Dehydration of Fructose to 5-Hydroxymethylfurfural: Effects of Acidity and Porosity of Different Catalysts in the Conversion, Selectivity, and Yield
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Catalysts
2.2. Characterization of the Catalysts
2.3. Catalytic Dehydration of Fructose
2.4. Analysis by High-Performance Liquid Chromatography (HPLC)
3. Results and Discussion
3.1. Crystalline and Amorphous Aluminosilicate Materials
3.2. Niobia and HPW Activities
3.3. Comparison with Other Catalysts for Aqueous Fructose Dehydration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sørensen, B. A history of renewable energy technology. Energy Policy 1991, 19, 8–12. [Google Scholar] [CrossRef]
- Bulut, U. The impacts of non-renewable and renewable energy on CO2 emissions in Turkey. Environ. Sci. Pollut. Res. 2017, 24, 15416–15426. [Google Scholar] [CrossRef]
- Christensen, C.H.; Rass-Hansen, J.; Marsden, C.C.; Taarning, E.; Egeblad, K. The Renewable Chemicals Industry. ChemSusChem 2008, 1, 283–289. [Google Scholar] [CrossRef]
- Kharaka, Y.K.; Dorsey, N.S. Environmental issues of petroleum exploration and production: Introduction. Environ. Geosci. 2005, 12, 61–63. [Google Scholar] [CrossRef] [Green Version]
- Koçak, E.; Şarkgüneşi, A. The impact of foreign direct investment on CO2 emissions in Turkey: New evidence from cointegration and bootstrap causality analysis. Environ. Sci. Pollut. Res. 2018, 25, 790–804. [Google Scholar] [CrossRef]
- Klass, D. Biomass as an Energy Resource: Concept and Markets. In Biomass for Renewable Energy, Fuels, and Chemicals; Elsevier: San Diego, CA, USA, 1998; pp. 29–50. [Google Scholar]
- Ližbetin, J.; Hlatká, M.; Bartuška, L. Issues Concerning Declared Energy Consumption and Greenhouse Gas Emissions of FAME Biofuels. Sustainability 2018, 10, 3025. [Google Scholar] [CrossRef]
- Ge, J.; Yoon, S.; Choi, N. Using Canola Oil Biodiesel as an Alternative Fuel in Diesel Engines: A Review. Appl. Sci. 2017, 7, 881. [Google Scholar] [CrossRef]
- van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Lucas, N.; Kokate, G.; Nagpure, A.; Chilukuri, S. Dehydration of fructose to 5-hydroxymethyl furfural over ordered AlSBA-15 catalysts. Microporous Mesoporous Mater. 2013, 181, 38–46. [Google Scholar] [CrossRef]
