Abstract
Activated carbons were obtained from three types of raw materials from the canning industry: peach, plum, and olive stones. The chemical composition and texture of the precursors and the physicochemical properties of the obtained carbons were analyzed. It was found that under the same conditions of preparation, the properties of the raw materials significantly affect the parameters of the activated carbons. The obtained carbons were oxidized with 12% nitric acid to form a larger amount of acidic oxygen-containing groups on their surface. The porous texture, the size, and the chemical nature of the surface of the activated carbons were analyzed. The adsorption capacity of the obtained activated carbons towards chlorhexidine gluconate contained in mouthwash was determined. It was found that the three carbons have a significant adsorption capacity towards chlorhexidine gluconate: 189.1 mg/g for carbon from peach stones, 189.1 mg/g for carbon from plum stones, and 156.7 mg/g for carbon from olive stones, respectively. It has been determined that the adsorption of chlorhexidine gluconate on the surface of the obtained activated carbons obeys the Langmuir model. It has been established that the adsorption capacity of the obtained activated carbons is influenced by their porous texture, size, and chemical nature of the surface.
1. Introduction
Proper treatment of wastewater is essential to ensure good water quality. The EU (European Union) has introduced new rules for water quality in order to achieve better protection for the people and the environment. If not treated properly, wastewater can cause water pollution, which has an impact on human health and the environment. Water quality in the EU has improved significantly over the last 30 years, due to improved EU legislation for urban wastewater treatment; however, new pollutants are constantly emerging. The EU aims to achieve zero water pollution by 2050. The Council has therefore adopted updated rules for urban wastewater treatment dating back to 1991 [1,2].
Despite being effective in the case of humans, chlorhexidine gluconate is toxic to aquatic organisms and hinders plant growth. However, there is no norm or regulation for the permissible limits that should be maintained before discharging the wastewater containing this compound into the inland surface water body. When chlorhexidine is discharged into municipal sewer pipes along with other types of municipal liquid wastes, there is a risk of killing the useful bacteria present in the sewers. Toxicity of chlorhexidine gluconate towards fish (Brachydanio rerio) is related to the lethal concentration LC 50 (96 h) = 10.4 mg/L and LC 0 = 10.0 mg/L and towards algae (Scenedesmus subspicatus) by the half maximal inhibitory concentration IC 50 (72 h) = 0.011 mg/L (OECD Test Guideline 201). Subacutetoxicity of chlorhexidine gluconate to green algae is also prominent, with IC 50 (10 days) = 4.4 mg/L. Hence, if it is discharged into the environment, it causes water pollution due to its ecotoxicological effects [3].
Adsorption is a well-established and powerful physicochemical technique for the treatment of domestic and industrial wastewater. Adsorption kinetics is often employed to study the reaction limiting stage, mechanism, and complete characterization of the adsorption process. Akin to adsorption isotherms, the adsorption experimental data are selected for their better fitting in various kinetic models. The model with the highest linear regression coefficient (R2) is considered to be the most suitable for describing the adsorption [4,5,6,7,8,9,10].
Due to their highly developed porous structure and large specific surface area, activated carbons show significant adsorption capacity for various pollutants in water, air, and natural products [1,2]. Activated carbon (AC) can be produced from various precursors, and in recent decades, special attention has been paid to the production of cheap activated carbon from various waste materials. As the demand for a sustainable society grows, biomass, which is a carbon-neutral resource, is recognized as a very important material in the circular economy. Agricultural by-products are very promising raw materials for the production of activated carbon due to their low cost and availability [1,2].
The main objectives of the present study are to determine the influence of the raw materials on the quality of the obtained activated carbons, establish their ability to extract chlorhexidine gluconate from an aqueous solution, and choose the most suitable to be used for this purpose. This will allow the utilization of waste biomass through processing by using an efficient one-stage low-cost method for the synthesis of activated carbons and their use for water purification from organic pollutants. The results obtained will contribute to environmental protection.
We want to highlight the fact that despite numerous studies related to the production of activated carbon, new and innovative publications in this field continue to be published. This is necessary due to the search for expanding the raw material base and the search for new possibilities for utilizing various organic wastes. The difference in the chemical composition and texture of various organic raw materials requires special studies to establish the optimal conditions for their processing and obtaining the highest quality activated carbons, depending on the method of application. In this sense, each study represents some kind of novelty. In our case, the situation is as follows:
- -
- A comprehensive approach is shown for selecting a suitable raw material (waste from the processing industry) for obtaining a high-quality activated carbon;
- -
- Three raw materials were used for the production of activated carbon, which are released in large quantities from the processing industry. The goal is to find a suitable opportunity for their use;
- -
- The possibility of selecting a suitable raw material based on the analysis of the content of lignin, cellulose, hemicellulose, and lipids is shown. It has been established that a significant influence on the suitability of the raw material for obtaining an activated carbon is the ratio of the amounts of lignin and cellulose in the precursor. In the selection of raw materials, a suitable texture was taken into account, which allows them to be successfully processed by an energy-saving, one-stage method combining carbonization and activation of the raw material;
- -
- The optimal processing conditions (temperature, temperature rise rate, and amount of the activating reagent: water vapor) of the selected raw materials were determined and indicated in the article;
- -
- The obtained activated carbons were subjected to oxidative treatment with HNO3 in order to give an acidic character to their surface and increase the amount of oxygen-containing functional groups. This treatment was chosen as appropriate considering the chemical nature of the chlorhexidine gluconate molecule;
- -
- The treatments of spent activated carbon have been carried out with the aim of regenerating their adsorption capacity. The treatment with water vapor at a higher temperature (500 °C) gives a satisfactory result. Based on the results obtained, conclusions have been made about the nature of the adsorption (physical or chemical) of chlorhexidine gluconate on the surface of the adsorbents. The adsorption capacity of the activated carbons obtained on the basis of the three selected raw materials towards chlorhexidine gluconate has been determined, and their applicability for this purpose has been established.
