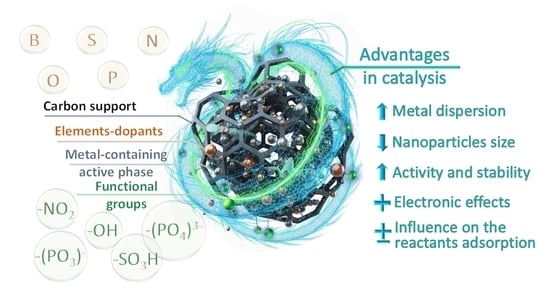
The Role of Surface Chemistry in Carbon-Supported Metal-Catalyzed Processes of Fine Organic Synthesis
Abstract
1. Introduction
- (i)
- High stability in acidic or basic medium;
- (ii)
- Possibility to adjust pore sizes for the reaction of interest;
- (iii)
- The presence of different functional groups on the surface, which are responsible for amphoteric properties and interaction with metal species;
- (iv)
- High thermal stability in an oxygen-free medium, which allows for easy reduction of metal-containing precursors;
- (v)
- Relatively low cost;
- (vi)
- Possibility of different shaping (granules, fibers, extrudates, etc.);
- (vii)
- Control over relative hydrophilicity/hydrophobicity by the elimination or addition of O-containing functional groups;
- (viii)
- Simplicity of metal regeneration via the burning of carbon.
- (i)
- Electronic (electron transfer at the metal-support interface);
- (ii)
- Geometric (changes in the shape of metal particles);
- (iii)
- Dispersion (decrease in the sizes of metal particles due to the interaction of metal precursor with functional groups on the carbon surface) [13].
- (i)
- Distinct electronic and physicochemical structure;
- (ii)
- Strong MSI, providing better resistance to aggregation and leaching;
- (iii)
- Low cost due to the 100% utilization of metal atoms.
- (i)
- Thermal decomposition of metal-containing precursors (metal complexes, metal–organic frameworks (MOFs), etc.). In many cases this method is used to simultaneously obtain carbon and confine metal atoms or particles;
- (ii)
- Adsorption of metals from solutions with further formation of metal particles during either in situ or ex situ (after catalyst separation) reduction;
- (iii)
- Incipient wetness impregnation (IWI).
2. Functionalization/Doping of Carbon Materials with Heteroatoms
- (i)
- Relative simplicity of doping;
- (ii)
- Tuning of electronic properties while introducing N atoms in the aromatic rings;
- (iii)
- Negligible difference in atomic radii of N and C, which prevents significant lattice mismatches;
- (iv)
- Ability to produce semiconducting materials for electronic application.
- (i)
- The number of defects or edges can be increased by a corresponding increase in the concentration of pyridinic N, since it is known that the pyridinic N prefers to occupy the edges or defects of the carbon materials [55];
- (ii)
- Thermal treatment of N-doped material allows the ratio between different forms of nitrogen to shift [54,63], i.e., a gradual increase in annealing temperature results in a decrease in the concentration of pyridinic N and a corresponding increase in the amount of pyrrolic N and then quaternary N (the most thermally stable nitrogen form).
3. Functionalized Carbons as Supports
3.1. O-Functionalized Carbons
3.2. N-Functionalized Carbons
3.3. S-Functionalized Carbons
3.4. P-Functionalized Carbons
3.5. B-Functionalized Carbons
4. Doped Graphenes, CNTs, and g-C3N4 as Supports
5. Carbon Supports Derived from MOFs and Other Polymers
6. Discussion
7. Conclusions and Outlook
- (i)
- Decrease in the mean diameters of metal NPs and an increase in their dispersion;
- (ii)
- Better uniformity of metal distribution on the support surface;
- (iii)
- Stabilization of single-atom state in the case of SACs;
- (iv)
- Increase in metal reducibility;
- (v)
- Formation of compounds from metals and heteroatoms (reactive MSI), i.e., the formation of metal sulfide NPs in the case of the S-doped carbon supports;
- (vi)
- Formation of reactive acid/base sites on the support surface, which may act in tandem with the metal active species;
- (vii)
- Tuning of the adsorption ability of the reactants on the catalyst surface by the regulation of its polarity and electronic properties, which may enhance catalytic activity and stability;
- (viii)
- Possibility to create separable and reusable homogeneous catalysts by grafting ligands on the surface of carbon supports.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | activated carbon |
| BC | B-doped carbon |
| BE | binding energy |
| BOMC | B-doped ordered mesoporous carbon |
| BPC | B-doped porous carbon |
| CNT | carbon nanotube |
| GHSV | gas hourly space velocity |
| g-C3N4 | graphitic carbon nitride |
| HDC | hydrodechlorination |
| HTC | hydrothermal carbonization |
| IWI | incipient wetness impregnation |
| MC | mesoporous carbon |
| MOF | metal–organic framework |
| MPC | magnetic porous carbon |
| MSI | metal support interaction |
| NC | N-doped carbon |
| NP | nanoparticle |
| NPC | N-doped porous carbon |
| NSC | carbon nanosheet |
| OMC | ordered mesoporous carbon |
| PC | porous carbon |
| PCBC | P-doped cotton stalk carbon material |
| PDC | plastic-derived carbon |
| PNS | pine nut shell |
| SAC | single-atom catalyst |
| SC | S-doped carbon |
| SSA | specific surface area |
| TPP | triphenylphosphine |
References
- Mironenko, R.M.; Belskaya, O.B.; Likholobov, V.A. Carbon Black as a Support in Palladium Catalysts for Hydrogenation of Organic Compounds. Solid Fuel Chem. 2020, 54, 362–367. [Google Scholar] [CrossRef]
- Podkolzin, S.G.; Chen, T.; Zheng, Y.; Yang, M.; Tadepalli, S.R.; Tampy, G.K.; Cherkauskas, J.P. Palladium Catalysts Supported on Carbon for Hydrogenation of Aromatic Hydrocarbons. U.S. Patent US 2020/0001274 A1, 2 January 2020. [Google Scholar]
- Duran-Uribe, E.S.; Sepúlveda-Escribano, A.; Ramos-Fernandez, E.V. Catalytic synergy: N,P modification of activated carbon for improved 1-chloro-4-nitrobenzene reduction. Carbon 2024, 227, 119262. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P. Selective oxidation of alcohols and aldehydes on metal catalysts. Catal. Today 2000, 57, 127–141. [Google Scholar] [CrossRef]
- Budarin, V.L.; Shuttleworth, P.S.; Clark, J.H.; Luque, R. Industrial Applications of C-C Coupling Reactions. Curr. Org. Synth. 2010, 7, 614–627. [Google Scholar] [CrossRef]
- Ayogu, J.I.; Onoabedje, E.A. Recent advances in transition metal-catalysed cross-coupling of (hetero)aryl halides and analogues under ligand-free conditions. Catal. Sci. Technol. 2019, 9, 5233–5255. [Google Scholar] [CrossRef]
- Hoque, N.; Saikia, B.K. Coal-Derived Carbon Materials: Pathways to Graphene, Carbon Nanotubes, Carbon Quantum Dots, and Nanodiamond for Energy and Environmental Solutions. ACS Omega 2025, 10, 53673–53701. [Google Scholar] [CrossRef] [PubMed]
- Mahene, W.L.; Kivevele, T.; Machunda, R. The role of textural properties and surface chemistry of activated carbon support in catalytic deoxygenation of triglycerides into renewable diesel. Catal. Commun. 2023, 181, 106737. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, P.; Kots, P.A.; Cohen, M.; Chen, Y.; Quinn, C.M.; deMello, M.D.; Boscoboinik, J.A.; Shaw, W.J.; Caratzoulas, S.; et al. Tuning the reactivity of carbon surfaces with oxygen-containing functional groups. Nat. Commun. 2023, 14, 2293. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, J.; Li, X. Controlled Synthesis of Carbon-Supported Pt-Based Electrocatalysts for Proton Exchange Membrane Fuel Cells. Electrochem. Energy Rev. 2022, 5, 13. [Google Scholar] [CrossRef]
- Gerber, I.C.; Serp, P. A Theory/Experience Description of Support Effects in Carbon-Supported Catalysts. Chem. Rev. 2020, 120, 1250–1349. [Google Scholar] [CrossRef]
- Das, A.; Mondal, S.; Hansda, K.M.; Adak, M.K.; Dhak, D. A critical review on the role of carbon supports of metal catalysts for selective catalytic hydrogenation of chloronitrobenzenes. Appl. Catal. A Gen. 2023, 649, 118955. [Google Scholar] [CrossRef]
- Campisi, S.; Chan-Thaw, C.E.; Villa, A. Understanding Heteroatom-Mediated Metal–Support Interactions in Functionalized Carbons: A Perspective Review. Appl. Sci. 2018, 8, 1159. [Google Scholar] [CrossRef]
- Jabeen, S.; Li, Y.; Wu, X.; Cheng, Y.; Liu, Y.; Yu, J.; Liu, N.; Huang, J.; Li, H. Exploring Carbon-based Materials as a tailored platform for Suzuki–Miyaura Coupling Reaction: A Review. Coord. Chem. Rev. 2025, 524, 216323. [Google Scholar] [CrossRef]
- Su, C.; Zou, S.; Li, J.; Wang, L.; Huang, J. Supporting Nano Catalysts for the Selective Hydrogenation of Biomass-derived Compounds. ChemSusChem 2024, 17, e202400602. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Pandey, A.K.; Pandey, S.K. Mechanistic insights into intermolecular and surface interactions for nanoscale metal–carbon-based coating heterostructures. Coord. Chem. Rev. 2025, 543, 216913. [Google Scholar] [CrossRef]
- Roy, S.; Ghosh, B.D.; Goh, K.L.; Ahn, H.J.; Chang, Y.-W. Super expanded freestanding 3D graphene foam as a versatile platform for CO2 capture and hydrogenation. Chem. Eng. J. 2023, 466, 143326. [Google Scholar] [CrossRef]
- Barra, A.; Carvalho, A.; Lopes, C.B.; Ruiz-Hitzky, E.; Nunes, C.; Ferreira, P. Reduced graphene oxide foams modified with caffeic acid for water decontamination: Capture and safe storage of Hg2+. J. Environ. Chem. Eng. 2024, 12, 114120. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Sun, C.; Duan, Y.; Wang, J.; Liu, Z.; Chen, X.; Zhu, M.; Song, P.; Liu, L. Fire-retardant superhydrophobic graphene-modified foam for oil/water separation. Chem. Eng. J. 2025, 525, 170752. [Google Scholar] [CrossRef]
