One-Pot Synthesis of Thiochromen-4-ones from 3-(Arylthio)propanoic Acids
Abstract
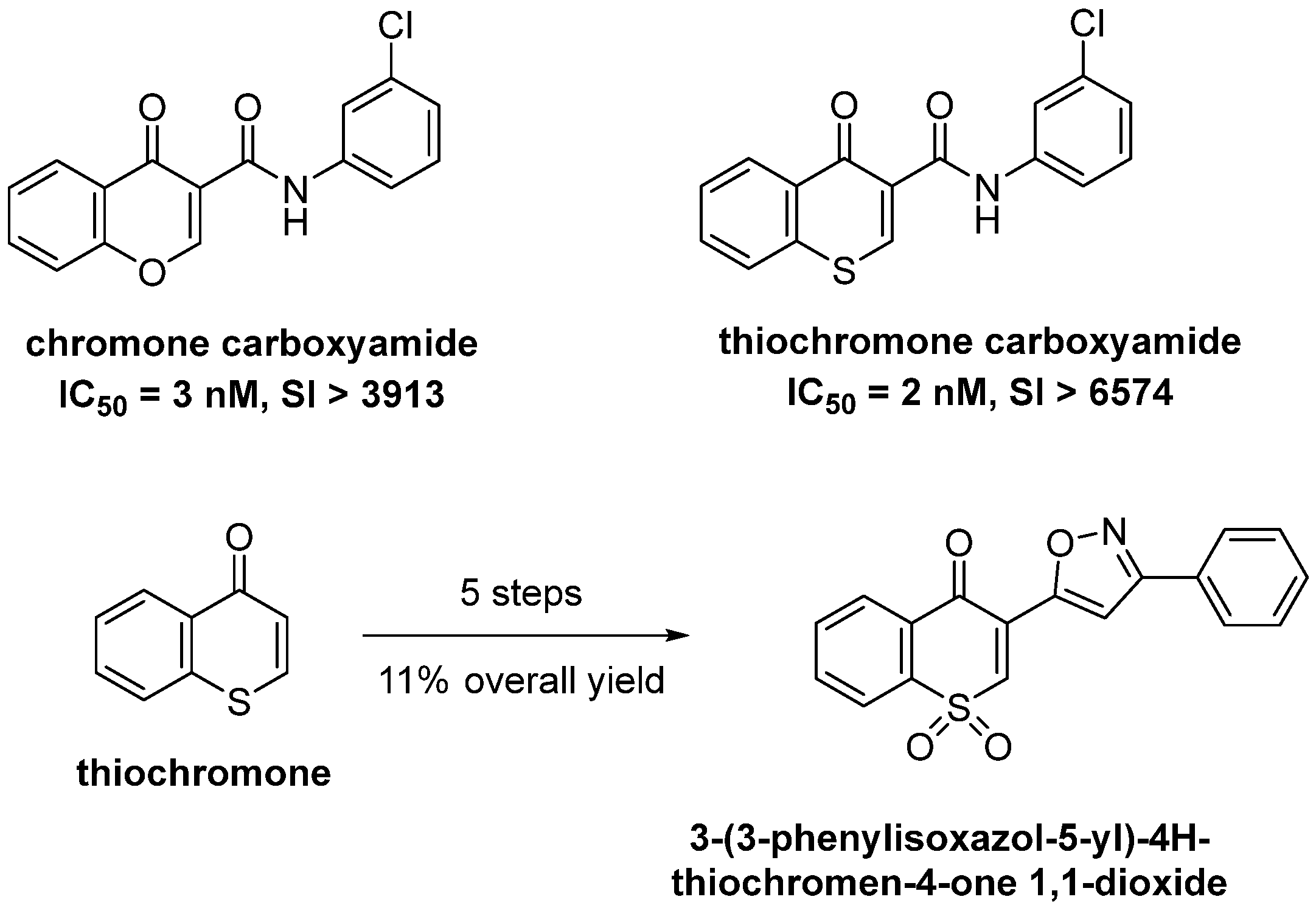
1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Materials
2.3. Preparation of Starting 3-(Arylthio)propanoic Acids 1
2.4. General Procedures
General Procedure A
2.5. Synthesis
2.5.1. Synthesis of 6-Methoxyl-4H-Thiochromen-4-one (3a)
2.5.2. Synthesis of 8-Methylthiochromen-4-one (3d)
2.5.3. Synthesis of 6,8-Dimethylthiochromen-4-one (3f)
2.5.4. Synthesis of 8-Isopropylthiochromen-4-one (3g)
2.5.5. Synthesis of 8-Methoxylthiochromen-4-one (3m)
2.5.6. Synthesis of 4H-Thiochromen-4-one (3b)
2.5.7. Synthesis of 6-Methylthiochromen-4-one (3c)
2.5.8. Synthesis of 6,7-Dimethylthiochromen-4-one (3e)
2.5.9. Synthesis of 6-tert-Butylthiochromen-4-one (3h)
2.5.10. Synthesis of 6-Fluorothiochromen-4-one (3i)
2.5.11. Synthesis of 6-Bromothiochromen-4-one (3j)
2.5.12. Synthesis of 6-Chlorothiochromen-4-one (3k)
2.5.13. Synthesis of 6-(Trifluoromethyl)thiochromen-4-one (3l)
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, M.; Dias, T.A.; Brito, A.; Proença, F. Biological importance of structurally diversified chromenes. Eur. J. Med. Chem. 2016, 123, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Budagumpi, S.; Pai, R.K.; Balakrishna, R.G. Chromones as a privileged scaffold in drug discovery: A review. Eur. J. Med. Chem. 2014, 78, 340–374. [Google Scholar] [CrossRef] [PubMed]
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Csepanyi, E.; Szabados-Furjesi, P.; Kiss-Szikszai, A.; Frensemeier, L.M.; Karst, U.; Lekli, I.; Haines, D.D.; Tosaki, A.; Bak, I. Antioxidant properties and oxidative transformation of different chromone derivatives. Molecules 2017, 22, 588. [Google Scholar] [CrossRef]
- Presley, C.C.; Valenciano, A.L.; Fernández-Murga, M.L.; Du, Y.; Shanaiah, N.; Cassera, M.B.; Goetz, M.; Clement, J.A.; Kingston, D.G.I. Antiplasmodial chromanes and chromenes from the monotypic plant species Koeberlinia spinosa. J. Nat. Prod. 2018, 81, 475–483. [Google Scholar] [CrossRef]
- Payen, L.; Honorat, M.; Guitton, J.; Gauthier, C.; Bouard, C.; Lecerf-Schmidt, F.; Peres, B.; Terreux, R.; Gervot, H.; Rioufol, C.; et al. MBL-II-141, a chromone derivative, enhances irinotecan (CPT-11) anticancer efficiency in ABCG2-positive xenografts. Oncotarget 2014, 5, 11957–11970. [Google Scholar]
