Abstract
Hydrogen sulfide (H2S), once regarded solely as a highly toxic gas, is now recognized as a crucial signaling molecule in plants, bacteria, and mammals. In humans, H2S signaling plays a role in numerous physiological and pathological processes, including vasodilation, neuromodulation, and cytoprotection. To exploit its biological functions and therapeutic potential, a wide range of H2S-releasing compounds, known as H2S donors, have been developed. These donors are designed to release H2S under physiological conditions in a controlled manner. Among them, self-reporting H2S donors are seen as a particularly innovative class, combining therapeutic delivery with real-time fluorescence-based detection. This dual functionality enables spatiotemporal monitoring of H2S release in biological environments, eliminating the need for additional sensors or probes that could disrupt cellular homeostasis. This review summarizes recent advancements in self-reporting H2S donor systems, organizing them based on their activation triggers, such as specific bioanalytes, enzymes, or external stimuli like light. The discussion covers their design strategies, performance in biological applications, and therapeutic potential. Key challenges are also highlighted, including the need for precise control of H2S release kinetics, accurate signal quantification, and improved biocompatibility. With continued refinement, self-reporting H2S donors offer great promise for creating multifunctional platforms that seamlessly integrate diagnostic imaging with therapeutic H2S delivery.
1. Introduction
Hydrogen sulfide (H2S) is a colorless gas that was first identified in the late 18th century by Swedish chemist Carl Wilhelm Scheele, who produced it by acidifying metal polysulfides [1]. For much of its early history, H2S was regarded primarily as a toxic pollutant, prompting toxicology studies in animals and humans as early as the 1800s to establish exposure limits and detection methods [2,3,4,5,6]. It was not until the mid-20th century, with the discovery of the transsulfuration pathway in mammals [7,8,9], that researchers began to explore its potential biological roles.
A major breakthrough occurred in 1996, when Abe and Kimura demonstrated a possible neuromodulatory role for H2S [10]. This discovery sparked a paradigm shift in H2S research, leading to decades of investigations into its physiological functions. Ultimately, these efforts established H2S as the third recognized gasotransmitter in humans, alongside nitric oxide (NO) and carbon monoxide (CO) [11,12,13,14].
1.1. H2S Physical Characteristics and Endogenous Production
At room temperature, H2S is a colorless gas with a distinct odor reminiscent of rotten eggs. It is highly soluble in water (80 mM at 37 °C) [15], where it exists in equilibrium between its diprotic form (H2S) and hydrosulfide ion (HS−). Due to the relative acidity of H2S (pKa1 = 6.98, pKa2 = 19) [15], the hydrosulfide form predominates at physiological pH, accounting for over 70% of the species present. In its diprotic form, H2S is lipophilic, allowing it to diffuse across cellular membranes and reach intracellular targets [16]. Conversely, the hydrosulfide ion, which is polar and abundant at neutral pH, acts as a strong nucleophile and reductant, capable of altering the structure and oxidation state of biologically important sulfur species—activities thought to underpin much of hydrogen sulfide’s biological effects [17,18,19].
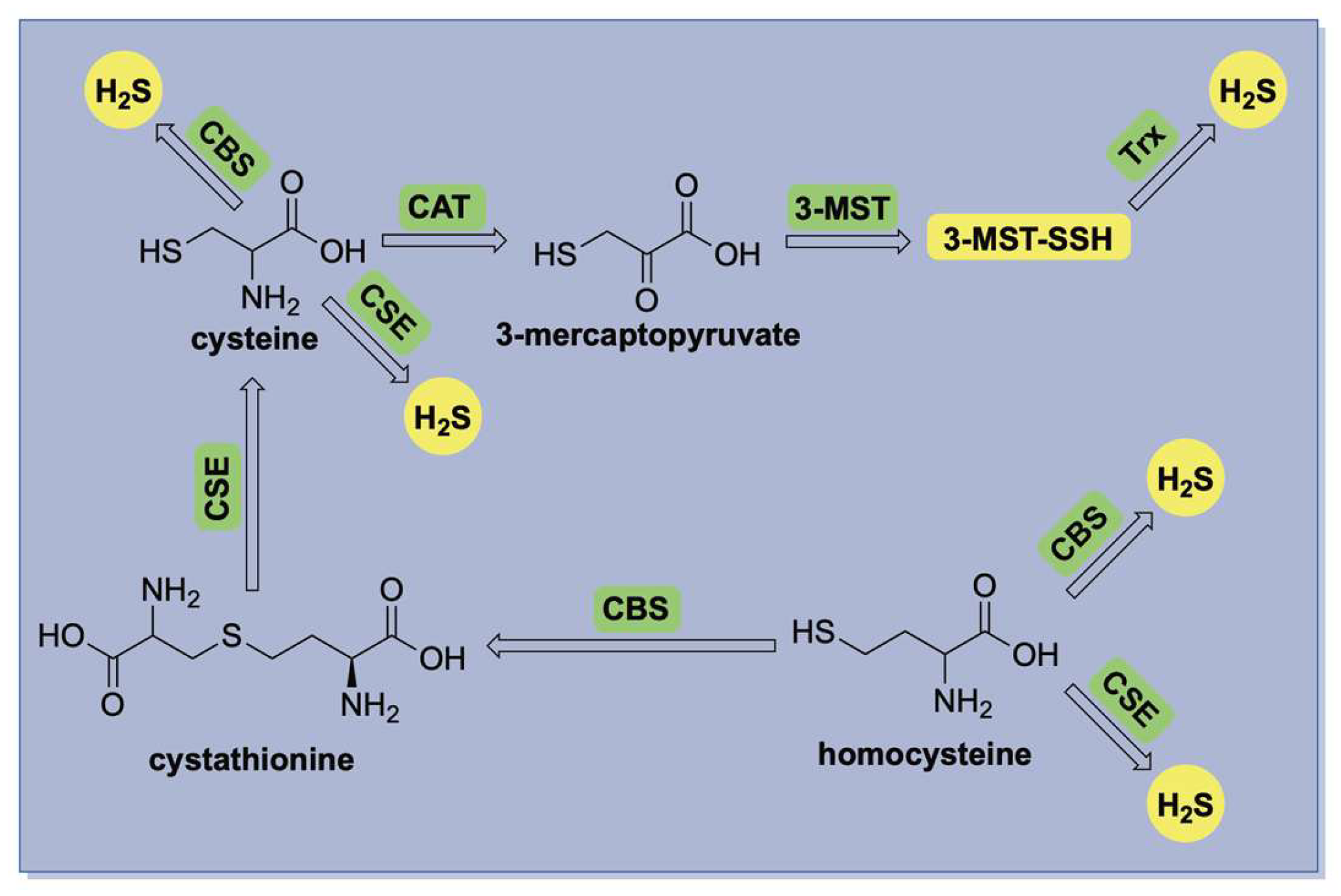
The biosynthesis of hydrogen sulfide (H2S) is primarily mediated by three enzymes: cystathionine-β-synthase (CBS) [20], cystathionine-γ-lyase (CSE) [21], and the 3-mercaptopyruvate sulfurtransferase (3-MST) in conjunction with cysteine aminotransferase (CAT) [22]. A summary of these pathways is illustrated in Figure 1. CBS and CSE are central components of the transsulfuration pathway, facilitating the conversion of homocysteine to cysteine via the intermediate cystathionine. Both enzymes are pyridoxal 5′-phosphate (PLP)-dependent and localized in the cytosol, where they produce H2S through the direct desulfurization of cysteine or homocysteine. In contrast, 3-MST is a PLP-independent enzyme predominantly located in mitochondria, where it catalyzes H2S production using 3-mercaptopyruvate as its substrate.
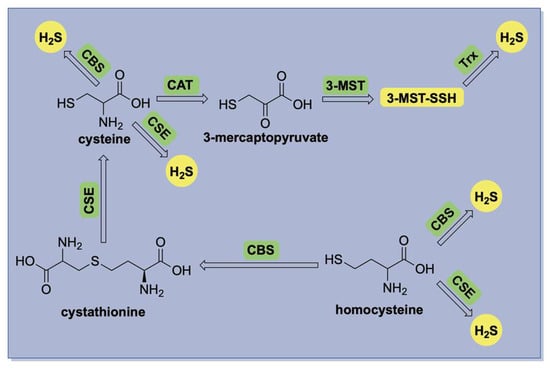
Figure 1.
A simplified illustration of enzymatic H2S production in mammalian systems.
1.2. H2S Biological Activity
As previously mentioned, the neuromodulatory effects of endogenous H2S were first reported by Abe and Kimura in 1996 [10]. Since this pioneering discovery, extensive research has expanded our understanding of H2S biology, revealing its involvement in processes such as vasodilation [23,24,25,26], angiogenesis [27], hormonal regulation [28], and cellular respiration [29,30,31]. Additionally, endogenous H2S has emerged as a promising protective agent against inflammation and oxidative stress [32,33,34], particularly within cardiovascular tissues, spurring interest in its potential as a therapeutic intervention for myocardial ischemia–reperfusion (MI/R) injury [35,36].
While H2S is a potent reductant capable of scavenging biologically relevant oxidants—including peroxynitrite (ONOO−), superoxide (O2•−), and hydrogen peroxide (H2O2)—its relatively low concentration compared to other cellular thiols suggests that its antioxidant effects are primarily mediated through targeted protein modifications rather than its direct quenching of oxidants [37]. Specifically, H2S promotes the persulfidation of oxidized cysteine residues—such as sulfenic acids (Protein–SOH), disulfides (Protein–SSR), and S-nitrosothiols (Protein–SNO)—converting them into persulfides (Protein–SSH), thereby modulating protein function and redox signaling [17,18,19]. For instance, H2S activates the Nrf2 pathway by persulfidating Keap1 at Cys151, thereby promoting the expression of cytoprotective enzymes such as heme oxygenase-1, NAD(P)H quinone oxidoreductase, and glutathione S-transferase [38,39,40]. Similarly, its vasodilatory effects stem from the persulfidation of ATP-sensitive potassium (KATP) channels, leading to channel activation and potassium influx [26,41]. Both pathways are well-documented and have been linked in the protective effects of H2S in disease prevention and mitigation.
Beyond its direct antioxidant and signaling functions, H2S also engages in crosstalk with other gasotransmitters [13]. Notably, it reacts with S-nitrosothiols to form thionitrous acid (HSNO), which can subsequently release nitric oxide (NO), a key signaling molecule with its own broad physiological roles [42]. This interaction underscores the interconnected nature of H2S and NO signaling pathways, as evidenced by findings that nitric oxide synthase (NOS) inhibition—blocking NO production from L-arginine—can abolish certain cardioprotective effects of H2S [43,44].
Despite significant advancements, much remains to be understood about the physiological and pathological roles of H2S, particularly its therapeutic potential. This knowledge gap has driven the search for improved biological delivery methods, moving beyond traditional approaches such as the direct use of hydrogen sulfide gas—which is highly toxic, foul-smelling, and challenging to handle—and inorganic sulfide salts, which generate a rapid, non-physiological bolus of H2S upon dissolution. These limitations prompted the development of H2S donors, small molecules capable of releasing H2S in a more controlled and biologically relevant manner [45,46,47].
1.3. H2S-Specific Tools for Exploring Its Chemical Biology
The first synthetic donor designed for this purpose was a phosphorodithioate-based compound introduced in 2008 (GYY4137) [48]. While the reactivity and biological effects of GYY4137 are well documented, concerns regarding its slow-release rate and low efficiency have been raised, as these factors complicate the interpretation of H2S’s precise biological functions. These shortcomings have led to the emergence of stimulus-responsive donors with tunable reactivity, designed to release H2S in response to precise biological triggers such as pH changes, redox fluctuations, or the presence of specific analytes or enzymes [45,46,47]. Such donors offer improved spatial and temporal control over H2S delivery, making them valuable tools for probing its biological and therapeutic effects in disease models.
2. Self-Reporting H2S Donors
Accurate tracking and quantification of H2S release from donors, especially in complex biological environments, remains a significant challenge. Conventional detection methods, including the methylene blue assay and gas chromatography, are suitable for in vitro buffer systems but are not practical for real-time monitoring, especially in live cells or tissues. To address this, reaction-based fluorescent probes have been developed, enabling non-destructive, real-time visualization of H2S delivery in biological samples [49,50].
A logical next step in streamlining this process involves the design of donors with built-in fluorescent reporting capabilities [51,52]. Such self-reporting systems eliminate the need for separate sensors, providing direct correlation between donor activation and H2S release. Furthermore, they offer valuable insights into the localization and concentration of H2S at its site of action.
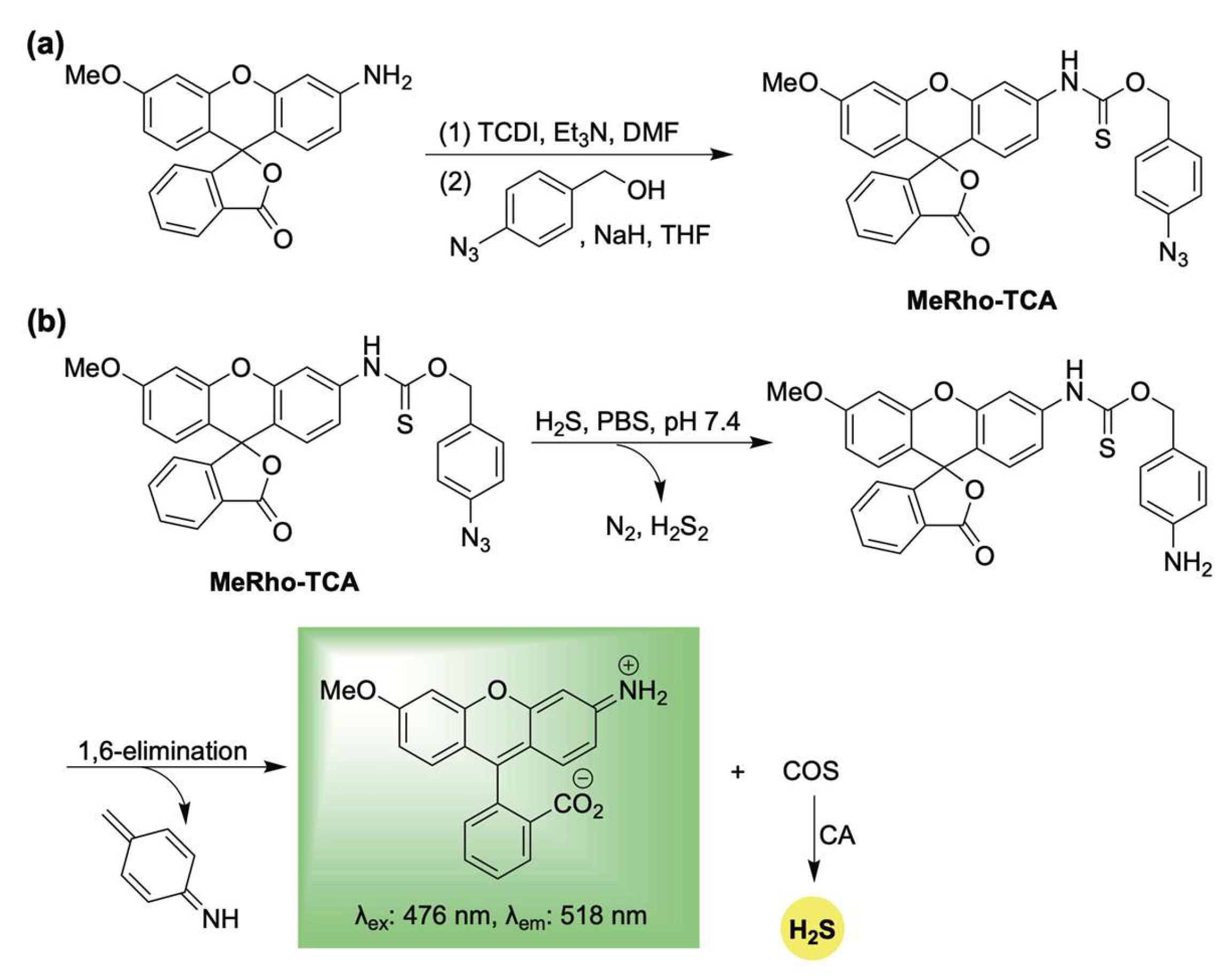
In response to this need, Pluth and colleagues developed the first fluorescent, self-reporting H2S donor system in 2016 [53]. Their design utilized a methylrhodol fluorophore quenched through the formation of a p-azidobenzylthiocarbamate linkage. This aryl azide functions as a bioorthogonal sensing unit, which, upon reaction with H2S, undergoes reduction to an unstable arylamine [54,55]. A subsequent 1,6-elimination releases carbonyl sulfide (COS), which is rapidly converted to H2S by the enzyme carbonic anhydrase (CA) [56], while concurrently restoring π-conjugation—and thereby fluorescence—of the reporter (Scheme 1). Using MeRho-TCA, the authors observed a 65-fold fluorescence enhancement after a 90 min incubation with H2S (50 equivalents). Importantly, MeRho-TCA demonstrated high selectivity for H2S over other potential interfering species, including glutathione, cysteine, homocysteine, thiosulfate, sulfite, sulfate, hydrogen peroxide, and nitric oxide.
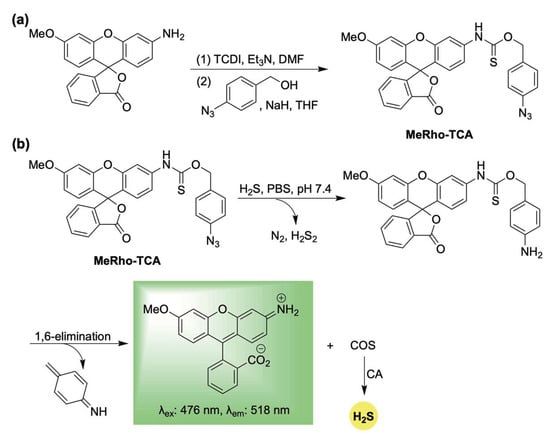
Scheme 1.
(a) Synthesis of MeRho-TCA. (b) Proposed mechanism for COS/H2S release from MetRho-TCA upon activation by H2S.
