Desulfurative Acetoxylation of Alkyl Benzyl Phenyl Sulfides
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental
2.2. Materials
2.3. General Procedures for Desulfurative Acetoxylation of Alkyl Phenyl Sulfides (GP1)

2.4. General Procedures for Desulfurative Acetoxylation of Alkyl Phenyl Sulfides (GP2)
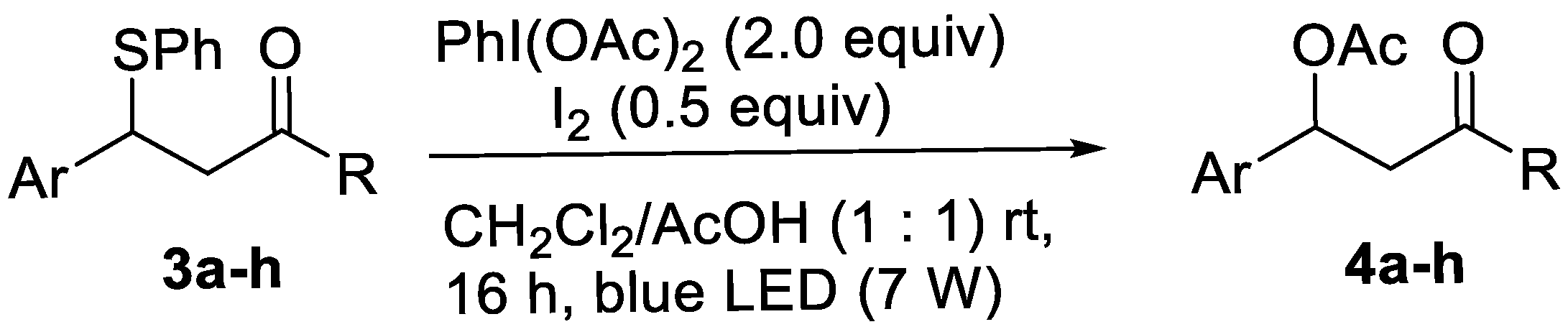
2.5. Analytical Data of Compounds Obtained
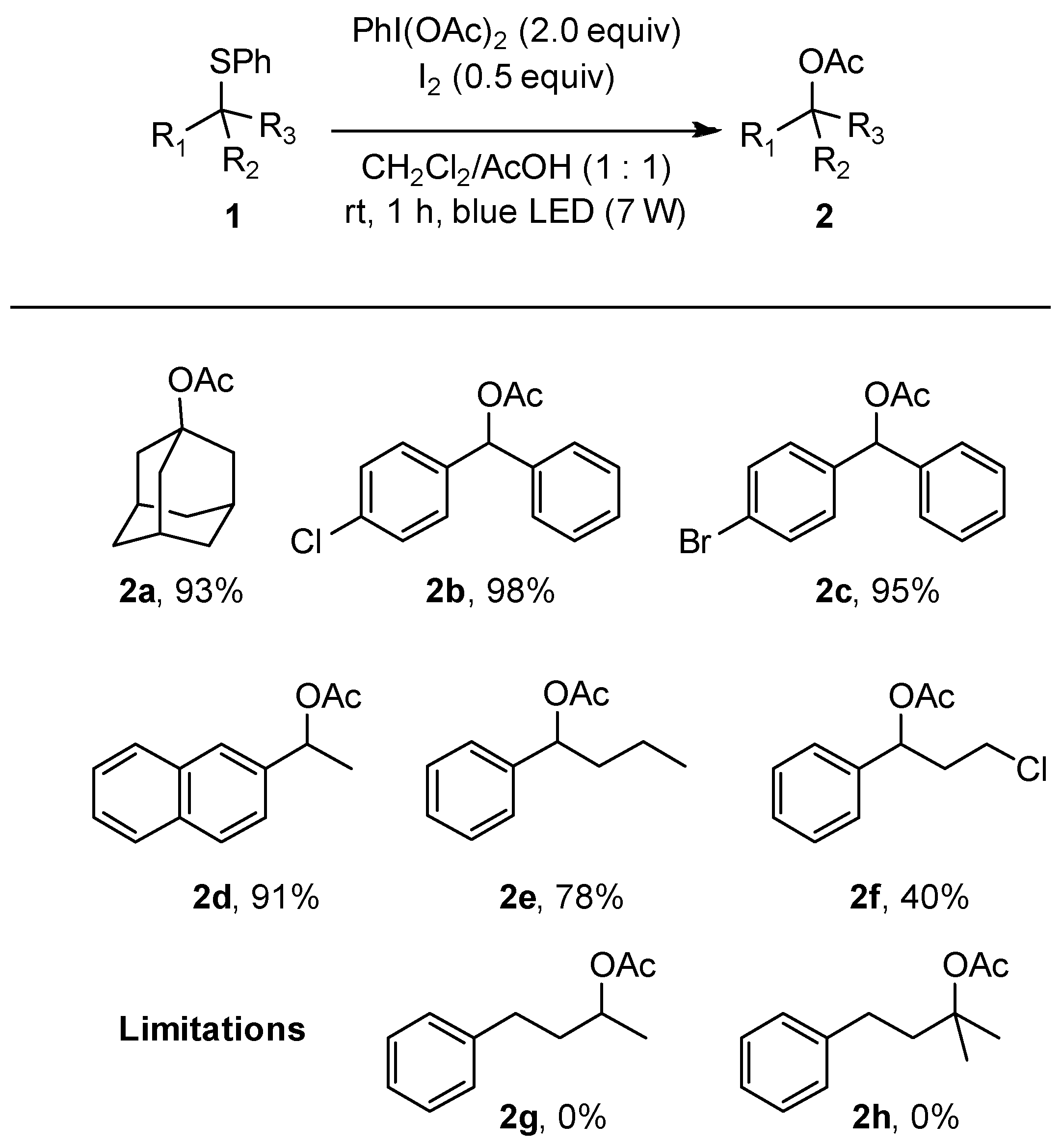
2.5.1. 1-Acetoxyadamantane 2a
2.5.2. (4-Chlorophenyl)(phenyl)methyl Acetate 2b
2.5.3. (4-Bromophenyl)(phenyl)methyl Acetate 2c
2.5.4. 1-(Naphthalen-2-yl)ethyl Acetate 2d
2.5.5. 1-Phenylbutyl Acetate 2e
2.5.6. 3-Chloro-1-phenylpropyl Acetate 2f
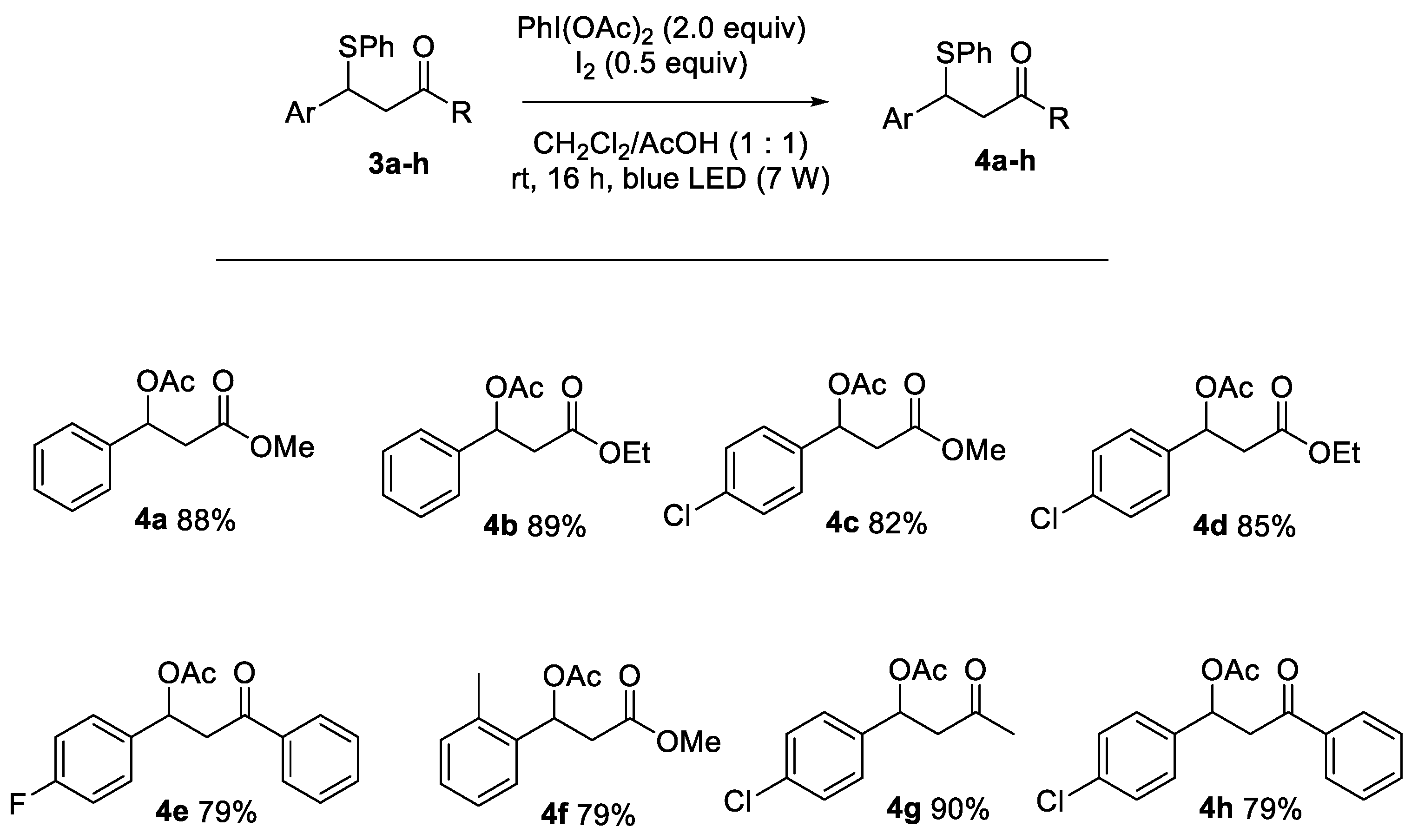
2.5.7. Methyl 3-Acetoxy-3-phenylpropanoate 4a
2.5.8. Ethyl 3-Acetoxy-3-phenylpropanoate 4b
2.5.9. Methyl 3-Acetoxy-3-(4-chlorophenyl)propanoate 4c
2.5.10. Ethyl 3-Acetoxy-3-(4-chlorophenyl)propanoate 4d
2.5.11. 1-(4-Fluorophenyl)-3-oxo-3-phenylpropyl Acetate 4e
2.5.12. Methyl-3-acetoxy-3-(o-tolyl)propanoate 4f
2.5.13. 1-(4-Chlorophenyl)-3-oxobutyl Acetate 4g
2.5.14. 1-(4-Chlorophenyl)-3-oxo-3-phenylpropyl Acetate 4h
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Grover, J.; Dutta, B.; Ghosh, D.; Shee, P.K.; Maiti, S.; Werz, D.B.; Maiti, D. Harnessing C-H acetoxylation: Agateway to oxygen-enriched organic frameworks. Chem. Sci. 2025, 16, 10141–10158. [Google Scholar] [CrossRef]
- Xu, Q.; Gu, X.; Liu, S.; Dou, Q.; Shi, M. The Use of Chiral BINAM NHC-Rh(III) Complexes in Enantioselective Hydrosilylation of 3-Oxo-3-Arylpropionic Acid Methyl or Ethyl Esters. J. Org. Chem. 2007, 72, 2240–2242. [Google Scholar] [CrossRef]
