Physical Mechanisms of Linear and Nonlinear Optical Responses in Ferrocene-Embedded Cycloparaphenylenes
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Electronic Structure
3.2. HOMO and LUMO Analysis
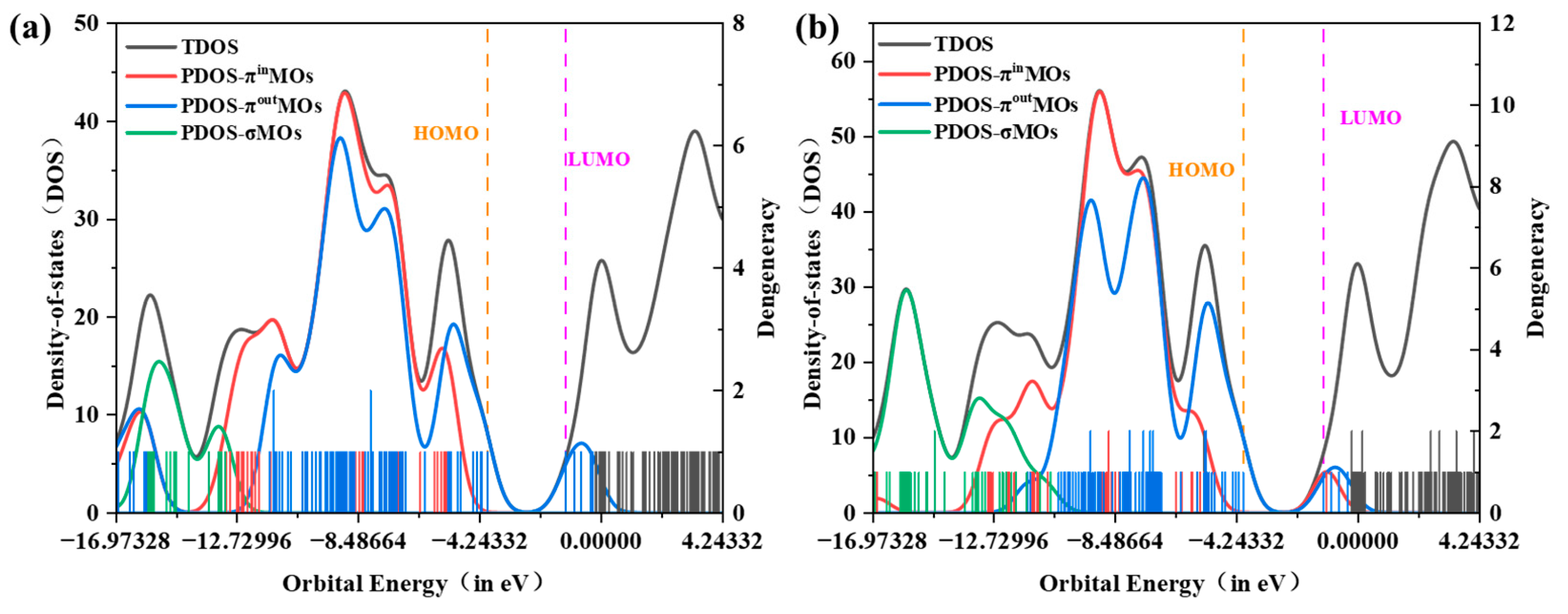
3.3. MO and DOS Analyses
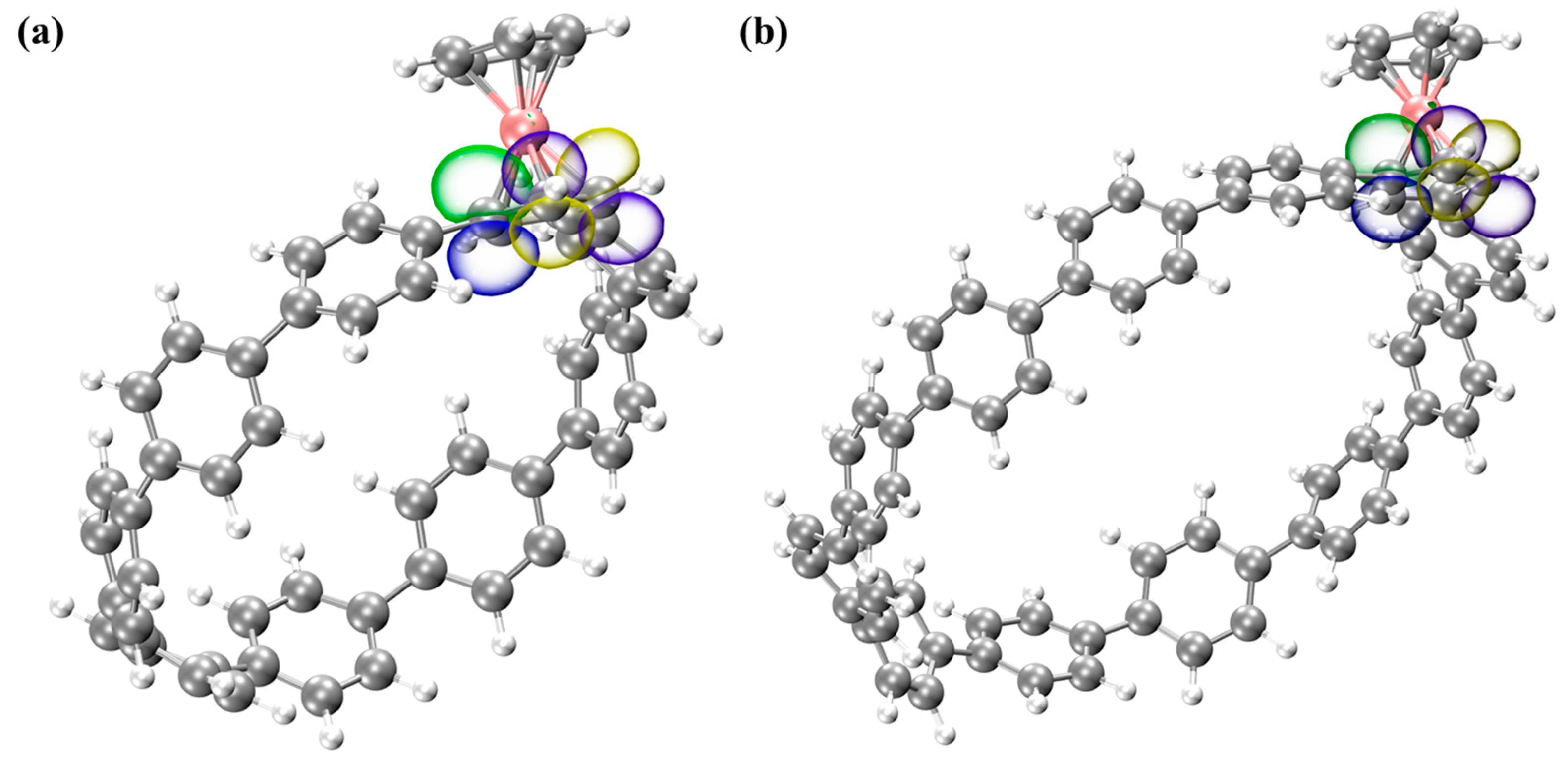
3.4. Natural Bond Orbital (NBO) Analysis
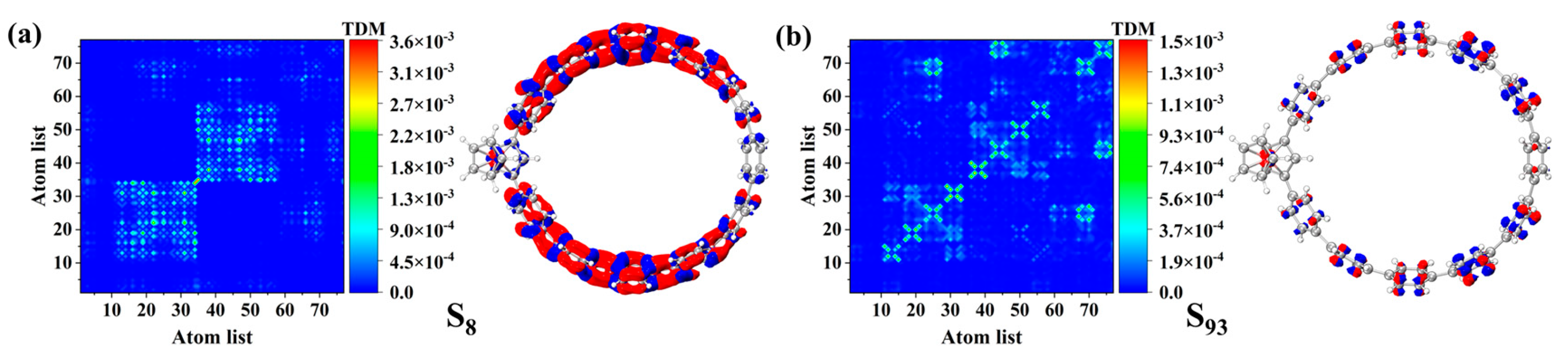
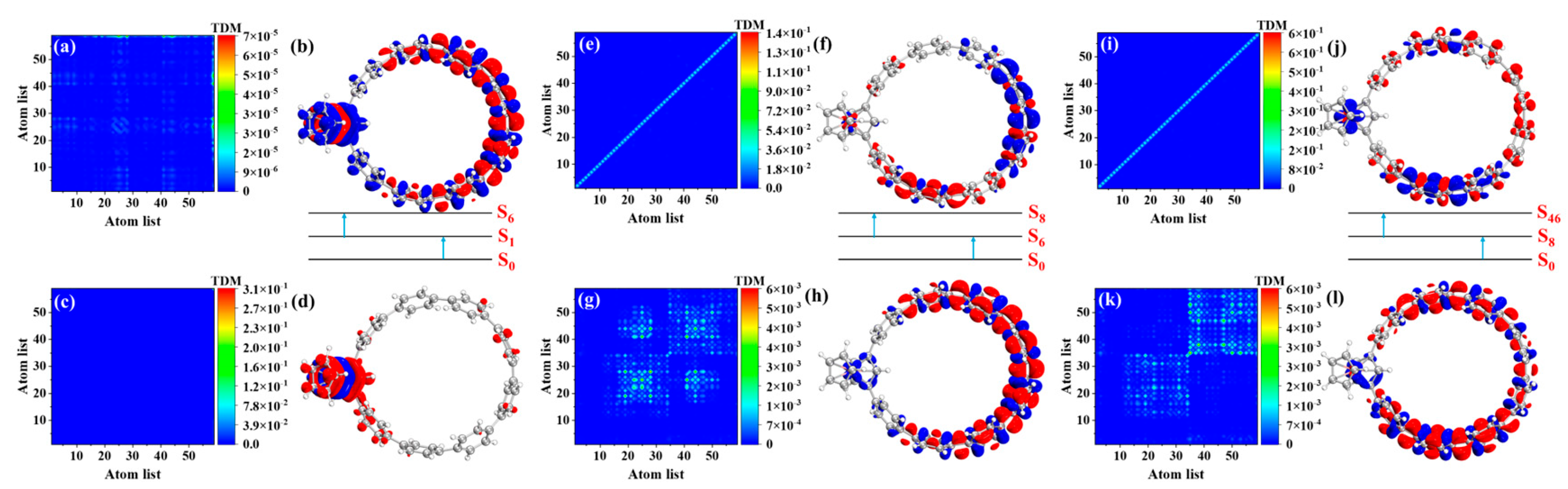
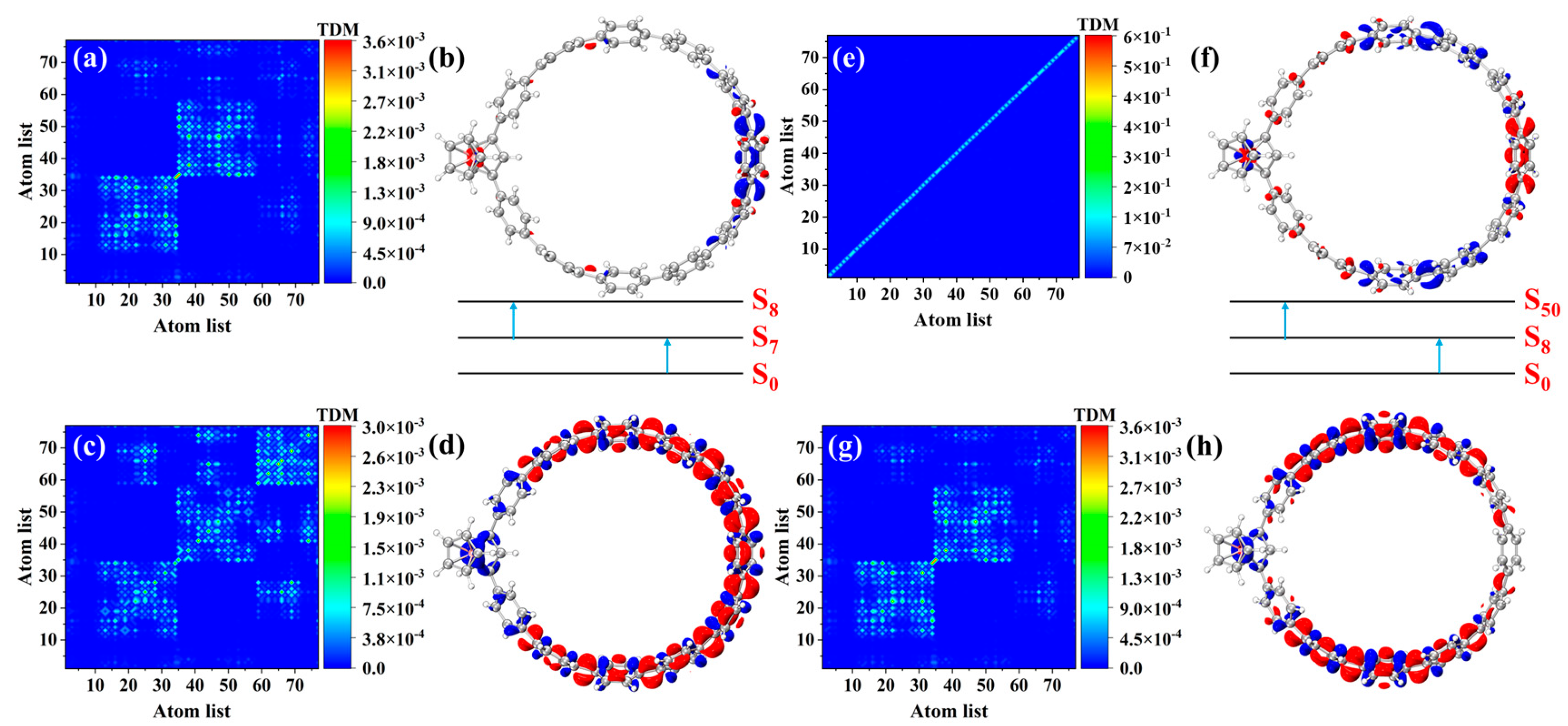
3.5. OPA and TPA Analyses of Fc-[8]CPP and Fc-[11]CPP
3.5.1. OPA Analyses
3.5.2. TPA Analyses
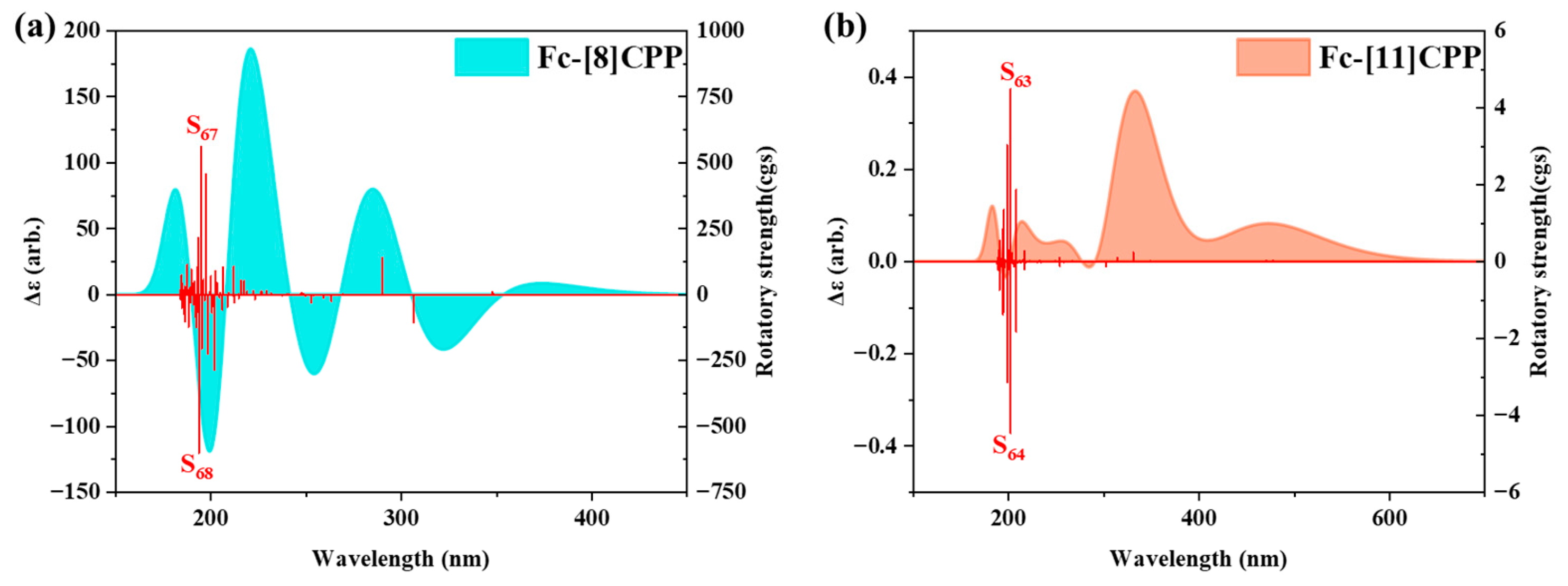
3.6. ECD Analysis of Fc-[8]CPP and Fc-[11]CPP
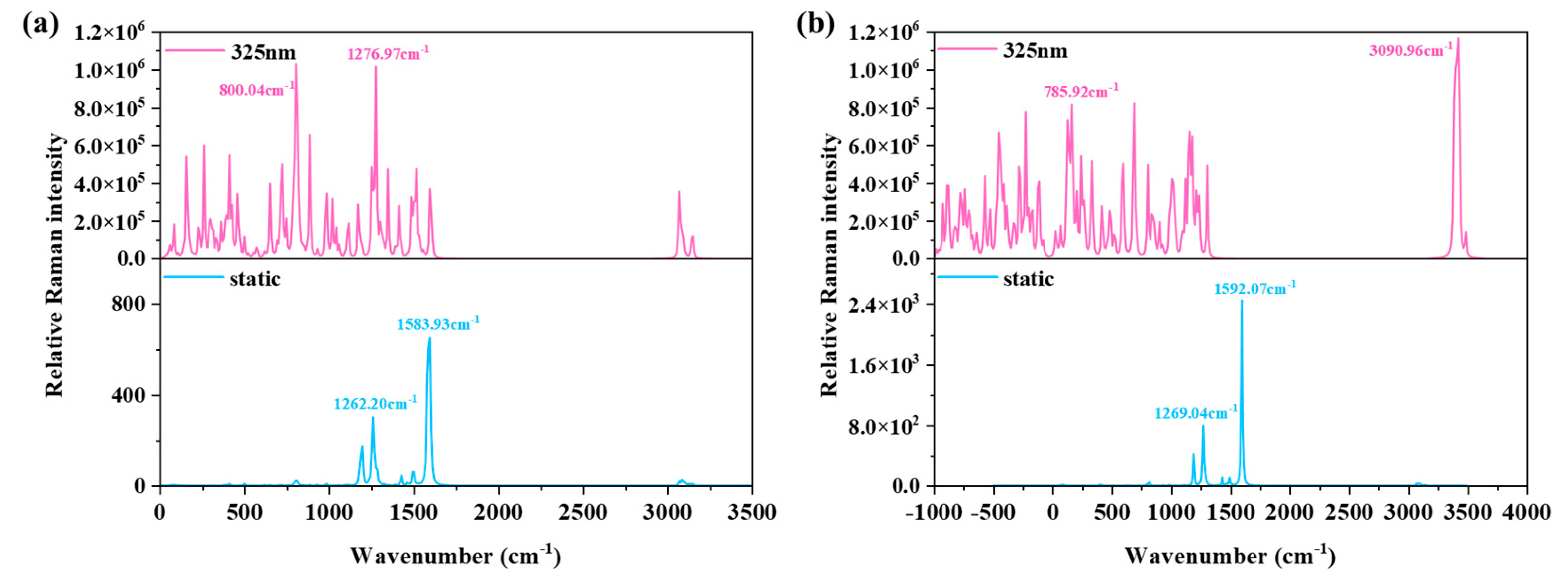
3.7. Raman Spectra and Resonance Raman Spectra of Fc-[8]CPP and Fc-[11]CPP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Segawa, Y.; Ito, H.; Itami, K. Structurally Uniform and Atomically Precise Carbon Nanostructures. Nat. Rev. Mater. 2016, 1, 15002. [Google Scholar] [CrossRef]
- Lewis, S.E. Cycloparaphenylenes and Related Nanohoops. Chem. Soc. Rev. 2015, 44, 2221–2304. [Google Scholar] [CrossRef] [PubMed]
- Jasti, R.; Bhattacharjee, J.; Neaton, J.B.; Bertozzi, C.R. Synthesis, Characterization, and Theory of [9]-, [12]-, and [18] Cy-cloparaphenylene: Carbon Nanohoop Structures. J. Am. Chem. Soc. 2008, 130, 17646–17647. [Google Scholar] [CrossRef]
- Van Raden, J.M.; White, B.M.; Zakharov, L.N.; Jasti, R. Nanohoop Rotaxanes from Active Metal Template Syntheses and Their Potential in Sensing Applications. Angew. Chem. Int. Ed. 2019, 58, 7341–7345. [Google Scholar] [CrossRef]
