Sweet, Sugar-Coated Hierarchical Platinum Nanostructures for Easy Support, Heterogenization and Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
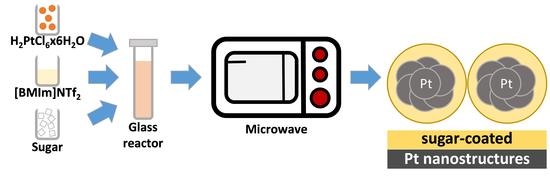
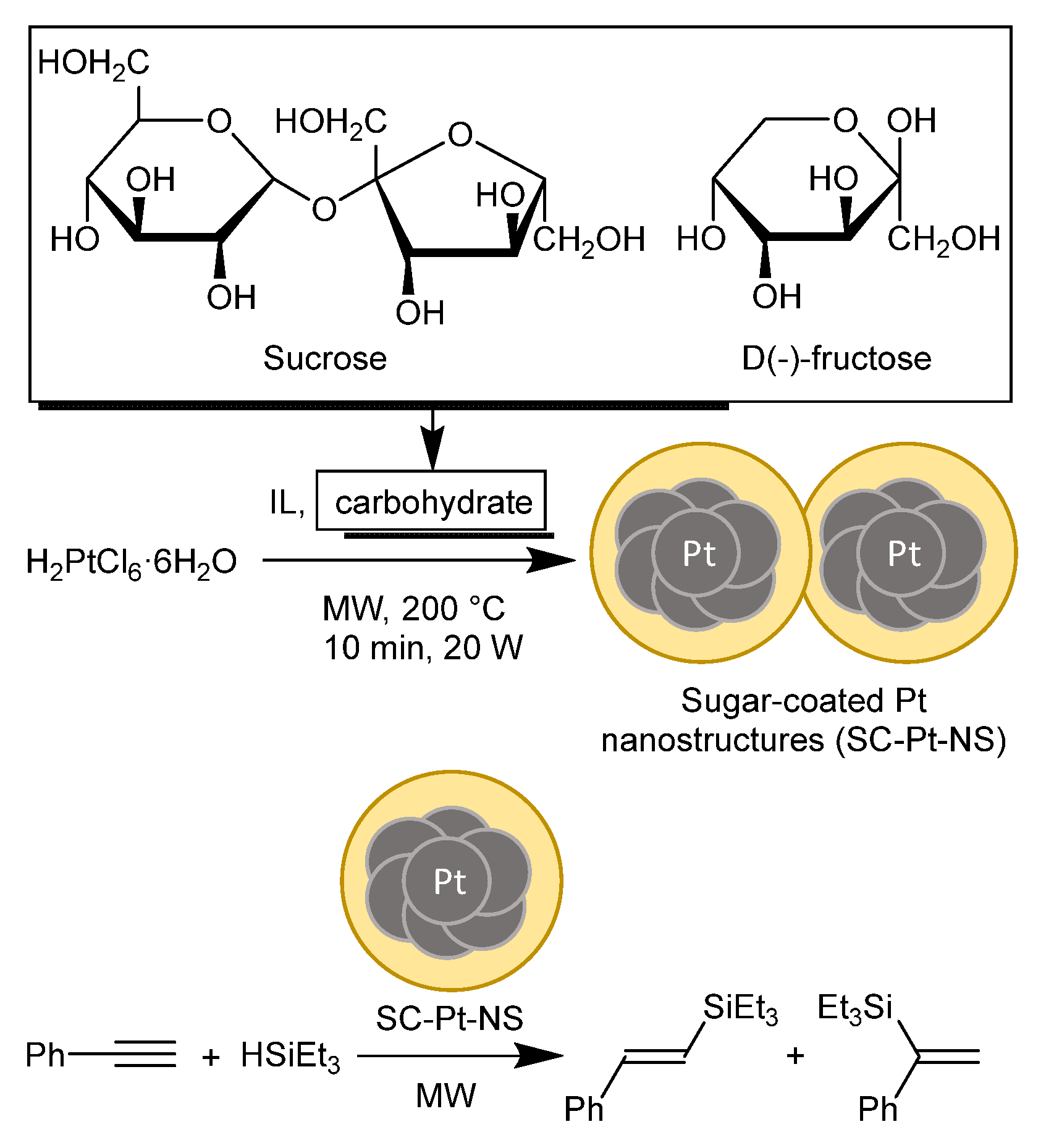
2.2. Synthesis of Sugar-Coated Pt Nanostructures (SC-Pt-NS)
2.3. Hydrosilylation Reactions
3. Results
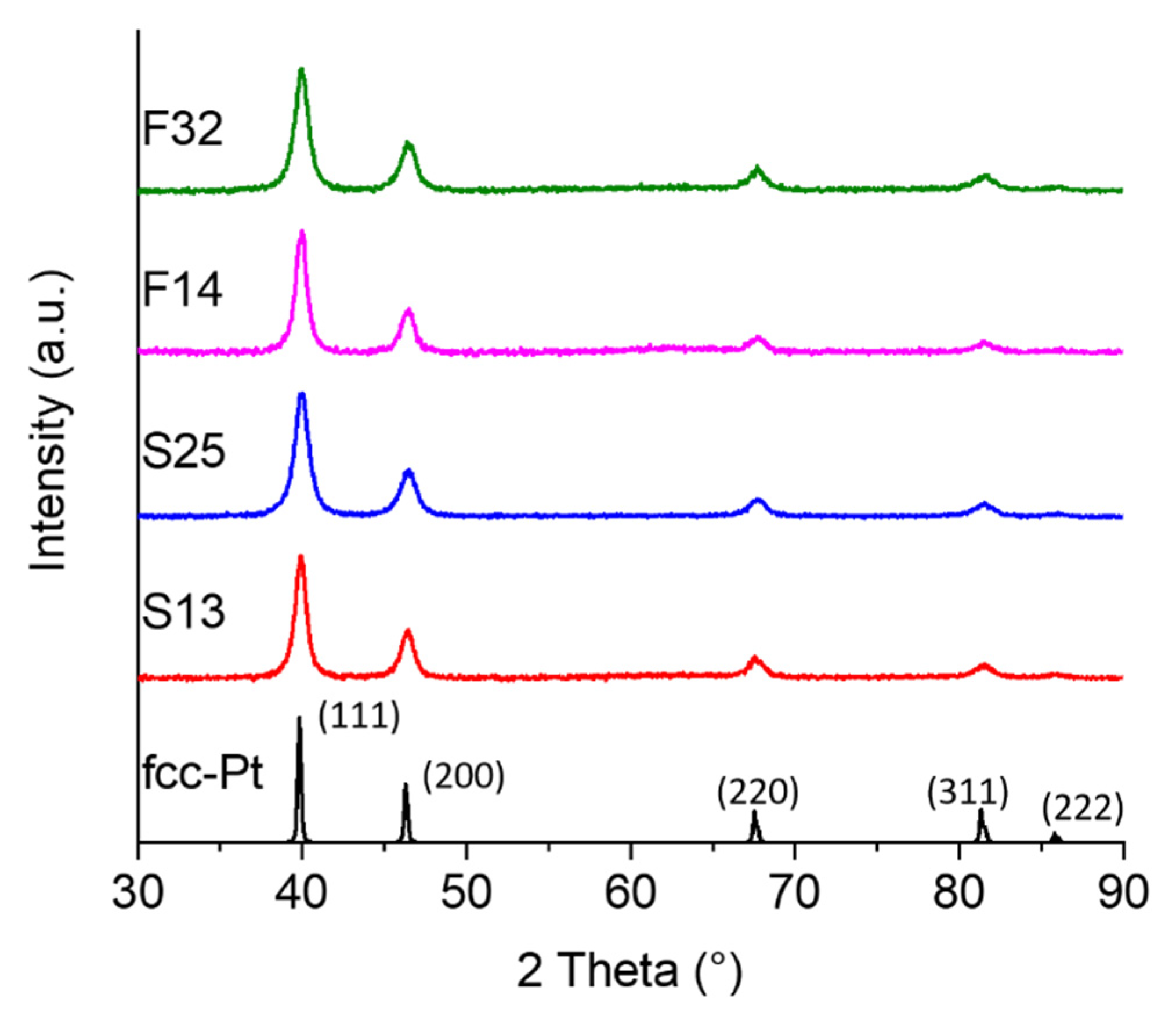
3.1. Synthesis and Characterization
3.2. Hydrosilylation Catalysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mizuno, N.; Misono, M. Heterogeneous Catalysis. Chem. Rev. 1998, 98, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, U.; Lefferts, L. (Eds.) Catalysis: An Integrated Textbook for Students; Wiley-VCH: Weinheim, Germany, 2018; ISBN 978-3-527-34159-7s. [Google Scholar]
- Beller, M.; Renken, A.; van Santen, R.A. (Eds.) Catalysis: From Principles to Applications; Wiley-VCH-Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; ISBN 978-3-527-32349-4. [Google Scholar]
- Yazgan, I.; Gümüş, A.; Gökkuş, K.; Demir, M.A.; Evecen, S.; Sönmez, H.A.; Miller, R.M.; Bakar, F.; Oral, A.; Popov, S.; et al. On the Effect of Modified Carbohydrates on the Size and Shape of Gold and Silver Nanostructures. J. Nanomater. 2020, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Mondal, D. A role for ultrasound in the fabrication of carbohydrate-supported nanomaterials. J. Utrasound 2019, 22, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, A.P.; Vocciante, M.; Salerno, M.; Soda, O.; Fabiano, B. A sustainable, top-down mechanosynthesis of carbohydrate-functionalized silver nanoparticles. React. Chem. Eng. 2022, 7, 888–897. [Google Scholar] [CrossRef]
- Li, K.; Liu, B. Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chem. Soc. Rev. 2014, 43, 6570–6597. [Google Scholar] [CrossRef]
- Wegner, S.; Janiak, C. Metal Nanoparticles in Ionic Liquids. Top. Curr. Chem. 2017, 375, 65. [Google Scholar] [CrossRef]
- Dupont, J.; Kollar, L. (Eds.) Ionic Liquids (ILs) in Organometallic Catalysis; Topics in Organometallic Chemistry 51; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-662-47857-8. [Google Scholar]
