Supported Eosin Y as a Photocatalyst for C-H Arylation of Furan in Batch and Flow
Abstract
:1. Introduction
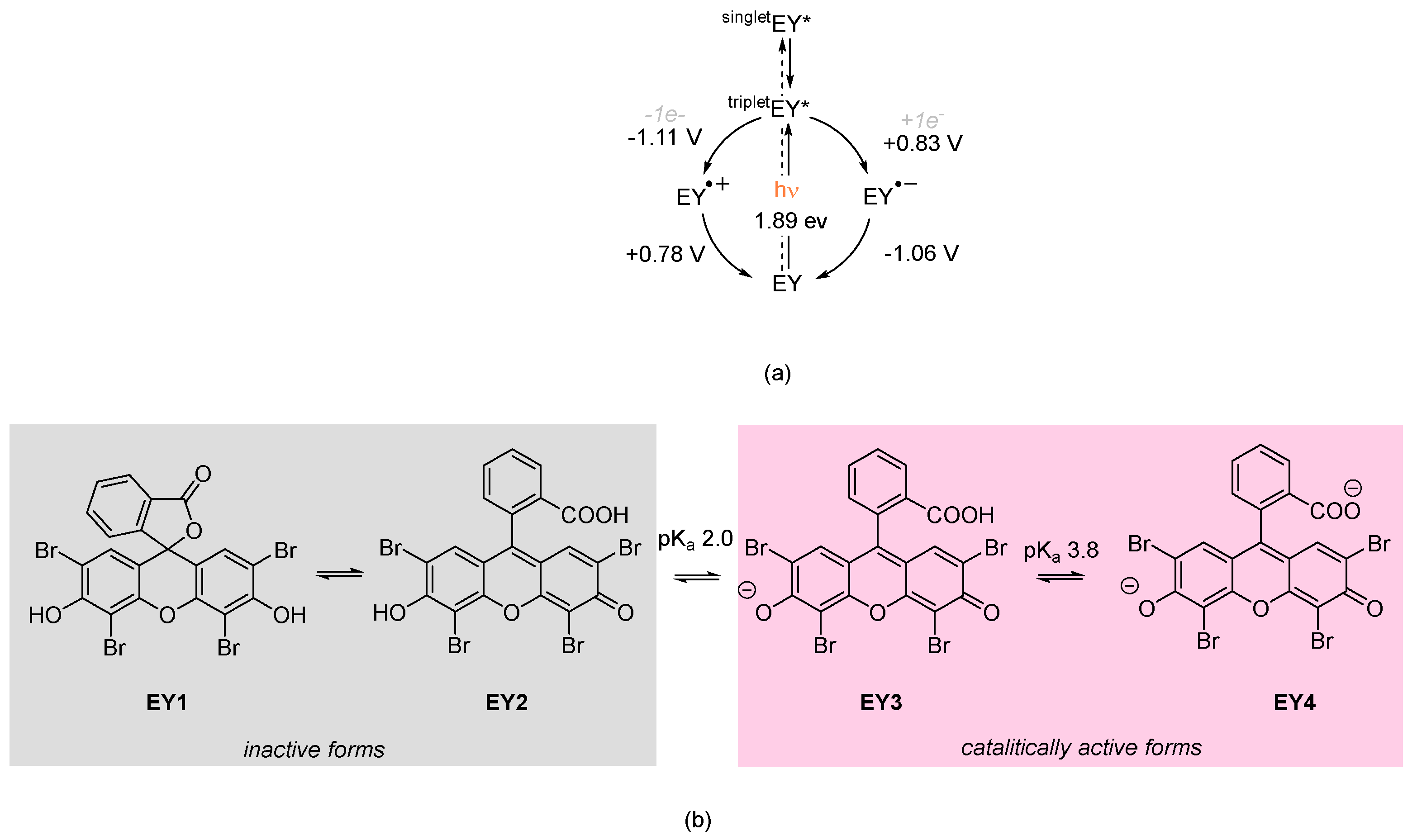
2. Results and Discussion
2.1. Synthesis of Polystyrene-Supported Eosin Y
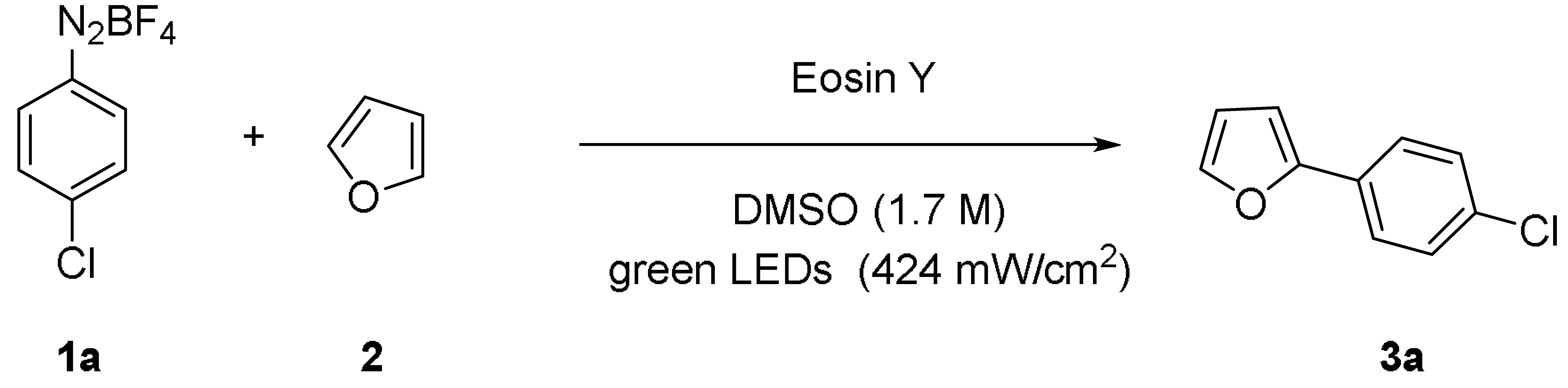
2.2. Application of Polystyrene-Supported Eosin Y in Photocatalysis
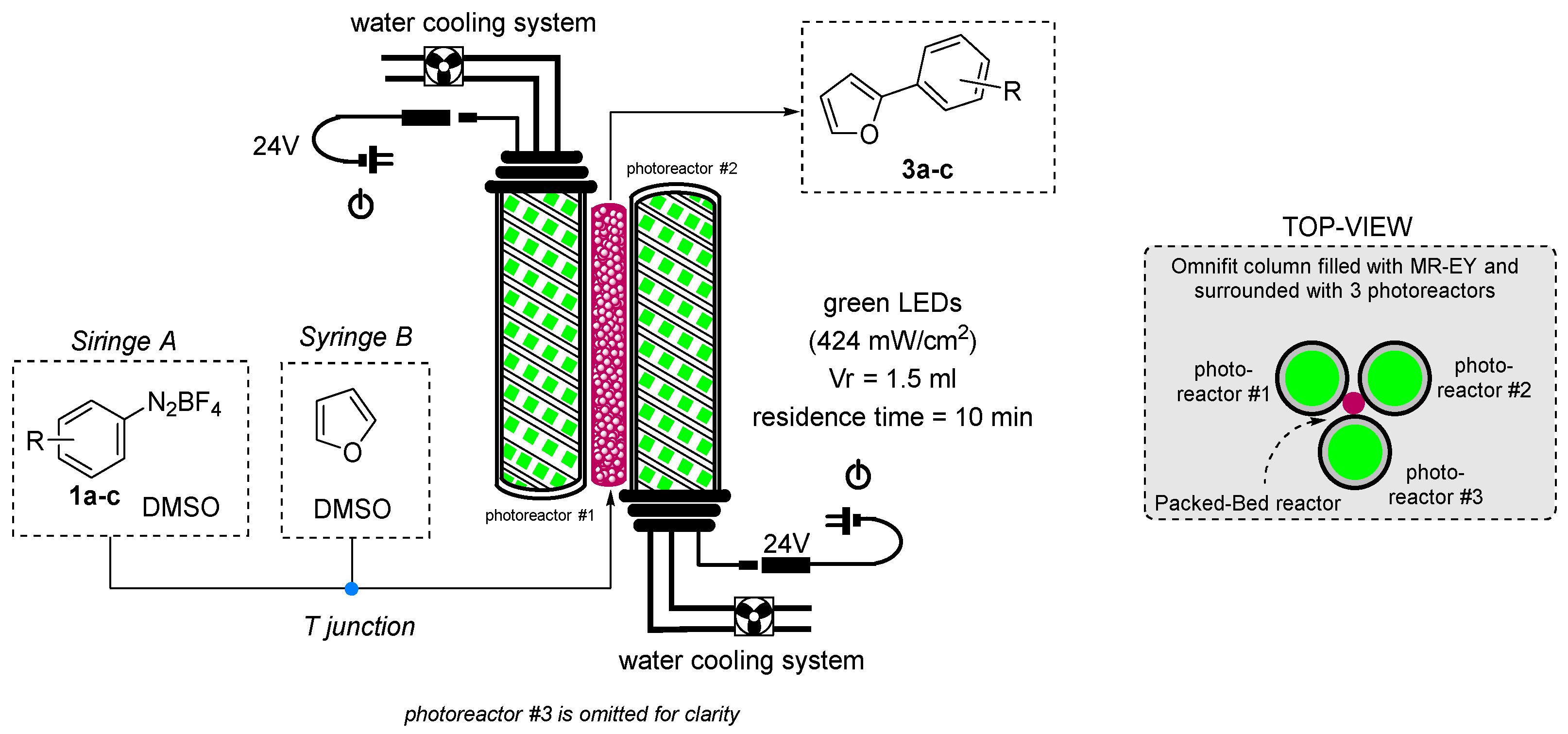
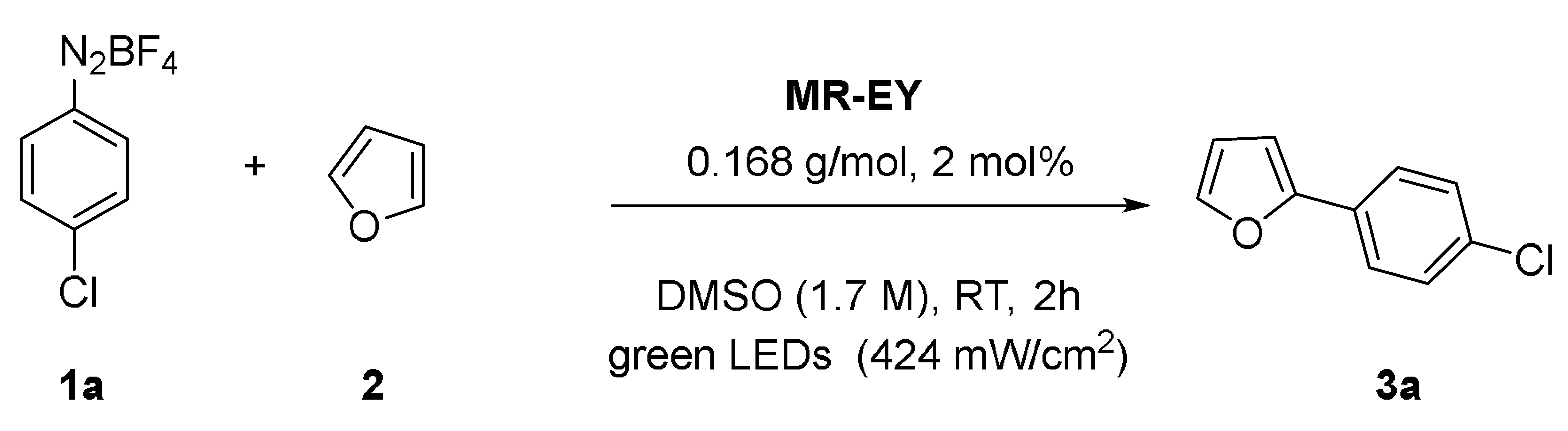
2.3. Recovery and Recycle and Flow Chemistry of Polystyrene-Supported Eosin Y
3. Experimental Section
3.1. General Description of Reagents and Equipment
3.2. Synthesis and Characterization of Solid-Supported Eosin Y Merrifield Resin (MR-EY)
3.3. General Procedure for the Direct C-H Arylation of Furan with Aryl Diazonium Salts under Batch Conditions
3.4. General Procedure for the Direct C-H Arylation of Furan with Aryl Diazonium Salts under Flow Conditions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nozik, A.J.; Miller, J. Introduction to solar photon conversion. Chem. Rev. 2010, 110, 6443–6445. [Google Scholar] [CrossRef] [PubMed]
- Ciamician, G. The Photochemistry of the Future. Science 1912, 36, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Nicewicz, D.A.; MacMillan, D.W.C. Merging Photoredox Catalysis with Organocatalysis: The Direct Asymmetric Alkylation of Aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, V.; Singh, P.P. Eosin Y catalysed photoredox synthesis: A review. Rsc. Adv. 2017, 7, 31377–31392. [Google Scholar] [CrossRef]