- China Raju, B.; Nageswara Rao, R.; Suman, P.; Yogeeswari, P.; Sriram, D.; Shaik, T.B.; Kalivendi, S.V. Synthesis, structure-activity relationship of novel substituted 4H-chromen-1,2,3,4-tetrahydropyrimidine-5-carboxylates as potential anti-mycobacterial and anticancer agents. Bioorg. Med. Chem. Lett. 2011, 21, 2855–2859. [Google Scholar] [CrossRef]
- Shaw, A.Y.; Chang, C.Y.; Liau, H.H.; Lu, P.J.; Chen, H.L.; Yang, C.N.; Li, H.Y. Synthesis of 2-styrylchromones as a novel class of antiproliferative agents targeting carcinoma cells. Eur. J. Med. Chem. 2009, 44, 2552–2562. [Google Scholar] [CrossRef]
- Li, N.-G.; Shi, Z.-H.; Tang, Y.-P.; Ma, H.-Y.; Yang, J.-P.; Li, B.-Q.; Wang, Z.-J.; Song, S.-L.; Duan, J.-A. Synthetic strategies in the construction of chromones. J. Heterocycl. Chem. 2010, 47, 785–799. [Google Scholar] [CrossRef]
- Sosnovskikh, V.Y. Synthesis and chemical properties of thiochromone and its 3-subsituted derivatives. Chem. Heterocycl. Compd. 2016, 52, 427–440. [Google Scholar] [CrossRef]
- Wang, H.-K.; Bastow, K.F.; Cosentino, L.M.; Lee, K.-H. Antitumor Agents. 166. Synthesis and Biological Evaluation of 5,6,7,8-Substituted-2-phenylthiochromen-4-ones. J. Med. Chem. 1996, 39, 1975. [Google Scholar]
- Schneller, S.W. Recent Developments in the Chemistry of Chromenes, Chromanones, and Chromones. Adv. Heterocycl. Chem. 1975, 18, 79. [Google Scholar]
- Guo, F.; Young, J.A.; Perez, M.S.; Hankerson, H.A.; Chavez, A.M. Progress on the Cu-Catalyzed 1,4-Conjugate Addition to Thiochromones. Catalysts 2023, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Mi, P.; Tan, Y.; Ye, S.; Lang, J.-J.; Lv, Y.; Jiang, J.; Chen, L.; Luo, J.; Lin, Y.; Yuan, Z.; et al. Discovery of C-3 isoxazole substituted thiochromone S,S-dioxide derivatives as potent and selective inhibitors for monoamine oxidase B (MAO-B). Eur. J. Med. Chem. 2024, 263, 115956. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, T.J.; Harvin, T.; Pickens-Flynn, T.; Austin, N.; Whitaker, S.H.; Tang Yuk Tutein, M.L.C.; Hukins, D.T.; Deese, N.; Guo, F. Conjugate Addition of Grignard Reagents to Thiochromones Catalyzed by Copper Salts: A Unified Approach to Both 2-Alkylthiochroman-4-One and Thioflavanone. Molecules 2020, 25, 2128. [Google Scholar] [CrossRef]