- Xu, D.; Lv, H.; Liu, B. Encapsulation of Metal Nanoparticle Catalysts Within Mesoporous Zeolites and Their Enhanced Catalytic Performances: A Review. Front. Chem. 2018, 6, 550. [Google Scholar] [CrossRef]
- Hosseiniamoli, H.; Bryant, G.; Kennedy, E.M.; Mathisen, K.; Nicholson, D.; Sankar, G.; Setiawan, A.; Stockenhuber, M. Understanding Structure–Function Relationships in Zeolite-Supported Pd Catalysts for Oxidation of Ventilation Air Methane. ACS Catal. 2018, 8, 5852–5863. [Google Scholar] [CrossRef]
- Marquardt, D.; Beckert, F.; Pennetreau, F.; Tölle, F.; Mülhaupt, R.; Riant, O.; Hermans, S.; Barthel, J.; Janiak, C. Hybrid materials of platinum nanoparticles and thiol-functionalized graphene derivatives. Carbon 2014, 66, 285–294. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.-C.; Feldmann, C. Polyol synthesis of nanoparticles: Status and options regarding metals, oxides, chalcogenides, and non-metal elements. Green Chem. 2015, 17, 4107–4132. [Google Scholar] [CrossRef]
- Fiévet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.-Y.; Sicard, L.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev 2018, 47, 5187–5233. [Google Scholar] [CrossRef]
- Adil, S.F.; Assal, M.E.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Liz-Marzán, L.M. Biogenic synthesis of metallic nanoparticles and prospects toward green chemistry. Dalton Trans. 2015, 44, 9709–9717. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Fievet, F.; Lagier, J.P.; Figlarz, M. Preparing Monodisperse Metal Powders in Micrometer and Submicrometer Sizes by the Polyol Process. MRS Bull. 1989, 14, 29–34. [Google Scholar] [CrossRef]
- Pentsak, E.O.; Galushko, A.S.; Cherepanova, V.A.; Ananikov, V.P. How to Make a Cocktail of Palladium Catalysts with Cola and Alcohol: Heteroatom Doping vs. Nanoscale Morphology of Carbon Supports. J. Nanomater. 2021, 11, 2599. [Google Scholar] [CrossRef]
- Ribeiro, C.A.; Albuquerque, L.J.; de Castro, C.E.; Batista, B.L.; de Souza, A.L.; Albuquerque, B.L.; Zilse, M.S.; Bellettini, I.C.; Giacomelli, F.C. One-pot synthesis of sugar-decorated gold nanoparticles with reduced cytotoxicity and enhanced cellular uptake. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123690. [Google Scholar] [CrossRef]