2. Materials and Methods
2.1. Preparation of Precursors
The novelty of the present work is the development of a new one-stage method, leading to the synthesis of adsorbents containing pores of different sizes, a large surface area, and a large amount of oxygen-containing functional groups on the surface. Due to the porous texture of the adsorbent, it is assumed that molecules of different sizes would have access to the adsorbent surface, which should lead to increased adsorption capacity.
The selected precursors, peach stones and plum stones, are taken from a can factory in Septemvri town, Bulgaria. Olive stones are taken from a Greek factory in the town of Lycovrisi.
2.2. Preparation of Activated Carbons from Peach Stones, Plum Stones, and Olive Stones
The novelty of the present work is the development of a new one-stage method, leading to the synthesis of adsorbents containing pores of different sizes, a large surface area, and a large amount of oxygen-containing functional groups on the surface. Due to the porous texture of the adsorbent, it is assumed that molecules of different sizes would have access to the adsorbent surface, which should lead to increased adsorption capacity.
The activated carbons are prepared by one-step pyrolysis in the presence of water vapor. The samples of 50 g are heated in a laboratory installation (stainless steel vertical reactor) in a flow of water vapor (120 mL/min) with a heating rate of 15 °C min−1 to a final carbonization temperature of 800 °C. The duration of treatment at the final temperature is 1 h. After treatment, the samples are left to cool down and treated with 10% nitric acid for 12 h, then washed with distilled water.
The samples are labeled in the text respectively: from peach stones—ACpeach, from plum stones—ACplum, from olive stones—AColive.
2.3. Methods of Carbon Characterization
The elemental analysis was performed on Vario Macro Cube (Elementar Analyzensysteme GmbH, Langenselbold, Germany) equipment for the determination of C, H, N, and S. Oxygen content was determined by the difference. Carbon was determined as CO2, Hydrogen was determined as H2O, whereas sulfur was determined as SO2.
A scanning electron microscope (JEOL JSM 6390, Jeol Ltd., Tokyo, Japan) with an accessory (INCA Oxford) for elemental analysis was used, allowing the determination of surface structure, morphology, and elemental composition of materials.
TG and DSC experiments were performed on a Simultaneous Thermal Analyzer STA 449 F3 (Netzsch GmbH, Selb, Germany) instrument.
The texture of the synthesized carbon material was characterized by N2 adsorption at −196 °C, carried out in an automatic volumetric apparatus, Quantachrome Autosorb iQ-C-XR/MP (Quantachrome Inc., Boynton Beach, FL, USA). Before the experiments, the sample was outgassed under vacuum at 350 °C overnight. The isotherms were used to calculate the specific surface area SBET, total pore volume Vtotal, and micropore volume using the Dubinin–Radushkevich method [11,12,13,14].
The amount of oxygen-containing surface functional groups with increasing acidity was determined by the Boehm method of titration with basic solutions of different base strength (NaHCO3, Na2CO3, NaOH, C2H5ONa) [15]. For this purpose, the samples were agitated for at least 16 h with 0.05 N solutions of four bases. The amount of OH− anions remaining in the solution is determined by adding an excess of standard HCl solution and back titration. The basic surface group content of the samples is determined with 0.05 N HCl. Every measurement was performed three times, and the average was taken [16].
Raman spectra were recorded using a Raman Spectrometer Senterra II (Bruker GmbH, Denkendorf, Germany). Samples were placed onto glass (approximately 10 mg) and analyzed using a vertical 20× objective in a 1800 backscattering arrangement. The Raman spectrometer parameters used to analyze the samples include the following: 532 nm laser wavelength and an exposure time of 100 s, resolution of 1 cm−1, and laser power of 6.5 mW.
Iodine adsorption in a water solution is a technique employed to determine the adsorption capacity of activated carbons. The iodine number indicates the porosity and surface area, and it is defined as the amount (mg) of iodine adsorbed by 1 g of carbon. The iodine adsorption was determined using the sodium thiosulfate volumetric method.
The procedure for pH measurements was performed as follows: 4.0 g of carbon (ground, not dried) was weighed into a 250 mL beaker, and 100 mL of distilled water was added. The beaker was covered with a watch glass and heated to boiling temperature for 5 min. The mixture was set aside, and the supernatant liquid was poured off as hot as possible but not below 60 °C. The decanted portion was set aside to cool down to 25 °C, and the pH value was measured to the nearest 0.1 pH unit (Standard NORIT).
2.4. Adsorption
The main methods for wastewater treatment are mechanical, physicochemical (adsorption and other methods), chemical treatment, biological (biochemical) purification, and ozonation (see Section 1 Introduction).
