Milk Coagulation Properties: A Study on Milk Protein Profile of Native and Improved Cattle Breeds/Types in Sri Lanka
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Analysis of Milk Coagulation Properties
2.2.1. Enzymatic Coagulation
2.2.2. Acid-Induced Coagulation Using Lactic Acid Bacteria (LAB)
2.2.3. Analysis of Milk Coagulation Properties
2.3. Analysis of Milk Protein Profile
2.4. Analysis of Ca Content of Milk
2.5. Analysis of Somatic Cell Score (SCS)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Milk Coagulation Property (MCP) Analysis
3.2. Correlation Analysis
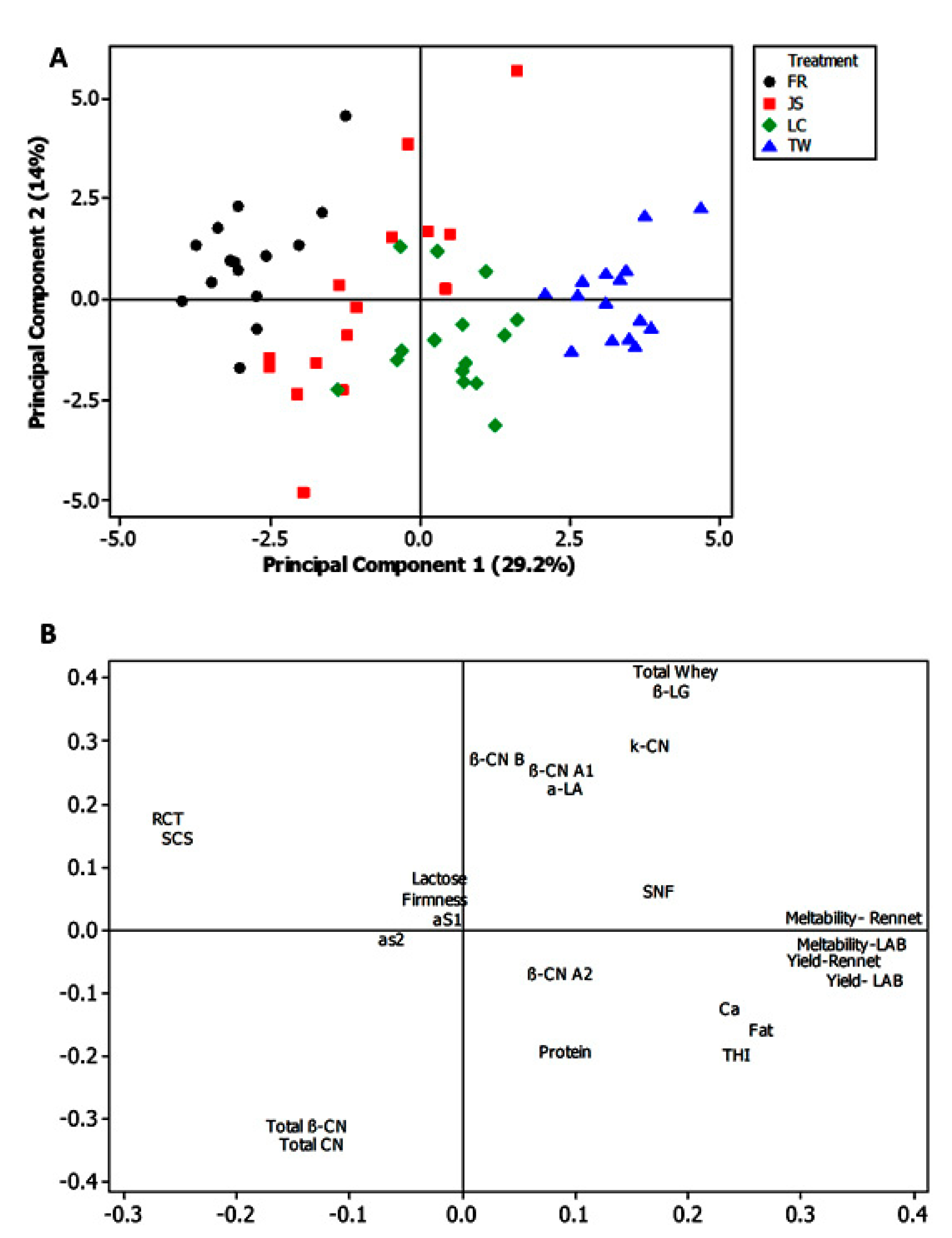
3.3. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beux, S.; Pereira, E.A.; Cassandro, M.; Nogueira, A.; Waszczynskyj, N. Milk coagulation properties and methods of detection. Cienc. Rural 2017, 47, e20161042. [Google Scholar] [CrossRef][Green Version]
- Ikonen, T.; Ojala, M.; Ruottinen, O. Associations between milk protein polymorphism and first lactation milk production traits in Finnish Ayrshire cows. J. Dairy Sci. 1999, 82, 1026–1033. [Google Scholar] [CrossRef]
- Joudu, I.; Henno, M.; Kaart, T.; Püssa, T.; Kärt, O. The effect of milk protein contents on the rennet coagulation properties of milk from individual dairy cows. Int. Dairy J. 2008, 18, 964–967. [Google Scholar] [CrossRef]
- Poulsen, N.A.; Bertelsen, H.; Jensen, H.; Gustavsson, F.; Glantz, M.; Månsson, H.L.; Andrén, A.; Paulsson, M.; Bendixen, C.; Buitenhuis, A. The occurrence of noncoagulating milk and the association of bovine milk coagulation properties with genetic variants of the caseins in 3 Scandinavian dairy breeds. J. Dairy Sci. 2013, 96, 4830–4842. [Google Scholar] [CrossRef]
- Hallén, E.; Allmere, T.; Lundén, A.; Andrén, A. Effect of genetic polymorphism of milk proteins on rheology of acid-induced milk gels. Int. Dairy J. 2009, 19, 399–404. [Google Scholar] [CrossRef]
- Wedholm, A.; Larsen, L.; Lindmark-Månsson, H.; Karlsson, A.; Andrén, A. Effect of protein composition on the cheese-making properties of milk from individual dairy cows. J. Dairy Sci. 2006, 89, 3296–3305. [Google Scholar] [CrossRef]
- De Marchi, M.; Dal Zotto, R.; Cassandro, M.; Bittante, G. Milk coagulation ability of five dairy cattle breeds. Int. J. Dairy Sci. 2007, 90, 3986–3992. [Google Scholar] [CrossRef]
- Tyrisevä, A.-M.; Ikonen, T.; Ojala, M. Repeatability estimates for milk coagulation traits and non-coagulation of milk in Finnish Ayrshire cows. J. Dairy Res. 2003, 70, 91–98. [Google Scholar] [CrossRef]
