Abstract
Periparturient diseases continue to be the greatest challenge to both farmers and dairy cows. They are associated with a decrease in productivity, lower profitability, and a negative impact on cows’ health as well as public health. This review article discusses the pathophysiology and diagnostic opportunities of mastitis, the most common disease of dairy cows. To better understand the disease, we dive deep into the causative agents, traditional paradigms, and the use of new technologies for diagnosis, treatment, and prevention of mastitis. This paper takes a systems biology approach by highlighting the relationship of mastitis with other diseases and introduces the use of omics sciences, specifically metabolomics and its analytical techniques. Concluding, this review is backed up by multiple studies that show how earlier identification of mastitis through predictive biomarkers can benefit the dairy industry and improve the overall animal health.
1. Introduction
Mastitis is a multifactorial inflammatory disease of the mammary gland. This intramammary infections (IMI) is most commonly caused by bacteria such as Escherichia coli, Staphylococcus aureus, and Streptococcus spp. and often times by fungi too. Mastitis is seen in dairy herds between the dry-off and early lactation period [1]. Other rare causative agents of mastitis can be trauma or toxic chemicals [2]. Mastitis presents chemical and physical alterations to the milk and abnormal mammary gland appearance. Depending on the signs and severity, mastitis can be classified as clinical mastitis (CM) or subclinical mastitis (SCM). Mastitis is commonly monitored by measuring the somatic cell count (SCC) in milk, which if exceed 200,000 cells/mL, the mammary gland is considered inflamed and this highly suggests infection [3], as presented in Figure 1.
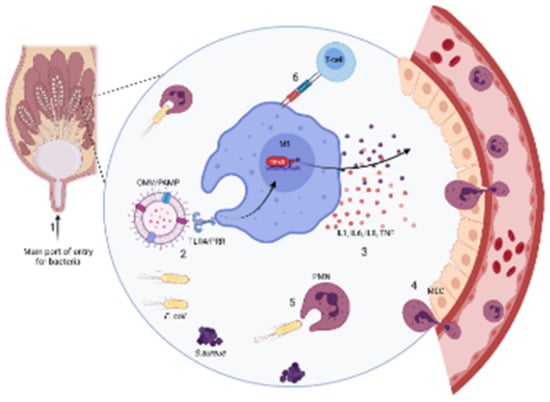
Figure 1.
Schematic presentation of mastitis pathogenesis. Typically, (1) once bacteria invade the teat end and ascend into the teat canal and alveoli, a local immune reaction starts; (2) bacterial by products, such as lipopolysaccharide (LPS) or outer membrane vesicle (OMV) of Gram-negative pathogens act as pathogen-associated molecular pattern (PAMP), which is recognized by pathogen recognition receptors (PRR), specifically Toll-like receptor 4 (TLR4), on macrophage type 1 (M1). After this contact, (3) humoral elements, such as cytokines (IL1, IL6, and TNF) and chemokines [interleukin(IL)-8] are released that alert other white blood cells, mainly polymorphonuclear (PMN) leuckocytes in the systemic circulation and trigger the release of acute phase proteins; (4) Once PMN have entered in the infected area, through mammary epithelial cells (MEC), (5) they engulf and kill bacteria through phagoctysis. If this inflammation is persistent, (6) then adaptive immunity is activated via the interaction of macrophages with lymphocytes, like T-regulatory cells.
This disease can significantly impact the health of dairy cattle and dairy farm profitability [4]. Indeed, the financial impact of mastitis is considerable, as it is estimated to cost a Canadian dairy farmer USD 662/year per cow [5]. In fact, reproductive disorders and mastitis are the most common reasons why dairy cows are prematurely culled from the herd [6]. The median incidence of CM has been estimated to be 19 cases per 100 cows/year [5], whereas the incidence of SCM is estimated to be up to 50 cases per 100 cows/year [7]. Farm management is a significant factor in the occurrence of IMI; thus, different farms will have different levels of CM or SCM [8]. Given the excessive costs and impact on dairy production, profitability and herd health, there is a strong global effort to lower mastitis incidence, reduce the negative impact and improve milk quality, production, and farmer finances.
The goals of this review are to present and discuss current and recent breakthroughs in mastitis diagnostic methods, as well as to introduce research on biomarker discovery and its possible application in dairy farms.
2. Disease Etiology and Pathogenesis
2.1. Disease Etiology and Traditional Bacterial Infections
In 1988, Watts listed 137 microorganisms as being causative for mastitis [9]. Later Zadoks and colleagues reported more than 200 pathogens are associated with this pathology [10]. Most mastitis cases in dairy cattle are thought to derive from contagious, environmental pathogens, or opportunistic microbes such as non-aureus Staphylococci (NAS) [11]. Following is a more detailed list of some of the most important agents causing IMI, presented in Table 1.

Table 1.
Classification of mastitis pathogens Adapted from Constable and colleagues (2017) [12].
2.1.1. Staphylococcus aureus
Staphylococcus aureus is currently one of the most problematic mastitis pathogens. It is more predominant in chronic or subclinical forms of the disease, causing mild, moderate, and severe infections that can cause sudden death [12]. It invades the mammary tissues and causes necrosis through the release of lipoteichoic acid (LTA) into the interstitial tissue of the mammary gland (MG) [13]. Staphylococcus aureus can also produce biofilms as a barrier against the host immune response. These infections are more common during early lactation and are associated with continuous losses in milk production [14,15]. LTA of Gram-positive bacteria, such as S. aureus, tend to elicit a weaker immune response. This is because S. aureus can dampen the activation of the NF-kB signaling pathway, not eliciting a strong proinflammatory cytokine expression [16,17]. Because of other variables such as cow age, infection spread, and treatment length, these infections cannot be fully controlled with antimicrobial treatments alone [18].
2.1.2. Escherichia coli
Escherichia coli is the primary pathogen that causes clinical signs of mastitis. This Gram-negative bacterium is found in the environment and causes acute to peracute infections resulting in a rapid, sometimes fatal, immune response [19]. Due to the systemic inflammation that follows E. coli infection, much more damage to the mammary gland and a substantial reduction in milk yield can occur, compared to infections by other pathogens [15]. The mammary gland operates under low oxygen pressures, making it a suitable environment for this coliform bacterium to thrive and flourish [20]. E. coli can be found in a non-pathogenic form in the udder, as well as gastrointestinal and reproductive tracts. Under non-favorable conditions, LPS released by Gram-negative bacteria such as E. coli can translocate into the systemic circulation and cause endotoxemia [21].
2.1.3. Streptococcus uberis
Streptococcus uberis, a common environmental pathogen that causes moderate clinical signs, which manifests as abnormal and visible changes in the mammary glands affected by CM [22]. It is a Gram-positive microorganism, present in pasture and free-stall systems, and because of its ubiquity in the environment, it can become a persistent causative agent of mastitis [22,23,24].
2.1.4. Klebsiella spp.
Klebsiella spp. are Gram-negative, environmental pathogens that cause 2–9% of clinical mastitis cases [25,26]. The importance of Klebsiella spp. is related to economic losses as these infections lower milk yield and increase veterinary bills. Furthermore, most cows that are positive for Klebsiella spp. are predisposed to life-threating mastitis and not a positive prognosis [27]. The pathogenicity of Klebsiella spp. infection is proposed to be mediated by many virulence genes and biofilm formation. Furthermore, 42% of all mastitis samples with Klebsiella spp. demonstrate antimicrobial resistance (AMR) [28,29].
2.1.5. Opportunistic Pathogens
Most opportunistic pathogens causing mastitis fall into the category of non-aureus staphylococci (NAS). These microbes are common residents of the teat skin. There are 48 known NAS, but few of them cause IMI. The best known of the causative NAS is Staphylococci chromogens. This microbe causes SCM but with only slight change to milk quality. An increase in NAS can occur because of poor hygiene, teat skin injuries, and inadequate milking procedures. A NAS infection does not often demonstrate any clinical signs. As a result, it can only be discovered from a positive bacteriological culture. Measures such as post-milking teat dipping with iodine along with improved milking hygiene, minimizing teat damages, and dry cow therapy can keep NAS infections under control [11]. NAS pathogens are becoming the predominant mastitis bacteria in many countries, and these microbes are often associated with recurrent infections due to their ability to form biofilms [30].
Mastitis-causing pathogens can be categorized into two groups: major or minor, based on their pathogenicity. The major pathogens comprise S. aureus, S. dysgalactiae, S. agalactiae, S. uberis and the Enterobacteriaceae. Cows infected with these pathogens require intensive care, and these bacteria are not easily eliminated. The minor pathogens include Corynebacterium spp. and the NAS spp. Minor pathogens do not cause visible changes to the udder and can be kept under control; however, they trigger an elevated SCC in the milk, leading to IMI and sudden death [31].
In Canada, most CM cases are caused by Escherichia coli and Staphylococcus aureus [1,32]. In the Netherlands and the US, E. coli was found to be predominant over all other microbes [33,34]. S. aureus, S. dysgalactiae, and E. coli are most frequently isolated in Norway and Sweden [35]. In New Zealand, S. uberis is the leading cause of CM and SCM [36], whereas, in Albania, S. aureus and S. agalactiae were found to be the main cause of SCM [37]. In China, Enterobacteriaceae were the most common pathogens leading to CM and SCM [38].
2.2. Pathogenesis
No matter the pathogen, whether it is environmental or contagious, the main route of mammary gland infection in mastitis is through the teat canal. The first anatomical structure that bacteria deal with is the teat end and teat canal. A sphincter muscle surrounds it and helps to maintain a tight closure, not to let milk escape. Keratin, a waxy material derived from the epithelial lining, also lines the teat canal. This serves as a physical obstruction usually present during the non-lactating period when the teat end is completely closed with this substance. Keratin contains antibacterial fatty acids with bacteriostatic and bactericidal activities. Their activity is more intense towards some bacteria than others [39,40]. When mastitis-causing pathogens pass this barrier, an innate immune response (IIR) is initiated.
This immune response is mediated at the beginning by innate immunity. It includes both cellular (e.g., polymorphonuclear neutrophils (PMN), macrophages, natural killer (NK) cells, dendritic cells, mammary epithelial cells (MEC), and humoral defenses (complement system, cytokines, lactoferrin, transferrin, lysozyme, acute phase proteins (APPs) as well as reactive oxygen species (ROS) and antimicrobial peptides [41]. Local mammary cell populations such as macrophages, dendritic cells, and epithelial cells have pathogen recognition receptors (PRRs) that interact with pathogen-associated molecular patterns (PAMPs). The latter are motifs or distinctive protein sequences on the surface of microbes (e.g., LPS or LTA) released by microorganisms when they replicate or degrade. Toll-like receptors (TLR) and nucleotide-binding and oligomerization domain-like receptors (NLR) are the two prominent families of PRRs [42]. LPS is recognized by TLR-4, whereas TLR-2 recognizes LTA. In case of a Gram-negative infection, LPS will stimulate innate immunity, being recognized by TLR-4. Other proteins, including cluster of differentiation 14 (CD14), myeloid differential protein 2 (MD2), and lipopolysaccharide-binding protein (LBP), help activate the nuclear factor kappa light chain enhancer of activated B cells (NF-κB) signaling pathway [43,44]. This activation triggers the production of pro-inflammatory cytokines and the acute phase response (APR), which initiates a ubiquitous and rapid innate immune response [45].
Peripheral neutrophils contacted by IL-8 rapidly migrate to the infected area to phagocytose and destroy the intruding bacteria. The high concentration of SCCs found in cows’ milk with CM or SCM is mostly made of neutrophils, indicating “a cellular battle” inside the mammary gland [46]. If microbes are eliminated, the host returns to a healthy, homeostatic state. If not, the adaptive immune system is activated. This response is mediated by T and B lymphocytes. T-cells are activated by encountering an antigen-presenting cell (APC), MHC II, plus various cytokines. Once activated, T-cells can exert their cytotoxic activity or activate B-cells, which produce antibodies to eliminate the bacterial intruder and create a memory of its specific antigen [47,48]. Depending on the type and scale of pathogen invasion, this infection is presented either in a clinical or subclinical form. This process is presented schematically in Figure 2.
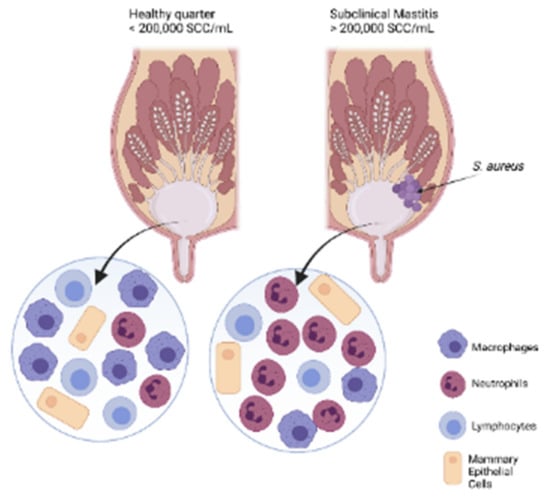
Figure 2.
Presence of somatic cells in healthy and infected quarters of a cow. In the milk of a healthy quarter there are present more macrophages, followed by a small percentage of lymphocytes, neutrophils, and epithelial cells, whereas an infected quarter, with clinical mastitis or subclinical mastitis, is overpopulated with neutrophils and few macrophages, lymphocytes, and epithilail cells.
2.2.1. Clinical Mastitis
Clinical mastitis is one of the most important diseases affecting dairy cows. Clinical mastitis manifests with clear visible external signs such as swelling, redness and/or pain in the udder, and systemic fever. There are also detectable changes to the milk and its components [49]. Variations in color, consistency, as well as presence of clots, blood, and chemical changes can occur in milk [46,50]. Other milk modifications may include increased conductivity, increased pH, and changes in water content.
Clinical mastitis is categorized based on its duration (subacute, acute, or peracute) and severity (mild, moderate, or severe) [51]. With mild/subacute CM, most changes are found in the milk’s color and consistency. Moderate/acute CM is characterized by changes in the milk and mammary gland, including redness, swelling, heat, and pain. Severe/peracute CM is characterized by abnormal milk and mammary gland along with systemic signs such as fever, loss of appetite, and inability or unwillingness to move [52,53].
2.2.2. Subclinical Mastitis
Subclinical mastitis is another form of mastitis but does not present with any noticeable symptoms or signs such as external changes in the udder or milk [54]. As the milk does not show any SCM changes, farmers unintentionally decrease the bulk-tank milk quality while mixing this milk with milk from healthy cows [55]. Adding SCM milk contributes to an increase in somatic cells, decreases milk quality, and introduces pathogens into the normal milk. Since there are no visible signs of the disease with SCM, it can continue to persist. The only way to discover it is by doing bacterial cultures and weekly tests to measure the presence of SCC in the milk throughout the lactation period [12].
Subclinical mastitis can be highly significant to a dairy farm as it can affect up to 50% of the herd [7,56]. This has made SCM detection and management an enormous challenge for the past, present, and future dairy industry. Currently, SCM and IMI are monitored through continuous measurements of SCC in the milk. If the SCC is lower than the threshold of 200,000 cells/mL, a quarter of the udder can be considered non-infected. Therefore, the optimal value of SCC for a herd should be no more than 100,000 SCC/mL of milk as this would guarantee that most of the herd is not infected [57,58].
