The Role of Exopolysaccharide-Producing Streptococcus thermophilus on Physical Properties of Stirred Skim Milk Gel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Microorganisms
2.2. Experimental Design
2.3. Milk Powder Analysis and Base Milk Preparation
2.4. Production of Stirred Skim Milk Gels
2.5. Properties of Stirred Skim Milk Gels
2.5.1. Exopolysaccharide Production and Serum Viscosity
2.5.2. Analysis of Physical Properties and Microstructure
2.5.3. Determination of Serum Release
2.6. Statistical Data Evaluation
3. Results and Discussion
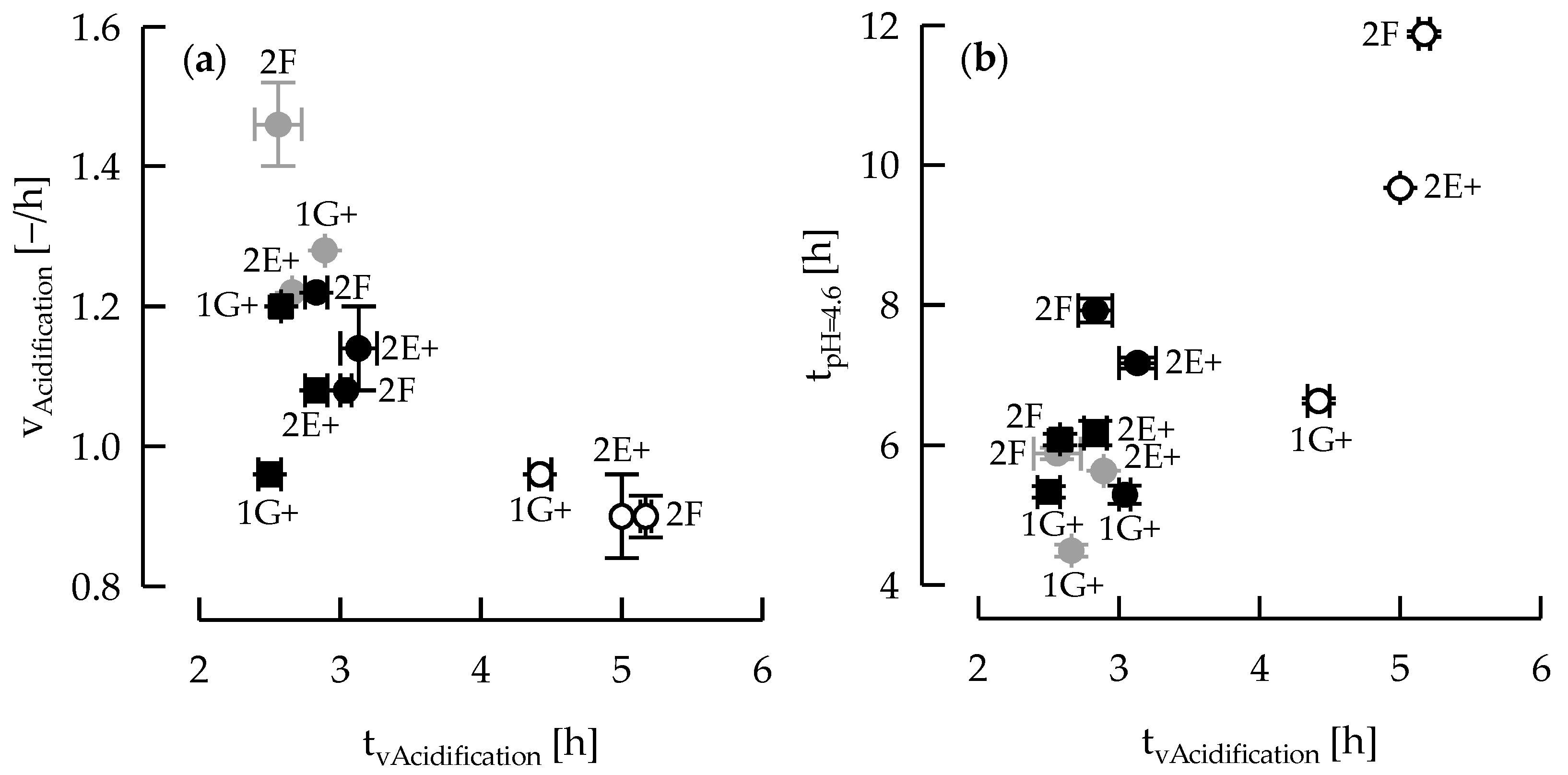
3.1. Acidification Kinetic and EPS Production
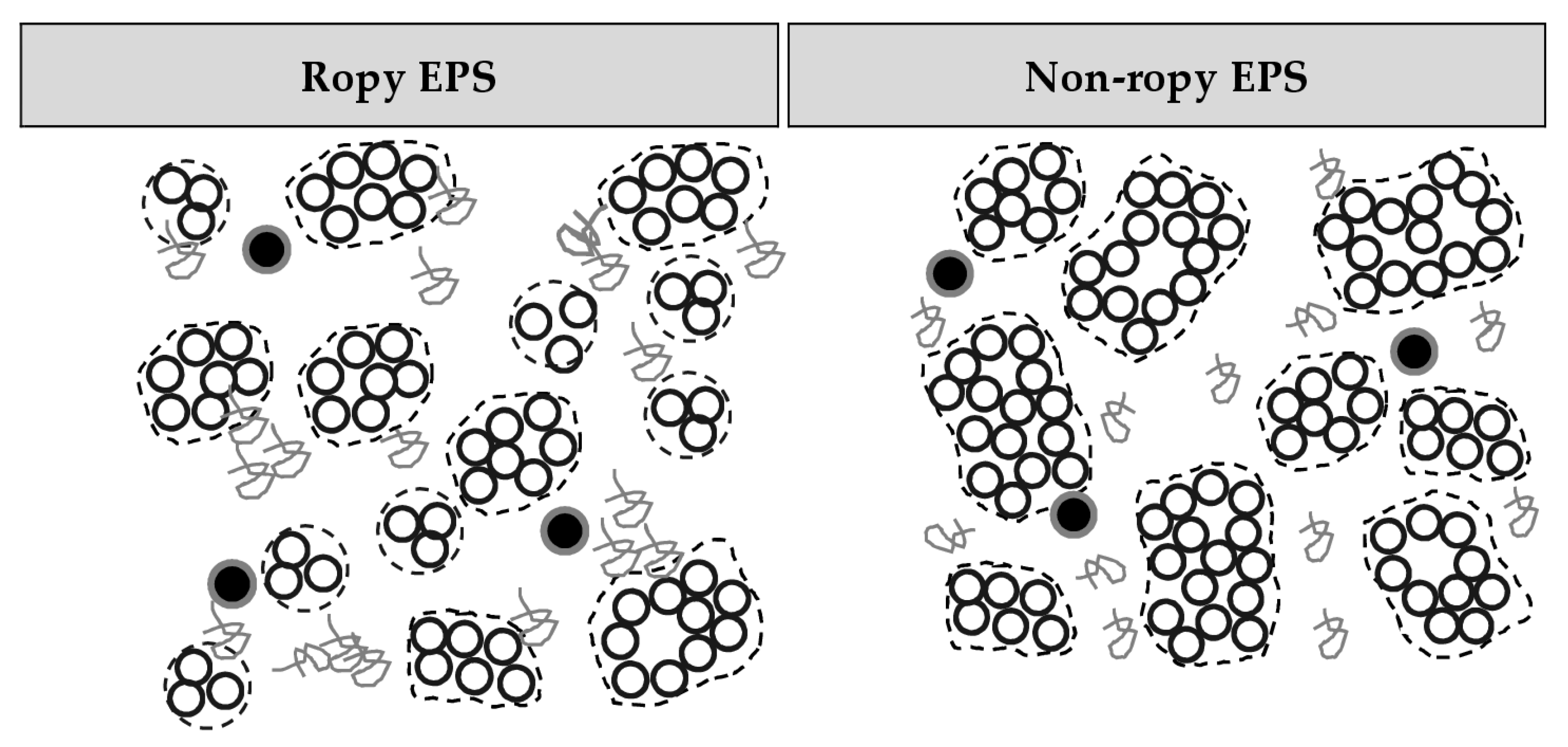
3.2. Casein: Whey Protein Ratio and Capsular EPS Affect Phase Separation
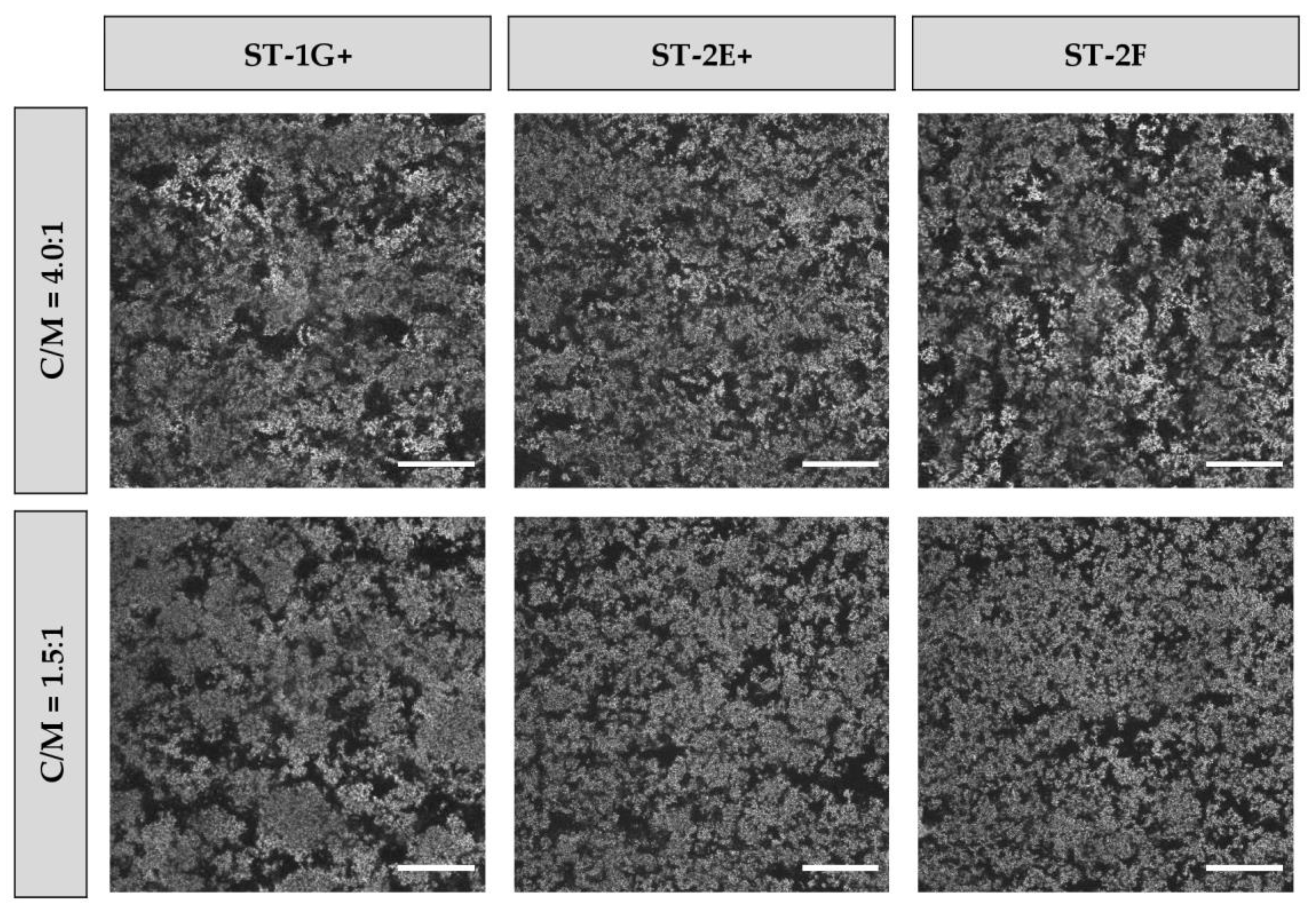
3.3. Particle Size and Microstructure
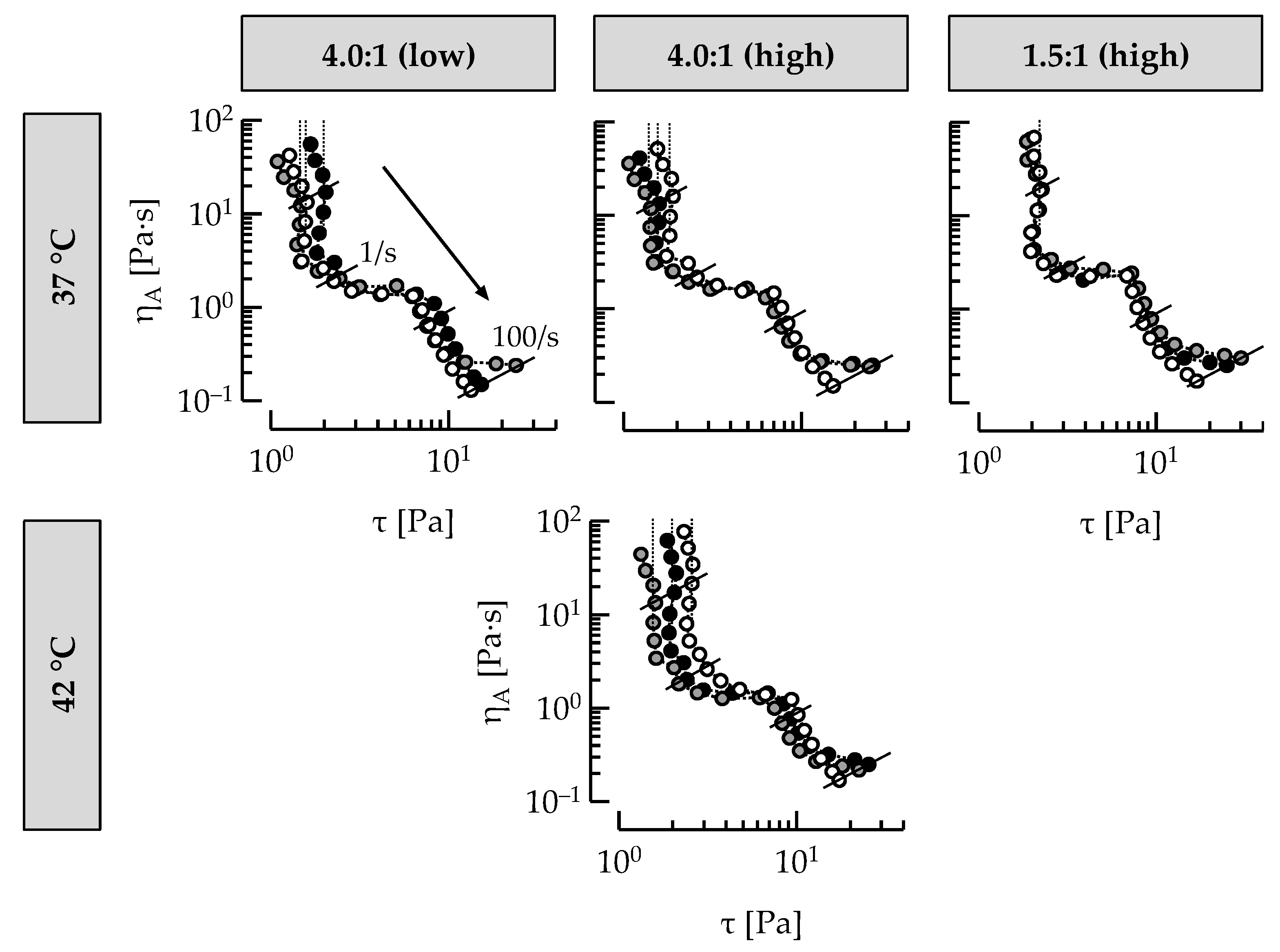
3.4. Casein: Whey Protein Ratio and Ropy EPS Affect Shear Viscosity
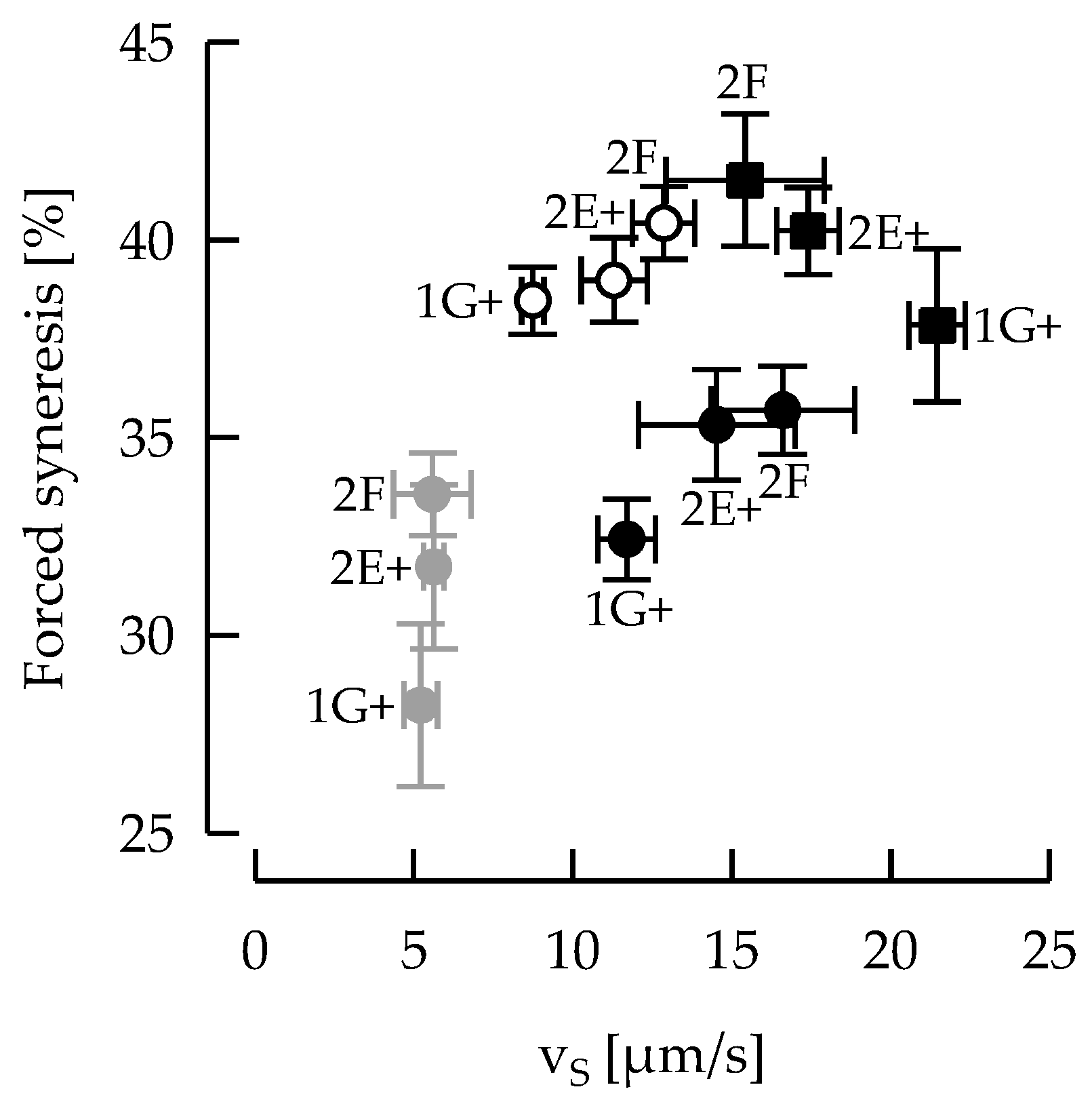
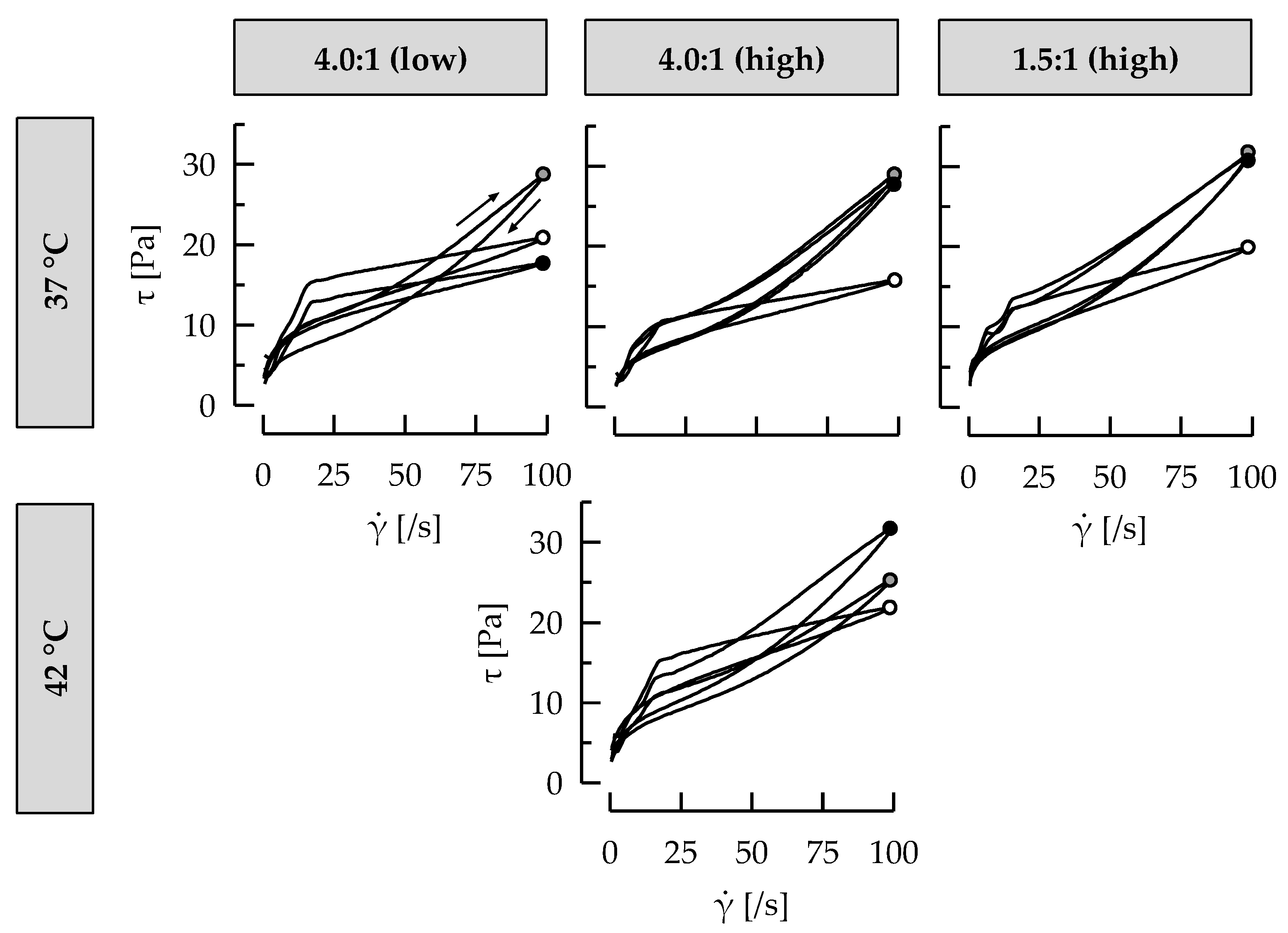
3.5. Ropy EPS Are Relevant for Shear Stability
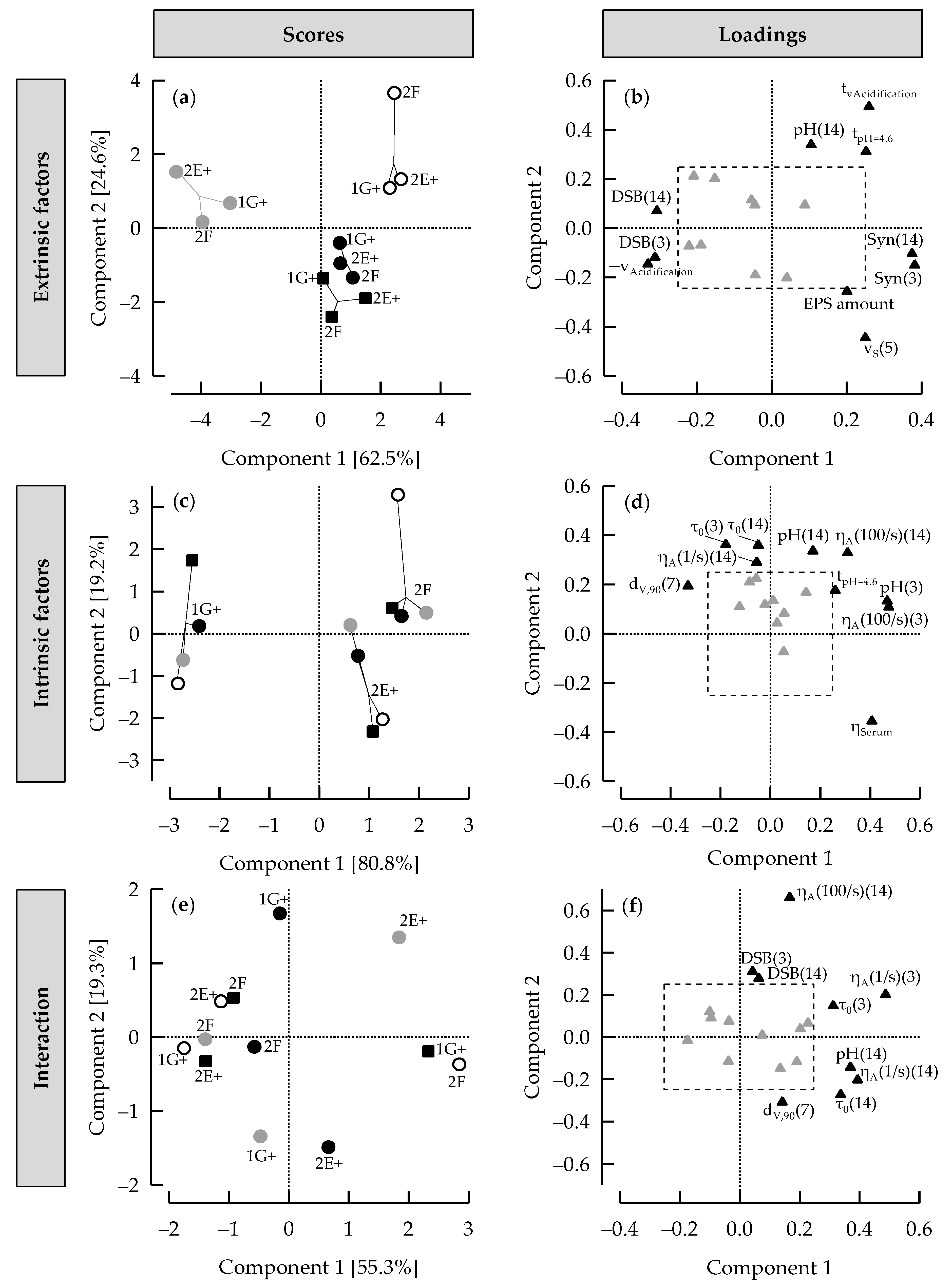
3.6. ASCA+ to Distinguish between Effects of Extrinsic and Intrinsic Factors on Stirred Gels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Dairy Science and Technology; CRC Press: New York, NY, USA, 2006. [Google Scholar]
- FAO/WHO. Codex Alimentarius International Food Standards: Codex Standard for Fermented Milks; Food and Agriculture Organisation of the United Nations/World Health Organisation: Geneva, Switzerland, 2003; CODEX STAN 243-2003. [Google Scholar]
