A Bacteria Sol–Gel Template Approach to Form Palladium Core–Shell Catalysts for Suzuki–Miyaura Reactions
Abstract
1. Introduction
2. Materials and Methods
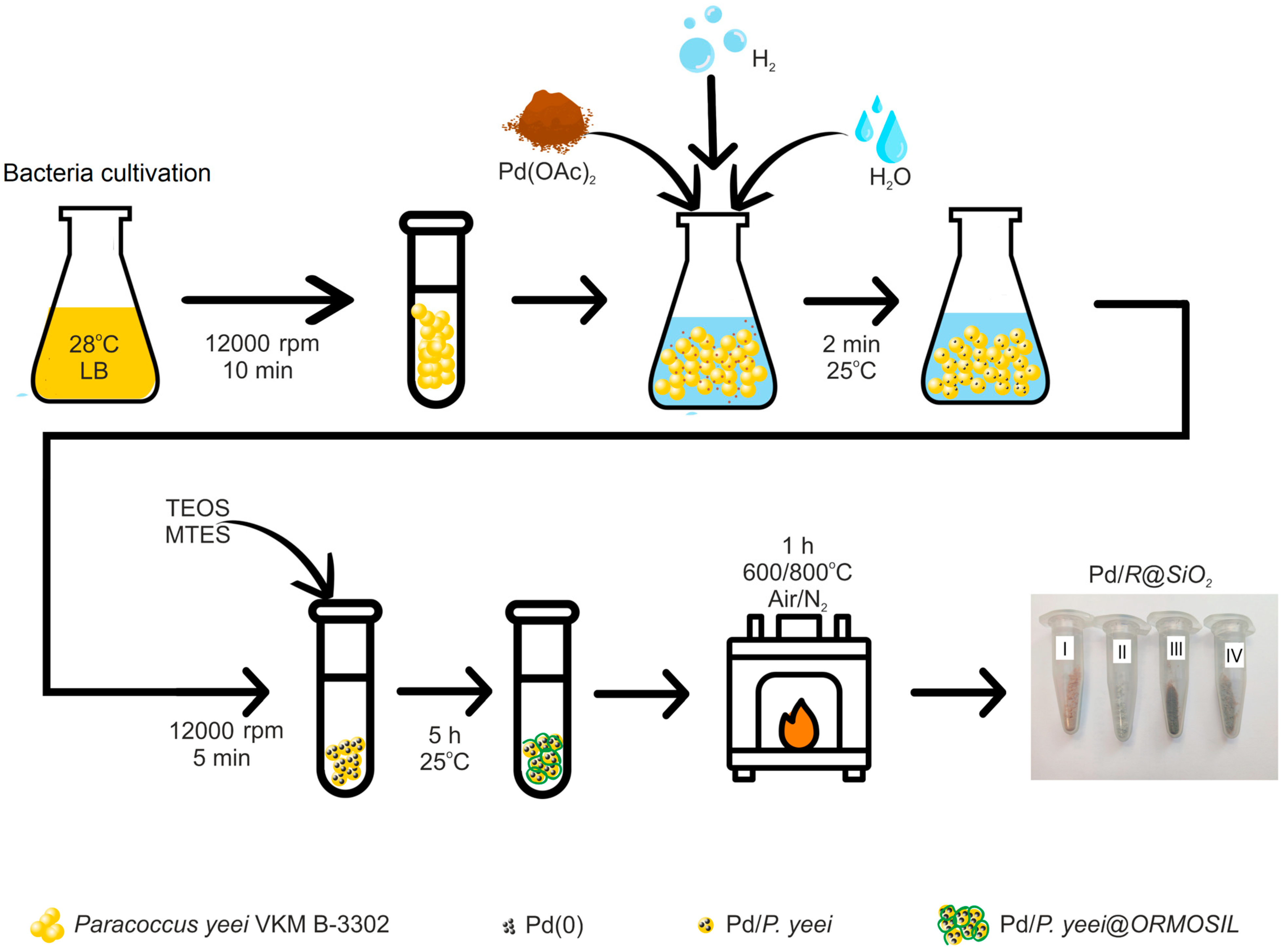
2.1. Bacterial Cell Cultivation
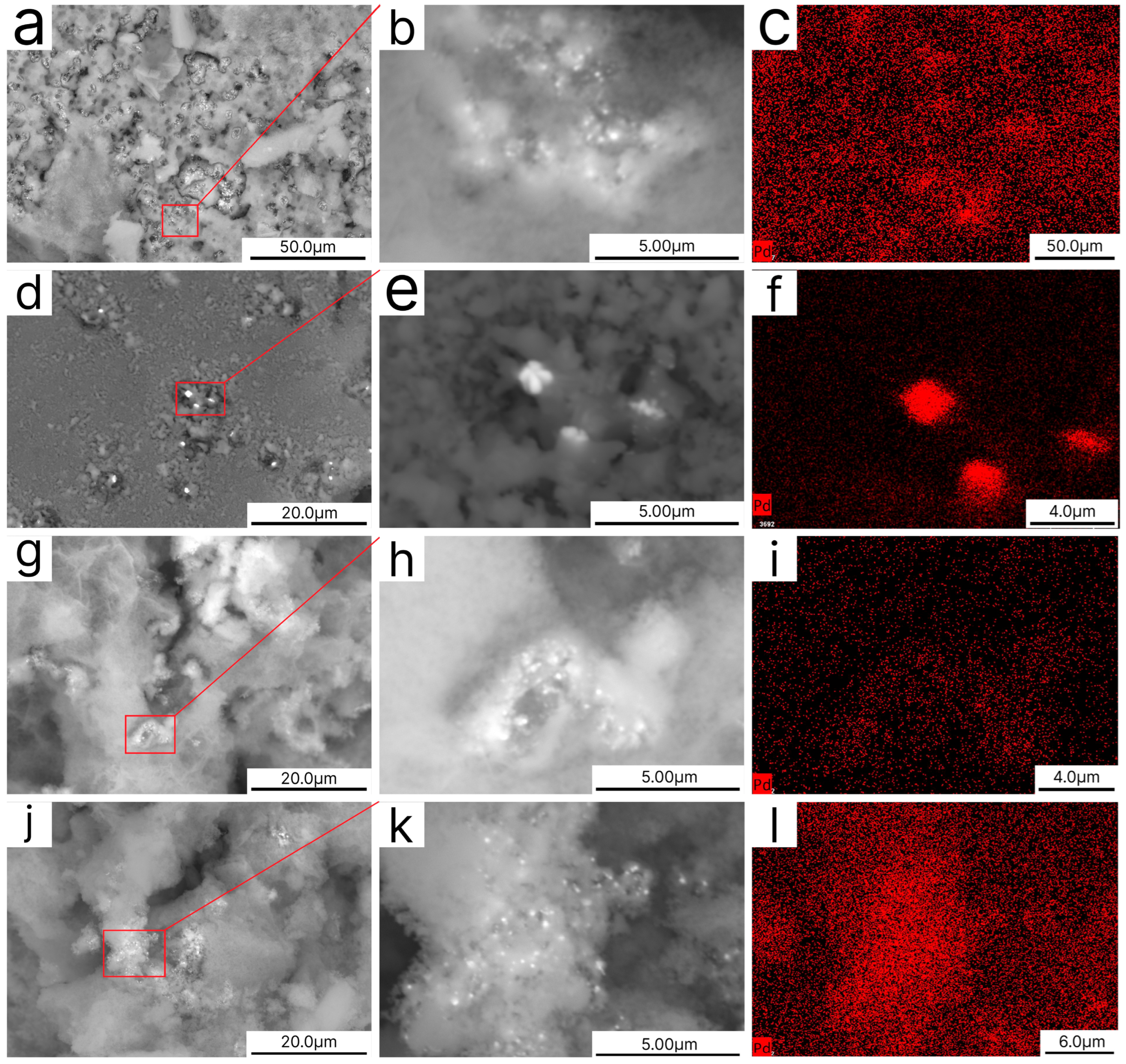
2.2. Scanning Electron Microscopy (SEM) Measurements
2.3. Transmission Electron Microscopy (TEM) Measurements
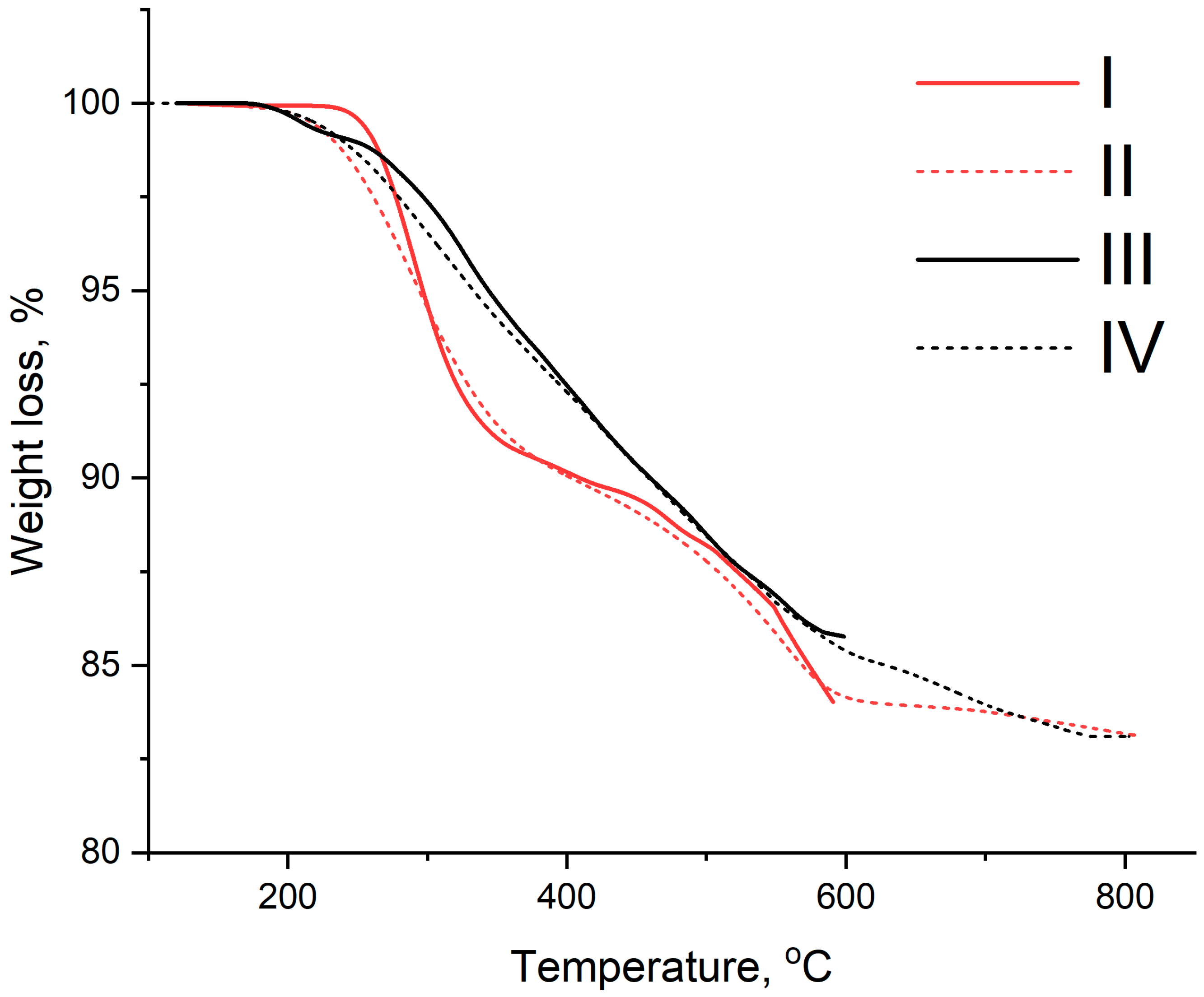
2.4. Thermogravimetric Analysis
2.5. Determination of Pd Content
2.6. Pd/R@SiO2 for Suzuki–Miyaura Reaction
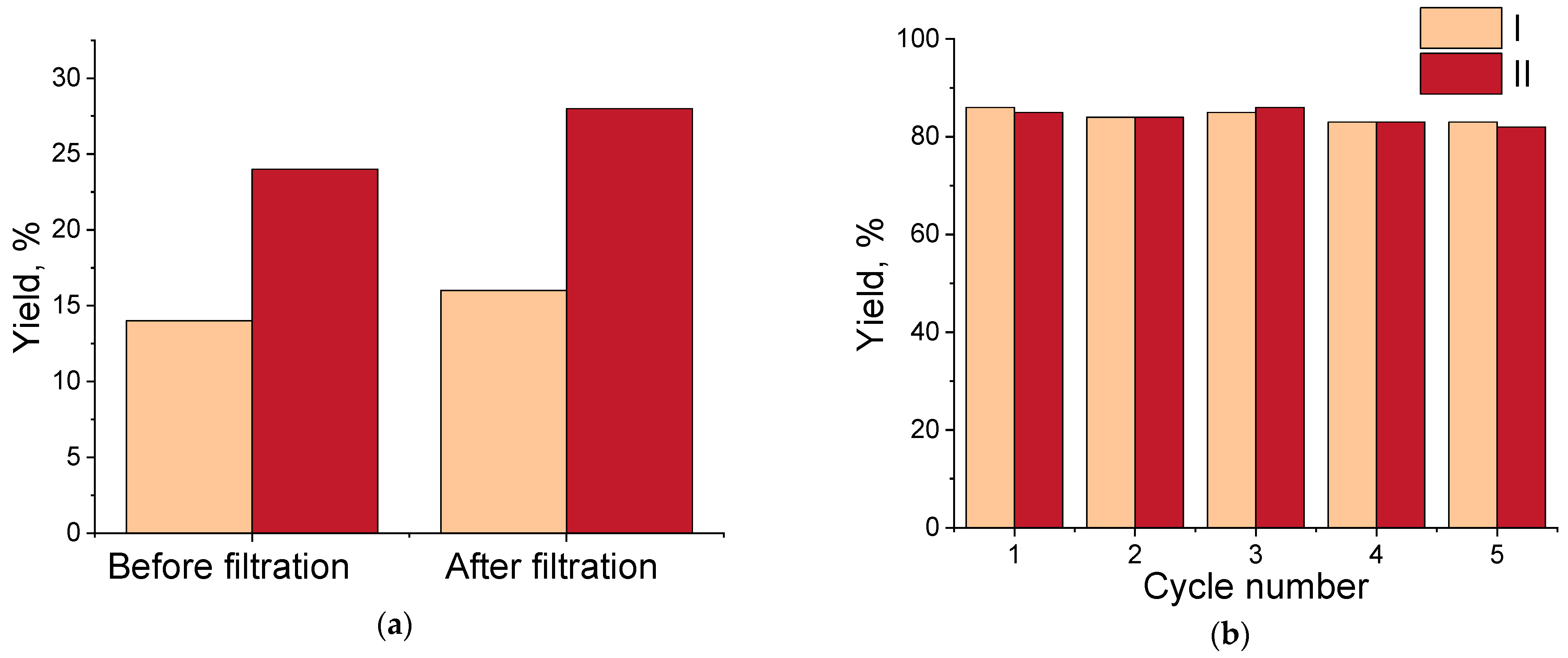
2.7. Pd/R@SiO2 Recycling Test
2.8. Pd/R@SiO2 Hot Filtration Test
3. Results
3.1. Catalyst Preparation
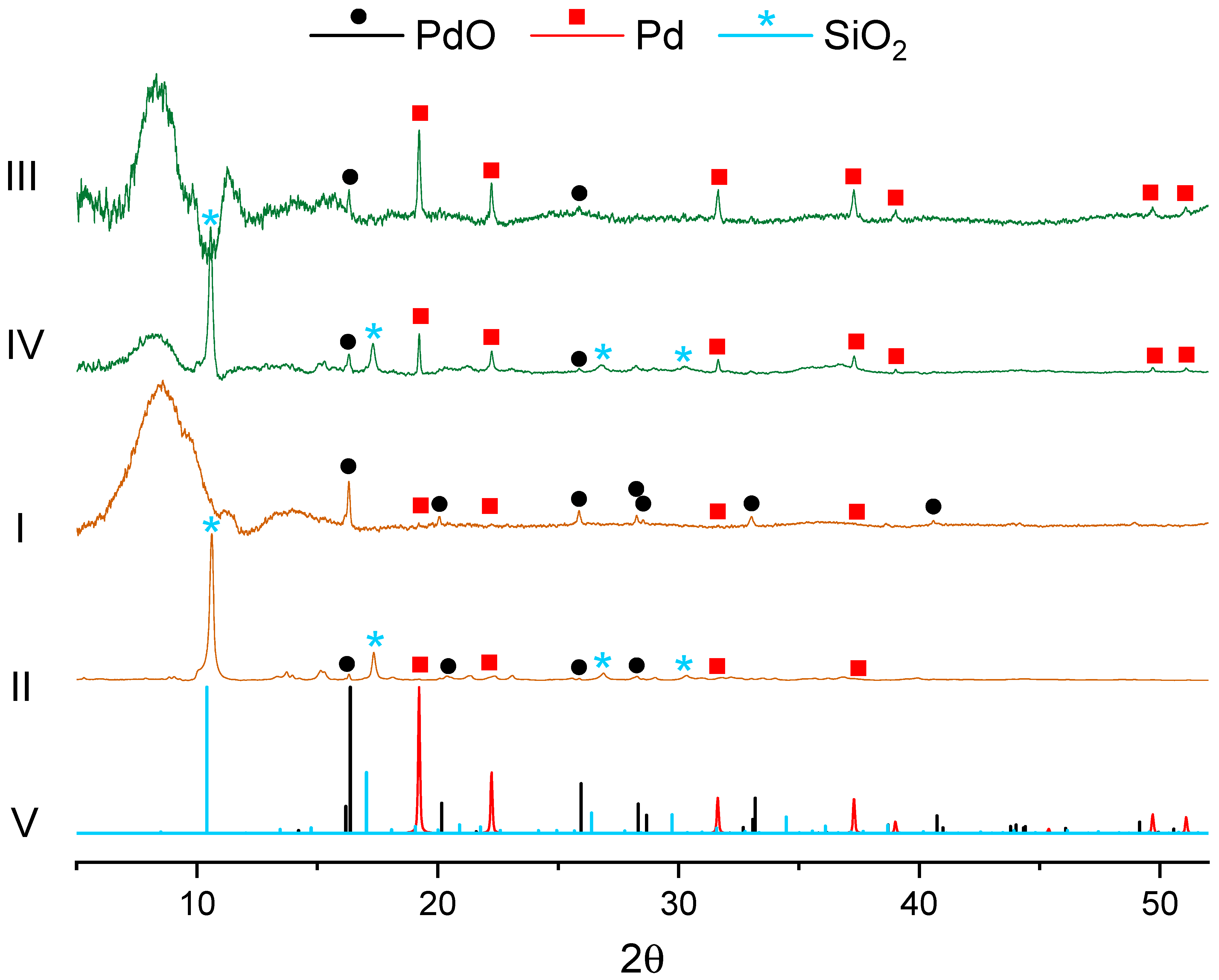
3.2. Catalyst Characterization
3.3. Catalytic Tests of (I–IV) Pd/R@SiO2 Catalysts in the Suzuki–Miyaura Reaction
4. Discussion
5. Conclusions
- The bacterial template effectively stabilizes the nanoparticle size, preventing significant agglomeration even after the calcination procedure;
- The sol–gel encapsulation does not significantly change the Pd nanoparticle size distribution, with sizes remaining in the 4–5 nm range before and after the process;
- The post-synthetic thermal treatment provides a viable pathway for modulating the nanoparticle size, offering a means to tailor the catalyst for reactions beyond cross-coupling;
- The resulting catalyst, with a low palladium loading of approximately 1 wt%, exhibits high activity in the Suzuki–Miyaura reaction, achieving competitive yields.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TEOS | Tetraethoxysilane |