All methods for water purification are effective; however, we selected adsorption as a well-established and powerful physicochemical technique for water treatment.
Chlorhexidine gluconate (Eludril care, 0.05% Chlorhexidine gluconate, Pierre Fabre, S.A., Castres, France) adsorption experiments are carried out with 0.05 g of adsorbent in 50 mL of chlorhexidine gluconate solution with concentrations of 5–100 mg/L. Stopped flasks containing the adsorbent and the adsorbate with different concentrations were agitated for predetermined intervals using a mechanical shaker at room temperature. After that, the suspensions were filtered with microporous filter paper. The adsorbed amount of the chlorhexidine gluconate was determined spectrophotometrically using a Pharo 300 UV spectrometer, Merck, Darmstadt, Germany (detected at 276 nm). pH was adjusted using 0.1 M NaOH and 0.1 M HCl.
3. Results and Discussion
3.1. Characterization of Nanoporous Activated Carbons
On the base of obtained results for iodine number and yield of carbon (the mass of carbon product divided by the mass of the raw materials, represented in %) adsorbents (Table 1), the activation temperature was optimized (800 °C) in order to obtain nanocarbons with acceptable yield and moderately high iodine capacity, which are much higher than the values in the case of samples, produced after water vapor activation at higher (850 °C) and lower temperatures (700–800 °C).

Table 1.
Characteristics of the process of carbon production.
The pore texture of the selected carbons is investigated by low-temperature N2 gas adsorption; the results are presented in Figure 1 and Table 2. Analysis of nitrogen adsorption data indicate that the activated carbons obtained from biomass waste (peach stones, plum stones, olive stones) possessed moderately high surface area (SBET) and developed pore structure. The part of the isotherms in the range of the relatively lower pressures (a steep increase with a tendency for saturation) is typical for microporous adsorbents. The N2 adsorption isotherms obtained correspond to IV type according to IUPAC classification (low pressure).
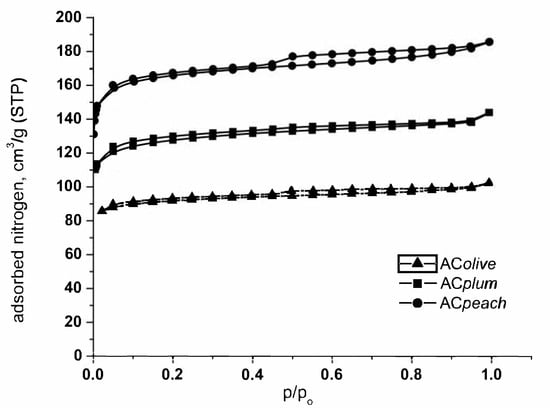
Figure 1.
N2 adsorption isotherms at −196 °C of the activated carbons from peach stones, olive stones, and plum stones.

Table 2.
Main textural parameters of the obtained activated carbons from N2 adsorption isotherms at 77 K.
The main textural parameters of the prepared carbons, obtained from the analysis of the low temperature nitrogen adsorption isotherms, are compiled in Table 2. Previous investigations connected with the pyrolysis of different biomass samples have shown that the composition of agricultural by-products has a strong influence on the final porous and chemical features of the solid product obtained after pyrolysis and subsequent activation. It was found that the high content of cellulose favors the production of carbon with a more developed porous structure, whereas lignin favors the formation of carbon with higher mechanical strength.
Chemical analysis of raw materials was carried out using the following methodology: Two wet chemical techniques were used to determine the different organic matter fractions of the raw materials—peach stone, plum stone, and olive stone. This analysis yielded four fractions: soluble in a toluene-ethanol mixture, soluble in 0.94 mol/L sulfuric acid, soluble in 13.5 mol/L sulfuric acid, and insoluble in 13.5 mol/L sulfuric acid. The lipids were solubilized by the first extraction step, proteins and hemicellulose by the second step, and cellulose by the third step. The lignin was left as a residual fraction.
It was determined that peach stones and plum stones contain a suitable combination of cellulose and lignin (about 37 and 30%, respectively), which ensures a developed porous structure of the final product. Evidently, data in Table 2 demonstrate that activated carbon from peach stones has a well-developed porous structure, with a prevailing content of micropores and a relatively high surface area of 729.7 m2/g for peach stones and 590.0 m2/g for plum stones, respectively.
Activated carbon from olive stones (317.7 m2/g) has a low content (13.95%) of cellulose and a higher content of lignin (30.48%). In accordance, the results demonstrate that carbon from olive stones is characterized by a not very well-developed porous structure.
It can be concluded that the composition of the precursor has a strong influence on the final porous and chemical features of a solid product obtained after pyrolysis and subsequent activation [11,12,13,14].
Pore size distribution [17] data are presented in Figure 2a–c and the results show the presence of micropores in the range of 1.5–2.0 nm and mesopores in the range of 2.0–3.0 nm.
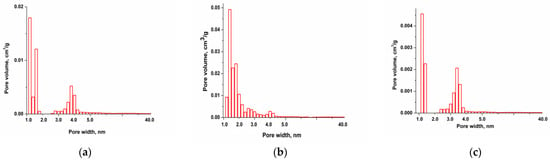
Figure 2.