3. Role of the Dry Period in the Life of Cows
3.1. Anatomical and Physiological Changes of the Mammary Gland
The dry or the nonlactating period is when metabolic and nutritional changes happen to the cow and the mammary gland [59]. This period prepares cows for the next calving and lactation cycle [60]. The dry period helps the udder tissue involute and regenerate to provide better milk yields after calving [61,62]. This period should be at least 40–60 days long; most farmers practice a 6- to 8-week (42–56 days) dry period. It has been shown that cows dried less than 40 days typically produce less milk than the previous lactation [60].
Involution, steady-state, and colostrogenesis are the three phases that the mammary gland goes through during the dry-off period. Involution that starts with milk cessation is proceeded by dry cow therapy (DCT), where the farmers routinely administer antibiotics, teat sealants, and vaccines as measures to eliminate current infections and prevent new ones from developing during late gestation. However, we must note that the use of antibiotics for prophylactic and metaphylactic purposes is prohibited in the EU as of 20 January 2022; however, certain conditions may apply [63]. After active and steady-state involution of the mammary gland during the dry off period, redevelopment and colostrogenesis or the production of colostrum begins.
Infection of the mammary gland during dry off period can be related to damage of the keratin barrier. Keratin is formed when the cells surrounding the teat canal lumen continue to desquamate (or shed). It obstructs the canal lumen between milkings, preventing bacterial penetration. Keratin loss or removal weakens the protective barrier. Without keratin, the teat canal cannot resist bacterial invasion. The method of infusing antibiotics into a mammary quarter to prevent mastitis at the initiation of dry off may damage the keratin barrier. The full insertion of the antibiotic treatment syringe cannula into the teat cistern may push portions of keratin colonized by bacteria. This may result in an intramammary infection with consequences on health of the cow and production on the next lactation cycle [63].
The critical period for a cow’s health is three weeks before and three weeks after calving. This is called the transition period. During this period, cows go through significant nutritional, metabolic, immune, and hormonal changes, making them more susceptible to periparturient diseases [63]. The periparturient period is when the fetus grows the most [64]. It requires several major metabolic adaptions (e.g., mobilization of energy and proteins from maternal body reserves) for the cow to support this high growth and milk production level. The transition period from gestation to lactation is linked with increased nutritional demands but at the same time with appetite depression, which puts the cow in a negative energy balance (NEB) and under metabolic stress [65,66]. To support their postpartum physiological requirements, cows typically experience lipolysis and ketogenesis, making them more prone to other metabolic diseases [67,68].
During the periparturient period, alterations in hormone levels can cause changes in energy balance metabolites, specifically with non-esterified fatty acids (NEFAs). Hormonal profiles related to parturition and lactation are also altered. For instance, estrogen begins to increase while progesterone declines just 1–2 days before calving. Furthermore, as lactation is initiated, serum levels of growth hormone (GH) and glucocorticoids increase while at the same time, they inhibit insulin and IGF-1 production, triggering mobilization of NEFAs [69]. Overproduction of the NEFAs leads to incomplete oxidation and accumulation in the liver due to the lack of re-esterification of the NEFAs back to triacylglycerides (TAGs). This predisposes cows to ketosis or fatty liver disease [70].
On the other hand, a high concentration of NEFAs initiates a pro-inflammatory response, which can benefit placental detachment during calving. The NEFAs can potentially support the host to fight infections or it can lead to self-harming if inflammation persists for a long time. Negative energy balance has been widely accepted as a failure to supply the energy requirements and sufficient dry matter intake (DMI) [70]. Furthermore, an increasing body of evidence, based on research done on dairy cows during the periparturient period, confirms that the energy shortfall is caused by the host’s reaction to systemic inflammation. It’s still a work in progress to pinpoint the source of this systemic inflammation throughout the transition period.
3.2. Susceptability to Intramammary Infections during the Dry Period
Many factors participate in the etiology of mastitis. Disease severity and extent depend on the balance between the host, the farm, and the pathogens [71]. A high incidence of IMI is strongly correlated with the immunosuppression that cows go through around parturition [72]. Pathogens then use this opportunity to attack and establish an infection [73]. The origin of these pathogens can be from the environment, the udder, or an existing infection [39]. Cows having dirty udders and farms with improper hygiene are more likely to develop clinical mastitis [74]. Sand bedding is preferred over organic material as sand does not predispose cattle to environmental mastitis [75]. It was estimated that the incidence of CM in conventional dairy farms in 2013 was 26.3 cases per 100 cows [1].
In contrast, organic dairy farms tend to have fewer cases of mastitis [26]. It is interesting to note that different studies report different SCC values between these two systems, with some stating that organic farms have higher SCC values [26]. This may be due to the fact that organic farms have different standards and feeding rations than conventional farms [76]. Additionally, SCC levels are affected by breed, stage of lactation, parity, and season [8]. Holstein cows are more likely to be culled when they present with high SCCs than Jersey cows [77]. Additionally, pure Holstein cows are more predisposed to become infected and to have a shorter lifespan than crossbreeds [78]. A dairy farmer’s financial interest is to make a profit by selecting cows that produce considerable amounts of milk. For many years, the dairy industry has focused on selecting cows for their production traits, but unintentionally, this has led to a negative impact on cow health and welfare. In particular, this has increased the average SCC in the milk and the number of mastitis cases while generating less milk yield and increased culling rates [79,80].
As far back as in the 1950s, it was first noticed that cows could be genetically selected for mastitis resistance and general health optimization [81,82]. Several studies have shown that susceptibility to IMI may be inherited. Subclinical mastitis has a low heritability from 0.03 to 0.17 [83,84,85], as does CM, with a heritability of 0.07 to 0.1 [79,86]. On the other hand, Svendsen and Heringstad reported that SCM cows with an SCC threshold between 50,000–200,000 SCC/mL shared a high genetic correlation (range = 0.89–0.92) [87].
Cows are susceptible to mastitis throughout lactation. However, they are most at risk two weeks postpartum, and as they grow older [33,88]. There is supporting evidence that many metabolic and immune alterations occur long before diagnosis of CM or SCM after parturition. Indeed, many researchers have reported that the infection is typically acquired during the dry-off period. It has been demonstrated that mastitis-positive cows identified within 30-100 days in lactation likely had the infection since the dry period [89,90]. This was later endorsed by Dervishi and colleagues in 2015 who found activation of innate immunity and other metabolic changes in cows that developed SCM after calving [91]. Furthermore, these cases increase persistence of IMI and recurrence in the herd [92].
4. Impact of Mammary Gland Infections on Dairy Herds
4.1. Mastitis—A Threat to the Dairy Industry Profitability
Mastitis costs the dairy industry dearly. In 1972, Foley and colleagues reported that the average cost to treat a mastitic cow was USD 30–50 per year [93]. They estimated that the total cost to the US economy was a loss of between USD 300 and 600 million per year. Since then, inflation has added to the estimated cost and the economic impact of mastitis. In 2018, it was reported that a Canadian dairy farmer would spend CAD 662 per cow in a year to treat or prevent mastitis [5]. These costs included the losses due to reduced milk production from SCM, treatment of CM, and prevention programs.
It is well known that one of the first consequences of an IMI infection is milk reduction [94]. On the other hand, even SCM cases are accompanied by yield losses. While not as high as CM, SCM is longer in duration [95]. The reduction in yield comes from mammary tissue destruction, so the MEC can no longer synthesize or secrete milk. Most SCM cases caused by S. aureus turn into a significant concern as the secretory tissue transforms into useless fibrotic tissue [96]. Knowing that almost 50% of a dairy herd can be affected by SCM and that 20–50% of the cows will experience more than one disorder around parturition, the scale of these losses is considerable [97]. Older cows produce less milk than those in the first lactation. At the limit of 200,000 SCC/mL, primiparous cows can lose 0.31 kg/d, while multiparous cows lose 0.58 kg/d [95]. Due to yield reductions, milk composition changes, especially the loss of nutrient value, the milk from SCM or CM cows become unconsumable, so it must be discarded. Another reason milk (quarter level or bulk tank) from SCM or CM-affected herds is often discarded is due to the presence of high levels of antibiotics, making it a concern for public safety [98]. A better solution for farmers is to remove or cull the infected cows and replace them with new ones. This further adds to the farm’s profitability [99]. Other costs due to CM or SCM are related to veterinary assistance, extra labor, and preventive measurements [5,100].
A growing concern for the dairy industry, veterinary medicine, and the public is the antibiotic presence, usage of antimicrobial growth promoters (AGP), and antibiotic resistance in livestock [101,102]. Antibiotic resistance and antibiotic presence are a burden not only for the farmer’s pocket and for the cow’s welfare but also for global health and economic issues. Most farmers use systemic or intramammary antibiotics after confirming the presence of elevated SCC. Non-specific and extensive antibiotic use has led to the global problem of antimicrobial resistance (AMR) [103]. Treatment of mastitis accounts for almost 80% of antibiotics used in dairy cows [104]. Antimicrobial resistance presents a significant concern because some bacterial populations develop resistance genes and can pass those to other bacterial communities.
Furthermore, the presence of antibiotic residues in the milk means that this milk cannot enter the human food chain or be fed to calves [105]. Resistant bacteria can pass onto humans. For example, through the consumption of unpasteurized milk, there have been a number of disease outbreaks [106,107]. The concept of One Health is becoming a central part of the agenda of health and veterinary discussions in the US, the European Union, and Canada [108]. For example, the establishment of Animal Antimicrobial Stewardship (AMS) Canada [109] and other national or international cohorts will help develop policies to prevent AMR while at the same time finding more effective treatments for farm animals and the environment.
4.2. Mastitis in Relation to Other Diseases
Cows are at a higher risk of developing one or more periparturient diseases after calving. Dry period is the most susceptible time to develop those diseases. Many of the periparturient diseases are then presented or manifested after calving. It has been suggested that bacterial endotoxins (LPS or LTA) might be implicated in the periparturient disease pathogenesis, given that the endotoxin produced by bacteria can translocate from the rumen, uterus, or mammary gland [21]. Indeed, several papers have shown a good correlation between increased SCC and other metabolic diseases diagnosed simultaneously [110,111,112,113]. Transition diseases are interlinked between one another [114]. For example, hypocalcemia makes a cow more predisposed to most periparturient diseases, while acidotic cows are more susceptible to mastitis, laminitis, milk fever, and left displaced abomasum (LDA). Retained placenta (RP) leads to metritis, milk fever, and LDA [27]. Ketotic cows have an increased concentration of NEFA. Beta-hydroxy butyrate (BHBA), in addition to being a diagnostic tool for ketosis, helps identify uterine infections and LDA.
Elevated levels of NEFA before parturition depresses feed intake, which impacts immune function and can lead to subclinical ketosis and metritis development. Metabolic changes and adaptations occurring during the transition period can be metaphorically compared to the domino effect—one falls over the other. Thus, incomplete oxidation of NEFA results in fatty liver and ketosis, which in ketotic cows with a high body condition score (BCS) increases the incidence of displaced abomasum (DA) [70]. The increased lipolysis and BHBA impair immune functions, consequently making cows prone to infectious diseases like mastitis and metritis. This immunosuppression, combined with hypocalcemia, leads to retention of fetal membranes or retained placenta [114].
Another initiator of this cascade can be the ruminal environment. As the diet changes, so do the bacterial population in the gut, favoring the release and translocation of the Gram-negative bacterial endotoxin, LPS, to enter the bloodstream, initiating an immune response [115,116,117]. To combat this, the host requires more energy, leading to a postpartum NEB. Recent studies have established that a decrease in the feed intake in SCM cows begins to appear at least four weeks before parturition and decreases after calving. This corresponds to a drop in milk production. These fluctuations are linked to the increased presence of TNF, which as a pro-inflammatory mediator, decreases appetite and inhibits prolactin, therefore, reducing milk yield [91]. Ketosis and fatty liver disease are the two most frequent disorders found simultaneously in dairy cows [27].
A study reported that SCM was preceded by the systemic presence of an inflammatory insult during the dry-off period, which may make cows more susceptible to other diseases [91]. The origin of the inflammation may be attributed to the translocation of LPS or pro-inflammatory cytokines into the systemic circulation [21,118]. In general, any metabolic disease, once it has occurred, presents a higher likelihood to develop again [119]. Moreover, cows that suffered once from CM are more predisposed to present it again throughout lactation [120,121]. These health disorders, including mastitis, poor reproductive performance, and metabolic perturbations, increase dairy farmers’ culling decisions [122].
Mastitis also impacts the reproductive health of dairy cows [123]. No matter when it happens during lactation, mastitis lowers a cow’s future reproductive performance and conception rates. In particular, cows experience more days open and more days to first service, more abortions, and higher culling rates [124,125]. Several meta-analyses have studied the relation between udder and uterus health. Some authors, initially, concluded that reproductive performance was not affected by IMI [126]. However, the latest study, which used far more data, proved that mastitis incidence was related to the incidence of uterine tract disorders [127].
Prepartum augmentation of NEFA increases the odds that a cow can develop mastitis, RP, ketosis, and DA after calving [128]. Clinically mastitic cows continue to have high levels of NEFA and BHBA and lowered glucose at the beginning of lactation [129]. These authors connected the susceptibility of mastitis to immune suppression through hyperketonemia on neutrophil recruitment [130]. Administration of LPS to the udder or naturally occurring IMI causes local and generalized immune alterations [131]. Most artificial-mastitis cases are induced at the beginning of lactation, while not many are assessed during the dry-off period. When a group of researchers applied LPS intravenously several times during the transition period in cows, they noticed increased levels of β-hydroxybutyric acid almost two weeks before parturition [116]. Later, the elevation of BHBA was found together with higher numbers of SCC at dry-off. This implies that endotoxin could initiate the elevation of BHBA [111]. After an LPS challenge post-calving, a few authors noted plasma changes characterized by increased insulin levels [132,133]. Another consistent finding is elevated plasma cortisol. This impacts insulin resistance, induced by pro-inflammatory cytokines such as IL-1, IL-6, and TNF [134,135]. These immune mediators are released upon exposure of host cells (leukocytes; MEC) to the pathogen and alert peripheral WBCs and the liver to initiate an APR [48]. The acute phase proteins released from the liver include serum amyloid A (SAA), haptoglobin (Hp), calcitonin gene-related peptide (CGRP), serum albumin, and lipopolysaccharide binding protein (LBP). These proteins were found to be elevated in cows two months before being diagnosed as mastitis positive [91]. High-density lipoprotein–SAA complexes make endotoxin neutralization possible and safe removal of endotoxin from the circulation. This rapid removal of endotoxin shifts out from the liver making it possible for a higher-than-average amount of lipids to pass through the liver, contributing to the deposition of fat to the liver or fatty liver disease [21,67,136].