| MTES | Methyltriethoxysilane |
| XRD | X-Ray Diffraction |
| TEM | Transmission Electronic Microscopy |
| SEM | Scanning Electronic Microscopy |
| EDX | Energy Dispersive X-Ray |
| TGA | Thermogravimetric Analysis |
| NP | Nanoparticle |
| BSE | Backscattered Electron |
| ICP-OES | Optical Emission Spectrometer with Inductively Coupled Plasma |
| GC-MS | Gas Chromatography–Mass Spectrometry |
References
- Kader, D.A.; Sidiq, M.K.; Taher, S.G.; Aziz, D.M. Recent advances in palladium-catalyzed Suzuki-Miyaura cross-coupling reactions: Exploration of catalytic systems, reaction parameters, and ligand influences: A review. J. Organomet. Chem. 2025, 15, 123569. [Google Scholar] [CrossRef]
- Farhang, M.; Akbarzadeh, A.R.; Rabbani, M.; Ghadiri, A.M. A retrospective-prospective review of Suzuki–Miyaura reaction: From cross-coupling reaction to pharmaceutical industry applications. Polyhedron 2022, 227, 116124. [Google Scholar] [CrossRef]
- Bourouina, A.; Meille, V.; de Bellefon, C. About Solid Phase vs. Liquid Phase in Suzuki-Miyaura Reaction. Catalysts 2019, 9, 60. [Google Scholar] [CrossRef]
- Li, Z.; Gelbaum, C.; Heaner, W.L.; Fisk, J.; Jaganathan, A.; Holden, B.; Pollet, P.; Liotta, C.L. Palladium-Catalyzed Suzuki Reactions in Water with No Added Ligand: Effects of Reaction Scale, Temperature, pH of Aqueous Phase, and Substrate Structure. Org. Process Res. Dev. 2016, 20, 1489–1499. [Google Scholar] [CrossRef]
