1. Introduction
Recently, multidisciplinary approaches have shown great potential in making progress in healthcare issues by integrating engineering, science, technology, and medicine. Biomedical engineering is a rapidly growing field that develops technologies to suit healthcare demands [
1]. This is apparent in every aspect of healthcare, from diagnosis and analysis to treatment and recovery. It has become widely accepted due to the rise in implanted medical devices, including artificial hips and pacemakers, and more cutting-edge technologies like drug delivery systems, stem cell engineering, and three-dimensional printing of biological organs, known as tissue engineering [
2].
Nanotechnology plays a pivotal role in the field of biomedical engineering through its provision of opportunities for the advancement of specific drug delivery systems and biosensors with enhanced specificity [
3,
4]. By their diminutive size, nanoparticles can effortlessly traverse the blood–brain barrier, rendering them highly desirable for employment within drug delivery systems and biosensors [
5]. Nanomaterials possess numerous advantages, including their expansive surface area, stability, lack of toxicity, and compatibility with living organisms, rendering them highly suitable for a diverse array of applications within the realm of biomedical engineering [
6]. In tissue engineering, nanoparticles can replicate tissue-specific microenvironments and effectively govern cellular properties, thereby addressing the limitations inherent in presently utilized biomaterials [
7]. Both organic and inorganic nanoparticles may be designed as efficacious biomolecule delivery systems, thereby enabling targeted therapeutic interventions [
8]. Nanotechnology also introduces novel functional materials and devices for employment in medical applications, including optical imaging for the diagnosis of diseases and the potential for therapeutic interventions. However, a thorough evaluation of the safety and toxicity of nanomaterials within the realm of biomedical applications is imperative [
8].
Mineral nanoparticles play a significant role in biomedical engineering [
9]. These nanoparticles can be functionalized with biological molecules and utilized in medical diagnostics, targeted drug delivery, tissue engineering, regenerative medicine, and biomedical textiles [
10]. More importantly, they can also be exploited in imaging, drug delivery, and disease therapy, demonstrating their capacity to target proteins specifically. The design and production of nanomaterials, including mineral nanoparticles, hold critical importance in attaining desired properties and optimal performance. Nanoparticles possess unique characteristics such as size, surface, and charge, influencing their biodistribution patterns and cell interaction. Specifically, hydroxyapatite nanoparticles present multifunctional properties and can be fabricated using various biomedical engineering techniques, offering multiple applications.
Hydroxyapatite nanoparticles, or HApNPs, are utilized as biomaterials in regenerative medicine and bone tissue engineering, serving as valuable tools for these applications [
11]. These nanoparticles exhibit favorable biocompatibility, facilitating cell adhesion and osseointegration [
11]. Diverse synthesis methods and compounds can be employed to fabricate HApNPs, enabling the customization of their physicochemical characteristics [
12]. Furthermore, their utilization in cancer therapy has shown promise, owing to their capacity to be loaded with anticancer drugs and activated to hinder cancer cell growth and metastasis [
13]. Extensive investigations have been conducted to comprehend the interaction between HApNPs and cell membranes and their internalization, yielding intriguing findings [
14]. By adjusting their shape, size, morphology, and crystalline phase, the properties of HApNPs can be tailored to amplify their biological response and enhance their capabilities in drug delivery [
15].
Ion-doped hydroxyapatite (iHA) is a specialized variant of hydroxyapatite that has garnered considerable attention in biomaterials and tissue engineering [
16]. Hydroxyapatite itself is an inherent mineral compound that is abundantly present in bone tissue, rendering it an optimal substance for biomedical applications [
17]. However, scientists have ascertained that introducing different ions into the hydroxyapatite structure can enhance its characteristics to better conform to specific biomedical requirements. Ion doping involves replacing specific calcium and phosphate ions in the HA crystal lattice with different types of ions such as magnesium, strontium, zinc, or silver. This progression modifies the physicochemical properties of hydroxyapatite, resulting in materials that exhibit enhanced biocompatibility, mechanical strength, and bioactivity [
18].
In biomedical engineering, to the best of our knowledge, the role of iHA is increasing in distinct biomedical aspects, including drug delivery, wound dressing, and tissue engineering. Considering each field, iHA plays various roles for better outcomes due to their unique and promoted physiochemical, mechanical, and biological properties. The main aim of this study was to highlight these roles in biomedical engineering. To do this, a comprehensive literature review was accomplished, focusing on the role of iHA in drug delivery, wound dressing, and tissue engineering.
2. Ion-Doped Hydroxyapatite (iHA)
Hydroxyapatite (HA) and ion-doped hydroxyapatite (iHA) have emerged as two distinct forms of calcium phosphate compounds that have garnered significant attention in the vast field of biomaterials. HA, an all-natural mineral in bones and teeth, has established itself as a highly esteemed material due to its exceptional biocompatibility and bioactivity. The crystal structure of hydroxyapatite is one of the most important properties of this material, which plays a key role in many biomedical and tissue engineering applications. Hydroxyapatite is a calcium phosphate compound with the chemical formula Ca
10(PO
4)
6(OH)
2, whose crystal structure belongs to the hexagonal group and has spatial symmetryP63/m [
19].
In this structure, calcium ions occupy two distinct crystallographic positions, referred to as Ca(I) and Ca(II). Phosphate ions PO43− are arranged in tetrahedral positions, while hydroxyl groups (–OH) align along the c-axis of the crystal in a parallel configuration. This orderly arrangement of ions contributes to the chemical stability and bioactive properties of the material. The crystal structure of hydroxyapatite gives it properties such as high biocompatibility, the ability to bind to hard tissues (such as bone and teeth), and the ability to be replaced in the human body. Furthermore, doping this structure with different ions can improve its physical and chemical properties for specific applications. The remarkable crystal structure of HA imparts it with unparalleled strength and stability, rendering it an exceedingly well-suited candidate for a wide array of biomedical applications, including but not limited to bone grafting, dental implants, and drug delivery systems. Researchers are exploring using different ions to enhance the properties of HA by creating iHA, which involves substituting calcium ions with metal ions like magnesium, zinc, strontium, or silver, aiming to improve HA’s physicochemical characteristics and biological performance.
Doping hydroxyapatite (HA) with various ions has gained significant attention as a chemical strategy to enhance its properties. Nevertheless, whether this approach can render HA competitive for commercial applications in biomedicine and beyond remains unclear. Notably, it is worth investigating whether material properties, such as grain size, morphology, surface charge, porosity, and topology, may exert more influence on controlling HA’s properties than the choice and concentration of the dopant.
IHA is a modified form in which calcium and phosphate atoms are substituted with other ions to improve their properties. Fluor ions can replace hydroxyl ions, producing fluorapatite with higher conductivity and thermal stability [
20]. Doping HA with elements like manganese can also affect its physical and structural properties [
21]. However, other factors like grain size, morphology, surface charge, and porosity influence HAp’s properties. Overall, ion-doped hydroxyapatite offers the potential for tailored properties to meet specific biomedical and tissue engineering needs.
The significance of incorporating ion doping into the synthesis of hydroxyapatite lies in its inherent capacity to augment the biological and mechanical properties of the material. Researchers can precisely customize its attributes to fulfill specific applications within the biomedical realm by skillfully integrating a diverse range of ions into hydroxyapatite’s structural framework. Ion doping exerts a profound influence over the physicochemical properties of the material, encompassing crucial aspects such as crystallinity, morphology, and thermal stability. This deliberate manipulation can yield substantial improvements in the biocompatibility and bioactivity of hydroxyapatite, ultimately rendering it even more aptly suited for employment in the arenas of bone regeneration and dental application.
Doping represents a practical means of regulating specific properties in HA, but other factors about the structure and composition of the material should ideally complement it. Furthermore, the investigation delves into the evolutionary trends associated with the type, frequency, and concurrency of dopants in HA over the period spanning 1990 to 2019, encompassing the entire Periodic Table. The findings of this analysis reveal an incessantly rising trend in the popularity of certain elements, such as Sr, Ce, and Se, while simultaneously uncovering a continuous decline in the popularity of other components over the past two decades. Notably, the selection of ions as dopants in HA is predominantly driven by broader patterns observed in materials science, effectively rendering doped HA a reflection of existing and past trends within this realm. By tracing the historical trajectory of these trends, examining this reflection can facilitate the extrapolation of future trends, albeit often necessitating the cessation of specific existing trends. The conceptual novelty of this analysis is highly noteworthy and warrants its application to a plethora of other materials.
4. iHA for Drug Delivery Systems
iHA has demonstrated considerable potential in the field of drug delivery systems. The main reason is its higher drug-loading capacity compared to HA. It was shown that the synthesis of mesoporous ion-doped hydroxyapatite provides a potential solution for more local drug delivery systems in biomedical applications due to their high surface area and pore volume, which allows for efficient drug loading and controlled release.
