A DFT Study on the Interaction of Doped Carbon Nanotubes with H2S, SO2 and Thiophene
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Optimized Parameters
3.2. Adsorption Energies
3.3. NBO Calculations
3.4. Reactivity Parameters
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Popov, V.N. Carbon nanotubes: Properties and application. Mat. Sci. Eng. R. Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Fu, K.; Lin, Y.; Huang, W. Functionalized carbon nanotubes: Properties and applications. Acc. Chem. Res. 2002, 35, 1096–1104. [Google Scholar] [CrossRef]
- Odom, T.W.; Huang, J.-L.; Kim, P.; Lieber, C.M. Atomic structure and electronic properties of single-walled carbon nanotubes. Nature 1998, 391, 62–64. [Google Scholar] [CrossRef]
- Chen, J.; Hamon, M.A.; Hu, H.; Chen, Y.; Rao, A.M.; Eklund, P.C.; Haddon, R.C. Solution properties of single-walled carbon nanotubes. Science 1998, 282, 95–98. [Google Scholar] [CrossRef]
- Iuima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar]
- Li, L.; Yang, H.; Miao, J.; Zhang, L.; Wang, H.Y.; Zeng, Z.; Huang, W.; Dong, X.; Liu, B. Unraveling oxygen evolution reaction on carbon-based electrocatalysts: Effect of oxygen doping on adsorption of oxygenated intermediates. ACS Energy Lett. 2017, 2, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Babu, D.J.; Bruns, M.; Schneider, R.; Gerthsen, D.; Schneider, J.J. Understanding the influence of N-doping on the CO2 adsorption characteristics in carbon nanomaterials. J. Phys. Chem. 2017, 121, 616–626. [Google Scholar] [CrossRef]
- Striolo, A.; Chialvo, A.A.; Gubbins, K.E. Water in carbon nanotubes: Adsorption isotherms and thermodynamic properties from molecular simulation. J. Chem. Phys. 2005, 122, 234712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.T. Hydrogen storage by alkali-doped carbon nanotubes–revisited. Carbon 2000, 38, 623–626. [Google Scholar] [CrossRef]
- Peng, S.; Cho, K. Ab initio study of doped carbon nanotube sensors. Nano Lett. 2003, 3, 513–517. [Google Scholar] [CrossRef]
- Hu, X.; Wu, Y.; Li, H.; Zhang, Z. Adsorption and activation of O2 on nitrogen-doped carbon nanotubes. J. Phys. Chem. C 2010, 114, 9603–9607. [Google Scholar] [CrossRef]
- Yoosefian, M. Powerful greenhouse gas nitrous oxide adsorption onto intrinsic and Pd doped Single walled carbon nanotube. Appl. Surf. Sci. 2017, 392, 225–230. [Google Scholar] [CrossRef]
- Cruz-Silva, E.; Cullen, D.A.; Gu, L.; Romo-Herrera, J.M.; Muñoz-Sandoval, E.; López-Urías, F.; Sumpter, B.G.; Meunier, V.; Charlier, J.-C.; Smith, D.J. Heterodoped Nanotubes: Theory, Synthesis, and Characterization of Phosphorus-Nitrogen Doped Multiwalled Carbon Nanotubes. ACS Nano 2008, 2, 441–448. [Google Scholar] [CrossRef]
- Ganji, M.; Ahmadian, N.; Goodarzi, M.; Khorrami, H. Molecular hydrogen interacting with Si-, S-and P-doped C60 fullerenes and carbon nanotube. J. Comp. Theor. Nanosci. 2011, 8, 1392–1399. [Google Scholar] [CrossRef]