However, its reliance on sulfide to trigger H2S release restricted its capacity to modulate cellular H2S levels as an effective donor. Instead, MeRho-TCA functioned primarily as an H2S-selective analyte replacement sensor, detecting H2S while maintaining cellular homeostasis by replenishing the consumed sulfide. Despite this limitation as a donor, its innovative self-reporting design set the stage for the development of next-generation donors that respond to biologically relevant stimuli and allow for more accurate monitoring of H2S delivery. The following sections will examine these advanced systems, organized by the specific reaction partners that mediate their activation.
2.1. Thiol-Activation
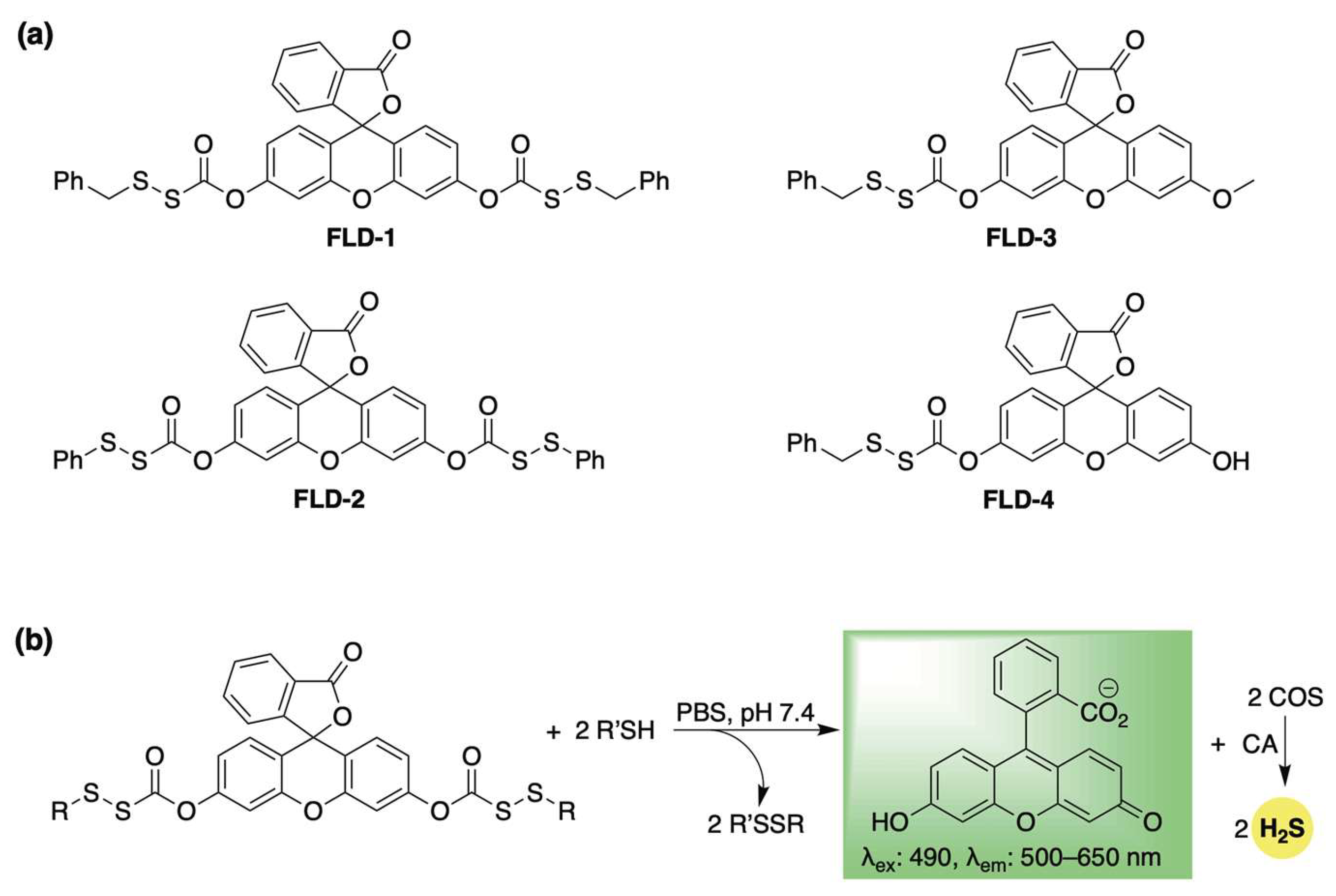
Pluth et al. developed a library of self-reporting H2S donors using fluorescein, which are selectively activated by biological thiols (FLD1–4, Scheme 2a) [57]. Their proposed mechanism involves a thiol–disulfide exchange with cysteine or glutathione, which triggers the uncaging of a sulfenyl thiocarbonate, which spontaneously breaks down to release COS while restoring the fully conjugated fluorescein reporter (Scheme 2b). COS is then transformed into H2S by CA. This design enables real-time tracking of H2S release while preventing the formation of electrophilic byproducts—a common issue among COS/H2S-based donors, which often yield a highly toxic quinone methide as a byproduct [58,59,60]. The authors validated their fluorescence response in the presence of cysteine and CA, and quantified H2S release using the methylene blue assay. For FLD-3, where H2S and fluorescein are released in a 1:1 ratio, a strong linear correlation was observed between fluorescence intensity and H2S concentration (R2 = 0.988), confirming that COS/H2S release can be accurately tracked using the fluorescein reporter. Additionally, the authors demonstrated that the H2S release rate from this series could be modulated by altering disulfide reactivity. Specifically, the more electrophilic phenyl sulfenyl thiocarbonate exhibited a significantly faster response than its benzyl counterpart. As expected, other biologically pertinent thiols—including N-acetylcysteine, homocysteine, and glutathione—were also effective in triggering the release of COS/H2S and fluorescein. Following these buffer-based studies, the H2S-releasing capability of FLD donors was further confirmed in live human cells using a Leica DMi8 fluorescence microscope (Wetzlar, Germany). HeLa cells treated with FLD-1 (50 µM) displayed strong fluorescence in the fluorescein channel, indicating successful cellular uptake, donor activation, and H2S release. Furthermore, FLD-1 demonstrated dose-dependent inhibition of lipopolysaccharide (LPS)-induced nitric oxide (NO) production in RAW 264.7 macrophage cells, highlighting its anti-inflammatory potential. Notably, this effect was absent in other components of the donor, emphasizing the potential role of H2S release in mediating anti-inflammatory responses.
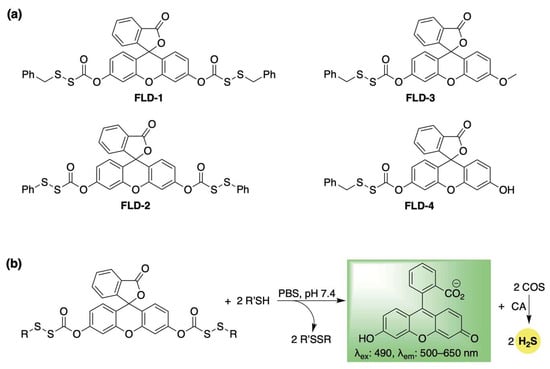
Scheme 2.
(a) Thiol-activated fluorogenic hydrogen sulfide donors (H2S), FLD1–FLD4. (b) Proposed mechanism for carbonyl sulfide (COS) and H2S release from FLD donors triggered by thiol–disulfide exchange.
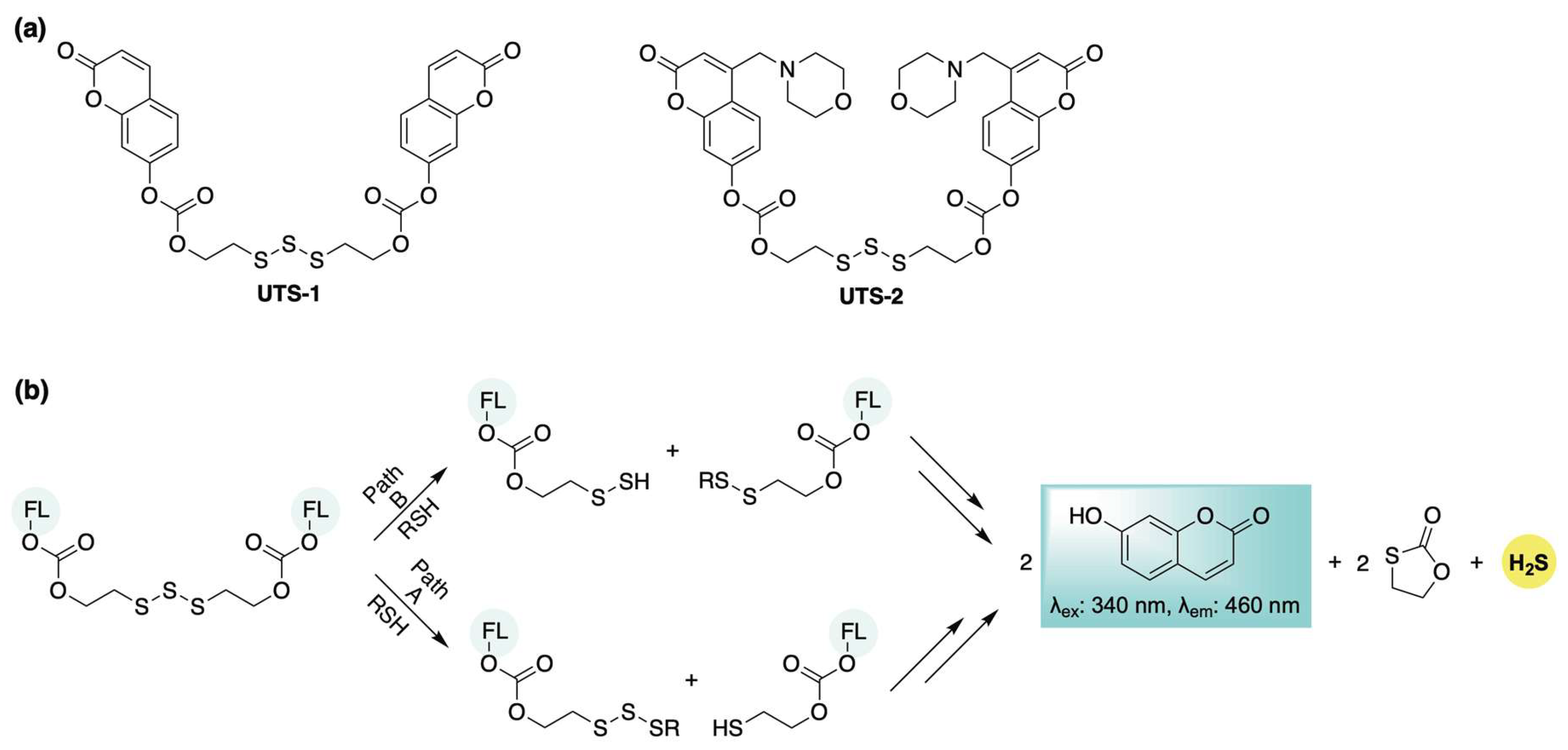
In 2020, Bhabak and colleagues introduced a biothiol-responsive, self-reporting H2S donor featuring an organotrisulfide moiety as the activation trigger. Upon thiol stimulation, this system releases 7-hydroxycoumarin—initially masked as an ester—resulting in the restoration of its fluorescence. (UTS-1, Scheme 3a) [61]. Building on this strategy, they also developed a lysosome-targeted variant (UTS-2, Scheme 3a) by incorporating a morpholino group into the fluorophore. Based on experimental and computational analyses, the authors proposed two possible mechanistic pathways for thiol attack on the trisulfide (Scheme 3b): Path A involves nucleophilic attack at the central sulfur, generating a free thiol that undergoes intramolecular cyclization to release 7-hydroxycoumarin. Subsequent thiol-mediated exchange reactions with the resulting trisulfide intermediate yield an additional fluorophore equivalent and release H2S. In Path B, thiol attack at the terminal sulfur produces a persulfide and a mixed disulfide. The persulfide can subsequently release H2S while generating an additional equivalent of disulfide. Continued thiol–disulfide exchange reactions involving the mixed disulfides ultimately result in the release of two equivalents of 7-hydroxycoumarin. Cellular H2S delivery from the donors was confirmed in HeLa cells via confocal fluorescence microscopy. Following a 1 h incubation with either UTS-1 or UTS-2, a strong blue fluorescence signal indicated successful donor activation. Co-localization experiments using LysoTracker™ (Invitrogen, Carlsbad, CA, USA) Red DND-99 further confirmed the lysosomal targeting capability of UTS-2. To validate intracellular H2S release, cells were co-incubated with H2S-sensitive probes NAP-1 and NAP-2 (lysosome-targeted). Enhanced fluorescence in both the blue channel (UTS-1 and UTS-2) and green channel (NAP-1 and NAP-2) showed significant overlap, supporting the effective intracellular delivery of both the fluorophore and H2S from the donors.
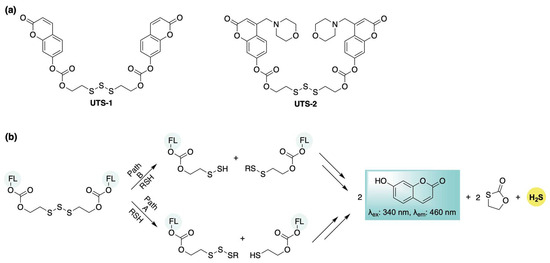
Scheme 3.
(a) Trisulfide-based H2S donors with self-reporting capabilities. (b) Proposed mechanisms for the parallel release of H2S and fluorophores.
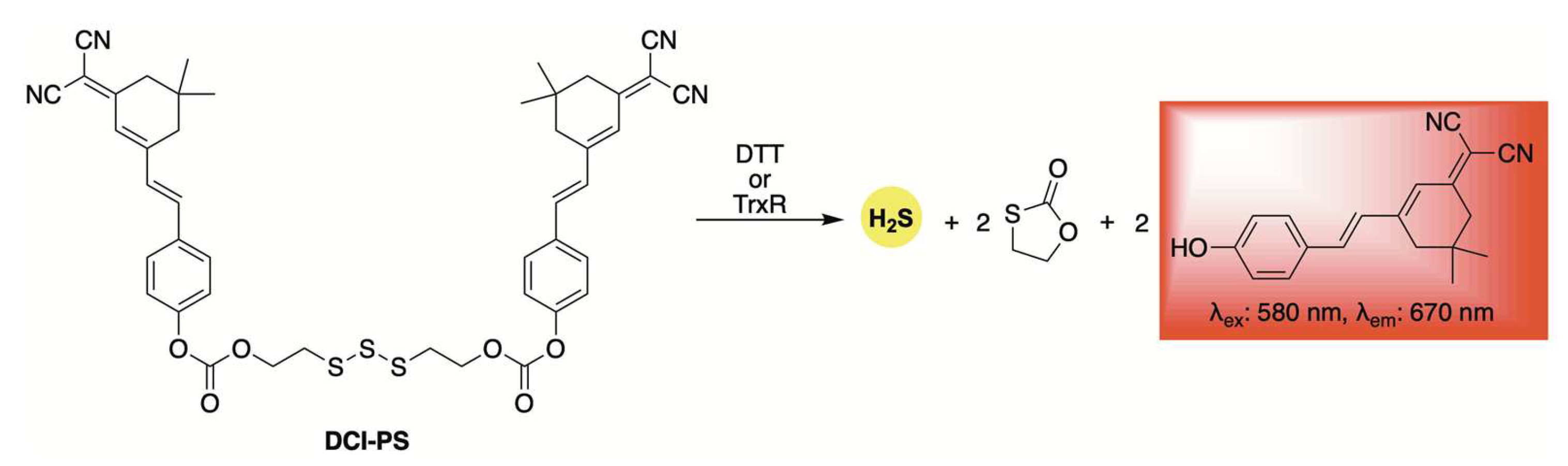
Using the same trisulfide scaffold, the Bhabak group developed a fluorogenic donor, DCI-PS, capable of releasing both H2S and a red-emitting fluorophore upon activation by biothiols (Scheme 4) [62]. Selectivity and kinetic studies revealed that DCI-PS exhibited greater reactivity toward dithiothreitol (DTT) compared to other abundant cellular biothiols, suggesting preferential activation by the enzyme thioredoxin reductase (TrxR) in a cellular context. This hypothesis was supported by confocal microscopy experiments, which showed a marked decrease in fluorescence in MDA-MB-231 human breast cancer cells following treatment with TrxR inhibitors.
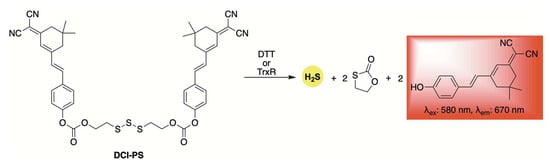
Scheme 4.
Structure of DCI-PS, a self-reporting donor activated by bithiols, enabling the co-release of H2S and a red-emitting fluorophore.
