Achillea fragrantissima Essential Oil, Wild Grown in Saudi Arabia and Egypt: Detailed Comparative Chemical Profiling, and Evaluation of Allelopathic, Antioxidant, and Antibacterial Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of the Plant Samples
2.2. Isolation of EO Samples via Hydrodistillation
2.3. EOs Analysis via the GC Flame Ionization Detector (GC-FID)
2.4. Gas Chromatography Coupled to Mass Spectrometry (GC–MS) Analysis of EOs
2.5. Biological Assays
2.5.1. Allelopathic Activity against the Weed, Chenopodium murale
2.5.2. Antioxidant Assessments
2.5.3. Antibacterial Effects on the Gram-Negative and Gram-Positive Bacteria
2.6. Data Treatment and Chemometric Analysis
3. Results and Discussions
3.1. Composition of EOs Derived from Saudi Arabian and Egyptian A. fragrantissima
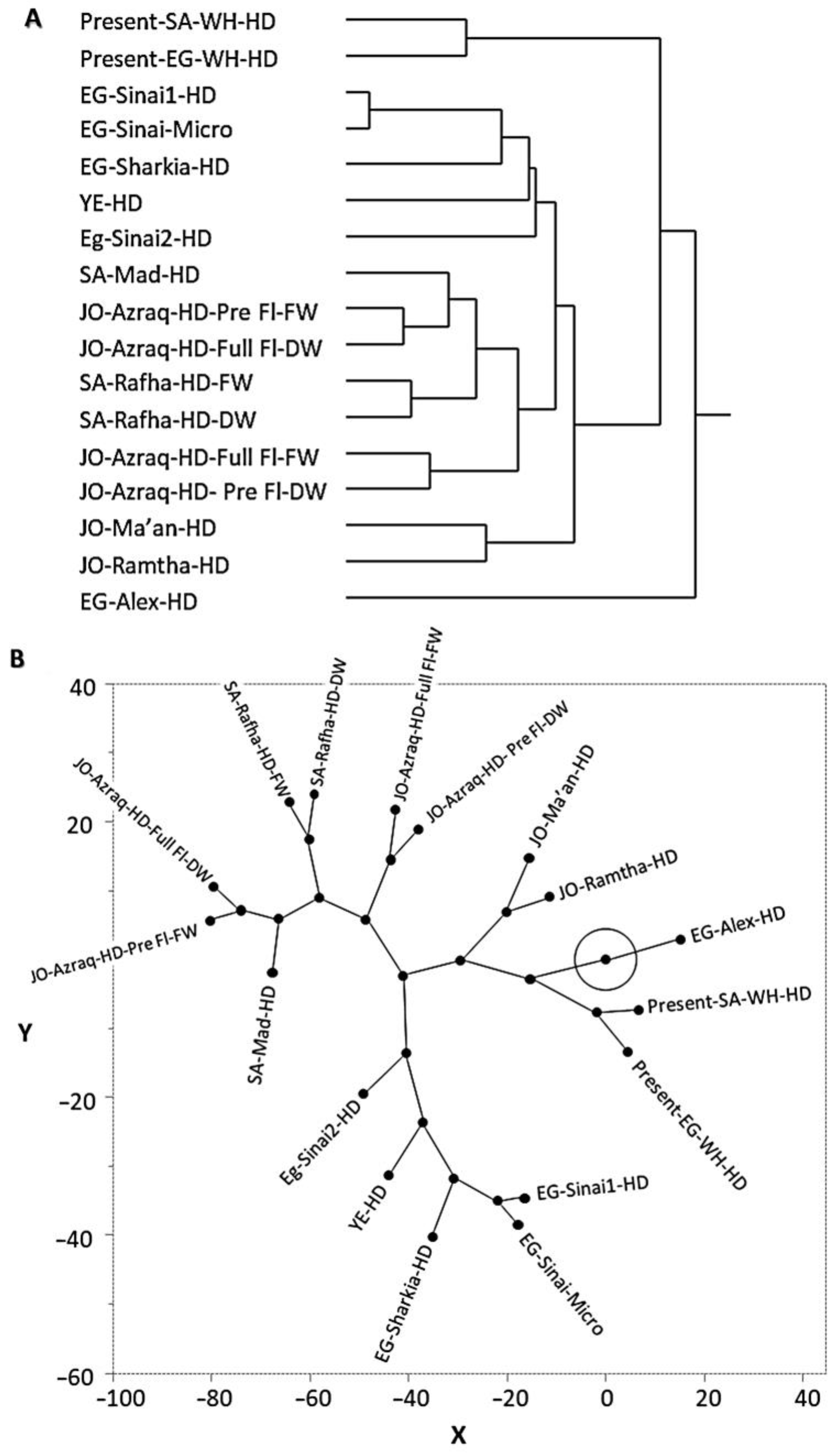
3.2. Multivariate Chemometric Analysis
3.3. Biological Activities of A. fragrantissima EOs
3.3.1. Allelopathic Activity
3.3.2. Antioxidant Potencies
3.3.3. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horváth, G.; Ács, K. Essential oils in the treatment of respiratory tract diseases highlighting their role in bacterial infections and their anti-inflammatory action: A review. Flavour Fragr. J. 2015, 30, 331–341. [Google Scholar] [CrossRef]
- Rehman, N.U.; Salkini, M.A.A.; Alanizi, H.M.; Alharbi, A.G.; Alqarni, M.H.; Abdel-Kader, M.S. Achillea fragrantissima Essential Oil: Composition and Detailed Pharmacodynamics Study of the Bronchodilator Activity. Separations 2022, 9, 334. [Google Scholar] [CrossRef]
- Luna, E.; Luna, I.; Scotti, L.; Monteiro, A.; Scotti, M.; de Moura, R. Active essential oils and their components in use against neglected diseases and arboviruses. Oxid. Med. Cell. Longev. 2019, 2019, 6587150. [Google Scholar] [CrossRef] [PubMed]
- Soltan, M.M.; Zaki, A.K. Antiviral screening of forty-two Egyptian medicinal plants. J. Ethnopharmacol. 2009, 126, 102–107. [Google Scholar] [CrossRef]
- Elshamy, A.; Abd-ElGawad, A.; Mohamed, T.; El Gendy, A.E.N.; Abd El Aty, A.A.; Saleh, I.; Moustafa, M.F.; Hussien, T.A.; Pare, P.W.; Hegazy, M.E.F. Extraction development for antimicrobial and phytotoxic essential oils from Asteraceae species: Achillea fragrantissima, Artemisia judaica and Tanacetum sinaicum. Flavour Fragr. J. 2021, 36, 352–364. [Google Scholar] [CrossRef]
- Palombo, E.A.; Semple, S.J. Antibacterial activity of traditional Australian medicinal plants. J. Ethnopharmacol. 2001, 77, 151–157. [Google Scholar] [CrossRef]
- Bartolotti, N.; Disouky, A.; Kalinski, A.; Elmann, A.; Lazarov, O. Phytochemicals from Achillea fragrantissima are modulators of AβPP metabolism. J. Alzheimer’s Dis. 2018, 66, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Navratilova, Z. Achillea fragrantissima: Pharmacology review. Clin. Oncol. 2019, 4, 1601. [Google Scholar]