- Mokoonlall, A.; Nöbel, S.; Hinrichs, J. Post-processing of fermented milk to stirred products: Reviewing the effects on gel structure. Trends Food Sci. Technol. 2016, 54, 26–36. [Google Scholar] [CrossRef]
- Román, S.; Sánchez-Siles, L.M.; Siegrist, M. The importance of food naturalness for consumers: Results of a systematic review. Trends Food Sci. Technol. 2017, 67, 44–57. [Google Scholar] [CrossRef]
- Mende, S.; Rohm, H.; Jaros, D. Influence of exopolysaccharides on the structure, texture, stability and sensory properties of yoghurt and related products. Int. Dairy J. 2016, 52, 57–71. [Google Scholar] [CrossRef]
- Zeidan, A.A.; Poulsen, V.K.; Janzen, T.; Buldo, P.; Derkx, P.M.F.; Øregaard, G.; Neves, A.R. Polysaccharide production by lactic acid bacteria: From genes to industrial applications. FEMS Microbiol. Rev. 2017, 41, S168–S200. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef] [PubMed]
- van de Velde, F.; de Hoog, E.H.A.; Oosterveld, A.; Tromp, R.H. Protein-polysaccharide interactions to alter texture. Annu. Rev. Food Sci. Technol. 2015, 6, 371–388. [Google Scholar] [CrossRef]
- Nguyen, A.T.B.; Nigen, M.; Jimenez, L.; Ait-Abderahim, H.; Cunault, C.; Marchesseau, S.; Picart-Palmade, L. A multi-scale approach to identify the role of heat treatment, milk protein composition and starter culture on the gel formation and the texture defects of acid milk gel. Food Hydrocoll. 2018, 85, 299–310. [Google Scholar] [CrossRef]
- Zhang, L.; Folkenberg, D.M.; Amigo, J.M.; Ipsen, R. Effect of exopolysaccharide-producing starter cultures and post-fermentation mechanical treatment on textural properties and microstructure of low-fat yoghurt. Int. Dairy J. 2016, 53, 10–19. [Google Scholar] [CrossRef]
