Abstract
Advancing catalysts for low-temperature NH3-SCR enhances their viability as a terminal flue gas denitration solution across diverse operating regimes. A high-performance, hydrothermally stable catalyst for low-temperature SCR was synthesized by depositing MnOx onto MgAlOx composite oxide supports. These supports, featuring varied Mg/Al ratios, originated from layered double hydroxide (LDH) precursors. The obtained catalyst with the Mg/Al ratio of 2 (Mn/Mg2AlOx) possesses relatively high concentrations of active oxygen species (Oα) and Mn4+ and exhibits remarkable catalytic performance. The Mn/Mg2AlOx catalyst exhibits a wide operating temperature range (100–300 °C) for denitration, achieving over 80% NOx conversion, along with robust water resistance. The temperature-programed surface reactions and NO oxidation reactions are performed to elucidate the promoting effect of water on N2 selectivity, which is not only due to inhibition of catalyst oxidation capacity at high temperature but also is related to the competing adsorption of water and NH3. In situ DRIFTS analysis confirmed that the NH3-SCR mechanism over Mn/Mg2AlOx adheres to the Eley–Rideal (E–R) pathway. These findings highlight the significant promise of Mn/MgAlOx catalysts for deployment as downstream denitration units within exhaust treatment systems.
1. Introduction
Generated during industrial fossil fuel combustion, nitrogen oxides (NOx) represent significant atmospheric contaminants. These compounds facilitate the formation of secondary pollutants, notably fine particulate matter (PM2.5) and ozone (O3) [1,2]. The selective catalytic reduction with ammonia (NH3-SCR) technique is a high-efficiency denitration method. As the fundamental component governing NH3-SCR system viability, the catalyst dictates operational practicality. Since the 1970s, V2O5-WO3(MoO3)/TiO2 has served as a highly effective medium-temperature (300–400 °C) catalyst in this technology [3,4]. Zeolite-based catalysts with unique pore structure and excellent catalytic performance have also received more and more attention and application in petrochemical, energy and environmental fields [5,6]. However, it has numerous inherent disadvantages. For example, V2O5 with biologic toxicity is prone to sublimation and shedding during actual operation as well as diffusion into the external environment with the fumes, causing serious harm to humans and the environment. Furthermore, the high quantities of SO2 and dust in the exhaust might nevertheless damage the V-based catalyst over a prolonged period of operation, leading to blockage or covering of the active sites and, subsequently, deactivation. The installation of NH3-SCR reactors at the end of a purification unit can reduce the impact of toxic flue gases on the catalyst, where the gas temperature is lower [7,8]. The hydrothermal stability of zeolite catalysts still needs to be improved, and the denitrification efficiency at low temperature is still not ideal [9]. Designing catalysts that are operational at low temperatures enables broader deployment of NH3-SCR as a terminal flue gas denitrification solution, ensuring adaptability across diverse operating conditions.
The multivalence and robust redox capacity of Mn-based catalysts enable “outstanding low-temperature performance”, making them well-suited for implementation in final-stage flue gas cleaning processes [10,11,12]. Stringent SO2 emission regulations (e.g., a limit of 35 mg/m3 in flue gas) present new opportunities for Mn-based catalysts in NOx removal applications. Even after desulfurization and the Cottrell process, the flue gas still contains some residual H2O, for example, the exhaust of waste-to-power (generating electricity through waste incineration), gas turbines and biomass combustion, which is the biggest obstruction to the application in fact of pure MnOx in terminal denitration systems. As a solution, one of the cutting-edge strategies for catalyst design is to effectively improve the water tolerance [13]. Gu et al. [14] synthesized Mn–Fe mixed oxides loaded with titanium silicalite-1 (TS-1) for NH3-SCR, which exhibited high water resistance and maintained about 94% NOx conversion, while 10% H2O was present for 12 h at 180 °C. The addition of Ti provided the active ingredient with additional surface acidic sites and stronger redox capacity. Zhao et al. [15] reported that a low-level MnCoOx doping on coal fly ash-derived 13X zeolite enables high-efficiency, low-temperature NH3-SCR, with over 80% NO conversion, 90% N2 selectivity and significant cost reduction. Pu et al. [16] developed a CNTs-functionalized CeO2/CNTs-GAC catalyst that achieves high NO conversion and superior SO2 tolerance in low-temperature NH3-SCR of NO. Xu et al. [17] reported that the inclusion of CNTs improved the H2O tolerance of the MnCe/GAC-CNTs catalyst by promoting the creation of mesopores and electron transfer. Previous reports [18,19] confirmed that the suitable support materials not only offer a lot of surface area for dispersing the active component, preventing the growth of large crystal particles, but also provide enough room for the catalytic reaction.
Layered double hydroxide (LDH)-derived MgAl composite oxides (MgAlOx) exhibit “substantial specific surface area and exceptional thermal stability”. These characteristics establish them as high-performance catalyst supports. Due to the interlayer confinement effect of LDHs, MgAlOx as support can prevent the aggregation of metal nanoparticles in favor of uniform dispersion [20,21]. The hydroxyl sites, basic sites and cation of MgAlOx combined with active components play a synergistic catalytic role [22,23]. For a long time, MgAlOx have been extensively studied for NOx adsorption. In NOx storage and reduction (NSR) testing, the newly designed Pt(Cu)/MgAlOx catalyst demonstrated exceptional low-temperature performance and robust sulfur tolerance [24]. Our research group [25] constructed Mn-loaded catalysts with MgAlOx derived from LDHs as the support for low-temperature NH3-SCR. Calcination at elevated temperatures induced strong MgAlOx–manganese interactions, stabilizing manganese as surface MnOx species while simultaneously doping Mn3+ into spinel-phase MgMn2O4, with both mechanisms collectively increasing surface chemisorbed oxygen. Our previous studies have proved that not only the different Mn salt precursors but also the properties of the MgAlOx support have an effect in significant ways on the catalytic performance. Gao et al. [26] systematically explored the role of different Mg/Al on the high-temperature CO2 adsorption capacity of MgAl-LDO catalysts and found that further modulation of the acid–base properties of the support could be achieved by appropriately adjusting the Mg/Al ratio in the interlayer of hydrotalcite precursors. Wu et al. [27] synthesized a series of CuMgFe-LDHs with varying Cu/Mg/Fe molar ratios and derived mixed oxide catalysts (LDO) for NH3-SCR denitration. The Cu0.5Mg2.5Fe1-LDO catalyst exhibited superior performance, achieving over 80% NOx conversion between 180 and 250 °C and demonstrating strong resistance to SO2. Wu et al. [28] also synthesized a series of NiTi-LDHs with varying Ni/Ti molar ratios for NH3-SCR denitration. The Ni4Ti1-LDO catalyst calcined at 400 °C demonstrated optimal performance, achieving over 90% NOx conversion between 240 and 360 °C with nearly 95% N2 selectivity. Hou et al. [29] reported the tunable synthesis of highly dispersed Ni–Mn layered double oxide catalysts from Ni–Mn LDH precursors, achieving optimal Ni/Mn ratios (Ni5Mn-LDO) that deliver excellent low-temperature NH3-SCR performance. Consequently, our focus shifts to enhancing MgAlOx support acidity through controlled Mg/Al ratio variation in LDH precursors. This approach enables the development of hydrothermally stable, low-temperature SCR catalysts with high activity.
This study systematically modulates Mg/Al ratios in MgAlOx composite oxides to investigate their influence on redox properties, surface oxygen vacancies, acidity and corresponding NH3-SCR performance. Ultimately, the optimal Mn/Mg2AlOx has a wide temperature window for denitration, removing 80% of NOx at 100–300 °C, as well as satisfactory water resistance activity. A multi-technique investigation (kinetics, XPS, H2-TPR, NH3-TPD, in situ DRIFTS) revealed the water-resistance behavior and denitration pathways of Mn/MgtAlOx catalysts.
2. Materials and Methods
2.1. Synthesis of the Mn/MgtAlOx Catalysts
Al(NO3)3·9H2O (A.R.) and Mg(NO3)2·6H2O (A.R.) are provided by Tianjin Damao Chemical Reagent Co., Ltd. (Tianjin, China). MnC4H6O6·4H2O (A.R.) and Na2CO3 (A.R.) are provided by Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). NaOH (A.R.) is provided by Sinopharm Chemical Reagent Co., Ltd. (Beijing, China).
