Synthesis of Silver Nanocluster-Loaded FAU Zeolites and the Application in Light Emitting Diode
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of R-FAU (R = Li, Na, K) Zeolite
2.3. Synthesis of Silver Clusters Loaded R-FAU (R = Li, Na, K) Zeolites
2.4. Fabrication of R-FAU/Ag (R = Li, Na, K) Phosphor-Based LED
2.5. Measurement
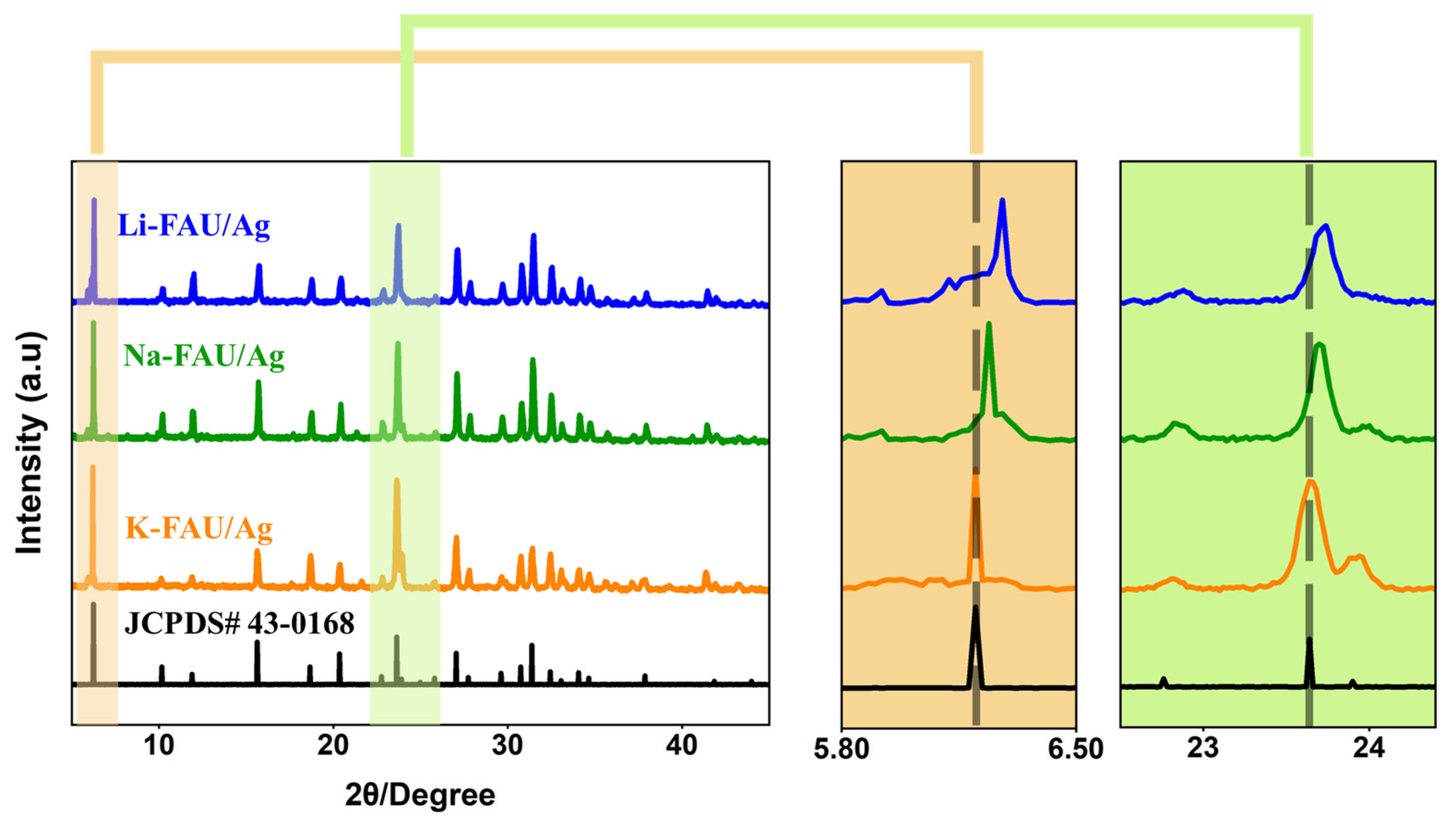
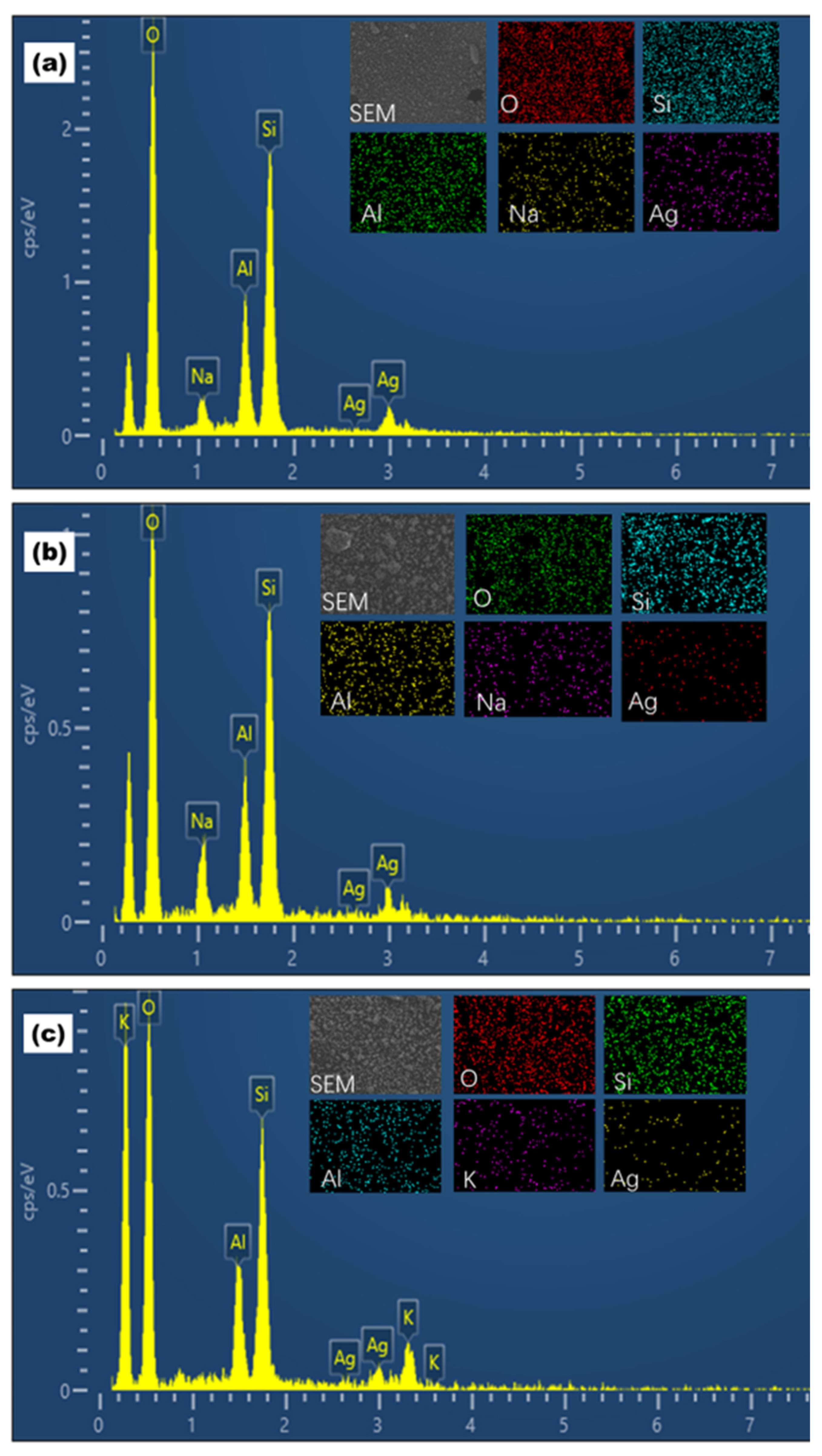
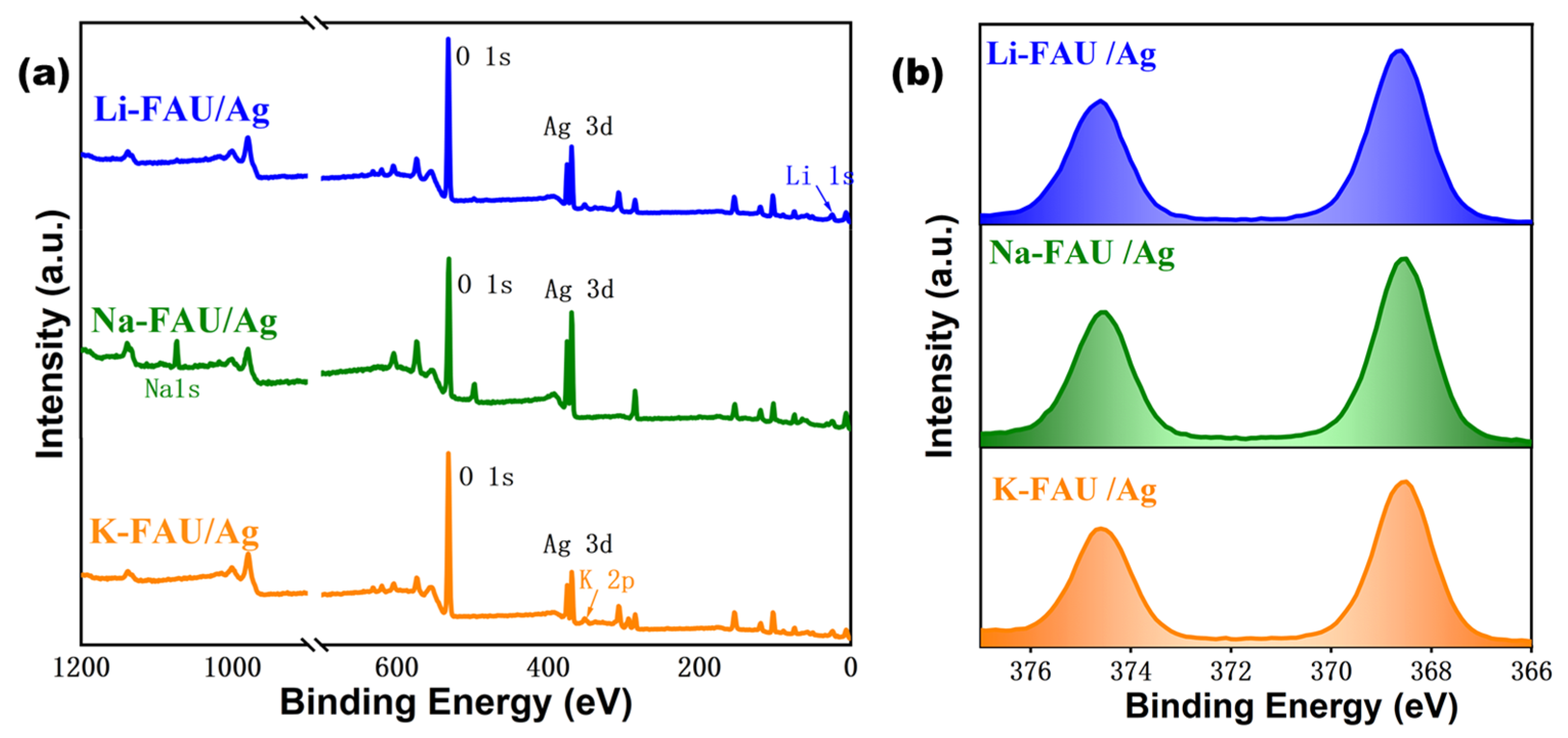
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Devaraj, V.; Lee, I.H.; Kim, M.; Nguyen, T.M.; Son, J.P.; Lee, J.-M.; Lee, D.; Kim, K.H.; Oh, J.-W. Unveiling facet effects in metallic nanoparticles to design an efficient plasmonic nanostructure. Curr. Appl. Phys. 2022, 44, 22–28. [Google Scholar] [CrossRef]
- Evstropiev, S.K.; Nikonorov, N.V.; Saratovskii, A.S. Double stabilization of silver molecular clusters in thin films. Res. Chem. Intermed. 2020, 46, 4033–4046. [Google Scholar] [CrossRef]
- Xie, Y.P.; Shen, Y.L.; Duan, G.X.; Han, J.; Zhang, L.P.; Lu, X. Silver nanoclusters: Synthesis, structures and photoluminescence. Mater. Chem. Front. 2020, 4, 2205–2222. [Google Scholar] [CrossRef]
- Jonckheere, D.; Coutino-Gonzalez, E.; Baekelant, W.; Bueken, B.; Reinsch, H.; Stassen, I.; Fenwick, O.; Richard, F.; Samorì, P.; Ameloot, R.; et al. Silver-induced reconstruction of an adeninate-based metal–organic framework for encapsulation of luminescent adenine-stabilized silver clusters. J. Mater. Chem. C 2016, 4, 4259–4268. [Google Scholar] [CrossRef]
- Quintanilla, M.; Liz-Marzan, L.M. Caged clusters shine brighter. Science 2018, 361, 645. [Google Scholar] [CrossRef]
- Diez, I.; Ras, R.H. Fluorescent silver nanoclusters. Nanoscale 2011, 3, 1963–1970. [Google Scholar] [CrossRef]
