Radiographic Analysis of Grammont-Style and Lateralized Reverse Shoulder Arthroplasty in Gleno-Humeral Osteoarthritis
Abstract
:1. Background
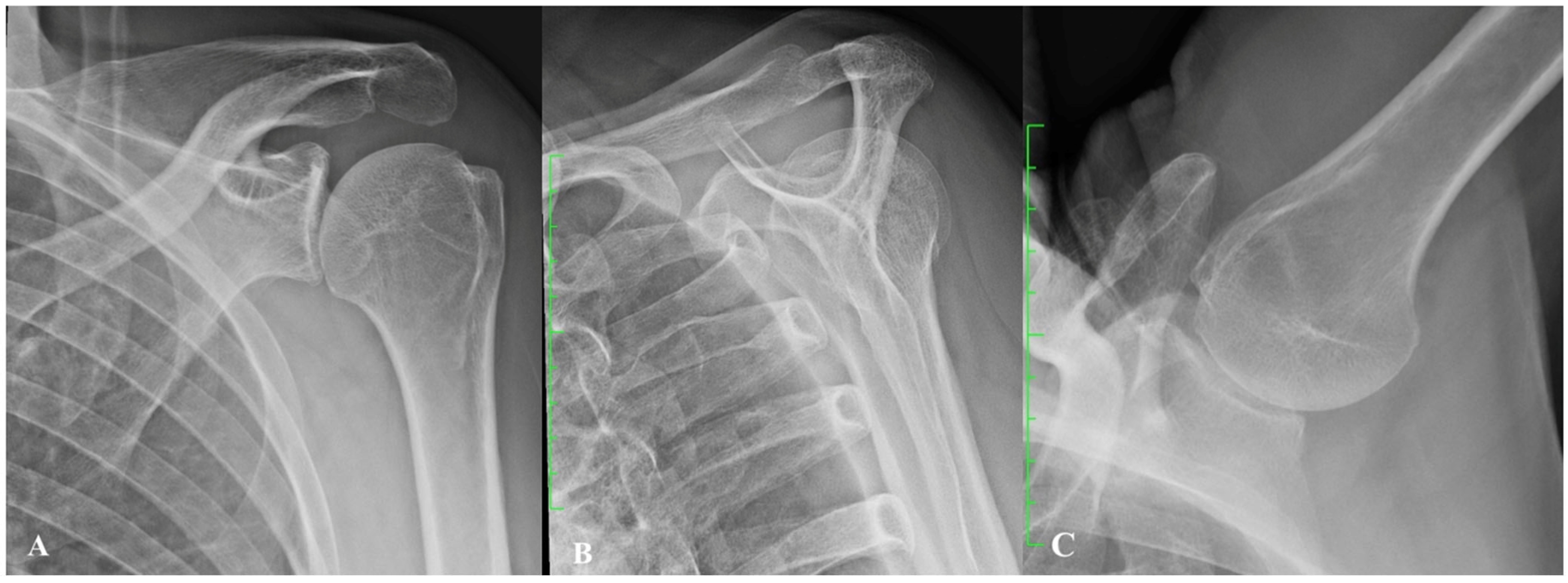
2. Gleno-Humeral Deformity in Primary Osteoarthritis and Cuff Tear Arthropathy
3. Biomechanics of Reverse Shoulder Arthroplasty
- Medialization and inferiorization of the joint CoR, achieved through medialization of the glenoid and the humerus with a 145° neck–shaft angle to increase the deltoid lever arm.
- Setting the CoR at the bone–prosthetic glenoid interface, thus reducing shear forces on the metal back.
- Distalization of the humerus, thus tensioning the deltoid to recover strength even from the onset of the motion.
- Semi-constrained design, obtained with a convex glenosphere and a concave humeral cup with the same curvature radius, creating a ball and socket joint and providing a stable fulcrum and ensuring static stability.
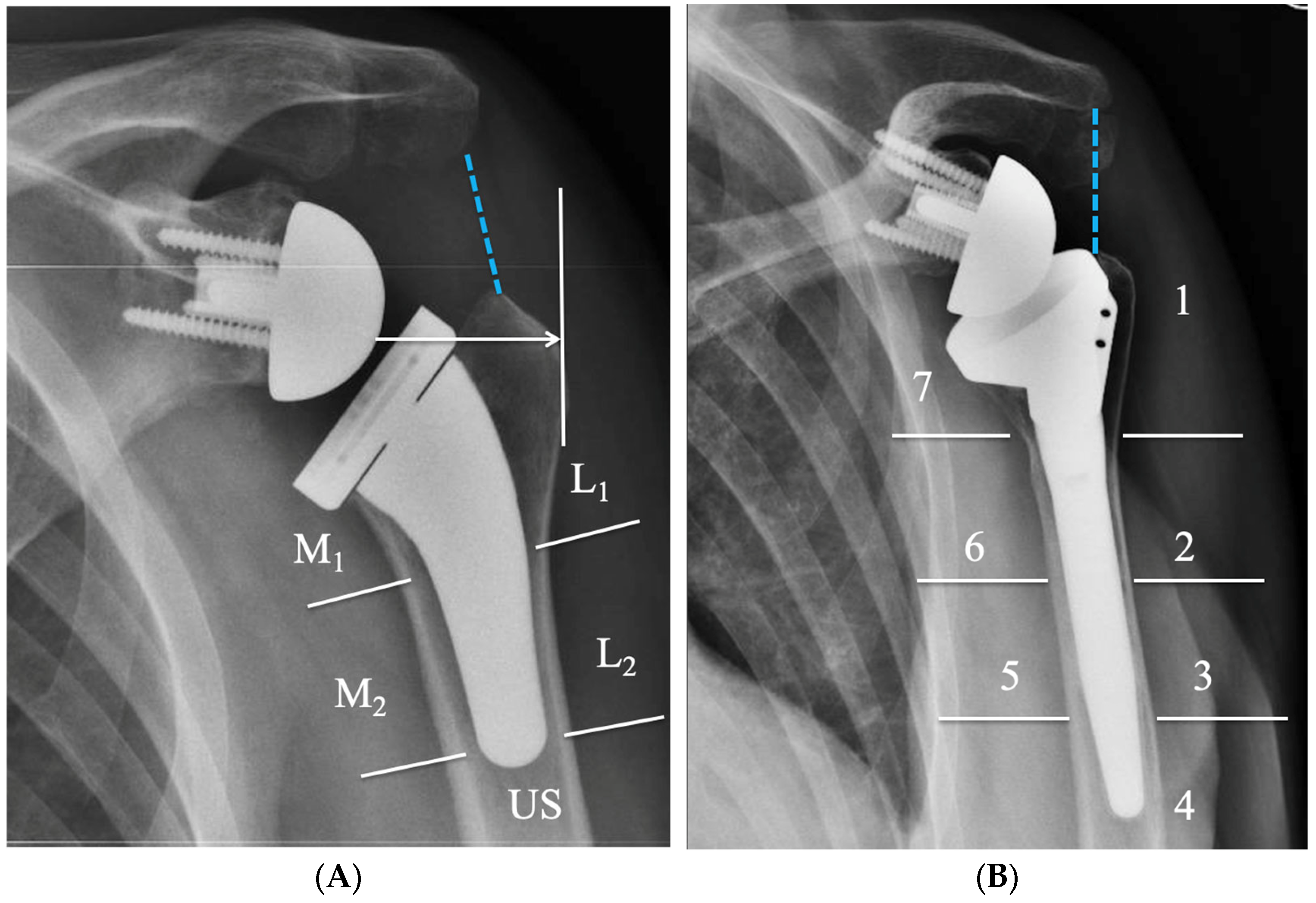
4. Radiographic Evaluation of Grammont-Style Reverse Arthroplasty
5. Radiographic Features of Lateralized Reverse Arthroplasty
6. Biomechanical Considerations of Lateralized Reverse Implants
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grammont, P.; Trouilloud, P.; Laffay, J.; Deries, X. Etude et Réalisation d’une Nouvelle Prothèse d’épaule. Rhumatologie 1987, 39, 407–418. [Google Scholar]
- Goetti, P.; Denard, P.J.; Collin, P.; Ibrahim, M.; Mazzolari, A.; Lädermann, A. Biomechanics of Anatomic and Reverse Shoulder Arthroplasty. EFORT Open Rev. 2021, 6, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Berliner, J.L.; Regalado-Magdos, A.; Ma, C.B.; Feeley, B.T. Biomechanics of Reverse Total Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2015, 24, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Frankle, M.; Levy, J.C.; Pupello, D.; Siegal, S.; Saleem, A.; Mighell, M.; Vasey, M. The Reverse Shoulder Prosthesis for Glenohumeral Arthritis Associated with Severe Rotator Cuff Deficiency. A Minimum Two-Year Follow-up Study of Sixty Patients Surgical Technique. J. Bone Jt. Surg. Am. 2006, 88 Pt 2 (Suppl. 1), 178–190. [Google Scholar] [CrossRef]
- Tashjian, R.Z.; Hillyard, B.; Childress, V.; Kawakami, J.; Presson, A.P.; Zhang, C.; Chalmers, P.N. Outcomes after a Grammont-Style Reverse Total Shoulder Arthroplasty? J. Shoulder Elb. Surg. 2021, 30, e10–e17. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Watkinson, D.; Hatzidakis, A.M.; Hovorka, I. Neer Award 2005: The Grammont Reverse Shoulder Prosthesis: Results in Cuff Tear Arthritis, Fracture Sequelae, and Revision Arthroplasty. J. Shoulder Elb. Surg. 2006, 15, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Rugg, C.M.; Coughlan, M.J.; Lansdown, D.A. Reverse Total Shoulder Arthroplasty: Biomechanics and Indications. Curr. Rev. Musculoskelet. Med. 2019, 12, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Kozak, T.; Bauer, S.; Walch, G.; Al-Karawi, S.; Blakeney, W. An Update on Reverse Total Shoulder Arthroplasty: Current Indications, New Designs, Same Old Problems. EFORT Open Rev. 2021, 6, 189–201. [Google Scholar] [CrossRef]
- National Joint Registry. 19th Annual Report 2022; NJR: London, UK, 2022. [Google Scholar]
- American Academy of Orthopaedic Surgeons. Shoulder & Elbow Registry (SER): 2020 Annual Report; American Academy of Orthopaedic Surgeons (AAOS): Rosemont, IL, USA, 2021. [Google Scholar]
- Harkness, E.F.; Macfarlane, G.J.; Silman, A.J.; McBeth, J. Is Musculoskeletal Pain More Common Now than 40 Years Ago?: Two Population-Based Cross-Sectional Studies. Rheumatology 2005, 44, 890–895. [Google Scholar] [CrossRef]
- Cadogan, A.; Laslett, M.; Hing, W.A.; McNair, P.J.; Coates, M.H. A Prospective Study of Shoulder Pain in Primary Care: Prevalence of Imaged Pathology and Response to Guided Diagnostic Blocks. BMC Musculoskelet. Disord. 2011, 12, 119. [Google Scholar] [CrossRef]