Mn/MgtAlOx (t = 1, 2, 3, 4) catalysts with varied Mg/Al molar ratios were prepared using a combination of the co-precipitation approach and the simple wet impregnation method. Taking Mn/Mg2AlOx as an example, 9.4 g of Al(NO3)3·9H2O and 12.8 g of Mg(NO3)2·6H2O were dissolved in 50 mL deionized water to form a mixed nitrate solution. At room temperature, a mixed base solution was prepared in a volumetric flask by dissolving 2 M NaOH and 1 M Na2CO3 solids with a molar ratio of (CO32−):(OH−) = 1:2. A beaker filled with 50 mL of deionized water was vigorously stirred as the two solutions were introduced drop by drop, and the pH was regulated at roughly 10 throughout the precipitation by adjusting the drop speed. The resulting mixture underwent aging at 60 °C for 18 h under continuous agitation. Subsequent filtration isolated the precipitate, which was then subjected to repeated deionized water washings until a neutral pH (~7) was attained. The filtered precipitate underwent drying at 120 °C for 12 h to yield the MgAl-LDH precursor (Mg/Al = 2). The resulting material was then calcined at 800 °C for 5 h with a heating rate of 2 °C/min to generate the Mg2AlOx support. Manganese acetate (MnC4H6O6·4H2O) was then impregnated onto this support following established protocols from prior research [25]. A series of 50 wt%Mn/MgtAlOx catalysts were prepared via the conventional impregnation method. Taking the Mn/Mg2AlOx as an example, a stoichiometric amount of MnC4H6O6·4H2O was added to an aqueous suspension of Mg2AlOx support and stirred to achieve homogeneous dispersion of the precursor. The resulting mixture was then evaporated in a water bath and dried overnight. Finally, the obtained material was calcined at 500 °C for 5 h with a heating rate of 2 °C/min to obtain the Mn/Mg2AlOx catalyst.
The actual elemental composition and Mg/Al molar ratios of the catalysts are provided in Table S2.
2.2. Catalytic Activity Test
Catalytic activity assessments employed a continuous-flow, fixed-bed quartz reactor (laboratory-built). The simulated flue gas contained 500 ppm NH3, 500 ppm NO, 5% O2 and N2 balance, with 5% H2O vapor introduced where applicable. Testing occurred at 40,000 h−1 gas hourly space velocity (GHSV). Catalysts underwent initial thermal conditioning at 350 °C for 20 min under reaction gas flow. Subsequently, the temperature was reduced to 50 °C at a controlled 5 °C·min−1 ramp rate, with 20 min stabilization periods at each intermediate measurement point. A chemiluminescence NOx analyzer (Thermo 42i-HL) (Thermo Fisher Scientific, Waltham, MA, USA)recorded NOx concentrations in the products in real time. N2O concentrations in effluent gases were quantified via gas chromatography (Agilent 7890B, Agilent Technologies, Santa Clara, CA, USA) equipped with an electron capture detector (ECD). Catalyst performance metrics—NOx conversion and N2 selectivity—were derived from Equations (1) and (2):
where and the subscript “in” and “out” denote, respectively, the concentration of the corresponding gas at the inlet and output.
2.3. Reaction Kinetics Testing
Identical reactor configurations assessed NH3-SCR kinetics. To eliminate internal/external diffusion limitations, differential reactor conditions were maintained (5–20% NOx conversion, GHSV = 200,000 h−1). Kinetic parameters were normalized using Equations (3) and (4):
where k denotes the reaction rate constant (mol·g−1·s−1), FNOx is the NOx molar flow rate (mol·s−1), w represents catalyst mass (g), and X signifies NOx conversion.
Ea corresponds to apparent activation energy (kJ·mol−1), A is the pre-exponential factor, R is the molar gas constant (8.314 J·mol−1·K−1), and T is the reaction temperature (K).
2.4. Catalyst Characterization
Phase composition analysis was performed using a Bruker X-ray diffractometer with Cu Kα radiation (λ = 1.5418 Å). XRD patterns were acquired across 5–90° 2θ at 6 °·min−1 scan rates. Average crystallite dimensions were derived via the Scherrer equation.
Textural properties of synthesized catalysts were characterized through N2 physisorption at −196 °C using a Micromeritics ASAP 2460 analyzer (Micromeritics Instrument Corporation, Norcross, GA, USA). Prior to the experiment, samples were degassed at 300 °C for 6 h to remove any adsorbed substances. Specific surface areas were determined via the Brunauer–Emmett–Teller (BET) method, while Barrett–Joyner–Halenda (BJH) analysis yielded pore size distributions and cumulative pore volumes.
XPS analysis was conducted using a Thermo Fisher Scientific K-Alpha spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) equipped with an Al Kα X-ray source. All binding energies were referenced to the adventitious carbon C 1s peak at 284.8 eV.
H2-TPR analyses employed a quartz flow reactor with inline TCD (FX6080, Lunan Ruihong Chemical Instrument Co., Ltd., Zaozhuang, China). Samples (50 mg) underwent 500 °C pretreatment for 30 min under N2 flow before cooling to ambient temperature. Reduction profiles were then acquired from 20 to 700 °C at 10 °C·min−1 in 5% H2/N2.
NH3-TPD analyses utilized a TCD-equipped quartz flow reactor (FX6080, Lunan Ruihong Chemical Instrument Co., Ltd.). Samples (50 mg) underwent N2 pretreatment (500 °C, 30 min) and cooling to ambient temperature. The NH3 adsorption was then conducted for 60 min under two distinct conditions: (a) under dry conditions using a 1250 ppm NH3/N2 gas mixture and (b) in the presence of water vapor using a 1250 ppm NH3/N2 and 5% H2O gas mixture. After 30 min N2 purging to remove physisorbed species, programmed desorption proceeded at 10 °C·min−1 (20–550 °C).
In situ DRIFTS analysis utilized a Nicolet is 50 FTIR spectrometer with MCT detection (Thermo Fisher Scientific, Waltham, MA, USA). Samples underwent 30 min pretreatment at 200 °C under 50 mL·min−1 N2 flow. Background spectra were acquired at target temperatures in N2 before introducing regulated gas streams (500 ppm NH3/N2 or 500 ppm NO/N2 + 5% O2), each supplied at a flow rate of 50 mL/min. Catalyst spectra were recorded after system stabilization.
3. Results and Discussion
3.1. Catalytic Performance of Catalysts
Catalytic activities for NH3-SCR over prepared Mn/MgtAlOx systems are shown in Figure 1. Temperature-dependent NOx removal efficiencies display characteristic volcano trends across all samples (Figure 1a). At Mg/Al = 1, Mn/MgAlOx exhibits notably low activity, reaching only 70% peak NOx conversion at 200 °C. Progressively higher Mg/Al ratios substantially enhance catalytic performance across all samples. Notably, Mn/Mg2AlOx exhibits substantially enhanced low-temperature activity, attaining 60% NOx conversion at 50 °C. The catalyst also demonstrates an expanded operational range, sustaining >80% NOx conversion between 100 and 300 °C. Regarding Mn/Mg3AlOx, the temperature interval is narrowest, even if the almost 100% NOx conversion may be attained at 200 °C. Catalytic activity undergoes marked deterioration when temperatures exceed 250 °C, as evidenced by declining NOx conversion rates. Mn/Mg4AlOx with elevated Mg/Al ratios exhibits constrained low-temperature activity but sustains stable high-temperature performance, maintaining >80% NOx conversion between 150 and 300 °C. Figure 1b shows that altering of Mg/Al improves the N2 selectivity of the various samples over the temperature range tested, as shown by the N2 selectivity of Mn/MgtAlOx being higher than 80% at low temperatures (150 °C), demonstrating the prepared catalysts are highly efficient in the reduction of NO to environmentally friendly N2 via NH3. Elevated temperatures progressively reduce N2 selectivity over Mn/MgtAlOx catalysts, attributable to excessive NH3 oxidation at high temperatures. A comprehensive evaluation of low-temperature activity, operational temperature breadth and N2 selectivity confirms Mn/Mg2AlOx as the optimal NH3-SCR catalyst. We also added Table S3 comparing NOx conversion, temperature window and H2O resistance with other Mn-based catalysts.
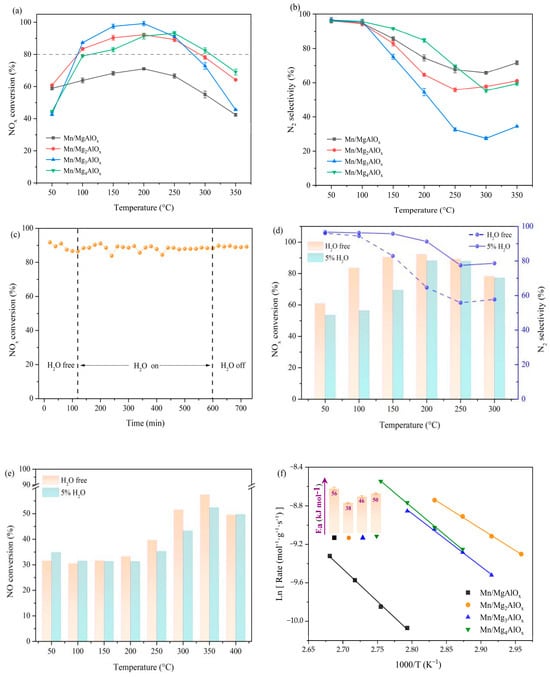
Figure 1.
