Visible-Light-Mediated Dehydrogenative Cross-Coupling of Azaarenes and Ethers
Abstract
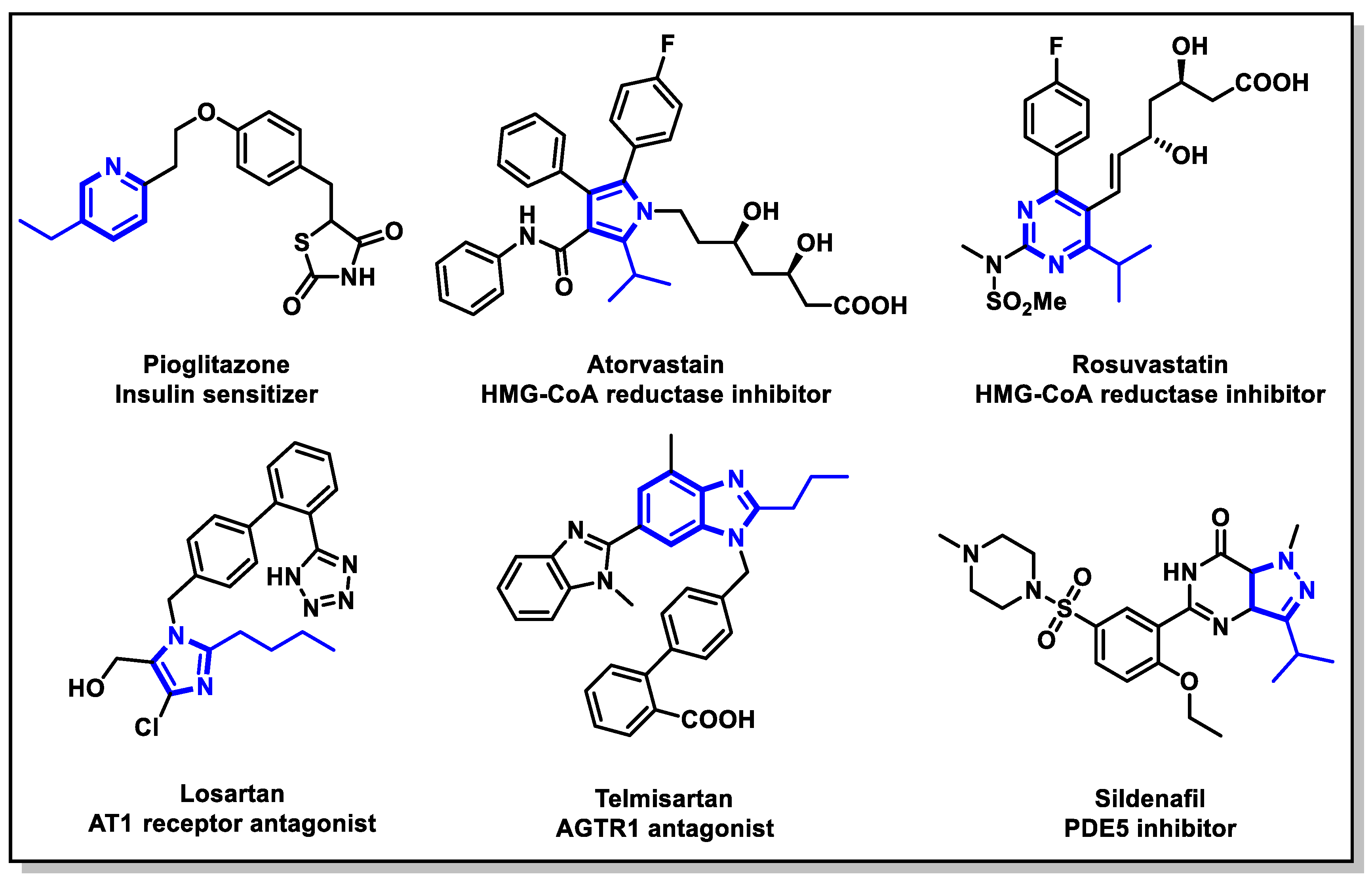
1. Introduction
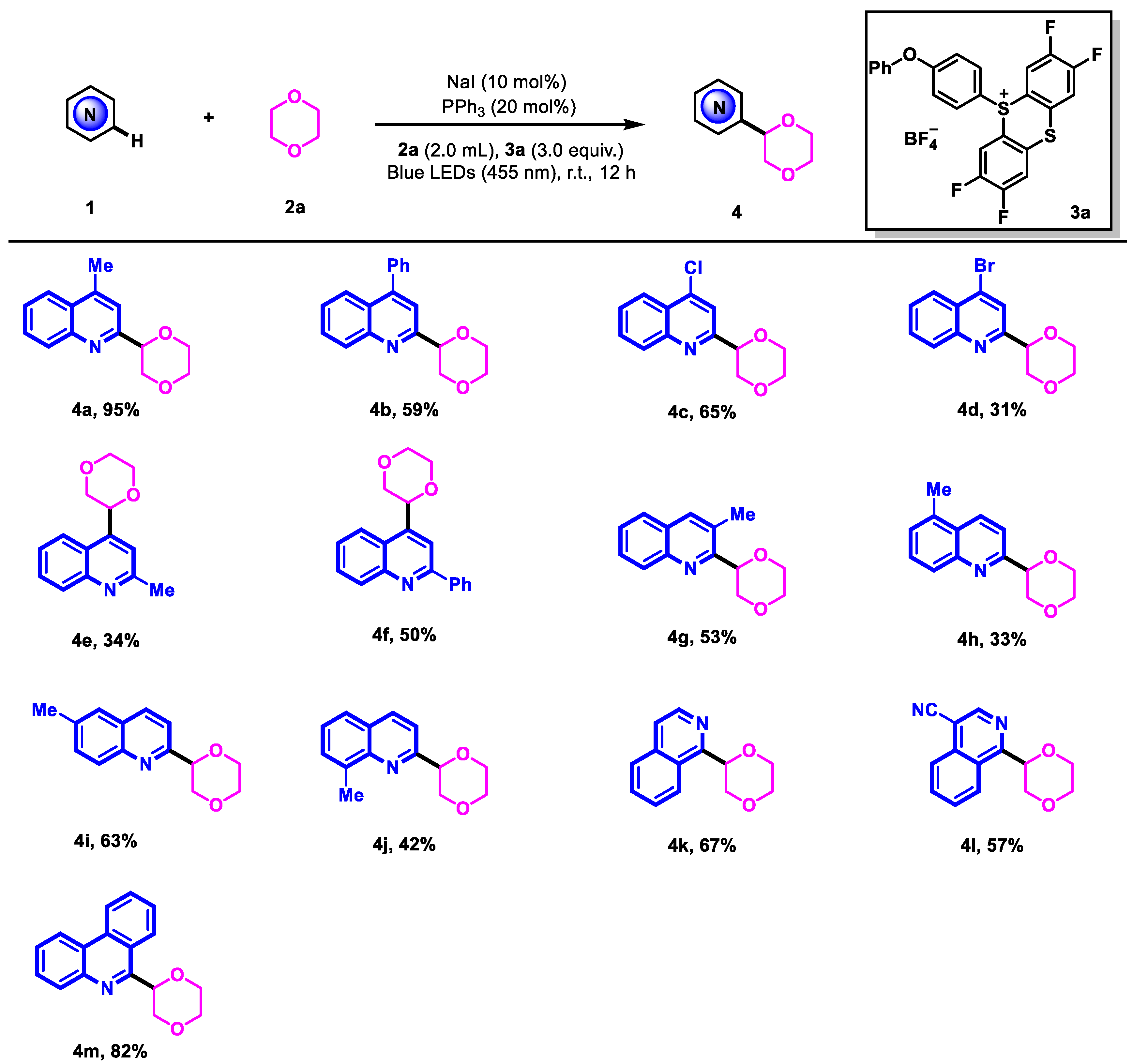
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, X.Y.; Zhao, Q.Q.; Chen, J.; Xiao, W.J.; Chen, J.R. When Light Meets Nitrogen-centered radicals: From reagents to catalysts. Acc. Chem. Res. 2020, 53, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Mcgrath, N.A.; Brichacek, M.; Njardarson, J.T. A Graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 2010, 87, 1348–1349. [Google Scholar] [CrossRef]
- Chan, K.L.; Teo, K.; Dumesnil, J.G. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis. Circulation 2010, 121, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Wysham, C.; Blevins, T.; Arakaki, R.; Colon, G.; Garcia, P.; Atisso, C.; Kuhstoss, D.; Lakshmanan, M. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care 2014, 37, 2159–2167. [Google Scholar] [CrossRef]
- Taylor, A.P.; Robinson, R.P.; Fobian, Y.M.; Blakemore, D.C.; Jones, L.H.; Fadeyi, O. Modern advances in heterocyclic chemistry in drug discovery. Org. Biomol. Chem. 2016, 14, 6611–6637. [Google Scholar] [CrossRef]
- Diez, J.; Querejeta, R.; Lopez, B.; González, A.; Larman, M.; Ubago, J.L.M. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation 2002, 105, 2512–2517. [Google Scholar] [CrossRef]
- Cao, X.; Wei, L.; Yang, J.; Song, H.; Wei, Y. A Visible-light-induced bromine radical initiates direct C–H alkylation of heteroaromatics. Org. Biomol. Chem. 2024, 22, 1157–1161. [Google Scholar] [CrossRef]
- Lu, C.; Histand, G.; Lin, D. Visible light-induced direct alkylation of the purine C8–H bond with ethers. Org. Biomol. Chem. 2023, 21, 3167–3171. [Google Scholar] [CrossRef]
- Liu, X.; Xie, D.; Yang, Q.; Song, Z.; Fu, Y.; Peng, Y. Ag-catalyzed cross-dehydrogenative-coupling for the synthesis of 1,4-dioxan-2-yl substituted quinazoline hybrids in an aqueous medium. Org. Biomol. Chem. 2024, 22, 7725–7735. [Google Scholar] [CrossRef]
- Pan, Z.-T.; Shen, L.-M.; Dagnaw, F.W.; Zhong, J.-J.; Jian, J.-X.; Tong, Q.-X. Minisci reaction of heteroarenes and unactivated C(sp3)-H alkanes via a photogenerated chlorin radical. Chem. Commun. 2023, 59, 1637–1640. [Google Scholar] [CrossRef]
- Xie, Z.; Cai, Y.; Hu, H.; Hu, H.; Lin, C.; Jiang, J.; Chen, Z.; Wang, L.; Pan, Y. Cu-catalyzed cross-dehydrogenative coupling reactions of (benzo)thiazoles with cyclic ethers. Org. Lett. 2013, 15, 4600–4603. [Google Scholar] [CrossRef] [PubMed]