- Khan, M.; Khan, M.; Abdullah, M.M.; Al-Wahaibi, L.H.; Alkhathlan, H.Z. Characterization of secondary metabolites of leaf and stem essential oils of Achillea fragrantissima from central region of Saudi Arabia. Arab. J. Chem. 2020, 13, 5254–5261. [Google Scholar] [CrossRef]
- Abdel-Azim, N.S.; Shams, K.A.; Shahat, A.; El Missiry, M.M.; Ismail, S.I.; Hammouda, F.M. Egyptian herbal drug industry: Challenges and future prospects. Res. J. Med. Plant 2011, 5, 136–144. [Google Scholar] [CrossRef]
- Eissa, T.; Palomino, O.; Carretero, M.; Gómez-Serranillos, M. Ethnopharmacological study of medicinal plants used in the treatment of CNS disorders in Sinai Peninsula, Egypt. J. Ethnopharmacol. 2014, 151, 317–332. [Google Scholar] [CrossRef] [PubMed]
- El-Ashmawy, I.M.; Al-Wabel, N.A.; Bayad, A.E. Achillea fragrantissima, rich in flavonoids and tannins, potentiates the activity of diminazine aceturate against Trypanosoma evansi in rats. Asian Pac. J. Trop. Med. 2016, 9, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Choucry, M.A. Chemical composition and anticancer activity of Achillea fragrantissima (Forssk.) Sch. Bip. (Asteraceae) essential oil from Egypt. J. Pharmacogn. Phytother. 2017, 9, 1–5. [Google Scholar]
- Farouk, A.; Ali, H.; Al-Khalifa, A.R.; Mohsen, M.; Fikry, R. Comparative study for the volatile constituents and the antioxidant activity of the essential oils of dried Achillea fragrantissima cultivated in Madinah Monawara, Saudi Arabia and Egypt. Int. J. Food Prop. 2019, 22, 395–404. [Google Scholar] [CrossRef]
- El-Shazly, A.; Hafez, S.; Wink, M. Comparative study of the essential oils and extracts of Achillea fragrantissima (Forssk.) Sch. Bip. and Achillea santolina L. (Asteraceae) from Egypt. Die Pharm.-Int. J. Pharm. Sci. 2004, 59, 226–230. [Google Scholar]
- Kaul, P.; Rajeswara Rao, B.; Bhattacharya, A.; Singh, K. Effect of weather parameters on yield and quality of the essential oil of rose-scented geranium (Pelargonium species). Agric. Sci. Dig. 1999, 19, 84–86. [Google Scholar]
- Al-Jaber, H.I.; Abu Zarga, M.H.; Al-Aboudi, A.F.; Al-Qudah, M.A.; Al-Shawabkeh, A.F.; Abaza, I.F.; Abuaisheh, M.N.; Afifi, F.U. Essential oil composition and anticholinesterase activity evaluation of Achillea fragrantissima growing wild in Jordan. J. Herbs Spices Med. Plants 2018, 24, 272–281. [Google Scholar] [CrossRef]
- Ahmed, W.; Aburjai, T.; Hudaib, M.; Al-Karablieh, N. Chemical composition of essential oils hydrodistilled from aerial parts of Achillea fragrantissima (Forssk.) Sch. Bip. and Achillea santolina L. (Asteraceae) growing in Jordan. J. Essent. Oil Bear. Plants 2020, 23, 15–25. [Google Scholar] [CrossRef]