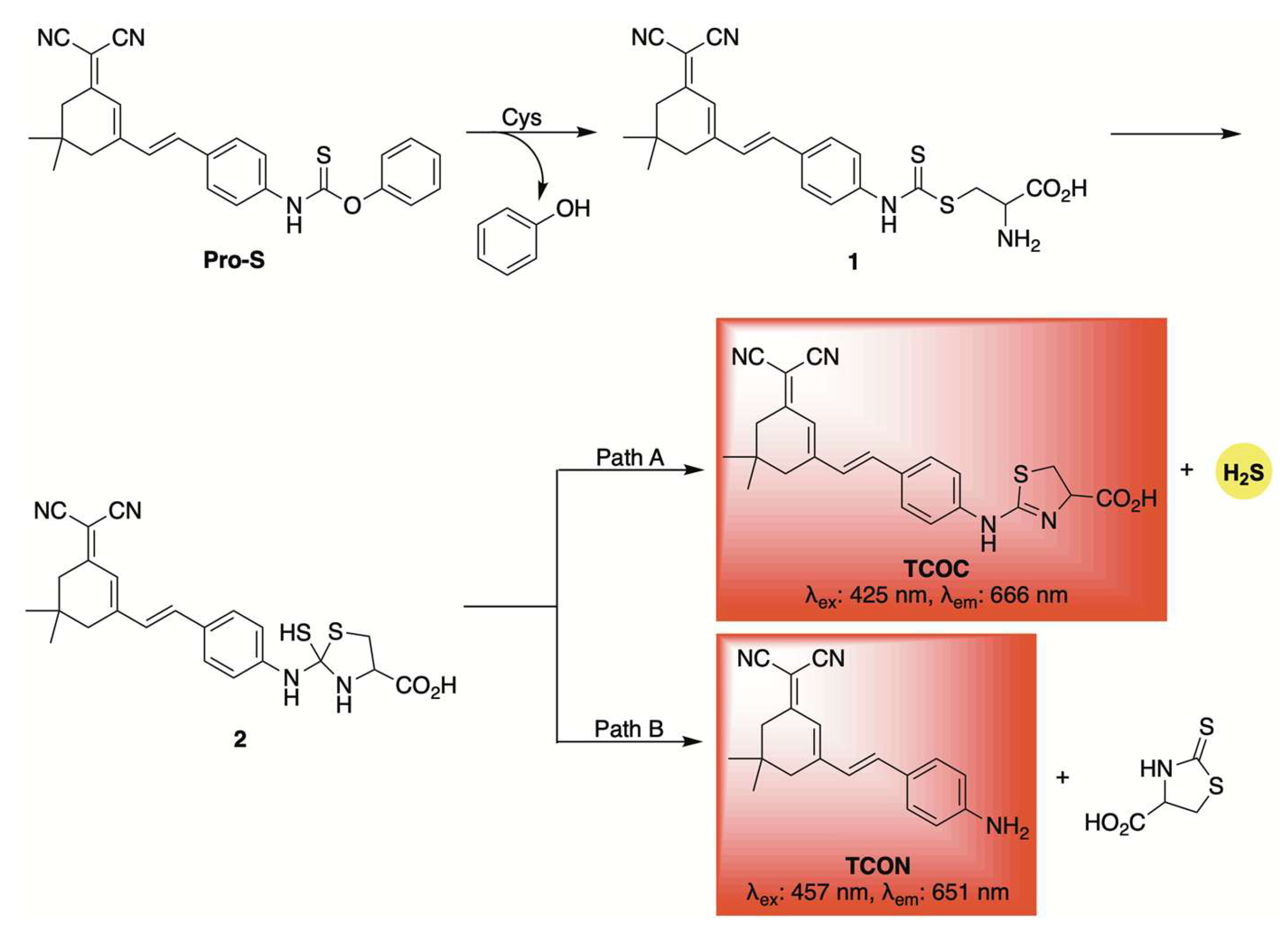
A similar fluorescent reporter was employed by Zhang and co-workers in the development of Pro-S, a self-reporting H2S donor activated by cysteine [63]. A proposed mechanism is depicted in Scheme 5. Initially, the nucleophilic thiol of cysteine likely attacks the carbon–sulfur double bond of Pro-S, displacing phenol and forming intermediate 1. Intramolecular cyclization, driven by the free amine on cysteine, then produces intermediate 2, which can decompose via two competing pathways: one leading to H2S release and formation of the fluorophore TCOC (Path A), and the other yielding the fluorophore TCON without H2S discharge (Path B). Due to these competing pathways, the observed fluorescence signal does not necessarily correlate with H2S release. Nonetheless, similar thiocarbamate and dithiocarbamate-based frameworks have been widely used for cysteine detection in various biological and disease models [64,65]. Their dual functionality—as reporters and H2S donors—adds a valuable dimension to their design. Pro-S proved effective for live-cell imaging, allowing visualization of H2S release in both cellular and zebrafish models. Moreover, it demonstrated potential anti-inflammatory properties, as shown by its ability to suppress pro-inflammatory mediators like TNF-α and IL-6 in RAW 264.7 macrophage cells.
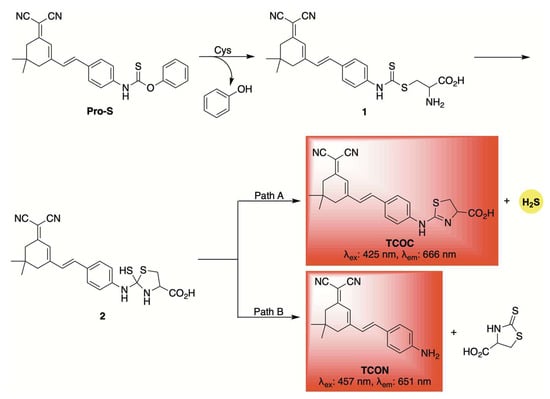
Scheme 5.
Proposed mechanism for cysteine-triggered release of both a fluorophore and H2S from the self-reporting donor Pro-S.
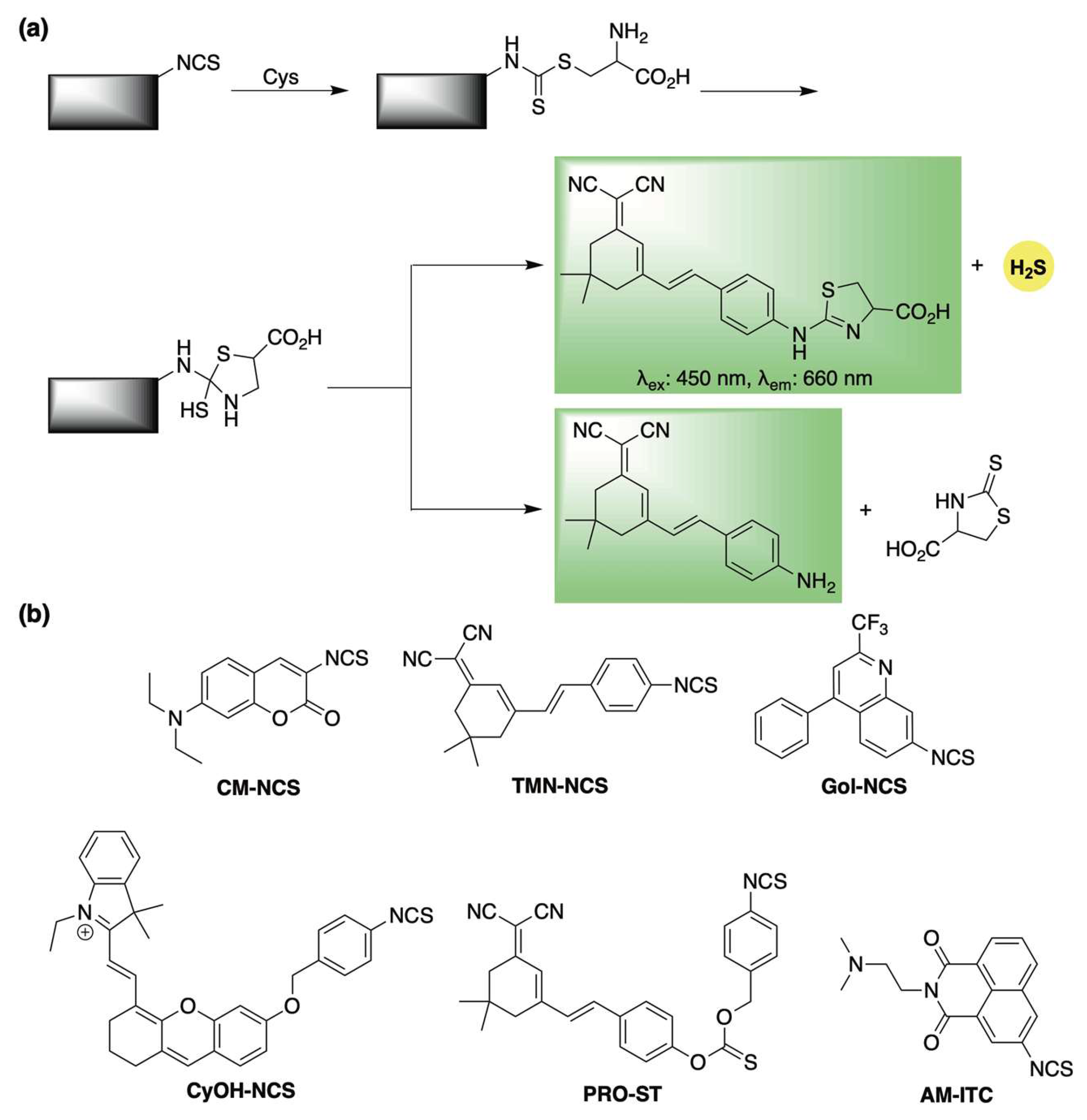
Isothiocyanates have increasingly been recognized as reliable cysteine-triggered H2S-releasing motifs [66,67]. They have also been employed in the design of self-reporting H2S donors through a mechanism analogous to the thiocarbamate framework described earlier (Scheme 6a). The first demonstration of this approach was reported in 2021 by Feng and co-workers, who incorporated a masked coumarin fluorophore onto an isothiocyanate scaffold, generating donor CM-NCS (Scheme 6b) [68]. Upon reaction with cysteine, the system released H2S accompanied by a pronounced fluorescence signal.
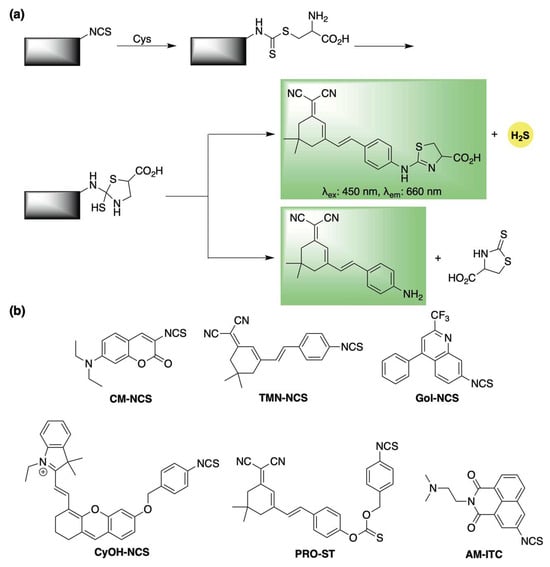
Scheme 6.
(a) Proposed mechanism for cysteine-triggered H2S release with fluorescence emission from isothiocyanate-based donors (b) Representative cysteine-responsive, self-reporting isothiocyanate-based H2S donors.
Building on this, Ge et al. enhanced the photophysical properties of the donor system by attaching a red-emitting dye to the isothiocyanate group to spawn donor TMN-NCS (Scheme 6b) [69]. TMN-NCS demonstrated high stability across a broad pH range and low cytotoxicity in human cells. Moreover, its improved fluorescence allowed for real-time imaging of cysteine activation in both live human cells and mice.
The isothiocyanate scaffold also served as the foundation for Gol-NCS, a Golgi-targeted cysteine sensor capable of releasing H2S (Scheme 6b) [70]. Gol-NCS showed excellent biocompatibility and enabled monitoring of cysteine dynamics in live cells and zebrafish. Additionally, its H2S-releasing capacity suggests it may help trigger protective cellular mechanisms in response to Golgi stress.
In 2022, Zhao and co-workers developed CyOH-NCS, an isothiocyanate-based donor that undergoes a cysteine-triggered 1,6-elimination to release a near-infrared hemicyanine dye (Scheme 6b) [71]. Similarly, this system involves two fluorophore formation pathways, only one of which is coupled with H2S release. CyOH-NCS showed strong selectivity for cysteine over other biothiols and was effective for imaging fluctuations in cysteine in living cells, zebrafish, and mice.
Zhang and colleagues also incorporated the isothiocyanate moiety in the design of PRO-ST (Scheme 6b) [72], Upon activation by cysteine, a subsequent 1,6-elimination triggers the release of a near-infrared dye, enabling real-time monitoring of COS/H2S release. Beyond its self-reporting capability, PRO-ST demonstrated notable anti-inflammatory activity in vitro, significantly reducing the expression of inflammatory mediators in LPS-stimulated macrophages. Furthermore, topical application of PRO-ST in a murine full-thickness wound model led to accelerated wound healing compared to controls, underscoring its promise for clinical use.
Most recently, Bhabak and colleagues introduced AM-ITC, a cysteine-responsive self-reporting donor designed for the co-delivery of H2S and the fluorescent anticancer drug amonafide (Scheme 6b) [73]. This isothiocyanate-based donor displayed reduced cytotoxicity compared to amonafide alone in both cancerous and healthy cells, reinforcing the potential of H2S donor-based codrug strategies to minimize side effects and improve chemoprotective outcomes.
2.2. ROS-Activation
Reactive oxygen species (ROS)—such as hydrogen peroxide, superoxide, and peroxynitrite—are widespread biomolecules primarily generated in the mitochondria as byproducts of cellular metabolism. While ROS can play essential roles in cell signaling [74], their excessive accumulation leads to oxidative damage to lipids, proteins, and nucleic acids, contributing to a wide range of oxidative stress-related diseases, including cancer, neurodegenerative disorders, and cardiovascular diseases [75,76,77,78,79]. Given the antioxidative properties of H2S, it is unsurprising that numerous ROS-activated, self-reporting H2S donors have been developed as dual-function agents capable of both imaging and alleviating cellular oxidative stress.
Inspired by the structural design of Pluth’s original analyte replacement sensor [53], a range of ROS-activated, self-reporting H2S donors have been developed based on benzylic thiocarbamate-containing scaffolds.
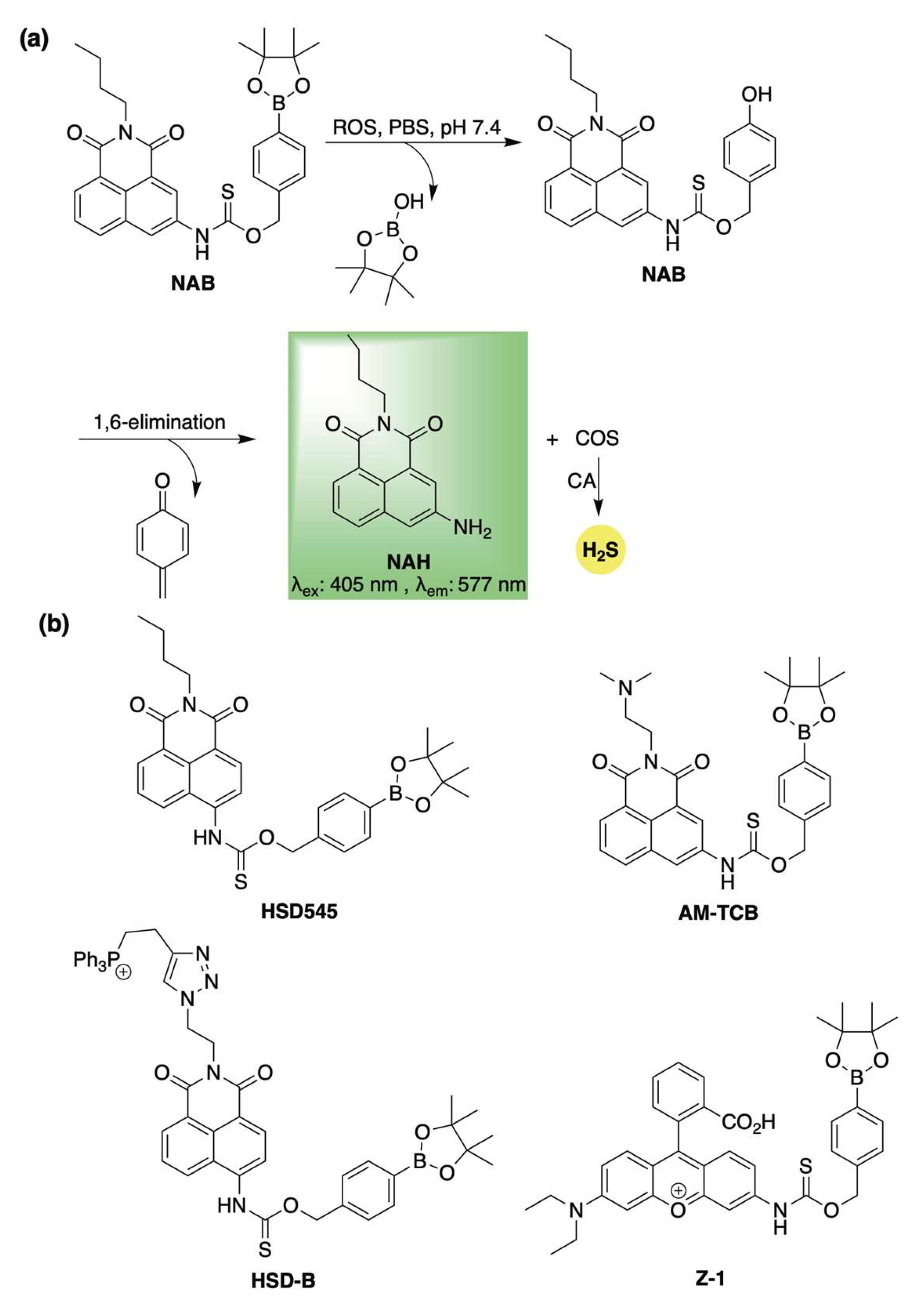
Among the first to leverage this concept, Ma and co-workers introduced NAB—an ROS-responsive H2S donor featuring an off–on fluorescent response [80]. NAB was designed by masking a naphthalimide-based fluorophore (NAH) as a thiocarbamate, linked through the benzylic position of an arylboronate group (Scheme 7a). Upon exposure to ROS, the boronate ester is oxidized to a phenol [81], triggering a 1,6-elimination cascade that releases COS—rapidly converted to H2S by CA—along with the fluorescent reporter (Scheme 7a). H2S release from NAB was confirmed using the methylene blue assay, although less than 20% efficiency was observed after 7 h. Despite the modest yield—likely a result of the oxidative conditions required for H2S release—NAB’s built-in fluorescence proved to be a valuable feature for real-time monitoring of reaction progress. Indeed, the authors showed that conversion of NAB to NAH could be easily tracked by fluorescence spectroscopy, with the initial rate of increase in fluorescence directly proportional to H2O2 concentration. This highlights NAB’s potential for both qualitative and quantitative H2O2 detection alongside controlled H2S release. Additionally, cell imaging studies confirmed selective activation of NAB in response to elevated ROS levels, enabling targeted H2S delivery. Moreover, its anti-inflammatory properties were further demonstrated in PMA-stimulated RAW264.7 macrophages, where NAB was shown to provide concentration-dependent cell rescue from oxidative stress.