- Correa, A. Iron-catalyzed direct α-arylation of ethers with azoles. Chem. Commun. 2015, 51, 13365–13368. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Li, Z.; Li, C.-J. Cross-dehydrogenative coupling: A sustainable reaction for C−C bond formations. Green Chem. 2021, 23, 6789–6862. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Li, J.; Li, C.-J. A cross-dehydrogenative C(sp3)-H heteroarylation via photo-induced catalytic chlorine radical generation. Nat. Commun. 2021, 12, 4010. [Google Scholar] [CrossRef]
- Antonchick, A.P.; Burgmann, L. Direct selective oxidative cross-coupling of simple alkanes with heteroarenes. Angew. Chem. Int. Ed. 2013, 52, 3267–3271. [Google Scholar] [CrossRef]
- He, T.; Yu, L.; Zhang, L.; Wang, L.; Wang, M. Direct C2-alkylation of azoles with alcohols and ethers through dehydrogenative cross-coupling under metal-dree conditions. Org. Lett. 2011, 13, 5016–5019. [Google Scholar] [CrossRef]
- Jin, L.; Jie, F.; Lu, G.; Cai, C. Di-tert-butyl peroxide (DTBP)-mediated oxidative cross-coupling of isochroman and indole derivatives. Adv. Synth. Catal. 2015, 357, 2105–2109. [Google Scholar] [CrossRef]
- Schlegel, M.; Qian, S.; Nicewicz, D.A. Aliphatic C-H functionalization using pyridine n-oxides as h-atom abstraction agents. ACS Catal. 2022, 12, 10499–10505. [Google Scholar] [CrossRef]
- Li, D.-S.; Liu, T.; Hong, Y.; Cao, C.-L.; Wu, J.; Deng, H.-P. Stop-dlow microtubing reactor-assisted visible light-induced hydrogen-evolution cross coupling of heteroarenes with C(sp3)-H bonds. ACS Catal. 2022, 12, 4473–4480. [Google Scholar] [CrossRef]
- DiRocco, D.A.; Dykstra, K.; Krska, S.; Vachal, P.; Conway, D.V.; Tudge, M. Late-stage functionalization of biologically active heterocycles through photoredox catalysis. Angew. Chem. Int. Ed. 2014, 53, 4802–4806. [Google Scholar] [CrossRef]
- Garza-Sanchez, R.A.; Tlahuext-Aca, A.; Tavakoli, G.; Glorius, F. Visible light-mediated direct decarboxylative C-H functionalization of heteroarenes. ACS Catal. 2017, 7, 4057–4061. [Google Scholar] [CrossRef]
- Gutiérrez-Bonet, Á.; Remeur, C.; Matsui, J.K.; Molander, G.A. Late-stage C-H alkylation of heterocycles and 1,4-quinones via oxidative homolysis of 1,4-dihydropyridines. J. Am. Chem. Soc. 2017, 139, 12251–12258. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.F.; Dixon, J.A.; O’Hara, F.; Funder, E.D.; Dixon, D.D.; Rodriguez, R.A.; Baxter, R.D.; Herlé, B.; Sach, N.; Collins, M.R.; et al. Practical and innate carbon-hydrogen functionalization of heterocycles. Nature 2012, 492, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Liu, Z.J.; Lei, L.; Fu, Y. Palladium-catalyzed C-H activation/cross-coupling of pyridine N-oxides with nonactivated secondary alkyl bromides. J. Am. Chem. Soc. 2013, 135, 616–619. [Google Scholar] [CrossRef]