- Wang, T.; Nolte, M.W.; Shanks, B.H. Catalytic dehydration of C 6 carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 2014, 16, 548–572. [Google Scholar] [CrossRef]
- Lv, G.; Deng, L.; Lu, B.; Li, J.; Hou, X.; Yang, Y. Efficient dehydration of fructose into 5-hydroxymethylfurfural in aqueous medium over silica-included heteropolyacids. J. Clean. Prod. 2017, 142, 2244–2251. [Google Scholar] [CrossRef]
- Kruger, J.S.; Choudhary, V.; Nikolakis, V.; Vlachos, D.G. Elucidating the Roles of Zeolite H-BEA in Aqueous-Phase Fructose Dehydration and HMF Rehydration. ACS Catal. 2013, 3, 1279–1291. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L. Sulfated zirconia as a solid acid catalyst for the dehydration of fructose to 5-hydroxymethylfurfural. Catal. Commun. 2009, 10, 1771–1775. [Google Scholar] [CrossRef]
- Haque, M.A.; Yang, X.; Ong, K.L.; Tang, W.-T.; Kwan, T.H.; Kulkarni, S.; Lin, C.S.K. Bioconversion of beverage waste to high fructose syrup as a value-added product. Food Bioprod. Process. 2017, 105, 179–187. [Google Scholar] [CrossRef]
- de Souza, R.L.; Yu, H.; Rataboul, F.; Essayem, N. 5-Hydroxymethylfurfural (5-HMF) Production from Hexoses: Limits of Heterogeneous Catalysis in Hydrothermal Conditions and Potential of Concentrated Aqueous Organic Acids as Reactive Solvent System. Challenges 2012, 3, 212–232. [Google Scholar] [CrossRef]
- Choudhary, V.; Burnett, R.I.; Vlachos, D.G.; Sandler, S.I. Dehydration of Glucose to 5-(Hydroxymethyl)furfural and Anhydroglucose: Thermodynamic Insights. J. Phys. Chem. C 2012, 116, 5116–5120. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, S.; Cen, Y.; Qin, Z.; Wang, J.; Fan, W. Ordered mesoporous Nb–W oxides for the conversion of glucose to fructose, mannose and 5-hydroxymethylfurfural. Appl. Catal. B Environ. 2017, 200, 611–619. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; van der Schaaf, J.; Schouten, J.C.; Nijhuis, T.A. The effect of solvent addition on fructose dehydration to 5-hydroxymethylfurfural in biphasic system over zeolites. J. Catal. 2012, 287, 68–75. [Google Scholar] [CrossRef]
- Thananatthanachon, T.; Rauchfuss, T.B. Efficient Production of the Liquid Fuel 2,5-Dimethylfuran from Fructose Using Formic Acid as a Reagent. Angew. Chem. 2010, 122, 6766–6768. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Baba, Y.; Noma, R.; Kitano, M.; Kondo, J.N.; Hayashi, S.; Hara, M. Nb2O5·nH2O as a Heterogeneous Catalyst with Water-Tolerant Lewis Acid Sites. J. Am. Chem. Soc. 2011, 133, 4224–4227. [Google Scholar] [CrossRef]