- Oh, J.H.; Chung, S.W.; Oh, C.H.; Kim, S.H.; Park, S.J.; Kim, K.W.; Park, J.H.; Lee, S.B.; Lee, J.J. The Prevalence of Shoulder Osteoarthritis in the Elderly Korean Population: Association with Risk Factors and Function. J. Shoulder Elb. Surg. 2011, 20, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Takagishi, K.; Shitara, H.; Ichinose, T.; Shimoyama, D.; Yamamoto, A.; Osawa, T.; Tajika, T. Prevalence of and Risk Factors for Shoulder Osteoarthritis in Japanese Middle-Aged and Elderly Populations. J. Shoulder Elb. Surg. 2014, 23, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, M.D.; Mercer, D.M.; Warme, W.J.; Bertelsen, A.L.; Matsen, F.A. Comparison of Patients Undergoing Primary Shoulder Arthroplasty before and after the Age of Fifty. J. Bone Jt. Surg. Am. 2010, 92, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of Osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Ibounig, T.; Simons, T.; Launonen, A.; Paavola, M. Glenohumeral Osteoarthritis: An Overview of Etiology and Diagnostics. Scand. J. Surg. 2021, 110, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Dieppe, P.A.; Lohmander, L.S. Pathogenesis and Management of Pain in Osteoarthritis. Lancet 2005, 365, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.; Watt, I.; Dieppe, P. Influence of Primary Generalised Osteoarthritis on Development of Secondary Osteoarthritis. Lancet 1983, 2, 8–11. [Google Scholar] [CrossRef]
- Fernández-Moreno, M.; Rego, I.; Carreira-Garcia, V.; Blanco, F.J. Genetics in Osteoarthritis. Curr. Genom. 2008, 9, 542–547. [Google Scholar] [CrossRef]
- Loughlin, J. Genetic Contribution to Osteoarthritis Development: Current State of Evidence. Curr. Opin. Rheumatol. 2015, 27, 284–288. [Google Scholar] [CrossRef]
- Chapman, K.; Valdes, A.M. Genetic Factors in OA Pathogenesis. Bone 2012, 51, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Adams, J.; Leddy, H.A.; McNulty, A.L.; O’Conor, C.J.; Guilak, F. The Mechanobiology of Articular Cartilage: Bearing the Burden of Osteoarthritis. Curr. Rheumatol. Rep. 2014, 16, 451. [Google Scholar] [CrossRef] [PubMed]
- Gürer, G.; Bozbas, G.T.; Tuncer, T.; Unubol, A.I.; Ucar, U.G.; Memetoglu, O.I. Frequency of Joint Hypermobility in Turkish Patients with Knee Osteoarthritis: A Cross Sectional Multicenter Study. Int. J. Rheum. Dis. 2018, 21, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
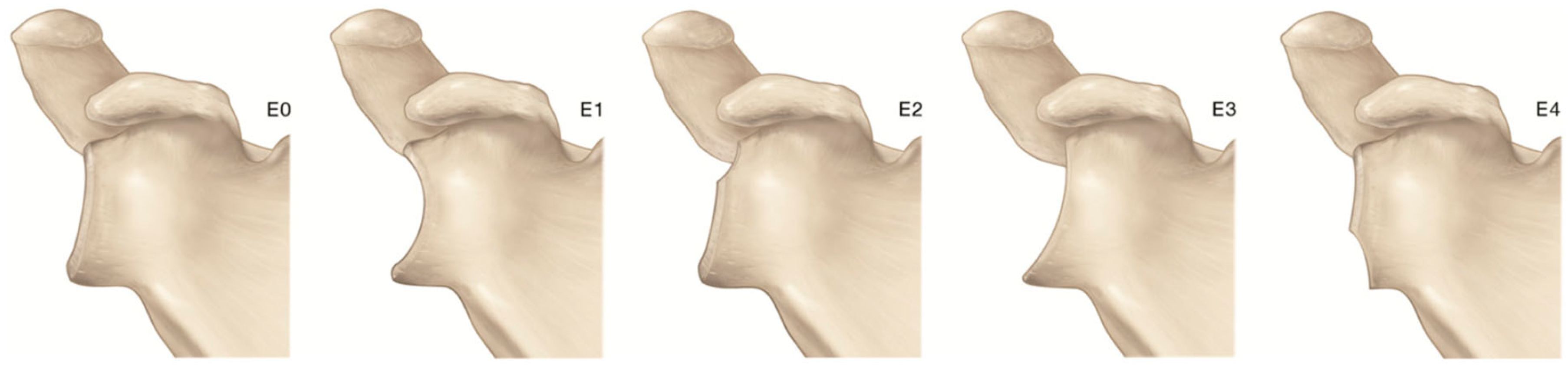
- Hamada, K.; Yamanaka, K.; Uchiyama, Y.; Mikasa, T.; Mikasa, M. A Radiographic Classification of Massive Rotator Cuff Tear Arthritis. Clin. Orthop. Relat. Res. 2011, 469, 2452–2460. [Google Scholar] [CrossRef] [PubMed]
- Samilson, R.L.; Prieto, V. Dislocation Arthropathy of the Shoulder. J. Bone Jt. Surg. Am. 1983, 65, 456–460. [Google Scholar] [CrossRef]
- Allain, J.; Goutallier, D.; Glorion, C. Long-Term Results of the Latarjet Procedure for the Treatment of Anterior Instability of the Shoulder. J. Bone Jt. Surg. Am. 1998, 80, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Gerber, C.; Maquieira, G.; Espinosa, N. Latissimus Dorsi Transfer for the Treatment of Irreparable Rotator Cuff Tears. J. Bone Jt. Surg. Am. 2006, 88, 113–120. [Google Scholar] [CrossRef]
- Guyette, T.M.; Bae, H.; Warren, R.F.; Craig, E.; Wickiewicz, T.L. Results of Arthroscopic Subacromial Decompression in Patients with Subacromial Impingement and Glenohumeral Degenerative Joint Disease. J. Shoulder Elb. Surg. 2002, 11, 299–304. [Google Scholar] [CrossRef]
- Hamada, K.; Fukuda, H.; Mikasa, M.; Kobayashi, Y. Roentgenographic Findings in Massive Rotator Cuff Tears. A Long-Term Observation. Clin. Orthop. Relat. Res. 1990, 24, 92–96. [Google Scholar] [CrossRef]
