Abstract
Essential oils (EOs) are advised by traditional medical systems for the treatment of a variety of disorders worldwide. In many ancient medical systems around the world, Polygonum herbs have been employed as remedies including P. equisetiforme Sm. The EO profile of P. equisetiforme and its bioactivities have yet to be discussed in depth. As a result, the current study aims to investigate the chemical profile and free radical scavenging capacity of P. equisetiforme EO. Hydrodistillation was used to obtain the EO from P. equisetiforme, and gas chromatography–mass spectrometry (GC-MS) was used for analysis. A total of forty-three compounds, including terpenes and sesquiterpenes as the main components (76.13% and 69.06%, respectively), were identified in the oil using the GC-MS analysis. The main constituents of the oil were hexahydrofarnesyl acetone (29.45%), 7-epi-selinene (14.45%), isospathulenol (8.35%), and n-docosane (6.79%). The chemosystematic significance of the plant was established via multivariate assessing, comprising principal component analysis (PCA), hierarchical clustering, and constellation plot, of the EO principal components of the various Polygonum plants. The P. equisetiforme exhibited different associations with the studied Polygonum spp. Then, the scavenging of the free radicals 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) was used to evaluate the radical scavenging abilities of EO compared with those of vitamin C, a reference antioxidant. P. equisetiforme EO exhibited the scavenging capacity of the DPPH and the ABTS free radical with respective IC50 values of 470.01 and 113.74 mg L−1 compared with vitamin C, and with IC50 values of 39.06 and 26.09 mg L−1, respectively. The in silico studies revealed that the oxygenated sesquiterpenes, especially ar-turmerone, hexahydrofarnesyl acetone, and 5E,9E-farnesyl acetone, exhibited the best fitting with hematopoietic cell kinase (Hck) and human Peroxiredoxin 5 proteins with ΔG values of −6.14 and −4.93, −6.83 and −5.34, and −7.08 and −5.47 kcal/mol, respectively. The major components’ combined or individual effects may be responsible for the antioxidant properties. Therefore, additional extensive studies are advised to characterize the essential compounds as radical scavenger agents, either individually or in combination.
1. Introduction
New therapeutic compounds can be found mostly in natural products and their derivatives [1]. Essential/volatile oils (EOs/VOs) are complex combinations of low-molecular-weight components that are extracted from plants using hydrodistillation, microwave, steam distillation, and solvent extraction [2]. The main components that give EOs their distinctive scent and biological effects are terpenes and phenylpropanoids [3,4]. Since the Middle Ages, EOs have been utilized extensively for fungicidal, bactericidal, virucidal, insecticidal, antiparasitic, medicinal, and cosmetic uses [5,6]. Traditional medical systems around the world recommend essential oils for a range of health issues [5]. They are frequently used as flavoring ingredients in foods, beverages, cosmetics, medications, and fragrances [5,6]. These days, they are particularly popular in the pharmaceutical, food industries, sanitary, and agricultural [6,7].
The polygonaceae family, including around 40 genera and 800 flowering plant species, is spread across tropical, subtropical, and temperate climates [8]. Around 150 plants belonging to the Polygonum genus are widely distributed in North Africa, Europe, and Western Asia [7,9]. Eight species of Egyptian flora have been identified as abundant, growing in Egypt’s coastal and inland deserts, Nile Delta, and Sinai. Worldwide, Polygonum plants have been used for a number of traditional ailments, including the treatment of snakebite, heart illness, diarrhea, dermatitis, influenza, hemorrhoids, bacterial and skin infections, dysentery, and insomnia [10]. Additionally, the Polygonum plants are a significant supplier of numerous nutrient components, including proteins and lipids [11]. These plants are also potential sources of pharmaceutical and biological metabolites, mainly flavonoids, phenols, tannins, terpenes, stilbenes, anthraquinones, polysaccharides, and glycolipids [12]. In some countries, P. equisetiforme appears to be popular with domestic animals for grazing and browsing, providing significant economic benefits to the rural inhabitants [13]. P. equisetiforme is widely used in folk medicines for treating cold, cough, and sore throat [14]. It is additionally employed as a tea flavoring [15]. The previous chemical characterization of the P. equisetiforme revealed that this plant is very rich in phenolic acids, and flavonoids [16] with significant antioxidant, hepatoprotective, antibacterial, and antifungal effects [17,18].
Peroxiredoxins, a family of peroxidases, work in concert with other antioxidants, including enzymatic and non-enzymatic antioxidants, to control the concentrations of reactive oxygen species (ROS) and to safeguard cells from oxidative damage. Peroxiredoxin 5 (PRDX5), one of this group of enzymes, has been reported to reduce peroxides and hydroperoxides and prevent their intracellular accumulation [19]. The SRC family of cytoplasmic tyrosine kinases (SFKs) includes hematopoietic cell kinase (HCK), which is expressed in cells of the myeloid and B-lymphocyte cell lineages. Numerous kinds of leukemia are linked to excessive HCK activation, which promotes cell growth and survival by physical linkage with oncogenic fusion proteins and through functional interactions with receptor tyrosine kinases. Additionally, elevated HCK activity is shown in a variety of solid tumors, such as breast and colon cancer, and it is associated with lower patient survival rates. HCK promotes macrophage polarization towards a wound-healing, tumor-promoting, alternatively activated state, as well as promoting a growth factor and pro-inflammatory cytokine release from myeloid cells [20].
The EO profiling and bioactivities of P. equisetiforme have not been described before. Thus, the current work was intended to investigate the chemical profile of P. equisetiforme EO extracted from the aerial parts of this plant as well as its antioxidant potential. Moreover, molecular docking studies of the major compounds of EO were carried out on human Peroxiredoxin 5 and hematopoietic cell kinase (Hck).