- Zeror, S.; Collin, J.; Fiaud, J.C.; Zouioueche, L.A. Enantioselective Ketoester Reductions in Water: A Comparison between Microorganism-and Ruthenium-Catalyzed Reactions. Tetrahedron Asymmetry 2010, 21, 1211–1215. [Google Scholar] [CrossRef]
- Mahmoodi, N.O.; Tajik, H.; Tabatabaeian, K.; Shahbazi, M. The Enantioselective β-Keto Ester Reductions by Saccharomyces Cerevisiae. J. Serbian Chem. Soc. 2006, 71, 889–894. [Google Scholar] [CrossRef]
- Ju, L.; Yao, J.; Wu, Z.; Liu, Z.; Zhang, Y. Palladium-Catalyzed Oxidative Acetoxylation of Benzylic C-H Bond Using Bidentate Auxiliary. J. Org. Chem. 2013, 78, 10821–10831. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.V.S.; Reddy, L.R.; Corey, E.J. Novel Acetoxylation and C-C Coupling Reactions at Unactivated Positions in α-Amino Acid Derivatives. Org. Lett. 2006, 8, 3391–3394. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.R.; Hull, K.L.; Sanford, M.S. A Highly Selective Catalytic Method for the Oxidative Functionalization of C-H Bonds. J. Am. Chem. Soc. 2004, 126, 2300–2301. [Google Scholar] [CrossRef]
- Guy, A.; Lemor, A.; Imbert, D.; Lemaire, M. Stereoselective Acetoxylation of Chiral Phenylacetic Esters. Tetrahedron Lett. 1989, 30, 327–330. [Google Scholar] [CrossRef]
- Baba, H.; Moriyama, K.; Togo, H. Benzylic-Acetoxylation of Alkylbenzenes with PhI(OAc)2 in the Presence of Catalytic Amounts of TsNH2 and I2. Tetrahedron Lett. 2011, 52, 4303–4307. [Google Scholar] [CrossRef]
- Concepcion, J.I.; Francisco, C.G.; Freire, R.; Salazar, J.A.; Suarez, E. Iodosobenzene Diacetate, an Efficient Reagent for the Oxidative Decarboxylation of Carboxylic Acids. J. Org. Chem. 1986, 51, 402–404. [Google Scholar] [CrossRef]
