Coconut Fatty Acid Distillate Ca-Soap with Different Calcium Sources: Effects of Varied Proportions of Protected and Unprotected Fat Supplementation in Dairy Rations
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Sample Preparation
2.3. Measurements of Protected CFAD Quality
2.4. In Vitro Fermentability and Digestibility Measurement
2.5. Microbial Population Measurement
2.6. Experimental Design and Data Analysis
3. Results
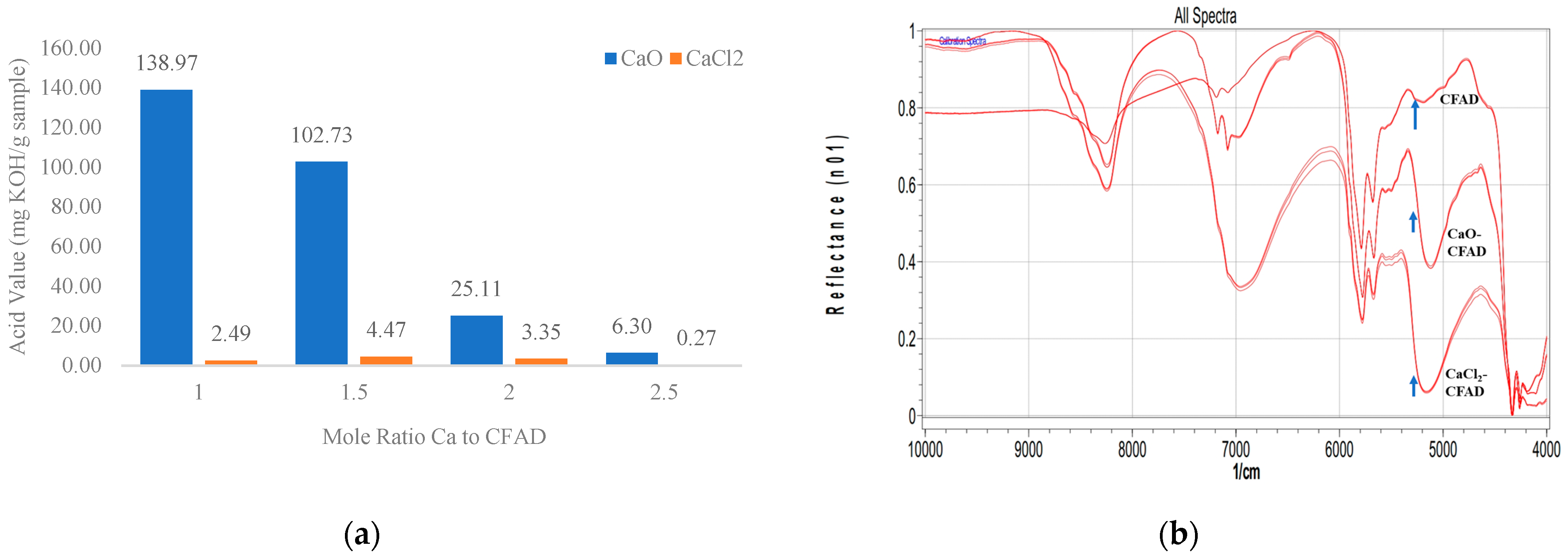
3.1. Evaluation of the Quality of Protected Ca-Soap CFAD
3.2. In Vitro Fermentability and Digestibility
3.3. Methane Production and Microbial Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahardjo, B.; Jiang, B. An analysis for providing safety in the cooking oil production process through FMECA approach. Int. J. Basic. Appl. Sci. 2016, 5, 151–156. [Google Scholar] [CrossRef]
- Statista. Coconut Production Worldwide in 2021, by Leading Country. Available online: https://www.statista.com/statistics/1040499/world-coconut-production-by-leading-producers/ (accessed on 11 November 2023).
