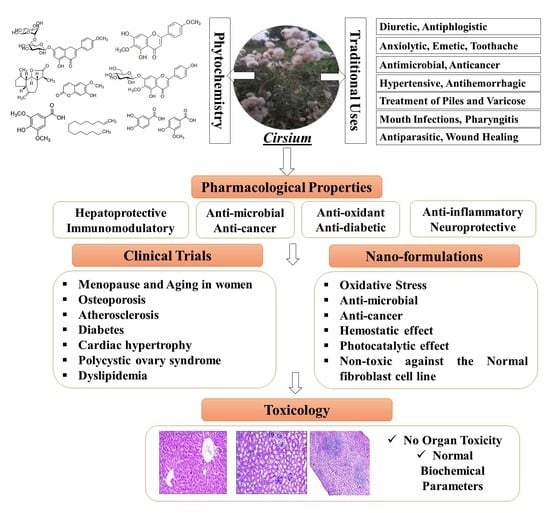
Traditional Uses, Phytochemical Composition, Pharmacological Properties, and the Biodiscovery Potential of the Genus Cirsium
Abstract
1. Introduction
2. Methodology
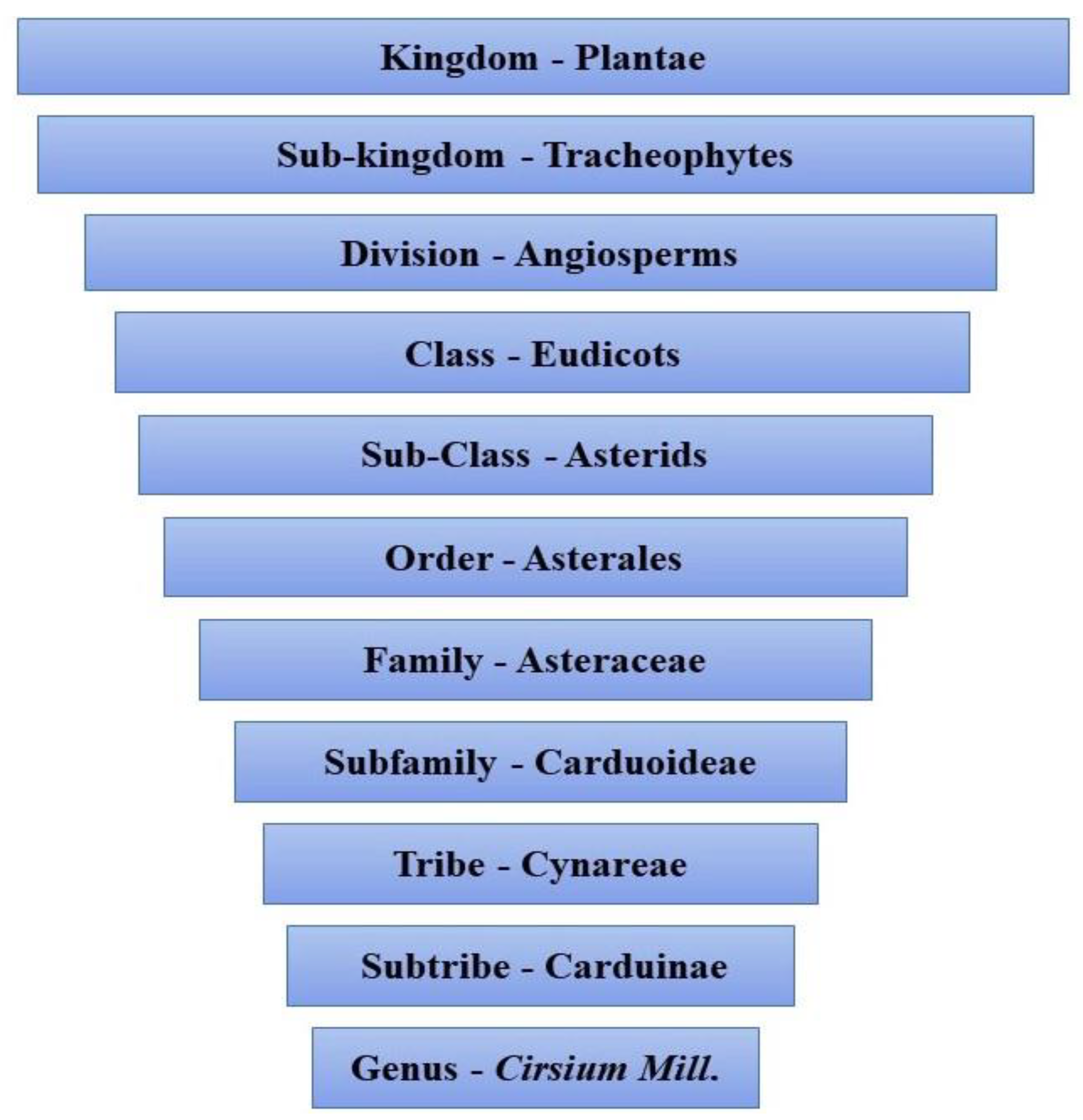
3. Cirsium
4. Botanical Description of Genus Cirsium
5. Traditional Uses
6. Culinary Uses of Cirsium
7. Phytochemical Composition
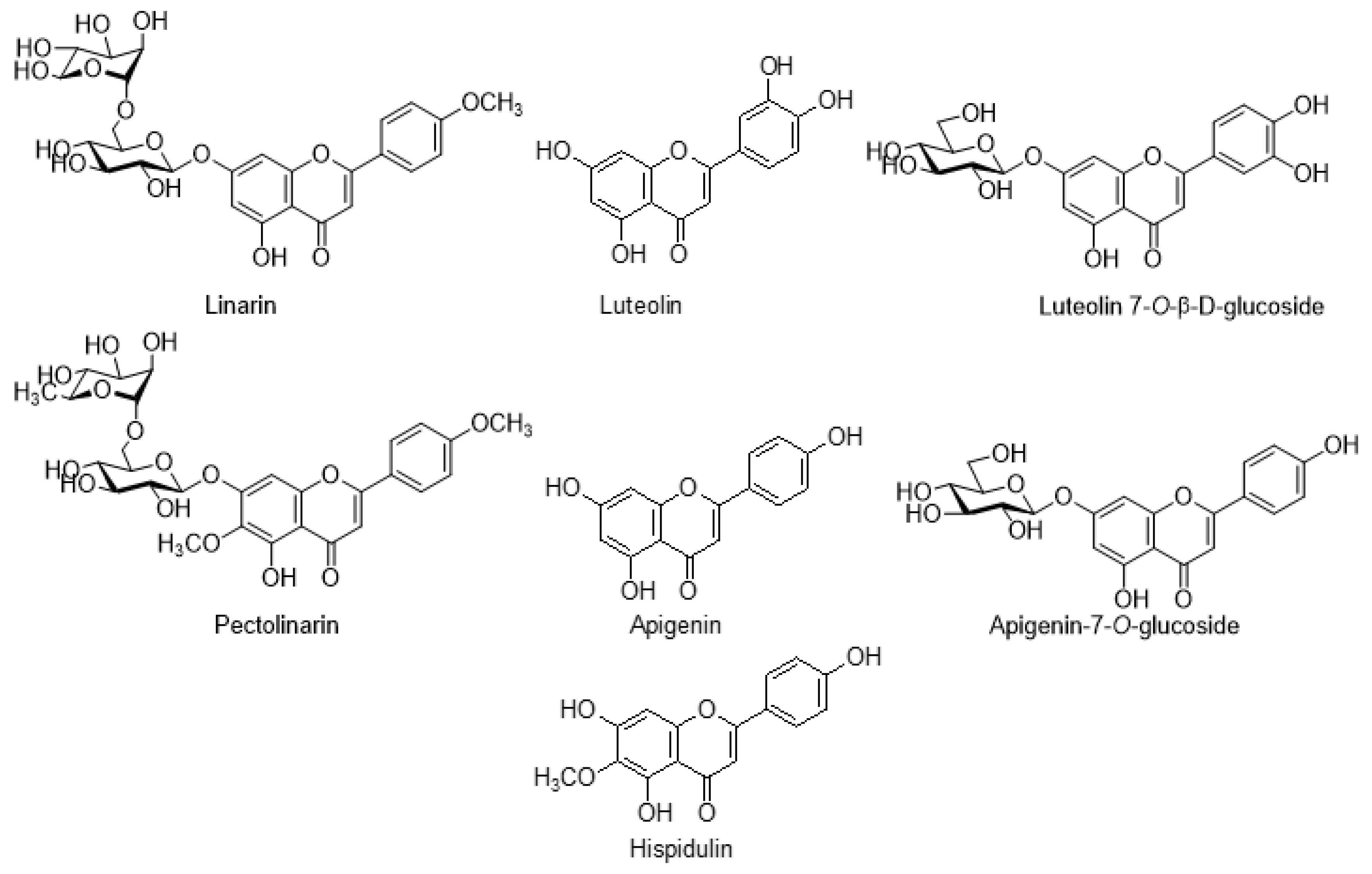
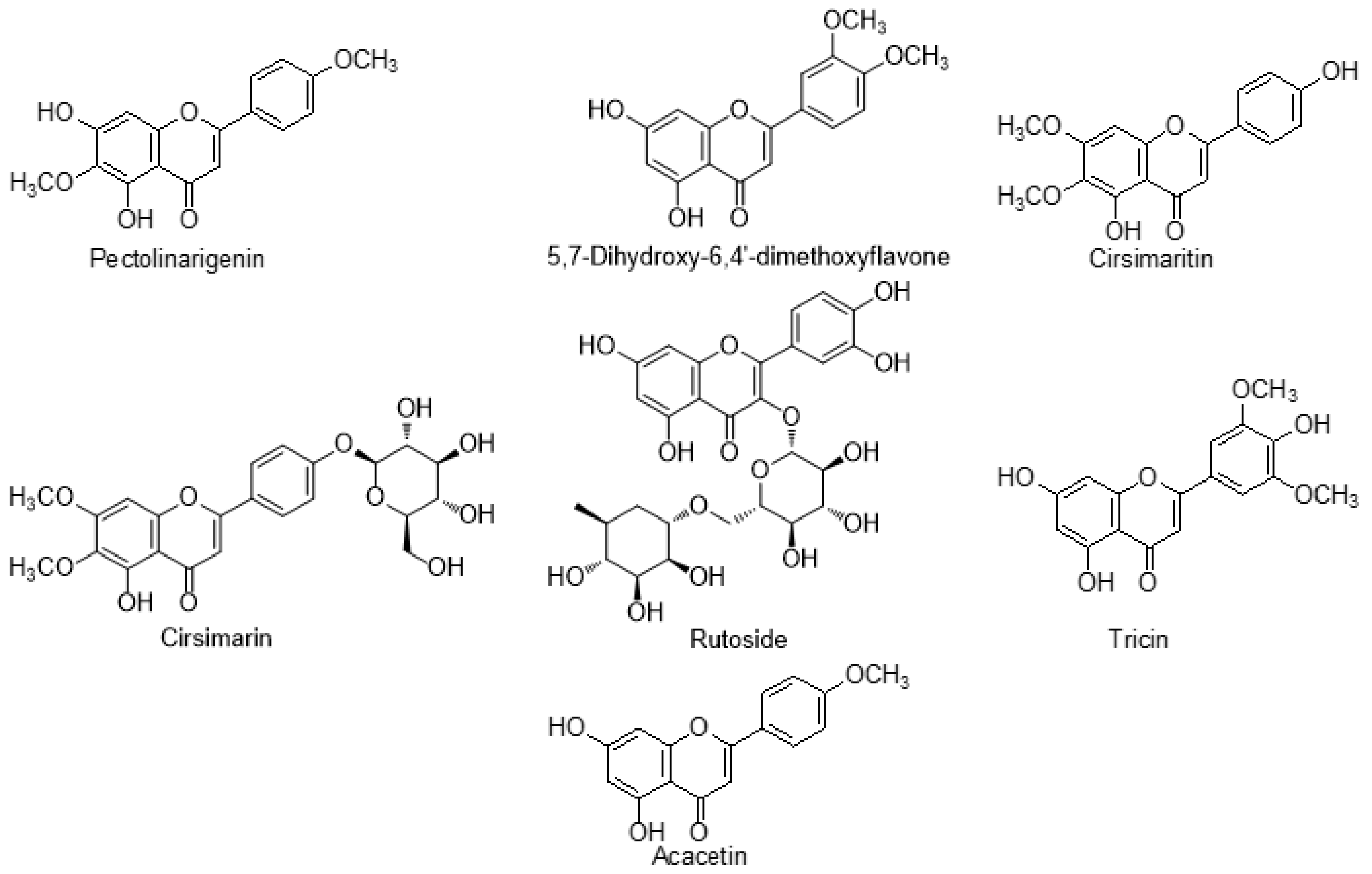
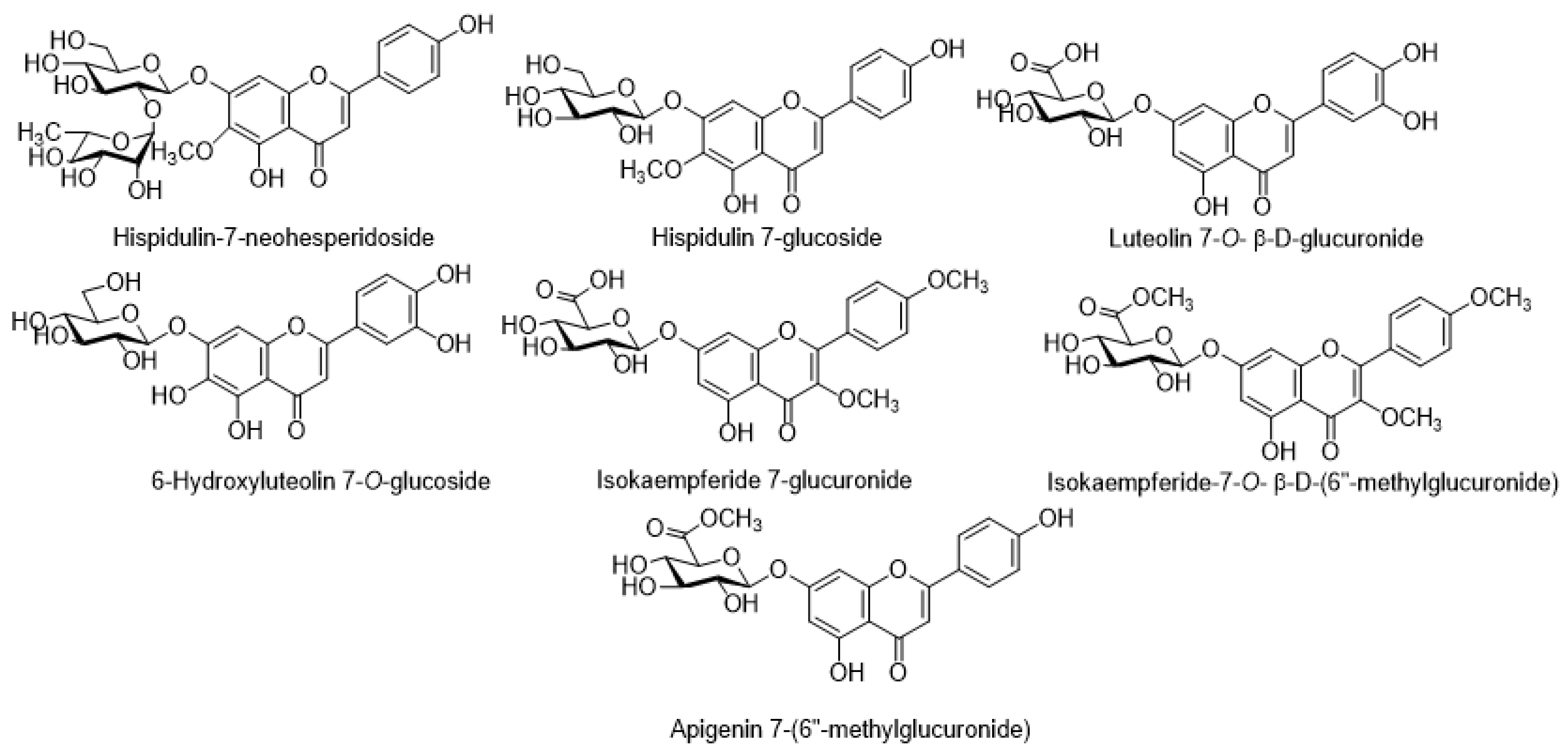
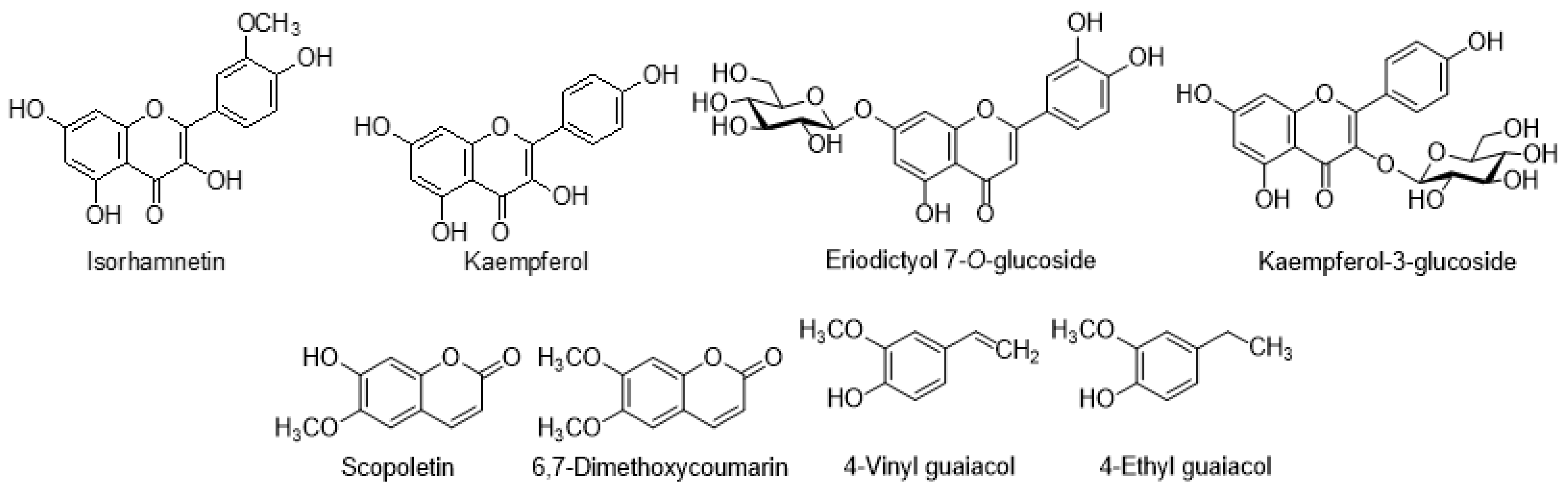
7.1. Flavonoids
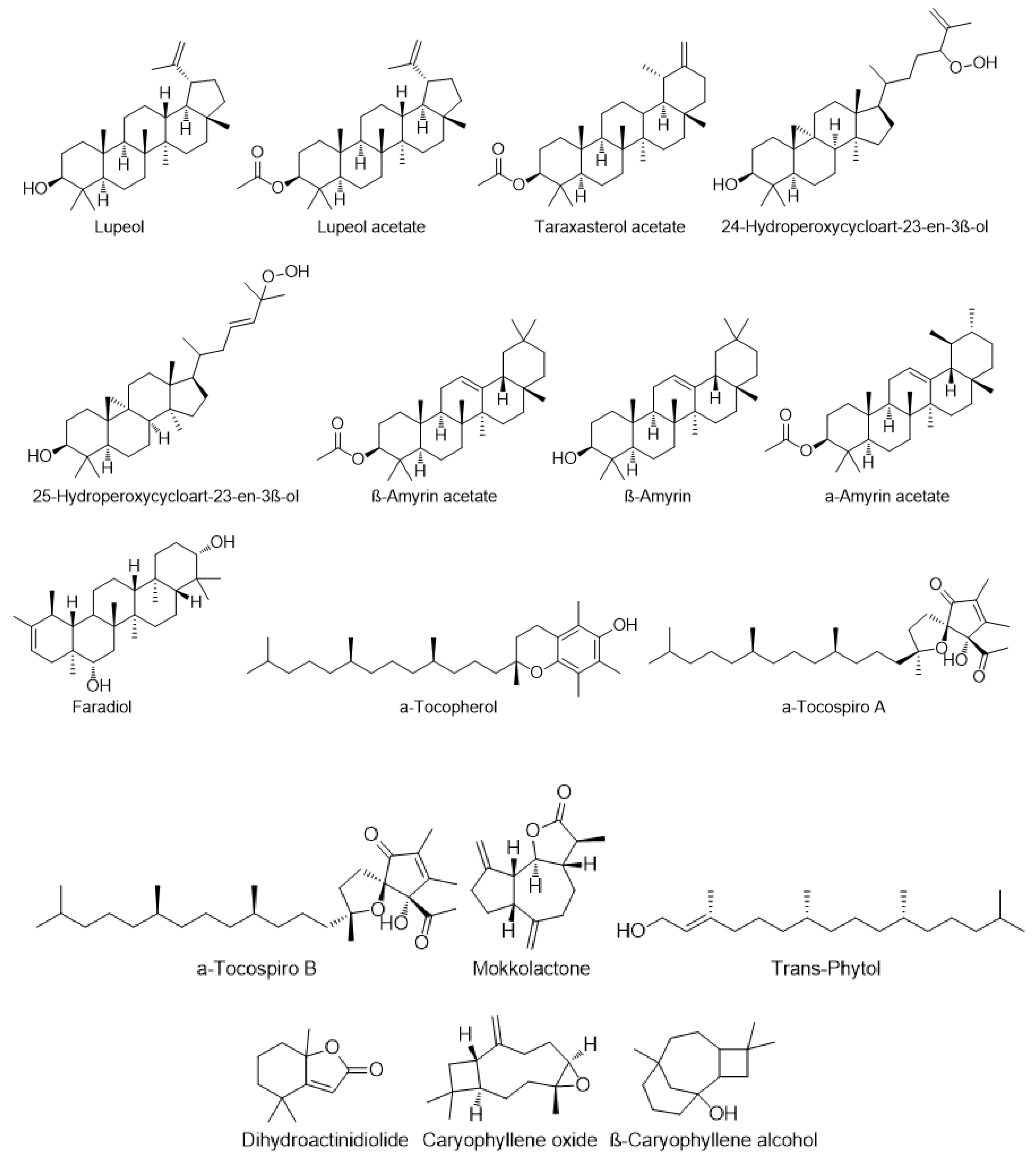
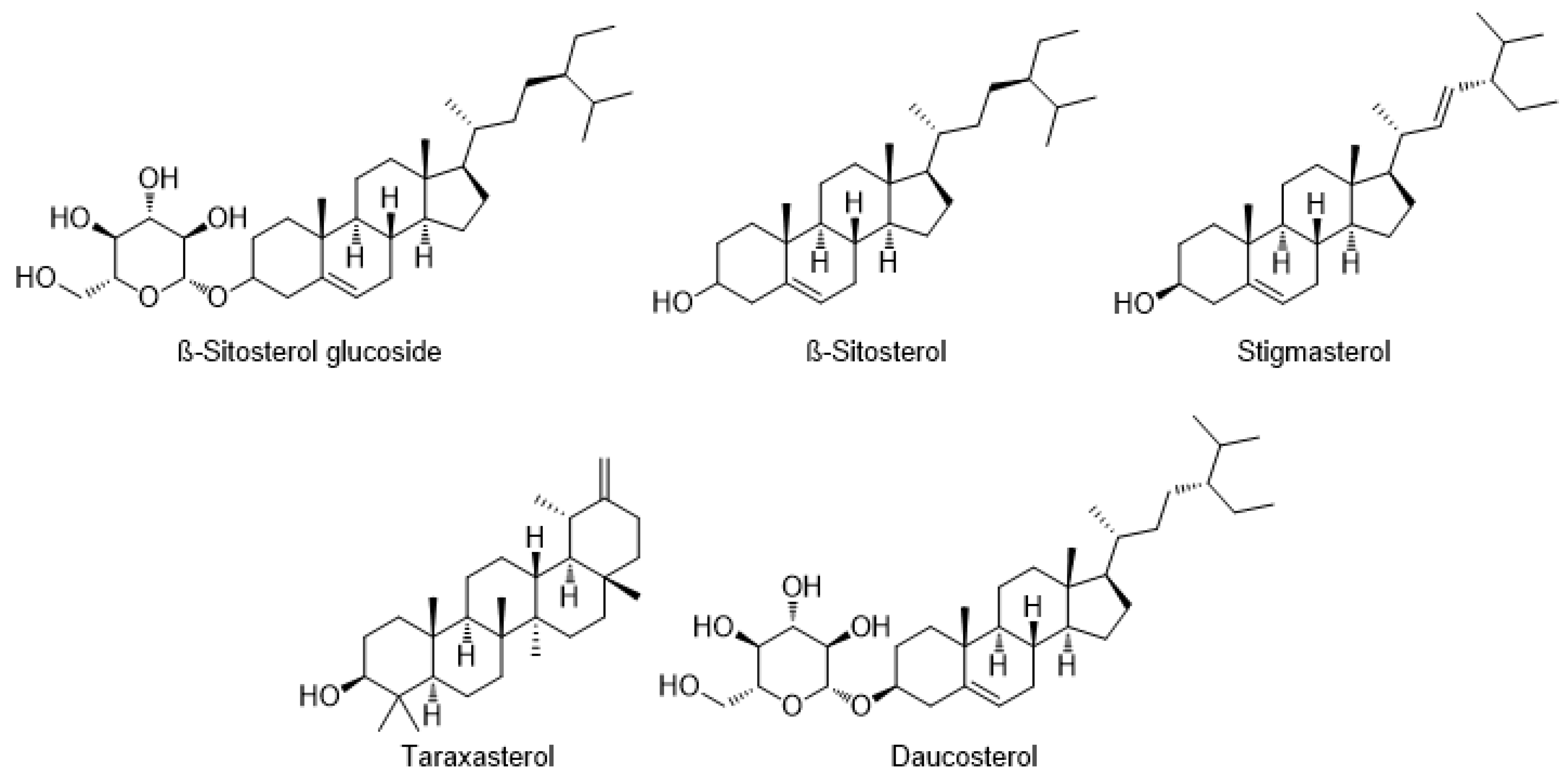
7.2. Terpenoids and Sterols
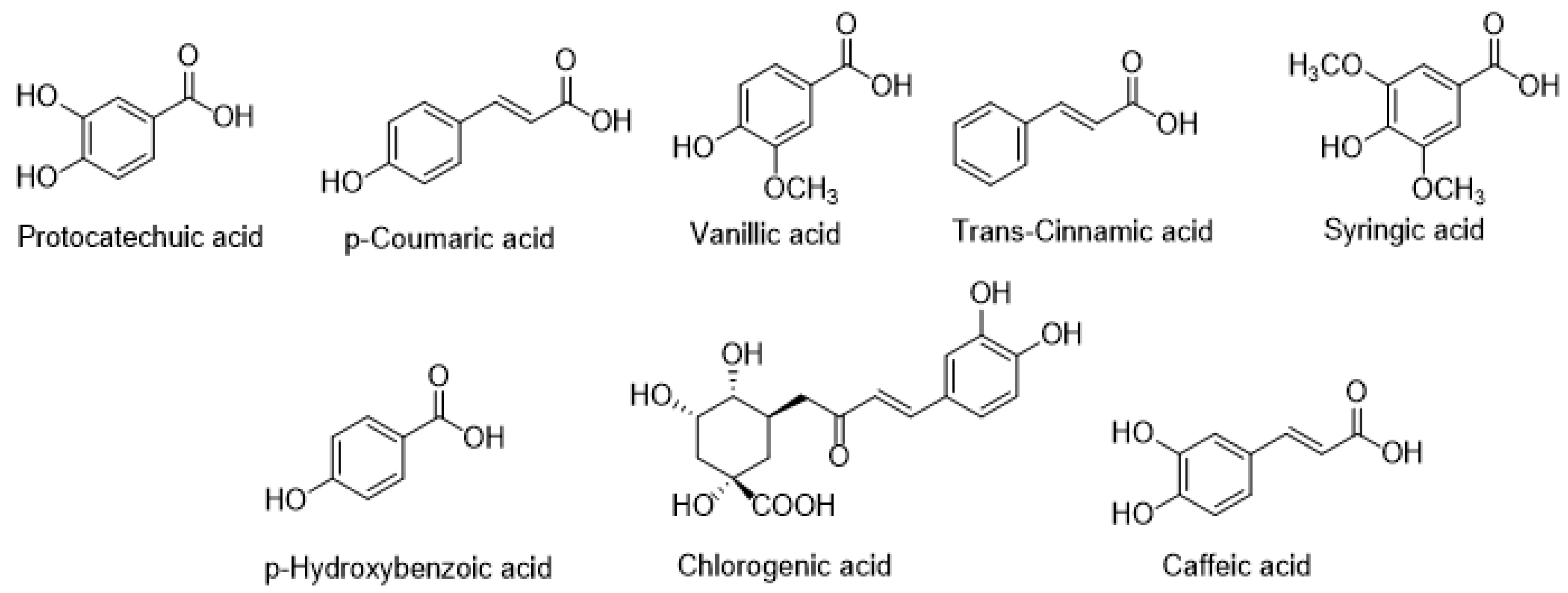
7.3. Phenolic Acids
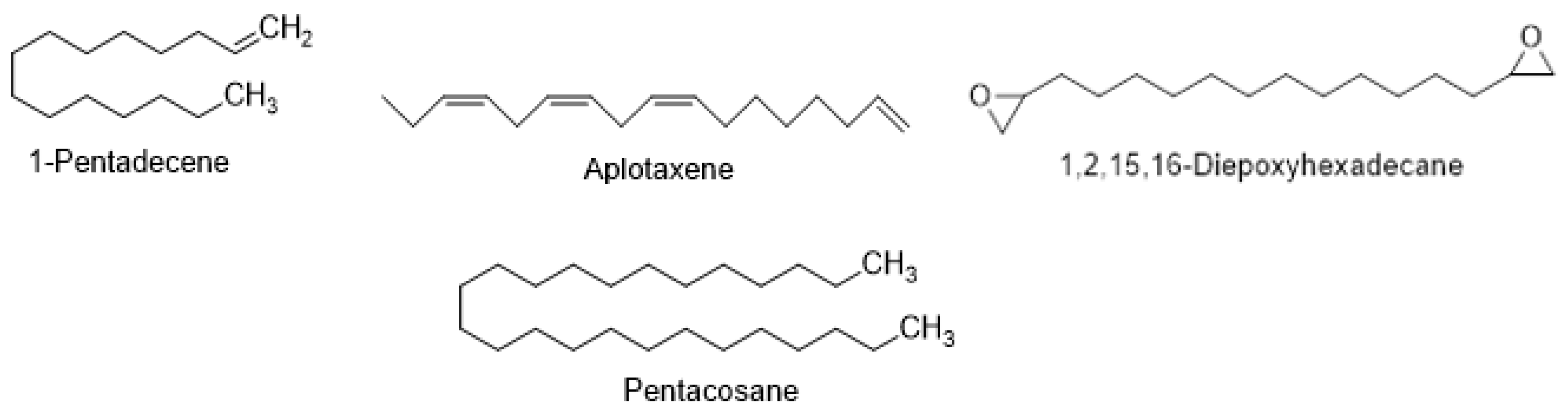
7.4. Polyacetylenes, Acetylenes, and Hydrocarbons
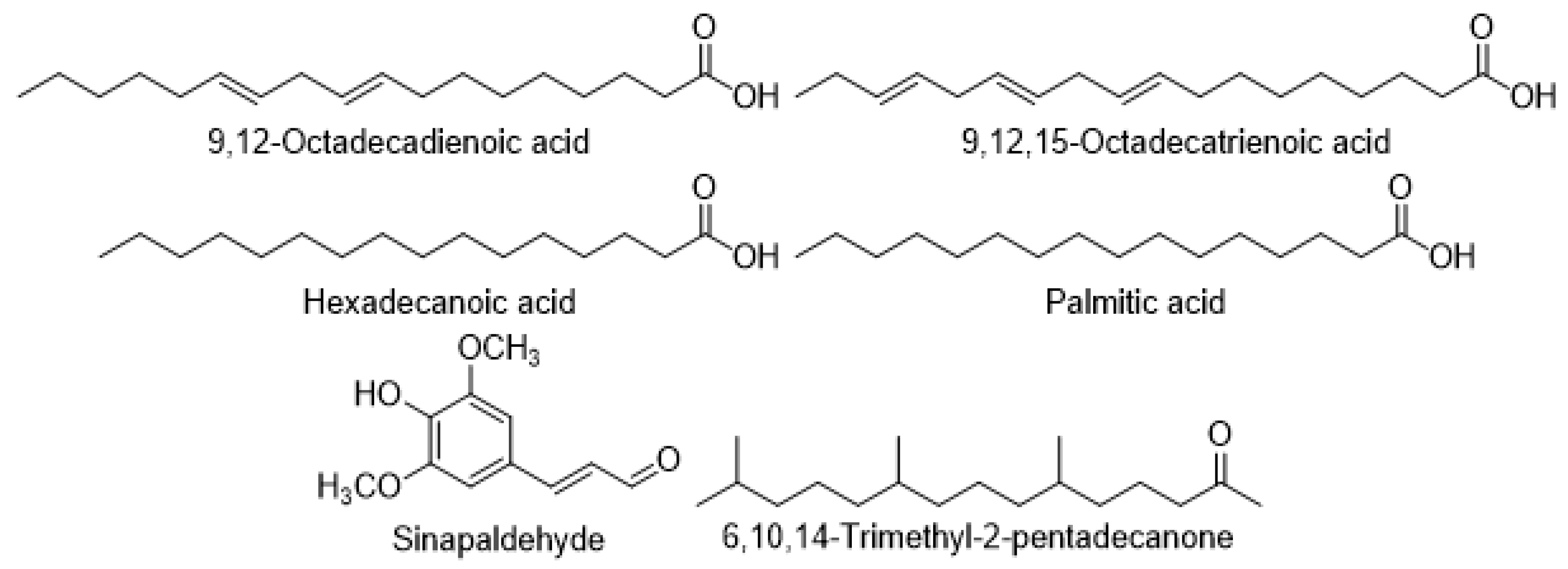
7.5. Fatty acids, Aldehydes, and Ketones
8. Essential Oil Composition
9. Pharmacological Studies
9.1. Antimicrobial Activity
9.2. Antioxidant Activity
9.3. Antiproliferative Activity
9.4. Analgesic and Anti-Inflammatory Activity
9.5. Hepatoprotective Activity
9.6. Immunomodulatory Activity
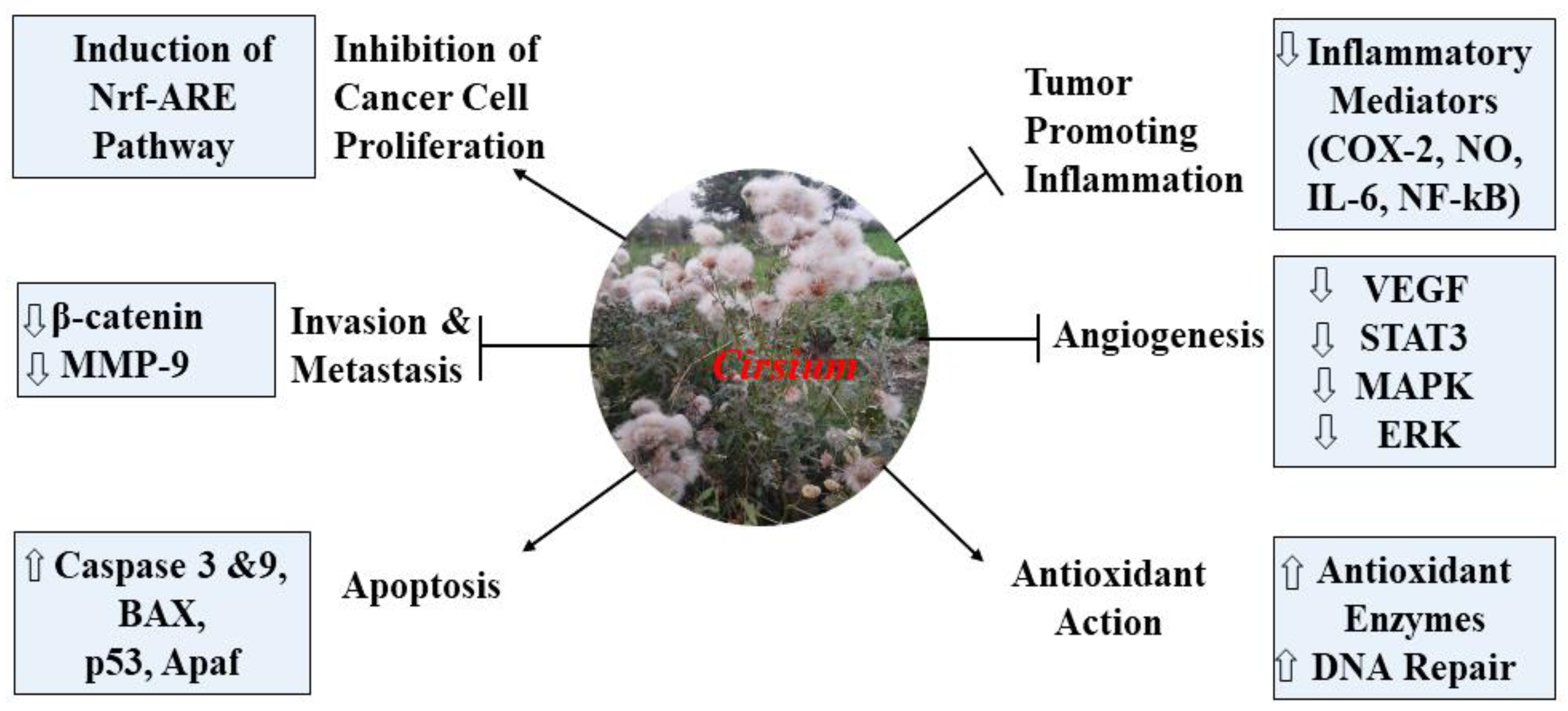
9.7. Anticancer Activity
9.8. Oviposition Stimulatory Activity
9.9. Anti-Anxiety Effect
9.10. Nephroprotective
9.11. Other Therapeutic Effects