- Wang, Y.L.; Su, K.H.; Zhang, J.P. Studying of B, N, S, Si and P Doped (5, 5) Carbon Nanotubes by the Density Functional Theory. In Advanced Materials Research; Trans Tech Publications Ltd.: Zurich, Switzerland, 2012; pp. 1488–1492. [Google Scholar]
- Garcia, A.G.; Baltazar, S.E.; Castro, A.H.R.; Robles, J.F.P.; Rubio, A. Influence of S and P doping in a graphene sheet. J. Comput. Theor. Nanosci. 2008, 5, 2221–2229. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Gao, J.; Liu, X.; Yang, Y.; Wu, S. Controllable nitrogen introduction into porous carbon with porosity retaining for investigating nitrogen doping effect on SO2 adsorption. Chem. Eng. J. 2016, 290, 116–124. [Google Scholar] [CrossRef]
- Tavakol, H.; Shahabi, D.; Keshavarzipour, F. Theoretical calculation of simple and doped CNTs with the potential adsorption of various ions for water desalination technologies. Struct. Chem. 2020, 31, 399–409. [Google Scholar] [CrossRef]
- Tavakol, H.; Hashemi, F.; Molavian, M.R. Theoretical investigation on the performance of simple and doped graphenes for the surface adsorption of various ions and water desalination. Struct. Chem. 2017, 28, 1687–1695. [Google Scholar] [CrossRef]
- Khan, A.A.; Schuler, M.M.; Prior, M.G. Effects of hydrogen sulfide exposure on lung mitochondrial respiratory chain enzymes in rats. Toxicol. Appl. Pharmacol. 1990, 103, 482–490. [Google Scholar] [CrossRef]
- Mori, F.; Tanji, K.; Wakabayashi, K. Thiophene, a sulfur-containing heterocyclic hydrocarbon, causes widespread neuronal degeneration in rats. Neuropathology 2000, 20, 283–288. [Google Scholar]
- Saadat, K.; Tavakol, H. An exceptional functionalization of doped fullerene observed via theoretical studies on the interactions of sulfur-doped fullerenes with halogens and halides. RSC Adv. 2015, 5, 55227–55237. [Google Scholar] [CrossRef]
- Tavakol, H.; Shahabi, D. DFT, QTAIM, and NBO study of adsorption of rare gases into and on the surface of sulfur-doped, single-wall carbon nanotubes. J. Phys. Chem. C 2015, 119, 6502–6510. [Google Scholar] [CrossRef]
- Tavakol, H.; Mollaei-Renani, A. DFT, AIM, and NBO study of the interaction of simple and sulfur-doped graphenes with molecular halogens, CH3OH, CH3SH, H2O, and H2S. Struct. Chem. 2014, 25, 1659–1667. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Revision D; Gaussian, Inc.: Wallingford, CT, UK, 2009; p. 1. [Google Scholar]
- Shahabi, D.; Tavakol, H. A DFT study on the catalytic ability of aluminum doped graphene for the initial steps of the conversion of methanol to gasoline. Comp. Theor. Chem. 2018, 1127, 8–15. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; He, Z.; Xu, Y.; Yu, W. Theoretical investigations on charge transport properties of tetrabenzo [a, d, j, m] coronene derivatives using different density functional theory functionals (B3LYP, M06-2X, and ω-B97X-D). J. Chem. Res. 2019, 43, 293–303. [Google Scholar] [CrossRef]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO Version 3.1. Available online: https://www.scienceopen.com (accessed on 30 June 2021).