- Karinen, R.; Vilonen, K.; Niemelä, M. Biorefining: Heterogeneously Catalyzed Reactions of Carbohydrates for the Production of Furfural and Hydroxymethylfurfural. ChemSusChem 2011, 4, 1002–1016. [Google Scholar] [CrossRef]
- Zhong, J.; Pérez-Ramírez, J.; Yan, N. Biomass valorisation over polyoxometalate-based catalysts. Green Chem. 2021, 23, 18–36. [Google Scholar] [CrossRef]
- Kreissl, H.T.; Nakagawa, K.; Peng, Y.-K.; Koito, Y.; Zheng, J.; Tsang, S.C.E. Niobium oxides: Correlation of acidity with structure and catalytic performance in sucrose conversion to 5-hydroxymethylfurfural. J. Catal. 2016, 338, 329–339. [Google Scholar] [CrossRef]
- Testa, M.L.; Miroddi, G.; Russo, M.; la Parola, V.; Marcì, G. Dehydration of Fructose to 5-HMF over Acidic TiO2 Catalysts. Materials 2020, 13, 1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valadares, D.S.; Clemente, M.C.H.; de Freitas, E.F.; Martins, G.A.V.; Dias, J.A.; Dias, S.C.L. Niobium on BEA Dealuminated Zeolite for High Selectivity Dehydration Reactions of Ethanol and Xylose into Diethyl Ether and Furfural. Nanomaterials 2020, 10, 1269. [Google Scholar] [CrossRef]
- Okuhara, T. Water-Tolerant Solid Acid Catalysts. Chem. Rev. 2002, 102, 3641–3666. [Google Scholar] [CrossRef]
- Assary, R.S.; Redfern, P.C.; Hammond, J.R.; Greeley, J.; Curtiss, L.A. Computational Studies of the Thermochemistry for Conversion of Glucose to Levulinic Acid. J. Phys. Chem. B 2010, 114, 9002–9009. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.L.; Almeida-Trapp, M.; Mithöfer, A.; Plass, W.; Gallo, J.M.R. Rationalizing the conversion of glucose and xylose catalyzed by a combination of Lewis and Brønsted acids. Catal. Today 2020, 344, 92–101. [Google Scholar] [CrossRef]
- Patil, S.K.R.; Lund, C.R.F. Formation and Growth of Humins via Aldol Addition and Condensation during Acid-Catalyzed Conversion of 5-Hydroxymethylfurfural. Energy Fuels 2011, 25, 4745–4755. [Google Scholar] [CrossRef]
- Dutta, S.; De, S.; Saha, B. Advances in biomass transformation to 5-hydroxymethylfurfural and mechanistic aspects. Biomass Bioenergy 2013, 55, 355–369. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; van der Schaaf, J.; Schouten, J.C.; Nijhuis, T.A. Fructose Dehydration to 5-Hydroxymethylfurfural over Solid Acid Catalysts in a Biphasic System. ChemSusChem 2012, 5, 1812–1819. [Google Scholar] [CrossRef]