Pore size distribution of ACplum (a), ACpeach (b), and AColive (c).
3.2. TG—DSC Analysis
The differential thermogravimetry and differential scanning calorimetry of carbon materials provided valuable data regarding the type of ongoing processes during heat treatment (Figure 3). The DSC and TG profiles show different processes taking place during the temperature treatment (20–900 °C) of the sample. The data show that the thermal destruction of peach stones occurs more slowly and at higher temperatures compared to olive and plum stones. Olive stones are destroyed faster and at a lower temperature. This is due to the different textures of biomass materials. It is more dense in the case of peach stones, and more loose in the case of olive stones. These differences in the behavior of different raw materials when heated are clearly shown by the DSC analysis. The exo effects that are reported at 280 and 320, and 360 and 380 °C, respectively, for olive and plum stones, are due to the synthesis reactions (proceeding with the release of heat) with the participation of radicals obtained from the destruction of the organic mass of these stones. For peach stones, these peaks are very weakly expressed due to the small amounts of radicals derived from their organic mass [18].
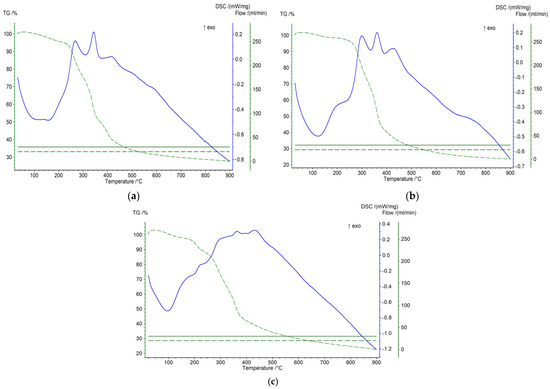
Figure 3.
TG-DSC analysis of the initial sample of olive stones (a), plum stones (b), and peach stones (c). (TG-green line and DSC-blue line).
The results obtained from TG and DSC analysis are valuable for obtaining information about the nature of the processes that occur during the thermal treatment of carbonizates. Based on these results, an appropriate temperature increase regime was determined, allowing the formation of a product with desired properties.
3.3. Raman Structural Analysis of ACpeach, AColive, ACplum
The analysis of the structure of the obtained carbonized carbon sample, including the evaluation of the ordering of the crystal structure, was possible due to research using Raman spectroscopy.
The Raman spectra of the carbon samples, which are displayed in Figure 4, show the presence of two distinctive bands—D-peak (1319 cm−1) and G-peak (1570 cm−1). The G-peak is due to the in-plane stretching vibration of sp2 carbon atoms. It is well known that the D-band is characteristic of disordered structures, and it is caused by sp2 carbon bond modes, edge effects, and structural defects [19].
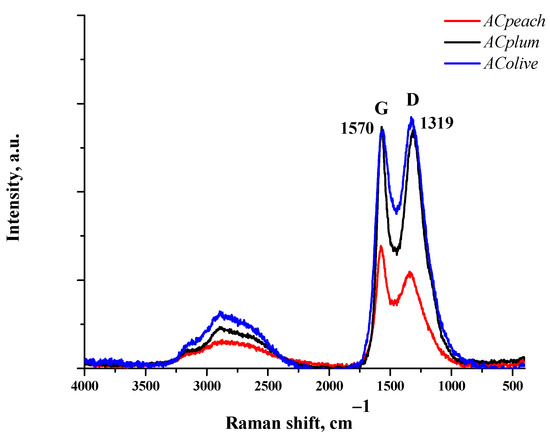
Figure 4.
Raman spectrum of ACpeach, AColive, and ACplum samples.
The results from Raman spectroscopy show that the amount of disordered carbon structures increases in the order of AColive < ACplum < ACpeach, according to the rise of the BET surface area.
3.4. Elemental Analysis of Carbons
The novelty of the present work is the development of a new one-stage method, leading to the synthesis of adsorbents containing pores of different sizes, a large surface area, and a large amount of oxygen-containing functional groups on the surface. Due to the porous texture of the adsorbent, it is assumed that molecules of different sizes would have access to the adsorbent surface, which should lead to increased adsorption capacity.
The chemical composition of the starting materials and activated carbons obtained from different agricultural by-products is presented in Table 3. The data show that the activated carbons obtained from peach stones have the largest content of carbon and the smallest content of hydrogen. This means that they have the most condensed and somewhat cross-linked structure. The activated carbons from olive stones are characterized by the smallest content of carbon and the largest content of hydrogen, which means that they have the least condensed and most loose structure. The oxygen content in the three carbons can be attributed mainly to oxygen formed as a result of the oxidative treatment of the obtained activated carbons with nitric acid.

Table 3.
Chemical composition of the raw materials and the solid products.
The differences in the ultimate analysis and elemental analysis of activated carbons produced from different raw materials under the same conditions indicate the dominant influence of the composition and structure of the precursors on their reactivity in the pyrolysis/activation reactions during steam pyrolysis.