- Geng, F.; Song, K.; Xing, J.Z.; Yuan, C.; Yan, S.; Yang, Q.; Chen, J.; Kong, B. Thio-glucose bound gold nanoparticles enhance radio-cytotoxic targeting of ovarian cancer. Nanotechnology 2011, 22, 285101. [Google Scholar] [CrossRef]
- Shrivas, K.; Nirmalkar, N.; Thakur, S.S.; Deb, M.K.; Shinde, S.S.; Shankar, R. Sucrose capped gold nanoparticles as a plasmonic chemical sensor based on non-covalent interactions: Application for selective detection of vitamins B1 and B6 in brown and white rice food samples. Food Chem. 2018, 250, 14–21. [Google Scholar] [CrossRef]
- Panigrahi, S.; Kundu, S.; Ghosh, S.; Nath, S.; Pal, T. General method of synthesis for metal nanoparticles. J. Nanopart. Res. 2004, 6, 411–414. [Google Scholar] [CrossRef]
- Panigrahi, S.; Kundu, S.; Ghosh, S.K.; Nath, S.; Pal, T. Sugar assisted evolution of mono- and bimetallic nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2005, 264, 133–138. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Welton, T. Ionic liquids in catalysis. Coord. Chem. Rev. 2004, 248, 2459–2477. [Google Scholar] [CrossRef]
- Wender, H.; Migowski, P.; Feil, A.F.; Teixeira, S.R.; Dupont, J. Sputtering deposition of nanoparticles onto liquid substrates: Recent advances and future trends. Coord. Chem. Rev. 2013, 257, 2468–2483. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic Liquids—New “Solutions” for Transition Metal Catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Consorti, C.S.; Suarez, P.A.Z.; de Souza, R.F.; Burrow, R.A.; Farrar, D.H.; Lough, A.J.; Loh, W.; Da Silva, L.H.M.; Dupont, J. Identification of 1,3-dialkylimidazolium salt supramolecular aggregates in solution. J. Phys. Chem. B 2005, 109, 4341–4349. [Google Scholar] [CrossRef]
- Dupont, J. On the solid, liquid and solution structural organization of imidazolium ionic liquids. J. Braz. Chem. Soc. 2004, 15, 341–350. [Google Scholar] [CrossRef]