NH3-SCR performance over Mn/MgtAlOx catalysts: (a) NOx conversion profiles; (b) N2 selectivity trends; (c) long-time tolerance to H2O of Mn/Mg2AlOx at 200 °C; (d) effect of H2O on SCR activity of Mn/Mg2AlOx; (e) effect of H2O on NO oxidation of Mn/Mg2AlOx; (f) Arrhenius plots.
We conducted comparative experiments on Mg2AlOx supports and Mn/γ-Al2O3 catalysts. As shown in Figure S2, the Mg2AlOx support exhibited a relatively low NOx conversion (approximately 10%) in the low-temperature range (100–250 °C). The Mn/Mg2AlOx catalyst demonstrated significantly superior low-temperature activity compared to Mn/γ-Al2O3. This highlights the high SCR activity contributed by manganese oxides and the crucial role of the LDH-derived Mg2AlOx support.
Literature [30] attributes SCR catalyst deactivation in humid reaction environments to competitive adsorption between H2O vapor and reactants (NH3, NO) at catalytic sites. Figure 1c presents the variation in the NOx conversion over time for the Mn/Mg2AlOx at 200 °C with a continuous flow of 5 vol% H2O into the reaction atmosphere for 8 h. First of all, the NOx conversion is stable at 92% for the first two hours under the standard reaction atmosphere. After introducing H2O for 8 h, the NOx conversion of Mn/Mg2AlOx decreases slightly by about 4%. With the suspension of H2O supply, the activity of Mn/Mg2AlOx can be recovered to the initial state, implying that the inhibitory action of water over catalyst is reversible. According to the results above, the Mn/Mg2AlOx catalyst exhibits excellent H2O resistance ability. Figure 1d illustrates the influence of 5 vol% H2O on Mn/Mg2AlOx-catalyzed NOx reduction across temperatures. Below 250 °C, water vapor suppresses catalytic activity relative to anhydrous conditions, evidenced by a 100 °C conversion decline from 83% to 56%. Above this threshold, hydrothermal effects become negligible. These findings demonstrate temperature-dependent water adsorption behavior. Strong H2O adsorption at low temperatures competitively inhibits catalytic sites, significantly suppressing activity. Above ~250 °C, diminished water adsorption attenuates this inhibitory effect, with some studies reporting enhanced NOx reduction efficiency under such conditions [31,32]. Moreover, the presence of water improved the N2 selectivity of the catalyst to a certain degree, which increased from 65% to 91% at 200 °C. The previous researchers [33] have proposed that the inhibition of N2O production by water vapor is also related to its inhibition of catalyst oxidation ability. Figure 1e exhibits the inhibition of NO oxidation over Mn/Mg2AlOx by H2O molecules, with a decrease in NO conversion when the reaction temperature exceeds 200 °C. As the NO is oxidized to NO2 by reactive oxygen species, it can trigger the “fast SCR” reaction, which has a lower activation energy and higher catalytic activity than typical NH3-SCR. However, over-oxidation capacity can entail excessive oxidation of NH3, causing the reductant to be rapidly consumed by O2, whereby the SCR reaction does not proceed properly, as well as generating byproducts of N2O, which reduces the NOx removal efficiency and narrows the operation temperature range of the catalyst. Accordingly, the N2 selectivity is somewhat improved when the oxidation capacity over Mn/Mg2AlOx is suppressed by H2O at high temperature, which is consistent with the findings of the activity tests. A comparison of the catalytic performance with othe catalysts reported in the literature is provided in the Supplementary Material (Table S3), which demonstrates the competitive activity and superior selectivity of our catalyst.
NH3 oxidation (NH3-SCO) acts as a kinetic competitor to NH3-SCR by depleting NH3 and lattice/surface oxygen, thereby narrowing the effective activity window at elevated temperatures. In our catalysts, water vapor moderates the oxidation strength, which both restrains NH3-SCO and aligns with the observed rise in N2 selectivity under humid conditions and the reduced NO-oxidation ability in H2O. Thus, while NH3-SCO can become relevant above ~300 °C, its contribution under the humid low-temperature regime studied here is limited, helping maintain high N2 selectivity.
Kinetic studies employed high space velocities (GHSV = 200,000 h−1) and low NOx conversions (<20%) to eliminate diffusion limitations. Figure 1f derives apparent activation energies (Ea) from Arrhenius plot slopes for various catalysts. Mn/Mg2AlOx exhibits the lowest Ea (36 kJ·mol−1), confirming Mg/Al ratio modulation effectively lowers reaction energy barriers and enhances catalytic activity.
3.2. Crystal Structure and Texture Property
XRD of the as-synthesized Mg-Al LDH (prior to calcination) confirmed a well-defined hydrotalcite structure for all Mg/Al ratios. The full detailed peaks/analysis of the LDH phase are available in the Supplementary Materials. The XRD patterns of MgtAlOx supports obtained from MgAl-CO3 LDH after calcination at 800 °C are shown in Figure 2a. The main component of MgtAlOx is MgO species (PDF#74-1225), with a small amount of MgAl2O4 spinel phase (PDF#86-2258) incorporated. The strength of diffraction peaks ascribed to MgO increases when Mg content increases, while peaks related to MgAl2O4 decrease in intensity. When the Mg/Al ratio increases to 4, only a weak diffraction peak assigned to MgAl2O4 can be detected at 36.8°. As shown in Figure 2b, Mn/MgtAlOx catalysts exhibit MnO2 (PDF#44-0992) as the main crystal structure, with traces of Mn3O4 (PDF#80-0382) and MgMn2O4 (PDF#72-1336) crystalline phases. The diffraction peaks of MgMn2O4 become more intensified with increasing Mg/Al ratios, especially at 2θ = 44.7°, where the change is more pronounced. In addition, the Mn/MgAlOx catalyst also involves Al2O3 (PDF#12-0539), showing weak diffraction peaks at 2θ = 16.3° and 25.7°. The absence of Al-associated reflections in other catalysts suggests either atomic-level dispersion of aluminum species or their existence in an XRD-amorphous phase.
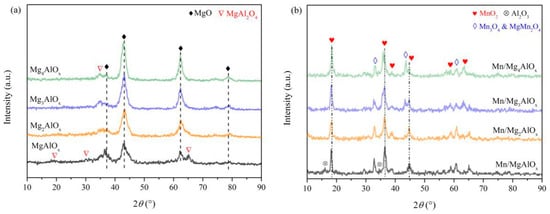
Figure 2.
(a) XRD patterns of MgtAlOx and (b) Mn/MgtAlOx.
N2 adsorption–desorption isotherms and pore size distributions for the Mn/MgtAlOx catalyst series appear in Figure 3. Per IUPAC classification, all Mn/MgtAlOx catalysts display characteristic type II isotherms featuring H3 hysteresis loops across defined relative pressure ranges. This behavior confirms macroporous architectures within the catalysts [34]. Notably, Mn/Mg2AlOx exhibits hysteresis loop closure at reduced relative pressure (p/p0 = 0.4), signaling enhanced microporosity. The broad pore size distributions in Figure 3b further reveal a hierarchical structure with pores spanning the micro-, meso-, and macroporous ranges. Although the average pore sizes are situated within the mesoporous regime (Table 1), the coexistence of larger and smaller pores is evident. As established in [35], coexisting micropores and mesopores increase accessible surface area, while macroporous domains facilitate mass transport. Consequently, optimal catalytic activity in Mn/Mg2AlOx requires a narrow pore size dispersion and a balanced microporous–mesoporous–macroporous hierarchy. Table 1 summarizes textural properties, revealing the minimal surface area of Mn/MgAlOx (45.4 m2·g−1), which is consistent with good crystallinity in XRD. On the contrary, Mn/Mg2AlOx exhibits the highest specific surface area of 88.0 m2·g−1 and the smallest average pore size of 9.01 nm, which favors the dispersed distribution of the active components within the channels [36]. It can be seen that the pore structure properties of the prepared materials are important factors to influence their catalytic activity [37].