- Otteson, C.E.; Levinn, C.M.; Van Raden, J.M.; Pluth, M.D.; Jasti, R. Nanohoop Rotaxane Design to Enhance the Selectivity of Reaction-Based Probes: A Proof-of-Principle Study. Org. Lett. 2021, 23, 4608–4612. [Google Scholar] [CrossRef]
- Hermann, M.; Wassy, D.; Esser, B. Conjugated Nanohoops Incorporating Donor, Acceptor, Hetero- or Polycyclic Aromatics. Angew. Chem. Int. Ed. Engl. 2021, 60, 15743–15766. [Google Scholar] [CrossRef]
- Yamago, S.; Kayahara, E.; Iwamoto, T. Organoplatinum-Mediated Synthesis of Cyclic π-Conjugated Molecules: Towards a New Era of Three-Dimensional Aromatic Compounds. Chem. Rec. 2014, 14, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Darzi, E.R.; Jasti, R. The Dynamic, Size-Dependent Properties of [5]–[12] Cycloparaphenylenes. Chem. Soc. Rev. 2015, 44, 6401–6410. [Google Scholar] [CrossRef]
- Zirakzadeh, A.; Herlein, A.; Groß, M.A.; Mereiter, K.; Wang, Y.; Weissensteiner, W. Halide-Mediated Ortho-Deprotonation Reactions Applied to the Synthesis of 1,2-and 1,3-Disubstituted Ferrocene Derivatives. Organometallics 2015, 34, 3820–3832. [Google Scholar] [CrossRef]
- Kayahara, E.; Kouyama, T.; Kato, T.; Yamago, S. Synthesis and Characterization of [n] CPP (N = 5, 6, 8, 10, and 12) Radical Cation and Dications: Size-Dependent Absorption, Spin, and Charge Delocalization. J. Am. Chem. Soc. 2016, 138, 338–344. [Google Scholar] [CrossRef]
- Zou, L.; Gao, Y.; Zhang, Q.; Ye, X.Y.; Xie, T.; Wang, L.W.; Ye, Y. Recent Progress in Asymmetric Domino Intramolecular Cycliza-tion/Cascade Reactions of Substituted Olefins. Chem. Asian J. 2023, 18, e202300617. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; von Delius, M. The Supramolecular Chemistry of Strained Carbon Nanohoops. Angew. Chem. Int. Ed. 2020, 59, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Shang, W.; Sun, H.; Liang, Q.; Kayahara, E.; Yamago, S.; Li, Y.; Zhang, D. Synthesis of Octatrimethylsi-lyl-[8]cycloparaphenylene for Multifunctionalized Cycloparaphenylene. J. Org. Chem. 2025, 90, 8959–8965. [Google Scholar] [CrossRef] [PubMed]