- Marquard, A.N.; Slaymaker, L.E.; Hamers, R.J.; Goldsmith, R.H. Investigation of activity, stability, and degradation mechanism of surface-supported Pd-PEPPSI complexes for Suzuki-Miyaura coupling. Mol. Catal. 2017, 429, 10–17. [Google Scholar] [CrossRef]
- Mpungose, P.P.; Vundla, Z.P.; Maguire, G.E.M.; Friedrich, H.B. The Current Status of Heterogeneous Palladium Catalysed Heck and Suzuki Cross-Coupling Reactions. Molecules 2018, 23, 1676. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Perez-Ramirez, J.; Gong, J.; Dewangan, N.; Hidajat, K.; Gates, B.C.; Kawi, S. Core-shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2. Chem. Soc. Rev. 2020, 49, 2937–3004. [Google Scholar] [CrossRef]
- Egan-Morriss, C.; Kimber, R.L.; Powell, N.A.; Lloyd, J.R. Biotechnological synthesis of Pd-based nanoparticle catalysts. Nanoscale Adv. 2022, 4, 654–679. [Google Scholar] [CrossRef]
- Roy, A.; Elzaki, A.; Tirth, V.; Kajoak, S.; Osman, H.; Algahtani, A.; Islam, S.; Faizo, N.L.; Khandaker, M.U.; Islam, M.N.; et al. Biological Synthesis of Nanocatalysts and Their Applications. Catalysts 2021, 11, 1494. [Google Scholar] [CrossRef]
- Qamar, S.U.R.; Ahmad, J.N. Nanoparticles: Mechanism of biosynthesis using plant extracts, bacteria, fungi, and their applications. J. Mol. Liq. 2021, 334, 116040. [Google Scholar] [CrossRef]