- Guo, S.; Chen, J.; Zhang, Y.; Liu, J. Graphene-Based Films: Fabrication, Interfacial Modification, and Applications. Nanomaterials 2021, 11, 2539. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, B.; Peng, Y.; Yuan, Y.; Tang, Z.; Meng, W.; Zhao, Y.; Wang, X. One-step sustainable preparation of laser induced S-doped graphene for assembly of high-performance supercapacitors. J. Clean. Prod. 2024, 450, 141956. [Google Scholar] [CrossRef]
- Zhao, M.; Jin, H.; Chen, Z.; Fang, R.; Feng, H.; He, D. Interface engineering of scalable graphene foam induces hydrophilic/aerophobic trimetallic (Fe, Co, Ni) nanosheets for efficient oxygen evolution. Surf. Interfaces 2025, 62, 106082. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Huang, J.; Jiang, H.; Liang, B.; Wang, B.; He, D.; Chen, H. Melamine foam-derived N-doped carbon framework and graphene-supported sulfurized polyacrylonitrile for high performance lithium--sulfur battery cathode. J. Energy Storage 2025, 118, 116330. [Google Scholar] [CrossRef]
- Raut, S.S.; Yadav, K.; Patro, T.U.; Kulkarni, P.S. Nano-engineered graphene based NiCo2O4-coated reticulated vitreous carbon foam (RVC@2.5%graphene@NCO) incorporated cylindrical reactor for continuous flow photodegradation of 2,3-DCP. J. Hazard. Mater. 2025, 496, 139268. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Ma, X.; Liu, X.; Zhang, R.; Wang, Y. Metal–Support Interaction of Carbon–Based Electrocatalysts for Oxygen Evolution Reaction. Nanoenergy Adv. 2023, 3, 48–72. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Sui, W.; Parvez, A.M.; Xu, T.; Si, C.; Hu, J. Carbon-based single-atom catalysts derived from biomass: Fabrication and application. Adv. Colloid Interface Sci. 2024, 329, 103176. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, X.; Peng, C.; Priest, C.; Mei, Y.; Wu, G. Carbon-Supported Single Metal Site Catalysts for Electrochemical CO2 Reduction to CO and Beyond. Small 2021, 17, 2005148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, W.; Cao, D.; Cheng, D. Carbon-based material-supported single-atom catalysts for energy conversion. iScience 2022, 25, 104367. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Gao, K.; Wang, X.; Zheng, H.; Cao, J.; Mi, L.; Huo, Q.; Yang, H.; Liu, J.; He, C. Subnanometric Ru clusters with upshifted D band center improve performance for alkaline hydrogen evolution reaction. Nat. Commun. 2022, 13, 3958. [Google Scholar] [CrossRef]
- Beermann, V.; Holtz, M.E.; Padgett, E.; de Araujo, J.F.; Muller, D.A.; Strasser, P. Real-time imaging of activation and degradation of carbon supported octahedral Pt–Ni alloy fuel cell catalysts at the nanoscale using in situ electrochemical liquid cell STEM. Energy Environ. Sci. 2019, 12, 2476–2485. [Google Scholar] [CrossRef]
- Karczmarska, A.; Adamek, M.; El Houbbadi, S.; Kowalczyk, P.; Laskowska, M. Carbon-Supported Noble-Metal Nanoparticles for Catalytic Applications—A Review. Crystals 2022, 12, 584. [Google Scholar] [CrossRef]
- Bie, C.; Yu, H.; Cheng, B.; Ho, W.; Fan, J.; Yu, J. Design, Fabrication, and Mechanism of Nitrogen-Doped Graphene-Based Photocatalyst. Adv. Mater. 2021, 33, 2003521. [Google Scholar] [CrossRef]
- Deng, C.; Xu, L.; Hu, K.; Chen, X.; Gao, R.; Zhang, L.; Wang, L.; Zhang, C. Research Advances on Nitrogen-Doped Carbon Materials in COx Hydrogenation. Atmosphere 2023, 14, 1510. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Noh, H.-J.; Baek, J.-B. Nitrogen-Doped Carbon Nanomaterials: Synthesis, Characteristics and Applications. Chem. Asian J. 2020, 15, 2282–2293. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, W.; Jiang, X.; Liu, H.; Ma, S.; Wang, B. Preparation of a novel nitrogen-containing graphitic mesoporous carbon for the removal of acid red 88. Sci. Rep. 2020, 10, 1353. [Google Scholar] [CrossRef]
- Pustahija, L.; Bandl, C.; Alem, S.A.A.; Kern, W. Surface Functionalization of Activated Carbon: Coupling of 3-(Aminopropyl)trimethoxysilane and (3-Glycidyloxypropyl)trimethoxysilane. C 2024, 10, 104. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, T.; Tai, J.; Ma, N. Recent Advances in Carbon-Based Catalysts for Heterogeneous Asymmetric Catalysis. Molecules 2025, 30, 2643. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhu, M.; Zhang, J.; Zhang, H.; Dai, B. Nitrogen functional groups on an activated carbon surface to effect the ruthenium catalysts in acetylene hydrochlorination. RSC Adv. 2015, 5, 86172–86178. [Google Scholar] [CrossRef]
- Gao, C.; Guo, M.; Liu, Y.; Zhang, D.; Gao, F.; Sun, L.; Li, J.; Chen, X.; Terrones, M.; Wang, Y. Surface modification methods and mechanisms in carbon nanotubes dispersion. Carbon 2023, 212, 118133. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Ramasamy, R.P. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Pavía, M.; Emo, M.; Hupont, S.; Mohamed, A.R.; Gal, S.; Estellé, P.; Vigolo, B. Covalent vs. Non covalent chemical modification of multiwalled carbon nanotubes based-nanofluids: Stability and thermal conductivity steadiness over temperature. J. Mol. Liq. 2024, 404, 124856. [Google Scholar] [CrossRef]
- Liu, Z.; Zhong, S.; Zhang, X.; Tian, K.; Qing, T.; Liu, X. Carbon nitride supported Co single-atom catalyst with low metal leaching for activation of peroxymonosulfate to degrade p-nitrophenol. J. Water Process Eng. 2025, 72, 107418. [Google Scholar] [CrossRef]
- Faisal, S.N.; Haque, E.; Noorbehesht, N.; Zhang, W.; Harris, A.T.; Church, T.L.; Minett, A.I. Pyridinic and graphitic nitrogen-rich graphene for high-performance supercapacitors and metal-free bifunctional electrocatalysts for ORR and OER. RSC Adv. 2017, 7, 17950–17958. [Google Scholar] [CrossRef]
- Valero-Romero, M.J.; Rodríguez-Cano, M.Á.; Palomo, J.; Rodríguez-Mirasol, J.; Cordero, T. Carbon-Based Materials as Catalyst Supports for Fischer–Tropsch Synthesis: A Review. Front. Mater. 2021, 7, 617432. [Google Scholar] [CrossRef]
- Kim, J.-H.; Hwang, S.Y.; Park, J.E.; Lee, G.B.; Kim, H.; Kim, S.; Hong, B.U. Impact of the oxygen functional group of nitric acid-treated activated carbon on KOH activation reaction. Carbon Lett. 2019, 29, 281–287. [Google Scholar] [CrossRef]
- Li, Y.; Lu, W.; Zhao, Z.; Zhao, M.; Lyu, Y.; Gong, L.; Zhu, H.; Ding, Y. Tuning surface oxygen group concentration of carbon supports to promote Fischer-Tropsch synthesis. Appl. Catal. A Gen. 2021, 613, 118017. [Google Scholar] [CrossRef]
- De Clippel, F.; Dusselier, M.; Van de Vyver, S.; Peng, L.; Jacobs, P.A.; Sels, B.F. Tailoring nanohybrids and nanocomposites for catalytic applications. Green Chem. 2013, 15, 1398–1430. [Google Scholar] [CrossRef]
- Tipo, R.; Chaichana, C.; Noda, R.; Chaiklangmuang, S. Influence of coal treatments on the Ni loading mechanism of Ni-loaded lignite char catalysts. RSC Adv. 2021, 11, 35624–35643. [Google Scholar] [CrossRef]
- Gamal, A.; Jlassi, K.; Ahmad, Y.H.; Tang, M.; Al-Qaradawi, S.Y.; Chehimi, M.M.; Ozoemena, K.I.; Abdullah, A.M. Carbon-supported catalysts for carbon dioxide methanation: A review. J. CO2 Util. 2024, 85, 102881. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-Doped Graphene by Chemical Vapor Deposition and Its Electrical Properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Ehrat, F.; Urban, P.; Teves, R.; Wyrwich, R.; Döblinger, M.; Feldmann, J.; Urban, A.S.; Stolarczyk, J.K. Effect of nitrogen atom positioning on the trade-off between emissive and photocatalytic properties of carbon dots. Nat. Commun. 2017, 8, 1401. [Google Scholar] [CrossRef]
- Labulo, A.H.; Omondi, B.; Nyamori, V.O. Graphene/pyrrolic-structured nitrogen-doped CNT nanocomposite supports for Pd-catalysed Heck coupling and chemoselective hydrogenation of nitroarenes. SN Appl. Sci. 2019, 1, 142. [Google Scholar] [CrossRef]
- Lei, G.; Ma, J.; Zhao, M.; Wu, S.; He, H.; Qi, H.; Peng, W.; Fan, X.; Zhang, G.; Zhang, F.; et al. Nitrogen-carbon materials base on pyrolytic graphene hydrogel for oxygen reduction. J. Colloid Interface Sci. 2021, 602, 274–281. [Google Scholar] [CrossRef]
- Lv, Q.; Si, W.; He, J.; Sun, L.; Zhang, C.; Wang, N.; Yang, Z.; Li, X.; Wang, X.; Deng, W.; et al. Selectively nitrogen-doped carbon materials as superior metal-free catalysts for oxygen reduction. Nat. Commun. 2018, 9, 3376. [Google Scholar] [CrossRef]
- Tian, K.; Wang, J.; Cao, L.; Yang, W.; Guo, W.; Liu, S.; Li, W.; Wang, F.; Li, X.; Xu, Z.; et al. Single-site pyrrolic-nitrogen-doped sp2-hybridized carbon materials and their pseudocapacitance. Nat. Commun. 2020, 11, 3884. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Ding, Y.; Zhang, P.; Wang, M.; Jia, Y.; Xu, Y.; Li, Y.; Fan, K.; Sun, L. Pyrrolic N or pyridinic N: The active center of N-doped carbon for CO2 reduction. Chin. J. Catal. 2022, 43, 2405–2413. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Zhang, X.; Yu, S.; Wang, S.; Sun, G. Enhanced metal-support interaction of nitrogen-doped carbon supported Pt nanoparticles with high activity for electrocatalytic oxygen reduction reaction. Carb. Neutrality 2025, 4, 10. [Google Scholar] [CrossRef]