- Wu, X.; See, J.W.T.; Xu, K.; Hirao, H.; Roger, J.; Hierso, J.-C.; Zhou, J. A general palladium-catalyzed method for alkylation of heteroarenes using secondary and tertiary alkyl halides. Angew. Chem., Int. Ed. 2014, 126, 13791–13795. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Singh, S.; Wu, Y.; Wang, Z.; Hao, W.; Verma, P.; Qiao, J.X.; Sunoj, R.B.; Yu, J.-Q. Pd-catalyzed γ-C(sp3)-H fluorination of free amines. J. Am. Chem. Soc. 2020, 142, 9966–9974. [Google Scholar] [CrossRef]
- Zhou, W.J.; Cao, G.M.; Shen, G.; Zhu, X.-Y.; Gui, Y.-Y.; Ye, J.-H.; Sun, L.; Liao, L.-L.; Li, J.; Yu, D.-G. Visible-light-driven palladium-catalyzed radical alkylation of C-H bonds with unactivated alkyl bromides. Angew. Chem. Int. Ed. 2017, 56, 15683–15687. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.; Li, C.J. Catalyst-free and redox-neutral innate trifluoromethylation and alkylation of aromatics enabled by light. J. Am. Chem. Soc. 2017, 139, 14315–14321. [Google Scholar] [CrossRef]
- Klauck, F.J.R.; James, M.J.; Glorius, F. Deaminative strategy for the visible-light-mediated generation of alkyl radicals. Angew. Chem. Int. Ed. 2017, 56, 12336–12339. [Google Scholar] [CrossRef]
- Li, G.X.; Morales-Rivera, C.A.; Wang, Y.; Gao, F.; He, G.; Liu, P.; Chen, G. Photoredox-mediated Minisci C-H alkylation of N-heteroarenes using boronic acids and hypervalent iodine. Chem. Sci. 2016, 7, 6407–6412. [Google Scholar] [CrossRef]
- Matsui, J.K.; Primer, D.N.; Molander, G.A. Metal-free C-H alkylation of heteroarenes with alkyltrifluoroborates: A general protocol for 1, 2 and 3 alkylation. Chem. Sci. 2017, 8, 3512–3522. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jin, J. Visible light-promoted aliphatic C–H arylation using selectfluor as a hydrogen atom transfer reagent. Org. Lett. 2019, 21, 6179–6184. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Y.; Wang, W. Direct Cα-heteroarylation of structurally diverse ethers via a mild N-hydroxysuccinimide mediated cross-dehydrogenative coupling reaction. Chem. Sci. 2017, 8, 4044–4050. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Li, Y.; Wu, Z.; Zhao, M.; Ji, X.; Liu, P.; Zhang, X. Synthesis of alkyl-substituted pyrazine N-oxides by transition-metal-free oxidative cross-coupling reactions. Asian J. Org. Chem. 2018, 7, 1118–11123. [Google Scholar] [CrossRef]