- Khanal, S.N.; Lucey, J.A. Effect of fermentation temperature on the properties of exopolysaccharides and the acid gelation behaviour for milk fermented by Streptococcus thermophilus strains DGCC7785 and ST-143. J. Dairy Sci. 2018, 101, 3799–3811. [Google Scholar] [CrossRef]
- Amatayakul, T.; Sherkat, F.; Shah, N.P. Physical characteristics of set yoghurt made with altered casein to whey protein ratios and EPS-producing starter cultures at 9 and 14% total solids. Food Hydrocoll. 2006, 20, 314–324. [Google Scholar] [CrossRef]
- Buldo, P.; Benfeldt, C.; Folkenberg, D.M.; Jensen, H.B.; Amigo, J.M.; Sieuwerts, S.; Thygesen, K.; van den Berg, F.; Ipsen, R. The role of exopolysaccharide-producing cultures and whey protein ingredients in yoghurt. LWT Food Sci. Technol. 2016, 72, 189–198. [Google Scholar] [CrossRef]
- Mende, S.; Peter, M.; Bartels, K.; Rohm, H.; Jaros, D. Addition of purified exopolysaccharide isolates from S. thermophilus to milk and their impact on the rheology of acid gels. Food Hydrocoll. 2013, 32, 178–185. [Google Scholar] [CrossRef]
- Folkenberg, D.M.; Dejmek, P.; Skriver, A.; Ipsen, R. Interactions between EPS-producing Streptococcus thermophilus strains in mixed yoghurt cultures. J. Dairy Res. 2006, 73, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Vaningelgem, F.; Zamfir, M.; Mozzi, F.; Adriany, T.; Vancanneyt, M.; Swings, J.; De Vuyst, L. Biodiversity of exopolysaccharides produced by Streptococcus thermophilus strains is reflected in their production and their molecular and functional characteristics. Appl. Environ. Microbiol. 