Abstract
Phlorotannins, bioactive compounds isolated from brown seaweeds, have garnered significant attention in recent years for their wide-ranging therapeutic properties, particularly their anti-inflammatory effects. Recent studies have identified phlorotannins as potent inhibitors of inflammatory pathways such as NF-κB, MAPK, JAK/STAT3, and NLRP3. Specifically, phlorotannins derived from seaweeds like Ecklonia cava, Ishige okamurae, and Sargassum horneri have been shown to inhibit the gene and protein expression of pro-inflammatory cytokines and other inflammatory mediators in both in vivo and in vitro conditions. Despite these promising findings, no commercial drugs derived from seaweed phlorotannins have yet been developed to treat inflammatory diseases, and reports of clinical trials remain rare, even in the context of functional food applications for chronic inflammatory conditions. To address this knowledge gap, the authors reviewed peer-reviewed research articles published in 2020 or later, focusing on the anti-inflammatory potential of phlorotannins. The insights provided in this review are expected to be valuable for industries such as functional food research groups and others involved in developing anti-inflammatory therapeutics.
1. Introduction
Inflammation is a complex biological response triggered by harmful stimuli such as pathogens, damaged cells, or irritants [1]. Inflammation plays a dual role: providing immediate defense and initiating tissue repair while also contributing to disease progression when chronic. Acute inflammation is typically beneficial, whereas dysregulated or chronic inflammation underlies various pathologies, including autoimmune diseases, neurodegenerative conditions, and cancer [2,3]. Recent studies in pathology and immunology have emphasized that inflammation functions not only as an adaptive response to harmful stimuli but also as a critical protective mechanism coordinated by the immune system [2,3,4].
Specifically, the inflammatory signalling cascade can be triggered or intensified by the loss of epithelial barrier function due to cell death, allowing microorganisms to break the epithelial barrier. Thus, cell death has been identified as both an initiator and amplifier of inflammation. While cell death-driven inflammation provides a backup defence mechanism against infections, disruptions in the regulation of cell death due to environmental or genetic factors can lead to excessive inflammation [5]. Therefore, taken together, prolonged exposure of internal organs to inflammation can lead to the development of chronic inflammation, the induction of autoimmune processes, and even the pathogenesis of cancer cells, contributing to various inflammatory diseases [4,6].
Recently, brown seaweeds have been studied for their potential health applications, including anti-inflammatory, antioxidant, and anti-cancer properties. Phlorotannins from brown seaweeds also impact immune function and allergy symptoms [7,8]. Moreover, their neuroprotective properties and ability to activate AMP-activated protein kinase (AMPK) highlight their role in reducing inflammation and oxidative stress [9]. By modulating pathways like NF-κB, MAPK, JAK/STAT3, and NLRP3 inflammasomes, phlorotannins emerge as promising candidates for future drug development [10].
Despite these promising findings, no commercial drugs derived from seaweed phlorotannins have yet been developed to treat inflammatory diseases, and reports of clinical trials remain rare, even in the context of functional food applications for chronic inflammatory conditions. To address this knowledge gap, the authors reviewed peer-reviewed research articles published in 2020 or later, focusing on the anti-inflammatory potential of phlorotannins. The insights provided in this review are expected to be valuable for industries such as functional food research groups and others involved in developing anti-inflammatory therapeutics. Compared to earlier reviews, this work distinguishes itself by focusing solely on literature published since 2020, with an emphasis on individual phlorotannins and their precise mechanisms of action rather than general bioactivity from crude extracts. This review also discusses synergistic effects with fucoidans and updates on novel extraction methods.
1.1. Structure of Phlorotannin and General Information
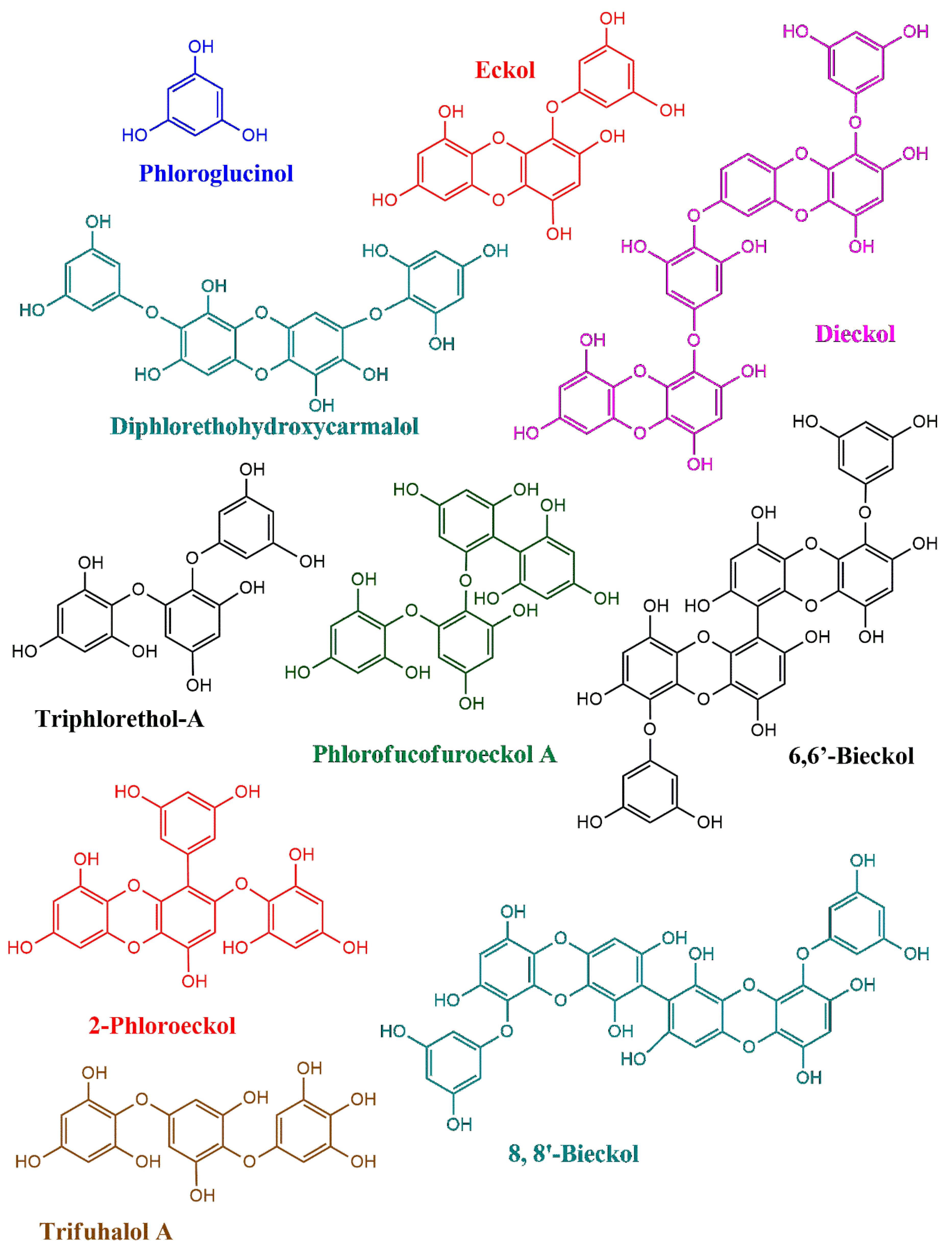
Phlorotannins are a group of polyphenolic compounds mostly reported in brown seaweeds (Phaeophyceae) (Figure 1) [11]. In general, red and green seaweeds are also found to carry low concentrations of phenols, whereas brown seaweeds are reported to be abundant in phlorotannins, with 1% to 14% dry biomass basis [12]. Between 1973 and 2003, Karl–Werner Glombitza and his collaborators conducted extensive studies on algal phlorotannins, forming the basis of our current understanding of their structural characteristics [13,14,15]. In his early work, Glombitza reported preliminary insights into the biosynthesis of phlorotannins. According to earlier reports, Glombitza suggested the possibility of extracting phloroglucinol, a key building block of phlorotannins, from members of the Fucales and Laminariales orders [15]. However, according to recent reports, phlorotannins are biosynthesized in most of the brown seaweed orders.
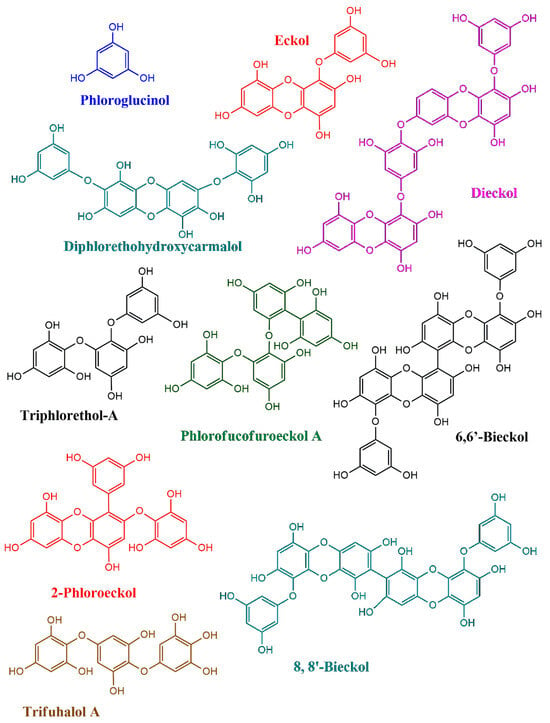
Figure 1.
The chemical structures of phlorotannins reported from brown seaweeds.
These secondary metabolites are derived from phloroglucinol (1,3,5-trihydroxybenzene), which serves as their basic monomeric unit. Phlorotannins are synthesized through the acetate-malonate pathway and are distinguished by their polymeric structure, which varies in size, complexity, and degree of polymerization [16]. This structural diversity of phlorotannins is responsible for their wide range of biological activities.
The fundamental structure of phlorotannins involves the repetitive linkage of phloroglucinol units through ether bonds, phenyl bonds, or both [17]. Based on the type of bonds and the arrangement of the phloroglucinol units, phlorotannins are classified into four major groups. These include phlorotannins with ether linkages (fuhalols and phlorethols/-C-O-C-ether bonds), those with phenyl linkages (fucols/-C-C- phenyl bonds), compounds containing both ether and phenyl linkages (fucophlorethols/both -C-C- and C-O-C- bonds), and those characterized by a dibenzodioxin linkage (eckols and carmalols) [18]. Furthermore, phlorotannins have a wide molecular weight range, from 126 Da (a single phloroglucinol unit) to over 1000 kDa in polymeric forms with linear or branched linkages [19].