2. Materials and Methods
2.1. Plant Collection, Authentication, and Preparation
The healthy, flowering aerial parts of P. equisetiforme were gathered from various populations growing within homogenous sandy habitat along the Mediterranean Sea Coast, near Baltim City (31°31′56.56″ N, 31°18′38.12″ E), during the flowering season in May 2022 (Figure 1). In this context, three composite samples from different patches of P. equisetiforme were collected for further preparation and analysis. According to Boulos [21] and Tackholm [22], plant authentication was carried out. At Mansoura University in Egypt’s Faculty of Science, a voucher specimen (Mans. 0161605004) was made and added to the herbarium. All the plant materials were carefully removed from the dust, dried for a short time at 25–28 °C in an open, shaded area, and then processed into powder using a grinder (IKA® MF 10 Basic Microfine Grinder Drive, Breisgau, Germany).

Figure 1.
Photographs of Polygonum equisetiforme Sm.: (A) an overview of the mature plant; (B) a close view of young branches.
2.2. Chemical and Drugs
Ethanol (99%), n-hexane, 2,2-Diphenyl-1-picrylhydrazyl (DPPH), and 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) were obtained from Sigma-Aldrich, Darmstadt, Germany. The C8-C22 n-alkane standards were bought from Sigma-Aldrich (St. Louis, MO, USA).
2.3. EO Extraction
The above-ground parts of P. equisetiformes (150 g) were hydrodistilled for three hours using a Clevenger apparatus in a glass round flask (5 L) containing 2000 mL water. Next, the extracted EO layer was separated using 3 drops of n-hexane before being immediately dried with 0.5 g of anhydrous Na2SO4. The same procedure was used to obtain three EO samples from three plant samples (weighing 150 g each). For GC-MS analysis and biological evaluations, three distinctive dark-brown glass vials containing the three EO samples were put in a refrigerator set at 4 °C.
2.4. EO Analysis by Gas Chromatography–Mass Spectroscopy (GC-MS)
The chemical components of each of the three EO samples were identified using the same methods and under the same circumstances as those previously described by [23,24]. For the sake of summarizing, the GC-MS analysis used the TRACE Ultra-Gas Chromatography and Thermo Scientific ISQTM EC single quadrupole mass spectrometer, all of which are manufactured by THERMO ScientificTM Corporate, Waltham, MA, USA. The GC-MS device was equipped with a TR-5 MS column that has an interior diameter of 30 m and a film that is 0.25 mm thick. With a flow rate of (1.0 mL min−1) and a ratio of split of (1:10), helium was used as the carrier gas. The temperature schedule was set at 60 °C for a single minute and then increased to 240 °C at an average of 4.0 °C per minute. Each EO sample was injected in a modest volume (1 µL in hexane), at a 1:10 (v/v) concentration, into the injector and detector at 210 °C. The mass spectral data were collected at 70 eV using electron ionization (EI), with a spectrum spanning m/z 40–450. The chemical composition was determined by employing the AMDIS (automated mass spectral deconvolution and identification) applications, in addition to access to the Wiley Spectral Library collection and the NIST Library database (Gaithersburg, MD, USA; Wiley, Hoboken, NJ, USA), which were used for calculating of the retention indices relative to n-alkanes (C8–C22) or assessment to the mass spectral data of authentic components. The monoterpenes, camphene, d-limonene, pinene (α- and β-), linalool, geraniol, α-terpineol, and citral, and the sesquiterpenes, elemene (α- and β-), α-copaene, germacrene D, β-caryophyllene, cubedol, α-eudesmol, and spathulenol, were used as the references authenticated components in the GC-MS analysis. Upon completion of the GC-MS analysis, the three EO samples were gathered and stored in a single black glass vial pending the beginning of the biological investigations.
2.5. DPPH and ABTS Scavenging Activity
According to Miguel [25], the isolated EO from P. equisetiforme was tested for its antioxidant efficacy by measuring its ability to scavenge DPPH radicals. Using ethanol as the solvent, an array of EO concentrations of 65.5, 125, 250, 500, and 1000 mg L−1 were created. Equal amounts of freshly made DPPH (0.3 mM) and EO were created in test tubes, vigorously mixed, and incubated for 20 min in the dark at room temperature. Subsequently, the spectrophotometer (Analytik Jena, Jena, Germany) was used to determine the absorbance at 517 nm. In accordance with Re et al. [26], the antioxidant activity of P. equisetiforme EO via the reduction of ABTS was performed. In the DPPH assay, the EO concentrations range of 65.5–1000 mg L−1 were prepared in ethanol. In brief, 2 mL of newly made ABTS were combined with around 0.2 mL of each concentration, and the mixture was then incubated for 6 min in the dark. A Spectronic 21D spectrophotometer (Milton Roy, CA, USA) was applied to compute the color absorbance at 734 nm. Furthermore, a standard scavenger drug as vitamin C was created as a positive control within a range of concentrations of 12.5–125 mg L−1. It was handled in the same way as the EO as previously mentioned. The following calculation was used to compute the ratio of scavenging efficiency in both methods.
where A sample and A control = the recorded absorbances of the sample and control, respectively.
Additionally, several quantities of vitamin C (12.5–125 mg L−1) were generated, and their antioxidant activity was assessed as previously stated for the EO. This was carried out to compare the effectiveness of the antioxidant with that of a standard antioxidant.
2.6. Data Treatments and Statistical Analysis
The data of free radical scavenging activity data based on three replications was treated with one-way ANOVA followed by Tukey’s HSD test using the CoStat program, version 6.311 (CoHort Software, Monterey, CA, USA. For chemometric analysis, we prepared a dataset of 37 chemical compounds that were identified as major identified compounds with an abundance of more than 2.5% of the total EO mass. These compounds were identified as major compounds within 11 Polygonum species including our studied plant, P. equisetiforme as well as P. odoratum, P. lapathifolium, P. arenarium, P. aviculare, P. arenastrum, P. bellardii, P. cognatum, P. persicaria, P. minus, and P. bistorta subsp. carneum. The dataset was subjected to multivariate analysis, principal component analysis (PCA), and hierarchical cluster analysis. These analyses were performed using the JMP® Pro 16.0.0 (SAS Institute Inc., Cary, NC, USA).