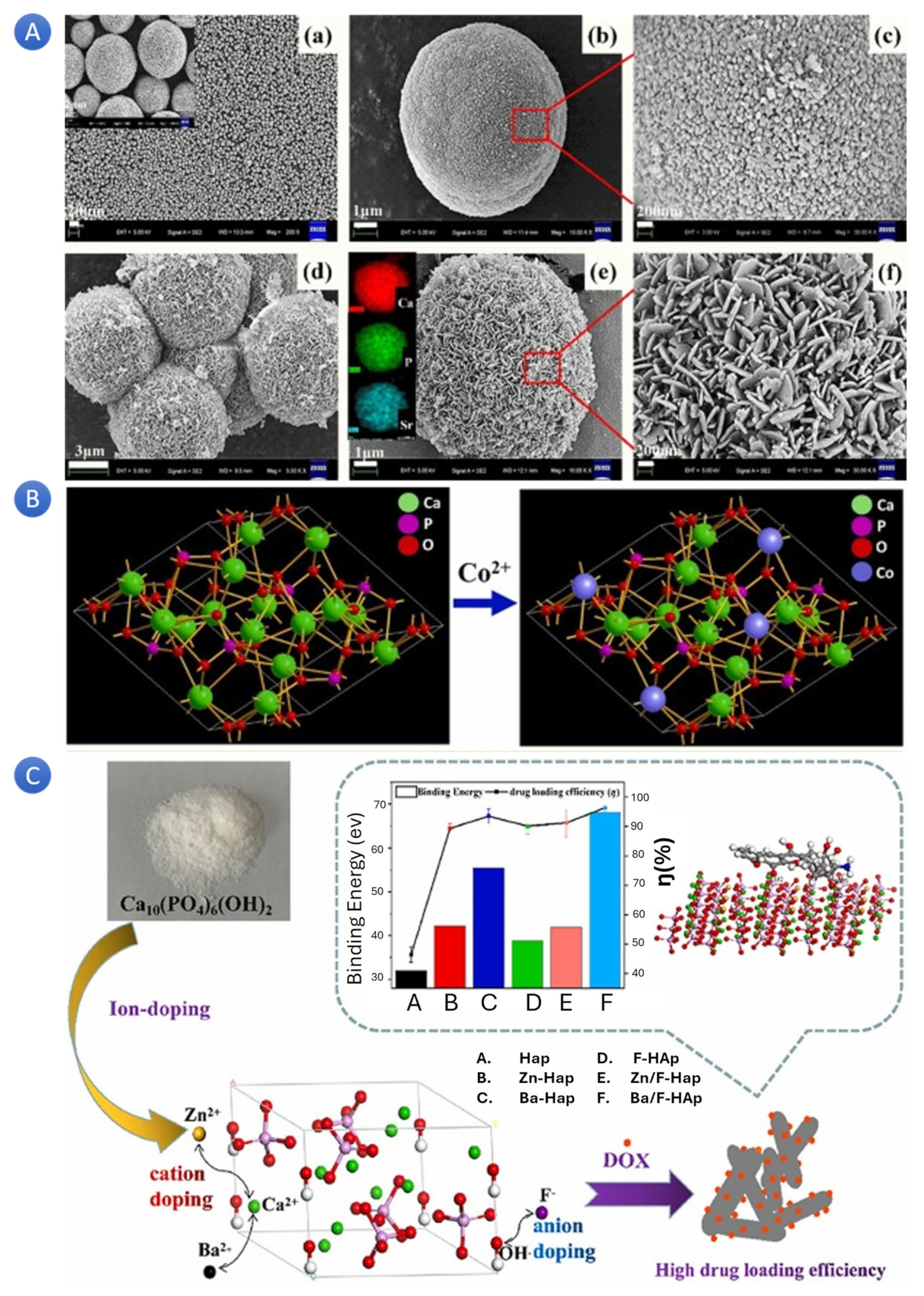
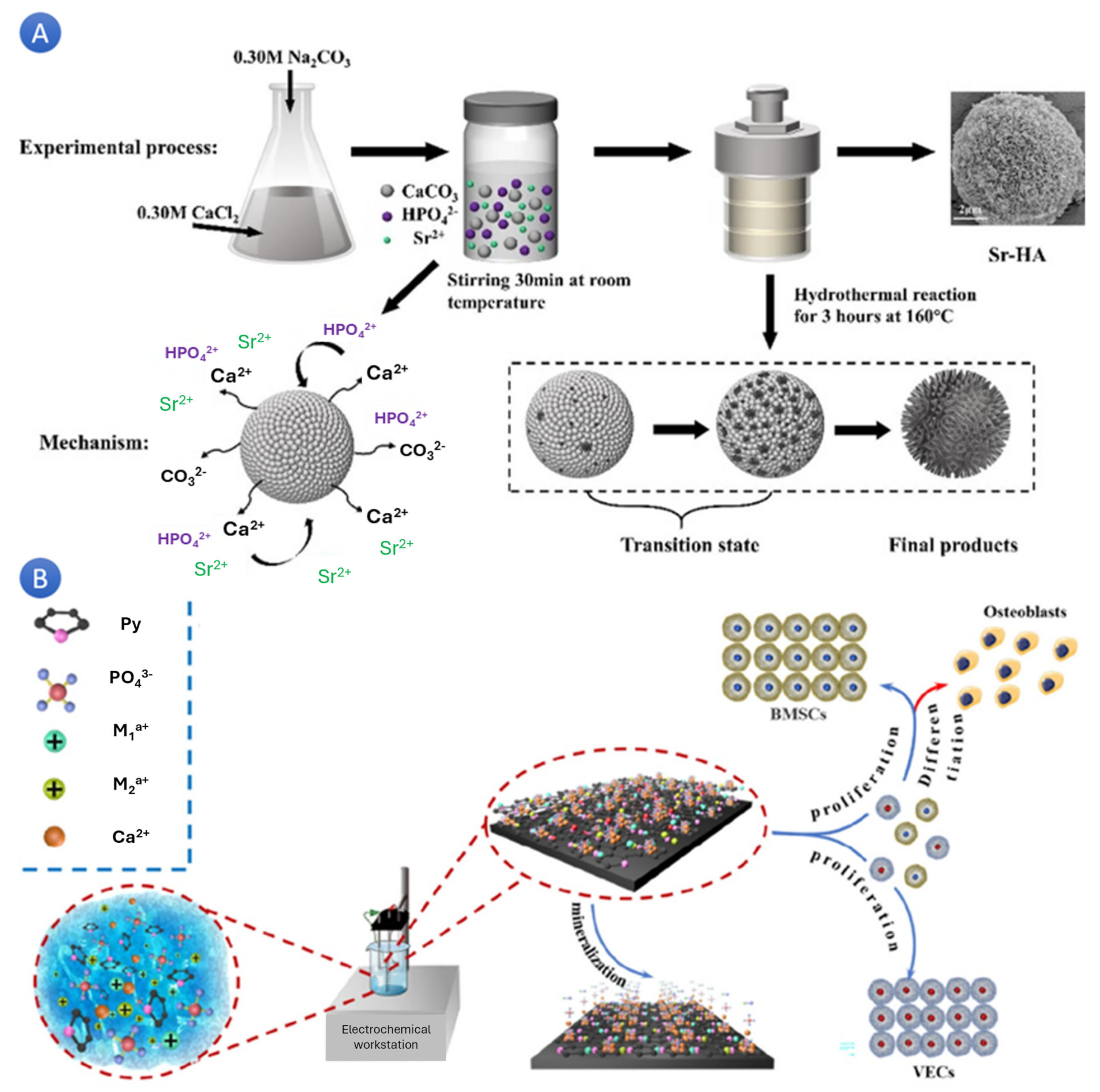
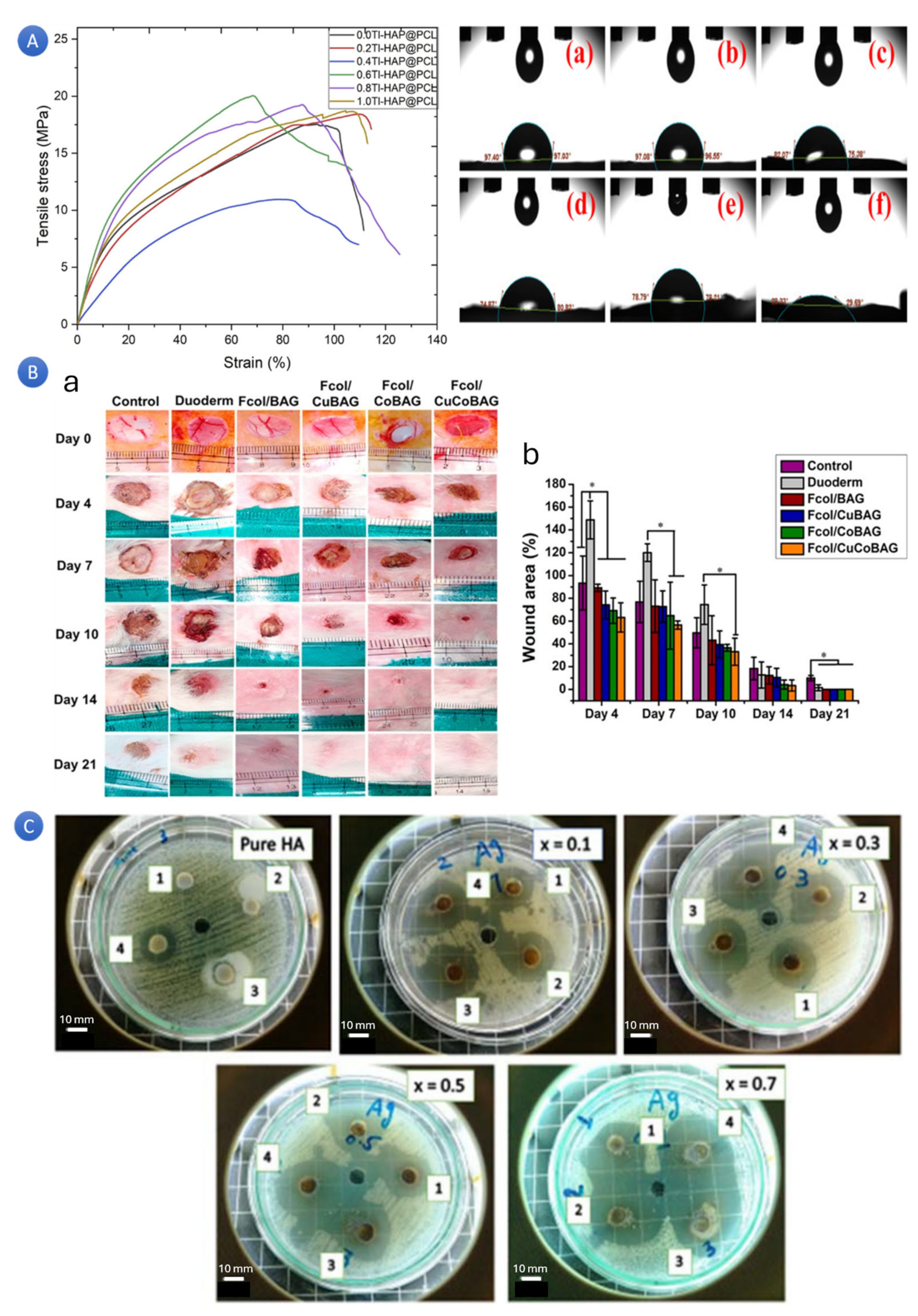
In a study, scientists successfully synthesized mesoporous strontium-doped hydroxyapatite (Sr-HA) microspheres for drug-delivery applications. The microspheres, fabricated through a solid-state reaction and subsequent hydrothermal treatment, exhibited a hierarchically mesoporous structure (
Figure 2A). These Sr-HA microspheres, with their excellent drug-carrying capacity and slow-release performance, were found to be cytocompatible in vitro. These successful applications of Sr-HA microspheres in drug delivery systems provide reassurance about their reliability and potential for promoting bone repair and regeneration [
29,
30]. In another similar study, the authors used a hydrothermal method to successfully prepare strontium-doped hydroxyapatite (Sr-HA) microspheres with interconnected porosity and pore channels. The primary objective of this investigation was to obtain Sr-HA microspheres with interconnected porosity and pore channels, which were effectively achieved by manipulating the Sr
2+ concentration through a hydrothermal approach. Notably, the microstructure and composition of the microspheres were carefully adjusted by the Sr
2+ concentration, leading to diverse morphologies and sizes. The incorporation of Sr
2+ augmented the microspheres’ biodegradability and enhanced their vancomycin loading capacity and sustained release properties, thus exhibiting their potential for biomedical applications [
31].
In a separate study, scientists have developed a versatile cobalt-doped hydroxyapatite (Co-HA) platform with promising potential in both biomedical imaging and therapeutic applications (
Figure 2B). Co-HA nanoparticles, synthesized through Co-precipitation, have shown an enhanced fluorescence activity and proved to be non-toxic when tested on breast cancer cell lines [
27]. The loading of DOX as a chemotherapeutic drug with a loading capacity of 2.0 mol% Co-HA further underscores its potential. The authors have also suggested exciting uses for DOX-loaded Co-HA, particularly in imaging-guided drug delivery systems. These potential applications of Co-HA in biomedical imaging are indeed promising and pave the way for its use in a wide range of therapeutic applications.