The high degree of hydroxylation enhances water solubility and radical scavenging capacity, contributing significantly to the bioactivities of phlorotannins [19]. These properties also influence their pharmacokinetics, including solubility, membrane permeability, and interaction with enzymes or receptors. Notably, phlorotannins exhibit multiple roles in brown algae, such as UV protection, structural integrity, antioxidative defense, and herbivore deterrence by acting as appetite suppressants [20].
Recent studies suggest that low-molecular-weight phlorotannins such as triphlorethol-A, and eckol are more bioavailable and show stronger intracellular anti-inflammatory effects, while high-molecular-weight forms may act as physical UV barriers or degrade into active fragments in vivo [7,21]. These effects are modulated by physicochemical traits such as polarity, hydroxyl orientation, and molecular size.
Phlorotannins are mainly introduced into the body through the diet, and their health effects largely depend on how well they are absorbed and metabolized. Although there is limited research specifically on phlorotannins, they are generally believed to function like other plant-based polyphenols [22]. Similar to other tannins, phlorotannins tend to bind strongly with proteins, and these interactions, with both food components and proteins in the digestive system, can influence their behaviour and effectiveness [23]. Due to the large size and complex structure, phlorotannins are not efficiently absorbed in the upper parts of the digestive tract. Furthermore, research has shown that, like phenolic compounds from land plants, phlorotannins are also affected by the conditions within the gastrointestinal environment, which can result in a reduction of both concentration and antioxidant effectiveness during the digestion process of phlorotannins [22]. Moreover, most of the phlorotannins pass through to the colon in an unaltered form [24,25]. In the colon, gut bacteria break them down into smaller phenolic substances that can be more easily taken up by the body [25,26]. However, only a small portion is estimated to be absorbed into the bloodstream.
The breakdown of phlorotannins involves multiple metabolic steps, including chemical transformations during digestion and microbial fermentation in the colon [27]. These changes influence how phlorotannins work inside the body. Because only a small amount is absorbed, phlorotannins are likely to have their strongest effects locally in the gut, where they may help support beneficial bacteria and contribute to digestive health. To make better use of phlorotannins in health and medicine, it is important to enhance their stability and how easily they are absorbed. New technologies, such as using nano-sized delivery systems and advanced methods for extraction and purification, show promise in improving how these compounds act in the body and increasing their potential for therapeutic applications. Given these constraints, improving the stability and bioavailability of phlorotannins is essential for enhancing their therapeutic efficacy. Novel delivery systems, such as nanoencapsulation and emulsification, along with advanced extraction and purification techniques, may help optimize their pharmacokinetic properties and facilitate clinical application.
1.2. Recent Eco-Friendly Extraction Methods Reported for Phlorotannin Isolation and Identification
Recent advances in green extraction technologies have positioned Natural Deep Eutectic Solvents (NADES) as a transformative alternative for the sustainable and efficient recovery of phlorotannins from brown algae [28]. NADES are composed of naturally derived, biodegradable constituents such as sugars, organic acids, amino acids, and quaternary ammonium salts, which can be custom-tailored to achieve desirable physicochemical properties, including viscosity, polarity, and hydrogen bonding capacity [28,29]. Unlike conventional organic solvents, NADES offer low volatility, minimal toxicity, and excellent solvating power for polyphenolic compounds, making them especially suitable for food-grade and pharmaceutical applications [30].
Several studies have substantiated the superiority of NADES in the extraction of phlorotannins. Santos et al. (2024) demonstrated that certain NADES combinations, such as lactic acid: fructose (5:1), L-lactic acid: glucose (5:1), and L-lactic acid: sodium acetate (7:1) found to significantly improve the yield, total phenolic content, and radical scavenging activity of phlorotannin-rich extracts from Sargassum muticum and Gelidium corneum [31]. In another study, Rukavina et al. (2021) reported the effectiveness of choline chloride (ChCl)-based NADES for extracting bioactive phenolic compounds from the halophyte Polygonum maritimum L. Compared to conventional solvents like acetone, NADES formulations, particularly those containing fructose and sucrose, exhibited superior bioactive properties while preserving key phenolic constituents [32]. In another study, water-rich NADES (WRNADES) were applied in a one-hour ultrasound-assisted extraction (UAE) from Saccharina latissima, resulting in polyphenol yields that surpassed traditional 6-h extractions with aqueous methanol and acetone. The most efficient WRNADES—betaine:1,3-butanediol (1:1)—demonstrated high extraction efficiency, scalability, and alignment with green chemistry principles [33]. Further supporting the potential of NADES, Obluchinskaya et al. (2023) developed an optimized NADES-UAE method using lactic acid:choline chloride (3:1) for the extraction of phlorotannins from Fucus vesiculosus. The process achieved a high yield (137.3 mg phloroglucinol equivalents/g dry algae) within 23 min and identified 32 different phlorotannins using HPLC-HRMS/MS. Notably, the antioxidant activity of NADES extracts was comparable to ethanol-based ones, demonstrating their effectiveness and suitability for bioactive compound recovery [28].
Importantly, NADES not only enhance extraction yield but also play a crucial role in stabilizing phlorotannin structures by minimizing oxidation and polymerization, thereby preserving their biological efficacy. Moreover, the food-grade nature of many NADES constituents provides a regulatory advantage, facilitating their integration into functional food formulations without the need for extensive purification or solvent removal. However, challenges remain regarding the scalability of NADES-based extraction, solvent recyclability, and standardization of extract composition factors that require systematic investigation.
2. Anti-Inflammatory Mechanisms Reported from Phlorotannin
2.1. NLRP3 Inhibitory Properties Reported from Phlorotannins
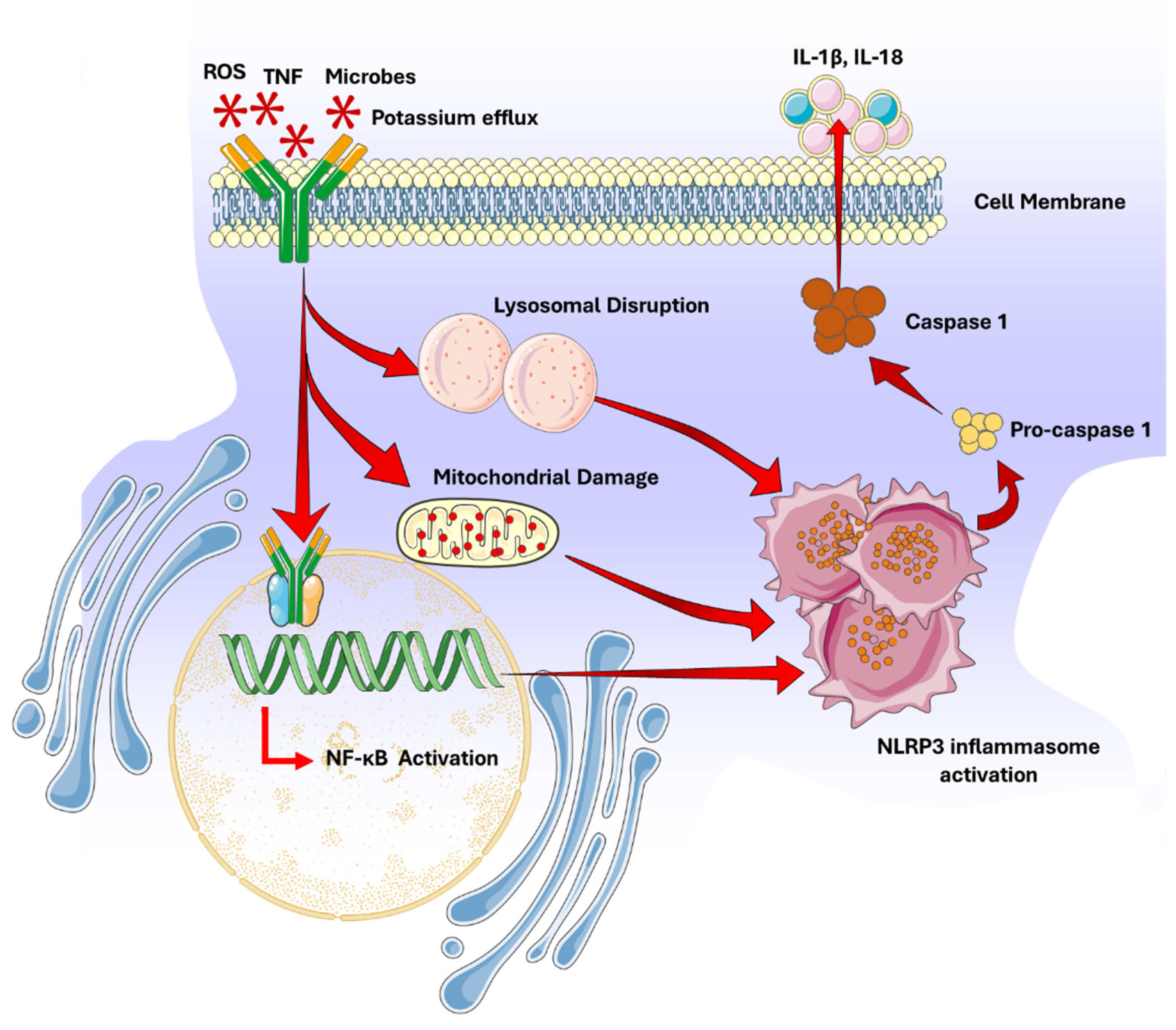
The NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) pathway comprises the activation of the NLRP3 inflammasome, a multiprotein complex essential for innate immunity and inflammatory responses [34]. NLRP3 functions as a sensor protein that detects various cellular stress signals and initiates inflammation. The NLRP3 pathway plays a critical role in addressing infections, tissue damage, metabolic imbalances, and cell death [35]. The NLRP3 inflammasome activation occurs in two steps: transcription (priming) and oligomerization (activation). Priming involves the upregulation of NLRP3 and pro-inflammatory cytokines such as interleukin-1β (IL-1β) and interleukin-18 (IL-18), triggered by the recognition of microbial products or danger signals via pattern recognition receptors (PRRs) like toll-like receptors (TLRs) [35,36,37]. Activation of NLRP3 signal cascade occurs through signals such as potassium efflux, reactive oxygen species (ROS), mitochondrial damage, or lysosomal destabilization, leading to the assembly of the inflammasome complex, including NLRP3, the adaptor protein ASC, and pro-caspase-1 [38,39].