5. Current Diagnostic Approaches
The inflammatory response seen in a cow’s udder can indicate the presence of mastitis, whereas the identification of the bacterial pathogen causing the disease confirms the intramammary infection [51]. Usually, CM can be diagnosed via an abnormal appearance of the udder. Redness, swelling, and warm when touched due to inflammation are examples of common symptoms. Additionally, the milk changes its appearance from white to yellow, as well as its consistency.
Subclinical diseases present a significant problem for monitoring the health of dairy herds. SCM cases are quite common in many conventional dairy farms. Two of the most routinely used methods to identify SCM cows are the California Mastitis Test (CMT) and Somatic Cell Count (SCC) test [137]. Estimation of SCCs is a traditional approach to diagnose mastitis. In addition to SCC measurements, several other methods are being used. The count of somatic cells or CM biomarkers, such as N-acetyl-beta-D glycosaminidase and lactate dehydrogenase enzymes have shown positive outcomes. However, scientists are always working to find better, faster, and cheaper methods. Immunoassays, hand-held biosensors, nucleic acid tests, and enzymatic assays as well as advances in genomics, proteomics, and metabolomics sciences has made it easier to detect mastitis at a much earlier time [137].
5.1. Laboratory Techniques
5.1.1. Somatic Cell Count
The somatic cell count or SCC indicates an IMI and gives an overview of a cow’s udder health [3]. Data suggests that clean udders or quarters have approximately 70,000 cells/mL, and SCC measurements equal to or greater than 200,000 cells/mL indicate a SCM cow [138]. According to the Dairy Farmers of Canada website, last updated August 2017, it is required that a sample of raw milk must contain less than 400,000 cells/mL. In the United States, as of August 2018, the allowed amount of SCC is 750,000 cells/mL. In the European Union, the limit is 400,000 cells/mL [139,140]. Normally, milk should not have more than 150,000 cells/mL, and if the SCC is greater than 200,000 cells/mL, it shows some level of abnormality, and that the immune system is activated [141]. There is a chance that high SCCs sometimes can be caused by factors other than an infection. High levels of animal stress, for example, can produce high SCCs, thus giving false-positive results [57,142].
Under normal health conditions, the somatic cells found in the mammary gland are the macrophages, which make up 66–68% of detected cells. Other somatic cells may include neutrophils, mononuclear, and epithelial cells. The local concentration of neutrophils increases as the IMI progresses. The only cells that demonstrate the presence of high SCCs are leukocytes, specifically high numbers of neutrophils, seen in almost 90% of SCM cases [143].
A random sampling procedure is typically followed by farmers when collecting milk samples for laboratory SCC evaluation. Somatic cells in milk can be analyzed in several ways, but most labs use flow cytometry or combine flow cytometry and fluorescence [144]. In Alberta, milk samples are processed by CanWest DHI, which has a standardized protocol. Samples can be taken from herd average of individual cows (HSCC) or bulk tank (BTSCC) [145]. Analyzing bulk tank milk is a convenient and inexpensive method to control milk quality and test for pathogen presence [146]. Measurement of BTSCC can help the farm evaluate its management policies, but the most accurate SCM detection in a cow is through quarter samples [147].
5.1.2. Bacterial Culturing
After confirmation of contamination, the causative agent should be identified for treatment purposes and good management practices [148]. The National Mastitis Council (2017) has described various methods for working with bacterial cultures. The most common method is still the standard plate count (SPC) with a healthy target of less than 5000 colony-forming units (CFUs)/mL. If the number of CFUs is over 20,000 per mL, then financial fees are levied on the farms [149].
Pathogen identification by plate culture focuses on discovering Staphylococcus aureus, Streptococcus agalactia, Escherichia coli, Mycoplasma spp., Corynebacterium spp. and NAS [150]. When taking milk samples for culture, attention must be paid to avoiding contamination. Factors such as a dirty stall or a contaminated environment, poor udder preparation, or incorrectly performing the procedure may lead to milk contamination and the presence of a remarkably high number of bacteria on the plate, leading to false-positive results [151,152].
5.1.3. Polymerase Chain Reaction (PCR)—Based Methods
Although culture plate identification of bacteria is considered the gold standard for CM and SCM, it is not necessarily the best method. Compared to the polymerase chain reaction (PCR), culture plate methods were able to identify mastitis pathogens on only 47% of the no-growth milk samples [153]. Nucleic acid-based detection or PCR has facilitated the detection of those pathogens that cannot be identified using standard bacterial culture plates. The superior sensitivity of PCR enables better farm management, too [154].
Electrospray ionization mass spectrometry ESI-MS is another common diagnostic method that can detect other microorganisms such as parasites, yeasts, and viruses, but PCR is still the preferred sequencing technique for bacterial identification [155,156]. Polymerase chain reaction methods are more rapid, but several times more expensive than conventional bacterial plate culture methods. However, it should be kept in mind that PCR methods can detect only the species included in the PCR kit [157].
Other molecular techniques include real-time quantitative PCR (qPCR), loop-mediated isothermal amplification (LAMP), and next-generation sequencing (NGS) methods [158,159,160]. As a secondary confirmatory test, matrix-assisted laser desorption/ionization time-of-flight (MALDI–TOF) mass spectrometry can be applied as a diagnostic technique for bacterial species identification in mastitis studies [161,162]. However, the cost of analysis, the required sample pre-treatment and the frequency of false-negative results mean that this approach is not readily adaptable for commercial use [163].
5.2. Cow-Side Tests
5.2.1. California Mastitis Test
The California Mastitis Test (CMT) is one of the oldest and easiest tests to use as a cow-side test. It evaluates the milk’s alkalinity using a detergent (alkyl aryl sulfonate) combined with a pH indicator, bromocresol purple [164]. If mastitis is present, this results in the formation of a purple viscous mass due to the nucleic acids and other constituents released from the lysis of somatic cells. The interpretation of the CMT might be subjective as it gives variable results to identify IMI. The CMT kit is composed of a plastic paddle and four cups to put the milk from each quarter of the udder. The reagent is added, then the milk in the cup is stirred until a mixture is formed. The test is most useful when there is a very high number of SCCs, with an average of 500,000 cells/mL [165]. The result of the CMT is read as negative (N) or trace (T) and then depending on the viscosity of the gel, with a grade from 1 to 3 to indicate the number of somatic cells, with three being the highest (>5 million SCC/mL) [166]. The CMT cannot identify whether the infection is from major or minor pathogens [137].
5.2.2. Electrical Conductivity Test
During inflammation, concentrations of many ions change. Increases in the concentration of ions such as potassium, magnesium, sodium, and calcium can increase the electrical conductivity (EC) in milk [137]. A high EC is based on high sodium and chloride concentrations in the milk and a loss of lactose and potassium. However, the concentration of ions in milk can be influenced by factors other than mastitis. Conductivity is also affected by the cow’s age and lactation stage [167].
Many dairy farmers use automatic milking systems (AMS), which helps them increase milk production and minimize labor costs [168,169]. Automatic milking systems are equipped with sensors that measure EC to detect mastitis [170]. One drawback of this process is that just a part of the milk is measured. Sensors that identify mastitis in AMS farms do not consider the measurement of foremilk [171,172]. Foremilk is the milk obtained in the first part of milking. This initial milk is discarded, whereas alveolar ejection is preceded by teat cleaning and stimulation. The time difference between cisternal and alveolar milk is between 50–100 s, and this can correlate negatively with EC and SCC [172,173,174]. It has been shown that milk sampled before ejection improves mastitis detection [175].
5.2.3. On-Farm Culture
Biplates and triplates are the main cow-side testing methods to confirm the presence of bacteria. The biplate contains two types of agars, one for Gram-negative and another one for Gram-positive bacteria. In contrast, the triplate type comprises three types of agars, which can differentiate Gram-negatives from Gram-positives and Gram-positive staphylococci from Gram-positive streptococci. The results of biplate or triplate tests are not as promising as one would hope. However, they can be used to discover whether the pathogen can grow in culture [176].
A milk sample is considered contaminated when three or more colonies are present. One quarter may be regarded as cured when the bacteria found present at the beginning of the plate test are no longer isolated from the milk sample. On the other hand, there are cases where cows with clinical mastitis have a bacteriologically negative sample. These results are present even when all the necessary protocols for collecting and performing the sample analysis were followed. The reasons might be due to a low concentration of the pathogen in the milk, presence of intracellular bacteria, or of growth-inhibitory substances in the milk. In cases where no pathogen can be detected, enzyme-linked immunosorbent assays (ELISA) methods can help identify S. aureus, E. coli, S. dysgalactiae, and S. agalactiae [151,176]. However, it is essential to realize that in some cases, microorganisms in quarters of milk are due to microbiological contamination during sampling rather than IMI, particularly in milk samples with low colony counts, <100 CFU/mL [177].
Different farms apply different on-farm techniques to monitor udder health. This includes measuring enzymes (N-acetyl-D glucosaminidase; lactate dehydrogenase), pH indicators, strip plates or portable SCC measurements (PortaCheck; BacSomatic; DeLaval Cell Counter) [11,137,178]. These conventional methods are focused on discovering mastitis at the time of occurrence.
The need to detect and prevent mastitis or IMI as soon as possible is important for disease mitigation and spread control. Emerging innovations using a combination of biotechnology and nanotechnology are making this possible. Thanks to the invention of nanotechnology-based biosensors and lab-on-a-chip technologies, high-throughput analysis using proteomics and metabolomics is now possible [179]. These systems will offer farmers an all-in-one method—from processing the sample to analyzing the sample and giving an accurate result right on the farm [180]. Most of the studies done with biosensor and biomarkers in the field of mastitis have been performed in milk. However, it was noted that milk samples negatively influenced biosensor detection performance and suggested that other biological fluids should be used [108].
6. Comparison of Mastitis Tests and Future Approaches
Eradicating mastitis infections is almost impossible. Much effort is still focused on eliminating existing infections and preventing new ones. The National Institute for Research in Dairying in 1970 created a 10-point control program [151]. According to this program, to have profitable udders, better dairy cow health and welfare, and positive treatment outcomes, the detection of udder infection should be done as early as possible [100,181]. Monitoring and preventive measurements require reliable and affordable prognostic and diagnostic methods. As discussed above, most of the diagnostic techniques are widely used, but many lack the necessary accuracy. Some are prohibitive in time and cost, while others are limited to detecting mastitis only when the cow is already severely infected. For example, CMT has been used for a very long time as a cow side test [182]. Still, its performance is questionable due to the variability in the execution of the test and the user’s ability to read it correctly [183]. Generally, CMT detects the presence of IMI 4 days after calving and is not able to work for SCM detection [165,184]. Compared to other diagnostic tests, the CMT has been proven less accurate (87.4–90.8%) and more time-consuming for a large herd [185]. On the other hand, with better sensitivity and specificity, counting somatic cells in milk can be more successful. The drawback to this approach is that it requires laboratory analysis, which limits its use as a real-time detection method and is associated with higher costs per test [186,187]. Portable devices, such as the DeLaval cell counter, Porta SCC and Fossomatic are useful for on-farm SCM evaluation [188,189]. Even though these portable systems are easy to use and fast, they lack sensitivity at low SCC [137].
Errors can occur while interpreting SCC data since this is influenced by several factors such as the presence of bacteria, diurnal variation, age, stage of lactation, and milk sample [190]. A group of authors determined that the accuracy of SCC should be higher than 85% to identify the bacterial species associated with IMI [191]. They also showed how SCC levels fluctuated between various mastitis microorganisms. Out of all quarters or composite milk samples that exceeded 200,000 SCC/mL, over one third were culture negative [191]. Furthermore, they found out that if the threshold to detect S. aureus is 150,000–200,000 cells/mL, 30.8% of all cows will remain undetected as they had lower SCC but were bacteriologically positive. By not identifying truly infected samples, SCC methods unintentionally create adverse outcomes for both the cow and the farm. The two most frequently used diagnostic methods, CMT and SCC, can detect abnormalities in the udder but cannot specify the causative agent. The inability to identify the pathogens leads to inappropriate treatments that increase antibiotic resistance leading to the spread of antibiotic-resistant strains [108]. Other methods to fight bacterial resistance to antibiotics or toxic compounds (RATC) and inflammation can be considered, such as probiotics, prebiotics, and proteobiotics [192,193,194].
There are many diagnostic approaches for mastitis detection, including SCC, CMT and EC. Other techniques such as sensor-based systems, immunoassays, and specific biomarkers from PCR, nucleotide sequencing, proteomics or metabolomics are just being introduced [51]. Higher diagnostic accuracy can often be achieved if we combine multiple methods [195]. A comparison between two of the most common used tests is summarized in Figure 3.
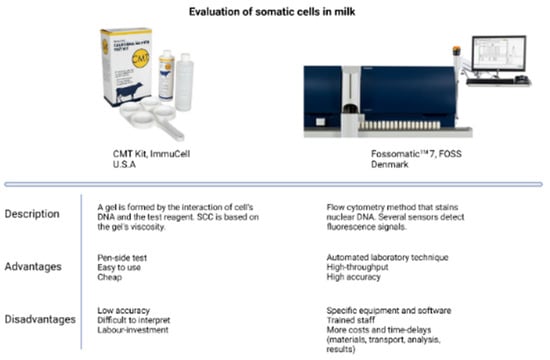
Figure 3.
Comparison of two of the most used tests to detect somatic cells in milk. CMT kit image courtesy of ImmuCell (USA); Fossomatic 7 image courtesy of FOSS (Denmark).
Future pen-side mastitis tests should take into consideration the need to detect cows susceptible for new or existing IMIs before calving, while at the same time being economical and user friendly. The development of better, faster, cheaper, and more convenient tests will encourage appropriate interventions to prevent transmission, reduce antimicrobial resistance, and minimize financial losses.
7. Metabolomics Investigation of Mastitis
7.1. Application of Metabolomics in Periparturient Diseases
The dairy farmers and milk industry’s primary goal is to breed cows for high milk production and quality. However, this goal is associated with a drawback—increased incidence of periparturient diseases [196,197]. Holstein cows, which make up 93% of Canadian herds produce about 10,753 L/milk for 305 days in milk (DIM) [6]. During the dry period and early lactation period, cows go through physiological, immunological, metabolic, nutritional changes, and adaptions that prepare them for calving and the next lactation [198,199]. As mentioned, our lab has observed such changes up to 8 weeks before calving and these continue until 8 weeks postpartum. Cows that do not adapt to these changes likely present either clinical or subclinical forms of diseases (ketosis, milk fever, retained placenta, fatty liver, metritis, mastitis, or laminitis). On average, almost 50% of dairy cows in a Canadian dairy herd present with more than one disease during the transition period [21,97,136].
