Dietary Factors and Production Season Effect on the Properties of Goat Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
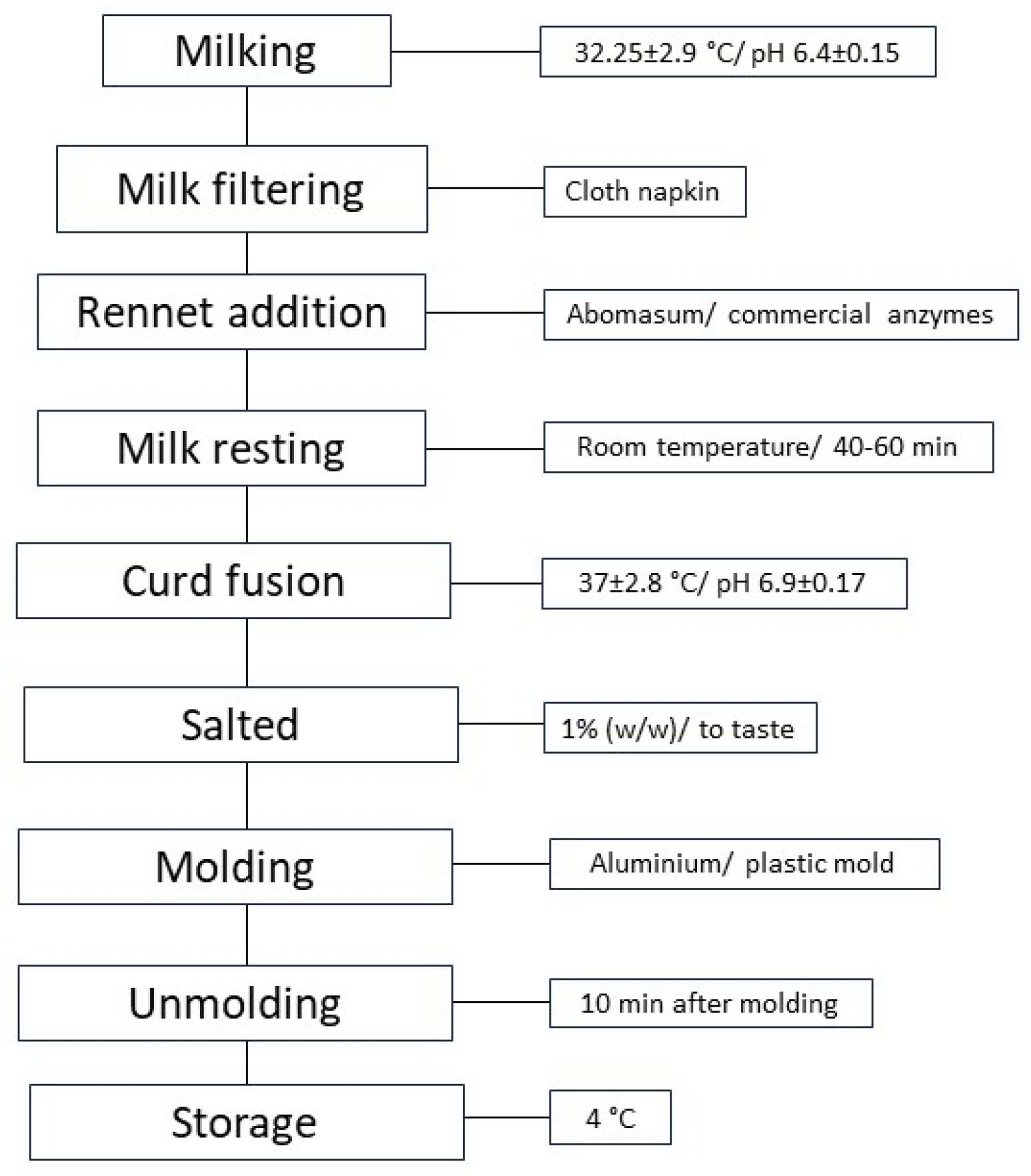
2.2. Production Process of Cheeses
2.3. Sampling
2.4. Chemical Composition
2.5. Texture Profile Analysis (TPA)
2.6. Microbiological Analysis
2.7. Statistical Analysis
3. Results and Discussion
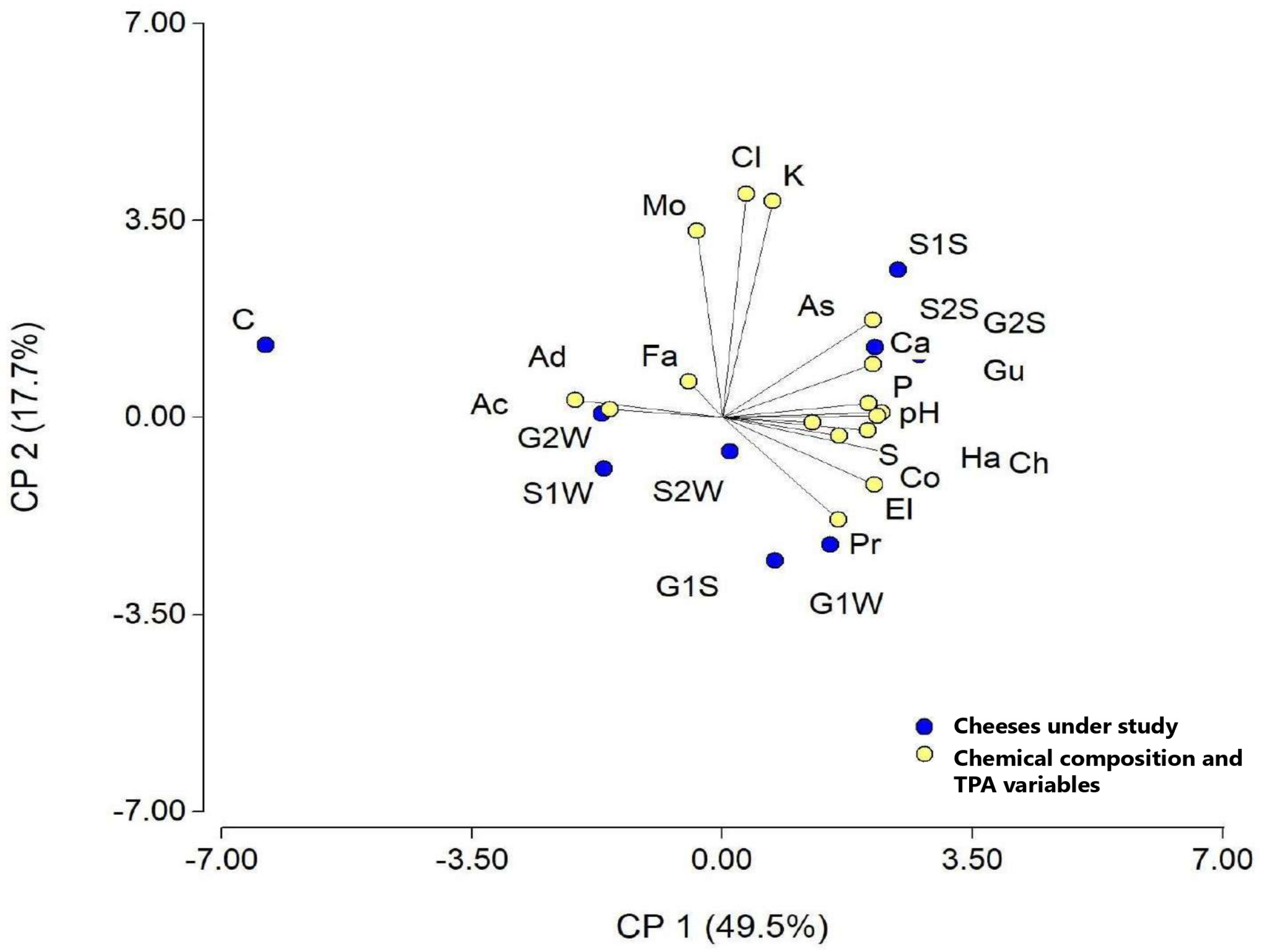
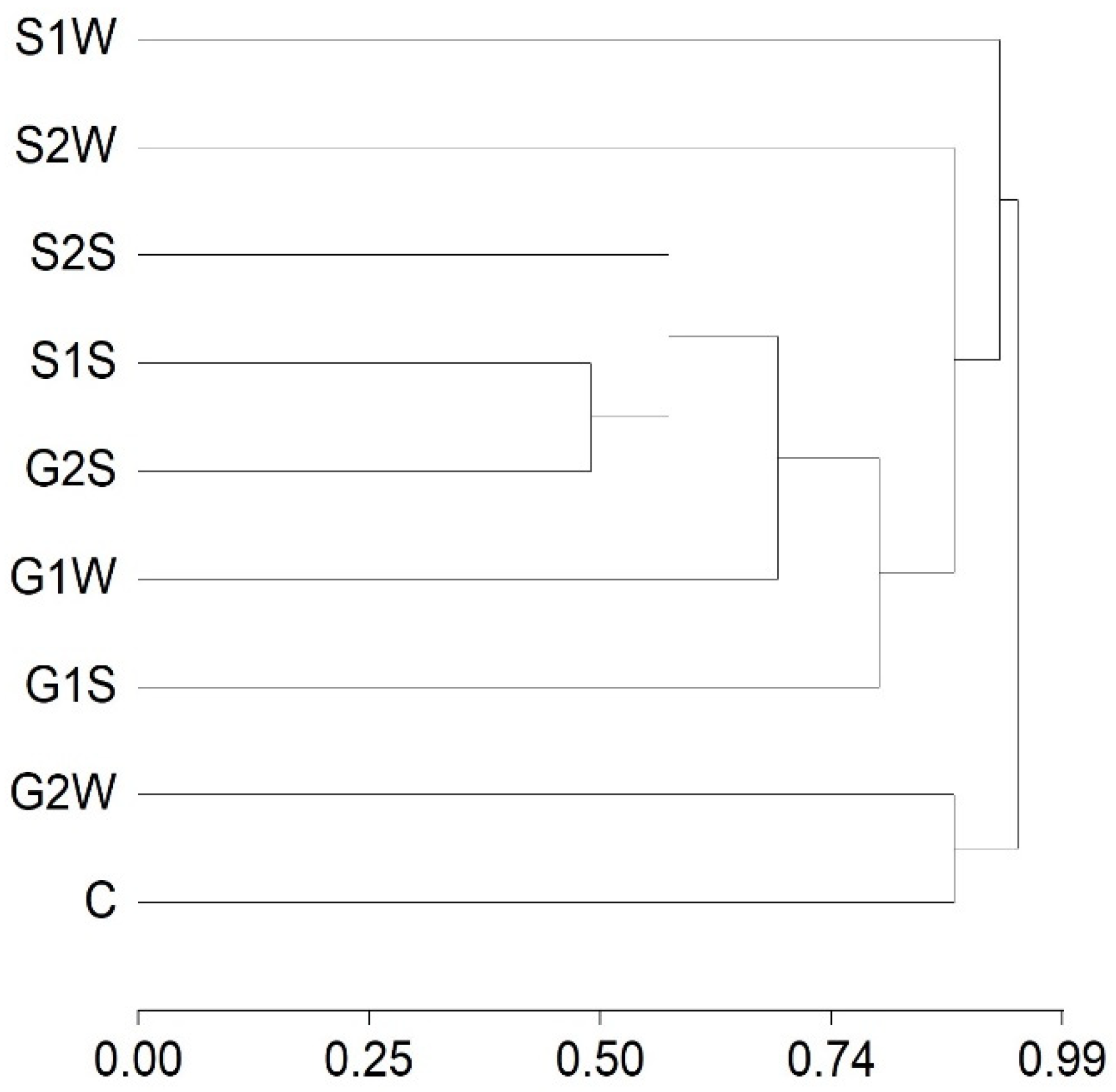
3.1. Chemical Composition
3.2. TPA Analysis
3.3. Microbiological Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skapetas, B.; Bampidis, V. Goat production in the world: Present situation and trends. Livest. Res. Rural. Dev. 2016, 28, 200. [Google Scholar]
- Pozzolan, M.; Stocco, G.; Dettori, M.; Bittante, G.; Vacca, G. Effect of goat milk composition on cheesemaking traits and daily cheese production. J. Dairy Sci. 2019, 102, 3947–3955. [Google Scholar]
- Rangel OS, C.; Campos ML, G.; Charles RA, V.; Chávez GM, L.; Palomo, L.L.; Contreras EJ, C.; Solanilla DJ, F.; Flores GA, C.; Rodríguez, H.R. Biological control of pathogens in artisanal cheeses. Int. Dairy J. 2023, 140, 105612. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H.; El-Shibiny, S. Conjugated Linoleic Acid in Milk from Grazing and Non-Grazing Dairy Goats. J. Food Sci. 2019, 84, S1448–S1456. [Google Scholar]