10. Herbal Formulations and Clinical Trials
11. Patents
12. Pharmacokinetics
13. Toxicology
14. Nanoformulations of Cirsium
15. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| ALT | Alanine aminotransferase |
| ARI | Aldose reductase inhibition |
| AST | Aspartate aminotransferase |
| Bel7402 | Human hepatocellular carcinoma |
| Cmax | Maximum plasma concentration |
| CCl4 | Carbon tetrachloride |
| COX-2 | Cyclooxygenase-2 |
| DPPH | 2,2-diphenyl-1-picryl-hydrazyl-hydrate |
| ED50 | Median effective dose |
| 5-FU | Fluorouracil |
| GABA | Gamma-aminobutyric acid |
| G1 | Growth 1 phase |
| G2/M | Growth 2 phase |
| HCT-8 | Human colon cancer cell line |
| HeLa cell | Henrietta Lacks cell |
| Hif-2α | Hypoxia-Inducible Factor-2α |
| HPLC-MS | High-performance liquid chromatography–mass spectrometry |
| IC50 | Half-maximal inhibitory concentration |
| IL-6 | Interleukin-6 |
| MBC | Minimum bacterial concentration |
| MCF-7 | Human breast cancer cell line |
| MDA-MB-231 | Human mammary carcinoma |
| MIC | Minimum inhibitory concentration |
| MMP3 | Matrix metalloproteinase-3 |
| MMP13 | Matrix metalloproteinase-13 |
| NF-κB | Nuclear factor kappa B |
| NO | Nitric oxide |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| p-Akt | Protein kinase B |
| p-ERK | Extracellular signal-regulated kinase |
| SOD | Superoxide dismutase |