- Lovell, T.C.; Colwell, C.E.; Zakharov, L.N.; Jasti, R. Symmetry Breaking and the Turn-on Fluorescence of Small, Highly Strained Carbon Nanohoops. Chem. Sci. 2019, 10, 3786–3790. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, J. Syntheses and Properties of Heteroatom-Doped Conjugated Nanohoops. Chin. J. Org. Chem. 2022, 42, 3437–3455. [Google Scholar] [CrossRef]
- Wu, D.; Cheng, W.; Ban, X.; Xia, J. Cycloparaphenylenes (CPPs): An Overview of Synthesis, Properties, and Potential Applications. J. Org. Chem. 2018, 7, 2161–2181. [Google Scholar] [CrossRef]
- Wang, L.; Nogami, J.; Nagashima, Y.; Tanaka, K. Enantioselective Synthesis of Axially Chiral Figure-Eight Spirocyclopara-phenylenes via Rh-Catalyzed Intermolecular [2 + 2 + 2] Cycloaddition. Asian J. Org. Chem. 2018, 7, 2161–2181. [Google Scholar]
- Hines, D.A.; Darzi, E.R.; Hirst, E.S.; Jasti, R.; Kamat, P.V. Carbon Nanohoops: Excited Singlet and Triplet Behavior of Aza [8]CPP and 1,15-Diaza [8]CPP. J. Phys. Chem. A 2015, 119, 8083–8089. [Google Scholar] [CrossRef]
- Brouillac, C.; McIntosh, N.; Heinrich, B.; Jeannin, O.; Sagazan, O.D.; Coulon, N.; Rault-Berthelot, J.; Cornil, J.; Jacques, E.; Quinton, C.; et al. Grafting Electron-Accepting Fragments on [4]cyclo-2,7-carbazole Scaffold: Tuning the Structural and Electronic Properties of Nanohoops. Adv. Sci. 2024, 11, 2309115. [Google Scholar] [CrossRef]