- Mansi, I.; Ali, N.A.A.; Mhaidat, N.M.; Hussain, K.; Al-Kaf, A.G.; Anwar, S.; Setzer, W.N. Chemical composition and biological activity of the essential oil isolated from the leaves of Achillea fragrantissima growing wild in Yemen. Pharmacogn. J. 2019, 11, 1077–1081. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Al-Namazi, A.A.; Assaeed, A.M.; Al-Huqail, A.A.; Dar, B.A.; Elshamy, A.I. Insights into the chemical composition of Ocimum forskaolii and O. americanum essential oils and their phytotoxicity. J. Essent. Oil Bear. Plants 2023, 26, 1–18. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.; El Gendy, A.; Elshamy, A.; Omer, E. Chemical composition of the essential oil of Trianthema portulacastrum L. Aerial parts and potential antimicrobial and phytotoxic activities of its extract. J. Essent. Oil Bear. Plants 2016, 19, 1684–1692. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Ammar, N.M.; Hassan, H.A.; Al-Rowaily, S.L.; Ragab, T.I.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M. Essential oil and its nanoemulsion of Araucaria heterophylla resin: Chemical characterization, anti-inflammatory, and antipyretic activities. Ind. Crops Prod. 2020, 148, 112272. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Assaeed, A.M.; Al-Rowaily, S.L.; Alshahri, M.S.; Bonanomi, G.; Elshamy, A.I. Influence of season and habitat on the essential oils composition, allelopathy, and antioxidant activities of Artemisia monosperma Delile. Separations 2023, 10, 263. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Almadiy, A.A.; Nenaah, G.E.; Al Assiuty, B.A.; Moussa, E.A.; Mira, N.M. Chemical composition and antibacterial activity of essential oils and major fractions of four Achillea species and their nanoemulsions against foodborne bacteria. LWT-Food Sci. Technol. 2016, 69, 529–537. [Google Scholar] [CrossRef]
- Nenaah, G.E. Bioactivity of powders and essential oils of three Asteraceae plants as post-harvest grain protectants against three major coleopteran pests. J. Asia-Pac. Entomol. 2014, 17, 701–709. [Google Scholar] [CrossRef]
- Nenaah, G.E.; Ibrahim, S.I.; Al-Assiuty, B.A. Chemical composition, insecticidal activity and persistence of three Asteraceae essential oils and their nanoemulsions against Callosobruchus maculatus (F.). J. Stored Prod. Res. 2015, 61, 9–16. [Google Scholar] [CrossRef]
- Zeedan, G.; Abdalhamed, A.; Ottai, M.; Abdelshafy, S.; Abdeen, E. Antimicrobial, antiviral activity and GC-MS analysis of essential oil extracted from Achillea fragrantissima plant growing in Sinai Peninsula. Egypt. J. Microb. Biochem. Technol. S 2014, 8, 1–7. [Google Scholar]
- Alsohaili, S. Seasonal variation in the chemical composition and antimicrobial activity of essential oil extracted from Achillea fragrantissima grown in northern-eastern Jordanian desert. J. Essent. Oil Bear. Plants 2018, 21, 139–145. [Google Scholar] [CrossRef]