- Romolini, G.; Steele, J.A.; Hofkens, J.; Roeffaers, M.B.J.; Coutino-Gonzalez, E. Tunable Luminescence from Stable Silver Nanoclusters Confined in Microporous Zeolites. Adv. Opt. Mater. 2021, 9, 2100527. [Google Scholar] [CrossRef]
- De Cremer, G.; Coutiño-Gonzalez, E.; Roeffaers, M.B.J.; Moens, B.; Ollevier, J.; Schoonheydt, R.; Jacobs, P.A.; De Schryver, F.C.; Hofkens, J.; De Vos, D.E.; et al. Characterization of fluorescence in heat-treated silver-exchanged zeolites. J. Am. Chem. Soc. 2009, 131, 3049–3056. [Google Scholar] [CrossRef]
- Aono, H.; Yahara, K.; Johan, E.; Itagaki, Y. Effect of coexisting lithium content on fluorescent properties of silver ion-exchanged LTA zeolite. J. Ceram. Soc. Jpn. 2020, 128, 670–676. [Google Scholar] [CrossRef]
- Ahmad, I.; Muhmood, T.; Rehman, A.; Zahid, M.; Abohashrh, M.; Nishat, S.; Raharjo, Y.; Zhou, Z.; Yang, X. Zeolite imidazole framework entrapped quantum dots (QDs@ ZIF-8): Encapsulation, properties, and applications. J. Taiwan. Inst. Chem. Eng. 2023, 149, 104993. [Google Scholar] [CrossRef]
- Coutino-Gonzalez, E.; Baekelant, W.; Grandjean, D.; Roeffaers, M.B.J.; Fron, E.; Aghakhani, M.S.; Bovet, N.; Van der Auweraer, M.; Lievens, P.; Vosch, T.; et al. Thermally Activated LTA (Li)-Ag Zeolites with Water-Responsive Photoluminescence Properties. J. Mater. Chem. C 2015, 3, 11857–11867. [Google Scholar] [CrossRef]
- Johan, E.; Kanda, Y.; Matsue, N.; Itagaki, Y.; Aono, H. Whitish fluorescence of partially Ag-exchanged zeolite Y affected by coexisting cations. J. Lumin. 2019, 213, 482–488. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, J.; Zhao, D.; Wang, Y.; Li, H. Stable and Highly Luminescent Silver Nanoclusters in the 13X Zeolite Enabled by Mg2+ Doping and Their Luminescence Tuning by Heating Temperature. Inorg. Chem. 2023, 62, 18299–18306. [Google Scholar] [CrossRef]