- Walch, G.; Edwards, T.B.; Boulahia, A.; Nové-Josserand, L.; Neyton, L.; Szabo, I. Arthroscopic Tenotomy of the Long Head of the Biceps in the Treatment of Rotator Cuff Tears: Clinical and Radiographic Results of 307 Cases. J. Shoulder Elb. Surg. 2005, 14, 238–246. [Google Scholar] [CrossRef]
- Habermeyer, P.; Magosch, P.; Luz, V.; Lichtenberg, S. Three-Dimensional Glenoid Deformity in Patients with Osteoarthritis: A Radiographic Analysis. J. Bone Jt. Surg. Am. 2006, 88, 1301–1307. [Google Scholar] [CrossRef]
- Friedman, R.J.; Hawthorne, K.B.; Genez, B.M. The Use of Computerized Tomography in the Measurement of Glenoid Version. J. Bone Jt. Surg. Am. 1992, 74, 1032–1037. [Google Scholar] [CrossRef]
- Bryce, C.D.; Davison, A.C.; Lewis, G.S.; Wang, L.; Flemming, D.J.; Armstrong, A.D. Two-Dimensional Glenoid Version Measurements Vary with Coronal and Sagittal Scapular Rotation. J. Bone Jt. Surg. Am. 2010, 92, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Bokor, D.J.; O’Sullivan, M.D.; Hazan, G.J. Variability of Measurement of Glenoid Version on Computed Tomography Scan. J. Shoulder Elb. Surg. 1999, 8, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, T.; De Wilde, L.; Victor, J. The Normal 3D Gleno-Humeral Relationship and Anatomy of the Glenoid Planes. J. Belg. Soc. Radiol. 2018, 102, 18. [Google Scholar] [CrossRef] [PubMed]
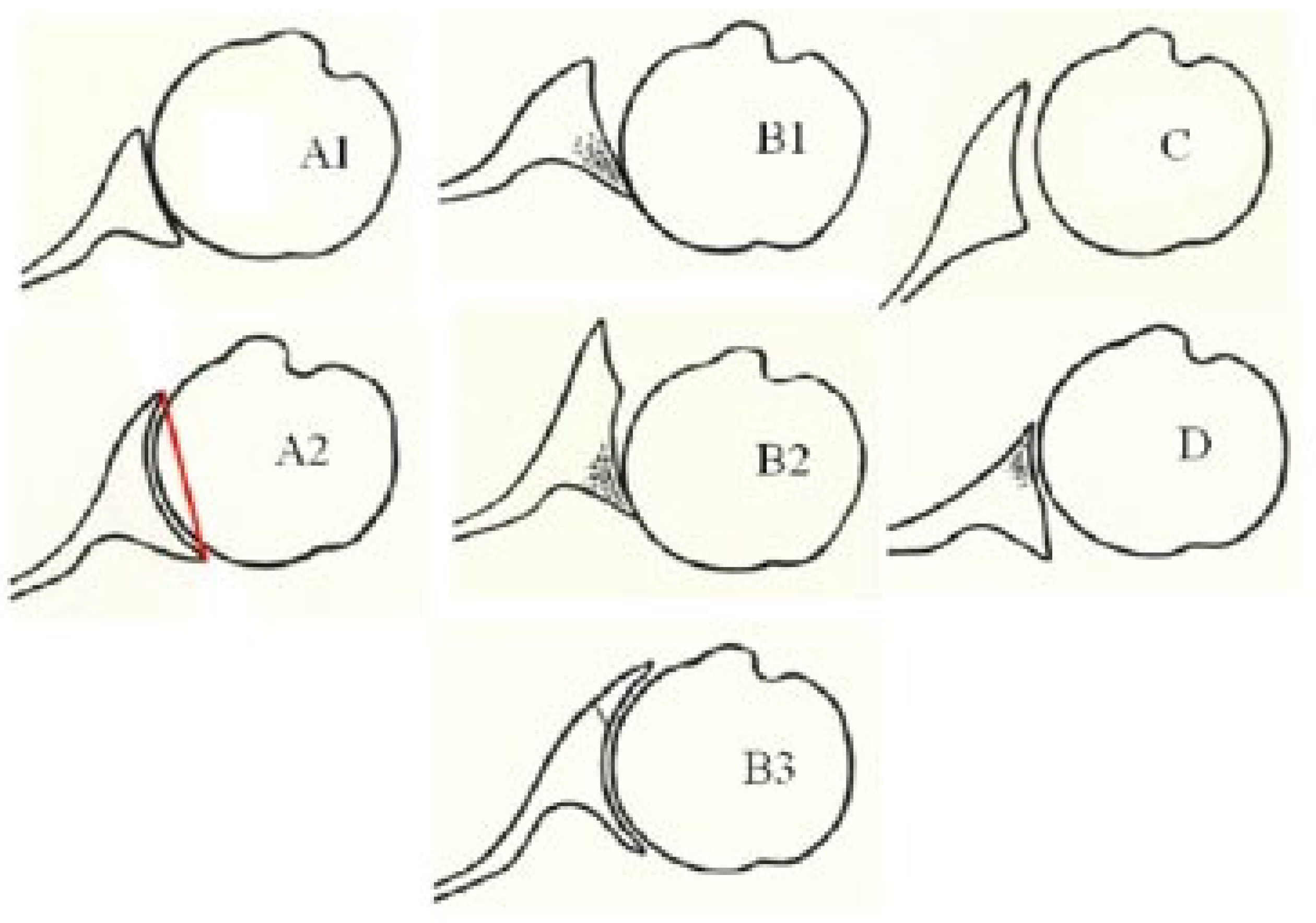
- Walch, G.; Badet, R.; Boulahia, A.; Khoury, A. Morphologic Study of the Glenoid in Primary Glenohumeral Osteoarthritis. J. Arthroplast. 1999, 14, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Kidder, J.F.; Rouleau, D.M.; DeFranco, M.J.; Pons-Villanueva, J.; Dynamidis, S. Revisited: Walch Classification of the Glenoid in Glenohumeral Osteoarthritis. Shoulder Elb. 2012, 4, 11–15. [Google Scholar] [CrossRef]
- Nowak, D.D.; Gardner, T.R.; Bigliani, L.U.; Levine, W.N.; Ahmad, C.S. Interobserver and Intraobserver Reliability of the Walch Classification in Primary Glenohumeral Arthritis. J. Shoulder Elb. Surg. 2010, 19, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Scalise, J.J.; Codsi, M.J.; Brems, J.J.; Iannotti, J.P. Inter-Rater Reliability of an Arthritic Glenoid Morphology Classification System. J. Shoulder Elb. Surg. 2008, 17, 575–577. [Google Scholar] [CrossRef]
- Scalise, J.J.; Codsi, M.J.; Bryan, J.; Brems, J.J.; Iannotti, J.P. The Influence of Three-Dimensional Computed Tomography Images of the Shoulder in Preoperative Planning for Total Shoulder Arthroplasty. J. Bone Jt. Surg. Am. 2008, 90, 2438–2445. [Google Scholar] [CrossRef]
- Budge, M.D.; Lewis, G.S.; Schaefer, E.; Coquia, S.; Flemming, D.J.; Armstrong, A.D. Comparison of Standard Two-Dimensional and Three-Dimensional Corrected Glenoid Version Measurements. J. Shoulder Elb. Surg. 2011, 20, 577–583. [Google Scholar] [CrossRef]
- Daggett, M.; Werner, B.; Gauci, M.O.; Chaoui, J.; Walch, G. Comparison of Glenoid Inclination Angle Using Different Clinical Imaging Modalities. J. Shoulder Elb. Surg. 2016, 25, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.W.; Powell, K.A.; Yum, J.K.; Brems, J.J.; Iannotti, J.P. Use of Three-Dimensional Computed Tomography for the Analysis of the Glenoid Anatomy. J. Shoulder Elb. Surg. 2005, 14, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Bercik, M.J.; Kruse, K.; Yalizis, M.; Gauci, M.-O.; Chaoui, J.; Walch, G. A Modification to the Walch Classification of the Glenoid in Primary Glenohumeral Osteoarthritis Using Three-Dimensional Imaging. J. Shoulder Elb. Surg. 2016, 25, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Frankle, M.A.; Teramoto, A.; Luo, Z.-P.; Levy, J.C.; Pupello, D. Glenoid Morphology in Reverse Shoulder Arthroplasty: Classification and Surgical Implications. J. Shoulder Elb. Surg. 2009, 18, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Visotsky, J.L.; Basamania, C.; Seebauer, L.; Rockwood, C.A.; Jensen, K.L. Cuff Tear Arthropathy: Pathogenesis, Classification, and Algorithm for Treatment. J. Bone Jt. Surg. Am. 2004, 86 (Suppl. 2), 35–40. [Google Scholar] [CrossRef]