- Sonawane, M.R.; Habale, D.; Nagare, B.J.; Gharde, R. Interaction of O2, CO2, NO2 and SO2 on Si-doped Carbon Nanotube. Int. J. Appl. Phys. Math. 2011, 1, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Shimoyama, I.; Baba, Y. Thiophene adsorption on phosphorus-and nitrogen-doped graphites: Control of desulfurization properties of carbon materials by heteroatom doping. Carbon. 2016, 98, 115–125. [Google Scholar] [CrossRef]
- Qu, Z.; Sun, F.; Liu, X.; Gao, J.; Qie, Z.; Zhao, G. The effect of nitrogen-containing functional groups on SO2 adsorption on carbon surface: Enhanced physical adsorption interactions. Surf. Sci. 2018, 677, 78–82. [Google Scholar] [CrossRef]
- Zhang, H.P.; Luo, X.G.; Song, H.T.; Lin, X.Y.; Lu, X.; Tang, Y. DFT study of adsorption and dissociation behavior of H2S on Fe-doped graphene. Appl. Surf. Sci. 2014, 317, 511–516. [Google Scholar] [CrossRef]

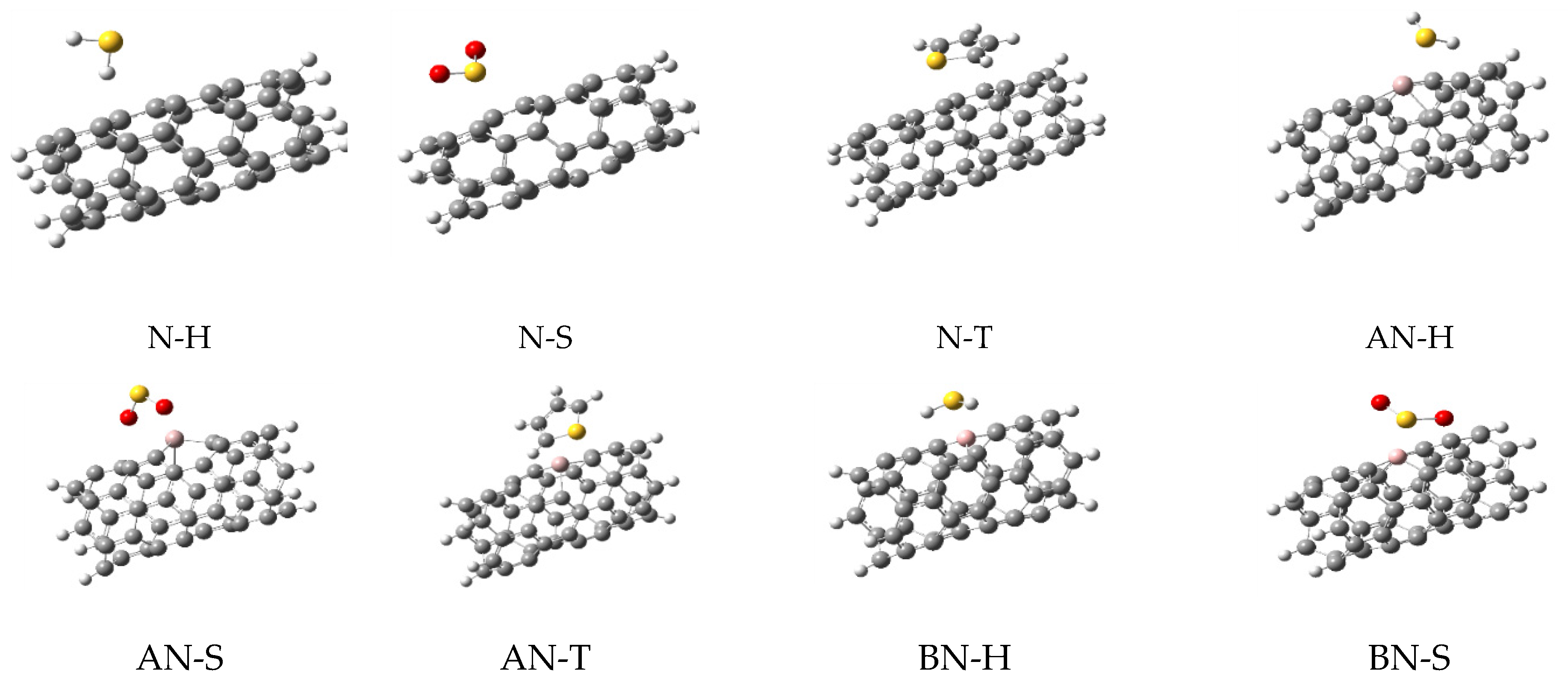
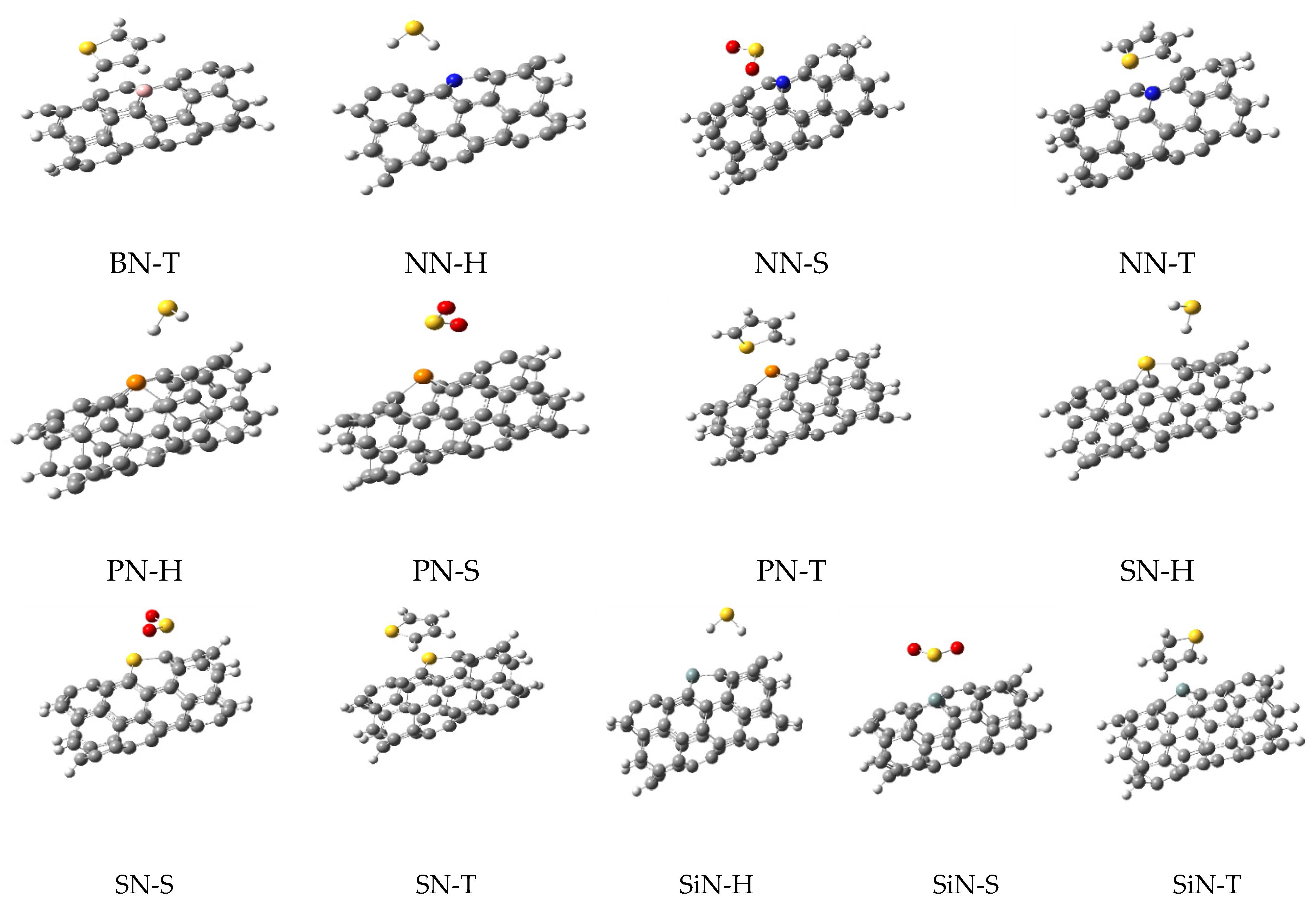
| N Alone | N-H2S | N-SO2 | N-Thiophene | ||||
|---|---|---|---|---|---|---|---|
| C-X (Av.) a | C-X (Av.) a | N-M b | C-X (Av.) a | N-M b | C-X (Av.) a | N-M b | |
| N | 1.439 | 1.442 | 2.391 | 1.444 | 2.974 | 1.444 | 3.243 |
| AN | 1.913 | 1.933 | 2.330 | 1.977 | 1.789 | 1.935 | 2.307 |
| BN | 1.526 | 1.590 | 2.163 | 1.594 | 1.448 | 1.538 | 2.804 |
| NN | 1.441 | 1.445 | 2.284 | 1.447 | 2.979 | 1.436 | 3.162 |
| PN | 1.870 | 1.871 | 2.567 | 1.867 | 3.300 | 1.869 | 3.255 |
| SN | 1.863 | 1.863 | 2.174 | 1.868 | 2.018 | 1.860 | 3.134 |
| SiN | 1.868 | 1.871 | 2.291 | 1.872 | 2.930 | 1.865 | 3.067 |