| VEGF | Vascular endothelial growth factor |
| CA-CuNP | Cirsium arvense-derived copper nanoparticles |
| CSC | Cirsium setosum Carbonisata |
| CD | Carbon dots |
References
- Asghar, M.; Younas, M.; Arshad, B.; Zaman, W.; Ayaz, A.; Rasheed, S.; Shah, A.H.; Ullah, F.; Saqib, S. Bioactive potential of cultivated Mentha arvensis l for preservation and production of health-oriented food. J. Anim. Plant. Sci. 2022, 32, 835–844. [Google Scholar]
- Cláudio, A.F.M.; Cognigni, A.; de Faria, E.L.; Silvestre, A.J.; Zirbs, R.; Freire, M.G.; Bica, K. Valorization of olive tree leaves: Extraction of oleanolic acid using aqueous solutions of surface-active ionic liquids. Sep. Purif. Technol. 2018, 204, 30–37. [Google Scholar] [CrossRef]
- Salehi, B.; Kumar, N.V.A.; Şener, B.; Sharifi-Rad, M.; Kılıç, M.; Mahady, G.B.; Vlaisavljevic, S.; Iriti, M.; Kobarfard, F.; Setzer, W.N.; et al. Medicinal Plants Used in the Treatment of Human Immunodeficiency Virus. Int. J. Mol. Sci. 2018, 19, 1459. [Google Scholar] [CrossRef]
- Marmitt, D.J.; Shahrajabian, M.H. Plant species used in Brazil and Asia regions with toxic properties. Phytotherapy Res. 2021, 35, 4703–4726. [Google Scholar] [CrossRef]
- Plants of the World Online: Cirsium Mill. Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:30001899-2 (accessed on 8 August 2022).