- Nogueira, J.S.M.; Santana, V.T.; Henrique, P.V.; de Aguiar, L.G.; Silva, J.P.A.; Mussatto, S.I.; Carneiro, L.M. Production of 5-Hydroxymethylfurfural from Direct Conversion of Cellulose Using Heteropolyacid/Nb2O5 as Catalyst. Catalysts 2020, 10, 1417. [Google Scholar] [CrossRef]
- Chang, C.-C.; Je Cho, H.; Yu, J.; Gorte, R.J.; Gulbinski, J.; Dauenhauer, P.; Fan, W. Lewis acid zeolites for tandem Diels–Alder cycloaddition and dehydration of biomass-derived dimethylfuran and ethylene to renewable p-xylene. Green Chem. 2016, 18, 1368–1376. [Google Scholar] [CrossRef]
- Primo, A.; Garcia, H. Zeolites as catalysts in oil refining. Chem. Soc. Rev. 2014, 43, 7548–7561. [Google Scholar] [CrossRef] [PubMed]
- Corma, A. From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis. Chem. Rev. 1997, 97, 2373–2420. [Google Scholar] [CrossRef] [PubMed]
- Corma, A. Inorganic Solid Acids and Their Use in Acid-Catalyzed Hydrocarbon Reactions. Chem. Rev. 1995, 95, 559–614. [Google Scholar] [CrossRef]
- Guisnet, M.R. Model reactions for characterizing the acidity of solid catalysts. Acc. Chem. Res. 1990, 23, 392–398. [Google Scholar] [CrossRef]
- Zhuravlev, L.T. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Yi, F.; Chen, Y.; Tao, Z.; Hu, C.; Yi, X.; Zheng, A.; Wen, X.; Yun, Y.; Yang, Y.; Li, Y. Origin of weak Lewis acids on silanol nests in dealuminated zeolite Beta. J. Catal. 2019, 380, 204–214. [Google Scholar] [CrossRef]
- Caratzoulas, S.; Davis, M.E.; Gorte, R.J.; Gounder, R.; Lobo, R.F.; Nikolakis, V.; Sandler, S.I.; Snyder, M.A.; Tsapatsis, M.; Vlachos, D.G. Challenges of and Insights into Acid-Catalyzed Transformations of Sugars. J. Phys. Chem. C 2014, 118, 22815–22833. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Weitz, E. An in Situ NMR Study of the Mechanism for the Catalytic Conversion of Fructose to 5-Hydroxymethylfurfural and then to Levulinic Acid Using 13C Labeled d -Fructose. ACS Catal. 2012, 2, 1211–1218. [Google Scholar] [CrossRef]
- Wang, M.; Xia, Y.; Zhao, L.; Song, C.; Peng, L.; Guo, X.; Xue, N.; Ding, W. Remarkable acceleration of the fructose dehydration over the adjacent Brønsted acid sites contained in an MFI-type zeolite channel. J. Catal. 2014, 319, 150–154. [Google Scholar] [CrossRef]