- Cao, Y.; Si, W.; Zhang, Y.; Hao, Q.; Lei, W.; Xia, X.; Li, J.; Wang, F. Nitrogen-doped graphene: Effect of graphitic-N on the electrochemical sensing properties towards acetaminophen. FlatChem 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Chen, Z.; Jaworski, A.; Chen, J.; Budnyak, T.M.; Szewczyk, I.; Rokicińska, A.; Dronskowski, R.; Hedin, N.; Kuśtrowski, P.; Slabon, A. Graphitic nitrogen in carbon catalysts is important for the reduction of nitrite as revealed by naturally abundant 15N NMR spectroscopy. Dalton Trans. 2021, 50, 6857–6866. [Google Scholar] [CrossRef]
- Li, F.; Li, M.; Luo, Y.; Li, M.; Li, X.; Zhang, J.; Wang, L. The Synergistic Effect of Pyridinic Nitrogen and Graphitic Nitrogen of Nitrogen-Doped Graphene Quantum Dots for Enhanced TiO2 Nanocomposites’ Photocatalytic Performance. Catalysts 2018, 8, 438. [Google Scholar] [CrossRef]
- Wang, N.; Lu, B.; Li, L.; Niu, W.; Tang, Z.; Kang, X.; Chen, S. Graphitic Nitrogen Is Responsible for Oxygen Electroreduction on Nitrogen-Doped Carbons in Alkaline Electrolytes: Insights from Activity Attenuation Studies and Theoretical Calculations. ACS Catal. 2018, 8, 6827–6836. [Google Scholar] [CrossRef]
- Zhou, N.; Wang, N.; Wu, Z.; Li, L. Probing Active Sites on Metal-Free, Nitrogen-Doped Carbons for Oxygen Electroreduction: A Review. Catalysts 2018, 8, 509. [Google Scholar] [CrossRef]
- Kumar Choutipalli, V.S.; Subramanian, V. Role of Graphitic Nitrogen and p-Conjugated Functional Groups in Selective Oxidation of Alcohols: A DFT based Mechanistic Elucidation. Chem. Asian J. 2019, 14, 4798–4806. [Google Scholar] [CrossRef]
- Sun, Y.; Xiao, Y.; Ren, L.; Cheng, Z.; Niu, Y.; Li, Z.; Zhang, S. Pyrrolic Nitrogen Boosted H2 Generation from an Aqueous Solution of HCHO at Room Temperature by Metal-Free Carbon Catalysts. J. Phys. Chem. Lett. 2024, 15, 4538–4545. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, Y.; Wang, C.; Liu, Z.; Xie, Z. Pyridinic nitrogen dominated doping on Pd/carbon catalysts for enhanced hydrogenation performance. Front. Chem. 2022, 10, 1046058. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Teng, P.-Y.; Yeh, C.-H.; Koshino, M.; Chiu, P.-W.; Suenaga, K. Structural and Chemical Dynamics of Pyridinic Nitrogen Defects in Graphene. Nano Lett. 2015, 15, 7408–7413. [Google Scholar] [CrossRef] [PubMed]
- Schmies, H.; Hornberger, E.; Anke, B.; Jurzinsky, T.; Nong, H.N.; Dionigi, F.; Kühl, S.; Drnec, J.; Lerch, M.; Cremers, C.; et al. Impact of Carbon Support Functionalization on the Electrochemical Stability of Pt Fuel Cell Catalysts. Chem. Mater. 2018, 30, 7287–7295. [Google Scholar] [CrossRef]
- Zhang, J.; Hong, Y.; Gao, W.; Xu, J.; Zhang, L.; Sun, B. Bamboo-like Carbon Nanotubes with the Theoretical Upper-Limit N-Doping Concentration for Advanced Nonradical Oxidation. ACS Appl. Nano Mater. 2023, 6, 22283–22290. [Google Scholar] [CrossRef]
- Suboch, A.N.; Podyacheva, O.Y. Pd Catalysts Supported on Bamboo-like Nitrogen-Doped Carbon Nanotubes for Hydrogen Production. Energies 2021, 14, 1501. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Liu, W.; Wei, Y.; Wang, Y.; Luo, S.; Hou, P.; Zhang, Y.; Yan, S.; Liu, X. Bamboo-like tubular nitrogen-doped carbon derived from g-C3N4 as an ultra-high performance anode of potassium-ion battery. Appl. Surf. Sci. 2023, 640, 158321. [Google Scholar] [CrossRef]
- Song, K.; Li, G.; Yu, J.; Zheng, T.; Wang, J. Bamboo-like nitrogen-doped carbon nanotubes directly grown from commercial carbon black for encapsulating FeCo nanoparticles as efficient oxygen reduction electrocatalysts. J. Colloid Interface Sci. 2025, 679 Pt B, 364–372. [Google Scholar] [CrossRef]
- Tong, Z.W.; Yuan, Y.F.; Yin, S.M.; Wang, B.X.; Guo, S.Y.; Mo, C.L. Bamboo-like carbon nanotube-carbon nanotube for high-performance sodium-ion batteries. Mater. Lett. 2022, 311, 131587. [Google Scholar] [CrossRef]
- Guo, P.; Li, H.W.; Huang, B.Y.; Ji, D.; Li, G.X.; Zhao, X.H. Precisely Engineered Nitrogen Dopants in Pd/NC Catalysts: Synergistic Pyridinic-Pyrrolic-Graphitic Triad for Alkaline Methanol Electrooxidation. Langmuir 2025, 41, 23582–23595. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-X.; Ying, J.; Liu, H.-W.; Yang, X.-Y. Pt–C interactions in carbon-supported Pt-based electrocatalysts. Front. Chem. Sci. Eng. 2023, 17, 1677–1697. [Google Scholar] [CrossRef]
- Wang, B.-H.; Chen, G.-H.; Hu, B.; Chen, L.; Wang, X.; Tian, S.; Hu, X.-S.; Li, Y.; Peng, C.; Yin, S.-F. Recent advances in tunable metal–support interactions for enhancing the photocatalytic nitrogen reduction reaction. EES Catal. 2024, 2, 180–201. [Google Scholar] [CrossRef]
- Li, B.; Sun, X.Y.; Su, D. Calibration of the basic strength of the nitrogen groups on the nanostructured carbon materials. Phys. Chem. Chem. Phys. 2015, 17, 6691–6694. [Google Scholar] [CrossRef]
- Kiuchi, H.; Shibuya, R.; Kondo, T.; Nakamura, J.; Niwa, H.; Miyawaki, J.; Kawai, M.; Oshima, M.; Harada, Y. Lewis Basicity of Nitrogen-Doped Graphite Observed by CO2 Chemisorption. Nanoscale Res. Lett. 2016, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.G.; Huš, M.; Baragau, I.-A.; Bowen, J.; Heil, T.; Nicolaev, A.; Abramiuc, L.E.; Sapelkin, A.; Sajjad, M.T.; Kellici, S. Engineering Nitrogen-Doped Carbon Quantum Dots: Tailoring Optical and Chemical Properties through Selection of Nitrogen Precursors. Small 2024, 20, 2310587. [Google Scholar] [CrossRef] [PubMed]
- Iwanow, M.; Gärtner, T.; Sieber, V.; König, B. Activated carbon as catalyst support: Precursors, preparation, modification and characterization. Beilstein J. Org. Chem. 2020, 16, 1188–1202. [Google Scholar] [CrossRef]
- Khalil, K.M.S.; Elhamdy, W.A.; Goda, M.N.; Said, A.E.-A.A. Biomass derived P-containing activated carbon as a novel green catalyst/support for methanol conversion to dimethyl ether alternative fuel. J. Environ. Chem. Eng. 2021, 9, 106572. [Google Scholar] [CrossRef]
- Chen, H.; Luo, X.; Huang, S.; Yu, F.; Li, D.; Chen, Y. Phosphorus-doped activated carbon as a platinum-based catalyst support for electrocatalytic hydrogen evolution reaction. J. Electroanal. Chem. 2023, 948, 117820. [Google Scholar] [CrossRef]
- Choi, S.S.; Choi, T.R.; Choi, H.-J. Surface Modification of Phosphoric Acid-activated Carbon in Spent Coffee Grounds to Enhance Cu(II) Adsorption from Aqueous Solutions. Appl. Chem. Eng. 2021, 32, 589–598. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Liu, Y.; Pu, L.; Chen, Z.; Den, S. The high-performance and mechanism of P-doped activated carbon as a catalyst for air-cathode microbial fuel cells. J. Mater. Chem. A 2015, 3, 21149–21158. [Google Scholar] [CrossRef]
- Mayes, R.T.; Fulvio, P.F.; Ma, Z.; Dai, S. Phosphorylated mesoporous carbon as a solid acid catalyst. Phys. Chem. Chem. Phys. 2011, 13, 2492–2494. [Google Scholar] [CrossRef]
- Zeng, X.-Y.; Wang, Y.; Li, R.-X.; Cao, H.-L.; Li, Y.-F.; Lü, J. Impacts of temperatures and phosphoric-acid modification to the physicochemical properties of biochar for excellent sulfadiazine adsorption. Biochar 2022, 4, 14. [Google Scholar] [CrossRef]
- Neme, I.; Gonfa, G.; Masi, C. Activated carbon from biomass precursors using phosphoric acid: A review. Heliyon 2022, 8, e11940. [Google Scholar] [CrossRef] [PubMed]
- Alhawtali, S.; El-Harbawi, M.; Al-Awadi, A.S.; El Blidi, L.; Alrashed, M.M.; Yin, C.-Y. Enhanced Adsorption of Methylene Blue Using Phosphoric Acid-Activated Hydrothermal Carbon Microspheres Synthesized from a Variety of Palm-Based Biowastes. Coatings 2023, 13, 1287. [Google Scholar] [CrossRef]
- El Farissi, H.; Beraich, A.; Lamsayah, M.; Talhaoui, A.; El Bachiri, A. The efficiency of carbon modified by phosphoric acid (H3PO4) used in the removal of two antibiotics amoxicillin and metronidazole from polluted water: Experimental and theoretical investigation. J. Mol. Liquid 2023, 391, 123237. [Google Scholar] [CrossRef]
- Murugan, M.; Dineshkumar, G.; Ganesan, M.; Sahoo, N.; Sivanantham, M. Influence of Phosphoric Acid Activation on Physiochemical Characteristics of Activated Carbons and Their Performance as Supercapacitor. Energy Storage 2024, 6, e70050. [Google Scholar] [CrossRef]
- Wang, B.; Yu, L.; Zhang, J.; Pu, Y.; Zhang, H.; Li, W. Phosphorus-doped carbon supports enhance gold-based catalysts for acetylene hydrochlorination. RSC Adv. 2014, 4, 15877–15885. [Google Scholar] [CrossRef]
- Zheng, Q.; Wei, X.; Li, X.; Liu, B.; Chen, A.; Tang, H.; Han, W. P-doped Carbon as the Efficient Support of Nickel Catalysts for Hydrodechlorination of Chlorodifluoromethane. ChemistrySelect 2020, 5, 13290–13294. [Google Scholar] [CrossRef]