- Poulsen, N.A.; Glantz, M.; Rosengaard, A.K.; Paulsson, M.; Larsen, L.B. Comparison of milk protein composition and rennet coagulation properties in native Swedish dairy cow breeds and high-yielding Swedish Red cows. J. Dairy Sci. 2017, 100, 8722–8734. [Google Scholar] [CrossRef]
- Jõudu, I.; Henno, M.; Värv, S.; Viinalass, H.; Püssa, T.; Kaart, T.; Arney, D.; Kärt, O. The effect of milk proteins ok milk coagulation properties in estonian dairy breeds. Vet. ir Zootech. 2009, 46, 14–19. [Google Scholar]
- Pazzola, M.; Vacca, G.M.; Noce, A.; Porcedda, M.; Onnis, M.; Manca, N.; Dettori, M.L. Exploring the Genotype at CSN3 Gene, Milk Composition, Coagulation and Cheese-Yield Traits of the Sardo-Modicana, an Autochthonous Cattle Breed from the Sardinia Region, Italy. Animals 2020, 10, 1995. [Google Scholar] [CrossRef]
- Teter, A.; Kędzierska-Matysek, M.; Barłowska, J.; Król, J.; Brodziak, A. Nutritional value and coagulation properties of milk from local cow breeds, including the selected macro-and micronutrients and trace elements. Mljekarstvo/Dairy 2020, 70, 210–220. [Google Scholar] [CrossRef]
- Bett, R.C.; Okeyo, M.A.; Malmfors, B.; Johansson, K.; Agaba, M.; Kugonza, D.R.; Bhuiyan, A.; Vercesi Filho, A.E.; Mariante, A.S.; Mujibi, F.D. Cattle breeds: Extinction or quasi-extant? Resources 2013, 2, 335–357. [Google Scholar] [CrossRef]
- Weerasingha, W.; Ranadheera, C.; Prasanna, P.; Silva, G.; Vidanarachchi, J. Probiotic Viability and Physicochemical Properties of Set-Yoghurt Made of Native and Improved Cow Milk. Trop. Agric. 2021, 32, 39–48. [Google Scholar] [CrossRef]
- Kübarsepp, I.; Henno, M.; Kärt, O.; Tupasela, T. A comparison of the methods for determination of the rennet coagulation properties of milk. Acta Agric. Scand. A Anim. 2005, 55, 145–148. [Google Scholar] [CrossRef]
- Lóopez, M.; Jordan, M.; Granados, M.; Fernandez, J.; Castillo, M.; Laencina, J. Viscosity changes during rennet coagulation of Murciano-Granadina goat milk. Int. J. Dairy Technol. 1999, 52, 102–106. [Google Scholar] [CrossRef]
- Calvo, M.M.; Balcones, E. Influence of heat treatment on rennet clotting properties of mixtures of cow’s, ewe’s, and goat’s milk and on cheese yield. J. Agric. Food Chem. 1998, 46, 2957–2962. [Google Scholar] [CrossRef]
- Wedholm, A. Variation in Milk Protein Composition and Its Importance for the Quality of Cheese Milk. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2008. Available online: https://pub.epsilon.slu.se/1701/1/AWKappa.pdf (accessed on 5 May 2022).