- Caroprese, M.; Marzano, A.; Marino, R.; Gliatta, G.; Muscio, A.; Sevi, A. Effects of Grazing and Indoor Feeding on Milk Quality and Immune Function in Dairy Goats. J. Dairy Res. 2018, 85, 147–155. [Google Scholar]
- Mele, M.; Buccioni, A.; Petacchi, F.; Antongiovanni, M. Effect of forage-to-concentrate ratio on milk composition and fatty acid profile in stall-fed goats. J. Dairy Sci. 2022, 105, 5123–5134. [Google Scholar]
- Sanz Sampelayo, M.R.; Chilliard, Y.; Schmidely, P.; Boza, J. Influence of type of diet on the fat constituents of goat and sheep milk. Small Rumin. Res. 2021, 40, 45–70. [Google Scholar] [CrossRef]
- Ramos, P.A.; Barreira, J.C.; Fernandes, Â.; Pereira, J.A.; Ferreira, I.C.; Sarmento, B. Influence of season on the characteristics of cheese from goat milk. J. Dairy Sci. 2023, 106, 2510–2521. [Google Scholar]
- Sanz Sampelayo, M.R.; Chilliard, Y.; Schmidely, P.; Boza, J. Seasonal variations in the sensory properties of goat cheese. J. Food Sci. 2021, 86, S1448–S1456. [Google Scholar]
- Montel, M.C.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Seasonal variations in the prevalence of Listeria monocytogenes in goat cheese. Int. J. Food Microbiol. 2022, 279, 77–87. [Google Scholar]