- Koul, B.; Poonia, A.K.; Yadav, D.; Jin, J.O. Microbe-Mediated Biosynthesis of Nanoparticles: Applications and Future Prospects. Biomolecules 2021, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Coccia, F.; Mascitti, A.; Caporali, S.; Polito, L.; Porcheddu, A.; Di Nicola, C.; Tonucci, L.; d’Alessandro, N. Wool-supported Pd and Rh nanoparticles for selective hydrogenation of maleic acid to succinic acid in batch and flow systems. RSC Adv. 2025, 15, 36760–36768. [Google Scholar] [CrossRef]
- Singh, M.; Sinha, A.; Qazi, A.; Soni, R.; Rana, V.; Tyagi, T. Bacteria and their mediated nanoparticles for the environmental sustainable development to support SDGs. In Nanoparticles Synthesis by Soil Microbes; Elsevier: Amsterdam, The Netherlands, 2025; pp. 253–270. [Google Scholar]
- Rybochkin, P.V.; Kamanina, O.A.; Lantsova, E.A.; Arlyapov, V.A.; Saverina, E.A. Characterization of the catalytic ability and surface properties of a heterogeneous biocatalyst obtained by the sol-gel method. J. Sol-Gel Sci. Technol. 2022, 108, 310–319. [Google Scholar] [CrossRef]
- Lantsova, E.A.; Rybochkin, P.V.; Saverina, E.A.; Kamanina, O.A. Biohybrid silicon-organic materials architecture obtained using various structure-affecting agents. J. Sol-Gel Sci. Technol. 2024, 110, 134–141. [Google Scholar] [CrossRef]
- Rybochkin, P.V.; Perchikov, R.N.; Karlinskii, B.Y.; Kamanina, O.A.; Arlyapov, V.A.; Kashin, A.S.; Ananikov, V.P. Aerobic bacteria-supported biohybrid palladium catalysts for efficient cross-coupling reactions. J. Catal. 2024, 429, 115238. [Google Scholar] [CrossRef]