- Janda, A.; Bell, A.T. Effects of Si/Al Ratio on the Distribution of Framework Al and on the Rates of Alkane Monomolecular Cracking and Dehydrogenation in H-MFI. J. Am. Chem. Soc. 2013, 135, 19193–19207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drago, R.S.; Dias, S.C.; Torrealba, M.; de Lima, L. Calorimetric and Spectroscopic Investigation of the Acidity of HZSM-5. J. Am. Chem. Soc. 1997, 119, 4444–4452. [Google Scholar] [CrossRef]
- Freitas, E.F.; Araújo, Á.A.L.; Paiva, M.F.; Dias, S.C.L.; Dias, J.A. Comparative acidity of BEA and Y zeolite composites with 12-tungstophosphoric and 12-tungstosilicic acids. Mol. Catal. 2018, 458, 152–160. [Google Scholar] [CrossRef]
- Loureiro Dias, S.C.; de Macedo, J.L.; Dias, J.A. Acidity measurements of zeolite Y by adsorption of several probes. Phys. Chem. Chem. Phys. 2003, 5, 5574–5579. [Google Scholar] [CrossRef]
- De Mattos, F.C.G.; de Carvalho, E.N.C.B.; de Freitas, E.F.; Paiva, M.F.; Ghesti, G.F.; de Macedo, J.L.; Dias, S.C.L.; Dias, J.A. Acidity and Characterization of 12-Tungstophosphoric Acid Supported on Silica-Alumina. J. Braz. Chem. Soc. 2016, 28, 336–347. [Google Scholar] [CrossRef]
- Caliman, E.; Dias, J.A.; Dias, S.C.L.; Garcia, F.A.C.; de Macedo, J.L.; Almeida, L.S. Preparation and characterization of H3PW12O40 supported on niobia. Microporous Mesoporous Mater. 2010, 132, 103–111. [Google Scholar] [CrossRef]
- Weingarten, R.; Cho, J.; Xing, R.; Conner, W.C.; Huber, G.W. Kinetics and Reaction Engineering of Levulinic Acid Production from Aqueous Glucose Solutions. ChemSusChem 2012, 5, 1280–1290. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Xie, Q.; Wang, J.; Hou, B.; Jia, L.; Cui, J.; Li, D. Conversion of fructose into furfural or 5-hydroxymethylfurfural over HY zeolites selectively in γ-butyrolactone. Appl. Catal. A Gen. 2019, 572, 51–60. [Google Scholar] [CrossRef]
- Singh, M.; Kamble, R.; Viswanadham, N. Effect of Crystal Size on Physico-Chemical Properties of ZSM-5. Catal. Lett. 2008, 120, 288–293. [Google Scholar] [CrossRef]
- Stanciakova, K.; Weckhuysen, B.M. Water–active site interactions in zeolites and their relevance in catalysis. Trends Chem. 2021, 3, 456–468. [Google Scholar] [CrossRef]