Once activated, the NLRP3 inflammasome cleaves pro-caspase-1 into active caspase-1, which processes pro-IL-1β and pro-IL-18 into their mature forms, facilitating their secretion and mediating inflammatory responses [36,37,40] (Figure 2) Dysregulation of the NLRP3 pathway is linked to various inflammatory conditions, including autoimmune thyroid diseases, gout, systemic lupus erythematosus, atherosclerosis, Alzheimer’s, type II diabetes, Parkinson’s, and certain cancers [39,41].
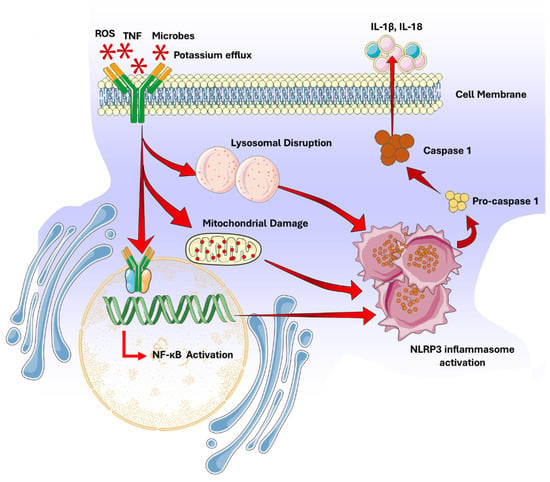
Figure 2.
NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) signaling pathway.
Several studies with seaweed recently reported the NLRP3 inhibitory activities of phlorotannins under different conditions. Ecklonia cava, a brown seaweed renowned for its bioactive compounds, is an abundant source of phlorotannins such as dieckol, eckol, and phlorofucofuroeckol A (Table 1). These compounds have demonstrated significant potential in modulating inflammatory responses and mitigating skin disorders. Recent studies have highlighted the therapeutic relevance of E. cava in both hyperpigmentation and inflammation-related conditions [42,43]. In a study by Byun et al. (2024) [42], the melanogenesis-inhibitory properties of phlorotannins derived from E. cava were evaluated using UV-exposed keratinocytes and animal skin. The findings revealed that phlorotannins, in combination with extracellular vesicles, alleviated UV-induced oxidative stress and inhibited the TXNIP/NLRP3/IL-18 pathway, key upstream regulators of melanogenesis. This co-treatment also restored skin structural integrity, demonstrating its potential as a therapeutic strategy for managing hyperpigmentation. In another investigation, Kim et al. (2023) [43] assessed the anti-inflammatory effects of E. cava extract in a murine model of dextran sulphate sodium-induced colitis. The extract significantly reduced disease severity, as evidenced by increased colon length, reduced spleen weight, and improved histological outcomes. Mechanistically, E. cava extract suppressed inflammatory cytokine upregulation, immune cell infiltration, and intestinal dysbiosis while promoting mucosal recovery. Its ability to inhibit the NLRP3 and nuclear factor kappa B pathways highlights its role as a natural therapeutic agent for inflammatory diseases. Furthermore, Oh et al. (2021) reported that E. cava extract and dieckol attenuated dexamethasone-induced muscle atrophy by reducing NLRP3 inflammasome formation and pyroptosis [44]. In an earlier study, Oh et al. (2020) demonstrated the potential of E. cava extract and pyrogallol-phloroglucinol-6,6-bieckol to attenuate pyroptosis in endothelial and vascular smooth muscle cells induced by high-fat diets and palmitate. According to the authors E. cava extract and pyrogallol-phloroglucinol-6,6-bieckol suppressed the TLR4/NF-κB pathway, reduced NLRP3 inflammasome formation, and normalized inflammatory markers such as IL-1β and IL-18. These treatments alleviated cellular dysfunction in the mouse aorta, a key factor in atherosclerosis [45]. In another study, Oh et al. (2020) evaluated the effects of dieckol, a component of E. cava extract, in a high-fat diet-induced animal model of non-alcoholic fatty liver disease (NAFLD). They found that E. cava extract or dieckol reduced NLRP3 expression, pyroptosis, triglyceride accumulation, and lipogenic gene expression, effectively attenuating NAFLD [46]. These findings emphasize the diverse therapeutic applications of E. cava and its phlorotannins in managing inflammatory and degenerative conditions.

Table 1.
NLRP3 inflammasome inhibitory properties reported from brown seaweed phlorotannins.
2.2. MAPK Inhibitory Properties Reported from Phlorotannins
Mitogen-activated protein kinases (MAPKs) are critical regulators of signal transduction, mediating responses to extracellular stimuli such as cytokines, stress, and growth factors. MAPK family members, including c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), p38 kinase, and, play a central role in inflammation [50,51]. Besides that, MAPK-related proteins were found to play major roles in UV-exposed skin damage, neurodegenerative diseases, apoptosis, and in oxidative stress. Thus, dysregulation of MAPK pathways is implicated in a range of diseases, demanding the search for targeted therapeutic agents to control MAPK signal cascade [52]. According to recent reports, phlorotannins, isolated from brown seaweeds, have emerged as potent inhibitors of MAPK signal cascade (Table 2). This section of the review discusses MAPK inhibitory properties reported from phlorotannins.
Studies have highlighted the MAPK inhibitory potential of brown seaweed phlorotannins in various models. Phlorotannins, such as diphlorethohydroxycarmalol (from Ishige okamurae) and eckmaxol (from Ecklonia maxima), have demonstrated significant anti-inflammatory and photoprotective properties by inhibiting MAPK activation. Diphlorethohydroxycarmalol has been shown to reduce collagenase activity and suppress pro-inflammatory cytokines by downregulating JNK, ERK, and p38 phosphorylation [53,54]. The protective effects of phlorotannins against UVB-induced DNA damage are attributed to their ability to induce the nucleotide excision repair system in HaCaT human keratinocytes [55]. Moreover, Wang et al. (2020) reported inhibitory activities diphlorethohydroxycarmalol against UVB-induced damage both in vitro in human dermal fibroblasts and in vivo in zebrafish [56]. Additionally, phlorotannins, such as 6,6′-bieckol, have demonstrated anti-photoaging effects by suppressing MAPK/AP-1 signaling in UVB-damaged keratinocytes (Table 1) [57]. In another study, Piao et al. (2021) attempted to investigate the protective effect of phloroglucinol (1,3,5-trihydroxybenzene), against UVB-exposed human HaCaT keratinocytes. According to the results, phloroglucinol attenuates UVB-induced 8-oxoguanine formation in human HaCaT keratinocytes through AKT and ERK-mediated NRF2/OGG1 signaling pathways [58].
Kang et al. (2023) further reported that E. cava extracts containing dieckol significantly decrease the expression of pro-inflammatory cytokines and inhibit the activation of MAPK pathways in both Lipopolysaccharide-stimulated RAW macrophages and an Ovalbumin-induced asthma mouse model [59]. Similarly, Byun et al. (2021) demonstrated that phlorotannins from E. cava mitigate inflammatory responses in the liver, attenuating the effects of NAFLD by regulating MAPK signaling and cytokine production [42]. Furthermore, phlorotannins in E. cava have been shown to promote collagen synthesis and protect against UVB-induced damage by inhibiting MAPK activation. Phlorotannins also enhance skin health by regulating the MAPK pathway in response to oxidative damage. Research on E. cava has demonstrated the ability of phlorotannins to promote collagen synthesis and protect against UVB-induced damage by inhibiting MAPK activation [60]. Similarly, Phlorofucofuroeckol A from E. cava was found to ameliorate the TGF-β1-induced fibrotic response of human tracheal fibroblasts via the downregulation of MAPKs and SMAD 2/3 pathways inactivated TGF-β receptor [61].
Phlorotannins like triphlorethol-A and dieckol were found to inhibit carcinoma cell proliferation and induce apoptosis by targeting MAPK and JAK/STAT pathways [62]. Research on triphlorethol-A from Ishige okamurae also shows promising anticancer effects via blocking JAK2/STAT3 and p38 MAPK/ERK signaling pathways in U251 human glioma cancer cells [62]. Additionally, I. okamurae extract rich in phlorotannins was found to attenuate amyloid beta peptide (Aβ25-35) induced neurotoxicity by suppressing MAPK activation, suggesting a neuroprotective role of phlorotannins [63]. Kim et al. (2021) also reported the protective effect of I. okamurae phlorotannins-rich extract (diphlorethohydroxy-carmalol and ishophloroglucin A) against methylglyoxal-induced nephrotoxicity. According to the authors, I. okamurae phlorotannins-rich extract was found to protect mesangial cells via inhibiting AGE formation, AGE-protein cross-linking, and breaking pre-formed cross-links [47]. Similarly, Cho et al. (2024) also reported the therapeutic potential due to its impact of diphlorethohydroxycarmalol and ishophloroglucin (I. okamurae) on MAPK signalling (ERK and JNK) pathways. According to the authors, phlorotannins exhibit inhibitory effects on osteoclastogenesis by suppressing key proteins involved in bone resorption, such as tartrate-resistant acid phosphatase, metallopeptidase-9 (MMP-9), and cathepsin K [48]. In another study, trifuhalol A, a phlorotannin isolated from Agarum cribrosum, inhibited the degranulation of immune cells and the biosynthesis of IL-33 and IgE in differentiated B cells and keratinocytes [64]. Additionally, in a recent study, Cao et al. (2024) also, reported the protective effect of Sargahydroquinoic acid (a hydroquinone derivative) from Sargassum macrocarpum against TNF-α and UV-induced skin aging in human dermal fibroblasts. According to the authors, Sargahydroquinoic acid effectively inhibited both the phosphorylation of Akt and the expression of total Akt levels in UV-irradiated dermal fibroblasts [65]. These observations demonstrate the potential applications of phlorotannins in brown seaweeds as MAPK inhibitors to treat different diseases.

Table 2.
MAPK inhibitory properties reported from brown seaweed phlorotannins.
Table 2.
