Abstract
MIL-101(Cr), a widely studied chromium-based metal–organic framework material consisting of chromium metal ions and terephthalic acid ligands, has attracted much attention due to its ultra-high specific surface area, large pore size, and excellent thermal, chemical, and aqueous stability. The outstanding properties and abundant unsaturated Lewis acid sites of this material have shown promising applications in aqueous phase adsorption, gas storage, separation, catalysis, drug delivery, and sensing. In this paper, we systematically review the synthesis technology and performance optimization strategy of MIL-101(Cr), discuss the advantages and limitations of various synthesis methods, such as traditional hydrothermal method, microwave-assisted hydrothermal method, template method, and solvent-thermal method, and summarize and analyze the optimization strategy of MIL-101 from the aspects of physical modification and chemical modification. In addition, this paper summarizes the latest application progress of MIL-101(Cr) in gas adsorption and separation, wastewater purification, pollutant removal, catalysis, and pharmaceutical delivery, and points out the current challenges and future development directions, to provide guidance and inspiration for the industrial application of MIL-101(Cr) and the development of new materials.
1. Introduction
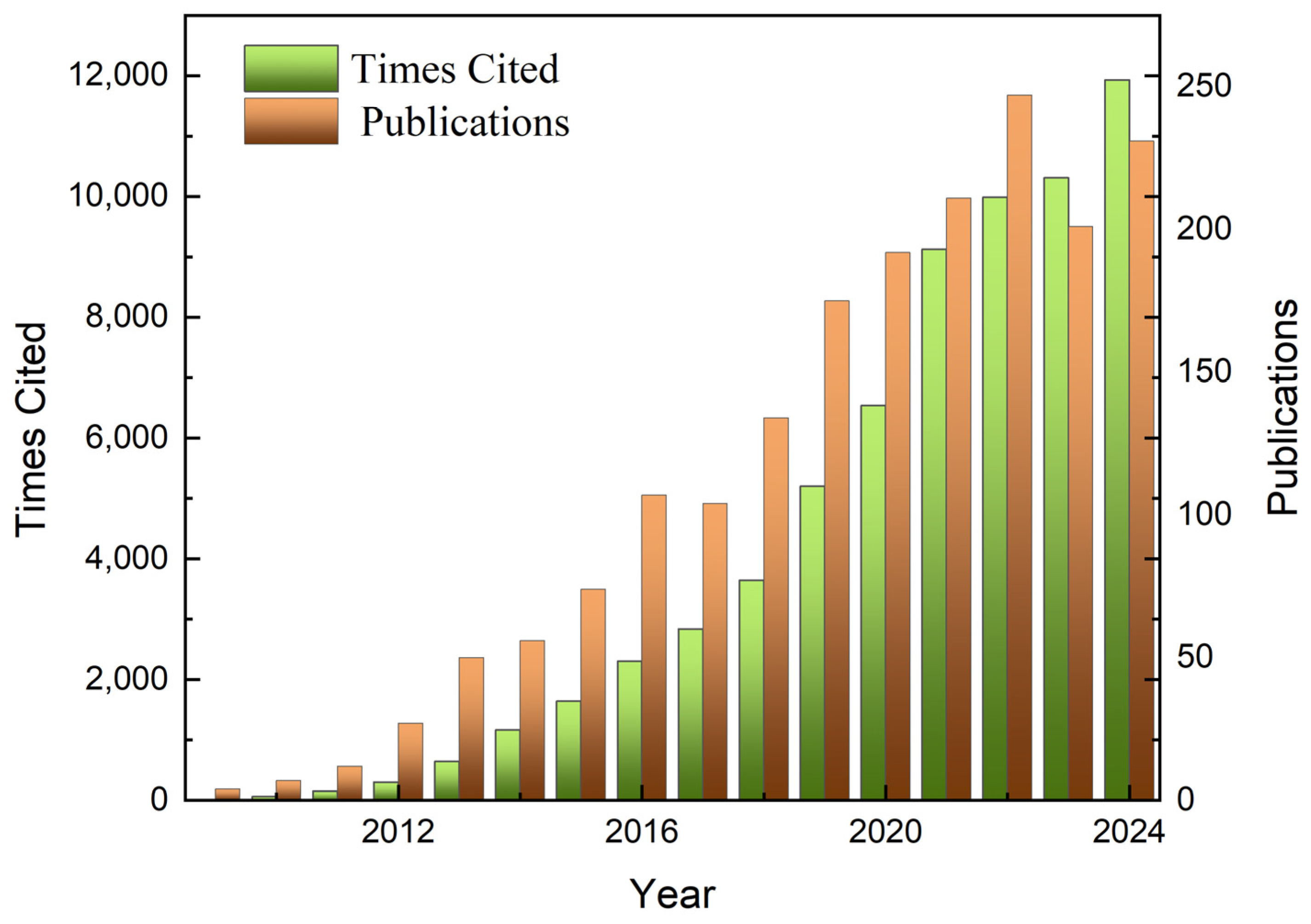
Metal–organic frameworks (MOFs) [1,2], also known as porous coordination polymers (PCPs), are a class of porous crystalline materials formed by the self-assembly of metal ions or metal clusters with organic ligands [3,4,5]. Due to their high specific surface area, high porosity, tunable pore size, and easy functionalization, MOFs show great potential for applications in many fields, such as gas storage and separation [6,7], adsorption, sensing [8,9], catalysis, proton conductivity [8], and drug delivery [10]. Over the past three decades, the number of scientific studies reported on the topology of MOFs and their potential applications has increased almost geometrically (Figure 1). An important feature of MOFs is their ultra-high porosity (up to 90% of the free volume) as well as their impressive Langmuir specific surface area (which can even exceed 10,000 m2 g−1) [11], which provides strong support for applications such as gas adsorption, sensing, and catalysis.
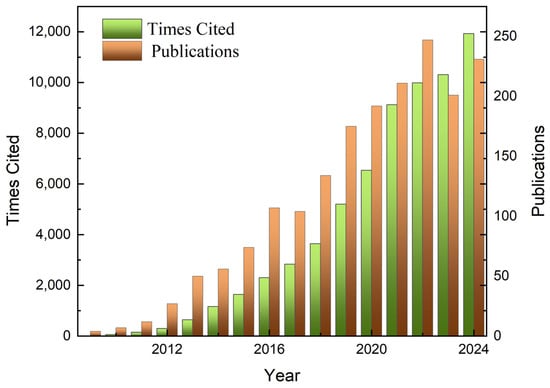
Figure 1.
Statistical data from scientific articles on MIL-101(Cr) since 2009.
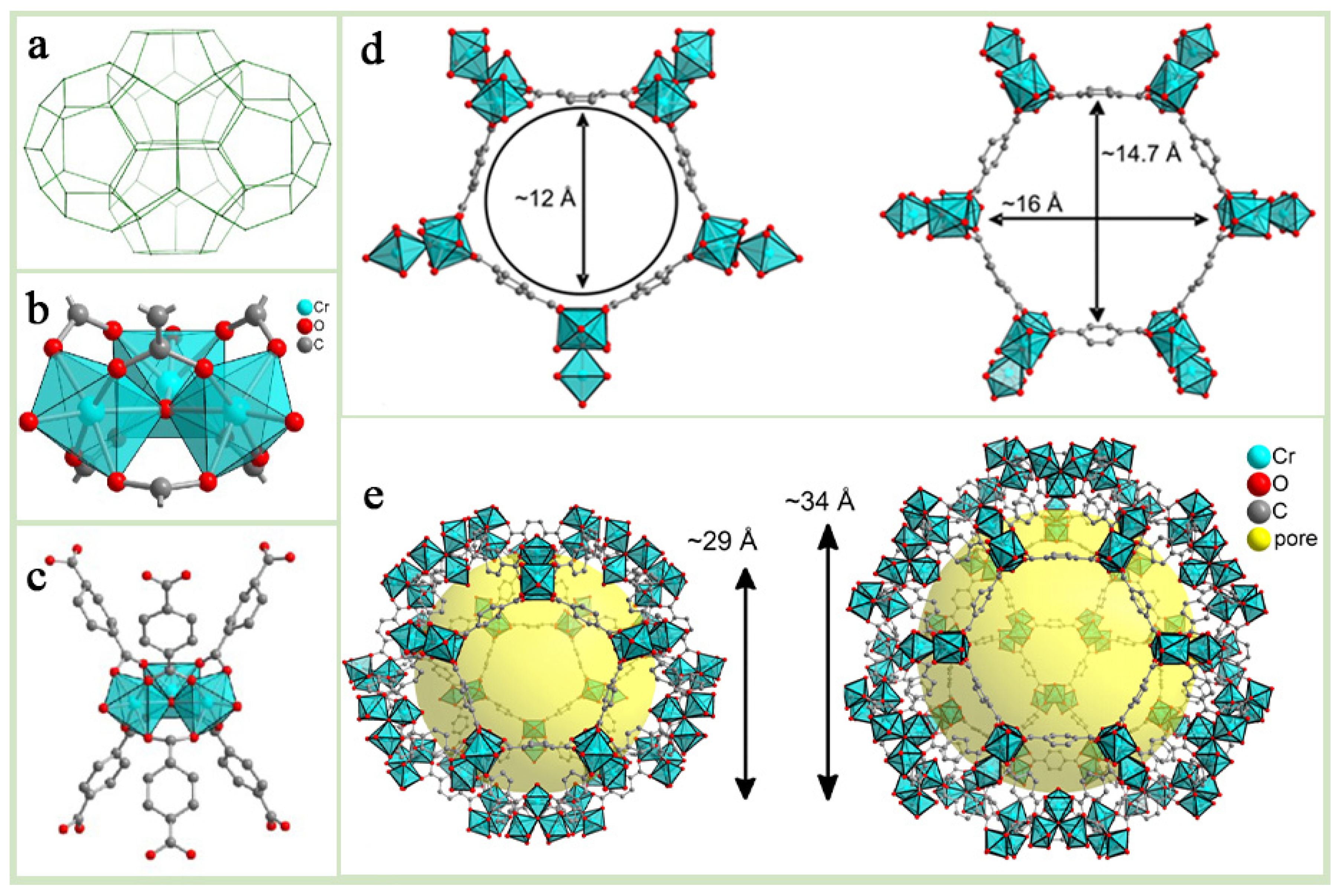
Among numerous MOFs, the Lavoisier Institute Materials (MIL) series developed at the Lavoisier Institute, have garnered significant attention for their remarkable structural diversity and outstanding properties [12,13,14]. Within the MIL series, M(III) terephthalates (where M = Cr, Fe, Al, V, Mn, In, etc., in decreasing order of importance), together with terephthalic acid derivatives and extended terephthalate analogs, form an important subclass. MIL-47/MIL-53 [15], MIL-88 [16], MIL-100 [17], and MIL-101 [18] are four of the best-known porous M(III) terephthalates, and these materials are particularly noteworthy in terms of potential applications [19]. For example, MIL-101(Fe) consists of Fe3+ octahedral chains with 1,4-benzenedicarboxylic acid, part of which Fe3+ can be converted to Fe2+ under certain conditions, thus showing good activation in catalytic applications. MIL-101(Cr), as one of the most representative materials in the MIL series, is formed by coordination of Cr3O ionic clusters with terephthalic acid (H2BDC) [20] (Figure 2b,c), and the chemical formula can be expressed as [Cr3(O)X(BDC)3(H2)2] (where X is either OH- or F-), which is structurally similar to the MTN zeolite topology (Figure 2a), with two different sizes of mesoporous cavities, 29 Å and 34 Å (Figure 2e). In addition, its maximum pore window diameter is about 16 Å and its BET specific surface area is up to about 4100 m2 g−1 (Figure 2d). The water of crystallization at the end of the MIL-101(Cr) framework can be removed under high-temperature or vacuum conditions to form unsaturated metal sites, which endows it with potential Lewis acid catalytic activity. Due to its high porosity, good physicochemical properties and stability, MIL-101(Cr) has been widely used in various fields such as electrocatalysis, photocatalysis, pollutant adsorption, mixed matrix membranes, and drug delivery [21,22,23,24,25,26,27].
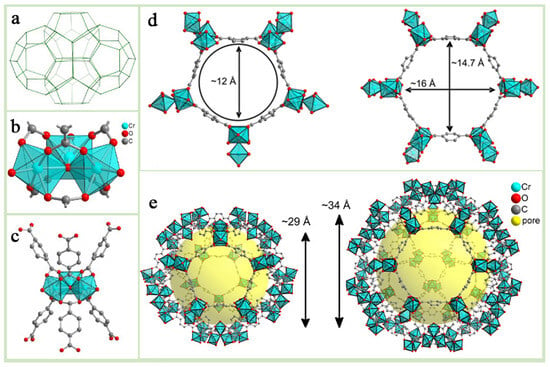
Figure 2.
Structural characteristics of MIL-101(Cr) metal–organic framework: (a) MIL-101(Cr) Crystal Bat Model Drawing. (b) Schematic representation of the aggregate structure of MIL-101(Cr). (c) Schematic of MIL-011(Cr) Trident Structure. (d) Schematic diagram of pore size: pentagonal windows(~12A) and hexagonal windows(~16A, ~14.7A) as largest windows in cages. (e) Schematic of MIL-101(Cr) polygonal structure model (various sizes including ~29A and ~34A).
In recent years, although there have been numerous studies on the synthesis and application of MIL-101(Cr) [28], there is still a lack of comprehensive and systematic reviews, especially in the context of the rapid development of synthesis methods and the emergence of new perspectives and strategies. This article provides a detailed introduction to the synthesis technology and performance optimization strategies of MIL-101(Cr) based on the existing literature and focuses on reviewing the progress of its application in multiple fields, such as adsorption, sensing, catalysis, etc. At the same time, we discuss the challenges it faces and put forward the outlook of its future industrial application, with a view of providing valuable systematic references for the researchers in the related fields.
2. The Synthesis Method of MIL-101(Cr)
MIL-101(Cr) has been synthesized by various methods, and each method has its unique advantages and limitations. Different synthesis and activation conditions have a significant effect on the morphology, specific surface area, yield, structural stability, and crystallinity of MIL-101(Cr) [29,30,31]. It is critical to select an appropriate method for the synthesis of MIL-101(Cr) by combining available experimental conditions, target applications, cost, and operating conditions. Traditional hydrothermal [32] and HF-free synthesis are the most widely used methods, but with the development of green chemistry and efficient synthesis techniques, microwave-assisted synthesis [33], template hydrothermal [34], solvothermal [35], and mechanochemical synthesis [36,37,38] are also gaining attention.
2.1. Hydrothermal Synthesis
2.1.1. Conventional Hydrothermal Method
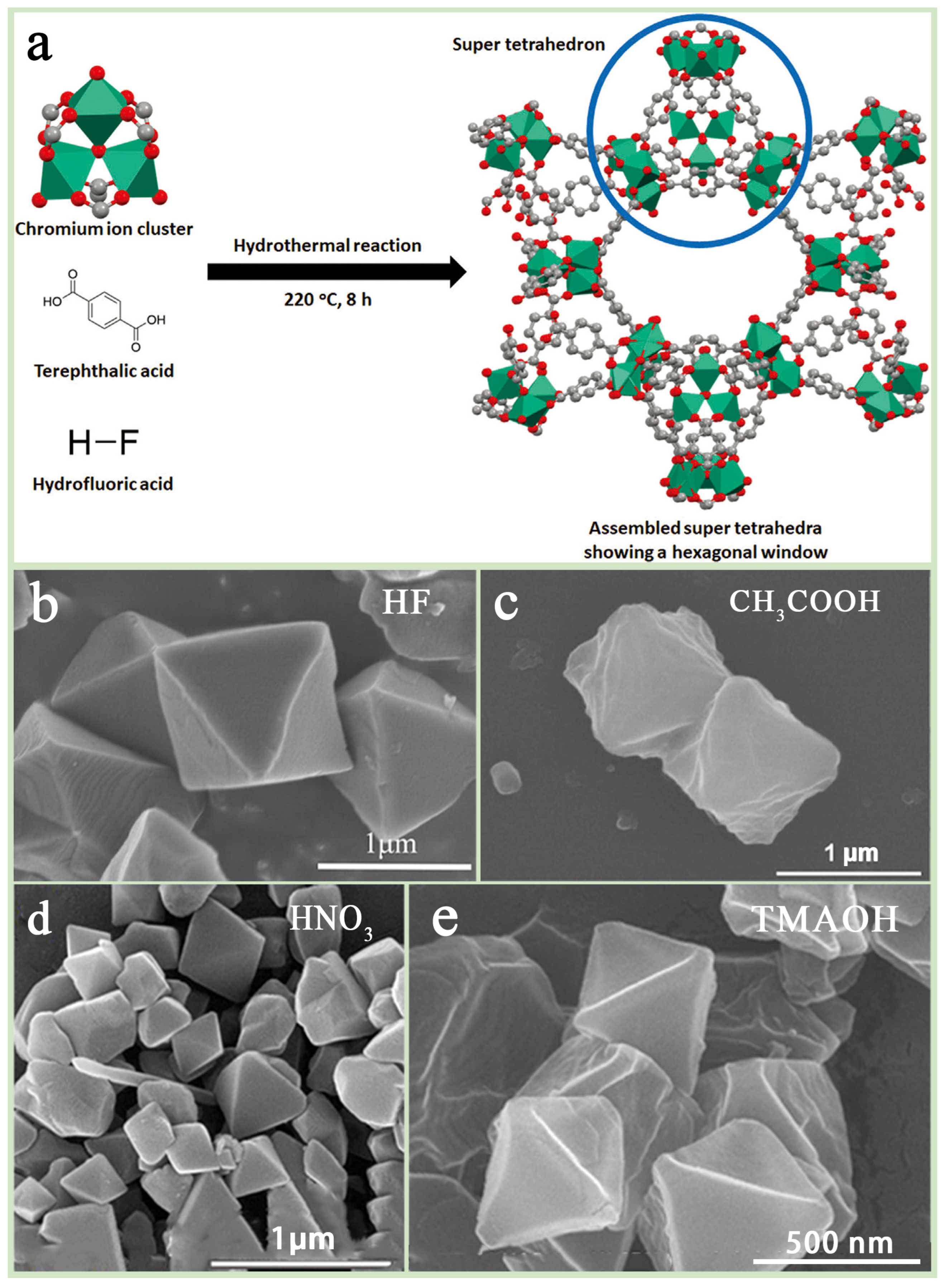
Hydrothermal synthesis is one of the most common methods of preparing MIL-101(Cr), usually using water as the solvent. The basic process consists of dissolving Cr(NO3)3-9H2O mixed with terephthalic acid (H2BDC) in deionized water, adding hydrogen fluoride (HF) as an additive, and reacting the product for 8 h at 220 °C in a stainless steel PTFE-lined hydrothermal kettle. Subsequently, the product was purified by ammonium fluoride and ethanol and, finally, dried in a vacuum oven to obtain MIL-101(Cr) (Figure 3a). In this process, HF plays a crucial role, not only promoting the coordination of ligands and framework formation but also improving the crystallinity of the material, as well as increasing the specific surface area and pore volume [39] (Figure 3b). However, the high toxicity and volatility of HF increase the safety requirements and cost during production, which has prompted researchers to explore the use of other additives (e.g., acetic acid (CH3COOH), nitric acid (HNO3), hydrochloric acid (HCl), etc.) to replace HF (Figure 3c,d). In addition, Pan et al. [40] utilized tetramethylammonium hydroxide (TMAOH) as an additive and improved the morphology and adsorption properties of MIL-101(Cr) by controlling its incorporation (Figure 3e), while Zhao et al. [41] used sodium acetate and synthesized regular octahedral MIL-101(Cr) crystals, which exhibited excellent adsorption properties after activation at 140 °C.
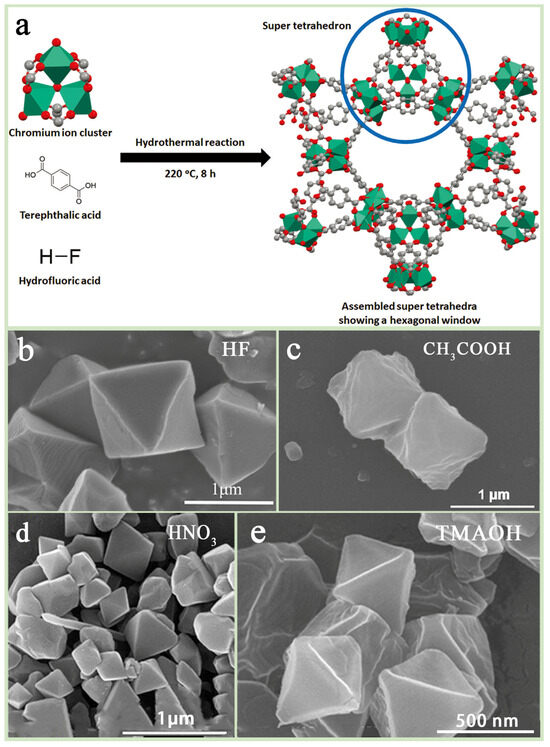
Figure 3.
(a) Flowchart of MIL-101(Cr) hydrothermal synthesis method [42] and SEM images of MIL-101(Cr) with different additives: (b) HF, (c) CH3COOH [43], (d) HNO3 [44], (e) TMAOH [40].
2.1.2. Microwave-Assisted Hydrothermal Method
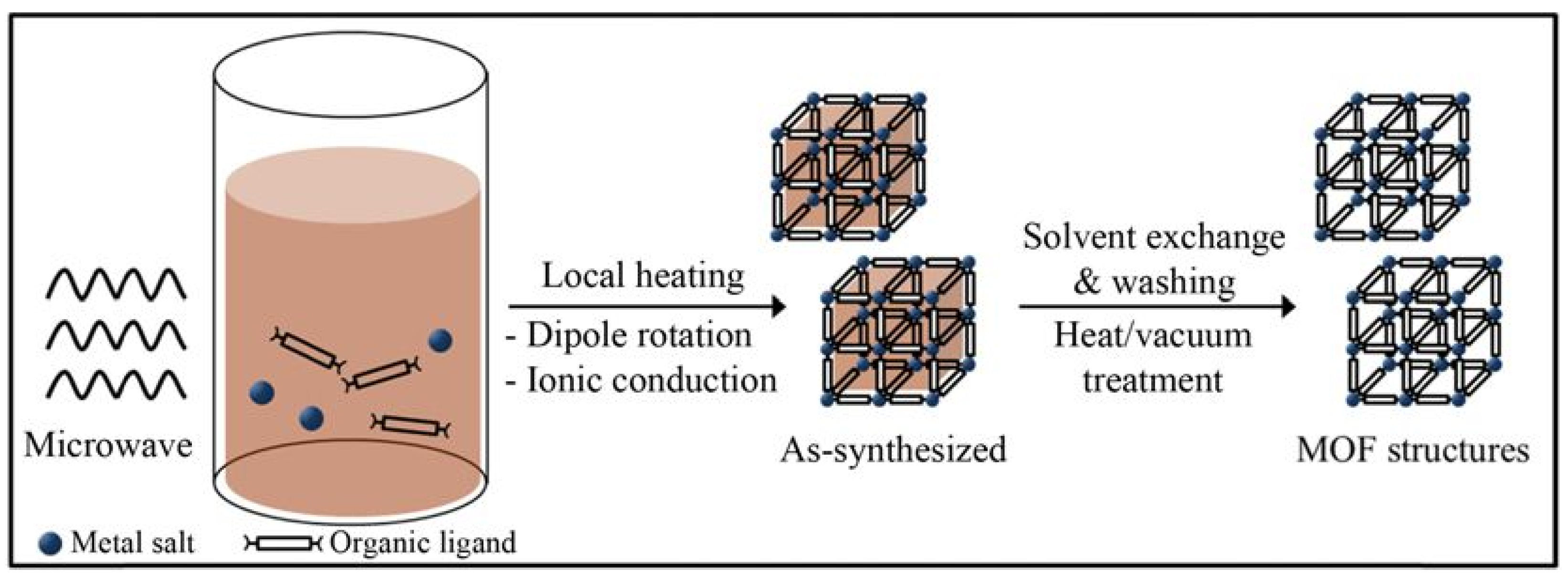
Microwave microwave-assisted hydrothermal method is a method of rapidly driving reactions in high-temperature and high-pressure aqueous solutions through microwave heating and has been widely used for the rapid synthesis of nanoporous materials under hydrothermal conditions (Figure 4). In addition to rapid crystallization, the potential advantages of this technology also include efficient energy transfer, phase selectivity, particle size distribution control, and simple morphology control [45,46,47]. The earliest reported microwave synthesis of MOFs was MIL-100 (Cr), which had comparable reaction rates to conventional hydrothermal synthesis [48]. The author extended the method to the synthesis of MIL-101(Cr) and successfully synthesized MIL-101(Cr) with similar physical, chemical, and structural properties to those synthesized using conventional hydrothermal methods in less than 1 h. After groundbreaking discoveries, the application of the microwave-assisted synthesis method in the synthesis process of MIL-101(Cr) has become increasingly widespread. Soltanolkottabi et al. [49] synthesized MIL-101(Cr) by a two-step method using microwave-assisted and electrothermal heating and found that when the pH was adjusted to 3 at the electrothermal heating stage, the obtained crystals of MIL-101(Cr) were octahedral and exhibited a CO2 adsorption capacity of 7.6 mmol g-1 Khan et al. [45] compared the effect of microwave-assisted and electrical heating methods for the synthesis of MIL-101(Cr) under different water concentration and pH conditions, and the results showed that the samples obtained by microwave-assisted method with smaller particle size and larger BET specific surface area and pore volume. This is because the reaction kinetics under the microwave field is shifted from “nucleation dominance” to “growth inhibition”, and the nature of the reaction is a competition between the nucleation rate and the growth rate. The instantaneous high concentration in the nucleation phase consumes most of the precursors, which leads to the stagnation of the growth phase due to the limitation of material transport, thus leading to the decrease in the synthesized particle size [49]. Zhao et al. [46] synthesized MIL-101(Cr) in octahedral morphology with a size of about 100 nm under microwave radiation conditions of 220 °C and 300 W, BET specific surface area of 3054 m2 g−1, and pore volume of 2.01 cm3 g−1 and showed benzene adsorption capacity of 16.5 mmol g−1. Yin et al. [50] synthesized standardized and homogeneous MIL-101(Cr) using microwave hydrothermal method in only 30 min, which greatly reduced the reaction time. Carretero-Cerdán et al. [51] synthesized amino-functionalized MIL-101(Cr)-NH2 using a one-step microwave-assisted method and found that among the various fluoride (LiF, NaF, KF, and CsF) modifiers replacing hydrofluoric acid (HF), NaF performed the best in terms of crystallinity and yield. High yields (>70%) and high crystallinity of MIL-101(Cr) and MIL-101(Cr)-NH2 were successfully obtained in less than 1 h. The test results showed that the microwave-assisted synthesis method significantly reduced the reaction time (from 12–72 h to 1 h) and increased the yields compared to the conventional solvothermal and hydrothermal synthesis methods, while replacing N,N- dimethylformamide (DMF) as solvent, which is more environmentally friendly. The significant improvement in reaction efficiency of microwave-assisted method is attributed to the coupling between the applied oscillating electric field and the permanent dipole moment of molecules in the synthesis medium, which rapidly transfers energy to the interior of the reactants and effectively induces molecular rotation, achieving instantaneous energy input at the molecular level and enabling the reaction system to reach high temperature in a short period of time. In addition, the thermal effect of microwaves can also generate thermal energy through the rapid rotation and collision of polar molecules, further improving the reaction efficiency. At the same time, the microwave-assisted volumetric heating mechanism (volumetric heating) realizes the simultaneous heating of the inside and outside of the reaction system, forming a reverse temperature field of “hot inside and cold outside”. This uniform heating not only enhances the reaction rate and causes the precursor solution to reach supersaturation in a very short time but also triggers homogeneous nucleation and formation of uniform nanoscale MIL-101(Cr) particles.
