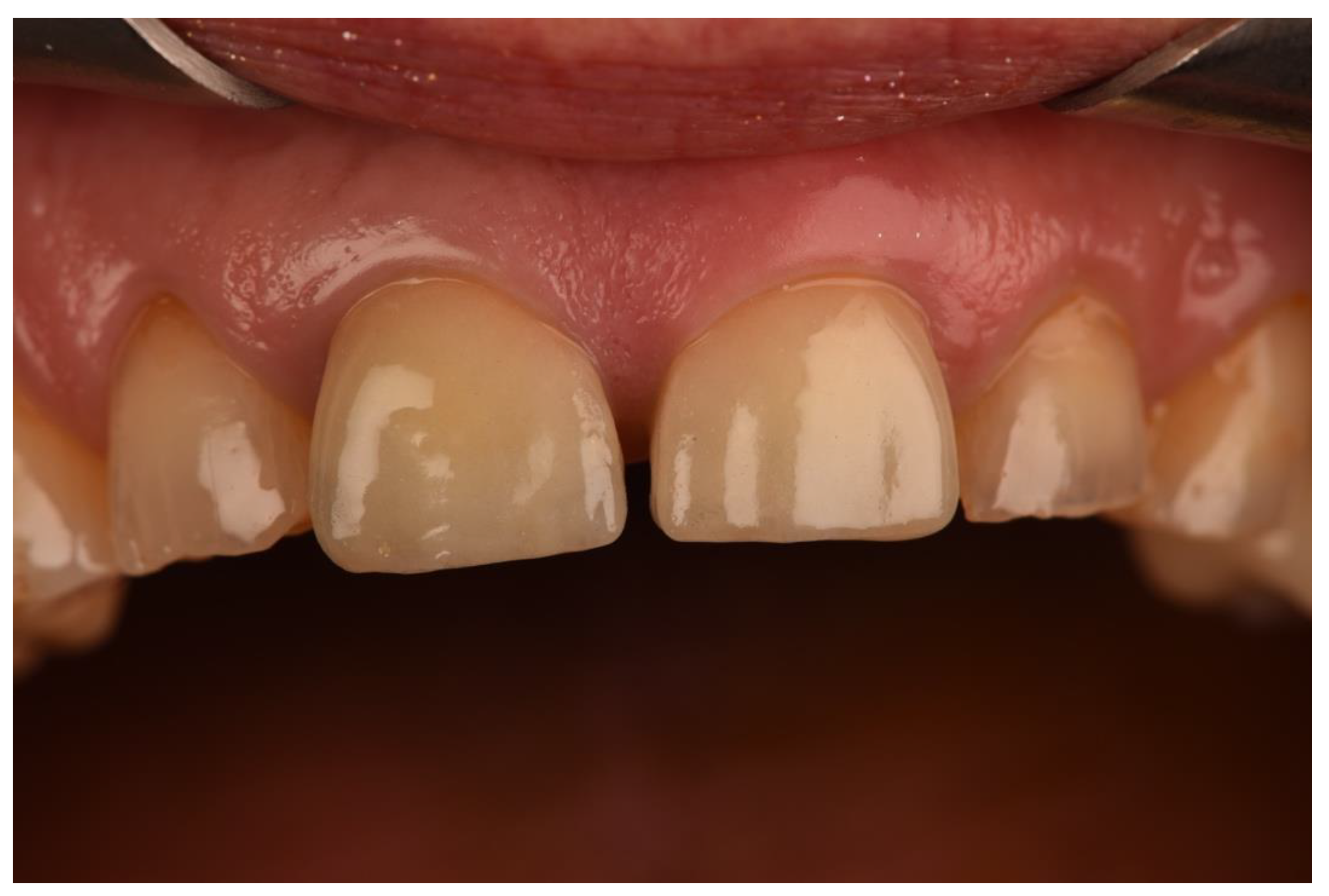
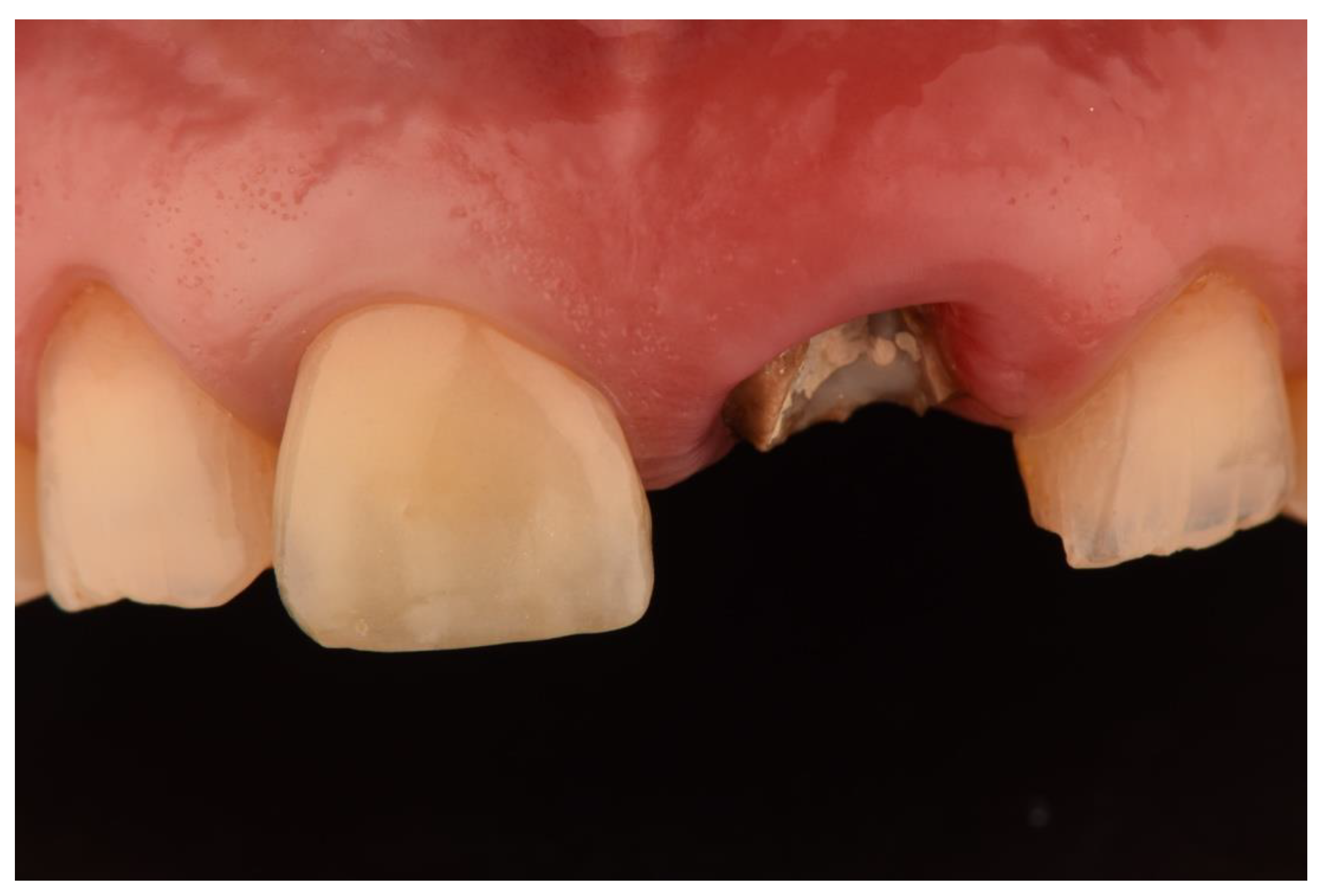
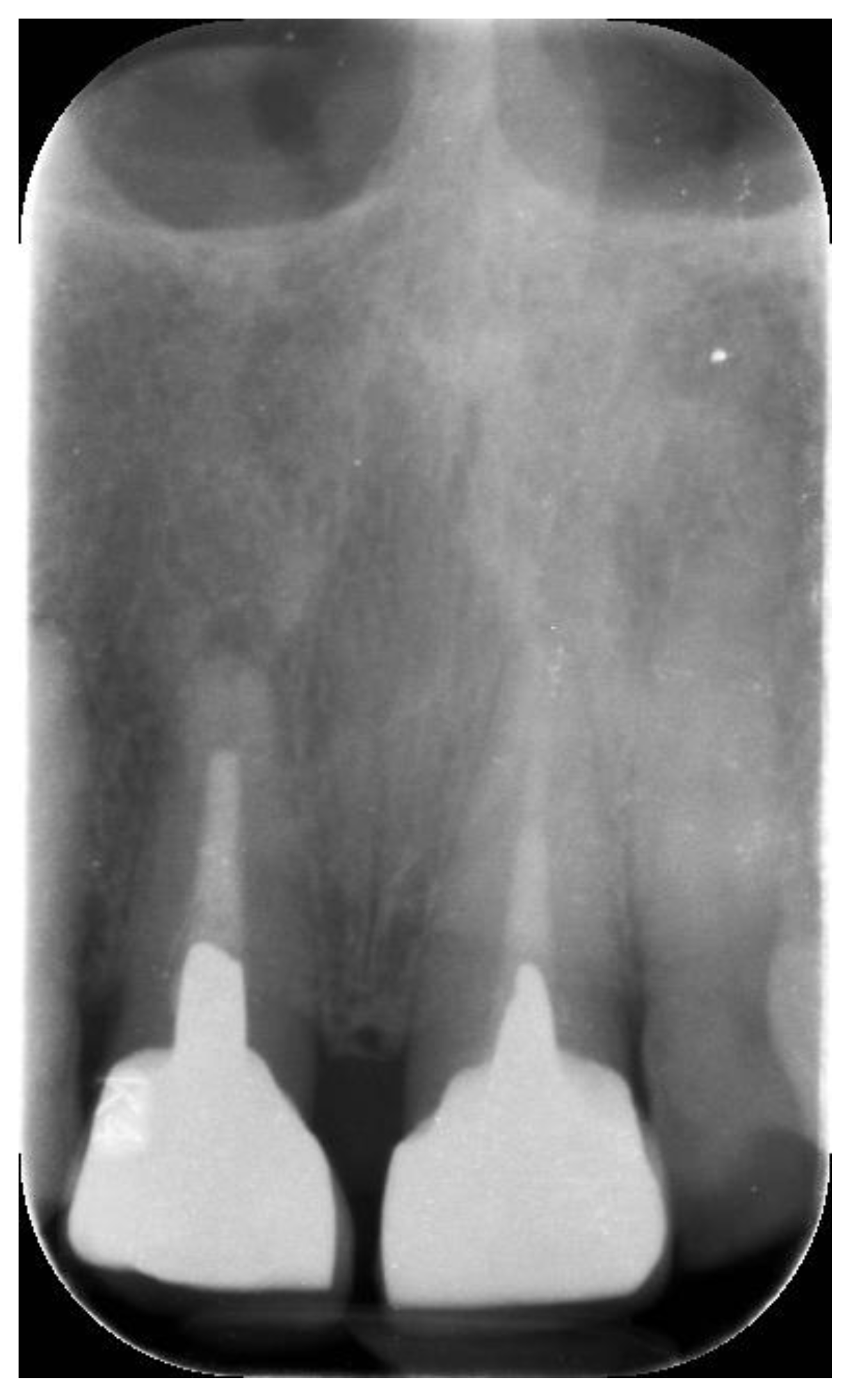

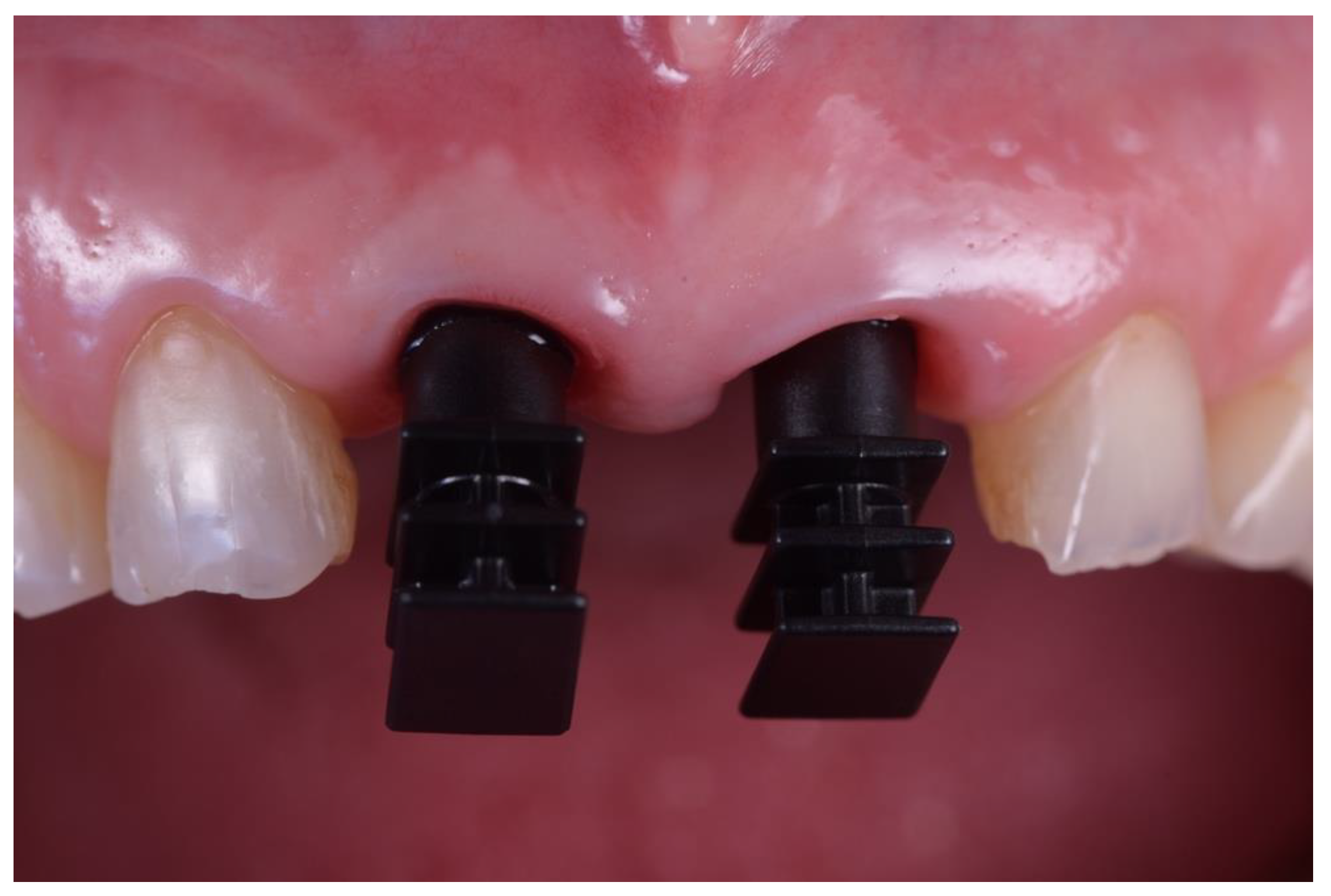
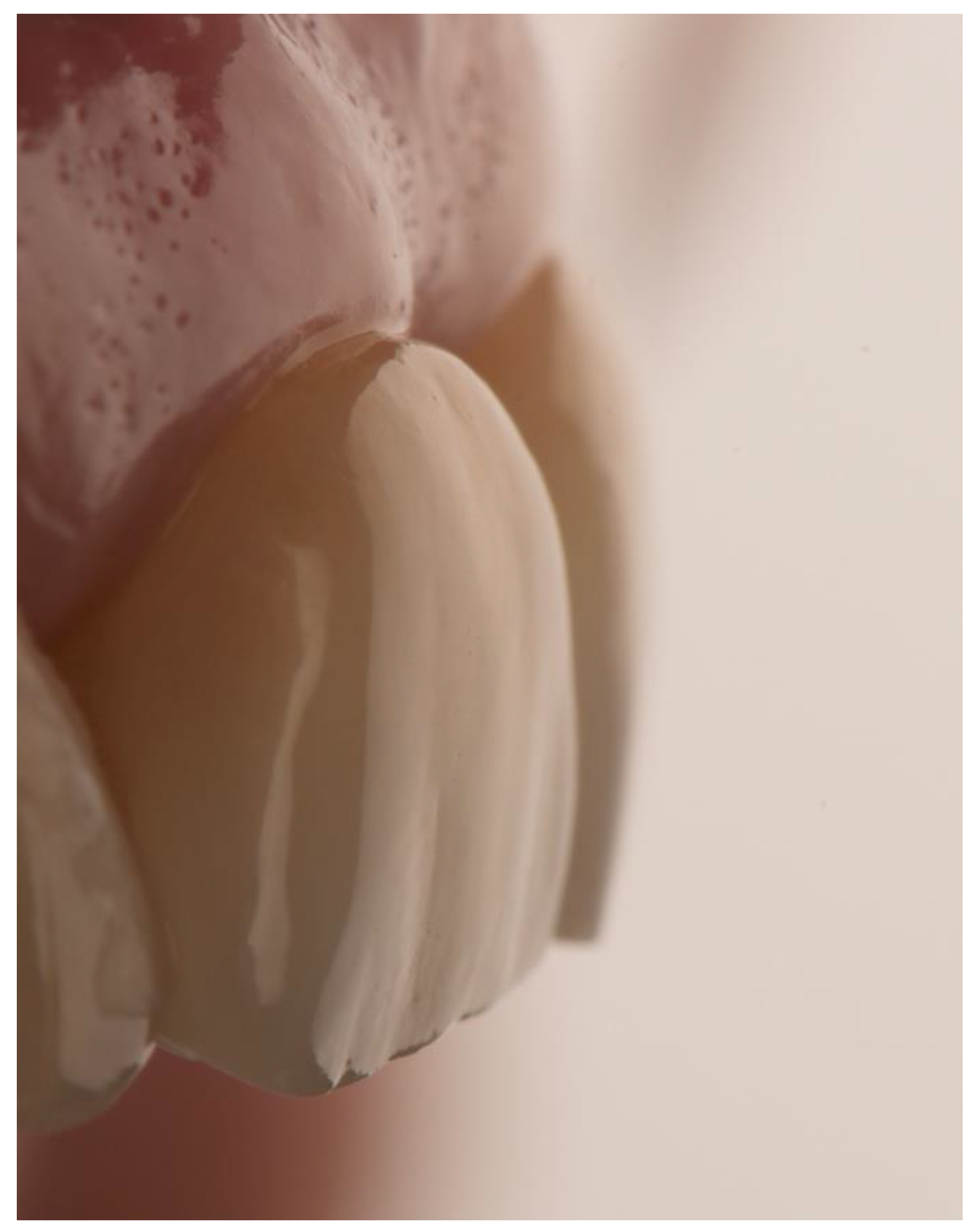
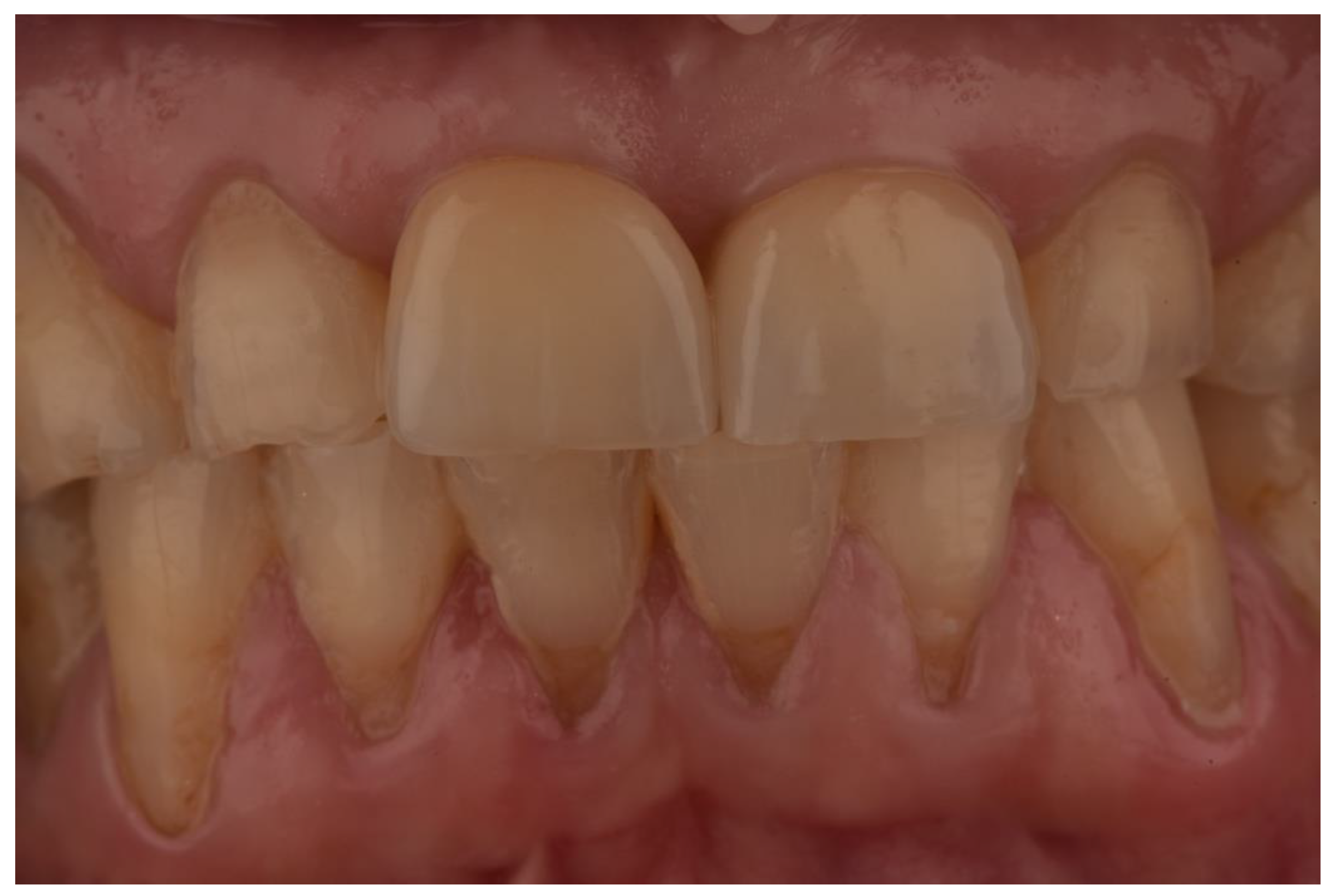
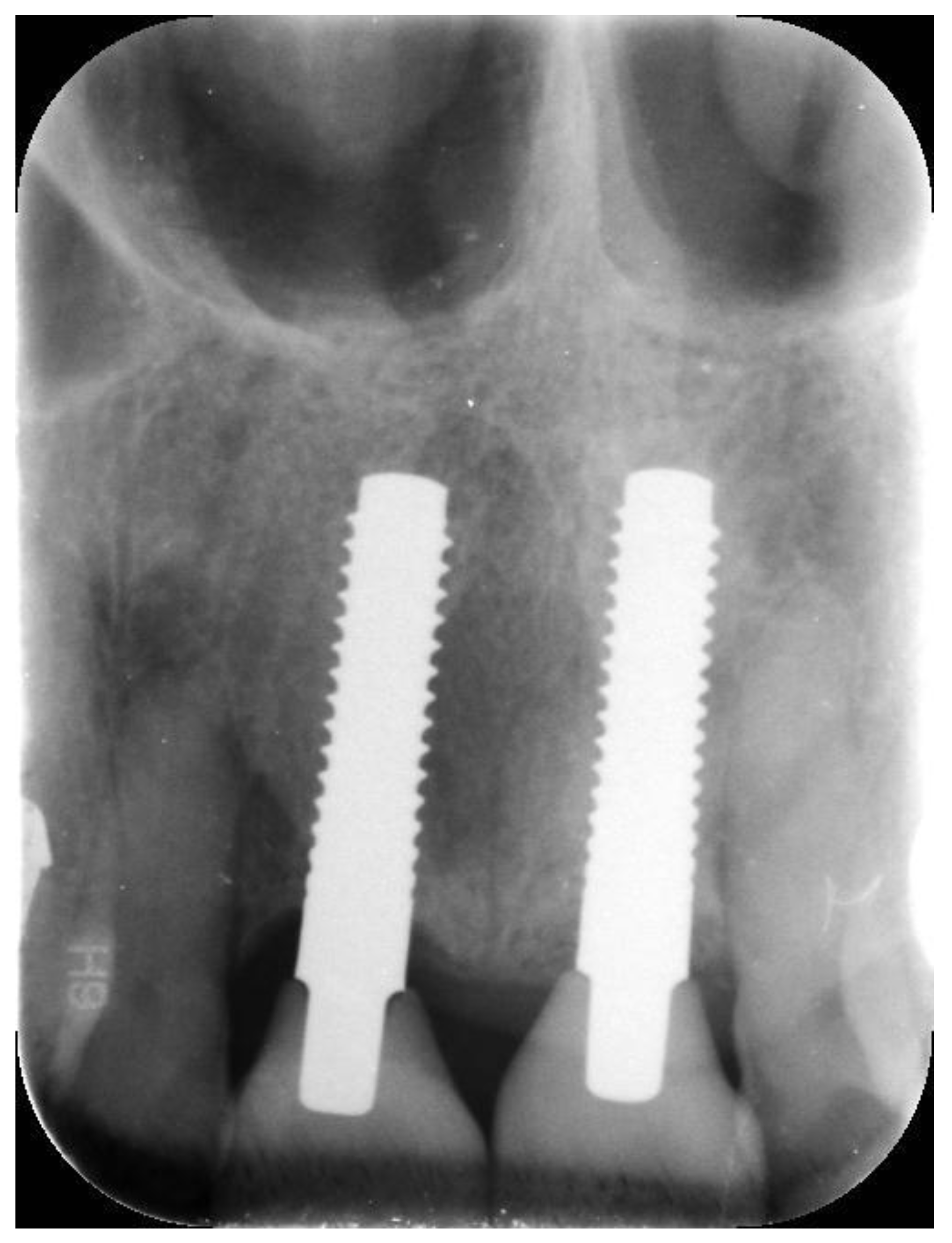
| N | Follow-Up (Months) | Age | Gender | Implant Position | Implant Diameter (mm) | Implant