- Hari, D.P.; Konig, B. Synthetic applications of eosin Y in photoredox catalysis. Chem. Commun. 2014, 50, 6688–6699. [Google Scholar] [CrossRef] [Green Version]
- Penzkofer, A.; Beidoun, A.; Daiber, M. Intersystem-Crossing and Excited-State Absorption in Eosin-Y Solutions Determined by Picosecond Double Pulse Transient Absorption-Measurements. J. Lumin. 1992, 51, 297–314. [Google Scholar] [CrossRef] [Green Version]
- Penzkofer, A.; Beidoun, A. Triplet-Triplet Absorption of Eosin-Y in Methanol Determined by Nanosecond Excimer-Laser Excitation and Picosecond Light Continuum Probing. Chem. Phys. 1993, 177, 203–216. [Google Scholar] [CrossRef] [Green Version]
- Penzkofer, A.; Beidoun, A.; Speiser, S. Singlet Excited-State Absorption of Eosin-Y. Chem. Phys. 1993, 170, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Majek, M.; Filace, F.; Wangelin, A.J.V. On the mechanism of photocatalytic reactions with eosin Y. Beilstein. J. Org. Chem. 2014, 10, 981–989. [Google Scholar] [CrossRef] [Green Version]
- Herbrik, F.; Camarero González, P.; Krstic, M.; Puglisi, A.; Benaglia, M.; Sanz, M.A.; Rossi, S. Eosin Y: Homogeneous Photocatalytic In-Flow Reactions and Solid-Supported Catalysts for In-Batch Synthetic Transformations. Appl. Sci. 2020, 10, 5596–5612. [Google Scholar] [CrossRef]
- Herbrik, F.; Rossi, S.; Sanz, M.A.; Puglisi, A.; Benaglia, M. Immobilized Eosin Y for the Photocatalytic Oxidation of Tetrahydroisoquinolines in Flow. ChemCatChem 2022. [Google Scholar] [CrossRef]