| N-H2S | N-SO2 | N-Thiophene | |||||||
| ΔEad (G) | ΔEad (W) | BSSE | ΔEad (G) | ΔEad (W) | BSSE | ΔEad (G) | ΔEad (W) | BSSE | |
| N | −1.56 | −1.49 | 0.91 | −4.41 | −4.11 | 2.20 | −2.56 | −2.67 | 2.20 |
| AN | −5.15 | −5.04 | 1.89 | −7.08 | −6.87 | 2.29 | −5.69 | −6.02 | 2.43 |
| BN | −4.12 | −4.05 | 2.01 | −5.69 | −5.44 | 2.08 | −4.93 | −5.18 | 2.38 |
| NN | −3.21 | −3.08 | 1.01 | −3.67 | −3.53 | 2.45 | −3.51 | −3.64 | 2.40 |
| PN | −2.69 | −2.57 | 0.98 | −3.93 | −3.78 | 1.91 | −3.71 | −3.76 | 1.75 |
| SN | −2.99 | −2.92 | 1.08 | −4.14 | −3.85 | 1.97 | −2.72 | −2.95 | 2.04 |
| SiN | −2.46 | −2.47 | 0.96 | −5.17 | −4.95 | 2.90 | −3.60 | −3.74 | 2.26 |
| ΔHad (G) | ΔHad (W) | Thermal correction | ΔHad (G) | ΔHad (W) | Thermal Correction | ΔHad (G) | ΔHad (W) | Thermal correction | |
| N | −1.24 | −1.16 | 1.28 | −3.92 | −3.64 | 0.77 | −2.01 | −2.11 | 1.55 |
| AN | −4.82 | −4.71 | 1.66 | −6.62 | −6.41 | 0.98 | −5.11 | −5.44 | 1.84 |
| BN | −3.75 | −3.68 | 1.94 | −5.18 | −4.92 | 1.11 | −4.37 | −4.62 | 1.69 |
| NN | −2.87 | −2.73 | 0.60 | −3.22 | −3.08 | 0.96 | −2.97 | −3.09 | 1.76 |
| PN | −2.33 | −2.21 | 1.13 | −3.41 | −3.27 | 0.23 | −3.12 | −3.18 | 1.58 |
| SN | −2.70 | −2.62 | 1.35 | −3.66 | −3.38 | 0.85 | −2.22 | −2.46 | 1.42 |
| SiN | −2.11 | −2.13 | 1.47 | −4.74 | −4.51 | 0.81 | −3.06 | −3.21 | 1.55 |
| ΔGad (G) | ΔGad (W) | Thermal correction | ΔGad (G) | ΔGad (W) | Thermal Correction | ΔGad (G) | ΔGad (W) | Thermal correction | |
| N | −0.13 | −0.06 | 2.39 | −2.40 | −2.13 | 2.99 | −0.36 | −0.47 | 3.20 |
| AN | −3.62 | −3.52 | 2.84 | −5.06 | −4.85 | 2.54 | −3.41 | −3.74 | 3.54 |
