A Brief Review of Cu-Based Catalysts for the Selective Liquid-Phase Hydrogenation of Furfural to Furfuryl Alcohol
Abstract
1. Introduction
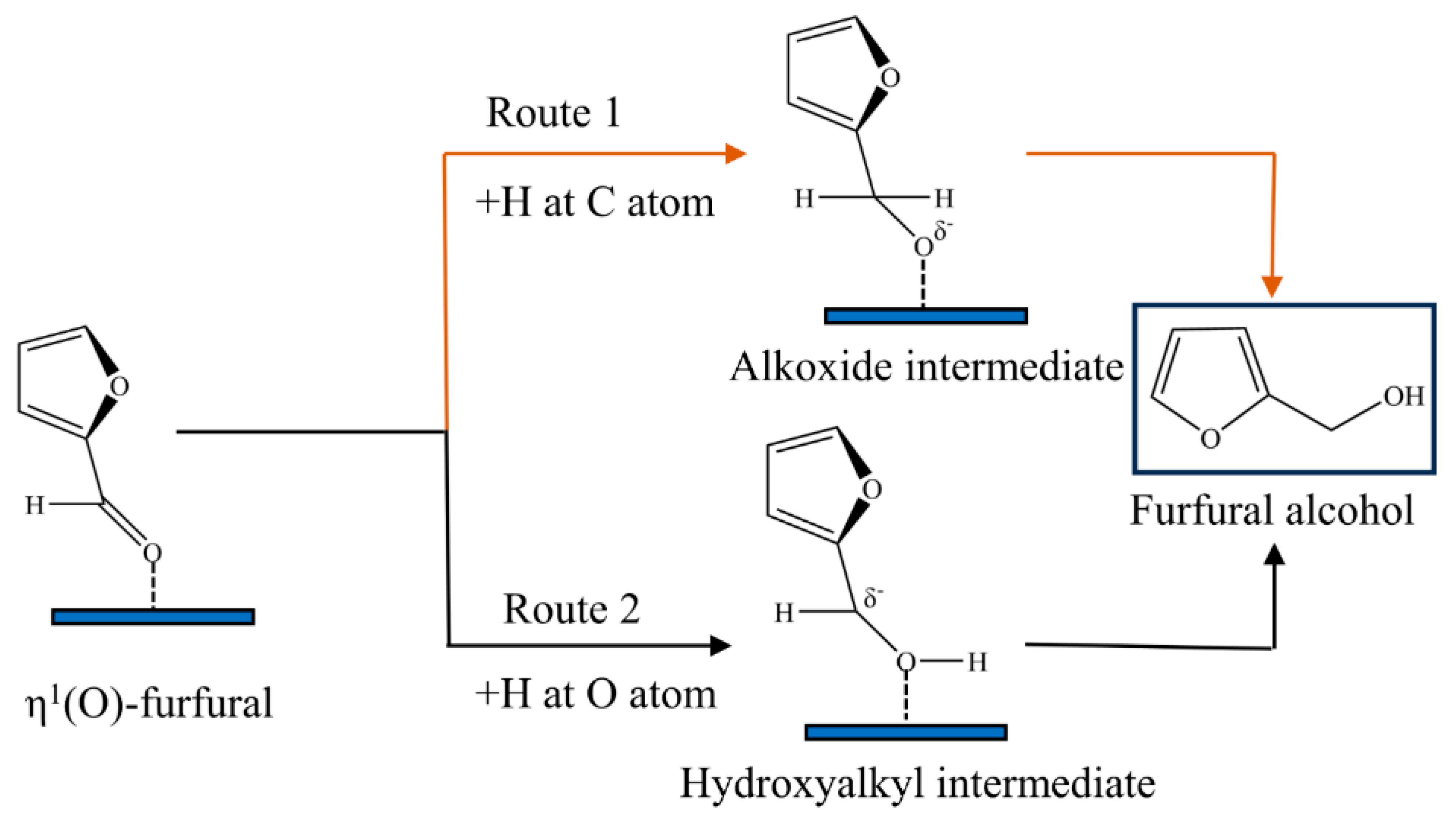
2. Reaction Mechanisms
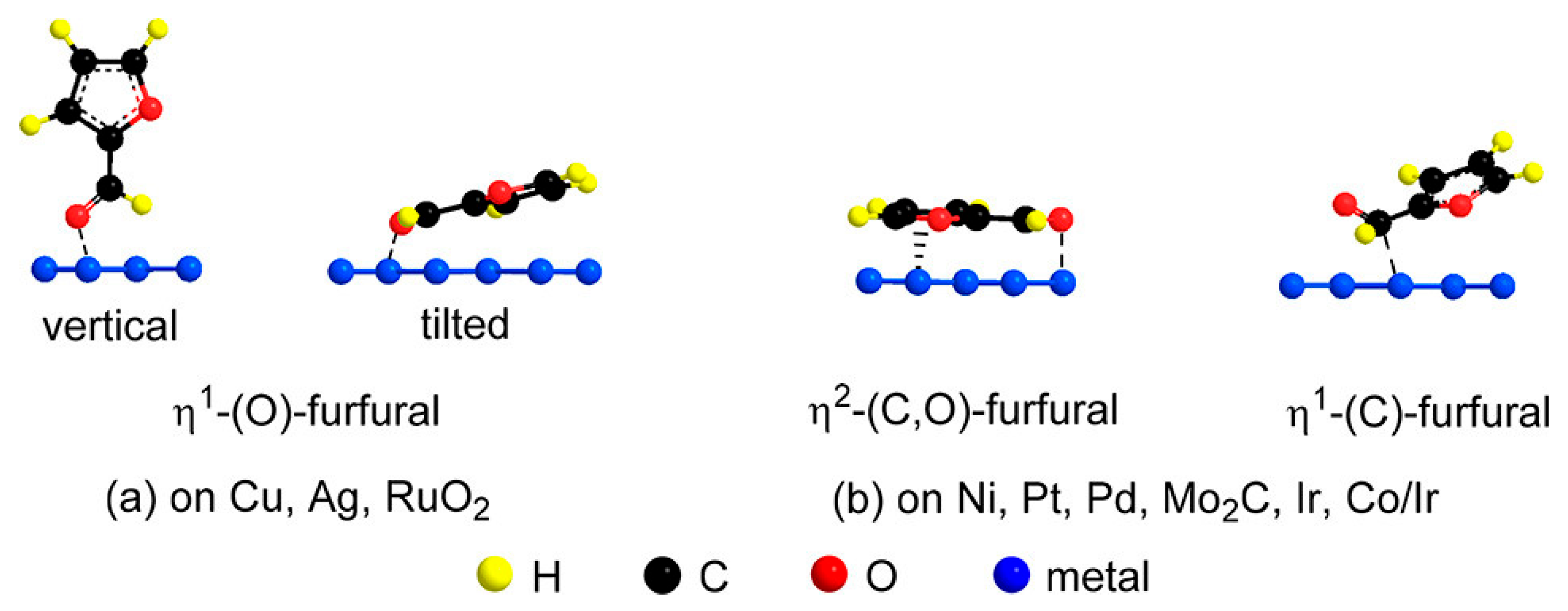
2.1. η1-(O)-aldehyde Configuration
2.2. η2-(C,O)-aldehyde Configuration
2.3. Solvent Effects
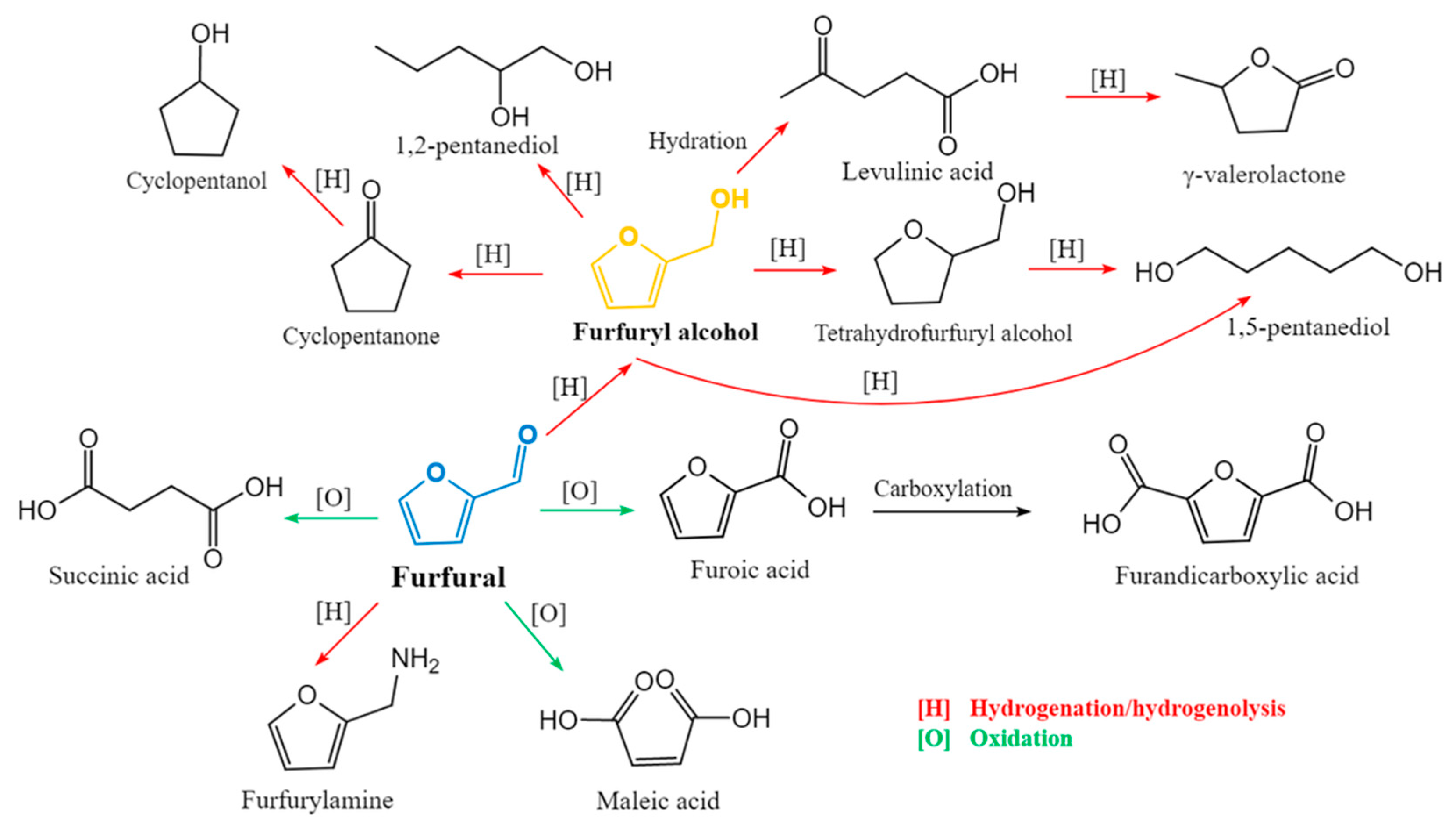
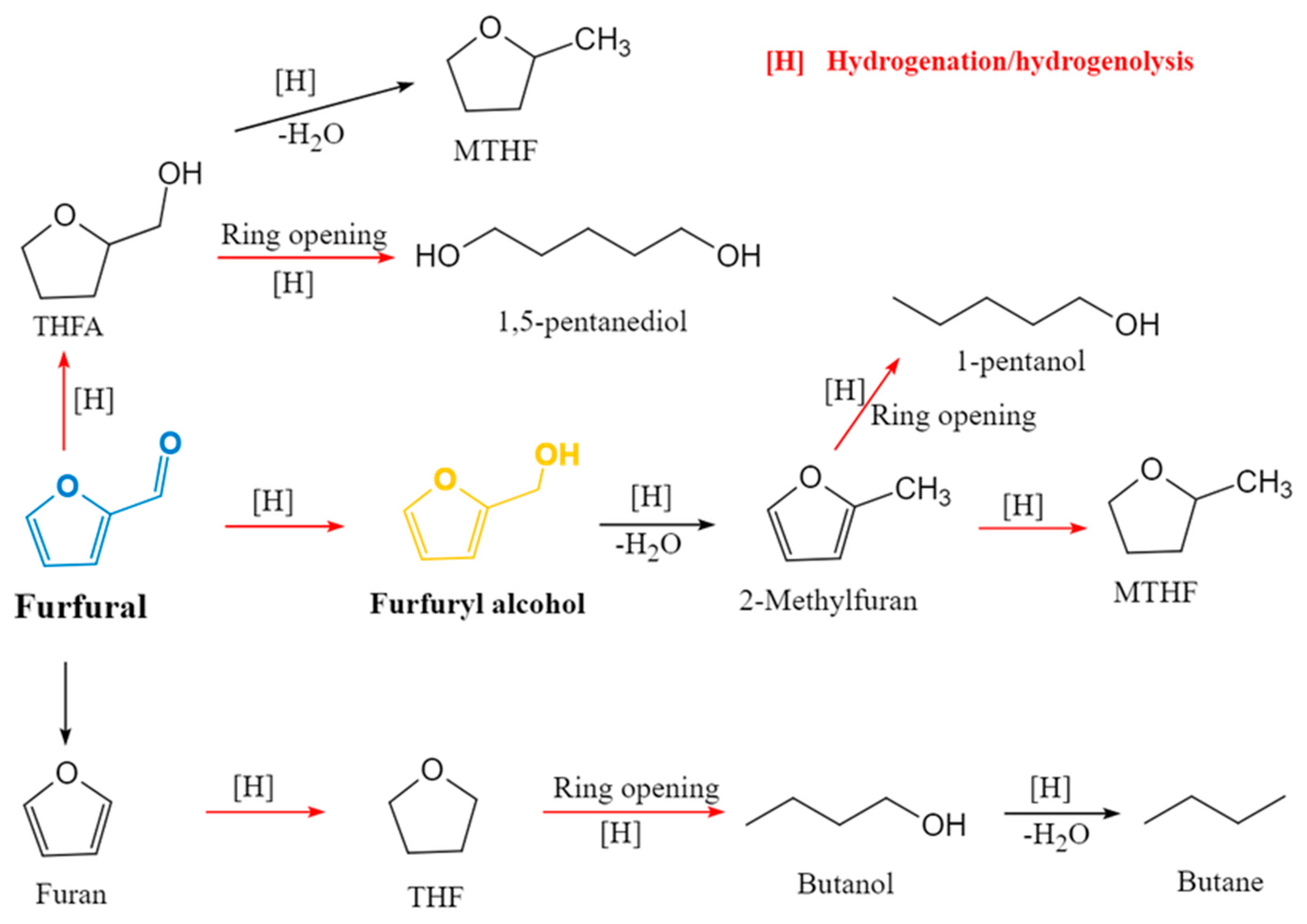
2.4. Side Reactions
3. Catalyst
3.1. Active Metal
3.1.1. Noble Metal
3.1.2. Fe, Co, and Ni
3.1.3. Cu
3.2. Support
3.2.1. Metal Oxide
3.2.2. SiO2
3.2.3. Molecular Sieve
3.2.4. Carbon-Containing Material
3.2.5. MOF
3.3. Promoter
3.3.1. Metal Promoter
3.3.2. Metal Oxide Promoter
3.3.3. Other Promoter
3.4. Preparation Method
3.4.1. Supported Cu Catalysts
3.4.2. Coated Cu Catalysts
3.4.3. Integrated Cu and Support
4. Characterization Method
5. Conclusions and Perspectives
5.1. Summary and Significance
5.2. Composition and Preparation Method for Better Catalyst Design
5.3. Using Novel Characterization Technique
5.4. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Nijnens, J.; Behrens, P.; Kraan, O.; Sprecher, B.; Kleijn, R. Energy transition will require substantially less mining than the current fossil system. Joule 2023, 7, 2408–2413. [Google Scholar] [CrossRef]
- Niu, Y.; Yan, Y.; Dong, J.; Yue, K.; Duan, X.; Hu, D.; Li, J.; Peng, L. Evidence for sustainably reducing secondary pollutants in a typical industrial city in China: Co-benefit from controlling sources with high reduction potential beyond industrial process. J. Hazard. Mater. 2024, 478, 135556. [Google Scholar] [CrossRef]
- Ali, H.; Ali, I.; Baz, K. Do industrialization and nonrenewable energy affect environmental quality? Evidence from top fossil fuel–consuming countries. Environ. Sci. Pollut. Res. 2023, 30, 109800–109809. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. The refinery of the future. Nature 2024, 629, 295–306. [Google Scholar] [CrossRef]
- Phang, F.J.F.; Chew, J.J.; Chakraborty, S.; Sunarso, J. Synthesis of hydrochars via wet torrefaction of biomass for sustainable energy production: A life cycle assessment study. ACS Sustain. Chem. Eng. 2025, 13, 2884–2892. [Google Scholar] [CrossRef]
- Ge, H.; Kuwahara, Y.; Okada, M.; Yamashita, H. Mild hydrodeoxygenation of aromatic ketones by Pd/HxWO3–y with plasmonic features assisted by visible-NIR light irradiation. ACS Sustain. Chem. Eng. 2024, 12, 2162–2171. [Google Scholar] [CrossRef]
- Gunukula, S.; Pendse, H.P.; DeSisto, W.J.; Wheeler, M.C. Heuristics to guide the development of sustainable, biomass-derived, platform chemical derivatives. ACS Sustain. Chem. Eng. 2018, 6, 5533–5539. [Google Scholar] [CrossRef]
- Liao, Y.; Koelewijn, S.F.; Van den Bossche, G.; Van Aelst, J.; Van den Bosch, S.; Renders, T.; Navare, K.; Nicolaï, T.; Van Aelst, K.; Maesen, M.; et al. A sustainable wood biorefinery for low–carbon footprint chemicals production. Science 2020, 367, 1385–1390. [Google Scholar] [CrossRef]
- Shi, N.; Liu, Q.; Zhang, Q.; Wang, T.; Ma, L. High yield production of 5-hydroxymethylfurfural from cellulose by high concentration of sulfates in biphasic system. Green Chem. 2013, 15, 1967–1974. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, S.; Li, Q.; Zhou, G.; Xia, H. Recent advances in the conversion of furfural into bio-chemicals through chemo-and bio-catalysis. RSC Adv. 2021, 11, 27042–27058. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhang, W.; Zhou, C.; Huang, J.; Su, Z.; Qiao, Z.; Qin, X.; Xiong, P.; Xiao, F.-S. Carbothermal shock synthesis of a PtCoCe ternary oxide catalyst for selective hydrogenolysis of furfural to 1,5-Pentanediol. ACS Catal. 2025, 15, 7731–7740. [Google Scholar] [CrossRef]
- Li, H.; Luo, M.; Zhu, T.; Wang, C.; Liao, Y.; Wang, H.; Ma, L. Valorization of furfural to cyclopentanone/cyclopentanol over platinum cluster catalysts: The ensemble effect. Appl. Catal. B Environ. Energy 2025, 366, 125052. [Google Scholar] [CrossRef]
- Di, J.; Li, Q.; Ma, C.; He, Y.-C. An efficient and sustainable furfurylamine production from biomass-derived furfural by a robust mutant ω-transaminase biocatalyst. Bioresour. Technol. 2023, 369, 128425. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, G.; Zhang, W.; Li, X.; Zhang, Z.; Yang, M.; Wu, Y.; Wang, D.; Li, Y. Oriented conversion of a LA/HMF mixture to GVL and FDCA in a biphasic solvent over a Ru single-atom/nanoparticle dual-site catalyst. ACS Catal. 2023, 13, 2268–2276. [Google Scholar] [CrossRef]
- Ramos, N.C.; Manyé Ibáñez, M.; Mittal, R.; Janik, M.J.; Holewinski, A. Combining renewable electricity and renewable carbon: Understanding reaction mechanisms of biomass-derived furanic compounds for design of catalytic nanomaterials. Acc. Chem. Res. 2023, 56, 2631–2641. [Google Scholar] [CrossRef]
- Ren, Z.; Yu, L.; Song, X.; Wang, S.; Li, H.; Wang, L.; Zhang, X.; Li, X.; Yang, Y.; Wang, Z.; et al. Atomically dispersed Sn catalysts toward selective oxidation of furfural to biodegradable polymer monomers. ACS Catal. 2025, 15, 3799–3809. [Google Scholar] [CrossRef]
- Liu, B.; Chen, X.; Xu, Y.; Qiao, C.; Lu, Z.; Tian, Y. A combo Zr–zeolite and Zr(OH)4 mixture composition for one–pot production of γ–valerolactone from furfural. Renew. Energy 2024, 229, 120751. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Li, M.-Y.; Shi, Y.-C.; Xu, W.-X.; Zhao, L.; Zhang, A.-Z. Exploring Cr(VI)-induced blood-brain barrier injury and neurotoxicity in zebrafish and snakehead fish, and inhibiting toxic effects of astaxanthin. Environ. Pollut. 2024, 355, 124280. [Google Scholar] [CrossRef]