- Bureš, P.; Wang, Y.-F.; Horová, L.; Suda, J. Genome Size Variation in Central European Species of Cirsium (Compositae) and their Natural Hybrids. Ann. Bot. 2004, 94, 353–363. [Google Scholar] [CrossRef]
- Kadota, Y. Species Diversification of Genus Cirsium (Asteraceae) in Japan. Korean J. Plant Taxon. 2007, 37, 335–349. [Google Scholar] [CrossRef]
- Ashmita, P.; Singh, L.; Kumar, D.; Antil, R.; Dahiya, P. Cirsium arvense: A Multi-potent Weed. Ann. Biol. 2020, 36, 442–447. [Google Scholar]
- Shahrajabian, M.H. Spear Thistle (Cirsium Vulgare L.) And Ramsons (Allium Ursinum L.), Impressive Health Benefits and High-Nutrient Medicinal Plants. Pharmacogn. Commun. 2021, 11, 168–171. [Google Scholar] [CrossRef]
- Luo, W.; Wu, B.; Tang, L.; Li, G.; Chen, H.; Yin, X. Recent research progress of Cirsium medicinal plants in China. J. Ethnopharmacol. 2021, 280, 114475. [Google Scholar] [CrossRef]
- Moore, R.J. The Thistles of Canada. Ottawa, Canada. 1974. Available online: https://archive.org/details/thistlesofcanada00moor/page/19/mode/1up?ref=ol&view=theater&q=cirsium (accessed on 24 August 2021).
- Mabberley, D.J. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classifications and Uses, 3rd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Karthikeyan, S.M.; Sanjappa, S.; Moorthy, S. Flowering Plants of India. In Dicotyledons; BSI: Kolkata, India, 2009. [Google Scholar]
- Bohm, B.A.; Stuessy, T.F. Flavonoids of the Sunflower Family (Asteraceae); Springer: Wien, Austria, 2001. [Google Scholar]
- Venner, L. Common Name: Bull Thistle, (Common Thistle, Spear Thistle, Scotch Thistle, Black Thistle, Plume Thistle). Available online: https://azkurs.org/common-name-bull-thistle-common-thistle-spear-thistle-scotch-t.html (accessed on 22 August 2021).
- Jordon-Thaden, I.E.; Louda, S.M. Chemistry of Cirsium and Carduus: A role in ecological risk assessment for biological control of weeds? Biochem. Syst. Ecol. 2003, 31, 1353–1396. [Google Scholar]
- Formisano, C.; Rigano, D.; Senatore, P.F.; De Feo, V.; Bruno, M.; Rosselli, S. Composition and allelopathic effect of essential oils of two thistles: Cirsium creticum (Lam.) D.’Urv. ssp. triumfetti (Lacaita) Werner and Carduus nutans L. J. Plant. Interact. 2007, 2, 115–120. [Google Scholar] [CrossRef]
- Chon, S.-U.; Kim, Y.-M.; Lee, J.-C. Herbicidal potential and quantification of causative allelochemicals from several Compositae weeds. Weed Res. 2003, 43, 444–450. [Google Scholar] [CrossRef]
- van der Kooi, C.J.; Pen, I.; Staal, M.; Stavenga, D.G.; Elzenga, J.T.M. Competition for pollinators and intra-communal spectral dissimilarity of flowers. Plant Biol. 2015, 18, 56–62. [Google Scholar] [CrossRef]
- Parus, A.; Grys, A. Cirsium oleraceum (L.) Scop-active substances and possible usage. Postapy. Fitoter. 2011, 2011, 100–105. [Google Scholar]
- Boğa, M.; Yılmaz, P.K.; Cebe, D.B.; Fatima, M.; Siddiqui, B.S.; Kolak, U. Chemical Constituents and Biological Activities of Cirsium leucopsis, C. sipyleum, and C. eriophorum. Z. Für Nat. C 2014, 69, 381–390. [Google Scholar] [CrossRef]
- Chakraborty, T.; Saha, S.; Bisht, N.S. First Report on the Ethnopharmacological Uses of Medicinal Plants by Monpa Tribe from the Zemithang Region of Arunachal Pradesh, Eastern Himalayas, India. Plants 2017, 6, 13. [Google Scholar] [CrossRef]
- Bin Lee, W.; Kwon, H.C.; Cho, O.R.; Lee, K.C.; Choi, S.U.; Baek, N.I.; Lee, K.R. Phytochemical constituents of Cirsium setidens Nakai and their cytotoxicity against human cancer cell lines. Arch. Pharm. Res. 2002, 25, 628–635. [Google Scholar]
- Loizzo, M.R.; Statti, G.A.; Tundis, R.; Conforti, F.; Ando’, S.; Menichini, F. Antimicrobial activity and cytotoxicity of Cirsium tenoreanum. Fitoterapia 2004, 75, 577–580. [Google Scholar] [CrossRef]
- Ishida, H.; Umino, T.; Tsuji, K.; Kosuge, T. Studies on antihemorrhagic substances in herbs classified as hemostatics in Chinese medicine. VII On the antihemorrhagic principle in Cirsium japonicum DC. Chem. Pharm. Bull. 1987, 35, 861–864. [Google Scholar] [CrossRef]
- Cheriet, T.; Ben-Bachir, B.; Thamri, O.; Seghiri, R.; Mancini, I. Isolation and Biological Properties of the Natural Flavonoids Pectolinarin and Pectolinarigenin—A Review. Antibiotics 2020, 9, 417. [Google Scholar] [CrossRef]
- He, X.-F.; He, Z.-W.; Jin, X.-J.; Pang, X.-Y.; Gao, J.-G.; Yao, X.-J.; Zhu, Y. Caryolane-type sesquiterpenes from Cirsium souliei. Phytochem. Lett. 2014, 10, 80–85. [Google Scholar] [CrossRef]
- Walesiuk, A.; Nazaruk, J.; Braszko, J.J. Pro-cognitive effects of Cirsium rivulare extracts in rats. J. Ethnopharmacol. 2010, 129, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Dang, S. Plants Profile for Cirsium vulgare, USDA Plant Database 1984. Available online: https://plants.usda.gov/home/plantProfile?symbol=CIVU (accessed on 14 August 2021).
- Nazaruk, J.; Gudej, J. Flavoniod compounds from the flowers of Cirsium rivulare (JACQ) All. Acta. Pol. Pharm. 2003, 60, 87–89. [Google Scholar] [PubMed]
- Singh, A.; Lal, M.; Samant, S.S. Diversity, indigenous uses and conservation prioritization of medicinal plants in Lahaul valley, proposed Cold Desert Biosphere Reserve, India. Int. J. Biodivers. Sci. Manag. 2009, 5, 132–154. [Google Scholar] [CrossRef]
- Singh, B.; Borthakur, S.K. Wild medicinal plants used by tribal communities of Meghalaya. JETBD 2011, 35, 331–339. [Google Scholar]
- Creeping Thistle Facts and Health Benefits, Heal Benefits Times (n.d.). Available online: https://www.healthbenefitstimes.com/creeping-thistle (accessed on 2 March 2022).
- Guarrera, P.M. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium). Fitoterapia 2005, 76, 1–25. [Google Scholar] [CrossRef]
- Khan, H.U.Z.; Khan, S.; Chen, Y.; Wan, P. In vitro antimicrobial activity of the chemical constituents of Cirsium arvense (L). Scop. J. Med. Plants Res. 2013, 7, 1894–1898. [Google Scholar]
- Paluch, A. Folk herbal medicine in Poland in the nineteenth and early twentieth centuries. Pol. Tow. Ludozn. Wrocław 1989, 10, 98–99. [Google Scholar]
- Grzycka, K.; Krzaczek, T.; Miłkowska, J. Research on the biological activity of selected species of flower plants. Ann. Univ. Mariae Curie Sklodowska Lublin Pol. Sect. D 1978, 33, 275–283. [Google Scholar]
- Ożarowski, A.; Jaroniewski, W. Rośliny Lecznicze i ich Praktyczne Zastosowanie (Medicinal Plants and Their Practical Applying), Wydawniczy Związków Zawodowych, Warsaw, n.d. Available online: https://scholar.google.co.in/scholar?hl=en&as_sdt=0%2C5&q=Ożarowski+A.%2C+Jaroniewski+W.+1989.+Rośliny+lecznicze+i+ich+praktyczne+zastosowanie+%28Medicinal+plants+and+their+practical+applying%29.+Warszawa%3A+Instytut+Wydawniczy+Związków+Zawodowych&btnG= (accessed on 19 August 2021).