Length (mm) | GBR | Placement Timing (IP/DP) | Loading Timing (IL/CL) | Mean MBL (mm) (T0–T1) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 96 | 42 | F | 11 | 4.1 | 12 | N | IP | IL | 1.9–3.48 |
| 2 | 96 | 59 | F | 21 | 4.1 | 14 | Y | IP | IL | 1.41–1.70 |
| 3 | 94 | 41 | M | 13 | 4.1 | 12 | N | IP | IL | 2.11–3.2 |
| 4 | 92 | 50 | M | 12 | 3.3 | 12 | N | DP | IL | 2.35–3.3 |
| 5 | 83 | 35 | M | 14 | 3.3 | 12 | N | DP | IL | 1.57–1.92 |
| 6 | 88 | 37 | F | 15 | 4.1 | 12 | N | IP | IL | 2.73–2.74 |
| 7 | 73 | 43 | F | 46 | 3.3 | 12 | N | DP | CL | 1.8–1.87 |
| 8 | 73 | 46 | F | 46 | 4.1 | 12 | N | DP | CL | 1.35–1.69 |
| 9 | 62 | 37 | F | 46 | 4.1 | 10 | N | DP | CL | 1.94–2.06 |
| 10 | 59 | 47 | M | 12 | 4.1 | 12 | Y | IP | CL | 0.36–2.03 |