- Ma, L.; Liu, T.; Li, J.; Yang, Q. Interaction characteristics and mechanism of Cr(VI)/Cr(III) with microplastics: Influence factor experiment and DFT calculation. J. Hazard. Mater. 2024, 476, 134957. [Google Scholar] [CrossRef] [PubMed]
- Jorqueira, D.S.S.; de Lima, L.F.; Moya, S.F.; Vilcocq, L.; Richard, D.; Fraga, M.A.; Suppino, R.S. Critical review of furfural and furfuryl alcohol production: Past, present, and future on heterogeneous catalysis. Appl. Catal. A Gen. 2023, 665, 119360. [Google Scholar] [CrossRef]
- Racha, A.; Samanta, C.; Sreekantan, S.; Marimuthu, B. Review on catalytic hydrogenation of biomass-derived furfural to furfuryl alcohol: Recent advances and future trends. Energy Fuels 2023, 37, 11475–11496. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, A.; Bao, W.; Zhang, L. Recent advances in catalytic hydrogenation of furfural over non-noble metal catalysts. Ind. Eng. Chem. Res. 2025, 64, 13966–13983. [Google Scholar] [CrossRef]
- Wang, J.; Quan, Y.; Ren, J. Effect of Cu loading on the structure and catalytic properties of Cu/SBA-15 for the hydrogenation of furfural to furfuryl alcohol. Sep. Purif. Technol. 2025, 362, 131568. [Google Scholar] [CrossRef]
- Mensah, J.; Jampaiah, D.; Ahmed, M.H.M.; Konarova, M.; Durndell, L.J.; Bhargava, S.K.; Lee, A.F.; Wilson, K. Liquid-phase furfural hydrogenation over Ni/Alumina catalysts. ACS Sustain. Chem. Eng. 2025, 13, 2893–2905. [Google Scholar] [CrossRef]
- Kuchkina, N.; Sorokina, S.; Lisichkin, D.; Bykov, A.; Bukalov, S.; Matveeva, V.; Shifrina, Z. Simultaneous synthesis of a microporous polymer framework with embedded Nickel nanoparticles for enhanced selective hydrogenation of furfural to furfuryl alcohol. ACS Appl. Polym. Mater. 2025, 7, 2017–2028. [Google Scholar] [CrossRef]
- Jaswal, A.; Singh, P.P.; Mondal, T. Furfural—A versatile, biomass-derived platform chemical for the production of renewable chemicals. Green Chem. 2022, 24, 510–551. [Google Scholar] [CrossRef]
- Sitthisa, S.; Sooknoi, T.; Ma, Y.; Balbuena, P.B.; Resasco, D.E. Kinetics and mechanism of hydrogenation of furfural on Cu/SiO2 catalysts. J. Catal. 2011, 277, 1–13. [Google Scholar] [CrossRef]
- Sitthisa, S.; An, W.; Resasco, D.E. Selective conversion of furfural to methylfuran over silica-supported NiFe bimetallic catalysts. J. Catal. 2011, 284, 90–101. [Google Scholar] [CrossRef]
- Sitthisa, S.; Pham, T.; Prasomsri, T.; Sooknoi, T.; Mallinson, R.G.; Resasco, D.E. Conversion of furfural and 2-methylpentanal on Pd/SiO2 and Pd–Cu/SiO2 catalysts. J. Catal. 2011, 280, 17–27. [Google Scholar] [CrossRef]
- Medlin, J.W. Understanding and controlling reactivity of unsaturated oxygenates and polyols on metal catalysts. ACS Catal. 2011, 1, 1284–1297. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, Y.; Yang, Y.; Li, Y.-W.; Jiao, H. Exploring furfural catalytic conversion on Cu(111) from computation. ACS Catal. 2015, 5, 4020–4032. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Govindarajan, N.; Chan, K. Understanding activity trends in furfural hydrogenation on transition metal surfaces. ACS Catal. 2022, 12, 12902–12910. [Google Scholar] [CrossRef]
- Vorotnikov, V.; Mpourmpakis, G.; Vlachos, D.G. DFT study of furfural conversion to furan, furfuryl alcohol, and 2-Methylfuran on Pd(111). ACS Catal. 2012, 2, 2496–2504. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nakazawa, H.; Watanabe, H.; Tomishige, K. Total Hydrogenation of Furfural over a Silica-Supported Nickel Catalyst Prepared by the Reduction of a Nickel Nitrate Precursor. ChemCatChem 2012, 4, 1791–1797. [Google Scholar] [CrossRef]
- Saha, B.; Racha, A.; Chaudhary, P.K.; Singh, B.K.; Samanta, C.; Newalkar, B.L. Enhanced production and techno-economic analysis of sustainable biofuel production via continuous hydrogenation of furfural using the Cu–ZnO–Al2O3 catalyst. ACS Sustain. Chem. Eng. 2025, 13, 3183–3199. [Google Scholar] [CrossRef]
- Arundhathi, R.; Reddy, P.L.; Samanta, C.; Newalkar, B.L. Chromium-free Cu@Mg/γ-Al2O3–An active catalyst for selective hydrogenation of furfural to furfuryl alcohol. RSC Adv. 2020, 10, 41120–41126. [Google Scholar] [CrossRef]
- Jia, P.; Lan, X.; Li, X.; Wang, T. Highly active and selective NiFe/SiO2 bimetallic catalyst with optimized solvent effect for the liquid-phase hydrogenation of furfural to furfuryl alcohol. ACS Sustain. Chem. Eng. 2018, 6, 13287–13295. [Google Scholar] [CrossRef]
- Singh, G.; Singh, L.; Gahtori, J.; Gupta, R.K.; Samanta, C.; Bal, R.; Bordoloi, A. Catalytic hydrogenation of furfural to furfuryl alcohol over chromium-free catalyst: Enhanced selectivity in the presence of solvent. Mol. Catal. 2021, 500, 111339. [Google Scholar] [CrossRef]
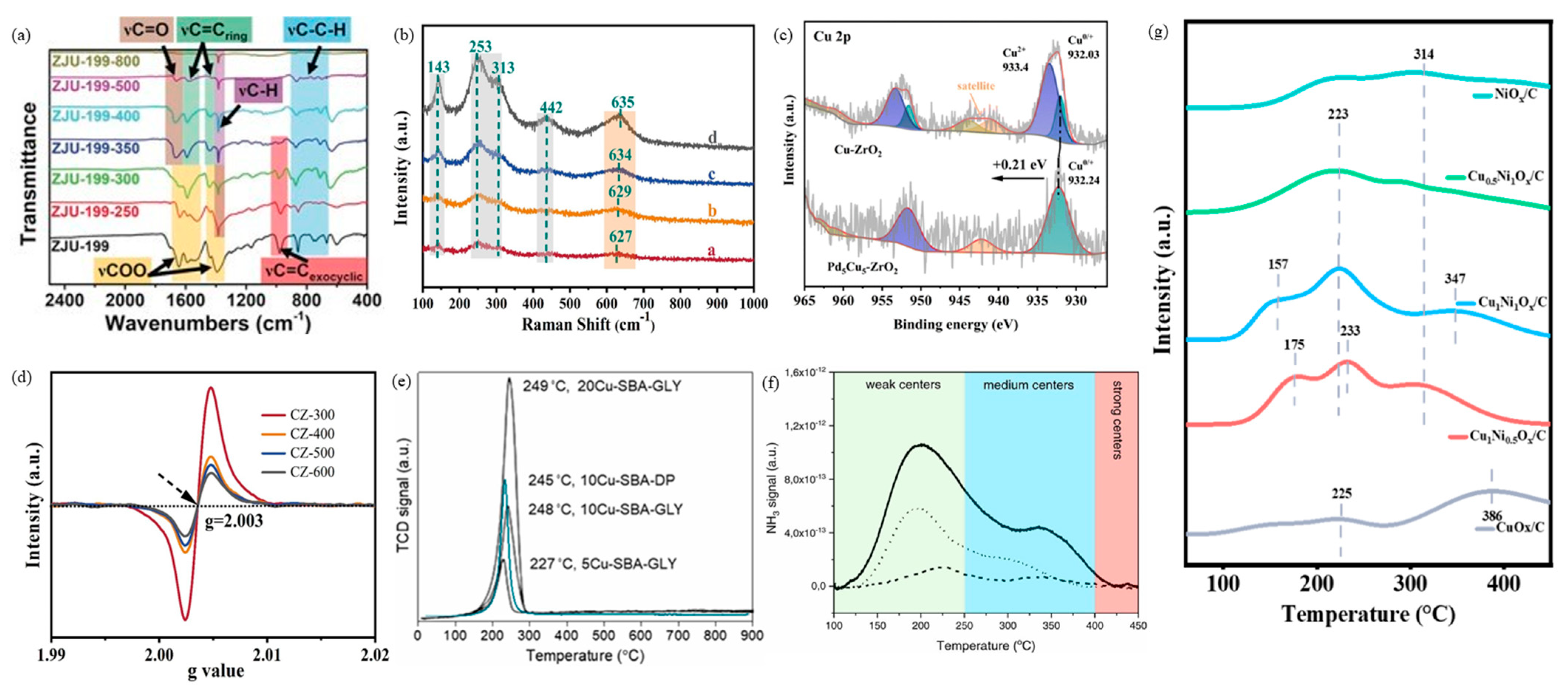
- Zhang, J.; Jia, Z.; Yu, S.; Liu, S.; Li, L.; Xie, C.; Wu, Q.; Zhang, Y.; Yu, H.; Liu, Y.; et al. Regulating the Cu0-Cu+ ratio to enhance metal-support interaction for selective hydrogenation of furfural under mild conditions. Chem. Eng. J. 2023, 468, 143755. [Google Scholar] [CrossRef]
- Islam, M.J.; Granollers Mesa, M.; Osatiashtiani, A.; Taylor, M.J.; Manayil, J.C.; Parlett, C.M.A.; Isaacs, M.A.; Kyriakou, G. The effect of metal precursor on copper phase dispersion and nanoparticle formation for the catalytic transformations of furfural. Appl. Catal. B Environ. 2020, 273, 119062. [Google Scholar] [CrossRef]