In a study with a focus on DOX release [
32], using a simple hydrothermal process and no organic additives, a different nanorod-like morphology ion-doped nanostructured HA (Zn
2+ (smaller than Ca
2+) and Ba
2+ (larger than Ca
2+) for cation doping, F
− as anion-doping, and Zn
2+/F
− as well as Ba
2+/F
− as cation–anion codoping, for comparison, were effectively created and employed as drug carriers to load the therapeutic anti-tumor medication DOX (
Figure 2C). It was claimed that with the addition of various ions, the specific surface area of HA rose; the Zn-doped HA had the largest surface area (101.86 m
2/g), but not the highest DOX loading efficiency. They suggested that pore shape, surface charge, other variables, and a sample’s exact surface area affect how much DOX it can load. The HA doped with Ba/F demonstrated the most noteworthy efficiency in terms of DOX loading (96.3%), with Ba-HA following closely behind (93.4%). Moreover, both of these variants exhibited pH-dependent sustained release behavior. The findings also suggested that the DOX loading capability of HA can be further enhanced, and the sustained release duration can be extended by carefully selecting suitable ions for HA doping. This discovery holds immense significance in advancing the clinical implementation of HA as a DOX drug carrier.
Figure 2.
(
A) Microscopic pictures of hydroxyapatite microspheres doped with strontium for drug delivery applications in different magnifications [
29] (Reprinted with permission). (
B) Fluorescence-conjugated nanostructured hydroxyapatite platform doped with cobalt for use in imaging-guided medication administration [
33] (Reprinted with permission). (
C) The effect of ions-doped hydroxyapatite on doxorubicin’s adsorption and release characteristics as a model anticancer medication [
32] (Reprinted with permission).
Figure 2.
(
A) Microscopic pictures of hydroxyapatite microspheres doped with strontium for drug delivery applications in different magnifications [
29] (Reprinted with permission). (
B) Fluorescence-conjugated nanostructured hydroxyapatite platform doped with cobalt for use in imaging-guided medication administration [
33] (Reprinted with permission). (
C) The effect of ions-doped hydroxyapatite on doxorubicin’s adsorption and release characteristics as a model anticancer medication [
32] (Reprinted with permission).
Moreover, Zn-HA has exhibited an improved loading and release rate for drugs like melatonin [
34] and amoxicillin, with the highest drug loading capacity observed at one mol% Zn-HA [
35]. These studies effectively accentuate the vast potential of iHA in drug delivery applications, which encompasses the facilitation of bone repair and regeneration, imaging-guided drug delivery, and the enhancement of drug loading and release properties. Based on the results, the produced Zn–HA (1 mol%) exhibited a more significant amoxicillin loading in the drug delivery test, with the drug loading of amoxicillin reaching 101.4 mg g
−1. Compared to pure HA (55.37%), the release rate of amoxicillin from the Zn–HA (1 mol%) in the first five hours was approximately 42.58%. Although more thorough pre-clinical research is required to confirm these findings, the Zn–HA is anticipated to become a potential drug carrier in the biomedical industry.
In exciting research, a carrier for the localized transportation of medication utilizing functionalized hydroxyapatite with silver doping has been devised [
36]. This system has been designed to release silver ions over an extended period. The study’s findings indicate that the newly created composite scaffolds exhibit antibacterial properties for up to one year. Moreover, these scaffolds also facilitate the controlled delivery of anesthetic drugs for two weeks. The results also showed that the silver ions from the prepared scaffolds were released at 0.001 ± 0.0005 wt%/h, considering the incorporated silver amount. In this study, Lidocaine hydrochloride was also incorporated into the scaffolds to provide controlled anesthetic drug delivery, which was observed for up to two weeks.
Another study discussed the construction of a drug delivery system using hydroxyapatite microspheres. The system was functionalized with strontium and loaded with berberine hydrochloride to enhance osteogenic activity and antibacterial properties. The findings revealed that including Sr did not influence HA’s physical phase and morphology. However, its chemical composition and crystallinity were modified. In addition, the Sr-HA microspheres exhibited favorable microscopic morphology and ion retardation. These characteristics established a base for enhancing the integrated capacity in subsequent endeavors. As demonstrated in sustained release and antibacterial tests, Berberine hydrochloride enhanced antibacterial performance after being loaded with 3% Sr iHA microspheres. In vitro cell experiments showed that Sr ions at different doping concentrations significantly promoted the differentiation of rat bone mesenchymal stem cells.
Scientists conducted research in which the authors assessed the efficacy of titanium pins coated with silver ion-doped calcium phosphate-based ceramic nanopowder in deterring bacterial colonization. The findings revealed that the silver-doped CA-based titanium pins exhibited a statistically significant decrease in the release of bacteria compared to both pure hydroxyapatite (HA)-coated pins and uncoated pins (p = 0.039 and p = 0.002, respectively). Compared to the broth media containing pure HA-coated and silver iHA-coated pins, the bacterial growth was significantly more significant in the broth containing uncoated pins after 24 h. Compared to the uncoated and pure HA-coated pins, the release of germs from the HA-coated pins doped with silver was statistically lower. No significant differences were observed between the HA-coated and uncoated pin groups.
It has been asserted that the amalgamation of hydroxyapatite (HA) nanoparticles and a drug carrier such as zirconium (Zr) is anticipated to enhance the effectiveness of HA nanoparticles in the treatment of lung cancer [
37]. In a scientific investigation, researchers synthesized nanoparticles of hydroxyapatite doped with zirconium (HAp-Zr). The nanoparticles were tagged using the radioisotope Scandium-46 ([46Sc]Sc-HAp-Zr) to assess their cellular uptake and biodistribution patterns. The findings demonstrated that the [46Sc]Sc-HAp-Zr nanoparticles accumulated in the A549 lung cancer cell line, and the doping of zirconium increased the internalization by the cells. It was also confirmed that combining HA nanoparticles with Zr enhanced the cellular internalization of the nanoparticles in the lung cancer cell line A549.
The primary objective of an additional investigation was to fabricate nanoscale magnesium-doped hydroxyapatite (Mg-HA) by utilizing magnesium nitrate as the source of magnesium (Mg) [
38]. Reflux condensation was employed to produce unadulterated HA and Mg-HA samples that were subsequently subjected to microwave irradiation. The X-ray diffraction (XRD) analyses corroborated that the incorporation of Mg did not instigate any alterations in the structure of hydroxyapatite. The photoluminescence spectra of the Mg-HA samples exhibited an augmented intensity as the concentration of Mg increased, suggesting the prospective utilization of these particles in imaging and drug delivery domains. The in vitro drug release investigation substantiated that the entrapped drug was gradually liberated from the Mg-HA scaffolds. The synthesis of luminescent Mg-incorporated hydroxyapatite through the reflux condensation method presents promising prospects in photoluminescence, in vitro drug release, and kinetic analyses.
In a study conducted by Sangeetha et al. [
39], a multifunctional platform was synthesized utilizing a microwave-assisted wet precipitation method of cobalt ferrite magnetite particles fixed in hydroxyapatite. The resulting platform displayed a core–shell morphology and achieved a maximum saturation magnetization of 3.9 emu/g after being subjected to a calcination process at 900 °C. The platform was combined with the chemotherapy drug doxorubicin by employing an adsorption technique, showcasing a controlled release for up to 98 h. These findings indicate that the magnetic hydroxyapatite nanoparticles modified with doxorubicin hold significant promise as a targeted drug delivery system for cancer treatment.
5. iHA for Tissue Engineering
In recent years, tissue engineering has garnered significant attention as a promising approach to regenerative medicine. One primary obstacle in this domain is the development of appropriate biomaterials that can replicate the natural extracellular matrix and support cellular growth and differentiation. Among the various alternatives, HA has emerged as a promising contender owing to its exceptional biocompatibility and resemblance to the mineral constituent of bone tissue. Nevertheless, pure HA exhibits restricted biological activity and necessitates modifications to enhance its therapeutic potential. A particular modification involves the introduction of ion (iodine–oxygen–oxygen) ions into HA, a technique that has displayed substantial potential in tissue engineering applications. Integrating ions into HA augments its mechanical properties and improves its bioactivity and osteoinductive potential.
IHA has surfaced as a promising biomaterial for diverse applications in tissue engineering. Thanks to its exceptional biocompatibility and similarity to the natural mineral of bone, iHA has been extensively explored for bone tissue regeneration. Its incorporation into scaffolds stimulates cell attachment, proliferation, and differentiation, thus fostering enhanced osteogenesis and improved bone repair. Additionally, iHA possesses distinctive characteristics that render it suitable for drug-delivery systems in tissue engineering.
Its porous structure facilitates the controlled release of bioactive molecules, growth factors, or therapeutic agents at the desired location. This attribute enables targeted treatment and heightens the effectiveness of therapies. Furthermore, iHA can be employed as a coating material for implants to enhance their osseointegration with surrounding tissues.