- Neubert, T.D.; Schmidt, Y.; Conroy, E.; Stamos, D. Radical mediated C-H functionalization of 3,6-dichloropyridazine: Efficient access to novel tetrahydropyridopyridazines. Org. Lett. 2015, 17, 2362–2365. [Google Scholar] [CrossRef]
- Jin, L.-K.; Wan, L.; Feng, J.; Cai, C. Nickel-catalyzed regioselective cross-dehydrogenative coupling of inactive C(sp3)-H bonds with indole derivatives. Org. Lett. 2015, 17, 4726–4729. [Google Scholar] [CrossRef]
- Devari, S.; Shah, B.A. Visible light-promoted C-H functionalization of ethers and electron-deficient arenes. Chem. Commun. 2016, 52, 1490–1493. [Google Scholar] [CrossRef]
- Liu, W.; Yang, X.; Zhou, Z.; Li, C. Simple and clean photo-induced methylation of heteroarenes with MeOH. Chem 2017, 2, 688–702. [Google Scholar] [CrossRef]
- McCallum, T.; Pitre, S.P.; Morin, M.; Scaiano, J.C.; Barriault, L. The photochemical alkylation and reduction of heteroarenes. Chem. Sci. 2017, 8, 7412–7418. [Google Scholar] [CrossRef]
- Ye, L.; Cai, S.H.; Wang, D.-X.; Wang, Y.-Q.; Lai, L.J.; Feng, C.; Loh, T.P. Photoredox catalysis induced bisindolylation of ethers/alcohols via sequential C-H and C-O bond cleavage. Org. Lett. 2017, 19, 6164–6167. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Tang, N.; Wu, Z.; Wang, D.; Ji, M.; Xu, Y.; Wang, M.; Zhu, C. Metal-free alcohol-directed regioselective heteroarylation of remote unactivated C(sp3)-H bonds. Nat. Commun. 2018, 9, 3343–3350. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Maity, P.; Ranu, B.C. Cobalt-catalyzed remote C-4 functionalization of 8-aminoquinoline amides with ethers via C-H activation under visible-light irradiation. Access to α-heteroarylated ether derivatives. Org. Lett. 2018, 20, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-X.; Hu, X.; He, G.; Chen, G. Photoredox-mediated remote C(sp3)-H heteroarylation of free alcohols. Chem. Sci. 2019, 10, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, L.; Huang, S.; Xu, K.; Zeng, C.-C. Electrochemical quinuclidine-mediated Minisci-type acylation of N-heterocycles with aldehydes. Chem. Commun. 2024, 60, 6174–6177. [Google Scholar] [CrossRef]
- Ding, H.; Xu, K.; Zeng, C. Nickel-catalyzed electrochemical Minisci acylation of aromatic N-heterocycles with α-keto acids via ligand-to-metal electron transfer pathway. J. Catal. 2020, 381, 38–43. [Google Scholar] [CrossRef]
- Dou, G.-Y.; Jiang, Y.-Y.; Xu, K.; Zeng, C.-C. Electrochemical Minisci-type trifluoromethylation of electron-deficient heterocycles mediated by bromide ions. Org. Chem. Front. 2019, 6, 2392–2397. [Google Scholar] [CrossRef]