| BN | −2.43 | −2.35 | 3.26 | −3.57 | −3.32 | 2.72 | −2.70 | −2.94 | 3.36 |
| NN | −0.46 | −0.31 | 3.01 | −1.71 | −1.57 | 2.47 | −1.35 | −1.47 | 3.38 |
| PN | −1.19 | −1.08 | 2.27 | −1.86 | −1.71 | 1.78 | −1.48 | −1.55 | 3.22 |
| SN | −1.42 | −1.35 | 2.63 | −2.06 | −1.78 | 2.45 | −0.61 | −0.85 | 3.03 |
| SiN | −0.92 | −0.95 | 2.66 | −3.16 | −2.93 | 2.39 | −1.40 | −1.54 | 3.21 |
| Nanotube Charges | N Alone | N-H2S | N-SO2 | N-Thiophene | ||||
| C (Av) a | X b | C (Av) a | X b | C (Av) a | X b | C (Av) a | X b | |
| N | 0.000 | 0.000 | −0.022 | −0.068 | −0.017 | −0.091 | −0.003 | −0.011 |
| AN | −0.498 | 1.673 | −0.493 | 1.568 | −0.445 | 1.750 | −0.536 | 1.747 |
| BN | −0.310 | 0.639 | −0.256 | 0.450 | −0.178 | 0.222 | −0.315 | 0.730 |
| NN | 0.219 | −0.381 | 0.223 | −0.414 | 0.205 | −0.422 | 0.231 | −0.381 |
| PN | −0.271 | 0.928 | −0.266 | 0.928 | −0.275 | 0.894 | −0.270 | 0.942 |
| SN | −0.195 | 0.844 | −0.223 | 0.851 | −0.233 | 0.953 | −0.194 | 0.856 |
| SiN | −0.391 | 1.170 | −0.391 | 1.146 | −0.386 | 1.157 | −0.398 | 1.203 |
| Adsorbate charges | N-H2S | N-SO2 | N-thiophene | |||||
| S | Y (Av) d | S | Y (Av) d | S | Y (Av) d | |||
| small molecule c | −0.340 | 0.170 | 1.286 | −0.643 | 0.358 | −0.416 | ||
| N | −0.333 | 0.164 | 1.296 | −0.673 | 0.360 | −0.413 | ||
| AN | −0.242 | 0.235 | 0.967 | −0.827 | 0.494 | −0.507 | ||
| BN | −0.034 | 0.226 | 1.074 | −0.724 | 0.372 | −0.405 | ||
| NN | −0.356 | 0.172 | 1.262 | −0.657 | 0.364 | −0.415 | ||
| PN | −0.354 | 0.176 | 1.303 | −0.660 | 0.352 | −0.415 | ||
| SN | −0.361 | 0.169 | 1.289 | −0.888 | 0.367 | −0.416 | ||