- Hari, D.P.; Schroll, P.; König, B. Metal-Free, Visible-Light-Mediated Direct C–H Arylation of Heteroarenes with Aryl Diazonium Salts. J. Am. Chem. Soc. 2012, 134, 2958–2961. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Dozzi, M.; Puglisi, A.; Pagani, M. 3D-printed, home-made, UV-LED photoreactor as a simple and economic tool to perform photochemical reactions in high school laboratories. Chem. Teach. Int. 2020, 2, 20190010. [Google Scholar] [CrossRef]
- Herbrik, F.; Sanz, M.A.; Puglisi, A.; Rossi, S.; Benaglia, M. Enantioselective Organophotocatalytic Telescoped Synthesis of a Chiral Privileged Active Pharmaceutical Ingredient. Chem. Eur. J. 2022, 28, e202200164. [Google Scholar] [CrossRef] [PubMed]
- Medici, F.; Resta, S.; Presenti, P.; Caruso, L.; Puglisi, A.; Raimondi, L.; Rossi, S.; Benaglia, M. Stereoselective Visible-Light Catalyzed Cyclization of Bis(enones): A Viable Approach to the Synthesis of Enantiomerically Enriched Cyclopentane Rings. Eur. J. Org. Chem. 2021, 4521–4524. [Google Scholar] [CrossRef]
- Bailey, T.R.; Young, D.C. Compounds, Compositions and Methods for Treating or Preventing Viral Infections and Associated Diseases. U.S. Patent 2004198741-A1, 7 October 2004. [Google Scholar]
- Zoller, J.; Fabry, D.C.; Rueping, M. Unexpected Dual Role of Titanium Dioxide in the Visible Light Heterogeneous Catalyzed C–H Arylation of Heteroarenes. ACS Catalysis 2015, 5, 39003904. [Google Scholar] [CrossRef]
- Liang, Y.F.; Steinbock, R.; Yang, L.; Ackermann, L. Continuous Visible-Light Photoflow Approach for a Manganese-Catalyzed (Het)Arene C-H Arylation. Angew. Chem. Int. Ed. 2018, 57, 10625–10629. [Google Scholar] [CrossRef]