- Nazaruk, J. Antioxidant activity and total phenolic content in Cirsium five species from north–east region of Poland. Fitoterapia 2008, 79, 194–196. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J.; Lee, Y.K.; Chung, M.J.; Kwon, K.H.; Lee, S. Determination of pectolinarin in Cirsium spp. using HPLC/UV analysis. J. Appl. Biol. Chem. 2016, 59, 107–112. [Google Scholar] [CrossRef]
- Chan, F.L.; Choi, H.; Chen, Z.; Chan, P.S.; Huang, Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett. 2000, 160, 219–228. [Google Scholar] [CrossRef]
- Ku, K.-L.; Tsai, C.-T.; Chang, W.-M.; Shen, M.-L.; Wu, C.-T.; Liao, H.-F. Hepatoprotective Effect of Cirsium arisanense Kitamura in Tacrine-Treated Hepatoma Hep 3B Cells and C57BL Mice. Am. J. Chin. Med. 2008, 36, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Orhan, D.D.; Ergun, F.; Yesilada, E.; Tsuchiya, K.; Takaishi, Y.; Kawazoe, K. Antioxidant activity of two flavonol glycosides from Cirsium hypoleucum DC. through bioassay-guided fractionation Wound Healing View project Studies on Biological Activities of Vitis vinifera Leaves. Turkish J. Pharm. Sci. 2007, 4, 1–14. [Google Scholar]
- Singh, V.; Chauhan, N.S. Traditional practices of herbal medicines in the Lahaul valleys, Himachal Himalayas. Indian J. Tradit. Knowl. 2005, 4, 208–220. [Google Scholar]
- Lee, S.J. Korean Folk Medicine; Seoul National University Press: Seoul, Korea, 1966. [Google Scholar]
- Demirtas, I.; Tufekci, A.R.; Yaglioglu, A.S.; Elmastas, M. Studies on the antioxidant and antiproliferative potentials of Cirsium arvense subsp. vestitum. J. Food Biochem. 2016, 41, e12299. [Google Scholar] [CrossRef]
- Sahli, R.; Rivière, C.; Dufloer, C.; Beaufay, C.; Neut, C.; Bero, J.; Hennebelle, T.; Roumy, V.; Ksouri, R.; Leclercq, J.; et al. Antiproliferative and Antibacterial Activities of Cirsium scabrum from Tunisia. Evid. Based Complement. Altern. Med. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Vizgirdas, R.S.; Rey-Vizgirdas, E.M. Wild Plants of the Sierra Nevada; University of Nevada Press: Reno, NV, USA, 2009. [Google Scholar]
- Fernández-Martínez, E.; Díaz-Espinoza, R.; Villavicencio-Nieto, M.A.; Pérez-Escandón, B.E.; Pérez-Hernández, N.; Macías, A.; Ortíz, M.I.; A Ponce-Monter, H. Preliminary phytochemical and biological study of Cirsium ehrenbergii. Proc. West. Pharmacol. Soc. 2007, 50, 162–164. [Google Scholar]
- Nazaruk, J.; Jakoniuk, P. Flavonoid composition and antimicrobial activity of Cirsium rivulare (Jacq.) All. flowers. J. Ethnopharmacol. 2005, 102, 208–212. [Google Scholar] [CrossRef]
- Khan, H.U.Z.; Ali, F.; Khan, U.S.; Ali, I. Phytochemical study on the constituents from Cirsium arvense. Mediterr. J. Chem. 2011, 2, 64–69. [Google Scholar] [CrossRef]
- Hossain, L.M.; Hossain, A.M.; Sadhu, K.S. HPLC Profiling and Evaluation of In-vitro Antioxidant Activity of Cirsium arvense L. (Family: Asteraceae). J. Pharmacogn. Phytochem. 2016, 5, 272–277. [Google Scholar]
- Liu, S.; Zhang, J.; Li, D.; Liu, W.; Luo, X.; Zhang, R.; Li, L.; Zhao, J. Anticancer activity and quantitative analysis of flavone of Cirsium japonicum DC. Nat. Prod. Res. 2007, 21, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Rodriguez, J.P.; Lee, K.H.; Park, J.Y.; Kang, K.S.; Hahm, D.-H.; Huh, C.K.; Lee, S.C.; Lee, S. Determination of flavonoids from Cirsium japonicum var. maackii and their inhibitory activities against aldose reductase. Appl. Biol. Chem. 2017, 60, 487–496. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kim, G.-H. Identification for Flavones in Different Parts of Cirsium japonicum. Prev. Nutr. Food Sci. 2003, 8, 330–335. [Google Scholar] [CrossRef]
- Malejko, J.; Nalewajko-Sieliwoniuk, E.; Nazaruk, J.; Siniło, J.; Kojło, A. Determination of the total polyphenolic content in Cirsium palustre (L.) leaves extracts with manganese(IV) chemiluminescence detection. Food Chem. 2014, 152, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Luo, X.; Li, D.; Zhang, J.; Qiu, D.; Liu, W.; She, L.; Yang, Z. Tumor inhibition and improved immunity in mice treated with flavone from Cirsium japonicum DC. Int. Immunopharmacol. 2006, 6, 1387–1393. [Google Scholar] [CrossRef]
- Vázquez, M.M.; Apan, T.O.R.; Lastra, A.L.; Bye, R. A Comparative Study of the Analgesic and Anti-Inflammatory Activities of Pectolinarin Isolated from Cirsium subcoriaceum and Linarin Isolated from Buddleia cordata. Planta Med. 1998, 64, 134–137. [Google Scholar] [CrossRef]
- Lim, H.; Son, K.H.; Chang, H.W.; Bae, K.; Kang, S.S.; Kim, H.P. Anti-inflammatory Activity of Pectolinarigenin and Pectolinarin Isolated from Cirsium chanroenicum. Biol. Pharm. Bull. 2008, 31, 2063–2067. [Google Scholar] [CrossRef]
- Yoo, Y.M.; Nam, J.H.; Kim, M.Y.; Choi, J.; Park, H.J. Pectolinarin and Pectolinarigenin of Cirsium setidens Prevent the Hepatic Injury in Rats Caused by D-Galactosamine via an Antioxidant Mechanism. Biol. Pharm. Bull. 2008, 31, 760–764. [Google Scholar] [CrossRef]
- Syrchina, A.I.; Semenov, A.A.; Zinchenko, S.V. Chemical investigation of Cirsium setosum. Chem. Nat. Compd. 1997, 33, 212. [Google Scholar] [CrossRef]
- Miyaichi, Y.; Matsuura, M.; Tomimori, T. Phenolic Compound from the Roots of Cirsium japonicum DC. Nat. Med. 1995, 49, 92–94. [Google Scholar]
- Kozyra, M.; Biernasiuk, A.; Malm, A.; Chowaniec, M. Chemical compositions and antibacterial activity of extracts obtained from the inflorescences of Cirsium canum (L.) all. Nat. Prod. Res. 2015, 29, 2059–2063. [Google Scholar] [CrossRef] [PubMed]
- Ganzera, M.; Pöcher, A.; Stuppner, H. Differentiation of Cirsium japonicum and C. setosum by TLC and HPLC-MS. Phytochem. Anal 2005, 16, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Kozyra, M.; Mardarowicz, M.; Kochmańska, J. Chemical composition and variability of the volatile components from inflorescences of Cirsium species. Nat. Prod. Res. 2015, 29, 1942–1944. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P. Aplotaxene derivatives from Cirsium helenioides. Phytochemistry 1992, 31, 2039–2041. [Google Scholar] [CrossRef]
- Gnonlonfin, G.J.B.; Sanni, A.; Brimer, L. Review Scopoletin–A Coumarin Phytoalexin with Medicinal Properties. Crit. Rev. Plant Sci. 2012, 31, 47–56. [Google Scholar] [CrossRef]
- Ma, Q.; Jiang, J.-G.; Yuan, X.; Qiu, K.; Zhu, W. Comparative antitumor and anti-inflammatory effects of flavonoids, saponins, polysaccharides, essential oil, coumarin and alkaloids from Cirsium japonicum DC. Food Chem. Toxicol. 2019, 125, 422–429. [Google Scholar] [CrossRef]
- Kuhnau, J. Flavonoids. A class of semi-essential food components: Their role in human nutrition. J. Nutr. Diet. 1976, 24, 117–191. [Google Scholar]
- Yuan, Z.; Duan, H.; Xu, Y.; Wang, A.; Gan, L.; Li, J.; Liu, M.; Shang, X. α-Tocospiro C, a novel cytotoxic α-tocopheroid from Cirsium setosum. Phytochem. Lett. 2014, 8, 116–120. [Google Scholar] [CrossRef]
- Choi, H.S. Chemical Composition of the Essential Oil from Cirsium setidens, a Korean Medicinal Plant. TACL 2015, 5, 94–102. [Google Scholar]
- Nazaruk, J.; Jablonski, J. Chemical constituents of chloroform and petroleum extracts from Cirsium palustre flower heads. Chem. Nat. Compd. 2011, 47, 654–655. [Google Scholar] [CrossRef]
- Nazaruk, J.E.; Karna Kalemba, D. The chemical composition of the essential oils of Cirsium palustre and C. rivulare and their antiproliferative effect. Nat. Prod. Commun. 2012, 7, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Dutta, A.; Chaudhuri, A.; Mukherjee, D.; Dey, S.; Halder, S.; Ghosh, J.; Sultana, S.S.; Biswas, G.; Lai, T.K.; et al. Successful Therapy of Murine Visceral Leishmaniasis with Astrakurkurone, a Triterpene Isolated from the Mushroom Astraeus hygrometricus, Involves the Induction of Protective Cell-Mediated Immunity and TLR9. Antimicrob. Agents Chemother. 2016, 60, 2696–2708. [Google Scholar] [CrossRef] [PubMed]
- Kozyra, M.; Głowniak, K. Phenolic acids in extracts obtained from the flowering herbs of Cirsium vulgare (Savi) Ten. growing in Poland. Acta Soc. Bot. Pol. 2013, 82, 325–329. [Google Scholar] [CrossRef]
- Arnason, T.; Swain, T.; Wat, C.K.; Graham, E.A.; Partington, S.; Towers, G.H.N.; Lam, J. Mosquito larvicidal activity of polyacetylenes from species in the Asteraceae. Biochem. Syst. Ecol. 1981, 9, 63–68. [Google Scholar] [CrossRef]
- Bohlmann, F.; Burkhardt, T.; Zdero, C. Naturally Occurring Acetylenes; Academic Press: London, UK, 1973. [Google Scholar]
- Baek, N.; Park, J.D.; Lee, Y.H.; Jeong, S.Y.; Kim, S.I. A Novel Polyacetylene from Cirsium spp. Pharm. Soc. Korea 1995, 39, 268–275. [Google Scholar]
- Guillet, G.; Philogène, B.J.R.; O’Meara, J.; Durst, T.; Arnason, J.T. Multiple modes of insecticidal action of three classes of polyacetylene derivatives from Rudbeckia hirta. Phytochemistry 1997, 46, 495–498. [Google Scholar] [CrossRef]
- Harborne, J.B. Classes and functions of secondary products from plants. In Chemicals from Plants; Walton, N.J., Brown, D.E., Eds.; Imperial College Press: London, UK, 1999; pp. 1–26. [Google Scholar]
- Shuqing, W.; Hanhong, X.; Shanhuan, Z.; Zhizhen, S.; Zhun, L. Phototoxicity of synthetic polyacetylenes against mosquito larvae (Culex quinquefasciatus), Kun Chong Xue Bao. Acta Entomol. Sin. 2000, 43, 264–270. [Google Scholar]
- Ying, W.; Masao, T.; Felicitas, K.; Matthias, H.; Kurt, H. Polyacetylenes fromArtemisia borealis and their biological activities. Phytochemistry 1990, 29, 3101–3105. [Google Scholar] [CrossRef]
- Wat, C.-K.; Prasad, S.; Graham, E.; Partington, S.; Arnason, T.; Towers, G.; Lam, J. Photosensitization of invertebrates by natural polyacetylenes. Biochem. Syst. Ecol. 1981, 9, 59–62. [Google Scholar] [CrossRef]
- Amiri, N.; Yadegari, M.; Hamedi, B. Essential Oil Composition of Cirsium arvense L. Produced in Different Climate and Soil Properties. Rec. Nat. Prod. 2018, 12, 251–262. [Google Scholar] [CrossRef]
- Dehjurian, A.; Lari, J.; Motavalizadehkakhky, A. Anti-Bacterial Activity of Extract and the Chemical Composition of Essential Oils in Cirsium arvense from Iran. J. Essent. Oil Bear. Plants 2017, 20, 1162–1166. [Google Scholar] [CrossRef]
- Zeng, Q.-H.; Zhao, J.-B.; Wang, J.-J.; Zhang, X.-W.; Jiang, J.-G. Comparative extraction processes, volatile compounds analysis and antioxidant activities of essential oils from Cirsium japonicum Fisch. ex DC and Cirsium setosum (Willd.) M.Bieb. LWT 2016, 68, 595–605. [Google Scholar] [CrossRef]