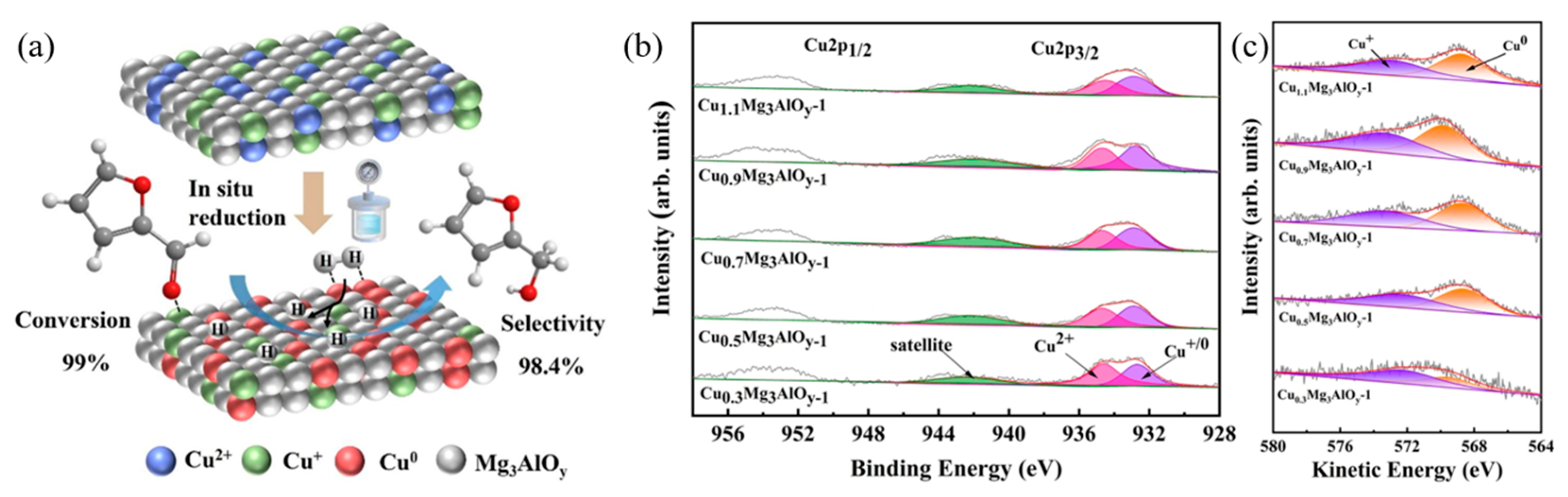
- Li, J.; Niu, X.; Zhu, Y. Synergistic effect of surface Cu0 and Cu+ species on improved selective hydrogenation of furfural to furfuryl alcohol over hydrotalcite-derived CuxMg3Al oxides. Appl. Surf. Sci. 2023, 635, 157774. [Google Scholar] [CrossRef]
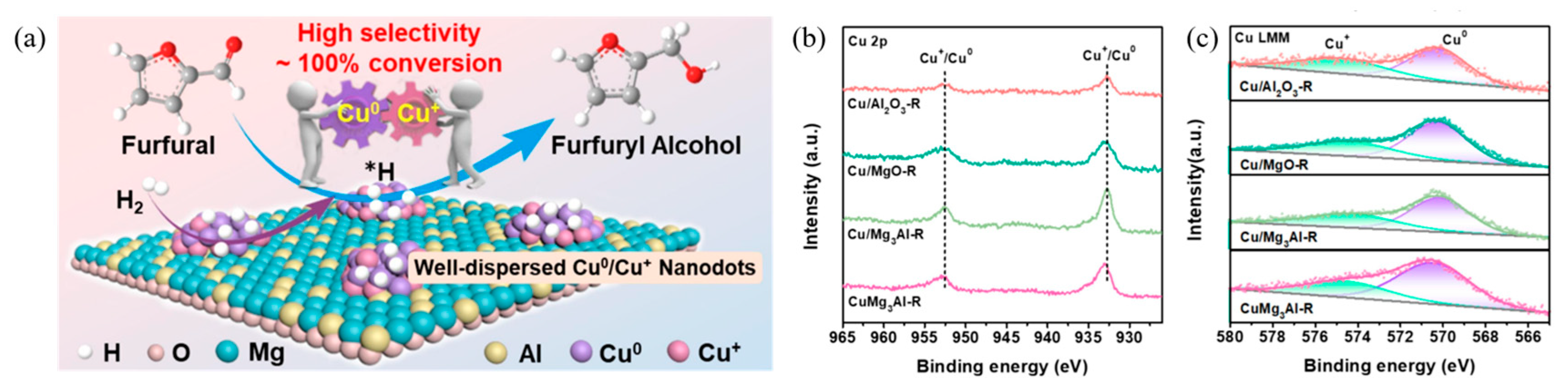
- Yi, W.-J.; Yang, J.; Gao, Y.; Zhou, X.; Zhang, W.; Zhang, M.; Liu, Z. Well-dispersed Cu0/Cu+ nanodots confined in hydrotalcite-derived oxide for highly efficient and selective furfural hydrogenation to furfuryl alcohol. Chem. Eng. J. 2025, 515, 163742. [Google Scholar] [CrossRef]
- Tan, J.; He, J.; Gao, K.; Zhu, S.; Cui, J.; Huang, L.; Zhu, Y.; Zhao, Y. Catalytic hydrogenation of furfural over Cu/CeO2 Catalyst: The effect of support morphology and exposed facet. Appl. Surf. Sci. 2022, 604, 154472. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, J.; Yi, W.-J.; Yang, J.; Jiang, K.; Yang, T.; Li, Z.; Zhang, M.; Liu, Z.; Wu, B. Unveiling the effects of support crystal phase on Cu/Al2O3 catalysts for furfural selective hydrogenation to furfuryl alcohol. Chem. Eng. J. 2024, 498, 155620. [Google Scholar] [CrossRef]
- Soares, W.L.S.; Feitosa, L.F.; Moreira, C.R.; Bertella, F.; Lopes, C.W.; de Farias, A.M.D.; Fraga, M.A. Tailoring Cu–SiO2 interaction through nanocatalyst architecture to assemble surface sites for furfural aqueous-phase hydrogenation to cycloketones. ACS Appl. Mater. Interfaces 2025, 17, 13146–13161. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Song, S.; Zhao, W.; Ding, T.; Tian, Y.; Li, X. Cis-trans adsorption configuration influenced high-efficient hydrogenation of furfural over zeolite-encapsulated PtCu bimetallic catalyst. Chem. Eng. J. 2024, 489, 151471. [Google Scholar] [CrossRef]
- Liang, W.; Xu, G.; Fu, Y. Accurate restricted transition-state shape selective hydrogenation of furfural over zeolite confined Cu catalyst. Chin. J. Catal. 2025, 74, 71–81. [Google Scholar] [CrossRef]
- Srivastava, S.; Mohanty, P.; Parikh, J.K.; Dalai, A.K.; Amritphale, S.S.; Khare, A.K. Cr-free Co–Cu/SBA-15 catalysts for hydrogenation of biomass-derived α-, β-unsaturated aldehyde to alcohol. Chin. J. Catal. 2015, 36, 933–942. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Tang, Q.; Jing, F.; Cao, Q.; Fang, W. Efficient activation of H2 on copper species immobilized by MCM-41 for selective hydrogenation of furfural at ambient pressure. Mol. Catal. 2021, 515, 111921. [Google Scholar] [CrossRef]
- Gong, W.; Chen, C.; Zhang, Y.; Zhou, H.; Wang, H.; Zhang, H.; Zhang, Y.; Wang, G.; Zhao, H. Efficient synthesis of furfuryl alcohol from H2-hydrogenation/transfer hydrogenation of furfural using sulfonate group modified Cu catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2172–2180. [Google Scholar] [CrossRef]
- Moyo, P.S.; Mehlana, G.; Matsinha, L.C.; Makhubela, B.C.E. Copper-based metal–organic framework: Synthesis, characterization and evaluation for the hydrogenation of furfural to furfuryl alcohol. J. Inorg. Organomet. Polym. Mater. 2025, 35, 2257–2273. [Google Scholar] [CrossRef]
- Li, P.; Ma, F.; Fu, M.; Lu, S.; Xia, X.; Li, C.; Gao, Y.; Li, F. Hydrogenation of furfural to furfuryl alcohol over MOF-derived Fe/Cu@C and Fe3O4/Cu@C catalysts. React. Chem. Eng. 2022, 7, 994–1004. [Google Scholar] [CrossRef]
- Zhao, H.; Liao, X.Q.; Cui, H.S.; Zhu, M.C.; Hao, F.; Xiong, W.; Luo, H.; Lv, Y.; Liu, P.L. Efficient Cu-Co bimetallic catalysts for the selective hydrogenation of furfural to furfuryl alcohol. Fuel 2023, 351, 128887. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, D. Aqueous phase catalytic hydrogenation of furfural to furfuryl alcohol over in-situ synthesized Cu–Zn/SiO2 catalysts. Mater. Chem. Phys. 2021, 260, 124152. [Google Scholar] [CrossRef]
- Cao, P.; Lin, L.; Qi, H.; Chen, R.; Wu, Z.; Li, N.; Zhang, T.; Luo, W. Zeolite-encapsulated Cu nanoparticles for the selective hydrogenation of furfural to furfuryl alcohol. ACS Catal. 2021, 11, 10246–10256. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Bing, Z.; Gao, Y.; Yang, T.; Liu, Q.; Zhang, M.; Liu, Z. Refining surface copper species on Cu/SiO2 catalysts to boost furfural hydrogenation to furfuryl alcohol. Molecules 2025, 30, 225. [Google Scholar] [CrossRef] [PubMed]
- Tu, R.; Liang, K.; Sun, Y.; Wu, Y.; Lv, W.; Jia, C.Q.; Jiang, E.; Wu, Y.; Fan, X.; Zhang, B.; et al. Ultra-Dilute high-entropy alloy catalyst with core-shell structure for high-active hydrogenation of furfural to furfuryl alcohol at mild temperature. Chem. Eng. J. 2023, 452, 139526. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z.; Liu, W.; Liang, T.; Dang, D.; Tian, X. In Situ hybridizing Cu3(BTC)2 and Titania to attain a high-performance Copper catalyst: Dual-functional role of metal-support interaction on the activity and selectivity. ChemCatChem 2021, 13, 3846–3856. [Google Scholar] [CrossRef]
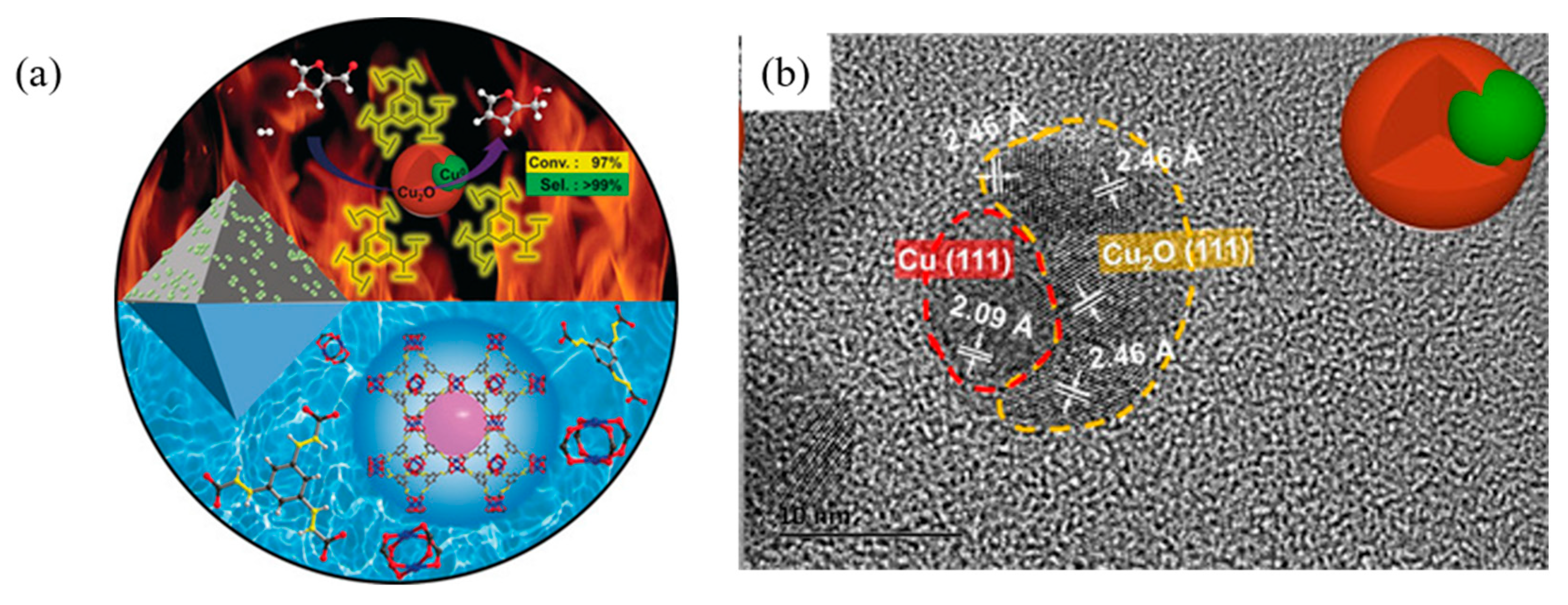
- Chen, K.; Ling, J.-L.; Wu, C.-D. In Situ generation and stabilization of accessible Cu/Cu2O heterojunctions inside organic frameworks for highly efficient catalysis. Angew. Chem. Int. Ed. 2020, 59, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Ling, J.; Wu, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Zheng, H.-Y.; Zhu, Y.-L.; Teng, B.-T.; Bai, Z.-Q.; Zhang, C.-H.; Xiang, H.-W.; Li, Y.-W. Towards understanding the reaction pathway in vapour phase hydrogenation of furfural to 2-methylfuran. J. Mol. Catal. A Chem. 2006, 246, 18–23. [Google Scholar] [CrossRef]