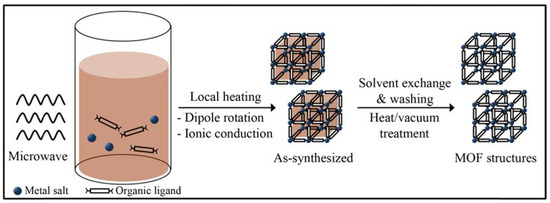
Figure 4.
Microwave-assisted solvothermal synthesis of MOF structures [48]. Copyright 2013 Korean Institute of Chemical Engineers, Seoul, Korea, Elsevier Ltd.
Although microwave-assisted hydrothermal synthesis has many advantages and has been widely used in the synthesis of MIL-101(Cr) in recent years, it still faces some challenges. Microwave heating accelerates the reaction process through instantaneous energy input, and the reaction time window is narrow, which can easily lead to the generation of by-products. In addition, laboratory-grade microwave reactors (such as single-mode cavities) are difficult to directly amplify, and when applied to large-scale production, industrial-grade multi-mode cavities often suffer from local overheating or widened particle size distribution due to uneven electromagnetic field distribution, which hinders commercial production.
2.1.3. Template Hydrothermal Method
The template method is based on the hydrothermal synthesis method, which aims to regulate the crystal morphology and size by using templates (hard templates such as silica, carbon nanotubes, or soft templates such as surfactants, polymers, etc.) with specific three-dimensional structures to guide the growth of MIL-101(Cr) crystals [52,53]. Utilizing templates to occupy the crystal space and removing the templates after the reaction, additional mesoporous or macroporous structures can be formed in the MIL-101(Cr) structure, resulting in the construction of MIL-101(Cr) with hierarchical pores [54,55]. For example, it has been found that nanoscale MIL-101(Cr) crystals with a wider range of sizes can be obtained in a shorter time by selecting expanded graphite (EG) as the guiding template. With the increase in EG addition, the crystal growth rate will be accelerated, and the crystal size will be smaller and more uniform. Yang et al. [54], using expanded graphite (EG) as a template, prepared nanoscale MIL-101(Cr) crystals with a BET specific surface area of 3751 m2 g−1 and a yield of 43%, with a reaction time of only 2 h. Huang et al. [52] used CTAB as a soft template to obtain irregular MIL-101(Cr) nanoparticles with a mesopore-to-macroporous ratio of 19:1. Chen et al. [53] employed MCM-41 with well-organized mesopores as a structural template guide to regulate the growth of MIL-101(Cr) crystals along a certain direction and limit the expansion of the framework. The hydroxyl groups in MCM-41 preferentially coordinated with the Cr3 centers in the MOFs, which resulted in the laminar arrangement of the MIL-101(Cr) nanocrystals on the substrate surface. Structural characterization further shows that the introduction of MCM-41 increases the microporous volume and specific surface area of MIL-101(Cr). Although the synthesis of MIL-101(Cr) by template hydrothermal method can effectively shorten the reaction time and obtain smaller-sized nanocrystals, there are still problems such as relatively low yield, unclear mechanism of action, relatively complicated post-treatment, incomplete removal of template agent, and so on. Therefore, further exploration and optimization are still needed.
2.2. Solvothermal Method
A solvothermal method is a method in which the reaction is carried out in a closed system with an organic solvent or a non-aqueous solvent as the medium. The method takes advantage of the good solubility of the solvent at high temperature and pressure to promote the full reaction of the reactants and to increase the crystallinity and porosity of the products [56,57]. This method is often used to ameliorate the limitations of conventional hydrothermal methods, such as long reaction times and low yields. Tan et al. [56] synthesized MIL-101(Cr) using a mixed solvent system of DMF and H2O at 140 °C. At a DMF/H2O volume ratio of 0.20, the product showed a BET specific surface area of 2453 m2 g−1 and a high yield of 83.3%. In addition, MIL-101(Cr), synthesized using a hybrid solvothermal method, also outperformed the conventional hydrothermal product in terms of water adsorption properties. Fallah et al. [58] synthesized MIL-101(Cr) and its composites with mordenite zeolite (MOR) using a solvothermal method, which showed higher thermal stability. Cao et al. [57] used an in situ growth restriction strategy to synthesize MIL-101(Cr) with different pore structures by changing the solvent dosage and studied in depth the mechanism of solvent effect on pore structure and the improvement of toluene adsorption performance. The experimental results show that the solvent effect can regulate the pore structure of MIL-101(Cr) by affecting the rate of crystal nucleation and growth. When the solvent dosage is low, the nucleation rate is faster than the growth rate, resulting in small crystal size and dense pore structure, high specific surface area, but small pore volume; when the solvent dosage is increased, the opposite is true. Although the solvothermal synthesis of MIL-101(Cr) has flexibility in low temperature regulation and morphology design, its toxic reagent dependence and high energy consumption and yield are still bottlenecks for industrialization. In the future, it is necessary to promote its practical application in the fields of adsorption, catalysis, and energy storage through the innovation of green processes, technological breakthroughs in scale-up, and a multifunctional design.
2.3. Mechanochemical Synthesis Method
Mechanochemical synthesis (MSS) is a green synthesis method that induces chemical reactions in solid feedstocks by mechanical forces (e.g., grinding, ball milling, etc.). The core advantages over conventional solvothermal methods are the absence of solvents, allowing the use of metal precursors with low solubility, short reaction times, low energy consumption, and the possibility of avoiding the use of toxic reagents such as hydrofluoric acid (HF), which makes it a suitable synthesis strategy for continuous industrial production [37,59,60,61]. The synthesis consists of two main stages: grinding and heating in a high-pressure heater for several hours [62]. Leng et al. prepared MIL-101 with a very high N content and a high specific surface area of 2764 m2 g−1 by ball milling the metal source and ligand for 30 min, followed by heating in an autoclave at 220 °C for 4 h. The specific surface area of MIL-101 is also very high. The authors compared it with MIL-101(Cr) synthesized using a solvothermal method and found that its crystal size was significantly smaller than that of the MIL-101(Cr) sample prepared under hydrothermal conditions. Mechanochemical methods have great potential for future development due to their unique and multiple advantages. However, it also faces some limitations, such as the crystallinity of the product that needs to be improved and the mechanical force that may lead to crystal agglomeration and structural damage. In the future, the mechanochemical/hydrothermal/solvent/microwave-coupled synthesis technique can be developed to balance the crystallinity of the products with the synthesis efficiency, and the efficient synthesis strategy of MIL-101(Cr) can be further explored [63,64].
Overall, the hydrothermal method is still the main method for the preparation of MIL-101(Cr) due to its simplicity and efficiency, while the microwave-assisted, template method, and solvothermal method provide more options for improving product quality, shortening the reaction time, and modulating the crystal structure (Table 1 compares several common methods for synthesizing MIL-101(Cr)). In addition to the above five synthetic methods, some new MIL-101(Cr) synthesis strategies, which are more efficient and environmentally friendly, have emerged in recent years, for example, the ionothermal method [65,66], ultrasonic radiation method, and so on. Hojatollah et al. [67] introduced a new method for the synthesis of MIL-101(Cr) by an ultrasonic radiation (UTS) technique, which accelerates the preparative reaction through the cavitation effect of ultrasound. Compared with the conventional hydrothermal and microwave methods, MIL-101(Cr) synthesized by ultrasonic radiation has more significant time and energy efficiency advantages, can achieve high yield synthesis in a shorter period of time, does not require the use of large quantities of hydrofluoric acid, which reduces the risk to the environment, and it is undoubtedly a faster, more efficient, and environmentally friendly synthesis method.

Table 1.
Comparison of synthesis methods for MIL-101(Cr).
3. Performance Optimization Strategies
Due to its unique porous structure, high specific surface area, tunable chemical properties, and modification strategies, MIL-101(Cr) shows broad application prospects in the fields of gas storage, adsorption and separation, catalysis, and sensing. However, the performance of MIL-101(Cr) in some applications still needs to be further optimized, so its physically regulated modification and chemical modification strategies have been widely studied and applied, and there is still much room for exploration and development in the future.
3.1. Physical Modification
3.1.1. Regulation of Particle Sizes, Morphology, and Pore Sizes
The highly developed microporous and mesoporous structure of MIL-101(Cr) is one of its most important characteristics, and thanks to its rich pore network, the specific surface area (SBET) of MIL-101(Cr) is as high as 4230 m2/g, which has a decisive impact on its performance and applications. The morphology and particle size also significantly influence the properties of MIL-101(Cr), with different morphologies and particle sizes leading to different optimal application areas [5,68]. For example, in catalysis, the octahedral structure equips MIL-101(Cr) with more coordination unsaturation sites, which plays a key role in the exposure of its catalytic sites. On the contrary, spherical MIL-101(Cr) exhibits higher performance in dye adsorption as it provides a more homogeneous surface and higher pore utilization, which effectively enhances the adsorption of target molecules. According to the application characteristics of MIL-101(Cr) in different fields, optimizing its particle size, morphology, and pore space to maximize the application energy efficiency is the key research direction of MIL-101(Cr) at this stage and even in the future.
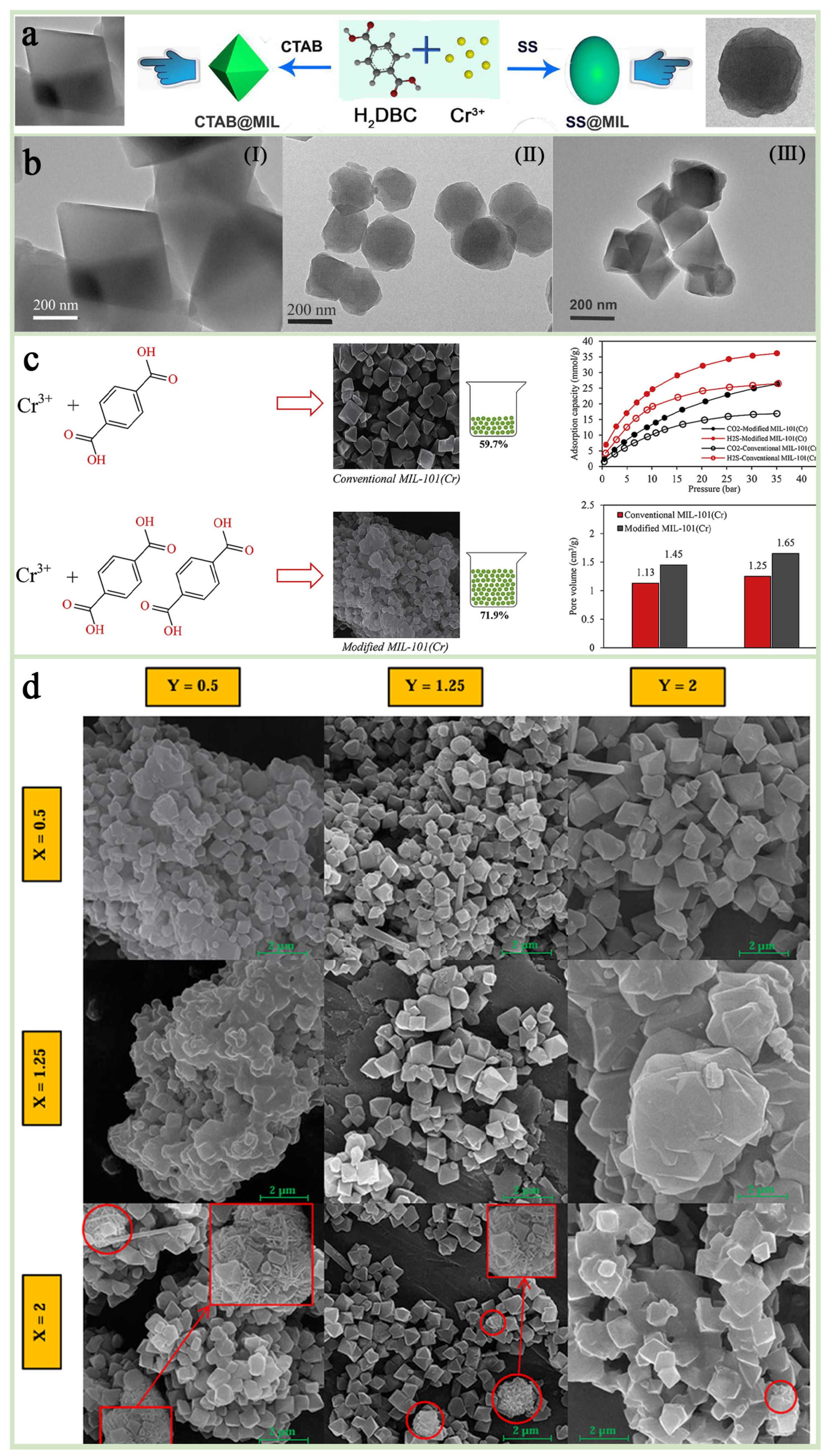
Through research, it has been found that precise control of the particle size, morphology (such as spherical, cubic, octahedral), and pores of MIL-101(Cr) can be achieved by adjusting the type and concentration of additives (such as acetic acid, NaOH, TMAOH, CTAB, etc.), or using templates and molds [42,69]. For example, NaOH additives were shown to reduce the particle size of MIL-101(Cr) to the nanometer scale (~90 nm on average), and the specific surface area could reach 4065 m2 g−1 [70,71]. Zhao et al. [72] successfully achieved the modulation of the pore structure of MIL-101(Cr) by adjusting the concentration of acetic acid. Ortho-octahedral crystals of MIL-101(Cr) with an average particle size of about 100 nm can be obtained at lower concentrations of acetic acid. And the use of NaOH can modulate the particle size of MIL-101(Cr) down to about 90 nm, with a specific surface area up to more than 4000 m2/g. Xu et al. [68] found that the addition of sodium stearate (SS) and cetyltrimethylammonium bromide (CTAB) could effectively modulate the morphological features of MIL-101(Cr) while significantly improving its adsorption capacity and selectivity for methyl orange (Figure 5a,b). In addition, the optimization of morphology and pore size can also be achieved by adjusting the ratio of components and reaction conditions (microwave power, reaction temperature, etc.) [73,74]. Zhou et al. [75] successfully prepared a MIL-101(Cr)-based segmented membrane with excellent pore size distribution and morphology by adjusting the ratios of acetic acid, ligand, and metal to balance the nucleation and growth process of MIL-101(Cr), which showed excellent performance in CO2/N2 separation at low pressure, successfully confirming the importance of systematic regulation of component ratios in enhancing the energy efficiency of MIL 101(Cr) applications. Alivand et al. [74] prepared a series of MIL-101@M-X-Y samples by adjusting the ratios (X) of ligand (H2BDC) to metal clusters (Cr) and the ratio (Y) of modifier (HNO3) to metal clusters during synthesis as a means to modulate the pore structure and surface properties of the material, and a MIL-101(Cr) material with higher adsorption performance and yield was successfully developed, which provides a highly efficient and cost-effective solution for the adsorption of industrial gases (Figure 5c,d).
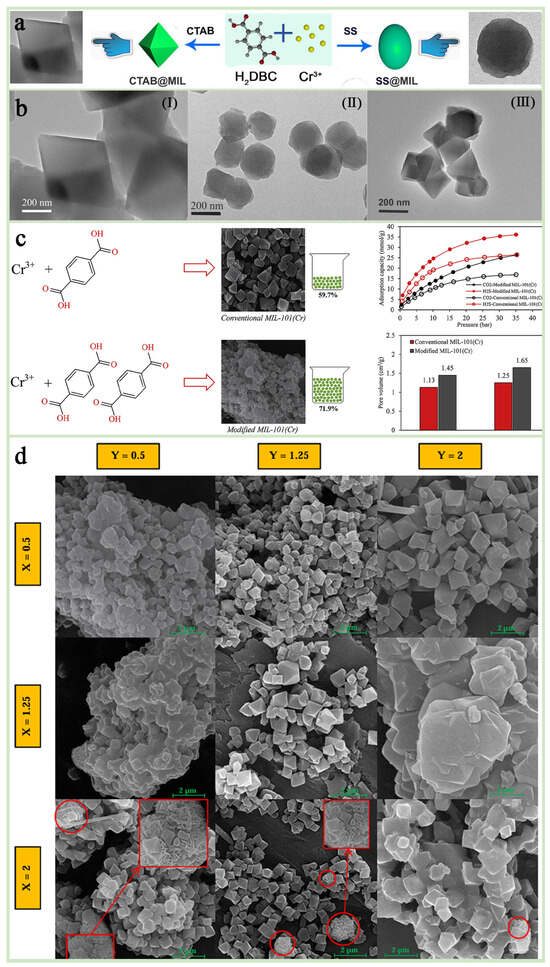
Figure 5.
(a) A synthetic scheme of different morphologies of MIL-101(Cr) crystal. (b) TEM images of (I) MIL-101(Cr), (II) SS@MIL-0.2, and (III) CTAB@MIL-0.2 [68]. Copyright Iranian Chemical Society 2020 (c) Regulatory strategies and data graphs. (d) FE-SEM images of different synthesized MIL-101@M-X-Y nanoadsorbents at the scale of 2 [74]. Copyright 2018 Elsevier Inc.
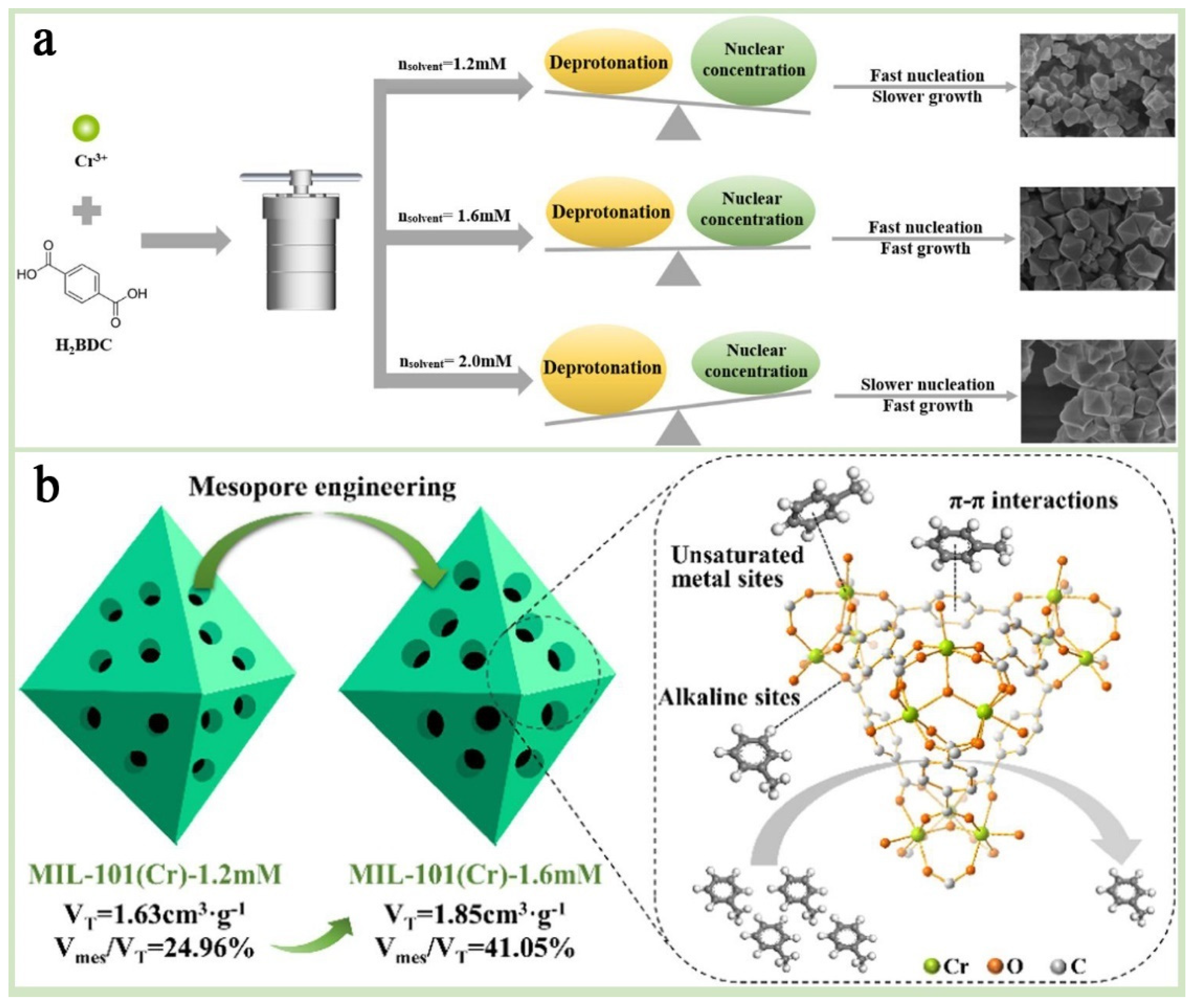
By using the solvent “squeezing” strategy and adjusting the type and quantity of solvents in the synthesis process of MIL-101(Cr), it is possible to introduce additional defect structures into its pores, thereby significantly improving the porosity. That is, during the synthesis process, the “squeezing” effect generated by the solvent molecules filling the pores of MIL-101(Cr) will affect the shape and size of the pores, causing them to expand and even form new pore structures inside the pores. These newly formed pores not only increase the total pore volume of the material but also may improve the connectivity between pores, making the pore structure of the entire material more uniform and optimized. Subsequently, the solvent can be gradually removed by heating, vacuuming, or other physical and chemical methods. Cao et al. [57] investigated the effect of solvent on the nucleation and growth rate of MIL-101(Cr) crystals in order to explore the regulatory effect of solvent dosage on MIL-101(Cr) pores. By modulating the pore structure, it was found that the adsorption rate of MIL-101(Cr)-1.6 mM in the pore diffusion stage was significantly increased, and the larger pore volume exposed more unsaturated metal sites and basic sites, which was applied to the adsorption of toluene, demonstrating a higher toluene adsorption capacity (Figure 6). In addition, this study found that temperature also had an effect on MIL-101 pores. In the adsorption of toluene by MIL-101(Cr), the process was susceptible to temperature because of the dominant role of van der Waals forces, and because the adsorption effect would be significantly weakened by the increase in temperature.
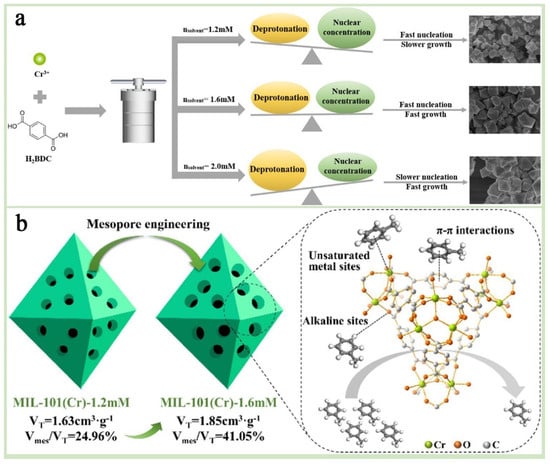
Figure 6.
(a) An illustration of the mechanism for structure- and morphology-controllable synthesis of MIL-101(Cr)-XmM nanocrystals. (b) Toluene adsorption mechanism of MIL-101(Cr)-XmM [57]. Copyright 2024 Elsevier Ltd.
In addition to the above methods used to regulate the morphology, particle size, and pore space of MIL-101(Cr), there are other potential manipulation measures that may also have an impact on its morphological characteristics. For example, the parameter settings in the experimental process, the centrifugal speed can effectively separate nanoparticles with different particle sizes or impurities in the pores and, also, achieve the regulation of the particle size of MIL-101(Cr). The modulation of the morphology, particle size, and pore space of MIL-101(Cr) has developed rapidly and has gradually expanded from the traditional optimization around synthetic methods to functionalized modifications, green synthesis, and composite design. The efficient preparation of MIL-101(Cr) with specific particle size, morphology, and porosity plays an important role in enhancing its performance in multiple applications. However, the modulation of MIL-101(Cr) morphology may involve the choice of a templating agent and the risk of structural damage during the removal process. The control of particle size may be affected by factors such as reaction time and temperature, making it difficult to achieve precise control. The regulation of pore space may require the adjustment of the ratio of ligand and metal source, which will be a laborious and tedious project, and may also lead to the destabilization of the crystal structure of MIL-101(Cr). In addition, multifactorial coupling effects must be considered, such as the mutual constraints that may arise when morphology and pore space are regulated simultaneously. Therefore, in the future, combining computational simulation to optimize the design, standardize the experiments, and booking the application space through science and technology to cope with the above challenges will be an important development direction in this field.