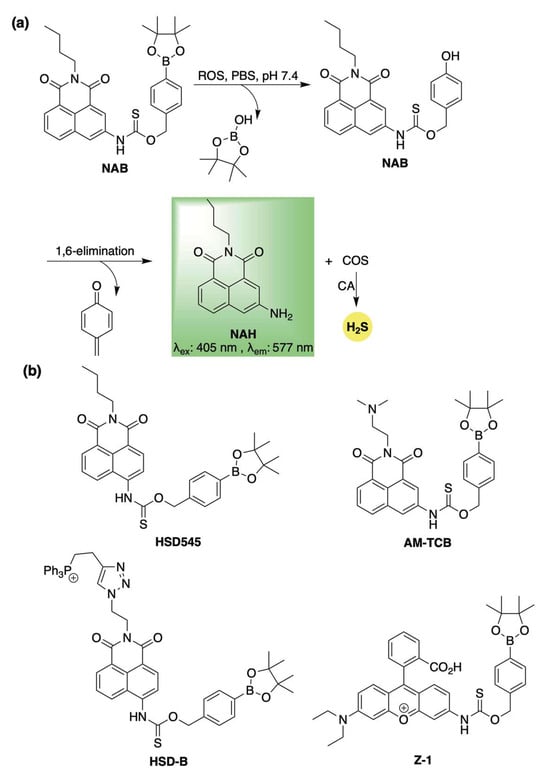
Scheme 7.
(a) Proposed mechanism for ROS-mediated activation of NAB, a self-reporting H2S donor. (b) Representative structures of thiocarbamate-based, ROS-responsive H2S donors that undergo a 1,6-elimination to simultaneously release COS/H2S and a fluorophore.
Using a similar design strategy, Zhang and co-workers soon after reported on donor HSD545 (Scheme 7b) [82]. Utilizing the same mechanism as NAB for the simultaneous release of H2S and a fluorophore, HSD545 likewise exhibited selectivity for ROS activation and conferred cellular protection in SMMC-7721 cells against H2O2-induced oxidative stress. Additionally, confocal imaging in an LPS-induced inflammation zebrafish model further confirmed the ROS-selective H2S release from HSD545 and demonstrated its effectiveness for real-time in vivo monitoring.
Building on their earlier work with a cysteine-activated, H2S-releasing amonafide codrug, Bhaback and co-workers demonstrated that this system could be adapted for ROS activation, exemplified by the development of AM-TCB (Scheme 7b) [83]. This prodrug exhibited selective reactivity toward ROS, releasing both H2S and the anticancer payload via the same self-immolative mechanism shown in Scheme 7a. In cellular studies, AM-TCB retained anticancer activity against HeLa and MDA-MB-231 cells, while also offering protective effects in noncancerous HEK-293 cells.
This ROS-responsive strategy has also facilitated the development of mitochondria-targeted, self-reporting H2S donors. The first example, reported by Ning et al., was an ROS-activated donor featuring a triphenylphosphonium group appended to a naphthalimide fluorophore via azide-alkyne click chemistry (HSD-B, Scheme 7b) [84]. HSD-B was shown to act as a ratiometric fluorescent probe, exhibiting an emission shift from 475 nm (without H2O2) to 540 nm (with H2O2), enhancing its sensitivity. Co-localization studies with MitoTracker™ Red in H9c2 cells confirmed mitochondrial accumulation and H2S release within that subcellular compartment. Protective effects of HSD-B were further demonstrated in H9c2 cardiomyocytes under hypoxia/reoxygenation-induced injury, suggesting potential therapeutic utility.
In 2023, Ye and co-workers introduced a second mitochondria-targeting, self-reporting H2S donor, Z-1 (Scheme 7b) [85]. This system, which similarly employs a boronate ester trigger, exhibited heightened sensitivity toward peroxynitrite (ONOO−)—a highly reactive ROS associated with oxidative stress, inflammation, and the progression of various diseases [86,87,88]. Co-localization with MitoTracker™ Green in HepG2 cells yielded a Pearson’s correlation coefficient of 0.7886, confirming mitochondrial localization and ROS-triggered H2S delivery. Therapeutic assessments in vitro and in vivo highlighted Z-1’s ability to suppress ferroptosis, attributed to both ONOO− scavenging and H2S release.
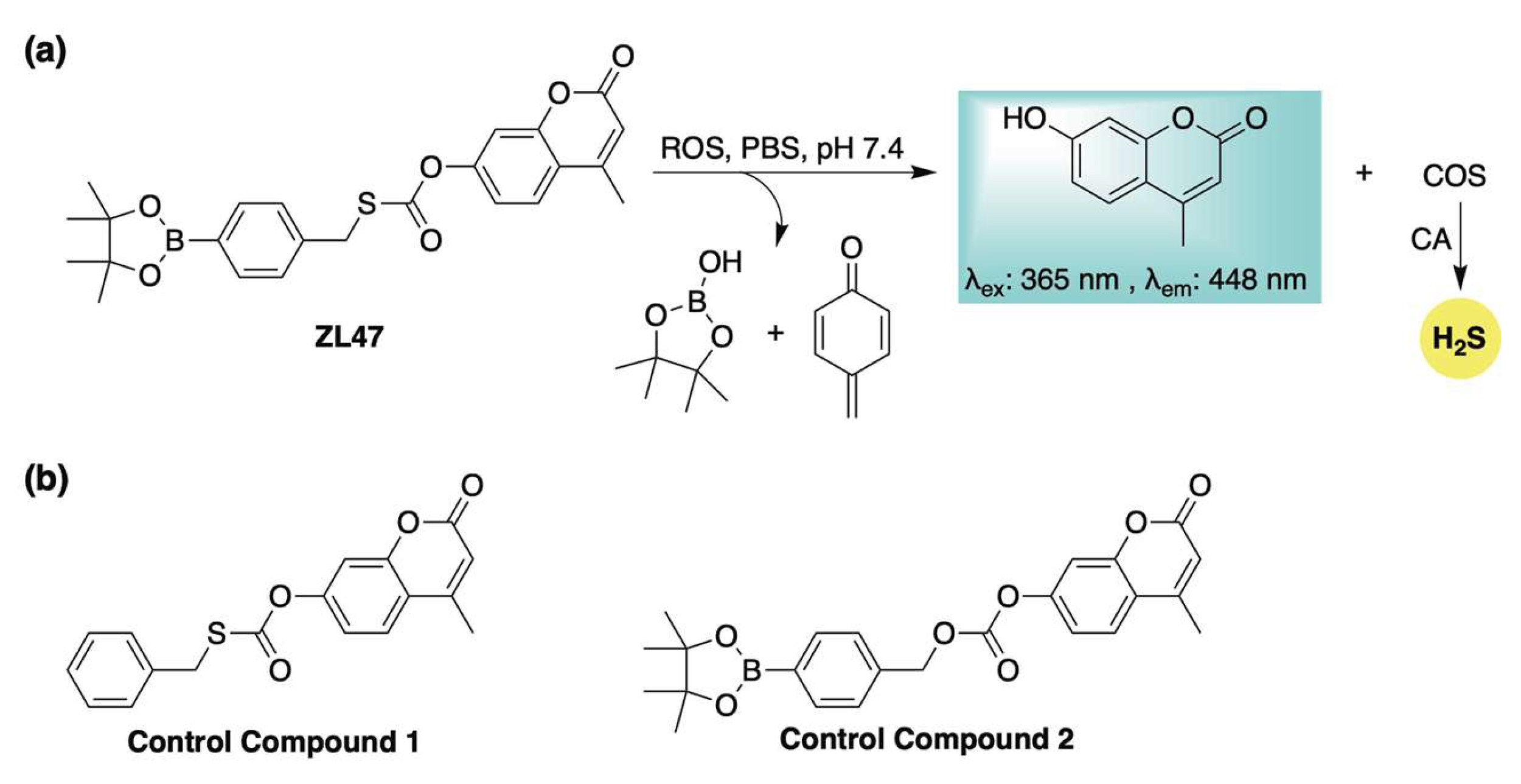
Building on these previous designs, our group developed an ROS-activated, COS-based donor system (ZL47, Scheme 8a) capable of real-time fluorescence reporting of H2S release [89]. Distinct from earlier ROS-responsive donors, ZL47 features an S-alkyl thiocarbonate moiety, which enhances reaction kinetics and improves donor efficiency. To validate its stability and elucidate its activation mechanism, two control compounds were synthesized and evaluated alongside ZL47 (Scheme 8b). Fluorescence assays monitoring the release of the coumarin reporter demonstrated that ZL47 was unreactive toward biothiols and metal ions but selectively responsive to ROS (KO2, ONOO−, and H2O2). H2S generation was confirmed via a methylene blue assay, which revealed that both H2O2 (to initiate activation) and carbonic anhydrase (to hydrolyze COS) were required for H2S formation. Notably, control compound 1 (Scheme 8b) failed to produce H2S under identical conditions, further supporting the proposed release mechanism of boronate oxidation followed by a 1,6-elimination (Scheme 8a). The biological activity of ZL47 and related controls was also evaluated in HeLa cells. While both ZL47 and control compound 2 (Scheme 8b) were capable of imaging elevated ROS levels, only ZL47 conferred cytoprotective effects. This benefit is likely due to its dual function—fluorescence signaling and antioxidant (COS/H2S) delivery—highlighting the promise of ROS-activated, self-reporting H2S donors as theranostic agents for oxidative stress-related pathologies.
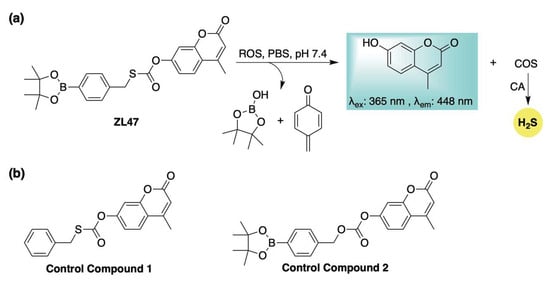
Scheme 8.
(a) Proposed mechanism for ROS-triggered activation of ZL47, a self-reporting H2S donor featuring a distinct S-alkyl thiocarbonate linkage. (b) Control compounds employed to assess the activation mechanism and biological function of ZL47.
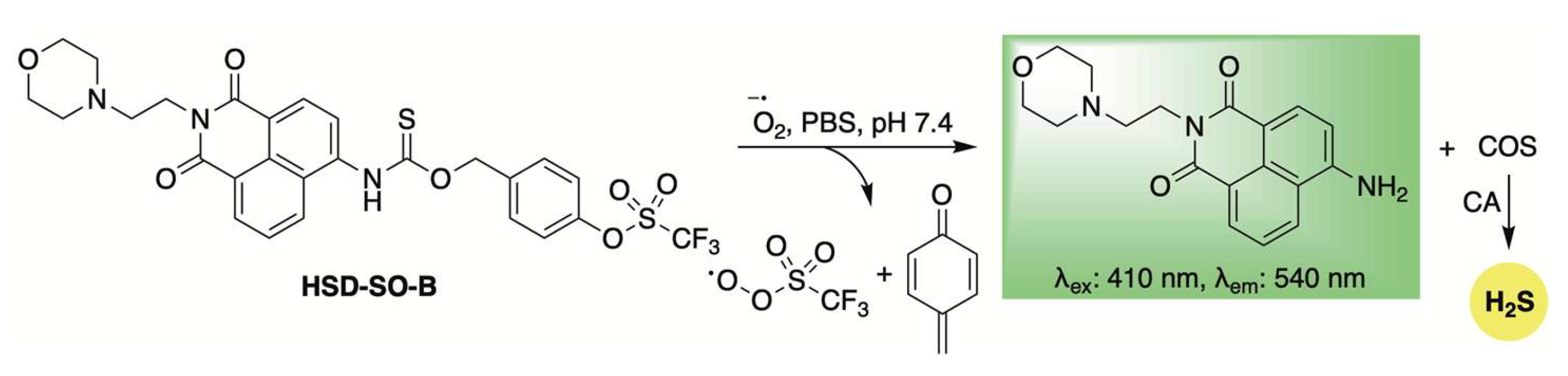
Expanding on the concept of ROS-activated, self-reporting H2S donors, Yao and colleagues developed the donor HSD-SO-B, which is selectively activated by superoxide (Scheme 9) [90]. This selectivity was achieved by masking a phenol group with a triflate moiety. Upon superoxide-triggered unmasking, the liberated phenol undergoes a 1,6-elimination reaction, releasing COS/H2S along with a ratiometric fluorescence shift. The self-reporting ability of HSD-SO-B was further evaluated in RAW264.7 cells treated with 2-methoxyestradiol, a superoxide anion inducer. In a diabetic wound mouse model, treatment with HSD-SO-B significantly accelerated wound closure compared to untreated controls and groups treated with superoxide dismutase (SOD). Histological examination showed improved re-epithelialization, increased collagen deposition, reduced infiltration of CD11b+ immune cells, and higher levels of CD31+ vascular endothelial cells in wounds treated with HSD-SO-B. These findings highlight the therapeutic promise of HSD-SO-B for diabetic wound healing and other conditions through its dual action of scavenging superoxide anions and promoting H2S release.
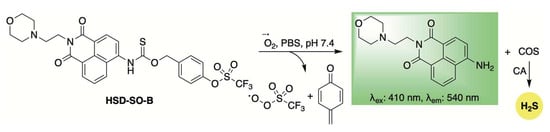
Scheme 9.
Mechanistic illustration of superoxide-induced activation of HSD-SO-B, resulting in H2S release accompanied by a fluorescence shift.
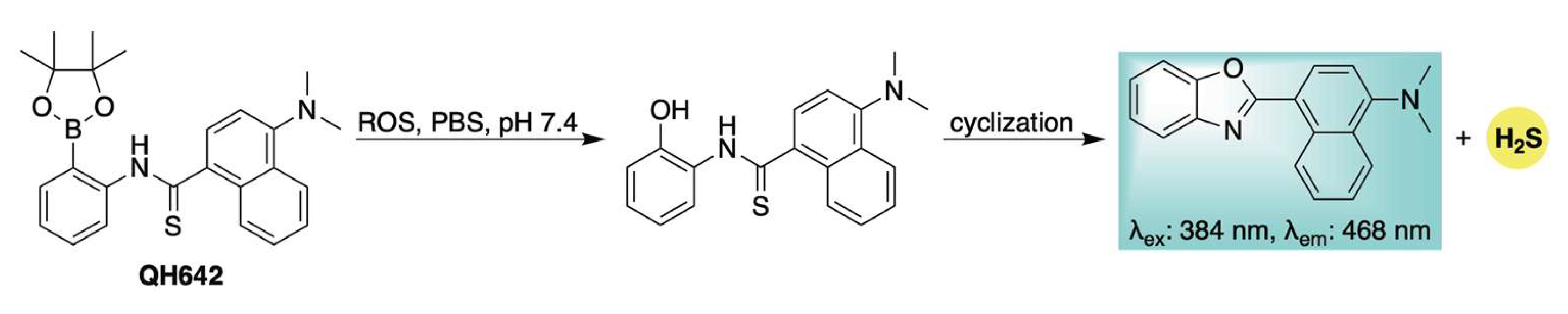
The previously described ROS-activated H2S donors all operate via a common 1,6-elimination mechanism, which generates a highly electrophilic and cytotoxic quinone methide byproduct [58,59]. To circumvent potential biological interference from this reactive species, our group developed a novel self-reporting H2S donor, QH642, which undergoes an ROS-triggered intramolecular cyclization to release H2S directly—bypassing initial COS generation—while simultaneously producing a fluorescent benzoxazole as the sole byproduct (Scheme 10) [91]. Confocal microscopy confirmed that ROS activation and H2S donation could be visualized in live HeLa cells through the formation of the benzoxazole fluorophore, directly linking fluorescence to H2S release from QH642. This was further corroborated using SS2 [92,93], an H2S-selective fluorescent probe, which confirmed H2S delivery from QH642 under conditions of cellular oxidative stress. A key advantage of this design is that fluorophore formation only occurs upon actual H2S release, minimizing the risk of false-positive signals. This feature enhances the reliability of monitoring the progression of H2S donation in complex biological systems and facilitates the study of its specific physiological roles.
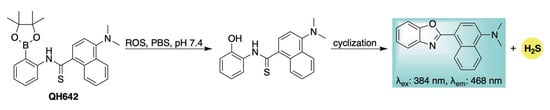
Scheme 10.
Mechanistic depiction of ROS-induced activation of QH642, leading to H2S release and generation of a fluorescent benzoxazole byproduct.
2.3. Light-Activation
Light irradiation offers several key advantages as an external stimulus, providing a noninvasive and cost-effective method of activation that can be finely tuned for precise spatial and temporal control over the release of bioactive molecules [94,95,96]. Unsurprisingly, light has been employed to trigger the release of H2S from small-molecule donors, and the incorporation of a caged fluorophore enables real-time monitoring of this release.
This approach was first demonstrated by Singh and co-workers, who utilized an excited-state intramolecular proton transfer (ESIPT) process to develop a ratiometric fluorescent sensor for H2S release (Scheme 11) [97]. As illustrated in Scheme 11, the authors proposed that upon light exposure, a photo-induced Favorskii rearrangement occurs, leading to the release of H2S along with a blue-shifted fluorogenic byproduct. Cellular uptake of the donor was confirmed in HeLa cells via confocal microscopy, where bright green fluorescence was initially observed. Ten minutes after photoirradiation, both green and blue fluorescence were detected, indicating partial decomposition of the donor. After 20 min, a complete shift from green to blue fluorescence was observed, suggesting the reaction had gone to completion and quantitative release of H2S was achieved.
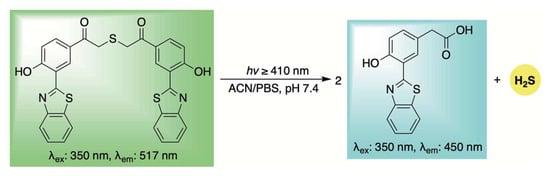
Scheme 11.