- Klein, S.M.; Dunning, P.; Mulieri, P.; Pupello, D.; Downes, K.; Frankle, M.A. Effects of Acquired Glenoid Bone Defects on Surgical Technique and Clinical Outcomes in Reverse Shoulder Arthroplasty. J. Bone Jt. Surg. Am. 2010, 92, 1144–1154. [Google Scholar] [CrossRef]
- Sirveaux, F.; Favard, L.; Oudet, D.; Huquet, D.; Walch, G.; Molé, D. Grammont Inverted Total Shoulder Arthroplasty in the Treatment of Glenohumeral Osteoarthritis with Massive Rupture of the Cuff. Results of a Multicentre Study of 80 Shoulders. J. Bone Jt. Surg. Br. 2004, 86, 388–395. [Google Scholar] [CrossRef]
- Beuckelaers, E.; Jacxsens, M.; Van Tongel, A.; De Wilde, L.F. Three-Dimensional Computed Tomography Scan Evaluation of the Pattern of Erosion in Type B Glenoids. J. Shoulder Elb. Surg. 2014, 23, 109–116. [Google Scholar] [CrossRef]
- Knowles, N.K.; Keener, J.D.; Ferreira, L.M.; Athwal, G.S. Quantification of the Position, Orientation, and Surface Area of Bone Loss in Type B2 Glenoids. J. Shoulder Elb. Surg. 2015, 24, 503–510. [Google Scholar] [CrossRef]
- Boileau, P.; Morin-Salvo, N.; Gauci, M.-O.; Seeto, B.L.; Chalmers, P.N.; Holzer, N.; Walch, G. Angled BIO-RSA (Bony-Increased Offset-Reverse Shoulder Arthroplasty): A Solution for the Management of Glenoid Bone Loss and Erosion. J. Shoulder Elb. Surg. 2017, 26, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K.W.; Ricchetti, E.T.; Iannotti, J.P. Surgical Management of the Biconcave (B2) Glenoid. Curr. Rev. Musculoskelet. Med. 2016, 9, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.E.; Ricchetti, E.T.; Huffman, G.R.; Iannotti, J.P.; Glaser, D.L. Addressing Glenoid Bone Deficiency and Asymmetric Posterior Erosion in Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2013, 22, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Scheiderer, B.; Murphy, M.; Savino, A.; Mehl, J.; Kia, C.; Obopilwe, E.; DiVenere, J.; Cote, M.P.; Denard, P.J.; et al. Biconcave Glenoids Show 3 Differently Oriented Posterior Erosion Patterns. J. Shoulder Elb. Surg. 2021, 30, 2620–2628. [Google Scholar] [CrossRef] [PubMed]
- Werthel, J.-D.; Walch, G.; Vegehan, E.; Deransart, P.; Sanchez-Sotelo, J.; Valenti, P. Lateralization in Reverse Shoulder Arthroplasty: A Descriptive Analysis of Different Implants in Current Practice. Int. Orthop. 2019, 43, 2349–2360. [Google Scholar] [CrossRef]
- Luthringer, T.A.; Larose, G.; Kwon, Y.; Zuckerman, J.D.; Virk, M.S. Reverse Total Shoulder Arthroplasty Biomechanical Considerations and the Concept of Lateralization. Bull. Hosp. Jt. Dis. 2022, 80, 65–74. [Google Scholar]
- Galvin, J.W.; Kim, R.; Ment, A.; Durso, J.; Joslin, P.M.N.; Lemos, J.L.; Novikov, D.; Curry, E.J.; Alley, M.C.; Parada, S.A.; et al. Outcomes and Complications of Primary Reverse Shoulder Arthroplasty with Minimum of 2 Years’ Follow-up: A Systematic Review and Meta-Analysis. J. Shoulder Elb. Surg. 2022, 31, e534–e544. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Watkinson, D.J.; Hatzidakis, A.M.; Balg, F. Grammont Reverse Prosthesis: Design, Rationale, and Biomechanics. J. Shoulder Elb. Surg. 2005, 14, S147–S161. [Google Scholar] [CrossRef] [PubMed]
- Routman, H.D.; Flurin, P.-H.; Wright, T.W.; Zuckerman, J.D.; Hamilton, M.A.; Roche, C.P. Reverse Shoulder Arthroplasty Prosthesis Design Classification System. Bull. Hosp. Jt. Dis. 2015, 73 (Suppl. 1), S5–S14. [Google Scholar]
- Wall, B.; Nové-Josserand, L.; O’Connor, D.P.; Edwards, T.B.; Walch, G. Reverse Total Shoulder Arthroplasty: A Review of Results According to Etiology. J. Bone Jt. Surg. Am. 2007, 89, 1476–1485. [Google Scholar] [CrossRef]