3.1.2. Preparation of Hybrid Composites
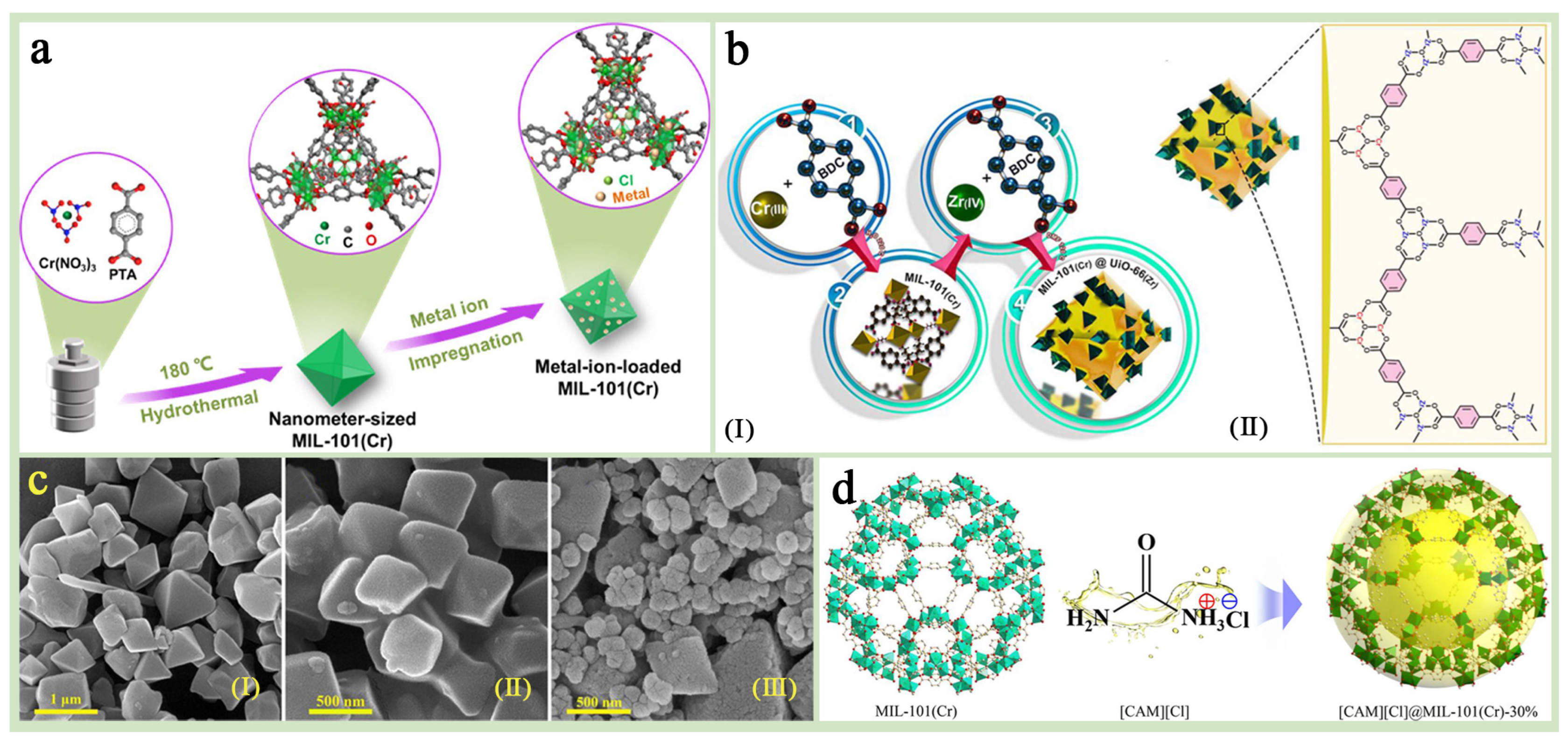
MIL-101(Cr) combined with other functional materials (blending method, in situ method) can form composites with excellent synergistic effects, which leads to the targeted optimization of MIL-101(Cr) properties [1,44,76,77,78]. Bimetallic MOFs formed by doping other metal ions or MOFs into MIL-101(Cr) as the main body have shown enhanced properties in various fields due to their synergistic and complementary effects and significantly enhanced metal activity centers [79,80] (Figure 7a). FRafati Jolodar et al. [81] prepared a bimetallic MOF (MIL-101(Cr-Al)) based on MIL-101(Cr) and used it for dynamic adsorption and separation of CO2 from natural gas. Comparing this bimetallic MIL-101(Cr-Al) composite with monometallic MIL-101(Cr), it was found that the bimetallic composite MIL-101(Cr-Al) outperformed the monometallic MIL-101(Cr) in terms of CO2 adsorption capacity and selectivity, and exhibited higher dynamic adsorption performance under high-pressure conditions. Fakhraie et al. [44] designed a novel core–shell structural composite, MIL-101(Cr)@UiO-66(Zr) (MU-4) nanocrystals, for the efficient selective separation of H2S and CO2. The small pore size of UiO-66(Zr) in the composite effectively synergized with the large pore size of MIL-101(Cr) to achieve efficient and stable adsorption and separation of acid gases (Figure 7b,c). IAST predictions show that the composite nanocrystals with this core–shell structure significantly outperform pure MIL-101(Cr) in terms of selectivity for a wide range of gases (H2S/CH4, H2S/N2, CO2/CH4, and CO2/N2), with improvement of 7.8%, 47.0%, 32.1%, and 59.9%, respectively, at high pressures. The design of this novel core–shell structure provides a new strategy for the development of high-performance MIL-101(Cr) substrates.
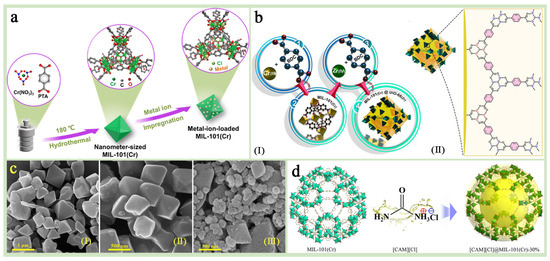
Figure 7.
(a) A schematic illustration of the metal–ion-loaded MIL-101(Cr) fabrication [80]. Copyright 2024 American Chemical Society (b) A schematic illustration of the fabrication process of the core–shell nanocrystals (I). A demonstration of the assembly of zirconium ions and terephthalic acid molecules on the surface of MIL-101(Cr) crystals (II). (c) FE-SEM images of (I) MIL-101(Cr), (II) UiO-66(Zr), and (III) MU-4 [44]. Copyright 2022 Elsevier B.V. (d) An illustration of the structure for [CAM][Cl]@MIL-101(Cr) composite (Cr polyhedra: green; ILs: yellow; C: gray; O, red; H atoms were omitted for clarity) [82]. Copyright 2023 Elsevier B.V.
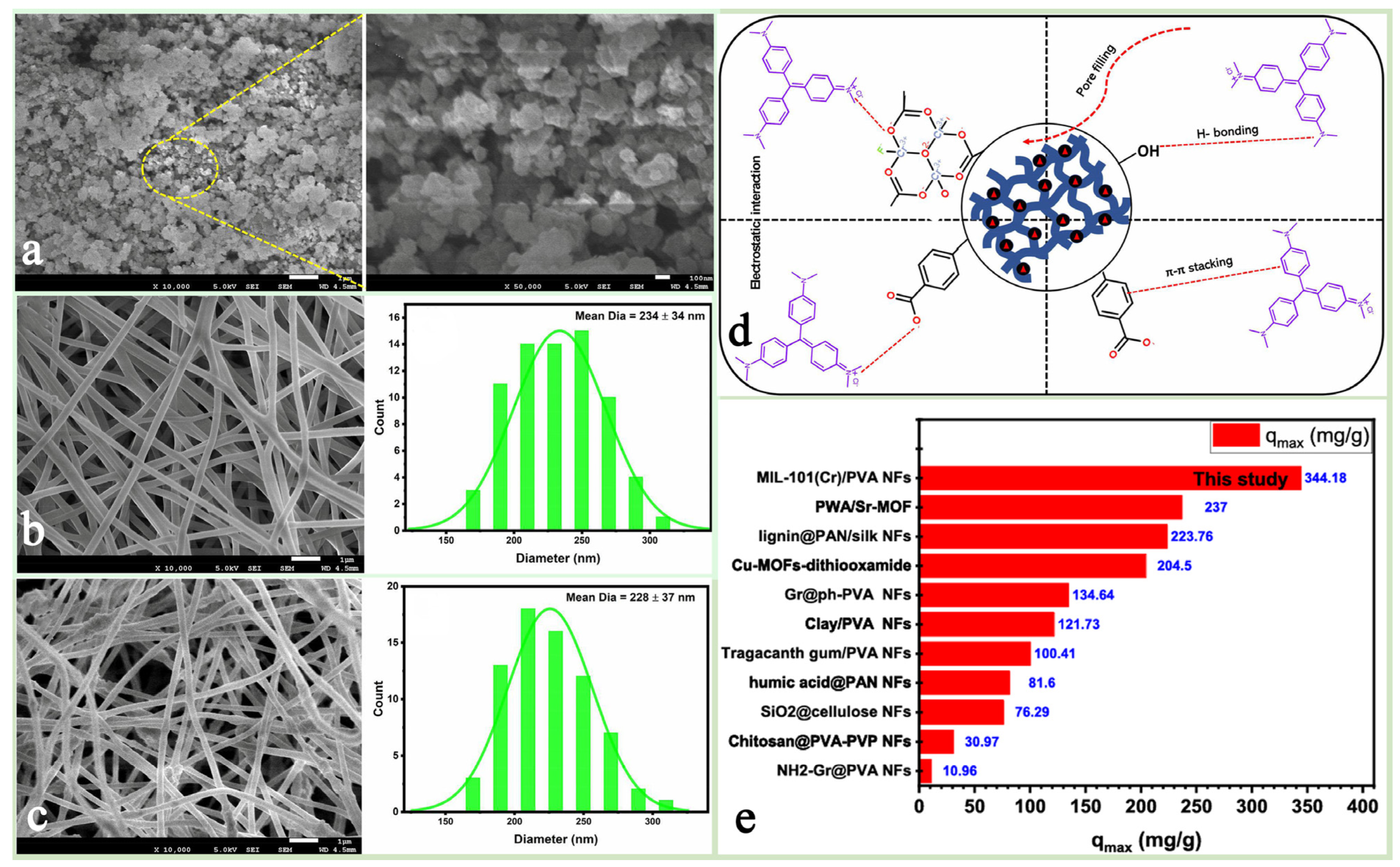
Modulating the pore size and surface chemistry of MIL-101(Cr) by doping functional complementary materials is an important strategy to optimize its properties [79]. Bazmi et al. [83] successfully prepared FAC@MIL-101(Cr) composites with micro- and mesoporous structure by embedding amine-functionalized activated carbon in MIL-101(Cr). Thanks to the enhanced interaction between MIL-101(Cr) and CO2 molecules due to the embedding of FAC, and the presence of mesopores facilitated the transport of CO2 molecules, the CO2 adsorption kinetics of the composite FAC-6@MIL-101(Cr) was three times higher than that of pure MIL-101(Cr), and the CO2/N2 selectivity and CO2 adsorption capacity were also significantly higher. Han et al. [82] loaded ionic liquids (ILs carboxamide chloride—[CAM][Cl]) into MIL-101(Cr) and prepared a composite material [CAM][Cl]@MIL-101(Cr)-30% whose NH3 adsorption capacity (44.6%) was significantly higher than that of pure MIL-101(Cr) (Figure 7d), achieving an efficient capture and selective separation of ammonia (NH3) and carbon dioxide (CO2). Thamer et al. [84] investigated a MIL-101(Cr)/poly(vinyl alcohol) (PVA) nanofiber composites (MIL-101(Cr)@PVA NFs) prepared based on the green electrospinning technique and used them for the removal of crystalline violet (CV), a cationic dye in water. Thanks to the aromatic ring structure and high specific surface area of MIL-101(Cr) itself, as well as the transverse and longitudinal distribution slits of PVA and the abundant hydroxyl (-OH) distribution on the surface, the MIL-101(Cr) particles were arranged in nanoneedles in the PVA nanofibers, which showed excellent electrostatic adsorption performance, stability, and reusability (Figure 8). All these studies successfully demonstrated the great application potential of the hybrid composite strategy in optimizing the properties of MIL-101(Cr).
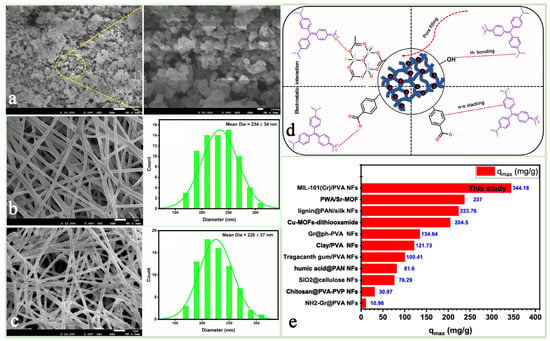
Figure 8.
FESEM images of (a) MIL-101(Cr); (b) SEM image and diameter distribution of PVA NFs; (c) SEM image and diameter distribution of MIL-101(Cr) @PVANFs. (d) Adsorption interaction between MIL-101(Cr)@PVA NFs and CV dye. (e) Adsorption performance of MIL-101(Cr)/PVA NFs versus other adsorbents for CV removal [84]. Copyright 2024 Society of Plastics Engineers.
Although MIL-101(Cr) composites can achieve improved functionality and performance due to the significant complementary effects of the components, they still face some key challenges in practical applications. For example, the composite of two components with different chemical properties can easily lead to poor interfacial compatibility and dispersion, which hinders the optimization of composite properties. Furthermore, due to the differences in the physicochemical properties of the components, the control of the scale-up process parameters is also a major challenge as inaccurate settings may trigger incomplete local reactions in the composites.
3.1.3. High-Temperature Calcination
High-temperature calcination of MIL-101(Cr) can effectively remove the terminal water molecules coordinated with Cr3+ in its structure, thus exposing the unsaturated Cr3+ sites and forming a strong Lewis acid center, which can significantly improve the energy efficiency of MIL-101(Cr) in the fields of catalysis and energy storage [85,86,87]. In addition, high-temperature calcination can effectively regulate the chemical composition, pore structure, and surface properties of MIL-101(Cr) by changing the parameter settings (e.g., temperature, heating rate, retention time, atmosphere, etc.), thus realizing the targeted design of its functions. For example, low-temperature calcination (200–350 °C) is mainly used to activate the sites while retaining the main structure of MOF; medium-high-temperature calcination (350–600 °C) is mostly used for the production of Cr-based metal oxides or partially carbonized materials, which is suitable for the preparation of catalytic materials; ultra-high-temperature calcination (>800 °C) can completely carbonize MIL-101(Cr) to produce derivative materials suitable for the energy storage field. Furthermore, the heating rate of calcination directly affects the integrity of the crystal structure, the exposure of the active sites, and the production of by-products. A slow temperature increase helps the orderly exposure of Cr3+ sites, while a fast temperature increase may lead to aggregation or sintering of active sites, which is detrimental to the catalytic efficiency of the derivatives. Under specific conditions, the use of two-stage warming (e.g., initial 2 °C min−1 to 300 °C and subsequent 10 °C min−1 to the target temperature) can ensure the gradual removal of water molecules while avoiding pore shrinkage caused by rapid warming. This strategy can maintain the excellent dispersion and mesoporous structure of the prepared MIL-101(Cr) derivatives. The calcination retention time determines the completion of the calcination process, which directly affects the crystallinity, porosity, and chemical stability of the material. Usually, short-time calcination only partially removes ligand water and surface adsorbates, which can retain the main structure of MOF, and long-time calcination (>4 h) can promote the complete oxidation or carbonization of organic ligands to form high-purity metal oxides. The type of atmosphere (oxidizing, inert, reducing) directly determines the chemical composition and surface functionalization of the calcined products. For example, oxidizing atmosphere calcination produces oxides, inert atmosphere preserves the carbon skeleton, and reducing atmosphere achieves the effect of modulating the metal valence state [88].
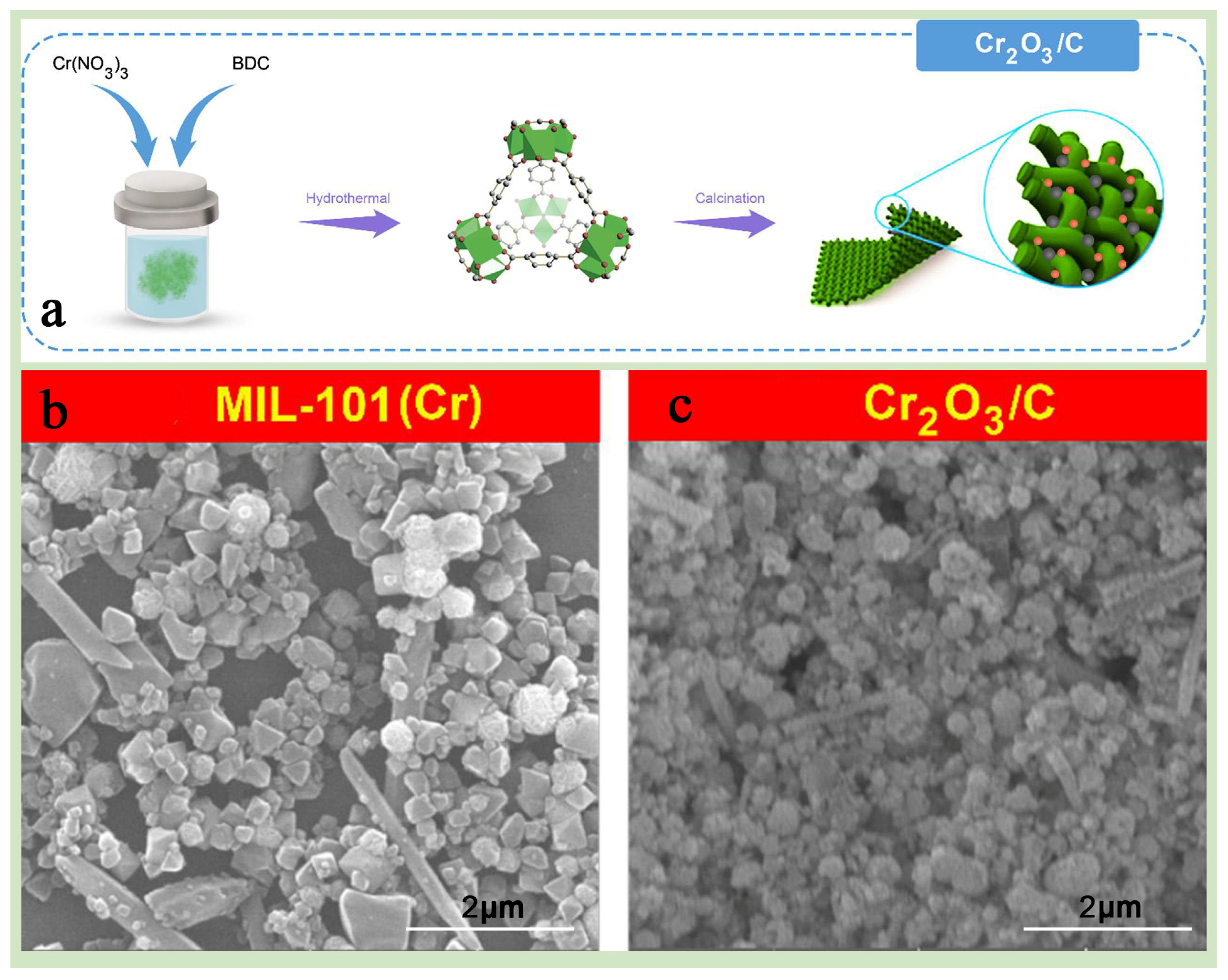
Farisabadi et al. [89] successfully prepared high-performance MIL-101(Cr)-derived carbon material Cr2O3/C by calcining MIL-101(Cr) at high temperature of 800 °C for 5 h at a heating rate of 5 °C min−1 and applied it to asymmetric supercapacitors (Figure 9a–c), which exhibited excellent specific capacity and multiplicity performance, successfully confirming the advantages of high-temperature calcination of MIL-101(Cr) for improving its electrochemical performance. Zhu et al. [88] prepared praseodymium (Pr)-doped Cr2O3 catalysts (M-PrCr) by pyrolyzing MIL-101(Cr) for 5 h at 400 °C under air atmosphere with a heating rate of 5 °C min−1, which were applied to the efficient catalytic oxidation of 1,2-dichloroethane (1,2-DCE) and exhibited excellent catalytic performance and durability at low temperatures, providing a promising strategy for preparing and optimizing MIL-101(Cr)-based catalytic materials.
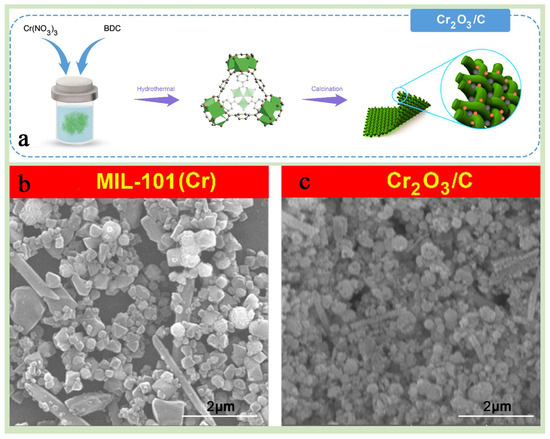
Figure 9.
(a) A schematic representation illustrating the fabrication process of materials. (b,c) SEM images of (b) MIL-101(Cr) and (c) Cr2O3/C [89]. Copyright 2018 Elsevier B.V.
Although the high-temperature pyrolysis MIL-101(Cr) strategy is more widely used in the field of energy storage and conversion, the inherent poor electrical conductivity of MIL-101(Cr) makes this its greatest application advantage not in the field of energy storage. So, figuring out how to multifacetedly enhance the application value of high-temperature pyrolysis MIL-101(Cr) will be a very promising research direction in the future.
3.2. Chemical Modification Strategies
3.2.1. Modified Functionalization
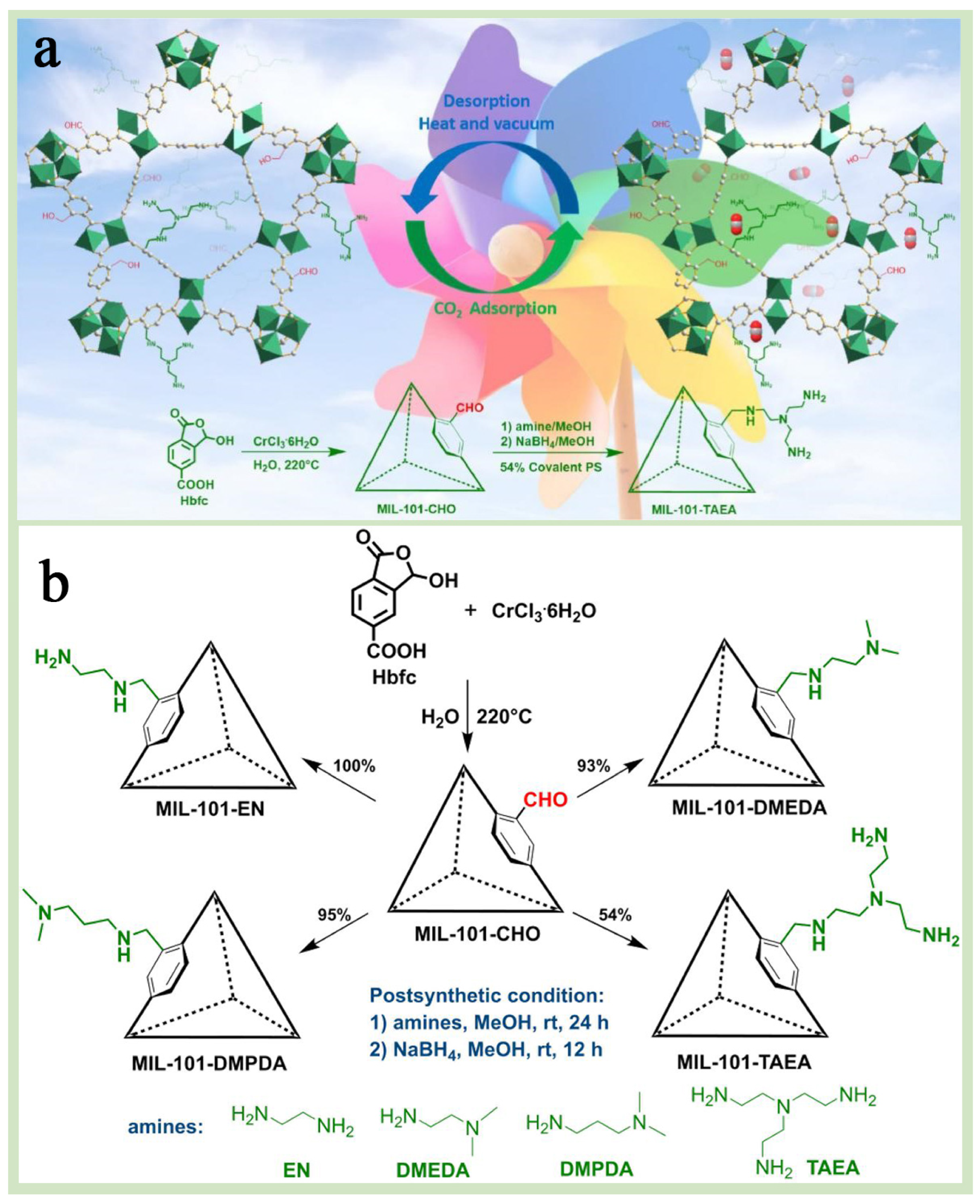
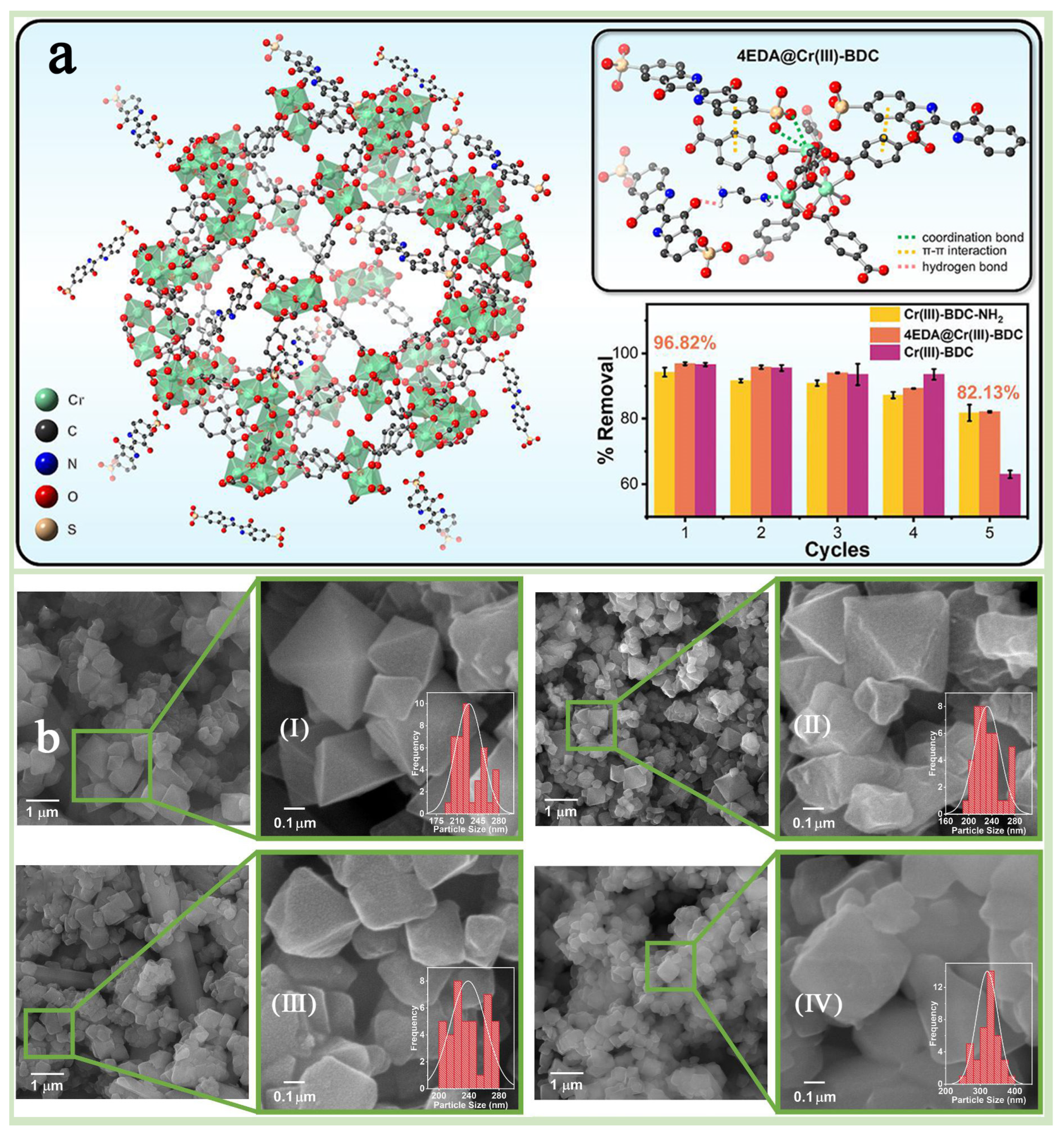
A chemical modification is an important means of enhancing the properties of MIL-101(Cr) and offers higher structural designability and scalability than physical modification [90,91]. MIL-101(Cr) functionalization modification is one of the most convenient and energy-efficient chemical modification strategies for the application, which can be achieved by adjusting the functional groups of grafting, thus conferring MIL-101(Cr) substrates with specific functional properties while keeping the crystal structure of MIL-101(Cr) unchanged [92,93]. Functionalization modification is subdivided into two types: one is a pre-synthesis modification, which refers to the direct use of functionalized ligands, such as the introduction of terephthalic acid with amino or hydroxyl groups during the synthesis process [51], and the other is post-synthesis modification, which refers to the introduction of functional groups such as amino, hydroxyl, and sulfhydryl groups through chemical reactions after the synthesis of MIL-101(Cr) [90,94]. Xi et al. [21] successfully synthesized MIL-101-CHO with formaldehyde moiety by pre-synthesis method and further converted it into four MIL-101(Cr)-containing amine groups by post-synthesis strategy (Figure 10a,b). The results showed that MIL-101-TAEA aminated with “N,N-bis(2-aminoethyl)ethylenediamine” had the largest specific surface area and pore volume, while the amination degree was the lowest, and the crystal structure of MIL-101(Cr) was retained. Dendy et al. [95] modified MIL-101(Cr) via ethylenediamine (EDA) using post-synthetic modification and focusing on the effect of amine groups and surface properties on the adsorption performance of the modified EDA@Cr(III)-BDCs, which were also compared with the unmodified and MIL-101(Cr)-NH2 generated by using pre-synthetic modification. The test results showed that EDA@Cr(III)-BDC exhibited higher stability and superior pore properties in multiple adsorption–desorption cycling experiments, which was attributed to the strong chemical bonding and electrostatic interactions formed between the amine groups in EDA and the Cr(III)-BDC frameworks, which made the material less susceptible to degradation or inactivation in an aqueous environment (Figure 11a). In addition, it was found that the amine group content in EDA@Cr(III)-BDC could be precisely regulated by varying the amount of EDA, which, in turn, adjusted its surface charge and adsorption properties (Figure 11b). Yang et al. [69] used a pre-synthesis method to introduce sulfonic acid groups (-SO3H) into the backbone of MIL-101(Cr), which enhanced the adsorption drive of MIL-101(Cr) for toxic dyes, resulting in the preparation of highly efficient selective dye adsorbents. In addition, the pore structure, specific surface area, and surface charge distribution of MIL-101-SO3H were precisely controlled by adjusting the molar ratio of H2BDC-SO3Na to H2BDC, thereby optimizing its adsorption performance of MIL-101-SO3H for various dyes. These studies successfully demonstrated the potential and flexible applicability of chemical functionalization modification strategies in optimizing the adsorption performance of MIL-101(Cr).