- Lu, G.-p.; Cai, C.; Lipshutz, B.H. Stille couplings in water at room temperature. Green. Chem. 2013, 15, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Glushkov, R.G.; Vozyakova, T.I.; Adamskaya, E.V.; Guskova, T.A.; Pushkina, T.V.; Radkevich, T.P.; Soloveva, N.P. Synthesis and antibacterial activity of furyl-substituted quinolonecarboxylic acids. Pharm. Chem J. 1998, 32, 8–12. [Google Scholar] [CrossRef]
- Hanson, P.; Jones, J.R.; Taylor, A.B.; Walton, P.H.; Timmsb, A.W. Sandmeyer Reactions. Part 7.1 An Investigation into the Reduction Steps of Sandmeyer Hydroxylation and Chlorination Reactions. J. Chem. Soc. Perkin. Trans. 2002, 2, 1135–1150. [Google Scholar] [CrossRef]
- Oliveira, C.C.; Dos Santos, E.A.; Nunes, J.H.; Correia, C.R. Stereoselective Arylation Of Substituted Cyclopentenes By Substrate-Directable Heck-Matsuda Reactions: A Concise Total Synthesis Of The Sphingosine 1-Phosphate Receptor (S1P(1)) Agonist VPC01091. J. Org. Chem. 2012, 77, 8182–8190. [Google Scholar] [CrossRef] [PubMed]
- Alwin, S.; Sahaya, S.X.; Ranjini, M.; Nabhiraj, P.Y.; Warrier, K.G.K.; Mohan, R.G. Surface modification of titania aerogel films by oxygen plasma treatment for enhanced dye adsorption. Thin. Solid. Films 2015, 595, 164–170. [Google Scholar] [CrossRef]
- Rastogi, S.K.; Zhao, Z.; Gildner, M.B.; Shoulders, B.A.; Velasquez, T.L.; Blumenthal, M.O.; Wang, L.; Li, X.; Hudnall, T.W.; Betancourt, T.; et al. Synthesis, optical properties and in vitro cell viability of novel spiropyrans and their photostationary states. Tetrahedron 2021, 80, 131854. [Google Scholar] [CrossRef]