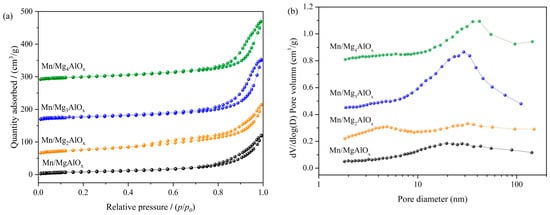
Figure 3.
(a) N2 adsorption and desorption isotherms and (b) BJH pore size distribution profiles for Mn/MgtAlOx catalysts.

Table 1.
The textural parameters and the quantitative data from XPS spectra of Mn/MgtAlOx catalysts.
3.3. Acidic Property
The chemisorption and activation of ammonia constitute a fundamental NH3-SCR mechanism stage, critically influenced by catalyst surface acidity during reactive intermediate formation [38]. Acid site concentration and strength across Mn/MgtAlOx surfaces were evaluated through NH3-TPD analysis (Figure 4). All catalysts display broad NH3 desorption profiles originating from Lewis acid sites, with spectral deconvolution revealing two superimposed desorption features. Peaks below 200 °C correspond to NH3 desorption from weak acid sites, indicating physisorbed or weakly chemisorbed ammonia. Desorption events between 200 and 350 °C reflect NH3 release from medium-strength acid sites with stronger chemical binding, consistent with established NH3-TPD interpretation frameworks [39]. It can be seen that there is no significant change in the position of the desorption peaks of catalysts with different Mg/Al ratios. Quantitative analysis reveals Mn/MgAlOx possesses the lowest acid site density, equivalent to 57% of Mn/Mg2AlOx’s total acidity. Weak and medium-strong acid sites constitute 51% and 49% of acid sites, respectively. Acid site deficiency constrains SCR reactivity, explaining the pronounced activity loss. Conversely, Mn/Mg2AlOx exhibits greater total acidity, comprising 36% weak and 64% medium-strong acid sites. This promotes NH3 adsorption, subsequently enhancing catalytic activity via the “NH4NO3 + NO” or “NH2 + NO” pathway. Consequently, superior medium-to-high temperature performance is achieved [40,41].
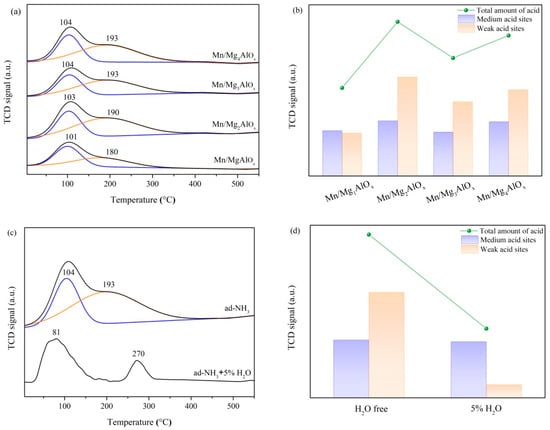
Figure 4.
(a) NH3-TPD spectra; (b) acid amount of Mn/MgtAlOx catalysts; (c) effect of H2O on NH3 adsorption; (d) effect of H2O on acid amount of Mn/Mg2AlOx.
Beyond suppressing catalytic oxidation capacity, water also competes with NH3 for adsorption sites during NH3-SCR [4]. Consequently, Figure 4c presents Mn/Mg2AlOx TPD profiles after 1 h NH3 exposure in humid atmospheres. Unlike dry conditions, the broad desorption feature resolves into distinct peaks at about 81 °C and 270 °C. Relative to anhydrous conditions, Mn/Mg2AlOx exhibits markedly reduced NH3 adsorption capacity in humid environments, especially at medium-strength acid sites. This indicates that competitive displacement by H2O restricts ammonia adsorption, consequently diminishing NH3 oxidation and suppressing N2O byproduct formation. The excellent water resistance of Mn/Mg2AlOx stems from its high surface area and macroporous architecture. At elevated temperatures, limited pore condensation of H2O minimally impacts NH3-SCR activity. Conversely, capillary-driven water accumulation in micropores at low temperatures blocks active sites, reducing catalytic efficiency [42]. In this way, the large pore volume and specific surface area of Mn/Mg2AlOx not only prevents the condensation of H2O molecules but also allows more active sites to be fully exposed.
3.4. Surface Element Analysis and Redox Property
Elemental composition and manganese oxidation states in Mn/MgtAlOx catalysts were characterized by XPS (Figure 5, Table 1). The Mn 2p spectrum (Figure 6a) displays characteristic spin-orbit splitting, with Mn 2p3/2 and Mn 2p1/2 peaks at ~641.5 eV and ~653.1 eV, respectively. Deconvolution of the Mn 2p3/2 region reveals three oxidation states: Mn2+ (~640.3 eV), Mn3+ (~641.4 eV) and Mn4+ (~642.5 eV). The latter species enhances reactive oxygen activation and mobility, as documented in catalysis literature [43,44]; thus, the relative ratio of Mn4+ by calculating the peak area ratio of Mn4+/(Mn2++Mn3++Mn4+) is shown. The relative ratio of Mn4+ in the over Mn/MgtAlOx serial catalysts follows an increased sequence of Mn/MgAlOx < Mn/Mg3AlOx < Mn/Mg4AlOx < Mn/Mg2AlOx, in harmony with their catalytic activity. Consequently, abundant surface Mn4+ in Mn/Mg2AlOx accelerates the SCR redox cycle, constituting the primary mechanism for its superior catalytic performance. Complementary analysis of O 1s spectra (Figure 5b) resolves two components: adsorbed oxygen species Oα (531.2–531.6 eV) and lattice oxygen Oβ (529.6 eV). Since Oα has a higher mobility than Oβ, it tends to be more reactive in the oxidation of NO to NO2. Table 1 reveals parallel trends in Oα and Mn4+ surface concentrations across catalysts. This correlation likely originates from enhanced oxygen vacancy formation during MnOx to MnO2 transformation [45,46], facilitating oxygen molecule activation. Subsequently, NO reacts with activated oxygen at Mn4+ sites, generating adsorbed NO2 or nitrate intermediates that undergo reaction with ammonia.
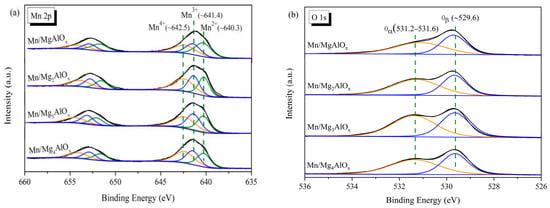
Figure 5.
XPS of Mn/MgtAlOx catalysts: (a) Mn 2p and (b) O 1s.
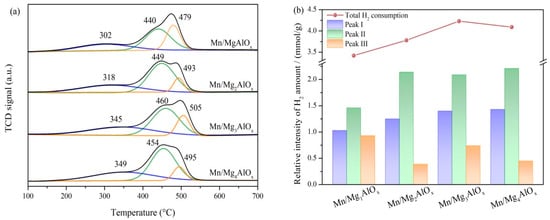
Figure 6.
(a) H2-TPR profiles; (b) H2 consumption of Mn/MgtAlOx catalysts.
Figure 6 displays the results of the H2-TPR investigation into the redox capabilities of the Mn/MgtAlOx catalysts. All samples show three reduction peaks at 100–700 °C, which are in relation to the gradual reduction of MnOx. Reduction profiles exhibit three characteristic peaks: the low-temperature peak (Peak I, 302–349 °C) is attributed to MnO2 to Mn2O3 transformation, the medium-temperature peak (Peak II, 440–460 °C) corresponds to Mn2O3 to Mn3O4 reduction, and the high-temperature peak (Peak III, 479–505 °C) originates from Mn3O4 to MnO phase transformation. Reduction peak temperatures directly reflect catalytic reducibility, with lower values indicating stronger reduction capability. Figure 6a demonstrates that reduction peaks broadly shift to higher temperatures with increasing Mg/Al ratios. Notably, the peaks associated with MnO2 species exhibit the most pronounced displacement. This pattern generally indicates strengthened interaction between highly dispersed manganese oxides and the MgtAlOx support. Elevated Mg2+ content within the catalyst enhances this effect, consequently increasing the reduction resistance of these MnOx species [47]. Furthermore, H2 consumption of samples is quantified by reference to the H2-TPR curves spectrum of the quantitative standard CuO. As presented in Figure 6b, the order of H2 consumption for the four samples is Mn/MgAlOx (3.42 mmol·g−1) < Mn/Mg2AlOx (3.78 mmol·g−1) < Mn/Mg4AlOx (4.09 mmol·g−1) < Mn/Mg3AlOx (4.23 mmol·g−1). Elevated Mg2+ content correlates with increased reduction peak intensity, suggesting greater formation of redox-active sites on the catalyst surface. Moderate formation of the MgMn2O4 phase can enhance the catalyst’s redox capacity [48]. However, excessively strong redox capability promotes undesirable side reactions, including ammonia over-oxidation, which negatively impacts denitration performance.