2004, 70, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Hamet, M.F.; Piermaria, J.A.; Abraham, A.G. Selection of EPS-producing Lactobacillus strains isolated from kefir grains and rheological characterization of the fermented milks. LWT Food Sci. Technol. 2015, 63, 129–135. [Google Scholar] [CrossRef]
- Mende, S.; Mentner, C.; Thomas, S.; Rohm, H.; Jaros, D. Exopolysaccharide production by three different strains of Streptococcus thermophilus and its effect on physical properties of acidified milk. Eng. Life Sci. 2012, 12, 466–474. [Google Scholar] [CrossRef]
- Jaros, D.; Haque, A.; Kneifel, W.; Rohm, H. Influence of the starter culture on the relationship between dry matter content and physical properties of stirred yogurt. Milchwiss. Milk Sci. Int. 2002, 57, 447–450. [Google Scholar]
- Skriver, A.; Roemer, H.; Qvist, K.B. Rheological characterization of stirred yoghurt: Viscometry. J. Texture Stud. 1993, 24, 185–198. [Google Scholar] [CrossRef]
- Bullard, J.; St-Gelais, D.; Turgeon, S.L. Production of set yoghurts using thermophilic starters composed of two strains with different growth biocompatibilities and producing different exopolysaccharides. Int. Dairy J. 2018, 79, 33–42. [Google Scholar] [CrossRef]
- Surber, G.; Mende, S.; Jaros, D.; Rohm, H. Clustering of Streptococcus thermophilus strains to establish a relation between exopolysaccharide characteristics and gel properties of acidified milk. Foods 2019, 8, 146. [Google Scholar] [CrossRef]
- EN ISO 8968-1:2014; ISO Milk and Milk Products—Determination of Nitrogen Content—Part 1: Kjeldahl Principle and Crude Protein Calculation. German Version. International Organization for Standardization: Geneva, Switzerland, 2014.