3.5. Oxygen Functional Group Content
Due to the large number of NH groups in the chlorhexidine gluconate molecule, it has an alkaline character, and it is positively charged. In order to modify the chemical properties of the surface, the obtained activated carbons were subjected to oxidative treatment with nitric acid. As a result, oxygen functional surface groups with an acidic character/carboxylic, lactone, and hydroxyl were formed, and the carbon surface acquired acidic character and a negative net charge. The chemical character of the surface was investigated by determining the presence of oxygen functional groups in the samples. The oxygen-containing functional groups are very important specific characteristics of the activated carbons because they have a strong effect on the adsorption properties. The identification and quantification of the oxygen groups in the prepared carbon materials are shown in Table 4. The Boehm titration reveals that various oxygen-containing groups with different chemical properties are present on the activated carbon surface.

Table 4.
Quantification of oxygen groups on activated carbon surface (mmol/g).
The content of all types of oxygen groups with a predominance of carbonyl groups was determined. More strongly acidic groups, such as carboxyl, lactones, and hydroxyl groups, were formed as a result of the oxidative treatment with nitric acid; they determine the acidic pH of the obtained activated carbons. Based on these results, an increased adsorption capacity of the obtained adsorbents can be expected as a result of chemical and electrostatic interactions with chlorhexidine gluconate molecules, which have an alkaline nature due to the high content of NH groups.
3.6. SEM Analysis
Figure 5 shows the SEM micrographs of the ACpeach, ACplum, and AColive. SEM images show that there are cracks in the samples, characteristic of well-developed cells of activated carbon. These cracks mainly occur between the layers aligned parallel to the pore surface, especially in the junction area of the cells.
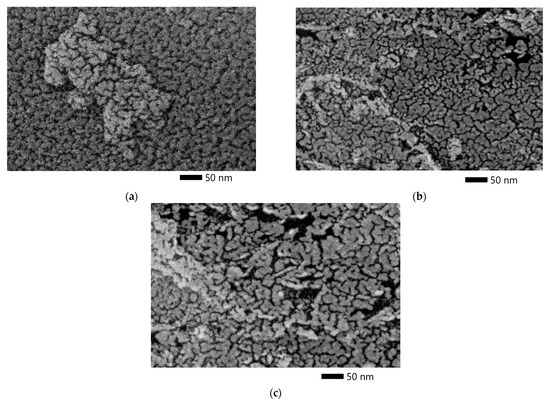
Figure 5.
SEM micrograph of a sample from a peach stone (a), plum stone (b), and olive stone (c).
The SEM micrographs show that ACpeach has the densest texture and the least amount of pores with a size greater than 50 nm macropores compared to the other two activated carbons.
3.7. Adsorption Investigations
The kinetics of activated carbon adsorption of chlorhexidine gluconate from aqueous solutions were studied for various concentrations ranging from 5 to 100 mg/L. The adsorption isotherms of chlorhexidine gluconate on activated carbons are presented in Figure S1 (Supplementary). They belong to type L of the Giles classification for adsorption from water solution [20,21], indicating that adsorption proceeds by the formation of a monolayer in the range of concentrations used. Our results show that the adsorption mechanism of chlorohexidine on the surface of all three adsorbents is similar. The experimental data fit well with the Langmuir equation:
where Ce (relative error of the measurement 1.0%) is the equilibrium pollutant concentration remaining in solution after adsorption (mg/L), Qe (relative error 2.01%) is the amount of pollutant bound to the adsorbent (mg/g), Qo (relative error 0.01%) is the maximum amount of the pollutant per unit weight of adsorbent (mg/g), and b (relative error 1.0%) is a constant related to the affinity of binding sites (L/mg).
Qe = QobCe/(1 + bCe)
The calculated experimental values of these parameters, together with the relative affinity of the adsorbates towards the surface of the adsorbents, parameter bQo, and the correlation coefficients, are presented in Table 5. The very good fittings, obtained for the sample, indicate that the Langmuir model is excellent for predicting the adsorptive behavior of these carbons toward chlorohexidine gluconate. The adsorption capacities for chlorhexidine gluconate of these carbons are very high, most probably due to the hydrophobic character of the carbon surface. The maximal adsorption capacities calculated from the Langmuir equation are 189.1 mg/g for (ACpeach), 156.7 mg/g for AColives, and 180.5 mg/g for ACplum, respectively.
log(x/m) = log Kf + (1/n) log Ce

Table 5.
Results obtained from the Langmuir and Freundlich equations applied to the adsorption isotherms of chlorhexidine gluconate on the studied carbons.
The linear form of Freundlich Equation (2) is applied to the experimental results presented in Table 5, whereas x is the amount of the adsorbed solute (mg), m is the weight of the adsorbent (g), Ce is the equilibrium concentration (mg/L), n is the slope, showing the variation of the adsorption with concentration, and Kf is the intercept, showing the adsorption capacity of the adsorbent.
The Kf values calculated from the Freundlich equation are 139.7 mg/g for (ACpeach), 112.4 mg/g for AColives, and 130 mg/g for ACplum, respectively.
The kinetic curves in Figure 6 show the adsorption of chlorhexidine gluconate on the synthesized activated carbons from solution with concentrations of 60–100 mg/L. Chlorhexidine gluconate adsorption increases sharply at a short contact time. The adsorption process is quite fast and efficiently illustrates that the adsorption of chlorhexidine gluconate is completed almost on the whole surface of activated carbons. Saturation of the activated carbon surface with the largest surface area occurs after a longer time.