- Kiyokawa, K.; Okumatsu, D.; Minakata, S. Hypervalent Iodine(III)-Mediated Decarboxylative Acetoxylation at Tertiary and Benzylic Carbon Centers. Beilstein J. Org. Chem. 2018, 14, 1046–1050. [Google Scholar] [CrossRef]
- Baldwin, J.E.; Adlington, R.M.; Russell, A.T.; Smith, M.L. Synthesis of a Biologically Active Analogue of Antibiotic A-32390A. J. Chem. Soc. Chem. Commun. 1994, 1, 85–86. [Google Scholar] [CrossRef]
- Shimada, H.; Kikuchi, S.; Okuda, S.; Haraguchi, K.; Tanaka, H. Nucleophilic substitution approach to 4′-substituted thymidines by employing 4′-benzenesulfonyl leaving group. Tetrahedron 2009, 65, 6008–6016. [Google Scholar] [CrossRef]
- Canestrari, D.; Lancianesi, S.; Badiola, E.; Strinna, C.; Ibrahim, H.; Adamo, M.F.A. Desulfurative Chlorination of Alkyl Phenyl Sulfides. Org. Lett. 2017, 19, 918–921. [Google Scholar] [CrossRef]
- Canestrari, D.; Cioffi, C.; Biancofiore, I.; Lancianesi, S.; Ghisu, L.; Ruether, M.; O’Brien, J.; Adamo, M.F.A.; Ibrahim, H. Sulphide as a Leaving Group: Highly Stereoselective Bromination of Alkyl Phenyl Sulphides. Chem. Sci. 2019, 10, 9042–9050. [Google Scholar] [CrossRef] [PubMed]
- Alletto, F.; Adamo, M.F.A. Enantiospecific On-Water Bromination: A Mild and Efficient Protocol for the Preparation of Alkyl Bromides. Green Chem. 2020, 22, 8692–8698. [Google Scholar] [CrossRef]
- Pineda, A.; Carr, J.; Rodriguez-Padron, D.; Lazaro Ronco, N.; Fox, K.; Gonzales-Arellano, C.; Gillick-Healy, M.W.; Kelly, B.G.; Adamo, M.F.A.; Luque, R. A continuous flow approach for the desulfurative bromination of sulfides. Sustain. Chem. Pharm. 2024, 38, 101490–101495. [Google Scholar] [CrossRef]