- Quigley, L.; O’Sullivan, O.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. Seasonal variations in the prevalence of Salmonella spp. in goat milk. J. Dairy Sci. 2023, 106, 2510–2521. [Google Scholar]
- Renes, E.; González, L.; Fresno, J.M.; Tornadijo, M.E.; Prieto, B. Seasonal changes in the microbial composition of artisanal cheeses. Food Microbiol. 2023, 74, 103–112. [Google Scholar]
- Legg, A.K.; Carr, A.J.; Bennett, R.J.; Johnston, K.A. Cheese: Chemistry, physics and microbiology. In Book General Aspects of Cheese Technology, 4th ed.; McSweeney, L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: San Diego, DA, USA, 2017; Volume 1, p. 643e675. [Google Scholar]
- Yoon, Y.; Lee, S.; Choi, K.H. Microbial benefits and risks of raw milk cheese. Food Control 2016, 63, 201–215. [Google Scholar] [CrossRef]
- EC. Regulation (EC) No. 853/2004 of the European Parliament and of the Council Laying Down Specific Hygiene Rules for the Hygiene of Foodstuffs. UNEP Law and Environment Assistance Platform (n.d.). 2004. Available online: https://leap.unep.org/countries/eu/national-legislation/regulation-ec-no8532004-european-parliament-and-council-laying (accessed on 11 September 2022).