| SiN | −0.340 | 0.165 | 1.216 | −0.725 | 0.364 | −0.415 | ||
| Complex | Donor | Acceptor | E2 | Donor | Acceptor | E2 | Donor | Acceptor | E2 | Sum a |
|---|---|---|---|---|---|---|---|---|---|---|
| N-H | LPC | σ*S-H | 0.55 | σS-H | LPC | 0.36 | σC-C | σ*S-H | 0.17 | 1.08 |
| N-S | LPC | Π*S-O | 1.65 | Π*c-c | σ*S-O | 0.83 | LPC | σ*S-O | 0.61 | 3.09 |
| N-T | Π*c-c | Π*c-c | 0.60 | Π*c-c | Π*c-c | 0.40 | Π*c-c | Π*c-c | 0.17 | 1.17 |
| AN-H | LPS | LP*Al | 1.62 | LPS | LP*Al | 1.34 | LPS | LP*Al | 0.61 | 3.57 |
| AN-S | LPO | LP*Al | 3.09 | LP*Al | σ*S-O | 1.39 | LPO | LP*Al | 1.09 | 5.57 |
| AN-T | Πc-c | LP*Al | 1.40 | Πc-c | LP*Al | 0.95 | Π*c-c | LP*Al | 0.89 | 3.24 |
| BN-H | LPS | LP*B | 1.32 | LPS | LP*B | 0.95 | CRS | LP*B | 0.61 | 2.88 |
| BN-S | LPO | σ*C-C | 1.75 | LPO | σ*C-C | 1.36 | LPO | RY*C | 0.56 | 3.67 |
| BN-T | Π*C-C | LP*B | 1.35 | ΠC-C | LP*B | 1.32 | Π*C-C | LP*B | 0.65 | 3.32 |
| NN-H | LPN | σ*S-H | 0.71 | ΠC-C | σ*S-H | 0.64 | σS-H | RY*C | 0.19 | 1.54 |
| NN-S | LPN | LP*S | 1.04 | ΠC-C | LP*S | 0.58 | σS-O | RY*C | 0.24 | 1.86 |
| NN-T | Π*C-C | Π*C-C | 0.70 | ΠC-C | Π*C-C | 0.57 | Π*C-C | Π*C-C | 0.42 | 1.69 |
| PN-H | ΠC-C | σ*S-H | 0.68 | Π*C-C | σ*S-H | 0.31 | Π*C-C | σ*S-H | 0.29 | 1.28 |
| PN-S | LPP | LP*S | 1.22 | ΠC-C | LP*S | 0.92 | LP*S | σ*C-P | 0.37 | 2.51 |
| PN-T | Π*C-C | Π*C-C | 1.13 | LPS | σ*C-P | 0.67 | ΠC-C | Π*C-C | 0.45 | 2.25 |
| SN-H | ΠC-C | σ*S-H | 0.84 | Π*C-C | σ*S-H | 0.36 | σS-H | Π*C-C | 0.24 | 1.44 |
| SN-S | LPO | σ*C-S | 1.56 | LPO | σ*C-S | 1.06 | LPO | σ*C-C | 0.32 | 2.94 |