- Shah, S.S.; Roche, A.M.; Sullivan, S.W.; Gaal, B.T.; Dalton, S.; Sharma, A.; King, J.J.; Grawe, B.M.; Namdari, S.; Lawler, M.; et al. The Modern Reverse Shoulder Arthroplasty and an Updated Systematic Review for Each Complication: Part II. JSES INTL 2021, 5, 121–137. [Google Scholar] [CrossRef]
- Henninger, H.B.; Barg, A.; Anderson, A.E.; Bachus, K.N.; Burks, R.T.; Tashjian, R.Z. Effect of Lateral Offset Center of Rotation in Reverse Total Shoulder Arthroplasty: A Biomechanical Study. J. Shoulder Elb. Surg. 2012, 21, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Collotte, P.; Gauci, M.-O.; Vieira, T.D.; Walch, G. Bony Increased-Offset Reverse Total Shoulder Arthroplasty (BIO-RSA) Associated with an Eccentric Glenosphere and an Onlay 135° Humeral Component: Clinical and Radiological Outcomes at a Minimum 2-Year Follow-Up. JSES Int. 2022, 6, 434–441. [Google Scholar] [CrossRef]
- Shah, S.S.; Gaal, B.T.; Roche, A.M.; Namdari, S.; Grawe, B.M.; Lawler, M.; Dalton, S.; King, J.J.; Helmkamp, J.; Garrigues, G.E.; et al. The Modern Reverse Shoulder Arthroplasty and an Updated Systematic Review for Each Complication: Part I. JSES INTL 2020, 4, 929–943. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Moineau, G.; Roussanne, Y.; O’Shea, K. Bony Increased-Offset Reversed Shoulder Arthroplasty: Minimizing Scapular Impingement While Maximizing Glenoid Fixation. Clin. Orthop. Relat. Res. 2011, 469, 2558. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.; Valenti, P.; Kany, J.; Elkholti, K.; Werthel, J.-D. Does Lateralisation of the Centre of Rotation in Reverse Shoulder Arthroplasty Avoid Scapular Notching? Clinical and Radiological Review of One Hundred and Forty Cases with Forty Five Months of Follow-Up. Int. Orthop. 2016, 40, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Mulieri, P.; Dunning, P.; Klein, S.; Pupello, D.; Frankle, M. Reverse Shoulder Arthroplasty for the Treatment of Irreparable Rotator Cuff Tear without Glenohumeral Arthritis. JBJS 2010, 92, 2544. [Google Scholar] [CrossRef] [PubMed]
- Dimock, R.; Fathi Elabd, M.; Imam, M.; Middleton, M.; Godenèche, A.; Narvani, A.A. Bony Increased-Offset Reverse Shoulder Arthroplasty: A Meta-Analysis of the Available Evidence. Shoulder Elb. 2021, 13, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Levy, J.C.; Frankle, M.A.; Cuff, D.; Keller, T.S.; Pupello, D.R.; Lee, W.E. Evaluation of Abduction Range of Motion and Avoidance of Inferior Scapular Impingement in a Reverse Shoulder Model. J. Shoulder Elb. Surg. 2008, 17, 608–615. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Comiskey, C.A.; Luo, Z.-P.; Pupello, D.R.; Frankle, M.A. Range of Impingement-Free Abduction and Adduction Deficit after Reverse Shoulder Arthroplasty. Hierarchy of Surgical and Implant-Design-Related Factors. J. Bone Jt. Surg. Am. 2008, 90, 2606–2615. [Google Scholar] [CrossRef]
- Werner, B.S.; Chaoui, J.; Walch, G. The Influence of Humeral Neck Shaft Angle and Glenoid Lateralization on Range of Motion in Reverse Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2017, 26, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.A.; Diep, P.; Roche, C.; Flurin, P.H.; Wright, T.W.; Zuckerman, J.D.; Routman, H. Effect of Reverse Shoulder Design Philosophy on Muscle Moment Arms. J. Orthop. Res. 2015, 33, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Denard, P.J.; Lederman, E.; Parsons, B.O.; Romeo, A.A. Finite Element Analysis of Glenoid-Sided Lateralization in Reverse Shoulder Arthroplasty. J. Orthop. Res. 2017, 35, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.W.; Langohr, G.D.G.; Johnson, J.A.; Athwal, G.S. Implant Design Variations in Reverse Total Shoulder Arthroplasty Influence the Required Deltoid Force and Resultant Joint Load. Clin. Orthop. Relat. Res. 2015, 473, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- Schnetzke, M.; Coda, S.; Raiss, P.; Walch, G.; Loew, M. Radiologic