- Kiciński, W.; Szala, M.; Bystrzejewski, M. Sulfur-doped porous carbons: Synthesis and applications. Carbon 2014, 68, 1–32. [Google Scholar] [CrossRef]
- Ito, Y.; Cong, W.; Fujita, T.; Tang, Z.; Chen, M. High Catalytic Activity of Ni-trogen and Sulfur Co-Doped Nanoporous Graphene in the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2015, 54, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Van Raden, J.M.; Louie, S.; Zakharov, L.N.; Jasti, R. 2, 2′-Bipyridyl-Embedded Cycloparaphenylenes as a General Strategy to Investigate Nanohoop-Based Coordination Complexes. J. Am. Chem. Soc. 2017, 139, 2936–2939. [Google Scholar] [CrossRef] [PubMed]
- Darzi, E.R.; Hirst, E.S.; Weber, C.D.; Zakharov, L.N.; Lonergan, M.C.; Jasti, R. Synthesis, Properties, and Design Principles of Donor–Acceptor Nanohoops. ACS Cent. Sci. 2015, 1, 335–342. [Google Scholar] [CrossRef]
- Peters, G.M.; Grover, G.; Maust, R.L.; Colwell, C.E.; Bates, H.; Edgell, W.N.; Jasti, R.; Kertesz, M.; Tovar, J.D. Linear and Radial Conjugation in Extended π-Electron Systems. J. Am. Chem. Soc. 2020, 142, 2293–2300. [Google Scholar] [CrossRef]
- Van Raden, J.M.; Louie, S.; Zakharov, L.N.; Jasti, R. Noncovalent Modification of Cycloparaphenylene by Catenane For-mation Using an Active Metal Template Strategy. Angew. Chem. 2023, 62, e202310613. [Google Scholar]
- Chen, M.; Unikela, K.S.; Ramalakshmi, R.; Li, B.; Darrigan, C.; Chrostowska, A.; Liu, S.Y. A BN-Doped Cycloparaphenylene Debuts. Angew. Chem. 2021, 60, 1556–1560. [Google Scholar] [CrossRef]