- Villegas de Gante, A.; Santos, M.A.; Cervantes, E.F. Los Quesos Mexicanos Tradicionales, 1st. ed.; Juan, P., Ed.; Universidad Autonoma de Chapingo: El Cooperativo, Mexico, 2016; ISBN 978-607-711-364-5. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; Kenneth, H., Ed.; Association of official Analytical Chemists, Inc: Arlington, VA, USA, 1990; Volume 1, pp. 69–80. ISBN 0-935584-42-0. [Google Scholar]
- Garcia OJ, D.; Flores GA, C.; Espinoza, V.J.; Ascacio VJ, A.; Nery FS, D.; Rodríguez, H.R. Morphological, physicochemical, techno-functional, phytochemical, and antioxidant evaluation of polyembryonic and non-polyembryonic maize sprouts. Biocatal. Agric. Biotechnol. 2023, 47, 102583. [Google Scholar]
- Tomar, O.; Akarca, G.; Gök, V.; Çağlar, M.Y. The effects of packaging materials on the fatty acid composition, organic acid content, and texture profiles of Tulum cheese. J. Food Sci. 2020, 85, 3134–3140. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (US). Bacteriological Analytical Manual (BAM). Laboratory Methods. Available from: Bacteriological Analytical Manual (BAM) FDA. Available online: https://www.fda.gov/food/laboratory-methods-food/bacteriological-analytical-manual-bam (accessed on 11 September 2021).
- Cuevas GP, F.; Heredia, C.P.Y.; Méndez RJ, I.; Hernández, M.A.; Reyes, D.R.; Vallejo, C.B.; González, C.A.F. Artisanal Sonoran cheese (Cocido cheese): An exploration of its production process, chemical composition and microbiological quality. J. Sci. Food Agric. 2017, 97, 4459–4466. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Lambert, G.; Schmidt, J.L.; Tourneur, C. La maîtrise du bioréacteur fromage. Biofutur 1985, 41, 23–50. [Google Scholar]
- Norma Oficial Mexicana NOM-223-SCFI/SAGARPA-2018, Queso-Denominacion, Especificaciones, Información Comercial y Métodos de Prueba. Available online: https://www.dof.gob.mx/normasOficiales/6973/seeco11_C/seeco11_C.html (accessed on 9 September 2021).