- Grandjean, D.; Coutino-Gonzalez, E.; Cuong, N.T.; Fron, E.; Baekelant, W.; Aghakhani, S.; Schlexer, P.; D’Acapito, F.; Banerjee, D.; Roeffaers, M.B.J.; et al. Origin of the bright photoluminescence of few-atom silver clusters confined in LTA zeolites. Science 2018, 361, 686–690. [Google Scholar] [CrossRef]
- Pan, L.; Ye, S.; Huang, R.; Wang, M.; Lin, P.; Zhang, H.; Wang, D. Luminescence Prop-erties and Theoretical Modeling of the Ag3 Cluster Confined inside the D6r Cavity of Faujasite Zeolite: Implications for the Design of Tunable Emission Phosphor. ACS Appl. Nano Mater. 2024, 7, 9934–9941. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Q.; Yue, C.; Wang, N.; Li, P.; Li, H. Emission-tunable silver clusters constrained within EMT zeolite. J. Mater. Chem. C 2023, 11, 17080–17086. [Google Scholar] [CrossRef]
- Xv, X.; Ye, S.; Pan, L.; Lin, P.; Liao, H.; Wang, D. Tailoring the Luminescence Properties of Silver Clusters Confined in Faujasite Zeolite through Framework Modification. Materials 2022, 15, 7431. [Google Scholar] [CrossRef]
- Wang, N.; Li, Q.; Yuan, J.; Zhao, D.; Yang, H.; Wang, Y. White light-emitting Ag+-doped H-SSZ-13 zeolite. Inorg. Chem. Commun. 2024, 168, 112875. [Google Scholar] [CrossRef]
- Tian, X.; Li, Q.; Yao, D.; Li, P.; Li, H.; Wang, Y. Tunable luminescence of silver-exchanged SOD zeolite thermally treated under mild conditions. J. Mater. Chem. C 2022, 10, 1666–1671. [Google Scholar] [CrossRef]
- Zhang, C.; Guan, S.; Li, H.-Y.; Dong, X.-Y.; Zang, S.-Q. Metal clusters confined in chiral zeolitic imidazolate framework for circularly polarized-luminescence inks. Nano Lett. 2024, 24, 2048–2056. [Google Scholar] [CrossRef]
- Haji-Hashemi, H.; Habibi, M.M.; Safarnejad, M.R.; Norouzi, P.; Ganjali, M.R. Label-free electrochemical immunosensor based on electrodeposited Prussian blue and gold nanoparticles for sensitive detection of citrus bacterial canker disease. Sens. Actuators B Chem. 2018, 275, 61–68. [Google Scholar] [CrossRef]