Synthetic scaffolds composed of calcium phosphate, predominantly HA, are extensively utilized in bone tissue engineering due to their osteoconductivity and bioactivity. HA, a natural constituent of bone, is known to enhance cell attachment by adsorbing a more significant amount of cell-adhesive plasma proteins, such as fibronectin and vitronectin, resulting in enhanced cell adhesion. Bacterial infection with bone grafts poses a considerable challenge that can lead to implant failure. Treating these infections is financially burdensome and laborious; therefore, an ideal bone scaffold should exhibit both biocompatibility and antibacterial activity. Zinc (Zn), the most abundant ion in bones, is often utilized as a substitute for Ca
2+ in HA [
40]. Furthermore, Zn
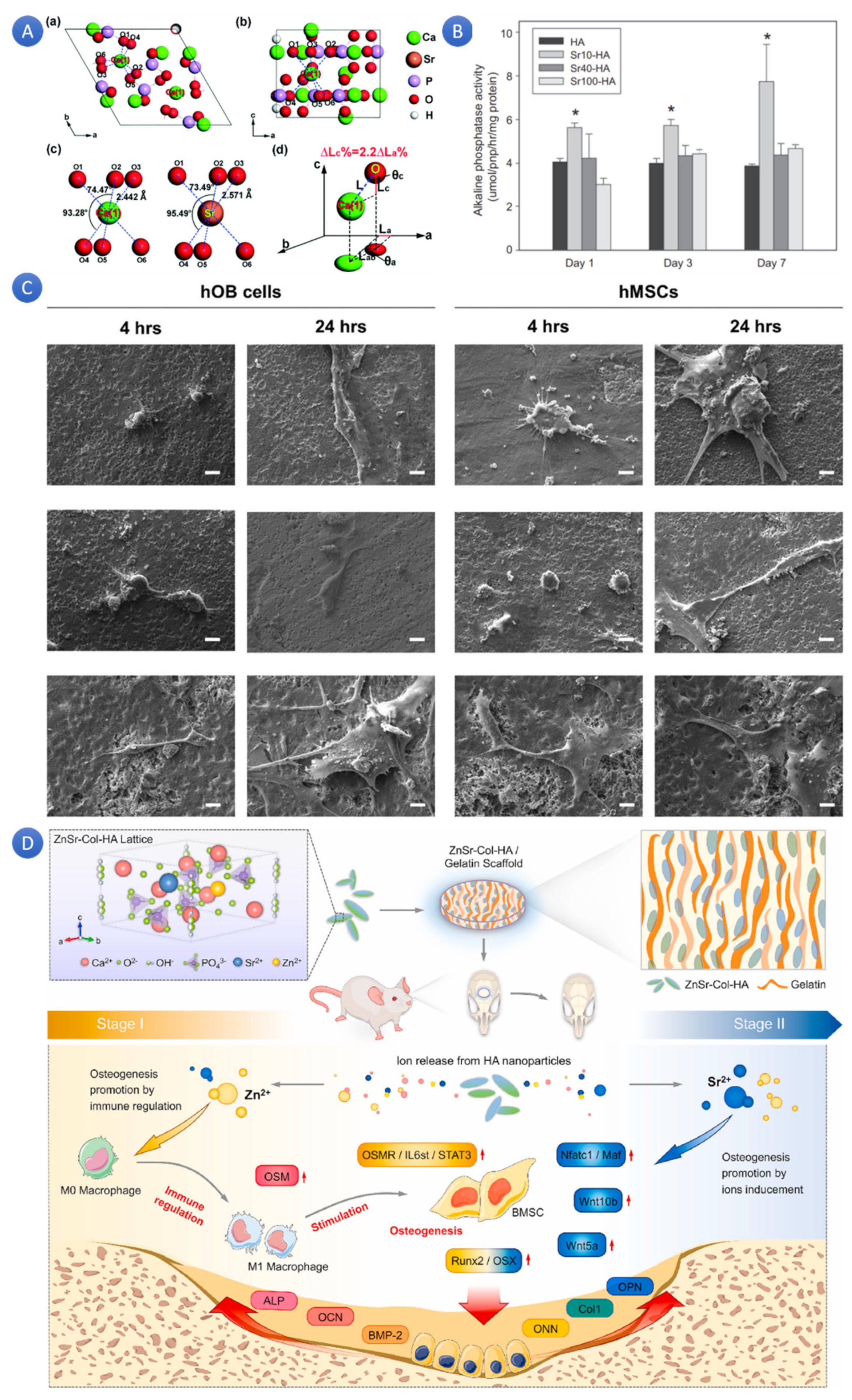
2+ plays a critical role in the immune system and is involved in numerous anabolic and catabolic processes that maintain cellular integrity. Additionally, Zn is essential for transcription and gene expression pathways. It was also shown that adding Sr to HA affects its biological properties in several ways. Sr/Ca substitutions in HA increased the lattice constants and promoted the growth of Sr-doped HA crystals, resulting in increased grain size [
41]. Sr-HA composites (
Figure 3A) have been shown to have good biocompatibility and promote cell proliferation, adhesion, and osteogenic differentiation [
42]. Sr-HA could enhance bone and teeth mineralization, osteogenesis, and angiogenesis while downgrading osteoclast activity (
Figure 3B) [
43]. Increasing Sr content in HA linearly affects crystal lattice parameters, unit cell volume, and density [
44]. Sr-doped HA scaffolds have better compactness and compressive strength and promote cell proliferation and osteoblast differentiation, making them suitable for bone tissue.
Adding Sr to HA enhances its biological properties, making it a promising material for bone repair, regeneration, and dental applications.
In a recent study, researchers attempted to synthesize strontium/zinc co-doped nano-hydroxyapatite (Sr/Zn-nHA) and integrate it with a poly(lactic-co-glycolic acid) (PLGA) scaffold to achieve bacterial inhibition and promote osteogenic potential for bone tissue engineering applications. The results demonstrated a reduction in colony-forming unit numbers dependent on the concentration of Zn, with the scaffold containing 4% Zn exhibiting the most effective antibacterial properties among all the Zn-containing scaffolds tested. Incorporating PLGA into Sr/Zn-nHA did not impact the antibacterial activity of Zn, and the scaffold consisting of 4% Sr/Zn-nHAp-PLGA demonstrated a remarkable 99.7% inhibition of bacterial growth. Additionally, the scaffold with co-doping of Sr/Zn supported the proliferation of osteoblast cells without any apparent cytotoxic effects, and the optimal doping percentage within the 4% Sr/Zn-nHAp-PLGA scaffold was found to be conducive to cell growth [
45]. It was also reported that Zn-nHA can also provide antibacterial activities during bone regeneration and control inflammation [
34].
In a parallel investigation on bone tissue engineering [
46], a composite material comprising tantalum and Sr-HA was developed via the sol–gel technique for coating a titanium substrate. To achieve this, surface modification was accomplished by employing 3% tantalum pentoxide (Ta
2O
5), 3% strontium (Sr), and a combination of 1.5% Ta
2O
5 and 1.5% Sr as additives, in conjunction with HA gel via the spin coating technique. Incorporating these additives played a significant role in producing a porous structure layer coating that facilitated cell growth. Based on the biological findings, the inclusion of Sr ions together with tantalum within the HA matrix led to a notable increase in cell proliferation after a 48 h study period. Moreover, the results obtained from the analysis of microstructure, crystal structure, binding energy, and cell proliferation demonstrated that the incorporation of 1.5% Ta
2O
5 and 1.5% Sr within the HA coating on the titanium substrate resulted in an optimized porous structure, which is expected to enhance bone ingrowth in surface-modified titanium implants.
In a similar study, scientists showed that replacing more than one ion with Ca
2+ or PO
43− groups in hydroxyapatite can form multi-ion-doped hydroxyapatite for bone tissue engineering applications. Yedecki and his colleagues developed a 3D porous PCL-PEG-PCL scaffold integrated with strontium, magnesium, and boron multi-doped hydroxyapatite (10% and 20 wt%) for bone regeneration [
47]. Results showed minimal degradation and improved water uptake in scaffolds with varying porosities, such as the HA/PCL-PEG-PCL composite. The inclusion of HA improved water adsorption and mechanical properties. The compressive strength of the scaffolds ranged from 9.32 to 24.27 MPa, with 20% 2Sr0.5BHA scaffolds demonstrating the highest strength. The scaffolds containing 2Sr0.5BHA showed higher cell viability than the control group and those with HA.
The point is not always about creating porosity or strengthening the mechanical properties of scaffolds. Some ions showed that these roles work as agents for better tissue reconstruction when released in the defect site (
Figure 3C). Doping has been approved as a good strategy for ion release from HA in scaffolds. Salam and Gibson [
42] created co-substituted lithium-ion (Li
+) and carbonate ion hydroxyapatite compositions using an aqueous precipitation technique. A novel method for accounting for charge balance—which has, up till now, been disregarded in the synthesis of Li-doped calcium phosphates—is the co-substitution of Li
+ and CO
32−. Li-substituted samples generated an apatite surface layer and burst release of Li ions when incubated in the culture media. In vitro, osteogenesis was triggered in C2C12 and Human Bone Marrow-Derived Mesenchymal Stem Cells by releasing Li ions from the samples. Topographical characteristics of the sample induced osteogenesis in vitro in hOB cells.
Other studies have shown how iHA helps improve the scaffolds’ osteogenic properties. Osteoimmunomodulation plays a significant role in bone regeneration through a specific mechanism, which is highly related to active inorganic ions (
Figure 3D). Therefore, attempts have been made to obtain osteo-immunomodulatory BHA-based bone grafting materials with optimized osteogenic properties by ion doping [
48]. Doping Zn/Sr dual ions and collagen (co-assembly) to HA (ZnSr-Col-HA) promoted osteogenesis and osteo-immunomodulation, improving bone regeneration. By encouraging osteogenic gene expressions, activating osteogenic and Wnt signals, and rousing macrophages, the ZnSr-Col-HA biomaterial design philosophy created a favorable auto-immune microenvironment. Additionally, ZnSr-Col-HA showed a procedural promoting effect on the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) in vitro. The osteo-immune microenvironment promoted early osteogenic gene expressions via the OSM signal pathway. The significant enhancing effects of ZnSr-Col-HA on the healing of critical-sized cranial defects were confirmed by in vivo experiments [
49]. Similarly to this, ZS/HA/Col scaffolds, which include zinc silicate, collagen, and nanohydroxyapatite, improved angiogenesis, and bone regeneration by stimulating monocytes’ p38 signaling pathway, encouraging BMSC migration and differentiation and the creation of vessels [
50]. Moreover, Zn-Sr-sintered TBC scaffolds with well-structured pores exhibit excellent osteogenic qualities, biocompatibility, and in vivo repair capabilities, which makes them perfect for bone mending [
51]. In another study focusing on osteogenic properties, Co-HA was synthesized by the wet chemical technique and increased cellular VEGF secretion via HIF-1α stabilization [
52]. Investigations into how Co-HA affected the MG-63 cells’ cell cycle progression and proliferation showed that Co-HA maintained the cells’ survival and proliferation up to a certain point. Additionally, this degree of doping also caused the bone cells to differentiate, as seen by improved nodule formation and increased expression of differentiation markers (Runx2 and Osterix). Additionally, it is mandatory to optimize the concentration and level of ion doping. For example, in another study, it was reported that the incorporation of 0.1% Co
2⁺ into hydroxyapatite (symbolized as Co
0.
1HA) significantly improved the proliferation of mesenchymal stem cells (BMSCs) and showed a higher survival rate compared to other Co-HA samples. For optimal activity of BMSCs, the Co
2⁺ concentration should be provided at about 0.9369 µg/L [
53].
It is possible to synthesize multi-ion-doped HA where each ion can be considered for specific mechanobiological functions. Scientists could synthesize ternary trace elements Si, Sr, and F multi-doped HA (Si + Sr + F-HA) with a known number of doped ions (Si: 56 ppm; Sr: 87 ppm; F: 190 ppm). Si, Sr, and F concentrations in hydroxyapatite (HA) could significantly affect osteoconduction and biocompatibility. Compared to HA alone, silicon-substituted HA (Si-HA) has been demonstrated to have higher biological activity and positive impacts on early bone growth processes. It has been discovered that Sr-HA stimulates osteoblast development, cell adhesion, and the synthesis of new bones [
54]. Furthermore, it has been demonstrated that co-doping strontium and selenium in HA (Se-Sr-HA) can stimulate the growth of new bones and prevent cancer from returning in individuals with osteosarcoma [
55]. Accordingly, incorporating Si, Sr, and F in HA could improve its biocompatibility and osteoconduction properties, making it a promising material for bone repair and regeneration applications.