- Sajid, M.; Rizwan Dilshad, M.; Saif Ur Rehman, M.; Liu, D.; Zhao, X. Catalytic conversion of xylose to furfural by p-Toluenesulfonic acid (pTSA) and chlorides: Process optimization and kinetic modeling. Molecules 2021, 26, 2208. [Google Scholar] [CrossRef]
- Yang, X.; Xiang, X.; Chen, H.; Zheng, H.; Li, Y.-W.; Zhu, Y. Efficient Synthesis of Furfuryl Alcohol and 2-Methylfuran from Furfural over Mineral-Derived Cu/ZnO Catalysts. ChemCatChem 2017, 9, 3023–3030. [Google Scholar] [CrossRef]
- Zhang, Z.; Pei, Z.; Chen, H.; Chen, K.; Hou, Z.; Lu, X.; Ouyang, P.; Fu, J. Catalytic in-situ hydrogenation of furfural over bimetallic Cu–Ni alloy catalysts in isopropanol. Ind. Eng. Chem. Res. 2018, 57, 4225–4230. [Google Scholar] [CrossRef]
- Nehra, P.; Betsy, K.; Vinod, C. Selective Hydrogenation of Furfural to Furfuryl Alcohol over Pd Supported on Ternary Oxide in Aqueous Medium Under Mild Conditions. ChemCatChem 2025, 17, e02092. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Zhang, C.; Arai, M.; Zhou, L.; Zhao, F. Selective hydrogenation of furfural to furfuryl alcohol over Pd/TiH2 catalyst. Mol. Catal. 2021, 508, 111599. [Google Scholar] [CrossRef]
- Kijeński, J.; Winiarek, P. Selective hydrogenation of α,β-unsaturated aldehydes over Pt catalysts deposited on monolayer supports. Appl. Catal. A Gen. 2000, 193, L1–L4. [Google Scholar] [CrossRef]
- Seretis, A.; Mertika, I.; Gabrielatou, E.; Patatsi, E.; Thanou, I.; Diamantopoulou, P.; Tzevelekidis, P.; Fakas, C.; Lilas, P.; Kanellopoulos, P.G.; et al. Novel hydrogenation reaction of renewable furfural into furfuryl alcohol using highly efficient and selective water-soluble platinum catalysts modified with phosphines and nitrogen-containing ligands in green aqueous media. Catal. Today 2025, 444, 115019. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, D.; Li, C.; Wang, S.; Hu, X.; Zheng, G.; Chen, G. Highly dispersed Pt on partial deligandation of Ce-MOFs for furfural selective hydrogenation. Appl. Catal. B Environ. 2023, 328, 122458. [Google Scholar] [CrossRef]
- Cui, X.; Surkus, A.E.; Junge, K.; Topf, C.; Radnik, J.; Kreyenschulte, C.; Beller, M. Highly selective hydrogenation of arenes using nanostructured ruthenium catalysts modified with a carbon-nitrogen matrix. Nat. Commun. 2016, 7, 11326. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Adeeva, V.; Sachtler, W.M.H. Stability of supported transition metal catalysts in the hydrogenation of nitriles. Appl. Catal. A Gen. 2000, 196, 73–85. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Widmann, D.; Olesen, S.E.; Chorkendorff, I.; Biskupek, J.; Behm, R.J. Selective CO methanation on Ru/TiO2 catalysts: Role and influence of metal–support interactions. ACS Catal. 2015, 5, 6753–6763. [Google Scholar] [CrossRef]
- Hájek, J.; Kumar, N.; Mäki-Arvela, P.; Salmi, T.; Murzin, D.Y. Selective hydrogenation of cinnamaldehyde over Ru/Y zeolite. J. Mol. Catal. A Chem. 2004, 217, 145–154. [Google Scholar] [CrossRef]
- Miyazawa, T.; Koso, S.; Kunimori, K.; Tomishige, K. Development of a Ru/C catalyst for glycerol hydrogenolysis in combination with an ion-exchange resin. Appl. Catal. A Gen. 2007, 318, 244–251. [Google Scholar] [CrossRef]
- Yang, J.; Ma, J.; Yuan, Q.; Zhang, P.; Guan, Y. Selective hydrogenation of furfural on Ru/Al-MIL-53: A comparative study on the effect of aromatic and aliphatic organic linkers. RSC Adv. 2016, 6, 92299–92304. [Google Scholar] [CrossRef]
- Merlo, A.B.; Bengoa, J.F.; Bianco, M.V.; Marchetti, S.G.; Vetere, V. Characterization of NiFe alloy phases in nanoparticles used as selective catalysts for the production of furfuryl alcohol from furfural hydrogenation. Mol. Catal. 2024, 569, 114614. [Google Scholar] [CrossRef]
- Lara, K.; Arias, D.; Sepulveda, C.; Buljan, A.; Lizana, I.; Delgado, E.J.; Pecchi, G.; Herrera, C. Furfural hydrogenation over reduced pure (LaBO3) and substituted (LaB0.5B’0.5O3) (B, B’: Fe, Co, Ni) perovskites. ChemCatChem 2024, 16, e202301507. [Google Scholar] [CrossRef]
- Ren, W.; Tian, J.; Wang, Z.; Zhang, M. Which is better for converting furfural into furfuryl alcohol: The strong metal-support interaction effect or the formation of alloy? Appl. Catal. A Gen. 2024, 673, 119609. [Google Scholar] [CrossRef]
- Manikandan, M.; Venugopal, A.K.; Nagpure, A.S.; Chilukuri, S.; Raja, T. Promotional effect of Fe on the performance of supported Cu catalyst for ambient pressure hydrogenation of furfural. RSC Adv. 2016, 6, 3888–3898. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, L.; Zhao, J.; Qiu, M.; Liu, J.; He, J.; Wang, L.; Lei, J.; Tian, W.; Rong, L. Facile synthesis of a high-efficiency NiFe bimetallic catalyst without pre-reduction for the selective hydrogenation reaction of furfural. Catal. Sci. Technol. 2023, 13, 457–467. [Google Scholar] [CrossRef]
- Audemar, M.; Ciotonea, C.; De Oliveira Vigier, K.; Royer, S.; Ungureanu, A.; Dragoi, B.; Dumitriu, E.; Jérôme, F. Selective hydrogenation of furfural to furfuryl alcohol in the presence of a recyclable Cobalt/SBA-15 catalyst. ChemSusChem 2015, 8, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yang, Y.; Chen, L.; Xu, M.; Zhang, X.; Wei, M. A control over hydrogenation selectivity of furfural via tuning exposed facet of Ni catalysts. ACS Catal. 2019, 9, 4226–4235. [Google Scholar] [CrossRef]
- Lin, W.; Chen, Y.; Zhang, Y.; Zhang, Y.; Wang, J.; Wang, L.; Xu, C.C.; Nie, R. Surface synergetic effects of Ni–ReOx for promoting the mild hydrogenation of furfural to tetrahydrofurfuryl alcohol. ACS Catal. 2023, 13, 11256–11267. [Google Scholar] [CrossRef]