MAPK inhibitory properties reported from brown seaweed phlorotannins.
| Seaweed/Phlorotannin | Signal Mechanism/Bioactivity | Reference |
|---|---|---|
| pyrogallol-phloroglucinol-6,6-bieckol | Inhibit pyroptosis in endothelial and vascular smooth muscle cells induced by high-fat diets and palmitate. | [45] |
| Diphlorethohydroxycarmalol | Downregulation of JNK, ERK, and p38 phosphorylation. | [48,53] |
| Dieckol | Downregulation of JNK, ERK, and p38 phosphorylation in macrophages. | [52] |
| Eckmaxol | Downregulation of JNK, ERK, and p38 phosphorylation. | [54] |
| Diphlorethohydroxycarmalol | Inhibit MAPK activation in UVB-exposed human dermal fibroblasts and in zebrafish. | [56] |
| 6,6′-bieckol | Reduce UVB-induced oxidative stress damage in HaCaT cells. | [57] |
| Phloroglucinol (1,3,5-trihydroxybenzene | Protect against UVB-exposed cell damage via AKT and ERK-mediated Nrf2/Ogg1 signaling pathways. | [58] |
| Dieckol | Inhibit the activation of MAPK pathways in Lipopolysaccharide-Stimulated RAW Macrophages and Ovalbumin-Induced Asthma Mouse Model. | [59] |
| Dieckol | Promote collagen synthesis and protect against UVB-induced damage by inhibiting MAPK activation. | [60] |
| Phlorofucofuroeckol A from E. cava | Ameliorates TGF-β1-induced fibrotic response of human tracheal fibroblasts via the downregulation of MAPKs and SMAD 2/3 pathways inactivated TGF-β receptor. | [61] |
| Triphlorethol-A | Promote apoptosis by modulating MAPK and ERK pathways. | [62] |
| I. okamurae extract | Attenuate amyloid beta peptide (Aβ25-35) induced neurotoxicity by suppressing MAPK activation. | [63] |
| Ishophloroglucin | Downregulation of JNK, ERK, and p38 phosphorylation. | [47,48] |
| Trifuhalol A | Inhibit the degranulation of immune cells and the biosynthesis of IL-33 and IgE in differentiated B cells and keratinocytes. | [64] |
| Sargahydroquinoic acid (a hydroquinone derivative) from Sargassum macrocarpum | Downregulation of JNK, ERK, and p38 phosphorylation. | [65] |
| Ecklonia cava extract | Suppressed both the expression of the fibrotic phenotypic marker and cell migration by inhibiting the activation of the MAPK and Smad2/3 signaling pathways in human vocal fold fibroblasts. | [66] |
| Diphlorethohydroxycarmalol | Suppress the inflammatory myopathy-related protein expression through p-JNK/p-p38 in C2C12 myoblasts. | [67] |
| Diphlorethohydroxycarmalol | p38 MAPK expression in H2O2-induced zebrafish mussels. | [68] |
| Diphlorethohydroxycarmalol | Downregulate methylglyoxal-induced JNK, ERK, and p38 phosphorylation and reduce renal damage in Mouse glomerular mesangial cells | [69] |
| Phlorofucofuroeckol A from E. cava | Downregulate MAPK protein expression in TNF-α/IFN-γ-stimulated HaCaT keratinocytes and 12-O-tetradecanoylphorbol 13-acetate-induced ear edema in BALB/c mice. | [70] |
2.3. NF-kB Inhibitory Properties Reported from Phlorotannins
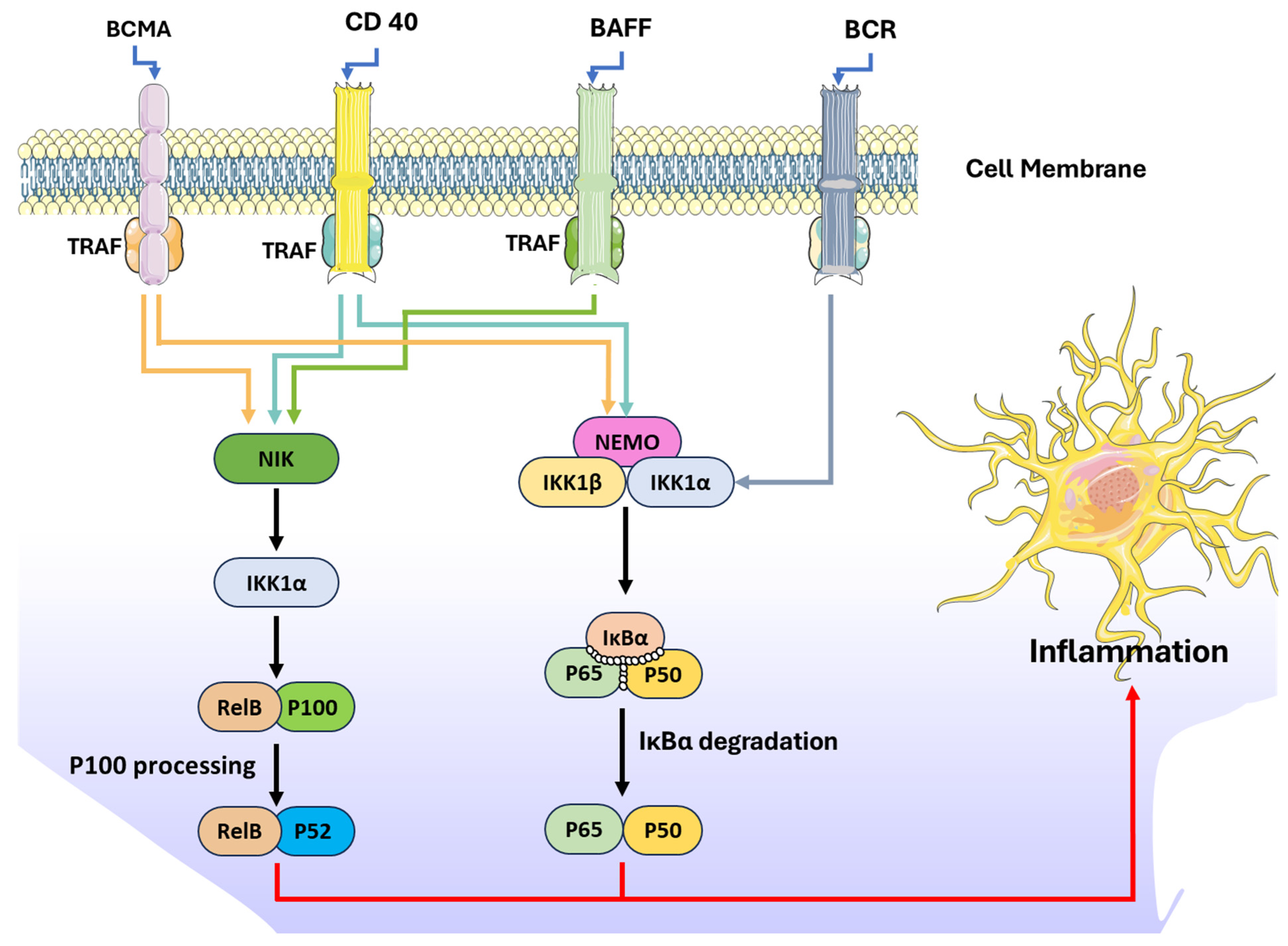
The nuclear factor kappa B (NF-κB) pathway plays a central role in regulating inflammatory responses and uncontrolled activation of NF-κB can lead to pathogenesis of inflammatory diseases including cancers. Furthermore, NF-kB proteins can regulate the expression of hundreds of genes, which regulate important physiological processes such as inflammation, immunity, proliferation, and cell death. The NF-κB signal cascade activates through two distinct mechanisms known as canonical and non-canonical pathways [71].
The canonical pathway is activated by numerous proinflammatory signals like IL-1β, TNF-α, and TLR ligands. The canonical pathway triggers the rapid but transient response, is crucial for the transcription of genes related to inflammation. Activation involves the phosphorylation and ubiquitination of IκB proteins, leading to their degradation and allowing the NF-κB complex (RelA/p50) to translocate into the nucleus to initiate gene transcription [72]. On the other hand, the non-canonical NF-κB pathway is a slower, more sustained pathway triggered by specific TNF receptor superfamily members, such as B cell-activating factor (BAFF), B-cell receptor (BCR), CD 40, and proliferation-inducing ligand (APRIL). Activation involves NF-κB-inducing kinase (NIK), which stabilizes and phosphorylates IKKα. This phosphorylation initiates the ubiquitination and processing of p100 into p52, facilitating the formation of the RelB-p52 complex that enters the nucleus [73,74] (Figure 3). This pathway is essential for immune cell development, lymphoid organ formation, and maintaining immune homeostasis. The regulation of NIK and its ubiquitination are pivotal for preventing aberrant activation [75]. Recent studies have demonstrated the potential of phlorotannins, polyphenolic compounds derived from brown seaweeds, to suppress NF-κB signal transduction. This section of the review summarizes the specific phlorotannins, their sources, and mechanisms of action, highlighting their therapeutic potential in combating inflammation and related diseases (Figure 3).
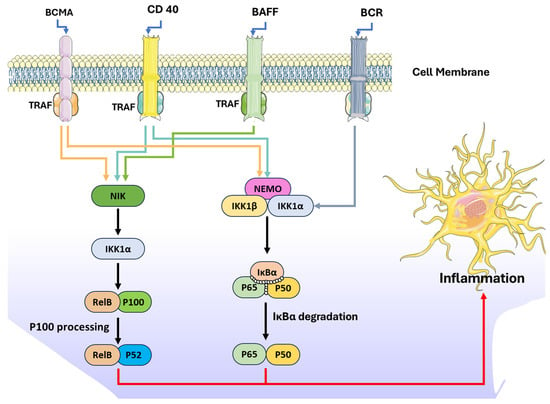
Figure 3.
NF-kB-mediated inflammatory pathways.