- Borawska, M.H.; Czechowska, S.K.; Markiewicz, R.; Socha, K.; Nazaruk, J.; Pałka, J.; Isidorov, V.A. Enhancement of antibacterial effects of extracts from Cirsium species using sodium picolinate and estimation of their toxicity. Nat. Prod. Res. 2010, 24, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Strawa, J.; Wajs-Bonikowska, A.; Leszczyńska, K.; Ściepuk, M.; Nazaruk, J.; Jakub, S.; Anna, W.B.; Leszczyńska, K.; Ściepuk, M.; Nazaruk, J. Chemical composition and antioxidant, antibacterial activity of Cirsium rivulare (Jacq) All. roots. Nat. Prod. Res. 2016, 30, 2730–2733. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Heo, S.-I.; Li, L.; Lee, M.J.; Wang, M.-H. Antioxidant and Hepatoprotective Activities of Cirsium setidens NAKAI against CCl4-Induced Liver Damage. Am. J. Chin. Med. 2008, 36, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Ji, Z. Chemical constituents from the aerial parts of Cirsium japonicum and their antifungal activities. Chin. J. Pestic. Sci. 2018, 20, 316–322. [Google Scholar]
- Nazaruk, J.; Czechowska, K.S.; Markiewicz, R.; Borawska, H.M. Polyphenolic compounds and in vitro antimicrobial and antioxidant activity of aqueous extracts from leaves of some Cirsium species. Nat. Prod. Res. 2008, 22, 1583–1588. [Google Scholar] [CrossRef]
- Shin, S.; Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Park, S.; Park, S.; Han, K.; Wang, M.-H. Phytochemical profile and antidiabetic effect of the bioactive fraction of Cirsium setidens in streptozotocin-induced type 2 diabetic mice. Process Biochem. 2022, 116, 60–71. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, S.; Kim, H.Y.; Cho, E.J. Cirsium japonicum var. maackii inhibits hydrogen peroxide-induced oxidative stress in SH-SY5Y cells. Korean J. Agric. Sci. 2021, 48, 119–131. [Google Scholar]
- Nalewajko-Sieliwoniuk, E.; Nazaruk, J.; Kotowska, J.; Kojło, A. Determination of the flavonoids/antioxidant levels in Cirsium oleraceum and Cirsium rivulare extracts with cerium(IV)–rhodamine 6G chemiluminescence detection. Talanta 2012, 96, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wang, L.H.; Jiang, J.G. Hepatoprotective effect of flavonoids from Cirsium japonicum DC on hepatotoxicity in comparison with silymarin. Food Funct. 2016, 7, 2179–2184. [Google Scholar] [CrossRef]
- Noh, Y.-H.; Hong, J.; Lee, J.-W.; Kim, S.-S.; Lee, J.Y.; Kang, I.-J.; Won, M.-H.; Jeong, Y.; Whang, W.K.; Myung, S.-C.; et al. A Complex of Cirsium japonicum var. maackii (Maxim.) Matisum. and Thymus vulgaris L. Improves Menopausal Symptoms and Supports Healthy Aging in Women. J. Med. Food 2022, 25, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Abdul, Q.A.; Byun, J.S.; Joung, E.-J.; Gwon, W.-G.; Lee, M.-S.; Kim, H.-R.; Choi, J.S. Protective effects of flavonoids isolated from Korean milk thistle Cirsium japonicum var. maackii (Maxim.) Matsum on tert -butyl hydroperoxide-induced hepatotoxicity in HepG2 cells. J. Ethnopharmacol. 2017, 209, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-W.; Chang, H.-C.; Ching, H.; Lien, J.-C.; Huang, H.-C.; Wu, C.-R. Antioxidant Effects and Phytochemical Properties of Seven Taiwanese Cirsium Species Extracts. Molecules 2021, 26, 3935. [Google Scholar] [CrossRef]
- Shin, M.-S.; Park, J.Y.; Lee, J.; Yoo, H.H.; Hahm, D.-H.; Lee, S.C.; Lee, S.; Hwang, G.S.; Jung, K.; Kang, K.S. Anti-inflammatory effects and corresponding mechanisms of cirsimaritin extracted from Cirsium japonicum var. maackii Maxim. Bioorganic Med. Chem. Lett. 2017, 27, 3076–3080. [Google Scholar] [CrossRef]
- Ghil, S.-H.; Kim, D.-Y.; Kang, S.-H. Ghil Cirsium japonicum extract induces apoptosis and anti-proliferation in the human breast cancer cell line MCF-7. Mol. Med. Rep. 2010, 3, 427–432. [Google Scholar] [CrossRef]
- Lin, P.-C.; Ji, L.-L.; Zhong, X.-J.; Li, J.-J.; Wang, X.; Shang, X.-Y.; Lin, S. Taraxastane-type triterpenoids from the medicinal and edible plant Cirsium setosum. Chin. J. Nat. Med. 2019, 17, 22–26. [Google Scholar] [CrossRef]
- Kim, B.R.; Seo, H.S.; Ku, J.M.; Kim, G.J.; Jeon, C.Y.; Park, J.H.; Jang, B.H.; Park, S.J.; Shin, Y.C.; Ko, S.G. Silibinin inhibits the production of pro-inflammatory cytokines through inhibition of NF-κB signaling pathway in HMC-1 human mast cells. Inflamm. Res. 2013, 62, 941–950. [Google Scholar] [CrossRef]
- Zhao, Z.-W.; Chang, J.-C.; Lin, L.-W.; Tsai, F.-H.; Chang, H.-C.; Wu, C.-R. Comparison of the Hepatoprotective Effects of Four Endemic Cirsium Species Extracts from Taiwan on CCl4-Induced Acute Liver Damage in C57BL/6 Mice. Int. J. Mol. Sci. 2018, 19, 1329. [Google Scholar] [CrossRef]
- Park, H.S.; Shim, S.M.; Kim, G.H. Silydianin in chloroform soluble fraction of Cirsium japonicum leaf inhibited adipocyte differentiation by regulating adipogenic transcription factors and enzymes. Appl. Biol. Chem. 2013, 56, 709–713. [Google Scholar] [CrossRef]
- Gurovic, M.S.V.; Castro, M.J.; Richmond, V.; Faraoni, M.B.; Maier, M.S.; Murray, A.P. Triterpenoids with acetylcholinesterase inhibition from Chuquiraga erinacea D. Don. subsp. erinacea (Asteraceae). Planta Med. 2010, 76, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Kang, L.J.; Jang, D.; Jeon, J.; Lee, H.; Choi, S.; Han, S.J.; Oh, E.; Nam, J.; Kim, C.S.; et al. Cirsium japonicum var. maackii and apigenin block Hif-2α-induced osteoarthritic cartilage destruction. J. Cell Mol. Med. 2019, 23, 5369–5379. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, H.Y.; Shibamoto, T.; Jang, T.S.; Lee, S.C.; Shim, J.S.; Hahm, D.-H.; Lee, H.-J.; Lee, S.; Kang, K.S. Beneficial effects of a medicinal herb, Cirsium japonicum var. maackii, extract and its major component, cirsimaritin on breast cancer metastasis in MDA-MB-231 breast cancer cells. Bioorganic Med. Chem. Lett. 2017, 27, 3968–3973. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Wu, Z.; Wu, M. Cirsium japonicum Flavones Enhance Adipocyte Differentiation and Glucose Uptake in 3T3-L1 Cells. Biol. Pharm. Bull. 2012, 35, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Park, J.J.; Min, B.S.; Jung, H.J.; Islam, M.N.; Choi, J.S. Inhibition of advanced glycation endproducts formation by Korean thistle, Cirsium maackii. Asian Pac. J. Trop. Med. 2015, 8, 1–5. [Google Scholar] [CrossRef]
- Luan, N.; Di Wei, W.; Wang, A.; Wu, X.L.; Qi, Y.; Li, J.J.; Zheng, J.Q.; Shang, X.Y. Four new taraxastane-type triterpenoic acids from Cirsium setosum. J. Asian Nat. Prod. Res. 2016, 18, 1015–1023. [Google Scholar] [CrossRef]
- Joy dela Pena, I.; Lee, H.L.; Yoon, S.Y.; de la Peña, J.B.; Kim, K.H.; Hong, E.Y.; Cheong, J.H. The ethanol extract of Cirsium japonicum increased chloride ion influx through stimulating GABA A receptor in human neuroblastoma cells and exhibited anxiolytic-like effects in mice. Drug Discov. Ther. 2013, 7, 18–23. [Google Scholar]
- De La Peña, J.B.I.; Kim, C.A.; Lee, H.L.; Yoon, S.Y.; Kim, H.J.; Hong, E.Y.; Kim, G.H.; Ryu, J.H.; Lee, Y.S.; Kim, K.M.; et al. Luteolin mediates the antidepressant-like effects of Cirsium japonicum in mice, possibly through modulation of the GABAA receptor. Arch. Pharmacal Res. 2013, 37, 263–269. [Google Scholar] [CrossRef]
- Liao, Z.; Chen, X.; Wu, M. Antidiabetic effect of flavones from Cirsium japonicum DC in diabetic rats. Arch. Pharmacal Res. 2010, 33, 353–362. [Google Scholar] [CrossRef]
- Meng, Y.H.; Wang, Q.H.; Yang, L. Pharmacological effects of Cirsium setosum in Hei Long Jiang. Inf. Tradit. Chin. Med. 2011, 28, 17–18. [Google Scholar]
- Chen, Q. Study on the Material Basis of Hemostatic Efficacy and the Mechanism of Hemostatic Synergism of Large Thistle Charcoal; Beijing University of Traditional Chinese Medicine: Beijing, China, 2014. [Google Scholar]
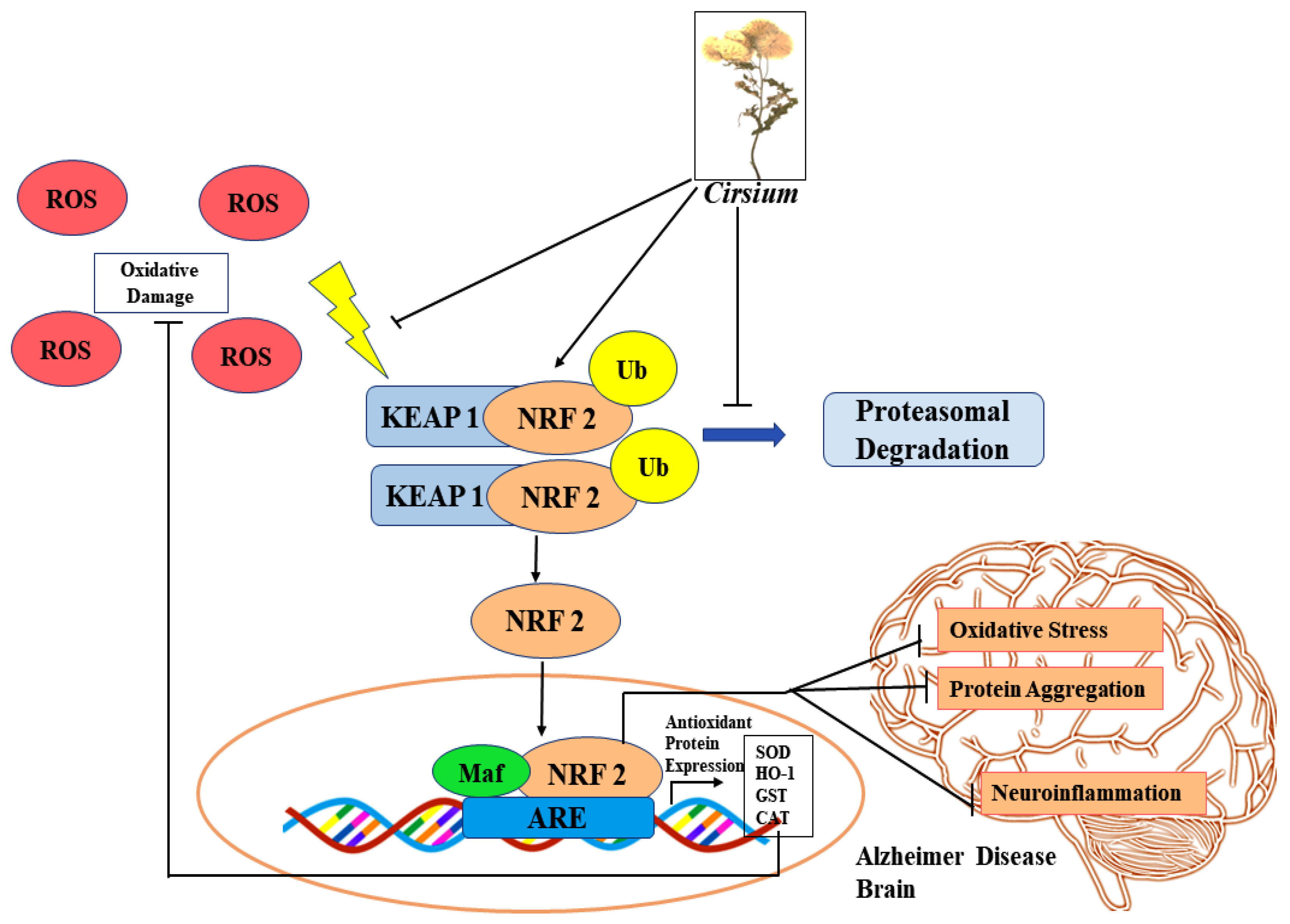
- Pang, Q.Q.; Kim, J.H.; Choi, J.M.; Song, J.L.; Lee, S.; Cho, E.J. Cirsium japonicum var. Maackii Improves Cognitive Impairment under Amyloid β25-35-Induced Alzheimer’s Disease Model. BioMed Res. Int. 2022, 2022, 1–11. [Google Scholar]
- Wang, Y. Study on the Hemostatic Material Basis and Mechanism of Cirsium Japonicum Charcoal; Beijing University of traditional Chinese Medicine: Beijing, China, 2018. [Google Scholar]
- Takaishi, Y.; Okuyama, T.; Nakano, K.; Murakami, K.; Tomimatsu, T.; Yamahara, J. Polyolefinic compounds from Cirsium nipponicum roots. Phytochemistry 1991, 30, 1539–1542. [Google Scholar] [CrossRef]
- Funk, C.D.; Hoshiko, S.; Matsumoto, T.; Rdmark, O.; Samuelsson, B. Characterization of the human 5-lipoxygenase gene. Proc. Natl. Acad. Sci. USA 1989, 86, 2587–2591. [Google Scholar] [CrossRef] [PubMed]
- Özçelik, B.; Deliorman Orhan, D.; Karaoğlu, T.; Ergun, F. Antimicrobial activities of various Cirsium hypoleucum extracts. Ann. Microbiol. 2005, 55, 135–138. [Google Scholar]
- Xu, Y.; Tang, J.; Guo, Q.; Xu, Y.; Yan, K.; Wu, L.; Xie, K.; Zhu, A.; Rong, X.; Ye, D.; et al. Traditional Chinese Medicine formula FTZ protects against polycystic ovary syndrome through modulating adiponectin-mediated fat-ovary crosstalk in mice. J. Ethnopharmacol. 2021, 268, 113587. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, M.; Wang, D.; Hu, Y.; Wang, R.; Diao, H.; Guo, J. FTZ protects against cardiac hypertrophy and oxidative injury via microRNA-214/SIRT3 signaling pathway. Biomed Pharm. 2022, 148, 112696. [Google Scholar] [CrossRef]
- Luo, D.; Li, J.; Chen, K.; Rong, X.; Guo, J. Untargeted Metabolomics Reveals the Protective Effect of Fufang Zhenshu Tiaozhi (FTZ) on Aging-Induced Osteoporosis in Mice. Front. Pharmacol. 2019, 9, 1483. [Google Scholar] [CrossRef]
- Wang, L.; Tao, W.; Luo, D.; Hu, Y.; Bei, W.; Guo, J. Potential synergistic effects of Chinese herbal prescription FTZ components detected in blood towards hepatic lipid-modulating targets. Complement. Ther. Med. 2014, 22, 887–893. [Google Scholar] [CrossRef]
- Hu, X.; Wang, M.; Bei, W.; Han, Z.; Guo, J. The Chinese herbal medicine FTZ attenuates insulin resistance via IRS1 and PI3K in vitro and in rats with metabolic syndrome. J. Transl. Med. 2014, 12, 47. [Google Scholar] [CrossRef]
- Song, L.; Zhang, D.; Guo, C.; Gu, Z.; Wang, L.; Yao, Y.S.; Wang, H.; Zeng, Z.; Wang, W.; Yang, Y.; et al. The traditional Chinese medicine formula Fufang-Zhenzhu-Tiaozhi protects myocardia from injury in diabetic minipigs with coronary heart disease. Biomed. Pharmacother. 2021, 137, 111343. [Google Scholar] [CrossRef] [PubMed]