Bone Adaptations on a Cementless Short-Stem Shoulder Prosthesis. J. Shoulder Elb. Surg. 2016, 25, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Nagels, J.; Stokdijk, M.; Rozing, P.M. Stress Shielding and Bone Resorption in Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2003, 12, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Berthold, D.P.; Morikawa, D.; Muench, L.N.; Baldino, J.B.; Cote, M.P.; Creighton, R.A.; Denard, P.J.; Gobezie, R.; Lederman, E.; Romeo, A.A.; et al. Negligible Correlation between Radiographic Measurements and Clinical Outcomes in Patients Following Primary Reverse Total Shoulder Arthroplasty. J. Clin. Med. 2021, 10, 809. [Google Scholar] [CrossRef] [PubMed]
- Lignel, A.; Berhouet, J.; Loirat, M.-A.; Collin, P.; Thomazeau, H.; Gallinet, D.; Boileau, P.; Favard, L. Reverse Shoulder Arthroplasty for Proximal Humerus Fractures: Is the Glenoid Implant Problematic? Orthop. Traumatol. Surg. Res. 2018, 104, 773–777. [Google Scholar] [CrossRef]
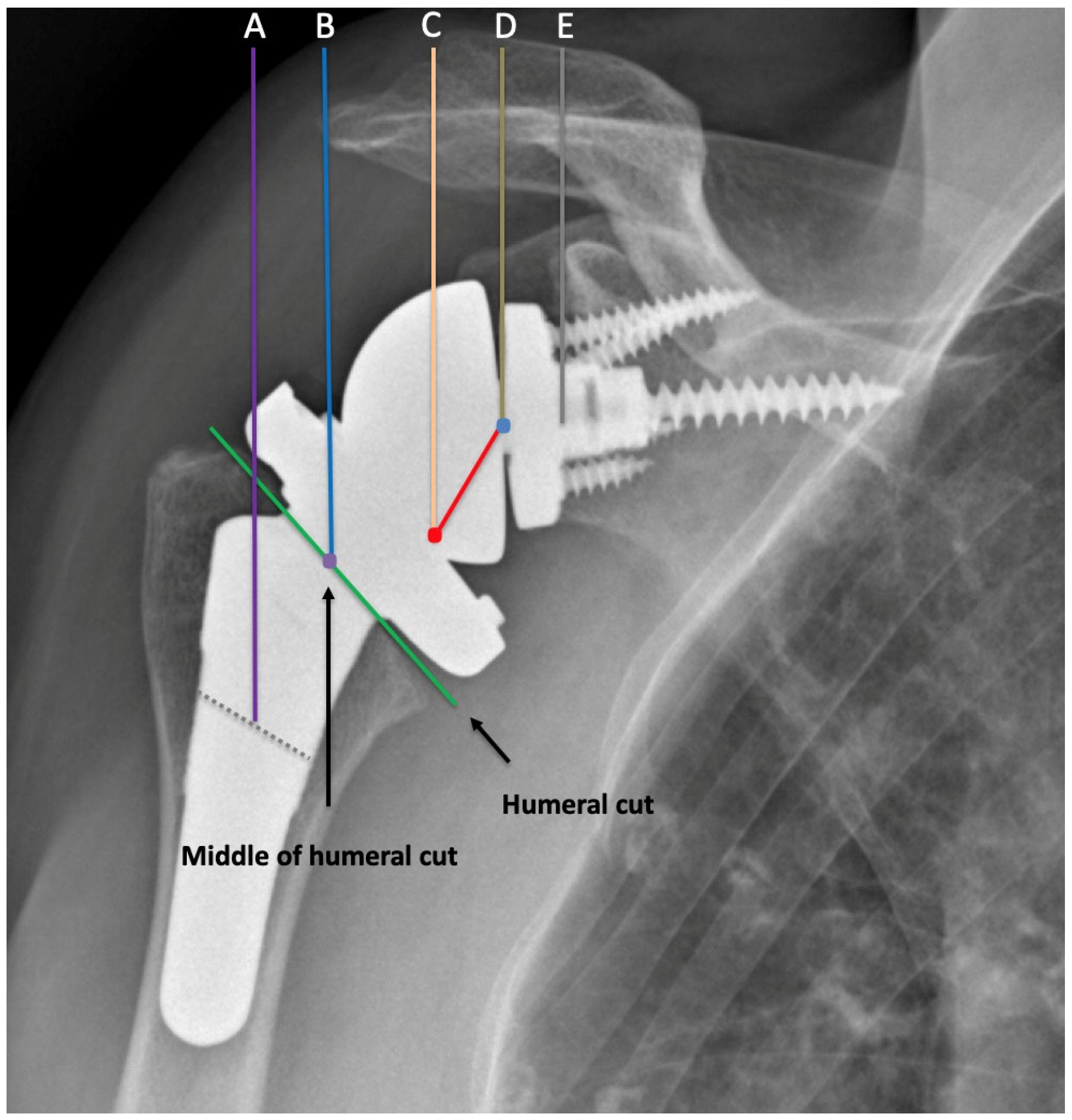
- Haidamous, G.; Lädermann, A.; Hartzler, R.U.; Parsons, B.O.; Lederman, E.S.; Tokish, J.M.; Denard, P.J. Radiographic Parameters Associated with Excellent versus Poor Range of Motion Outcomes Following Reverse Shoulder Arthroplasty. Shoulder Elb. 2022, 14, 39–47. [Google Scholar] [CrossRef]
- Nyffeler, R.W.; Werner, C.M.L.; Gerber, C. Biomechanical Relevance of Glenoid Component Positioning in the Reverse Delta III Total Shoulder Prosthesis. J. Shoulder Elb. Surg. 2005, 14, 524–528. [Google Scholar] [CrossRef]
- Li, X.; Dines, J.S.; Warren, R.F.; Craig, E.V.; Dines, D.M. Inferior Glenosphere Placement Reduces Scapular Notching in Reverse Total Shoulder Arthroplasty. Orthopedics 2015, 38, e88–e93. [Google Scholar] [CrossRef] [PubMed]
- Maurer, A.; Fucentese, S.F.; Pfirrmann, C.W.A.; Wirth, S.H.; Djahangiri, A.; Jost, B.; Gerber, C. Assessment of Glenoid Inclination on Routine Clinical Radiographs and Computed Tomography Examinations of the Shoulder. J. Shoulder Elb. Surg. 2012, 21, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Gauci, M.-O.; Wagner, E.R.; Clowez, G.; Chaoui, J.; Chelli, M.; Walch, G. The Reverse Shoulder Arthroplasty Angle: A New Measurement of Glenoid Inclination for Reverse Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2019, 28, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Werthel, J.-D.; Villard, A.; Kazum, E.; Deransart, P.; Ramirez, O. Accuracy of Reverse Shoulder Arthroplasty Angle According to the Size of the Baseplate. J. Shoulder Elb. Surg. 2023, 32, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Falaise, V.; Levigne, C.; Favard, L. Scapular Notching in Reverse Shoulder Arthroplasties: The Influence of Glenometaphyseal Angle. Orthop. Traumatol. Surg. Res. 2011, 97, S131–S137. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Walker, M.; Willis, M.; Pupello, D.R.; Frankle, M.A. Effects of Tilt and Glenosphere Eccentricity on Baseplate/Bone Interface Forces in a Computational Model, Validated by a Mechanical Model, of Reverse Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2011, 20, 732–739. [Google Scholar] [CrossRef]
- Laver, L.; Garrigues, G.E. Avoiding Superior Tilt in Reverse Shoulder Arthroplasty: A Review of the Literature and Technical Recommendations. J. Shoulder Elb. Surg. 2014, 23, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Roche, C.P.; Stroud, N.J.; Martin, B.L.; Steiler, C.A.; Flurin, P.-H.; Wright, T.W.; DiPaola, M.J.; Zuckerman, J.D. The Impact of Scapular Notching on Reverse Shoulder Glenoid Fixation. J. Shoulder Elb. Surg. 2013, 22, 963–970. [Google Scholar] [CrossRef]
- Mollon, B.; Mahure, S.A.; Roche, C.P.; Zuckerman, J.D. Impact of Scapular Notching on Clinical Outcomes after Reverse Total Shoulder Arthroplasty: An Analysis of 476 Shoulders. J. Shoulder Elb. Surg. 2017, 26, 1253–1261. [Google Scholar] [CrossRef]