- Patra, A.K.; Yu, Z. Effects of coconut and fish oils on ruminal methanogenesis, fermentation, and abundance and diversity of microbial populations in vitro. J. Dairy Sci. 2013, 96, 1782–1792. [Google Scholar] [CrossRef]
- Jackman, J.A.; Boyd, R.D.; Elrod, C.C. Medium-chain fatty acids and monoglycerides as feed additives for pig production: Towards gut health improvement and feed pathogen mitigation. J. Anim. Sci. Biotechnol. 2020, 11, 44. [Google Scholar] [CrossRef]
- Burdick, M.; Zhou, M.; Guan, L.L.; Oba, M. Effects of medium-chain fatty acid supplementation on performance and rumen fermentation of lactating Holstein dairy cows. Animal 2022, 16, 100491. [Google Scholar] [CrossRef]
- Nguyen, S.H.; Hegarty, R.S. Effects of defaunation and dietary coconut oil distillate on fermentation, digesta kinetics and methane production of Brahman heifers. J. Anim. Physiol. Anim. Nutr. 2017, 101, 984–993. [Google Scholar] [CrossRef]
- Hollmann, M.; Powers, W.J.; Fogiel, A.C.; Liesman, J.S.; Beede, D.K. Response profiles of enteric methane emissions and lactational performance during habituation to dietary coconut oil in dairy cows. J. Dairy Sci. 2013, 96, 1769–1781. [Google Scholar] [CrossRef]
- Delgado, D.C.; González, R.; Galindo, J.; Dihigo, L.E.; Cairo, J.; Almeida, M. Effect of the coconut oil on the consumption, digestion of nutrients and methane production in sheep fed with forage and concentrate. Cuba. J. Agric. Sci. 2012, 46, 381–384. [Google Scholar]
- Patra, A.K. A meta-analysis of the effect of dietary fat on enteric methane production, digestibility and rumen fermentation in sheep, and a comparison of these responses between cattle and sheep. Livest. Sci. 2014, 162, 97–103. [Google Scholar] [CrossRef]
- Yanza, Y.R.; Szumacher-Strabel, M.; Jayanegara, A.; Kasenta, A.M.; Gao, M.; Huang, H.; Patra, A.K.; Warzych, E.; Cieślak, A. The effects of dietary medium-chain fatty acids on ruminal methanogenesis and fermentation in vitro and in vivo: A meta-analysis. J. Anim. Physiol. Anim. Nutr. 2021, 105, 874–889. [Google Scholar] [CrossRef]
- Panyakaew, P.; Goel, G.; Lourenço, M.; Yuangklang, C.; Fievez, V. Medium-chain fatty acids from coconut or krabok oil inhibit in vitro rumen methanogenesis and conversion of non-conjugated dienoic biohydrogenation intermediates. Anim. Feed. Sci. Technol. 2013, 180, 18–25. [Google Scholar] [CrossRef]
- Wulandari; Widyobroto, B.P.; Noviandi, C.T.; Agus, A. In vitro digestibility and ruminal fermentation profile of ruminant diet in response to substitution of mixture feedstuff protected. Livest. Res. Rural Dev. 2020, 32, 12. [Google Scholar]
- dos Santos Neto, J.M.; Silva, J.O.; Meschiatti, M.A.P.; de Souza, J.; Negrão, J.A.; Lock, A.L.; Santos, F.A.P. Increasing levels of calcium salts of palm fatty acids affect production responses during the immediate postpartum and carryover periods in dairy cows. J. Dairy Sci. 2022, 105, 9652–9665. [Google Scholar] [CrossRef]
- Jenkins, T.C. Lipid Metabolism in the Rumen. J. Dairy Sci. 1993, 76, 3851–3863. [Google Scholar] [CrossRef]
- Hassan, F.; Arshad, M.A.; Ebeid, H.M.; Rehman, M.S.; Khan, M.S.; Shahid, S.; Yang, C. Phytogenic Additives Can Modulate Rumen Microbiome to Mediate Fermentation Kinetics and Methanogenesis through Exploiting Diet–Microbe Interaction. Front. Vet. Sci. 2020, 7, 575801. [Google Scholar] [CrossRef]