For orthopedic and dental applications, plasma-sprayed hydroxyapatite (HA) coated metallic implants have shown several intrinsic drawbacks, including brittleness, low fracture toughness, poor tensile strength, a sluggish osseointegration rate, and inadequate antibacterial qualities. To address these problems, the Taguchi statistical approach was employed to envision and create precise compositions of Sr, Zn, Ag, and F multi-ion doped HA [
56]. Due to differences in ionic radii with cations, it was demonstrated that Sr and Zn contributed the most to changing HA’s crystallite size and crystallinity; however, F and Ag had more effects on crystallite size than crystallinity. A different investigation showed that compared to pure HAp, Sr, and Zn up to 5% and Ag and F up to 2.5 wt% had better cell proliferation and increased bioactivity [
57]. Superior results were obtained regarding crystallinity, crystallite size, hardness, bioactivity, and cytotoxicity with the chosen composition of multi-ion-doped HAp. In another study [
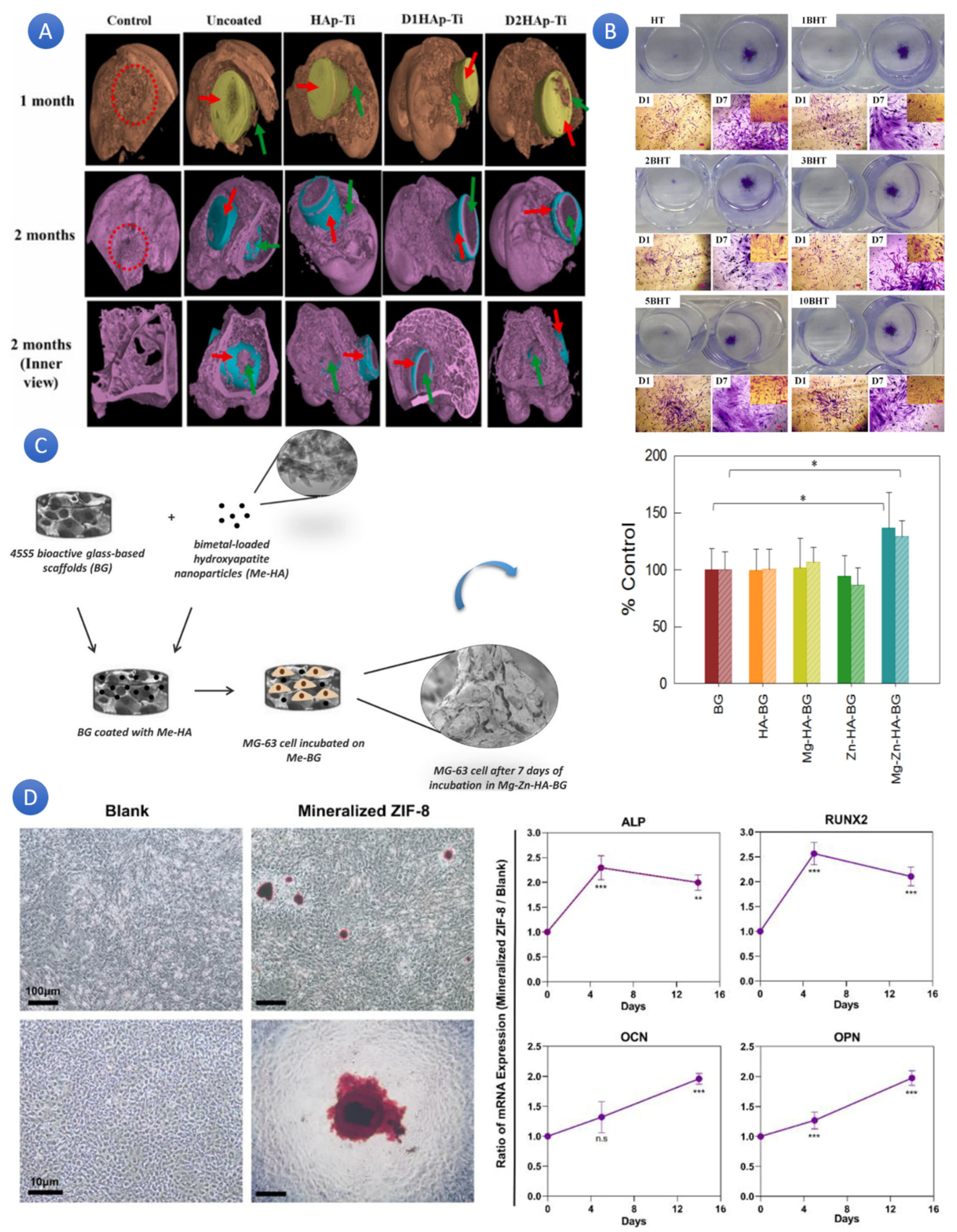
58], the viability of metallic implants coated using plasma spray, with a particular focus on two compositions (based on Sr–Zn–F-HAp–(D1HAp-Ti) and Sr–Zn–Ag–HA (D2HAp-Ti)) was investigated. The findings showed that the HA–10%CS and HA–20%CS coatings had a crack-free morphology, whereas the pure HA coating’s surface had microcracks. Crystallinity, surface roughness, porosity, microhardness, and Young’s modulus increased for HA–10%CS and HA–20%CS coatings with the sequential rise in CS content in HA relative to pure HA coating. Compared to HA, the HA–10%CS and HA–20%CS coatings were more favorable and showed better hemocompatibility (
Figure 4A). The erythrocytes were not negatively affected by the HA–10%CS and HA–20%CS coatings, and the hemolysis rate (HR) was within the safe range (<5%) for implant materials. This investigation showed that applying HA–10%CS and HA–20%CS coatings to the surface of Ti6Al4V alloy is a viable method of enhancing its performance for bio-implant applications.
Biphasic hydroxyapatite comprises two phases: beta-tricalcium phosphate (β-TCP) and HA. This combination produces a mixture that may be used as a synthetic bone graft because of its adjustable resorption, bioactivity, and inherent osteoinduction qualities. The material’s biphasic nature enables the degradation rate to be matched with the pace at which bone heals, and its similarity to genuine bone makes it a viable substitute for autologous bone transplantation. Research has demonstrated that biphasic hydroxyapatite, with its bioactivity and degradation patterns that allow for simultaneous bone growth, is a viable bone replacement for bone augmentation in dental implant therapy [
59]. As well as HA, biphasic HA/β-TCP could show better biological and physical properties after doping. To investigate boron absorption and correlate structural changes in incremental boron addition (0 to 10 mol%), researchers synthesized boron-doped hydroxyapatite/tricalcium phosphates (BHTs) [
60]. Based on the outcomes, by promoting bioactivity, cell proliferation, and osteoblast development, boron-doping modifies the biological characteristics of biphasic HA/TCP (
Figure 4B) [
60,
61,
62,
63]. Comparing boron-doped HA/TCP materials to pure HA/TCP, the former demonstrated greater hardness, reduced particle size, and enhanced crystallinity [
64]. Alkaline phosphatase activity, protein adsorption, intracellular calcium and phosphate storage, and calcium phosphate deposition improved by adding boron. The mechanical characteristics of HA/TCP were not adversely affected by boron doping. Additionally, it was shown that boron-doped HA/TCP composites were biocompatible and could shield cells from harm caused by reactive oxygen species [
65]. Findings from the research suggest that boron doping could enhance the mechanobiology properties of HA/TCP and become a promising candidate for substitute applications and bone tissue engineering.
Figure 4.
(
A) The osteogenesis of Ti6Al4V coatings doped with ternary ions via plasma spraying for orthopedic purposes (Defect site is shown by the red ring; implant material is shown by the red arrow; fresh bone development surrounding the implant material is shown by the green arrow). [
58] (Reprinted with permission). (
B) ALP staining demonstrating how β-Tricalcium phosphate/Boron-doped Biphasic Hydroxyapatite improves bone tissue engineering [
60] (Reprinted with permission). (
C) The percentage of control (uncoated BG) in the WST-8 test findings for MG-63 cell cultures after 2 (simple colored bar) and 7 (dashed bars) days in the presence of Mg-HA-BG, Zn-HA-BG, and MgZn-HA-BG scaffolds (* means significant difference) [
61] (Reprinted with permission). (
D) In vitro osteogenic induction studies employing rBMSCs co-cultured in the osteogenic induction solution-free regular media with mineralized ZIF-8. (
Left) In contrast to rBMSCs cultured in a standard medium (Alizarin red S staining), typical digital photos of rBMSCs treated with incubation with mineralized ZIF-8 showed the evident mineralization of ECM that included mineralized crystalloids after 14 days. (
Right) When rBMSCs were exposed to mineralized ZIF-8 incubation, they showed a marked up-regulation of early (Runx2/Alp) and late (Ocn/Opn) indicators for osteogenesis after 5 and 14 days (the more *, the more significant differences) [
62] (Reprinted with permission). “ns” stands for non-significant differences, and “***” means significant difference.
Figure 4.
(
A) The osteogenesis of Ti6Al4V coatings doped with ternary ions via plasma spraying for orthopedic purposes (Defect site is shown by the red ring; implant material is shown by the red arrow; fresh bone development surrounding the implant material is shown by the green arrow). [
58] (Reprinted with permission). (
B) ALP staining demonstrating how β-Tricalcium phosphate/Boron-doped Biphasic Hydroxyapatite improves bone tissue engineering [
60] (Reprinted with permission). (
C) The percentage of control (uncoated BG) in the WST-8 test findings for MG-63 cell cultures after 2 (simple colored bar) and 7 (dashed bars) days in the presence of Mg-HA-BG, Zn-HA-BG, and MgZn-HA-BG scaffolds (* means significant difference) [
61] (Reprinted with permission). (
D) In vitro osteogenic induction studies employing rBMSCs co-cultured in the osteogenic induction solution-free regular media with mineralized ZIF-8. (
Left) In contrast to rBMSCs cultured in a standard medium (Alizarin red S staining), typical digital photos of rBMSCs treated with incubation with mineralized ZIF-8 showed the evident mineralization of ECM that included mineralized crystalloids after 14 days. (
Right) When rBMSCs were exposed to mineralized ZIF-8 incubation, they showed a marked up-regulation of early (Runx2/Alp) and late (Ocn/Opn) indicators for osteogenesis after 5 and 14 days (the more *, the more significant differences) [
62] (Reprinted with permission). “ns” stands for non-significant differences, and “***” means significant difference.
![Chemistry 07 00137 g004 Chemistry 07 00137 g004]()
Scientists demonstrated that by coating bioactive glass with ion-doped hydroxyapatite, their biological properties could be modified and made more suitable for tissue engineering. In a study, 45S5 composition bioactive glass (BG) scaffolds were coated with hydroxyapatite nanoparticles loaded with Mg
2+, Zn
2+, and both Mg
2+/Zn
2+ ions, and these scaffolds were investigated as potential materials for tissue engineering applications (
Figure 4C) [
61]. The results of the mineralization assay showed that, in contrast to mono-metal-loaded scaffolds, bi-metal-loaded Mg-Zn-HA-BG scaffolds showed higher/faster bioactivity. The mineralization of HA-BG, Zn-HA-BG, and Mg-HA-BG was comparable to that of uncoated scaffolds. Furthermore, MG-63 cell proliferation increased within 48 h and seven days for Mg-Zn-HA-BG scaffolds. SEM pictures corroborated these findings by showing more contact between these scaffolds and the cells than with scaffolds coated with HA and loaded with a single metal. The conventional scaffolds with 45S5 BG composition performed better biologically, thanks to the nanocrystalline Mg-Zn-HA coatings.