2.7. Molecular Docking Studies
Docking studies have been carried out using Molecular Operating Environment (MOE) software version 2015.10 [27]. The catalytic domains of human Peroxiredoxin 5 (PDB ID: 1HD2) [28] and hematopoietic cell kinase (Hck), one of Src family tyrosine kinase (PDB ID: 2HCK) [29], were obtained from the protein data bank. The crystal structures were cleaned of unwanted solvents, ligands, and cofactors, and the default MOE “QuickPrep” module settings were employed. The “Site Finder” option was then used to generate the probable binding pockets containing the crucial residues. For validation of the docking process, the co-crystallized ligands were re-docked in the active binding sites. The database file (.mdb) for the energy-minimized compounds was generated and docked in the ligand sites. The best conformers were studied in both 2D and 3D representations. S-scores with RMSD values ≤ 2 Å are inserted in the results section.
3. Results and Discussions
3.1. Chemical Profiling of P. equisetiforme EO
The hydrodistillation of P. equisetiforme materials yielded 0.8% (v/w) dark yellow oil with a strong odor. The yield of P. equisetiforme EO was about equal to the documented yields from P. aviculare (0.9%), P. cognatum (0.8), P. lapathifolium (0.9%), P. bellardii (0.8%), P. persicaria (0.8%), and P. arenastrum (0.9%). However, this yield was larger than the EO produced from P. arenarium (0.6%) [30] and P. minus (0.30%) [31]. On the contrary, P. bistorta subsp. Carneum’s flowers have been shown to produce EO with a yield of 0.12% [32], which is higher than the EO produced by P. equisetiforme in the current study and by other Polygonum plants [30,31].
Totaling 96.48% of the total oil mass, 43 components from P. equisetiformes EO were identified. All the identified components from the GC-MS experiments (Figure 2) were added to Table 1 along with their retention times (Rt), relative abundances, and Kovats indices (KI). Nine categories of compounds were assigned, including the following: oxygen-containing mono- (0.85%), sesqui- (51.98%), and diterpenes (1.29%); non-oxygenated mono- (3.85%), sesqui- (17.08%), and diterpenes (1.08%); non-oxygenated hydrocarbons (14.38%); and carotenoid-derived compounds (1.18%).
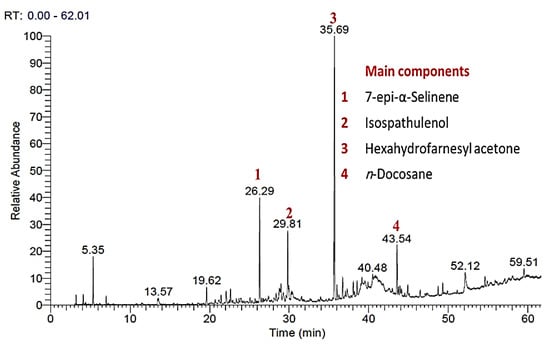
Figure 2.
The GC-MS chromatogram of P. equisetiforme essential oil. The major component peaks were numbered from 1 to 4.

Table 1.
Essential oil chemical composition characterization of P. equisetiforme.
The chemical characterization of P. equisetiformes EO revealed that sesquiterpenes are the essential category of compounds with a relative abundance of 51.98 including non-oxygenated and oxygenated forms. Hexahydrofarnesyl acetone is a major sesquiterpene in the EO of P. equisetiforme, with a relative content of 29.45, despite being reported to be minor in the EO of Polygonum species as P. bistorta subsp. carneum [32]. With a relative abundance of 14.45, the 7-epi-selinene was assigned to be the main component in all identified sesquiterpene, particularly the sesquiterpene hydrocarbons. While isospathulenol, with a relative abundance of 8.35, has been identified as the most prominent oxygenated sesquiterpene. According to data from EOs generated from the Polygonum species: P. aviculare, P. persicaria, P. lapathifolium, P. arenarium, P. bellardii, P. arenastrum, and P. cognatum, the sesquiterpene abundance in the current investigation was in full accordance with those data.
The recorded data for P. minus [31] and P. bistorta subsp. carneum [32], which include the hydrocarbons as majors, contradicted this finding. The two major sesquiterpenes, 7-epi-selinene and isospathulenol, were not found in any of the Polygonum plant’s EOs [30,31,32]. Meanwhile, they were discovered as major constituents of EOs of other plants such as Ruilopezia bracteosa [33], Teucrium yemense [34], Piper lepturum var. angustifolium [35], Piper oradendron [36], and others.
The current findings include non-oxygenated hydrocarbons as majors and minors of oxygenated forms with a significant abundance of 19.17%. Among all identified hydrocarbons, n-docosane (6.79%) and n-pentacosane (1.67%) had the highest abundances. In accordance with the present findings, the hydrocarbons were identified as the predominant components in the EOs of a number of Polygonum species, including P. minus [31], P. cognatum [30], and P. bistorta subsp. carneum [32].
Monoterpenes and compounds-derived carotenoids were all detected as traces in relative quantities of 4.70% and 1.18%, respectively. As traces in the EOs of various Polygonum plants, monoterpenes, and carotenoid-derived compounds have also been reported [30,31,32]. Apart from d-limonene as the main identified monoterpene with a relative abundance of 2.55, the other monoterpenes were identified with minor abundances ranging from 0.32 to 0.53%. Also, only one carotenoid-derived compound, (E)-α-ionone (rel. conc. 1.18%), was assigned.
The diterpenes were discovered as a minor category of constituents among terpenoid compounds, with a relative abundance of 2.37%. All of the defined diterpenes were represented by phytol and phytane, with respective percentages of 1.29% and 1.08%. The larger, biosynthesized isoprenoids, such as the diterpenes, are usually non-volatile or hardly volatile, notwithstanding the fact that most isoprenoids, such as isoprenes and mono- and sesqui-terpenes, are extremely volatile chemicals [37,38]. Thus, the enriched EOs with diterpenes are extremely uncommon in the plant kingdom [39]. The minor diterpene constituents in the EO of this plant is fully agreed with this fact and with the scarcity of diterpenes the in EOs of Polygonum species [30,31,32]. Phytol was earlier recognized to be essential in the EO of P. hydropiper [30].