- Handojo, L.A.; Indarto, A.; Shofinita, D.; Saadi, M.R.; Yulistia, D.; Hasyyati, F.I. Calcium Soap from Palm Fatty Acid Distillate for Ruminant Feed: Reaction Method. Int. J. Eng. Adv. Technol. 2019, 8, 422–425. [Google Scholar]
- Handojo, L.A.; Indarto, A.; Shofinita, D.; Meitha, A.; Nabila, R.; Triharyogi, H. Calcium soap from palm fatty acid distillate (PFAD) for ruminant feed: Quality of calcium source. MATEC Web Conf. 2018, 156, 02007. [Google Scholar] [CrossRef]
- Kumar, R.; Sivaiah, K.; Reddy, Y.R.; Ekambram, B.; Reddy, T.J.; Reddy, G.V.N. Effect of supplementation of dietary protected lipids on intake and nutrient utilization in Deccani lambs. Trop. Anim. Health Prod. 2006, 38, 151–158. [Google Scholar] [CrossRef]
- Grabska, J.; Ishigaki, M.; Beć, K.B.; Wójcik, M.J.; Ozaki, Y. Correlations between Structure and Near-Infrared Spectra of Saturated and Unsaturated Carboxylic Acids. Insight from Anharmonic Density Functional Theory Calculations. J. Phys. Chem. A 2017, 121, 3437–3451. [Google Scholar] [CrossRef]
- Handojo, A.; Indarto, D.; Shofinita, M.R.; Saadi, D.; Yulistia, D.; Hasyyati, F.I. Calcium soap from palm fatty acid distillate for ruminant feed: Analysis of Product Quality (FTIR). IJSBB 2018, 2, 1–5. [Google Scholar]
- Anzhany, D.; Toharmat, T.; Despal. Ration to Produce Milk High in Conjugated Linoleic Acid (CLA) at Smallholder Dairy Farm: An In Vitro Reconstruction. Am. J. Anim. Vet. Sci. 2022, 17, 130–138. [Google Scholar]
- Wardeh, F.M. Models for Estimating Energy and Protein Utilization for Feeds. Ph.D. Thesis, Utah State University, Logan, UT, USA, 1981. [Google Scholar]
- Mertens, D.R. Creating a System for Meeting the Fiber Requirements of Dairy Cows. J. Dairy Sci. 1997, 80, 1463–1481. [Google Scholar] [CrossRef] [PubMed]
- Zahera, R.; Sari, L.A.; Permana, I.G.; Despal. The use of near-infrared reflectance spectroscopy (NIRS) to predict dairy fibre feeds in vitro digestibility. IOP Conf. Ser. Earth Environ. Sci. 2022, 951, 012100. [Google Scholar] [CrossRef]
- Tilley, J.M.A.; Terry, R.A. A two stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Despal, D.; Irmadani, D.; Permana, I.G.; Zahera, R.; Nuraina, N. Effect of Different Unsaturated Fatty Acids Sources on in Vitro Fermentability and Digestibility of Ration in Dairy Cattle. Online J. Anim. Feed. Res. 2022, 12, 154–159. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Anim. Res. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Putra, L.O.; Suharti, S.; Sarwono, K.A.; Sutikno, S.; Fitri, A.; Astuti, W.D.; Rohmatussolihat, R.; Widyastuti, Y.; Ridwan, R.; Fidriyanto, R.; et al. The effects of heat-moisture treatment on resistant starch levels in cassava and on fermentation, methanogenesis, and microbial populations in ruminants. Vet. World 2023, 16, 811–819. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Ogimoto, K.; Imai, S. Atlas of Rumen Microbiology; Societe Press: Tokyo, Japan, 1981. [Google Scholar]
- Putri, E.M.; Zain, M.; Warly, L.; Hermon, H. Effects of rumen-degradable-to-undegradable protein ratio in ruminant diet on in vitro digestibility, rumen fermentation, and microbial protein synthesis. Vet. World 2021, 14, 640–648. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.; Edwards, R.; Greenhalgh, J.; Morgan, C.; Sinclair, L.; Wilkinson, R. Animal Nutrition, 7th ed.; Preason Education Limited: England, UK, 2010. [Google Scholar]