Rapid condensation of mesenchymal stem cells (MSCs) and strong contact between MSCs and the matrix on the nanosurfaces may contribute to these events. These interactions most likely result from coordinated signal cascades and biological processes influenced by the extracellular matrix. Researchers examined the proliferation of human adipose tissue-derived mesenchymal stem cells (hMSC) on metal iHA nanocoated surfaces (Zn, Ag, and Cu) [
66]. They postulated that because metal ions are known to have various chemical and surface characteristics, they may consequently translate into unique biological characteristics. They, therefore, investigated how each affected substrate–cell interaction, cellular behavior, osteogenic differentiation potential, and cell survival. According to the findings, nanocoated surfaces containing Zn, Ag, and Cu metal ions showed hMSC survival, osteogenic differentiation, and cell adhesion capabilities greater than commercial HAP. These findings demonstrate that Zn, Ag, and Cu metal ions influenced the biocompatibility of alien material.
It was hypothesized that modifying synthetic material-based scaffolds by ion-doped-HA can improve their biological properties. Numerous studies have investigated the impact of ion-doped hydroxyapatite crystals on the osteogenic properties of polycaprolactone (PCL) composite scaffolds. ZIF-8 has been shown to significantly enhance the biocompatibility and osteoinductivity of PCL scaffolds in both in vitro and in vivo settings (
Figure 4D) [
62]. Analogously, it has been demonstrated that the integration of nHA with poly(aryl ether ketone) (PAEK-COOH) composites encouraged cell growth and caused osteogenic differentiation in cells [
67]. Moreover, it has been discovered that replacing calcium in CaP scaffolds with strontium and magnesium improves the differentiation of hMSCs and encourages the production of bone [
68]. Furthermore, it has been demonstrated that adding nHA and Sr-HA to polymeric blends of PLLA, PCL, and PHBV improved the scaffolds’ ability to promote osteogenesis in vitro [
69]. Lastly, it has been discovered that adding hydroxyapatite (HA) and collagen type I (COL) to PCL scaffolds enhanced the osteogenic differentiation and bone formation of hADSCs, or mesenchymal stem cells generated from adipose tissue [
70].
Vascular endothelial growth factor (VEGF) pathway activation produced by biomaterials is becoming more widely acknowledged as a viable tissue engineering alternative for angiogenesis. In research, wet chemical synthesis was used to create bivalent nickel (Ni
2+) ion-doped nHA (Ni-HA) to improve proangiogenic and osteoconductive qualities [
71]. This is while it has been shown that the HapNPs containing nickel induced the expression of VEGF and HIF-1α, vascular endothelial growth factor, suggesting that nickel plays a role in generating VEGF in cells [
72]. The mechanism of the effect of nickel on the bone tissue engineering of nanohydroxyapatite involves the activation of the VEGF pathway for angiogenesis. Ni
2+ doping influenced the cell viability, proliferation, and differentiation of MG-63 in a concentration-dependent way and cellular VEGF expression induction manifold compared to the group with no Ni
2+ [
71]. Additionally, the involvement of HIF-1α in cellular VEGF production was observed [
73]. The production of VEGF and other critical signaling pathways involved in angiogenesis, such as endothelial nitric oxide synthase (eNOS) and fibroblast growth factor-2 (FGF-2), is stimulated by the presence of nickel ions in the doped nHA [
74]. Nickel-doped nHA stimulates angiogenic factors, which improve endothelial cell migration, proliferation, survival, and the development of capillary-like structures [
75]. These results imply that doping nHA with nickel can improve its proangiogenic characteristics, making it a viable biomaterial for stimulating angiogenesis in tissue engineering and bone healing applications.
Ion doping for HA provided strong properties in developing electrically polarized HA-based ceramics that could adapt more easily to bone chemistry and the original benefits of electrical polarization treatment. Scientists used the single and binary forms of MgO and SrO as dopants to assess the effects of these dopants on the adhesion of osteoblast cells and their proliferation on polarized HA surfaces [
76]. The authors stated that this approach demonstrated good cellular adherence and notable spreading on ceramic coated with both Sr
2+ and Mg
2+ doped HA samples (negatively charged surfaces) compared to the ceramic coated with undoped HAp. It also showed better cell viability after seven days. More interestingly, positively charged iHA surfaces showed low and limited cellular growth compared to the neutral surfaces. Sr
2+ doping into the HA lattice adjusts the electrostatic interactions involved in fibronectin (FN) adsorption on HA, leading to enhanced FN adhesion [
77]. The flipping of OH-ions, the interchange of proton vacancies, and the hopping of OH-vacancies along the c-axis are the processes of ion conduction and polarization in HA the observed polarization and ion conductivities during electrical poling in HA result from these processes [
78].
6. iHA for Wound Healing
Wound healing, as well as tissue engineering, could benefit from the use of iHA. Before that, it is necessary to highlight the potential of HA in wound healing. Based on the literature, HA showed its potential in fabricating injectable granular hydrogels with advanced healing properties, such as exudate absorption, mass exchange, and cell migration [
79]. The primary role of HA in wound dressings comprises improving mechanical properties, water uptake capacity, oxygen permeability, degradation, and cell proliferation acceleration [
80]. The central aspect of HA in wound dressing turns back to its capacity in loading therapeutic agents like antibacterial agents for local delivery [
81]. In the case of iHA, it can be concluded that, in contrast with HA, it can depict a larger drug loading capacity for in situ drug release. This aspect was discussed in
Section 3 in more detail.
Fewer studies have used iHA for wound dressing than for tissue engineering. However, iHA could help scientists develop more ideas for wound dressings in general.
The hydrophobic characteristic of the biodegradable polycaprolactone (PCL) polymer, which has been intensively employed for wound dressing development, prevents it from adhering firmly, even with its straightforward production technique, high viscoelastic capabilities, and solubility in a variety of solvents (such as acetone or toluene). Nevertheless, this disadvantage can be overcome by adding inorganic chemicals like HA. Tellurium and vanadium ions can be added to HA nanoparticles embedded in a PCL-based electrospun patch to enhance and optimize their characteristics for wound healing applications [
82]. The mechanical behavior progressed upon incorporating Te ions from 7.45 to 10.38 MPa in tensile strength. The presence of iHA showed an increase in the hydrophilic properties of the final patch. Likewise, the introduction of thallium (Tl) ions into PCL scaffolds to modify HA has resulted in increased mechanical properties, reduced contact angle, and better roughness, rendering them effective wound dressings with encouraging cell survival (
Figure 5A) [
83]. This study confirms that the capacity to manipulate a co-phase allows it to modify the mechanical properties, stability, elasticity, porosity, particle size, and crystallinity to create optimum compositions for the intended use.
It is well known that reactive oxygen species (ROS) release, bioactivity control, and rough materials have a higher bioactivity than smooth, nonporous composites, which may all be altered by varying the composition and degree of roughness [
86]. It was reported that with increased cell survival and the creation of porosity, non-oriented fibers, co-doping HA with aluminum/vanadate (Al/V) ions in PCL nanofibrous scaffolds, have also shown promise for wound healing applications [
87].
As previously mentioned, iHA has a higher antibacterial agent loading capacity and controls drug release than HA or other nanoparticle types. Distinct metal ions can be used as dopants. For instance, in a study, a novel cerium/gallium-doped wound dressing was fabricated using phosphate glass fibers by the melt-quenching and melt-spinning process [
88]. Gallium addition reduced the degradation rate, and cerium-PGF and gallium-PGF could regulate the release of ions with antibacterial qualities. Using bioactive molecules obtained from organic sources combined with inorganic materials (natural sources) for better wound healing and tissue reconstruction has attracted the attention of scientists. In the research, four wound dressings were fabricated in which distinct concentrations of Cu and Co (as dopants) were employed (
Figure 5B) [
84]. These ions were doped to bioactive glass and embedded with derived collagen from fish (Rohu) skin (Fcol) at 3% and 5% concentrations. Based on the results, treatment with Fcol/CoBAG and Fcol/CuCoBAG microfibers promoted the healing of skin wounds in rabbits because it increased wound maturity, wound closure, and the controlled deposition of extracellular matrix components—mature elastin and collagen. Notably, efficient neo-vascularization was observed during the early stages of the healing process. It has been reported that using Cu
2+ and Co
2+ could promote blood vessel development, which is essential for tissue regeneration to be successful [
89]. These findings confirm that iHA can be an excellent candidate for delivering these ions in an appropriate concentration.
In chronic wounds, bacterial colonization is a frequent consequence. In wounds, bacteria develop into biofilms that appear to increase until they obstruct healing by causing ordered angiogenesis, inactivating growth factors essential to the healing process, and promoting persistent inflammation [
90]. Doping antibacterial ions like Ag and cu to HA could increase their antibacterial impact during wound healing (
Figure 5C) [
85,
91,
92]. Many research investigations are documenting encouraging outcomes regarding using bioactive glasses (BGs) in restoring and renewing soft tissues and facilitating wound healing. By developing novel compositions of BGs that possess the unique ability to release ions that initiate specific biological responses, the potential applications of BGs are being extended even further in repairing soft tissues [
93]. In the case of chronic and infected wounds, scientists could propose a new methylcellulose-based wound dressing integrated with copper and zinc-doped borosilicate and borate bioactive glasses [
94]. Copper was chosen to create angiogenic properties, and zinc to introduce antibacterial effects. It was discovered that the regulated release of therapeutic ions could actively suppress or trigger immunological responses, depending on the eventual application, by employing BGs with varying compositions and concentrations. Freeze-dried methylcellulose-based foams with Cu-doped borate BG particles showed high porosity and improved wettability. They showed good mechanical properties and bioactivity. Cell biology tests showed material biocompatibility and superior antibacterial effects against
E. coli and
S. aureus bacteria. Electrospun mats with BG particles showed superior cell attachment and tensile strain.
Zn has been another promising ion with antibacterial activities that effectively heal wounds. Zn-doped mesoporous bioactive glass nanoparticles, derived through the sol–gel process, have demonstrated promising results in wound healing [
95]. These nanoparticles have exhibited favorable compatibility with blood, possess antibacterial properties, and have the capability to enhance the proliferation of fibroblast cells [
96]. The inclusion of zinc within the composition of the glass has resulted in alterations in several properties, including the glass transition temperature, the size of the particles, the zeta potential, and the specific surface area [
40]. Moreover, when these bioactive glass nanoparticles were incubated in simulated body fluid, they facilitated the formation of a layer similar to apatite on their surface [
97]. Consequently, zinc ions have been shown to prevent HA from forming on the particles when added to hydroxyapatite. This is advantageous since it allows for the prolonged release of Zn
2+ ions at low concentrations, perhaps preventing toxicity. Additionally, Zn-HA has shown angiogenesis stimulation and antimicrobial activity, which are critical for wound healing.
7. iHA for Coating Implants
Orthopedic implants, which are artificial devices used to replace or support damaged or missing joints, bones, or other body parts, play a crucial role in the healthcare of numerous patients. Nevertheless, the occurrence of infection linked to these medical implants continues to be a significant and noteworthy challenge in contemporary trauma and orthopedic surgery. This persistent problem has a profound impact on patient outcomes, leading to prolonged hospital stays, increased healthcare costs, and, in some cases, even necessitating the removal or revision of the implant itself.