- Alsohaili, S.A.; Al-fawwaz, A.T. Composition and antimicrobial activity of Achillea fragrantissima essential oil using food model media. Eur. Sci. J. 2014, 10, 156–165. [Google Scholar]
- Aćimović, M.; Lončar, B.; Stanković Jeremić, J.; Cvetković, M.; Pezo, L.; Pezo, M.; Todosijević, M.; Tešević, V. Weather conditions influence on lavandin essential oil and hydrolate quality. Horticulturae 2022, 8, 281. [Google Scholar] [CrossRef]
- Mehalaine, S.; Chenchouni, H. Quantifying how climatic factors influence essential oil yield in wild-growing plants. Arab. J. Geosci. 2021, 14, 1257. [Google Scholar] [CrossRef]
- Chang, X.; Alderson, P.; Wright, C. Effect of temperature integration on the growth and volatile oil content of basil (Ocimum basilicum L.). J. Hortic. Sci. Biotechnol. 2005, 80, 593–598. [Google Scholar] [CrossRef]
- El-Sherei, M.; Khaleel, A.; Motaal, A.A.; Abd-Elbaki, P. Effect of seasonal variation on the composition of the essential oil of Solidago canadensis cultivated in Egypt. J. Essent. Oil Bear. Plants 2014, 17, 891–898. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, H.; Wen, S.; Qiu, F.; Liu, X. Effects of harvest season and storage time on the essential oil of the linalool chemotype of Cinnamomum camphora. J. Essent. Oil Bear. Plants 2019, 22, 1379–1385. [Google Scholar] [CrossRef]
- Karalija, E.; Dahija, S.; Tarkowski, P.; Zeljković, S.Ć. Influence of Climate-Related Environmental Stresses on Economically Important Essential Oils of Mediterranean Salvia sp. Front Plant Sci. 2022, 13, 864807. [Google Scholar] [CrossRef]
- Thinh, B.B.; Chac, L.D.; Hanh, D.H.; Korneeva, A.A.; Hung, N.; Igoli, J.O. Effect of extraction method on yield, chemical composition and antimicrobial activity of essential oil from the fruits of Amomum villosum var. xanthioides. J. Essent. Oil Bear. Plants 2022, 25, 28–37. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Hwang, M.H.; Sacks, E.J.; Yu, C.Y.; Kim, S.H.; Chung, I.M. Screening of allelochemicals in miscanthus sacchariflorus extracts and assessment of their effects on germination and seedling growth of common weeds. Plants 2020, 9, 1313. [Google Scholar] [CrossRef]
- Omezzine, F.; Ladhari, A.; Haouala, R. Physiological and biochemical mechanisms of allelochemicals in aqueous extracts of diploid and mixoploid Trigonella foenum-graecum L. S. Afr. J. Bot. 2014, 93, 167–178. [Google Scholar] [CrossRef]
- Wang, K.; Wang, T.; Ren, C.; Dou, P.; Miao, Z.; Liu, X.; Huang, D.; Wang, K. Aqueous extracts of three herbs allelopathically inhibit lettuce germination but promote seedling growth at low concentrations. Plants 2022, 11, 486. [Google Scholar] [CrossRef]
- Staszek, P.; Krasuska, U.; Ciacka, K.; Gniazdowska, A. ROS metabolism perturbation as an element of mode of action of allelochemicals. Antioxidants 2021, 10, 1648. [Google Scholar] [CrossRef] [PubMed]