3.5. In Situ DRIFTs Analysis
To obtain insight into the reaction process of catalysts, the adsorption behavior of reactants is studied. Figure 7a,b show in situ DRIFTS spectra of NH3 adsorption over Mn/MgtAlOx at 100 °C. After the addition of NH3, it is clear that both catalysts have comparable characteristics. Infrared bands at approximately 1188 and 1616 cm−1 correspond to NH3 adsorbed at Lewis acid sites (predominantly Mn4+ cations, with a contribution from Al3+ sites of the support) [49,50]. Conversely, the weaker band near 1427 cm−1 arises from NH4+ species bound to Brønsted acid sites. Additionally, N-H stretching vibrations of Lewis-adsorbed NH3 generate peaks at 3172, 3253 and 3361 cm−1 [51,52]. O-H stretching vibrations at 3693 and 3747 cm−1 produce negative bands, arising from interactions between adsorbed NH3 and surface hydroxyl groups. This process consumes hydroxyl groups while generating ionic NH4+ species [53]. Furthermore, spectral band intensities progressively increase during extended NH3 exposure to catalysts, attaining saturation within 30 min.
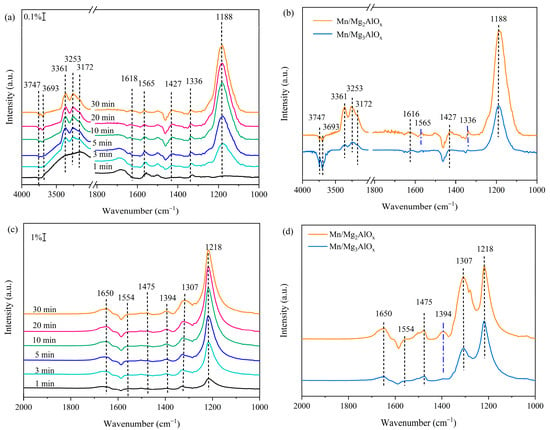
Figure 7.
(a,b) In situ DRIFTS spectra of NH3 adsorption and (c,d) NO + O2 adsorption over Mn/Mg2AlOx and Mn/Mg3AlOx catalysts ((a,c): Mn/Mg2AlOx. (b,d): yellow for Mn/Mg2AlOx and blue for Mn/Mg3AlOx correspond to 30 min of exposure).
Figure 7b reveals that Lewis acid sites (predominantly Mn4+ cations, with a contribution from Al3+ sites of the support) dominate over Brønsted sites in both catalysts, suggesting that adsorbed NH3 constitutes the predominant ammonia species. Distinct from Mn/Mg3AlOx, infrared bands at 1336 and 1565 cm−1 confirm surface NH2 species on Mn/Mg2AlOx [54,55]. Reactive oxygen species oxidize adsorbed NH3 to form NH2−, establishing this intermediate as critical for the reaction mechanism. The detection of NH2− on Mn/Mg2AlOx signifies concurrent NH3 adsorption and activation. This surface process mediates subsequent reactions with NO, yielding N2 and H2O, thereby enhancing NOx removal efficiency. Relative to Mn/Mg3AlOx, Mn/Mg2AlOx exhibits markedly more intense NH3 absorption bands, signifying enhanced surface acidity and ammonia adsorption capacity following Mg/Al ratio modification. These observations align with NH3-TPD results. Figure 7c,d present in situ DRIFTS spectra for NO + O2 co-adsorption on Mn/MgtAlOx at 100 °C. With the introduction of NOx, absorption peaks belonging to a variety of nitrate species appeared on the catalyst’s surface. The peaks locating at 1218, 1307, 1475 and 1554 cm−1 are ascribed to bridging nitrate, monodentate nitrate, linear nitrite and bidentate nitrate species, respectively, while the newly appearing peak at 1650 cm−1 is ascribed to the gaseous NO2 [56,57,58]. Mn/Mg2AlOx exhibits an additional absorption band at 1394 cm−1, assigned to monodentate nitrate [59], which is not observed on Mn/Mg3AlOx. Nitrate-related band intensities increase progressively over time. Figure 7d demonstrates that all nitrate species signals are substantially stronger on Mn/Mg2AlOx versus Mn/Mg3AlOx, revealing that Mg/Al ratio modulation enhances NOx adsorption and activation. This augmented NO + O2 co-adsorption generates greater surface nitrate concentrations, thereby accelerating the “fast SCR” pathway [60].
To elucidate NH3-NOx reaction pathways on Mn/Mg2AlOx, in situ DRIFTS spectra were acquired at 100 °C using sequential steps: 1 h NH3 pre-adsorption, 30 min N2 purge, and NO + O2 exposure, with results shown in Figure 8a,b. Post-adsorption spectral features confirm Lewis-bound NH3 (1188, 1616 cm−1), Brønsted-bound NH4+ (1427 cm−1) and NH2− intermediates (1336, 1565 cm−1) on the catalyst. All ammonia-associated bands diminish within 3 min of NO + O2 introduction, indicating efficient acid site-mediated adsorption and activation of ammonia species for subsequent NOx reactions [61]. Subsequent spectral features reveal nitrate species deposition, including bridged nitrate (1218 cm−1), monodentate nitrate (1307 and 1394 cm−1) and gaseous NO2 (1650 cm−1) [62,63]. Nitrate-associated band intensities steadily intensify during prolonged exposure, reflecting progressive accumulation on the catalyst surface. These observations collectively demonstrate that all surface ammonia species participate in NH3-SCR reactions over Mn/Mg2AlOx via the Eley–Rideal (E–R) mechanism.
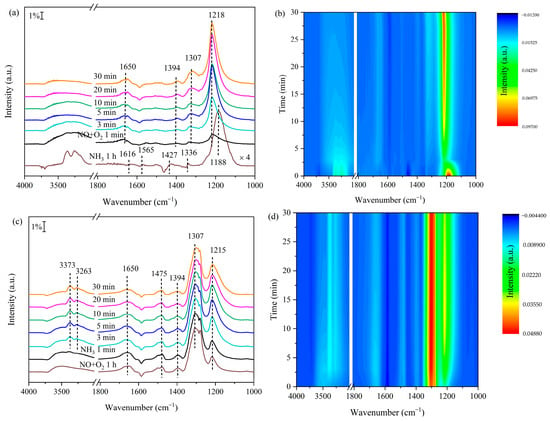
Figure 8.
(a) In situ DRIFTS spectra of the reaction between NO + O2 and pre-adsorbed NH3 on Mn/Mg2AlOx at 100 °C and (b) the corresponding mapping results; (c) reaction between NH3 and pre-adsorbed NO + O2 on Mn/Mg2AlOx at 100 °C and (d) the corresponding mapping results.
Figure 8c,d show in situ DRIFTS spectra of the adsorbed state species on the surface of Mn/Mg2AlOx produced by first pre-adsorbing NOx at 100 °C for 1 h, then purging with N2 for 30 min and finally feeding NH3. Following NOx saturation, spectral features emerge for bridging nitrate (1215 cm−1), monodentate nitrate (1307 and 1394 cm−1), linear nitrite (1475 cm−1) and gaseous NO2 (1650 cm−1). Upon NH3 introduction, only linear nitrite and gaseous NO2 bands diminish over time, indicating partial nitrate reactivity with ammonia. Conversely, monodentate nitrate peaks remain stable, demonstrating surface accumulation. Bridging nitrate signals intensify progressively due to spectral overlap between accumulating Lewis-adsorbed NH3 (1210 cm−1) and pre-existing bridging nitrate species [64]. Infrared bands at 3263 and 3373 cm−1 correspond to N-H stretching vibrations of Lewis-bound NH3 species. Stability analyses reveal that nitrate species exhibit greater surface persistence than ammonia on Mn/Mg2AlOx, with NH3 reactions involving pre-adsorbed NOx proving kinetically challenging. Consequently, NH3-SCR over Mn/Mg2AlOx predominantly follows the Eley–Rideal (E–R) mechanism, involving adsorbed NH3 reacting with gaseous NOx species [65,66].