ESIPT-driven photo-release of H2S with a simultaneous fluorescence shift for real-time tracking.
The O-nitrobenzyl group is a well-established photo-labile protecting group that has been extensively utilized in the design of light-responsive prodrugs, including H2S donors [98,99,100]. Upon irradiation at 365 nm, this moiety undergoes a hydrogen atom transfer and subsequent rearrangement that expels a leaving group at the benzylic position [101,102,103]. Leveraging these properties, O-nitrobenzyl-protected, fluorescence-reporting H2S donors have been reported.
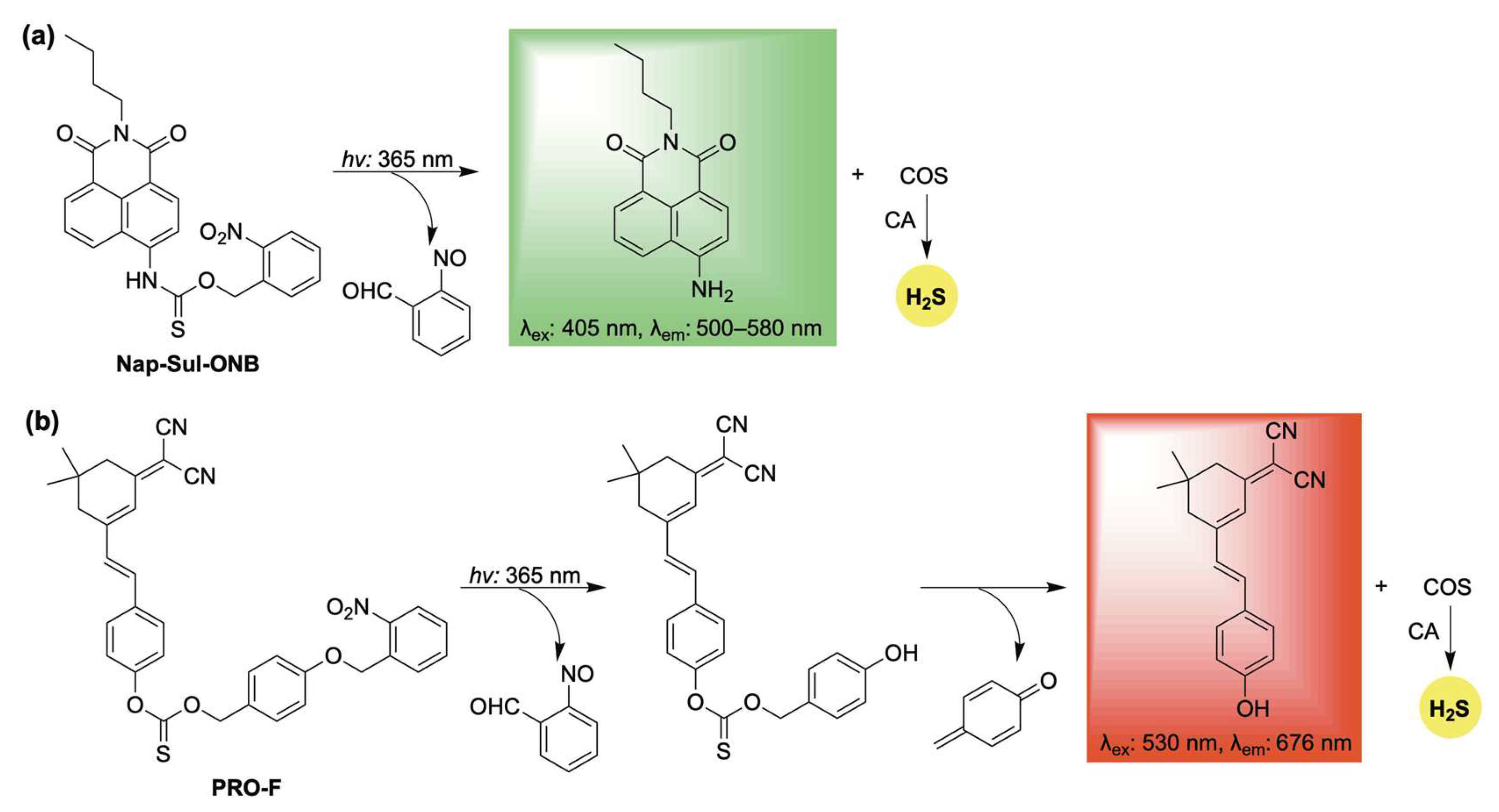
One such example, Nap-Sul-ONB, was developed by Gou and co-workers through the conjugation of an O-nitrobenzyl group to a naphthalimide fluorophore via a thiocarbamate linker (Scheme 12a) [104]. Upon UV irradiation, Nap-Sul-ONB is proposed to undergo a Norrish type II reaction, producing 2-nitrosobenzaldehyde and a thiocarbamic acid intermediate, which further decomposes to release COS along with a fluorescent naphthalimide signal (Scheme 12a). Cell imaging studies verified that Nap-Sul-ONB is activated by UV light. Moreover, the donor exhibited UV-dependent antioxidative effects against both endogenous and exogenous ROS, attributed to the controlled release of H2S.
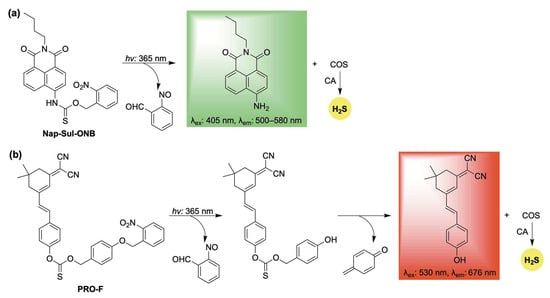
Scheme 12.
Photoactivated self-reporting donors: (a) Nap-Sul-ONB and (b) PRO-F, along with their proposed mechanisms for H2S release coupled with fluorescence.
Similarly, the O-nitrobenzyl protecting group was utilized in the design of PRO-F, a self-reporting H2S donor featuring near-infrared fluorescence (Scheme 12b) [105]. PRO-F incorporates an additional linker that undergoes a 1,6-elimination following photoactivation, enabling the simultaneous release of H2S and the fluorophore reporter (Scheme 12b). H2S release from PRO-F was verified using the methylene blue assay, and its intracellular delivery was confirmed with the H2S-selective probe WSP-5. Building on this validation, PRO-F was evaluated in a diabetic mouse model, where it demonstrated the ability to mitigate ROS and inflammation—key factors that impair wound healing under pathological conditions.
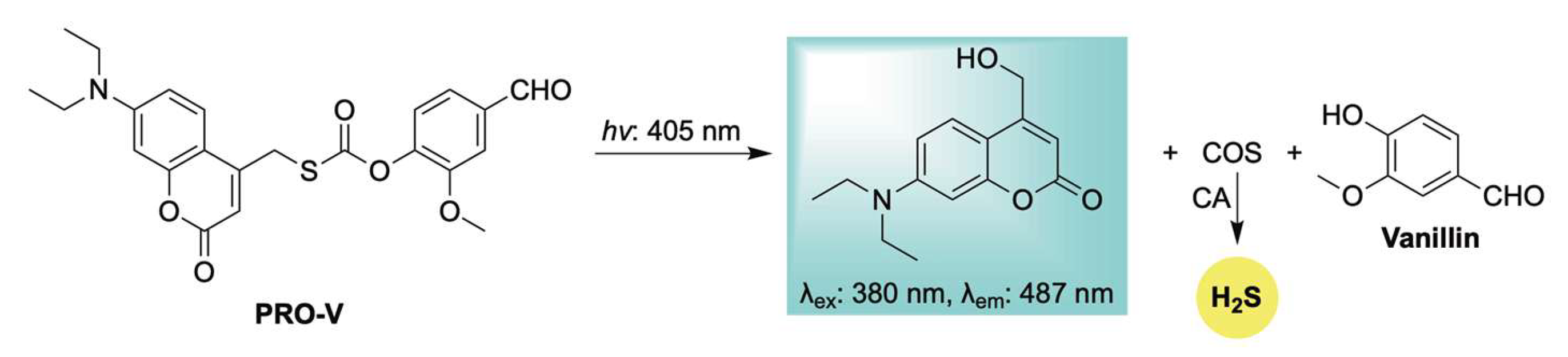
In 2025, Zhang and colleagues advanced their work on photoactivated H2S donors by introducing a new class of photocaged COS/H2S moieties featuring diethylaminocoumarin as a light-sensitive protecting group (Scheme 13) [106]. Upon blue light irradiation, these compounds release H2S through carbonic anhydrase–mediated COS hydrolysis, accompanied by fluorescence changes. The authors further demonstrated the platform’s versatility by conjugating it with pharmaceutical agents to generate light-activated, H2S-releasing hybrid prodrugs. One such example, Pro-V—a vanillin-conjugated prodrug—exhibited notable antitumor activity by inhibiting melanoma cell proliferation and promoting apoptosis. Despite these encouraging outcomes, the limited tissue penetration of blue light highlights the need for future efforts to develop photocages with red- or NIR-shifted activation profiles for broader clinical utility.
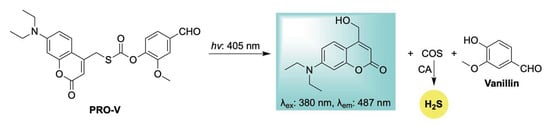
Scheme 13.
Proposed mechanism for blue-triggered co-release of H2S and the parent drug vanillin, accompanied by a coumarin-based fluorescent reporter to enable real-time tracking of donor progress.
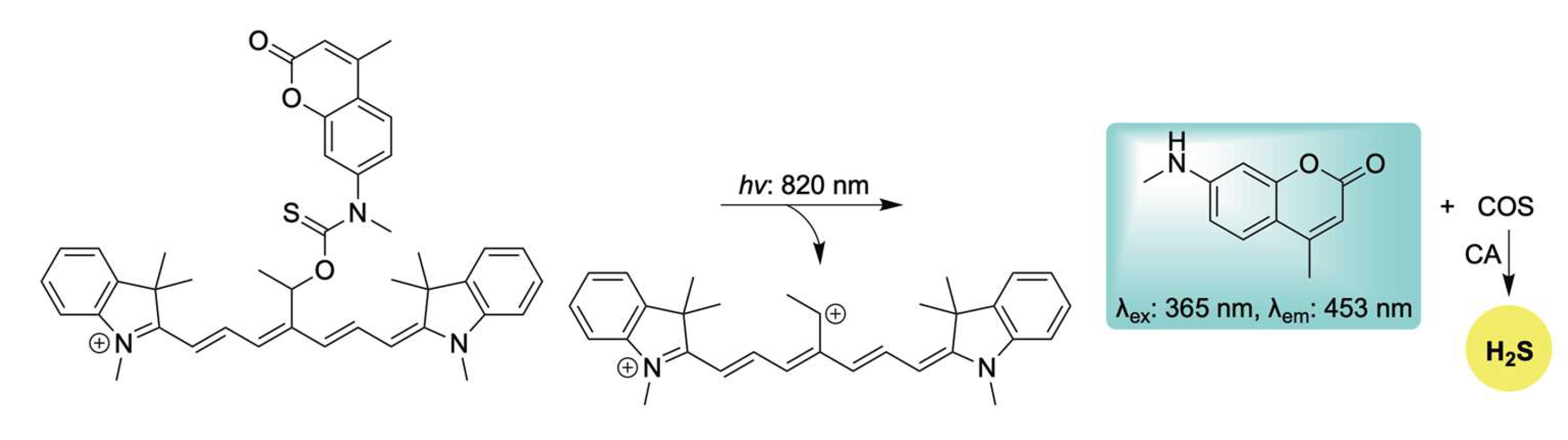
To this end, Štacko and colleagues introduced a novel Cy7-based photocage capable of controlled H2S release in tandem with an amine-based fluorescent reporter upon activation with near-infrared (NIR) light (Scheme 14) [107]. Moreover, by exploiting the intrinsic mitochondrial-targeting properties of cyanine dyes, the authors achieved precise co-delivery of H2S and the fluorophore specifically to mitochondria. H2S donation was verified in buffer using the methylene blue assay, while mitochondrial localization and delivery were validated in HeLa cells using MitoTracker™ DR. This adaptable platform holds significant potential for targeting other subcellular organelles for the co-delivery of H2S with amine-containing therapeutic agents, activated by tissue-penetrating NIR light.
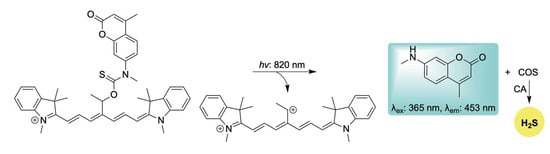
Scheme 14.
Proposed mechanism for the NIR-triggered release of H2S and accompanying coumarin-based fluorescent reporter.
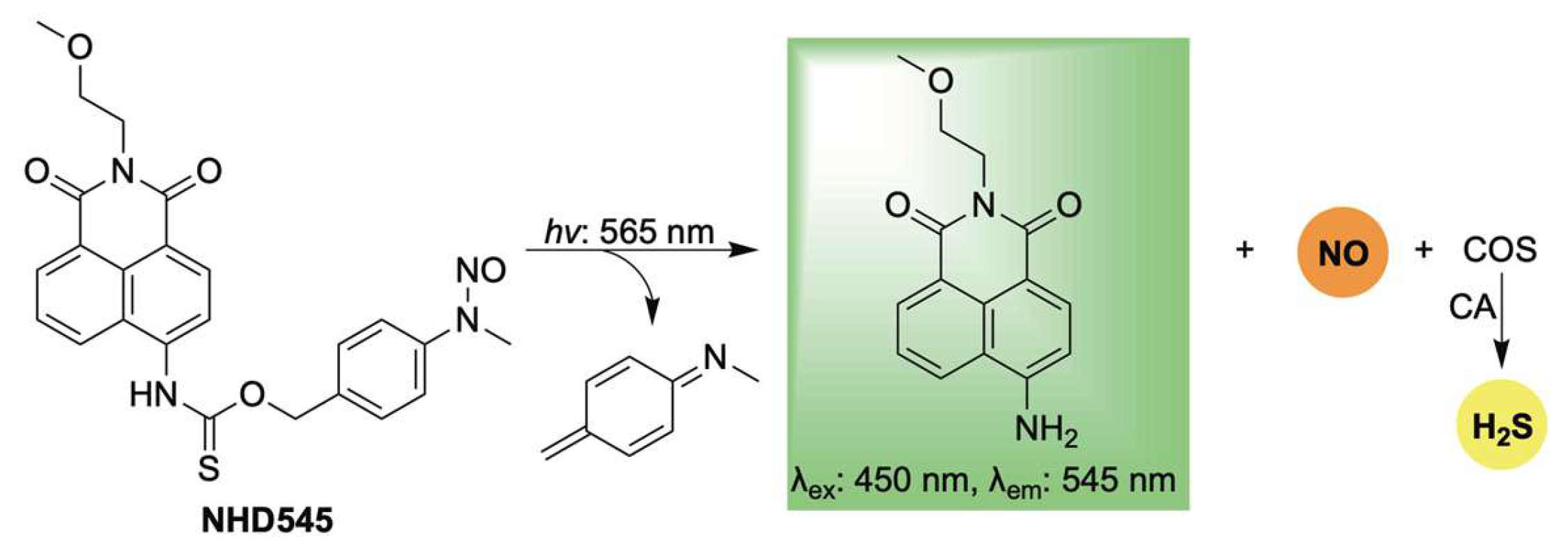
Lastly, Jiang, Li, and Zhang reported a novel photoresponsive dual-gas donor, NHD545, capable of simultaneously releasing NO and H2S via COS hydrolysis, with real-time monitoring enabled by turn-on fluorescence (Scheme 15) [108]. A key advantage of NHD545 is its ability to deliver both gasotransmitters from a single molecule, eliminating the need for separate donors. Moreover, the dual release is accompanied by enhanced fluorescence, allowing for straightforward qualitative analysis without auxiliary techniques specific to both gases. NHD545 exhibited notable anti-inflammatory effects, with both NO and H2S contributing to the regulation of superoxide dismutase (SOD) and lactate dehydrogenase (LDH) levels—highlighting their synergistic roles in inflammation modulation. In vivo studies in zebrafish further confirmed UV-triggered release of both gases, underscoring the potential of NHD545 for investigating the cooperative biological functions of nitric oxide and hydrogen sulfide.
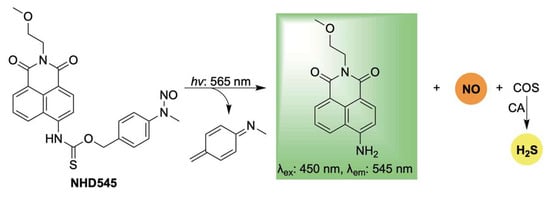
Scheme 15.
Proposed mechanism for light-activated dual release of NO and H2S, in conjunction with turn-on fluorescence.
2.4. Enzyme-Activation
Enzymes have been extensively utilized for prodrug activation and targeted drug delivery [109]. Compared to other stimuli, they offer remarkable specificity and rapid kinetics. Additionally, their catalytic nature enables activation without depleting biologically important analytes or disrupting cellular homeostasis.
Among these, esterases—ubiquitous enzymes that hydrolyze ester bonds—have been harnessed to develop efficient H2S donors with tunable release profiles [110,111]. However, to our knowledge, previously reported esterase-activated donors lack self-reporting capabilities for tracking H2S delivery.