- Santonja, M.; Bousquet-Mélou, A.; Greff, S.; Ormeño, E.; Fernandez, C. Allelopathic effects of volatile organic compounds released from Pinus halepensis needles and roots. Ecol. Evol. 2019, 9, 8201–8213. [Google Scholar] [CrossRef] [PubMed]
- Zámboriné Németh, É.; Thi Nguyen, H. Thujone, a widely debated volatile compound: What do we know about it? Phytochem. Rev. 2020, 19, 405–423. [Google Scholar] [CrossRef]
- Menelaou, M.A.; Macias, F.A.; Weidenhamer, J.D.; Williamson, G.B.; Fischer, N.H. Sesquiterpenes from Chrysoma pauciflosculosa. Spectrosc. Lett. 1995, 28, 1061–1074. [Google Scholar] [CrossRef]
- Ferreira, J.; Janick, J. Allelopathic plants. XVI. Artemisia species. Allelopath. J. 2004, 14, 167–176. [Google Scholar]
- Verma, R.S.; Joshi, N.; Padalia, R.C.; Goswami, P.; Singh, V.R.; Chauhan, A.; Verma, S.K.; Iqbal, H.; Verma, R.K.; Chanda, D. Chemical composition and allelopathic, antibacterial, antifungal and in vitro acetylcholinesterase inhibitory activities of yarrow (Achillea millefolium L.) native to India. Ind. Crops Prod. 2017, 104, 144–155. [Google Scholar] [CrossRef]
- Wei, C.; Zhou, S.; Li, W.; Jiang, C.; Yang, W.; Han, C.; Zhang, C.; Shao, H. Chemical composition and allelopathic, phytotoxic and pesticidal activities of Atriplex cana Ledeb.(Amaranthaceae) essential oil. Chem. Biodivers. 2019, 16, e1800595. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; Barbosa de Moraes, A.A.; Santana da Costa, K.; Pereira Galúcio, J.M.; Taube, P.S.; Leal Costa, C.M.; Neves Cruz, J.; de Aguiar Andrade, E.H.; Guerreiro de Faria, L.J. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Valgimigli, L. Essential oils: An overview on origins, chemistry, properties and uses. In Essential Oils as Natural Food Additives: Composition, Applications, Antioxidant and Antimicrobial Properties; Nova Science Pub Inc.: New York, NY, USA, 2012; pp. 1–24. [Google Scholar]
- Foti, M.C. Antioxidant properties of phenols. J. Pharm. Pharmacol. 2007, 59, 1673–1685. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Pandini, J.; Pinto, F.; Scur, M.; Santana, C.; Costa, W.; Temponi, L. Chemical composition, antimicrobial and antioxidant potential of the essential oil of Guarea kunthiana A. juss. Braz. J. Biol. 2017, 78, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Guleria, S.; Kumar, A.; Tiku, A.K. Chemical composition and fungitoxic activity of essential oil of Thuja orientalis L. grown in the north-western Himalaya. Z. Naturforschung C 2008, 63, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Guleria, S.; Tiku, A.; Gupta, S.; Singh, G.; Koul, A.; Razdan, V. Chemical composition, antioxidant activity and inhibitory effects of essential oil of Eucalyptus teretecornis grown in north-western Himalaya against Alternaria alternata. J. Plant Biochem. Biotechnol. 2012, 21, 44–50. [Google Scholar] [CrossRef]