3.2. Chemosystematic Significance via Multivariate Analysis
The major compounds of the P. equisetiformes EO (>2.5%) and those of the other Polygonum species, including P. odoratum, P. lapathifolium, P. arenarium, P. aviculare, P. arenastrum, P. bellardii, P. cognatum, P. persicaria [30], P. minus [31], and P. bistorta subsp. carneum [32], were used to construct the chemo-systematic significance of the species. The foundation of this work was the multivariate analysis of the principal components of the various Polygonum plants, including principal component analysis (PCA) (Figure 3), hierarchical clustering, and constellation plot (Figure 4). Also, the correlation between the different studies Polygonum species and their essential oils compounds are presented in Figure S1.
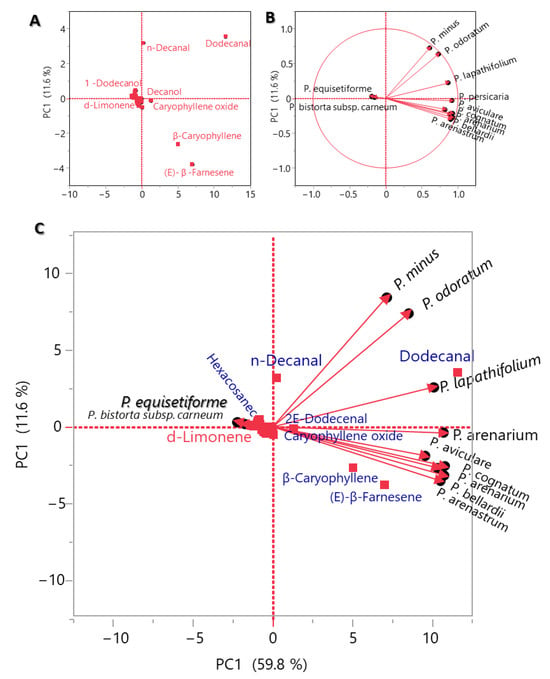
Figure 3.
PCA of main constituents of EO of P. equisetiforme and the Polygonum species: the PCA space observation (A), the correlation circle (B), and the biplot diagram (C).
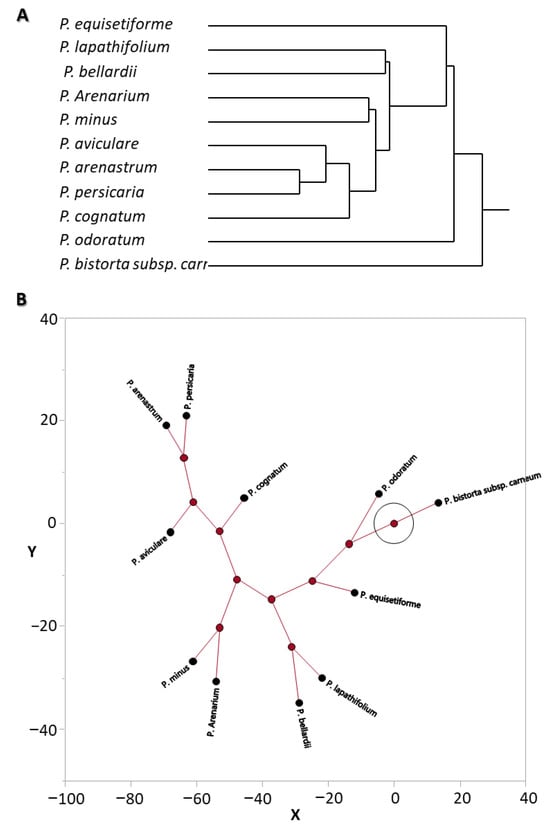
Figure 4.
Cluster analysis of the P. equisetiforme and another 10 Polygonum species based on the abundance of the essential oil compounds: (A) hierarchical clustering and (B) constellation plot.
The PCA analysis showed that P. equisetiforme has a weak association (+11.5%) with the other Polygonum species. The findings demonstrated that the P. equisetiformes EO has a distinctive phenomenon of the existence of sesquiterpenes as the primary components with plenty of hexahydrofarnesyl acetone, 7-epi-selinene, and isospathulenol. This outcome was found in the same line of EOs generated from P. lapathifolium, P. arenarium, P. aviculare, P. arenastrum, P. bellardii, P. cognatum, P. persicaria [30], and P. minus [31]. The main components in some of these Polygonum species have been identified as sesquiterpenes, but the main compounds were different than the EO of P. equisetiforme.
The main constituents of the EOs of P. lapathifolium, P. arenarium, P. aviculare, P. arenastrum, P. bellardii, P. cognatum, and P. persicaria have been found to be β-caryophyllene and (E)-β-farnesene [30]. Caryophyllene oxide was also identified as an essential sesquiterpene in the plants P. lapathifolium, P. arenarium, P. bellardii, and P. persicaria [30]. On the other hand, the abundance of sabinene, γ-terpinene, geranyl acetate, and α-terpineol in P. aviculare, P. bellardii, P. cognatum, and P. arenastrum EOs did not match with the scarcity of monoterpenoids in P. equisetiformes EO in any way [30]. In contrast to the P. equisetiformes EO, which was characterized by the presence of the hydrocarbons as minors, the Polygonum species were distinguished by an abundance of the hydrocarbons, such as dodecanal, n-decanal, tetradecanoic acid, and hexadecanoic acid [30]. The ten Polygonum were evaluated using a hierarchical clustering and constellation plot (Figure 4), which provided groupings of plants based on the correlation. Dodecanal, (E)-β-farnesene, β-caryophyllene, caryophyllene oxide, and β-bisabolene abundances were observed to significantly correlate with each other in P. lapathifolium and P. bellardii [30]. P. arenarium and P. minus displayed a substantial association depending on the presence of oxygenated hydrocarbons like n-decanal and decanol [31]. Similar to this, a strong association between P. arenarium and P. persicaria was established based on the abundance of dodecanal, (E)-β-farnesene, β-caryophyllene, and caryophyllene oxide [31]. In conclusion, P. equisetiforme has varying correlations with the other Polygonum plants, but all our findings are in agreement that the EOs contain high amounts of sesquiterpenes and hydrocarbons.