- Liao, H.; Ye, S.; Lin, P.; Pan, L.; Wang, D. In situ growth of lanthanides-doped nanoparticles inside zeolites with enhanced up conversion emission for gallic acid detection. J. Colloid. Interface Sci. 2023, 652, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, X.; Yang, Z.; Li, H. Size effect of encapsulated metal within zeolite: Biomass, CO2 and methane utilization. J. Catal. 2023, 417, 368–378. [Google Scholar] [CrossRef]
- Li, Q.; Tian, X.; Yuan, J.; Zhao, D.; Wang, Y.; Li, H. Tunable luminescence of silver nanoclusters confined in SOD/FAU zeolites and selective sensing for organic amine. Inorg. Chem. 2023, 62, 2430–2439. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Luo, Z.; Yu, Y.; Yao, Q.; Xie, J. Luminescent noble metal nanoclusters as an emerging optical probe for sensor development. Chem.–Asian J. 2013, 8, 858–871. [Google Scholar] [CrossRef]
- De Cremer, G.; Sels, B.F.; Roeffaers, M.B.J.; Coutin, E.; De Vos, D.E.; Vosch, T.; Hofkens, J. Optical encoding of silver zeolite microcarriers. Adv. Mater. 2010, 22, 957–960. [Google Scholar] [CrossRef]
- Yao, D.; Yang, J.; Xie, Y.; Wang, Y.; Wang, Y.; Li, H. Warm white-light phosphor based on a single-phase of Ag+/Eu3+/Zn2+ loading SOD zeolites with application to white LEDs. J. Alloys Compd. 2020, 823, 153778. [Google Scholar] [CrossRef]
- Chakraborty, I.; Pradeep, T. Atomically precise clusters of noble metals: Emerging link between atoms and nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef]
- Yao, D.; Xu, S.; Wang, Y.; Li, H. White-emitting phosphors with high color-rendering index based on silver cluster-loaded zeolites and their application to near-UV LED-based white LEDs. Mater. Chem. Front. 2019, 3, 1080–1084. [Google Scholar] [CrossRef]