- Lu, C.; Chen, P.; Sheng, H.; Li, C.; Wang, J. Physical Mechanism on Linear Spectrum and Nonlinear Spectrum in Double Helical Carbon Nanomolecule-Infinitene. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 282, 121674. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Omachi, H.; Matsuura, S.; Miyata, Y.; Kitaura, R.; Segawa, Y.; Itami, K.; Shinohara, H. Size-selective Com-plexation and Extraction of Endohedral Metallofullerenes with Cycloparaphenylene. Angew. Chem. 2014, 126, 3166–3170. [Google Scholar] [CrossRef]
- Chang, X.; Xu, Y.; von Delius, M. Recent Advances in Supramolecular Fullerene Chemistry. Chem. Soc. Rev. 2024, 53, 47–83. [Google Scholar] [CrossRef]
- Paul, A.; Borrelli, R.; Bouyanfif, H.; Gottis, S.; Sauvage, F. Tunable Redox Potential, Optical Properties, and Enhanced Stability of Modified Ferrocene-Based Complexes. ACS Omega 2019, 4, 14780–14789. [Google Scholar] [CrossRef]
- Gray, H.B.; Sohn, Y.S.; Hendrickson, N. Hendrickson, Electronic structure of metallocenes. J. Am. Chem. Soc. 2002, 93, 3603–3612. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, J.; Lan, B.; Chen, X.; Kono, H.; Xu, H.; Yan, J.; Li, W.; Yagi, A.; Yuan, Y. Ferrocene-Based Conjugated Macrocycles: Shotgun Synthesis, Size-Dependent Properties and Tunable Fluorescence Intensity. Org. Chem. Front. 2024, 11, 5130–5137. [Google Scholar] [CrossRef]
- Lan, B.; Xu, J.; Zhu, L.; Chen, X.; Kono, H.; Wang, P.; Zuo, X.; Yan, J.; Yagi, A.; Zheng, Y. Side-Chain Type Ferrocene Macrocycles. Precis. Chem. 2024, 2, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Why Is Ferrocene so Exceptional? Eur. J. Inorg. Chem. 2017, 2017, 6–29. [Google Scholar] [CrossRef]
- Cullen, W.R.; Woollins, J.D. Ferrocene-Containing Metal Complexes. Coord. Chem. Rev. 1981, 39, 1–30. [Google Scholar] [CrossRef]
- Inkpen, M.S.; Scheerer, S.; Linseis, M.; White, A.J.; Winter, R.F.; Albrecht, T.; Long, N.J. Oligomeric Ferrocene Rings. Nat. Chem. 2016, 8, 825–830. [Google Scholar] [CrossRef]