At the moment, one of the main reasons for implant failure is infections related to surgical implants. Antibiotic resistance is a significant challenge in orthopedic implant infections [
98]. Strains of
Staphylococcus aureus that infect implants exhibit elevated rates of resistance to antibiotics, and there is a concerning escalation of antibiotic resistance in other species, including
Staphylococcus epidermidis (
S. epidermidis). This surge in pathogenic organisms impervious to antibiotics, coupled with the heightened prevalence of infection, necessitates exploring a material-centric perspective on antimicrobials for implant-related infections. Given that the initial stage of implant-related infection involves the colonization of bacteria (comprising adherence and initial multiplication), it becomes imperative to prevent bacterial attachment to the implant by implementing surface treatments. Acknowledging the significance of this approach in thwarting the initial stage of implant-related infections is crucial.
In several therapeutic applications, the synthesis of silver-doped hydroxyapatite as an antibacterial agent has promise for preventing post-surgical infections. Scientists tried to coat implants with antibacterial ions to prevent bacterial colonization after implanting. To achieve this, Ag-HA was employed in distinct concentrations. Compared to the uncoated implant, the antibacterial test showed that the Ag-doped HA-coated implant showed higher
Staphylococcus aureus growth inhibition, and increasing the silver content significantly enhanced the antibacterial activity. In a similar study, the same coating was used for titanium pins (66 titanium pins) using the electrospray method, and their potential to prevent bacterial colonization was assessed [
99]. Compared to the broth media containing pure HA-coated and Ag-HA-coated pins, the results showed that at 24 h, bacterial growth was significantly more significant in broth containing uncoated pins (
p = 0.036 and
p = 0.009, respectively). Compared to pure HA-coated pins and uncoated pins, the release of germs from silver-doped HA-coated pins was significantly lower (
p = 0.039 and
p = 0.002, respectively). There were no discernible variations between the HA-coated and untreated pin groups. The minimum inhibitory concentration limits for the silver ion-doped powder for coagulase-negative Staphylococcus were 8 μg/mL. No free silver ions were found in the broth medium. In another study, scientists showed that titanium knee prostheses coated with Ag-doped calcium phosphate increased the resistance against bacterial colonization compared to uncoated prostheses [
100]. A theory for how Ag-HA works is that the released Ag binds to important enzyme functional groups (
Figure 6A). The bacterial plasma or cytoplasmic membrane, which is linked to several significant enzymes, is a prime target location for Ag ions because they trigger the release of K
+ ions from bacteria [
101].
Metallic biomaterials are utilized more extensively than any other material family in medical devices for human applications. The functionality and durability of an implant material are influenced by its corrosion resistance, which is a crucial factor governing biocompatibility. The prevailing principle for metallic biomaterials, excluding biodegradable metals, has been that more excellent corrosion resistance correlates with higher biocompatibility. However, the harsh body environment presents multiple challenges regarding corrosion control. Therefore, the objective of the study [
102] was to investigate the corrosion resistance and in vitro bioactivity of a manganese-doped hydroxyapatite (Mn-HA) film that was electroplated onto NaOH-treated titanium (Ti) (
Figure 6B). The NaOH treatment process was implemented on the Ti surface to enhance the adhesion of the Mn-HA coating. Results from the bond strength test demonstrated that the adhesion of the Mn-HA coating was significantly improved compared to the HA coating. Moreover, the potentiodynamic polarization test revealed that the MnHAp-coated surface exhibited superior corrosion resistance to the surface with only HAp. Furthermore, the bioactivity test, which involved immersing the coatings in simulated body fluid, demonstrated that the MnHA coating facilitated rapid bone-like apatite nucleation and growth. In addition, osteoblast cellular tests indicated that the MnHA coating exhibited better in vitro biocompatibility enhancement for Ti than the HA coating.
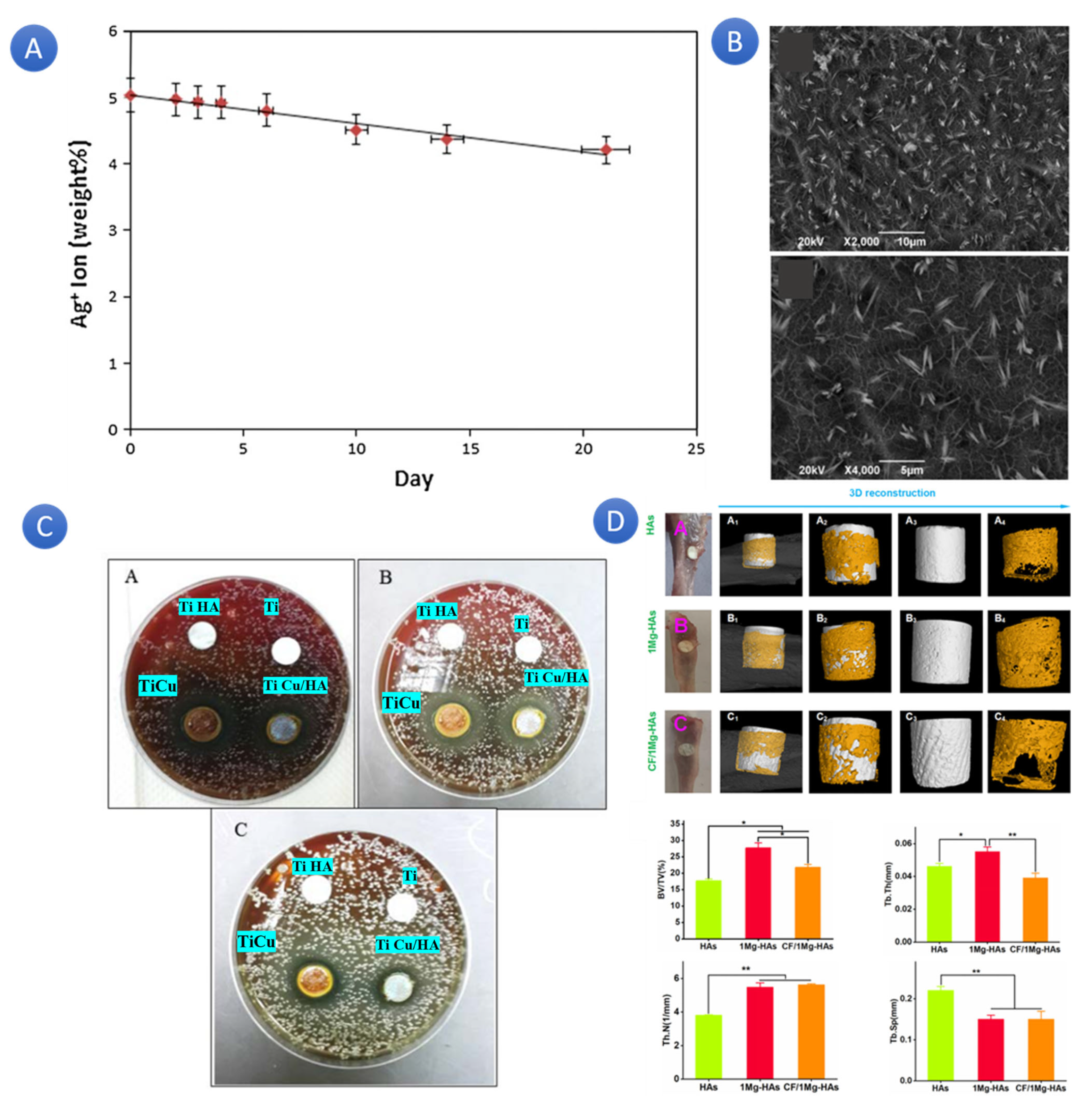
In a similar study, a Ti implant was coated with Cu-doped HA (Ti, Ti Cu, Ti HA, and Ti Cu/HA) as a dental implant. The exciting results show that the Cu-doped HA coating significantly improved the implant’s antibacterial activity against Prophyromonas gingivalis bacteria. This promising finding opens up new possibilities for enhancing the safety and efficacy of dental implants, instilling a sense of optimism in the field of dental care (
Figure 6C) [
103].
The coating process was performed using the electrophoretic deposition technique. One of the therapeutic options for the rehabilitation of patients who are entirely or partially edentulous is the dental implant. Ti-6Al-4V alloy and commercially pure titanium (Cp-Ti) are common materials in dental implants. However, Cp-Ti is not a good combination because of its weak mechanical characteristics. Therefore, it has been suggested that Ti-6Al-4V alloy enhances the mechanical qualities. The findings of this study indicated that the process of altering the surface properties of the Ti-6Al-7Nb alloy through the incorporation of Cu-doped HA could potentially serve as a favorable technique for the development of a coating that possesses the ability to effectively manage and mitigate the occurrence of infections in the vicinity of dental implants. It was reported that a certain amount of ion doping significantly impacted osteogenic differentiation at different levels and could end up in bone marrow mesenchymal stem cells’ osteogenic differentiation (
Figure 6D) [
104].
Another concern regarding implants is the interaction between plasma proteins and the implant materials. When implantable materials are inserted into the host’s anatomical site, they immediately come into contact with blood. As a result, it has been observed that plasma proteins quickly adhere to the surfaces of biomaterials based on their chemical and physical properties and those of the biomaterial itself. This occurrence leads to osseointegration, which refers to the formation of new bone tissue on the implant’s surface. This process occurs after the implant is placed, allowing for a direct and close relationship between the living bone and implant material without fibrous tissue. Various factors can either positively or negatively affect osseointegration. For instance, the design of the implant, the treatment of its surface, its wettability, and its chemical properties can be adjusted to enhance osseointegration. Numerous studies in the literature support using microrough surfaces instead of smooth ones and using hydrophilic rather than hydrophobic ones to promote osseointegration [
105].
Therefore, researchers endeavored to examine the impact of synthesis conditions on the antimicrobial properties of Ag-HA NP, specifically concerning
E. coli and
S. epidermidis [
106]. They also tried to assess the biocompatibility of Ag-HA NP and its ability to adsorb two crucial blood plasma proteins, human serum albumin (HSA) and fibrinogen (Fib). The study’s findings indicated that the pH of the synthesis greatly influenced the Ag content of Ag-HA NPs and the subsequent release of Ag
+ from the NPs when in solution. Consequently, this had a notable effect on the efficacy of the antimicrobial properties and the cytotoxicity towards murine preosteoblast cells (MC3T3-E1). Moreover, it was observed that HSA exhibited a higher adsorption rate when compared to Fib on a molar basis. Notably, the adsorption of HSA caused a significant alteration in its conformation, transitioning from a primarily α-helix structure with minor β-sheet content in solution to a more excellent β-sheet structure when adsorbed. Consequently, the melting temperature (Tm) of HSA underwent a considerable shift from 76 °C in solution to approximately 65–66 °C when adsorbed. On the other hand, Fib experienced a modest decrease in α-helix content and a slight increase in β-sheet content upon adsorption, with its Tm remaining unchanged at approximately 60 °C. These variances in the behavior of HSA and Fib can be attributed to the significantly smaller size of HSA, which allows for a higher molecular packing density on the surface, thereby inducing more pronounced conformational changes. The protein adsorption behavior observed on Ag-HA was similar to that on pure HAP. Consequently, it was demonstrated that Ag-HA NPs possess antimicrobial activity without adversely affecting biocompatibility or blood plasma protein adsorption, providing reassurance about the safety of these materials.