- Mellmer, M.A.; Sanpitakseree, C.; Demir, B.; Ma, K.; Elliott, W.A.; Bai, P.; Johnson, R.L.; Walker, T.W.; Shanks, B.H.; Rioux, R.M.; et al. Effects of chloride ions in acid-catalyzed biomass dehydration reactions in polar aprotic solvents. Nat. Commun. 2019, 10, 1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, I.; Ziolek, M. Niobium Compounds: Preparation, Characterization, and Application in Heterogeneous Catalysis. Chem. Rev. 1999, 99, 3603–3624. [Google Scholar] [CrossRef] [PubMed]
- Maurer, S. Structural and acidic characterization of niobia aerogels. J. Catal. 1992, 135, 125–134. [Google Scholar] [CrossRef]
- Wachs, I.E.; Jehng, J.-M.; Deo, G.; Hu, H.; Arora, N. Redox properties of niobium oxide catalysts. Catal. Today 1996, 28, 199–205. [Google Scholar] [CrossRef]
- Jehng, J.M.; Wachs, I.E. Molecular structures of supported niobium oxide catalysts under in situ conditions. J. Phys. Chem. 1991, 95, 7373–7379. [Google Scholar] [CrossRef]
- Batamack, P.; Vincent, R.; Fraissard, J. Niobium oxide acidity studied by proton broad-line NMR at 4 K and MAS NMR at room temperature. Catal. Lett. 1996, 36, 81–86. [Google Scholar] [CrossRef]
- Tanabe, K. Niobic acid as an unusual acidic solid material. Mater. Chem. Phys. 1987, 17, 217–225. [Google Scholar] [CrossRef]
- Tanabe, K. Catalytic application of niobium compounds. Catal. Today 2003, 78, 65–77. [Google Scholar] [CrossRef]
- Martins, J.B.L.; Fialho, T.A.S. Interaction of pyridine on Nb2O5. J. Mol. Struct. 2005, 732, 1–5. [Google Scholar] [CrossRef]
- García-Sancho, C.; Rubio-Caballero, J.M.; Mérida-Robles, J.M.; Moreno-Tost, R.; Santamaría-González, J.; Maireles-Torres, P. Mesoporous Nb2O5 as solid acid catalyst for dehydration of d-xylose into furfural. Catal. Today 2014, 234, 119–124. [Google Scholar] [CrossRef]
- do Prado, N.T.; Souza, T.E.; Machado, A.R.T.; Souza, P.P.; Monteiro, R.S.; Oliveira, L.C.A. Enhanced catalytic activity for fructose conversion on nanostructured niobium oxide after hydrothermal treatment: Effect of morphology and porous structure. J. Mol. Catal. A Chem. 2016, 422, 23–34. [Google Scholar] [CrossRef]
- Wang, F.; Wu, H.-Z.; Liu, C.-L.; Yang, R.-Z.; Dong, W.-S. Catalytic dehydration of fructose to 5-hydroxymethylfurfural over Nb2O5 catalyst in organic solvent. Carbohydr. Res. 2013, 368, 78–83. [Google Scholar] [CrossRef]