- Baekelant, W.; Aghakhani, S.; Fron, E.; Martin, C.; Woong-Kim, C.; Steele, J.A.; De Baerdemaeker, T.; d’Acapito, F.; Chernysov, D.; van der Auweraer, M.; et al. Luminescent silver–lithium-zeolite phosphors for near-ultraviolet LED applications. J. Mater. Chem. C 2019, 7, 14366–14374. [Google Scholar] [CrossRef]
- Pan, L.; Ye, S.; Xv, X.; Lin, P.; Huang, R.; Wang, D. Zeolite-Encaged Luminescent Silver Nanoclusters. Materials 2023, 16, 3736. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ye, S.; Liao, H.; Liu, J.; Wang, D. Formation of luminescent silver-clusters and efficient energy transfer to Eu3+ in faujasite NaX zeolite. J. Solid. State Chem. 2020, 285, 121227. [Google Scholar] [CrossRef]
- Coutiño-Gonzalez, E.; Baekelant, W.; Steele, J.A.; Kim, C.W.; Roeffaers, M.B.J.; Hofkens, J. Silver clusters in zeolites: From self-assembly to ground-breaking luminescent properties. Acc. Chem. Res. 2017, 50, 2353–2361. [Google Scholar] [CrossRef]
- JCPDS# 43-0168; Zeolite Y, (Na) [Sodium Aluminum Silicate Hydrate]. International Centre for Diffraction Data (ICDD): Newtown Square, PA, USA, 1991.
- Fenwick, O.; Coutiño-Gonzalez, E.; Richard, F.; Bonacchi, S.; Baekelant, W.; de Vos, D.; Roeffaers, M.B.J.; Hofkens, J.; Samorì, P. X-Ray-Induced Growth Dynamics of Luminescent Silver Clusters in Zeolites. Small 2020, 16, 2002063. [Google Scholar] [CrossRef]
- Yu, J.; Ye, S.; Liao, H.; Xv, X.; Wang, D. Luminescence Control of Silver Nanoclusters by Tailoring Extra-Framework Cations in FAU-Y Zeolite: Implications for Tunable Emission. ACS Appl. Nano Mater. 2021, 4, 13692–13699. [Google Scholar] [CrossRef]
- Price, L.; Leung, K.M.; Sartbaeva, A. Local and average structural changes in zeolite A upon ion exchange. Magnetochemistry 2017, 3, 42. [Google Scholar] [CrossRef]