- Kwart, H.R.; Miller, K. Chlorinolysis of Sulfur-Carbon Bonds in Aryl-Alkyl Sulfides. J. Am. Chem. Soc. 1956, 78, 5008–5011. [Google Scholar] [CrossRef]
- Mori, K.; Takaishi, H. Synthesis of monocerin, an antifungal, insecticidal and phytotoxic heptaketide metabolite of Exserohilum monoceras. Tetrahedron 1989, 45, 1639–1646. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Wang, C.J. Organocatalytic Asymmetric Sulfa-Michael Addition of Thiols to α,β-Unsaturated Hexafluoroisopropyl Esters: Expeditious Access to (R)-Thiazesim. Org. Lett. 2013, 15, 3448–3451. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, Q.; Liu, Y.; Wang, Q. 4-(N,N-Dimethylamino)Pyridine Hydrochloride as a Recyclable Catalyst for Acylation of Inert Alcohols: Substrate Scope and Reaction Mechanism. Org. Lett. 2014, 16, 236–239. [Google Scholar] [CrossRef]
- O’Neill, M.; Beecher, D.; Mangan, D.; Rowan, A.S.; Monte, A.; Sroka, S.; Modregger, J.; Hundle, B.; Moody, T.S. A Novel Lipase Enzyme Panel Exhibiting Superior Activity and Selectivity over Lipase B from Candida Antarctica for the Kinetic Resolution of Secondary Alcohols. Tetrahedron Asymmetry 2012, 23, 583–586. [Google Scholar] [CrossRef]
- Engström, K.; Vallin, M.; Hult, K.; Bäckvall, J.E. Kinetic Resolution of Diarylmethanols Using a Mutated Variant of Lipase CALB. Tetrahedron 2012, 68, 7613–7618. [Google Scholar] [CrossRef]