- Cais-Sokolińska, D.; Pikul, J. Cheese meltability as assessed by the Tube Test and Schreiber Test depending on fat contents and storage time, based on curd-ripened fried cheese. Czech J. Food Sci. 2009, 27, 301–308. [Google Scholar] [CrossRef]
- Johansson, M.; Åkerstedt, M.; Li, S.; Zamaratskaia, G.; Lundh, Å.S. Casein breakdown in bovine milk by a field strain of Staphylococcus aureus. J. Food Prot. 2013, 76, 1638–1642. [Google Scholar] [CrossRef]
- Kira, C.S.; Maihara, V.A. Determination of major and minor elements in dairy products through inductively coupled plasma optical emission spectrometry after wet partial digestion and neutron activation analysis. Food Chem. 2007, 100, 390–395. [Google Scholar] [CrossRef]
- Hallén, E.; Wedholm, A.; Andrén, A.; Lundén, A. Effect of β-casein, κ-casein and β-lactoglobulin genotypes on concentration of milk protein variants. J. Anim. Breed. Genet. 2008, 125, 119–129. [Google Scholar] [CrossRef]
- Islam, M.; Alam, M.; Islam, M.; Khan, M.; Ekeberg, D.; Rukke, E.; Vegarud, G. Principal milk components in buffalo, holstein cross, indigenous cattle and Red Chittagong Cattle from Bangladesh. Asian-Australas. J. Anim. Sci. 2014, 27, 886. [Google Scholar] [CrossRef] [PubMed]
- Niero, G.; Franzoi, M.; Manuelian, C.L.; Visentin, G.; Penasa, M.; De Marchi, M. Protein profile of cow milk from multibreed herds and its relationship with milk coagulation properties. Ital. J. Anim. Sci. 2021, 20, 2232–2242. [Google Scholar] [CrossRef]
- Wedholm, A.; Hallén, E.; Bach Larsen, L.; Lindmark-Månsson, H.; Hans Karlsson, A.; Allmere, T. Comparison of milk protein composition in a Swedish and a Danish dairy herd using reversed phase HPLC. Acta Agric. Scand. A Anim. 2006, 56, 8–15. [Google Scholar] [CrossRef]
- Tyrisevä, A.M.; Vahlsten, T.; Ruottinen, O.; Ojala, M. Milk coagulation ability and prevalence of noncoagulating milk in Finnish dairy cows. In Proceedings of the 7th World Congress on Genetics Applied to Livestock Production, Montpellier, France, 19–23 August 2002; Session 9; Institut National de la Recherche Agronomique (INRA): Paris, France, 2002. [Google Scholar]
- Ikonen, T. Possibilities of Genetic Improvement of Milk Coagulation Properties of Dairy Cows. Ph.D. Thesis, Faculty of Agriculture and Forestry of the University of Helsinki, Helsinki, Finland, 2000. [Google Scholar]