- Kamanina, O.A.; Rybochkin, P.V.; Borzova, D.V.; Soromotin, V.N.; Galushko, A.S.; Kashin, A.S.; Ivanova, N.M.; Zvonarev, A.N.; Suzina, N.E.; Holicheva, A.A.; et al. Sustainable catalysts in a short time: Harnessing bacteria for swift palladium nanoparticle production. Nanoscale 2025, 17, 5289–5300. [Google Scholar] [CrossRef]
- Kamanina, O.A.; Fedoseeva, D.G.; Rogova, T.V.; Ponamoreva, O.N.; Blokhin, I.V.; Machulin, A.V.; Alferov, V.A. Synthesis of organosilicon sol-gel matrices and preparation of heterogeneous biocatalysts based on them. Russ. J. Appl. Chem. 2014, 87, 761–766. [Google Scholar] [CrossRef]
- Ponamoreva, O.N.; Kamanina, O.A.; Alferov, V.A.; Machulin, A.V.; Rogova, T.V.; Arlyapov, V.A.; Alferov, S.V.; Suzina, N.E.; Ivanova, E.P. Yeast-based self-organized hybrid bio-silica sol-gels for the design of biosensors. Biosens. Bioelectron. 2015, 67, 321–326. [Google Scholar] [CrossRef]
- Palomo, J.; Caspers, M.; Urakawa, A. Nanostructured Rh/SiC@SiO2 core@shell catalysts for microwave-assisted dry reforming of methane. Catal. Today 2025, 460, 115464. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Saybulina, E.R.; Trenikhin, M.V.; Izmailov, R.R.; Lotsman, K.A.; Rodygin, K.S.; Ananikov, V.P. Comparing Separation vs. Fresh Start to Assess Reusability of Pd/C Catalyst in Liquid-Phase Hydrogenation. ChemCatChem 2021, 13, 3656–3661. [Google Scholar] [CrossRef]
- Puri, L.; Hu, Y.; Naterer, G. Critical review of the role of ash content and composition in biomass pyrolysis. Front. Fuels 2024, 2, 1378361. [Google Scholar] [CrossRef]