- Kumar, R.; Shard, A.; Bharti, R.; Thopate, Y.; Sinha, A.K. Palladium-Catalyzed Dehydrative Heck Olefination of Secondary Aryl Alcohols in Ionic Liquids: Towards a Waste-Free Strategy for Tandem Synthesis of Stilbenoids. Angew. Chem. Int. Ed. 2012, 51, 2636–2639. [Google Scholar] [CrossRef]
- Kim, H.; Choi, Y.K.; Lee, J.; Lee, E.; Park, J.; Kim, M.J. Ionic-Surfactant-Coated Burkholderia Cepacia Lipase as a Highly Active and Enantioselective Catalyst for the Dynamic Kinetic Resolution of Secondary Alcohols. Angew. Chem. Int. Ed. 2011, 50, 10944–10948. [Google Scholar] [CrossRef]
- Rodriguez, A.; Moran, W.J. Palladium-Catalyzed Three-Component Coupling Reactions: 1,1-Difunctionalization of Activated Alkenes. Eur. J. Org. Chem. 2009, 2009, 1313–1316. [Google Scholar] [CrossRef]
- Brem, J.; Liljeblad, A.; Paizs, C.; Toşa, M.I.; Irimie, F.D.; Kanerva, L.T. Lipases A and B from Candida Antarctica in the Enantioselective Acylation of Ethyl 3-Heteroaryl-3-Hydroxypropanoates: Aspects on the Preparation and Enantiopreference. Tetrahedron Asymmetry 2011, 22, 315–322. [Google Scholar] [CrossRef]
- Chen, X.S.; Hou, C.J.; Qin, C.; Liu, H.; Liu, Y.J.; Huang, D.Z.; Hu, X.P. Ir-Catalyzed Asymmetric Hydrogenation of β-Keto Esters with Chiral Ferrocenyl P,N,N-Ligands. RSC Adv. 2017, 7, 12871–12875. [Google Scholar] [CrossRef]
- Murakami, M.; Mukaiyama, T. A new method for the generation of a boron enolate of an ester, a new synthesis of 2-deoxy-D-ribose. Chem. Lett. 1982, 11, 241–244. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Shimamura, T.; Iida, D.; Matsuo, J.I.; Mukaiyama, T. Aldol Reaction of Enol Esters Catalyzed by Cationic Species Paired with Tetrakis(Pentafluorophenyl)Borate. Chem. Pharm. Bull. 2000, 48, 1838–1840. [Google Scholar] [CrossRef] [PubMed]