3.3. Radical Scavenging Activity
Both the DPPH and ABTS techniques were used to evaluate the EO of P. equisetiforme in comparison to vitamin C, a commonly prescribed antioxidant drug (Figure 5). With an increase in EO concentration, it was found that the DPPH radical scavenging increased. The DPPH colors were reduced within the EO concentration at 65.5 and 1000 mg L−1 by 20.10% and 67.16%, respectively. On the opposite side, the ABTS colors were lowered by ratios of 38.22% and 73.49%, respectively, using the same oil concentration. However, the concentrations of 12.5 and 125 mg L−1 of vitamin C, respectively, showed a reduction in the DPPH and ABTS colors of 42.60% and 84.03% (Figure 5). As a result, this EO behaves like a dose-dependent free radical scavenger.
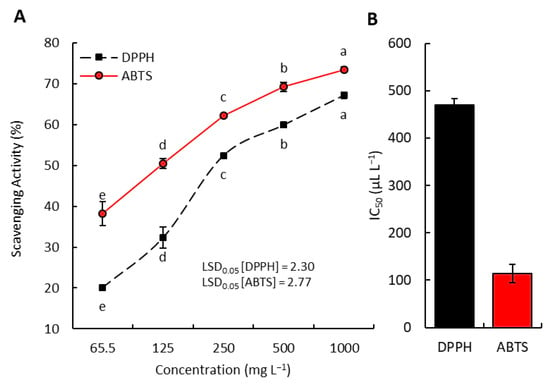
Figure 5.
Antioxidant activity of P. equisetiforme EO: (A) concentration–scavenging activity relation; (B) inhibitory concentration of 50% (IC50). Different letters of each line mean significant difference at p < 0.05.
Aggregate results revealed that P. equisetiforme EO has an efficient DPPH free radical scavenging capacity with an IC50 value of 470.01 mg L−1. At an IC50 value of 113.74 mg L−1, this oil was found to have a considerable ability to scavenge ABTS radicals. Meanwhile, the findings showed that vitamin C, the reference antioxidant used in this study, exhibits IC50 values of 39.06 mg L−1 for DPPH and 26.09 mg L−1 for ABTS, respectively (Figure 6).
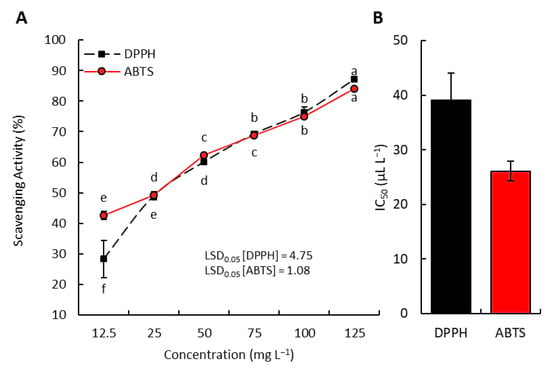
Figure 6.
Antioxidant activity of vitamin C (standard antioxidant): (A) concentration–scavenging activity relation; (B) inhibitory concentration of 50% (IC50). Different letters of each line mean significant difference at p < 0.05.
Since each of the richer OH, NH, and SH components contains an active hydrogen atom, the one-step H-atom transfer (HAT) technique offers a potential mechanism for their function as antioxidants [40]. It was also reported that the two assays’ mechanisms depend on an equal combination of hydrogen atom transfer (HAT) and electron transfer (ET), while the DPPH reacted with free OH in aromatic acids with an unsubstituted OH group but the ABTS did not distinguish between phenolic OH types [41,42]. This fact is the main strategy of the action mechanism in both DPPH and ABTS techniques. From our findings, the oxygenated constituents represented the main components of P. equisetiforme EO, and thus this oil acts as an antioxidant agent. The aliphatic hydroxylated compounds did not react as the action of aromatic acids due to the difficulty of the reaction of the aliphatic OH. So, the increasing of oxygenated components in the EOs lead to an increase in the chance of reaction among the OH groups enabling the radical scavenging ability [43].
Herein, the influence of essential components, such as hexahydrofarnesyl acetone, 7-epi-α-selinene, isospathulenol, and n-docosane, may be responsible for the free radical scavenging features of P. equisetiforme EO. These main compounds, along with the minor components, might function individually or together in a synergistic manner [44,45]. Hexahydrofarnesyl acetone has been identified as a main sesquiterpene found in multiple plants, including Launaea species [46], Kickxia aegyptiaca [47], Heliotropium curassavicum [48], and Bassia muricata [49], which are all sources of powerful antioxidant essential oils. The EOs derived from Eugenia uniflora and Cymbopogon schoenanthus were reported to have a significant antioxidant effect due to the presence of 7-epi-α-selinene as one of its main components [50,51]. Spathulenol and its isomer are very significant antioxidant agents in plants’ EOs [52]. To enable further investigation into the action of these main compounds as antioxidant agents, an in-silico study using MOE software was performed into hematopoietic cell kinase (Hck) and human Peroxiredoxin 5.
3.4. Molecular Docking Studies
The main metabolites of the EO have been docked into hematopoietic cell kinase (Hck) and human Peroxiredoxin 5 using MOE software. The results revealed remarkable affinities of the oxygenated sesquiterpenes toward both proteins as presented in Table 2. Compared to the reference compound, ascorbic acid, all studied compounds showed good binding affinity to Hck with a range of ΔG at −4.93 to −7.08 kcal/mol. Farnesyl acetone and hexahydrofarnesyl acetone revealed the best binding affinity with ΔG = −7.08 and −6.83 kcal/mol in comparison to ascorbic acid with ΔG = −5.12 kcal/mol.

Table 2.
Results of docking studies of the main identified compounds of the essential oil to the active sites of hematopoietic cell kinase (Hck) and human Peroxiredoxin 5. a binding energy.