- Lee, C.; Hristov, A.N.; Heyler, K.S.; Cassidy, T.W.; Long, M.; Corl, B.A.; Karnati, S.K.R. Effects of dietary protein concentration and coconut oil supplementation on nitrogen utilization and production in dairy cows. J. Dairy Sci. 2011, 94, 5544–5557. [Google Scholar] [CrossRef]
- Hidayah, N.; Suharti, S.; Wiryawan, K.G. In Vitro rumen fermentation of ration supplemented with protected vegetable oils. Med. Pet. 2014, 37, 129–135. [Google Scholar] [CrossRef][Green Version]
- Handojo, L.A.; Indarto, A.; Shofinita, D.; Saadi, M.R.; Yulistia, D.; Hasyyati, F.I. Calcium soap from palm fatty acid distillate for ruminant feed: Calcium oxide particles size. IMATEC Web of Conf. 2019, 268, 03001. [Google Scholar] [CrossRef]
- Evangelista, C.; Basiricò, L.; Bernabucci, U. An overview on the use of near infrared spectroscopy (nirs) on farms for the management of dairy cows. Agriculture 2021, 11, 296. [Google Scholar] [CrossRef]
- Mentink, R.L.; Hoffman, P.C.; Bauman, L.M. Utility of near-infrared reflectance spectroscopy to predict nutrient composition and in vitro digestibility of total mixed rations. J. Dairy Sci. 2006, 89, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Coppa, M.; Martin, B.; Agabriel, C.; Chassaing, C.; Sibra, C.; Constant, I.; Graulet, B.; Andueza, D. Authentication of cow feeding and geographic origin on milk using visible and near-infrared spectroscopy. J. Dairy Sci. 2012, 95, 5544–5551. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, S.; Xie, T.; Wang, Q.; Wang, Z.; Yang, H.; Li, S.; Wang, W. Effect of Unsaturated Fatty Acid Ratio In Vitro on Rumen Fermentation, Methane Concentration, and Microbial Profile. Fermentation 2022, 8, 540. [Google Scholar] [CrossRef]
- Suharti, S.; Nasution, A.R.; Wiryawan, K.G. In vitro rumen fermentation characteristics and fatty acid profiles added with calcium soap of canola/flaxseed oil. Med. Pet. 2017, 40, 171–177. [Google Scholar] [CrossRef]
- Gleason, C.B.; Beckett, L.M.; White, R.R. Rumen fermentation and epithelial gene expression responses to diet ingredients designed to differ in ruminally degradable protein and fiber supplies. Sci. Rep. 2022, 12, 2933. [Google Scholar] [CrossRef]
- Cherdthong, A.; Wanapat, M. Manipulation of in vitro ruminal fermentation and digestibility by dried rumen digesta. Livest. Sci. 2013, 153, 94–100. [Google Scholar] [CrossRef]
- Behan, A.A.; Loh, T.C.; Fakurazi, S.; Kaka, U.; Kaka, A.; Samsudin, A.A. Effects of supplementation of rumen protected fats on rumen ecology and digestibility of nutrients in sheep. Animals 2019, 9, 400. [Google Scholar] [CrossRef]
- Shen, J.; Zheng, W.; Xu, Y.; Yu, Z. The inhibition of high ammonia to in vitro rumen fermentation is pH dependent. Front. Vet. Sci. 2023, 10, 1163021. [Google Scholar] [CrossRef]
- Adelusi, O.O.; Onwuka, C.F.I.; Anifowose, I.O.; Ojo, V.O.A.; Yusuf, K.O. Effects of mixtures of coconut and palm kernel oil on the rumen fermentation parameters and microbial population of cattle. Livest. Res. Rural Dev. 2023, 27, 136. [Google Scholar]
- Anele, U.Y.; Crumel, X.; Olagunju, L.; Compart, D.P. Effects of Yucca schidigera Based Feed Additive on In Vitro Dry Matter Digestibility, Efficiency of Microbial Production, and Greenhouse Gas Emissions of Four Dairy Diets. Dairy 2022, 3, 326–332. [Google Scholar] [CrossRef]
- dos Santos Neto, J.M.; de Souza, J.; Lock, A.L. Effects of calcium salts of palm fatty acids on nutrient digestibility and production responses of lactating dairy cows: A meta-analysis and meta-regression. J. Dairy Sci. 2021, 104, 9752–9768. [Google Scholar] [CrossRef] [PubMed]