- Hisatome, M.; Yoshihashi, M.; Yamakawa, K.; Iitaka, Y. X-Ray Crystal Structure of 7, 16, 24, 33-Tetrathia [3] Paracyclo [3](1, 3) Ferroceno [3] Paracyclo [3](1, 3) Ferrocenophane. Bull. Chem. Soc. Jpn. 1987, 60, 3474–3476. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Wu, Y.; Fu, H.; Yao, J. A novel redox-fluorescence switch based on a triad containing ferrocene and perylene diimide units. Org. Lett. 2008, 10, 3065–3068. [Google Scholar] [CrossRef]
- Segawa, Y.; Omachi, H.; Itami, K. Theoretical Studies on the Structures and Strain Energies of Cycloparaphenylenes. Org. Lett. 2010, 12, 2262–2265. [Google Scholar] [CrossRef]
- Iwamoto, T.; Watanabe, Y.; Sakamoto, Y.; Suzuki, T.; Yamago, S. Selective and Random Syntheses of [n] Cyclopara-phenylenes (N = 8–13) and Size Dependence of Their Electronic Properties. J. Am. Chem. Soc. 2011, 133, 8354–8361. [Google Scholar] [CrossRef]
- Kamin, A.A.; Clayton, T.D.; Otteson, C.E.; Gannon, P.M.; Krajewski, S.; Kaminsky, W.; Jasti, R.; Xiao, D.J. Synthesis and Metalation of Polycatechol Nanohoops Derived from Fluorocycloparaphenylenes. Chem. Sci. 2023, 14, 9724–9732. [Google Scholar] [CrossRef]
- Li, K.; Xu, Z.; Deng, H.; Zhou, Z.; Dang, Y.; Sun, Z. Dimeric Cycloparaphenylenes with a Rigid Aromatic Linker. Angew. Chem. Int. Ed. 2021, 60, 7649–7653. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 16; Revision A. 03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.0. 16; Semichem, Inc.: Shawnee Mission, KS, USA, 2016.
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Di chroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO, Version 3.1; Gaussian, Inc.: Pittsburgh, PA, USA, 2003.