- Chen, J.; Jia, W.; Yu, X.; Sun, Y.; Zhang, J.; Yang, S.; Tang, X.; Peng, L.; Zeng, X.; Lin, L. Insight into the Co2+/Co3+ sites for the selective reduction of furfural to furfuryl alcohol. Fuel 2023, 332, 126137. [Google Scholar] [CrossRef]
- Shao, Y.; Wu, J.; Zheng, Z.; Fan, M.; Sun, K.; Bkangmo Kontchouo, F.M.; Zhang, L.; Zhang, S.; Hu, G.; Hu, X. Alloying cobalt in Co–Fe–Al catalyst for achieving the selective conversion of furfural to cyclopentanone. Renew. Energy 2022, 195, 957–971. [Google Scholar] [CrossRef]
- Ratthiwal, J.; Lazaro, N.; Pineda, A.; Esposito, R.; Alothman, Z.A.; Reubroycharoen, P.; Luque, R. Furfural conversion over calcined Ti and Fe metal-organic frameworks under continuous flow conditions. Catal. Commun. 2023, 177, 106649. [Google Scholar] [CrossRef]
- Gong, J.; Yue, H.; Zhao, Y.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.; Ma, X. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0–Cu+ sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef]
- Paris, C.; Karelovic, A.; Manrique, R.; Le Bras, S.; Devred, F.; Vykoukal, V.; Styskalik, A.; Eloy, P.; Debecker, D.P. CO2 hydrogenation to methanol with Ga- and Zn-doped mesoporous Cu/SiO2 catalysts prepared by the aerosol-assisted Sol-Gel process. ChemSusChem 2020, 13, 6409–6417. [Google Scholar] [CrossRef]
- Lee, J.; Seo, J.H.; Nguyen-Huy, C.; Yang, E.; Lee, J.G.; Lee, H.; Jang, E.J.; Kwak, J.H.; Lee, J.H.; Lee, H.; et al. Cu2O(100) surface as an active site for catalytic furfural hydrogenation. Appl. Catal. B Environ. 2021, 282, 119576. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Márquez-Rodríguez, I.; Moreno-Tost, R.; Santamaría-González, J.; Mérida-Robles, J.; Maireles-Torres, P. Gas-phase hydrogenation of furfural over Cu/CeO2 catalysts. Catal. Today 2017, 279, 327–338. [Google Scholar] [CrossRef]
- Rao, R.; Dandekar, A.; Baker, R.T.K.; Vannice, M.A. Properties of copper chromite catalysts in hydrogenation reactions. J. Catal. 1997, 171, 406–419. [Google Scholar] [CrossRef]
- Shoosri, T.; Chotiwilaiwan, P.; Rattanapornchaiwat, T.; Teerawatananond, T.; Miyake, T.; Panpranot, J.; Weerachawanasak, P. Bimetallic copper-and nickel-rich Cu–Ni phyllosilicate catalysts for the liquid phase selective hydrogenation of furfural to furfuryl alcohol. RSC Adv. 2024, 14, 38232–38244. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, W.-X. Sabatier principle of metal-support interaction for design of ultrastable metal nanocatalysts. Science 2021, 374, 1360–1365. [Google Scholar] [CrossRef]
- Wang, T.; Hu, J.; Ouyang, R.; Wang, Y.; Huang, Y.; Hu, S.; Li, W.-X. Nature of metal-support interaction for metal catalysts on oxide supports. Science 2024, 386, 915–920. [Google Scholar] [CrossRef]
- Ghashghaee, M.; Sadjadi, S.; Shirvani, S.; Farzaneh, V. A novel consecutive approach for the preparation of Cu–MgO catalysts with high activity for hydrogenation of furfural to furfuryl alcohol. Catal. Lett. 2017, 147, 318–327. [Google Scholar] [CrossRef]
- Li, F.; Cao, B.; Ma, R.; Liang, J.; Song, H.; Song, H. Performance of Cu/TiO2-SiO2 catalysts in hydrogenation of furfural to furfuryl alcohol. Can. J. Chem. Eng. 2016, 94, 1368–1374. [Google Scholar] [CrossRef]
- Jackson, M.A.; White, M.G.; Haasch, R.T.; Peterson, S.C.; Blackburn, J.A. Hydrogenation of furfural at the dynamic Cu surface of CuOCeO2/Al2O3 in a vapor phase packed bed reactor. Mol. Catal. 2018, 445, 124–132. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, X.; Jia, Z.; Han, Q.; Yu, S.; Liu, S.; Li, L.; Wu, Q.; Yu, H.; Liu, Y.; et al. Bi-functional active sites Cu/MgO-La2O3 catalysts the selective hydrogenation of furfural to furfuryl alcohol. Chem. Eng. J. 2024, 500, 157400. [Google Scholar] [CrossRef]
- Chen, B.; Hu, J.; Yu, Z.; Zhu, L.; Wu, C.; Yu, H. Improving the activity and selectivity of Ir/SiO2 for the selective hydrogenation of unsaturated carbonyl compounds by SnO2 promotion. Ind. Eng. Chem. Res. 2025, 64, 1181–1188. [Google Scholar] [CrossRef]
- Li, C.; Liu, Q.; Huang, D.; Wang, J.; Xi, Y.; Huang, Z.; Li, F. Catalytic tandem CO2 hydrogenation and hydroformylation for high-yield synthesis of C2+ alcohols. ACS Catal. 2025, 15, 11440–11451. [Google Scholar] [CrossRef]
- Coeck, R.; Claes, N.; Cuypers, T.; Bals, S.; De Vos, D.E. The sustainable and catalytic synthesis of N,N-alkylated fatty amines from fatty acids and esters. Green Chem. 2025, 27, 1410–1422. [Google Scholar] [CrossRef]
- Du, H.; Ma, X.; Jiang, M.; Yan, P.; Conrad Zhang, Z. Highly efficient Cu/SiO2 catalyst derived from ethanolamine modification for furfural hydrogenation. Appl. Catal. A Gen. 2020, 598, 117598. [Google Scholar] [CrossRef]
- Ruan, L.; Zhu, L.; Zhang, X.; Guo, G.; Shang, C.; Chen, B.; Guo, Z. Porous SiO2 nanosphere-supported PtCuCo trimetallic nanoparticles for highly efficient and selective furfural hydrogenation. Fuel 2023, 335, 126935. [Google Scholar] [CrossRef]
- Kerstens, D.; Smeyers, B.; Van Waeyenberg, J.; Zhang, Q.; Yu, J.; Sels, B.F. State of the art and perspectives of hierarchical zeolites: Practical overview of synthesis methods and use in catalysis. Adv. Mater. 2020, 32, 2004690. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J. New stories of zeolite structures: Their descriptions, determinations, predictions, and evaluations. Chem. Rev. 2014, 114, 7268–7316. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yue, Q.; Deng, Y.; Zhao, D. Ordered mesoporous materials based on interfacial assembly and engineering. Adv. Mater. 2013, 25, 5129–5152. [Google Scholar] [CrossRef] [PubMed]