- In Lee, J. Synthetic Approaches to 2-Alkylthiochroman-4-ones and Thioflavanones. Bull. Korean Chem. Soc. 2021, 42, 852–862. [Google Scholar] [CrossRef]
- Bass, S.A.; Parker, D.M.; Bellinger, T.J.; Eaton, A.S.; Dibble, A.S.; Koroma, K.L.; Sekyi, S.A.; Pollard, D.A.; Guo, F. Development of Conjugate Addition of Lithium Dialkylcuprates to Thiochromones: Synthesis of 2-Alkylthiochroman-4-ones and Additional Synthetic Applications. Molecules 2018, 23, 1728. [Google Scholar] [CrossRef]
- Guo, F.; Jeffries, M.C.; Graves, B.N.; Graham, S.A.; Pollard, D.A.; Pang, G.; Chen, H.Y. A rapid entry into thioflavanones via conjugate additions of diarylcuprates to thiochromones. Tetrahedron 2017, 73, 5745–5750. [Google Scholar] [CrossRef]
- Choi, E.J.; Lee, J.I.; Kim, G.H. Evaluation of the anticancer activities of thioflavanone and thioflavone in human breast cancer cell lines. Int. J. Mol. Med. 2012, 29, 252–256. [Google Scholar]
- Song, Y.-L.; Wu, F.; Zhang, C.-C.; Liang, G.-C.; Zhou, G.; Yu, J.-J. Ionic liquid catalyzed synthesis of 2-(indole-3-yl)-thiochroman-4- ones and their novel antifungal activities. Bioorg. Med. Chem. Lett. 2015, 25, 259–261. [Google Scholar] [CrossRef]
- Vargas, E.; Echeverri, F.; Vélez, I.D.; Robledo, S.M.; Quiňones, W. Synthesis and Evaluation of Thiochroman-4-One Derivatives as Potential Leishmanicidal Agents. Molecules 2017, 22, 2041. [Google Scholar] [CrossRef]
- Nakazumi, H.; Endo, T.; Nakaue, T.; Kitao, T. Synthesis of 4,10-dihydro-4,10-dioxo-1H[1]benzothiopyrano[3,2-B]pyridine and 7-oxo-7,13-dihydro[1]benzothiopyrano[2,3-b]-1,5-benzodiazepine. J. Heterocycl. Chem. 1985, 22, 89. [Google Scholar] [CrossRef]
- Willy, B.; Müller, T.J.J. A novel consecutive three-component Coupling-Addition-SNAr (CASNAR) synthesis of 4H-thiochromen-4-ones. Syn. Lett. 2009, 8, 1255–1260. [Google Scholar] [CrossRef]
- Willy, B.; Frank, W.; Müller, T.J.J. Microwave-assisted three-component coupling-addition-SNAr (CASNAR) sequences to annelated 4H-thiopyran-4-ones. Org. Biomol. Chem. 2010, 8, 90. [Google Scholar] [CrossRef]