Many diseases in humans and cattle can be detected or diagnosed by the perturbation of just one metabolite. For example, subclinical ketosis is still defined as an increased level of ketone bodies in the blood, especially BHBA. Ketosis occurs due to deficiencies in energy intake, and it is not detectable via visible physiological changes [200,201]. With the advancements in omics technologies, other kinds of chemical or protein biomarkers are being discovered that potentially offer greater sensitivity and specificity than traditional cell-based or gross property measurements [202]. For example, studies from our lab have identified that inflammatory mediators such as interleukin (IL)-1, IL-6, TNF, SAA and Hp are elevated at the beginning of the dry period, up to 8 weeks before calving in several periparturient diseases [91,110,111,113,203]. Those systemic findings of inflammation present another point of view regarding pathomechanisms of such diseases. In particular, those results show that post-calving disease is preceded by a systemic inflammatory insult weeks before presenting any clinical signs or physiological changes. There is mounting evidence that can attribute the origin of this insult to the presence of endotoxin in the circulation that can be translocated from the rumen, reproductive tract, or mammary gland, thereby initiating an immune response [21,136]. Furthermore, immunosuppression during the transition period can be caused by these pathogenic bacteria (i.e., Staphylococcus aureus; Escherichia coli). As proven on humans, this kind of immunosuppression can slow or even prevent an immune response [204,205]. One of those mechanisms causing immunosuppression is the impairment of neutrophil functions and extravasation [206,207].
Metabolomics, which offers a route to measure an animal’s chemical phenotype, is being used to understand the underlying metabolic changes associated with the transition period in dairy cattle and how it relates to disease manifestation. Metabolomics can identify potential metabolite biomarkers to identify animals susceptible to several periparturient diseases. In blood, three metabolites carnitine (C0), propionyl carnitine (C3), and lysophosphatidylcholine acyl C14:0 (LPC a C14:0) were found to be able to predict which cows would be susceptible to develop one or more diseases (retained placenta, mastitis, metritis, or laminitis) up to 4 weeks before calving [208]. Another study showed that up to 67 metabolites were expressed differently 21 days prepartum than the day of calving [209]. Many other metabolites (amino acids (AA), acylcarnitines (AC), phosphatidylcholines (PC), Lyso-PC, and metal ions) each specific to a given disease have been identified and measured with high predictive accuracy for a number of conditions using blood [210,211,212,213,214] or milk [215,216]. Biomarkers for animals at risk for developing ketosis were identified in urine [217] and blood [214]. Likewise, biomarkers for those at risk to develop periparturient diseases were found in serum [218,219,220], urine [221], or milk [222].
7.2. Application of Metabolomics for Mastitis Biomarker Discovery
One of the first metabolomic studies to look at mastitis was conducted in 2005 [223]. GC-MS demonstrated that it was possible to differentiate milk from healthy or mastitic samples chemically with what is now called an electronic nose. Other studies concluded that if the concentrations of specific volatile metabolites were high in milk, it meant that they were infected, and the volatiles corresponded to metabolic by-products from bacterial pathogens [224,225]. NMR studies conducted in milk found that lactate, acetate, BHBA, butyrate, and isoleucine were in a higher concentration in high SCC samples [226]. On the other hand, for the same samples, lactose, hippurate, and fumarate were at lower levels than in milk with low SCC levels [226]. A mastitis-induced experiment studied how oxylipin profiles in milk and mammary tissues changed and influenced the disease [227]. Hydroxy octadecadienoic acid (HODE) and oxo octadecadienoic acid derived from arachidonic acid and linoleic acid were higher in S. uberis mastitis. These results show that oxylipids are implicated in the inflammatory state within the mammary gland. Several authors have noted the increased milk concentrations of prostaglandins and thromboxane in mastitis samples [228,229]. These pro- and anti-inflammatory oxylipids may affect the host’s ability to eliminate the pathogen [230]. In another study with skimmed milk, using untargeted LC-MS, 690 metabolites were identified [231]. They challenged the cows with a S. uberis strain and collected milk samples 0, 36, 42, 57, 81, and 312 h after infusion. The bacterial load peak was noted at 36h, whereas most of the metabolite changes in milk occurred after 81h. They noticed increased levels of bile acids (taurochenodeoxycholic acid (C26H45NO6S), taurocholic acid (C26H45NO7S), glycocholate (C26H43NO6), glycodeoxycholate (C26H43NO5), and cholate (C24H40O5). Those bile acids support antimicrobial [232] and anti-inflammatory activities, facilitated through the farnesoid X receptor pathway [233], which inhibits the activation of the NF-kB signaling pathway [234,235]. Thomas and colleagues’ results revealed that high levels of bile acids in milk decreased the levels of pro-inflammatory cytokines [231]. Other authors have noticed alterations in metabolic pathways pre-and/or postpartum in SCM and CM cows compared to healthy cows. These results show extensive evidence of bacterial activities.
Several other studies have demonstrated that mastitis is preceded by alterations of metabolic pathways in the blood corresponding to inflammatory insults in the prepartum period [91,236,237]. Several serum metabolites were used to distinguish SCM cows from healthy cows up to 8 weeks before their due date using targeted GC-MS. Alterations in amino acid metabolism continued up to 8 weeks postpartum. The best indicators between the two groups were valine, serine, tyrosine, and phenylalanine [236]. Besides, distinguishing between CM cows and healthy cows could be achieved by quantifying about a half dozen metabolites, including N-methyl ethanolamine phosphate, choline, phosphorylcholine, free carnitine, trimethyl lysine, tyrosine, and proline. The most significant discriminator was 3′-sialyl lactose in serum [237]. This particular saccharide was more elevated than the control group at −21 days, probably to boost innate immunity [238]. This compound is known to protect calves against infections [239]. Lactate was also increased in this study, which correlates with what was found by other authors [91,240,241]. The shift in metabolite levels can be due to acute inflammation, as shown by the increased APPs [91,208,236,237,242]. A recent study from our group confirmed lactate in blood as a biomarker of high somatic cells 4 weeks before parturition and traditional diagnosis [220]. Detecting urine changes confirmed the hypothesis that SCM cows were preceded, associated, and followed by alterations of urinary metabolites [118].
Further validation of these findings over a larger number of cows and more diverse farm management settings can help us develop a better view of the pathology of mastitis. It might also help develop more robust pen-side tests to facilitate the identification and treatment of susceptible cows to improve overall dairy herd health.
8. Predictive Biomarkers: Opportunity for the Dairy Industry
Given that not all dairy cows are equally susceptible to mastitis [181,243], there is a need to develop a pen-side test with a panel of metabolites that can distinguish between cows that are more susceptible to developing mastitis from healthy cows. MS-based metabolomics approaches are highly sensitive and high-throughput instruments that allow identification and validation of biomarkers from biological biofluids. Current challenges for the dairy industry lay on high culling rates, treatment costs and tests that only screen for mastitis in milk during lactation. Considering the existing literature and approaches, we speculate that new studies focusing on finding predictive biomarkers during the dry-off period for the risk of SCM will bring many advantages on cow’s health, dairy industry and food safety.
9. Conclusions
Currently all types of mastitis are monitored and diagnosed after parturition where the incidence is known to be higher. A series of longitudinal studies and recent developments in the area of biomarkers for periparturient diseases has shed light into the pathobiology of mastitis. In this review article we present the interconnection of mastitis and other diseases occurring around calving as well as the opportunity to utilize metabolomics for predictive biomarkers. As noticed, several labs have identified potential biomarkers in blood, urine, and milk either postpartum or prepartum.
Dairy industry is profoundly impacted by the consequences of mastitis, therefore, prepartum diagnosis and monitoring of mastitis is of uttermost importance. Further large-scale validation and production of predictive biomarkers is necessary.
Author Contributions
Conceptualization, B.N.A., D.S.W. and K.H.; resources, B.N.A. and D.S.W.; writing—original draft preparation, K.H.; writing—review and editing, B.N.A. and D.S.W.; supervision, B.N.A. and D.S.W.; project administration, B.N.A.; funding acquisition, B.N.A. and D.S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This literature review was funded by Agriculture Funding Consortium and Alberta Milk, Canada, project #2018F003R.
Institutional Review Board Statement
This is a review article, and no animals or humans were subjected to experimentation.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data are available for this review article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thompson-Crispi, K.A.; Miglior, F.; Mallard, B.A. Incidence Rates of Clinical Mastitis among Canadian Holsteins Classified as High, Average, or Low Immune Responders. Clin. Vaccine Immunol. 2013, 20, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Reyher, K.K.; Dohoo, I.R. Diagnosing Intramammary Infections: Evaluation of Composite Milk Samples to Detect Intramammary Infections. J. Dairy Sci. 2011, 94, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Dohoo, I.R.; Leslie, K.E. Evaluation of Changes in Somatic Cell Counts as Indicators of New Intramammary Infections. Prev. Vet. Med. 1991, 10, 225–237. [Google Scholar] [CrossRef]
- Ruegg, P.L.; Petersson-Wolfe, C.S. Mastitis in Dairy Cows. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, ix–x. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, M.; Haine, D.; Kelton, D.F.; Barkema, H.W.; Hogeveen, H.; Keefe, G.P.; Dufour, S. Herd-Level Mastitis-Associated Costs on Canadian Dairy Farms. Front. Vet. Sci. 2018, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Culling and Replacement Rates in Dairy Herds in Canada; Canadian Dairy Information Centre (CDIC). Available online: https://agriculture.canada.ca/en/canadas-agriculture-sectors/animal-industry/canadian-dairy-information-centre/dairy-statistics-and-market-information/dairy-animal-genetics/culling-and-replacement-rates-dairy-herds-canada (accessed on 30 June 2021).
- Busanello, M.; Rossi, R.S.; Cassoli, L.D.; Pantoja, J.C.F.; Machado, P.F. Estimation of Prevalence and Incidence of Subclinical Mastitis in a Large Population of Brazilian Dairy Herds. J. Dairy Sci. 2017, 100, 6545–6553. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, J.; Piepers, S.; Supré, K.; De Vliegher, S. Pathogen-Specific Incidence Rate of Clinical Mastitis in Flemish Dairy Herds, Severity, and Association with Herd Hygiene. J. Dairy Sci. 2014, 97, 6926–6934. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.L. Etiological Agents of Bovine Mastitis. Vet. Microbiol. 1988, 16, 41–66. [Google Scholar] [CrossRef]
- Zadoks, R.N.; Middleton, J.R.; McDougall, S.; Katholm, J.; Schukken, Y.H. Molecular Epidemiology of Mastitis Pathogens of Dairy Cattle and Comparative Relevance to Humans. J. Mammary Gland. Biol. Neoplasia 2011, 16, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Moroni, P.; Nydam, D.V.; Ospina, P.A.; Scillieri-Smith, J.C.; Virkler, P.D.; Watters, R.D.; Welcome, F.L.; Zurakowski, M.J.; Ducharme, N.G.; Yeager, A.E. Diseases of the Teats and Udder. In Rebhun’s Diseases of Dairy Cattle; Elsevier: Amsterdam, The Netherlands, 2018; pp. 389–465. [Google Scholar] [CrossRef]
- Schukken, Y.H.; Günther, J.; Fitzpatrick, J.; Fontaine, M.C.; Goetze, L.; Holst, O.; Leigh, J.; Petzl, W.; Schuberth, H.-J.; Sipka, A.; et al. Host-Response Patterns of Intramammary Infections in Dairy Cows. Vet. Immunol. Immunopathol. 2011, 144, 270–289. [Google Scholar] [CrossRef] [PubMed]