- Comin, A.; Cassandro, M.; Chessa, S.; Ojala, M.; Dal Zotto, R.; De Marchi, M.; Carnier, P.; Gallo, L.; Pagnacco, G.; Bittante, G. Effects of composite β-and κ-casein genotypes on milk coagulation, quality, and yield traits in Italian Holstein cows. Int. J. Dairy Sci. 2008, 91, 4022–4027. [Google Scholar] [CrossRef]
- Muller-renaud, S.; Dupont, D.; Dulieu, P. Quantification of κ-casein in milk by an optical immunosensor. Food Agric. Immunol. 2003, 15, 265–277. [Google Scholar] [CrossRef]
- Gai, N.; Uniacke-Lowe, T.; O’Regan, J.; Faulkner, H.; Kelly, A.L. Effect of Protein Genotypes on Physicochemical Properties and Protein Functionality of Bovine Milk: A Review. Foods 2021, 10, 2409. [Google Scholar] [CrossRef]
- Bohmanova, J.; Misztal, I.; Cole, J. Temperature-humidity indices as indicators of milk production losses due to heat stress. Int. J. Dairy Sci. 2007, 90, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, X.; Li, Z.; Deng, L. Analysis of 17 elements in cow, goat, buffalo, yak, and camel milk by inductively coupled plasma mass spectrometry (ICP-MS). RSC Adv. 2020, 10, 6736–6742. [Google Scholar] [CrossRef]
- Soyeurt, H.; Bruwier, D.; Romnee, J.-M.; Gengler, N.; Bertozzi, C.; Veselko, D.; Dardenne, P. Potential estimation of major mineral contents in cow milk using mid-infrared spectrometry. Int. J. Dairy Sci. 2009, 92, 2444–2454. [Google Scholar] [CrossRef] [PubMed]
- Toffanin, V.; De Marchi, M.; Lopez-Villalobos, N.; Cassandro, M. Effectiveness of mid-infrared spectroscopy for prediction of the contents of calcium and phosphorus, and titratable acidity of milk and their relationship with milk quality and coagulation properties. Int. Dairy J. 2015, 41, 68–73. [Google Scholar] [CrossRef]
- Jensen, H.; Poulsen, N.; Andersen, K.; Hammershøj, M.; Poulsen, H.; Larsen, L. Distinct composition of bovine milk from Jersey and Holstein-Friesian cows with good, poor, or noncoagulation properties as reflected in protein genetic variants and isoforms. Int. J. Dairy Sci. 2012, 95, 6905–6917. [Google Scholar] [CrossRef]
- Malacarne, M.; Franceschi, P.; Formaggioni, P.; Sandri, S.; Mariani, P.; Summer, A. Influence of micellar calcium and phosphorus on rennet coagulation properties of cows milk. J. Dairy Res. 2014, 81, 129–136. [Google Scholar] [CrossRef]
- Abeykoon, C.; Rathnayake, R.; Johansson, M.; Silva, G.; Ranadheera, C.; Lundh, Å.; Vidanarachchi, J. Milk coagulation properties and milk protein genetic variants of three cattle breeds/types in Sri Lanka. Procedia Food Sci. 2016, 6, 348–351. [Google Scholar] [CrossRef]