| SN-T | ΠC-C | σ*C-S | 0.56 | Π*C-C | Π*C-C | 0.34 | Π*C-C | Π*C-C | 0.23 | 1.13 |
| SiN-H | σC-Si | σ*S-H | 0.57 | ΠC-C | σ*S-H | 0.38 | Π*C-C | σ*S-H | 0.20 | 1.15 |
| SiN-S | LPS | σ*C-Si | 1.82 | LPS | σ*C-Si | 1.02 | LPS | LP*Si | 0.81 | 3.65 |
| SiN-T | Π*C-C | Π*C-C | 1.19 | Π*C-C | Π*C-C | 0.42 | Π*C-C | LP*Si | 0.28 | 1.89 |
| Complex | E (HOMO) | E (LUMO) | Eg | μ | η | S | ω |
|---|---|---|---|---|---|---|---|
| N | −0.234 | −0.073 | 0.161 | −0.154 | 0.080 | 12.454 | 0.147 |
| N-H | −0.237 | −0.077 | 0.160 | −0.157 | 0.080 | 12.508 | 0.155 |
| N-S | −0.242 | −0.093 | 0.149 | −0.168 | 0.074 | 13.426 | 0.189 |
| N-T | −0.233 | −0.072 | 0.160 | −0.152 | 0.080 | 12.467 | 0.145 |
| AN | −0.252 | −0.055 | 0.197 | −0.154 | 0.099 | 10.149 | 0.120 |
| AN-H | −0.245 | −0.053 | 0.192 | −0.149 | 0.096 | 10.440 | 0.116 |
| AN-S | −0.249 | −0.079 | 0.169 | −0.164 | 0.085 | 11.801 | 0.159 |
| AN-T | −0.240 | −0.040 | 0.200 | −0.140 | 0.100 | 9.994 | 0.097 |
| BN | −0.217 | −0.061 | 0.155 | −0.139 | 0.078 | 12.870 | 0.125 |
| BN-H | −0.240 | −0.048 | 0.192 | −0.144 | 0.096 | 10.440 | 0.108 |
| BN-S | −0.237 | −0.075 | 0.162 | −0.156 | 0.081 | 12.318 | 0.149 |
| BN-T | −0.232 | −0.059 | 0.174 | −0.145 | 0.087 | 11.527 | 0.122 |
| NN | −0.244 | −0.067 | 0.178 | −0.155 | 0.089 | 11.268 | 0.136 |
| NN-H | −0.251 | −0.069 | 0.182 | −0.160 | 0.091 | 10.984 | 0.140 |
| NN-S | −0.250 | −0.079 | 0.171 | −0.165 | 0.086 | 11.667 | 0.158 |
| NN-T | −0.242 | −0.062 | 0.180 | −0.152 | 0.090 | 11.083 | 0.128 |
| PN | −0.248 | −0.063 | 0.184 | −0.156 | 0.092 | 10.854 | 0.131 |