- Golovin, A.; Devyatkin, V. Effect of Protected Vegetable Fats on Nutrient Digestibility and Productivity of Dairy Cows. In Fundamental and Applied Scientific Research in the Development of Agriculture in the Far East; Springer: Cham, Switzerland, 2021; pp. 367–376. [Google Scholar]
- Lourenço, M.; Ramos-Morales, E.; Wallace, R.J. The role of microbes in rumen lipolysis and biohydrogenation and their manipulation. Animal 2010, 4, 1008–1023. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, Y.; Tani, M.; Matsushita, Y.; Otsuka, H.; Kobayashi, Y. Effects of lauric acid on physical, chemical and microbial characteristics in the rumen of steers on a high grain diet. Anim. Sci. J. 2007, 78, 387–394. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Nguyen, S.H.; Bremner, G.; Cameron, M.; Hegarty, R.S. Methane emissions, ruminal characteristics and nitrogen utilisation changes after refaunation of protozoa-free sheep. Small Rumin. Res. 2016, 144, 48–55. [Google Scholar] [CrossRef]
- Hristov, A.N.; Vander Pol, M.; Agle, M.; Zaman, S.; Schneider, C.; Ndegwa, P.; Vaddella, V.K.; Johnson, K.; Shingfield, K.J.; Karnati, S.K.R. Effect of lauric acid and coconut oil on ruminal fermentation, digestion, ammonia losses from manure, and milk fatty acid composition in lactating cows. J. Dairy Sci. 2009, 92, 5561–5582. [Google Scholar] [CrossRef]
- Volmer, J.G.; McRae, H.; Morrison, M. The evolving role of methanogenic archaea in mammalian microbiomes. Front. Microbiol. 2023, 14, 1268451. [Google Scholar] [CrossRef]
- Qin, W.Z.; Li, C.Y.; Kim, J.K.; Ju, J.G.; Song, M.K. Effects of defaunation on fermentation characteristics and methane production by rumen microbes in vitro when incubated with starchy feed sources. Asian-Australas. J. Anim. Sci. 2012, 25, 1381–1388. [Google Scholar] [CrossRef]
| Ingredients | % DM 1 | Ash * | EE 2* | CP 3* | CF 4* | NFE 5 | TDN 6# | NDF 7* | NFC 8# |
|---|---|---|---|---|---|---|---|---|---|
| ------------------------% DM ------------------------------ | |||||||||
| Napier grass | 40 | 14.35 | 3.32 | 12.99 | 36.36 | 42.13 | 54.53 | 57.97 | 11.39 |
| Concentrates | 60 | 9.77 | 3.96 | 15.17 | 11.44 | 59.66 | 72.38 | 32.20 | 38.90 |
| Total | 100 | 11.60 | 3.70 | 14.30 | 21.41 | 52.65 | 65.24 | 42.51 | 27.89 |
| Target | Name | Sequence (5′-3′) | Amount Added (µ) |
|---|---|---|---|
| Total bacteria | 1114-f 1275-r | CGGCAACGAGCGCAACCC CCATTGTAGCACGTGTGTAGCC | 0.6 0.6 |
| Methanogens | q-mcrA-f q-mcra-r | TTCGGTGGATCDCARAGRGC GBARGTCGWAWCCGTAGAATCC | 1.2 1.2 |
| Butyrivibrio fibrisolvens | ButFib 2F ButFib 2R | ACCGCATAAGCGCACGGA CGGGTCCATCTTGTACCGATAAAT | 0.2 0.1 |
| Genus bacteroides | AllBac 296-f AllBac 412-r | GAGAGGAAGGTCCCCCAC CGCTACTTGGCTGGTTCAG | 0.2 3.6 |
| Streptococcus bovis | StrBoy 2F StrBoy 2R | TTCCTAGAGATAGGAAGTTTCTTCGG ATGATGGCAACTAACAATAGGGGT | 8.8 8.8 |
| Treatments | C=O Str. Second Overtone of COOH Wavenumber = 5263 cm−1 |
|---|---|
| CFAD 1 | 0.8199 |
| CaO-CFAD 2 | 0.5453 |
| CaCl2-CFAD 3 | 0.1079 |
| Parameters | Level | Unprotected-to-Protected CFAD 1 Ratio | Average | ||||
|---|---|---|---|---|---|---|---|
| 100:0 | 75:25 | 50:50 | 25:75 | 0:100 | |||
| pH | 0% | 6.93 ± 0.06 | 6.97 ± 0.06 | 6.90 ± 0.10 | 6.97 ± 0.06 | 6.97 ± 0.06 | 6.95 ± 0.03 |
| 1% | 6.87 ± 0.06 | 6.97 ± 0.15 | 6.97 ± 0.15 | 6.90 ± 0.10 | 6.90 ± 0.10 | 6.92 ± 0.04 | |
| 2% | 6.93 ± 0.06 | 6.93 ± 0.12 | 6.93 ± 0.06 | 6.97 ± 0.15 | 6.90 ± 0.10 | 6.93 ± 0.02 | |