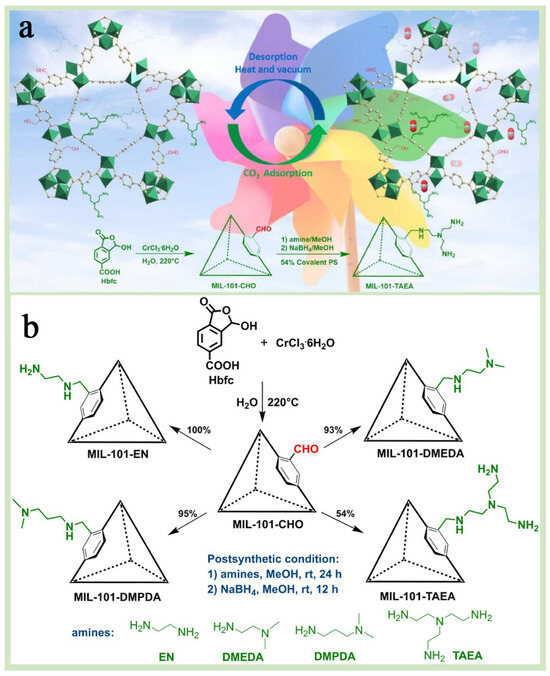
Figure 10.
(a) A schematic diagram of the CO2 adsorption and desorption cycles of MIL-101(Cr) material. (b) The synthesis route of MIL-101-CHO and amine functionalized MIL-101(Cr) [21]. Copyright 2024 Elsevier B.V.
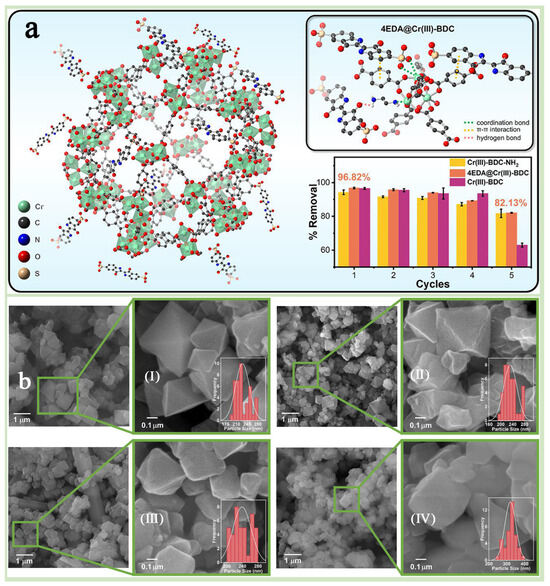
Figure 11.
(a) The model image for molecules of EDA@Cr(III)-BDC and the reusability of three MOFs. (b) FESEM images and average particle size distributions of Cr(III)-BDC (I), 2EDA@Cr(III)-BDC (II), 4EDA@Cr(III)-BDC (III), 10EDA@Cr(III)-BDC (IV) [95]. Copyright 2025 Elsevier B.V.
Functionalization can not only effectively optimize the adsorption performance and surface functional properties of MIL-101(Cr) but also significantly improve the electrocatalytic activity and electrical conductivity of MIL-101(Cr), increasing its application value in the field of energy storage [96,97]. For example, the introduction of Lewis basic nitrogen-containing functional groups (e.g., amino-NH2) into MIL-101(Cr) can effectively improve its surface charge distribution, enhance the interaction with the reactants, and stabilize the reaction intermediates, thus reducing the reaction activation energy and realizing the enhancement of catalytic performance. In addition, amines can act as electron donors to improve the conductivity of the materials by promoting electron transfer and enhancing the charge carrier concentration. Wang et al. [97] used NH2-MIL-101(Cr) as a heterogeneous photocatalyst and investigated its catalytic energy efficiency when hydrogen peroxide (H2O2) was used as a green oxidizing agent under mild conditions. It was found that NH2-MIL-101(Cr) was able to efficiently convert H2O2 to ROS, demonstrating higher styrene conversion and selectivity compared to MIL-101(Cr). This is attributed to the fact that the amino group can further enhance the interaction of the Cr site with H2O2 by facilitating the one-electron transfer process, thus improving the activity and selectivity of the reaction. Apart from -NH2, there are a number of functional groups that also show excellent value in optimizing the catalytic performance of MIL-101(Cr), such as halogen atom functional groups (fluorine (-F), chlorine (-Cl) and bromine (-Br)), which are a class of atoms with specific chemical properties, strong electronegativity and reactivity, and halogen-modified MIL-101(Cr) has superior chemical stability and catalytic activity [98,99,100]. There are also acidic groups, such as carboxyl groups (-COOH) and sulfur-containing sulfonic acid groups (-SO3H), which can significantly enhance the acidity of the material and provide protons for the reaction, and have an important potential for facilitating the conduct of catalytic reactions by acidic mechanisms. In addition, the introduction of sulfonic acid groups can also improve the hydrophilicity of MIL-101(Cr) and promote the diffusion, adsorption, and full contact with the electrolyte of catalytically active substances, which is expected to enable MIL-101(Cr) to exhibit higher conversion efficiency in aqueous catalytic reactions such as the catalytic hydrolysis of cellulose, thus promoting the research and development of high-performance MIL-101(Cr)-based catalytic materials.
Although functionalization modification of MIL-101(Cr) can effectively enhance its functionality, there are some potential challenges. For example, direct functionalization may lead to a large number of functional groups entering the mesoporous structure and occupying the pore channels, thus reducing the adsorption performance of MIL-101(Cr). In addition, certain methods of functionalization may affect the long-term stability of the material, e.g., partially modified MIL-101(Cr) may be inactivated in practical applications due to changes in environmental conditions. Further some of the functionalization methods require complex experimental conditions and expensive reagents, which increase the difficulty of preparation. Therefore, based on the above challenges and the diverse functionalization methods of MIL-101(Cr), weighing the combined effects of different methods on the adsorption performance, catalytic activity, and stability and selecting the best strategy are the key to achieving the targeted optimization of MIL-101(Cr) performance.
3.2.2. Ion Exchange
The ion-exchange strategy involves chemically replacing some or all of the chromium ions (Cr3+) in the MIL-101(Cr) framework with other metal ions (e.g., Fe3+, Co2+, Ni2+, Zn2 +, etc.). This strategy usually involves immersing MIL-101(Cr) in a solution containing the target metal ions and realizing the replacement of the metal ions through an ion exchange reaction [101]. Vu et al. [102] partially replaced chromium (Cr) with iron (Fe) in MIL-101(Cr) by homocrystalline substitution and investigated its catalytic energy efficiency as a novel heterogeneous photo-Fenton catalyst for the degradation of reactive dyes (e.g., Reactive Red 195, RR195). It was shown that pure MIL-101(Cr) had almost no catalytic activity, whereas Fe-Cr-MIL-101 exhibited significant photo-Fenton catalytic activity under simulated sunlight irradiation, and the degradation rate of RR195 reached 98% in 100 min. The significant improvement of the catalytic performance can be attributed to the catalytic decomposition of H2O2 by Fe(III) on the surface of Fe-Cr-MIL-101, and at the same time, H2O2, in turn, successfully captured the photogenerated electrons generated by the excited state of Fe-Cr-MIL-101, which synergistically generated a large number of hydroxyl radicals, which, in turn, considerably improved the degradation efficiency and stability. This study successfully demonstrated the potential of the ion substitution strategy in the targeted optimization of the performance of MIL-101(Cr) and the great scope for future development. However, despite the many advantages of the ion-exchange strategy, it still suffers from some shortcomings and limitations in terms of selectivity, exchange capacity, regeneration and waste treatment, kinetics, material stability, cost, and operating conditions. These limitations may affect the effectiveness and efficiency of MIL-101(Cr) in some applications. Therefore, in practical applications, it is necessary to select suitable ion exchange materials and optimize operating conditions according to specific needs in order to overcome these deficiencies.
The optimization strategies of physical modulation and chemical modifications of MIL-101(Cr) have their own advantages. Chemical modification mainly regulates its chemical properties by introducing functional groups or replacing metal ions, while physical modification optimizes its physical properties by regulating the pore structure, morphology, or preparing hybrid materials. These modification strategies can be used individually or in combination to meet different application requirements, providing a broad development space for the application of MIL-101(Cr) in gas storage and separation, seawater desalination [103] and catalysis. In addition to several types of optimization strategies introduced above, many other novel optimization strategies have emerged in recent years, such as the introduction of specific guest chemicals into MIL-101(Cr) to enhance the electrical conductivity, luminescence, and magnetism of MIL-101(Cr) [104]; the grafting of polymer chains on the surface of MIL-101(Cr) through chemical reactions; the self-assembly technique on the surface of MIL-101(Cr) to form a monomolecular layer; or the use of a dual exchange strategy of continuous ligand exchange and metal complexation process for systematic optimization of MIL-101(Cr) properties [99,104,105,106].
4. Application Progress
Due to unique advantages such as ultra-high specific surface area and excellent stability, MIL-101(Cr) has achieved remarkable results in adsorption [83], sensing [107], and many other fields [108,109,110] (As Table 2 summarizes the progress and advantages of MIL-101(Cr) applications in a number of areas), as follows:
4.1. Gas Adsorption and Separation
Thanks to its excellent physicochemical properties, MIL-101(Cr) stands out among many types of MOFs and shows superior application advantages in gas storage and separation applications [21,22,23,24]. Yang et al. [25] compared MIL-101(Cr), ZIF-8, and UiO-66 and found that MIL-101(Cr) adsorbed carbon dioxide (CO2) significantly better than the other MOF materials, reaching 29.4 mmol g−1. Llewellyn et al. [111] compared MIL-101(Cr) with MIL-100(Cr) for gas adsorption and found that the maximum adsorption of CO2 by MIL-101(Cr) could reach 40 mmol g−1 and its adsorption of hydrogen (H2) was also superior compared to that of MIL-100(Cr). Furthermore, Hong et al. [112] showed that the adsorption capacity of MIL-101(Cr) for CO2 was 37% higher than that of the commonly used adsorbent material zeolite monomer, and the adsorption efficiency was 1.5 times higher, which makes it an environmentally friendly and durable adsorbent material with great potential for development. The extremely high specific surface area (4100 m2 g−1) of MIL-101(Cr) and the abundance of large pore sizes (30–35 Å), which can provide a large number of adsorption sites for gas molecules, as well as its good chemical stability under various environmental conditions (e.g., acidic, alkaline, high temperature, etc.), ensures that it will not undergo structural damage or performance degradation during the process of gas separation and adsorption and storage; the diverse physical and chemical modification strategies make it possible to realize selective adsorption of specific gases through treatment, especially in the case of CO2, H2, methane (CH4), and other gases, showing excellent application efficacy [113,114]. Menezes et al. [115] investigated the adsorption properties of MIL-101(Cr) for CO2 and CH4 under high-pressure conditions with the aim of providing an efficient material for CO2/CH4 separation in natural gas purification processes. The data of this study show that MIL-101(Cr) has high adsorption capacity and selectivity for CO2 under high-pressure conditions, as well as good thermodynamic stability and reversibility, and maintains stable performance after six adsorption–desorption cycles, which has considerable potential for practical applications.
Membrane technology, with its advantages of low energy consumption, no solvent evaporation, and small footprint, is suitable for practical applications at different scales and is becoming increasingly valuable in the field of carbon-based gas separation and adsorption. The proper pore size distribution of separation membranes is crucial for gas separation, and it is usually necessary to match specific molecular dynamics diameters to achieve efficient and selective adsorption separations.MIL-101 membranes have been widely used for CO2/N2 separations due to their tunable structural features (e.g., porosity, functional groups, and pore size, etc.). Zhou et al. [75] balanced the nucleation and growth process of MIL-101(Cr) by adjusting the ratios of acetic acid, ligand, and metal, and successfully prepared a high-performance MIL-101(Cr) functionalized separation membrane (F-NH2-MIL-101(Cr) with a suitable pore size distribution (average pore size of about 0.56 nm) and functional groups, and their applications in CO2/N2 separation were explored. The results showed that under the optimal preparation conditions, the membrane exhibited high CO2 permeability (6104 GPU) and CO2/N2 selectivity (37), which outperformed most of the reported MOF membranes. Additionally, the membrane remained structurally stable throughout the 50 h test period, which has potential for long-term practical applications.
4.2. Wastewater Purification, Dye Adsorption
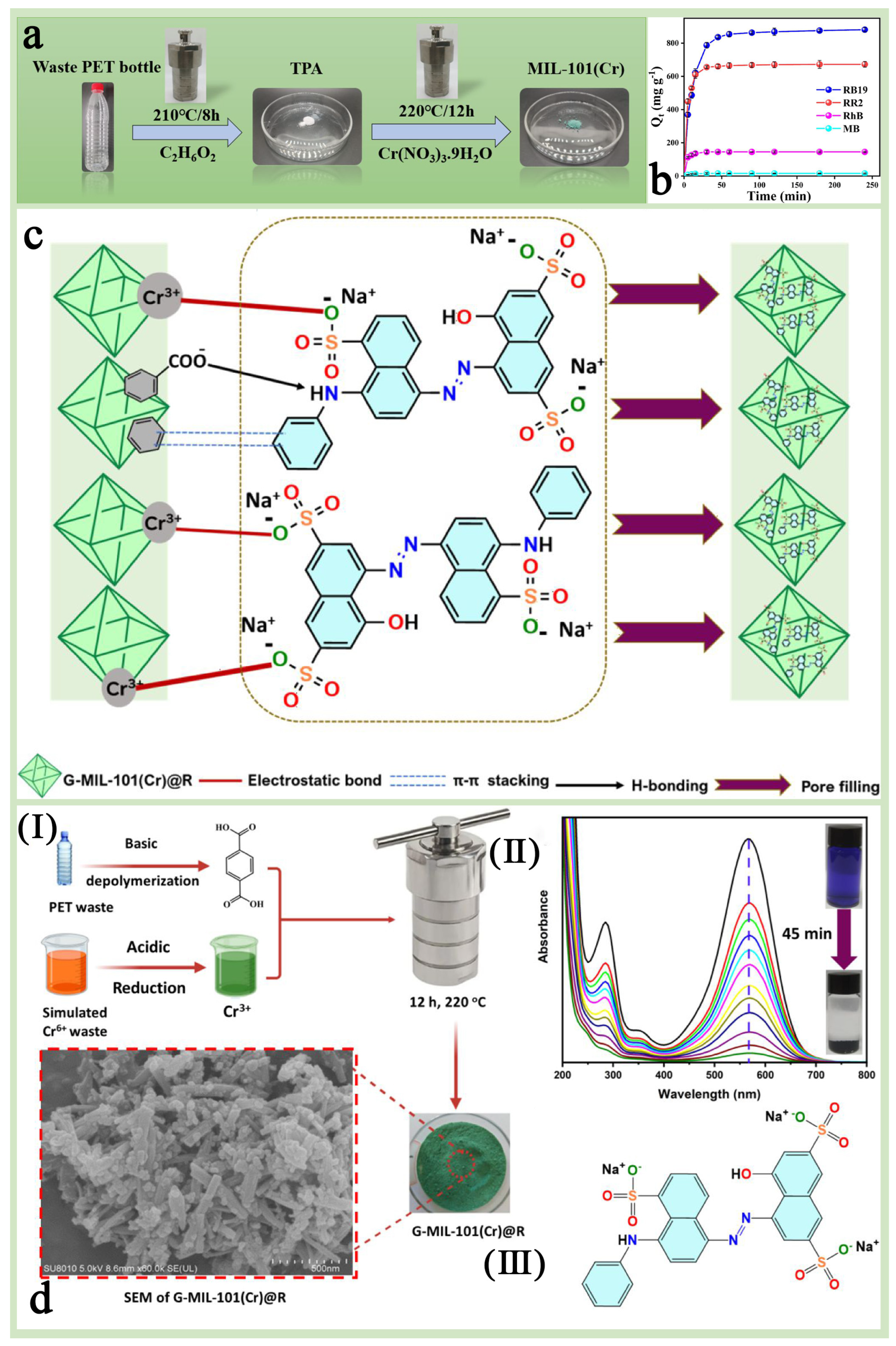
Harmful dyes in industrial wastewater are an important ecological problem, especially in wastewater discharged from the textile industry. These dyes are not only difficult to decompose but may also be carcinogenic and mutagenic, so effective treatment technologies are needed to remove these pollutants. MIL-101(Cr) has important applications in the treatment of organic dyes and wastewater due to its large number of well-distributed pore channels, outstanding chemical stability, abundance of unsaturated chromium (Cr) sites, and tunable surface properties [116,117,118,119,120,121]. In addition, MIL-101(Cr), synthesized through a green strategy, is expected to achieve efficient purification of waste dyes while closely following the concept of sustainable development [122]. Cheng et al. [123] synthesized MIL-101(Cr) without hydrofluoric acid (HF) using terephthalic acid (TPA) from discarded PET plastic bottles as an organic ligand and tested its adsorption performance on anionic dyes such as reactive red 2 (RR2) and reactive blue 19 (RB19) (Figure 12a). The results showed that the adsorption capacity of MIL-101(Cr) for RR2 and RB19 reached 662.87 mg/g and 863.67 mg/g, respectively (Figure 12b), and the adsorption efficiency remained high after several regeneration cycles (the regeneration rates were 91.18% and 91.44%, respectively, in the fourth cycle), which demonstrated a good cyclic regeneration capacity and practical application value. Keshta et al. [110] similarly prepared MIL-101(Cr) using a green synthesis method based on organic and inorganic waste recycling and applied it to the removal of the anionic dye Acid Blue 92 (AB-92) from water (Figure 12c,d). The results showed that this green synthesized G-MIL-101(Cr)@R had excellent adsorption performance, sustainability, and reusability, and the adsorption efficiency remained above 70% after five cycles without the use of regeneration solvents, which provided a highly efficient and environmentally friendly solution for the treatment of organic dyes in wastewater.
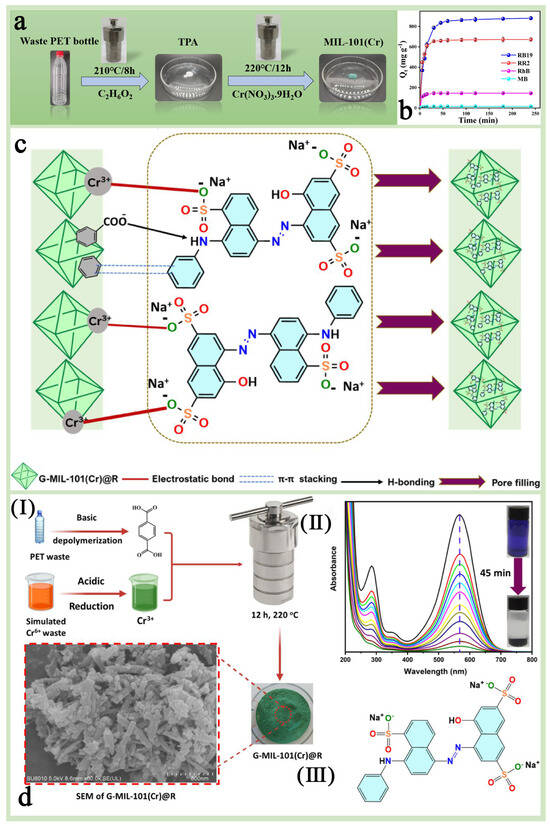
Figure 12.
(a) The schematic presentation of the depolymerization of waste PET bottles and the synthesis of PET-derived MIL-101(Cr). (b) Adsorption capacity of MIL-101(Cr) toward different dyes [123]. Copyright 2024 Elsevier B.V. (c) The plausible mechanism of the AB-92 dye adsorption over G-MIL-101(Cr)@R. (d) A schematic illustration for G-MIL-101(Cr)@R synthesis (I), UV–Vis absorption removal of AB-92 over 25 mg of G-MIL-101(Cr)@R (II), and the chemical structure of AB-92 (III) [110]. Copyright 2023 Elsevier B.V.