- Lévigne, C.; Garret, J.; Boileau, P.; Alami, G.; Favard, L.; Walch, G. Scapular Notching in Reverse Shoulder Arthroplasty: Is It Important to Avoid It and How? Clin. Orthop. Relat. Res. 2011, 469, 2512. [Google Scholar] [CrossRef]
- Melis, B.; DeFranco, M.; Lädermann, A.; Molé, D.; Favard, L.; Nérot, C.; Maynou, C.; Walch, G. An Evaluation of the Radiological Changes around the Grammont Reverse Geometry Shoulder Arthroplasty after Eight to 12 Years. J. Bone Jt. Surg. Br. 2011, 93, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Lopiz, Y.; Galán-Olleros, M.; Rodriguez-Rodriguez, L.; García-Fernández, C.; Marco, F. Radiographic Changes around the Glenoid Component in Primary Reverse Shoulder Arthroplasty at Mid-Term Follow-Up. J. Shoulder Elb. Surg. 2021, 30, e378–e391. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.C.; Anderson, C.; Samson, A. Classification of Postoperative Acromial Fractures Following Reverse Shoulder Arthroplasty. J. Bone Jt. Surg. Am. 2013, 95, e104. [Google Scholar] [CrossRef] [PubMed]
- Lädermann, A.; Chiu, J.C.-H.; Cunningham, G.; Hervé, A.; Piotton, S.; Bothorel, H.; Collin, P. Do Short Stems Influence the Cervico-Diaphyseal Angle and the Medullary Filling after Reverse Shoulder Arthroplasties? Orthop. Traumatol. Surg. Res. 2020, 106, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Tross, A.K.; Woolson, T.E.; Nolte, P.C.; Schnetzke, M.; Loew, M.; Millett, P.J. Primary Reverse Shoulder Replacement with a Short Stem: A Systematic Literature Review. JSES Rev. Rep. Tech. 2021, 1, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, S.M.; Norris, T.R. Radiographic Changes and Clinical Outcomes Associated with an Adjustable Diaphyseal Press-Fit Humeral Stem in Primary Reverse Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2017, 26, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sotelo, J.; Wright, T.W.; O’Driscoll, S.W.; Cofield, R.H.; Rowland, C.M. Radiographic Assessment of Uncemented Humeral Components in Total Shoulder Arthroplasty. J. Arthroplast. 2001, 16, 180–187. [Google Scholar] [CrossRef]
- Sperling, J.W.; Cofield, R.H.; O’Driscoll, S.W.; Torchia, M.E.; Rowland, C.M. Radiographic Assessment of Ingrowth Total Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2000, 9, 507–513. [Google Scholar] [CrossRef]
- Werner, B.S.; Jacquot, A.; Molé, D.; Walch, G. Is Radiographic Measurement of Acromiohumeral Distance on Anteroposterior View after Reverse Shoulder Arthroplasty Reliable? J. Shoulder Elb. Surg. 2016, 25, e276–e280. [Google Scholar] [CrossRef]
- Kim, D.-H.; Choi, H.-U.; Choi, B.-C.; Kim, J.-H.; Cho, C.-H. Postoperative Acromiohumeral Interval Affects Shoulder Range of Motions Following Reverse Total Shoulder Arthroplasty. Sci. Rep. 2022, 12, 21011. [Google Scholar] [CrossRef]
- Lädermann, A.; Williams, M.D.; Melis, B.; Hoffmeyer, P.; Walch, G. Objective Evaluation of Lengthening in Reverse Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2009, 18, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Lädermann, A.; Edwards, T.B.; Walch, G. Arm Lengthening after Reverse Shoulder Arthroplasty: A Review. Int. Orthop. 2014, 38, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, A.; Blasco, L.; Marcoin, A.; De Boissieu, P.; Siboni, R.; Nérot, C.; Ohl, X. Geometric Modification of the Humeral Position after Total Reverse Shoulder Arthroplasty: What Is the Optimal Lowering of the Humerus? J. Shoulder Elb. Surg. 2018, 27, 2207–2213. [Google Scholar] [CrossRef]
- Harman, M.; Frankle, M.; Vasey, M.; Banks, S. Initial Glenoid Component Fixation in “Reverse” Total Shoulder Arthroplasty: A Biomechanical Evaluation. J. Shoulder Elb. Surg. 2005, 14, S162–S167. [Google Scholar] [CrossRef] [PubMed]