3.6. The Reaction Mechanism of NOx over the Mn/Mg2AlOx
Based on the preceding in situ DRIFTS analysis, primary Eley–Rideal (E–R) mechanisms on the catalyst surface involve gaseous NH3 first adsorbing at Lewis acid sites (predominantly the Mn4+ cations, with a secondary contribution from Al3+ sites on the support), followed by Mn4+-mediated activation to NH2− intermediates. These species subsequently reduce gaseous NO to environmentally benign N2 and H2O (Equations (6)–(8)). However, excessive NH3 dehydrogenation yielding NH radicals initiates detrimental side reactions. NH reacts with NO to form N2O (reducing N2 selectivity), while NH over-oxidation generates NO, thereby compromising overall NOx removal efficiency (Equations (9)–(11)). The reactive oxygen species then further oxidize the metal ions in the low-valence states (Mn3+, Mn2+) to metal ions in the high-valence state (Mn4+), which are then used to initiate additional reduction processes (Equations (12) and (13)). Elevated Mn4+ concentrations and ROS abundance within Mn/Mg2AlOx enhance the kinetics of NH3 dehydrogenation and NO oxidation processes, thus elevating SCR performance [67,68].
4. Conclusions
In summary, a novel series of Mn/MgtAlOx catalysts derived from LDH precursors were developed for low-temperature NH3-SCR. The optimized Mn/Mg2AlOx catalyst demonstrated superior NOx conversion within a broad temperature window (100–300 °C) and outstanding water resistance. More importantly, this study unveils that the exceptional performance is not attributable to a single factor but to a synergistic effect arising from its hierarchical porosity (facilitating mass transfer), balanced acidic properties (favoring NH3 adsorption) and abundant surface Mn4+ and active oxygen species (promoting the redox cycle). Furthermore, in situ DRIFTS studies confirmed that the reaction follows the Eley–Rideal mechanism and provided molecular-level insight into the unique promoting effect of H2O on N2 selectivity by suppressing the over-oxidation of NH3.
Despite the promising results, several challenges remain to be addressed before practical application. Firstly, the resistance to SO2 poisoning, a ubiquitous component in flue gas, must be thoroughly investigated in the future. Secondly, the long-term stability under real flue gas conditions containing both H2O and SO2 is essential to evaluate. Finally, scaling up the catalyst and formulating it into a monolithic structure will be a critical step towards industrial implementation. This work provides a fundamental understanding and a robust catalyst platform, paving the way for the development of high-performance, water-resistant SCR catalysts for terminal denitrification applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry7050154/s1, Figure S1. (a) XRD patterns of MgAl-CO3 LDH and (b) enlarged regions in the range of 8–15° [69]; Figure S2. NOx conversion profiles of Mn/Mg2AlOx, Mg2AlOx, Mn/γ-Al2O3; Table S1. Structure parameters of MgAl-CO3 LDH; Table S2. Catalyst composition of Mn/MgtAlOx; Table S3. The catalytic performance of other Mn-based catalysts reported in the literature [70,71,72,73,74].
Author Contributions
Conceptualization, Z.W. and B.W.; methodology, Z.W., W.L. and L.W.; validation, R.J., B.W. and Z.W.; formal analysis, R.J. and B.W.; investigation, R.J. and J.Z.; resources, Z.W., W.L. and L.W.; writing—original draft preparation, B.W.; writing—review and editing, Z.W. and R.J.; visualization, R.J.; supervision, Z.W.; funding acquisition, W.L., J.Z., L.W. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Shandong Province (ZR2023MB100, ZR2021MB063, ZR2024QE354), National Natural Science Foundation of China (No. 21777055) and Innovation ability improvement project of technology-based small and medium-sized enterprises in Shandong Province (2022TSGC2043, 2021TSGC1358).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Peel, J.; Haeuber, R.; Garcia, V.; Russell, A.; Neas, L. Impact of nitrogen and climate change interactions on ambient air pollution and human health. Biogeochemistry 2012, 114, 121–134. [Google Scholar] [CrossRef]
- Chen, W.; Zou, R.; Wang, X. Toward an atomic-level understanding of the catalytic mechanism of selective catalytic reduction of NOx with NH3. ACS Catal. 2022, 12, 14347–14375. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Tang, W.; Kakwani, R.; Hou, Y.; Feng, G. Urea decomposition and implication for NOx reduction with Cu-zeolite and vanadia-selective catalytic reduction. Chem. Eng. Technol. 2020, 43, 1758–1764. [Google Scholar] [CrossRef]
- Damma, D.; Ettireddy, P.; Reddy, B.; Smirniotis, P. A review of low temperature NH3-SCR for removal of NOx. Catalysts 2019, 9, 349. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tonkyn, R.G.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Fickel, D.W.; D’Addio, E.; Lauterbach, J.A.; Lobo, R.F. The ammonia selective catalytic reduction activity of copper-exchanged small-pore zeolites. Appl. Catal. B Environ. 2011, 102, 441–448. [Google Scholar] [CrossRef]
- Fu, M.; Li, C.; Lu, P.; Qu, L.; Zhang, M.; Zhou, Y.; Yu, M.; Fang, Y. A review on selective catalytic reduction of NOx by supported catalysts at 100–300 °C-catalysts, mechanism, kinetics. Catal. Sci. Technol. 2014, 4, 14–25. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.; Yu, Q.; Han, X.; Bian, M.; Zhang, Y.; Yi, T. Optimization and comprehensive mechanism of environment-friendly bimetal oxides catalysts for efficient removal of NO in ultra-low temperature flue gas. Sep. Purifi. Technol. 2023, 311, 123324. [Google Scholar] [CrossRef]
- Wang, Z.; Lan, J.; Haneda, M.; Liu, Z. Selective catalytic reduction of NOx with NH3 over a novel Co-Ce-Ti catalyst. Catal. Today 2021, 376, 222–228. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Zhao, S.; Li, C.; Li, J.; Shi, Y.; Meng, X. A review on selective catalytic reduction of NOx by NH3 over Mn-based catalysts at low temperatures: Catalysts, mechanisms, kinetics and DFT calculations. Catalysts 2017, 7, 199. [Google Scholar] [CrossRef]
- Damma, D.; Boningari, T.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. Direct Decomposition of NOx over TiO2 Supported Transition Metal Oxides at Low Temperatures. Ind. Eng. Chem. Res. 2018, 57, 16615–16621. [Google Scholar] [CrossRef]
- Komaty, S.; Andijani, M.; Wang, N.; Navarro de Miguel, J.C.; Kumar Veeranmaril, S.; Hedhili, M.N.; Silva, C.I.Q.; Wang, Y.; Abou-Daher, M.; Han, Y.; et al. Enhancing Water Tolerance and N2 Selectivity in NH3-SCR Catalysts by Protecting Mn Oxide Nanoparticles in a Silicalite-1 Layer. Environ. Sci. Technol. 2024, 58, 15279–15287. [Google Scholar] [CrossRef]
- Gu, J.; Duan, R.; Chen, W.; Chen, Y.; Liu, L.; Wang, X. Promoting effect of Ti species in MnOx-FeOx/silicalite-1 for the low-temperature NH3-SCR reaction. Catalysts 2020, 10, 566. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, Y.; Jiang, J.; Liu, X.; Zhu, T. Exploring the promotion effect of low MnCoO doping for low-temperature NH3-SCR of 13X zeolite synthesized from coal fly ash. Sep. Purif. Technol. 2025, 357, 130064. [Google Scholar] [CrossRef]