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Chen, X.; Lu, C.; Wang, L.; Wang, J. Angle-Resolved One and Two-Photon Absorption Spectrum in Twisted Bilayer Graphene Quantum Dots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 271, 120894. [Google Scholar] [CrossRef]
- Li, N.; Zhang, L.; Wang, J. Modulation of chiral spectral deflection by van der Waals force-induced molecular electropolarization in catenane oligomers. RSC Adv. 2023, 13, 11055–11061. [Google Scholar] [CrossRef]
- Li, Y.; Gai, X.; Zhao, B.; Wang, J. Chiral Nonlinear Luminescence Study of Pentaoxonium Salt Molecules Driven by Internal Electric Field Induced by Orbital Polarization. J. Petrochem. Univ. 2025, 38, 65. [Google Scholar]
- Liu, Z.; Lu, T.; Chen, Q. Vibrational Spectra Molecular Vibrational Behaviors of All-Carboatomic Rings cy-clo [18]carbon Its Analogues. Chem. Asian J. 2021, 16, 56–63. [Google Scholar] [CrossRef]
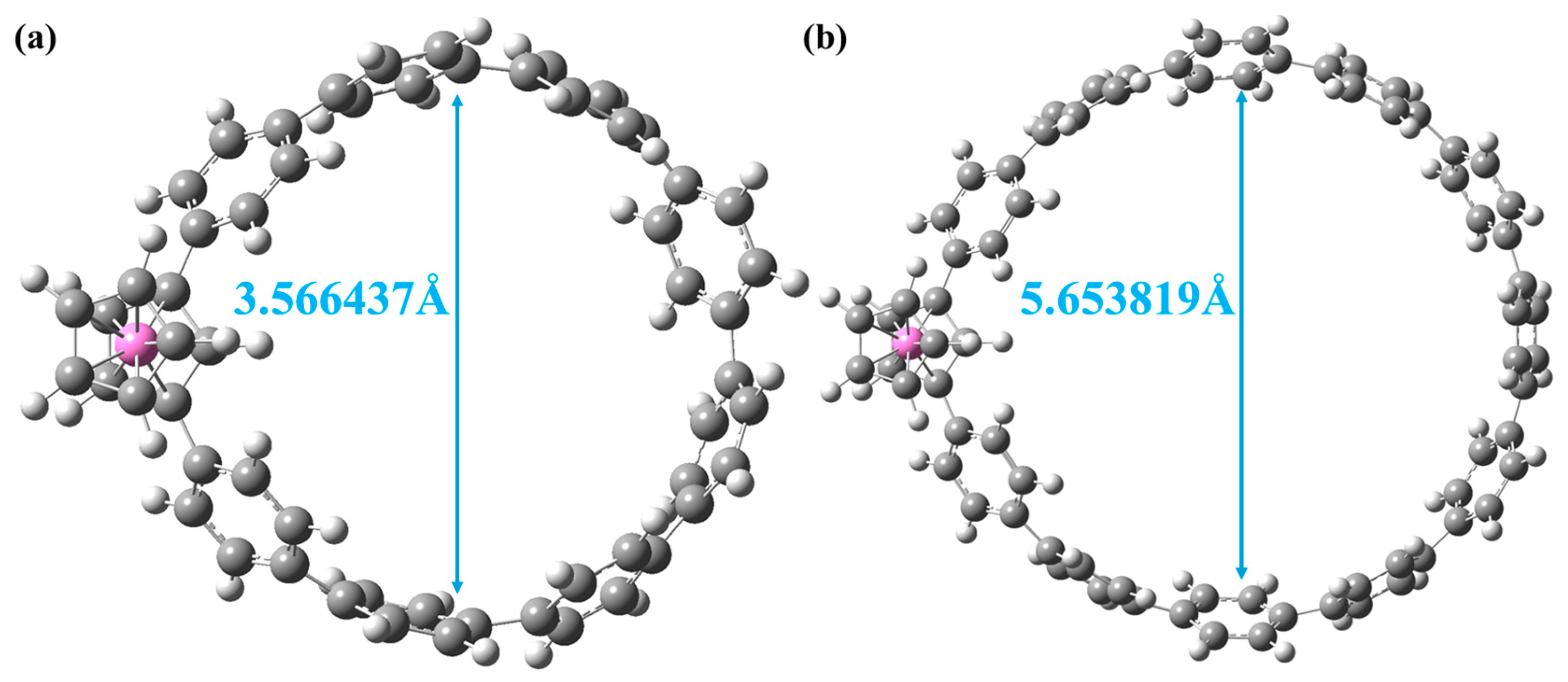

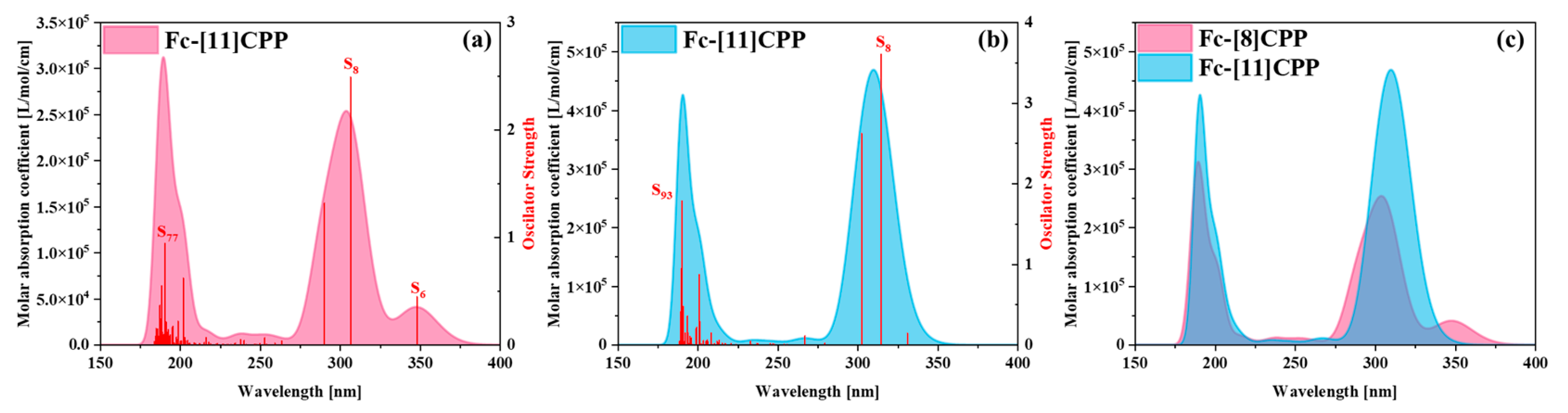


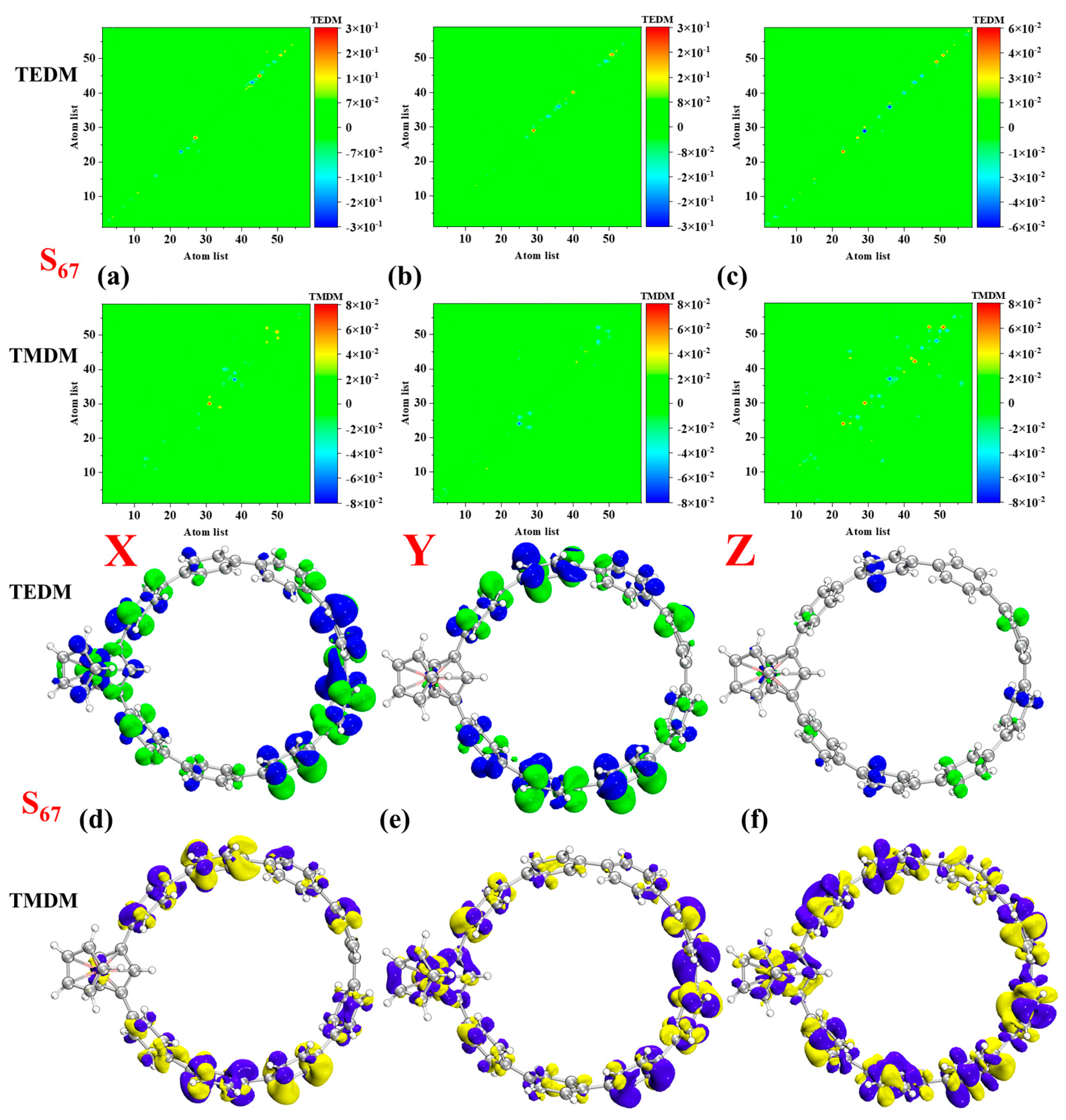
| Molecule | Excited States | Oscillator Strength | Excited Energy (eV) | H(Å) | D(Å) | t (Å) | Sr |
|---|---|---|---|---|---|---|---|
| Fc-[8]CPP | S8 | 2.4957 | 4.048 | 6.191 | 0.223 | −3.922 | 0.70482 |
| Fc-[8]CPP | S77 | 0.9466 | 6.510 | 6.449 | 0.830 | −3.607 | 0.27158 |