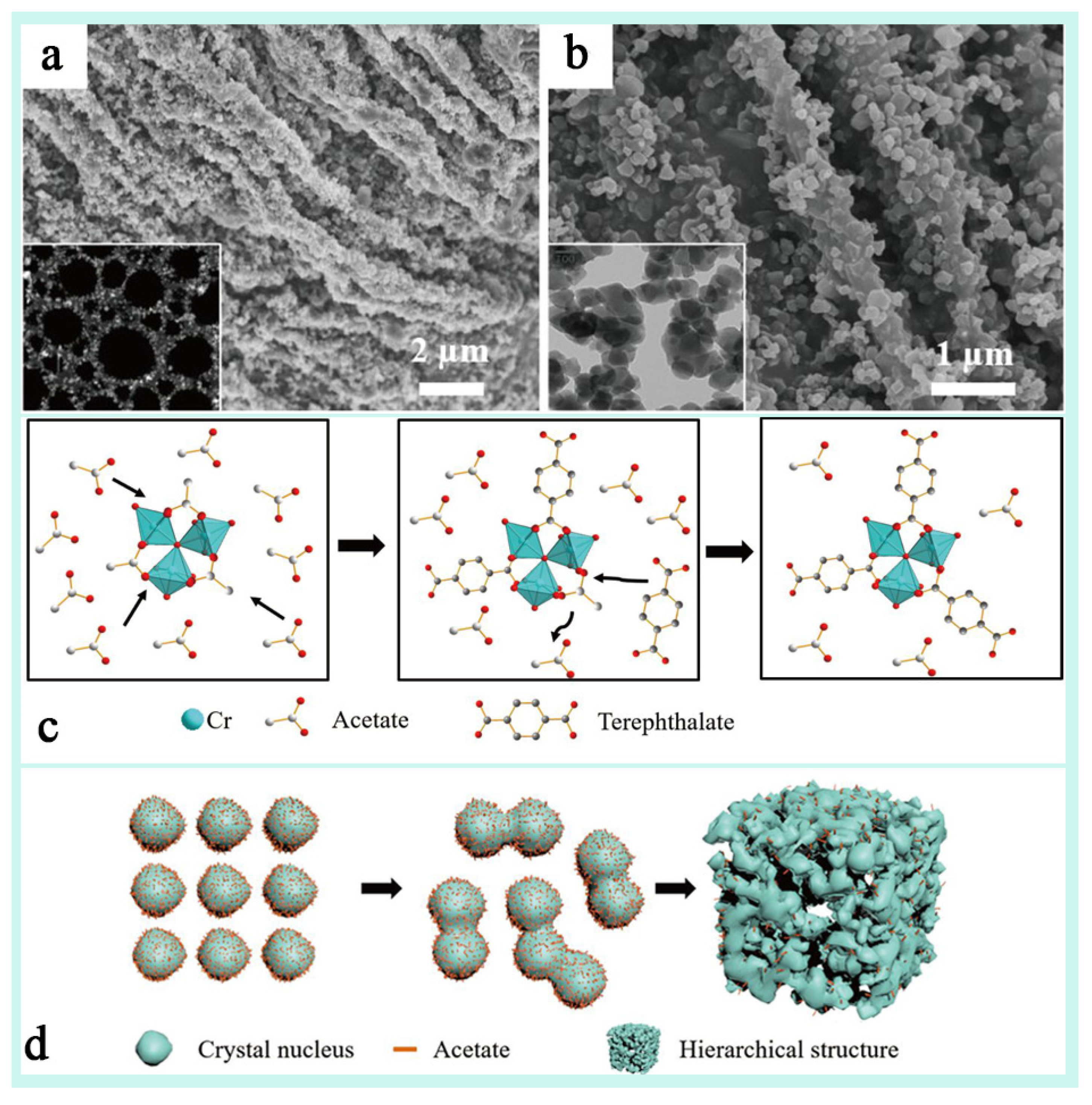
Different synthesis conditions (e.g., mineralizer, defective initiator, reaction temperature, etc.), crystal morphology (spherical vs. octahedral), and pore structure (mesoporous, microporous, etc.) have significantly affected the dye adsorption properties of MIL-101(Cr) [122,124,125]. Gökırmak Söğüt et al. [126] explored the effect of the ortho-octahedral MIL-101(Cr) and its spherical nano-MIL-101(Cr) adsorption properties and mechanisms in the removal of anionic dyes (Congo red, CR) and cationic dyes (thioflavin T, TFT) from aqueous solutions. This study showed that the effect of crystal morphology on the adsorption performance of the dyes was mainly in terms of the specific surface area, pore structure, adsorption kinetics, electrostatic interactions, and adsorption selectivity. MIL-101(Cr) with octahedral structure usually has higher adsorption capacity and stronger electrostatic interactions due to its larger pore size and pore depth but slower adsorption rate, whereas nano-MIL-101(Cr) with a spherical structure has faster adsorption rate and better reusability due to smaller particle size and better dispersion, but the adsorption capacity may be slightly lower. Therefore, in practical applications, a suitable crystal morphology of MIL-101(Cr) can be selected according to the nature of the target dyes and the treatment requirements, which can lead to the preparation of energy-efficient dye adsorbent materials. Zhao et al. [72] introduced a nano-HP-MIL-101(Cr) synthesized by a template-free method with a hierarchical porous structure of microporous to mesoporous to microporous structure (Figure 13a,b), and it was found that the hierarchical porous structure significantly enhanced the adsorption capacity and catalytic activity of HP-MIL-101(Cr) for macromolecular dyes. The crystal structure of HP-MIL-101(Cr) was found to be very similar to that of the original MIL-101(Cr) by characterization, and in addition, a columnar structure consisting of tightly aggregated nanocrystals of multiple morphologies was demonstrated (Figure 13c,d), which formed a macroscopic channel that was very conducive to adsorption. This study successfully validated the multifaceted tunability of MIL-101(Cr) and its great potential for development in the field of adsorption.
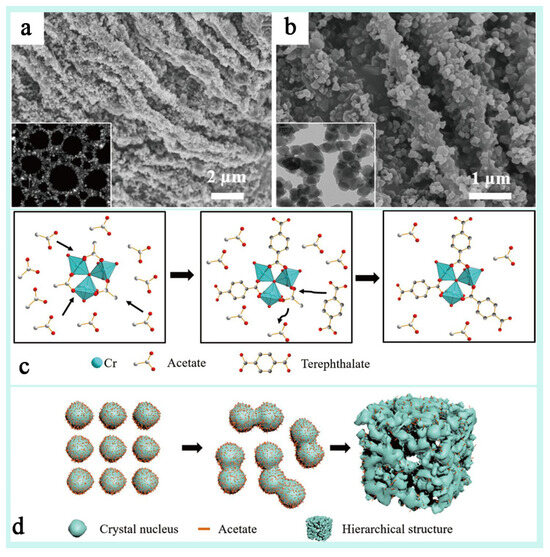
Figure 13.
(a,b) SEM and TEM (inset) images of (hierarchically porous) HP-MIL-101(Cr) under various magnifications. (c) A schematic illustration of the formation mechanism of HP-MIL-101(Cr) with monocarboxylic acid: the replacement of acetate with the terephthalate ligand. (d) The nanofusion procedure of the crystal nucleus [72]. Copyright Science China Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020.
4.3. Sensing Applications
MIL-101(Cr) has shown a wide range of potential applications in electrochemical, biological, immunological, and fluorescent sensors due to its unique structure and properties [127,128,129,130]. In humidity sensing, the high specific surface area and porosity enable MIL-101(Cr) to rapidly adsorb water molecules in the environment, which significantly increases the proton conduction path and improves the conductivity [131,132]. This property makes the sensor very sensitive to humidity changes and able to respond quickly under low-humidity conditions. Benseghir et al. [133] deposited electrically conductive MIL-101(Cr) complexed with poly(3,4-ethylenedioxythiophene) (PEDOT) on fork finger electrodes (IDEs) by an electric field-assisted spatially selective deposition method to enhance their conductivity and humidity sensing performance. The results show that electric field-assisted deposition is more conducive to maximizing the advantages of stability and hydrophilicity of MIL-101(Cr) as a sensor substrate and, at the same time, enables MIL-101(Cr) PEDOT to form oriented and aligned nanoparticle chains on the IDE, which, in turn, markedly improves the conductivity and adsorption area of the material. Together, these advantages make MIL-101(Cr) an ideal humidity sensing material, providing an important foundation for the development of high-performance MOF-based sensors.
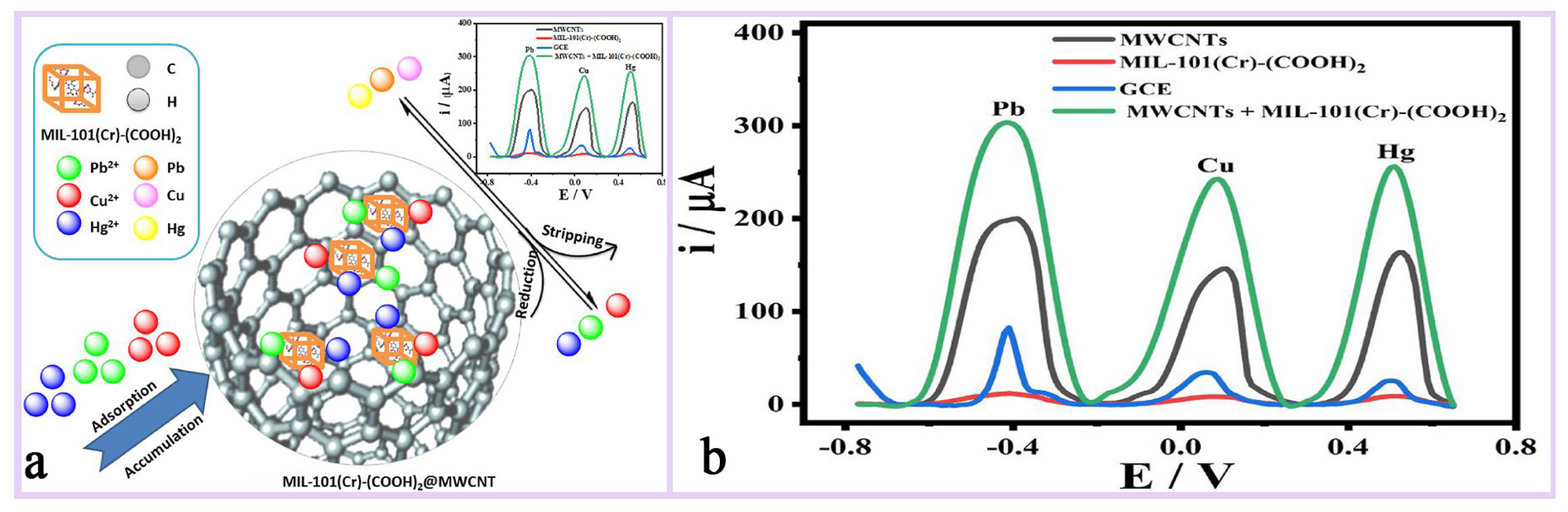
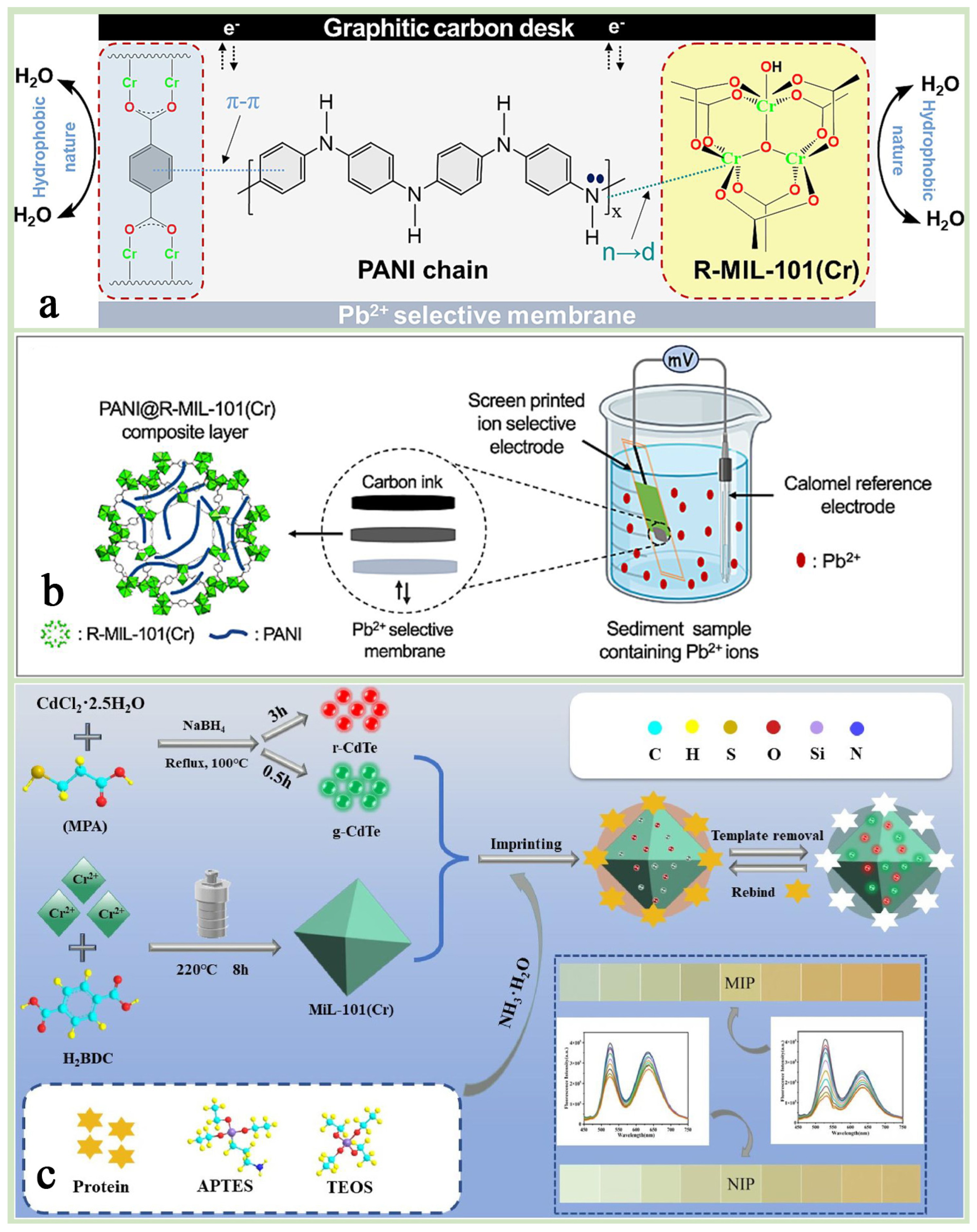
By adjusting its pore size and surface chemistry, MIL-101(Cr) can achieve selective recognition of specific ions or molecules and improve the selectivity of the sensor, such as its use for the detection of heavy metal ions (lead ion Pb2+, mercury ion Hg2+), biomolecules (e.g., glucose, DNA), etc., demonstrating high sensitivity and low detection limits [134,135]. Rafea et al. [135] designed a composite material MIL-101(Cr)-(COOH)2@MWCNTs based on MIL-101(Cr)-(COOH)2 and multi-walled carbon nanotubes (MWCNTs), which was used as a modified lead-glass carbon electrode (GCE) for the simultaneous detection of (Pb2+), copper (Cu2+), and mercury (Hg2+)) by an electrochemical sensor (Figure 14a,b). It was found that the sensor achieved high sensitivity for the detection of these heavy metal ions by differential pulsed anodic dissolution voltammetry (DPASV) and demonstrated good potential for application in a variety of real water samples. Keshta et al. [8] synthesized R-MIL-101(Cr) by recycling PET bottles and Cr6+ wastes and successfully prepared a high-performance ion-selective electrode for Pb2+ by compositing it with PANI. The PANI@R-MIL-101(Cr) composite material enables fast, sensitive, and stable Pb2+ detection through its efficient ion–electron conversion mechanism and excellent structural stability. Its long-life characteristics are mainly attributed to the high crystallinity and hydrophobicity of MIL-101(Cr) and the electrical conductivity of PANI, which together enable the sensor to maintain its high performance over a long period of time (Figure 15a,b).
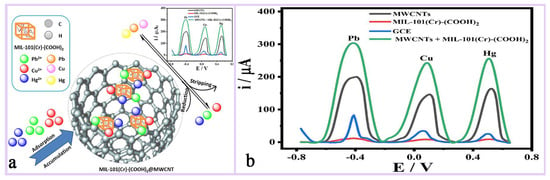
Figure 14.
(a) The working mechanism diagram of MIL-101(Cr)-(COOH)2. (b) DPASV curves for the stripping of 10 μM Pb, Cu and Hg deposited in 5 g/L HNO3. The deposition time was 60 s at −1.1 V. The curves show comparative study between MWCNTs, GCE, MOF, and MOF@MWCNTs [135]. Copyright 2024 Elsevier B.V.
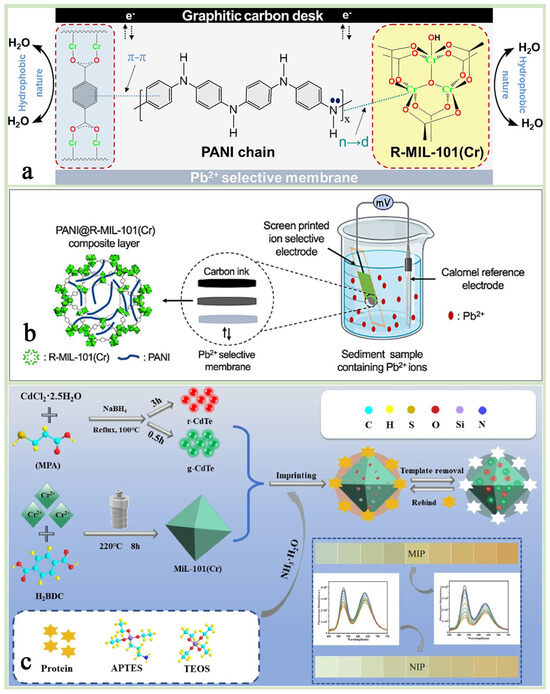
Figure 15.
(a) A schematic representation for the mechanism and intermolecular interactions between PANI chains and R-MIL-101(Cr) in the PANI@R-MIL-101(Cr) composite. (b) A schematic diagram of the sediments of the three ions in the sample species [8]. Copyright 2024 Elsevier B.V. (c) A schematic illustration for the syntheses process of g-CdTe/r-CdTe@MIL-101(Cr)@MIP [26]. Copyright 2023. Published by Elsevier B.V.
The excellent chemical stability and chemical compatibility of MIL-101(Cr) allow it to be stabilized in biological or chemical environments without inducing significant immune reactions. Moreover, MIL-101(Cr) prepared by green synthesis method exhibits low toxicity in the process of use compared to some other MOFs, so there is a great potential for the development in the fields of food, biological and chemical sensing, and detection [80,136,137]. Zhang et al. [138] successfully prepared a novel electrochemical biosensing material mediated by a MIL-101(Cr) molecular cage anchored on two-dimensional Ti3C2Tx MXene nanosheets for the detection of xanthine. The composite material combines the advantages of MXene’s high conductivity and MOF’s high specific surface area, which significantly enhances the performance of the sensor. This material not only has important application prospects in biomedical detection (e.g., disease diagnosis) and food industry detection (e.g., freshness detection of meat) but also provides a new design idea for the development of high-performance and low-cost electrochemical biosensors. Haghighi et al. [139] reported a MIL-101(Cr)-based quartz crystal microbalance sensor for the detection of volatile organic compounds (VOCs) at room temperature, especially toluene and o-xylene. The sensor utilized the high specific surface area, porosity, and π-π stacking interactions of MIL-101(Cr) to enhance the selectivity for aromatic hydrocarbons, and achieved high sensitivity, fast response, and low detection limit detection for toluene and o-xylene, which provided a new idea and methodology for the development of a new type of high-efficiency VOCs sensor.
In the field of fluorescent sensors, the porous structure of MIL-101(Cr) can adsorb fluorescent molecules or quantum dots to achieve fluorescence burst or enhancement through energy transfer or electron transfer mechanisms, thus realizing the detection of target molecules. Hu et al. [26] prepared a dual-emission fluorescent molecularly imprinted sensor (g-CdTe/r-CdTe@MIL-101(Cr)@MIP) using MIL-101(Cr) as a support material to enhance the imprinting sites, which combines the high specific surface area of MIL-101(Cr) and fluorescence properties of quantum dots to achieve high sensitivity and high protein selective detection (Figure 15c). In addition, the study innovatively integrated the sensor with a smartphone to realize portable and rapid detection in the field. This sensor, incorporating modern technology, provides a promising new approach for future on-site protein analysis, which is expected to be widely used in the fields of biomedicine, environmental monitoring, and food safety.
In addition to its excellent performance in the above sensing fields, MIL-101(Cr) also has great potential for further development in the field of immunosensors and drug carriers. MIL-101(Cr)’s high specific surface area, adjustable pore size, high stability, and reusability make it an excellent drug carrier sensor, which can immobilize the antigen or antibody molecules efficiently and enhance the binding efficiency and stability of antigens and antibodies, thus significantly improving the service life of the immunosensor and reducing the cost of detection. In addition, MIL-101(Cr) has been studied for the loading and slow release of drugs such as acetaminophen, progesterone, and ibuprofen, but there are limitations to its clinical application due to the presence of chromium in the framework [140,141,142,143,144].
4.4. Catalytic Applications
MIL-101(Cr) has a wide range of applications in a number of catalytic reactions due to its high porosity and unsaturated metal sites [43,97,145,146,147]. Tripathi et al. [27] synthesized MIL-101(Cr) catalyst by a simple hydrothermal method and utilized its large specific surface area, adjustable porosity, and easy separation from the reaction system to achieve the efficient synthesis of biphenyl and its derivatives. The regeneration ability of the catalyst is an important characteristic for its practical application, which can significantly reduce the cost in industrial production. The stability and porous structure of MIL-101(Cr) enabled the catalytic material to maintain good catalytic activity in five cycles, with yields of 97%, 95%, 93%, 92%, and 86%, respectively, which demonstrated high stability and reusability. The chromium ions (Cr3+) in pure MIL-101(Cr) can act as Lewis acidic sites to promote the breaking of carbon–halogen bonds in the reactants and provide active intermediates for subsequent reactions. It can also activate reactants by forming Lewis acid-base complexes with carbon atoms in the reactants (especially those adjacent to halogens).
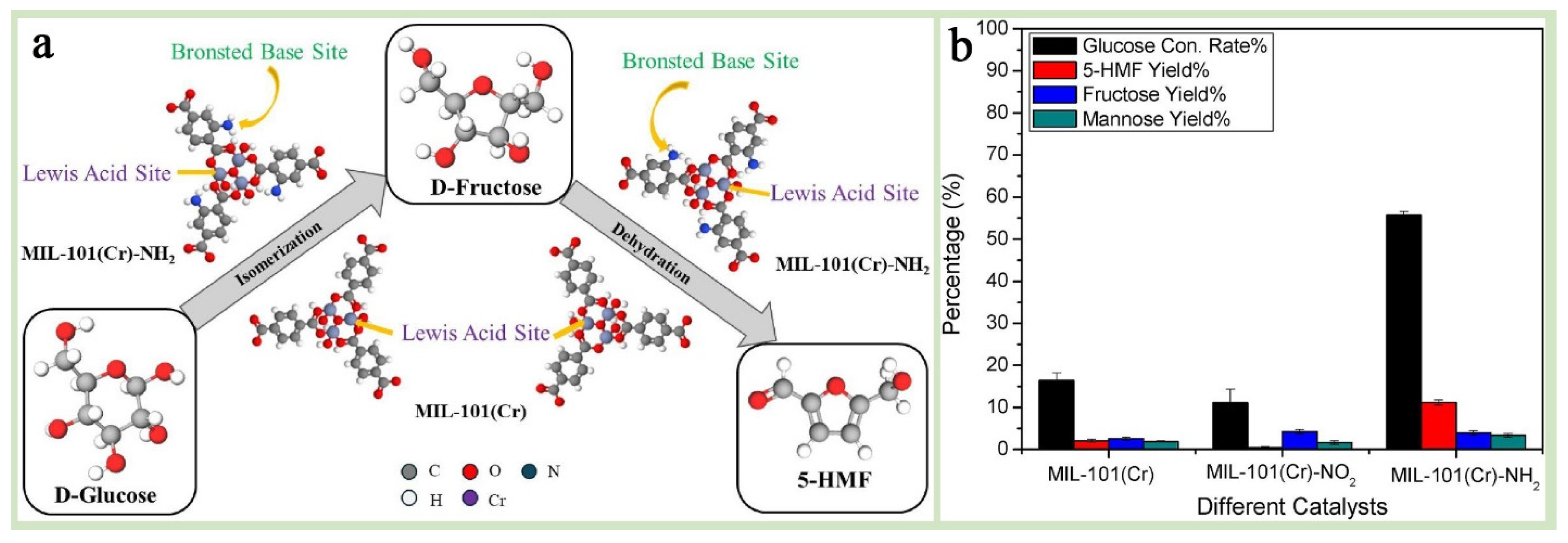
Benefiting from the above advantages, MIL-101(Cr) shows excellent application potential in catalyzing some specific reactants (e.g., aryl halides or p-hydroxy aryl halides, etc.), degrading organic pollutants, and catalyzing CO2 photoreduction. However, limited by the low intrinsic photogenerated carrier complex efficiency and electrical conductivity, their energy efficiency for single applications in most catalytic fields is poor [148]. Functionalization modification of its surface or preparation of advantageous and complementary functional heterocomplexes are the most widely used forms of MIL-101(Cr) in this field [43,91]. For example, in organic synthesis and electrocatalysis, modified MIL-101(Cr)-functionalized materials (e.g., MIL-101-SO3H, MIL-101(Cr)-NO2, and MIL-101(Cr)-NH2) exhibit excellent catalytic activities in reactions such as olefin oxidation, styrene oxidation, esterification, CO2 cycloaddition, hydroxyalkylation, electrolysis of water, and coupling [38,149,150,151,152]. Dharmapriya et al. [91] investigated the catalytic performance of two metal–organic framework (MOF) catalysts, MIL-101(Cr) and MIL-101(Cr)-NH2, in the dehydration of glucose to 5-hydroxymethylfurfural (5-HMF) and revealed the significant enhancement of the catalytic performance by the NH2 group, which provides a theoretical basis for the design of more efficient MIL-101(Cr) catalysts (Figure 16a,b). Zhang et al. [153] successfully introduced silver nanoparticles (Ag NPs) into the cage-like structure of MIL-101(Cr) using a “dual-solvent-photoreduction” strategy, in which hydrophobic n-hexane was used as the solvent instead of pure water. Through the advantageous complementary effect, the stability and fixed pores of MIL-101(Cr) effectively limited the growth and shedding of Ag NPs, while Ag NPs with excellent catalytic activity and a unique localized surface plasmon resonance effect perfectly compensated for the poor electrical conductivity of MIL-101(Cr). Using this hybridized material as an efficient photocatalyst, the efficiency of the nitrogen reduction reaction (PNRR) and the stability of the catalyst were significantly improved. This study not only confirms the development potential of MIL-101(Cr)-based hybrid materials in the field of catalysis but also successfully demonstrates the superiority of uniformly introducing functional materials into the interior of MIL-101(Cr) pores compared to the surface attachment strategy, providing strong inspiration for researchers to further optimize the synthesis details and schemes of MIL-101-based materials.
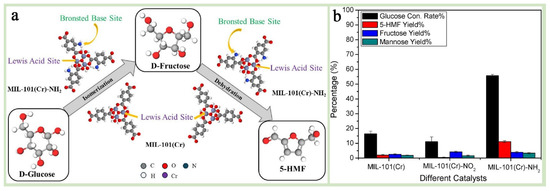
Figure 16.
(a) The proposed graphical summary of a reaction pathway for glucose dehydration into 5-HMF over MIL101(Cr) and MIL-101(Cr)-NH2. (b) The selection of the most suitable chromium-based MOFs for glucose dehydration among the three synthesized catalysts, namely, MIL-101(Cr), MIL-101(Cr)-NO2, and MIL-101(Cr)-NH2 [91]. Copyright 2024 Elsevier Inc.