- Saxer, S.; Miescher, S.S.; Lacroix, C. Characterization of the microflora of industrial Mexican cheeses produced without added chemical preservatives. LWT 2013, 53, 314–320. [Google Scholar] [CrossRef]
- Tadjine, D.; Boudalia, S.; Bousbia, A.; Khelifa, R.; Mebirouk, B.L.; Tadjine, A.; Chemmam, M. Pasteurization effects on yield and physicochemical parameters of cheese in cow and goat milk. Food Sci. Technol. 2020, 40, 580–587. [Google Scholar] [CrossRef]
- Lucey, J.; Kelly, J. Cheese yield. Int. J. Dairy Technol 1994, 47, 1–14. [Google Scholar] [CrossRef]
- Kucevic, D.; Pihler, I.; Plaversusic, M.; Vukovic, T. The composition of goat milk in different types of farmings. Biotechnol. Anim. Husb. 2016, 32, 403–412. [Google Scholar] [CrossRef]
- Plessas, S.; Bosnea, L.; Psarianos, C.; Koutinas, A.A.; Marchant, R.; Banat, I.M. Lactic acid production by mixed cultures of Kluyveromyces marxianus, Lactobacillus delbrueckii ssp. bulgaricus and Lactobacillus helveticus. Bioresour. Technol. 2008, 99, 5951–5955. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, L.; Fornasari, M.E.; Gatti, M.; Lazzi, C.; Neviani, E.; Giraffa, G. Grana Padano cheese whey starters: Microbial composition and strain distribution. Int. J. Food Microbiol. 2008, 127, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W. Goat Milk—Chemistry and Nutrition. In Handbook of Milk of Non-Bovine Mammals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 42–83. [Google Scholar]
- Ledesma, L.; Fresno, M.; Álvarez, S.; Darias, J.; Rodríguez, E.; Díaz, C. Cambios de la composición mineral de quesos de cabra en función de la dieta y el cuajo usado. Arch. Zootec. 2007, 56, 719–723. [Google Scholar]
- Herman, L.E.; Bolóivar, M.D.; Toledo, L.V.M.; Cuevas GL, F.; Lope NM, C.; Barron ZJ, A.; Díaz, R.P.; Ramírez, R.E. Minerals multi-element analysis and its relationship with geographical origin of artisanal Mexican goat cheeses. Food Sci. Technol. Int. 2019, 39, 517–525. [Google Scholar] [CrossRef]
- Suárez, C.C.G. Un Queso con Historia. Bordeando el Monte 58. Secretaria del Medio Ambiente. Available online: https://sma.gob.mx/wp-content/uploads/2021/09/Bordeando_58.pdf (accessed on 7 January 2023).
- Maldonado JJ, A.; Granados RL, D.; Hernández, M.O.; Pastor LF, J.; Isidro RL, M.; Salinas, G.H.; Torres, H.G. Uso de un alimento integral como complemento a cabras locales en pastoreo: Respuesta en producción y composición química de la leche. Nova Sci. 2017, 9, 55–75. [Google Scholar]
- Armienta, T.; Gilberto, T. Perfil Mineral del Suelo, Forraje y Tejidos del Ganado en Agostaderos del Estado de Nuevo León. Doctoral Dissertation, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, Mexico, 1995. Available online: http://eprints.uanl.mx/id/eprint/5657 (accessed on 22 March 2022).
- Chacón, V.A. Aspectos nutricionales de la leche de cabra (Capra hircus) y sus variaciones en el proceso agroindustrial. Agron. Mesoam. 2005, 16, 239–252. [Google Scholar] [CrossRef]
- Raynal, K.; Remuf, F. The Effect of heating on physicochemical and renneting properties of milk, a comparison between caprine, ovine and bovine milk. Int. Dairy J. 1998, 8, 695–706. [Google Scholar] [CrossRef]
- Pavia, M.; Trujillo, A.J.; Guamis, B.; Ferragut, V. Evolución de la composición y textura de un queso de oveja en la maduración. Alimentaria 1999, 306, 43–47. [Google Scholar]
- McMahon, D.J.; Paulson, B.; Oberg, C.J. Influence of Calcium, pH, and Moisture on Protein Matrix Structure and Functionality in Direct-Acidified Nonfat Mozzarella Cheese. J. Dairy Sci. 2005, 88, 3754–3763. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, S.; Rodríguez, V.; Ruiz, M.E.; Fresno, M. Correlaciones de textura y color instrumental con la composición química de quesos de cabra canarios. Arch. Zootec. 2007, 56, 663–666. [Google Scholar]
- Walstra, P.; Geurts, T.J.; Noomen, A.; Jellema, A.; Van Boekel MA, J.S. Dairy Technology: Principles of Milk: Properties and Processes, 1st ed.; Marcel Dekker: New York, NY, USA, 1999; Chapter 1. [Google Scholar]
- Adda, J.; Gripon, J.C.; Vassal, L. The chemistry of flavour and texture generation in cheese. Food Chem. 1982, 9, 115–129. [Google Scholar] [CrossRef]
- Guzmán, L.E.; Tejada, C.; de la Ossa, Y.J.; Rivera, C.A. Análisis comparativo de perfiles de textura de quesos frescos de leche de cabra y vaca. Biotecnol. Sect. Agropecu. Agroind 2015, 13, 139–147. [Google Scholar] [CrossRef]
- Diezhandino, I.; Fernández, D.; Sacristán, N.; Combarros-Fuertes, P.; Prieto, B.; Fresno, J.M. Rheological, textural, colour and sensory characteristics of a Spanish blue cheese (Valdeón cheese). LWT 2015, 65, 1118–1125. [Google Scholar] [CrossRef]
- Reyes-Estrada, O.; Murillo-Ortiz, M.; Herrera-Torres, E.; Gurrola-Reyes, J.N.; Carrete-Carreón, F.O. Cambios estacionales en consumo, composición química y degradabilidad ruminal de la dieta seleccionada por novillos en pastoreo. Ecosistemas Recur. Agropecu. 2015, 1, 97–106. [Google Scholar]
- Norma Oficial Mexicana NOM-243-SSA1-2010, Productos y Servicios. Leche, Fórmula Láctea, Producto Lácteo Combinado y derivados lácteos. Disposiciones y Especificaciones Sanitarias. Métodos de Prueba. Available online: https://dof.gob.mx/normasOficiales/4156/salud2a/salud2a.htm (accessed on 11 September 2023).