| 3% | 6.97 ± 0.06 | 6.97 ± 0.15 | 6.97 ± 0.15 | 6.93 ± 0.06 | 6.90 ± 0.10 | 6.95 ± 0.03 | |
| Average | 6.93 ± 0.04 | 6.96 ± 0.02 | 6.94 ± 0.03 | 6.94 ± 0.03 | 6.92 ± 0.03 | ||
| Ammonia (mM) | 0% | 7.69 ± 4.41 | 7.57 ± 3.95 | 7.85 ± 3.87 | 7.78 ± 1.94 | 7.42 ± 2.78 | 7.66 ± 0.17 |
| 1% | 7.96 ± 3.59 | 6.56 ± 1.93 | 6.98 ± 2.75 | 8.71 ± 3.06 | 8.08 ± 3.08 | 7.66 ± 0.87 | |
| 2% | 8.43 ± 1.57 | 6.87 ± 1.93 | 8.64 ± 4.06 | 7.25 ± 1.6 | 7.62 ± 3.53 | 7.76 ± 0.76 | |
| 3% | 7.47 ± 1.60 | 8.15 ± 1.37 | 8.71 ± 2.96 | 7.76 ± 2.92 | 8.43 ± 3.06 | 8.10 ± 0.50 | |
| Average | 7.88 ± 0.41 | 7.29 ± 0.71 | 8.04 ± 0.81 | 7.87 ± 0.61 | 7.89 ± 0.46 | ||
| Total VFAs 2 (mM) | 0% | 108.12 ± 13.87 | 113.84 ± 9.97 | 106.12 ± 10.35 | 111.02 ± 7.83 | 114.70 ± 3.12 | 110.76 ± 3.66 |
| 1% | 101.85 ± 9.35 | 116.14 ± 10.42 | 113.75 ± 11.49 | 111.49 ± 9.25 | 111.95 ± 7.50 | 111.04 ± 5.45 | |
| 2% | 117.71 ± 20.01 | 114.86 ± 14.00 | 111.18 ± 6.67 | 110.98 ± 13.15 | 107.18 ± 7.95 | 112.38 ± 4.03 | |
| 3% | 121.55 ± 12.61 | 108.97 ± 13.73 | 109.69 ± 12.15 | 114.36 ± 10.24 | 115.99 ± 8.16 | 114.11 ± 5.12 | |
| Average | 112.31 ± 8.97 | 113.45 ± 3.13 | 110.19 ± 3.18 | 111.96 ± 1.61 | 112.45 ± 3.90 | ||
| Parameters | Level | Unprotected-to-Protected CFAD 1 Ratio | Average | ||||
|---|---|---|---|---|---|---|---|
| 100:0 | 75:25 | 50:50 | 25:75 | 0:100 | |||
| IVDMD 2 (%) | 0% | 62.48 ± 1.21 | 63.92 ± 0.27 | 63.83 ± 0.23 | 62.71 ± 0.98 | 63.56 ± 1.22 | 63.30 ± 0.66 a |
| 1% | 62.84 ± 2.31 | 61.64 ± 0.55 | 62.72 ± 1.34 | 61.27 ± 0.62 | 62.38 ± 3.11 | 62.17 ± 0.69 ab | |
| 2% | 60.25 ± 2.01 | 60.86 ± 0.52 | 60.80 ± 2.95 | 61.22 ± 2.38 | 63.45 ± 1.67 | 61.32 ± 1.24 b | |
| 3% | 56.60 ± 3.30 | 57.94 ± 1.29 | 59.06 ± 0.61 | 62.42 ± 2.42 | 62.22 ± 1.88 | 59.65 ± 2.59 c | |
| Average | 60.54 ± 2.21 b | 61.09 ± 0.66 ab | 61.60 ± 1.28 ab | 61.90 ± 1.60 ab | 62.90 ± 0.70 b | ||
| IVDMO 3 (%) | 0% | 59.67 ± 1.21 | 61.72 ± 0.27 | 61.47 ± 0.23 | 60.07 ± 0.98 | 61.06 ± 1.22 | 60.80 ± 0.89 a |
| 1% | 60.07 ± 2.31 | 58.59 ± 0.55 | 59.55 ± 1.34 | 58.39 ± 0.62 | 59.62 ± 3.11 | 59.24 ± 0.72 ab | |
| 2% | 56.75 ± 2.01 | 57.63 ± 0.52 | 57.63 ± 2.95 | 58.09 ± 2.38 | 60.80 ± 1.67 | 58.18 ± 1.54 bc | |
| 3% | 52.74 ± 3.30 | 57.39 ± 1.29 | 56.76 ± 0.61 | 59.72 ± 2.42 | 59.31 ± 1.88 | 57.18 ± 2.78 c | |
| Average | 57.30 ± 3.39 | 58.83 ± 1.99 | 58.85 ± 2.10 | 59.07 ± 0.98 | 60.20 ± 0.86 | ||
| Parameters | Level | Unprotected-to-Protected CFAD 1 Ratio | Average | ||||
|---|---|---|---|---|---|---|---|
| 100:0 | 75:25 | 50:50 | 25:75 | 0:100 | |||
| Acetate (%) | 0% | 59.89 ± 5.79 | 56.71 ± 8.50 | 60.57 ± 12.72 | 61.00 ± 7.95 | 63.10 ± 6.03 | 60.25 ± 2.31 |
| 1% | 57.73 ± 9.79 | 54.86 ± 9.44 | 55.98 ± 4.01 | 65.72 ± 3.74 | 55.55 ± 6.16 | 57.97 ± 4.46 | |
| 2% | 56.41 ± 6.21 | 62.63 ± 4.69 | 62.65 ± 7.30 | 56.22 ± 4.32 | 57.09 ± 8.30 | 59.00 ± 3.34 | |
| 3% | 53.09 ± 5.82 | 53.57 ± 6.25 | 58.48 ± 4.82 | 61.15 ± 8.23 | 57.00 ± 8.37 | 56.66 ± 3.39 | |
| Average | 56.78 ± 2.85 | 56.94 ± 4.01 | 59.42 ± 2.86 | 61.02 ± 3.88 | 58.19 ± 3.35 | ||
| Propionate (%) | 0% | 22.61 ± 1.85 | 24.25 ± 6.82 | 19.26 ± 2.7 | 20.12 ± 3.23 | 21.16 ± 2.36 | 21.48 ± 1.99 |
| 1% | 23.76 ± 2.69 | 22.05 ± 3.02 | 23.42 ± 2.5 | 21.04 ± 2.7 | 24.29 ± 5.24 | 22.91 ± 1.34 | |
| 2% | 23.74 ± 2.72 | 21.18 ± 5.33 | 20.17 ± 1.09 | 23.05 ± 4.36 | 22.39 ± 5.61 | 22.11 ± 1.44 | |
| 3% | 25.76 ± 6.54 | 26.66 ± 7.98 | 23.52 ± 1.15 | 22.01 ± 2.28 | 25.07 ± 2.37 | 24.60 ± 1.85 | |