- Pretto, D.; De Marchi, M.; Penasa, M.; Cassandro, M. Effect of milk composition and coagulation traits on Grana Padano cheese yield under field conditions. J. Dairy Res. 2013, 80, 1–5. [Google Scholar] [CrossRef]
- Formaggioni, P.; Summer, A.; Franceschi, P.; Malacarne, M.; Mariani, P. Cheese yield: Factors of variation and predictive formulas. A review focused particularly on grana type cheeses. Ann. Fac. Med. Vet. Univ. Pisa. 2008, 28, 211–232. [Google Scholar]
- Gustavsson, F.; Glantz, M.; Buitenhuis, A.J.; Lindmark-Månsson, H.; Stålhammar, H.; Andrén, A.; Paulsson, M. Factors influencing chymosin-induced gelation of milk from individual dairy cows: Major effects of casein micelle size and calcium. Int. Dairy J. 2014, 39, 201–208. [Google Scholar] [CrossRef]
- Franceschi, P.; Malacarne, M.; Formaggioni, P.; Faccia, M.; Summer, A. Quantification of the Effect of the Cattle Breed on Milk Cheese Yield: Comparison between Italian Brown Swiss and Italian Friesian. Animals 2020, 10, 1331. [Google Scholar] [CrossRef]
- Van Hekken, D.L.; Tunick, M.H.; Malin, E.L.; Holsinger, V.H. Rheology and melt characterization of low-fat and full fat Mozzarella cheese made from microfluidized milk. LWT 2007, 40, 89–98. [Google Scholar] [CrossRef]
- Ikonen, T.; Morri, S.; Tyrisevä, A.-M.; Ruottinen, O.; Ojala, M. Genetic and phenotypic correlations between milk coagulation properties, milk production traits, somatic cell count, casein content, and pH of milk. Int. J. Dairy Sci. 2004, 87, 458–467. [Google Scholar] [CrossRef]
- Cecchinato, A.; Penasa, M.; Gotet, C.C.; De Marchi, M.; Bittante, G. Factors affecting coagulation properties of Mediterranean buffalo milk. Int. J. Dairy Sci. 2012, 95, 1709–1713. [Google Scholar] [CrossRef] [PubMed]
- Von Oloffs, K.; Schulte-Coerne, H.; Pabst, K.; Gravert, H.J.Z. Die Bedeutung der Proteinvarianten für genetische Unterschiede in der Käsereitauglichkeit der Milch.(The relevance of protein variants to genetic differences in cheese making properties in milk). Zuchtungskunde 1992, 64, 20–26. [Google Scholar]
- Cassandro, M.; Comin, A.; Ojala, M.; Dal Zotto, R.; De Marchi, M.; Gallo, L.; Carnier, P.; Bittante, G. Genetic parameters of milk coagulation properties and their relationships with milk yield and quality traits in Italian Holstein cows. Int. J. Dairy Sci. 2008, 91, 371–376. [Google Scholar] [CrossRef]
- Hallén, E.; Lundén, A.; Tyrisevä, A.-M.; Westerlind, M.; Andrén, A. Composition of poorly and non-coagulating bovine milk and effect of calcium addition. J. Dairy Res. 2010, 77, 398–403. [Google Scholar] [CrossRef]