- Pu, Y.; Wang, P.; Jiang, W.; Dai, Z.; Yang, L.; Jiang, X.; Jiang, Z.; Yao, L. A novel CNTs functionalized CeO2/CNTs-GAC catalyst with high NO conversion and SO2 tolerance for low temperature selective catalytic reduction of NO by NH3. Chemosphere 2021, 284, 131377. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, P.; Pu, Y.; Jiang, L.; Yang, L.; Jiang, W.; Yao, L. MnCe/GAC-CNTs catalyst with high activity, SO2 and H2O tolerance for low-temperature NH3-SCR. Sep. Purifi. Technol. 2023, 305, 122498. [Google Scholar] [CrossRef]
- Xu, G.; Guo, X.; Cheng, X.; Yu, J.; Fang, B. A review of Mn-based catalysts for low-temperature NH3-SCR: NOx removal and H2O/SO2 resistance. Nanoscale 2021, 13, 7052–7080. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, G.; Han, W.; Tang, Z. The water resistance enhanced strategy of Mn based SCR catalyst by construction of TiO2 shell and superhydrophobic coating. Chem. Eng. J. 2021, 426, 131334. [Google Scholar] [CrossRef]
- Jabłońska, M.; Palkovits, R. Nitrogen oxide removal over hydrotalcite-derived mixed metal oxides. Catal. Sci. Technol. 2016, 6, 49–72. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Large-scale synthesis of highly dispersed layered double hydroxide powders containing delaminated single layer nanosheets. Chem. Commun. 2013, 49, 6301–6303. [Google Scholar] [CrossRef]
- Huang, L.; Tang, F.; Liu, P.; Xiong, W.; Jia, S.; Hao, F.; Lv, Y.; Luo, H. Highly efficient and selective conversion of guaiacol to cyclohexanol over Ni-Fe/MgAlOx: Understanding the synergistic effect between Ni-Fe alloy and basic sites. Fuel 2022, 327, 125115. [Google Scholar] [CrossRef]
- Kumar, S.; Choudhary, P.; Sharma, D.; Sajwan, D.; Kumar, V.; Krishnan, V. Tailored Engineering of Layered Double Hydroxide Catalysts for Biomass Valorization: A Way Towards Waste to Wealth. ChemSusChem 2024, 17, e202400737. [Google Scholar] [CrossRef]
- Cui, Y.; Yan, Q.; Zhang, C.; Qiu, L.; Wang, Q. Pt/Ba/Co1Mg2Al1Ox with dual adsorption sites: A novel NOx storage and reduction catalyst. Catal. Commun. 2017, 101, 125–128. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Yang, Z.; Li, H.; Sheng, H.; Liu, W.; Li, Q.; Wang, L. Highly active MnOx supported on the MgAlOx oxides derived from LDHs for low temperature NH3-SCR. Fuel 2022, 329, 125519. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Z.; Wu, J.; Yi, X.; Zheng, A.; Umar, A.; O’Hare, D.; Wang, Q. Comprehensive investigation of CO2 adsorption on Mg-Al-CO3 LDH-derived mixed metal oxides. J. Mater. Chem. A 2013, 1, 12782. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Liu, L.; Du, Y.; Wu, X. Superior CuMgFe mixed oxide catalysts engineered by tuning the redox cycle for enhancing NOx removal performance. J. Environ. Chem. Eng. 2022, 10, 108824. [Google Scholar] [CrossRef]
- Wu, X.; Wang, R.; Du, Y.; Li, X.; Meng, H.; Xie, X. NOx removal by selective catalytic reduction with ammonia over hydrotalcite-derived NiTi mixed oxide. New J. Chem. 2019, 43, 2640–2648. [Google Scholar] [CrossRef]
- Hou, B.; Du, Y.; Liu, X.; Ci, C.; Wu, X.; Xie, X. Tunable preparation of highly dispersed NixMn-LDO catalysts derived from NixMn-LDHs precursors and application in low-temperature NH3-SCR reactions. RSC Adv. 2019, 9, 24377–24385. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Shi, L.; Fang, C.; Li, H.; Gao, R.; Huang, L.; Zhang, J. In situ supported MnOx-CeOx on carbon nanotubes for the low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2013, 5, 1127–1136. [Google Scholar] [CrossRef]
- Ma, L.; Li, Z.; Zhao, H.; Zhang, T.; Yan, N.; Li, J. Understanding the Water Effect for Selective Catalytic Reduction of NOx with NH3 over Cu-SSZ-13 Catalysts. ACS EST Eng. 2022, 2, 1684–1696. [Google Scholar] [CrossRef]
- Xiong, S.; Peng, Y.; Wang, D.; Huang, N.; Zhang, Q.; Yang, S.; Chen, J.; Li, J. The role of the Cu dopant on a Mn3O4 spinel SCR catalyst: Improvement of low-temperature activity and sulfur resistance. Chem. Eng. J. 2020, 387, 124090. [Google Scholar] [CrossRef]
- Obalová, L.; Karásková, K.; Jirátová, K.; Kovanda, F. Effect of potassium in calcined Co-Mn-Al layered double hydroxide on the catalytic decomposition of N2O. Appl. Catal. B Environ. 2009, 90, 132–140. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Ai, L.; Liu, W.; Li, Q.; Wang, X.; Wang, L. High performance of K-supported Pr2Sn2O7 pyrochlore catalysts for soot oxidation. Fuel 2022, 317, 123467. [Google Scholar] [CrossRef]
- Son, H.J.; Jang, H.; Nam, S.-E.; Kim, J.; Lee, C.S. Highly interconnected, trimodal porous TiO2 with an inverse-opal structure for photocatalytic membrane water treatment. Sep. Purif. Technol. 2025, 378, 134646. [Google Scholar] [CrossRef]
- Pappas, D.K.; Boningari, T.; Boolchand, P.; Smirniotis, P.G. Novel manganese oxide confined interweaved titania nanotubes for the low-temperature Selective Catalytic Reduction (SCR) of NOx by NH3. J. Catal. 2016, 334, 1–13. [Google Scholar] [CrossRef]
- Damma, D.; Pappas, D.K.; Boningari, T.; Smirniotis, P.G. Study of Ce, Sb, and Y exchanged titania nanotubes and superior catalytic performance for the selective catalytic reduction of NOx. Appl. Catal. B Environ. 2021, 287, 119939. [Google Scholar] [CrossRef]
- Boningari, T.; Ettireddy, P.R.; Somogyvari, A.; Liu, Y.; Vorontsov, A.; McDonald, C.A.; Smirniotis, P.G. Influence of elevated surface texture hydrated titania on Ce-doped Mn/TiO2 catalysts for the low-temperature SCR of NO under oxygen-rich conditions. J. Catal. 2015, 325, 145–155. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Yang, X.; Chen, Y.; Hu, X.; Wu, X. CeMn/TiO2 catalysts prepared by different methods for enhanced low-temperature NH3-SCR catalytic performance. Chem. Eng. Sci. 2021, 238, 116588. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, X.; Härelind, H.; Weng, D.; Wang, B.; Si, Z. NH3-SCR reaction mechanisms of NbO/Ce0.75Zr0.25O2 catalyst: DRIFTS and kinetics studies. J. Mol. Catal. A Chem. 2016, 423, 172–180. [Google Scholar] [CrossRef]
- Yu, J.; Si, Z.; Chen, L.; Wu, X.; Weng, D. Selective catalytic reduction of NO by ammonia over phosphate-containing Ce0.75Zr0.25O2 solids. Appl. Catal. B Environ. 2015, 163, 223–232. [Google Scholar] [CrossRef]
- Szymaszek, A.; Samojeden, B.; Motak, M. The Deactivation of Industrial SCR Catalysts-A Short Review. Energies 2020, 13, 3870. [Google Scholar] [CrossRef]
- Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, Y.; Wang, L.; Lu, G. A highly effective catalyst of Sm-Mn mixed oxide for the selective catalytic reduction of NOx with ammonia: Effect of the calcination temperature. J. Mol. Catal. A Chem. 2016, 420, 272–281. [Google Scholar] [CrossRef]
- Raja, S.; Alphin, M.S.; Sivachandiran, L.; Singh, P.; Damma, D.; Smirniotis, P.G. TiO2-carbon nanotubes composite supported MnOx-CuO catalyst for low-temperature NH3-SCR of NO: Investigation of SO2 and H2O tolerance. Fuel 2022, 307, 121886. [Google Scholar] [CrossRef]
- Sereewatthanawut, I.; Khajonvittayakul, C.; Swadchaipong, N.; Tongnan, V.; Maneesard, P.; Ampairojanawong, R.; Makdee, A.; Kangsadan, T.; Hartley, M.; Hartley, U.W. Enhanced catalytic performance of MnO2 nanowires for soot combustion by cobalt incorporation. Mater. Adv. 2025, 6, 6416–6426. [Google Scholar] [CrossRef]
- Payan, A.; Jafarihaghighi, F.; Soltan, J. Enhanced catalytic performance of α-MnO2 via coupling with a vacuum ultraviolet photoreactor: A catalytic ozonation approach targeting VOC oxidation. Process Saf. Environ. Prot. 2025, 201, 107499. [Google Scholar] [CrossRef]