- Shenghua, P.; Ziqin, Z.; Shuyu, T.; Huixia, Z.; Xianglu, R.; Jiao, G. An integrated fecal microbiome and metabolome in the aged mice reveal anti-aging effects from the intestines and biochemical mechanism of FuFang zhenshu TiaoZhi (FTZ). Biomed. Pharm. 2020, 121, 109421. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, J.; Li, S.; Lin, Y.; Xiao, X.; Guo, J. Comprehensive chemical analysis of Zhenshu Tiaozhi formula and its effect on ameliorating glucolipid metabolic disorders in diabetic rats. Biomed. Pharmacother. 2021, 133, 111060. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Cheng, J.; Huang, X.; Huang, B.; Shao, X.; Zhao, J.; Lan, D.; Zhu, Q.; Yan, M.; Zhang, Y.; et al. The Chinese medicine Fufang Zhenzhu Tiaozhi capsule protects against atherosclerosis by suppressing EndMT via modulating Akt1/β-catenin signaling pathway. J. Ethnopharmacol. 2022, 293, 115261. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-Q.; Tan, H.-B.; Zhang, X.-Y.; Zhang, Y.-Z.; Lin, Q.-Y.; Huang, M.-Y.; Lin, Z.-Y.; Mo, J.-Z.; Zhang, Y.; Lan, T.; et al. The Chinese medicine Fufang Zhenzhu Tiaozhi capsule protects against renal injury and inflammation in mice with diabetic kidney disease. J. Ethnopharmacol. 2022, 292, 115165. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiang, L.; Piao, S.; Gong, X.; Zhou, W.; Feng, W.; Li, H.; Li, L.; Wei, A.; Zhu, Q.; et al. The Efficacy and Safety of Chinese Medicine Fufang Zhenzhu Tiaozhi Capsule (FTZ) in the Treatment of Diabetic Coronary Heart Disease: Study Protocol for Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Diabetes Metab. Syndr. Obesity Targets Ther. 2021, 14, 2651–2659. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, M.; Shin, S.; Woo, J.; Son, D.; Ryu, D.; Yoo, J.; Park, D.; Jung, E. Effect of Cirsium japonicum Flower Extract on Skin Aging Induced by Glycation. Molecules 2022, 27, 2093. [Google Scholar] [CrossRef]
- Kim, K.M.; Son, H.D.; Shin, W.S.; Jung, S.E.; Park, H. Composition Containing Cirsium japonicum Extract as Active Ingredient for Stimulating Melanogenesis. U.S. Patent 2022/0287956, 15 September 2022. [Google Scholar]
- Oku, H.; Kamiyama, Y.; Inafuku, M. Method for Treating Fatty Liver. U.S. Patent 15/903,823, 19 May 2020. [Google Scholar]
- Mori, S.; Ichii, Y.; Tanaka, N.; Yorozu, H.; Kanazawa, S.; Nishizawa, Y. Lipolysis Acceleration Method. U.S. Patent 5,698,199, 16 December 1997. [Google Scholar]
- Kim, J.W.; Park, Y.C.; Lee, K.H.; Hyungwoo, K.I.M. Composition for Preventing, Ameliorating or Treating Acne Symptoms Using Natural Extracts as Active Ingredients. U.S. Patent 11,154,580, 26 October 2021. [Google Scholar]
- Shimotoyodome, Y.; Ito, S.; Sugai, Y.; Hashimoto, H.; Ishikawa, J. Ceramide Production Enhancer and Moisturizer. U.S. Patent 9,682,029, 20 June 2017. [Google Scholar]
- Wang, H.C.; Bao, Y.R.; Wang, S.; Li, T.J.; Meng, X.S. Simultaneous determination of eight bioactive components of Cirsium setosum flavonoids in rat plasma using triple quadrupole LC/MS and its application to a pharmacokinetic study. Biomed. Chromatogr. 2019, 33, e4632. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, M.; Cheng, X.; Liang, C.; Diao, X.; Zhang, L. Ultrahigh-performance liquid chromatography coupled with triple quadrupole and time-of-flight mass spectrometry for the screening and identification of the main flavonoids and their metabolites in rats after oral administration of Cirsium japonicum DC extract. Rapid Commun. Mass Spectrom. 2018, 32, 1451–1461. [Google Scholar]
- Zhang, Z.; Jia, P.; Zhang, X.; Zhang, Q.; Yang, H.; Shi, H.; Zhang, L. LC–MS/MS determination and pharmacokinetic study of seven flavonoids in rat plasma after oral administration of Cirsium japonicum DC. extract. J. Ethnopharmacol. 2014, 158, 66–75. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, Y.-H.; Kim, D.-B.; Bang, W.-S.; Lee, O.-H. Acute and 4-Week Repeated-Dose Oral Toxicity Studies of Cirsium setidens in Rats. Molecules 2014, 19, 7138–7151. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.M.; Bae, E.; Kim, G.H. Single-Dose Oral Acute Toxicity and Mutagenic Effects of Methanol Extracts of Cirsium japonicum. J. Food Biochem. 2011, 35, 845–858. [Google Scholar] [CrossRef]
- Shin, S.; Saravanakumar, K.; Mariadoss, A.V.A.; Hu, X.; Sathiyaseelan, A.; Wang, M.H. Functionalization of selenium nanoparticles using the methanolic extract of Cirsium setidens and its antibacterial, antioxidant, and cytotoxicity activities. J. Nanostructure Chem. 2022, 12, 23–32. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Ungureanu, C.; Besliu, D.; Lazea-Stoyanova, A.; Iosif, L. Bio-active nanomaterials phyto-generated from weed herb Cirsium arvense. Optoelectron. Adv. Mater. Rapid Commun. 2020, 14, 459–465. [Google Scholar]
- Tahir, K.; Nazir, S.; Li, B.; Khan, A.U.; Khan, Z.U.H.; Ahmad, A.; Khan, Q.U.; Zhao, Y. Enhanced visible light photocatalytic inactivation of Escherichia coli using silver nanoparticles as photocatalyst. J. Photochem. Photobiol. B Biol. 2015, 153, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, M.; Norouzi, B. Synthesis of cobalt oxide nanoparticles using Cirsium vulgare leaves extract and evaluation of electrocatalytic effects on oxidation of l-cysteine. Ionics 2020, 26, 1951–1961. [Google Scholar] [CrossRef]
- Khan, Z.U.H.; Khan, A.; Shah, A.; Wan, P.; Chen, Y.; Khan, G.M.; Khan, A.U.; Tahir, K.; Muhammad, N.; Khan, H.U. Enhanced photocatalytic and electrocatalytic applications of green synthesized silver nanoparticles. J. Mol. Liq. 2016, 220, 248–257. [Google Scholar] [CrossRef]
- Rehman, K.U.; Khan, A.U.; Tahir, K.; Nazir, S.; Albalawi, K.; Hassan, H.M.; Alabbad, E.A.; Refat, M.S.; Al-Shehri, H.S.; Aldawsari, A.M. Facile synthesis of copper oxide nanoparticles (CuONPs) using green method to promote photocatalytic and biocidal applications. J. Mol. Liq. 2022, 360, 119453. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, M.; Cheng, J.; Wu, S.; Xiong, W.; Kong, H.; Zhao, Y.; Qu, H. Hemostatic effect of novel carbon dots derived from Cirsium setosum Carbonisata. RSC Adv. 2018, 8, 37707–37714. [Google Scholar] [CrossRef]

| S. No | Species of Cirsium | Geographical Distribution | Traditional Uses | Common Name | Major Phytoconstituents | Pharmacological Applications | References |
|---|---|---|---|---|---|---|---|
| 1 | Cirsium arvense | Europe, Asia, Northern Africa, India | Pharyngitis, astringent, tonic, tumor, diuretic, toothache, diaphoretic | Canada thistle, Creeping thistle, Field thistle, Californian thistle | Acacetin, Ciryneol C, Hispidulin, Pectolinarigenin, Luteolin, Tracin, Scopoletin, Apigenin, Citronellol | Antimicrobial, antifungal, anticancer, antidiabetic, neuroprotective, anti-inflammatory | [5,8] |
| 2 | Cirsium oleraceum | Europe to West Siberia and Kazakhstan | Anxiolytic, diuretic, astringent, antiphlogistic, Antitumor | Cabbage thistle, Siberian thistle | Thymol, Carvacrol, Luteolin, Apigenin, Methylkaempferol | Antioxidant, antimicrobial, anti-glioma effect | [20] |
| 3 | Cirsium englerianum | Ethiopia | Dermal infections, cough, snake bite, hematuria, diarrhea anthrax, anti-scabies | - | Alkaloids, Quinones, Terpenoids, Phenolics, and Flavonoids | Antioxidant, antimicrobial | [5] |
| 4 | Cirsium eriophorum | China South-Central, East Himalaya, Myanmar, Tibet | Detoxification and cure of hepatic infections | Woolly thistle | Vanillic acid, Balanophonin, Apigenin, Kaempferol, Taraxasterol, Sitosterol, Linoleic acid | Antioxidant, acetylcholinesterase inhibitory activity | [21] |
| 5 | Cirsium wallichii | Afghanistan, East Himalaya, Nepal, Pakistan, West Himalaya | Pyrexia, bleeding relief, burning sensation, and stomach inflammation | Wallich’s Thistle, Plume thistles | Acetyljacoline, Fumaric acid | Antimicrobial, antifungal, antioxidant | [5] |
| 6 | Cirsium verutum | Assam, East Himalaya, Myanmar, Nepal, Pakistan, Tibet, Vietnam, West Himalaya | Typhoid, bleeding, chest pain, measles, purgative, pharyngitis, dyspepsia, dysentery, tuberculosis | Common thistle, Creeping thistle, Plume thistle | Lupeol, Taraxasterol acetate, Pectolinarigenin, Cirsitakaoside, Cirsitakaogenin, Pectolinarin | Antimicrobial, antifungal | [22] |
| 7 | Cirsium setidens | Korea | Pyrexia, detoxify, and improve blood circulation | Ungungqwui’ in Korea, Thistles in English | Linarin, Phytol, Syringin, Pectolinarigenin, Cyclocitral, Pentylfuran, Trans-β-Ionone Rutin, Setidenosides, Isorhamnetin | Antimicrobial, antifungal, anticancer, neuroprotective, anti-inflammatory Antidiabetic, osteogenic agent | [23] |
| 8 | Cirsium tenoreanum | Italy | Treatment of varicose | Cardo di Tenore | Kaempferol, Apigenin, Quercetin-3-O-galactoside | Antimicrobial, antiproliferative | [24] |
| 9 | Cirsium vulgare | Europe to Siberia and Arabian Peninsula, West Himalaya | Anxiolytic | Spear or bull thistle | Quercetin, Apigenin, Kaempferol, and Luteolin | Antioxidant, antimicrobial | [9] |
| 10 | Cirsium japonicum | China, Korea, Japan | Hemorrhages, cancer, hypertension, and hepatitis | Japanese thistle | Linarin, Luteolin, Coumaric acid Pectolinarin, Ciryneol, Syringin, Cirsimaritin Pectolinarigenin, Lupenyl acetate | Anticancer, anti-Alzheimer. anti-inflammatory, antimicrobial | [25] |
| Category of Phytoconstituent | Name of Phytoconstituents | Species | References |
|---|---|---|---|
| Flavanoids | Linarin | C. arvense | [46] |
| C. japonicum | [62,64] | ||
| C. setosum | [64] | ||
| C. rivulare | [65] | ||
| C. canum | [63] | ||
| Scopoletin | C. arvense | [51] | |
| Pectolinarigenin | C. chanroenicum | [59] | |
| C. setidens | [60] | ||
| Pectolinarigenin-7-O-glucopyranoside | C. arvense | [51] | |
| Acacetin | C. arvense | [51] | |
| 6,7-Dimethoxycoumarin | C. arvense | [51] | |
| Tracin | C. arvense | [51] | |
| Hispidulin | C. arvense | [35] | |
| C. japonicum | [54,55] | ||
| C. rivulare | [30] | ||
| Hispidulin-7-neohesperidoside | C. japonicum | [64] | |
| Luteolin | C. arvense | [35] | |
| C. japonicum | [55,64] | ||
| C. canum | [63] | ||
| C. palustre | [56] | ||
| C. rivulare | [62] | ||
| Luteolin 7-O-β-D-glucuronide | C. scabrum | [26] | |
| Luteolin 7-O-β-D-glucoside | C. scabrum | [26] | |
| C. canum | [63] | ||
| C. palustre | [56] | ||
| Eriodictyol 7-O-glucoside | C. palustre | [56] | |
| Pectolinarin | C. japonicum | [53,57,64] | |
| C. rivulare | [50] | ||
| C. subcoriaceum | [58] | ||
| C. chanroenicum | [59] | ||
| C. setidens | [60] | ||
| C. japonicum | [25] | ||
| Isokaempferide 7-O-β-D-(6″-methylglucuronide | C. rivulare | [50] | |
| Isokaempferide 7-glucuronide | C. rivulare | [50] | |
| Apigenin | C. canum | [63] | |
| C. setosum | [61] | ||
| C. rivulare | [30] | ||
| C. japonicum | [55] | ||
| Apigenin 7-(6″-methylglucuronide) | C. rivulare | [30,50] | |
| Apigenin 7-glucoside | C. canum | [63] | |
| C. rivulare | [30] | ||
| Kaempferol | C. canum | [63] | |
| Kaempferol 3-galactoside | C. rivulare | [50] | |
| Kaempferol 3-glucoside | C. canum | [63] | |
| Kaempferol 3- β-D-glucopyranoside (Astragalin) | C. setosum | [61] | |
| 4-Vinyl guaiacol | C. creticum | [17] | |
| 4-Ethyl guaiacol | C. creticum | [17] | |
| 5,7-Dihydroxy-6,4′-dimethoxyflavone | C. japonicum | [53,57] | |
| Cirsimaritin | C. japonicum | [49] | |
| Cirsimarin | C. japonicum | [49] | |
| Rutoside | C. canum | [68] | |
| 6-Hydroxyluteolin 7-O-glucoside | C. palustre | [56] | |
| Tricin | C. rivulare | [69] | |
| Isorhamnetin | C. helenioides | [66] | |