- Hacène, M.; Lamia, A.; Mohammed, N.Z.; Ali, A.H. Proteolysis, microbiology, volatiles and sensory evaluation of Algerian traditional cheese Bouhezza made using goat’s raw milk. Int. J. Food Prop 2017, 20, S3246–S3265. [Google Scholar]
- Gursoy, O.; Küçükçetin, A.; Gökçe, Ö.; Ergin, F.; Kocatürk, K. Physicochemistry, microbiology, fatty acids composition and volatile profile of traditional Söğle tulum (goat’s skin bag) cheese. An. Acad. Bras. Ciências 2018, 90, 3661–3674. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Statistics and Geography (INEGI) (2023) Coahuila de Zaragoza. Clima. Coahuila de Zaragoza. Available online: https://cuentame.inegi.org.mx/monografias/informacion/coah/territorio/clima.aspx?tema=me&e=05 (accessed on 8 September 2023).
- Normanno, G.; Corrente, M.; La Salandra, G.; Dambrosio, A.; Quaglia, N.C.; Parisi, A.; Greco, G.; Bellacicco, A.L.; Virgilio, S.; Celano, G.V. Methicillin-resistant Staphylococcus aureus (MRSA) in foods of animal origin product in Italy. Int. J. Food Microbiol. 2007, 117, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Rozos, G.; Skoufos, I.; Fotou, K.; Alexopoulos, A.; Tsinas, A.; Bezirtzoglou, E.; Tzora, A.; Voidarou, C. Safety Issues Regarding the Detection of Antibiotics Residues, Microbial Indicators and Somatic Cell Counts in Ewes’ and Goats’ Milk Reared in Two Different Farming Systems. Appl. Sci. 2022, 12, 1009. [Google Scholar] [CrossRef]
- Wanniatie, V.; Sudarwanto, M.B.; Purnawarman, T.; Jayanegara, A. Comparison of Microbiological Quality between Organic and Conventional Goat Milk: A Study Case in Bogor, Indonesia. Adv. Anim. Vet. Sci. 2019, 7, 593–598. [Google Scholar] [CrossRef]
| Cheese | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| G1W | G2W | S1W | S2W | G1S | G2S | S1S | S2S | C | |
| Physicochemical parameters | |||||||||
| Moisture (%) | 53.15 ± 1.2 d e | 50.92 ± 1.6 f | 54.90 ± 0.6 c d | 54.02 ± 1.0 d | 51.71 ± 0. 5 e f | 56.92 ± 0.3 b c | 64.35 ± 0.8 a | 57.05 ± 0.2 b | 62.87 ± 0.4 a |
| Protein (%) | 18.25 ± 0.7 a | 11.81 ± 0.3 e f | 12.05 ± 0.7 d e f | 12.75 ± 0.4 c d e | 14.38 ± 0.2 b c | 13.61 ± 0.8 c d | 12.71 ± 0.6 c d e | 15.90 ± 0.5 b | 10.81 ± 0.5 f |
| Fat (%) | 10 ± 0 e | 14 ± 0 c | 15 ± 0 b | 15 ± 0 b | 15 ± 0 b | 16 ± 0 a | 13 ± 0 d | 13 ± 0 d | 14 ± 0 c |
| Ash (%) | 2.80 ± 0.10 b | 2.71 ± 0.15 b | 2.05 ± 0.03 c | 2.53 ± 0.03 b c | 2.08 ± 0.1 c | 3.46 ± 0.4 a | 3.53 ± 0.3 a | 4.01 ± 0.2 a | 1.35 ± 0 d |
| Acidity (%) | 0.69 ± 0.1 c | 0.99 ± 0.1 b | 0.21 ± 0.05 d | 0.18 ± 0 d | 0.27 ± 0 d | 0.21 ± 0.1 d | 0.15 ± 0.1 d | 0.18 ± 0.1 d | 1.71 ± 0.1 a |
| pH | 5.77 ± 0.9 e | 5.63 ± 0.5 e | 6.17 ± 0.6 d | 6.33 ± 0.2 c | 6.33 ± 0.1 c | 6.7 ± 0.1 a b | 6.8 ± 0.1 a | 6.23 ± 0.1 c | 4.1 ± 0.1 f |