- Qiu, G.; Wang, X.; Huang, C.; Li, Y.; Chen, B. Niobium phosphotungstates: Excellent solid acid catalysts for the dehydration of fructose to 5-hydroxymethylfurfural under mild conditions. RSC Adv. 2018, 8, 32423–32433. [Google Scholar] [CrossRef] [Green Version]
- Kourieh, R.; Rakic, V.; Bennici, S.; Auroux, A. Relation between surface acidity and reactivity in fructose conversion into 5-HMF using tungstated zirconia catalysts. Catal. Commun. 2013, 30, 5–13. [Google Scholar] [CrossRef]
- Rac, V.; Rakić, V.; Stošić, D.; Otman, O.; Auroux, A. Hierarchical ZSM-5, Beta and USY zeolites: Acidity assessment by gas and aqueous phase calorimetry and catalytic activity in fructose dehydration reaction. Microporous Mesoporous Mater. 2014, 194, 126–134. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Zhu, L.; Meng, X.; Xiao, F.-S. Efficient conversion of fructose to 5-hydroxymethylfurfural over sulfated porous carbon catalyst. J. Energy Chem. 2013, 22, 241–244. [Google Scholar] [CrossRef]
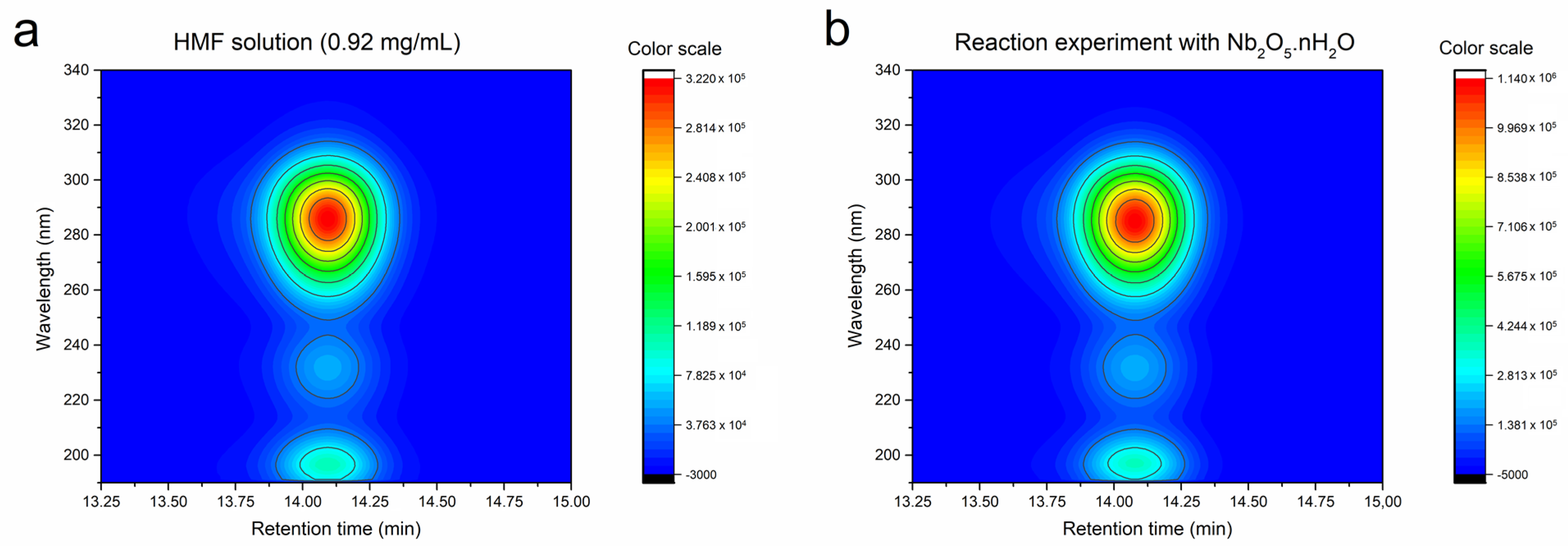
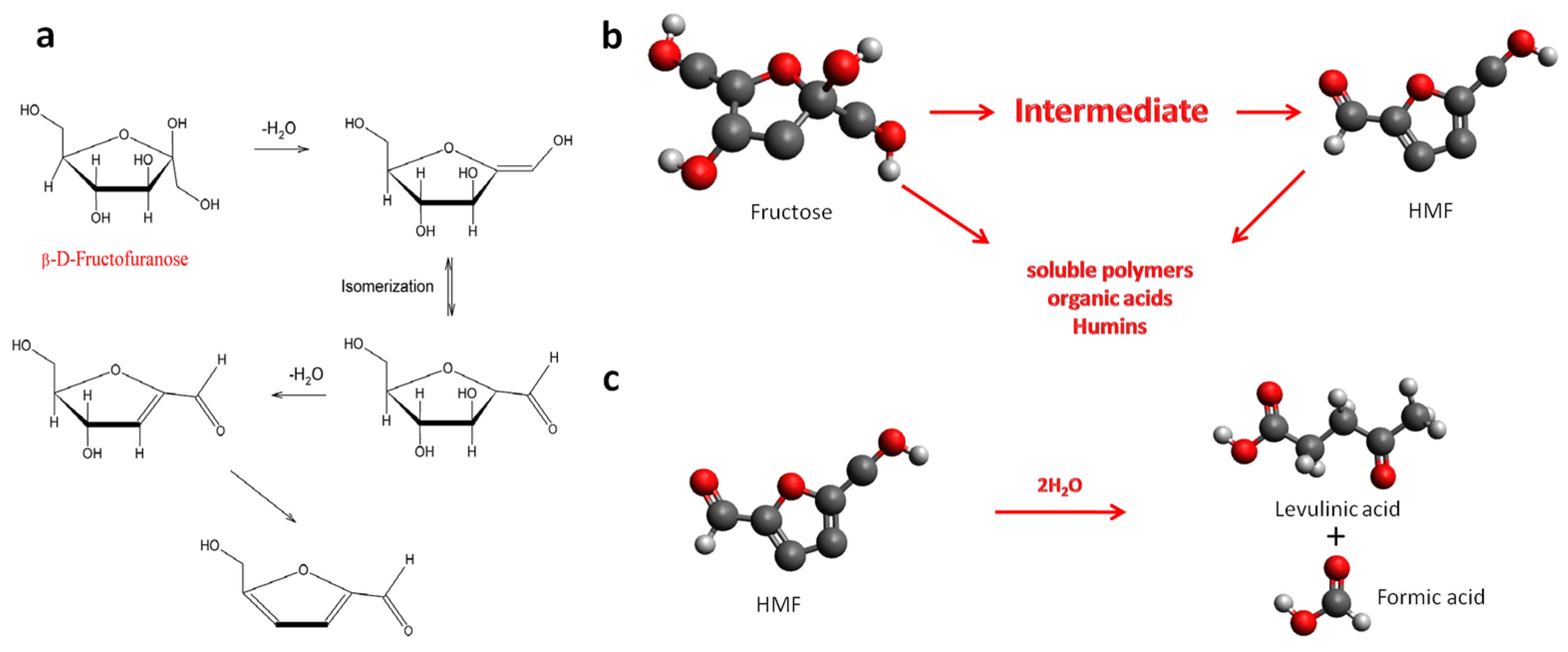


| Catalyst | Conversion (%) b | Selectivity (%) | Yield (%) | CB (%) c |
|---|---|---|---|---|
| No catalyst | 24.9 | 3.7 | 0.9 | 76.0 |
| HBEA | 17.2 | 6.6 | 1.1 | 84.0 |
| HY | 12.0 | 8.8 | 1.1 | 89.1 |
| HZSM-5 | 13.7 | 17.2 | 2.4 | 88.6 |
| SiO2-Al2O3 | 15.4 | 11.1 | 1.7 | 86.3 |
| Nb2O5 (amorphous) | 47.5 | 28.9 | 13.7 | 66.2 |
| 20%HPW/Nb2O5 | 53.9 | 13.4 | 7.2 | 53.3 |
| HPW | 19.3 | 34.6 | 6.7 | 87.4 |
| Catalyst | −ΔH1 (kJ/mol) | −ΔH2 (kJ/mol) | n1 (mmol/g) | n2 (mmol/g) | nT (mmol/g) | αPy (Py/nm2) |
|---|---|---|---|---|---|---|
| SiO2-Al2O3 | 81 | 44 | 0.20 | 0.65 | 0.85 | 1.05 |
| HY | 143 | 74 | 0.11 | 0.22 | 0.33 | 0.24 |
| HBEA | 150 | 63 | 0.15 | 0.38 | 0.53 | 0.42 |
| HZSM-5 | 170 | 36 | 0.05 | 0.53 | 0.58 | 0.80 |
| Nb2O5 | 88 | 50 | 0.06 | 0.15 | 0.21 | 1.00 |
| 20%HPW/Nb2O5 | 85 | 45 | 0.09 | 0.19 | 0.28 | 2.48 |
| Catalyst | SBET a (m2 g−1) | Vp b (cm3 g−1) | Vμp c (cm3 g−1) | DXRD d (nm) |
|---|---|---|---|---|
| SiO2-Al2O3 | 489 | 0.69 | - | n.a. |
| HY | 813 | 0.34 | 0.32 | 69 |
| HBEA | 754 | 0.79 | 0.23 | 22 |
| HZSM-5 | 438 | 0.26 | 0.11 | 118 |
| Nb2O5 | 122 | 0.13 | 0.02 | n.a. |
| 20%HPW/Nb2O5 | 52 | 0.06 | 0.01 | n.a. |
| Catalyst | TON (Fructose) | TON (HMF) |