- Jensen, H.; Holland, J.; Poulsen, N.; Larsen, L. Milk protein genetic variants and isoforms identified in bovine milk representing extremes in coagulation properties. Int. J. Dairy Sci. 2012, 95, 2891–2903. [Google Scholar] [CrossRef]
- Bobbo, T.; Cipolat-Gotet, C.; Bittante, G.; Cecchinato, A. The nonlinear effect of somatic cell count on milk composition, coagulation properties, curd firmness modeling, cheese yield, and curd nutrient recovery. Int. J. Dairy Sci. 2016, 99, 5104–5119. [Google Scholar] [CrossRef] [PubMed]

| Breed/Type | Milk Protein Profile (as % of the Total Protein) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| α-LA | β-LG | αs2-CN | αS1-CN | κ-CN | β-CN B | β-CN A1 | β-CN A2 | Total β-CN | Total Whey Protein | Total CN | |
| Jersey | 0.98 ± 0.4 a | 6.35 ± 2.6 a | 5.44 ± 1.0 a | 32.02 ± 3.8 a | 3.47 ± 1.7 b | 5.07 ± 1.3 a | 18.59 ± 5.8 a | 36.25 ± 8.9 a | 48.75 ± 5.4 a | 7.26 ± 2.8 a | 89.68 ± 4.2 a |
| Friesian | 0.87 ± 0.4 a | 5.15 ± 1.8 a | 5.68 ± 1.7 a | 33.63 ± 3.8 a | 4.88 ± 1.3 ab | 4.43 ± 0.8 a | 20.87 ± 2.4 a | 37.18 ± 9.8 a | 46.58 ± 3.9 a | 6.01 ± 2.0 a | 90.78 ± 2.6 a |
| Lankan Cattle | 0.95 ± 0.4 a | 5.53 ± 0.9 a | 5.61 ± 1.6 a | 34.02 ± 2.2 a | 3.89 ± 1.3 b | 3.93 ± 1.3 a | 17.71 ± 5.7 a | 38.86 ± 7.1 a | 46.33 ± 2.9 a | 6.48 ± 1.2 a | 89.85 ± 2.3 a |
| Thamankaduwa White | 1.04 ± 0.3 a | 6.57 ± 1.3 a | 5.12 ± 1.4 a | 33.36 ± 1.7 a | 6.03 ± 1.8 a | 4.84 ± 1.1 a | 21.73 ± 0.0 a | 40.54 ± 4.9 a | 45.22 ± 2.9 a | 7.61 ± 1.3 a | 89.73 ± 2.1 a |
| Animal Breed/Type | Milk Coagulation Properties | |||||
|---|---|---|---|---|---|---|
| RCT (min) | Firmness (%) | Coagulation Yield (%) | Meltability Ratio | |||
| Rennet | LAB | Rennet | LAB | |||
| Jersey | 3.79 ± 0.9 ab | 56.3 a | 64.9 ± 7.3 bc | 66.6 ± 2.6 c | 1.69 ± 0.2 b | 1.79 ± 0.4 b |
| Friesian | 4.14 ± 0.2 a | 53.8 a | 63.5 ± 4.5 c | 65.2 ± 2.3 b | 1.10 ± 0.0 c | 1.08 ± 0.0 c |
| Lankan Cattle | 3.37 ± 0.3 bc | 61.5 a | 69.7 ± 4.4 b | 70.2 ± 3.8 a | 1.20 ± 0.0 c | 1.20 ± 0.0 c |
| Thamankaduwa White | 3.24 ± 0.3 c | 64.3 a | 78.9 ± 3.2 b | 80.0 ± 2.6 a | 5.26 ± 0.3 a | 5.19 ± 0.3 a |
| Breed/Cattle Type | Fat (%) | Protein (%) | Lactose (%) | Solids Non-Fat (%) |
|---|---|---|---|---|
| Thamankaduwa White | 4.56 ± 0.50 a | 3.20 ± 0.32 a | 4.28 ± 0.18 a | 7.89 ± 0.15 a |
| Lankan | 4.32 ± 1.32 a | 3.14 ± 0.26 a | 4.29 ± 0.25 a | 7.80 ± 0.46 a |
| Jersey | 4.36 ± 0.82 b | 3.18 ± 0.28 a | 4.11 ± 0.79 a | 7.37 ± 1.47 b |