| Ca (%) | 1.49 ± 0.2 b c d | 1.42 ± 0.07 c d | 1.25 ± 0.02 d e | 1.54 ± 0.02 b c d | 0.94 ± 0 e | 1.88 ± 0.2 b | 1.67 ± 0.1 b c | 2.36 ± 0.1 a | 0.23 ± 0 f |
| P (%) | 0.40 ± 0.01 b | 0.27 ± 0.02 b c | 0.23 ± 0 e | 0.30 ± 0 d | 0.27 ± 0 d e | 0.37 ± 0 a b | 0.33 ± 0 a | 0.48 ± 0 a | 0.11 ± 0 f |
| K (%) | 0.20 ± 0.02 d e | 0.28 ± 0.02 b c | 0.23 ± 0 c d | 0.26 ± 0 c d | 0.14 ± 0 e | 0.34 ± 0 a b | 0.38 ± 0 a | 0.36 ± 0 a | 0.27 ± 0 c |
| S (%) | 0.17 ± 0.01a | 0.12 ± 0.01 c | 0.11 ± 0 c d | 0.12 ± 0 c | 0.11 ± 0 c d | 0.15 ± 0 a b | 0.13 ± 0 b c | 0.16 ± 0 a b | 0.08 ± 0 d |
| Cl (%) | 0.12 ± 0.01 d | 0.6 ± 0.04 b | 0.21 ± 0 c d | 0.29 ± 0 c | 0.09 ± 0 d | 0.69 ± 0.1 b | 1.0 ± 0.1 a | 0.63 ± 0 b | 0.57 ± 0 b |
| Texture parameters | |||||||||
| Hardness (N) | 8.25 ± 0.05 b | 4.27 ± 0.8 c d | 3.58 ± 0.55 c d | 6.49 ± 0.16 b c | 14.35 ± 1.38 a | 14.08 ± 2.34 a | 13.01 ± 1.3 a | 3.28 ± 0.14 d | 4.13 ± 0.99 c d |
| Adhesiveness (mj) | 0.19 ± 0.17 a | 0.5 ± 0.23 a | 0.44 ± 0.6 a | 0.16 ± 0.04 a | 0.06 ± 0.09 a | 0.27 ± 0.31 a | 0.08 ± 0.08 a | 0.12 ± 0.04 a | 0.35 ± 0.20 a |
| Elasticity | 0.93 ± 0.01 a | 0.88 ± 0.06 a | 0.93 ± 0.02 a | 0.92 ± 0.02 a | 0.95 ± 0 a | 0.91 ± 0.04 a | 0.95 ± 0.01 a | 0.93 ± 0.01 a | 0.56 ± 0.08 b |
| Cohesiveness | 0.80 ± 0.04 a | 0.71 ± 0.12 a | 0.68 ± 0.17 a | 0.74 ± 0.02 a | 0.75 ± 0.02 a | 0.72 ± 0.07 a | 0.81 ± 0.02 a | 0.86 ± 0.03 a | 0.36 ± 0.04 b |
| Chewiness (N) | 6.24 ± 0.30 b | 2.88 ± 0.87 c | 2.56 ± 0.07 c d | 4.93 ± 0.35 b | 10.18 ± 1.01 a | 9.12 ± 1.18 a | 9.92 ± 0.85 a | 2.64 ± 0.03 c d | 0.80 ± 0.01 d |
| Gumminess (N) | 6.55 ± 0.36 a b c | 2.97 ± 0.84 c d | 2.77 ± 0.07 c d | 5.01 ± 0.18 b c d | 10.72 ± 1.06 a | 9.97 ± 1.03 a | 10.48 ± 0.87 a | 7.83 ± 4.1 a b | 1.45 ± 0.21 d |
| Microbiological Counts (Log cfu g−1) | |||||||||
| Total coliforms | 7.5 ± 0.05 a | 5.40 ± 0.03 c | 1 ± 0 h | 1.5 ± 0.20 g | 4.50 ± 0.03 e | 4.50 ± 0.2 e | 5.96 ± 0.02 b | 4.89 ± 0.04 d | 3.76 ± 0.06 f |
| Mold and yeast | 7.52 ± 0.05 a | 7.42 ± 0.05 a | 2.39 ± 0.03 g | 4.85 ± 0.01 d | 5.59 ± 0.02 b | 3.74 ± 0.26 f | 5.25 ± 0.02 c | 4.98 ± 0.07 c d | 4.12 ± 0.12 e |
| Staphylococcus | 6.53 ± 0.06 e | 5.73 ± 0.11 a b | 2.16 ± 0.16 e | 4.72 ± 0.02 b c d | 5.39 ± 0.05 a b c | 5.50 ± 0.04 a b c | 3.62 ± 1.62 d e | 4.98 ± 0.07 b c d | 4.09 ± 0.09 c d |
| LAB | 4.67 ± 0 e | 6.07 ± 0.04 c | 1.77 ± 0.0 g | 4.06 ± 0.06 f | 6.24 ± 0.10 b c | 6.31 ± 0.03 b | 6.34 ± 0.05 b | 5.05 ± 0.10 d | 6.82 ± 0.07 a |
| Cheese Population | |||
|---|---|---|---|
| Seasonality Contrast 1 | Feeding Regiment Contrast 2 | Manufacturing Technology Contrast 3 | |
| G1W, G2W, S1W, and S2W vs. G1S, G2S, S1S, and S2S | G1W, G2W, G1S, and G2S vs. S1W, S2W, S1S, and S2S | C vs. G1W, G2W, S1W, S2W, G1S, G2S, S1S, and S2S | |
| Physicochemical parameters | |||