- EN ISO/DIS 8968-4:2013; ISO Milk and Milk Products—Determination of Nitrogen Content—Part 4: Determination of Protein and Non Protein Nitrogen Content and True Protein Content Calculation (Reference Method). German Version. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 17997-1:2004; ISO Milk—Determination of Casein-Nitrogen Content—Part 1: Indirect Method (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2004.
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Jaros, D.; Heidig, C.; Rohm, H. Enzymatic modification through microbial transglutaminase enhances the viscosity of stirred yogurt. J. Texture Stud. 2007, 38, 179–198. [Google Scholar] [CrossRef]
- Thiel, M.; Féraud, B.; Govaerts, B. ASCA+ and APCA+: Extensions of ASCA and APCA in the analysis of unbalanced multifactorial designs. J. Chemom. 2017, 31, 2895. [Google Scholar] [CrossRef]
- Saccenti, E.; Smilde, A.K.; Camacho, J. Group-Wise ANOVA Simultaneous Component analysis for designed omics experiments. Metabolomics 2018, 14, 1–18. [Google Scholar] [CrossRef]
- Oliveira, M.N.; Sodini, I.; Remeuf, F.; Corrieu, G. Effect of milk supplementation and culture composition on acidification, textural properties and microbiological stability of fermented milks containing probiotic bacteria. Int. Dairy J. 2001, 11, 935–942. [Google Scholar] [CrossRef]
- Purwandari, U.; Shah, N.P.; Vasiljevic, T. Effects of exopolysaccharide-producing strains of Streptococcus thermophilus on technological and rheological properties of set-type yoghurt. Int. Dairy J. 2007, 17, 1344–1352. [Google Scholar] [CrossRef]