| Friesian | 3.15 ± 1.21 c | 3.06 ± 0.18 a | 4.35 ± 0.39 a | 7.29 ± 0.25 b |
| Coagulation Property | α-LA | β-LG | αs2 CN | αS1 CN | κ-CN | β-CN B | β-CN A1 | β-CN A2 | Total β-CN | Total Whey | Total CN | Total Protein | Total Fat | Ca Content | SCS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RCT | −0.19 | −0.01 | 0.03 | 0.02 | −0.45 ** | −0.15 | −0.40 * | 0.02 | 0.15 | 0.17 | 0.20 | −0.21 | −0.31 * | −0.49 ** | 0.52 ** |
| Firmness | 0.20 | 0.13 | 0.17 | −0.17 | 0.24 | 0.2 | 0.23 | 0.16 | 0.02 | 0.08 | 0.35 * | 0.33 * | 0.16 | 0.24 | −0.15 |
| Coagulation Yield | |||||||||||||||
| Rennet Yield | 0.24 | 0.39 | −0.14 | 0.02 | 0.69 ** | 0.51 ** | 0.37 | 0.15 | −0.22 | −0.40 * | 0.49 ** | 0.44 * | 0.40 * | 0.43 * | −0.43 * |
| LAB yield | 0.27 | 0.34 | −0.21 | 0.09 | 0.77 ** | 0.62 ** | 0.26 | 0.27 | −0.29 | −0.41 * | 0.30 * | 0.33 * | 0.34 * | 0.51 ** | −0.43 * |
| Meltability | |||||||||||||||
| Rennet | 0.20 | 0.41 | −0.10 | 0.01 | 0.46 ** | 0.16 | 0.10 | 0.18 | −0.28 | 0.43 | −0.13 | 0.11 | 0.50 ** | 0.35 * | −0.39 |
| LAB | 0.21 | 0.38 | −0.10 | −0.04 | 0.43 * | 0.18 | 0.03 | 0.18 | −0.23 | 0.40 | −0.14 | 0.16 | 0.41 * | 0.34 * | −0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weerasingha, V.; Priyashantha, H.; Ranadheera, C.S.; Prasanna, P.; Silva, P.; Vidanarachchi, J.K.; Johansson, M. Milk Coagulation Properties: A Study on Milk Protein Profile of Native and Improved Cattle Breeds/Types in Sri Lanka. Dairy 2022, 3, 710-721. https://doi.org/10.3390/dairy3040049
Weerasingha V, Priyashantha H, Ranadheera CS, Prasanna P, Silva P, Vidanarachchi JK, Johansson M. Milk Coagulation Properties: A Study on Milk Protein Profile of Native and Improved Cattle Breeds/Types in Sri Lanka. Dairy. 2022; 3(4):710-721. https://doi.org/10.3390/dairy3040049
Chicago/Turabian StyleWeerasingha, Viraj, Hasitha Priyashantha, Chaminda Senaka Ranadheera, Pradeep Prasanna, Pradeepa Silva, Janak K. Vidanarachchi, and Monika Johansson. 2022. "Milk Coagulation Properties: A Study on Milk Protein Profile of Native and Improved Cattle Breeds/Types in Sri Lanka" Dairy 3, no. 4: 710-721. https://doi.org/10.3390/dairy3040049
APA StyleWeerasingha, V., Priyashantha, H., Ranadheera, C. S., Prasanna, P., Silva, P., Vidanarachchi, J. K., & Johansson, M. (2022). Milk Coagulation Properties: A Study on Milk Protein Profile of Native and Improved Cattle Breeds/Types in Sri Lanka. Dairy, 3(4), 710-721. https://doi.org/10.3390/dairy3040049