|---|---|---|
| HBEA | 18,013 | 1152 |
| HY | 20,184 | 1850 |
| SiO2-Al2O3 | 10,056 | 1110 |
| HZSM-5 | 13,111 | 2297 |
| Nb2O5 (amorphous) | 125,550 | 36,211 |
| 20%HPW/Nb2O5 | 110,869 | 14,884 |
| HPW | 10,301 | 3576 |
| Catalyst | HMF Yield (%) | Temperature (°C) | Time (h) | Ratio a (%) | Reference |
|---|---|---|---|---|---|
| Nb2O5 | 13.7 | 120 | 2 | 10 | This work |
| Nb2O5 | 7.3 | 120 | 2 | 10 | [66] |
| NbO(OH)3 | 10.0 | 150 | 2 | 5 | [16] |
| NbPW (0.6 Nb/P) | 7.8 | 80 | 3 | 33 | [67] |
| Cs2HPW12O40 | 4.0 | 150 | 2 | 5 | [16] |
| ZrO2 | 8.0 | 130 | 4 | 13.3 | [68] |
| 16.8% WO3/ZrO2 | 12.0 | 130 | 4 | 13.3 | [68] |
| 12% WO3/ZrO2 | 7.0 | 150 | 2 | 5 | [16] |
| ZSM-5 (hierarchical) | 9.8 | 130 | 4 | 13.3 | [69] |
| BEA (hierarchical) | 3.2 | 130 | 4 | 13.3 | [69] |
| USY (hierarchical) | 8.3 | 130 | 4 | 13.3 | [69] |
| Porous Carbon-SO3H | 2.5 | 110 | 4 | 10 | [70] |
| TiO2-SO3H | 4.0 | 140 | 1 | 11.1 | [26] |
| HCl or H2SO4 b | 40 | 120 | - | b | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, J.P.V.; Campos, P.T.A.; Paiva, M.F.; Linares, J.J.; Dias, S.C.L.; Dias, J.A. Dehydration of Fructose to 5-Hydroxymethylfurfural: Effects of Acidity and Porosity of Different Catalysts in the Conversion, Selectivity, and Yield. Chemistry 2021, 3, 1189-1202. https://doi.org/10.3390/chemistry3040087
Lima JPV, Campos PTA, Paiva MF, Linares JJ, Dias SCL, Dias JA. Dehydration of Fructose to 5-Hydroxymethylfurfural: Effects of Acidity and Porosity of Different Catalysts in the Conversion, Selectivity, and Yield. Chemistry. 2021; 3(4):1189-1202. https://doi.org/10.3390/chemistry3040087
Chicago/Turabian StyleLima, João Pedro Vieira, Pablo Teles Aragão Campos, Mateus Freitas Paiva, José J. Linares, Sílvia C. L. Dias, and José A. Dias. 2021. "Dehydration of Fructose to 5-Hydroxymethylfurfural: Effects of Acidity and Porosity of Different Catalysts in the Conversion, Selectivity, and Yield" Chemistry 3, no. 4: 1189-1202. https://doi.org/10.3390/chemistry3040087
APA StyleLima, J. P. V., Campos, P. T. A., Paiva, M. F., Linares, J. J., Dias, S. C. L., & Dias, J. A. (2021). Dehydration of Fructose to 5-Hydroxymethylfurfural: Effects of Acidity and Porosity of Different Catalysts in the Conversion, Selectivity, and Yield. Chemistry, 3(4), 1189-1202. https://doi.org/10.3390/chemistry3040087