- Dego, O.K.; van Dijk, J.E.; Nederbragt, H. Factors Involved in the Early Pathogenesis of Bovine Staphylococcus Aureus Mastitis with Emphasis on Bacterial Adhesion and Invasion. A Review. Vet. Q. 2002, 24, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Perssonwaller, K.; Bengtsson, B.; Lindberg, A.; Nyman, A.; Ericssonunnerstad, H. Incidence of Mastitis and Bacterial Findings at Clinical Mastitis in Swedish Primiparous Cows—Influence of Breed and Stage of Lactation. Vet. Microbiol. 2009, 134, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, A.-M.; Liski, E.; Pyörälä, S.; Taponen, S. Pathogen-Specific Production Losses in Bovine Mastitis. J. Dairy Sci. 2018, 101, 9493–9504. [Google Scholar] [CrossRef] [PubMed]
- Lara-Zárate, L.; López-Meza, J.E.; Ochoa-Zarzosa, A. Staphylococcus Aureus Inhibits Nuclear Factor Kappa B Activation Mediated by Prolactin in Bovine Mammary Epithelial Cells. Microb. Pathog. 2011, 51, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, A.E.J.; van den Borne, B.H.P.; Wall, S.K.; Wellnitz, O.; Bruckmaier, R.M.; Spadavecchia, C. Experimentally Induced Subclinical Mastitis: Are Lipopolysaccharide and Lipoteichoic Acid Eliciting Similar Pain Responses? Acta Vet. Scand. 2017, 59, 40. [Google Scholar] [CrossRef]
- Barkema, H.W.; Schukken, Y.H.; Zadoks, R.N. Invited Review: The Role of Cow, Pathogen, and Treatment Regimen in the Therapeutic Success of Bovine Staphylococcus Aureus Mastitis. J. Dairy Sci. 2006, 89, 1877–1895. [Google Scholar] [CrossRef]
- Pyörälä, S.; Hovinen, M.; Simojoki, H.; Fitzpatrick, J.; Eckersall, P.D.; Orro, T. Acute Phase Proteins in Milk in Naturally Acquired Bovine Mastitis Caused by Different Pathogens. Vet. Rec. 2011, 168, 535. [Google Scholar] [CrossRef]
- Hogan, J.; Larry Smith, K. Coliform Mastitis. Vet. Res. 2003, 34, 507–519. [Google Scholar] [CrossRef]
- Eckel, E.F.; Ametaj, B.N. Invited Review: Role of Bacterial Endotoxins in the Etiopathogenesis of Periparturient Diseases of Transition Dairy Cows. J. Dairy Sci. 2016, 99, 5967–5990. [Google Scholar] [CrossRef]
- Leigh, J.A. Streptococcus Uberis: A Permanent Barrier to the Control of Bovine Mastitis? Vet. J. 1999, 157, 225–238. [Google Scholar] [CrossRef]
- Bradley, A.J.; Leach, K.A.; Breen, J.E.; Green, L.E.; Green, M.J. Survey of the Incidence and Aetiology of Mastitis on Dairy Farms in England and Wales. Vet. Rec. 2007, 160, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Rato, M.G.; Nerlich, A.; Bergmann, R.; Bexiga, R.; Nunes, S.F.; Vilela, C.L.; Santos-Sanches, I.; Chhatwal, G.S. Virulence Gene Pool Detected in Bovine Group C Streptococcus Dysgalactiae Subsp. Dysgalactiae Isolates by Use of a Group A S. Pyogenes Virulence Microarray. J. Clin. Microbiol. 2011, 49, 2470–2479. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Hulland, C.; Ruegg, P.L. Characterization of Clinical Mastitis Occurring in Cows on 50 Large Dairy Herds in Wisconsin. J. Dairy Sci. 2013, 96, 7538–7549. [Google Scholar] [CrossRef] [PubMed]
- Levison, L.J.; Miller-Cushon, E.K.; Tucker, A.L.; Bergeron, R.; Leslie, K.E.; Barkema, H.W.; DeVries, T.J. Incidence Rate of Pathogen-Specific Clinical Mastitis on Conventional and Organic Canadian Dairy Farms. J. Dairy Sci. 2016, 99, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Gröhn, Y.T.; Erb, H.N.; McCulloch, C.E.; Saloniemi, H.S. Epidemiology of Metabolic Disorders in Dairy Cattle: Association Among Host Characteristics, Disease, and Production. J. Dairy Sci. 1989, 72, 1876–1885. [Google Scholar] [CrossRef]
- Schönborn, S.; Wente, N.; Paduch, J.-H.; Krömker, V. In Vitro Ability of Mastitis Causing Pathogens to Form Biofilms. J. Dairy Res. 2017, 84, 198–201. [Google Scholar] [CrossRef]
- Massé, J.; Dufour, S.; Archambault, M. Characterization of Klebsiella Isolates Obtained from Clinical Mastitis Cases in Dairy Cattle. J. Dairy Sci. 2020, 103, 3392–3400. [Google Scholar] [CrossRef]
- Tremblay, Y.D.N.; Lamarche, D.; Chever, P.; Haine, D.; Messier, S.; Jacques, M. Characterization of the Ability of Coagulase-Negative Staphylococci Isolated from the Milk of Canadian Farms to Form Biofilms. J. Dairy Sci. 2013, 96, 234–246. [Google Scholar] [CrossRef]
- Reyher, K.K.; Dohoo, I.R.; Scholl, D.T.; Keefe, G.P. Evaluation of Minor Pathogen Intramammary Infection, Susceptibility Parameters, and Somatic Cell Counts on the Development of New Intramammary Infections with Major Mastitis Pathogens. J. Dairy Sci. 2012, 95, 3766–3780. [Google Scholar] [CrossRef]
- Sargeant, J.M.; Scott, H.M.; Leslie, K.E.; Ireland, M.J.; Bashiri, A. Clinical Mastitis in Dairy Cattle in Ontario: Frequency of Occurrence and Bacteriological Isolates. Can. Vet. J. 1998, 39, 33–38. [Google Scholar]
- Barkema, H.W.; Schukken, Y.H.; Lam, T.J.G.M.; Beiboer, M.L.; Wilmink, H.; Benedictus, G.; Brand, A. Incidence of Clinical Mastitis in Dairy Herds Grouped in Three Categories by Bulk Milk Somatic Cell Counts. J. Dairy Sci. 1998, 81, 411–419. [Google Scholar] [CrossRef]
- Roberson, J.R.; Warnick, L.D.; Moore, G. Mild to Moderate Clinical Mastitis: Efficacy of Intramammary Amoxicillin, Frequent Milk-Out, a Combined Intramammary Amoxicillin, and Frequent Milk-Out Treatment Versus No Treatment. J. Dairy Sci. 2004, 87, 583–592. [Google Scholar] [CrossRef]
- Reksen, O.; Sølverød, L.; Branscum, A.J.; Østerås, O. Relationships Between Milk Culture Results and Treatment for Clinical Mastitis or Culling in Norwegian Dairy Cattle. J. Dairy Sci. 2006, 89, 2928–2937. [Google Scholar] [CrossRef]
- McDougall, S. Prevalence of Clinical Mastitis in 38 Waikato Dairy Herds in Early Lactation. N. Z. Vet. J. 1999, 47, 143–149. [Google Scholar] [CrossRef]
- Kopali, A.; Shoshi, N.; Koleci, X. Prevalence of Subclinical Mastitis in Dairy Cows: A Case Study of the Livestock Complex, Tirana (Albania). Res. Opin. Anim. Vet. Sci. 2011, 593–596. [Google Scholar]
- He, W.; Ma, S.; Lei, L.; He, J.; Li, X.; Tao, J.; Wang, X.; Song, S.; Wang, Y.; Wang, Y.; et al. Prevalence, Etiology, and Economic Impact of Clinical Mastitis on Large Dairy Farms in China. Vet. Microbiol. 2020, 242, 108570. [Google Scholar] [CrossRef] [PubMed]
- Breen, J.E.; Green, M.J.; Bradley, A.J. Quarter and Cow Risk Factors Associated with the Occurrence of Clinical Mastitis in Dairy Cows in the United Kingdom. J. Dairy Sci. 2009, 92, 2551–2561. [Google Scholar] [CrossRef]
- Sordillo, L.M. Mammary Gland Immunobiology and Resistance to Mastitis. Vet. Clin. N. Am.-Food Anim. Pract. 2018, 34, 507–523. [Google Scholar] [CrossRef]
- Rainard, P.; Riollet, C. Innate Immunity of the Bovine Mammary Gland. Vet. Res. 2006, 37, 369–400. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Leopold, S.J.; Cranendonk, D.R.; van der Poll, T. Host Innate Immune Responses to Sepsis. Virulence 2014, 5, 36–44. [Google Scholar] [CrossRef]
- Miyake, K. Innate Immune Sensing of Pathogens and Danger Signals by Cell Surface Toll-like Receptors. Semin. Immunol. 2007, 19, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Weber, T.E.; Baumgard, L.H.; Gabler, N.K. Growth and Development Symposium: Endotoxin, Inflammation, and Intestinal Function in Livestock1,2. J. Anim. Sci. 2012, 90, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Brenaut, P.; Lefèvre, L.; Rau, A.; Laloë, D.; Pisoni, G.; Moroni, P.; Bevilacqua, C.; Martin, P. Contribution of Mammary Epithelial Cells to the Immune Response during Early Stages of a Bacterial Infection to Staphylococcus Aureus. Vet. Res. 2014, 45, 16. [Google Scholar] [CrossRef]
- Zhao, X.; Lacasse, P. Mammary Tissue Damage during Bovine Mastitis: Causes and Control1. J. Anim. Sci. 2008, 86 (Suppl. 13), 57–65. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Streicher, K.L. Mammary Gland Immunity and Mastitis Susceptibility. J. Mammary Gland. Biol. Neoplasia 2002, 7, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Ezzat Alnakip, M.; Quintela-Baluja, M.; Böhme, K.; Fernández-No, I.; Caamaño-Antelo, S.; Calo-Mata, P.; Barros-Velázquez, J. The Immunology of Mammary Gland of Dairy Ruminants between Healthy and Inflammatory Conditions. J. Vet. Med. 2014, 2014, 659801. [Google Scholar] [CrossRef] [PubMed]
- Leslie, K.E.; Petersson-Wolfe, C.S. Assessment and Management of Pain in Dairy Cows with Clinical Mastitis. Vet. Clin. N. Am.-Food Anim. Pract. 2012, 28, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Roberson, J.R. Treatment of Clinical Mastitis. Vet. Clin. N. Am.-Food Anim. Pract. 2012, 28, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Adkins, P.R.F.; Middleton, J.R. Methods for Diagnosing Mastitis. Vet. Clin. N. Am.-Food Anim. Pract. 2018, 34, 479–491. [Google Scholar] [CrossRef]
- Ruegg, P.L. Managing Mastitis and Producing Quality Milk. In Dairy Production Medicine; Risco, C.A., Retamal, P.M., Eds.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Suojala, L.; Kaartinen, L.; Pyörälä, S. Treatment for Bovine Escherichia Coli Mastitis—An Evidence-Based Approach. J. Vet. Pharmacol. Ther. 2013, 36, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Forsbäck, L.; Lindmark-Månsson, H.; Andrén, A.; Åkerstedt, M.; Svennersten-Sjaunja, K. Udder Quarter Milk Composition at Different Levels of Somatic Cell Count in Cow Composite Milk. Animal 2009, 3, 710–717. [Google Scholar] [CrossRef]
- Leitner, G.; Silanikove, N.; Merin, U. Estimate of Milk and Curd Yield Loss of Sheep and Goats with Intrammamary Infection and Its Relation to Somatic Cell Count. Small Rumin. Res. 2008, 74, 221–225. [Google Scholar] [CrossRef]
- Pitkälä, A.; Haveri, M.; Pyörälä, S.; Myllys, V.; Honkanen-Buzalski, T. Bovine Mastitis in Finland 2001—Prevalence, Distribution of Bacteria, and Antimicrobial Resistance. J. Dairy Sci. 2004, 87, 2433–2441. [Google Scholar] [CrossRef]
- Schukken, Y.H.; Wilson, D.J.; Welcome, F.; Garrison-Tikofsky, L.; Gonzalez, R.N. Monitoring Udder Health and Milk Quality Using Somatic Cell Counts. Vet. Res. 2003, 34, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Rhoda, D.A.; Pantoja, J.C.F. Using Mastitis Records and Somatic Cell Count Data. Vet. Clin. N. Am.-Food Anim. Pract. 2012, 28, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Dingwell, R.T.; Kelton, D.F.; Leslie, K.E. Management of the Dry Cow in Control of Peripartum Disease and Mastitis. Vet. Clin. N. Am.-Food Anim. Pract. 2003, 19, 235–265. [Google Scholar] [CrossRef]
- Jones, G. Proper Dry Cow Management Critical for Mastitis Control Drying-Off. Va. Tech. 2009, 404–212, 1–6. [Google Scholar]
- Capuco, A.V.; Akers, R.M.; Smith, J.J. Mammary Growth in Holstein Cows During the Dry Period: Quantification of Nucleic Acids and Histology. J. Dairy Sci. 1997, 80, 477–487. [Google Scholar] [CrossRef]
- Kuhn, M.T.; Hutchison, J.L.; Norman, H.D. Minimum Days Dry to Maximize Milk Yield in Subsequent Lactation. Anim. Res. 2005, 54, 351–367. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Moyes, K.M. Factors Contributing to Immunosuppression in the Dairy Cow during the Periparturient Period. Jpn. J. Vet. Res. 2015, 63 (Suppl. 1), S15–S24. [Google Scholar]
- Dingwell, R.T.; Leslie, K.E.; Schukken, Y.H.; Sargeant, J.M.; Timms, L.L.; Duffield, T.F.; Keefe, G.P.; Kelton, D.F.; Lissemore, K.D.; Conklin, J. Association of Cow and Quarter-Level Factors at Drying-off with New Intramammary Infections during the Dry Period. Prev. Vet. Med. 2004, 63, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Butler, W.R.; Smith, R.D. Interrelationships Between Energy Balance and Postpartum Reproductive Function in Dairy Cattle. J. Dairy Sci. 1989, 72, 767–783. [Google Scholar] [CrossRef]
- Bell, A.W. Regulation of Organic Nutrient Metabolism during Transition from Late Pregnancy to Early Lactation. J. Anim. Sci. 1995, 73, 2804. [Google Scholar] [CrossRef] [PubMed]
- Ametaj, B. A New Understanding of the Causes of Fatty Liver in Dairy Cows. Adv. Dairy Technol. 2005, 17, 97–112. [Google Scholar]
- Von Keyserlingk, M.A.G.; Rushen, J.; de Passillé, A.M.; Weary, D.M. Invited Review: The Welfare of Dairy Cattle—Key Concepts and the Role of Science. J. Dairy Sci. 2009, 92, 4101–4111. [Google Scholar] [CrossRef]
- Lucy, M.C.; Jiang, H.; Kobayashi, Y. Changes in the Somatotrophic Axis Associated with the Initiation of Lactation. J. Dairy Sci. 2001, 84, E113–E119. [Google Scholar] [CrossRef]
- Ingvartsen, K.L. Feeding- and Management-Related Diseases in the Transition Cow. Anim. Feed. Sci. Technol. 2006, 126, 175–213. [Google Scholar] [CrossRef]
- De Vliegher, S.; Ohnstad, I.; Piepers, S. Management and Prevention of Mastitis: A Multifactorial Approach with a Focus on Milking, Bedding and Data-Management. J. Integr. Agric. 2018, 17, 1214–1233. [Google Scholar] [CrossRef]
- Sordillo, L.M. Factors Affecting Mammary Gland Immunity and Mastitis Susceptibility. Livest. Prod. Sci. 2005, 98, 89–99. [Google Scholar] [CrossRef]
- Contreras, G.A.; Rodríguez, J.M. Mastitis: Comparative Etiology and Epidemiology. J. Mammary Gland. Biol. Neoplasia 2011, 16, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.S.; Smith, K.L.; Hoblet, K.H.; Todhunter, D.A.; Schoenberger, P.S.; Hueston, W.D.; Pritchard, D.E.; Bowman, G.L.; Heider, L.E.; Brockett, B.L. Bacterial Counts in Bedding Materials Used on Nine Commercial Dairies. J. Dairy Sci. 1989, 72, 250–258. [Google Scholar] [CrossRef]
- Hogan, J.S.; Bogacz, V.L.; Thompson, L.M.; Romig, S.; Schoenberger, P.S.; Weiss, W.P.; Smith, K.L. Bacterial Counts Associated with Sawdust and Recycled Manure Bedding Treated with Commercial Conditioners. J. Dairy Sci. 1999, 82, 1690–1695. [Google Scholar] [CrossRef]