- Xing, B.; Li, L.; Ni, C.; Hu, J. Pentafluoroethylation of arenediazonium tetrafluoroborates using on-site generated tetrafluoroethylene. chin. J. Chem. 2019, 37, 1131–1136. [Google Scholar]
- Sherborne, G.J.; Gevondian, A.G.; Funes-Ardoiz, I.; Dahiya, A.; Fricke, C.; Schoenebeck, F. Modular and selective arylation of aryl germanes (C-GeEt3) over C-Bpin, C-SiR3 and halogens enabled by light-activated gold catalysis. Angew. Chem. Int. Ed. 2020, 59, 15543–15548. [Google Scholar] [CrossRef]
- Çeken, B.; Kízíl, M. Synthesis and DNA-cleaving activity of a series of substituted arenediazonium ions. Russ. J. Bioorg. Chem. 2008, 34, 488–498. [Google Scholar] [CrossRef]


| Entry | 1a:2 | Catalyst | Loading (mol%) | Time (h) | Yield 1 (%) |
|---|---|---|---|---|---|
| 1 | 1:10 | - | - | 24 | - |
| 2 | 1:10 | Eosin Y | 2 | 24 | 55 |
| 3 | 1:10 | MR-EY | 2 | 2 | 44 |
| 4 | 1:10 | MR-EY | 2 | 4 | 47 |
| 5 | 1:10 | MR-EY | 2 | 16 | 53 |
| 6 | 1:10 | MR-EY | 2 | 24 | 52 |
| 7 | 1:10 | MR-EY | 5 | 2 | 53 |
| 8 | 1:10 | MR-EY | 10 | 2 | 55 |
| 9 | 1:20 | MR-EY | 2 | 2 | 75 |
| 10 | 1:30 | MR-EY | 2 | 2 | 75 |

| Entry | 1 | R | 1:2 | Yield 3 1 (%) |
|---|---|---|---|---|
| 1 | b 2 | 4-OMe | 1:10 | 18 |
| 2 | b 2 | 4-OMe | 1:20 | 40 |
| 3 | c 2 | 4-NO2 | 1:20 | 67 |
| 4 | d 3 | 4-nBu | 1:20 | 62 |
| 5 | e 4 | 3,4,5-(OMe)3 | 1:20 | 47 |
| 6 | f 5 | 3,5-Cl2 | 1:20 | 38 |
| 7 | g 6 | 2-OMe | 1:20 | 46 |