- Li, D.; Li, J.; Zhao, F.; Wang, G.; Qin, Q.; Hao, Y. The influence of fermentation condition on production and molecular mass of EPS produced by Streptococcus thermophilus 05-34 in milk-based medium. Food Chem. 2016, 197, 367–372. [Google Scholar] [CrossRef]
- Shene, C.; Canquil, N.; Bravo, S.; Rubilar, M. Production of the exopolysaccharides by Streptococcus thermophilus: Effect of growth conditions on fermentation kinetics and intrinsic viscosity. Int. J. Food Microbiol. 2008, 124, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Remeuf, F.; Mohammed, S.; Sodini, I.; Tissier, J.P. Preliminary observations on the effects of milk fortification and heating on microstructure and physical properties of stirred yogurt. Int. Dairy J. 2003, 13, 773–782. [Google Scholar] [CrossRef]
- Hassan, A.N.N.; Frank, J.F.; Schmidt, K.A.A.; Shalabi, S.I.I. Textural properties of yogurt made with encapsulated non-ropy lactic cultures. J. Dairy Sci. 1996, 79, 2098–2103. [Google Scholar] [CrossRef]
- Amatayakul, T.; Halmos, A.L.; Sherkat, F.; Shah, N.P. Physical characteristics of yoghurts made using exopolysaccharide-producing starter cultures and varying casein to whey protein ratios. Int. Dairy J. 2006, 16, 40–51. [Google Scholar] [CrossRef]
- Mende, S.; Dong, T.; Rathemacher, A.; Rohm, H.; Jaros, D. Physicochemical characterisation of the exopolysaccharides of Streptococcus thermophilus ST-143. Int. J. Food Sci. Technol. 2014, 49, 1254–1263. [Google Scholar] [CrossRef]