In another investigation, the conduct of HAp, both with and without the presence of Ag and Sr, was evaluated using the galvanostatic pulsed electrochemical deposition method [
107]. The findings indicated that including Ag and Sr positively affected the HAp. Furthermore, examinations of wettability through contact angle and surface free energy assessments revealed that all surfaces displayed hydrophilic properties. The behavior of MC3T3-E1 in vitro demonstrated that adding Sr to the HA coatings, either as a sole doping agent or in conjunction with Ag, improved cytocompatibility regarding cell proliferation and osteogenic differentiation. Additionally, the authors concluded that the pulse deposition technique proved an effective electrochemical method for producing undoped and doped HA coatings with desirable in vitro characteristics, and incorporating Ag and Sr as co-doped elements led to a higher deposition rate, resulting in thicker layers. Finally, all suggested coatings enhanced the electrochemical performance of the Ti substrate in SBF media.
8. iHA for Multimodal Imaging
Recently, it has been demonstrated that specific HA hybrid composites can effectively serve as biomarkers and therapeutic agents in theragnostics [
108]. HA, which serves as a material for bone restoration, has been extensively discussed in
Section 5,
Section 6 and
Section 7. This investigation includes HA doped with cobalt, zinc, manganese, copper, and silver. However, numerous studies are working on HA in a calcium-deficient form. To enhance the osteoconductive and antimicrobial properties of HA, it has been co-doped with cerium and strontium [
109]. Lanthanides, also known as rare earth elements, are suitable for substituting Ca
2+ ions [
110]. These elements’ distinctive magnetic and optical characteristics, which arise from their 4f-electronic configuration, make them appropriate for producing magnetic resonance (MR) agents and highly sensitive diagnostic bioassays [
111]. The f-f transition, which the Laporte rule prohibits, can be partially achieved by mixing the 4f
n configuration with the 4f
n−15d
1 configuration of opposite parity or through intramolecular charge transfer [
112]. Depending on the transition, precisely the relative spacing of the initial and final energy states of photo-excited carriers, photoluminescence can occur through either down-conversion or up-conversion processes.
Multimodal imaging has garnered significant interest in recent years, representing an approach that combines multiple imaging techniques in a single diagnostic step. For instance, computer tomography, magnetic resonance imaging, and photoluminescence tomography can be sequentially or simultaneously applied to the same sample in specific equipment configurations. This approach offers a notable advantage in diagnosing complex diseases like tumors. By utilizing different imaging modalities, the localization of findings within the patient’s body can be improved, and the specificity of diagnostic results can be increased. Furthermore, multimodal imaging proves patient-friendly by saving time and potentially rendering repeated consultations obsolete. However, due to the involvement of various imaging modalities, a multimodal contrast agent that can respond to the different types of radiation or (electro-)magnetic fields associated with these modalities is necessary.
One novel and promising strategy for a multimodal contrast agent is to use doped HA as a ceramic nanoparticle-sized contrast agent or marker for biomedical luminescence imaging [
113,
114]. Some examples of rare earth elements (REEs) that have been used as dopants to create single-modal HA contrast agents include terbium (Tb
3+) and europium (Eu
3+).
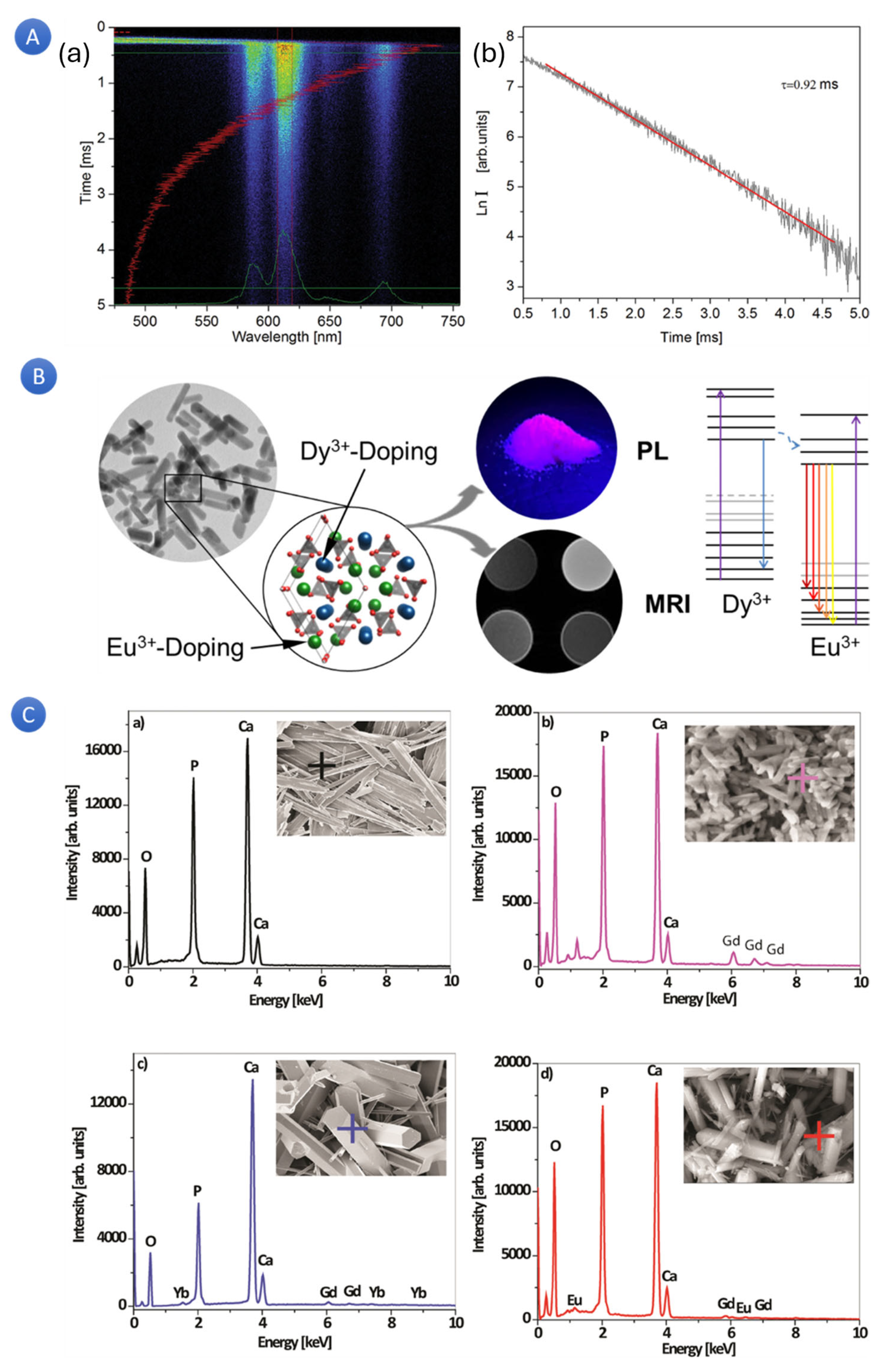
Utilizing the apatite structure’s flexibility, researchers doped hydroxyapatite (HA) nano- and microparticles with various combinations of rare earth ions (RE
3+ = gadolinium (Gd)), europium (Eu), Ytterbium (Yb), and Terbium (Tm)) to enhance their magnetic and optical properties and make them suitable for use in multimodal imaging-based diagnostics and preventive medicine (
Figure 7A) [
113,
115]. According to the results, an increasing concentration of defects resulted in a shift in the energy band gap and the formation of a faint blue luminescence (λex = 370 nm), as evidenced by the HA: RE
3+ optical characterization. The distinctive transitions of Tm
3+ and Eu
3+ were seen in the “up” and “down” conversion spectra of the HA: Gd/Yb/Tm and HA: Gd/Eu powders, respectively. Moreover, all the HA: RE
3+ powders showed paramagnetic behavior, unlike diamagnetic HAp. Cell viability studies of the powdered HA: Gd/Yb/Tm and HA: Gd/Eu showed high biocompatibility in human dental pulp stem cell cultures.
The paramagnetic behavior of rare earth elements is another significant property that can be utilized for multimodal imaging. In the context of MRI, magnetizable materials are employed as contrast agents. Over the years, ions such as gadolinium (Gd
3+) and dysprosium (Dy
3+) have been utilized to synthesize aqueous paramagnetic contrast agent complexes administered during MRI examinations [
19]. In a particular investigation [
116], luminescent and magnetic HA were prepared through the process of doping with europium (Eu
3+) and dysprosium (Dy
3+), respectively. The co-doping of Eu
3+ and Dy
3+ was implemented to combine the desired physical properties. As per the researchers’ findings, Eu-doped HA (Eu: HA) demonstrated persistent photoluminescent (PL) properties dependent on the concentration of the dopant. On the other hand, Dy-doped HA (Dy: HA) displayed paramagnetic behavior due to the substantial magnetic moment of Dy
3+. The nanoparticles of co-doped HA (Eu:Dy: HA) combined these properties within a single crystal. Interestingly, multimodal co-doped HA exhibited enhanced PL properties due to energy transfer from the Dy
3+ sensitizer to the Eu
3+ activator ions. Eu:Dy: HA demonstrated significant transverse relaxation effects, with a maximum transverse relativity of 83.3 L/(mmol·s). Owing to their adjustable PL, magnetic properties, and cytocompatibility, Eu:-, Dy:-, and Eu:Dy: HA represented promising biocompatible ceramic materials for luminescence imaging. These materials could simultaneously function as contrast agents for MRI in permanent implants or functional coatings.
In another related study [
117], researchers introduced a prospective multimodal agent based on nanosized HA co-doped with Eu
3+ and Gd
3+ ions. The introduction of HA: Eu
3+/Gd
3+ led to the observation of tuneable photoluminescence intensity, which varied depending on the concentrations of Eu
3+ and Gd
3+. Additionally, the magnetization increased when higher Gd3+ doping was applied. Moreover, the co-doping of Gd
3+ ions resulted in an enhancement of Eu
3+ emission intensity. As a result, HA: Eu
3+/Gd
3+ presents itself as a potentially promising candidate for bioimaging applications.
Our research on doping HA with paramagnetic properties led to significant changes in particle size and shape, as shown in
Figure 7C. However, regardless of the doping, the particles maintained their elongated structure, appearing as plates, rods, needles, or hexagonal prisms of varying dimensions. As the concentration of dopants increased, the magnetization level and coercivity of the iHA gradually augmented. Incorporating rare earth ion dopants in place of calcium modified the material’s lattice parameters and bandgap. The iHA particles exhibited a compelling combination of magnetism and fluorescence, indicating their potential as a multimodal imaging agent. Cell compatibility testing further confirmed this potential, which reassured us of the safety of the synthesized iHA particles when administered in vitro to various cell types obtained from patients.