- Vollmer, C.; Redel, E.; Abu-Shandi, K.; Thomann, R.; Manyar, H.; Hardacre, C.; Janiak, C. Microwave irradiation for the facile synthesis of transition-metal nanoparticles (NPs) in ionic liquids (ILs) from metal-carbonyl precursors and Ru-, Rh-, and Ir-NP/IL dispersions as biphasic liquid-liquid hydrogenation nanocatalysts for cyclohexene. Chem. Eur. J. 2010, 16, 3849–3858. [Google Scholar] [CrossRef]
- Dupont, J.; Scholten, J.D. On the structural and surface properties of transition-metal nanoparticles in ionic liquids. Chem. Soc. Rev. 2010, 39, 1780–1804. [Google Scholar] [CrossRef]
- Redel, E.; Thomann, R.; Janiak, C. Use of ionic liquids (ILs) for the IL-anion size-dependent formation of Cr, Mo and W nanoparticles from metal carbonyl M(CO)6 precursors. Chem. Commun. 2008, 27, 1789–1791. [Google Scholar] [CrossRef]
- Krämer, J.; Redel, E.; Thomann, R.; Janiak, C. Use of Ionic Liquids for the Synthesis of Iron, Ruthenium, and Osmium Nanoparticles from Their Metal Carbonyl Precursors. Organometallics 2008, 27, 1976–1978. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Transition metal nanoparticles in ionic liquids: Synthesis and stabilization. J. Mol. Liq. 2019, 276, 826–849. [Google Scholar] [CrossRef]
- Redel, E.; Krämer, J.; Thomann, R.; Janiak, C. Synthesis of Co, Rh and Ir nanoparticles from metal carbonyls in ionic liquids and their use as biphasic liquid–liquid hydrogenation nanocatalysts for cyclohexene. J. Organomet. Chem. 2009, 694, 1069–1075. [Google Scholar] [CrossRef]
- Cravotto, G.; Gaudino, E.C.; Boffa, L.; Lévêque, J.-M.; Estager, J.; Bonrath, W. Preparation of second generation ionic liquids by efficient solvent-free alkylation of N-heterocycles with chloroalkanes. Molecules 2008, 13, 149–156. [Google Scholar] [CrossRef]
- Jiang, J.; Yu, S.-H.; Yao, W.-T.; Ge, H.; Zhang, G.-Z. Morphogenesis and Crystallization of Bi2S3 Nanostructures by an Ionic Liquid-Assisted Templating Route: Synthesis, Formation Mechanism, and Properties. Chem. Mater. 2005, 17, 6094–6100. [Google Scholar] [CrossRef]
- Yee, P.; Shah, J.K.; Maginn, E.J. State of hydrophobic and hydrophilic ionic liquids in aqueous solutions: Are the ions fully dissociated? J. Phys. Chem. B 2013, 117, 12556–12566. [Google Scholar] [CrossRef]