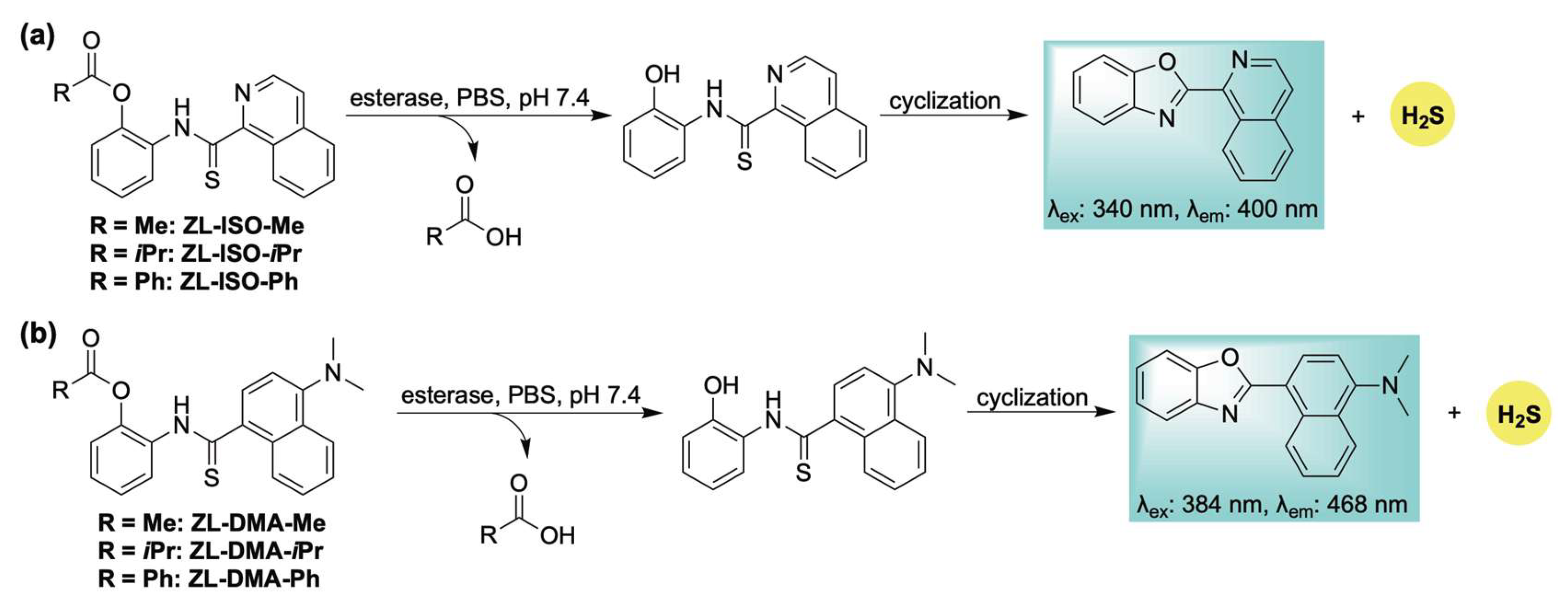
To address this limitation, we designed a library of esterase-responsive H2S donors that generate benzoxazole-based fluorophores as byproducts, enabling real-time monitoring of gas release [112]. Building on our previously reported ROS-activated system (Scheme 10), we hypothesized that incorporating an ester functionality would yield an esterase-sensitive scaffold capable of self-reporting H2S release through the formation of an isoquinoline-based fluorophore (Scheme 16a). To tune the release kinetics, a series of donors featuring ester groups with varying steric and electronic properties was synthesized. Methylene blue assays revealed distinct H2S release rates across the series (methyl > isopropyl > phenyl), consistent with the expected steric and electronic influences. These findings were corroborated by fluorescence measurements of the corresponding fluorophore. H2S release in live human cells was confirmed using the H2S-selective probe SS2. However, the self-reporting capability of this series was limited in cellular environments due to the poor photophysical properties of the isoquinoline fluorophore, necessitating high concentrations (500 µM) for effective visualization in cellulo. To address this limitation, an additional series was developed wherein intramolecular cyclization produced a fluorescent byproduct with bathochromically shifted excitation (384 nm) and emission (468 nm) wavelengths (Scheme 16b). Although kinetic analysis indicated slower H2S release—likely due to the same electronic effects responsible for enhancing fluorophore performance—this new scaffold enabled direct visualization of donor activation and H2S release in live human cells via confocal microscopy.
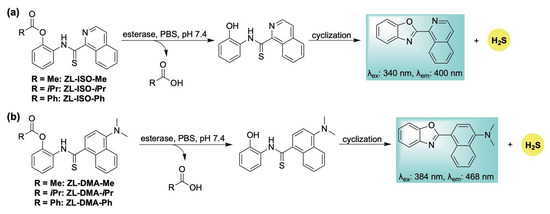
Scheme 16.
Esterase-responsive H2S donors: (a) ZL-ISO-Me, ZL-ISO-iPr, and ZL-ISO-Ph; and (b) ZL-DMA-Me, ZL-DMA-iPr, and ZL-DMA-Ph, along with their proposed mechanisms for H2S release and concomitant fluorophore generation.
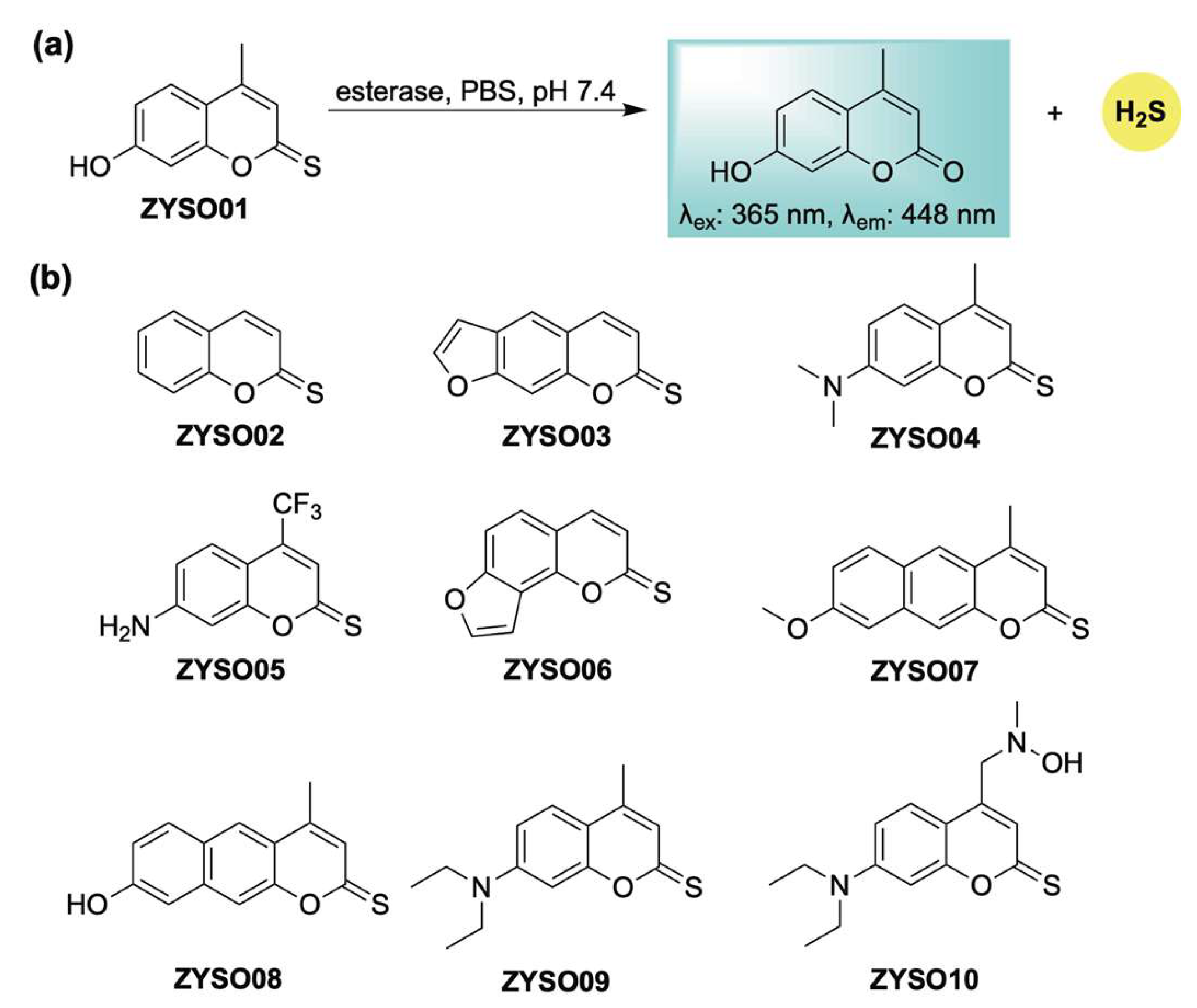
Following our initial report, Guo and Zheng developed an esterase-sensitive thiocoumarin series with self-reporting capabilities (Scheme 17) [113]. This strategy relies on esterase-catalyzed hydrolysis of thionoesters, enabling real-time fluorescent tracking of H2S liberation (Scheme 17a). In vivo evaluation in a mouse model demonstrated favorable organ distribution, with fluorescence imaging revealing pronounced accumulation of the donor in the kidneys, brain, and liver. These thiocoumarin derivatives also offered therapeutic benefits attributed to both H2S and the coumarin scaffold—especially in the treatment of cerebral infarction. In particular, ZYSO01 and its analogs (Scheme 17b) were assessed using a transient middle cerebral artery occlusion (tMCAO) model. These compounds significantly reduced infarct size compared to controls, an effect attributed to the combined pharmacological actions of H2S and the coumarin core. Biochemical analysis further indicated that this donor platform had a favorable safety profile, exhibiting reduced hepatotoxicity relative to traditional coumarin derivatives, as evidenced by lower markers of liver and kidney dysfunction in treated animals.
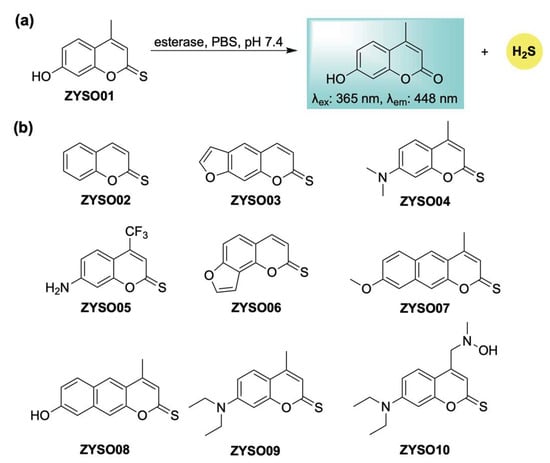
Scheme 17.
(a) Proposed mechanism for esterase-mediated hydrolysis of thiocoumarin-based donors, resulting in H2S release in conjunction with turn-on fluorescence. (b) Representative thiocoumarin derivatives examined in this study.
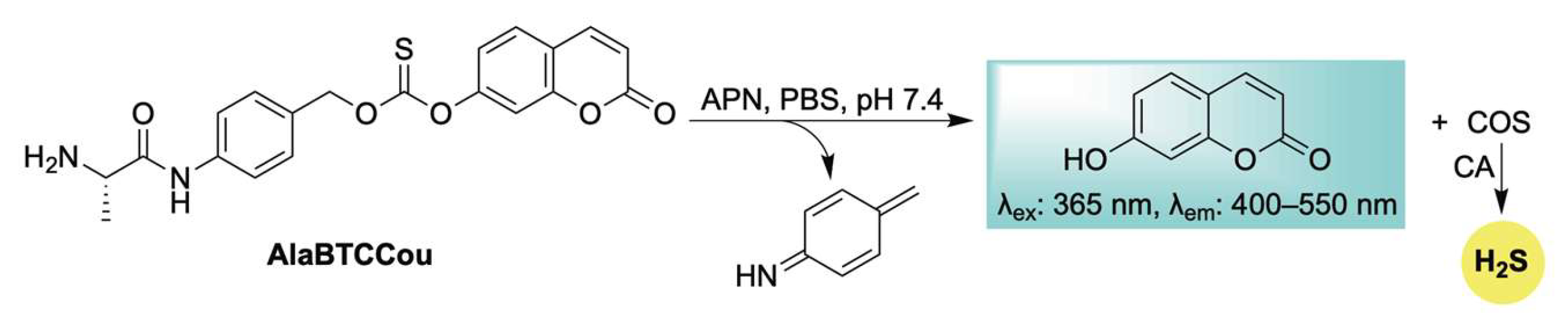
Expanding the repertoire of enzyme-responsive H2S donors with built-in fluorescence reporting, Zhao, Yuan, and Wang introduced AlaBTCCou—a self-reporting COS/H2S donor activated by aminopeptidase N (APN) (Scheme 18) [114]. Upon APN-mediated cleavage, a 1,6-elimination cascade—as seen throughout this review—is triggered, resulting in the simultaneous release of COS and a coumarin fluorophore (Scheme 18). Due to the elevated expression of APN at inflamed sites, the authors showed that targeting this enzyme enables a dual-purpose approach for both imaging and therapeutic applications. For example, in LPS-stimulated RAW 264.7 cells, AlaBTCCou was effectively activated, leading to H2S release accompanied by turn-on fluorescence. In addition, AlaBTCCou was shown to inhibit the excessive production of nitric oxide (NO), a hallmark of inflammation, emphasizing its anti-inflammatory properties and potential for tracking inflamed sites.
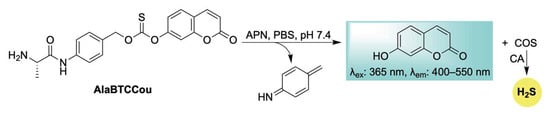
Scheme 18.
Proposed mechanism for APN-triggered activation of AlaBTCCou, leading to COS/H2S release in conjunction with self-reporting fluorescence.
2.5. Bioorthogonal-Activation
Bioorthogonal chemistry refers to chemical reactions that can occur within living systems without disrupting native biochemical processes [115,116,117]. While often challenging synthetically to identify suitable reaction partners that meet these criteria, this activation strategy does offer several notable advantages, including exceptional selectivity, precise spatiotemporal control, high modularity in design, and strong compatibility with in vivo applications. Owing to these benefits, bioorthogonal approaches have naturally been incorporated into the design of H2S donors [118].
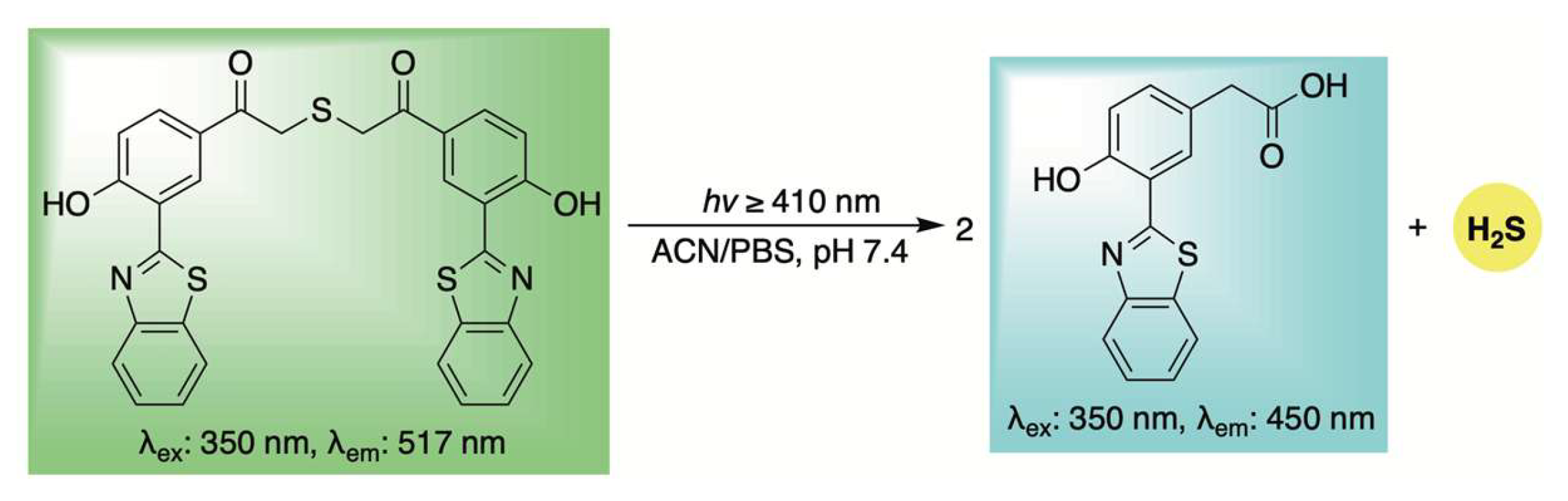
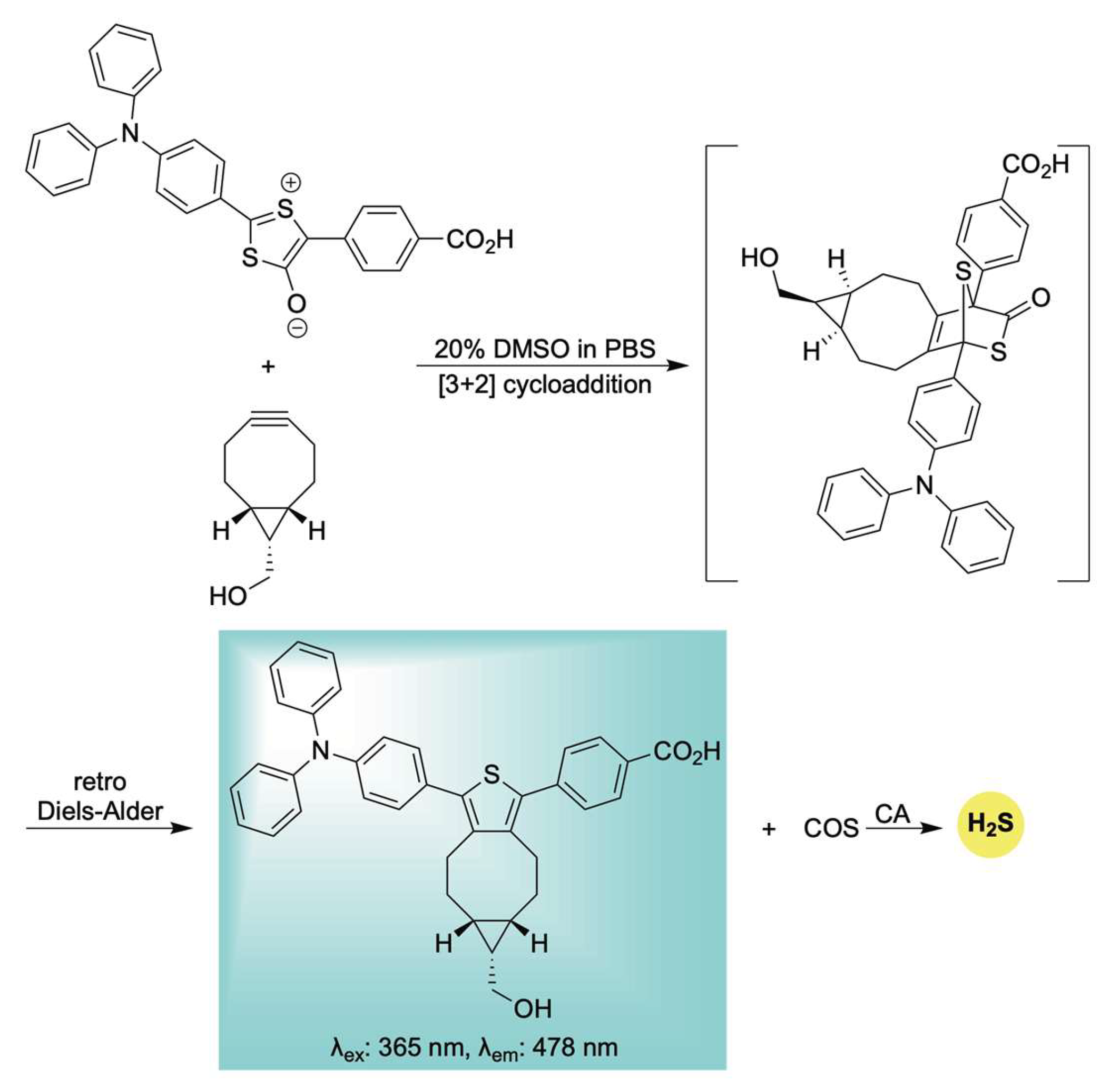
Building upon this earlier work, Liang et al. developed a unique delivery platform utilizing a bioorthogonal click-and-release mechanism to afford a fluorescent thiophene cycloadduct in conjunction with COS/H2S delivery (Scheme 19) [119]. The incorporation of a triphenylamine (TPA) unit served to red-shift the emission of the resulting thiophene fluorophore via a strong push–pull electronic effect. Interestingly, the TPA moiety also appeared to promote mitochondrial targeting, likely due to its lipophilic and cationic character—properties reminiscent of the more conventional triphenylphosphonium moiety [120]. Biocompatibility assays demonstrated low toxicity toward H9c2 cells, confirming the suitability of this system for use in live-cell environments. Notably, the mitochondria-targeted release of H2S conferred significant protection against H2O2-induced oxidative stress and mitochondrial membrane damage. These findings underscore the system’s potential as a multifunctional tool for H2S biology, offering both effective mitochondrial delivery and real-time monitoring of its physiological effects.