- Shin, H.S.; Choi, H.C.; Jung, Y.; Kim, S.B.; Song, H.J.; Shin, H.J. Chemical and size effects of nanocomposites of silver and polyvinyl pyrrolidone determined by X-ray photoemission spectroscopy. Chem. Phys. Lett. 2004, 383, 418–422. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Yan, C.; Chen, G. Solvent-free preparation of hierarchical 4A zeolite monoliths: Role of experimental conditions. J. Cryst. Growth 2019, 528, 125286. [Google Scholar] [CrossRef]
- Altantzis, T.; Coutino-Gonzalez, E.; Baekelant, W.; Martinez, G.T.; Abakumov, A.M.; Van Tendeloo, G.; Roeffaers, M.B.J.; Bals, S.; Hofkens, J. Direct observation of luminescent silver clusters confined in faujasite zeolites. ACS Nano 2016, 10, 7604–7611. [Google Scholar] [CrossRef]
- Yu, J.; Ye, S.; Shi, Y.; Liao, H.; Xv, X.; Wang, D. Confinement of highly luminescent silver nanoclusters in FAUY Zeolite: A study on the formation effects of silver nanoclusters and the sensitization of Tb3+. ACS Appl. Nano Mater. 2021, 4, 6290–6298. [Google Scholar] [CrossRef]
- Yang, J.; Jin, R. Advances in enhancing luminescence of atomically precise Ag nanoclusters. J. Phys. Chem. C 2020, 125, 2619–2625. [Google Scholar] [CrossRef]
- Wang, L.; Zhong, W.; Gao, W.; Liu, W.; Shang, L. Dynamic multicolor luminescent anti-counterfeiting based on spiropyran-engineered gold nanoclusters. Chem. Eng. J. 2024, 479, 147490. [Google Scholar] [CrossRef]
- International Commission on Illumination (CIE). The CIE 1931 Color Spaces; CIE Technical Report; CIE: Vienna, Austria, 1931. [Google Scholar]
- Ploch, N.L.; Einfeldt, S.; Frentrup, M.; Rass, J.; Wernicke, T.; Knauer, A.; Kueller, V.; Weyers, M.; Kneissl, M. Investigation of the temperature dependent efficiency droop in UV LEDs. Semicond. Sci. Technol. 2013, 28, 125021. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Jing, Z.; Yung, W.K.; Fan, J. Bayesian based lifetime prediction for high power white LEDs. Expert. Syst. Appl. 2021, 185, 115627. [Google Scholar] [CrossRef]