- Ruegg, P.L. Management of Mastitis on Organic and Conventional Dairy Farms1. J. Anim. Sci. 2009, 87 (Suppl. 13), 43–55. [Google Scholar] [CrossRef]
- Bannerman, D.D.; Kauf, A.C.W.; Paape, M.J.; Springer, H.R.; Goff, J.P. Comparison of Holstein and Jersey Innate Immune Responses to Escherichia Coli Intramammary Infection. J. Dairy Sci. 2008, 91, 2225–2235. [Google Scholar] [CrossRef]
- Dezetter, C.; Bareille, N.; Billon, D.; Côrtes, C.; Lechartier, C.; Seegers, H. Changes in Animal Performance and Profitability of Holstein Dairy Operations after Introduction of Crossbreeding with Montbéliarde, Normande, and Scandinavian Red. J. Dairy Sci. 2017, 100, 8239–8264. [Google Scholar] [CrossRef] [PubMed]
- Heringstad, B.; Chang, Y.M.; Gianola, D.; Klemetsdal, G. Genetic Analysis of Clinical Mastitis, Milk Fever, Ketosis, and Retained Placenta in Three Lactations of Norwegian Red Cows. J. Dairy Sci. 2005, 88, 3273–3281. [Google Scholar] [CrossRef]
- Negussie, E.; Strandén, I.; Mäntysaari, E.A. Genetic Association of Clinical Mastitis with Test-Day Somatic Cell Score and Milk Yield During First Lactation of Finnish Ayrshire Cows. J. Dairy Sci. 2008, 91, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Lush, J.L. Inheritance of Susceptibility to Mastitis. J. Dairy Sci. 1950, 33, 121–125. [Google Scholar] [CrossRef]
- Shook, G.E. Selection for Disease Resistance. J. Dairy Sci. 1989, 72, 1349–1362. [Google Scholar] [CrossRef]
- De Haas, Y.; Ouweltjes, W.; ten Napel, J.; Windig, J.J.; de Jong, G. Alternative Somatic Cell Count Traits as Mastitis Indicators for Genetic Selection. J. Dairy Sci. 2008, 91, 2501–2511. [Google Scholar] [CrossRef] [PubMed]
- Urioste, J.I.; Franzén, J.; Windig, J.J.; Strandberg, E. Genetic Relationships among Mastitis and Alternative Somatic Cell Count Traits in the First 3 Lactations of Swedish Holsteins. J. Dairy Sci. 2012, 95, 3428–3434. [Google Scholar] [CrossRef] [PubMed]
- Narayana, S.G.; Miglior, F.; Naqvi, S.A.; Malchiodi, F.; Martin, P.; Barkema, H.W. Genetic Analysis of Subclinical Mastitis in Early Lactation of Heifers Using Both Linear and Threshold Models. J. Dairy Sci. 2018, 101, 11120–11131. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, D.; Bennewitz, J.; Stamer, E.; Junge, W.; Kalm, E.; Thaller, G. Genetic Analysis of Mastitis Data with Different Models. J. Dairy Sci. 2011, 94, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, M.; Heringstad, B. Somatic Cell Count as an Indicator of Sub-Clinical Mastitis. Genetic Parameters and Correlations with Clinical Mastitis. Interbull Bull. 2006, 35, 12. [Google Scholar]
- Olde Riekerink, R.G.M.; Barkema, H.W.; Kelton, D.F.; Scholl, D.T. Incidence Rate of Clinical Mastitis on Canadian Dairy Farms. J. Dairy Sci. 2008, 91, 1366–1377. [Google Scholar] [CrossRef]
- Green, M.J.; Green, L.E.; Medley, G.F.; Schukken, Y.H.; Bradley, A.J. Influence of Dry Period Bacterial Intramammary Infection on Clinical Mastitis in Dairy Cows. J. Dairy Sci. 2002, 85, 2589–2599. [Google Scholar] [CrossRef]
- Bradley, A.; Breen, J.; Green, M. Management: Mastitis Pattern Analysis—A Fresh Look at the Analysis of Bovine Mastitis: Part 2—Clinical Mastitis Data. Livestock 2008, 13, 30–35. [Google Scholar] [CrossRef]
- Dervishi, E.; Zhang, G.; Hailemariam, D.; Dunn, S.M.; Ametaj, B.N. Innate Immunity and Carbohydrate Metabolism Alterations Precede Occurrence of Subclinical Mastitis in Transition Dairy Cows. J. Anim. Sci. Technol. 2015, 57, 46. [Google Scholar] [CrossRef]
- Jamali, H.; Barkema, H.W.; Jacques, M.; Lavallée-Bourget, E.-M.; Malouin, F.; Saini, V.; Stryhn, H.; Dufour, S. Invited Review: Incidence, Risk Factors, and Effects of Clinical Mastitis Recurrence in Dairy Cows. J. Dairy Sci. 2018, 101, 4729–4746. [Google Scholar] [CrossRef]
- Dairy Cattle: Principles, Practices, Problems, Profits; Foley, R.C., Ed.; Lea & Febiger: Philadelphia, PA, USA, 1972. [Google Scholar]
- Hertl, J.A.; Schukken, Y.H.; Welcome, F.L.; Tauer, L.W.; Gröhn, Y.T. Effects of Pathogen-Specific Clinical Mastitis on Probability of Conception in Holstein Dairy Cows. J. Dairy Sci. 2014, 97, 6942–6954. [Google Scholar] [CrossRef]
- Halasa, T.; Nielen, M.; De Roos, A.P.W.; Van Hoorne, R.; de Jong, G.; Lam, T.J.G.M.; van Werven, T.; Hogeveen, H. Production Loss Due to New Subclinical Mastitis in Dutch Dairy Cows Estimated with a Test-Day Model. J. Dairy Sci. 2009, 92, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Botaro, B.G.; Cortinhas, C.S.; Dibbern, A.G.; e Silva, L.F.P.; Benites, N.R.; dos Santos, M.V. Staphylococcus Aureus Intramammary Infection Affects Milk Yield and SCC of Dairy Cows. Trop. Anim. Health Prod. 2015, 47, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, S. Monitoring Metabolic Health of Dairy Cattle in the Transition Period. J. Reprod. Dev. 2010, 56, S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, P.L. Investigation of Mastitis Problems on Farms. Vet. Clin. N. Am.-Food Anim. Pract. 2003, 19, 47–73. [Google Scholar] [CrossRef]
- Hogeveen, H.; Huijps, K.; Lam, T. Economic Aspects of Mastitis: New Developments. N. Z. Vet. J. 2011, 59, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Halasa, T.; Huijps, K.; Østerås, O.; Hogeveen, H. Economic Effects of Bovine Mastitis and Mastitis Management: A Review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef]
- Brown, K.; Uwiera, R.R.E.; Kalmokoff, M.L.; Brooks, S.P.J.; Inglis, G.D. Antimicrobial Growth Promoter Use in Livestock: A Requirement to Understand Their Modes of Action to Develop Effective Alternatives. Int. J. Antimicrob. Agents 2017, 49, 12–24. [Google Scholar] [CrossRef]
- Oliver, S.P.; Murinda, S.E. Antimicrobial Resistance of Mastitis Pathogens. Vet. Clin. N. Am.-Food Anim. Pract. 2012, 28, 165–185. [Google Scholar] [CrossRef]
- Aga, D.S.; Lenczewski, M.; Snow, D.; Muurinen, J.; Sallach, J.B.; Wallace, J.S. Challenges in the Measurement of Antibiotics and in Evaluating Their Impacts in Agroecosystems: A Critical Review. J. Environ. Qual. 2016, 45, 407–419. [Google Scholar] [CrossRef]
- Pol, M.; Ruegg, P.L. Treatment Practices and Quantification of Antimicrobial Drug Usage in Conventional and Organic Dairy Farms in Wisconsin. J. Dairy Sci. 2007, 90, 249–261. [Google Scholar] [CrossRef]
- Maynou, G.; Migura-Garcia, L.; Chester-Jones, H.; Ziegler, D.; Bach, A.; Terré, M. Effects of Feeding Pasteurized Waste Milk to Dairy Calves on Phenotypes and Genotypes of Antimicrobial Resistance in Fecal Escherichia Coli Isolates before and after Weaning. J. Dairy Sci. 2017, 100, 7967–7979. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J.V. Does the Use of Antimicrobial Agents in Veterinary Medicine and Animal Husbandry Select Antibiotic-Resistant Bacteria That Infect Man and Compromise Antimicrobial Chemotherapy? J. Antimicrob. Chemother. 1996, 38, 1–3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oliver, S.P.; Jayarao, B.M.; Almeida, R.A. Foodborne Pathogens in Milk and the Dairy Farm Environment: Food Safety and Public Health Implications. Foodborne Pathog. Dis. 2005, 2, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.A.M.; Martins, V.C.; Cardoso, F.A.; Germano, J.; Rodrigues, M.; Duarte, C.; Bexiga, R.; Cardoso, S.; Freitas, P.P. Biosensors for On-Farm Diagnosis of Mastitis. Front. Bioeng. Biotechnol. 2019, 7, 186. [Google Scholar] [CrossRef]
- Otto, S.J.G.; Szkotnicki, J.; McElwain, C.; So, I.; Weese, J.S.; Prescott, J.F. Building the Antimicrobial Stewardship Leadership Plan for Animal Health in Canada (Workshop, Ottawa, 3–4 October 2017). Can. Vet. J. 2018, 59, 746–748. [Google Scholar] [PubMed]
- Zhang, G.; Hailemariam, D.; Dervishi, E.; Deng, Q.; Goldansaz, S.; Dunn, S.; Ametaj, B. Alterations of Innate Immunity Reactants in Transition Dairy Cows before Clinical Signs of Lameness. Animals 2015, 5, 717–747. [Google Scholar] [CrossRef]
- Zhang, G.; Hailemariam, D.; Dervishi, E.; Goldansaz, S.A.; Deng, Q.; Dunn, S.M.; Ametaj, B.N. Dairy Cows Affected by Ketosis Show Alterations in Innate Immunity and Lipid and Carbohydrate Metabolism during the Dry off Period and Postpartum. Res. Vet. Sci. 2016, 107, 246–256. [Google Scholar] [CrossRef]
- Dervishi, E.; Zhang, G.; Hailemariam, D.; Dunn, S.M.; Ametaj, B.N. Occurrence of Retained Placenta Is Preceded by an Inflammatory State and Alterations of Energy Metabolism in Transition Dairy Cows. J. Anim. Sci. Biotechnol. 2016, 7, 26. [Google Scholar] [CrossRef]
- Dervishi, E.; Zhang, G.; Hailemariam, D.; Goldansaz, S.A.; Deng, Q.; Dunn, S.M.; Ametaj, B.N. Alterations in Innate Immunity Reactants and Carbohydrate and Lipid Metabolism Precede Occurrence of Metritis in Transition Dairy Cows. Res. Vet. Sci. 2016, 104, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, F.J.; Doherty, M.L. Production Diseases of the Transition Cow. Vet. J. 2008, 176, 3–9. [Google Scholar] [CrossRef]
- Emmanuel, D.G.V.; Madsen, K.L.; Churchill, T.A.; Dunn, S.M.; Ametaj, B.N. Acidosis and Lipopolysaccharide from Escherichia Coli B:055 Cause Hyperpermeability of Rumen and Colon Tissues. J. Dairy Sci. 2007, 90, 5552–5557. [Google Scholar] [CrossRef] [PubMed]
- Zebeli, Q.; Dunn, S.M.; Ametaj, B.N. Perturbations of Plasma Metabolites Correlated with the Rise of Rumen Endotoxin in Dairy Cows Fed Diets Rich in Easily Degradable Carbohydrates. J. Dairy Sci. 2011, 94, 2374–2382. [Google Scholar] [CrossRef]
- Saleem, F.; Ametaj, B.N.; Bouatra, S.; Mandal, R.; Zebeli, Q.; Dunn, S.M.; Wishart, D.S. A Metabolomics Approach to Uncover the Effects of Grain Diets on Rumen Health in Dairy Cows. J. Dairy Sci. 2012, 95, 6606–6623. [Google Scholar] [CrossRef]
- Zwierzchowski, G.; Zhang, G.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. Mass-Spec-Based Urinary Metabotyping around Parturition Identifies Screening Biomarkers for Subclinical Mastitis in Dairy Cows. Res. Vet. Sci. 2020, 129, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.F. The Effect of Nutritional Management of the Dairy Cow on Reproductive Efficiency. Anim. Reprod. Sci. 2006, 96, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.J.; Schukken, Y.H.; van Vliet, J.H.; Grommers, F.J.; Tielen, M.J.; Brand, A. Effect of Natural Infection with Minor Pathogens on Susceptibility to Natural Infection with Major Pathogens in the Bovine Mammary Gland. Am. J. Vet. Res. 1997, 58, 17–22. [Google Scholar] [PubMed]
- Zadoks, R.N.; Allore, H.G.; Barkema, H.W.; Sampimon, O.C.; Wellenberg, G.J.; Gröhn, Y.T.; Schukken, Y.H. Cow- and Quarter-Level Risk Factors for Streptococcus Uberis and Staphylococcus Aureus Mastitis. J. Dairy Sci. 2001, 84, 2649–2663. [Google Scholar] [CrossRef]
- De Vries, A. Economic Trade-Offs between Genetic Improvement and Longevity in Dairy Cattle. J. Dairy Sci. 2017, 100, 4184–4192. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, A.; Frago, F.; Shafii, B.; Dalton, J.C.; Price, W.J.; McGuire, M.A. Effect of Clinical Mastitis and Other Diseases on Reproductive Performance of Holstein Cows. Anim. Reprod. Sci. 2009, 112, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.R.; Schrick, F.N.; Lewis, M.J.; Dowlen, H.H.; Oliver, S.P. Influence of Clinical Mastitis During Early Lactation on Reproductive Performance of Jersey Cows. J. Dairy Sci. 1998, 81, 1285–1290. [Google Scholar] [CrossRef]
- Schrick, F.N.; Hockett, M.E.; Saxton, A.M.; Lewis, M.J.; Dowlen, H.H.; Oliver, S.P. Influence of Subclinical Mastitis During Early Lactation on Reproductive Parameters. J. Dairy Sci. 2001, 84, 1407–1412. [Google Scholar] [CrossRef]
- Fourichon, C.; Seegers, H.; Malher, X. Effect of Disease on Reproduction in the Dairy Cow: A Meta-Analysis. Theriogenology 2000, 53, 1729–1759. [Google Scholar] [CrossRef]
- Dolecheck, K.A.; García-Guerra, A.; Moraes, L.E. Quantifying the Effects of Mastitis on the Reproductive Performance of Dairy Cows: A Meta-Analysis. J. Dairy Sci. 2019, 102, 8454–8477. [Google Scholar] [CrossRef] [PubMed]
- Cameron, R.E.B.; Dyk, P.B.; Herdt, T.H.; Kaneene, J.B.; Miller, R.; Bucholtz, H.F.; Liesman, J.S.; Vandehaar, M.J.; Emery, R.S. Dry Cow Diet, Management, and Energy Balance as Risk Factors for Displaced Abomasum in High Producing Dairy Herds. J. Dairy Sci. 1998, 81, 132–139. [Google Scholar] [CrossRef]
- Moyes, K.M.; Larsen, T.; Friggens, N.C.; Drackley, J.K.; Ingvartsen, K.L. Identification of Potential Markers in Blood for the Development of Subclinical and Clinical Mastitis in Dairy Cattle at Parturition and during Early Lactation. J. Dairy Sci. 2009, 92, 5419–5428. [Google Scholar] [CrossRef] [PubMed]
- Zarrin, M.; Wellnitz, O.; van Dorland, H.A.; Bruckmaier, R.M. Induced Hyperketonemia Affects the Mammary Immune Response during Lipopolysaccharide Challenge in Dairy Cows. J. Dairy Sci. 2014, 97, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Wellnitz, O.; Bruckmaier, R.M. The Innate Immune Response of the Bovine Mammary Gland to Bacterial Infection. Vet. J. 2012, 192, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Waldron, M.R.; Kulick, A.E.; Bell, A.W.; Overton, T.R. Acute Experimental Mastitis Is Not Causal Toward the Development of Energy-Related Metabolic Disorders in Early Postpartum Dairy Cows. J. Dairy Sci. 2006, 89, 596–610. [Google Scholar] [CrossRef]