- Gao, Y.-Y.; Wu, Z.-G.; Yu, L.; Wang, Y.; Pan, Y. Alkyl carbazates for electrochemical deoxygenative functionalization of heteroarenes. Angew. Chem. Int. Ed. 2020, 59, 10859. [Google Scholar] [CrossRef]
- Mo, Y.-M.; Lu, Z.-H.; Rughoobur, G.; Patil, P.; Gershenfeld, N.; Akinwande, A.I.; Buchwald, S.L.; Jensen, K.F. Microfluidic electrochemistry for single-electron transfer redox-neutral reactions. Science 2020, 368, 1352–1357. [Google Scholar] [CrossRef]
- He, H.; Strater, Z.M.; Lambert, T.H. Electrophotocatalytic C-H functionalization of ethers with high regioselectivity. J. Am. Chem. Soc. 2020, 142, 1698–1703. [Google Scholar] [CrossRef]
- Niu, L.; Jiang, C.; Liang, Y.; Liu, D.; Bu, F.; Shi, R.; Chen, H.; Chowdhury, A.D.; Lei, A. Manganese-catalyzed oxidative azidation of C(sp3)-H bonds under electrophotocatalytic conditions. J. Am. Chem. Soc. 2020, 142, 17693–17702. [Google Scholar] [CrossRef]
- Xu, P.; Chen, P.-Y.; Xu, H.-C. Scalable photoelectrochemical dehydrogenative cross-coupling of heteroarenes with aliphatic c-h bonds. Angew. Chem. Int. Ed. 2020, 59, 14275–14280. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, L.; Quadri, L.L.; Merli, D.; Ravelli, D. Photoelectrochemical cross-dehydrogenative coupling of benzothiazoles with strong aliphatic C-H bonds. Chem. Commun. 2021, 57, 4424–4427. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Mac Millan, D.W.C. Direct α-arylation of ethers through the combination of photoredox-mediated C-H functionalization and the Minisci reaction. Angew. Chem. Int. Ed. 2015, 54, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ji, X.; Han, T.; Deng, G.-J.; Huang, H. LiBr-promoted photoredox Minisci-type alkylations of quinolines with ethers. Adv. Synth. Catal. 2019, 361, 5643–5647. [Google Scholar] [CrossRef]
- Vijeta, A.; Reisner, E. Carbon nitride as a heterogeneous visible-light photocatalyst for the Minisci reaction and coupling to H2 production. Chem. Commun. 2019, 55, 14007–14010. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Z.-L.; Wang, Z.-W.; Xu, C.-Y.; Huang, L.; Wang, S.-C.; Zhang, H.; Lei, A.-W. Electrochemical arylation of electron-deficient arenes through reductive activation. Angew. Chem. Int. Ed. 2019, 58, 15747–15751. [Google Scholar] [CrossRef]
- Reid, J.P.; Rupert, S.J.; Sigman, M.S.; Phipps, R.J. Predictive multivariate linear regression analysis guides successful catalytic enantioselective Minisci reactions of diazines. J. Am. Chem. Soc. 2019, 141, 19178–19185. [Google Scholar] [CrossRef]