Phlorotannins, such as phloroglucinol, eckol, dieckol, phlorofucofuroeckol A, and diphlorethohydroxycarmalol (DPHC) isolated from brown seaweeds (See Figure 1) found to possess strong NF-kB inhibitory properties under in vivo and in vitro conditions [76,77] (Table 3). Fucus vesiculosus, is a well-known source of phlorotannins such as fucofurodiphlorethol, fucofurotriphlorethol and fucofuropentaphlorethol, which are found to exhibit promising anti-inflammatory activity by modulating NF-kB-related protein expressions [78]. Ethanolic extract separated from Sargassum horneri also showed a protective effect against TNF-α/IFN-γ-induced inflammation in human keratinocytes and TPA-induced ear edema in mice and LPS-induced inflammation in RAW 264.7 macrophages via blocking NF-κB expression [79,80]. S. macrocarpum contains sargahydroquinoic acid which has been reported to inhibit NF-κB activation by blocking the degradation of inhibitor κB-α (IκBα) in LPS-stimulated murine macrophage RAW 264.7 cells [81]. In another study, treatment with Eisenia bicyclis phlorotannins was found to downregulate the gene expression levels of NF-κB in LPS-stimulated THP-1 macrophages [82]. Moreover, 80% methanolic extract of E. bicyclis rich in phlorotannins such as Phloroglucinol, 7-Phloroeckol, Phlorofucofuroeckol A, Dieckol reported to ameliorate dextran sulfate sodium-induced colitis in mice through the Ahr pathway via downregulating NF-κB gene expression [83]. Ishige okamurae produces DPHC, which blocks TNF-α-induced NF-κB signaling in muscle cells, offering protection against inflammatory myopathies. According to the authors, DPHC suppressed the inflammatory myopathy-related protein expression through NF-κB (p-IκB-α/p-p65NF-κB) signaling cascade [67]. Additionally, in another study Lee et al. (2024) also reported the protective effect of DPHC against H2O2-induced zebrafish muscle tissues [68]. E. cava is another well-known brown seaweed recognized for its potential NF-κB inhibitory properties. Recently, several studies have highlighted the protective effects of phlorotannins isolated from E. cava against NF-κB-mediated inflammatory responses. Specifically, phlorotannins such as 7-phloroeckol, eckol, dieckol, and phlorofucofuroeckol have been found to inhibit NF-κB protein expression in TNF-α/IFN-γ-stimulated HaCaT keratinocytes, 12-O-tetradecanoylphorbol-13-acetate-induced ear edema in BALB/c mice, and alcohol-induced oxidative stress in HepG2/CYP2E1 cells, as well as in other in vivo and in vitro models [77,84,85].

Table 3.
NF-kB inhibitory properties reported from brown seaweed phlorotannins.
2.4. JAK/STAT3 Inhibitory Properties Reported from Phlorotannins
The Janus kinase and signal transducers and activators of transcription 3 (JAK/STAT3) pathway is an important signaling mechanism that controls a variety of biological processes [89]. The JAK/STAT3 pathway serves as a bridge between extracellular signals and gene expression, enabling cells to respond to environmental cues [90]. Under normal conditions, JAK/STAT3 promotes cell cycle progression, tissue repair, and immune regulation. According to previous studies, cytokines (IL-3, IL-4, IL-5, IL-6, IL-7, IL-10, IL-11, IL-12, IL-15, IL-21, IL-22, and TNF-α), hormones, and growth factors binding to their multimeric receptor are responsible for the upstream activation of JAK/STAT3 [89,90,91,92,93].
JAKs are activated when cytokines or growth factors bind to specific cell surface receptors as mentioned above. These binding triggers JAK autophosphorylation and receptor phosphorylation, creating docking sites for STAT3. Subsequently, JAKs phosphorylate STAT3 at a specific tyrosine residue (typically at tyrosine 705), causing it to dimerize and translocate into the nucleus [94]. Once inside the nucleus, STAT3 binds to DNA and regulates the transcription of target genes involved in processes such as inflammation, apoptosis inhibition, and cell proliferation [92,93].
Even though the JAK/STAT3 pathway is essential for typical cellular function, dysregulation of JAK/STAT3 signaling is the root cause of many disease conditions. Specifically, hyperactivation of JAK/STAT3 is a hallmark of many cancers, as dysregulated JAK/STAT3 supports the proliferation, survival, invasiveness, and metastasis of cancer cells, while strongly suppressing the antitumor immune responses [95,96,97]. Additionally, chronic activation of JAK/STAT3 signaling is associated with autoimmune diseases such as rheumatoid arthritis, psoriasis, and inflammatory bowel disease [98].
Recently, a number of studies have shown the potential of phlorotannins to inhibit JAK/STAT3 signal cascade. Specifically, phlorotannins, which can downregulate the expression of cytokines such as IL-3, IL-4, IL-5, IL-6, IL-7, IL-10, IL-11, IL-12, IL-15, IL-21, IL-22, and TNF-α, can be considered as potential JAK/STAT3 inhibitors (Table 4).

Table 4.
JAK/STAT3 inhibitory properties reported from brown seaweed phlorotannins.
Eckol is a phlorotannin with diverse bioactivities, as confirmed by numerous studies conducted under various experimental conditions. Notably, eckol has shown significant potential as a candidate for developing JAK/STAT3 inhibitors. In a recent study, Liao et al. (2023) reported its inhibitory effects on dextran sodium sulfate-upregulated TLR4/STAT3/NF-κB signaling in colonic tissues at the molecular level, highlighting its anti-inflammatory and immunomodulatory roles in chronic ulcerative colitis in mice [99]. Furthermore, eckol isolated from E. cava was found to suppress immunoglobulin E-mediated mast cell activation and passive cutaneous anaphylaxis in mice by downregulating cytokines such as IL-4, IL-5, and IL-6 [96].
In another study, Son et al. (2020) reported the inhibitory effects of a phlorotannin-rich fraction from E. cava on IL-6 and IL-10 mRNA expression in high-fat diet-induced obese mice [88]. In a recent study, ishophloroglucin A identified from I. okamurae was found to inhibit JAK/STAT3 protein expression in ishophloroglucin A administrated ob/ob mice models [100]. Kim et al. (2023) also reported the IL-6 inhibitory properties of trifuhalol A isolated from Agarum cribrosum, against the Ovalbumin-Induced Allergic Asthma Model [101]. Similarly, Bong et al. (2022) also reported the potential of trifuhalol A to ameliorate allergic inflammation by reducing the expression of IL-33, IL-4, and IL-13 in house dust mite-treated dorsal skin of mice [64]. In another recent study, a synthetic phloroglucinol derivative was also found to inhibit LPS-stimulated IL-6 and TNF-α production in BV-2 cells, suggesting the potential to develop commercial drugs for JAK/STAT3 inhibitors [102]. Taken together, these results suggest the potential of brown seaweed phlorotannins to develop into JAK/STAT3 inhibitors to treat inflammatory diseases including different types of cancers.
3. Synergistic Anti-Inflammatory Effects Reported Between Phlorotannins and Other Algal Components
In addition to their individual anti-inflammatory properties, recent studies have revealed that phlorotannins exhibit enhanced bioactivity when combined with other marine-derived secondary metabolites, such as sulfated polysaccharides like fucoidan [103,104,105].
In a recent study, Obluchinskaya et al. (2022) reported the positive correlation of anti-inflammatory activities between the phlorotannins and fucoidan isolated from five different brown seaweeds [104]. Similarly, Chouh et al. (2022) demonstrated that fucoidan and phlorotannin-rich extracts from brown algae exhibit complementary antioxidant and anti-inflammatory effects, suggesting that combining these bioactive compounds could further enhance therapeutic outcomes [104,106]. In addition, several studies have investigated the interactions between phlorotannins and other secondary metabolite-rich extracts in terms of synergistic or additive anti-inflammatory activity. Specifically, Han et al. (2021) reported that a mixture of phlorotannin and fucoidan from E. cava prevents the Aβ-induced cognitive decline with mitochondrial and cholinergic activation [107]. This synergism appears to be mediated through concurrent modulation of inflammatory signaling pathways, particularly by suppressing the phosphorylation of key kinases in the MAPK family proteins, including Akt and JNK. Similarly, another study by Kim et al. (2021) demonstrated that three seaweed extracts containing polyphenols and polysaccharides from Undaria pinnatifida sporophyll, Codium fragile, and Gracilaria verrucosa were found to have anti-inflammatory and antidiabetic effects in C2C12 muscle cells [108]. Besides that, a combination of phlorotannins isolated from E. bicyclis and Lactobacillus casei was found to be highly effective in preventing ulcerative colitis [83]. According to the authors, the reduction in the expression of inflammatory cytokines and the restoration of the Firmicutes-to-Bacteroidetes ratio observed in experimental findings highlight the therapeutic potential of these combinations. Furthermore, this evidence suggests that these synergistic formulations could provide more effective strategies for developing functional foods and marine-derived therapeutics aimed at chronic inflammatory disorders, including metabolic syndrome and neurodegenerative diseases.
4. Conclusions
Phlorotannins and phlorotannin-rich extracts from brown seaweeds demonstrate remarkable potential as bioactive compounds, particularly for their anti-inflammatory and immunomodulatory properties. Recent studies reveal their ability to modulate key molecular pathways such as the NLRP3 inflammasome, NF-κB, MAPK, and JAK/STAT3, positioning them as promising natural candidates for novel therapeutic development. However, challenges such as low natural abundance (1–14% of dry biomass), poor bioavailability, metabolic instability, and regulatory limitations continue to hinder their translational potential. Taken together, these emerging insights underscore the need for a more comprehensive understanding of phlorotannin efficacy, not as isolated compounds, but within the broader context of whole algal extracts, synergistic formulations, and advanced, sustainable extraction methods. Without incorporating these multidimensional factors, any evaluation of their clinical relevance or industrial scalability remains incomplete. Therefore, future research should aim to elucidate structure–function relationships, validate efficacy through clinical trials, and optimize scalable extraction techniques, all while integrating synergistic and technological perspectives. This approach will be crucial for unlocking their full potential in functional foods, nutraceuticals, and pharmaceutical applications.
Author Contributions
K.H.I.N.M.H.: Data curation, Formal analysis, Methodology, Writing—original draft, Writing—review and editing; D.P.N.: Data curation, Formal analysis, Methodology, Software, Validation, L.W.: Data curation, Methodology, Writing—original draft, Writing—review and editing; K.K.A.S.: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant received from the Research Council, University of Sri Jayewardenepura, Sri Lanka. Grant number RC/URG/FOT/2024/59.
Conflicts of Interest
The authors declare no competing financial interest.