- Vernay, M.C.M.B.; Wellnitz, O.; Kreipe, L.; van Dorland, H.A.; Bruckmaier, R.M. Local and Systemic Response to Intramammary Lipopolysaccharide Challenge during Long-Term Manipulated Plasma Glucose and Insulin Concentrations in Dairy Cows. J. Dairy Sci. 2012, 95, 2540–2549. [Google Scholar] [CrossRef]
- Waldron, M.R.; Nishida, T.; Nonnecke, B.J.; Overton, T.R. Effect of Lipopolysaccharide on Indices of Peripheral and Hepatic Metabolism in Lactating Cows. J. Dairy Sci. 2003, 86, 3447–3459. [Google Scholar] [CrossRef]
- Huszenicza, G.; Jánosi, S.; Gáspárdy, A.; Kulcsár, M. Endocrine Aspects in Pathogenesis of Mastitis in Postpartum Dairy Cows. Anim. Reprod. Sci. 2004, 82–83, 389–400. [Google Scholar] [CrossRef]
- Ametaj, B.N.; Zebeli, Q.; Iqbal, S. Nutrition, Microbiota, and Endotoxin-Related Diseases in Dairy Cows. Rev. Bras. Zootec. 2010, 39, 433–444. [Google Scholar] [CrossRef]
- Viguier, C.; Arora, S.; Gilmartin, N.; Welbeck, K.; O’Kennedy, R. Mastitis Detection: Current Trends and Future Perspectives. Trends Biotechnol. 2009, 27, 486–493. [Google Scholar] [CrossRef]
- Koeck, A.; Miglior, F.; Kelton, D.F.; Schenkel, F.S. Alternative Somatic Cell Count Traits to Improve Mastitis Resistance in Canadian Holsteins. J. Dairy Sci. 2012, 95, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.A.; Hillerton, J.E. The Effect of Selective Dry Cow Treatment on New Intramammary Infections. J. Dairy Sci. 2002, 85, 112–121. [Google Scholar] [CrossRef]
- Beli, E. Evaluation Of Albanian Raw Milk Quality Situation By Using Somatic Cell Counts. J. Multidiscip. Eng. Sci. Technol. (JMEST) 2016, 3, 5971–5974. [Google Scholar]
- Vissio, C.; Dieser, S.A.; Agnelli, H.L.; Odierno, L.M.; Larriestra, A.J. Accuracy of the Composite Somatic Cell Count to Detect Intra-Mammary Infection in Dairy Cows Using Latent Class Analysis. Prev. Vet. Med. 2014, 113, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, P.L.; Pantoja, J.C.F. Understanding and Using Somatic Cell Counts to Improve Milk Quality. Ir. J. Agric. Food Res. 2013, 52, 101–117. [Google Scholar]
- Pilla, R.; Schwarz, D.; König, S.; Piccinini, R. Microscopic Differential Cell Counting to Identify Inflammatory Reactions in Dairy Cow Quarter Milk Samples. J. Dairy Sci. 2012, 95, 4410–4420. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, T. Potential for Broad Applications of Flow Cytometry and Fluorescence Techniques in Microbiological and Somatic Cell Analyses of Milk. Int. J. Food Microbiol. 2003, 85, 269–279. [Google Scholar] [CrossRef]
- Dufour, S.; Fréchette, A.; Barkema, H.W.; Mussell, A.; Scholl, D.T. Invited Review: Effect of Udder Health Management Practices on Herd Somatic Cell Count. J. Dairy Sci. 2011, 94, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Bauman, C.A.; Barkema, H.W.; Dubuc, J.; Keefe, G.P.; Kelton, D.F. Canadian National Dairy Study: Herd-Level Milk Quality. J. Dairy Sci. 2018, 101, 2679–2691. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.; Wall, S.; Stephan, R.; Corti, S.; Bruckmaier, R. Milk Somatic Cell Count, Lactate Dehydrogenase Activity, and Immunoglobulin G Concentration Associated with Mastitis Caused by Different Pathogens: A Field Study. SAT 2017, 159, 283–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hope, A. Laboratory Handbook on Bovine Mastitis. Aust. Vet. J. 2000, 78, 488. [Google Scholar] [CrossRef]
- Murphy, S.C.; Martin, N.H.; Barbano, D.M.; Wiedmann, M. Influence of Raw Milk Quality on Processed Dairy Products: How Do Raw Milk Quality Test Results Relate to Product Quality and Yield? J. Dairy Sci. 2016, 99, 10128–10149. [Google Scholar] [CrossRef] [PubMed]
- Klaas, I.C.; Zadoks, R.N. An Update on Environmental Mastitis: Challenging Perceptions. Transbound. Emerg. Dis. 2018, 65, 166–185. [Google Scholar] [CrossRef]
- Diseases of the Mammary Gland. In Veterinary Medicine; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1904–2001. [CrossRef]
- Ashraf, A.; Imran, M. Diagnosis of Bovine Mastitis: From Laboratory to Farm. Trop. Anim. Health Prod. 2018, 50, 1193–1202. [Google Scholar] [CrossRef]
- Bexiga, R.; Koskinen, M.T.; Holopainen, J.; Carneiro, C.; Pereira, H.; Ellis, K.A.; Vilela, C.L. Diagnosis of Intramammary Infection in Samples Yielding Negative Results or Minor Pathogens in Conventional Bacterial Culturing. J. Dairy Res. 2011, 78, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Lui, C.; Cady, N.; Batt, C. Nucleic Acid-Based Detection of Bacterial Pathogens Using Integrated Microfluidic Platform Systems. Sensors 2009, 9, 3713–3744. [Google Scholar] [CrossRef]
- Perreten, V.; Endimiani, A.; Thomann, A.; Wipf, J.R.K.; Rossano, A.; Bodmer, M.; Raemy, A.; Sannes-Lowery, K.A.; Ecker, D.J.; Sampath, R.; et al. Evaluation of PCR Electrospray-Ionization Mass Spectrometry for Rapid Molecular Diagnosis of Bovine Mastitis. J. Dairy Sci. 2013, 96, 3611–3620. [Google Scholar] [CrossRef]
- Lange, C.C.; Brito, M.A.V.P.; Reis, D.R.L.; Machado, M.A.; Guimarães, A.S.; Azevedo, A.L.S.; Salles, É.B.; Alvim, M.C.T.; Silva, F.S.; Meurer, I.R. Species-Level Identification of Staphylococci Isolated from Bovine Mastitis in Brazil Using Partial 16S RRNA Sequencing. Vet. Microbiol. 2015, 176, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Cantekïn, Z.; Ergün, Y.; Doğruer, G.; Saribay, M.K.; Solmaz, H. Süt Sığırlarında Sub-Klinik Mastitisin Tanısında Kültür ve PCR Yöntemlerinin Karşılaştırılması. Kafkas Univ. Vet. Fak. Derg. 2015, 21, 277–282. [Google Scholar] [CrossRef]
- Graber, H.U.; Casey, M.G.; Naskova, J.; Steiner, A.; Schaeren, W. Development of a Highly Sensitive and Specific Assay to Detect Staphylococcus Aureus in Bovine Mastitic Milk. J. Dairy Sci. 2007, 90, 4661–4669. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, P.; Zhou, S.; Zhang, L. Loop-Mediated Isothermal Amplification (LAMP): A Novel Rapid Detection Platform for Pathogens. Microb. Pathog. 2017, 107, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Anis, E.; Hawkins, I.K.; Ilha, M.R.S.; Woldemeskel, M.W.; Saliki, J.T.; Wilkes, R.P. Evaluation of Targeted Next-Generation Sequencing for Detection of Bovine Pathogens in Clinical Samples. J. Clin. Microbiol. 2018, 56, e00399-18. [Google Scholar] [CrossRef]
- Gonçalves, J.L.; Tomazi, T.; Barreiro, J.R.; de Campos Braga, P.A.; Ferreira, C.R.; Araújo Junior, J.P.; Eberlin, M.N.; dos Santos, M.V. Identification of Corynebacterium Spp. Isolated from Bovine Intramammary Infections by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. Vet. Microbiol. 2014, 173, 147–151. [Google Scholar] [CrossRef]
- Cameron, M.; Barkema, H.W.; De Buck, J.; De Vliegher, S.; Chaffer, M.; Lewis, J.; Keefe, G.P. Identification of Bovine-Associated Coagulase-Negative Staphylococci by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry Using a Direct Transfer Protocol. J. Dairy Sci. 2017, 100, 2137–2147. [Google Scholar] [CrossRef]
- Barreiro, J.R.; Gonçalves, J.L.; Braga, P.A.C.; Dibbern, A.G.; Eberlin, M.N.; Veiga dos Santos, M. Non-Culture-Based Identification of Mastitis-Causing Bacteria by MALDI-TOF Mass Spectrometry. J. Dairy Sci. 2017, 100, 2928–2934. [Google Scholar] [CrossRef]
- Schalm, O.W.; Noorlander, D.O. Experiments and Observations Leading to Development of the California Mastitis Test. J. Am. Vet. Med. Assoc. 1957, 130, 199–204. [Google Scholar]
- Sargeant, J.M.; Leslie, K.E.; Shirley, J.E.; Pulkrabek, B.J.; Lim, G.H. Sensitivity and Specificity of Somatic Cell Count and California Mastitis Test for Identifying Intramammary Infection in Early Lactation. J. Dairy Sci. 2001, 84, 2018–2024. [Google Scholar] [CrossRef]
- Gordon, W.A.; Morris, H.A.; Packard, V. Methods to Detect Abnormal Milk—A Review. J. Food Prot. 1980, 43, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Pyörälä, S. Indicators of Inflammation in the Diagnosis of Mastitis. Vet. Res. 2003, 34, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Hovinen, M.; Pyörälä, S. Invited Review: Udder Health of Dairy Cows in Automatic Milking. J. Dairy Sci. 2011, 94, 547–562. [Google Scholar] [CrossRef] [PubMed]
- John, A.J.; Garcia, S.C.; Kerrisk, K.L.; Freeman, M.J.; Islam, M.R.; Clark, C.E.F. Short Communication: The Diurnal Intake and Behavior of Dairy Cows When Access to a Feed of Consistent Nutritive Value Is Restricted. J. Dairy Sci. 2017, 100, 9279–9284. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.; Clark, C.E.F.; Lyons, N.A.; Thomson, P.C.; Kerrisk, K.L.; García, S.C. Early Detection of Clinical Mastitis from Electrical Conductivity Data in an Automatic Milking System. Anim. Prod. Sci. 2017, 57, 1226. [Google Scholar] [CrossRef]
- Bruckmaier, R.M.; Blum, J.W. Oxytocin Release and Milk Removal in Ruminants. J. Dairy Sci. 1998, 81, 939–949. [Google Scholar] [CrossRef]
- Lehmann, M.; Wall, S.K.; Wellnitz, O.; Bruckmaier, R.M. Changes in Milk L-Lactate, Lactate Dehydrogenase, Serum Albumin, and IgG during Milk Ejection and Their Association with Somatic Cell Count. J. Dairy Sci. 2015, 82, 129–134. [Google Scholar] [CrossRef]
- Bruckmaier, R.M.; Weiss, D.; Wiedemann, M.; Schmitz, S.; Wendl, G. Changes of Physicochemical Indicators during Mastitis and the Effects of Milk Ejection on Their Sensitivity. J. Dairy Sci. 2004, 71, 316–321. [Google Scholar] [CrossRef]
- Bansal, B.K.; Hamann, J.; Grabowski, N.T.; Singh, K.B. Variation in the Composition of Selected Milk Fraction Samples from Healthy and Mastitic Quarters, and Its Significance for Mastitis Diagnosis. J. Dairy Sci. 2005, 72, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.; Bruckmaier, R.M.; Thomson, P.C.; House, J.; García, S.C. Suitability of Somatic Cell Count, Electrical Conductivity, and Lactate Dehydrogenase Activity in Foremilk before versus after Alveolar Milk Ejection for Mastitis Detection. J. Dairy Sci. 2019, 102, 9200–9212. [Google Scholar] [CrossRef]
- Royster, E.; Godden, S.; Goulart, D.; Dahlke, A.; Rapnicki, P.; Timmerman, J. Evaluation of the Minnesota Easy Culture System II Bi-Plate and Tri-Plate for Identification of Common Mastitis Pathogens in Milk. J. Dairy Sci. 2014, 97, 3648–3659. [Google Scholar] [CrossRef] [PubMed]
- Dohoo, I.R.; Smith, J.; Andersen, S.; Kelton, D.F.; Godden, S. Diagnosing Intramammary Infections: Evaluation of Definitions Based on a Single Milk Sample. J. Dairy Sci. 2011, 94, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, S.A.; Megahed, A.A.; Ebeid, M.H.; Constable, P.D. Ability of Milk PH to Predict Subclinical Mastitis and Intramammary Infection in Quarters from Lactating Dairy Cattle. J. Dairy Sci. 2019, 102, 1417–1427. [Google Scholar] [CrossRef]
- Boyd-Moss, M.; Baratchi, S.; Di Venere, M.; Khoshmanesh, K. Self-Contained Microfluidic Systems: A Review. Lab Chip 2016, 16, 3177–3192. [Google Scholar] [CrossRef]
- Sang, S.; Zhang, W.; Zhao, Y. Review on the Design Art of Biosensors. In State of the Art in Biosensors-General Aspects; Rinken, T., Ed.; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Ruegg, P.L. A 100-Year Review: Mastitis Detection, Management, and Prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed]
- Luedecke, L.O.; Forster, T.L.; Ashworth, U.S. Relationship Between California Mastitis Test Reaction and Leucocyte Count, Catalase Activity, and A-Esterase Activity of Milk from Opposite Quarters. J. Dairy Sci. 1967, 50, 1592–1596. [Google Scholar] [CrossRef]
- Lam, T.; Olde Riekerink, R.; Sampimon, O.; Smith, H. Mastitis Diagnostics and Performance Monitoring: A Practical Approach. Ir. Vet. J. 2009, 62, S34. [Google Scholar] [CrossRef] [PubMed]
- Dingwell, R.T.; Leslie, K.E.; Schukken, Y.H.; Sargeant, J.M.; Timms, L.L. Evaluation of the California Mastitis Test to Detect an Intramammary Infection with a Major Pathogen in Early Lactation Dairy Cows. Can. Vet. J. 2003, 44, 413–415. [Google Scholar] [PubMed]
- Rossi, R.S.; Amarante, A.F.; Correia, L.B.N.; Guerra, S.T.; Nobrega, D.B.; Latosinski, G.S.; Rossi, B.F.; Rall, V.L.M.; Pantoja, J.C.F. Diagnostic Accuracy of Somaticell, California Mastitis Test, and Microbiological Examination of Composite Milk to Detect Streptococcus Agalactiae Intramammary Infections. J. Dairy Sci. 2018, 101, 10220–10229. [Google Scholar] [CrossRef] [PubMed]
- Labohm, R.; Götz, E.; Luhofer, G.; Hess, R.G.; Bostedt, H. Factors Influencing the Somatic Milk-Cell-Count in Dairy Cows. 1. Influence of Bacteriological Findings, Stage and Number of Lactation. Milchwissenschaft 1998, 53, 63–66. [Google Scholar]
- Hillerton, E. Detecting Mastitis Cow-Side. In Proceedings of the National Mastitis Council Annual Meeting Proceedings (2000), Atlanta, GA, USA, 13–16 February 2000; pp. 48–53. [Google Scholar]
- Leslie, K.; Barratt, K.; Petersson, C.; Bashiri, A. An Evaluation of the Portascc® Test as a Measure of Udder Health Status Dairy Cows (an Excerpt From a Technical Report). Viewed 2015, 20, 2060–2061. [Google Scholar]