- Rong, H.; Wang, T.; Zhou, M.; Wang, H.; Hou, H.; Xue, Y. Combustion Characteristics and Slagging during Co-Combustion of Rice Husk and Sewage Sludge Blends. Energies 2017, 10, 438. [Google Scholar] [CrossRef]
- Kalishyn, Y.Y.; Ordynskyi, V.V.; Ishchenko, M.V.; Bychko, I.B.; Kaidanovych, Z.V.; Trypolskyi, A.I.; Strizhak, P.E. Synthesis and Thermal Stability of Palladium Nanoparticles Supported on γ-Al2O3. Curr. Nanomater. 2020, 5, 79–90. [Google Scholar] [CrossRef]
- Rahmati, M.; Safdari, M.-S.; Fletcher, T.H.; Argyle, M.D.; Bartholomew, C.H. Chemical and Thermal Sintering of Supported Metals with Emphasis on Cobalt Catalysts During Fischer–Tropsch Synthesis. Chem. Rev. 2020, 120, 4455–4533. [Google Scholar] [CrossRef]
- Moulijn, J.A.; Van Diepen, A.; Kapteijn, F. Catalyst deactivation: Is it predictable?: What to do? Appl. Catal. A Gen. 2001, 212, 3–16. [Google Scholar] [CrossRef]
- Rostovshchikova, T.N.; Shilina, M.I.; Gurevich, S.A.; Yavsin, D.A.; Veselov, G.B.; Stoyanovskii, V.O.; Vedyagin, A.A. Studies on High-Temperature Evolution of Low-Loaded Pd Three-Way Catalysts Prepared by Laser Electrodispersion. Materials 2023, 16, 3501. [Google Scholar] [CrossRef]
- Seo, C.Y.; Chen, X.; Sun, K.; Allard, L.F.; Fisher, G.B.; Schwank, J.W. Palladium redispersion at high temperature within the Pd@SiO2 core@shell structure. Catal. Commun. 2018, 108, 73–76. [Google Scholar] [CrossRef]