The acetonic carbonyl of both compounds formed hydrogen bonds with the crucial amino acids Lys 295 and Met 341. Additionally, hydrophobic interactions were formed between an aliphatic chain of both compounds and the amino acids residues Leu 273 and Val 281 (Figure 7).
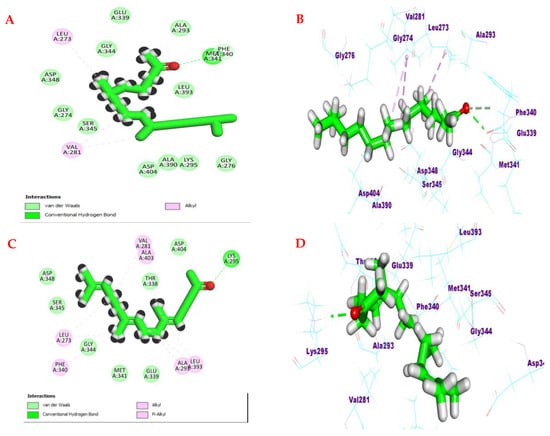
Figure 7.
(A,C) Two-dimensional binding modes and (B,D) three-dimensional binding modes of compounds hexahydrofarnesyl acetone and farnesyl acetone in the active site of hematopoietic cell kinase (Hck) (PDB ID: 2HCK).
Similarly, the investigated compounds exhibited remarkable binding affinity to human Peroxiredoxin 5 with ΔG range −4.46 to −5.47 kcal/mol compared to the ascorbic acid ΔG = −4.32 kcal/mol. In the same way, oxygenated sesquiterpenes, including ar-turmerone, hexahydrofarnesyl acetone, and farnesyl acetone, showed the highest affinity with ΔG = −5.47, 5.34, and −4.93 kcal/mol, respectively. With the crucial amino acid Gly 46, the carbonyl group of three compounds formed hydrogen bonds. Besides, other hydrophilic interactions were formed between the amino acids and three compounds (Figure 8).
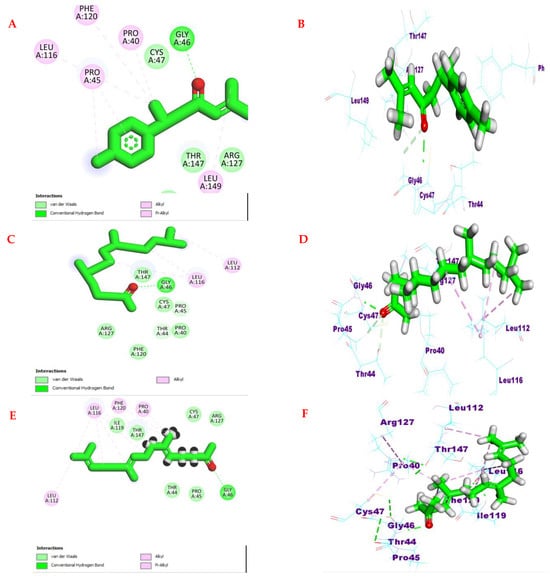
Figure 8.
(A,C,E) Two-dimensional binding modes and (B,D,F) three-dimensional binding modes of compounds ar-turmerone, hexahydrofarnesyl acetone, and farnesyl acetone in the active site ofhuman Peroxiredoxin 5 (PDB ID: 1HD2).
This study pointed out that the antioxidant effect of EO is not restricted only to the phenolic compounds but also to the non-phenolic terpenoids. Baschieri and his coworkers reported that the non-phenolic terpenoids have termination-enhancing antioxidant activity. This antioxidant performance requires higher concentrations than that of phenols. Also, it is much less predictable due to non-concentration linearity [53].
4. Conclusions
The EO of P. equisetiforme was extracted via the hydrodistillation technique. The current GC-MS profiling of P. equisetiforme EO afforded the identification of terpenes as main constituents. Hexahydrofarnesyl acetone, 7-epi-selinene, isospathulenol, and n-docosane were assigned as the major compounds. The chemometric systematic significance of P. equisetiforme with different polygonum plants depending upon the PCA and AHC revealed that this plant has a characteristic chemical fingerprint among all studied plants. The free radical scavenging assaying results revealed that P. equisetiforme EO has efficient DPPH and ABTS radical scavenging abilities compared to vitamin C as a standard antioxidant. The plausible chemo-biological study revealed the significant antioxidant action contribution of the main EO compounds, either singly or in combination. This result was deduced via the MOE study that exhibited the significance of the oxygenated sesquiterpenes especially ar-turmerone, hexahydrofarnesyl acetone, and 5E,9E-farnesyl acetone. The here-presented data highlight the need for additional research on the biological functions of this EO and its constituent parts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry5040151/s1, Figure S1: Correlation matrix between the different studies Polygonum species and their essential oils compounds.