- Kim, H.S.; Woo, S.M.; Kang, G.M.; You, S.-H.; Lee, S.-S.; Park, S.; Park, J.-H.; Cho, Y.; Lee, K.R.; Lee, K.-S.; et al. Phosphorus-Doped Highly Crystalline Carbon for High Platinum Stability and Robust Support in Proton-Exchange Membrane Fuel Cells. Small Methods 2025, early view. [Google Scholar] [CrossRef]
- Chida, K.; Yoshii, T.; Kawaguchi, R.; Inoue, M.; Tani, F.; Sobue, T.; Ohtani, S.; Kato, K.; Ogoshi, T.; Nakahata, S.; et al. Rational bottom-up synthesis of sulphur-rich porous carbons for single-atomic platinum catalyst supports. Green Chem. 2024, 26, 8758–8767. [Google Scholar] [CrossRef]
- Yin, P.; Yan, Q.-Q.; Liang, H.-W. Strong Metal-Support Interactions Through Sulfur-Anchoring of Metal Catalysts on Carbon Supports. Angew. Chem. Int. Ed. 2023, 62, e202302819. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Huang, M.; Ma, H.-L.; Zhang, Z.-Q.; Gao, J.-M.; Zhu, Y.-L.; Han, X.-J.; Guo, X.-Y. Preparation of a Carbon-Based Solid Acid Catalyst by Sulfonating Activated Carbon in a Chemical Reduction Process. Molecules 2010, 15, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gao, M.; Geng, J.; Cheng, Y.; Wang, X.; Wu, C.; Wang, Q.; Liu, S.; Cheung, S.M. Catalytic performance and deactivation mechanism of a one-step sulfonated carbon-based solid-acid catalyst in an esterification reaction. Renew. Energy 2021, 164, 824–832. [Google Scholar] [CrossRef]
- Mateo, W.; Lei, H.; Villota, E.; Qian, M.; Zhao, Y.; Huo, E.; Zhang, Q.; Lin, X.; Wang, C.; Huang, Z. Synthesis and characterization of sulfonated activated carbon as a catalyst for bio-jet fuel production from biomass and waste plastics. Bioresour. Technol. 2020, 297, 122411. [Google Scholar] [CrossRef]
- Sangsiri, P.; Laosiripojana, N.; Laosiripojana, W.; Daorattanachai, P. Activity of a Sulfonated Carbon-Based Catalyst Derived from Organosolv Lignin toward Esterification of Stearic Acid under Near-Critical Alcohol Conditions. ACS Omega 2022, 7, 40025–40033. [Google Scholar] [CrossRef] [PubMed]
- Da Luz Corrêa, A.P.; Bastos, R.R.C.; da Rocha, G.N.; Zamian, F.J.R.; da Conceição, L.R.V. Preparation of sulfonated carbon-based catalysts from murumuru kernel shell and their performance in the esterification reaction. RSC Adv. 2020, 10, 20245–20256. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Kim, K.; Park, J.; Sung, Y.-E. Role and Potential of Metal Sulfide Catalysts in Lithium-Sulfur Battery Applications. ChemCatChem 2019, 11, 2373–2387. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. Metal Sulfide Photocatalysts for Hydrogen Generation: A Review of Recent Advances. Catalysts 2022, 12, 1316. [Google Scholar] [CrossRef]
- Chen, S.; Liu, X.; Xiong, J.; Mi, L.; Song, X.-Z.; Li, Y. Defect and interface engineering in metal sulfide catalysts for the electrocatalytic nitrogen reduction reaction: A review. J. Mater. Chem. A 2022, 10, 6927–6949. [Google Scholar] [CrossRef]
- He, R.; Huang, X.; Feng, L. Recent Progress in Transition-Metal Sulfide Catalyst Regulation for Improved Oxygen Evolution Reaction. Energy Fuels 2022, 36, 6675–6694. [Google Scholar] [CrossRef]
- Wu, Z.; Xiong, Z.; Lai, B. Metal sulfide-based catalysts in advanced oxidation processes for water decontamination. Environ. Funct. Mater. 2022, 1, 298–315. [Google Scholar] [CrossRef]
- Szymanski, G.S.; Suzuki, Y.; Ohba, T.; Sulikowski, B.; Góra-Marek, K.; Tarach, K.A.; Koter, S.; Kowalczyk, P.; Ilnicka, A.; Zięba, M.; et al. Linking the Defective Structure of Boron-Doped Carbon Nano-Onions with Their Catalytic Properties: Experimental and Theoretical Studies. ACS Appl. Mater. Interfaces 2021, 13, 51628–51642. [Google Scholar] [CrossRef]
- Suo, N.; Huang, H.; Wu, A.M.; Cao, G.Z.; Zhang, G.F. A Novel Method of Synthesizing Boron-doped Carbon Catalysts. Fuel Cells 2018, 18, 681–687. [Google Scholar] [CrossRef]
- Khalili, D.; Karimi, Y.; Khoy, A.; Zare, M. Extended insight into the catalytic activity of boron-doped graphitic carbon nitride for the synthesis of bis-pyrazolyl methanes and pyranopyrazoles. Sci. Rep. 2025, 15, 27303. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, M.; Tao, W.; Han, L.; Zhang, J.; Zhao, Q. A Palladium Catalyst Supported on Boron-Doped Porous Carbon for Efficient Dehydrogenation of Formic Acid. Nanomaterials 2024, 14, 549. [Google Scholar] [CrossRef]
- Yi, H.; Huo, X.; Gu, J.; Wei, L.; Sun, Z.; Du, F.; Dai, C.; Wu, X.; Liu, Z.; Ren, J. Boron doping positively enhances the catalytic activity of carbon materials for the removal of bisphenol A. RSC Adv. 2022, 12, 21780–21792. [Google Scholar] [CrossRef]
- Chang, Y.; Li, J.; Ma, J.; Liu, Y.; Xing, R.; Wang, Y.; Zhang, G. Oxygenated boron-doped carbon via polymer dehalogenation as an electrocatalyst for high-efficiency O2 reduction to H2O2. Sci. China Mater. 2022, 65, 1276–1284. [Google Scholar] [CrossRef]
- Li, J.; Shi, C.; Bao, A.; Jia, J. Development of Boron-Doped Mesoporous Carbon Materials for Use in CO2 Capture and Electrochemical Generation of H2O2. ACS Omega 2021, 6, 8438–8446. [Google Scholar] [CrossRef]
- Duman, B.; Fiçicilar, B. Development of low-cost nitrogen- and boron-doped carbon black cathode catalysts for the improvement of hydrogen-bromine flow battery cathode kinetics. J. Appl. Electrochem. 2023, 53, 1421–1431. [Google Scholar] [CrossRef]
- Maboya, W.K.; Maubane-Nkadimeng, M.S.; Jijana, A.N.; Mmako, H.K. Nitrogen inclusion in carbon nanotubes initiated by boron doping and chlorination: Their use as electrocatalysts for oxygen reduction reaction. Front. Mater. 2022, 9, 886471. [Google Scholar] [CrossRef]
- Yao, R.; Gu, J.; He, H.; Yu, T. Improved Electrocatalytic Activity and Durability of Pt Nanoparticles Supported on Boron-Doped Carbon Black. Catalysts 2020, 10, 862. [Google Scholar] [CrossRef]
- Byeon, A.; Choi, J.W.; Lee, H.W.; Yun, W.C.; Zhang, W.; Hwang, C.-K.; Lee, S.Y.; Han, S.S.; Kim, J.M.; Lee, J.W. CO2-derived edge-boron-doped hierarchical porous carbon catalysts for highly effective electrochemical H2O2 production. Appl. Catal. B Environ. 2023, 329, 122557. [Google Scholar] [CrossRef]
- Choi, J.W.; Byeon, A.; Kim, S.; Hwang, C.-K.; Zhang, W.; Lee, J.; Yun, W.C.; Paek, S.Y.; Kim, J.H.; Jeong, G.; et al. Mesoporous Boron-Doped Carbon with Curved B4C Active Sites for Highly Efficient H2O2 Electrosynthesis in Neutral Media and Air-Supplied Environments. Adv. Mater. 2025, 37, 2415712. [Google Scholar] [CrossRef]
- Kobayashi, R.; Ishii, T.; Imashiro, Y.; Ozaki, J. Synthesis of P- and N-doped carbon catalysts for the oxygen reduction reaction via controlled phosphoric acid treatment of folic acid. Beilstein J. Nanotechnol. 2019, 10, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Takada, R.; Li, X.; Narimatsu, K.; Miyake, K.; Uchida, Y.; Nishiyama, N. Undemanding synthesis of N,P co-doped carbon nanosheets for the hydrogen evolution reaction: Combining experimental quantitative analysis and DFT calculation corroboration. J. Mater. Chem. A 2025, 13, 13884–13897. [Google Scholar] [CrossRef]
- Cruz-Silva, E.; Cullen, D.A.; Gu, L.; Romo-Herrera, J.M.; Muñoz-Sandoval, E.; López-Urías, F.; Sumpter, B.G.; Meunier, V.; Charlier, J.C.; Smith, D.J.; et al. Heterodoped nanotubes: Theory, synthesis, and characterization of phosphorus-nitrogen doped multiwalled carbon nanotubes. ACS Nano 2008, 2, 441–448. [Google Scholar] [CrossRef]
- Xie, X.; Shi, J.; Pu, Y.; Wang, Z.; Zhang, L.-L.; Wang, J.-X.; Wang, D. Cellulose derived nitrogen and phosphorus co-doped carbon-based catalysts for catalytic reduction of p-nitrophenol. J. Colloid Interface Sci. 2020, 571, 100–108. [Google Scholar] [CrossRef]
- Hussain, N.; Alawn, S.; Alshamsi, H.; Sahib, I. Green Synthesis of S- and N-Codoped Carbon Nanospheres and Application as Adsorbent of Pb (II) from Aqueous Solution. Int. J. Chem. Eng. 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Bahadur, R.; Wijerathne, B.; Vinu, A. Multiple Heteroatom Doped Nanoporous Biocarbon for Supercapacitor and Zinc-ion Capacitor. ChemSusChem 2024, 17, e202400999. [Google Scholar] [CrossRef]
- Miao, X.; Tian, F.; Bai, M.; Zhang, Y.; Wang, W.; Zhao, Z.; Shao, X.; Ji, X. Highly Efficient Hierarchical Porous Carbon Supported Pd-Based Catalysts for Additive-Free Dehydrogenation of Formic Acid. Catalysts 2022, 12, 240. [Google Scholar] [CrossRef]
- Smiljanić, M.; Srejić, I.; Georgijević, J.P.; Maksić, A.; Bele, M.; Hodnik, N. Recent progress in the development of advanced support materials for electrocatalysis. Front. Chem. 2023, 11, 1304063. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, Y.; Liang, M.; Niu, R.; Ge, Y.; Zou, Y.; Dong, X. Effect of the Microstructure of Carbon Supports on the Oxygen Reduction Properties of the Loaded Non-Noble Metal Catalysts. Nanomaterials 2025, 15, 1327. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Cang, Y.; Li, Q.; Fan, X.; Lin, H. Noble Metal Single-Atom Coordinated to Nitrogen, Oxygen, and Carbon as Electrocatalysts for Oxygen Evolution. Catalysts 2023, 13, 1378. [Google Scholar] [CrossRef]