| Steroids | Stigmasterol | C. arvense | [46] |
| Steroidal glucoside | Daucosterol | C. arvense | [46] |
| Alkaloids | Benzymidazole | C. arvense | [46] |
| Terpenes | α-Tocopherol | C. setidens | [23] |
| C. arvense | [35] | ||
| α-Tocospiro A, B and C | C. setosum | [70] | |
| 4(15),10(14)-Guaiadien-12,6-olide | C. setidens | [23] | |
| Trans-Phytol | C. setidens | [23,71] | |
| Dihydroactinidiolide | C. creticum | [17] | |
| Triterpenes | Lupeol | C. scabrum | [47] |
| Lupeol acetate | C. palustre | [72] | |
| Taraxasterol acetate | C. scabrum | [47] | |
| 25-Hydroperoxycycloart-23-en-3β-ol | C. scabrum | [47] | |
| C. setidens | [23] | ||
| β-Amyrin | C. palustre | [72] | |
| Faradiol | C. palustre | [72] | |
| Sesquiterpenes | Caryophyllene oxide | C.setidens | [71] |
| β-Caryophyllene alcohol | C.setidens | [71] | |
| Cyclic ether | Ciryneol | C. arvense | [51] |
| 1,2,15,16-Diepoxyhexadecane | C. setidens | [71] | |
| Fatty acids | 9, 12, 15-Octadecatrienoic acid | C. setidens | [23] |
| 9, 12-Octadecadienoic acid | C. setidens | [23] | |
| Hexadecanoic acid | C. setidens | [23] | |
| C. creticum | [17] | ||
| C. palustre | [72] | ||
| Palmitic acid | C. japonicum | [41] | |
| Sterols | Acylglycosyl β-sitosterol | C. setidens | [23] |
| β-Sitosterol glucoside | C. setidens | [23] | |
| Taraxasterol | C. setosum | [61] | |
| Glycerol | Monogalactosyldiacyl glycerol | C. setidens | [23] |
| C. helenioides | [66] | ||
| C. palustre | [73] | ||
| C. rivulare | [73] | ||
| Dihydroaplotaxene | C. helenioides | [66] | |
| Tetrahydroaplotaxene | C. helenioides | [66] | |
| Pentacosane | C. setidens | [71] | |
| Aldehydes | Sinapaldehyde | C. helenioides | [66] |
| Ketones | 6,10,14-Trimethyl-2-pentadecanone | C. setidens | [71] |
| Phenolic acids | Chlorogenic acid | C. canum | [63] |
| C. palustre | [56] | ||
| Caffeic acid | C. canum | [63] | |
| p-Coumaric acid | C. canum | [63] | |
| Protocatechuic acid | C. canum | [63] | |
| p-Hydroxybenzoic acid | C. canum | [63] | |
| Vanillic acid | C. canum | [63] | |
| Syringic acid | C. canum | [63] | |
| Trans-Cinnamic acid | C. canum | [63] |
| S. No | Cirsium Species | Application | Model | Detailed Information | References |
|---|---|---|---|---|---|
| 1. | C. scabrum | In vitro | S. aureus, Dermabacter hominis | Moderate activity | [47] |
| 2. | C. canum | In vitro | Gram-positive Bacteria | Inhibitory activity against S. aureus and S. pneumoniae | [63] |
| 3. | C. arvense | In vitro | S. aureus, S. typhi | Zone of inhibition: 9–32 mm | [35] |
| 4. | C. oleraceum C. palustre C. rivulare C. vulgare C. arvense | In vitro | S. aureus P. aeruginosa B. subtilis C. albicans Micrococcus luteus E. coli | Minimum inhibitory concentration range from 3.12–50 mg/mL | [50,89] |
| 5. | C. hypoleucum | In vitro | S. aureus | Inhibitory activity against S. aureus at 32 μg/mL | [92] |
| 6. | C. setidens | In vitro | - | DPPH activity: IC50 value of 45.14 g/mL | [93] |
| 7. | C. japonicum | In vitro | Neuronal cells | More levels of heme oxygenase, thioredoxin reductase, antioxidative enzymes | [94] |
| 8. | C. arvense | In vitro | - | DPPH activity:118 µg/mL | [46,52] |
| 9. | C. palustre | In vitro | - | CAF(Flower) > CAR (Root) > CAL (Leaf) > CAS (Stem) | [56] |
| 10. | C. leucopsis C. sipyleum C. eriophorum | In vitro | - | DPPH inhibition: 4–38.98 % | [95] |
| 11. | C. oleraceum C. rivulare | In vitro | - | ABTS scavenging activity: >85% | [96] |
| 12. | C. setidens | In vivo | Wister albino rats | DPPH inhibition: 2.15–30% | [21] |
| 13. | C. arvense C. oleraceum C. palustre C. rivulare | In vitro | - | Total antioxidant activity: 0.98 to 2.71 mM/L | [39] |
| S. No | Cirsium Species | Application | Model | Detailed Information | References |
|---|---|---|---|---|---|
| 1. | C. scabrum | In vitro | J774 cancerous cell line | IC50 = 11.53 μg/mL | [47] |
| 2. | C. rivulare | In vitro | MCF-7 and MDA-MBA-breast cancer cell line | IC50 = 110 to 140 μg/mL | [73] |
| 3. | C. setosum | In vitro | HCT8 colon cancer cells | IC50 = 0.03 μM | [70] |
| 4. | C. tenoreanum | In vitro | MCF7 breast cancer cells | 73% cell death | [24] |
| 5. | C. arvense | In vitro | HeLa and C6 cell lines | CAR > CAF > CAL | [46] |
| 6. | C. setidens | In vitro | Lung, skin, ovarian, and colon cancer cells | ED50 = 2.66 to 11.25 μM | [23] |
| 7. | C. japonicum | In vitro | Breast cancer cells | Reduction in angiogenesis by lowering the production of VEGF, Akt, and ERK in MDA-MB-231 cells | [100] |
| 8. | C. japonicum | In vitro | MCF-7 cells | Arresting the cell cycle in the G1 phase and induced apoptosis | [101] |
| 9. | C. chanroenicum | In vitro | RAW macrophage cells and murine leukemia cells | Inhibition of cyclooxygenase and leukotriene production | [59] |
| 10. | C. subcoriaceum | In vivo | Murine model | ED30 = 25 mg/kg | [58] |
| 11. | C. japonicum | In vitro | Macrophage cell line Mast cell line | Reduction in pro-inflammatory cytokines, NO and NF-κB in Mast Cells | [102,103] |
| Activity | Cirsium Species | Application | Model | Detailed Information | References |
|---|---|---|---|---|---|
| Oviposition stimulatory | C. japonicum | In vitro | Ostrinia zealis | Extract potently induced oviposition by females | [41] |
| Allelopathy | C. creticum | In vivo | Radish Lettuce Cress | Inhibitory activity on germination | [17] |
| Enzyme inhibition activity | C. japonicum | In vitro and In vivo | Chondrocytes | Decrease the levels of Hif-2α, metalloproteinases, and cyclooxygenases | [104] |
| Flavonoids of C. japonicum | In vitro | Aldose reductase inhibitor | IC50 values of 0.21 μg/mL and 0.77 μM | [54] | |
| C. leucopsis C. sipyleum C. eriophorum | In vitro | Acetyl-and butyryl-cholinesterase inhibitory activity | 16–57% Inhibition | [105,106] | |
| C. japonicum | In vivo | Murine model | Reduction in the levels of lipoprotein lipase and fatty acid synthetase | [106] | |
| Hepatoprotective | C. japonicum Cirsii herba | In vivo | C57BL/6 Mice | Decrease in liver necrosis restored the hepatic antioxidant enzymes and malondialdehyde | [107] |
| C. arisanense | In vitro and In vivo | Hep 3B Cells and Mice | Reduction in Hepatitis B surface antigen. Declined the levels of SGOT and SGPT | [42] | |
| C. setidens | In vivo | Mice | Decrement in hepatic damage in rats induced due to CCl4 and hepatic ballooning degeneration | [21,83] | |
| Nephroprotective | C. japonicum | In vivo | Murine Model | Decrease the levels of Cholesterol and triglycerides | [108] |
| C. japonicum | In vitro | 3T3-L1 Cells | Enhancement in insulin-stimulated glucose uptake | [109] | |
| Immunomodulatory | C. japonicum | In vivo | Murine model | Induction of humoral and cellular immune responses. Activation of complement pathway and Natural killer cell activity | [57] |
| S. No | Title of Patent | Applicant | Published Application Number |
|---|---|---|---|
| 1. | Composition containing Cirsium japonicum extract as active ingredient for stimulating melanogenesis | Biospectrum Inc. | US20210361559A1 |
| 2. | Immunoregulatory composition containing Cirsium maritimum extract | Kochi Prefectural University Corp Kochi | JP6882730B2 |
| 3. | Organic extract of plant of genus Cirsium, and application and composition thereof | Zhejiang Wolwo Bio Pharmaceutical Co., Ltd. | CN112022892A |
| 4. | Method for treating fatty liver | NPO Amami Functional Foods Study Group University of the Ryukyus Amino UP Co., Ltd. | US10653740B2 |
| 5. | Ceramide production enhancer and moisturizer | Kao Corp | US9682029B2 |
| 6. | Composition for preventing, ameliorating, or treating acne symptoms using natural extracts as active ingredients | Celim Biotech Co., Ltd. | US11154580B1 |
| 7. | Preparation composition for external use for skin and bath agent composition | Kao Corp | JPH09208483A |
| 8. | Composition for bubble bath | Kao Corp | JPH10147516A |
| 9. | Adiponectin secretion promoting agent | NPO Amami Functional Foods Study Group Tokunoshima Town Osaka University NUC | US20210283207A1 |
| 10. | Lipolysis acceleration method | Kao Corp | US5698199A |
| 11. | Fat accumulation inhibitor, drug, prophylactic or therapeutic agent for fatty liver, food or drink, and method for producing fat accumulation inhibitor | NPO Amami Functional Foods Study Group Amino UP Chemical Co., Ltd. University of the Ryukyus | US20160184378A1 |
| Model | Administration Method | Quantitative Method | Details | References |
|---|---|---|---|---|
| Sprague-Dawley rats | Oral | UHPLC-Q-TOF-MS | Quercetin, Luteolin, Diosmetin, Cirsimarin, Linarin, Apigenin, Cirsimaritin, Pectolinarin, Tilianin, Hispedulin, Pectolinarigenin, Acacetin were detected | [139] |
| Sprague-Dawley rats | Oral | LC-MS | Maximum Cmax for quercetin = 513.2 ng/mL, while the minimum Cmax of diosmetin = 231.2 ng/mL AUC0–t value of Compounds (Higher Bioavailability) Quercetin = 6071 ng·h/mL Wogonin = 3789 ng·h/mL Naringin = 2808 ng·h/mL Acacetin = 2636 ng·h/mL Rutin = 1884 ng·h/mL AUC0–t value of Compounds (Lower Bioavailability) Diosmetin = 238.0 ng·h/mL | [138] |
| Sprague-Dawley rats | Oral | LC-MS/MS | Cmax of detected Compounds (ng/mL) Pectolinarin = 876 Diosmetin = 37 Pectolinarigenin = 6 Linarin = 86 Hispidulin = 32 Acacetin = 19 Apigenin = 148 Tmax of Pectolinarin, linarin, pectolinarigenin, hispidulin, diosmetin, acacetin = 5 min Tmax of Apigenin = 360 min | [140] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggarwal, G.; Kaur, G.; Bhardwaj, G.; Mutreja, V.; Sohal, H.S.; Nayik, G.A.; Bhardwaj, A.; Sharma, A. Traditional Uses, Phytochemical Composition, Pharmacological Properties, and the Biodiscovery Potential of the Genus Cirsium. Chemistry 2022, 4, 1161-1192. https://doi.org/10.3390/chemistry4040079
Aggarwal G, Kaur G, Bhardwaj G, Mutreja V, Sohal HS, Nayik GA, Bhardwaj A, Sharma A. Traditional Uses, Phytochemical Composition, Pharmacological Properties, and the Biodiscovery Potential of the Genus Cirsium. Chemistry. 2022; 4(4):1161-1192. https://doi.org/10.3390/chemistry4040079
Chicago/Turabian StyleAggarwal, Gaurav, Gurpreet Kaur, Garima Bhardwaj, Vishal Mutreja, Harvinder Singh Sohal, Gulzar Ahmad Nayik, Anikesh Bhardwaj, and Ajay Sharma. 2022. "Traditional Uses, Phytochemical Composition, Pharmacological Properties, and the Biodiscovery Potential of the Genus Cirsium" Chemistry 4, no. 4: 1161-1192. https://doi.org/10.3390/chemistry4040079
APA StyleAggarwal, G., Kaur, G., Bhardwaj, G., Mutreja, V., Sohal, H. S., Nayik, G. A., Bhardwaj, A., & Sharma, A. (2022). Traditional Uses, Phytochemical Composition, Pharmacological Properties, and the Biodiscovery Potential of the Genus Cirsium. Chemistry, 4(4), 1161-1192. https://doi.org/10.3390/chemistry4040079