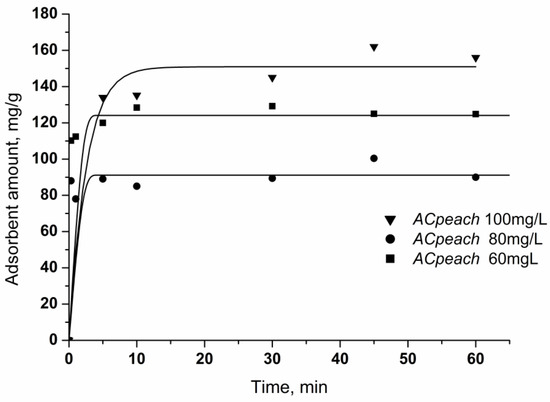
Figure 6.
Effect of treatment time on the adsorption of chlorhexidine gluconate. Conditions: carbon concentration, 50 mg/50 mL, chlorhexidine gluconate concentration: 60, 80, 100 mg/L.
With increasing initial concentration of the solution, the amount of adsorbed chlorhexidine gluconate increases while keeping the time constant. In the case of solutions with concentrations of 60 and 80 mg/L, the extraction of all chlorhexidine gluconate molecules is observed without complete saturation of the surface. In the case of adsorption from a solution with a concentration of 100 mg/L, a certain delay in surface saturation is observed, which is probably due to complete saturation of the surface and increased penetration into the pores of the adsorbent, which takes a little more time.
The influence of different doses (0.02–0.5 g per 50 mL solution) on the removal of chlorhexidine gluconate at fixed initial concentrations of 100 mg/L is shown in Figure 7. The increase in carbon dose leads to the enhancement of the percent removal of chlorhexidine gluconate.
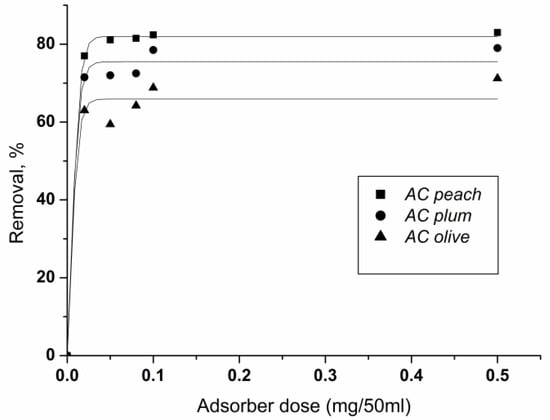
Figure 7.
Effect of carbon dosage on the removal of chlorhexidine gluconate adsorption onto ACpeach, AColive, and ACplum. Conditions: adsorbent amount 0.02–0.5 g per 50 mL solution, concentration 100 mg/L, time of treatment 60 min, 25 °C.
At equal doses of adsorbents, the activated carbons with the highest surface area extract the largest percentage of chlorhexidine. The result shows the essential role of the adsorption surface for the extent of adsorbate extraction.
The values of maximal adsorption capacity for our sample are much higher than those from the scarce literature data for adsorption of chlorhexidine gluconate, reported by other researchers (Table 6).

Table 6.
Monolayer adsorption capacity of chlorhexidine gluconate in various adsorbents obtained from the Langmuir model.
Chlorhexidine gluconate is an organic pollutant with ecotoxicological effects that is often found in wastewater, but the literature data for its removal are scarce and relatively not very new [23].
Photocatalytic degradation of chlorhexidine digluconate is conducted only in the presence of TiO2 photocatalyst [24], whereas in water, chlorhexidine digluconate is stable for more than 7 days [25].
Moreover, it is still the focus of researchers who are trying to find effective adsorbents for the extraction of chlorhexidine gluconate from water.
In this sense, a high-quality novel activated carbon was produced by an original method, on the basis of waste biomass, and successfully applied for the removal of chlorhexidine gluconate.
3.8. Effect of pH of the Solution
Since it influences the electrostatic interactions between the adsorbent and the adsorbate, pH is one of the most important factors in the adsorption process. The adsorption of chlorhexidine gluconate on the activated carbons as a function of pH was studied over a pH range 2–12 (Figure 8).
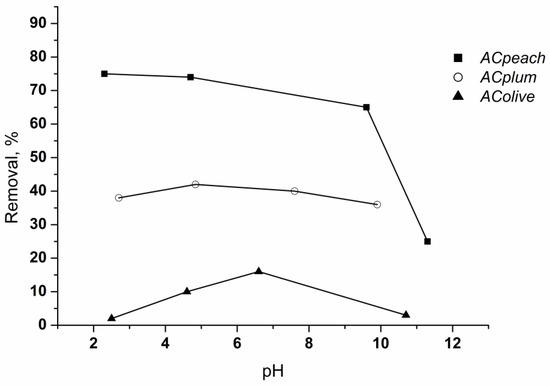
Figure 8.
Effect of pH on the retention of chlorhexidine gluconate on carbon materials. Conditions: time of treatment—60 min, activated carbon amount—0.05 g/50 mL, chlorhexidine gluconate concentration—80 mg/L, 25 °C.