| 11 | 48 | 48 | M | 44 | 4.1 | 12 | N | IP | CL | 2.75–2.86 |
| 12 | 48 | 48 | M | 45 | 4.1 | 12 | N | IP | CL | 2.34–3.24 |
| 13 | 48 | 48 | M | 46 | 4.1 | 12 | N | IP | CL | 3.5–4.19 |
| 14 | 42 | 31 | M | 46 | 4.1 | 10 | N | DP | CL | 1.23–2.03 |
| 15 | 42 | 34 | M | 34 | 3.3 | 12 | N | IP | CL | 1.68–2.32 |
| 16 | 41 | 65 | F | 24 | 3.3 | 12 | Y | IP | IL | 2.1–2.82 |
| 17 | 40 | 64 | F | 21 | 3.3 | 14 | Y | IP | IL | 2.19–3.45 |
| 18 | 38 | 65 | F | 11 | 4.1 | 14 | N | IP | IL | 1.48–2.17 |
| 19 | 33 | 54 | F | 26 | 3.3 | 10 | N | IP | CL | 1.32–1.66 |
| 20 | 30 | 63 | F | 31 | 3.3 | 14 | N | IP | IL | 1.72–1.86 |
| 21 | 27 | 52 | M | 22 | 3.3 | 14 | Y | IP | CL | 1.49–2.53 |