- Redel, E.; Thomann, R.; Janiak, C. First correlation of nanoparticle size-dependent formation with the ionic liquid anion molecular volume. Inorg. Chem. 2008, 47, 14–16. [Google Scholar] [CrossRef]
- Troegel, D.; Stohrer, J. Recent advances and actual challenges in late transition metal catalyzed hydrosilylation of olefins from an industrial point of view. Coord. Chem. Rev. 2011, 255, 1440–1459. [Google Scholar] [CrossRef]
- Alonso, F.; Buitrago, R.; Moglie, Y.; Ruiz-Martínez, J.; Sepúlveda-Escribano, A.; Yus, M. Hydrosilylation of alkynes catalysed by platinum on titania. J. Organomet. Chem. 2011, 696, 368–372. [Google Scholar] [CrossRef] [Green Version]
- Lipke, M.C.; Liberman-Martin, A.L.; Tilley, T.D. Electrophilic Activation of Silicon-Hydrogen Bonds in Catalytic Hydrosilations. Angew. Chem. Int. Ed. 2017, 56, 2260–2294. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, L.D.; Wang, H.; Junge, K.; Cui, X.; Beller, M. Recent Advances in Catalytic Hydrosilylations: Developments beyond Traditional Platinum Catalysts. Angew. Chem. Int. Ed. 2021, 60, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Naganawa, Y.; Inomata, K.; Sato, K.; Nakajima, Y. Hydrosilylation reactions of functionalized alkenes. Tetrahedron Lett. 2020, 61, 151513. [Google Scholar] [CrossRef]
- Nakajima, Y.; Shimada, S. Hydrosilylation reaction of olefins: Recent advances and perspectives. RSC Adv. 2015, 5, 20603–20616. [Google Scholar] [CrossRef]
- Komiyama, T.; Minami, Y.; Hiyama, T. Recent Advances in Transition-Metal-Catalyzed Synthetic Transformations of Organosilicon Reagents. ACS Catal. 2017, 7, 631–651. [Google Scholar] [CrossRef]
- Rivero-Crespo, M.; Oliver-Meseguer, J.; Kapłońska, K.; Kuśtrowski, P.; Pardo, E.; Cerón-Carrasco, J.P.; Leyva-Pérez, A. Cyclic metal(oid) clusters control platinum-catalysed hydrosilylation reactions: From soluble to zeolite and MOF catalysts. Chem. Sci. 2020, 11, 8113–8124. [Google Scholar] [CrossRef]
- Chalk, A.J.; Harrod, J.F. Homogeneous Catalysis. II. The Mechanism of the Hydrosilation of Olefins Catalyzed by Group VIII Metal Complexes 1. J. Am. Chem. Soc. 1965, 87, 16–21. [Google Scholar] [CrossRef]