Data show that the adsorption is maximal at pH 5–7, and then decreases as the pH of the solution becomes more basic. The reason for this is that chlorhexidine gluconate is of a basic character (positively charged). The fall in the uptake at pH > 6 is due to the repulsive interactions that appear between the cationic form of the adsorbate and the charges on the carbon surfaces [26,27]. The start of a decrease at higher pH values of chlorhexidine gluconate adsorption on the adsorbent obtained from olive stones is due to the higher content of acidic oxygen groups, which are active centers for the adsorption of alkaline chlorhexidine gluconate molecules, as well as the very small amount of groups with an alkaline character on its surface.
3.9. Desorption Measurements
The interactions between the carbon surface and chlorhexidine gluconate are of two main types, physical and chemical, and they are related to physisorption and chemisorption, respectively. A study of the character of the adsorption of chlorhexidine gluconate on the activated carbon surface is carried out. For this purpose, we tried to desorb chlorhexidine gluconate from chlorhexidine gluconate-loaded adsorbent: (i) with hot water and (ii) with water vapor treatment at 500 °C for 1 h. The results in Table 7 show that a small fraction (6%) of the chlorhexidine gluconate is weakly adsorbed in unstable quasi-chemical complexes on the carbon surface area, which are recovered as a result of the water boiling. The remaining chlorhexidine gluconate molecules are chemically adsorbed and retained in the pores of the carbon. We have some successful desorption and regeneration experiments, with 74–76% recovery (by adsorption capacity) and 80–90% regeneration (by surface area) using treatment with water vapor.

Table 7.
Textural parameters of the obtained activated carbons with adsorbed chlorhexidine gluconate and after desorption with water vapor, from N2 adsorption isotherms at 77 K.
The adsorption capacity towards chlorhexidine gluconate of regenerated samples with water vapor at 500 °C for 1 h was determined. The obtained results are 144.5 (76% regeneration = 144.5/189.5), 137.3 (76% regeneration = 144.5/189.5), and 115.7 (74% regeneration = 115.7/156.7) mg/g, respectively.
4. Conclusions
It was found that activated carbons obtained from various wastes from the processing industry (peach, plum and olive stones) by one-step pyrolysis in the presence of water vapor, show very good abilities to efficiently extract chlorhexidine gluconate from an aqueous solution with adsorption capacities in the following order: activated carbon from peach stones 189.1 mg/g, activated carbon from plum stones 180.5 mg/g, activated carbon from olive stones 156.7 mg/g.
The adsorption behavior is well described by Langmuir isotherms, which shows that the Langmuir model is suitable for predicting the adsorption behavior of the studied activated carbons towards chlorhexidine gluconate.
The data show that an important indicator of the applicability of the raw material for the selected processing is their chemical composition (most notably the ratio between the lignin and cellulose content) and texture.
The main role of the adsorption capacity of the samples are the surface area and the content of meso- and micro-pores with larger size as well as the preliminary oxidative treatment with nitric acid to form an appropriate chemical nature of the surface (a large number of oxygen functional groups with an acidic nature and an appropriate pH) of the obtained activated carbons, consistent with the alkaline nature of the chlorhexidine gluconate molecule.
The significant adsorption capacity towards chlorhexidine gluconate of the activated carbons obtained from various raw materials, obtained from the processing industry, opens up the possibility of their effective use.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/chemistry7030091/s1, Figure S1 Linear Langmuir plots of chlorhexidine gluconate adsorption on AC peach stones (a), olives stones (b), plum stones (c).
Author Contributions
Conceptualization, I.S., B.T., A.K., B.P., P.D. and N.P.; methodology, I.S., B.T., A.K., B.P., P.D. and N.P.; investigation, I.S., A.K., B.T. and B.P.; writing—original draft preparation, I.S., A.K., B.P., B.T., P.D. and N.P.; writing—review and editing, I.S., B.T., P.D. and N.P.; funding acquisition, I.S., B.T., P.D. and N.P. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge financial support for this work by the Bulgarian National Science Fund, grant number KP-06-M77/2 and Project BG05M2OP001-1.002-0019: “Clean technologies for sustainable environment—water, waste, energy for circular economy”, financed by the Operational programme “Science and Education for Smart Growth” 2014–2020, co-financed by the European union through the European structural and investment funds. The authors are also grateful to the funding by Project BG-RRP-2.017-0006-C01 from the Recovery Plan for Europe (NextGenerationEU), and IC-PL/05/2024-2025 Project (Bulgarian Academy of Sciences).