- Guleria, S.; Tiku, A.; Koul, A.; Gupta, S.; Singh, G.; Razdan, V. Antioxidant and antimicrobial properties of the essential oil and extracts of Zanthoxylum alatum grown in North-Western Himalaya. Sci. World J. 2013, 2013, 790580. [Google Scholar] [CrossRef]



| No | Compound Name | KI | Collected from | Type | Identification | |||
|---|---|---|---|---|---|---|---|---|
| Publ. | Exp. | Saudi Arabia | Egypt | |||||
| 1 | Santolinatriene | 905 | 905 | 0.3 ± 0.01 * | 2.0 ± 0.06 | MH | MS, KI | |
| 2 | α-Pinene | 936 | 938 | ND | 0.3 ± 0.01 | MH | MS, KI | |
| 3 | Yomogi alcohol | 996 | 994 | 0.6 ± 0.02 | 6.2 ± 0.11 | OM | MS, KI | |
| 4 | p-Cymene | 1026 | 1025 | 0.3 ± 0.01 | 0.1 ± 0.00 | MH | MS, KI | |
| 5 | Santolina alcohol | 1038 | 1040 | 4.0 ± 0.15 | 6.2 ± 0.21 | OM | MS, KI | |
| 6 | Artemisia ketone | 1062 | 1061 | 4.9 ± 0.14 | 9.00 ± 0.23 | OM | MS, KI | |
| 7 | Artemesia alcohol | 1083 | 1084 | 0.7 ± 0.01 | 3.3 ± 0.09 | OM | MS, KI | |
| 8 | α-Thujone | 1105 | 1103 | 12.0 ± 0.26 | 8.6 ± 0.22 | OM | MS, KI | |
| 9 | β-Thujone | 1115 | 1116 | 4.7 ± 0.13 | 5.8 ± 0.18 | OM | MS, KI | |
| 10 | 3-Thujanone | 1124 | 1126 | 3.0 ± 0.10 | 1.8 ± 0.06 | OM | MS, KI | |
| 11 | cis-Sabinol | 1140 | 1143 | 0.3 ± 0.01 | 0.5 ± 0.02 | OM | MS, KI | |
| 12 | Lavandulol | 1171 | 1173 | 4.2 ± 0.17 | 3.6 ± 0.08 | OM | MS, KI | |
| 13 | 4-Terpineol | 1198 | 1197 | 2.6 ± 0.05 | 17.4 ± 0.31 | OM | MS, KI | |
| 14 | cis-Geraniol | 1255 | 1253 | 0.8 ± 0.03 | - | OM | MS, KI | |
| 15 | Myrcenyl acetate | 1261 | 1260 | 10.3 ± 0.29 | 9.1 ± 0.23 | OM | MS, KI | |
| 16 | Sabinyl acetate | 1291 | 1293 | 0.2 ± 0.01 | - | OM | MS, KI | |
| 17 | p-Cymen-7-ol (Cumic alcohol) | 1287 | 1289 | 2.3 ± 0.06 | 1.5 ± 0.08 | OM | MS, KI | |
| 18 | Benzene acetic acid, ethyl ester | 1246 | 1244 | ND | 0.3 ± 0.01 | OH | MS, KI | |
| 19 | Nerol acetate | 1365 | 1367 | 1.4 ± 0.04 | - | OM | MS, KI | |
| 20 | Germacrene D | 1480 | 1483 | 2.8 ± 0.07 | 1.1 ± 0.03 | SH | MS, KI | |
| 21 | Valencene | 1493 | 1491 | 1.4 ± 0.04 | 1.1 ± 0.03 | SH | MS, KI | |
| 22 | Spathulenol | 1516 | 1514 | 2.0 ± 0.05 | - | OS | MS, KI | |
| 23 | α-Sesquiphellandrene (Zingiberene) | 1526 | 1525 | 11.6 ± 0.10 | 7.0 ± 0.12 | SH | MS, KI | |
| 24 | Caryophyllene oxide | 1573 | 1573 | 3.4 ± 0.07 | 2.4 ± 0.05 | OS | MS, KI | |
| 25 | trans-Sesquisabinene hydrate | 1577 | 1575 | 0.8 ± 0.03 | ND | OS | MS, KI | |
| 26 | Isoaromadendrene epoxide | 1594 | 1596 | 0.2 ± 0.01 | ND | OS | MS, KI | |
| 27 | Neoclovenoxid-alcohol | 1608 | 1609 | 1.2 ± 0.05 | ND | OS | MS, KI | |
| 28 | Salvial-4(14)-en-1-one | 1612 | 1611 | 1.6 ± 0.05 | 0.3 ± 0.01 | OS | MS, KI | |
| 29 | Aromadendrene oxide-(2) | 1631 | 1630 | 0.4 ± 0.01 | ND | OS | MS, KI | |