- Meister, T.K.; Riener, K.; Gigler, P.; Stohrer, J.; Herrmann, W.A.; Kühn, F.E. Platinum Catalysis Revisited—Unraveling Principles of Catalytic Olefin Hydrosilylation. ACS Catal. 2016, 6, 1274–1284. [Google Scholar] [CrossRef]
- Abramov, A.; Diaz Diaz, D. Katalysatoren immobilisieren. Nachr. Chem. 2022, 70, 75–78. [Google Scholar] [CrossRef]
- Lambert, J.M. The nature of platinum in silicones for biomedical and healthcare use. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 78, 167–180. [Google Scholar] [CrossRef]
- Brook, M.A. Platinum in silicone breast implants. Biomaterials. 2006, 27, 3274–3286. [Google Scholar] [CrossRef]
- Wixtrom, R.; Glicksman, C.; Kadin, M.; Lawrence, M.; Haws, M.; Ferenz, S.; Sung, J.; McGuire, P. Heavy Metals in Breast Implant Capsules and Breast Tissue: Findings from the Systemic Symptoms in Women-Biospecimen Analysis Study: Part 2. Aesthet. Surg. J. 2022, 42, 1067–1076. [Google Scholar] [CrossRef]
- Kukawka, R.; Pawlowska-Zygarowicz, A.; Dutkiewicz, M.; Maciejewski, H.; Smiglak, M. New approach to hydrosilylation reaction in ionic liquids as solvent in microreactor system. RSC Adv. 2016, 6, 61860–61868. [Google Scholar] [CrossRef]
- Zielinski, W.; Kukawka, R.; Maciejewski, H.; Smiglak, M. Ionic Liquids as Solvents for Rhodium and Platinum Catalysts Used in Hydrosilylation Reaction. Molecules. 2016, 21, 1115. [Google Scholar] [CrossRef]
- Geldbach, T.J.; Zhao, D.; Castillo, N.C.; Laurenczy, G.; Weyershausen, B.; Dyson, P.J. Biphasic hydrosilylation in ionic liquids: A process set for industrial implementation. J. Am. Chem. Soc. 2006, 128, 9773–9780. [Google Scholar] [CrossRef]
- Taccardi, N.; Fekete, M.; Berger, M.E.; Stanjek, V.; Schulz, P.S.; Wasserscheid, P. Catalyst recycling in monophasic Pt-catalyzed hydrosilylation reactions using ionic liquids. Appl. Catal. A Gen. 2011, 399, 69–74. [Google Scholar] [CrossRef]
- Hofmann, N.; Bauer, A.; Frey, T.; Auer, M.; Stanjek, V.; Schulz, P.S.; Taccardi, N.; Wasserscheid, P. Liquid-Liquid Biphasic, Platinum-Catalyzed Hydrosilylation of Allyl Chloride with Trichlorosilane using an Ionic Liquid Catalyst Phase in a Continuous Loop Reactor. Adv. Synth. Catal. 2008, 350, 2599–2609. [Google Scholar] [CrossRef]