| Moisture (%) | 53.25 vs. 57.51 ** | 53.18 vs. 57.58 ** | 62.87 vs. 55.38 ** |
| Protein (%) | ns | 14.51 vs. 13.35 ** | 10.81 vs. 13.93 ** |
| Fat (%) | 13.5 vs. 14.25 ** | 13.75 vs. 14 ** | 14 vs. 13.88 ** |
| Ash (%) | 2.52 vs. 3.27 ** | 2.76 vs. 3.03 ** | 1.35 vs. 2.9 ** |
| Acidity (%) | 0.52 vs. 0.20 ** | 0.54 vs. 0.18 ** | 1.71 vs. 0.36 ** |
| pH | 5.98 vs. 6.52 ** | 6.11 vs. 6.38 ** | 4.1 vs. 6.25 ** |
| Ca (%) | 1.42 vs. 1.71 ** | 1.43 vs. 1.70 ** | 0.23 vs. 1.57 ** |
| P (%) | 0.30 vs. 0.36 ** | ns | 0.11 vs. 0.33 ** |
| K (%) | 0.24 vs. 0.30 ** | 0.24 vs. 0.31 ** | ns |
| S (%) | ns | 0.14 vs.0.13 * | 0.08 vs. 0.14 ** |
| Cl (%) | 0.31 vs. 0.60 ** | 0.38 vs. 0.53 ** | 0.57 vs. 0.45 ** |
| Texture parameters | |||
| Hardness (N) | 5.65 vs. 11.18 ** | 10.24 vs. 6.59 ** | 4.13 vs. 8.41 ** |
| Adhesiveness (mj) | ns | ns | ns |
| Elasticity | ns | ns | 0.56 vs. 0.93 ** |
| Cohesiveness | 0.73 vs. 0.79 * | ns | 0.36 vs. 0.76 ** |
| Chewiness (N) | 4.15 vs. 7.97 ** | 7.11 vs. 5.01 ** | 0.80 vs. 6.06 ** |
| Gumminess (N) | 4.33 vs. 9.75 ** | ns | 1.45 vs. 7.04 ** |
| Microbiological counts | |||
| Total coliforms (Log cfu g−1) | 3.69 vs. 4.96 ** | 5.32 vs. 3.34 ** | 3.76 vs. 4.33 ** |
| Molds and yeast (Log cfu g−1) | 5.55 vs. 4.89 ** | 6.07 vs. 4.37 ** | 4.12 vs. 5.22 ** |
| Staphylococcus (Log cfu g−1) | ns | 5.79 vs. 3.87 ** | 4.09 vs. 4.83 * |
| LAB (Log cfu g−1) | 4.14 vs. 5.99 ** | 5.82 vs. 4.30 ** | 6.82 vs. 5.06 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel-Ortega, S.d.C.; Campos-Múzquiz, L.G.; Charles-Rodríguez, A.V.; Palomo-Ligas, L.; Solanilla-Duque, J.F.; Flores-Gallegos, A.C.; Rodríguez-Herrera, R. Dietary Factors and Production Season Effect on the Properties of Goat Cheese. Dairy 2024, 5, 346-359. https://doi.org/10.3390/dairy5030028
Rangel-Ortega SdC, Campos-Múzquiz LG, Charles-Rodríguez AV, Palomo-Ligas L, Solanilla-Duque JF, Flores-Gallegos AC, Rodríguez-Herrera R. Dietary Factors and Production Season Effect on the Properties of Goat Cheese. Dairy. 2024; 5(3):346-359. https://doi.org/10.3390/dairy5030028
Chicago/Turabian StyleRangel-Ortega, Sarahí del Carmen, Lizeth Guadalupe Campos-Múzquiz, Ana Verónica Charles-Rodríguez, Lissethe Palomo-Ligas, José Fernando Solanilla-Duque, Adriana Carolina Flores-Gallegos, and Raúl Rodríguez-Herrera. 2024. "Dietary Factors and Production Season Effect on the Properties of Goat Cheese" Dairy 5, no. 3: 346-359. https://doi.org/10.3390/dairy5030028
APA StyleRangel-Ortega, S. d. C., Campos-Múzquiz, L. G., Charles-Rodríguez, A. V., Palomo-Ligas, L., Solanilla-Duque, J. F., Flores-Gallegos, A. C., & Rodríguez-Herrera, R. (2024). Dietary Factors and Production Season Effect on the Properties of Goat Cheese. Dairy, 5(3), 346-359. https://doi.org/10.3390/dairy5030028