- Matsen, F.A.; Iannotti, J.P.; Churchill, R.S.; De Wilde, L.; Edwards, T.B.; Evans, M.C.; Fehringer, E.V.; Groh, G.I.; Kelly, J.D.; Kilian, C.M.; et al. One and Two-Year Clinical Outcomes for a Polyethylene Glenoid with a Fluted Peg: One Thousand Two Hundred Seventy Individual Patients from Eleven Centers. Int. Orthop. 2019, 43, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Morin-Salvo, N.; Bessière, C.; Chelli, M.; Gauci, M.-O.; Lemmex, D.B. Bony Increased-Offset-Reverse Shoulder Arthroplasty: 5 to 10 Years’ Follow-Up. J. Shoulder Elb. Surg. 2020, 29, 2111–2122. [Google Scholar] [CrossRef] [PubMed]
- Boutsiadis, A.; Lenoir, H.; Denard, P.J.; Panisset, J.-C.; Brossard, P.; Delsol, P.; Guichard, F.; Barth, J. The Lateralization and Distalization Shoulder Angles Are Important Determinants of Clinical Outcomes in Reverse Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2018, 27, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Corbaz, J.; Athwal, G.S.; Walch, G.; Blakeney, W.G. Lateralization in Reverse Shoulder Arthroplasty. J. Clin. Med. 2021, 10, 5380. [Google Scholar] [CrossRef]
- Slowinski, J.J.; Bauer, J.A.; Feng, L.; Schoch, N.; Sperling, J.W.; Duquin, T.R. Computed Tomography–Based Three-Dimensional Modeling of Glenoid Bone Preservation with Augmented Baseplates. Semin. Arthroplast. JSES 2023, 33, 162–168. [Google Scholar] [CrossRef]
- Freislederer, F.; Toft, F.; Audigé, L.; Marzel, A.; Endell, D.; Scheibel, M. Lateralized vs. Classic Grammont-Style Reverse Shoulder Arthroplasty for Cuff Deficiency Hamada Stage 1–3: Does the Design Make a Difference? J. Shoulder Elb. Surg. 2022, 31, 341–351. [Google Scholar] [CrossRef]
- Merolla, G.; Walch, G.; Ascione, F.; Paladini, P.; Fabbri, E.; Padolino, A.; Porcellini, G. Grammont Humeral Design versus Onlay Curved-Stem Reverse Shoulder Arthroplasty: Comparison of Clinical and Radiographic Outcomes with Minimum 2-Year Follow-Up. J. Shoulder Elb. Surg. 2018, 27, 701–710. [Google Scholar] [CrossRef]
- Lawrence, C.; Williams, G.R.; Namdari, S. Influence of Glenosphere Design on Outcomes and Complications of Reverse Arthroplasty: A Systematic Review. Clin. Orthop. Surg. 2016, 8, 288–297. [Google Scholar] [CrossRef]
- Mahendraraj, K.A.; Abboud, J.; Armstrong, A.; Austin, L.; Brolin, T.; Entezari, V.; Friedman, L.; Garrigues, G.E.; Grawe, B.; et al.; ASES Complications of RSA Research Group Predictors of Acromial and Scapular Stress Fracture after Reverse Shoulder Arthroplasty: A Study by the ASES Complications of RSA Multicenter Research Group. J. Shoulder Elb. Surg. 2021, 30, 2296–2305. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merolla, G.; Sircana, G.; Padolino, A.; Fauci, F.; Augusti, C.A.; Saporito, M.; Paladini, P. Radiographic Analysis of Grammont-Style and Lateralized Reverse Shoulder Arthroplasty in Gleno-Humeral Osteoarthritis. Prosthesis 2023, 5, 1075-1092. https://doi.org/10.3390/prosthesis5040075
Merolla G, Sircana G, Padolino A, Fauci F, Augusti CA, Saporito M, Paladini P. Radiographic Analysis of Grammont-Style and Lateralized Reverse Shoulder Arthroplasty in Gleno-Humeral Osteoarthritis. Prosthesis. 2023; 5(4):1075-1092. https://doi.org/10.3390/prosthesis5040075
Chicago/Turabian StyleMerolla, Giovanni, Giuseppe Sircana, Antonio Padolino, Francesco Fauci, Carlo Alberto Augusti, Marco Saporito, and Paolo Paladini. 2023. "Radiographic Analysis of Grammont-Style and Lateralized Reverse Shoulder Arthroplasty in Gleno-Humeral Osteoarthritis" Prosthesis 5, no. 4: 1075-1092. https://doi.org/10.3390/prosthesis5040075
APA StyleMerolla, G., Sircana, G., Padolino, A., Fauci, F., Augusti, C. A., Saporito, M., & Paladini, P. (2023). Radiographic Analysis of Grammont-Style and Lateralized Reverse Shoulder Arthroplasty in Gleno-Humeral Osteoarthritis. Prosthesis, 5(4), 1075-1092. https://doi.org/10.3390/prosthesis5040075