| Fc-[11]CPP | S8 | 3.6081 | 3.945 | 8.216 | 0.060 | −4.490 | 0.73501 |
| Fc-[11]CPP | S93 | 1.7844 | 6.526 | 8.371 | 0.092 | −5.180 | 0.34518 |
| Molecule | State | Process | Integral Value (Debye) |
|---|---|---|---|
| Fc-[8]CPP | S6 | 0.016 × 0.038 | |
| Fc-[8]CPP | S8 | 4.473 × 139.474 | |
| Fc-[8]CPP | S46 | 21.156 × 536.952 | |
| Fc-[11]CPP | S8 | 1.492 × 0.299 | |
| Fc-[11]CPP | S50 | 31.987 × 861.072 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Wang, Q.; Zou, Y.; Jin, Y.; Wang, J. Physical Mechanisms of Linear and Nonlinear Optical Responses in Ferrocene-Embedded Cycloparaphenylenes. Chemistry 2025, 7, 136. https://doi.org/10.3390/chemistry7050136
Zhang G, Wang Q, Zou Y, Jin Y, Wang J. Physical Mechanisms of Linear and Nonlinear Optical Responses in Ferrocene-Embedded Cycloparaphenylenes. Chemistry. 2025; 7(5):136. https://doi.org/10.3390/chemistry7050136
Chicago/Turabian StyleZhang, Gang, Qianqian Wang, Yi Zou, Ying Jin, and Jingang Wang. 2025. "Physical Mechanisms of Linear and Nonlinear Optical Responses in Ferrocene-Embedded Cycloparaphenylenes" Chemistry 7, no. 5: 136. https://doi.org/10.3390/chemistry7050136
APA StyleZhang, G., Wang, Q., Zou, Y., Jin, Y., & Wang, J. (2025). Physical Mechanisms of Linear and Nonlinear Optical Responses in Ferrocene-Embedded Cycloparaphenylenes. Chemistry, 7(5), 136. https://doi.org/10.3390/chemistry7050136