- Foley, D.A.; Doecke, C.W.; Buser, J.Y.; Merritt, J.M.; Murphy, L.; Kissane, M.; Collins, S.G.; Maguire, A.R.; Kaerner, A. ReactNMR and ReactIR as Reaction Monitoring and Mechanistic Elucidation Tools: The NCS Mediated Cascade Reaction of α-Thioamides to α-Thio-β-chloroacrylamides. J. Org. Chem. 2011, 76, 9360–9640. [Google Scholar] [CrossRef] [PubMed]
- Kiyokawa, K.; Watanabe, T.; Fra, L.; Kojima, T.; Minakata, S. Hypervalent Iodine(III)-Mediated Decarboxylative Ritter-Type Amination Leading to the Production of α-Tertiary Amine Derivatives. J. Org. Chem. 2017, 82, 11711–11720. [Google Scholar] [CrossRef]
- Borowiecki, P.; Bretner, M. Studies on the chemoenzymatic synthesis of (R-) and (S-) methyl 3-aryl-3-hydroxyproprionates: The influence of toluene-pretreatment of lipase preparations on enantioselective transesterification. Tetrahedron Asymmetry 2013, 24, 925–936. [Google Scholar] [CrossRef]
- Nagai, H.; Morita, Y.; Shimizu, Y.; Kanai, M. Ligand-Promoted, Boron-Mediated Chemoselective Carboxylic Acid Aldol Reaction. Org. Lett. 2016, 18, 2276–2279. [Google Scholar] [CrossRef] [PubMed]
- Varala, R.; Seema, V.; Dubasi, N. Phenyliodine(III)diacetate (PIDA): Applications in organic synthesis. Organics 2023, 4, 1–40. [Google Scholar] [CrossRef]
- Kang, B.; Wei, L.; Jiang, H.; Qi, C. Cyclic Diphenylchloronium-Salt-Triggered Coupling of Sulfides with Nucleophiles: Modular Assembly of Pharmaceuticals. Org. Lett. 2025, 27, 3655–3660. [Google Scholar] [CrossRef] [PubMed]

 | |||
|---|---|---|---|
| Entry | Oxidant | Solvent | 1a:2a:3a b |
| 1 | PIDA | CH2Cl2 | 40:60:0 |