| 22 | 27 | 69 | F | 36 | 4.1 | 12 | Y | DP | CL | 1.72–3.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeo, E.; Scaringi, R.; Lops, D.; Palazzolo, A. Single Crown Restorations Supported by One-Piece Zirconia Dental Implants: Case Series with a Mean Follow-Up of 58 Months. Prosthesis 2023, 5, 1060-1074. https://doi.org/10.3390/prosthesis5040074
Romeo E, Scaringi R, Lops D, Palazzolo A. Single Crown Restorations Supported by One-Piece Zirconia Dental Implants: Case Series with a Mean Follow-Up of 58 Months. Prosthesis. 2023; 5(4):1060-1074. https://doi.org/10.3390/prosthesis5040074
Chicago/Turabian StyleRomeo, Eugenio, Riccardo Scaringi, Diego Lops, and Antonino Palazzolo. 2023. "Single Crown Restorations Supported by One-Piece Zirconia Dental Implants: Case Series with a Mean Follow-Up of 58 Months" Prosthesis 5, no. 4: 1060-1074. https://doi.org/10.3390/prosthesis5040074
APA StyleRomeo, E., Scaringi, R., Lops, D., & Palazzolo, A. (2023). Single Crown Restorations Supported by One-Piece Zirconia Dental Implants: Case Series with a Mean Follow-Up of 58 Months. Prosthesis, 5(4), 1060-1074. https://doi.org/10.3390/prosthesis5040074