- Guo, J.; Ding, R.; Li, Y.; Lu, Y.; Yan, Z.; Chen, Z.; He, Y.; Yang, Q.; Guo, X.; Zhang, Y.; et al. Semi-ionic C-F optimizes metal-support interaction of Rh/F, N-codoped porous carbon for efficient all-pH hydrogen production. Nano Energy 2025, 139, 110975. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, L.; Lin, S.; Dong, P.; Fu, X.; Wang, Y.; Liu, Q.; Wu, M. Carbon-Supported Fe-Based Catalyst for Thermal-Catalytic CO2 Hydrogenation into C2+ Alcohols: The Effect of Carbon Support Porosity on Catalytic Performance. Molecules 2024, 29, 4628. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Meng, S.; Hei, J.; Li, C.; Yin, X.; Wu, F. Constructing carbon supported copper-based catalysts for efficient CO2 hydrogenation to methanol. RSC Adv. 2023, 13, 14554–14564. [Google Scholar] [CrossRef] [PubMed]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, J.; Duyar, M.S.; Ordomsky, V.V.; Khodakov, A.Y.; Liu, J. Carbon based catalysts for Fischer–Tropsch synthesis. Chem. Soc. Rev. 2021, 50, 2337–2366. [Google Scholar] [CrossRef]
- Tian, L.; Liu, S.; Tahir, N.; Huang, L.; Tao, H.; Zhao, D.; Wang, J.; Cai, J. Preparation of metal-modified carbon-based catalyst and experimental study on catalytic pyrolysis of distillers dried grains with soluble. J. Anal. Appl. Pyrolysis 2024, 183, 106771. [Google Scholar] [CrossRef]
- Moseenkov, S.I.; Kuznetsov, V.L.; Zolotarev, N.A.; Kolesov, B.A.; Prosvirin, I.P.; Ishchenko, A.V.; Zavorin, A.V. Investigation of Amorphous Carbon in Nanostructured Carbon Materials (A Comparative Study by TEM, XPS, Raman Spectroscopy and XRD). Materials 2023, 16, 1112. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Iakunkov, A.; Boulanger, N.; Lazar, O.A.; Enachescu, M.; Grimm, A.; Talyzin, A.V. Activated carbons with extremely high surface area produced from cones, bark and wood using the same procedure. RSC Adv. 2023, 13, 14543–14553. [Google Scholar] [CrossRef]
- Stepacheva, A.A.; Markova, M.E.; Lugovoy, Y.V.; Kosivtsov, Y.Y.; Matveeva, V.G.; Sulman, M.G. Plant-Biomass-Derived Carbon Materials as Catalyst Support, A Brief Review. Catalysts 2023, 13, 655. [Google Scholar] [CrossRef]
- Demiral, I.; Samdan, C.; Demiral, H. Enrichment of the surface functional groups of activated carbon by modification method. Surf. Interf. 2021, 22, 100873. [Google Scholar] [CrossRef]
- Cuong, D.V.; Wu, J.-C.; Khan, E.; Laing, G.D.; Ok, Y.S.; Hou, C.-H. Integrated 3D pore architecture design of biobased engineered catalysts and adsorbents: Preparation, chemical doping, and environmental applications. Environ. Sci. Adv. 2023, 2, 1167–1188. [Google Scholar] [CrossRef]
- Crawford, C.J.; Qiao, Y.; Liu, Y.; Huang, D.; Yan, W.; Seeberger, P.H.; Oscarson, S.; Chen, S. Defining the Qualities of High-Quality Palladium on Carbon Catalysts for Hydrogenolysis. Org. Process Res. Dev. 2021, 25, 1573–1578. [Google Scholar] [CrossRef]
- Alshammari, H.M.; Aldosari, O.F.; Alotaibi, M.H.; Alotaibi, R.L.; Alhumaimess, M.S.; Morad, M.H.; Adil, S.F.; Shaik, M.R.; Islam, M.S.; Khan, M.; et al. Facile Synthesis and Characterization of Palladium@Carbon Catalyst for the Suzuki-Miyaura and Mizoroki-Heck Coupling Reactions. Appl. Sci. 2021, 11, 4822. [Google Scholar] [CrossRef]
- Kumar, M.M.; Prabhudesai, V.S.; Vinu, R. Lignin Depolymerization to Guaiacol and Vanillin Derivatives via Catalytic Transfer Hydrogenolysis using Pd-Lewis Metal Oxide Supported on Activated Carbon Catalysts. Mol. Catal. 2023, 549, 113474. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Boruah, B.; Raja, D.; Vinu, R. Microwave assisted co-pyrolysis of biomasses with polypropylene and polystyrene for high quality bio-oil production. Fuel Process. Technol. 2018, 175, 64–75. [Google Scholar] [CrossRef]
- Stucchi, M.; Capelli, S.; Villa, A.; Vandegehuchte, B.D.; Prati, L. Effect of carbon oxygen functionalization on the activity of Pd/C catalysts in hydrogenation reactions. ChemCatChem 2024, 16, e202301639. [Google Scholar] [CrossRef]
- Bateni, H.; Prabhu, P.T.; Gebur, H.E.; Tessonnier, J.-P. Bottom-Up Synthesis Strategies Enabling the Investigation of Metal Catalyst-Carbon Support Interactions. C 2022, 8, 37. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, W.; Sun, J.; Li, S.; Bai, G.; Li, S.; Sun, C.; Pang, S. Synthesis of flowerlike carbon nanosheets from hydrothermally carbonized glucose: An in situ self-generating template strategy. RSC Adv. 2019, 9, 37355–37364. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, X.; Qiu, W.; Song, J.; Nan, J.; Bai, G.; Pang, S. Effects of Surface Oxygen-Containing Groups of the Flowerlike Carbon Nanosheets on Palladium Dispersion, Catalytic Activity and Stability in Hydrogenolytic Debenzylation of Tetraacetyldibenzylhexaazaisowurtzitane. Catalysts 2021, 11, 441. [Google Scholar] [CrossRef]
- You, P.; Wu, L.; Zhou, L.; Xu, Y.; Qin, R. Impact of Oxygen Containing Groups on Pd/C in the Catalytic Hydrogenation of Acetophenone and Phenylacetylene. Catalysts 2024, 14, 545. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Z.-X.; Guo, H.-T.; Li, J.; Liu, Z.-L.; Wang, P.-F.; Wang, L.-L.; Yi, T.-F. Critical Role of Carbon Substrates in Optimizing Ru-Based HER Catalysts: From Dimensional Insights to Metal-Support Interactions Engineering. Adv. Funct. Mater. 2025, early view. [Google Scholar] [CrossRef]
- Ruiz-Bernal, Z.; Lillo-Ródenas, M.Á.; Román-Martínez, M.C. Efficient and labor-saving Ru/C catalysts for the transformation of levulinic acid into γ-valerolactone under mild reaction conditions. React. Chem. Eng. 2024, 9, 461–474. [Google Scholar] [CrossRef]
- Ruiz-Bernal, Z.; Lillo-Ródenas, M.Á.; Román-Martínez, M.C. Effect of the carbon surface chemistry on the metal speciation in Ru/C catalysts. Impact on the transformation of levulinic acid to γ-valerolactone. Appl. Surf. Sci. 2025, 681, 161554. [Google Scholar] [CrossRef]
- Tane, E.G.; Ruiz-Bernal, Z.; López-Serrano, C.; Lillo-Ródenas, M.A.; Román-Martínez, M.C. Carbon supported Ru, Ni and RuNi catalysts for the transformation of levulinic acid to γ-valerolactone. Activity of carbon metal-free samples and mechanistic insight based on the redox properties of the oxygen surface groups. Catal. Today 2025, 458, 115366. [Google Scholar] [CrossRef]
- Ni, J.; Shi, S.; Zhang, C.; Fang, B.; Wang, X.; Lin, J.; Liang, S.; Lin, B.; Jiang, L. Enhanced catalytic performance of the carbon-supported Ru ammonia synthesis catalyst by an introduction of oxygen functional groups via gas-phase oxidation. J. Catal. 2022, 409, 78–86. [Google Scholar] [CrossRef]
- Huang, R.; Guan, C.; Guo, Q.; Jiang, J. Ball-milling treated superfine powdered activated carbon triggers Mn(VII) activation for enhanced oxidation of organic contaminants. Water Res. 2025, 286, 124255. [Google Scholar] [CrossRef]
- Librando, I.L.; Mahmoud, A.G.; Carabineiro, S.A.C.; Guedes da Silva, M.F.C.; Geraldes, C.F.G.C.; Pombeiro, A.J.L. The Catalytic Activity of Carbon-Supported Cu(I)-Phosphine Complexes for the Microwave-Assisted Synthesis of 1,2,3-Triazoles. Catalysts 2021, 11, 185. [Google Scholar] [CrossRef]
- Qiao, X.; Zhao, Z.-H.; Zhang, J. Progress in mercury-free catalysts for acetylene hydrochlorination. Catal. Sci. Technol. 2024, 14, 3838–3852. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, X.; Zhang, J.; Huang, J. A Self-Disperse Copper-Based Catalyst Synthesized via a Dry Mixing Method for Acetylene Hydrochlorination. Catalysts 2024, 14, 207. [Google Scholar] [CrossRef]
- Kaiser, S.K.; Surin, I.; Amorós-Pérez, A.; Büchele, S.; Krumeich, F.; Clark, A.H.; Román-Martínez, M.C.; Lillo-Ródenas, M.A.; Pérez-Ramírez, J. Design of carbon supports for metal-catalyzed acetylene hydrochlorination. Nat. Commun. 2021, 12, 4016. [Google Scholar] [CrossRef] [PubMed]
- Giulimondi, V.; Ruiz-Ferrando, A.; Giannakakis, G.; Surin, I.; Agrachev, M.; Jeschke, G.; Krumeich, F.; López, N.; Clark, A.H.; Pérez-Ramírez, J. Evidence of bifunctionality of carbons and metal atoms in catalyzed acetylene hydrochlorination. Nat. Commun. 2023, 14, 5557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, B.; Yue, Y.; Di, S.; Zhai, Y.; He, H.; Sheng, G.; Lai, H.; Zhu, Y.; Guo, L.; et al. Towards a greener approach for the preparation of highly active gold/carbon catalyst for the hydrochlorination of ethyne. J. Catal. 2018, 365, 153–162. [Google Scholar] [CrossRef]
- Wang, B.; Yue, Y.; Wang, S.; Shao, S.; Chen, Z.; Fang, X.; Pang, X.; Pan, Z.; Zhao, J.; Li, X. Stabilizing supported gold catalysts in acetylene hydrochlorination by constructing an acetylene–deficient reaction phase. Green Energy Environ. 2021, 6, 9–14. [Google Scholar] [CrossRef]
- Wang, S.; Wan, L.; Jin, C.; Wang, T.; Zhuge, K.; Yue, Y.; Cai, H.; Wang, B.; Chang, R.; Zhao, J.; et al. Mechanistic insight into acetylene hydrochlorination with Ru-ligands via dual activation promoted by metal-ligands cooperative catalysis. Appl. Catal. A Gen. 2023, 665, 119382. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Liu, Y.; Chen, Y.; You, Z.; Zhang, L.; Liang, Y.; He, Z. A well-performing N, S double doping-carbon/metal composite catalyst prepared from used adsorbent for highly efficient degradation of floatation reagents. J. Clean. Prod. 2023, 428, 139408. [Google Scholar] [CrossRef]