Institutional Review Board Statement
The study was conducted in accordance with the European Code of Conduct for Research Integrity and the Declaration of Helsinki and approved by the Ethics Committee of the Institute of Organic Chemistry with Centre of Phytochemistry (protocol code IOCCP-005 and date of approval 23 April 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; Taylor & Francis: Routledge, UK; CRC Press: Hoboken, NJ, USA, 2005; ISBN 978-0-42-911418-2. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Ania, C.O. Surface chemistry of activated carbons and its characterization. In Activated Carbon Surfaces in Environmental Remediation; Bandosz, T.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 159–230. ISBN 9780080455952. [Google Scholar]
- Banerjee, D.; Basu, S.; Sarkar, U. Removal of chlorhexidine gluconate in presence of a cationic surfactant using acid functionalized activated carbon: Validation of multicomponent models. J. Environ. Chem. Eng. 2020, 8, 104154. [Google Scholar] [CrossRef]
- Berenjian, A.; Chan, N.; Malmiri, H.J. Volatile organic compounds removal methods: A review. Am. J. Biochem. Biotechnol. 2012, 8, 220–229. [Google Scholar] [CrossRef]
- Kulshrestha, R.; Sisodiya, A.; Tiwari, S. Overview of Methods and Processes Used in Wastewater Treatment. In Current Status of Fresh Water Microbiology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 289–301. [Google Scholar]
- Aksu, Z.; Yener, A. Comparative adsorption/biosorption study of mono-chlorinated phenols onto various sorbents. Waste Manag. 2001, 21, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Sandu, T.; Sarbu, A.; Caprarescu, S.; Stoica, E.B.; Iordache, T.-V.; Chiiac, A.-L. Polymer Membranes as Innovative Means of Quality Restoring for Wastewater Bearing Heavy Metals. Membranes 2022, 12, 1179. [Google Scholar] [CrossRef] [PubMed]
- Tsyntsarski, B.; Petrova, B.; Budinova, T.; Petrov, N.; Sarbu, A.; Sandu, T.; Ferhat Yardim, M.; Sirkecioglu, A. Removal of detergents by zeolites and membranes. Bulg. Chem. Commun. 2014, 46, 157–164. Available online: http://www.bcc.bas.bg/BCC_Volumes/Volume_46_Number_1_2014/22.pdf (accessed on 9 June 2024).
- Boustila, H.; Boutillara, Y.; Velasco, L.-F.; Djellali, A.; Tazibet, S. Tailoring Activated Carbon Properties for Pb(II) and Cr(VI) Removal from Water in Continuous Mode. Chem. Eng. Technol. 2022, 45, 258–265. [Google Scholar] [CrossRef]
- Tzvetkov, G.; Mihaylova, S.; Stoitchkova, K.; Tzvetkov, P.; Spassov, T. Mechanochemical and chemical activation of lignocellulosic material to prepare powdered activated carbons for adsorption applications. Powder Technol. 2016, 299, 41–50. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmet, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouqueol, J.; Sing, K. Adsorption by Powders and Porous Solids—Principles, Methodology and Applications; Elsevier: Amsterdam, The Netherlands, 1999; ISBN 978-0-12-598920-6. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 978-0-08-044463-5. [Google Scholar] [CrossRef]
- Chen, S.G.; Yang, R.T. Theoretical basis for the potential theory adsorption isotherms: The Dubinin-Radushkevich and Dubinin-Astakhov equation. Langmuir 1994, 10, 4244–4249. [Google Scholar] [CrossRef]
- Boehm, H.P. Chemical Identification of Surface Groups. In Advances in Catalysis and Related Subjects; Eley, D.D., Pines, H., Weisz, P.B., Eds.; Academic Press: New York, NY, USA, 1966; Volume 16, pp. 179–188. [Google Scholar] [CrossRef]
- Papirer, E.; Sheng, L.; Donnet, J.B. Contribution to the study of basic surface groups on carbons. Carbon 1987, 25, 243–247. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramirez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–3. [Google Scholar] [CrossRef]
- Núñez-Decap, M.; Wechsler-Pizarro, A.; Vidal-Vega, M. Mechanical, physical, thermal and morphological properties of polypropylene composite materials developed with particles of peach and cherry stones. Sustain. Mater. Technol. 2021, 29, e00300. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Giles, C.H.; D’Silva, A.P.; Easton, I.A. A general treatment and classification of the solute adsorption isotherm part. II. Experimental interpretation. J. Colloid Interface Sci. 1974, 47, 766–778. [Google Scholar] [CrossRef]
- Gimenez-Martın, E.; Lopez-Andrade, M.; Ontiveros-Ortega, A.; Espinosa-Jimenez, M. Adsorption of chlorhexidine onto cellulosic fibers. Cellulose 2009, 16, 467–479. [Google Scholar] [CrossRef]
- Nakamura, T.; Tanada, S.; Kawasaki, N.; Hara, T.; Imaki, M.; Miyoshi, T. Adsorption Characteristics of Chiorhexidine onto Activated Carbon for Medical Waste Water Treatment. Seikatsu Eisei 1993, 37, 249–255. Available online: https://www.jstage.jst.go.jp/article/seikatsueisei1957/37/6/37_6_249/_pdf (accessed on 18 December 2024).
- Ishigami, S.; Hase, S.; Nakashima, H.; Yamada, H.; Dohgomori, H.; Natsugoe, S.; Aikou, T. Intravenous chlorhexidine gluconate causing acute respiratory distress syndrome. Clin. Toxicol. 2001, 39, 77–80. [Google Scholar] [CrossRef]
- Mucklow, E.S. Accidental feeding of a dilute antiseptic solution (chlorhexidine 0.05% with cetrimide 1%) to five babies. Hum. Toxicol. 1988, 7, 567–569. [Google Scholar] [CrossRef]
- Leyla-Ramos, R.; Bernal-Jacome, L.; Mendoza-Barron, J.; Herna24ndez-Orta, M. Kinetic modeling of pentachlorophenol adsorption onto granular activated carbon. J. Taiwan Inst. Chem. Eng. 2009, 40, 622–629. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Zheng, T.; Wang, P.; Jiang, J.-P.; Li, N. Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. 2010, 157, 348–356. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).