| 8 | h 2 | 3-NO2 | 1:20 | 52 |
| 9 | i 7 | 2-Cl,4-NO2 | 1:20 | 36 |
| Entry | Run | Yield 3a 1 (%) |
|---|---|---|
| 1 | 1 | 70 |
| 2 | 2 | 45 |
| 3 | 3 | 40 |
| Entry | Method | Substrate | Product | Yield 3 1 (%) | Productivity 2 (mmol/h) | STY 3 (mmol mL −1 h −1) | Relative Factor 4 |
|---|---|---|---|---|---|---|---|
| 1 | batch | 1a | 3a | 75 5 | 0.25 | 0.07 | 1 |
| 2 | flow | 1a | 3a | 55 | 0.89 | 0.61 | 8.8 |
| 3 | batch | 1b | 3b | 40 6 | 0.13 | 0.037 | 1 |
| 4 | flow | 1b | 3b | 42 | 0.68 | 0.47 | 12.6 |
| 5 | batch | 1c | 3c | 67 7 | 0.22 | 0.062 | 1 |
| 6 | flow | 1c | 3c | 34 | 0.65 | 0.38 | 6.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, S.; Herbrik, F.; Resta, S.; Puglisi, A. Supported Eosin Y as a Photocatalyst for C-H Arylation of Furan in Batch and Flow. Molecules 2022, 27, 5096. https://doi.org/10.3390/molecules27165096
Rossi S, Herbrik F, Resta S, Puglisi A. Supported Eosin Y as a Photocatalyst for C-H Arylation of Furan in Batch and Flow. Molecules. 2022; 27(16):5096. https://doi.org/10.3390/molecules27165096
Chicago/Turabian StyleRossi, Sergio, Fabian Herbrik, Simonetta Resta, and Alessandra Puglisi. 2022. "Supported Eosin Y as a Photocatalyst for C-H Arylation of Furan in Batch and Flow" Molecules 27, no. 16: 5096. https://doi.org/10.3390/molecules27165096
APA StyleRossi, S., Herbrik, F., Resta, S., & Puglisi, A. (2022). Supported Eosin Y as a Photocatalyst for C-H Arylation of Furan in Batch and Flow. Molecules, 27(16), 5096. https://doi.org/10.3390/molecules27165096