| Average | 23.97 ± 1.31 | 23.54 ± 2.45 | 21.59 ± 2.2 | 21.56 ± 1.26 | 23.23 ± 1.78 | ||
| n-Butyrate (%) | 0% | 11.39 ± 4.02 | 12.29 ± 4.56 | 12.48 ± 5.63 | 13.01 ± 3.08 | 10.96 ± 4.01 | 12.03 ± 0.83 |
| 1% | 12.02 ± 6.96 | 13.68 ± 2.91 | 12.34 ± 2.17 | 8.33 ± 2.68 | 12.73 ± 2.94 | 11.82 ± 2.05 | |
| 2% | 13.78 ± 3.74 | 10.78 ± 0.81 | 10.83 ± 3.3 | 12.54 ± 4.1 | 11.72 ± 3.86 | 11.93 ± 1.26 | |
| 3% | 13.85 ± 4.86 | 13.52 ± 0.82 | 13.22 ± 4.28 | 12.18 ± 5.22 | 12.64 ± 5.07 | 13.08 ± 0.67 | |
| Average | 12.76 ± 1.25 | 12.56 ± 1.34 | 12.22 ± 1 | 11.51 ± 2.15 | 12.01 ± 0.84 | ||
| Iso-Butyrate (%) | 0% | 2.18 ± 1.07 | 2.59 ± 1.26 | 2.65 ± 1.36 | 1.98 ± 0.87 | 1.69 ± 0.64 | 2.22 ± 0.41 |
| 1% | 1.82 ± 0.78 | 3.39 ± 1.56 | 2.94 ± 0.78 | 1.67 ± 0.96 | 3.12 ± 1.9 | 2.59 ± 0.79 | |
| 2% | 1.33 ± 0.41 | 1.96 ± 0.55 | 2.27 ± 1.21 | 3.01 ± 1.56 | 3.13 ± 2.28 | 2.34 ± 0.75 | |
| 3% | 3.02 ± 2.07 | 2.48 ± 1.26 | 1.65 ± 0.27 | 1.68 ± 0.49 | 2.03 ± 0.67 | 2.17 ± 0.58 | |
| Average | 2.09 ± 0.71 | 2.61 ± 0.59 | 2.38 ± 0.56 | 2.09 ± 0.63 | 2.49 ± 0.75 | ||
| Iso-Valerate (%) | 0% | 2.10 ± 1.07 | 2.58 ± 1.63 | 2.52 ± 1.23 | 2.17 ± 1.08 | 2.01 ± 1.16 | 2.28 ± 0.26 |
| 1% | 2.59 ± 1.99 | 3.43 ± 1.55 | 2.97 ± 0.94 | 1.50 ± 0.16 | 2.11 ± 0.47 | 2.52 ± 0.75 | |
| 2% | 2.76 ± 2.14 | 1.99 ± 0.85 | 2.31 ± 1.59 | 2.86 ± 0.71 | 3.09 ± 1.71 | 2.60 ± 0.44 | |
| 3% | 2.58 ± 2.05 | 2.16 ± 0.78 | 1.79 ± 0.62 | 1.70 ± 0.5 | 1.95 ± 0.51 | 2.04 ± 0.35 | |
| Average | 2.51 ± 0.29 | 2.54 ± 0.64 | 2.4 ± 0.49 | 2.06 ± 0.6 | 2.29 ± 0.54 | ||
| n-Valerate (%) | 0% | 1.84 ± 0.99 | 1.58 ± 1.93 | 2.51 ± 2.15 | 1.72 ± 1.24 | 1.08 ± 0.09 | 1.75 ± 0.52 |
| 1% | 2.07 ± 1.79 | 2.58 ± 0.89 | 2.35 ± 0.4 | 1.75 ± 0.7 | 2.2 ± 0.96 | 2.19 ± 0.31 | |
| 2% | 1.99 ± 1.3 | 1.45 ± 0.34 | 1.77 ± 1.49 | 2.32 ± 0.21 | 2.58 ± 1.53 | 2.02 ± 0.44 | |
| 3% | 1.70 ± 0.77 | 1.62 ± 0.47 | 1.35 ± 0.12 | 1.27 ± 0.49 | 1.31 ± 0.17 | 1.45 ± 0.19 | |
| Average | 1.90 ± 0.16 | 1.81 ± 0.52 | 2.00 ± 0.54 | 1.77 ± 0.43 | 1.79 ± 0.71 | ||
| A:P 2 Ratio | 0% | 2.65 ± 0.22 | 2.47 ± 0.75 | 3.24 ± 1.02 | 3.12 ± 0.85 | 3.02 ± 0.54 | 2.90 ± 0.32 |
| 1% | 2.43 ± 0.38 | 2.55 ± 0.74 | 2.41 ± 0.38 | 3.17 ± 0.51 | 2.39 ± 0.75 | 2.59 ± 0.33 | |
| 2% | 2.40 ± 0.35 | 3.15 ± 1.15 | 3.11 ± 0.39 | 2.51 ± 0.6 | 2.69 ± 0.88 | 2.77 ± 0.34 | |
| 3% | 2.14 ± 0.50 | 2.18 ± 0.88 | 2.49 ± 0.25 | 2.82 ± 0.6 | 2.31 ± 0.53 | 2.39 ± 0.28 | |
| Average | 2.41 ± 0.21 | 2.59 ± 0.41 | 2.81 ± 0.42 | 2.9 ± 0.3 | 2.6 ± 0.32 | ||
| CH4 (mol/100 mol) | 0% | 25.29 ± 1.02 | 23.77 ± 4.22 | 26.95 ± 4.27 | 27.12 ± 3.24 | 26.96 ± 1.78 | 26.02 ± 1.46 |
| 1% | 24.25 ± 1.55 | 24.09 ± 4.07 | 23.68 ± 1.45 | 27.12 ± 1.97 | 23.41 ± 4.07 | 24.51 ± 1.50 | |
| 2% | 24.37 ± 1.64 | 26.67 ± 3.55 | 26.98 ± 1.96 | 23.97 ± 4.05 | 24.22 ± 6.03 | 25.24 ± 1.46 | |
| 3% | 22.35 ± 3.12 | 22.18 ± 4.89 | 25.13 ± 0.68 | 26.34 ± 2.24 | 23.81 ± 2.37 | 23.96 ± 1.79 | |
| Average | 24.06 ± 1.23 | 24.18 ± 1.86 | 25.69 ± 1.59 | 26.14 ± 1.49 | 24.60 ± 1.61 | ||
| Parameters | Level | Unprotected-to-Protected CFAD 1 Ratio | Average | ||||
|---|---|---|---|---|---|---|---|
| 100:0 | 75:25 | 50:50 | 25:75 | 0:100 | |||
| Butyrivibrio fibrisolvens (2−ΔΔCT) | 0 | 1.17 ± 0.6 | 0.96 ± 0.36 | 0.96 ± 0.36 | 1.43 ± 0.15 | 1.11 ± 0.7 | 1.13 ± 0.12 |
| 1 | 1.21 ± 0.76 | 0.65 ± 0.4 | 1.75 ± 0.59 | 1.15 ± 0.46 | 1.20 ± 0.30 | 1.19 ± 0.26 | |
| 2 | 1.39 ± 0.74 | 1.58 ± 0.21 | 0.91 ± 0.24 | 0.97 ± 0.35 | 1.02 ± 0.35 | 1.17 ± 0.27 | |