- Lokteva, E.S.; Golubina, E.V. Metal-support interactions in the design of heterogeneous catalysts for redox processes. Pure Appl. Chem. 2019, 91, 609–631. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Huang, Z.; Yang, Y.; Xia, C.; Li, F. One-Pot Synthesis of Pd Nanoparticle Catalysts Supported on N-Doped Carbon and Application in the Domino Carbonylation. ACS Catal. 2013, 3, 839–845. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, M.; Dai, B. Cobalt-nitrogen-activated carbon as catalyst in acetylene hydrochlorination. Catal. Commun. 2017, 98, 22–25. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, C.; Meng, X.; Liu, Y.; Fan, J.; Lan, G.; Li, Y. Synergistic effect between nitrogen-doped sites and metal chloride for carbon supported extra-low mercury catalysts in acetylene hydrochlorination. Chin. J. Chem. Eng. 2025, 79, 145–154. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, H.; Zhang, J.; Qiu, Y.; Zhang, Z.; Xu, Q.; Meng, G.; Yan, W.; Gu, L.; Li, Y. Decreasing the coordinated N atoms in a single atom Cu catalyst to achieve selective transfer hydrogenation of alkynes. Chem. Sci. 2021, 12, 14599–14605. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, X.; Sun, Y.; Hua, D.; Yang, S.; Sun, L.; Li, T.; Chen, L. Co-pyrolysis induced strong metal-support interaction in N-doped carbon supported Ni catalyst for the hydrogenolysis of lignin. Chem. Eng. J. 2023, 473, 145182. [Google Scholar] [CrossRef]
- Sreenivasulu, M.; Khouqeer, G.A.; Shetti, R.S.; AbdelAll, N.; Alodhayb, A.N.; Shetti, N.P. Co-Incorporated S-Doped Graphitic Carbon Derived from Orange Peel Biowaste: An Efficient Electrocatalyst for Sustainable Water Splitting and Urea Oxidation. Energy Fuels 2025, 39, 10610–10627. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Nan, H.; Shen, S.-C.; Chen, M.-X.; Liang, H.-W.; Huang, C.-Q.; Yao, T.; Chu, S.-Q.; Li, W.-X.; Yu, S.-H. Incorporating Sulfur Atoms into Palladium Catalysts by Reactive Metal–Support Interaction for Selective Hydrogenation. CCS Chem. 2022, 4, 3051–3063. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, C.; Yao, C.; Ma, D.; Chen, Y.; Tian, M.; Xie, H.; Pan, L.; Zhen, Y.; Chen, R.; et al. Sulfur-doped activated carbon supported platinum species as robust catalysts for nitrobenzene hydrogenation to p-Aminophenol. Mol. Catal. 2023, 545, 113216. [Google Scholar] [CrossRef]
- Lu, C.; Wang, M.; Feng, Z.; Qi, Y.; Feng, F.; Ma, L.; Zhang, Q.; Li, X. Phosphorus-carbon framework over the activated carbon supported palladium nanoparticles for the chemoselective hydrogenation of para-chloronitrobenzene. Catal. Sci. Technol. 2017, 7, 1581–1589. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Yan, H.; Yang, C.; Wu, R.; Wang, J.; Wei, Y.; Sun, H.; Liang, C. Pd catalysts supported on P-doped carbon derived from cotton stalk for acetylene hydrochlorination. J. Alloys Compd. 2025, 1044, 184312. [Google Scholar] [CrossRef]
- Konovalova, D.A.; Larichev, Y.V.; Stepanenko, S.A.; Shivtsov, D.M.; Borodina, O.A.; Kvon, R.I.; Yeletsky, P.M.; Koskin, A.P.; Yakovlev, V.A. P-Doped-Activated Carbon-Supported Pd Catalysts for Synthesis and Dehydrogenation of Tetradecahydrophenazine as a LOHC Substrate. Energy Fuels 2025, 39, 14782–14797. [Google Scholar] [CrossRef]
- Shivtsov, D.M.; Koskin, A.P.; Stepanenko, S.A.; Ilyina, E.V.; Ayupov, A.B.; Bedilo, A.F.; Yakovlev, V.A. Hydrogen Productionby N-Heterocycle Dehydrogenation over Pd Supported on Aerogel Prepared Mg-Al Oxides. Catalysts 2023, 13, 334. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, S.; Shi, W.; Zhang, B.; Wang, J.; Kim, Y.A.; Endo, M.; Su, D.S. Efficient and highly selective boron-doped carbon materials-catalyzed reduction of nitroarenes. Chem. Commun. 2015, 51, 13086–13089. [Google Scholar] [CrossRef]
- Su, Y.; Yao, C.; Zhang, Q.; Xu, L.; Wang, H.; Liu, J.; Hou, S. Palladium Nanoparticles Supported on B-Doped Carbon Nanocage as Electrocatalyst toward Ethanol Oxidation Reaction. ChemElectroChem 2019, 6, 5211–5219. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Boron-Doped Carbon nanoparticles supported palladium as an efficient hydrogen evolution electrode in PEM water electrolysis. Renew. Energy. 2020, 146, 2281–2290. [Google Scholar] [CrossRef]
- Zhu, F.; Zhu, M.; Kang, L. B-doped activated carbon as a support for a high-performance Zn-based catalyst in acetylene acetoxylation. Green Energy Environ. 2022, 7, 221–228. [Google Scholar] [CrossRef]
- Li, R.; Zhao, J.; Han, D.; Li, X. One-step synthesis of B-doped mesoporous carbon as supports of Pd nanoparticles for liquid phase catalytic hydrodechlorination. Catal. Commun. 2017, 97, 116–119. [Google Scholar] [CrossRef]
- Gardenö, D.; Bouša, D.; Sofer, Z.; Schneider, J.; Floreková, J.; Ashtiani, S.J.; Friess, K. Hydrogen separation via graphene oxide/single-walled carbon nanotubes composite membranes doped with Pd2+ ions. Sep. Purif. Technol. 2025, 378 Pt 3, 134773. [Google Scholar] [CrossRef]
- Kancharla, S.; Sasaki, K. Selective extraction of precious metals from simulated automotive catalyst waste and their conversion to carbon supported PdPt nanoparticle catalyst. Colloids Surf. A Physicochem. Eng. Asp. 2023, 665, 131179. [Google Scholar] [CrossRef]
- Shu, S.; Song, T.; Wang, C.; Dai, H.; Duan, L. [2+1] Cycloadditions Modulate the Hydrophobicity of Ni-N4 Single Atom Catalysts for Efficient CO2 Electroreduction. Angew. Chem. Int. Ed. 2024, 63, e202405650. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, B.; Zhuo, J.; Ma, X.; Tian, Y.; Wang, W.; Liu, Y.; Hou, J. Dual regulation of interfacial proton transport and alloy electronic structure in Cu-In-(COOH)CNTs catalysts for selective CO2 electroreduction. J. Environ. Chem. Eng. 2025, 13, 118231. [Google Scholar] [CrossRef]
- Bogdanovskaya, V.; Vernigor, I.; Radina, M.; Andreev, V.; Korchagin, O.; Novikov, V. Carbon Nanotube Modified by (O, N, P) Atoms as Effective Catalysts for Electroreduction of Oxygen in Alkaline Media. Catalysts 2020, 10, 892. [Google Scholar] [CrossRef]
- Niu, F.; Pang, Y.; Liu, W.; Shi, Z.; Cui, Y.; Yang, Z.; Yang, S.; Yin, Y. Strong metal-support interaction induced by oxygen vacancies to enhance the activity and durability of Pt/TiO2/hollow carbon nanospheres ORR catalyst. J. Alloys Compd. 2025, 1010, 177039. [Google Scholar] [CrossRef]
- Meng, J.; Bu, L.; Wang, D. Molybdenum-modified platinum nanoparticles confined on Ni-encapsulated carbon nanotubes with strong metal-support interaction for efficient hydrogen evolution catalysis. Chem. Eng. Sci. 2025, 317, 122041. [Google Scholar] [CrossRef]
- Wang, H.; Luan, X.; Li, H.; Zong, Y.; Xiao, W.; Xu, G.; Chen, D.; Fu, G.; Wu, Z.; Wang, L. Ru-M (Fe, Co, Ni) onto Nitrogen-doped Two-dimensional Carbon Nanosheets through Microwave Approach with Strong Metal-support Interactions for overall Water-splitting. Chem. Eng. J. 2024, 502, 158063. [Google Scholar] [CrossRef]
- Lyu, J.; Kudiiarov, V.; Lider, A. An Overview of the Recent Progress in Modifications of Carbon Nanotubes for Hydrogen Adsorption. Nanomaterials 2020, 10, 255. [Google Scholar] [CrossRef]
- Ye, B.; Kim, S.-I.; Lee, M.; Ezazi, M.; Kim, H.-D.; Kwon, G.; Lee, D.H. Synthesis of oxygen functionalized carbon nanotubes and their application for selective catalytic reduction of NOx with NH3. RSC Adv. 2020, 10, 16700–16708. [Google Scholar] [CrossRef] [PubMed]
- Nejabat, F.; Rayati, S.; Bagheri, S. Carbon nanotube as catalyst support for monometallic and bimetallic Nanostructures: Unexpectedly high efficiency of supported bimetallic bare metals. Appl. Surf. Sci. 2025, 705, 163511. [Google Scholar] [CrossRef]
- Tao, Z.; Shen, D.; Liu, Y.; Zhang, X.; Zhang, G. Enhanced Stability and Activity of Nitrogen-Doped Carbon Nanotube-Supported Ni Catalysts for Methane Dry Reforming. Catalysts 2025, 15, 559. [Google Scholar] [CrossRef]
- Ning, X.; Li, Y.; Dong, B.; Wang, H.; Yu, H.; Peng, F.; Yang, Y. Electron transfer dependent catalysis of Pt on N-doped carbon nanotubes: Effects of synthesis method on metal-support interaction. J. Catal. 2017, 348, 100–109. [Google Scholar] [CrossRef]
- John, J.; Gravel, E.; Namboothiri, I.N.N.; Doris, E. Advances in carbon nanotube-noble metal catalyzed organic transformations. Nanotechnol. Rev. 2012, 1, 515–539. [Google Scholar] [CrossRef]
- Cornelio, B.; Rance, G.A.; Laronze-Cochard, M.; Fontana, A.; Sapi, J.; Khlobystov, A.N. Palladium nanoparticles on carbon nanotubes as catalysts of cross-coupling reactions. J. Mater. Chem. A 2013, 1, 8737–8744. [Google Scholar] [CrossRef]
- Ligi, M.C.; Flis, A.; Biagiotti, G.; Serrano, G.; Michał Pietrusiewicz, K.; Cicchi, S. Heterogeneous Organo- and Metal Catalysis Using Phosphine Oxide Derivatives Anchored on Multiwalled Carbon Nanotubes. C 2020, 6, 57. [Google Scholar] [CrossRef]