- Robinson, R.K.; Lucey, J.A.; Tamime, A.Y. Manufacture of Yoghurt. In Fermented Milks; Tamime, A.Y., Ed.; Blackwell Publishing: Oxford, UK, 2006; pp. 53–75. [Google Scholar]
- Knudsen, J.C.; Karlsson, A.O.; Ipsen, R.; Skibsted, L.H. Rheology of stirred acidified skim milk gels with different particle interactions. Colloids Surf. A Physicochem. Eng. Asp. 2006, 274, 56–61. [Google Scholar] [CrossRef]
- Hahn, C.; Müller, E.; Wille, S.; Weiss, J.; Atamer, Z.; Hinrichs, J. Control of microgel particle growth in fresh cheese (concentrated fermented milk) with an exopolysaccharide-producing starter culture. Int. Dairy J. 2014, 36, 46–54. [Google Scholar] [CrossRef]
- Folkenberg, D.M.; Dejmek, P.; Skriver, A.; Skov Guldager, H.; Ipsen, R. Sensory and Rheological screening of exopolysaccharide producing strains of bacterial yoghurt cultures. Int. Dairy J. 2006, 16, 111–118. [Google Scholar] [CrossRef]
- Bertinetto, C.; Engel, J.; Jansen, J. ANOVA Simultaneous component analysis: A tutorial review. Anal. Chim. Acta X 2020, 6, 100061. [Google Scholar] [CrossRef]
- Smilde, A.K.; Jansen, J.J.; Hoefsloot, H.C.J.; Lamers, R.J.A.N.; van der Greef, J.; Timmerman, M.E. ANOVA-simultaneous component analysis (ASCA): A new tool for analysing designed metabolomics data. Bioinformatics 2005, 21, 3043–3048. [Google Scholar] [CrossRef]
- Saccenti, E.; Camacho, J. Multivariate exploratory data analysis using component models. Compr. Foodomics 2021, 241–268. [Google Scholar] [CrossRef]

| Casein: Whey Protein Ratio (Acidification Activity of Direct Starter) | ||||
|---|---|---|---|---|
| Temperature [°C] | Strain | 4.0:1 (Low) | 4.0:1 (High) | 1.5:1 (High) |
| 37 | ST-1G+ | 34 c ± 1.4 | 29 d ± 3.2 | 42 b ± 4.5 |