- Ferronatto, J.A.; Ferronatto, T.C.; Schneider, M.; Pessoa, L.F.; Blagitz, M.G.; Heinemann, M.B.; Della Libera, A.M.M.P.; Souza, F.N. Diagnosing Mastitis in Early Lactation: Use of Somaticell®, California Mastitis Test and Somatic Cell Count. Ital. J. Anim. Sci. 2018, 17, 723–729. [Google Scholar] [CrossRef]
- Olde Riekerink, R.G.M.; Barkema, H.W.; Veenstra, W.; Berg, F.E.; Stryhn, H.; Zadoks, R.N. Somatic Cell Count During and Between Milkings. J. Dairy Sci. 2007, 90, 3733–3741. [Google Scholar] [CrossRef] [PubMed]
- Petzer, I.-M.; Karzis, J.; Donkin, E.F.; Webb, E.C.; Etter, E.M.C. Somatic Cell Count Thresholds in Composite and Quarter Milk Samples as Indicator of Bovine Intramammary Infection Status. Onderstepoort J. Vet. Res. 2017, 84, a1269. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Odhiambo, J.F.; Farooq, U.; Lam, T.; Dunn, S.M.; Ametaj, B.N. Intravaginal Probiotics Modulated Metabolic Status and Improved Milk Production and Composition of Transition Dairy Cows. J. Anim. Sci. 2016, 94, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Jiminez, J.A.; Uwiera, T.C.; Abbott, D.W.; Uwiera, R.R.E.; Inglis, G.D. Butyrate Supplementation at High Concentrations Alters Enteric Bacterial Communities and Reduces Intestinal Inflammation in Mice Infected with Citrobacter Rodentium. mSphere 2017, 2, e00243-17. [Google Scholar] [CrossRef]
- Tarsillo, B.; Priefer, R. Proteobiotics as a New Antimicrobial Therapy. Microb. Pathog. 2020, 142, 104093. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dhama, K.; Tiwari, R.; Iqbal Yatoo, M.; Khurana, S.K.; Khandia, R.; Munjal, A.; Munuswamy, P.; Kumar, M.A.; Singh, M.; et al. Technological Interventions and Advances in the Diagnosis of Intramammary Infections in Animals with Emphasis on Bovine Population—A Review. Vet. Q. 2019, 39, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Dobson, H.; Smith, R.; Royal, M.; Knight, C.; Sheldon, I. The High-Producing Dairy Cow and Its Reproductive Performance. Reprod. Domest. Anim. 2007, 42, 17–23. [Google Scholar] [CrossRef]
- Sundrum, A. Metabolic Disorders in the Transition Period Indicate That the Dairy Cows’ Ability to Adapt Is Overstressed. Animals 2015, 5, 978–1020. [Google Scholar] [CrossRef]
- Drackley, J.K. Biology of Dairy Cows During the Transition Period: The Final Frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Raphael, W. Significance of Metabolic Stress, Lipid Mobilization, and Inflammation on Transition Cow Disorders. Vet. Clin. N. Am.-Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef] [PubMed]
- David Baird, G. Primary Ketosis in the High-Producing Dairy Cow: Clinical and Subclinical Disorders, Treatment, Prevention, and Outlook. J. Dairy Sci. 1982, 65, 1–10. [Google Scholar] [CrossRef]
- Brunner, N.; Groeger, S.; Canelas Raposo, J.; Bruckmaier, R.M.; Gross, J.J. Prevalence of Subclinical Ketosis and Production Diseases in Dairy Cows in Central and South America, Africa, Asia, Australia, New Zealand, and Eastern Europe1. Transl. Anim. Sci. 2019, 3, 84–92. [Google Scholar] [CrossRef]
- Serkova, N.J.; Niemann, C.U. Pattern Recognition and Biomarker Validation Using Quantitative 1 H-NMR-Based Metabolomics. Expert Rev. Mol. Diagn. 2006, 6, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Dervishi, E.; Ametaj, B.N. Milk Fever in Dairy Cows Is Preceded by Activation of Innate Immunity and Alterations in Carbohydrate Metabolism Prior to Disease Occurrence. Res. Vet. Sci. 2018, 117, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Thammavongsa, V.; Kim, H.K.; Missiakas, D.; Schneewind, O. Staphylococcal Manipulation of Host Immune Responses. Nat. Rev. Microbiol. 2015, 13, 529–543. [Google Scholar] [CrossRef]
- Do Vale, A.; Cabanes, D.; Sousa, S. Bacterial Toxins as Pathogen Weapons Against Phagocytes. Front. Microbiol. 2016, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Loughman, J.A.; Hunstad, D.A. Attenuation of Human Neutrophil Migration and Function by Uropathogenic Bacteria. Microbes Infect. 2011, 13, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.E.; Loughman, J.A.; Hunstad, D.A. YbcL of Uropathogenic Escherichia Coli Suppresses Transepithelial Neutrophil Migration. Infect. Immun. 2012, 80, 4123–4132. [Google Scholar] [CrossRef] [PubMed]
- Hailemariam, D.; Mandal, R.; Saleem, F.; Dunn, S.M.; Wishart, D.S.; Ametaj, B.N. Identification of Predictive Biomarkers of Disease State in Transition Dairy Cows. J. Dairy Sci. 2014, 97, 2680–2693. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.Z.; Shen, L.H.; Jiang, J.; Huang, Y.X.; Bai, L.P.; Yu, S.M.; Yao, X.P.; Ren, Z.H.; Yang, Y.X.; Cao, S.Z. Plasma Metabolite Changes in Dairy Cows during Parturition Identified Using Untargeted Metabolomics. J. Dairy Sci. 2019, 102, 4639–4650. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, C.; Xia, C.; Zhang, H.; Sun, L.; Gao, Y. Plasma Metabolic Profiling of Dairy Cows Affected with Clinical Ketosis Using LC/MS Technology. Vet. Q. 2014, 34, 152–158. [Google Scholar] [CrossRef]
- Sun, L.W.; Zhang, H.Y.; Wu, L.; Shu, S.; Xia, C.; Xu, C.; Zheng, J.S. 1H-Nuclear Magnetic Resonance-Based Plasma Metabolic Profiling of Dairy Cows with Clinical and Subclinical Ketosis. J. Dairy Sci. 2014, 97, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Shu, S.; Xia, C.; Wang, P.; Sun, Y.; Xu, C.; Li, C. Mass Spectral Analysis of Urine Proteomic Profiles of Dairy Cows Suffering from Clinical Ketosis. Vet. Q. 2015, 35, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Deng, Q.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. DI/LC-MS/MS-Based Metabolic Profiling for Identification of Early Predictive Serum Biomarkers of Metritis in Transition Dairy Cows. J. Agric. Food Chem. 2017, 65, 8510–8521. [Google Scholar] [CrossRef]
- Zhang, G.; Dervishi, E.; Dunn, S.M.; Mandal, R.; Liu, P.; Han, B.; Wishart, D.S.; Ametaj, B.N. Metabotyping Reveals Distinct Metabolic Alterations in Ketotic Cows and Identifies Early Predictive Serum Biomarkers for the Risk of Disease. Metabolomics 2017, 13, 43. [Google Scholar] [CrossRef]
- Klein, M.S.; Almstetter, M.F.; Schlamberger, G.; Nürnberger, N.; Dettmer, K.; Oefner, P.J.; Meyer, H.H.D.; Wiedemann, S.; Gronwald, W. Nuclear Magnetic Resonance and Mass Spectrometry-Based Milk Metabolomics in Dairy Cows during Early and Late Lactation. J. Dairy Sci. 2010, 93, 1539–1550. [Google Scholar] [CrossRef]
- Klein, M.S.; Buttchereit, N.; Miemczyk, S.P.; Immervoll, A.-K.; Louis, C.; Wiedemann, S.; Junge, W.; Thaller, G.; Oefner, P.J.; Gronwald, W. NMR Metabolomic Analysis of Dairy Cows Reveals Milk Glycerophosphocholine to Phosphocholine Ratio as Prognostic Biomarker for Risk of Ketosis. J. Proteome Res. 2012, 11, 1373–1381. [Google Scholar] [CrossRef]
- Zhang, G.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. A Multi-Platform Metabolomics Approach Identifies Urinary Metabolite Signatures That Differentiate Ketotic From Healthy Dairy Cows. Front. Vet. Sci. 2021, 8, 595983. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, L.; Shu, S.; Zhu, K.; Xu, C.; Wang, J.; Wang, H. Nuclear Magnetic Resonance-Based Serum Metabolic Profiling of Dairy Cows with Footrot. J. Vet. Med. Sci. 2016, 78, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Dervishi, E.; Zhang, G.; Zwierzchowski, G.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. Serum Metabolic Fingerprinting of Pre-Lameness Dairy Cows by GC–MS Reveals Typical Profiles That Can Identify Susceptible Cows. J. Proteom. 2020, 213, 103620. [Google Scholar] [CrossRef] [PubMed]
- Haxhiaj, K.; Li, Z.; Johnson, M.; Dunn, S.M.; Wishart, D.S.; Ametaj, B.N. Blood Metabolomic Phenotyping of Dry Cows Could Predict the High Milk Somatic Cells in Early Lactation—Preliminary Results. Dairy 2022, 3, 5. [Google Scholar] [CrossRef]
- Zhang, G.; Dervishi, E.; Zwierzchowski, G.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. Urinary Metabolomics around Parturition Identifies Metabolite Alterations in Dairy Cows Affected Postpartum by Lameness: Preliminary Study. Dairy 2020, 1, 2. [Google Scholar] [CrossRef]
- Zwierzchowski, G.; Zhang, G.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. Milk Metabotyping Identifies Metabolite Alterations in the Whole Raw Milk of Dairy Cows with Lameness. J. Agric. Food Chem. 2020, 68, 4507–4514. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, Å.; Persson Waller, K.; Svennersten-Sjaunja, K.; Haugen, J.-E.; Lundby, F.; Lind, O. Detection of Mastitic Milk Using a Gas-Sensor Array System (Electronic Nose). Int. Dairy J. 2005, 15, 1193–1201. [Google Scholar] [CrossRef]
- Hettinga, K.A.; van Valenberg, H.J.F.; Lam, T.J.G.M.; van Hooijdonk, A.C.M. Detection of Mastitis Pathogens by Analysis of Volatile Bacterial Metabolites. J. Dairy Sci. 2008, 91, 3834–3839. [Google Scholar] [CrossRef]
- Hettinga, K.A.; van Valenberg, H.J.F.; Lam, T.J.G.M.; van Hooijdonk, A.C.M. The Origin of the Volatile Metabolites Found in Mastitis Milk. Vet. Microbiol. 2009, 137, 384–387. [Google Scholar] [CrossRef]
- Sundekilde, U.K.; Poulsen, N.A.; Larsen, L.B.; Bertram, H.C. Nuclear Magnetic Resonance Metabonomics Reveals Strong Association between Milk Metabolites and Somatic Cell Count in Bovine Milk. J. Dairy Sci. 2013, 96, 290–299. [Google Scholar] [CrossRef]
- Ryman, V.E.; Pighetti, G.M.; Lippolis, J.D.; Gandy, J.C.; Applegate, C.M.; Sordillo, L.M. Quantification of Bovine Oxylipids during Intramammary Streptococcus Uberis Infection. Prostaglandins Other Lipid Mediat. 2015, 121, 207–217. [Google Scholar] [CrossRef]
- Giri, S.N.; Chen, Z.; Carroll, E.J.; Mueller, R.; Schiedt, M.J.; Panico, L. Role of Prostaglandins in Pathogenesis of Bovine Mastitis Induced by Escherichia Coli Endotoxin. Am. J. Vet. Res. 1984, 45, 586–591. [Google Scholar] [PubMed]
- Atroshi, F.; Työppönen, J.; Sankari, S.; Kangasniemi, R.; Parantainen, J. Possible Roles of Vitamin E and Glutathione Metabolism in Bovine Mastitis. Int. J. Vitam. Nutr. Res. 1987, 57, 37–43. [Google Scholar] [PubMed]
- Aitken, S.L.; Corl, C.M.; Sordillo, L.M. Immunopathology of Mastitis: Insights into Disease Recognition and Resolution. J. Mammary Gland. Biol. Neoplasia 2011, 16, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.C.; Mudaliar, M.; Tassi, R.; McNeilly, T.N.; Burchmore, R.; Burgess, K.; Herzyk, P.; Zadoks, R.N.; Eckersall, P.D. Mastitomics, the Integrated Omics of Bovine Milk in an Experimental Model of Streptococcus Uberis Mastitis: 3. Untargeted Metabolomics. Mol. BioSyst. 2016, 12, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F.; Eckmann, L. How Bile Acids Confer Gut Mucosal Protection against Bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 4333–4334. [Google Scholar] [CrossRef]
- Calmus, Y.; Poupon, R. Shaping Macrophages Function and Innate Immunity by Bile Acids: Mechanisms and Implication in Cholestatic Liver Diseases. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xie, W.; Krasowski, M.D. PXR: A Xenobiotic Receptor of Diverse Function Implicated in Pharmacogenetics. Pharmacogenomics 2008, 9, 1695–1709. [Google Scholar] [CrossRef]
- Sipka, S.; Bruckner, G. The Immunomodulatory Role of Bile Acids. Int. Arch. Allergy Immunol. 2014, 165, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dervishi, E.; Zhang, G.; Dunn, S.M.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. GC–MS Metabolomics Identifies Metabolite Alterations That Precede Subclinical Mastitis in the Blood of Transition Dairy Cows. J. Proteome Res. 2017, 16, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Zandkarimi, F.; Vanegas, J.; Fern, X.; Maier, C.S.; Bobe, G. Metabotypes with Elevated Protein and Lipid Catabolism and Inflammation Precede Clinical Mastitis in Prepartal Transition Dairy Cows. J. Dairy Sci. 2018, 101, 5531–5548. [Google Scholar] [CrossRef] [PubMed]
- Ten Bruggencate, S.J.; Bovee-Oudenhoven, I.M.; Feitsma, A.L.; van Hoffen, E.; Schoterman, M.H. Functional Role and Mechanisms of Sialyllactose and Other Sialylated Milk Oligosaccharides. Nutr. Rev. 2014, 72, 377–389. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawase, H.; Kimura, K.; Watanabe, Y.; Ohtani, M.; Arai, I.; Urashima, T. Concentrations of Sialyloligosaccharides in Bovine Colostrum and Milk during the Prepartum and Early Lactation. J. Dairy Sci. 2003, 86, 1315–1320. [Google Scholar] [CrossRef]
- Hamann, J.; Krömker, V. Potential of Specific Milk Composition Variables for Cow Health Management. Livest. Prod. Sci. 1997, 48, 201–208. [Google Scholar] [CrossRef]
- Davis, S.R.; Farr, V.C.; Prosser, C.G.; Nicholas, G.D.; Turner, S.-A.; Lee, J.; Hart, A.L. Milk L-Lactate Concentration Is Increased during Mastitis. J. Dairy Res. 2004, 71, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Welderufael, B.G.; Løvendahl, P.; de Koning, D.-J.; Janss, L.L.G.; Fikse, W.F. Genome-Wide Association Study for Susceptibility to and Recoverability From Mastitis in Danish Holstein Cows. Front. Genet. 2018, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Gröhn, Y.T.; Wilson, D.J.; González, R.N.; Hertl, J.A.; Schulte, H.; Bennett, G.; Schukken, Y.H. Effect of Pathogen-Specific Clinical Mastitis on Milk Yield in Dairy Cows. J. Dairy Sci. 2004, 87, 3358–3374. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).