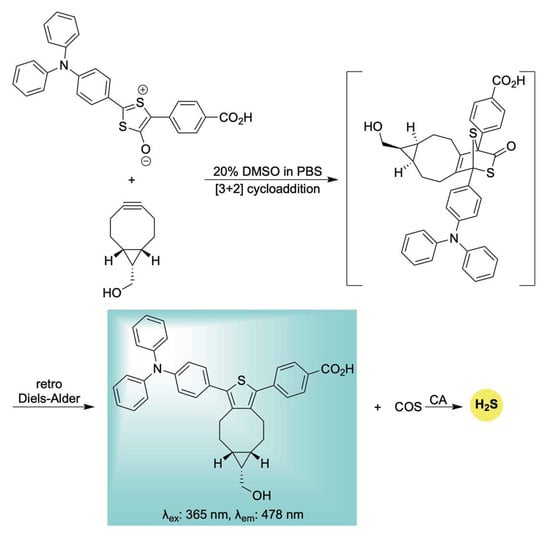
Scheme 19.
Proposed mechanism for the biorthogonal-triggered co-release of COS/H2S in conjunction with self-reporting fluorescence.
3. Conclusions and Outlook
The development of self-reporting, stimuli-responsive H2S donors has significantly advanced the study and manipulation of reactive sulfur species in living systems. As discussed throughout this review, a diverse array of donor architectures has been developed, each offering unique strengths in terms of activation mechanisms, release kinetics, targeting precision, and fluorescent self-reporting capabilities. Together, these donors have become powerful tools for elucidating the complex roles of H2S in cellular signaling and redox regulation, while also underscoring the therapeutic promise of controlled exogenous H2S delivery.
Despite significant progress, several challenges persist. Numerous donor systems still face challenges such as inefficient H2S release and insufficient control over where and when release occurs in vivo. Given the intricate nature of sulfur signaling pathways, there is a pressing need for enhanced selectivity and tunability—particularly for multifunctional donors intended for therapeutic co-delivery or theranostic use. Advancing modular donor designs, incorporating disease-relevant activation triggers, and coupling these systems with high-resolution imaging modalities will be crucial for overcoming current limitations and expanding their utility.
Looking ahead, the next generation of self-reporting H2S donors are expected to push the boundaries of precision medicine. Innovations in targeting strategies, such as organelle-specific or disease–environment–responsive systems, alongside advances in non-invasive activation methods (e.g., near-infrared light or ultrasound) are expected to enhance control and specificity. Additionally, the incorporation of multi-modal activation strategies and multichannel fluorescence sensing will enable superior spatiotemporal resolution, paving the way for powerful new platforms in basic research, diagnostics, and therapeutic development. Moreover, interdisciplinary collaboration between chemists, biologists, and clinicians will be critical for translating these tools from benchtop to bedside. By overcoming existing limitations and harnessing emerging technologies, self-reporting H2S donors have the potential to provide deeper insights into sulfide-mediated biological processes and open new avenues for disease diagnosis and therapy.
Author Contributions
Conceptualization, C.Z. and J.C.L.; investigation, C.Z. and J.C.L.; writing—original draft preparation, C.Z.; writing—review and editing, J.C.L.; supervision, J.C.L.; funding acquisition, J.C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Science Foundation (Grant No. 2143826).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Szabo, C. A Timeline of Hydrogen Sulfide (H2S) Research: From Environmental Toxin to Biological Mediator. Biochem. Pharmacol. 2018, 149, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.W.; Davenport, S.J. Hydrogen Sulphide Literature. Public Health Rep. 1924, 39, 1–13. [Google Scholar] [CrossRef]
- Beauchamp, R.O.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A.; Leber, P. A Critical Review of the Literature on Hydrogen Sulfide Toxicity. CRC Crit. Rev. Toxicol. 1984, 13, 25–97. [Google Scholar] [CrossRef] [PubMed]
- Reiffenstein, R.J.; Hulbert, W.C.; Roth, S.H. Toxicology of Hydrogen Sulfide. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 109–134. [Google Scholar] [CrossRef]
- Knight, L.D.; Presnell, S.E. Death by Sewer Gas: Case Report of a Double Fatality and Review of the Literature. Am. J. Forensic Med. Pathol. 2005, 26, 181–185. [Google Scholar] [CrossRef]
- Smith, R. A Short History of Hydrogen Sulfide. Am. Sci. 2010, 98, 6. [Google Scholar] [CrossRef]
- Du Vigneaud, V.; Loring, H.S.; Craft, H.A. The Oxidation of the Sulfur of Homocystine, Methionine, and S-Methylcysteine in the Animal Body. J. Biol. Chem. 1934, 105, 481–488. [Google Scholar] [CrossRef]
- Du Vigneaud, V.; Brown, G.B.; Chandler, J.P. The Synthesis of ll-S-(β-Amino-β-Carboxyethyl) Homocysteine and the Replacement by It of Cys-Tine in the Diet. J. Biol. Chem. 1942, 143, 59–64. [Google Scholar] [CrossRef]
- Binkley, F.; Du Vigneaud, V. The Formation of Cysteine from Homocysteine and Serine by Liver Tissue of Rats. J. Biol. Chem. 1942, 144, 507–511. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The Possible Role of Hydrogen Sulfide as an Endogenous Neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef]
- Wang, R. Two’s Company, Three’s a Crowd: Can H2S Be the Third Endogenous Gaseous Transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef]
- Wang, R. The Gasotransmitter Role of Hydrogen Sulfide. Antioxid. Redox Signal. 2003, 5, 493–501. [Google Scholar] [CrossRef]
- Wang, R. Shared Signaling Pathways among Gasotransmitters. Proc. Natl. Acad. Sci. USA 2012, 109, 8801–8802. [Google Scholar] [CrossRef]
- Papapetropoulos, A.; Foresti, R.; Ferdinandy, P. Pharmacology of the ‘Gasotransmitters’ NO, CO and H2S: Translational Opportunities. Br. J. Pharmacol. 2015, 172, 1395–1396. [Google Scholar] [CrossRef]
- Hughes, M.N.; Centelles, M.N.; Moore, K.P. Making and Working with Hydrogen Sulfide. Free Radic. Biol. Med. 2009, 47, 1346–1353. [Google Scholar] [CrossRef]
- Cuevasanta, E.; Denicola, A.; Alvarez, B.; Möller, M.N. Solubility and Permeation of Hydrogen Sulfide in Lipid Membranes. PLoS ONE 2012, 7, e34562. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R. Persulfidation (S-Sulfhydration) and H2S. In Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide; Moore, P.K., Whiteman, M., Eds.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2015; Volume 230, pp. 29–59. ISBN 978-3-319-18143-1. [Google Scholar]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling Through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef] [PubMed]
- Vignane, T.; Filipovic, M.R. Emerging Chemical Biology of Protein Persulfidation. Antioxid. Redox Signal. 2023, 39, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.W.; Kraus, J.P. Cystathionine β-Synthase: Structure, Function, Regulation, and Location of Homocystinuria-Causing Mutations. J. Biol. Chem. 2004, 279, 29871–29874. [Google Scholar] [CrossRef]
- Pan, L.L.; Liu, X.H.; Gong, Q.H.; Yang, H.B.; Zhu, Y.Z. Role of Cystathionine γ-Lyase/Hydrogen Sulfide Pathway in Cardiovascular Disease: A Novel Therapeutic Strategy? Antioxid. Redox Signal. 2012, 17, 106–118. [Google Scholar] [CrossRef]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate Sulfurtransferase Produces Hydrogen Sulfide and Bound Sulfane Sulfur in the Brain. Antioxid. Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef]
- Bhatia, M. Hydrogen Sulfide as a Vasodilator. IUBMB Life 2005, 57, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Popescu, C.; Vlădulescu-Trandafir, A.-I.; Onose, G. Signaling Paradigms of H2S-Induced Vasodilation: A Comprehensive Review. Antioxidants 2024, 13, 1158. [Google Scholar] [CrossRef] [PubMed]
- White, B.J.O.; Smith, P.A.; Dunn, W.R. Hydrogen Sulphide–Mediated Vasodilatation Involves the Release of Neurotransmitters from Sensory Nerves in Pressurized Mesenteric Small Arteries Isolated from Rats. Br. J. Pharmacol. 2013, 168, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W. The Vasorelaxant Effect of H2S as a Novel Endogenous Gaseous KATP Channel Opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Köhn, C.; Dubrovska, G.; Huang, Y.; Gollasch, M. Hydrogen Sulfide: Potent Regulator of Vascular Tone and Stimulator of Angiogenesis. Int. J. Biomed. Sci. 2012, 8, 81–86. [Google Scholar]
- Zhu, X.-Y.; Gu, H.; Ni, X. Hydrogen Sulfide in the Endocrine and Reproductive Systems. Expert Rev. Clin. Pharmacol. 2011, 4, 75–82. [Google Scholar] [CrossRef]
- Guo, W.; Kan, J.; Cheng, Z.; Chen, J.; Shen, Y.; Xu, J.; Wu, D.; Zhu, Y. Hydrogen Sulfide as an Endogenous Modulator in Mitochondria and Mitochondria Dysfunction. Oxidative Med. Cell. Longev. 2012, 2012, 878052. [Google Scholar] [CrossRef][Green Version]
- Huang, D.; Jing, G.; Zhu, S. Regulation of Mitochondrial Respiration by Hydrogen Sulfide. Antioxidants 2023, 12, 1644. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H.; Kashfi, K. Effects of Hydrogen Sulfide on Mitochondrial Function and Cellular Bioenergetics. Redox Biol. 2021, 38, 101772. [Google Scholar] [CrossRef]
- Li, M.; Mao, J.; Zhu, Y. New Therapeutic Approaches Using Hydrogen Sulfide Donors in Inflammation and Immune Response. Antioxid. Redox Signal. 2021, 35, 341–356. [Google Scholar] [CrossRef]
- Whiteman, M.; Winyard, P.G. Hydrogen Sulfide and Inflammation: The Good, the Bad, the Ugly and the Promising. Expert Rev. Clin. Pharmacol. 2011, 4, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Blackler, R.W.; Chan, M.V.; Da Silva, G.J.; Elsheikh, W.; Flannigan, K.L.; Gamaniek, I.; Manko, A.; Wang, L.; Motta, J.-P.; et al. Anti-Inflammatory and Cytoprotective Actions of Hydrogen Sulfide: Translation to Therapeutics. Antioxid. Redox Signal. 2015, 22, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Corvino, A.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Scognamiglio, A.; Caliendo, G.; Fiorino, F.; Severino, B. Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases. Antioxidants 2021, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Lukesh, J.C. H2S Donors with Cytoprotective Effects in Models of MI/R Injury and Chemotherapy-Induced Cardiotoxicity. Antioxidants 2023, 12, 650. [Google Scholar] [CrossRef]
- Carballal, S.; Trujillo, M.; Cuevasanta, E.; Bartesaghi, S.; Möller, M.N.; Folkes, L.K.; García-Bereguiaín, M.A.; Gutiérrez-Merino, C.; Wardman, P.; Denicola, A.; et al. Reactivity of Hydrogen Sulfide with Peroxynitrite and Other Oxidants of Biological Interest. Free Radic. Biol. Med. 2011, 50, 196–205. [Google Scholar] [CrossRef]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen Sulfide Mediates Cardioprotection Through Nrf2 Signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef]
- Qiu, Y.; Wu, Y.; Meng, M.; Luo, M.; Zhao, H.; Sun, H.; Gao, S. GYY4137 Protects against Myocardial Ischemia/Reperfusion Injury via Activation of the PHLPP-1/Akt/Nrf2 Signaling Pathway in Diabetic Mice. J. Surg. Res. 2018, 225, 29–39. [Google Scholar] [CrossRef]
- Hu, Q.; Yammani, R.D.; Brown-Harding, H.; Soto-Pantoja, D.R.; Poole, L.B.; Lukesh, J.C. Mitigation of Doxorubicin-Induced Cardiotoxicity with an H2O2-Activated, H2S-Donating Hybrid Prodrug. Redox Biol. 2022, 53, 102338. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Qian, L.-L.; Wang, R.-X. Hydrogen Sulfide-Induced Vasodilation: The Involvement of Vascular Potassium Channels. Front. Pharmacol. 2022, 13, 911704. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Miljkovic, J.L.; Nauser, T.; Royzen, M.; Klos, K.; Shubina, T.; Koppenol, W.H.; Lippard, S.J.; Ivanović-Burmazović, I. Chemical Characterization of the Smallest S-Nitrosothiol, HSNO; Cellular Cross-Talk of H2S and S-Nitrosothiols. J. Am. Chem. Soc. 2012, 134, 12016–12027. [Google Scholar] [CrossRef]
- Chatzianastasiou, A.; Bibli, S.-I.; Andreadou, I.; Efentakis, P.; Kaludercic, N.; Wood, M.E.; Whiteman, M.; Di Lisa, F.; Daiber, A.; Manolopoulos, V.G.; et al. Cardioprotection by H2S Donors: Nitric Oxide-Dependent and -Independent Mechanisms. J. Pharmacol. Exp. Ther. 2016, 358, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Sojitra, B.; Bulani, Y.; Putcha, U.K.; Kanwal, A.; Gupta, P.; Kuncha, M.; Banerjee, S.K. Nitric Oxide Synthase Inhibition Abrogates Hydrogen Sulfide-Induced Cardioprotection in Mice. Mol. Cell. Biochem. 2012, 360, 61–69. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A Review of Hydrogen Sulfide (H2S) Donors: Chemistry and Potential Therapeutic Applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hamsath, A.; Neill, D.L.; Wang, Y.; Yang, C.; Xian, M. Strategies for the Design of Donors and Precursors of Reactive Sulfur Species. Chem. A Eur. J. 2019, 25, 4005–4016. [Google Scholar] [CrossRef] [PubMed]
- Levinn, C.M.; Cerda, M.M.; Pluth, M.D. Activatable Small-Molecule Hydrogen Sulfide Donors. Antioxid. Redox Signal. 2020, 32, 96–109. [Google Scholar] [CrossRef]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.-H.; Moore, P.K. Characterization of a Novel, Water-Soluble Hydrogen Sulfide–Releasing Molecule (GYY4137): New Insights into the Biology of Hydrogen Sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef]
- Chan, J.; Dodani, S.C.; Chang, C.J. Reaction-Based Small-Molecule Fluorescent Probes for Chemoselective Bioimaging. Nat. Chem. 2012, 4, 973–984. [Google Scholar] [CrossRef]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical Probes for Molecular Imaging and Detection of Hydrogen Sulfide and Reactive Sulfur Species in Biological Systems. Chem. Soc. Rev. 2015, 44, 4596–4618. [Google Scholar] [CrossRef]
- Chen, G.; Yu, J.; Wu, L.; Ji, X.; Xu, J.; Wang, C.; Ma, S.; Miao, Q.; Wang, L.; Wang, C.; et al. Fluorescent Small Molecule Donors. Chem. Soc. Rev. 2024, 53, 6345–6398. [Google Scholar] [CrossRef]
- Misra, R.; Bhuyan, H.J.; Dutta, A.; Bhabak, K.P. Recent Developments on Activatable Turn-On Fluorogenic Donors of Hydrogen Sulfide (H2S). ChemMedChem 2024, 19, e202400251. [Google Scholar] [CrossRef] [PubMed]
- Steiger, A.K.; Pardue, S.; Kevil, C.G.; Pluth, M.D. Self-Immolative Thiocarbamates Provide Access to Triggered H2S Donors and Analyte Replacement Fluorescent Probes. J. Am. Chem. Soc. 2016, 138, 7256–7259. [Google Scholar] [CrossRef]
- Lippert, A.R.; New, E.J.; Chang, C.J. Reaction-Based Fluorescent Probes for Selective Imaging of Hydrogen Sulfide in Living Cells. J. Am. Chem. Soc. 2011, 133, 10078–10080. [Google Scholar] [CrossRef] [PubMed]
- Henthorn, H.A.; Pluth, M.D. Mechanistic Insights into the H2S-Mediated Reduction of Aryl Azides Commonly Used in H2S Detection. J. Am. Chem. Soc. 2015, 137, 15330–15336. [Google Scholar] [CrossRef]