4.5. Other Applications
Apart from separation and adsorption, drug delivery, detection and sensing [80,134], and catalysis, MIL-101(Cr) shows great potential for applications in other areas such as mixed-matrix membranes [154] and corrosion inhibitors [155]:
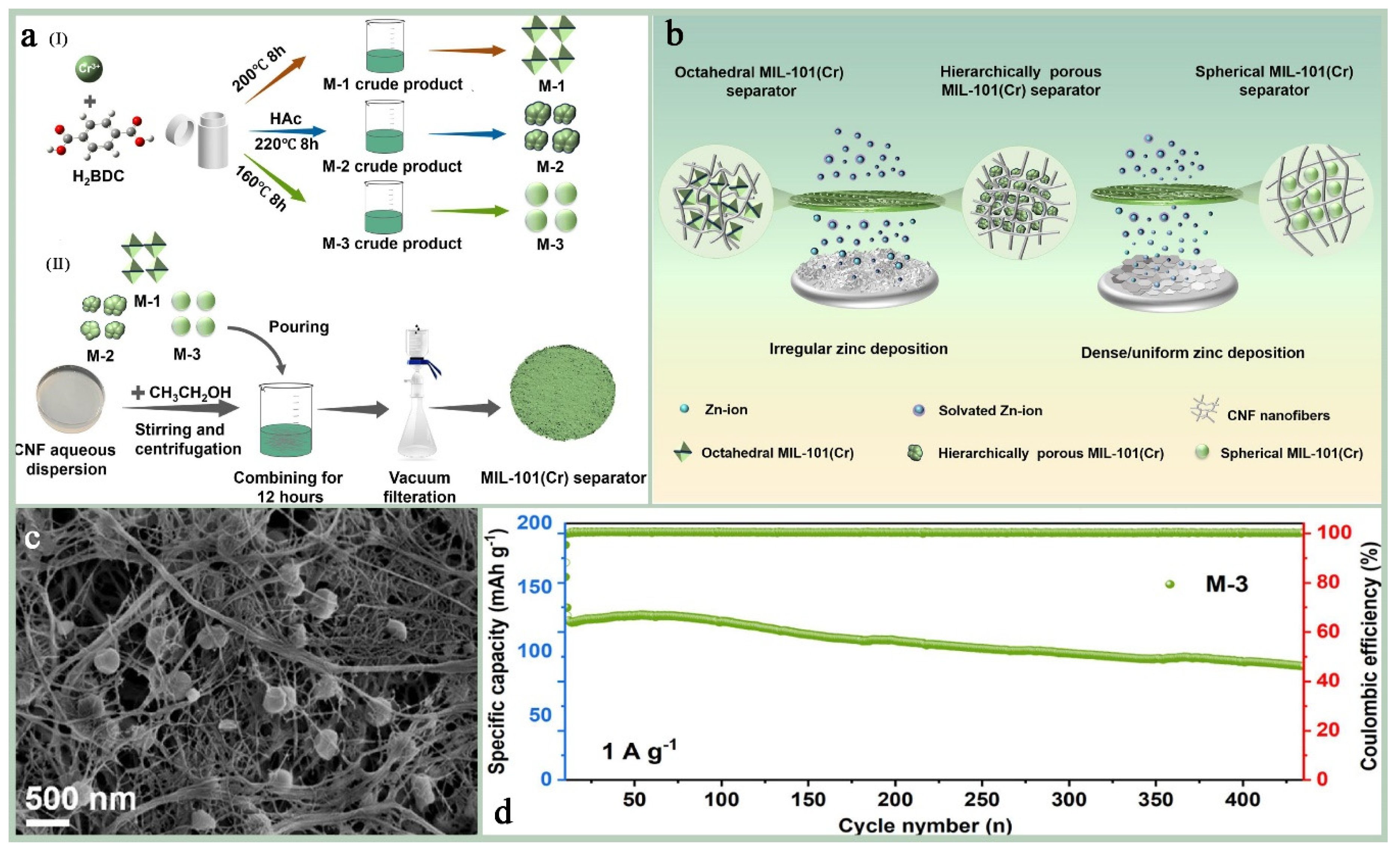
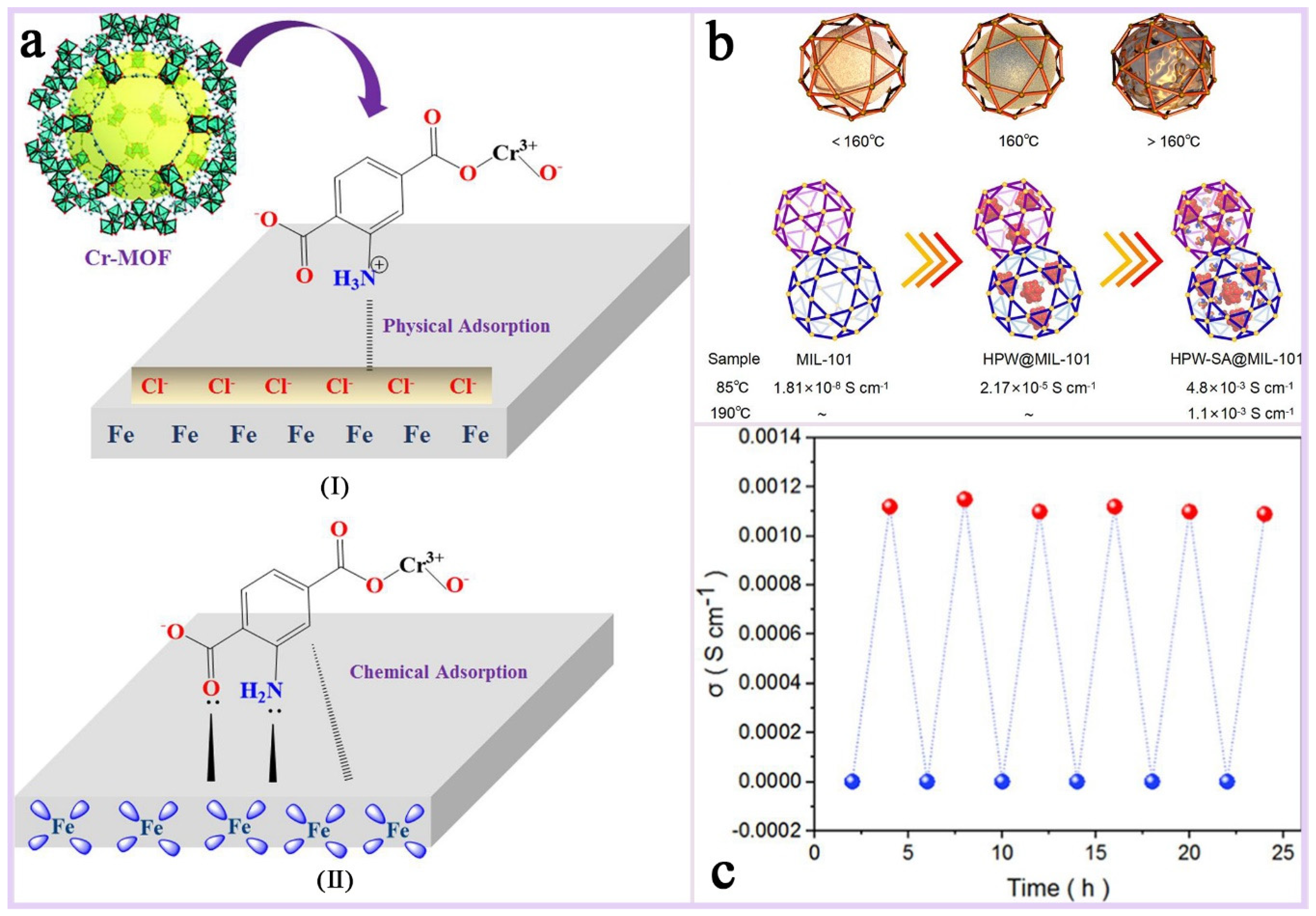
Mixed-matrix membranes (MMMs): MMMs are membranes made from a blend of two or more different materials, often combining the advantages of organic and inorganic materials. These membranes show great potential for applications in gas/liquid separation, catalytic carriers, electrochemical applications, environmental remediation, and pharmaceuticals. The performance of hybrid matrix membranes depends on the synergistic effect of its component materials, and the selectivity and permeability of the membrane can be optimized by careful design and selection of the materials to meet specific application requirements.MIL-101(Cr) has a very high specific surface area, which can provide a large number of active sites for hybrid matrix membranes [156]; its unique multi-microporous and mesoporous structure allows for its incorporation into polymer matrix as a dispersed phase conducive to improving the permeability and selectivity of the membrane [157]; most importantly, MIL-101(Cr) has excellent stability and mechanical strength, its most prominent advantage as a membrane substrate, which can give MMMs excellent environmental adaptability and resistance to chemical attack; and furthermore, MIL-101(Cr) and its functionalized materials show excellent electrical conductivity in high-temperature and high-humidity environments, and its plasmonic conductivity can reach 10−1 S cm−1 at specific temperatures, which is a plasmonic conductive substrate that has a large potential for applications in extreme environments [158,159,160]. Zhao et al. [5] investigated the effect of different-shape MIL-101(Cr) as a diaphragm material in aqueous zinc ion batteries (AZIBs) on the performance of the batteries (Figure 17a,b). The results show that the hybrid matrix membrane (MIL-101(Cr)@CNF) prepared by combining spherical MIL-101(Cr) with cellulose nanofibers (CNF) exhibits significant advantages in enhancing ionic conductivity, inhibiting the growth of zinc dendrites, and improving the cycling stability of the battery. The spherical MIL-101(Cr), i.e., M-3, was uniformly dispersed in CNF and filled the irregular voids between fibers (Figure 17c), and the ionic conductivity of this hybrid matrix membrane was as high as 7.50 mS/cm, which was significantly higher than that of other diaphragms. And in the Zn//MnO2 full-cell test, the M-3 diaphragm maintained 83.6% capacity retention after 400 cycles, with a Coulombic efficiency close to 100% (Figure 17d). Sun et al. [159] encapsulated polymetallic oxides (POM) and acid-base adducts (HPW-SA) in MIL-101(Cr) to improve their proton conductivity performance in high-temperature proton exchange membrane fuel cells. It was found that HPW-SA@MIL-101 exhibited excellent proton conductivity at high temperatures (160–190 °C). Its proton conductivity reached 1.1 × 10−3 S cm−1 at 190 °C, which is higher than most of the reported high-temperature proton-conducting materials (Figure 18b). And after six heating and cooling cycle tests, the proton conductivity performance of HPW-SA@MIL-101 did not decrease significantly, showing good reproducibility and stability (Figure 18c). Devautour-Vinot et al. [158] optimized the proton-conducting properties of mesoporous MIL-101(Cr) by introducing sulfonic acid groups and guest molecules (e.g., sulfuric acid) into it, and the results of the study showed that its performance was significantly enhanced under humidity conditions, even surpassing the best currently reported proton-conducting MOF materials.
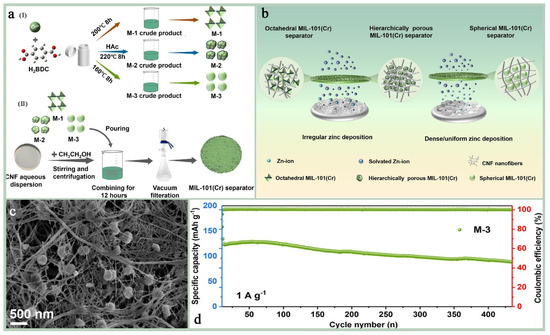
Figure 17.
(a) Schematic diagrams for the preparation of (I) MIL-101(Cr) with different morphologies; (II) MIL-101(Cr)@CNF separators. (b) A schematic diagram of zinc deposition on MIL-101(Cr)@CNF separators prepared by MIL-101(Cr) with different morphologies. (c) SEM images of spherical MIL-101(Cr)@CNF separators (d) Long-term cycling performance and Coulombic efficiency at a current density of 1 A g−1 [5]. Copyright 2025 Elsevier B.V.
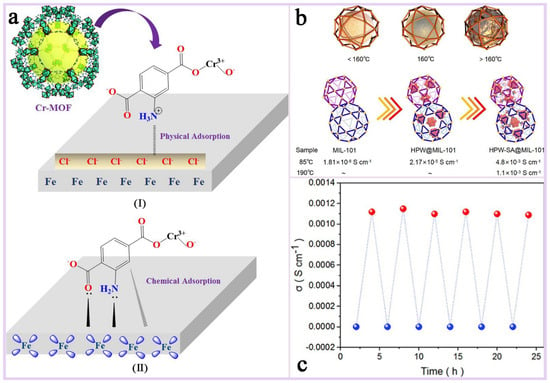
Figure 18.
(a) A schematic representation of adsorption types (I) physical and (II) chemical of Cr(III)-MOF on the MS surface [92]. Copyright © 2022, Emerald Publishing Limited. (b) A schematic diagram of the microscopic phase transition of HPW-SA@MIL-101(when the temperature rises to 160 °C, HPW-SA undergoes a phase change inside the MIL-101 Frame, and as the temperature continues to increase, the fluidity of liquid HPW-SA will increase) and the comparison of the conductivities of MIL-101, HPW@MIL-101, and HPW-SA@MIL-101. (c) HPW-SA@MIL-101’s anhydrous proton conductivity diagram of heating and cooling (red: 190 °C, blue: 25 °C) [159]. Copyright 2022 American Chemical Society.
Environmentally friendly corrosion inhibitors: Carbon steel (MS) is widely used in petrochemical and other fields due to its low cost and high strength, but it is prone to corrosion in acidic environments. In order to extend its service life, researchers have developed a variety of organic corrosion inhibitors, which usually contain heteroatoms such as oxygen, sulfur, nitrogen, etc., that are able to form a protective layer through charge interactions with the metal surface. MOF is considered to be an environmentally friendly corrosion inhibitor material with great potential for development due to its porous nature and high specific surface area [161,162,163]. MIL-101(Cr(III)) stands out among many MOFs due to its excellent thermal and chemical stability, as well as rich functionalizable properties, and shows a great application value in the field of corrosion prevention [164,165]. Hassan et al. [92] investigated the performance of aminated MIL-101(Cr(III)) as a corrosion inhibitor on MS in 1 M hydrochloric acid solution (Figure 18a). The characterization revealed that the aromatic rings (π-electrons) and the lone pair electrons of heteroatoms (e.g., nitrogen atoms) in the molecule of MIL-101(Cr(III)) could interact with the iron atoms on the surface of the carbon steel to form chemical bonds. This chemisorption effectively enhances the adsorption strength of Cr(III)-MOF on the metal surface, thus improving the corrosion inhibition effect. In addition, the amino functional group in Cr(III)-MOF is protonated in an acidic environment, and the positively charged protonated molecules formed can interact with negatively charged chloride ions (Cl−) through electrostatic force and adsorb on the carbon steel surface, forming a physical barrier to prevent corrosive mediums (e.g., H+ and Cl−) from coming into contact with the metal surface. The synergistic effect of physical and chemical adsorption allows MIL-101(Cr(III)) to exhibit highly effective corrosion inhibition, which is especially prominent in acidic environments.

Table 2.
Summary of MIL-101(Cr) applications.
Table 2.
Summary of MIL-101(Cr) applications.
| Areas of Application | Specific Application | Manifestations | Refs. |
|---|---|---|---|
| Gas adsorption and separation | Adsorption and separation of gases such as CO2, H2, CH4, NH2, Toluene, etc. | The high specific surface area (4100 m2/g), rich pore structure, and excellent stability make it have an excellent adsorption effect on many gases, especially CO2; its adsorption capacity can reach 40 mmol/g, which is 37% higher than that of the commonly used zeolite adsorbent material. | [83,113,166,167,168] |
| Dye adsorption and wastewater purification | Wastewater purification, dye adsorption treatment for anionic dyes (e.g., Activated Red 2, Activated Blue 19, Acid Blue 92, etc.) and cationic dyes (thioflavin). | Abundant pore channels make its adsorption capacity high: Activated Red 2 (662.87 mg/g), Activated Blue 19 (863.67 mg/g); outstanding chemical stability makes its cycle regeneration ability strong, and the regeneration rate is as high as 90% after many cycles. | [116,117,118,119,120,121,169] |
| Energy applications | Catalyzer (photocatalytic degradation of organic pollutants, CO2 photoreduction, photocatalysis, electrocatalysis, catalysis of organic synthesis), electrode material (batteries, capacitors) | The high porosity and unsaturated metal sites enable it to perform well in catalytic reactions; the functionalized modified materials (e.g., MIL-101(Cr)-SO3H, MIL-101(Cr)-NH2) exhibit excellent catalytic activity in a variety of organic synthesis reactions; and the composites with the functional materials can be used to improve the photo- and electrocatalytic properties. | [43,97,145,146,147,170,171,172,173] |
| Sensing | Humidity sensors, electrochemical sensors, biosensors, immunosensors, fluorescent sensors | It has been widely used in the field of sensing, covering a wide range of areas; its excellent chemical and thermal stability makes it mostly used in formaldehyde detection, microcystin LR detection, 2,4-dinitrophenol detection, etc., demonstrating good sensing performance and selectivity. | [26,127,128,129,130,131,133,134,135,174] |
| Drug delivery | Loading and extended release of acetaminophen, progesterone, ibuprofen, and other drugs | High specific surface area and adjustable pore size make it an excellent drug carrier; excellent chemical stability and biocompatibility make it very promising for biomedical applications; however, clinical applications are limited by the presence of chromium. | [140,141,142,143,144,174] |
| Proton conductivity | Plasmonic conductive materials for high-temperature and high-humidity environments | Plasmonic conductivity can reach 10−1 S/cm at specific temperatures; its conductivity and stability can be further optimized by chemical impregnation modification or composite modification. | [175] |
| Mixed matrix membrane | Improvement of membrane permeability and selectivity, treatment of water contamination | Its excellent chemical stability and porosity make it an important substrate for membranes in various fields; compounding with PVDF, Matrimid, and other polymers can significantly improve the mechanical strength and gas separation performance of membranes. | [156,157,158,159,160] |
| Environmentally friendly corrosion inhibitor | Forms a protective layer on the surface of carbon steel and other corrosion-prone substrates to achieve corrosion inhibition | The aromatic ring (π-electron) and the lone pair of electrons of heteroatoms (e.g., nitrogen atoms) in MIL-101(Cr) can interact with the iron atoms on the surface of carbon steel to effectively play a corrosion inhibition effect; a rich variety of modification and modification means can effectively adjust the chemical properties of the surface of MIL-101(Cr) to realize highly efficient directional corrosion protection. | [92,164,165] |
5. Conclusions and Outlook
This review systematically examines the synthetic methods, performance optimization strategies, and application progress of metal–organic framework material MIL-101(Cr) in various fields. MIL-101(Cr), with its ultra-high specific surface area, excellent pore structure, superior stability, and unsaturated Lewis acidic sites, has demonstrated great potential for applications in gas adsorption and separation, catalysis, dye removal, drug delivery, and so on. In this paper, we summarize the various synthesis techniques for MIL-101(Cr), including the conventional hydrothermal method, microwave-assisted hydrothermal method, template method, solvothermal method, and mechanochemical synthesis method. Each of these five methods has its own advantages and disadvantages, and has significant effects on the morphology, specific surface area, and pore structure of the material [176,177,178]. According to the specific application requirements as well as the experimental equipment and conditions, the selection of appropriate synthesis methods is the key to realizing the innovation of MIL-101(Cr) research and application. By adjusting the additives, component ratios, and reaction conditions, researchers have successfully realized the precise regulation of MIL-101(Cr) particle size, morphology, and pore structure. Furthermore, with the continuous innovation and advancement of physical modification and chemical modification strategies, the energy efficiency, potential,l and scope of application of MIL-101(Cr)-based materials are expected to be further expanded. In the future, MIL-101(Cr) is expected to become an excellent base material for application innovation in many fields and to promote the breakthrough and development of related technologies.
Although MIL-101(Cr) shows many excellent properties, its large-scale industrial synthesis and application still face many challenges: (1) Chromium (Cr) may have certain toxicity and potential carcinogenicity in specific oxidation states and exposure environments, which can pose certain dangers to ecosystems and human health. In the future, we should further explore the long-term toxicity effects of MIL-101(Cr) in different environments and biological systems and optimize its preparation process to reduce potential toxicity. (2) Additives commonly used in the synthesis process (e.g., HF, HCl, HNO3, etc.) not only burden the environment but also increase the cost of large-scale production, so the development of greener, more environmentally friendly, and more efficient synthesis methods will be the focus of the research to promote the scale-up synthesis of MIL-101(Cr). (3) MIL-101(Cr) production costs determine the development prospects of its large-scale production, and its production costs are mainly composed of raw materials, solvents, energy consumption, and equipment investment. It has been shown that the cost of raw materials accounts for the highest proportion of the total cost, so the selection of low-cost raw material components is the key to cost reduction. With the use of biological derivative ligands instead of conventional organic ligands, the long-term cost is expected to drop to less than USD 10 per kilogram. Selection of novel synthesis methods, such as microwave and mechanochemical methods, to simplify the process and reduce the use of solvents can also further reduce production costs. (4) Nevertheless, MIL-101(Cr) has a high specific surface area and abundant pore channels, but it is still challenging to precisely regulate the selective permeability and cycling efficiency of its pores. For example, the pore size may restrict the transport of certain molecules, and achieving such regulation requires the design of a large number of controlled experiments, which is a time-consuming and laborious process. Furthermore, due to the inherent defects, the efficiency of MIL-101(Cr) in applications such as photocatalysis and electrocatalysis still needs to be improved, so optimizing the existing strategies and developing novel ones are also the key exploratory directions for realizing large-scale synthesis and applications of MIL-101(Cr) in the future.
In conclusion, in response to the above challenges, future research should focus on the following directions: first, innovative synthesis techniques to develop more environmentally friendly and low-cost synthesis processes; second, optimization of performance strategies to enhance the efficiency of its application in the fields of catalysis and separation through structural design and functionalization modification; and third, the development of novel composites to make up for the deficiencies of MIL-101(Cr) by combining the advantages of other materials. The potential value of MIL-101(Cr) in the fields of environmental protection, wastewater treatment, green catalysis, drug delivery, and sustainable development has yet to be explored in depth, and promoting its transformation from laboratory research to practical and commercial applications is a key research direction in the future. This research direction is not only of practical significance but also highly prospective, and is expected to bring breakthroughs in related fields.