- Palani, T.; Park, K.; Song, K.H.; Lee, S. Palladium-catalyzed synthesis of (Z)-3-arylthioacrylic acids and thiochromenones. Adv. Synth. Catal. 2013, 355, 1160. [Google Scholar] [CrossRef]
- Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar]
- Giles, P.R.; Marson, C.M. On the Reaction of Thiochroman-4-one and 3-(Hydroxymethylene)thiochroman-4-one with N-chlorosuccinimide. Aust. J. Chem. 1992, 45, 439–443. [Google Scholar]
- Jia, W.Y.-J.; Li, W.; Liu, Y.; Zhang, D.-J.; Zhang, P.; Gong, P. Synthesis and in vitro anti-hepatitis B virus activity of 6H-[1]benzothiopyrano[4,3-b]quinolin-9-ols. Bioorg. Med. Chem. 2009, 17, 4569–4574. [Google Scholar] [CrossRef] [PubMed]

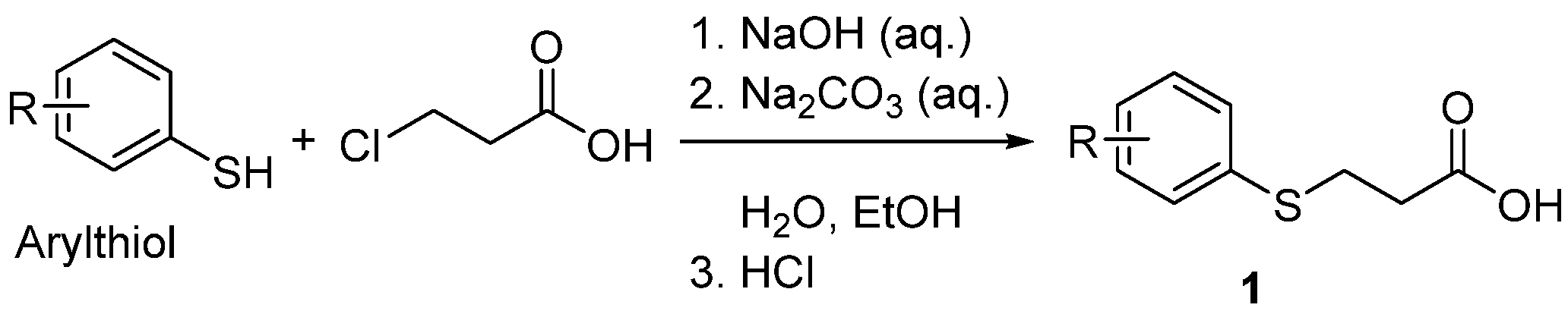

 | ||||||
|---|---|---|---|---|---|---|
| entry | acid a | T (°C) | Time (hr) | 2a yield (%) b | 2b (%) | 3a (%) b |
| 1 | H2SO4 | 0 to RT | 12 | 0 | 45 | 0 |
| 2 | H3PO4 | 0 to RT | 12 | 0 | 40 | 0 |
| 3 | PPA | 0 to RT | 12 | 0 | 0 | |
| 4 | PPA | 50 | 2 | 20 | 0 | |
| 5 | PPA | 50 | 4 | 25 | 0 | |
| 6 | PPA | 50 | 12 | 83 | 0 | |
| 7 | PPA | 100 | 5 | 35 | 30 | |
| 8 | PPA | 100 | 12 | 81 | ||
| 9 | - | 100 | 12 | 0 | ||
| 10 | PPA | 100 | 12 | 79 c | ||
| 11 | PPA | 100 | 12 | 75 d | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simpkins, K.S.; Guo, M.Y.; Smith, T.D.; Hankerson, H.A.; Guo, F. One-Pot Synthesis of Thiochromen-4-ones from 3-(Arylthio)propanoic Acids. Chemistry 2025, 7, 163. https://doi.org/10.3390/chemistry7050163
Simpkins KS, Guo MY, Smith TD, Hankerson HA, Guo F. One-Pot Synthesis of Thiochromen-4-ones from 3-(Arylthio)propanoic Acids. Chemistry. 2025; 7(5):163. https://doi.org/10.3390/chemistry7050163
Chicago/Turabian StyleSimpkins, Kahlia S., Maggie Y. Guo, Toniyah D. Smith, Holden A. Hankerson, and Fenghai Guo. 2025. "One-Pot Synthesis of Thiochromen-4-ones from 3-(Arylthio)propanoic Acids" Chemistry 7, no. 5: 163. https://doi.org/10.3390/chemistry7050163
APA StyleSimpkins, K. S., Guo, M. Y., Smith, T. D., Hankerson, H. A., & Guo, F. (2025). One-Pot Synthesis of Thiochromen-4-ones from 3-(Arylthio)propanoic Acids. Chemistry, 7(5), 163. https://doi.org/10.3390/chemistry7050163