- Santa Cruz-Navarro, D.; Torres-Rodríguez, M.; Gutiérrez-Arzaluz, M.; Mugica-Álvarez, V.; Pergher, S.B. Comparative study of Cu/ZSM-5 catalysts synthesized by two Ion-exchange methods. Crystals 2022, 12, 545. [Google Scholar] [CrossRef]
- Yu, Z.; Tian, H.; Sun, K.; Shao, Y.; Zhang, L.; Zhang, S.; Duan, P.; Liu, Q.; Niu, S.; Dong, D.; et al. Impacts of externally added Brønsted and Lewis acid on conversion of furfural to cyclopentanone over Ni/SiC catalyst. Mol. Catal. 2020, 496, 111187. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Ma, Y.; She, G.; Zhou, P.; Zhu, L.; Zhang, Z. Enable biomass-derived alcohols mediated alkylation and transfer hydrogenation. Nat. Commun. 2024, 15, 7012. [Google Scholar] [CrossRef]
- Yao, Y.; Yin, C.; Ma, C.; Li, Y.; Wang, Y.; Jiang, R.; He, W.; Xiang, Z.; Liu, Y.; Li, X.; et al. Aromatic ethers induced electronic structure reconstruction on encapsulated Nickel catalysts for high-performance catalytic hydrogenation. ACS Appl. Mater. Interfaces 2024, 16, 14680–14693. [Google Scholar] [CrossRef] [PubMed]
- Xi, N.; Chen, S.; Bao, R.; Wang, Q.; Lin, Y.; Yue, J.; Wang, R.; Yang, C.; Yin, W.; Qiu, T. Layered carbon encapsulated CuOx nanopaticles for selective hydrogenation of furfural to furfuryl alcohol. Mol. Catal. 2024, 565, 114364. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Skobelev, I.Y.; Ivanchikova, I.D.; Kovalenko, K.A.; Fedin, V.P.; Sorokin, A.B. Hydrocarbon oxidation over Fe- and Cr-containing metal-organic frameworks MIL-100 and MIL-101–a comparative study. Catal. Today 2014, 238, 54–61. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, G.; Zhu, Q.; Kong, X. CuZn@N-doped graphene layer for upgrading of furfural to furfuryl alcohol. Mol. Catal. 2022, 517, 112066. [Google Scholar] [CrossRef]
- Rodiansono; Astuti, M.D.; Santoso, U.T.; Shimazu, S. Hydrogenation of biomass-derived furfural over highly dispersed-Aluminium hydroxide supported Ni-Sn(3.0) alloy catalysts. Procedia Chem. 2015, 16, 531–539. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, Y.; Li, S.; Gao, L.; Xiao, G. Metal-organic frameworks derived bimetallic Cu-Co catalyst for efficient and selective hydrogenation of biomass-derived furfural to furfuryl alcohol. Mol. Catal. 2017, 436, 128–137. [Google Scholar] [CrossRef]
- Alba-Rubio, A.C.; Cecilia, J.A.; Jiménez-Gómez, C.P.; García-Sancho, C.; Cassidy, A.; Moreno-Tost, R.; Maireles-Torres, P. The role of MnOx in Cu-MnOx/SiO2 catalysts for the gas-phase hydrogenation of furfural. Mol. Catal. 2023, 546, 113224. [Google Scholar] [CrossRef]
- Sulmonetti, T.P.; Pang, S.H.; Claure, M.T.; Lee, S.; Cullen, D.A.; Agrawal, P.K.; Jones, C.W. Vapor phase hydrogenation of furfural over nickel mixed metal oxide catalysts derived from layered double hydroxides. Appl. Catal. A Gen. 2016, 517, 187–195. [Google Scholar] [CrossRef]
- Song, T.; Qi, Y.; Zhao, C.; Wu, P.; Li, X. Sorbitol-derived carbon overlayers encapsulated Cu nanoparticles on SiO2: Stable and efficient for the continuous hydrogenation of ethylene carbonate. iScience 2022, 25, 105239. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, X.; Gutiérrez, O.Y.; Ji, J.; Jin, P.; Wang, S.; Xu, Y.; Zhao, Y.; Wang, S.; Ma, X.; et al. Roles of Cu+ and Cu0 sites in liquid-phase hydrogenation of esters on core-shell CuZnx@C catalysts. Appl. Catal. B Environ. 2020, 267, 118698. [Google Scholar] [CrossRef]
- Li, Y.; Guo, X.; Liu, D.; Mu, X.; Chen, X.; Shi, Y. Selective Conversion of Furfural to Cyclopentanone or Cyclopentanol Using Co-Ni Catalyst in Water. Catalysts 2018, 8, 193. [Google Scholar] [CrossRef]
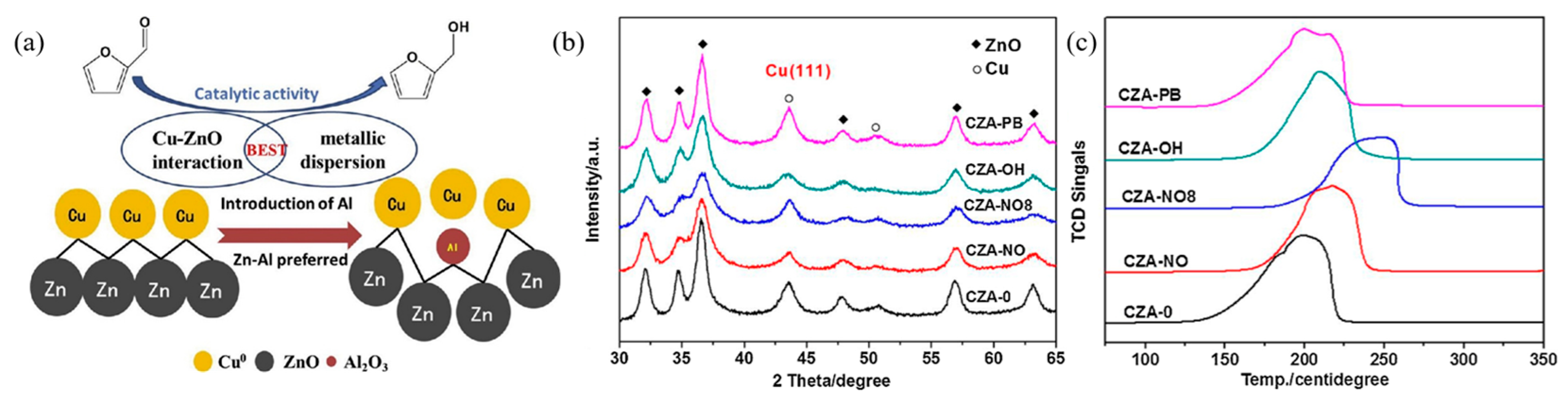
- Yang, X.; Meng, Q.; Ding, G.; Wang, Y.; Chen, H.; Zhu, Y.l.; Li, Y.W. Construction of novel Cu/ZnO-Al2O3 composites for furfural hydrogenation: The role of Al components. Appl. Catal. A Gen. 2018, 561, 78–86. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, R.; Yang, X.; Fang, Y.-X. Potassium carbonate (K2CO3)-assisted Copper-catalyzed liquid- phase hydrogenation of furfural: Striking promotion synergy enables a superior high furfuryl alcohol yield at mild reaction conditions. Ind. Eng. Chem. Res. 2022, 61, 16643–16652. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, X.; Zhu, Z.; Tan, L.; Yuan, H.; Wu, J.; Zhu, C.; Xu, J. In Situ synthesis of Nitrogen-doped carbon coated copper: Boosting superhydrophobicity, conductivity, and oxidation resistance. ACS Appl. Mater. Interfaces 2024, 16, 68703–68711. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Song, P.; Zhang, X.; Song, Y.; Guo, W.; Yao, Z. Highly efficient activation of molecular oxygen by Cu@N doped carbon for antibiotic degradation. Chem. Eng. J. 2025, 507, 160769. [Google Scholar] [CrossRef]
- Guo, X.; Yu, B.; Wang, Z.; Li, S.; Chen, X.; Yang, Y. Selective hydrogenation of furfural to furfuryl alcohol over Cu/CeCoOx in aqueous phase. Mol. Catal. 2022, 529, 112553. [Google Scholar] [CrossRef]
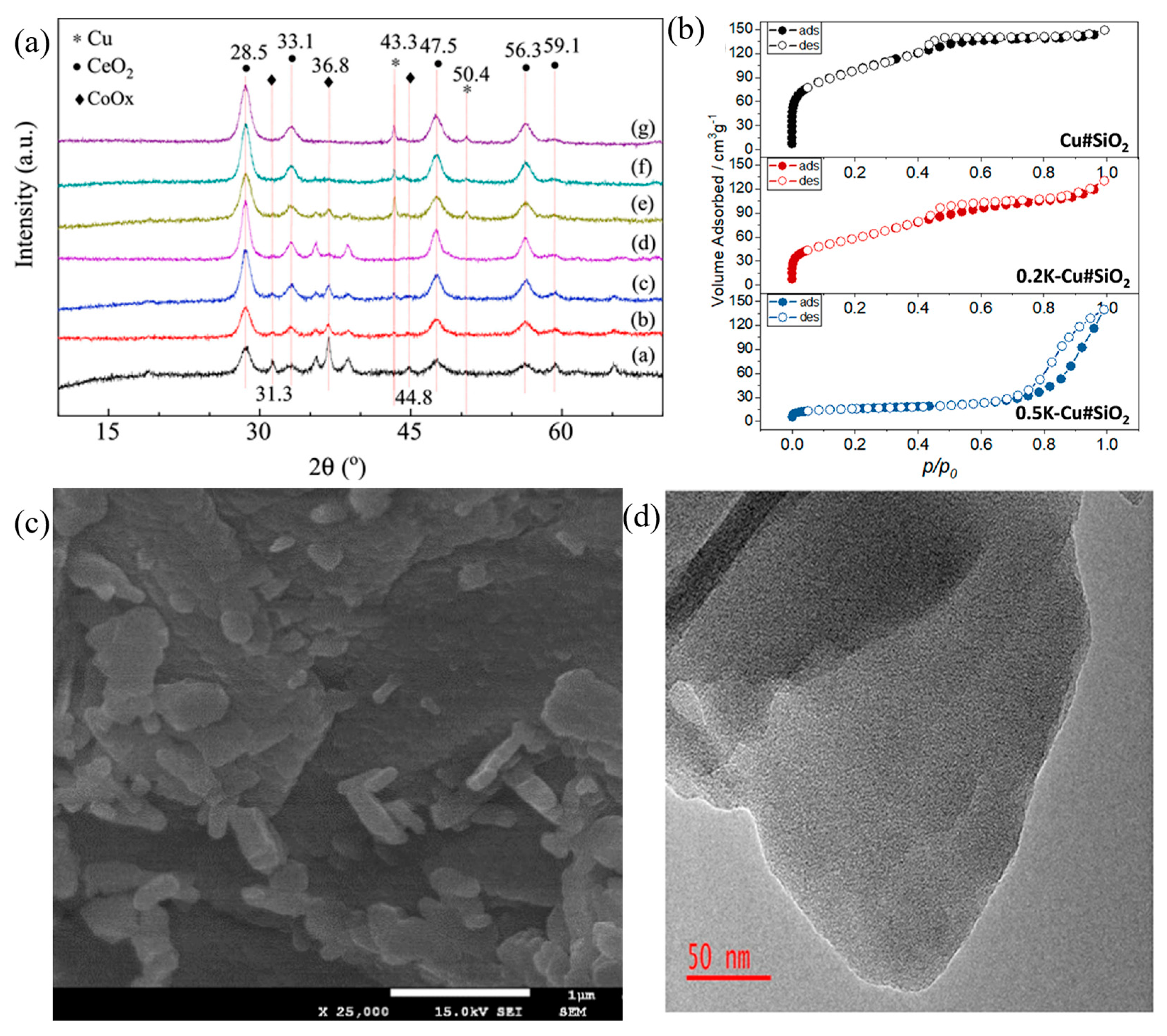
- Wang, X.; Liang, T.; Zheng, Z.; Guo, R.; Zhang, Z.; Miao, L.; Yang, X. MOF-silica hybrid derived high performance K-Cu#SiO2 catalyst for furfural valorization: The functional role of potassium acetate (KAc) in hybridization and copper electronic state. Appl. Catal. A Gen. 2022, 640, 118603. [Google Scholar] [CrossRef]