| 3 | 0.87 ± 0.33 | 1.24 ± 0.31 | 0.92 ± 0.20 | 0.79 ± 0.16 | 1.14 ± 0.27 | 0.99 ± 0.18 | |
| Average | 1.16 ± 0.19 | 1.11 ± 0.22 | 1.14 ± 0.36 | 1.09 ± 0.25 | 1.12 ± 0.08 | ||
| Genus bacteroides (2−ΔΔCT) | 0 | 1.00 ± 0.06 | 0.89 ± 0.18 | 1.08 ± 0.09 | 1.40 ± 0.09 | 1.45 ± 0.53 | 1.17 ± 0.19 |
| 1 | 0.89 ± 0.06 | 1.07 ± 0.18 | 0.81 ± 0.09 | 1.51 ± 0.09 | 1.17 ± 0.53 | 1.09 ± 0.25 | |
| 2 | 0.69 ± 0.22 | 0.88 ± 0.24 | 1.33 ± 0.32 | 1.09 ± 0.32 | 1.09 ± 0.16 | 1.02 ± 0.27 | |
| 3 | 0.64 ± 0.15 | 0.75 ± 0.20 | 0.76 ± 0.33 | 1.22 ± 0.28 | 1.29 ± 0.23 | 0.93 ± 0.24 | |
| Average | 0.81 ± 0.15 b | 0.90 ± 0.17 b | 1.00 ± 0.37 b | 1.31 ± 0.07a | 1.25 ± 0.03 a | ||
| Streptococcus bovis (2−ΔΔCT) | 0 | 1.37 ± 0.83 | 1.23 ± 0.53 | 1.38 ± 0.4 | 0.99 ± 0.86 | 1.35 ± 0.94 | 1.27 ± 0.16 |
| 1 | 1.81 ± 0.98 | 1.29 ± 0.01 | 1.18 ± 0.48 | 0.88 ± 0.35 | 0.95 ± 0.16 | 1.22 ± 0.37 | |
| 2 | 1.66 ± 0.39 | 1.01 ± 0.31 | 0.94 ± 0.92 | 1.86 ± 2.61 | 1.59 ± 0.99 | 1.41 ± 0.41 | |
| 3 | 1.18 ± 0.26 | 1.99 ± 0.3 | 0.45 ± 0.47 | 0.38 ± 0.29 | 1.72 ± 1.41 | 1.14 ± 0.73 | |
| Average | 1.51 ± 0.28 | 1.38 ± 0.43 | 0.99 ± 0.40 | 1.03 ± 0.61 | 1.40 ± 0.34 | ||
| Methanogens (2−ΔΔCT) | 0 | 1.46 ± 0.78 | 1.47 ± 0.56 | 1.53 ± 0.34 | 1.47 ± 0.56 | 1.15 ± 0.68 | 1.42 ± 0.15 a |
| 1 | 0.92 ± 0.37 | 1.55 ± 0.55 | 1.34 ± 0.33 | 1.23 ± 0.43 | 0.81 ± 0.79 | 1.17 ± 0.31 ab | |
| 2 | 1.37 ± 0.31 | 1.27 ± 0.11 | 0.94 ± 0.35 | 1.01 ± 0.13 | 1.03 ± 0.68 | 1.12 ± 0.19 ab | |
| 3 | 1.32 ± 0.51 | 0.87 ± 0.76 | 0.89 ± 0.23 | 0.80 ± 0.24 | 0.99 ± 0.43 | 0.97 ± 0.2 b | |
| Average | 1.27 ± 0.24 | 1.29 ± 0.3 | 1.17 ± 0.31 | 1.13 ± 0.29 | 0.99 ± 0.14 | ||
| Total Protozoa (log cell/mL) | 0 | 6.19 ± 0.13 | 6.19 ± 0.12 | 6.27 ± 0.01 | 6.22 ± 0.08 | 6.19 ± 0.09 | 6.21 ± 0.03 a |
| 1 | 6.18 ± 0.07 | 6.24 ± 0.08 | 6.16 ± 0.1 | 6.22 ± 0.05 | 6.16 ± 0.13 | 6.19 ± 0.04 ab | |
| 2 | 6.06 ± 0.15 | 6.03 ± 0.13 | 6.17 ± 0.02 | 6.19 ± 0.01 | 6.24 ± 0.09 | 6.14 ± 0.09 ab | |
| 3 | 5.96 ± 0.19 | 6.07 ± 0.13 | 6.12 ± 0.05 | 6.18 ± 0.06 | 6.10 ± 0.07 | 6.09 ± 0.08 b | |
| Average | 6.10 ± 0.11 | 6.13 ± 0.1 | 6.18 ± 0.06 | 6.2 ± 0.02 | 6.17 ± 0.06 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahera, R.; Pratiwi, M.I.; Fitri, A.; Koike, S.; Permana, I.G.; Despal. Coconut Fatty Acid Distillate Ca-Soap with Different Calcium Sources: Effects of Varied Proportions of Protected and Unprotected Fat Supplementation in Dairy Rations. Dairy 2024, 5, 542-554. https://doi.org/10.3390/dairy5030041
Zahera R, Pratiwi MI, Fitri A, Koike S, Permana IG, Despal. Coconut Fatty Acid Distillate Ca-Soap with Different Calcium Sources: Effects of Varied Proportions of Protected and Unprotected Fat Supplementation in Dairy Rations. Dairy. 2024; 5(3):542-554. https://doi.org/10.3390/dairy5030041
Chicago/Turabian StyleZahera, Rika, Mega Indah Pratiwi, Ainissya Fitri, Satoshi Koike, Idat Galih Permana, and Despal. 2024. "Coconut Fatty Acid Distillate Ca-Soap with Different Calcium Sources: Effects of Varied Proportions of Protected and Unprotected Fat Supplementation in Dairy Rations" Dairy 5, no. 3: 542-554. https://doi.org/10.3390/dairy5030041
APA StyleZahera, R., Pratiwi, M. I., Fitri, A., Koike, S., Permana, I. G., & Despal. (2024). Coconut Fatty Acid Distillate Ca-Soap with Different Calcium Sources: Effects of Varied Proportions of Protected and Unprotected Fat Supplementation in Dairy Rations. Dairy, 5(3), 542-554. https://doi.org/10.3390/dairy5030041