- He, Z.; Dong, B.; Wang, W.; Yang, G.; Cao, Y.; Wang, H.; Yang, Y.; Wang, Q.; Peng, F.; Yu, H. Elucidating Interaction between Palladium and N-Doped Carbon Nanotubes: Effect of Electronic Property on Activity for Nitrobenzene Hydrogenation. ACS Catal. 2019, 9, 2893–2901. [Google Scholar] [CrossRef]
- Wang, W.; Yang, G.; Wang, Q.; Cao, Y.; Wang, H.; Yu, H. Modifying carbon nanotubes supported palladium nanoparticles via regulating the electronic metal–carbon interaction for phenol hydrogenation. Chem. Eng. J. 2022, 436, 131758. [Google Scholar] [CrossRef]
- Shamilov, R.R.; Muzipov, Z.M.; Sagdeev, D.O.; Kholin, K.V.; Saifina, A.F.; Gubaidullin, A.T.; Galyametdinov, Y.G. Photocatalytic Materials Based on g-C3N4 Obtained by the One-Pot Calcination Method. C 2023, 9, 85. [Google Scholar] [CrossRef]
- Li, Z.; Lin, R.; Liu, Z.; Li, D.; Wang, H.; Li, Q. Novel graphitic carbon nitride/graphite carbon/palladium nanocomposite as a high-performance electrocatalyst for the ethanol oxidation reaction. Electrochim. Acta 2016, 191, 606–615. [Google Scholar] [CrossRef]
- Kim, H.-E.; Lee, I.H.; Cho, J.; Shin, S.; Ham, H.C.; Kim, J.Y.; Lee, H. Palladium Single-Atom Catalysts Supported on C@C3N4 for Electrochemical Reactions. ChemElectroChem 2019, 6, 4757–4764. [Google Scholar] [CrossRef]
- Park, H.; Lee, J.H.; Kim, E.H.; Kim, K.Y.; Choi, Y.H.; Youn, D.H.; Lee, J.S. Highly Active and Stable Palladium Catalyst on g-C3N4 Support for Direct Formic Acid Synthesis under Neutral Condition. Chem. Commun. 2016, 52, 14302–14305. [Google Scholar] [CrossRef]
- Pieta, I.S.; Gieroba, B.; Kalisz, G.; Pieta, P.; Nowakowski, R.; Naushad, M.; Rathi, A.; Gawande, M.B.; Sroka-Bartnicka, A.; Zboril, R. Developing Benign Ni/g-C3N4 Catalysts for CO2 Hydrogenation: Activity and Toxicity Study. Ind. Eng. Chem. Res. 2022, 61, 10496–10510. [Google Scholar] [CrossRef]
- Yang, T.; Mao, X.; Zhang, Y.; Wu, X.; Wang, L.; Chu, M.; Pao, C.-W.; Yang, S.; Xu, Y.; Huang, X. Coordination tailoring of Cu single sites on C3N4 realizes selective CO2 hydrogenation at low temperature. Nat. Commun. 2021, 12, 6022. [Google Scholar] [CrossRef]
- Singh, R.; Wang, L.; Sun, H.; Huang, J. CO2 Hydrogenation Using Size-dependent Ru Particles Supported on g-C3N4. Carbon Capture Sci. Technol. 2024, 13, 100248. [Google Scholar] [CrossRef]
- Kyriakos, P.; Hristoforou, E.; Belessiotis, G.V. Graphitic Carbon Nitride (g-C3N4) in Photocatalytic Hydrogen Production: Critical Overview and Recent Advances. Energies 2024, 17, 3159. [Google Scholar] [CrossRef]
- Khan, M.E. State-of-the-art developments in carbon-based metal nanocomposites as a catalyst: Photocatalysis. Nanoscale Adv. 2021, 3, 1887–1900. [Google Scholar] [CrossRef]
- Wudil, Y.S.; Ahmad, U.F.; Gondal, M.A.; Al-Osta, M.A.; Almohammedi, A.; Sa’id, R.S.; Hrahsheh, F.; Haruna, K.; Mohamed, M.J.S. Tuning of graphitic carbon nitride (g-C3N4) for photocatalysis: A critical review. Arab. J. Chem. 2023, 16, 104542. [Google Scholar] [CrossRef]
- Pei, J.; Li, H.; Yu, D.; Zhang, D. g-C3N4-Based Heterojunction for Enhanced Photocatalytic Performance: A Review of Fabrications, Applications, and Perspectives. Catalysts 2024, 14, 825. [Google Scholar] [CrossRef]
- Yan, Y.; Meng, Q.; Tian, L.; Cai, Y.; Zhang, Y.; Chen, Y. Engineering of g-C3N4 for Photocatalytic Hydrogen Production: A Review. Int. J. Mol. Sci. 2024, 25, 8842. [Google Scholar] [CrossRef]
- Ismail, M.; Zhu, Y.; Hajra; Du, C.; Yang, L.; Jin, M.; Ma, X.; Cao, C.; Zou, M. A novel three-dimensional graphitic carbon nitride/palladium phosphide heterojunction photocatalyst for enhanced hydrogen production and treatment of industrial dye-polluted water under visible light irradiation. J. Environ. Chem. Eng. 2025, 13, 116413. [Google Scholar] [CrossRef]
- Jeyalakshmi, V.; Wu, S.; Qin, S.; Sarma, B.B.; Doronkin, D.E.; Zhou, X.; Kolařík, J.; Schmuk, P. Pd single atoms on g-C3N4 photocatalysts: Minimum loading for maximum activity. Chem. Sci. 2025, 16, 4788–4795. [Google Scholar] [CrossRef]
- Jahanshahi, R.; Moghadam, H.H.; Sobhani, S.; Sansano, J.M. ZnCo2O4@g-C3N4@Cu as a new and highly efficient heterogeneous photocatalyst for visible light induced cyanation and Mizoroki–Heck cross coupling reactions. RSC Adv. 2024, 14, 26424–26436. [Google Scholar] [CrossRef]
- Wang, N.; Ma, L.; Wang, J.; Zhang, Y.; Jiang, R. Graphitic Carbon Nitride (g-C3N4) Supported Palladium Species: An Efficient Heterogeneous Photocatalyst Surpassing Homogeneous Thermal Heating Systems for Suzuki Coupling. ChemPlusChem 2019, 84, 1164–1168. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, X.; Cheng, J.; Yang, X.; Lin, W.; Hou, Y.; Zhang, G.; Wang, X. Heterogeneous metallaphotocatalytic Cross-Coupling reactions by a carbon Nitride-Nickel catalyst. J. Catal. 2024, 433, 115461. [Google Scholar] [CrossRef]
- Yu, Q.; Lin, X.; Li, X.; Chen, J. Photocatalytic Stille Cross-coupling on Gold/g-C3N4 Nano-heterojunction. Chem. Res. Chin. Univ. 2020, 36, 1013–1016. [Google Scholar] [CrossRef]
- Ji, H.-T.; Tang, Y.-Q.; Wang, Y.-H.; Wang, J.-S.; Xu, Y.-D.; Zeng, Y.-Y.; Li, T.; Gong, S.-F.; He, W.-M. Dual Ce@g-C3N4-Photoredox/Chlorine Catalysis: Cross-Dehydrogenative Coupling of N-Heteroarenes and Alkanes/Ethers with H2 Evolution. Org. Lett. 2024, 26, 9822–9827. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Li, M.; Shan, Y.; Wang, X. Configured droplet reactor by Pd/g-C3N4 for the Suzuki-Miyaura cross-coupling reaction under water condition. Chin. J. Chem. Eng. 2025, 81, 232–240. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Cheng, S.; Zhou, X.; Shang, N.; Gao, S.; Wang, C. Pd supported on graphene modified g-C3N4 hybrid: A highly efficient catalyst for hydrogenation of nitroarenes. Appl. Organomet. Chem. 2020, 34, e5684. [Google Scholar] [CrossRef]
- Hu, F.; Leng, L.; Zhang, M.; Chen, W.; Yu, Y.; Wang, J.; Horton, J.H.; Li, Z. Direct Synthesis of Atomically Dispersed Palladium Atoms Supported on Graphitic Carbon Nitride for Efficient Selective Hydrogenation Reactions. ACS Appl. Mater. Interfaces 2020, 12, 54146–54154. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Han, F.; Wang, L.; Huang, X.; Cao, Y.; He, P.; Yang, H.; Chen, J.; Li, H. Ru/g-C3N4 as an efficient catalyst for selective hydrogenation of aromatic diamines to alicyclic diamines. RSC Adv. 2020, 10, 16515–16525. [Google Scholar] [CrossRef]
- Mishra, A.A.; Chaurasia, S.R.; Bhanage, B.M. Ru–g-C3N4 as highly active heterogeneous catalyst for transfer hydrogenation of α–keto amide into β–aminol or α–hydroxyl amide. New J. Chem. 2020, 44, 10578–10585. [Google Scholar] [CrossRef]
- Song, J.; Cai, X.; Chen, Z.; Wang, T.; Xi, S.; Hu, Q.; Yan, N.; Loh, K.P. Expedient alkyne semi-hydrogenation by using a bimetallic AgCu–C3N4 single atom catalyst. Chem. Sci. 2024, 15, 10577–10584. [Google Scholar] [CrossRef]
- Shahpal, A. Harnessing palladium-decorated g-C3N4 nanosheets for selective catalytic benzyl alcohol synthesis. Mol. Catal. 2024, 567, 114453. [Google Scholar] [CrossRef]
- Gao, X.; Li, Z.; Yan, W.; Peng, X. Palladium/graphitic carbon nitride catalyst for selective oxygen transfer in Wacker oxidation. J. Saudi Chem. Soc. 2020, 24, 663–672. [Google Scholar] [CrossRef]
- Gong, J.; Chen, X.; Tang, T. Recent progress in controlled carbonization of (waste) polymers. Progr. Polym. Sci. 2019, 94, 1–32. [Google Scholar] [CrossRef]
- Blanchard, R.; Mekonnen, T. Valorization of plastic waste via chemical activation and carbonization into activated carbon for functional material applications. RSC Appl. Polym. 2024, 2, 557–582. [Google Scholar] [CrossRef]
- Gu, S.; He, J.; Zhu, Y.; Wang, Z.; Chen, D.; Yu, G.; Pan, C.; Guan, J.; Tao, K. Facile Carbonization of Microporous Organic Polymers into Hierarchically Porous Carbons Targeted for Effective CO2 Uptake at Low Pressures. ACS Appl. Mater. Interfaces 2016, 8, 18383–18392. [Google Scholar] [CrossRef]
- Kou, J.; Sun, L.-B. Nitrogen-Doped Porous Carbons Derived from Carbonization of a Nitrogen-Containing Polymer: Efficient Adsorbents for Selective CO2 Capture. Ind. Eng. Chem. Res. 2016, 55, 10916–10925. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, W.; Wang, J.; Pan, X. Nitrogen-containing nanoporous carbons synthesized from direct carbonization of non-porous coordination polymers for CO2 capture. J. Porous Mater. 2023, 30, 1273–1282. [Google Scholar] [CrossRef]
- Lee, J.-S.; Briggs, M.; Hasell, T.; Cooper, A. Hyperporous Carbons from Hypercrosslinked Polymers. Adv. Mater. 2016, 28, 9804–9810. [Google Scholar] [CrossRef]
- Nabae, Y.; Nagata, S.; Ohnishi, K.; Liu, Y.; Sheng, L.; Wang, X.-L.; Hayakawa, T. Block Copolymer Templated Carbonization of Nitrogen Containing Polymer to Create Fine and Mesoporous Carbon for Oxygen Reduction Catalyst. J. Polym. Sci. Part A Polym. Chem. 2016, 55, 464–470. [Google Scholar] [CrossRef]
- Guo, K.; Liu, D.; Zhang, Y.; Feng, H.; Li, Q. A full-spectrum-responsive metal-organic-framework-derived carbon-supported bimetallic catalyst for photothermal catalytic methane dry reforming. Chem. Eng. J. 2025, 519, 165155. [Google Scholar] [CrossRef]