- Tan, W.; Wang, C.; Yu, S.; Li, Y.; Xie, S.; Gao, F.; Dong, L.; Liu, F. Revealing the effect of paired redox-acid sites on metal oxide catalysts for efficient NOx removal by NH3-SCR. J. Hazard. Mater. 2021, 416, 125826. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; He, F.; Liu, X.; Qi, K.; Xie, J.; Li, F.; Yu, C. Low temperature NH3-SCR of NO over an unexpected Mn-based catalyst: Promotional effect of Mg doping. Appl. Surf. Sci. 2018, 427, 45–55. [Google Scholar] [CrossRef]
- Guo, R.; Wang, S.; Pan, W.; Li, M.-Y.; Sun, P.; Liu, S.-M.; Sun, X.; Liu, S.-W.; Liu, J. Different Poisoning Effects of K and Mg on the Mn/TiO2 Catalyst for Selective Catalytic Reduction of NOx with NH3: A Mechanistic Study. J. Phys. Chem. C 2017, 121, 7881–7891. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Xu, H.; Shen, K.; Zhou, C.; Jin, B.; Sun, K. Novel ultrasonic-modified MnOx/TiO2 for low-temperature selective catalytic reduction (SCR) of NO with ammonia. J. Colloid Interf. Sci. 2011, 361, 212–218. [Google Scholar] [CrossRef]
- Zha, K.; Feng, C.; Han, L.; Li, H.; Yan, T.; Kuboon, S.; Shi, L.; Zhang, D. Promotional effects of Fe on manganese oxide octahedral molecular sieves for alkali-resistant catalytic reduction of NOx: XAFS and in situ DRIFTs study. Chem. Eng. J. 2020, 381, 122764. [Google Scholar] [CrossRef]
- Zha, K.; Kang, L.; Feng, C.; Han, L.; Li, H.; Yan, T.; Maitarad, P.; Shi, L.; Zhang, D. Improved NOx reduction in the presence of alkali metals by using hollandite Mn-Ti oxide promoted Cu-SAPO-34 catalysts. Environ. Sci. Nano 2018, 5, 1408–1419. [Google Scholar] [CrossRef]
- Giordanino, F.; Borfecchia, E.; Lomachenko, K.A.; Lazzarini, A.; Agostini, G.; Gallo, E.; Soldatov, A.V.; Beato, P.; Bordiga, S.; Lamberti, C. Interaction of NH3 with Cu-SSZ-13 Catalyst: A Complementary FTIR, XANES, and XES Study. J. Phys. Chem. Lett. 2014, 5, 1552–1559. [Google Scholar] [CrossRef]
- Chen, W.; Ma, Y.; Qu, Z.; Liu, Q.; Huang, W.; Hu, X.; Yan, N. Mechanism of the selective catalytic oxidation of slip ammonia over Ru-modified Ce-Zr complexes determined by in situ diffuse reflectance infrared Fourier transform spectroscopy. Environ. Sci. Technol. 2014, 48, 12199–12205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, L.; Huang, L.; Zhang, J.; Gao, R.; Zhang, D. Rational Design of High-Performance DeNOx Catalysts Based on MnxCo3–xO4 Nanocages Derived from Metal–Organic Frameworks. ACS Catal. 2014, 4, 1753–1763. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, F.; Li, Z.; Niu, X.; Zhu, Y. Synergistic effect between the redox property and acidity on enhancing the low temperature NH3-SCR activity for NO removal over the Co0.2CexMn0.8-xTi10 (x = 0–0.40) oxides catalysts. Chem. Eng. J. 2018, 354, 393–406. [Google Scholar] [CrossRef]
- Wang, X.; Duan, R.; Liu, W.; Wang, D.; Wang, B.; Xu, Y.; Niu, C.; Shi, J.-W. The insight into the role of CeO2 in improving low-temperature catalytic performance and SO2 tolerance of MnCoCeOx microflowers for the NH3-SCR of NOx. Appl. Surf. Sci. 2020, 510, 145517. [Google Scholar] [CrossRef]
- Xue, H.; Guo, X.; Meng, T.; Mao, D.; Ma, Z. NH3-SCR of NO over M/ZSM-5 (M = Mn, Co, Cu) catalysts: An in-situ DRIFTS study. Surf. Interfaces 2022, 29, 101722. [Google Scholar] [CrossRef]
- Yadav, S.; Ahmad, M.; Siddiqi, K.S. Metal-ion directed synthesis of N2O2 type chelate complexes of Ni(II), Cu(II) and Zn(II): Spectral, thermal and single crystal studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 98, 240–246. [Google Scholar] [CrossRef]
- Boningari, T.; Pappas, D.K.; Smirniotis, P.G. Metal oxide-confined interweaved titania nanotubes M/TNT (M = Mn, Cu, Ce, Fe, V, Cr, and Co) for the selective catalytic reduction of NOx in the presence of excess oxygen. J. Catal. 2018, 365, 320–333. [Google Scholar] [CrossRef]
- Luo, W.; Rong, J.; Zhao, W.; Kang, K.; Long, L.; Yao, X. Morphology and crystal-plane dependence of CeO2-TiO2 catalysts: Activity and mechanism for the selective catalytic reduction of NO with NH3. Chem. Eng. J. 2022, 444, 136488. [Google Scholar] [CrossRef]
- Ni, S.; Tang, X.; Yi, H.; Gao, F.; Wang, C.; Shi, Y.; Zhang, R.; Zhu, W. Novel Mn-Ce bi-oxides loaded on 3D monolithic nickel foam for low-temperature NH3-SCR de-NOx: Preparation optimization and reaction mechanism. J. Rare Earth 2022, 40, 268–278. [Google Scholar]
- Yuan, H.; Chen, J.; Guo, Y.; Wang, H.; Hu, P. Insight into the Superior Catalytic Activity of MnO2 for Low-Content NO Oxidation at Room Temperature. J. Phys. Chem. C 2018, 122, 25365–25373. [Google Scholar] [CrossRef]
- Chen, L.; Niu, X.; Li, Z.; Dong, Y.; Zhang, Z.; Yuan, F.; Zhu, Y. Promoting catalytic performances of Ni-Mn spinel for NH3-SCR by treatment with SO2 and H2O. Catal. Commun. 2016, 85, 48–51. [Google Scholar] [CrossRef]
- Dong, Y.; Jin, B.; Liu, S.; Gao, J.; Wang, K.; Su, F. Abundant Oxygen Vacancies Induced by the Mechanochemical Process Boost the Low-Temperature Catalytic Performance of MnO2 in NH3-SCR. Catalysts 2022, 12, 1291. [Google Scholar] [CrossRef]
- Xue, H.; Guo, X.; Mao, D.; Meng, T.; Yu, J.; Ma, Z. Phosphotungstic Acid-Modified MnOx for Selective Catalytic Reduction of NOx with NH3. Catalysts 2022, 12, 1248. [Google Scholar] [CrossRef]
- Tang, X.; Li, J.; Sun, L.; Hao, J. Origination of N2O from NO reduction by NH3 over β-MnO2 and α-Mn2O3. Appl. Catal. B Environ. 2010, 99, 156–162. [Google Scholar] [CrossRef]
- Yang, S.; Fu, Y.; Liao, Y.; Xiong, S.; Qu, Z.; Yan, N.; Li, J. Competition of selective catalytic reduction and non selective catalytic reduction over MnOx/TiO2 for NO removal: The relationship between gaseous NO concentration and N2O selectivity. Catal. Sci. Technol. 2014, 4, 224–232. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, Z.; Yu, J.; Jaroniec, M. Effect of microstructure and surface hydroxyls on the catalytic activity of Au/AlOOH for formaldehyde removal at room temperature. J. Colloid Interf. Sci. 2017, 501, 164–174. [Google Scholar] [CrossRef]
- Xie, S.; Li, L.; Jin, L.; Wu, Y.; Liu, H.; Qin, Q.; Wei, X.; Liu, J.; Dong, L.; Li, B. Low temperature high activity of M (M=Ce, Fe, Co, Ni) doped M-Mn/TiO2 catalysts for NH3-SCR and in situ DRIFTS for investigating the reaction mechanism. Appl. Surf. Sci. 2020, 515, 146014. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, W.; Li, Z.; Sun, Y.; Shi, M.; Nan, Z.; Song, R.; Wang, L.; Guan, J. Effect of Metal Complexing on Mn-Fe/TS-1 Catalysts for Selective Catalytic Reduction of NO with NH3. Molecules 2023, 28, 3068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shi, T.; Wang, Y.; Hao, Y.; Gao, Y.; Li, H.; Jia, L.; Liu, F.; Zeng, S. Orchestrating dual adsorption sites and unravelling Ce-Mn interaction and reaction mechanisms for efficient NH3-SCR. J. Catal. 2024, 429, 115260. [Google Scholar] [CrossRef]
- Qin, Q.; Zhu, C.; Mo, D.; Chen, Z.; Dong, L.; Li, B.; Zhou, L. In situ doping derivative construction of MnOx/Mn-ZrO2-C for efficient degradation of NOx. Mol. Catal. 2025, 583, 115243. [Google Scholar] [CrossRef]
- Xu, Y.; Lai, W.; Jiang, L.; Jiang, W.; Dai, Z.; Yang, L.; Yao, L. Highly dispersed Mn and Cu on LDO/Z composite catalysts with strong H2O resistance for low-temperature NH3-SCR. Sep. Purif. Technol. 2025, 364, 132434. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).