| Concentration | 6.3% | 6.7% | 7.1% | 7.7% | 8.3% | 9.1% | 10% | 11.1% | 12.5% |
|---|---|---|---|---|---|---|---|---|---|
| Brightness (cd/m2) | 35.41 | 56.4 | 68.20 | 57.77 | 48.81 | 32.48 | 5.62 | 0.48 | 0.21 |
| Light spot diameter (mm) | 6.2 | 16.2 | 18.3 | 18.1 | 17.7 | 16.4 | 15.7 | 6.2 | 4.3 |
| Parameter | x | y | Color Temperature | Peak Wavelength | Intensity | Efficacy | Conversion Efficiency | Ra |
|---|---|---|---|---|---|---|---|---|
| K | nm | mcd | lm/W | |||||
| Li-FAU/Ag-LED | 0.2651 | 0.4073 | 7873 | 527 | 94.9 | 19.7 | 59.30% | 46.5 |
| Na-FAU/Ag-LED | 0.2979 | 0.4666 | 6387 | 538 | 63.7 | 13.2 | 39.80% | 52.2 |
| K-FAU/Ag-LED | 0.3076 | 0.4893 | 6087 | 545 | 74.2 | 15.6 | 47% | 62.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, T.; Huang, R.; Zhang, H.; Ye, S.; Wang, D. Synthesis of Silver Nanocluster-Loaded FAU Zeolites and the Application in Light Emitting Diode. Chemistry 2025, 7, 90. https://doi.org/10.3390/chemistry7030090
Zheng T, Huang R, Zhang H, Ye S, Wang D. Synthesis of Silver Nanocluster-Loaded FAU Zeolites and the Application in Light Emitting Diode. Chemistry. 2025; 7(3):90. https://doi.org/10.3390/chemistry7030090
Chicago/Turabian StyleZheng, Tianning, Ruihao Huang, Haoran Zhang, Song Ye, and Deping Wang. 2025. "Synthesis of Silver Nanocluster-Loaded FAU Zeolites and the Application in Light Emitting Diode" Chemistry 7, no. 3: 90. https://doi.org/10.3390/chemistry7030090
APA StyleZheng, T., Huang, R., Zhang, H., Ye, S., & Wang, D. (2025). Synthesis of Silver Nanocluster-Loaded FAU Zeolites and the Application in Light Emitting Diode. Chemistry, 7(3), 90. https://doi.org/10.3390/chemistry7030090