References
- Gusev, E.; Zhuravleva, Y. Inflammation: A New Look at an Old Problem. Int. J. Mol. Sci. 2022, 23, 4596. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chenli, Z. Inflammation. In Textbook of Pathologic Anatomy; Chen, K., Liang, L., Li, M., Pan, Y., Eds.; Springer Nature: Singapore, 2024; pp. 75–105. [Google Scholar] [CrossRef]
- Deng, L.; He, S.; Guo, N.; Tian, W.; Zhang, W.; Luo, L. Molecular mechanisms of ferroptosis and relevance to inflammation. Inflamm. Res. 2023, 72, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Bardelcikova, A.; Soltys, J.; Mojzis, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef]
- Massironi, S.; Vigano, C.; Palermo, A.; Pirola, L.; Mulinacci, G.; Allocca, M.; Peyrin-Biroulet, L.; Danese, S. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2023, 8, 579–590. [Google Scholar] [CrossRef]
- Barbosa, M.; Lopes, G.; Andrade, P.B.; Valentão, P. Bioprospecting of brown seaweeds for biotechnological applications: Phlorotannin actions in inflammation and allergy network. Trends Food Sci. Technol. 2019, 86, 153–171. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Kwon, O.I.; Hwang, H.J.; Shin, H.C.; Yang, S. Therapeutic effects of phlorotannins in the treatment of neurodegenerative disorders. Front. Mol. Neurosci. 2023, 16, 1193590. [Google Scholar] [CrossRef]
- Gheda, S.; Naby, M.A.; Mohamed, T.; Pereira, L.; Khamis, A. Antidiabetic and antioxidant activity of phlorotannins extracted from the brown seaweed Cystoseira compressa in streptozotocin-induced diabetic rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 22886–22901. [Google Scholar] [CrossRef]
- Kim, A.T.; Park, Y. Trifuhalol A, a phlorotannin from the brown algae Agarum cribrosum, reduces adipogenesis of human primary adipocytes through Wnt/beta-catenin and AMPK-dependent pathways. Curr. Res. Food Sci. 2023, 7, 100646. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Maheswari, V.; Babu, P.A.S. Phlorotannin and its Derivatives, a Potential Antiviral Molecule from Brown Seaweeds, an Overview. Russ. J. Mar. Biol. 2022, 48, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Glombitza, K.W.; Rosener, H.U.; Vilter, H.; Rauwald, W. Antibiotics from Algae. 8. Phloroglucinol from Phaeophyceae (author’s transl). Planta Medica 1973, 24, 301–303. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Pauli, K. Fucols and Phlorethols from the Brown Alga Scytothamnus australis Hook. et Harv. (Chnoosporaceae). Bot. Mar. 2003, 46, 315–320. [Google Scholar] [CrossRef]
- Glombitza, K.W. Highly Hydroxylated Phenols of the Phaeophyceae. In Marine Natural Products Chemistry; Faulkner, D.J., Fenical, W.H., Eds.; Springer: Boston, MA, USA, 1977; pp. 191–204. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.; Ahn, G. Marine algal flavonoids and phlorotannins; an intriguing frontier of biofunctional secondary metabolites. Crit. Rev. Biotechnol. 2022, 42, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Pereira, C.S.G.P.; Silva, A.; Barciela, P.; Jorge, A.O.S.; Perez-Vazquez, A.; Pereira, A.G.; Barreira, J.C.M.; Oliveira, M.B.P.P.; Prieto, M.A. Metabolite Profiling of Macroalgae: Biosynthesis and Beneficial Biological Properties of Active Compounds. Mar. Drugs 2024, 22, 478. [Google Scholar] [CrossRef]
- Catarino, M.D.; Pires, S.M.G.; Silva, S.; Costa, F.; Braga, S.S.; Pinto, D.C.G.A.; Silva, A.M.S.; Cardoso, S.M. Overview of Phlorotannins’ Constituents in Fucales. Mar. Drugs 2022, 20, 754. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; El Zokm, G.M.; El Sikaily, A.M.; Selim, A.I.; Ismail, G.A. Chemodiversity and bioactivity assessment of phlorotannins from some Phaeophyta species from the Red Sea. J. Appl. Phycol. 2023, 35, 1769–1788. [Google Scholar] [CrossRef]
- Begum, R.; Howlader, S.; Mamun-Or-Rashid, A.N.M.; Rafiquzzaman, S.M.; Ashraf, G.M.; Albadrani, G.M.; Sayed, A.A.; Peluso, I.; Abdel-Daim, M.M.; Uddin, M.S. Antioxidant and Signal-Modulating Effects of Brown Seaweed-Derived Compounds against Oxidative Stress-Associated Pathology. Oxid. Med. Cell Longev. 2021, 2021, 9974890. [Google Scholar] [CrossRef]
- Zheng, H.; Zhao, Y.; Guo, L. A Bioactive Substance Derived from Brown Seaweeds: Phlorotannins. Mar. Drugs 2022, 20, 742. [Google Scholar] [CrossRef]
- Catarino, M.D.; Marçal, C.; Bonifácio-Lopes, T.; Campos, D.; Mateus, N.; Silva, A.M.S.; Pintado, M.M.; Cardoso, S.M. Impact of Phlorotannin Extracts from Fucus vesiculosus on Human Gut Microbiota. Mar. Drugs 2021, 19, 375. [Google Scholar] [CrossRef]
- Ford, L.; Curry, C.; Campbell, M.; Theodoridou, K.; Sheldrake, G.; Dick, J.; Stella, L.; Walsh, P.J. Effect of Phlorotannins from Brown Seaweeds on the In Vitro Digestibility of Pig Feed. Animals 2020, 10, 2193. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Garcia, C.J.; Garcia-Villalba, R.; Silva, A.M.S.; Campos, D.A.; Manuela Pintado, M.; Neves, B.; Cardoso, S.M.; Tomas-Barberan, F.A. Exploring the fate of phlorotannins from Laminaria digitata across the gastrointestinal tract: Insights into susceptibility and bioactivity prior and post gastrointestinal digestion. Food Res. Int. 2024, 191, 114641. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Cassani, L.; Gomez-Zavaglia, A.; Jimenez-Lopez, C.; Lourenco-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Seaweed-based natural ingredients: Stability of phlorotannins during extraction, storage, passage through the gastrointestinal tract and potential incorporation into functional foods. Food Res. Int. 2020, 137, 109676. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Ji, Y.; Anegboonlap, P.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.; Rowland, I. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br. J. Nutr. 2016, 115, 1240–1253. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shevyrin, V.A.; Kovaleva, E.G.; Flisyuk, E.V.; Shikov, A.N. Optimization of Extraction of Phlorotannins from the Arctic Fucus vesiculosus Using Natural Deep Eutectic Solvents and Their HPLC Profiling with Tandem High-Resolution Mass Spectrometry. Mar. Drugs 2023, 21, 263. [Google Scholar] [CrossRef]
- Percevault, L.; Limanton, E.; Gauffre, F.; Lagrost, C.; Paquin, L. Extraction of Plant and Algal Polyphenols Using Eutectic Solvents. In Deep Eutectic Solvents for Medicine, Gas Solubilization and Extraction of Natural Substances; Fourmentin, S., Costa Gomes, M., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2021; pp. 241–306. [Google Scholar] [CrossRef]
- Misan, A.; Nadpal, J.; Stupar, A.; Pojic, M.; Mandic, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef]
- Santos, J.M.; Jesus, B.C.; Ribeiro, H.; Martins, A.; Marto, J.; Fitas, M.; Pinto, P.; Alves, C.; Silva, J.; Pedrosa, R.; et al. Extraction of macroalgae phenolic compounds for cosmetic application using eutectic solvents. Algal Res. 2024, 79, 103438. [Google Scholar] [CrossRef]
- Rukavina, I.; Rodrigues, M.J.; Pereira, C.G.; Mansinhos, I.; Romano, A.; Ślusarczyk, S.; Matkowski, A.; Custódio, L. Greener Is Better: First Approach for the Use of Natural Deep Eutectic Solvents (NADES) to Extract Antioxidants from the Medicinal Halophyte Polygonum maritimum L. Molecules 2021, 26, 6136. [Google Scholar] [CrossRef]
- Zeb, L.; Gerhardt, A.S.; Johannesen, B.A.; Underhaug, J.; Jordheim, M. Ultrasonic-Assisted Water-Rich Natural Deep Eutectic Solvents for Sustainable Polyphenol Extraction from Seaweed: A Case Study on Cultivated Saccharina latissima. ACS Sustain. Chem. Eng. 2024, 12, 14921–14929. [Google Scholar] [CrossRef]
- Xu, J.; Nunez, G. The NLRP3 inflammasome: Activation and regulation. Trends Biochem. Sci. 2023, 48, 331–344. [Google Scholar] [CrossRef]
- Bai, B.; Yang, Y.; Wang, Q.; Li, M.; Tian, C.; Liu, Y.; Aung, L.H.H.; Li, P.F.; Yu, T.; Chu, X.M. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, J.; Bi, L. Role of the NLRP3 inflammasome in autoimmune diseases. Biomed. Pharmacother. 2020, 130, 110542. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Manan, A.; Kim, J.; Choi, S. NLRP3 inflammasome: A key player in the pathogenesis of life-style disorders. Exp. Mol. Med. 2024, 56, 1488–1500. [Google Scholar] [CrossRef]
- Akbal, A.; Dernst, A.; Lovotti, M.; Mangan, M.S.J.; McManus, R.M.; Latz, E. How location and cellular signaling combine to activate the NLRP3 inflammasome. Cell Mol. Immunol. 2022, 19, 1201–1214. [Google Scholar] [CrossRef]
- Lee, A.H.; Shin, H.Y.; Park, J.H.; Koo, S.Y.; Kim, S.M.; Yang, S.H. Fucoxanthin from microalgae Phaeodactylum tricornutum inhibits pro-inflammatory cytokines by regulating both NF-kappaB and NLRP3 inflammasome activation. Sci. Rep. 2021, 11, 543. [Google Scholar] [CrossRef]
- Ren, W.; Sun, Y.; Zhao, L.; Shi, X. NLRP3 inflammasome and its role in autoimmune diseases: A promising therapeutic target. Biomed. Pharmacother. 2024, 175, 116679. [Google Scholar] [CrossRef]