Author Contributions
Conceptualization, T.Z. and Y.C.; resources, T.Z. and Y.C.; writing—original draft preparation, J.C. and M.T.; writing—review and editing, J.C., S.N. and P.X.; supervision, T.Z. and Y.C.; funding acquisition, T.Z. and Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the National Natural Science Foundation of China (52073086, 51802094), the Science and Technology Innovation Program of Hunan Province (2023RC1070), the Natural Science Foundation of Hunan Province (2024JJ7164, 2023JJ60447), the Postgraduate Scientific Research Innovation Project of Hunan Province (CX20240908), and Scientific Research and Innovation Foundation of Hunan University of Technology (CX2413).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dapaah, M.F.; Niu, Q.; Yu, Y.-y.; Jia, H.; Cheng, L. Tunning MIL-101(Cr) MOF polymorphism towards efficient adsorption and localized Fenton-like degradation of para-nitroaniline by Fe incorporation. Chem. Eng. J. 2024, 486, 150162. [Google Scholar] [CrossRef]
- Ege, F.; Pahinkar, D.G. Experimental investigation of water adsorption in MIL-101(Cr)-coated microchannels. Chem. Eng. J. 2023, 472, 144960. [Google Scholar] [CrossRef]
- Liang, X.; Deliormanlı, A.M.; Zeng, Q. Poly(ionic liquids-acrylic acid)-modified MIL-101(Cr) metal−organic frameworks: Preparation and efficient adsorption of europium. J. Rare Earths, 2024; In Press. [Google Scholar] [CrossRef]
- Rico-Barragán, A.A.; Álvarez, J.R.; Ovando-Medina, V.M.; Karina Rojas-Mayorga, C.; Alejandro Aguayo-Villarreal, I.; Luna-Triguero, A.; Dávila-Guzmán, N.E. Unraveling the toluene adsorption mechanism of MIL-101(Cr) derived from PET waste for COV removal applications. Mater. Today Sustain. 2024, 27, 100925. [Google Scholar] [CrossRef]
- Zhao, T.; Nie, S.; Xiao, P.; Peng, S.; Chen, J.; Yu, J.; Luo, F.; Chen, Y. Exploring the influence of MIL-101(Cr) morphologies on the efficacy of cellulose separators for zinc ion battery performance. J. Membr. Sci. 2025, 720, 123788. [Google Scholar] [CrossRef]
- Fakhraie, S.; Rajabi, H.R.; Ghasemy, E.; Rashidi, A.; Orooji, Y.; Hadizadeh, M.H.; Maklavany, D. Exceptional CO2 and H2S adsorption by tuning micro/mesopore ratios with embedded graphene oxide/N-doped carbon quantum dots in MIL-101(Cr): Experimental and computational insights. J. Colloid Interface Sci. 2025, 683, 769–783. [Google Scholar] [CrossRef]
- Yang, K.; Cheng, S.; Yao, Z.; Li, S.; Yang, Y. Amine-grafted MIL-101(Cr) and Cu-BTC via post-synthetic modification for synergistic enhancement of CO2 uptake. Solid State Sci. 2024, 153, 107572. [Google Scholar] [CrossRef]
- Keshta, B.E.; Yu, H.; Wang, L.; Uddin, M.A.; El-Attar, H.G.; Keshta, A.E.; Gemeay, A.H.; Hassan, F.; Eid, S.M. Cost-effective synthesis of MIL-101(Cr) from recyclable wastes and composite with polyaniline as an ion-to-electron transducer for potentiometric Pb2+ sensing. Chem. Eng. J. 2024, 485, 150049. [Google Scholar] [CrossRef]
- Li, J.; Sun, Q.; Jambal, T.; Dorjgotov, D.; Su, N.; Wu, Q.; Jirimutu. Rapid identification of adulterated camel meat by MIL-101(Cr)-based fluorescent sensing platform: Surface potential adjustment to optimize detection performance. Microchem. J. 2024, 200, 110476. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, J.; Tian, D.; Su, W.; Liu, C.; Zhang, J.; Xie, H.; Lu, M. Adsorption behavior and mechanism of NH2-MIL-101(Cr)@COFs@SA composite adsorbent for tetracycline removal. Polymer 2024, 312, 127631. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh Porosity in Metal-Organic Frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Liu, C.; Luo, X.; Wu, C.; Zheng, Y.; Zhuo, G.; Zhen, G. Potential application of room temperature synthesized MIL-100(Fe) in enhancing methane production in microbial electrolysis Cells-Anaerobic digestion treating Protein-Rich wastewater. Chem. Eng. J. 2024, 500, 156904. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Yu, X.; Shen, X.; Xu, L.; Zhang, Y.; Shi, J.; Gan, L. Alginate/MIL-88B(Fe) derived magnetic biochar bead for non-radical degradation of organic pollutant with low peroxymonosulfate consumption. Chem. Eng. J. 2024, 502, 157892. [Google Scholar] [CrossRef]
- Huang, L.; Guo, R.; Mao, Y.; Xu, Z.; Chi, Y. In situ encapsulation of capsaicinoids in MIL-88A as a food-grade nanopreservative for meat safety. Food Chem. 2024, 460, 140738. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, T.; Sun, Z. Citric acid-induced defective MIL-53(Fe/Ce) for efficient amoxicillin degradation in electro-Fenton system. Sep. Purif. Technol. 2025, 363, 131993. [Google Scholar] [CrossRef]
- Ni, W.; Zhang, S.; Cheng, L.; Luo, T.; Yang, H.; Duan, T.; Yan, D. Construction of MIL-88(Fe) derived MOF/TiO2 photoanode for efficient photoelectrochemical water splitting. J. Environ. Chem. Eng. 2024, 12, 113683. [Google Scholar] [CrossRef]
- Chen, G.; Song, R.; Wang, H.; Huang, L.; Wang, L.; Lei, J. Construction of hierarchical porous MIL-100(Fe) for horseradish peroxidase immobilization and its degradation performance of bisphenol A. J. Clean. Prod. 2024, 481, 144178. [Google Scholar] [CrossRef]
- Li, Z.; Lu, J.; Pan, R.; Fu, Q.; Zhang, T.-Y.; Xu, B. Band gap regulation of MIL-101(Fe) via pyrazine-based ligands substitution for enhanced visible-light adsorption and its photo-Fenton-like application. J. Environ. Sci. 2024, 155, 762–772. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, J.; Guo, K.; Zhang, L.; Du, G.; You, H.; Huang, J.; Tu, M.; Li, H.; Peng, Y.; et al. Deciphering the role of ultra-low-loaded rhodium in NiFe-MIL-53 for superior oxygen evolution reaction. J. Energy Chem. 2025, 100, 77–86. [Google Scholar] [CrossRef]
- Zhaochun, Y.; JiaCheng, Y.; Wen, Z.; Wenhui, Y.; Jianyu, G.; Li, L.; Hong, X. The preparation and adsorption performance of Co-doped MIL-101(Cr) for low-concentration C3F8. Chem. Eng. Sci. 2023, 282, 119302. [Google Scholar] [CrossRef]
- Xi, F.-G.; Dong, Z.-Y.; Li, Y.; Lu, Y.-S.; Pan, C.-X.; Liu, C.-Q.; Qiao, Y.-S. Preparation of isoreticular MIL-101(Cr) based on pre- and post-synthetic strategy and study for carbon dioxide capture. J. Mol. Struct. 2025, 1321, 139802. [Google Scholar] [CrossRef]
- Takhvar, A.; Akbari, S.; Souri, E.; Ahmadkhaniha, R.; Morsali, A.; Khoshayand, M.R.; Amini, M.; Taheri, A. Metal–Organic Frameworks MIL-101(Fe) and MIL-53(Al) as Efficient Adsorbents for Dispersive Micro-Solid-Phase Extraction of Sorafenib in Plasma and Wastewater, Coupled with HPLC-UV Analysis. J. Chromatogr. Sci. 2024, 63, bmaf003. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.-Z.; Xiong, X.-H.; Wang, S.-H.; Zhang, L.; Meng, L.-L.; Yan, L.; Fan, Y.-N.; Yan, T.-A.; Liu, D.-H.; Wei, Z.-W.; et al. MIL-101-Cr/Fe/Fe-NH2 for Efficient Separation of CH4 and C3H8 from Simulated Natural Gas. ACS Appl. Mater. Interfaces 2022, 14, 45444–45450. [Google Scholar] [CrossRef]
- Mahdipoor, H.R.; Ebrahimi, R.; Ganji Babakhani, E.; Halladj, R.; Safari, N.; Ganji, H. Investigating the selective adsorption of CO2 by MIL-101(Cr)-NH2 and modeling the equilibrium data using a new three-parameter isotherm. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 131971. [Google Scholar] [CrossRef]
- Yang, X.; Arami-Niya, A.; Lyu, J.; Guo, X. Net, Excess, and Absolute Adsorption of N2, CH4, and CO2 on Metal–Organic Frameworks of ZIF-8, MIL-101(Cr), and UiO-66 at 282–361 K and up to 12 MPa. J. Chem. Eng. Data 2021, 66, 404–414. [Google Scholar] [CrossRef]
- Hu, W.; Feng, S.; Pei, F.; Du, B.; Liu, B.; Mu, X.; Tong, Z. A novel smartphone-integrated binary-emission molecularly imprinted fluorescence sensor embedded with MIL-101(Cr) for sensitive and real-time detection of protein. Talanta 2023, 260, 124563. [Google Scholar] [CrossRef]
- Tripathi, J.; Gupta, M.; Yadav, A.; Waghmode, K.; More, P. Metal–organic framework MIL-101(Cr): An efficient catalyst for the synthesis of biphenyls and biphenyl diols. Res. Chem. Intermed. 2024, 50, 1645–1660. [Google Scholar] [CrossRef]
- Zou, M.; Dong, M.; Zhao, T. Advances in Metal-Organic Frameworks MIL-101(Cr). Int. J. Mol. Sci. 2022, 23, 9396. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Kong, Y.; Wang, F. Hydrate-based adsorption-hydration hybrid approach enhances methane storage in wet MIL-101(Cr)@AC under mild condition. Chem. Eng. J. 2023, 472, 145068. [Google Scholar] [CrossRef]
- Jiménez-Laines, G.; Flores, E.; García-Peña, N.G.; Chan-Espinoza, J.A.; Alvarado-Gil, J.J.; Rodríguez-Gattorno, G. Double step heating synthesis of MIL-101(Cr) composites for water harvesting applications. Microporous Mesoporous Mater. 2023, 362, 112782. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, R.; Zhou, Y.; Ma, W.; Fan, J.; Deng, X.; Zhu, C.; Yu, A.; Xu, G.; Li, D.-S.; et al. Sulfonate-functionalized MIL-101 with high lithium adsorption capacity and selectivity. Mater. Today Energy 2025, 49, 101817. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Qin, F.; Song, Z.; Liu, W. Synthesis of monodisperse spherical nano alumina by hydrothermal method and its mechanism study. Ceram. Int. 2024, 50, 39467–39474. [Google Scholar] [CrossRef]
- Franco, A.; Balestra, S.R.G.; Hamad, S.; Carrillo-Carrión, C. Full potential of microwave-assisted processes: From synthesis of high-quality MIL-101(Fe) catalyst to furfural valorization. Appl. Mater. Today 2024, 39, 102266. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, L.; Wang, J.; Gu, X.; Yu, H.; Huang, X.; Zhao, H.; Yang, J.; Gao, X.; Lin, Y. A rGO/Cu0.7Fe0.3S2 composite electrode for asymmetric supercapacitor prepared via MIL-101 template strategy. Diam. Relat. Mater. 2023, 140, 110508. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, R.-w.; Yan, G.-y.; Liang, Z.-y.; Hu, W.-n.; Xia, Y.-z.; Huang, R.-k. Solvothermal synthesis of TiO2@MIL-101(Cr) for efficient photocatalytic fuel denitrification. J. Fuel Chem. Technol. 2022, 50, 456–463. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, J.; Zhang, P.; Dai, S. Mechanochemical synthesis of metal–organic frameworks. Polyhedron 2019, 162, 59–64. [Google Scholar] [CrossRef]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Mechanochemistry: Toward green synthesis of metal–organic frameworks. Mater. Today 2021, 46, 109–124. [Google Scholar] [CrossRef]
- Leng, K.; Sun, Y.; Li, X.; Sun, S.; Xu, W. Rapid Synthesis of Metal–Organic Frameworks MIL-101(Cr) Without the Addition of Solvent and Hydrofluoric Acid. Cryst. Growth Des. 2016, 16, 1168–1171. [Google Scholar] [CrossRef]
- Ferey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surble, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Pan, T.-t.; Wang, Y.-q.; Liu, F.; Liu, C.-s.; Li, W.-x. Stable Metal-Organic Frameworks based mixed tetramethylammonium hydroxide for toluene adsorption. J. Solid State Chem. 2022, 306, 122732. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Q.; Wang, Z.; Wu, T.; Zhang, M.J.M.; Materials, M. Synthesis of MIL-101(Cr) and its water adsorption performance. Microporous Mesoporous Mater. 2020, 297, 110044. [Google Scholar] [CrossRef]
- Wee, M.G.V.; Chinnappan, A.; Ramakrishna, S. Elucidating Improvements to MIL-101(Cr)’s Porosity and Particle Size Distributions based on Innovations and Fine-Tuning in Synthesis Procedures. Adv. Mater. Interfaces 2023, 10, 2300065. [Google Scholar] [CrossRef]
- Yang, M.; Bao, Y.-S.; Zhou, M.-L.; Wang, S.; Cui, Y.-H.; Liu, W.; Li, L.-C.; Meng, L.-X.; Zhang, Y.-Y.; Han, Z.-B. An Efficient Bifunctional Core–Shell MIL-101(Cr)@MOF-867 Composite to Catalyze Deacetalization–Knoevenagel Tandem Reaction. Catal. Lett. 2023, 153, 3561–3568. [Google Scholar] [CrossRef]
- Fakhraie, S.; Rajabi, H.R.; Rashidi, A. Fabrication and application of novel core–shell MIL-101(Cr)@UiO-66(Zr) nanocrystals for highly selective separation of H2S and CO2. Chem. Eng. J. 2023, 452, 139001. [Google Scholar] [CrossRef]
- Khan, N.A.; Kang, I.J.; Seok, H.Y.; Jhung, S.H. Facile synthesis of nano-sized metal-organic frameworks, chromium-benzenedicarboxylate, MIL-101. Chem. Eng. J. 2011, 166, 1152–1157. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Huang, S.; Xia, Q.; Li, Z. Adsorption and Diffusion of Benzene on Chromium-Based Metal Organic Framework MIL-101 Synthesized by Microwave Irradiation. Ind. Eng. Chem. Res. 2011, 50, 2254–2261. [Google Scholar] [CrossRef]
- Uma, K.; Pan, G.-T.; Yang, T.C.-K. The Preparation of Porous Sol-Gel Silica with Metal Organic Framework MIL-101(Cr) by Microwave-Assisted Hydrothermal Method for Adsorption Chillers. Materials 2017, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-R.; Kim, J.; Ahn, W.-S. Synthesis of metal-organic frameworks: A mini review. Korean J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Soltanolkottabi, F.; Talaie, M.R.; Aghamiri, S.; Tangestaninejad, S. Introducing a dual-step procedure comprising microwave and electrical heating stages for the morphology-controlled synthesis of chromium-benzene dicarboxylate, MIL-101(Cr), applicable for CO2 adsorption. J. Environ. Manag. 2019, 250, 109416. [Google Scholar] [CrossRef]
- Yin, B.; Sun, L.; Tang, S.; Zhou, H. Preparation of Metal–Organic Framework/Polyvinylidene Fluoride Mixed Matrix Membranes for Water Treatment. Ind. Eng. Chem. Res. 2020, 59, 19689–19697. [Google Scholar] [CrossRef]
- Carretero-Cerdán, A.; Carrasco, S.; Sanz-Marco, A.; Jaworski, A.; Martín-Matute, B. One-step microwave-assisted synthesis of amino-functionalized chromium(III) terephthalate MIL-101-NH2. Mater. Today Chem. 2023, 31, 101618. [Google Scholar] [CrossRef]
- Huang, X.-X.; Qiu, L.-G.; Zhang, W.; Yuan, Y.-P.; Jiang, X.; Xie, A.-J.; Shen, Y.-H.; Zhu, J.-F. Hierarchically mesostructured MIL-101 metal–organic frameworks: Supramolecular template-directed synthesis and accelerated adsorption kinetics for dye removal. CrystEngComm 2012, 14, 1613–1617. [Google Scholar] [CrossRef]
- Chen, C.; Feng, N.; Guo, Q.; Li, Z.; Li, X.; Ding, J.; Wang, L.; Wan, H.; Guan, G. Template-directed fabrication of MIL-101(Cr)/mesoporous silica composite: Layer-packed structure and enhanced performance for CO2 capture. J. Colloid Interface Sci. 2018, 513, 891–902. [Google Scholar] [CrossRef]
- Yang, L.-T.; Qiu, L.-G.; Hu, S.-M.; Jiang, X.; Xie, A.-J.; Shen, Y.-H. Rapid hydrothermal synthesis of MIL-101(Cr) metal–organic framework nanocrystals using expanded graphite as a structure-directing template. Inorg. Chem. Commun. 2013, 35, 265–267. [Google Scholar] [CrossRef]
- Xi, J.; Li, H.; Xi, J.; Tan, S.; Zheng, J.; Tan, Z. Preparation of high porosity biochar materials by template method: A review. Environ. Sci. Pollut. Res. Int. 2020, 27, 20675–20684. [Google Scholar] [CrossRef]
- Tan, B.; Luo, Y.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Mixed-Solvothermal Synthesis of MIL-101(Cr) and Its Water Adsorption/Desorption Performance. Ind. Eng. Chem. Res. 2019, 58, 2983–2990. [Google Scholar] [CrossRef]
- Cao, J.; Li, Y.; Qi, M.; Ma, X.; He, F.; Wang, Y.; Zhao, D. Engineering porosity of MIL-101(Cr) using solvation effect. J. Environ. Chem. Eng. 2024, 12, 112753. [Google Scholar] [CrossRef]
- Fallah, M.; Sohrabnezhad, S. Study of synthesis of mordenite zeolite/MIL-101(Cr) metal–organic framework compounds with various methods as bi-functional adsorbent. Adv. Powder Technol. 2019, 30, 336–346. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Borysiuk, S.; Choma, J.; Jaroniec, M. Mechanochemical synthesis of highly porous materials. Mater. Horiz. 2020, 7, 1457–1473. [Google Scholar] [CrossRef]
- Tanaka, S. Chapter 10—Mechanochemical synthesis of MOFs. In Metal-Organic Frameworks for Biomedical Applications; Mozafari, M., Ed.; Woodhead Publishing: Oxford, UK, 2020; pp. 197–222. [Google Scholar]
- Chong, K.C.; Ho, P.S.; Lai, S.O.; Lee, S.S.; Lau, W.J.; Lu, S.-Y.; Ooi, B.S. Solvent-Free Synthesis of MIL-101(Cr) for CO2 Gas Adsorption: The Effect of Metal Precursor and Molar Ratio. Sustainability 2022, 14, 1152. [Google Scholar] [CrossRef]
- San Ho, P.; Chong, K.C.; Lai, S.O.; Mah, S.K.; Lee, S.S.; Lu, S.-Y.; Lau, W.J.; Ooi, B.S. Synthesis of MIL-101(Cr) metal organic framework by green synthesis for CO2 gas adsorption. IOP Conf. Ser. Earth Environ. Sci. 2021, 945, 012074. [Google Scholar]
- Wang, W.; Chai, M.; Bin Zulkifli, M.Y.; Xu, K.; Chen, Y.; Wang, L.; Chen, V.; Hou, J. Metal–organic framework composites from a mechanochemical process. Mol. Syst. Des. Eng. 2023, 8, 560–579. [Google Scholar] [CrossRef]
- Jin, C.; Shi, S.; Liao, S.; Liu, S.; Xia, S.; Luo, Y.; Wang, S.; Wang, H.; Chen, C. Post-Synthetic Ligand Exchange by Mechanochemistry: Toward Green, Efficient, and Large-Scale Preparation of Functional Metal–Organic Frameworks. Chem. Mater. 2023, 35, 4489–4497. [Google Scholar] [CrossRef]
- Vaid, T.P.; Kelley, S.P.; Rogers, R.D. Structure-directing effects of ionic liquids in the ionothermal synthesis of metal–organic frameworks. IUCrJ 2017, 4, 380–392. [Google Scholar] [CrossRef]
- Fujie, K.; Kitagawa, H. Ionic liquid transported into metal–organic frameworks. Coord. Chem. Rev. 2016, 307, 382–390. [Google Scholar] [CrossRef]
- Nikseresht, A.; Normohamadi, H. Development and optimization of ultrasound-assisted synthesis of MIL-101(Cr) using response surface methodology. Appl. Chem. Day 2024, 19, 223–246. [Google Scholar]
- Xu, W.; Ye, L.; Li, W.; Zhang, Z. Modulation of MIL-101(Cr) morphology and selective removal of dye from water. J. Iran. Chem. Soc. 2021, 18, 159–166. [Google Scholar] [CrossRef]
- Yang, J.-M.; Zhang, W.; Zhang, R.-Z.; Tong, M.-X. Modulation of the driving forces for adsorption on MIL-101 analogues by decoration with sulfonic acid functional groups: Superior selective adsorption of hazardous anionic dyes. Dalton Trans. 2020, 49, 6651–6660. [Google Scholar] [CrossRef]
- Zhao, T.; Li, S.-H.; Shen, L.; Wang, Y.; Yang, X.-Y. The sized controlled synthesis of MIL-101(Cr) with enhanced CO2 adsorption property. Inorg. Chem. Commun. 2018, 96, 47–51. [Google Scholar] [CrossRef]
- Jiang, D.; Burrows, A.D.; Edler, K.J. Size-controlled synthesis of MIL-101(Cr) nanoparticles with enhanced selectivity for CO2 over N2. CrystEngComm 2011, 13, 6916–6919. [Google Scholar] [CrossRef]
- Zhao, T.; Li, S.; Xiao, Y.-X.; Janiak, C.; Chang, G.; Tian, G.; Yang, X.-Y. Template-free synthesis to micro-meso-macroporous hierarchy in nanostructured MIL-101(Cr) with enhanced catalytic activity. Sci. China Mater. 2021, 64, 252–258. [Google Scholar] [CrossRef]
- Xu, W.; Li, W.; Lu, L.; Zhang, W.; Kang, J.; Li, B. Morphology-control of metal-organic framework crystal for effective removal of dyes from water. J. Solid State Chem. 2019, 279, 120950. [Google Scholar] [CrossRef]
- Alivand, M.S.; Shafiei-Alavijeh, M.; Tehrani, N.H.M.H.; Ghasemy, E.; Rashidi, A.; Fakhraie, S. Facile and high-yield synthesis of improved MIL-101(Cr) metal-organic framework with exceptional CO2 and H2S uptake; the impact of excess ligand-cluster. Microporous Mesoporous Mater. 2019, 279, 153–164. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, Y.; Liu, X.; Wang, Z. Highly permeable MOF NH2-MIL-101(Cr) fragment membranes for CO2/N2 separation. J. Membr. Sci. 2025, 722, 123918. [Google Scholar] [CrossRef]
- Liu, Q.; Lai, L.; Li, Z.; Hu, J.; Zhang, L.; Younas, W.; Mao, G. Controllable Synthesis of MIL-101(Cr)@TiO2 Core–Shell Nanocomposites for Enhanced Photocatalytic Activity. ACS Appl. Nano Mater. 2023, 6, 11764–11771. [Google Scholar] [CrossRef]
- Guo, D.; Huang, Q.; Zhao, R.; Guo, W.; Fan, K.; Han, Z.; Zhao, Z.; Nie, D. MIL-101(Cr)@Fe3O4 nanocomposites as magnetic solid-phase extraction adsorbent for the determination of multiple mycotoxins in agricultural products by ultra-high-performance liquid chromatography tandem mass spectrometry. Food Control 2023, 146, 109540. [Google Scholar] [CrossRef]
- Andani, A.M.; Tabatabaie, T.; Farhadi, S.; Ramavandi, B. MIL-101(Cr)–cobalt ferrite magnetic nanocomposite: Synthesis, characterization and applications for the sonocatalytic degradation of organic dye pollutants. RSC Adv. 2020, 10, 32845–32855. [Google Scholar] [CrossRef]
- Sharafinia, S.; Rashidi, A. MIL-101(Cr) hybrid nanoporous carbon derived MOF as a nano-adsorbent for dye removal using RSM-CCD. Arab. J. Chem. 2023, 16, 105288. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, F.; Liu, S.; Shen, F.; Peng, S. Nanometer-Sized MIL-101(Cr) Loaded with Metal Ions for Applications as a Nitrite Sensor with Enhanced Electrochemical Properties. ACS Appl. Nano Mater. 2024, 7, 16620–16629. [Google Scholar] [CrossRef]
- Rafati Jolodar, A.; Abdollahi, M.; Fatemi, S.; Mansoubi, H. Enhancing carbon dioxide separation from natural gas in dynamic adsorption by a new type of bimetallic MOF; MIL-101(Cr-Al). Sep. Purif. Technol. 2024, 334, 125990. [Google Scholar] [CrossRef]
- Han, G.; Li, F.; Guo, M.; Fan, H.; Guo, Q.; Yu, G. MIL-101(Cr) loaded simple ILs for efficient ammonia capture and selective separation. Chem. Eng. J. 2023, 471, 144545. [Google Scholar] [CrossRef]
- Bazmi, M.; Rashidi, A.; Naderifar, A.; Tabarkhoon, F.; Alivand, M.S.; Tabarkhoon, F.; Farahani, M.V.; Esrafili, M.D. Simultaneous enhancement of CO2 adsorption capacity and kinetics on a novel micro-mesoporous MIL-101(Cr)-based composite: Experimental and DFT study. J. CO2 Util. 2024, 83, 102809. [Google Scholar] [CrossRef]
- Thamer, B.M.; Al-aizari, F.A.; Abdo, H.S.; El-Newehy, M.H. Facile green fabrication of MIL-101(Cr)/PVA nanofiber composite as effective, stable, and reusable adsorbent for cationic dye removal. Polym. Eng. Sci. 2024, 64, 5375–5391. [Google Scholar] [CrossRef]
- Chang, H.; Abdulkareem-Alsultan, G.; Taufiq-Yap, Y.H.; Mohd Izham, S.; Sivasangar, S. Development of porous MIL-101 derived catalyst application for green diesel production. Fuel 2024, 355, 129459. [Google Scholar] [CrossRef]
- Nie, H.; Li, Z.; Zhang, X.; Wen, J.; Feng, Y.; Yu, Y.; Jia, L. Preparation and Hg0 Removal Performance of MIL-101(Cr)-Derived Carbon Matrix Composites. Polymers 2025, 17, 413. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-q.; Li, Y.-x.; Fu, X.; Yue, J.-y.; Cao, J.-w.; Ma, X.-b.; Liu, F.; Wang, J.-w. Catalytic oxidation of toluene by binary metal oxide Cr2O3/CeO2 from MIL-101(Cr). J. Solid State Chem. 2023, 328, 124334. [Google Scholar] [CrossRef]
- Zhu, P.; Hu, Z.; Chen, S. Praseodymium-Doped Cr2O3 Prepared by In Situ Pyrolysis of MIL-101(Cr) for Highly Efficient Catalytic Oxidation of 1,2-Dichloroethane. Molecules 2024, 29, 3417. [Google Scholar] [CrossRef] [PubMed]
- Farisabadi, A.; Moradi, M.; Hajati, S.; Kiani, M.A.; Espinos, J.P. Controlled thermolysis of MIL-101(Fe, Cr) for synthesis of FexOy/porous carbon as negative electrode and Cr2O3/porous carbon as positive electrode of supercapacitor. Appl. Surf. Sci. 2019, 469, 192–203. [Google Scholar] [CrossRef]
- Burlak, P.V.; Kovalenko, K.A.; Fedin, V.P. Preparation of heterogeneous catalysts by the post-synthetic modification of mesoporous metal-organic framework MIL-101. Russ. Chem. Bull. 2023, 72, 624–634. [Google Scholar] [CrossRef]