| 30 | .tau.-Cadinol | 1640 | 1638 | 1.9 ± 0.07 | 1.0 ± 0.03 | OS | MS, KI | |
| 31 | Alloaromadendrene oxide-(1) | 1641 | 1640 | 5.9 ± 0.14 | 3.3 ± 0.08 | OS | MS, KI | |
| 32 | α-Eudesmol | 1653 | 1652 | 0.6 ± 0.02 | ND | OS | MS, KI | |
| 33 | α-Bisabolol | 1685 | 1686 | 1.4 ± 0.06 | 0.6 ± 0.01 | OS | MS, KI | |
| 34 | Ledene oxide | 1682 | 1681 | 5.9 ± 0.21 | 2.9 ± 0.15 | OS | MS, KI | |
| 35 | Davanol acetate | 1689 | 1691 | 0.3 ± 0.01 | ND | OS | MS, KI | |
| 36 | Hexahydrofarnesyl acetone | 1845 | 1845 | 0.4 ± 0.02 | ND | OS | MS, KI | |
| 37 | n-Docosane | 2200 | 2201 | 0.7 ± 0.02 | ND | OH | MS, KI | |
| 38 | n-Heptacosane | 2700 | 2700 | 1.0 ± 0.04 | 1.0 ± 0.02 | OH | MS, KI | |
| 39 | n-Nonacosane | 2900 | 2900 | 0.4 ± 0.01 | ND | OH | MS, KI | |
| 40 | cis-Vaccenic acid | 2141 | 2140 | 0.4 ± 0.01 | ND | OH | MS, KI | |
| Monoterpenes | Hydrocarbons (MH) | 0.6 | 2.4 | |||||
| Oxygenated (OM) | 52.0 | 73.0 | ||||||
| Sesquiterpenes | Hydrocarbons (SH) | 15.8 | 9.2 | |||||
| Oxygenated (OS) | 26.3 | 10.5 | ||||||
| Oxygenated hydrocarbons (OH) | 2.1 | 1.0 | ||||||
| Total | 96.9 | 96.1 | ||||||
| Treatment | Conc. (mg/L) | Scavenging Activity (%) | |||
|---|---|---|---|---|---|
| DPPH | IC50 (mg/L) | ABTS | IC50 (mg/L) | ||
| Egyptian A. fragrantissima | 5 | 10.93 ± 0.29 HI | 30.94 | 9.58 ± 0.21 I | 39.02 |
| 10 | 19.27 ± 0.38 G | 14.74 ± 0.34 H | |||
| 20 | 32.13 ± 0.91 E | 26.21 ± 0.78 F | |||
| 30 | 53.91 ± 1.57 C | 40.68 ± 1.06 D | |||
| 40 | 62.35 ± 1.89 B | 53.39 ± 1.62 C | |||
| 50 | 76.37 ± 2.16 A | 61.05 ± 2.05 B | |||
| LSD0.05 | 4.09 *** | ||||
| F-value | 263.54 | ||||
| Saudi Arabian A. fragrantissima | 5 | 13.58 ± 0.41 HI | 28.72 | 11.727 ± 0.36 I | |
| 10 | 22.67 ± 0.69 G | 16.34 ± 0.50 H | |||
| 20 | 35.08 ± 1.06 E | 27.92 ± 0.85 F | |||
| 30 | 56.56 ± 1.71 C | 42.97 ± 1.30 D | 37.13 | ||
| 40 | 67.41 ± 2.04 B | 56.08 ± 1.70 C | |||
| 50 | 79.02 ± 2.39 A | 63.20 ± 1.92 B | |||
| LSD0.05 | 4.32 *** | ||||
| F-value | 243.94 | ||||
| Ascorbic acid | 1 | 4.80 ± 0.11 EF | 11.78 | 2.14 ± 0.09 F | |
| 2.5 | 14.02 ± 0.42 E | 10.36 ± 0.32 E | |||
| 5 | 40.74 ± 1.23 D | 37.08 ± 1.04 D | |||
| 10 | 53.28 ± 1.65 BC | 45.62 ± 1.36 CD | |||
| 15 | 59.57 ± 1.84 A | 55.91 ± 1.82 B | 13.03 | ||
| 20 | 72.72 ± 2.17 A | 69.06 ± 2.35 A | |||
| LSD0.05 | 9.98 *** | ||||
| F-value | 57.92 | ||||
| Microbes | EO (10 mg mL−1) | Standard Antibiotic IZ (10 mg L−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Egyptian | Saudi | Ampicillin | Chloramphenicol | Gentamicin | Tetracycline | |||
| IZ | MIC | IZ | MIC | |||||
| Gram-negative bacteria | ||||||||
| Escherichia coli | 25.01 ± 0.75 A | 0.052 | 26.13 ± 0.71 A | 0.049 | 21.08 ± 0.55 C | 11.31 ± 0.31 D | 25.93 ± 0.76 A | 22.10 ± 0.58 A |