- Byun, K.-A.; Park, Y.; Oh, S.; Batsukh, S.; Son, K.H.; Byun, K. Co-Treatment with Phlorotannin and Extracellular Vesicles from Ecklonia cava Inhibits UV-Induced Melanogenesis. Antioxidants 2024, 13, 408. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Kim, H.-Y.; Jang, J.-T.; Hong, S. Preventive Effect of Ecklonia cava Extract on DSS-Induced Colitis by Elevating Intestinal Barrier Function and Improving Pathogenic Inflammation. Molecules 2023, 28, 8099. [Google Scholar] [CrossRef]
- Oh, S.; Yang, J.; Park, C.; Son, K.; Byun, K. Dieckol Attenuated Glucocorticoid-Induced Muscle Atrophy by Decreasing NLRP3 Inflammasome and Pyroptosis. Int. J. Mol. Sci. 2021, 22, 8057. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Son, M.; Park, C.-H.; Jang, J.T.; Son, K.H.; Byun, K. The Reducing Effects of Pyrogallol-Phloroglucinol-6,6-Bieckol on High-Fat Diet-Induced Pyroptosis in Endothelial and Vascular Smooth Muscle Cells of Mice Aortas. Mar. Drugs 2020, 18, 648. [Google Scholar] [CrossRef]
- Oh, S.; Son, M.; Byun, K.-A.; Jang, J.T.; Choi, C.H.; Son, K.H.; Byun, K. Attenuating Effects of Dieckol on High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease by Decreasing the NLRP3 Inflammasome and Pyroptosis. Mar. Drugs 2021, 19, 318. [Google Scholar] [CrossRef]
- Kim, M.; Cho, C.; Lee, C.; Ryu, B.; Kim, S.; Hur, J.; Lee, S.-H. Ishige okamurae Ameliorates Methylglyoxal-Induced Nephrotoxicity via Reducing Oxidative Stress, RAGE Protein Expression, and Modulating MAPK, Nrf2/ARE Signaling Pathway in Mouse Glomerular Mesangial Cells. Foods 2021, 10, 2000. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, H.S.; Ahn, J.; Ryu, B.; Jea, J.G.; Lee, K.; Kim, K.; Ahn, G.; Lee, W.; Choi, K.M.; et al. Effect of Ishige okamurae Extract on Osteoclastogenesis In Vitro and In Vivo. Mar. Drugs 2024, 22, 137. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Kim, H.-J.; Heo, H.J. Ecklonia cava Attenuates PM2.5-Induced Cognitive Decline through Mitochondrial Activation and Anti-Inflammatory Effect. Mar. Drugs 2021, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Upadhyay, T.; Singh, S.; Chigurupati, S.; Alsubayiel, A.M.; Mani, V.; Vargas-De-La-Cruz, C.; Uivarosan, D.; Bustea, C.; Sava, C.; et al. Polyphenols Targeting MAPK Mediated Oxidative Stress and Inflammation in Rheumatoid Arthritis. Molecules 2021, 26, 6570. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Kim, H.-S.; Asanka Sanjeewa, K.K.; Han, E.J.; Jee, Y.; Ahn, G.; Rho, J.-R.; Jeon, Y.-J. Loliolide, isolated from Sargassum horneri; abate LPS-induced inflammation via TLR mediated NF-κB, MAPK pathways in macrophages. Algal Res. 2021, 56, 102297. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, H.-S.; Jayawardena, T.U.; Ryu, B.; Yang, H.-W.; Ahn, G.; Lee, W.; Jeon, Y.-J. Dieckol: An algal polyphenol attenuates urban fine dust-induced inflammation in RAW 264.7 cells via the activation of anti-inflammatory and antioxidant signaling pathways. J. Appl. Phycol. 2019, 32, 2387–2396. [Google Scholar] [CrossRef]
- Wang, L.; Kim, H.S.; Je, J.-G.; Oh, J.Y.; Kim, Y.-S.; Cha, S.-H.; Jeon, Y.-J. Protective Effect of Diphlorethohydroxycarmalol Isolated from Ishige okamurae Against Particulate Matter-Induced Skin Damage by Regulation of NF-κB, AP-1, and MAPKs Signaling Pathways In Vitro in Human Dermal Fibroblasts. Molecules 2020, 25, 1055. [Google Scholar] [CrossRef]
- Nagahawatta, D.P.; Liyanage, N.M.; Jayawardhana, H.H.A.C.K.; Jayawardena, T.U.; Lee, H.-G.; Heo, M.-S.; Jeon, Y.-J. Eckmaxol Isolated from Ecklonia maxima Attenuates Particulate-Matter-Induced Inflammation in MH-S Lung Macrophage. Mar. Drugs 2022, 20, 766. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Hewage, S.R.; Han, X.; Kang, K.A.; Kang, H.K.; Lee, N.H.; Hyun, J.W. Protective Effect of Diphlorethohydroxycarmalol against Ultraviolet B Radiation-Induced DNA Damage by Inducing the Nucleotide Excision Repair System in HaCaT Human Keratinocytes. Mar. Drugs 2015, 13, 5629–5641. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kim, H.S.; Oh, J.Y.; Je, J.G.; Jeon, Y.J.; Ryu, B. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against UVB-induced damage in vitro in human dermal fibroblasts and in vivo in zebrafish. Food Chem. Toxicol. 2020, 136, 110963. [Google Scholar] [CrossRef] [PubMed]
- He, Y.L.; Xiao, Z.; Yang, S.; Zhou, C.; Sun, S.; Hong, P.; Qian, Z.J. A Phlorotanin, 6,6′-bieckol from Ecklonia cava, Against Photoaging by Inhibiting MMP-1, -3 and -9 Expression on UVB-induced HaCaT Keratinocytes. Photochem. Photobiol. 2022, 98, 1131–1139. [Google Scholar] [CrossRef]
- Piao, M.J.; Kim, K.C.; Kang, K.A.; Fernando, P.; Herath, H.; Hyun, J.W. Phloroglucinol Attenuates Ultraviolet B-Induced 8-Oxoguanine Formation in Human HaCaT Keratinocytes through Akt and Erk-Mediated Nrf2/Ogg1 Signaling Pathways. Biomol. Ther. 2021, 29, 90–97. [Google Scholar] [CrossRef]
- Kang, H.; Park, C.-H.; Kwon, S.-O.; Lee, S.-G. ED Formula, a Complex of Ecklonia cava and Chrysanthemum indicum, Ameliorates Airway Inflammation in Lipopolysaccharide-Stimulated RAW Macrophages and Ovalbumin-Induced Asthma Mouse Model. Pharmaceuticals 2023, 16, 1185. [Google Scholar] [CrossRef]
- Wang, L.; Je, J.-G.; Yang, H.-W.; Jeon, Y.-J.; Lee, S. Dieckol, an Algae-Derived Phenolic Compound, Suppresses UVB-Induced Skin Damage in Human Dermal Fibroblasts and Its Underlying Mechanisms. Antioxidants 2021, 10, 352. [Google Scholar] [CrossRef]
- Heo, S.Y.; Jeong, M.S.; Lee, H.S.; Kim, Y.J.; Park, S.H.; Jung, W.K. Phlorofucofuroeckol A from Ecklonia cava ameliorates TGF-beta1-induced fibrotic response of human tracheal fibroblasts via the downregulation of MAPKs and SMAD 2/3 pathways inactivated TGF-beta receptor. Biochem. Biophys. Res. Commun. 2020, 522, 626–632. [Google Scholar] [CrossRef]
- Yuan, Z.; Yang, Z.; Li, W.; Wu, A.; Su, Z.; Jiang, B.; Ganesan, S. Triphlorethol-A attenuates U251 human glioma cancer cell proliferation and ameliorates apoptosis through JAK2/STAT3 and p38 MAPK/ERK signaling pathways. J. Biochem. Mol. Toxicol. 2022, 36, e23138. [Google Scholar] [CrossRef]
- Kwon, O.Y.; Lee, S.H. Ameliorating Activity of Ishige okamurae on the Amyloid Beta-Induced Cognitive Deficits and Neurotoxicity through Regulating ERK, p38 MAPK, and JNK Signaling in Alzheimer’s Disease-Like Mice Model. Mol. Nutr. Food Res. 2020, 64, e1901220. [Google Scholar] [CrossRef]
- Bong, S.K.; Park, N.J.; Lee, S.H.; Lee, J.W.; Kim, A.T.; Liu, X.; Kim, S.M.; Yang, M.H.; Kim, Y.K.; Kim, S.N. Trifuhalol A Suppresses Allergic Inflammation through Dual Inhibition of TAK1 and MK2 Mediated by IgE and IL-33. Int. J. Mol. Sci. 2022, 23, 10163. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lee, B.; Lee, B.-H.; Lee, S.; Kim, H.-R. Sargahydroquinoic acid from Sargassum macrocarpum attenuates TNF-α and UV-induced skin aging in human dermal fibroblasts. Algal Res. 2024, 78, 103410. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, H.S.; Oh, S.J.; Hwang, C.W.; Jung, W.K. Phlorotannins ameliorate extracellular matrix production in human vocal fold fibroblasts and prevent vocal fold fibrosis via aerosol inhalation in a laser-induced fibrosis model. J. Tissue Eng. Regen. Med. 2020, 14, 1918–1928. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Ahn, G.; Kim, H.-S.; Je, J.-G.; Kim, K.-N.; Jeon, Y.-J. Diphlorethohydroxycarmalol (DPHC) Isolated from the Brown Alga Ishige okamurae Acts on Inflammatory Myopathy as an Inhibitory Agent of TNF-α. Mar. Drugs 2020, 18, 529. [Google Scholar] [CrossRef]
- Lee, H.-G.; Kim, S.-Y.; Kim, K.-N.; Jeon, Y.-J. DPHC from Ishige okamurae mitigates oxidative stress-induced myopathy by regulating MuRF-1/MAFbx signaling in C2C12 cells. Food Biosci. 2024, 61, 104859. [Google Scholar] [CrossRef]
- Cho, C.H.; Yoo, G.; Kim, M.; Lee, C.J.; Choi, I.-W.; Ryu, B.; Kim, B.-M.; Lee, S.-H. Diphlorethohydroxycarmalol, a phlorotannin contained in brown edible seaweed Ishige okamurae, prevents AGE-related diabetic nephropathy by suppression of AGE-RAGE interaction. Food Biosci. 2023, 53, 102659. [Google Scholar] [CrossRef]
- Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Han, E.-J.; Han, H.-J.; Kim, K.-N.; Wang, L.; Heo, S.-J.; Jung, K.-S.; Ahn, G. Phlorofucofuroeckol-A refined by edible brown algae Ecklonia cava indicates anti-inflammatory effects on TNF-α/IFN-γ-stimulated HaCaT keratinocytes and 12-O-tetradecanoylphorbol 13-acetate-induced ear edema in BALB/c mice. J. Funct. Foods 2023, 109, 105786. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-kappaB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes. Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef]