| PN-H | −0.248 | −0.068 | 0.180 | −0.158 | 0.090 | 11.131 | 0.139 |
| PN-S | −0.252 | −0.097 | 0.155 | −0.174 | 0.078 | 12.902 | 0.196 |
| PN-T | −0.245 | −0.056 | 0.189 | −0.150 | 0.094 | 10.590 | 0.120 |
| SN | −0.234 | −0.068 | 0.166 | −0.151 | 0.083 | 12.056 | 0.138 |
| SN-H | −0.239 | −0.075 | 0.164 | −0.157 | 0.082 | 12.192 | 0.150 |
| SN-S | −0.250 | −0.099 | 0.152 | −0.174 | 0.076 | 13.165 | 0.200 |
| SN-T | −0.232 | −0.067 | 0.165 | −0.149 | 0.083 | 12.104 | 0.135 |
| SiN | −0.234 | −0.083 | 0.150 | −0.158 | 0.075 | 13.293 | 0.167 |
| SiN-H | −0.241 | −0.092 | 0.149 | −0.166 | 0.075 | 13.405 | 0.185 |
| SiN-S | −0.245 | −0.100 | 0.145 | −0.172 | 0.073 | 13.759 | 0.204 |
| SiN-T | −0.232 | −0.082 | 0.151 | −0.157 | 0.075 | 13.280 | 0.164 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavakol, H.; Haghshenas, H. A DFT Study on the Interaction of Doped Carbon Nanotubes with H2S, SO2 and Thiophene. Quantum Rep. 2021, 3, 366-375. https://doi.org/10.3390/quantum3030023
Tavakol H, Haghshenas H. A DFT Study on the Interaction of Doped Carbon Nanotubes with H2S, SO2 and Thiophene. Quantum Reports. 2021; 3(3):366-375. https://doi.org/10.3390/quantum3030023
Chicago/Turabian StyleTavakol, Hossein, and Hamed Haghshenas. 2021. "A DFT Study on the Interaction of Doped Carbon Nanotubes with H2S, SO2 and Thiophene" Quantum Reports 3, no. 3: 366-375. https://doi.org/10.3390/quantum3030023
APA StyleTavakol, H., & Haghshenas, H. (2021). A DFT Study on the Interaction of Doped Carbon Nanotubes with H2S, SO2 and Thiophene. Quantum Reports, 3(3), 366-375. https://doi.org/10.3390/quantum3030023