- Marquardt, D.; Barthel, J.; Braun, M.; Ganter, C.; Janiak, C. Weakly-coordinated stable platinum nanocrystals. CrystEngComm 2012, 14, 7607–7615. [Google Scholar] [CrossRef]
- Dobó, D.G.; Sipos, D.; Sápi, A.; London, G.; Juhász, K.; Kukovecz, Á.; Kónya, Z. Tuning the Activity and Selectivity of Phenylacetylene Hydrosilylation with Triethylsilane in the Liquid Phase over Size Controlled Pt Nanoparticles. Catalysts 2018, 8, 22. [Google Scholar] [CrossRef]
- Chauhan, M.; Hauck, B.J.; Keller, L.P.; Boudjouk, P. Hydrosilylation of alkynes catalyzed by platinum on carbon. J. Organomet. Chem. 2002, 645, 1–13. [Google Scholar] [CrossRef]
- Fernández, G.; Pleixats, R. Soluble Pt Nanoparticles Stabilized by a Tris-imidazolium Tetrafluoroborate as Efficient and Recyclable Catalyst for the Stereoselective Hydrosilylation of Alkynes. ChemistrySelect 2018, 3, 11486–11493. [Google Scholar] [CrossRef]
- Chauhan, B.P.S.; Sarkar, A. Functionalized vinylsilanes via highly efficient and recyclable Pt-nanoparticle catalysed hydrosilylation of alkynes. Dalton Trans. 2017, 46, 8709–8715. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Chen, J.; Xiao, Y.; Zhang, J. Platinum nanoparticles confined in imidazolium-based ionic polymer for assembling a microfluidic reactor with enhanced catalytic activity. Appl. Catal. A: Gen. 2019, 585, 117186. [Google Scholar] [CrossRef]
- Hu, W.; Xie, H.; Yue, H.; Prinsen, P.; Luque, R. Super-microporous silica-supported platinum catalyst for highly regioselective hydrosilylation. Catal. Commun. 2017, 97, 51–55. [Google Scholar] [CrossRef]
- Tierney, J.P.; Lidström, P. Microwave Assisted Organic Synthesis; Blackwell Publishing: Oxford, UK, 2005. [Google Scholar] [CrossRef]
- Hill, J.M.; Marchant, T.R. Modelling microwave heating. Appl. Math. Model. 1996, 20, 3–15. [Google Scholar] [CrossRef]
- Max, J.-J.; Chapados, C. Glucose and fructose hydrates in aqueous solution by IR spectroscopy. J. Phys. Chem. A 2007, 111, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Yong, L.; Kirleis, K.; Butenschön, H. Stereodivergent Formation of Alkenylsilanes:syn oranti Hydrosilylation of Alkynes Catalyzed by a Cyclopentadienylcobalt(I) Chelate Bearing a Pendant Phosphane Tether. Adv. Synth. Catal. 2006, 348, 833–836. [Google Scholar] [CrossRef]