| 2 | PIDA | CH2Cl2/AcOH (1:1) | 0:100:0 |
| 3 c | PIDA | CH2Cl2/AcOH (1:1) | 50:50:0 |
| 4 d | PIDA | CH2Cl2/AcOH (1:1) | 40:60:0 |
| 5 | – | CH2Cl2/AcOH (1:1) | 100:0:0 |
 | ||||
|---|---|---|---|---|
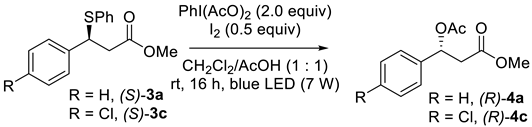
| Entry | Substrate | Conversion of 3 | ee of 4 b | er |
| 1 | (S) -3a | 100 | 18% | 59:41 |
| 2 | (S) -3c | 100 | 28% | 64:36 |
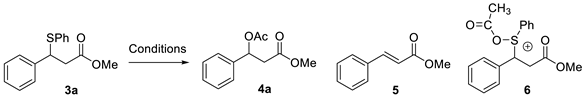 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | PIDA | I2 | Light | Time | 3a | 4a | 5 | 6 |
| 1 | Yes | Yes | Yes | 1 h | 0% | 37% | 6% | 43% |
| 2 | Yes | No | No | 4 h | 88% | 0% | 12% | 0% |
| 3 | Yes | No | Yes | 4 h | 63% | 4% | 21% | 12% |
| 4 | Yes | Yes | No | 2 h | 0% | 58% | 15% | 21% |
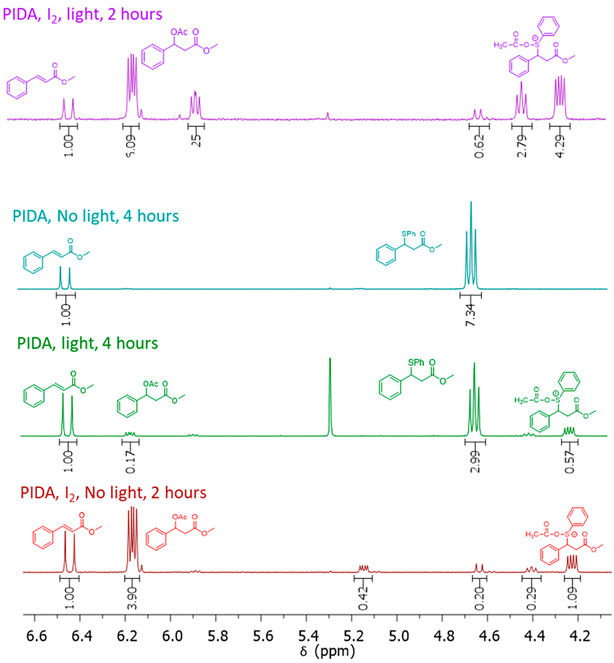 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canestrari, D.; Boddu, U.R.; Pallikonda, G.; Adamo, M.F.A. Desulfurative Acetoxylation of Alkyl Benzyl Phenyl Sulfides. Chemistry 2025, 7, 131. https://doi.org/10.3390/chemistry7040131
Canestrari D, Boddu UR, Pallikonda G, Adamo MFA. Desulfurative Acetoxylation of Alkyl Benzyl Phenyl Sulfides. Chemistry. 2025; 7(4):131. https://doi.org/10.3390/chemistry7040131
Chicago/Turabian StyleCanestrari, Daniele, Umamaheswara Rao Boddu, Gangaram Pallikonda, and Mauro F. A. Adamo. 2025. "Desulfurative Acetoxylation of Alkyl Benzyl Phenyl Sulfides" Chemistry 7, no. 4: 131. https://doi.org/10.3390/chemistry7040131
APA StyleCanestrari, D., Boddu, U. R., Pallikonda, G., & Adamo, M. F. A. (2025). Desulfurative Acetoxylation of Alkyl Benzyl Phenyl Sulfides. Chemistry, 7(4), 131. https://doi.org/10.3390/chemistry7040131