- Cheng, W.-M.; Shang, R.; Fu, Y. Photoredox/Brønsted acid co-catalysis enabling decarboxylative coupling of amino acid and peptide redox-active esters with N-heteroarenes. ACS Catal. 2017, 7, 907–911. [Google Scholar] [CrossRef]
- Luo, M.-P.; Gua, Y.-J.; Wang, S.-G. Photocatalytic enantioselective Minisci reaction of β-carbolines and application to natural product synthesis. Chem. Sci. 2023, 14, 251–256. [Google Scholar] [CrossRef]
- Colgan, A.C.; Rupert, S.J.; Gibson, D.C.; Chuentragool, P.; Lahdenperä, A.S.K.; Ermanis, K.; Phipps, R.J. Hydrogen atom transfer driven enantioselective Minisci reaction of alcohols. Angew. Chem. Int. Ed. 2022, 61, e202200266. [Google Scholar] [CrossRef]
- Fu, M.-C.; Shang, R.; Zhao, B.; Wang, B.; Fu, Y. Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide. Science 2019, 363, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Laze, L.; Quevedo-Flores, B.; Bosque, I.; Gonzalez-Gomez, J.C. Alkanes in Minisci-type reaction under photocatalytic conditions with hydrogen evolution. Org. Lett. 2023, 25, 8541–8546. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, L.; Guo, J.; Shao, Y.; Song, Y.; Ding, Y.; Zhu, L.; Yao, X. Light-promoted Minisci coupling reaction of ethers and aza aromatics catalyzed by Au/TiO2 heterogeneous photocatalyst. ChemCatChem 2021, 13, 1–9. [Google Scholar] [CrossRef]
- Guo, F.; Guo, Y.; Sun, Q.; Zhang, T.; Wang, Y.; Fang, L. Photoinduced HCl-mediated cross-dehydrogenative coupling of quinolines with alcohols and ethers. J. Org. Chem. 2024, 89, 14204–14208. [Google Scholar] [CrossRef]
- Guo, K.; Chen, L.; Qiu, J.-K.; Yuan, X.; Qin, L.-Z.; Zhang, X.; Sun, Q.; Duan, X. A Method for Alkylation of Nitrogenous Heterocyclic Compounds in a Visible-Light-Mediated Microreactor. CN Patent CN113620934 A, 9 November 2021. [Google Scholar]
- Cai, Y.; Roy, T.K.; Zähringer, T.J.B.; Lansbergen, B.; Kerzig, C.; Ritter, T. Arylthianthrenium salts for triplet energy transfer catalysis. J. Am. Chem. Soc. 2024, 146, 30474–30482. [Google Scholar] [CrossRef]
- Ni, S.; Halder, R.; Ahmadli, D.; Reijerse, E.J.; Cornella, J.; Ritter, T. C-heteroatom coupling with electron-rich aryls enabled by nickel catalysis and light. Nat. Catal. 2024, 7, 733–741. [Google Scholar] [CrossRef]
- Zhao, D.; Petzold, R.; Yan, J.; Muri, D.; Ritter, T. Tritiation of aryl thianthrenium salts with a molecular palladium catalyst. Nature 2021, 600, 444–449. [Google Scholar] [CrossRef]
- Cheng, Q.; Bai, Z.; Tewari, S.; Ritter, T. Bifunctional sulfilimines enable synthesis of multiple N-heterocycles from alkenes. Nat. Chem. 2022, 14, 898–904. [Google Scholar] [CrossRef]