Author Contributions
Conceptualization, A.M.A.-E. and A.I.E.; formal analysis, A.M.A.-E., R.F.A., A.F.E., A.E.-N.G.E.G., S.A.E.-N., A.I.E., and Y.A.E.-A.; investigation, A.M.A.-E., R.F.A., A.F.E., A.E.-N.G.E.G., S.A.E.-N., A.I.E., T.C.S. and Y.A.E.-A.; writing—original draft preparation, A.M.A.-E., R.F.A., A.F.E. and A.I.E.; writing—review and editing, A.M.A.-E., R.F.A., A.F.E., A.E.-N.G.E.G., S.A.E.-N., A.I.E., T.C.S. and Y.A.E.-A.; visualization, A.M.A.-E. and A.I.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by The Researchers Supporting Project number (RSPD2023R676) King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to The Researchers Supporting Project number (RSPD2023R676) King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, T.; Lupo, A.T., Jr.; Chinn, J.W., Jr.; Kang, R.K. Biological activity of essential oils and their constituents. Stud. Nat. Prod. Chem. 2000, 21, 571–631. [Google Scholar]
- Carson, C.F.; Hammer, K.A. Chemistry and bioactivity of essential oils. In Lipids and Essential Oils as Antimicrobial Agents; Thormar, A., Ed.; Wiley & Sons, Ltd.: Chichester, UK, 2011; pp. 203–238. [Google Scholar]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Boughalleb, F.; Bouhamda, T.; Abdellaoui, R.; Nasri, N. Unexploited Polygonum equisetiforme seeds: Potential source of useful natural bioactive products. Ind. Crop. Prod. 2018, 122, 349–357. [Google Scholar] [CrossRef]
- Budel, J.M.; Farago, P.V.; Duarte, M.d.R.; Takeda, I.J. Morpho-anatomical study of the cladodes of Homalocladium platycladum (FJ Muell.) LH Bailey (Polygonaceae). Rev. Bras. Farmacogn. 2007, 17, 39–43. [Google Scholar] [CrossRef]
- Koochak, H.; Seyyednejad, S.M.; Motamedi, H. Preliminary study on the antibacterial activity of some medicinal plants of Khuzestan (Iran). Asian Pac. J. Trop. Med. 2010, 3, 180–184. [Google Scholar] [CrossRef]
- Chen, K.-T.; Zhou, W.-L.; Liu, J.-W.; Zu, M.; He, Z.-N.; Du, G.-H.; Chen, W.-W.; Liu, A.-L. Active neuraminidase constituents of Polygonum cuspidatum against influenza A (H1N1) influenza virus. China J. Chin. Mater. Med. 2012, 37, 3068–3073. [Google Scholar]
- Kuhnlein, H.V.; Turner, N.J. Traditional Plant Foods of Canadian Indigenous Peoples: Nutrition, Botany, and Use; Taylor & Francis: New York, NY, USA, 1991; Volume 8. [Google Scholar]
- Yang, X.; Zou, Y.; Ye, J.; Bao, Z.; Ding, Z. Chemical studies on the plants of Polygonum. Yun Chem. Technol. 2003, 30, 31–33. [Google Scholar]
- Mouldi, G. Natural vegetation cover dynamic under grazing-rotation managements in desert rangelands of Tunisia. Arid Ecosyst. 2014, 20, 66–75. [Google Scholar]
- Khafagi, I.K.; Dewedar, A. The efficiency of random versus ethno-directed research in the evaluation of Sinai medicinal plants for bioactive compounds. J. Ethnopharmacol. 2000, 71, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Facciola, S. Cornucopia: A Source Book of Edible Plants; Kampong Publications: Vista, CA, USA, 1990. [Google Scholar]
- Hussein, S.; Usama, E.-M.; Tantawy, M.; Kawashty, S.; Saleh, N. Phenolics of selected species of Persicaria and Polygonum (Polygonaceae) in Egypt. Arab. J. Chem. 2017, 10, 76–81. [Google Scholar] [CrossRef]
- El-Toumy, S.A.; Salib, J.Y.; Shafik, N.H.; Elkarim, A.; Farag, A. New flavonoids from the aerial parts of Polygonum equisetiforme SM (Polygonaceae). Int. J. Pharm. Pharm. Sci. 2017, 9, 166–170. [Google Scholar] [CrossRef]
- El-Toumy, S.A.H.; Salib, J.Y.; Shafik, N.H.; Abd Elkarim, A.S.; Salama, A.; Omara, E.A.A.; Micky, J. Evaluation of hepatoprotective activity of Polygonum equisetiforme methanolic extract. J. Appl. Pharm. Sci. 2019, 9, 054–059. [Google Scholar]
- Seo, M.S.; Kang, S.W.; Kim, K.; Baines, I.C.; Lee, T.H.; Rhee, S.G. Identification of a new type of mammalian Peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J. Biol. Chem. 2000, 275, 20346–20354. [Google Scholar] [CrossRef]
- Poh, A.R.; O’Donoghue, R.J.; Ernst, M. Hematopoietic cell kinase (HCK) as a therapeutic target in immune and cancer cells. Oncotarget 2015, 6, 15752–15771. [Google Scholar] [CrossRef]
- Boulos, L. Flora of Egypt; Al-Hadara Publishing: Cairo, Egypt, 1999; Volume 1. [Google Scholar]
- Tackholm, V. Students’ Flora of Egypt, 2nd ed.; Cairo University Press: Cairo, Egypt, 1974. [Google Scholar]
- El Gendy, A.E.-N.G.; Essa, A.F.; El-Rashedy, A.A.; Elgamal, A.M.; Khalaf, D.D.; Hassan, E.M.; Abd-ElGawad, A.M.; Elgorban, A.M.; Zaghloul, N.S.; Alamery, S.F. Antiviral potentialities of chemical characterized essential oils of Acacia nilotica bark and fruits against hepatitis A and herpes simplex viruses: In vitro, in silico, and molecular dynamics studies. Plants 2022, 11, 2889. [Google Scholar] [CrossRef]
- Abdelhameed, M.F.; Asaad, G.F.; Ragab, T.I.; Ahmed, R.F.; El Gendy, A.E.-N.G.; Abd El-Rahman, S.S.; Elgamal, A.M.; Elshamy, A.I. Oral and topical anti-inflammatory and antipyretic potentialities of Araucaria bidiwillii shoot essential oil and its nanoemulsion in relation to chemical composition. Molecules 2021, 26, 5833. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE), 2015. Chemical Computing Group. Canada, Montreal. Available online: http://www.chemcomp.com (accessed on 6 September 2023).