| Klebsiella pneumonia | 21.07 ± 0.59 B | 0.062 | 21.67 ± 0.64 B | 0.061 | 7.73 ± 0.15 DE | 11.07 ± 0.28 D | 21.13 ± 0.51 C | 21.64 ± 0.61 A |
| Pseudomonas aeruginosa | 17.86 ± 0.51 C | 0.092 | 18.52 ± 0.57 C | 0.085 | 6.52 ± 0.13 E | 10.64 ± 0.23 D | 11.38 ± 0.36 D | 0.00 D |
| Salmonella typhimurium | 13.84 ± 0.36 D | 0.126 | 13.09 ± 0.41 D | 0.127 | 0.00 F | 0.00 E | 0.00 E | 10.90 ± 0.32 C |
| Gram-positive bacteria | ||||||||
| Bacillus subtilis | 22.81 ± 0.62 AB | 0.051 | 21.45 ± 0.58 B | 0.053 | 8.55 ± 0.17 D | 20.15 ± 0.61 B | 20.68 ± 0.52 C | 11.36 ± 0.28 C |
| Staphylococcus aureus | 20.50 ± 0.58 B | 0.052 | 22.06 ± 0.63 B | 0.048 | 28.98 ± 0.92 A | 15.09 ± 0.45 C | 24.07 ± 0.63 B | 18.67 ± 0.48 B |
| Staphylococcus epidermidis | 24.14 ± 0.76 A | 1.410 | 25.38 ± 0.82 A | 0.051 | 22.09 ± 0.64 C | 24.76 ± 0.73 A | 24.50 ± 0.58 B | 20.53 ± 0.52 AB |
| Enterobacter cloacae | 9.92 ± 0.21 D | 0.051 | 10.23 ± 0.09 E | 0.049 | 24.66 ± 0.81 B | 19.41 ± 0.52 B | 20.05 ± 0.61 C | 18.06 ± 0.37 B |
| LSD0.05 | 2.54 *** | 1.11 *** | 1.22 *** | 1.32 *** | 1.09 *** | 2.54 *** | ||
| F-value | 42.94 | 284.86 | 667.73 | 354.30 | 460.54 | 84.79 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-ElGawad, A.M.; Ahmed, R.F.; Elshamy, A.I.; Sadek, E.G.; Assaeed, A.M.; Bonanomi, G.; El Gendy, A.E.-N.G.; El-Amier, Y.A. Achillea fragrantissima Essential Oil, Wild Grown in Saudi Arabia and Egypt: Detailed Comparative Chemical Profiling, and Evaluation of Allelopathic, Antioxidant, and Antibacterial Activities. Chemistry 2023, 5, 2347-2361. https://doi.org/10.3390/chemistry5040155
Abd-ElGawad AM, Ahmed RF, Elshamy AI, Sadek EG, Assaeed AM, Bonanomi G, El Gendy AE-NG, El-Amier YA. Achillea fragrantissima Essential Oil, Wild Grown in Saudi Arabia and Egypt: Detailed Comparative Chemical Profiling, and Evaluation of Allelopathic, Antioxidant, and Antibacterial Activities. Chemistry. 2023; 5(4):2347-2361. https://doi.org/10.3390/chemistry5040155
Chicago/Turabian StyleAbd-ElGawad, Ahmed M., Rania F. Ahmed, Abdelsamed I. Elshamy, Eslam G. Sadek, Abdulaziz M. Assaeed, Giuliano Bonanomi, Abd El-Nasser G. El Gendy, and Yasser A. El-Amier. 2023. "Achillea fragrantissima Essential Oil, Wild Grown in Saudi Arabia and Egypt: Detailed Comparative Chemical Profiling, and Evaluation of Allelopathic, Antioxidant, and Antibacterial Activities" Chemistry 5, no. 4: 2347-2361. https://doi.org/10.3390/chemistry5040155
APA StyleAbd-ElGawad, A. M., Ahmed, R. F., Elshamy, A. I., Sadek, E. G., Assaeed, A. M., Bonanomi, G., El Gendy, A. E.-N. G., & El-Amier, Y. A. (2023). Achillea fragrantissima Essential Oil, Wild Grown in Saudi Arabia and Egypt: Detailed Comparative Chemical Profiling, and Evaluation of Allelopathic, Antioxidant, and Antibacterial Activities. Chemistry, 5(4), 2347-2361. https://doi.org/10.3390/chemistry5040155