- Xu, J.; Han, X.; Zhu, L.; Wang, W.; Ruan, L.; Yang, Z.; Ye, H.; Chen, B.H. Revealing the intrinsic relationship between nano/electronic structure of CuCo/NC (NC drived from ZIF-67) and their catalytic performance for furfural selective hydrogenation. J. Catal. 2025, 447, 116140. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Yang, H.; Chong, S.; Liu, G.; Zhang, Y.; Wang, K. Effect of the metal-support interaction in the Cu/ZnO catalyst on its performance in the hydrogenation of furfural to furfuryl alcohol. J. Fuel Chem. Technol. 2024, 52, 1045–1056. [Google Scholar] [CrossRef]
- Kaim, J.; Śliwa, M.; Kuterasiński, Ł.; Samson, K.; Ruggiero-Mikołajczyk, M.; Zimowska, M.; Mordarski, G.; Karcz, R.; Podobiński, J.; Datka, J.; et al. Gas-phase hydrogenation and decarbonylation of furfuryl aldehyde over catalysts containing Cu and Ni. Catal. Today 2024, 441, 114897. [Google Scholar] [CrossRef]
- Kumar, A.; Shivhare, A.; Bal, R.; Srivastava, R. Metal and solvent-dependent activity of spinel-based catalysts for the selective hydrogenation and rearrangement of furfural. Sustain. Energy Fuels 2021, 5, 3191–3204. [Google Scholar] [CrossRef]
- Yang, X.; Chen, H.; Meng, Q.; Zheng, H.; Zhu, Y.; Li, Y.W. Insights into influence of nanoparticle size and metal–support interactions of Cu/ZnO catalysts on activity for furfural hydrogenation. Catal. Sci. Technol. 2017, 7, 5625–5634. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Tan, J.; Zhang, Y.; Cui, J.; Wang, C.; Fang, L.; Zhu, Y.; Huang, L.; Shi, H.; et al. Understanding the synergistic catalysis of balanced Cu0-Cu+ sites and oxygen vacancies in Cu/ZrO2 catalysts for the efficient hydrogenation of furfural. Chem. Eng. J. 2024, 501, 157796. [Google Scholar] [CrossRef]
- Yu, D.; Yu, X.; Zhang, Y.; Wang, K. Deactivation mechanism of Cu/SiO2 catalyst in gas phase hydrogenation of furfural to furfuryl alcohol. J. Fuel Chem. Technol. 2023, 51, 1751–1760. [Google Scholar] [CrossRef]
- Costa, M.M.; Coelho, E.C.D.A.; Moya, S.F.; Suppino, R.S. Cascade reactions of furfural conversion with different bifunctional niobia-supported metal catalysts. React. Kinet. Mech. Catal. 2025, 1–23. [Google Scholar] [CrossRef]
- Zhang, C.; Xing, Z.; Li, Y.; Xu, T.; Sun, Y.; Bai, J. Alloying palladium with copper on zirconium dioxide for accelerating the selective catalytic transfer hydrogenation of furfural to furfuryl alcohol. New J. Chem. 2025, 49, 6684–6693. [Google Scholar] [CrossRef]
- Chen, S.; de Souza, P.M.; Ciotonea, C.; Marinova, M.; Dumeignil, F.; Royer, S.; Wojcieszak, R. Micro-/mesopores confined ultrasmall Cu nanoparticles in SBA-15 as a highly efficient and robust catalyst for furfural hydrogenation to furfuryl alcohol. Appl. Catal. A Gen. 2022, 633, 118527. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Rao, Z.; Liu, H.; Zhang, R.; Jia, W.; Zhang, J.; Sun, Y.; Peng, L. High-performance CuNi-alloy catalysts for efficient solvent-free hydrogenation furfural to furfuryl alcohol. Appl. Catal. B Environ. Energy 2025, 371, 125228. [Google Scholar] [CrossRef]
- Pholjaroen, B.; Li, N.; Huang, Y.; Li, L.; Wang, A.; Zhang, T. Selective hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-pentanediol over vanadium modified Ir/SiO2 catalyst. Catal. Today 2015, 245, 93–99. [Google Scholar] [CrossRef]
- Tang, Q.; Sun, X.; Duan, Z.; Xiao, Z.; Liu, X. Efficient hydrogenation of biomass-derived furfural to tetrahydrofurfuryl alcohol over a non-noble Ni/CeO2 catalyst under mild conditions. Mol. Catal. 2025, 584, 115261. [Google Scholar] [CrossRef]
- Sahraie, N.R.; Kramm, U.I.; Steinberg, J.; Zhang, Y.; Thomas, A.; Reier, T.; Paraknowitsch, J.-P.; Strasser, P. Quantifying the density and utilization of active sites in non-precious metal oxygen electroreduction catalysts. Nat. Commun. 2015, 6, 8618. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Tsoncheva, T.; Dimitrov, M.; Minchev, C.; Knözinger, H. Characterization of Cu/MCM-41 and Cu/MCM-48 mesoporous catalysts by FTIR spectroscopy of adsorbed CO. Appl. Catal. A Gen. 2003, 241, 331–340. [Google Scholar] [CrossRef]
- Liao, X.; Zhao, H.; Liu, R.; Luo, H.; Lv, Y.; Liu, P. Highly efficient and selective hydrogenation of furfural to furfuryl alcohol and cyclopentanone over Cu-Ni bimetallic Catalysts: The crucial role of CuNi alloys and Cu+ species. J. Catal. 2024, 436, 115603. [Google Scholar] [CrossRef]
- Rehr, J.J.; Albers, R.C. Theoretical approaches to x-ray absorption fine structure. Rev. Mod. Phys. 2000, 72, 621–654. [Google Scholar] [CrossRef]
- Wang, M.; Árnadóttir, L.; Xu, Z.J.; Feng, Z. In Situ X-ray absorption spectroscopy studies of nanoscale electrocatalysts. Nano-Micro Lett. 2019, 11, 47. [Google Scholar] [CrossRef]
- Sharma, R.V.; Das, U.; Sammynaiken, R.; Dalai, A.K. Liquid phase chemo-selective catalytic hydrogenation of furfural to furfuryl alcohol. Appl. Catal. A Gen. 2013, 454, 127–136. [Google Scholar] [CrossRef]
- Kment, Š.; Bakandritsos, A.; Tantis, I.; Kmentová, H.; Zuo, Y.; Henrotte, O.; Naldoni, A.; Otyepka, M.; Varma, R.S.; Zbořil, R. Single atom catalysts based on earth-abundant metals for energy-related applications. Chem. Rev. 2024, 124, 11767–11847. [Google Scholar] [CrossRef]
- Islam, M.J.; Granollers Mesa, M.; Osatiashtiani, A.; Manayil, J.C.; Isaacs, M.A.; Taylor, M.J.; Tsatsos, S.; Kyriakou, G. PdCu single atom alloys supported on alumina for the selective hydrogenation of furfural. Appl. Catal. B Environ. 2021, 299, 120652. [Google Scholar] [CrossRef]