- Cai, Y.; Ritter, T. Meerwein-type bromoarylation with arylthianthrenium salts. Angew. Chem. Int. Ed. 2022, 61, e202209882. [Google Scholar] [CrossRef]
- He, Z.; Dydio, P. Photoinduced Cu(II)-Mediated Decarboxylative Thianthrenation of Aryl and Heteroaryl Carboxylic Acids. Angew. Chem. Int. Ed. 2024, 63, e202410616. [Google Scholar] [CrossRef]
- Berger, F.; Plutschack, M.B.; Riegger, J.; Yu, W.; Speicher, S.; Ho, M.; Frank, N.; Ritter, T. Site-Selective and Versatile Aromatic C-H Functionalization by Thianthrenation. Nature 2019, 567, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, X.; Guo, H.-M.; Wu, X. Photoredox/persistent radical cation dual catalysis for alkoxy radical generation from alcohols. Org. Chem Front. 2022, 9, 3532–3539. [Google Scholar] [CrossRef]




 | ||
|---|---|---|
| Entry | Deviation from Standard Conditions | Yield (%) b |
| 1 | none | 95 |
| 2 | MeCN instead of 1,4–dioxane as solvent | 65 |
| 3 | DCM instead of 1,4–dioxane as solvent | 33 |
| 4 | EA instead of 1,4–dioxane as solvent | 58 |
| 5 | 1,4–dioxane (1.0 mL) | 62 |
| 6 | 1,4–dioxane (3.0 mL) | 84 |
| 7 | 3b/3c/3d/3e/3f instead of 3a as additive | N.R. |
| 8 | 10 mol% PPh3 instead of 20 mol% PPh3 | 34 |
| 9 | 20 mol% NaI instead of 10 mol% NaI | 76 |
| 10 | 390 nm/550 nm light instead of 455 nm light | N.R. |
| 11 | Without NaI or PPh3 | N.R. |
| 12 | Without light | N.R. |
| 13 | Without 3a | N.R. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Chen, W.; Chen, X.; Zhou, Y.; Han, B.; Wang, Y.; Jia, H.; Guo, K.; Qiu, J.; Wang, J.; et al. Visible-Light-Mediated Dehydrogenative Cross-Coupling of Azaarenes and Ethers. Chemistry 2025, 7, 103. https://doi.org/10.3390/chemistry7040103
Song J, Chen W, Chen X, Zhou Y, Han B, Wang Y, Jia H, Guo K, Qiu J, Wang J, et al. Visible-Light-Mediated Dehydrogenative Cross-Coupling of Azaarenes and Ethers. Chemistry. 2025; 7(4):103. https://doi.org/10.3390/chemistry7040103
Chicago/Turabian StyleSong, Junsong, Wanyu Chen, Xin Chen, Yi Zhou, Bin Han, Yao Wang, Honghua Jia, Kai Guo, Jiangkai Qiu, Jian Wang, and et al. 2025. "Visible-Light-Mediated Dehydrogenative Cross-Coupling of Azaarenes and Ethers" Chemistry 7, no. 4: 103. https://doi.org/10.3390/chemistry7040103
APA StyleSong, J., Chen, W., Chen, X., Zhou, Y., Han, B., Wang, Y., Jia, H., Guo, K., Qiu, J., Wang, J., & Ma, C. (2025). Visible-Light-Mediated Dehydrogenative Cross-Coupling of Azaarenes and Ethers. Chemistry, 7(4), 103. https://doi.org/10.3390/chemistry7040103