- Valerio, A.; Trindade, F.J.; Penacchio, R.F.S.; Cisi, B.; Damasceno, S.; Estradiote, M.B.; Rodella, C.B.; Ferlauto, A.S.; Kycia, S.W.; Morelhao, S.L. Implications of size dispersion on X-ray scattering of crystalline nanoparticles: CeO2 as a case study. J. Appl. Crystallogr. 2024, 57, 793–807. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Tsybulya, S.V. Influence of Nanoparticles Size on XRD Patterns for Small Monodisperse Nanoparticles of Cu0 and TiO2 Anatase. Ind. Eng. Chem. Res. 2018, 57, 2526–2536. [Google Scholar] [CrossRef]
- Dastoom, Z. Production of Ni0.5Co0.5Fe2O4/activated carbon@chitosan magnetic nanobiocomposite as a novel adsorbent of methylene blue in aqueous solutions. Sci. Rep. 2023, 13, 6137. [Google Scholar] [CrossRef]
- Usoltsev, O.; Stoian, D.; Skorynina, A.; Kozyr, E.; Njoroge, P.N.; Pellegrini, R.; Groppo, E.; van Bokhoven, J.A.; Bugaev, A. Restructuring of Palladium Nanoparticles during Oxidation by Molecular Oxygen. Small 2024, 20, e2401184. [Google Scholar] [CrossRef] [PubMed]
- Johansson Seechurn, C.C.C.; Sperger, T.; Scrase, T.G.; Schoenebeck, F.; Colacot, T.J. Understanding the Unusual Reduction Mechanism of Pd(II) to Pd(I): Uncovering Hidden Species and Implications in Catalytic Cross-Coupling Reactions. J. Am. Chem. Soc. 2017, 139, 5194–5200. [Google Scholar] [CrossRef]
- Amoroso, F.; Colussi, S.; Del Zotto, A.; Llorca, J.; Trovarelli, A. PdO hydrate as an efficient and recyclable catalyst for the Suzuki–Miyaura reaction in water/ethanol at room temperature. Catal. Commun. 2011, 12, 563–567. [Google Scholar] [CrossRef]