- Boyce, B.F.; Li, J.; Yao, Z.; Xing, L. Nuclear Factor-Kappa B Regulation of Osteoclastogenesis and Osteoblastogenesis. Endocrinol. Metab. 2023, 38, 504–521. [Google Scholar] [CrossRef]
- Akhter, A.; Street, L.; Ghosh, S.; Burns, B.F.; Elyamany, G.; Shabani-Rad, M.T.; Stewart, D.A.; Mansoor, A. Concomitant high expression of Toll-like receptor (TLR) and B-cell receptor (BCR) signalling molecules has clinical implications in mantle cell lymphoma. Hematol. Oncol. 2017, 35, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Iacobazzi, D.; Convertini, P.; Todisco, S.; Santarsiero, A.; Iacobazzi, V.; Infantino, V. New Insights into NF-κB Signaling in Innate Immunity: Focus on Immunometabolic Crosstalks. Biology 2023, 12, 776. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Amarante, S.J.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Brown Algae Phlorotannins: A Marine Alternative to Break the Oxidative Stress, Inflammation and Cancer Network. Foods 2021, 10, 1478. [Google Scholar] [CrossRef]
- Lin, L.; Yang, S.; Xiao, Z.; Hong, P.; Sun, S.; Zhou, C.; Qian, Z.-J. The Inhibition Effect of the Seaweed Polyphenol, 7-Phloro-Eckol from Ecklonia Cava on Alcohol-Induced Oxidative Stress in HepG2/CYP2E1 Cells. Mar. Drugs 2021, 19, 158. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Silva, A.; Cruz, M.T.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Phlorotannins from Fucus vesiculosus: Modulation of Inflammatory Response by Blocking NF-κB Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 6897. [Google Scholar] [CrossRef]
- Han, E.J.; Kim, H.-S.; Jung, K.; Asanka Sanjeewa, K.K.; Iresha Nadeeka Madushani Herath, K.H.; Lee, W.; Jee, Y.; Jeon, Y.-J.; Lee, J.; Kim, T.; et al. Sargassum horneri ethanol extract ameliorates TNF-α/IFN-γ-induced inflammation in human keratinocytes and TPA-induced ear edema in mice. Food Biosci. 2021, 39, 100831. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, H.-S.; Kim, S.-Y.; Ahn, G.; Kim, H.-J.; Fu, X.; Jee, Y.; Jeon, Y.-J. Ethanol extract separated from Sargassum horneri (Turner) abate LPS-induced inflammation in RAW 264.7 macrophages. Fish. Aquat. Sci. 2019, 22, 6. [Google Scholar] [CrossRef]
- Joung, E.J.; Cao, L.; Lee, B.; Gwon, W.G.; Park, S.H.; Kim, H.R. Sargahydroquinoic Acid, a Cyclooxygenase-2 Inhibitor, Attenuates Inflammatory Responses by Regulating NF-kappaB Inactivation and Nrf2 Activation in Lipopolysaccharide-Stimulated Cells. Inflammation 2021, 44, 2120–2131. [Google Scholar] [CrossRef]
- Raja, R.; Hemaiswarya, S.; Arunkumar, K.; Mathiyazhagan, N.; Kandasamy, S.; Arun, A.; Ramasamy, P. Efficacy of Eisenia bicyclis phlorotannins in the treatment of diabetes and reducing inflammation. Food Biosci. 2023, 52, 102381. [Google Scholar] [CrossRef]
- Go, Y.G.; Wang, Q.; Park, J.; Lee, H.-J.; Kim, H. Phlorotannins Isolated from Eisenia bicyclis and Lactobacillus casei Ameliorate Dextran Sulfate Sodium-Induced Colitis in Mice through the AhR Pathway. Appl. Sci. 2024, 14, 2835. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, H.S.; Lee, W.; Han, E.J.; Kim, S.Y.; Fernando, I.P.S.; Ahn, G.; Kim, K.N. Eckol from Ecklonia cava ameliorates TNF-alpha/IFN-gamma-induced inflammatory responses via regulating MAPKs and NF-kappaB signaling pathway in HaCaT cells. Int. Immunopharmacol. 2020, 82, 106146. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Park, J.-H.; Park, S.-A.; Joo, N.-R.; Lee, B.H.; Lee, K.B.; Oh, S.-M. Dieckol or phlorofucofuroeckol extracted from Ecklonia cava suppresses lipopolysaccharide-mediated human breast cancer cell migration and invasion. J. Appl. Phycol. 2019, 32, 631–640. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of Phlorotannins Extraction from Fucus vesiculosus and Evaluation of Their Potential to Prevent Metabolic Disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, S.Y.; Lee, H.G.; Je, J.G.; Jee, Y.; Jeon, Y.J. Sargassum horneri (Turner) inhibit urban particulate matter-induced inflammation in MH-S lung macrophages via blocking TLRs mediated NF-kappaB and MAPK activation. J. Ethnopharmacol. 2020, 249, 112363. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Oh, S.; Choi, J.; Jang, J.T.; Choi, C.H.; Park, K.Y.; Son, K.H.; Byun, K. The Phlorotannin-Rich Fraction of Ecklonia cava Extract Attenuated the Expressions of the Markers Related with Inflammation and Leptin Resistance in Adipose Tissue. Int. J. Endocrinol. 2020, 2020, 9142134. [Google Scholar] [CrossRef] [PubMed]
- Stark, G.R.; Darnell, J.E., Jr. The JAK-STAT pathway at twenty. Immunity 2012, 36, 503–514. [Google Scholar] [CrossRef]
- Xue, C.; Yao, Q.; Gu, X.; Shi, Q.; Yuan, X.; Chu, Q.; Bao, Z.; Lu, J.; Li, L. Evolving cognition of the JAK-STAT signaling pathway: Autoimmune disorders and cancer. Signal Transduct. Target. Ther. 2023, 8, 204. [Google Scholar] [CrossRef]
- Huynh, J.; Etemadi, N.; Hollande, F.; Ernst, M.; Buchert, M. The JAK/STAT3 axis: A comprehensive drug target for solid malignancies. Semin. Cancer Biol. 2017, 45, 13–22. [Google Scholar] [CrossRef]
- Ceyzeriat, K.; Abjean, L.; Carrillo-de Sauvage, M.A.; Ben Haim, L.; Escartin, C. The complex STATes of astrocyte reactivity: How are they controlled by the JAK-STAT3 pathway? Neuroscience 2016, 330, 205–218. [Google Scholar] [CrossRef]
- Imada, K.; Leonard, W.J. The Jak-STAT pathway. Mol. Immunol. 2000, 37, 1–11. [Google Scholar] [CrossRef]
- Teng, Y.; Ross, J.L.; Cowell, J.K. The involvement of JAK-STAT3 in cell motility, invasion, and metastasis. JAKSTAT 2014, 3, e28086. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Han, E.J.; Kim, H.-S.; Sanjeewa, K.K.A.; Herath, K.H.I.N.M.; Jeon, Y.-J.; Jee, Y.; Lee, J.; Kim, T.; Shim, S.-Y.; Ahn, G. Eckol from Ecklonia cava Suppresses Immunoglobulin E-mediated Mast Cell Activation and Passive Cutaneous Anaphylaxis in Mice. Nutrients 2020, 12, 1361. [Google Scholar] [CrossRef]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Jia, J.; Sheng, J.; Zhang, S.; Huang, K.; Li, H.; He, F. Protective and anti-inflammatory role of REG1A in inflammatory bowel disease induced by JAK/STAT3 signaling axis. Int. Immunopharmacol. 2021, 92, 107304. [Google Scholar] [CrossRef]
- Liao, M.; Wei, S.; Hu, X.; Liu, J.; Wang, J. Protective Effect and Mechanisms of Eckol on Chronic Ulcerative Colitis Induced by Dextran Sulfate Sodium in Mice. Mar. Drugs 2023, 21, 376. [Google Scholar] [CrossRef]
- Kang, N.; Oh, S.; Kim, S.-Y.; Ahn, H.; Son, M.; Heo, S.-J.; Byun, K.; Jeon, Y.-J. Anti-obesity effects of Ishophloroglucin A from the brown seaweed Ishige okamurae (Yendo) via regulation of leptin signal in ob/ob mice. Algal Res. 2022, 61, 102533. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.H.; Zhang, S.; Bong, S.-K.; Kim, A.T.; Lee, H.; Liu, X.; Kim, S.M.; Kim, S.-N. Anti-Allergic Inflammatory Effect of Agarum cribrosum and Its Phlorotannin Component, Trifuhalol A, against the Ovalbumin-Induced Allergic Asthma Model. Curr. Issues Mol. Biol. 2023, 45, 8882–8893. [Google Scholar] [CrossRef]
- Kim, J.; Won Choi, J.; Jeong Kim, H.; Kim, B.; Kim, Y.; Hwejin Lee, E.; Kim, R.; Kim, J.; Park, J.; Jeong, Y.; et al. Phloroglucinol Derivatives Exert Anti-Inflammatory Effects and Attenuate Cognitive Impairment in LPS-Induced Mouse Model. ChemMedChem 2024, 19, e202400056. [Google Scholar] [CrossRef]
- Barbosa, M.; Valentao, P.; Ferreres, F.; Gil-Izquierdo, A.; Andrade, P.B. In vitro multifunctionality of phlorotannin extracts from edible Fucus species on targets underpinning neurodegeneration. Food Chem. 2020, 333, 127456. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shikov, A.N. In Vitro Anti-Inflammatory Activities of Fucoidans from Five Species of Brown Seaweeds. Mar. Drugs 2022, 20, 606. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, B.; Baroutian, S.; Li, J.; Zhang, B.; Ying, T.; Lu, J. Combination of marine bioactive compounds and extracts for the prevention and treatment of chronic diseases. Front. Nutr. 2022, 9, 1047026. [Google Scholar] [CrossRef] [PubMed]
- Chouh, A.; Nouadri, T.; Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phlorotannins of the Brown Algae Sargassum vulgare from the Mediterranean Sea Coast. Antioxidants 2022, 11, 1055. [Google Scholar] [CrossRef] [PubMed]
- Han, H.J.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Kim, D.-O.; Kim, G.-H.; Heo, H.J. Mixture of Phlorotannin and Fucoidan from Ecklonia cava Prevents the Aβ-Induced Cognitive Decline with Mitochondrial and Cholinergic Activation. Mar. Drugs 2021, 19, 434. [Google Scholar] [CrossRef]
- Kim, E.; Cui, J.; Kang, I.; Zhang, G.; Lee, Y. Potential Antidiabetic Effects of Seaweed Extracts by Upregulating Glucose Utilization and Alleviating Inflammation in C2C12 Myotubes. Int. J. Environ. Res. Public Health 2021, 18, 1367. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).