- Gao, Y.; Yi, W.; Yang, J.; Jiang, K.; Yang, T.; Li, Z.; Zhang, M.; Liu, Z.; Wu, B. Effect of calcination atmosphere on the performance of Cu/Al2O3 catalyst for the selective hydrogenation of furfural to furfuryl alcohol. Molecules 2024, 29, 2753. [Google Scholar] [CrossRef]
- Du, H.; Ma, X.; Yan, P.; Jiang, M.; Zhao, Z.; Zhang, Z.C. Catalytic furfural hydrogenation to furfuryl alcohol over Cu/SiO2 catalysts: A comparative study of the preparation methods. Fuel Process. Technol. 2019, 193, 221–231. [Google Scholar] [CrossRef]
- Meira, D.M.; Monte, M.; Fernández-García, M.; Meunier, F.; Mathon, O.; Pascarelli, S.; Agostini, G. A flexible cell for in situ combined XAS-DRIFTS-MS experiments. J. Synchrotron Radiat. 2019, 26, 801–810. [Google Scholar] [CrossRef] [PubMed]


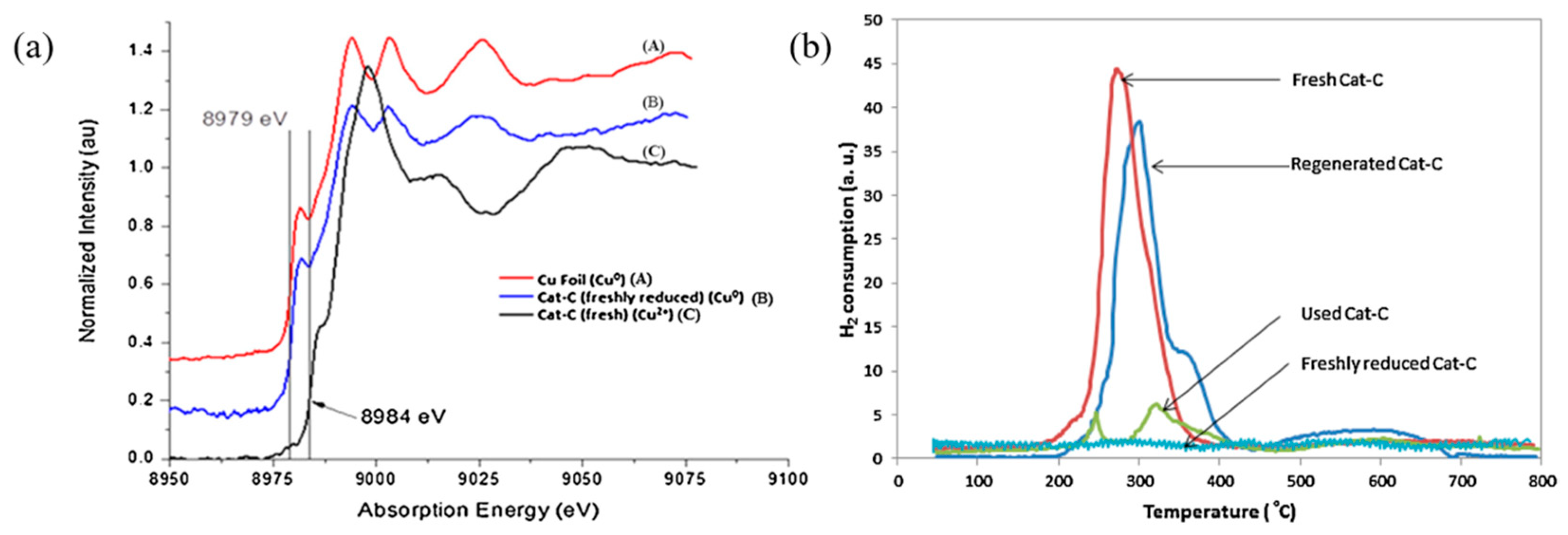
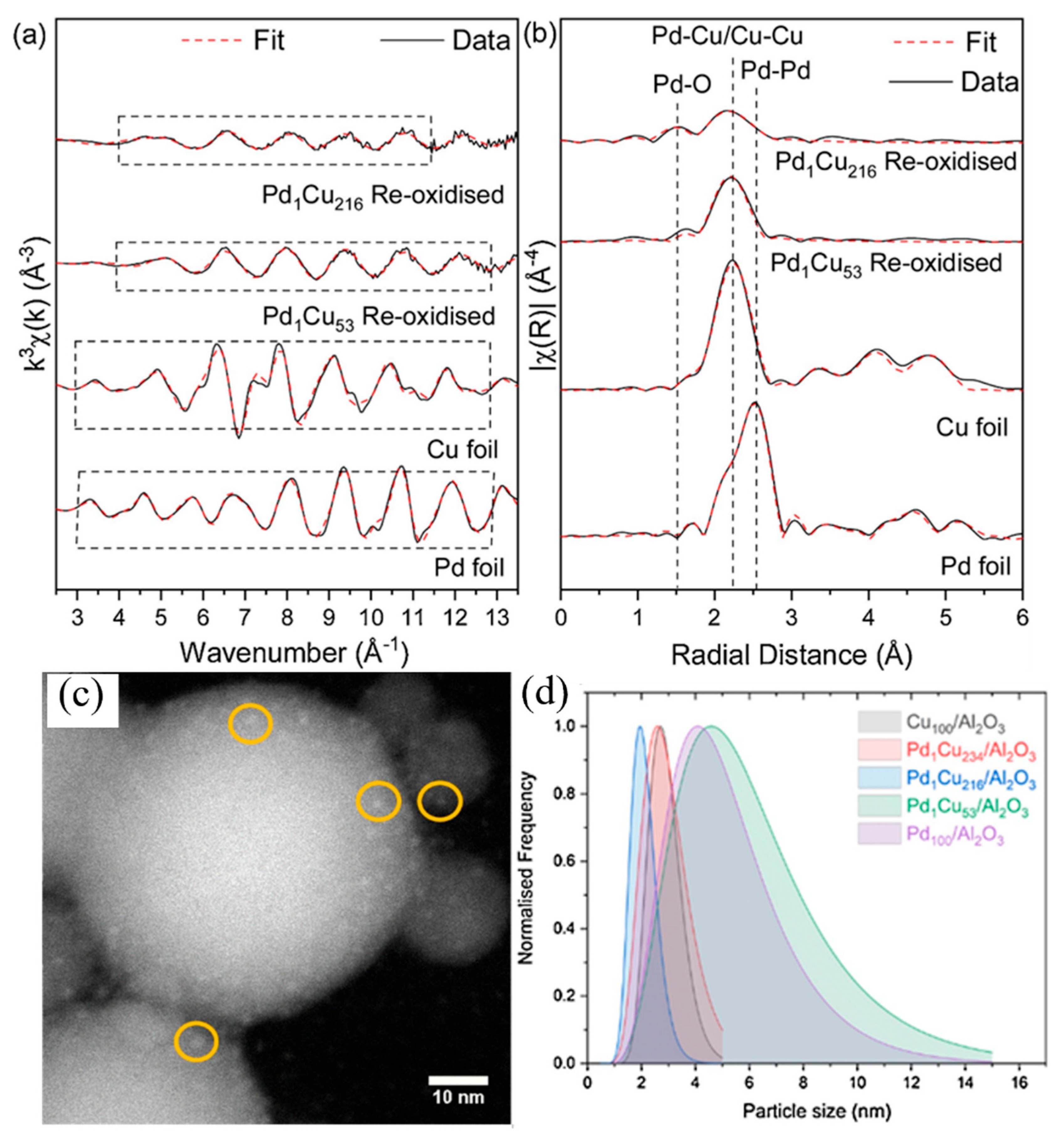
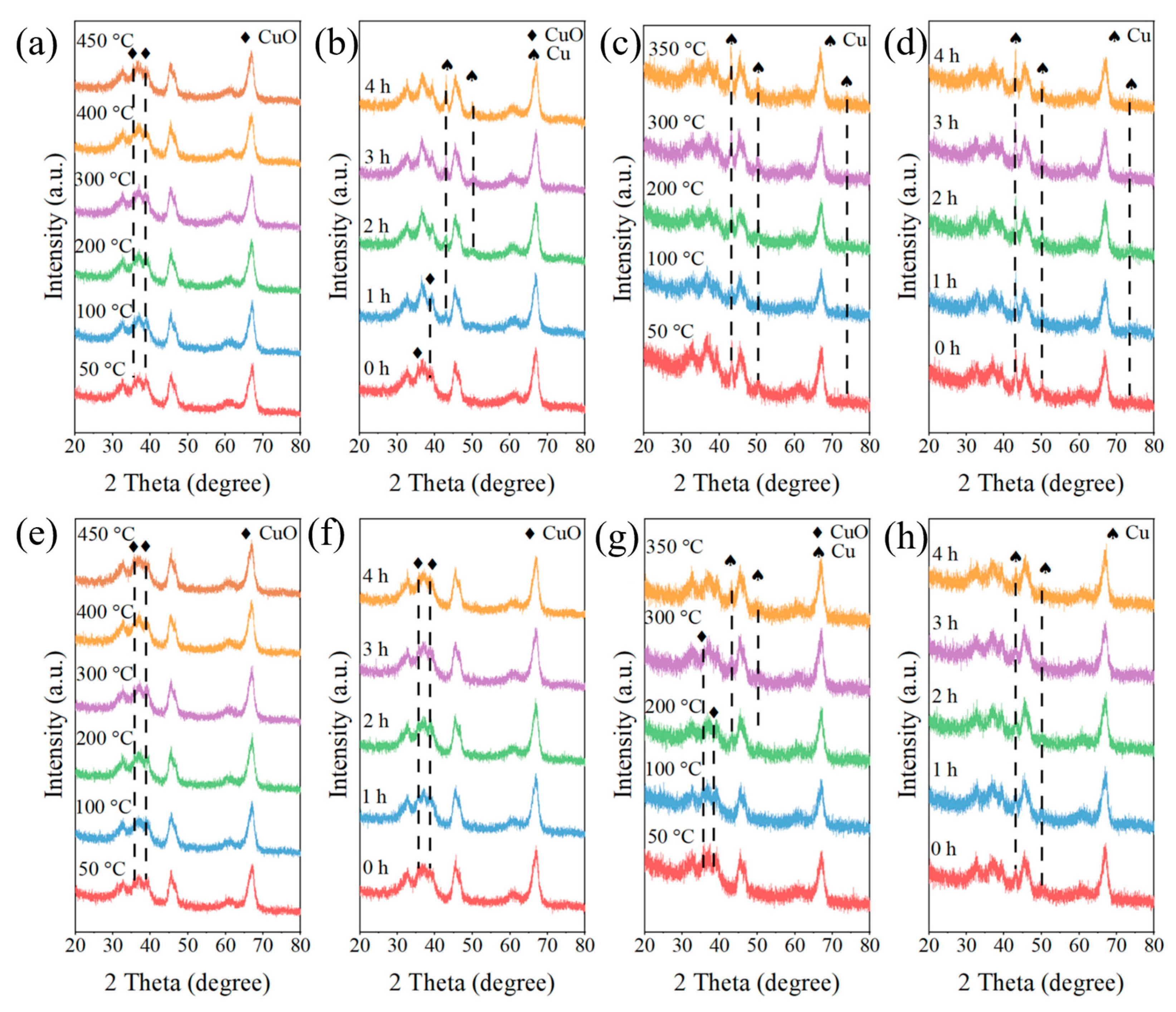


| Catalyst | Metal Loading (wt%) | Solvent | Temperature (°C) | Time (h) | H2 Pressure (bar) | FF Conversion (%) | FA Selectivity (%) | FA Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Cu/Zn/Al2O3 (CZAl) | 10 | H2O | 100 | 4 | 10 | 100 | ~99 | 99 | [40] |
| Cu/Zn/Al2O3 (CZAl) | 10 | Ethanol | 100 | 4 | 10 | 99 | ~99 | 98.01 | |
| Cu/Zn/Al2O3 (CZAl) | 10 | Methanol | 100 | 4 | 10 | 99 | ~95 | 94.05 | |
| Cu/Zn/Al2O3 (CZAl) | 10 | 2-Propanol | 100 | 4 | 10 | 19 | ~90 | 17.1 | |
| Cu/Zn/Al2O3 (CZAl) | 10 | DMF | 100 | 4 | 10 | 98 | ~99 | 97.02 | |
| Cu/Zn/Al2O3 (CZAl) | 10 | Acetone | 100 | 4 | 10 | 98 | ~80 | 78.4 | |
| Cu/Zn/Al2O3 (CZAl) | 10 | Toluene | 100 | 4 | 10 | 77 | ~99 | 76.23 | |
| Cu/Zn/Al2O3 (CZAl) | 10 | Cyclohexane | 100 | 4 | 10 | 4 | ~99 | 4.0 | |
| Cu/MgO | 5 | Isopropyl alcohol | 109.85 | 2.3 | 20 | 99.9 | 99.9 | 99.98 | [41] |
| Cu/Al2O3 (Acetate) | 1 | MeOH | 50 | 7 | 1.5 | 24.2 ± 1.2 | 96.0 ± 4.8 | 23.23 | [42] |
| Cu/Al2O3 (Acetate) | 5 | MeOH | 50 | 7 | 1.5 | 47.7 ± 2.4 | 97.6 ± 4.9 | 46.56 | |
| Cu/Al2O3 (Sulfate) | 1 | MeOH | 50 | 7 | 1.5 | 2.2 ± 0.1 | 5.1 ± 0.3 | 0.11 | |
| Cu/Al2O3 (Sulfate) | 5 | MeOH | 50 | 7 | 1.5 | 7.8 ± 0.4 | 0.8 ± 0.1 | 0.062 | |
| Cu0.9Mg3AlOy | - | Isopropanol | 120 | 1.5 | 16 | 99 | 98.4 | 97.42 | [43] |
| CuMg3Al-R | 20 | i-PrOH | 130 | 3 | 20 | 100 | 99.3 | 99.3 | [44] |
| Cu/CeO2-R (nanorod) | 5 | γ-butyrolactone | 100 | 4 | 20 | 97.5 | 96.2 | 93.8 | [45] |
| Cu/η-Al2O3 | 5 | Isopropanol | 150 | 2 | 10 | 99.7 | 94 | 93.7 | [46] |
| NPCu@SiO2 | - | H2O | 150 | 4 | 30 | 62 | 31 | 19.2 | [47] |
| SiO2@Cu | 7.6 | H2O | 150 | 4 | 30 | 84 | 17 | 14.3 | |
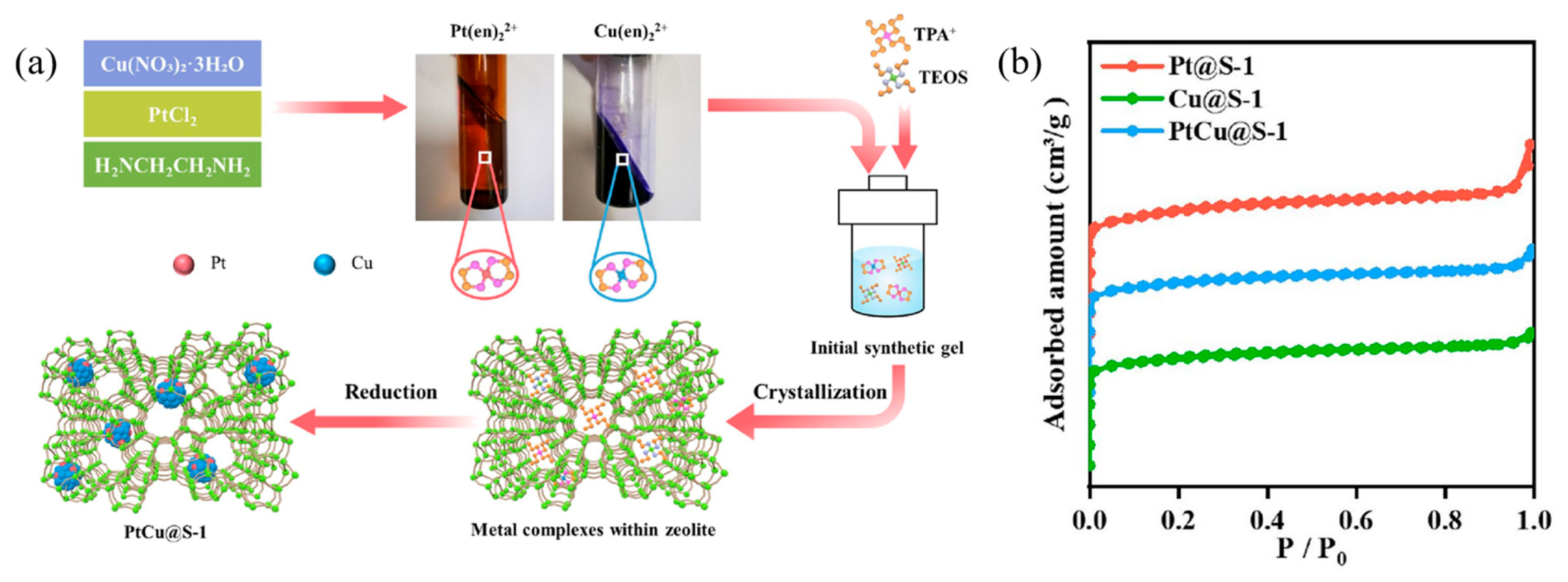
| PtCu@S-1 (S-1, silicalite-1) | 0.8 | THF | 160 | 6 | 20 | 99.9 | 100 | 99.9 | [48] |
| Cu@MFI | - | H2O | 70 | 5 | 40 | 100 | 100 | 100 | [49] |
| Co-Cu/SBA-15 | 10 | Isopropanol | 170 | 4 | 20 | 99 | 80 | 79.2 | [50] |
| Cu/MCM-41 | 5 | i-PrOH | 120 | 4 | 1 | 100 | 100 | 100 | [51] |
| Cu/AC-SO3H | 16 | 2-propanol | 105 | 2 | 4 | 100 | 100 | 100 | [52] |
| [Cu2(L1)2·5DMF·4H2O]n | - | Methanol | 140 | 24 | 50 | 76 | 100 | 76 | [53] |
| Fe3O4/Cu@C | 27.4 | n-butanol | 180 | 4 | 10 | 98.5 | 89.4 | 88.1 | [54] |
| Fe/Cu@C | 30.4 | i-propanol | 300 | 4 | 10 | ~60% | ~90 | 54 | [54] |
| Cu3Co1/MgOx | 20 | Isopropanol | 110 | 2 | 20 | 100 | 99 | 99 | [55] |
| Cu2Zn/SiO2 | 13 | Deionized water | 120 | 4 | 25 | 81.9 | 94.8 | 77.6 | [56] |
| Na–Cu@TS-1 | 2.1 | Isopropanol | 110 | 2 | 10 | 93 | 98.1 | 91.2 | [57] |
| Cu/SiO2-AE | 5 | Isopropanol | 90 | 2 | 10 | 55.2 | 99.9 | 55.1 | [58] |
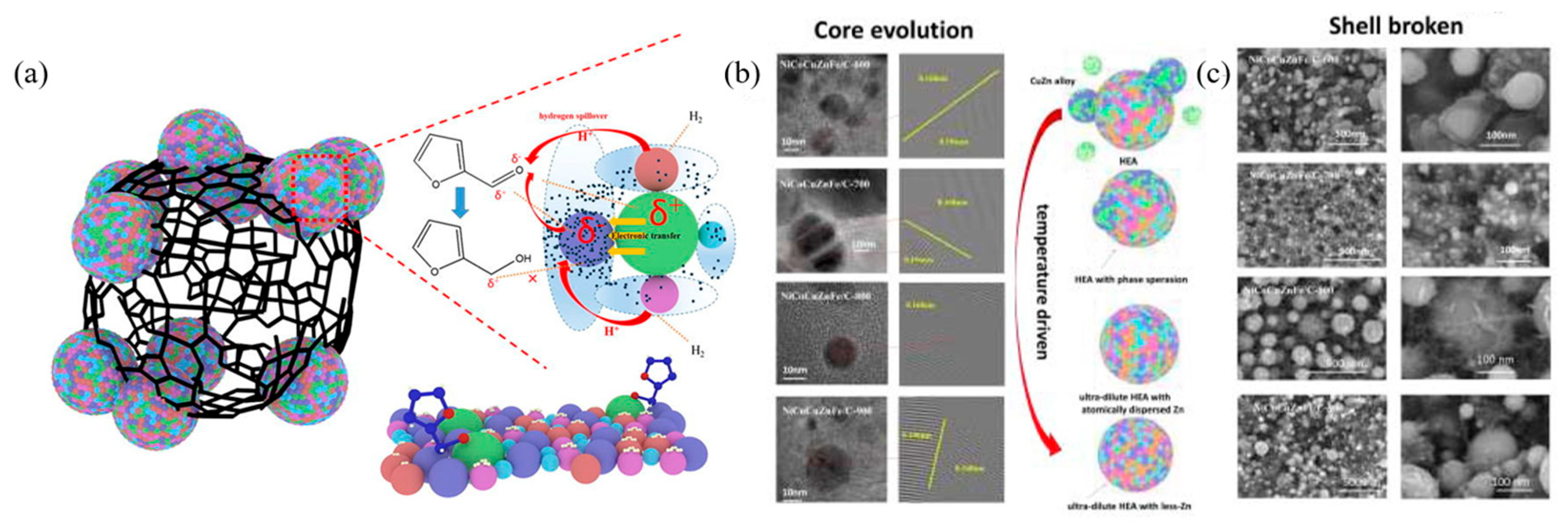
| NiCoCuZnFe/C-800 | - | Isopropanol | 90 | 9 | 30 | 87.32 | 100 | 87.32 | [59] |
| CuO#TiO2 | 12 | Ethanol | 140 | 1 | 20 | 99 | 99 | 99 | [60] |
| ZJU-199-350 | - | Isopropanol | 130 | 3 | 10 | 97 | 99 | 96 | [61] |
| Support | Advantage | Disadvantage |
|---|---|---|
| Metal oxide | Low cost, easy to regulate, well-studied for application | Relatively undeveloped porous structure |
| SiO2 | Low cost, high chemical inertness | Irregular structure, poor porosity |
| Microporous molecular sieves | Well-defined topological framework with uniform micropores, high hydrothermal stability | Strong surface acidity, limited pore size |
| Mesoporous molecular sieves | Ordered mesoporous structure, relatively facile preparation | Structural hydrothermal instability |
| Carbon-containing material | Structural diversity, facile functionalization | Severe side reactions |
| MOF | Developed pore structure, high surface area, and abundant metal anchoring sites | Expensive organic ligands, easy decomposability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.; Gao, Y.; Li, C.; Zhang, M.; Liu, Z. A Brief Review of Cu-Based Catalysts for the Selective Liquid-Phase Hydrogenation of Furfural to Furfuryl Alcohol. Chemistry 2025, 7, 153. https://doi.org/10.3390/chemistry7050153
Lin T, Gao Y, Li C, Zhang M, Liu Z. A Brief Review of Cu-Based Catalysts for the Selective Liquid-Phase Hydrogenation of Furfural to Furfuryl Alcohol. Chemistry. 2025; 7(5):153. https://doi.org/10.3390/chemistry7050153
Chicago/Turabian StyleLin, Tiantian, Yongzhen Gao, Chao Li, Meng Zhang, and Zhongyi Liu. 2025. "A Brief Review of Cu-Based Catalysts for the Selective Liquid-Phase Hydrogenation of Furfural to Furfuryl Alcohol" Chemistry 7, no. 5: 153. https://doi.org/10.3390/chemistry7050153
APA StyleLin, T., Gao, Y., Li, C., Zhang, M., & Liu, Z. (2025). A Brief Review of Cu-Based Catalysts for the Selective Liquid-Phase Hydrogenation of Furfural to Furfuryl Alcohol. Chemistry, 7(5), 153. https://doi.org/10.3390/chemistry7050153