- Dharmapriya, T.N.; Huang, P.-J. Investigating the catalytic influence of MIL-101(Cr) and MIL-101(Cr)-NH2 on glucose dehydration into 5-hydroxymethylfurfural and modelled through response surface methodology. Microporous Mesoporous Mater. 2025, 381, 113335. [Google Scholar] [CrossRef]
- Hassan, H.M.; Alsohaimi, I.H.; El-Aassar, M.R.; El-Hashemy, M.A.; Alraddadid, T.S. Electrochemical study on using aminated MIL–101(Cr) as corrosion inhibitor for mild steel in acidic environment. Anti-Corros. Methods Mater. 2023, 70, 34–41. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Z.; Li, G. Preparation of MIL-101(Cr)–NH2@TAPB-DVA-COF based membrane solid-phase extraction for efficient enrichment and sensitive determination of trace aromatic disinfection by-products in juice drinks. Talanta 2024, 273, 125901. [Google Scholar] [CrossRef]
- Vigroux, A.; Lherbet, C.; Fabing, I.; Barthelemy, M.-C.; Laurent, C.; Hoffmann, P. A Post-Synthetic Modification Approach to Expand MIL-101-NH2 Functionalization. Preprints 2025. [Google Scholar] [CrossRef]
- Dendy, D.; Lestari, W.W.; Anshori, I.; Surawijaya, A.; Handayani, M.; Wahyuningsih, S.; Saraswati, T.E.; Ridho Suharbiansah, R.S. Enhanced indigo carmine adsorption using ethylenediamine-modified MIL-101(Cr) materials. Mater. Chem. Phys. 2025, 334, 130465. [Google Scholar] [CrossRef]
- Nikseresht, A.; Ghoochi, F.; Mohammadi, M. Postsynthetic Modification of Amine-Functionalized MIL-101(Cr) Metal–Organic Frameworks with an EDTA–Zn(II) Complex as an Effective Heterogeneous Catalyst for Hantzsch Synthesis of Polyhydroquinolines. ACS Omega 2024, 9, 28114–28128. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Cai, B.-C.; Zhou, Y.-J.; Zhou, C.-W.; Wu, M.-M.; Zhou, X.-C.; Wang, F.-L.; Zhou, X.-P.; Li, D. Mesoporous metal–organic framework NH2-MIL-101(Cr) as an efficient photocatalyst for the epoxidation of styrene. Dalton Trans. 2024, 53, 15297–15304. [Google Scholar] [CrossRef] [PubMed]
- Buragohain, A.; Couck, S.; Van Der Voort, P.; Denayer, J.F.; Biswas, S. Synthesis, characterization and sorption properties of functionalized Cr-MIL-101-X (X=–F,–Cl,–Br,–CH3,–C6H4,–F2,–(CH3) 2) materials. J. Solid State Chem. 2016, 238, 195–202. [Google Scholar] [CrossRef]
- Díaz-Ramírez, M.L.; Sánchez-González, E.; Álvarez, J.R.; González-Martínez, G.A.; Horike, S.; Kadota, K.; Sumida, K.; González-Zamora, E.; Springuel-Huet, M.-A.; Gutiérrez-Alejandre, A.; et al. Partially fluorinated MIL-101(Cr): From a miniscule structure modification to a huge chemical environment transformation inspected by 129Xe NMR. J. Mater. Chem. A 2019, 7, 15101–15112. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, X.; Zhai, Q.; Wang, H.; Jiang, H.; Ren, Y. Metal–Organic Framework Surface Functionalization Enhancing the Activity and Stability of Palladium Nanoparticles for Carbon–Halogen Bond Activation. Inorg. Chem. 2022, 61, 6995–7004. [Google Scholar] [CrossRef]
- Zorainy, M.Y.; Titi, H.M.; Kaliaguine, S.; Boffito, D.C. Multivariate metal–organic framework MTV-MIL-101 via post-synthetic cation exchange: Is it truly achievable? Dalton Trans. 2022, 51, 3280–3294. [Google Scholar] [CrossRef]
- Vu, T.A.; Le, G.H.; Dao, C.D.; Dang, L.Q.; Nguyen, K.T.; Dang, P.T.; Tran, H.T.K.; Duong, Q.T.; Nguyen, T.V.; Lee, G.D. Isomorphous substitution of Cr by Fe in MIL-101 framework and its application as a novel heterogeneous photo-Fenton catalyst for reactive dye degradation. RSC Adv. 2014, 4, 41185–41194. [Google Scholar] [CrossRef]
- Han, B.; Chakraborty, A. Synergistic ionic liquid encapsulated MIL-101(Cr) metal-organic frameworks for an innovative adsorption desalination system. J. Clean. Prod. 2024, 474, 143565. [Google Scholar] [CrossRef]
- Chen, H.; Peng, B.; Zhang, P.; Yang, Y.; Hu, X. “Turn-on” fluorescence sensing for sensitively detecting Cr(vi) via a guest exchange process in Cu NCs@MIL-101 composites. Anal. Methods 2024, 16, 4835–4842. [Google Scholar] [CrossRef]
- Park, J.; Feng, D.; Zhou, H.-C. Dual Exchange in PCN-333: A Facile Strategy to Chemically Robust Mesoporous Chromium Metal–Organic Framework with Functional Groups. J. Am. Chem. Soc. 2015, 137, 11801–11809. [Google Scholar] [CrossRef]
- Hu, Y.; Fang, Z.; Wan, X.; Ma, X.; Wang, Y.; Dong, M.; Ye, Z.; Peng, X. Ferrocene Dicarboxylic Acid Ligand-Exchanged Hollow MIL-101(Cr) Nanospheres for Solar-Driven Atmospheric Water Harvesting. ACS Sustain. Chem. Eng. 2022, 10, 6446–6455. [Google Scholar] [CrossRef]
- Li, S.; Dong, K.; Cai, M.; Li, X.; Chen, X. A plasmonic S-scheme Au/MIL-101(Fe)/BiOBr photocatalyst for efficient synchronous decontamination of Cr(VI) and norfloxacin antibiotic. eScience 2024, 4, 100208. [Google Scholar] [CrossRef]
- Gajipara, D.H.; Kalla, S.; Murthy, Z.V.P. MIL-101(Cr) incorporated hollow fiber membrane for rubber wastewater treatment using membrane bioreactor. J. Ind. Eng. Chem. 2024, 134, 181–193. [Google Scholar] [CrossRef]
- Keshta, B.E.; Yu, H.; Wang, L.; El-Attar, H.G.; El Aziz, F.A.; Mahmoud, Y.A.G.; Gemeay, A.H.; Hassan, F. Green preparation of antibacterial agents: MIL-101(Cr) synthesized from PET bottles recycling and its functionalization with silver nanoparticles. Mater. Today Commun. 2024, 38, 107731. [Google Scholar] [CrossRef]
- Keshta, B.E.; Yu, H.; Wang, L.; Gemeay, A.H. Cutting-edge in the green synthesis of MIL-101(Cr) MOF based on organic and inorganic waste recycling with extraordinary removal for anionic dye. Sep. Purif. Technol. 2024, 332, 125744. [Google Scholar] [CrossRef]
- Llewellyn, P.L.; Bourrelly, S.; Serre, C.; Vimont, A.; Daturi, M.; Hamon, L.; De Weireld, G.; Chang, J.-S.; Hong, D.-Y.; Kyu Hwang, Y.; et al. High Uptakes of CO2 and CH4 in Mesoporous Metal—Organic Frameworks MIL-100 and MIL-101. Langmuir 2008, 24, 7245–7250. [Google Scholar] [CrossRef]
- Hong, W.Y.; Perera, S.P.; Burrows, A.D. Comparison of MIL-101(Cr) metal-organic framework and 13X zeolite monoliths for CO2 capture. Microporous Mesoporous Mater. 2020, 308, 110525. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Mi, J.; Jin, J.; Meng, H. Dual hydrophobic modification on MIL-101(Cr) with outstanding toluene removal under high relative humidity. Chem. Eng. J. 2023, 451, 139000. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, J.; Liu, Z.; Lei, G.; Xi, G.; Zhang, Y.; Liu, H.; Guo, X.; Ren, H. Integration of [Cr3O(HCOO)6(H2O)3]NO3 into MIL-101(Cr) for enhanced separation of CO2/N2 mixture. Appl. Surf. Sci. 2024, 665, 160378. [Google Scholar] [CrossRef]
- Menezes, T.R.; Santos, K.M.C.; Silva, T.S.L.; Santos, K.S.; Ramos, A.L.; Borges, G.R.; Franceschi, E.; Dariva, C.; De Conto, J.F.; Egues, S.M.; et al. Carbon dioxide and methane capture in metal-organic framework MIL-101(Cr) at high pressure. Gas Sci. Eng. 2023, 119, 205136. [Google Scholar] [CrossRef]
- Lv, T.; Liu, F.; Xiao, M.; Liu, Y.; Wang, L.; Gao, G. Synthesis of reusable hierarchical Pore PVDF-MIL-101(Cr) foam for Solid phase extraction of fluoroquinolones from water and its adsorption behavior for anionic and cationic dyes. J. Chromatogr. A 2025, 1740, 465577. [Google Scholar] [CrossRef]
- Keshta, B.E.; Yu, H.; Wang, L.; Yi, H.; Jian, S.; Uddin, M.D.A.; Ouyang, C.; Wang, Y.; Yuan, X.; Zhang, Y.; et al. Preparation of unsaturated MIL-101(Cr) with Lewis acid sites for the extraordinary adsorption of anionic dyes. npj Clean Water 2025, 8, 15. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, M.; Xin, X.; Ye, Q.; Zhang, K.; Wang, Z. Core-Shell Composite MIL-101(Cr)@TiO2 for Organic Dye Pollutants and Vehicle Exhaust. Molecules 2023, 28, 5530. [Google Scholar] [CrossRef]
- Cui, G.-Y.; Zhang, W.; Yang, J.-M. Selective adsorptive removal of anionic dyes from aqueous solutions using MIL-101@GO: Effect of GO. Colloids Surf. A Physicochem. Eng. Asp. 2023, 667, 131364. [Google Scholar] [CrossRef]
- Fathi Hasanbarogh, A.; Ghasemi, N.; Ezzatzadeh, E. Kinetic and thermodynamic studies of Methylene Blue adsorption process from aqueous solutions by MIL-101(Cr)@ZnO nanostructure. Int. J. Environ. Anal. Chem. 2024, 104, 4911–4927. [Google Scholar] [CrossRef]
- Ren, X.; Wang, C.-C.; Li, Y.; Wang, P.; Gao, S. Defective SO3H-MIL-101(Cr) for capturing different cationic metal ions: Performances and mechanisms. J. Hazard. Mater. 2023, 445, 130552. [Google Scholar] [CrossRef]
- Zhao, T.; Zhu, H.; Dong, M.; Zou, M.; Tang, S.; Luo, M.; Li, X. Low-Temperature and Additive-Free Synthesis of Spherical MIL-101(Cr) with Enhanced Dye Adsorption Performance. Inorganics 2022, 10, 33. [Google Scholar] [CrossRef]
- Cheng, S.; Li, Y.; Yu, Z.; Su, Y. Efficient adsorption removal of anionic dyes by waste PET-derived MIL-101(Cr). Sep. Purif. Technol. 2025, 354, 128985. [Google Scholar] [CrossRef]
- Li, M.; Cao, P.; Dong, W.; Zhao, J.; Li, D. Fast and Efficient Removal of Anionic Dyes from Water by Hierarchical Porous MIL-101(Cr). J. Inorg. Organomet. Polym. Mater. 2025, 35, 196–205. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, R.-Z.; Huang, Y.-Q.; Yang, J.-M. Effect of the Synergetic Interplay between the Electrostatic Interactions, Size of the Dye Molecules, and Adsorption Sites of MIL-101(Cr) on the Adsorption of Organic Dyes from Aqueous Solutions. Cryst. Growth Des. 2018, 18, 7533–7540. [Google Scholar] [CrossRef]
- Gökırmak Söğüt, E. Superior Adsorption Efficiency of MIL-101(Cr) and Nano-MIL-101(Cr) in Anionic and Cationic Dye Removal from Aqueous Solution. ChemistrySelect 2023, 8, e202205000. [Google Scholar] [CrossRef]
- Haghighi, E.; Zeinali, S. Formaldehyde detection using quartz crystal microbalance (QCM) nanosensor coated by nanoporous MIL-101(Cr) film. Microporous Mesoporous Mater. 2020, 300, 110065. [Google Scholar] [CrossRef]
- Zhang, K.; Dai, K.; Bai, R.; Ma, Y.; Deng, Y.; Li, D.; Zhang, X.; Hu, R.; Yang, Y. A competitive microcystin-LR immunosensor based on Au NPs@metal-organic framework (MIL-101). Chin. Chem. Lett. 2019, 30, 664–667. [Google Scholar] [CrossRef]
- Manohara Reddy, Y.V.; Shin, J.H.; Hwang, J.; Kweon, D.H.; Choi, C.H.; Park, K.; Kim, S.K.; Madhavi, G.; Yi, H.; Park, J.P. Fine-tuning of MXene-nickel oxide-reduced graphene oxide nanocomposite bioelectrode: Sensor for the detection of influenza virus and viral protein. Biosens. Bioelectron. 2022, 214, 114511. [Google Scholar] [CrossRef]
- Yang, J.; Kou, Y.K. Sulfo-modified MIL-101 with immobilized carbon quantum dots as a fluorescence sensing platform for highly sensitive detection of DNP. Inorganica Chim. Acta 2021, 519, 120276. [Google Scholar] [CrossRef]
- Conti, P.P.; Iacomi, P.; Nicolas, M.; Maurin, G.; Devautour-Vinot, S. MIL-101(Cr)@QCM and MIL-101(Cr)@IDE as Sorbent-Based Humidity Sensors for Indoor Air Monitoring. ACS Appl. Mater. Interfaces 2023, 15, 33675–33681. [Google Scholar] [CrossRef]
- Ge, L.; Feng, Y.; Wu, J.; Wang, R.; Ge, T. Performance evaluation of MIL-101(Cr) based desiccant-coated heat exchangers for efficient dehumidification. Energy 2024, 289, 130049. [Google Scholar] [CrossRef]
- Benseghir, Y.; Tsang, M.Y.; Schöfbeck, F.; Hetey, D.; Kitao, T.; Uemura, T.; Shiozawa, H.; Reithofer, M.R.; Chin, J.M. Electric-field assisted spatioselective deposition of MIL-101(Cr)PEDOT to enhance electrical conductivity and humidity sensing performance. J. Colloid Interface Sci. 2025, 678, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xing, H.; Ma, L.; Wang, F.; Zhang, M.; Li, Z.; Wan, Z.; Cui, X.; Yang, G. A sensitive electrochemical sensor for the detection of Pb2+ and Cu2+ based on NH2-MIL-101(Cr)@ZIF-8/MWCNTs/GCE. Microchem. J. 2024, 207, 112165. [Google Scholar] [CrossRef]
- Rafea, O.A.S.; Abdel-Aziz, A.M.; Sayed, M.A.; Abdelhameed, R.M.; Badr, I.H.A. Enhanced simultaneous voltammetric detection of lead, copper, and mercury using a MIL-101(Cr)-(COOH)2@MWCNTs modified glassy carbon electrode. Anal. Chim. Acta 2025, 1338, 343600. [Google Scholar] [CrossRef] [PubMed]
- Massah, R.T.; Zambou Jiokeng, S.L.; Liang, J.; Njanja, E.; Ma Ntep, T.M.; Spiess, A.; Rademacher, L.; Janiak, C.; Tonle, I.K. Sensitive Electrochemical Sensor Based On an Aminated MIL-101(Cr) MOF for the Detection of Tartrazine. ACS Omega 2022, 7, 19420–19427. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.; Wang, W.; Min, K.; Ling, W.; Ma, W.; Zhang, W.; Hou, X.; Wei, L.; Liu, Q.; et al. Dose-Dependent Effect on Plant Growth of Exposure to Metal–Organic Framework MIL-101(Cr). Environ. Sci. Technol. 2024, 58, 8009–8019. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Chen, Y.; Li, S.; Li, F.; Wu, X.; Gui, T.; Cao, Z.; Wang, Y. MIL-101(Cr) molecular cage anchored on 2D Ti3C2TX MXene nanosheets as high-performance electrochemical sensing platform for detection of xanthine. Microchim. Acta 2023, 190, 267. [Google Scholar] [CrossRef]
- Haghighi, E.; Zeinali, S. A Highly Sensitive Toluene and Xylene QCM Nanosensor Using Nanoporous MIL-101(Cr) as a Sensing Layer. ChemistrySelect 2023, 8, e202204307. [Google Scholar] [CrossRef]
- Silva, I.M.P.; Carvalho, M.A.; Oliveira, C.S.; Profirio, D.M.; Ferreira, R.B.; Corbi, P.P.; Formiga, A.L.B. Enhanced performance of a metal-organic framework analogue to MIL-101(Cr) containing amine groups for ibuprofen and nimesulide controlled release. Inorg. Chem. Commun. 2016, 70, 47–50. [Google Scholar] [CrossRef]
- Tian, Y.; Deng, P.; Wu, Y.; Ding, Z.; Li, G.; Liu, J.; He, Q. A Simple and Efficient Molecularly Imprinted Electrochemical Sensor for the Selective Determination of Tryptophan. Biomolecules 2019, 9, 294. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, W.; Ma, C.; Li, Z. Fabrication of an electrochemical biosensor array for simultaneous detection of L-glutamate and acetylcholine. J. Biomed. Nanotechnol. 2013, 9, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yi, H.; Liu, Y.; Wang, Z.; Liu, S.; He, N.; Liu, H.; Deng, Y. One-Step Synthesis of DNA Templated Water-Soluble Au–Ag Bimetallic Nanoclusters for Ratiometric Fluorescence Detection of DNA. J. Biomed. Nanotechnol. 2018, 14, 150–160. [Google Scholar] [CrossRef]
- Pederneira, N.; Aina, P.O.; Rownaghi, A.A.; Rezaei, F. Performance of MIL-101(Cr) and MIL-101(Cr)-Pore Expanded as Drug Carriers for Ibuprofen and 5-Fluorouracil Delivery. ACS Appl. Bio Mater. 2024, 7, 1041–1051. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Tahami, S. Recent developments in MIL-101 metal organic framework for heterogeneous catalysis. Rev. Chem. Eng. 2023, 39, 707–728. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Dong, L.; Yin, Z.; Tian, Z.; Yang, W.; Yang, Z. MIL-101(Cr)-decorated Ti/TiO2 anode for electrochemical oxidation of aromatic pollutants from water. Chin. Chem. Lett. 2023, 34, 107404. [Google Scholar] [CrossRef]
- Tripathi, J.; Sangale, M.; Ghaywat, P.; Gawali, A.; Yadav, A.; Waghmode, K.; More, P. Metal-organic framework MIL-101(Cr): An efficient catalyst for the synthesis of dihydro-pyrimidinones. Inorganica Chim. Acta 2024, 565, 121989. [Google Scholar] [CrossRef]
- Wang, C.; Hou, S.; Ma, M.; Ji, Z.; Wang, P.; Wang, Y.; Su, Y.; Zhou, Y.; Li, M. Recent development of MIL-101(Cr) for photocatalysis: A mini review. J. Water Process Eng. 2024, 66, 106040. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Chen, X.; Dai, S.; Bao, Z.; Yang, Q.; Ren, Q.; Zhang, Z. Cooperative Interplay of Brønsted Acid and Lewis Acid Sites in MIL-101(Cr) for Cross-Dehydrogenative Coupling of C–H Bonds. ACS Appl. Mater. Interfaces 2021, 13, 10845–10854. [Google Scholar] [CrossRef]
- Henschel, A.; Gedrich, K.; Kraehnert, R.; Kaskel, S. Catalytic properties of MIL-101. Chem. Commun. 2008, 35, 4192–4194. [Google Scholar] [CrossRef]
- Santiago-Portillo, A.; Blandez, J.F.; Navalón, S.; Álvaro, M.; García, H. Influence of the organic linker substituent on the catalytic activity of MIL-101(Cr) for the oxidative coupling of benzylamines to imines. Catal. Sci. Technol. 2017, 7, 1351–1362. [Google Scholar] [CrossRef]
- Dai, H.; Cao, N.; Yang, L.; Su, J.; Luo, W.; Cheng, G. AgPd nanoparticles supported on MIL-101 as high performance catalysts for catalytic dehydrogenation of formic acid. J. Mater. Chem. A 2014, 2, 11060–11064. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Su, S.; Tan, M.; Liu, G.; Wang, Y.; Luo, M. Ag nanoparticles in the cages of MIL-101(Cr) as an efficient and stable photocatalyst for nitrogen reduction reaction. Catal. Sci. Technol. 2023, 13, 705–713. [Google Scholar] [CrossRef]
- Esposito, E.; Carta, M.; Fuoco, A.; Monteleone, M.; Comesaña-Gándara, B.; Gkaniatsou, E.; Sicard, C.; Wang, S.; Serre, C.; McKeown, N.B.; et al. Single and mixed gas permeability studies on mixed matrix membranes composed of MIL-101(Cr) or MIL-177(Ti) and highly permeable polymers of intrinsic microporosity. J. Membr. Sci. 2024, 697, 122475. [Google Scholar] [CrossRef]
- Swain, J.; Priyadarshini, A.; Sahu, J.R.; Sinha, J.K.; Dave, S.; Sahu, R. Chapter 9—Developments in corrosion inhibition through MOFs. In Electrochemical Applications of Metal-Organic Frameworks; Dave, S., Sahu, R., Tripathy, B.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 205–218. [Google Scholar]
- Unlu, D. Water Desalination by Pervaporation Using MIL-101(Cr) and MIL-101(Cr)@GODoped PVA Hybrid Membranes. Water Air Soil Pollut. 2023, 234, 96. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Zhao, J.; Zhang, H.; Liu, L.; Sun, X.; Li, G.; Liang, H. Interaction between MIL-101(Cr) and natural organic matter in an integrated MOF-UF system. Sep. Purif. Technol. 2023, 314, 123476. [Google Scholar] [CrossRef]
- Devautour-Vinot, S.; Sanil, E.S.; Geneste, A.; Ortiz, V.; Yot, P.G.; Chang, J.S.; Maurin, G. Guest-Assisted Proton Conduction in the Sulfonic Mesoporous MIL-101 MOF. Chem. Asian J. 2019, 14, 3561–3565. [Google Scholar] [CrossRef]
- Sun, X.; Bai, X.; Wang, Q.; Lu, Y.; Liu, S. Phase-Changeable Polyoxometalate-Based Acid–Base Adduct for High-Temperature Proton Conduction. ACS Appl. Energy Mater. 2022, 5, 7523–7529. [Google Scholar] [CrossRef]
- Kachhadiya, D.D.; Murthy, Z.V.P. Separation of n-butanol from aqueous mixtures using TiO2 and h-BN functionalized MIL-101(Cr) incorporated PVDF mixed matrix membranes. Sep. Purif. Technol. 2023, 306, 122613. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, Y.; Yuan, Y.; Zhou, X.; Liu, Y.; Meng, X. Recent advances of metal–organic frameworks in corrosion protection: From synthesis to applications. Chem. Eng. J. 2022, 430, 132823. [Google Scholar] [CrossRef]
- Anadebe, V.C.; Chukwuike, V.I.; Barik, R.C. Metal Functionalized Organic Compounds/Metal-Organic Frameworks (MOFs) as Corrosion Inhibitors. In Functionalized Nanomaterials for Corrosion Mitigation: Synthesis, Characterization, and Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2022; Volume 1418, pp. 155–167. [Google Scholar]
- Al Kiey, S.A.; El-Shahat, M.; Abdelhameed, R.M. Role of different metal precursors based MOFs for boosting anti-corrosion performance of mild steel in acid media. Mater. Today Sustain. 2023, 23, 100460. [Google Scholar] [CrossRef]
- Ren, B.; Li, Y.; Meng, D.; Li, J.; Gao, S.; Cao, R. Encapsulating polyaniline within porous MIL-101 for high-performance corrosion protection. J. Colloid Interface Sci. 2020, 579, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, H.; Meng, S.; Wang, F.; Mei, T.; Cai, X.; Ran, Y. Smart epoxy coating: G-C3N4 nanosheets loaded MOFs for enhanced anti-corrosion and UV resistance. Chem. Eng. J. 2024, 488, 150731. [Google Scholar] [CrossRef]
- Rico-Barragán, A.A.; Álvarez, J.R.; Pioquinto-García, S.; Rodríguez-Hernández, J.; Rivas-García, P.; Dávila-Guzmán, N.E. Cleaner production of metal-organic framework MIL-101(Cr) for toluene adsorption. Sustain. Prod. Consum. 2023, 40, 159–168. [Google Scholar] [CrossRef]
- Tehrani, N.H.M.H.; Alivand, M.S.; Kamali, A.; Esrafili, M.D.; Shafiei-Alavijeh, M.; Ahmadi, R.; Samipoorgiri, M.; Tavakoli, O.; Rashidi, A. Seed-mediated synthesis of a modified micro-mesoporous MIL-101(Cr) for improved benzene and toluene adsorption at room conditions. J. Environ. Chem. Eng. 2023, 11, 109558. [Google Scholar] [CrossRef]
- Habib, N.; Durak, Ö.; Uzun, A.; Keskin, S. Incorporation of a pyrrolidinium-based ionic liquid/MIL-101(Cr) composite into Pebax sets a new benchmark for CO2/N2 selectivity. Sep. Purif. Technol. 2023, 312, 123346. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, M.K.; Kumar, V.; Huu Do, H.; Thi Kim Le, A.; Van Nguyen, A.; Gwag, J.S.; Nam, P.C.; Trung, L.G. Optimal adsorption of pefloxacin antibiotics from aqueous solutions: Improved performance with metal-organic framework MIL-101(Cr). Colloids Surf. A Physicochem. Eng. Asp. 2024, 689, 133642. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, X.; Liu, P.; Chen, B.; Ma, J. “Blockchain-Like” MIL-101(Cr)/Carbon Black Electrodes for Unprecedented Defluorination by Capacitive Deionization. Small 2023, 19, 2205619. [Google Scholar] [CrossRef]
- Wu, M.; Huang, M.; Zhang, B.; Li, Y.; Liu, S.; Wang, H.; Fan, M.; Li, B.; Dong, L.; Chen, G. Construction of 3D porous BiOBr/MIL-101(Cr) Z-scheme heterostructure for boosted photocatalytic degradation of tetracycline hydrochloride. Sep. Purif. Technol. 2023, 307, 122744. [Google Scholar] [CrossRef]
- Kudiiarov, V.N.; Kurdyumov, N.; Elman, R.R.; Svyatkin, L.A.; Terenteva, D.V.; Semyonov, O. Microstructure and hydrogen storage properties of MgH2/MIL-101(Cr) composite. J. Alloys Compd. 2024, 976, 173093. [Google Scholar] [CrossRef]
- Su, S.; Li, X.; Liu, Z.; Ding, W.; Cao, Y.; Yang, Y.; Su, Q.; Luo, M. Microchemical environmental regulation of POMs@MIL-101(Cr) promote photocatalytic nitrogen to ammonia. J. Colloid Interface Sci. 2023, 646, 547–554. [Google Scholar] [CrossRef]
- Li, Y.-F.; Zou, C.-J.; Liu, X.-B.; Gan, F.; Fang, P.-P. Highly Sensitive and Selective Detection of Pharmaceuticals on Au/MIL-101(Cr) by SERS. Anal. Chem. 2023, 95, 7933–7940. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qian, L.; Liu, L.; Liu, Z.; Zhang, H.; Yang, L.; Zhang, D.; Chen, Z.; Fang, P.; He, C. Extra highways for proton diffusion in TiO2@MIL-101-Cr/Nafion composite membranes with high single-cell performance. J. Power Sources 2023, 564, 232906. [Google Scholar] [CrossRef]
- Lu, J.; Jv, A.; Zhao, H.; Che, G.; Feng, B.; Zhang, Y.; Liu, C.; Guan, R. Fc-COOH modified MIL-101(Cr) as enhanced photocatalyst to efficiently degrade tetracycline under visible light. Sep. Purif. Technol. 2024, 346, 127442. [Google Scholar] [CrossRef]
- Zhao, K.; Li, X.; Cheng, G.; Liu, L.; Chen, R.; Jiao, Y.; Liu, Y.; Zhu, G. Rapid and selective removal of bisphenol S from environmental samples by surface-imprinted polymer synthesized based on metal-organic framework MIL-101(Cr). J. Environ. Chem. Eng. 2024, 12, 113569. [Google Scholar] [CrossRef]
- Mohmmad, A.; Mosavian, M.T.H.; Moosavi, F. Pharmaceutically active compounds removal from aqueous solutions by MIL-101(Cr)-NH2: A molecular dynamics study. Ecotoxicol. Environ. Saf. 2024, 278, 116333. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).