- Del Zotto, A.; Zuccaccia, D. Metallic palladium, PdO, and palladium supported on metal oxides for the Suzuki–Miyaura cross-coupling reaction: A unified view of the process of formation of the catalytically active species in solution. Catal. Sci. Technol. 2017, 7, 3934–3951. [Google Scholar] [CrossRef]

| I (600 °C, O2) | II (800 °C, O2) | III (600 °C, N2) | IV (800 °C, N2) | |
|---|---|---|---|---|
 | 84% | 85% | 95% | 89% |
 | 72% | 75% | 84% | 80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soromotin, V.N.; Rybochkin, P.V.; Pertseva, V.A.; Kamanina, O.A. A Bacteria Sol–Gel Template Approach to Form Palladium Core–Shell Catalysts for Suzuki–Miyaura Reactions. Chemistry 2025, 7, 188. https://doi.org/10.3390/chemistry7060188
Soromotin VN, Rybochkin PV, Pertseva VA, Kamanina OA. A Bacteria Sol–Gel Template Approach to Form Palladium Core–Shell Catalysts for Suzuki–Miyaura Reactions. Chemistry. 2025; 7(6):188. https://doi.org/10.3390/chemistry7060188
Chicago/Turabian StyleSoromotin, Vitaliy N., Pavel V. Rybochkin, Violetta A. Pertseva, and Olga A. Kamanina. 2025. "A Bacteria Sol–Gel Template Approach to Form Palladium Core–Shell Catalysts for Suzuki–Miyaura Reactions" Chemistry 7, no. 6: 188. https://doi.org/10.3390/chemistry7060188
APA StyleSoromotin, V. N., Rybochkin, P. V., Pertseva, V. A., & Kamanina, O. A. (2025). A Bacteria Sol–Gel Template Approach to Form Palladium Core–Shell Catalysts for Suzuki–Miyaura Reactions. Chemistry, 7(6), 188. https://doi.org/10.3390/chemistry7060188