- Declercq, J.-P.; Evrard, C.; Clippe, A.; Vander Stricht, D.; Bernard, A.; Knoops, B. Crystal structure of human Peroxiredoxin 5, a novel type of mammalian Peroxiredoxin at 1.5 Å resolution. J. Mol. Biol. 2001, 311, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Sicheri, F.; Moarefi, I.; Kuriyan, J. Crystal structure of the Src family tyrosine kinase Hck. Nature 1997, 385, 602–609. [Google Scholar] [CrossRef]
- Demirpolat, A. Chemical Composition of Essential Oils of Seven Polygonum Species from Turkey: A Chemotaxonomic Approach. Molecules 2022, 27, 9053. [Google Scholar] [CrossRef] [PubMed]
- Yaacob, K.B. Essential Oil of Polygonum minus Huds. J. Essent. Oil Res. 1990, 2, 167–172. [Google Scholar] [CrossRef]
- Iskender, N.Y.; Güleç, C.A.; Yücel, M.; Sinek, K.; Yayli, N. Analysis of the essential oil from the flower of Polygonum bistorta L. subsp. carneum (Koch). Asian J. Chem. 2011, 23, 1940–1942. [Google Scholar]
- Alarcón, L.; Peña, A.; Velasco, J.; Baptista, J.G.; Rojas, L.; Aparicio, R.; Usubillaga, A. Chemical composition and antibacterial activity of the essential oil of Ruilopezia bracteosa. Nat. Prod. Commun. 2015, 10, 655–656. [Google Scholar] [CrossRef]
- Ali, N.A.A.; Chhetri, B.K.; Dosoky, N.S.; Shari, K.; Al-Fahad, A.J.; Wessjohann, L.; Setzer, W.N. Antimicrobial, antioxidant, and cytotoxic activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) essential oils. Medicines 2017, 4, 17. [Google Scholar] [CrossRef]
- Pereira, R.A.; Ramos, Y.J.; de Queiroz, G.A.; Guimarães, E.F.; Defaveri, A.C.A.; de Lima Moreira, D. Chemodiversity of Essential Oils in Piper L.(Piperaceae) Species from the Restinga of Marambaia Island, Rio de Janeiro-RJ, Brazil. Rev. Virtual Química 2021, 13, 1203–1215. [Google Scholar] [CrossRef]
- Martínez-Arévalo, J.V.; Cruz, S.M.; Apel, M.A.; Henriques, A.T.; Cáceres, A. Essential Oil of Piper oradendron from the Pacific Slope of Guatemala. Nat. Prod. Commun. 2019, 14, 79–81. [Google Scholar]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A model for exploring labdane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front. Chem. 2014, 2, 35. [Google Scholar] [CrossRef]
- Thulasiram, H.V.; Erickson, H.K.; Poulter, C.D. Chimeras of two isoprenoid synthases catalyze all four coupling reactions in isoprenoid biosynthesis. Science 2007, 316, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Essa, A.F.; El-Hawary, S.S.; Abd-El Gawad, A.M.; Kubacy, T.M.; AM El-Khrisy, E.E.D.; Elshamy, A.I.; Younis, I.Y. Prevalence of diterpenes in essential oil of Euphorbia mauritanica L.: Detailed chemical profile, antioxidant, cytotoxic and phytotoxic activities. Chem. Biodivers. 2021, 18, e2100238. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhao, M.; Xiao, C.; Zhao, Q.; Su, G. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides: Importance of controlling reaction pH and time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef] [PubMed]
- El-Kashak, W.A.; Elshamy, A.I.; Mohamed, T.A.; El Gendy, A.E.-N.G.; Saleh, I.A.; Umeyama, A. Rumpictuside A: Unusual 9, 10-anthraquinone glucoside from Rumex pictus Forssk. Carbohydr. Res. 2017, 448, 74–78. [Google Scholar] [CrossRef]
- Hammad, H.M.; Albu, C.; Matar, S.A.; Litescu, S.-C.; Al Jaber, H.; Abualraghib, A.; Afifi, F.U. Biological activities of the hydro-alchoholic and aqueous extracts of Achillea biebersteinii Afan.(Asteraceae) grown in Jordan. Afr. J. Pharm. Pharmacol. 2013, 7, 1686–1694. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1, 8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef]
- Tak, J.H.; Jovel, E.; Isman, M.B. Comparative and synergistic activity of Rosmarinus officinalis L. essential oil constituents against the larvae and an ovarian cell line of the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae). Pest Manag. Sci. 2016, 72, 474–480. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Abd-ElGawad, A.M.; El-Amier, Y.A.; El Gendy, A.E.N.G.; Al-Rowaily, S.L. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragr. J. 2019, 34, 316–328. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; El-Amier, Y.A.; Bonanomi, G.; Gendy, A.E.-N.G.E.; Elgorban, A.M.; Alamery, S.F.; Elshamy, A.I. Chemical composition of Kickxia aegyptiaca essential oil and its potential antioxidant and antimicrobial activities. Plants 2022, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.M.; Elshamy, A.I.; Al-Rowaily, S.L.; El-Amier, Y.A. Habitat affects the chemical profile, allelopathy, and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant Heliotropium curassavicum. Plants 2019, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.; El Gendy, A.E.-N.; El-Amier, Y.; Gaara, A.; Omer, E.; Al-Rowaily, S.; Assaeed, A.; Al-Rashed, S.; Elshamy, A. Essential oil of Bassia muricata: Chemical characterization, antioxidant activity, and allelopathic effect on the weed Chenopodium murale. Saudi J. Biol. Sci. 2020, 27, 1900–1906. [Google Scholar] [CrossRef]
- Cipriano, R.R.; Maia, B.H.; Deschamps, C. Chemical variability of essential oils of Eugenia uniflora L. genotypes and their antioxidant activity. An. Acad. Bras. Ciências 2021, 93, e20181299. [Google Scholar] [CrossRef]
- Yagi, S.; Babiker, R.; Tzanova, T.; Schohn, H. Chemical composition, antiproliferative, antioxidant and antibacterial activities of essential oils from aromatic plants growing in Sudan. Asian Pac. J. Trop. Med. 2016, 9, 763–770. [Google Scholar] [CrossRef]
- Gourine, N.; Yousfi, M.; Bombarda, I.; Nadjemi, B.; Stocker, P.; Gaydou, E. Antioxidant activities and chemical composition of essential oil of Pistacia atlantica from Algeria. Ind. Crop. Prod. 2010, 31, 203–208. [Google Scholar] [CrossRef]
- Baschieri, A.; Ajvazi, M.D.; Tonfack, J.L.F.; Valgimigli, L.; Amorati, R. Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem. 2017, 232, 656–663. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).