- Dong, Z.; Le, X.; Liu, Y.; Dong, C.; Ma, J. Metal organic framework derived magnetic porous carbon composite supported gold and palladium nanoparticles as highly efficient and recyclable catalysts for reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol. J. Mater. Chem. A 2014, 2, 18775–18785. [Google Scholar] [CrossRef]
- Thakur, S.; Gogate, P.R. Synthesis of Pd/C catalyst using formaldehyde reduction method and application for ultrasound assisted transfer hydrogenation of corn oil. Chem. Eng. Process. Process Intensif. 2020, 152, 107939. [Google Scholar] [CrossRef]
- Wen, J.; Chen, Y.; Ji, S.; Zhang, J.; Wang, D.; Li, Y. Metal-organic frameworks-derived nitrogen-doped carbon supported nanostructured PtNi catalyst for enhanced hydrosilylation of 1-octene. Nano Res. 2019, 12, 2584–2588. [Google Scholar] [CrossRef]
- Ke, Y.-H.; Yu, X.-M.; Wang, X.; Liu, H.; Yuan, H. Mofs-derived transition metal carbon-based compounds supported Au-Pt catalyst for the catalytic oxidation of glycerol to glyceric acid. Appl. Surf. Sci. 2025, 682, 161674. [Google Scholar] [CrossRef]
- Veerakumar, P.; Pounraj, T.; Lin, K. Simple Preparation of Porous Carbon Supported Ruthenium: Propitious Catalytic Activity in the Reduction of Ferrocyanate(III) and Cationic Dye. ACS Omega 2018, 3, 12609–12621. [Google Scholar] [CrossRef]
- Wang, H.; Gu, X.-K.; Zheng, X.; Pan, H.; Zhu, J.; Chen, S.; Cao, L.; Li, W.-X.; Lu, J. Disentangling the size-dependent geometric and electronic effects of palladium nanocatalysts beyond selectivity. Sci. Adv. 2019, 5, eaat6413. [Google Scholar] [CrossRef]
- Lai, N.S.; Tew, Y.S.; Zhong, X.; Yin, J.; Li, J.; Yan, B.; Wang, X. Artificial Intelligence (AI) Workflow for Catalyst Design and Optimization. Ind. Eng. Chem. Res. 2023, 62, 17835–17848. [Google Scholar] [CrossRef]
- Wen, X.; Geng, X.; Su, G.; Li, Y.; Li, Q.; Yi, Y.; Liu, L. Machine learning-driven design of single-atom catalysts for carbon dioxide valorization to high-value chemicals: A review of photocatalysis, electrocatalysis, and thermocatalysis. Green Chem. 2025, 27, 4898–4925. [Google Scholar] [CrossRef]
- Zhang, T.; Cheng, H.; Nian, Y.; Zhang, J.; Li, Q.; Han, Y. Application of generative artificial intelligence in catalysis. Chin. J. Chem. Eng. 2025, 84, 86–95. [Google Scholar] [CrossRef]

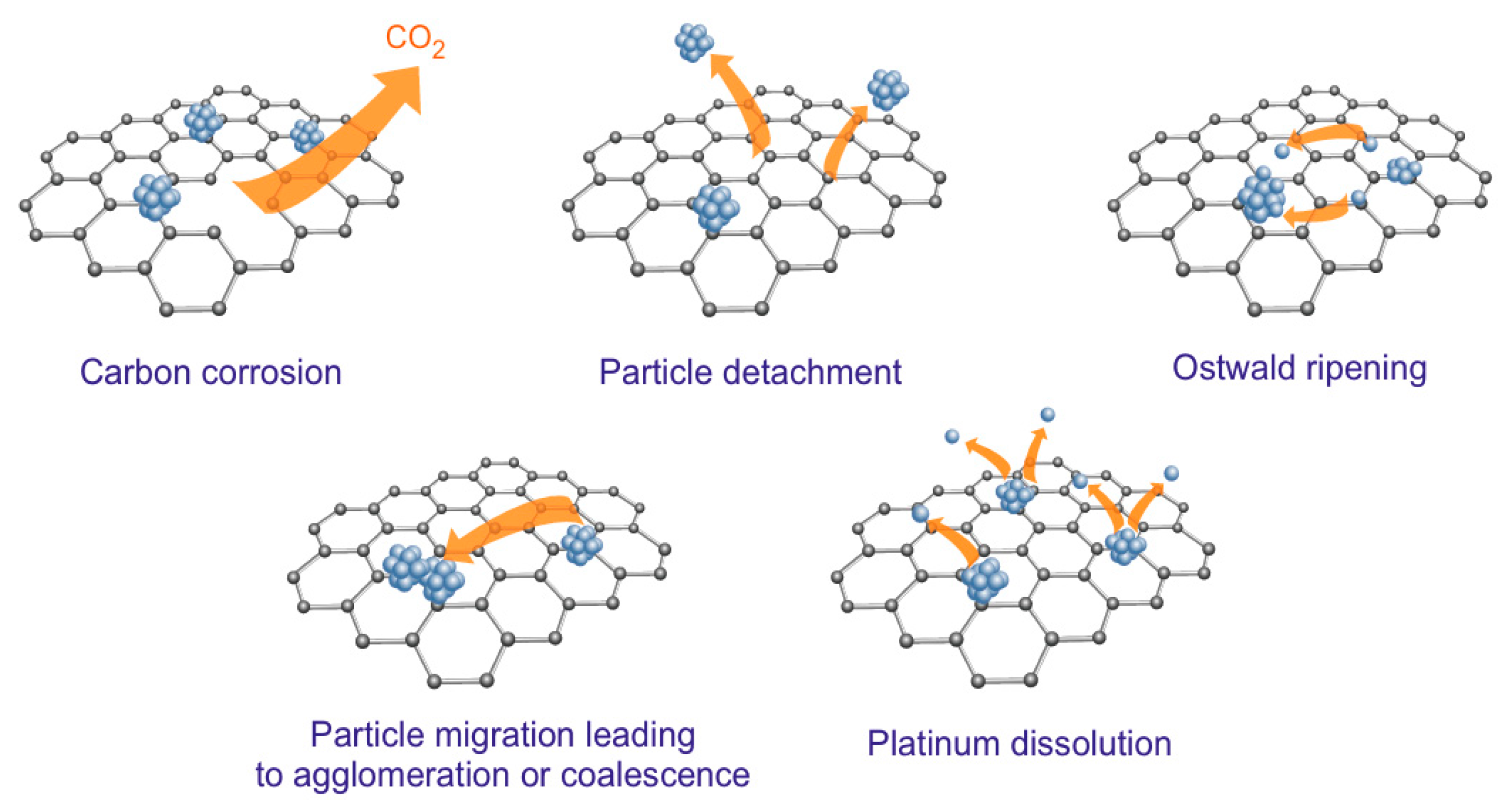


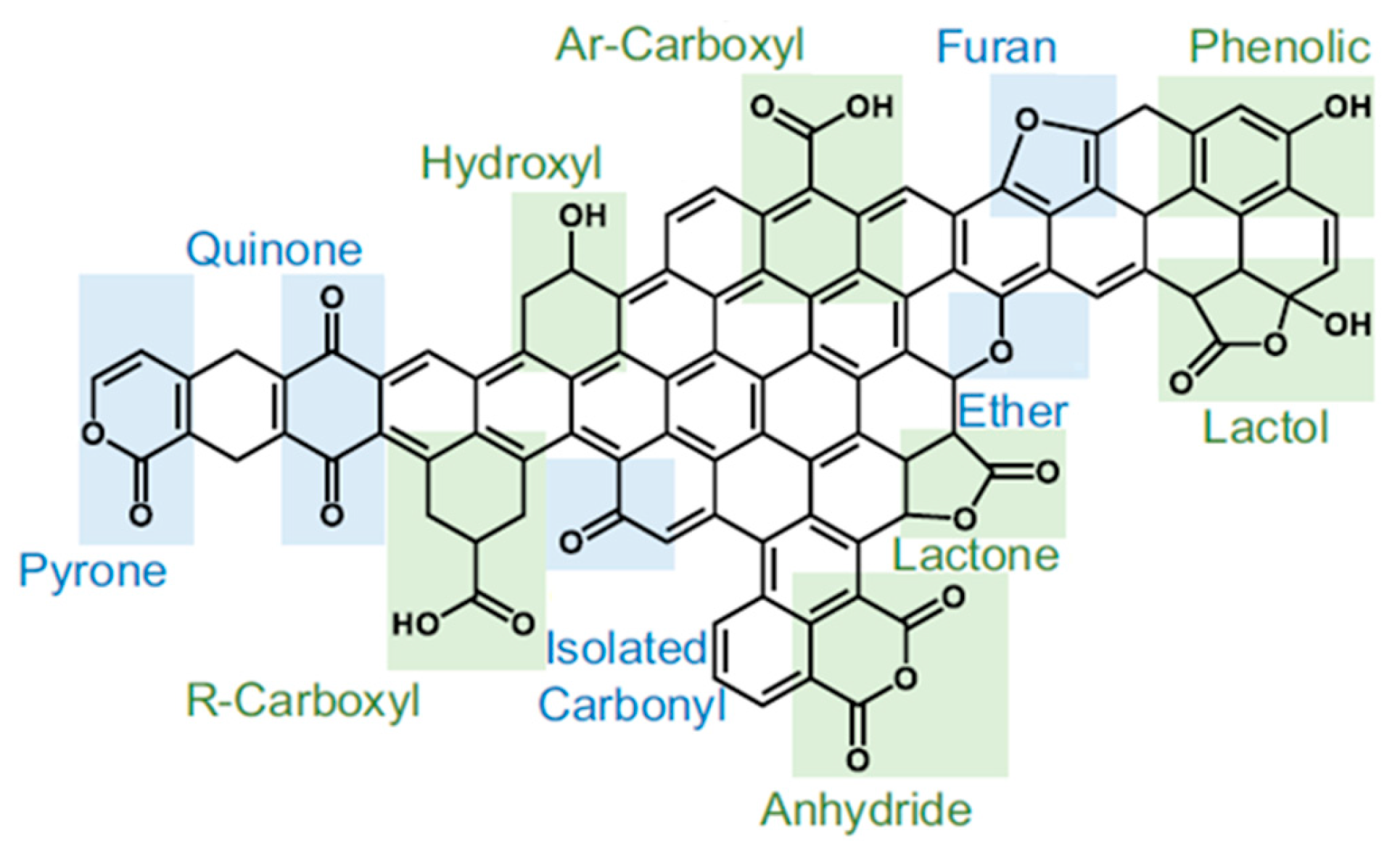
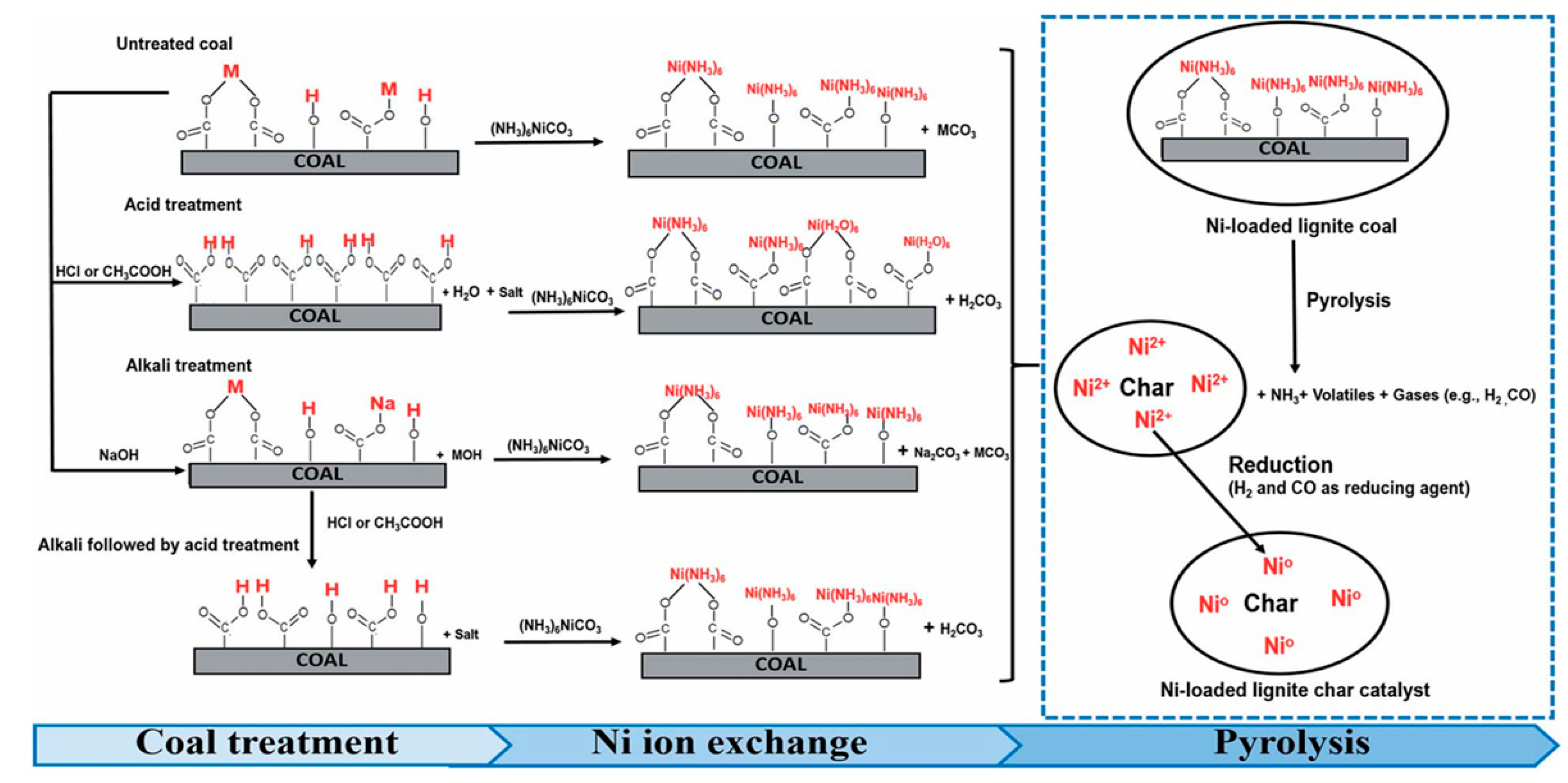







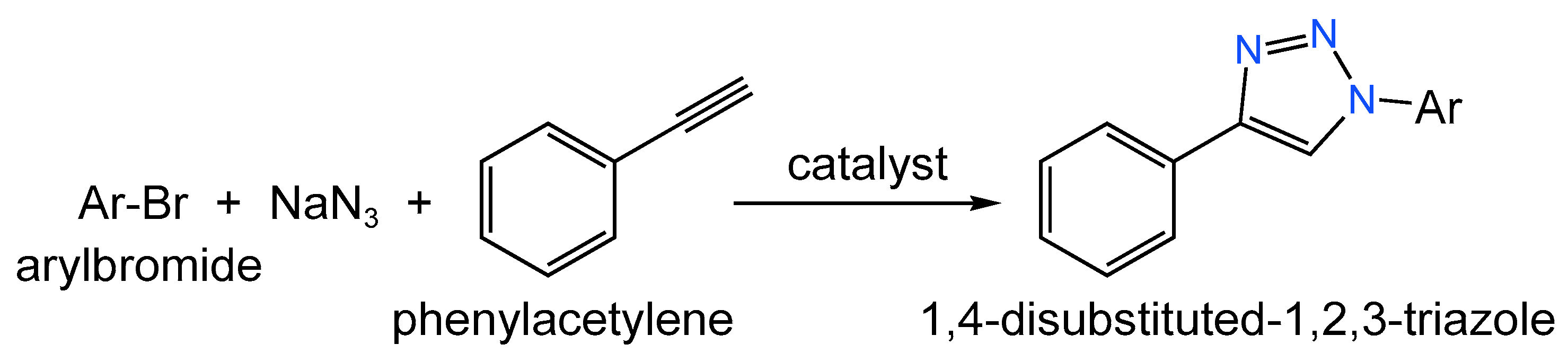
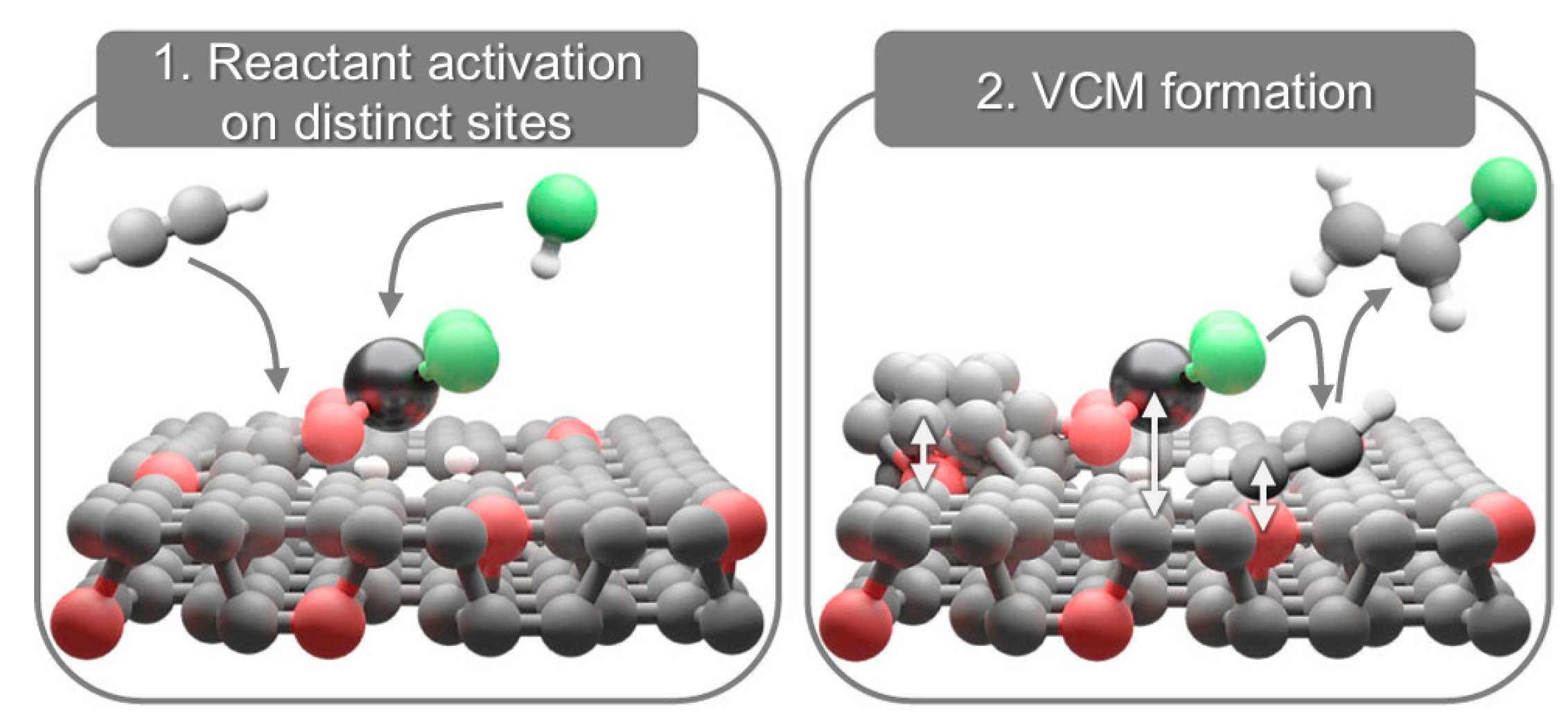





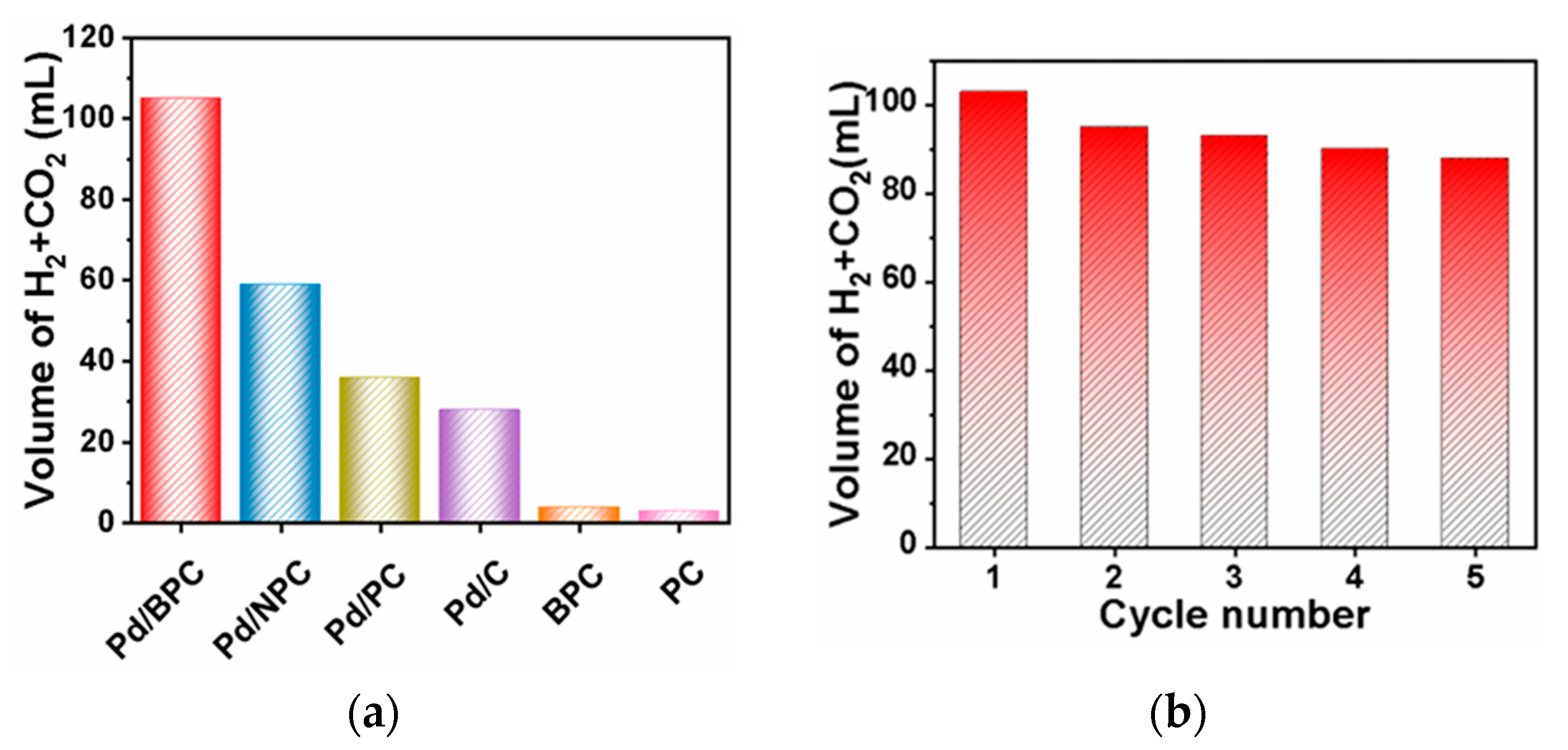
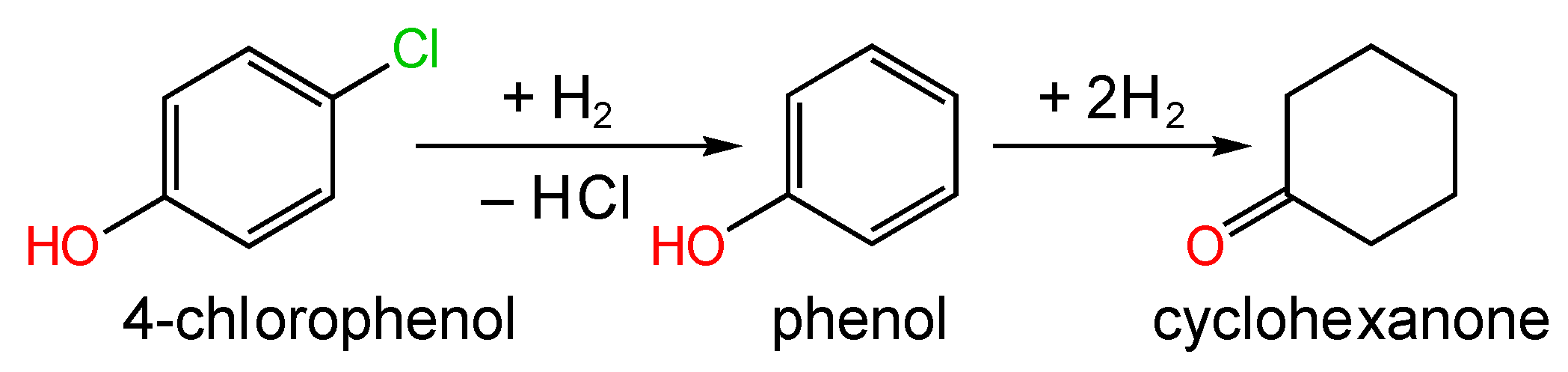





| Catalyst (Metal Content) | Method of Carbon Modification/Synthesis | Heteroatom Content, % | Sizes of Metal Particles, nm | BE of Pd, eV | Reducibility (Ratio of Pd0 and Pd2+) | Ref. |
|---|---|---|---|---|---|---|
| O-doped carbon supports | ||||||
| Pd/GNP (1 wt.%) | NA | 3.3 (XPS) | 3.5 ± 1.3 | ND | ND | [143] |
| Pd/GNP-Ox-4 (1 wt.%) | oxidation of GNP with HNO3 | 7.3 (XPS) | 3.8 ± 1.2 | 335.3 (Pd0) 337.7 (Pd2+) | Pd0/Pd2+ = 5.56 | [143] |
| Pd/GNP-Ox-6 (1 wt.%) | oxidation of GNP with H2O2 | 5.7 (XPS) | 3.9 ± 1.3 | 335.3 (Pd0) 337.5 (Pd2+) | Pd0/Pd2+ = 2.30 | [143] |
| Pd/MC-ox200 (4.76 wt.%) | reoxidation of MC with HNO3 followed by thermal treatment | 25.6 (elemental analysis) | 3.31 | ND | ND | [144] |
| Pd/MC-ox400 (4.80 wt.%) | 20.4 (elemental analysis) | 3.37 | ND | ND | [144] | |
| Pd/MC-ox800 (4.55 wt.%) | 4.7 (elemental analysis) | 3.57 | ND | ND | [144] | |
| Pd/NSC-600 (5 wt.%) | NA | 5.8 (elemental analysis) | 5.0 | 335.8 (Pd0) 337.8 (Pd2+) | Pd2+/Pd0 = 3.83 | [146] |
| Pd/NSC (5 wt.%) | NA | 7.6 (elemental analysis) | 2.8 | 335.9 (Pd0) 337.8 (Pd2+) | Pd2+/Pd0 = 4.18 | [146] |
| Pd/NSCox-2 (5 wt.%) | oxidation of NSC with HNO3 | 18.0 (elemental analysis) | 2.0 | 335.9 (Pd0) 337.8 (Pd2+) | Pd2+/Pd0 = 8.62 | [146] |
| 60 °C-H2-Pd/C (5 wt.%) | NA | 12.1 (XPS) | 3.2 ± 0.8 | 335.7 (Pd0) | NA | [147] |
| 400 °C-H2-Pd/C (5 wt.%) | NA | 11.0 (XPS) | 7.0 ± 1.3 | 335.5 (Pd0) | NA | [147] |
| 800 °C-H2-Pd/C (5 wt.%) | NA | 6.5 (XPS) | 22 ± 6 | 335.8 (Pd0) | NA | [147] |
| Pd/MPC (5.11 wt.%) | calcination of Fe-MIL-88A | ND | about 5 nm | 335.3 (Pd0) | NA | [234] |
| N-doped carbon supports | ||||||
| Pd@CN400 (Pd content not indicated) | direct carbonization of Pd-NHC complexes | 7.15 (elemental analysis) | 8.2 ± 0.8 | 335.5 (Pd0) | NA | [164] |
| Pd@CN800 (24 wt.%) | 4.45 (elemental analysis) | 12.3 ± 1.1 | 335.5 (Pd0) | NA | [164] | |
| Pd/CNTs (2.54 wt.%) | NA | NA | 3.1 ± 0.2 | about 335.9 for Pd0 | about 50% of Pd2+ | [197] |
| Pd/N@CNTs (2.27 wt.%) | chemical vapor deposition of pyridine to CNTs | 7.08 (XPS) | 2.7 ± 0.4 | 336.1 (Pd0) 338.3 (Pd2+) | 54.6% of Pd2+ | [197] |
| Pd/N@CNTs-900 (2.52 wt.%) | 5.25 (XPS) | 2.9 ± 0.3 | 336.2 (Pd0) 337.7 (Pd2+) | 46.45% of Pd2+ | [197] | |
| Pd/N@CNTs-1100 (2.52 wt.%) | 2.92 (XPS) | 2.9 ± 0.1 | 336.1 (Pd0) 337.9 (Pd2+) | 34.8% of Pd2+ | [197] | |
| Pd/N@CNTs-1300 (2.52 wt.%) | 1.84 (XPS) | 2.8 ± 0.2 | 336.0 (Pd0) 337.8 (Pd2+) | 38.9% of Pd2+ | [197] | |
| Pd/p-CNTs (4.70 wt.%) | defluorination of FCNTs | NA | 3.11 ± 0.78 | 335.7 (Pd0) ca. 337.8 (Pd2+) | ND | [198] |
| Pd/N@CNTs (4.59 wt.%) | defluorination of FCNTs, decomposition of pyridine | 7.98 (XPS) | 2.60 ± 0.46 | ca. 335.9 (Pd0) ca. 337.8 (Pd2+) | ND | [198] |
| Pd/N&S@CNTs (4.89 wt.%) | defluorination of FCNTs, decomposition of both pyridine and thiophene | 5.25 of N (XPS) 3.72 of S (XPS) | 2.24 ± 0.74 | 336.0 (Pd0) ca. 338.0 (Pd2+) | ND | [198] |
| Pd/N&P@CNTs (4.67 wt.%) | defluorination of FCNTs, decomposition of both pyridine and TPP | 7.60 of N (XPS) 2.86 of P (XPS) | 2.68 ± 0.54 | 335.9 (Pd0) ca. 338.0 (Pd2+) | ND | [198] |
| Pd/g-C3N4 (9.83 wt.%) | NA | ND | 3.82 | 335.2 (Pd0) 337.3 (Pd2+) | ND | [218] |
| Pd-CNNS/rGO20 (8.38 wt.%) | deposition of g-C3N4 nanosheets to the rGO | ND | 1.31 ± 0.02 | 336.2 (Pd0) | NA | [219] |
| Pd1/C3N4 (0.18 wt.%) | NA | ND | single atoms | 337.2 (Pd0) | NA | [220] |
| S-doped carbon supports | ||||||
| PdxS/SC-300 (5 wt.%) | carbonization of 2,2′ bithiophene | 13.2 (elemental analysis) | 0.90 ± 0.24 | 337.2 (Pd2+) | Pd0/Pd2+ = 0 | [170] |
| PdxS/SC-500 (5 wt.%) | 5.8 ± 1.8 | 335.6 (Pd0) | Pd0/Pd2+ = 3.06 | [170] | ||
| PdxS/SC-700 (5 wt.%) | 14.1 ± 6.2 | 335.6 (Pd0) | Pd0/Pd2+ = 3.75 | [170] | ||
| Pd/S@CNTs (4.50 wt.%) | doping of defluorinated FCNTs with sublimed S | 7.58 (XPS) | 2.18 ± 0.38 | 336.1 (Pd0) ca. 338.0 (Pd2+) | ND | [198] |
| P-doped carbon supports | ||||||
|
Pd/C
(2 wt.%) | NA | NA | 14.1 | 335.7 (Pd0) 337.8 (Pd2+) | Pd0/Pd2+ = 4.03 | [172] |
|
Pd/C-EG
(2 wt.%) | NA | NA | 14.3 | 335.7 (Pd0) 337.8 (Pd2+) | Pd0/Pd2+ = 2.26 | [172] |
|
Pd/C-P-EG
(2 wt.%) |
AC covered with P-doped carbon layer | 0.55 | 8.7 | 335.5 (Pd0) 337.5 (Pd2+) | Pd0/Pd2+ = 2.22 | [172] |
| Pd/CBC (0.49 wt.%) | NA | NA | ND | 335.8 (Pd0) 337.4 (Pd2+) | 79.72% of Pd2+ | [173] |
| Pd/PCBC (0.48 wt.%) | activation of biomass charcoal with H3PO4 | ND | ND | 335.8 (Pd0) 337.7 (Pd2+) | 92.73% of Pd2+ | [173] |
|
Pd/PNS
(10.3 wt.%) | activation of carbon with H3PO4 followed by thermal treatment | 4.3 (elemental analysis) | 4.4 ± 2.5 | 335.6 (Pd0) 337.8 (Pd2+) | 38% of Pd2+ | [174] |
|
Pd/PNS-700
(10.4 wt.%) | 8.6 (elemental analysis) | 5.4 ± 2.9 | 335.6 (Pd0) 337.8 (Pd2+) | 50% of Pd2+ | [174] | |
| Pd/P@CNTs (4.93 wt.%) | defluorination of FCNTs, decomposition of TPP | 3.41 (XPS) | 2.58 ± 0.39 | ca. 336.0 (Pd0) ca. 337.8 (Pd2+) | ND | [198] |
| B-doped carbon supports | ||||||
| Pd/BPC (3 wt.%) | treatment of PC with boric acid | ND | 3.6 | 335.7 (Pd0) 337.2 (Pd2+) | ND | [109] |
| Pd/B3-CNPs (29.34 wt.%) | thermal decomposition of acetylene in the presence of ferrocene and boric acid | 4.98 (EDS) | 66.63 | 335.6 (Pd0) ca. 337.0 (Pd2+) | ND | [178] |
| Pd@BOMC (1 wt.%) | HTC of boric acid and resorcinol/hexamethylenetetramine, self-assembly with Pluronic F127 | 2.5 of N (XPS) 0.85 of B (XPS) 11.85 of O (XPS) | 3 | 335.6 (Pd0) 337.5 (Pd2+) | ND | [180] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikoshvili, L.Z.; Bakhvalova, E.S.; Sulman, M.G. The Role of Surface Chemistry in Carbon-Supported Metal-Catalyzed Processes of Fine Organic Synthesis. Chemistry 2025, 7, 198. https://doi.org/10.3390/chemistry7060198
Nikoshvili LZ, Bakhvalova ES, Sulman MG. The Role of Surface Chemistry in Carbon-Supported Metal-Catalyzed Processes of Fine Organic Synthesis. Chemistry. 2025; 7(6):198. https://doi.org/10.3390/chemistry7060198
Chicago/Turabian StyleNikoshvili, Linda Zh., Elena S. Bakhvalova, and Mikhail G. Sulman. 2025. "The Role of Surface Chemistry in Carbon-Supported Metal-Catalyzed Processes of Fine Organic Synthesis" Chemistry 7, no. 6: 198. https://doi.org/10.3390/chemistry7060198
APA StyleNikoshvili, L. Z., Bakhvalova, E. S., & Sulman, M. G. (2025). The Role of Surface Chemistry in Carbon-Supported Metal-Catalyzed Processes of Fine Organic Synthesis. Chemistry, 7(6), 198. https://doi.org/10.3390/chemistry7060198