- Levinn, C.M.; Cerda, M.M.; Pluth, M.D. Development and Application of Carbonyl Sulfide-Based Donors for H2S Delivery. Acc. Chem. Res. 2019, 52, 2723–2731. [Google Scholar] [CrossRef]
- Zhao, Y.; Cerda, M.M.; Pluth, M.D. Fluorogenic Hydrogen Sulfide (H2S) Donors Based on Sulfenyl Thiocarbonates Enable H2S Tracking and Quantification. Chem. Sci. 2019, 10, 1873–1878. [Google Scholar] [CrossRef]
- Monks, T.J.; Hanzlik, R.P.; Cohen, G.M.; Ross, D.; Graham, D.G. Quinone Chemistry and Toxicity. Toxicol. Appl. Pharmacol. 1992, 112, 2–16. [Google Scholar] [CrossRef]
- Monks, T.; Jones, D. The Metabolism and Toxicity of Quinones, Quinonimines, Quinone Methides, and Quinone-Thioethers. Curr. Drug Metab. 2002, 3, 425–438. [Google Scholar] [CrossRef]
- Pluth, M.D. Moving Past Quinone-Methides: Recent Advances Toward Minimizing Electrophilic Byproducts from COS/H2S Donors. Curr. Top. Med. Chem. 2021, 21, 2882–2889. [Google Scholar] [CrossRef]
- Mahato, S.K.; Bhattacherjee, D.; Bhabak, K.P. The Biothiol-Triggered Organotrisulfide-Based Self-Immolative Fluorogenic Donors of Hydrogen Sulfide Enable Lysosomal Trafficking. Chem. Commun. 2020, 56, 7769–7772. [Google Scholar] [CrossRef] [PubMed]
- Mahato, S.K.; Bhattacherjee, D.; Barman, P.; Bhabak, K.P. Thioredoxin Reductase-Triggered Fluorogenic Donor of Hydrogen Sulfide: A Model Study with a Symmetrical Organopolysulfide Probe with Turn-On Near-Infrared Fluorescent Emission. J. Mater. Chem. B 2022, 10, 2183–2193. [Google Scholar] [CrossRef]
- Zhao, X.; Ning, L.; Zhou, X.; Song, Z.; Zhang, J.; Guan, F.; Yang, X.-F. An Activatable Near-Infrared Fluorescence Hydrogen Sulfide (H2S) Donor for Imaging H2S Release and Inhibiting Inflammation in Cells. Anal. Chem. 2021, 93, 4894–4901. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Bai, X.; Li, P.; Su, D.; Zhang, W.; Zhang, W.; Tang, B. Highly Specific Cys Fluorescence Probe for Living Mouse Brain Imaging via Evading Reaction with Other Biothiols. Anal. Chem. 2019, 91, 8591–8594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, C.; Chen, Y.; Cai, X.; Sheng, W.; Zhu, H.; Jia, P.; Li, Z.; Huang, S.; Zhu, B. Visualization of the Cysteine Level During Golgi Stress Using a Novel Golgi-Targeting Highly Specific Fluorescent Probe. Chem. Commun. 2020, 56, 1807–1810. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Testai, L.; Citi, V.; Marino, A.; Bellagambi, F.G.; Ghimenti, S.; Breschi, M.C.; Calderone, V. Pharmacological Characterization of the Vascular Effects of Aryl Isothiocyanates: Is Hydrogen Sulfide the Real Player? Vasc. Pharmacol. 2014, 60, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, X.; Lu, Y.; Liang, D.; Huang, D. Isothiocyanates as H2S Donors Triggered by Cysteine: Reaction Mechanism and Structure and Activity Relationship. Org. Lett. 2019, 21, 5977–5980. [Google Scholar] [CrossRef]
- Hong, J.; Feng, G. Isothiocyanate Can Be Used as a Highly Specific Recognition Site for Fluorescent Cysteine Probes. Sens. Actuators B Chem. 2021, 326, 129016. [Google Scholar] [CrossRef]
- Ge, C.; Wang, H.; Ni, T.; Yang, Z.; Chang, K. Red-Emitting Fluorescent Turn-on Probe with Specific Isothiocyanate Recognition Site for Cysteine Imaging in Living Systems. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 259, 119826. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, C.; Rong, X.; Zhang, Y.; Su, M.; Wang, X.; Liu, M.; Zhang, X.; Sheng, W.; Zhu, B. A New Isothiocyanate-Based Golgi-Targeting Fluorescent Probe for Cys and Its Bioimaging Applications During the Golgi Stress Response. Bioorganic Chem. 2022, 122, 105741. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Fu, Y.; Meng, M.; Jin, H.; Zhao, W. An Isothiocyanate-Functionalized Self-Immolative Near-Infrared Fluorescence Probe for Cysteine Sensing and Bioimaging in Living Systems. Sens. Actuators B Chem. 2022, 366, 132013. [Google Scholar] [CrossRef]
- Zhao, X.; Ding, M.; Ning, L.; Yuan, F.; Li, J.; Guo, Y.; Mu, Y.; Zhang, J. Biothiol-Triggered H2S Release from a Near-Infrared Fluorescent H2S Donor Promotes Cutaneous Wound Healing. Acta Mater. Medica 2022, 1, 476–485. [Google Scholar] [CrossRef]
- Mahato, S.K.; Barman, P.; Badirujjaman, M.; Bhabak, K.P. Cysteine-Responsive Prodrug of the Anti-Cancer Drug Amonafide: Fluorogenic Adjuvant Drug Delivery with Hydrogen Sulfide (H2S). Chem. Commun. 2023, 59, 4802–4805. [Google Scholar] [CrossRef]
- Averill-Bates, D. Reactive Oxygen Species and Cell Signaling. Review. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Cunningham-Bussel, A. Beyond Oxidative Stress: An Immunologist’s Guide to Reactive Oxygen Species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting Cancer Cells by ROS-Mediated Mechanisms: A Radical Therapeutic Approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative Diseases and Oxidative Stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.; Fang, Y.; Shi, W.; Li, X.; Chen, W.; Xian, M.; Ma, H. Reactive Oxygen Species-Triggered Off-On Fluorescence Donor for Imaging Hydrogen Sulfide Delivery in Living Cells. Chem. Sci. 2019, 10, 7690–7694. [Google Scholar] [CrossRef]
- Lippert, A.R.; Van De Bittner, G.C.; Chang, C.J. Boronate Oxidation as a Bioorthogonal Reaction Approach for Studying the Chemistry of Hydrogen Peroxide in Living Systems. Acc. Chem. Res. 2011, 44, 793–804. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z. A Fluorogenic H2S Donor Activated by Reactive Oxygen Species for Real-Time Monitoring in Cells and In Vivo. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120243. [Google Scholar] [CrossRef] [PubMed]
- Sufian, A.; Badirujjaman, M.; Barman, P.; Bhabak, K.P. Dual-Stimuli-Activatable Hybrid Prodrug for the Self-Immolative Delivery of an Anticancer Agent and Hydrogen Sulfide with Turn-On Fluorescence. Chem. A Eur. J. 2023, 29, e202302197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Hu, P.; Wang, Y.; Tang, Q.; Zheng, Q.; Wang, Z.; He, Y. A Reactive Oxygen Species (ROS) Activated Hydrogen Sulfide (H2S) Donor with Self-Reporting Fluorescence. ACS Sens. 2020, 5, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, M.; Zhao, F.; Song, X.; Ye, Y. ONOO−-Triggered Fluorescence H2S Donor for Mitigating Drug-Induced Liver Injury. Sens. Actuators B Chem. 2023, 378, 133131. [Google Scholar] [CrossRef]
- Li, J.; Lim, C.S.; Kim, G.; Kim, H.M.; Yoon, J. Highly Selective and Sensitive Two-Photon Fluorescence Probe for Endogenous Peroxynitrite Detection and Its Applications in Living Cells and Tissues. Anal. Chem. 2017, 89, 8496–8500. [Google Scholar] [CrossRef]
- Luo, X.; Cheng, Z.; Wang, R.; Yu, F. Indication of Dynamic Peroxynitrite Fluctuations in the Rat Epilepsy Model with a Near-Infrared Two-Photon Fluorescent Probe. Anal. Chem. 2021, 93, 2490–2499. [Google Scholar] [CrossRef]
- Sun, Q.; Xu, J.; Ji, C.; Shaibani, M.S.S.; Li, Z.; Lim, K.; Zhang, C.; Li, L.; Liu, Z. Ultrafast Detection of Peroxynitrite in Parkinson’s Disease Models Using a Near-Infrared Fluorescent Probe. Anal. Chem. 2020, 92, 4038–4045. [Google Scholar] [CrossRef]
- Zhu, C.; Suarez, S.I.; Lukesh, J.C. Illuminating and Alleviating Cellular Oxidative Stress with an ROS-Activated, H2S-Donating Theranostic. Tetrahedron Lett. 2021, 69, 152944. [Google Scholar] [CrossRef]
- Zhang, N.; Fu, T.; Li, T.; Zhong, P.; Li, L.; Peng, M.; Li, Z.; Zhang, L.; Wang, H.; Hu, P.; et al. A Superoxide Anion Responsive and Self-Reporting Fluorescent H2S Donor for the Treatment of Diabetic Wound. Free Radic. Biol. Med. 2025, 231, 109–119. [Google Scholar] [CrossRef]
- Hu, Q.; Zhu, C.; Hankins, R.A.; Murmello, A.R.; Marrs, G.S.; Lukesh, J.C. An ROS-Responsive Donor That Self-Reports Its H2S Delivery by Forming a Benzoxazole-Based Fluorophore. J. Am. Chem. Soc. 2023, 145, 25486–25494. [Google Scholar] [CrossRef]
- Tian, H.; Qian, J.; Bai, H.; Sun, Q.; Zhang, L.; Zhang, W. Micelle-Induced Multiple Performance Improvement of Fluorescent Probes for H2S Detection. Anal. Chim. Acta 2013, 768, 136–142. [Google Scholar] [CrossRef]
- Choi, S.-A.; Park, C.S.; Kwon, O.S.; Giong, H.-K.; Lee, J.-S.; Ha, T.H.; Lee, C.-S. Structural Effects of Naphthalimide-Based Fluorescent Sensor for Hydrogen Sulfide and Imaging in Live Zebrafish. Sci. Rep. 2016, 6, 26203. [Google Scholar] [CrossRef]
- Ai, X.; Mu, J.; Xing, B. Recent Advances of Light-Mediated Theranostics. Theranostics 2016, 6, 2439–2457. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, H.; Gu, X.; Tang, B.Z. Photoactivatable Biomedical Materials Based on Luminogens with Aggregation-Induced Emission (AIE) Characteristics. Adv. Healthc. Mater. 2021, 10, 2101177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Moralès, O.; Mordon, S.; Delhem, N.; Boleslawski, E. Could Photodynamic Therapy Be a Promising Therapeutic Modality in Hepatocellular Carcinoma Patients? A Critical Review of Experimental and Clinical Studies. Cancers 2021, 13, 5176. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, Y.; Das, J.; Chaudhuri, A.; Karmakar, A.; Maiti, T.K.; Singh, N.D.P. Light Triggered Uncaging of Hydrogen Sulfide (H2S) with Real-Time Monitoring. Chem. Commun. 2018, 54, 3106–3109. [Google Scholar] [CrossRef]
- Dormán, G.; Prestwich, G.D. Using Photolabile Ligands in Drug Discovery and Development. Trends Biotechnol. 2000, 18, 64–77. [Google Scholar] [CrossRef]
- Edler, M.; Mayrbrugger, S.; Fian, A.; Trimmel, G.; Radl, S.; Kern, W.; Griesser, T. Wavelength Selective Refractive Index Modulation in a ROMP Derived Polymer Bearing Phenyl- and Ortho-Nitrobenzyl Ester Groups. J. Mater. Chem. C 2013, 1, 3931. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Bolton, S.G.; Pluth, M.D. Light-Activated COS/H2S Donation from Photocaged Thiocarbamates. Org. Lett. 2017, 19, 2278–2281. [Google Scholar] [CrossRef]
- Il’ichev, Y.V.; Schwörer, M.A.; Wirz, J. Photochemical Reaction Mechanisms of 2-Nitrobenzyl Compounds: Methyl Ethers and Caged ATP. J. Am. Chem. Soc. 2004, 126, 4581–4595. [Google Scholar] [CrossRef]
- Aujard, I.; Benbrahim, C.; Gouget, M.; Ruel, O.; Baudin, J.-B.; Neveu, P.; Jullien, L. O-Nitrobenzyl Photolabile Protecting Groups with Red-Shifted Absorption: Syntheses and Uncaging Cross-Sections for One- and Two-Photon Excitation. Chem. Eur. J. 2006, 12, 6865–6879. [Google Scholar] [CrossRef]
- Bochet, C.G. Photolabile Protecting Groups and Linkers. J. Chem. Soc. Perkin Trans. 1 2002, 125–142. [Google Scholar] [CrossRef]
- Hua, W.; Zhao, J.; Gou, S. A Naphthalimide Derivative Can Release COS and Form H2S in a Light-Controlled Manner and Protect Cells against ROS with Real-Time Monitoring Ability. Analyst 2020, 145, 3878–3884. [Google Scholar] [CrossRef]
- Yuan, F.; He, X.; Lu, Y.; Ning, L.; Zhao, X.; Zhang, S.; Guan, F.; Guo, Y.; Zhang, J. Photoactivated Hydrogen Sulfide Donor with a Near-Infrared Fluorescence Report System for Accelerated Chronic Wound Healing. Anal. Chem. 2023, 95, 6931–6939. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Guo, A.; Wang, L.; Ning, L.; Guo, Y.; Zhang, J. Tunable Light-Activated Platform for Controlled Hydrogen Sulfide Release with Tracking. Angew. Chem. Int. Ed. 2025, 64, e202501685. [Google Scholar] [CrossRef] [PubMed]
- Hanc, K.; Janeková, H.; Štacko, P. Concurrent Subcellular Delivery of Hydrogen Sulfide and a Payload with Near-Infrared Light. JACS Au 2024, 4, 2687–2694. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.; Lin, Z.; Cheng, Y.; Tang, Y.; Chen, Q.; Jiang, L.; Li, L.; Zhang, Z. A Photo-Triggered Dual-Gas Donor of Nitric Oxide and Hydrogen Sulfide with Fluorescence for Real-Time Monitoring of Its Release. Analyst 2025, 150, 378–385. [Google Scholar] [CrossRef]
- Yang, Y.; Aloysius, H.; Inoyama, D.; Chen, Y.; Hu, L. Enzyme-Mediated Hydrolytic Activation of Prodrugs. Acta Pharm. Sin. B 2011, 1, 143–159. [Google Scholar] [CrossRef]
- Chauhan, P.; Bora, P.; Ravikumar, G.; Jos, S.; Chakrapani, H. Esterase Activated Carbonyl Sulfide/Hydrogen Sulfide (H2S) Donors. Org. Lett. 2017, 19, 62–65. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, B.; Ji, K.; Pan, Z.; Chittavong, V.; Wang, B. Esterase-Sensitive Prodrugs with Tunable Release Rates and Direct Generation of Hydrogen Sulfide. Angew. Chem. Int. Ed. 2016, 55, 4514–4518. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, C.; Weaver, D.E.; Lukesh, J.C. Esterase-Activated Hydrogen Sulfide Donors with Self-Reporting Fluorescence Properties and Highly Tunable Rates of Delivery. ACS Chem. Biol. 2024, 19, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bai, X.; Peng, W.; Liu, J.; Jia, Z.; Cheng, M.; Li, J.; Guo, W.; Zheng, Y. A Thiocoumarin Based Self-Reporting Sulfide Prodrug Strategy with a Favorable Safety Profile. Eur. J. Med. Chem. 2025, 289, 117426. [Google Scholar] [CrossRef] [PubMed]
- Rong, F.; Bao, W.; Li, G.; Ge, Y.; Zhu, W.; Hao, B.; Zhao, Y.; Yuan, Y.; Wang, Y. Aminopeptidase N-Activated Self-Immolative Hydrogen Sulfide Donor for Inflammatory Response-Specific Wound Healing. Angew. Chem. Int. Ed. 2025, 64, e202423527. [Google Scholar] [CrossRef] [PubMed]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem. Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef]
- Prescher, J.A.; Dube, D.H.; Bertozzi, C.R. Chemical Remodelling of Cell Surfaces in Living Animals. Nature 2004, 430, 873–877. [Google Scholar] [CrossRef]
- Nguyen, S.S.; Prescher, J.A. Developing Bioorthogonal Probes to Span a Spectrum of Reactivities. Nat. Rev. Chem. 2020, 4, 476–489. [Google Scholar] [CrossRef]
- Steiger, A.K.; Yang, Y.; Royzen, M.; Pluth, M.D. Bio-Orthogonal “Click-and-Release” Donation of Caged Carbonyl Sulfide (COS) and Hydrogen Sulfide (H2S). Chem. Commun. 2017, 53, 1378–1380. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, R.; Tang, C.; Zhang, C.; Xu, W.; Wu, L.; Wang, Y.; Ye, D.; Liang, Y. Design and Development of a Bioorthogonal, Visualizable and Mitochondria-Targeted Hydrogen Sulfide (H2S) Delivery System. Angew. Chem. Int. Ed. 2022, 61, e202112734. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).