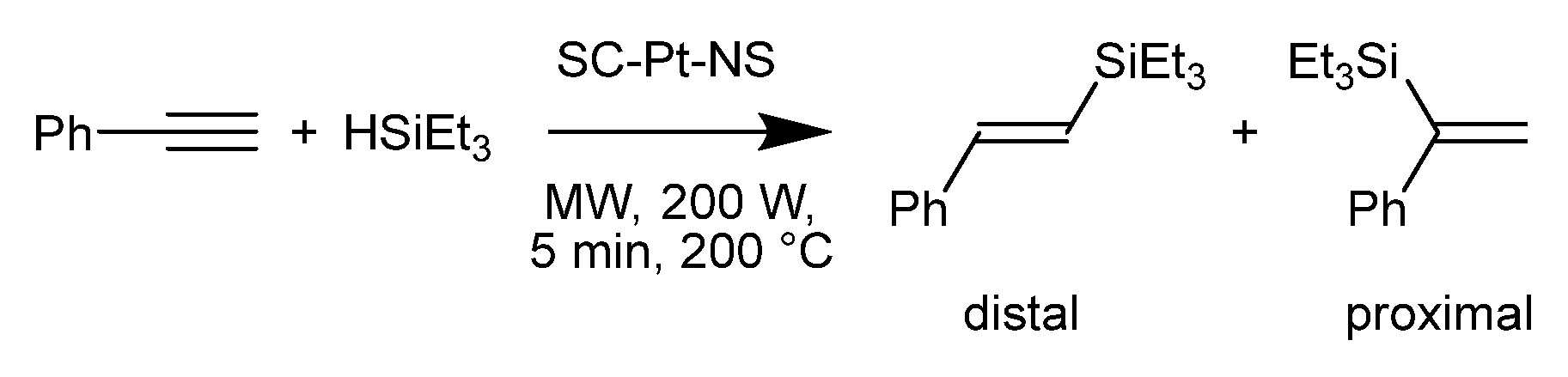
| Catalyst with References | Time (h) | Temp. (°C) | Substrate/Pt 1 | Conversion (%) 2 | d/p Ratio 3 |
|---|---|---|---|---|---|
| SC-Pt-NS | |||||
| S13 | 0.083 | 200 | 7500 | 98 | 2.1 |
| S25 | 0.083 | 200 | 3800 | 99 | 1.4 |
| F14 | 0.083 | 200 | 6900 | 98 | 1.7 |
| F32 | 0.083 | 200 | 3500 | 99 | 1.4 |
| Comparison | |||||
| Pt-free 4 | 0.083 | 200 | – | 0 | – |
| H2PtCl6·6H2O | 0.083 | 200 | 2200 | 98 | 1.9 |
| Pt-NP | 0.083 | 200 | 1100 | 91 | 1.9 |
| 7.0 nm Pt/SBA-15 [62] | 6 | 70 | 390 | 6.8 | 1.8 |
| Pt1/NaY [49] | 24 | 110 | 2440 | 82 | 0.3 |
| Pt/C [63] | 4.5 | 70 | 4880 | 91 | 3.3 |
| Pt-NP [64] | 1.3 | 60 | 200 | 82 | 4.9 |
| Pt-NP [64] | 24 | 60 | 200 | 94 | 9.0 |
| Pt-NP [65] | 10 | rt | 1000 | 98 | 6.7 |
| C-Pt/ImIP-2BrB [66] | 4 | 80 | 2000 | 79 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woitassek, D.; Moya-Cancino, J.G.; Sun, Y.; Song, Y.; Woschko, D.; Roitsch, S.; Janiak, C. Sweet, Sugar-Coated Hierarchical Platinum Nanostructures for Easy Support, Heterogenization and Separation. Chemistry 2022, 4, 1147-1160. https://doi.org/10.3390/chemistry4040078
Woitassek D, Moya-Cancino JG, Sun Y, Song Y, Woschko D, Roitsch S, Janiak C. Sweet, Sugar-Coated Hierarchical Platinum Nanostructures for Easy Support, Heterogenization and Separation. Chemistry. 2022; 4(4):1147-1160. https://doi.org/10.3390/chemistry4040078
Chicago/Turabian StyleWoitassek, Dennis, José G. Moya-Cancino, Yangyang Sun, Yefan Song, Dennis Woschko, Stefan Roitsch, and Christoph Janiak. 2022. "Sweet, Sugar-Coated Hierarchical Platinum Nanostructures for Easy Support, Heterogenization and Separation" Chemistry 4, no. 4: 1147-1160. https://doi.org/10.3390/chemistry4040078
APA StyleWoitassek, D., Moya-Cancino, J. G., Sun, Y., Song, Y., Woschko, D., Roitsch, S., & Janiak, C. (2022). Sweet, Sugar-Coated Hierarchical Platinum Nanostructures for Easy Support, Heterogenization and Separation. Chemistry, 4(4), 1147-1160. https://doi.org/10.3390/chemistry4040078