| ST-2E+ | 23 e ± 1.0 | 24 e ± 1.3 | 22 e ± 1.3 | |
| ST-2F | 33 c ± 3.6 | 24 e ± 0.6 | 23 e ± 1.1 | |
| 42 | ST-1G+ | 50 a ± 4.4 | ||
| ST-2E+ | 33 c,d ± 0.8 | |||
| ST-2F | 37 c ± 2.2 | |||
| Casein: Whey Protein Ratio (Acidification Activity of Direct Starter) | ||||
|---|---|---|---|---|
| Temperature [°C] | Strain | 4.0:1 (Low) | 4.0:1 (High) | 1.5:1 (High) |
| 37 | ST-1G+ | 126 g ± 11.7 | 164 e,f,g ± 23.9 | 199 d,e,f ± 11.2 |
| ST-2E+ | 204 d,e,f ± 20.1 | 206 d,e ± 15.4 | 389 a ± 51.7 | |
| ST-2F | 137 f,g ± 9.3 | 237 c,d ± 11.8 | 303 b,c ± 47.7 | |
| 42 | ST-1G+ | 206 d,e ± 18.7 | ||
| ST-2E+ | 217 d,e ± 17.6 | |||
| ST-2F | 344 a,b ± 43.3 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surber, G.; Rohm, H.; Jaros, D. The Role of Exopolysaccharide-Producing Streptococcus thermophilus on Physical Properties of Stirred Skim Milk Gel. Dairy 2022, 3, 761-775. https://doi.org/10.3390/dairy3040052
Surber G, Rohm H, Jaros D. The Role of Exopolysaccharide-Producing Streptococcus thermophilus on Physical Properties of Stirred Skim Milk Gel. Dairy. 2022; 3(4):761-775. https://doi.org/10.3390/dairy3040052
Chicago/Turabian StyleSurber, Georg, Harald Rohm, and Doris Jaros. 2022. "The Role of Exopolysaccharide-Producing Streptococcus thermophilus on Physical Properties of Stirred Skim Milk Gel" Dairy 3, no. 4: 761-775. https://doi.org/10.3390/dairy3040052
APA StyleSurber, G., Rohm, H., & Jaros, D. (2022). The Role of Exopolysaccharide-Producing Streptococcus thermophilus on Physical Properties of Stirred Skim Milk Gel. Dairy, 3(4), 761-775. https://doi.org/10.3390/dairy3040052







